
- Gross motor development
- Fine motor development
- Speech and Language
- Social, emotional and behavioural
- Hearing and Vision
- Developmental Delay
- Autism Spectrum Disorder
- Child Protection
- HEADSSS Assessment
- Palliative Care
- Pierre Robin Sequence
- Down Syndrome
- Childhood Eczema
- Diabetic Ketoacidosis
- Hyperthyroidism
- Hypothyroidism
- Adrenal Cortical Insufficiency
- Anaphylaxis
- Approach to the seriously unwell child
- Basic Life Support
- Brief Resolved Unexplained Event (ALTE)
- Febrile Seizures
- Fluid Management
- Paediatric Shock
- Vital Signs and GCS
- Burns assessment
- Supracondylar fracture
- Clavicle fracture
- Cervical fracture
- Bite injuries
- Otitis Externa
- Mastoiditis
- Acute otitis media
- Otitis media with effusion
- Nasal trauma
- Peri-orbital cellulitis
- Foreign Bodies
- Epiglottitis
- Tonsillitis
- Peritonsillar Abscess
- Glandular Fever
- Laryngomalacia
- Gastro-Oesophageal Reflux Disease
- Coeliac Disease
- Cow’s Milk Protein Allergy
- Mesenteric Adenitis
- Gastroenteritis
- Crohns Disease
- Ulcerative Colitis
- Whooping Cough
- Bronchiolitis
- Bronchiectasis
- Cystic Fibrosis
- COVID-19 (coronavirus disease 2019)
- Cardiac Physiology in CHDs
- Foetal vs Adult circulation
- ECG interpretation
- Infective Endocarditis
- Acute Rheumatic Fever
- Patent Ductus Arteriosus
- Atrial Septal Defect
- Tetralogy of Fallot
- Transposition of the Great Arteries
- Ventricular Septal Defect
- Atrioventricular Septal Defects (AVSD)
- Tricuspid atresia
- Total Anomalous Pulmonary Venous Drainage
- Hypoplastic left heart syndrome
- Early onset neonatal sepsis
- Late-Onset Neonatal Sepsis
- Meconium Aspiration Syndrome
- Necrotising Enterocolitis
- Retinopathy of Prematurity
- The preterm infant
- Acute Lymphoblastic Leukaemia
- Acute Myeloid Leukaemia
- Sickle Cell Disease
- Haemophilia
- Ewing Sarcoma
- Nephroblastoma
- Neuroblastoma
- Osteosarcoma
- Primary Brain Tumours
- Oncological Emergencies
- Under construction
- Nephrotic Syndrome
- Kidney Stones
- Urinary Tract Infection
- Acute Appendicitis
- Gastroschisis
- Hirschsprung’s disease
- Inguinal Hernia
- Intussusception
- Omphalocele
- Pyloric stenosis
- Cryptorchidism
- Hypospadias
- Balanitis xerotica obliterans (BXO)
- Testicular torsion
- Epididymitis
- Paraphimosis
- Osteomyelitis
- Septic Arthritis
- Bone tumours
- Open fractures
- Principles of fracture management
- Hydrocephalus
- Intracranial infections
- Peri-operative care
- Cardiovascular Exam
- Respiratory exam
- Abdominal Exam
- Newborn Examination (NIPE)
Original Author(s): Dr Phil Jordan and Dr Umberto Piaggio Last updated: 16th February 2021 Revisions: 19
- 1 Introduction
- 2.1 Physiological jaundice
- 2.2 Pathological jaundice
- 3 Risk factors and history
- 4 Clinical Presentation
- 5.1 Bilirubin
- 5.2 Further investigations
- 5.3 As needed
- 6.1 Phototherapy
- 6.2 Fluid intake
- 6.3 Exchange Transfusion
- 6.4 IV Immunglobulin
- 7 Complications
- 8 Prognosis
- 9 References

Introduction
Jaundice is t he yellow colouring of skin and sclera caused by the accumulation of bilirubin in the skin and mucous membranes.
Neonatal jaundice occurs in 60% of term infants and 80% of preterm infants [1] and is caused by hyperbilirubinaemia that is unconjugated (divided into physiological or pathological) or conjugated (always pathological). High levels of unconjugated bilirubin have acute harmful effects as well as long term damage if left untreated, such as kernicterus .
10% of breast fed babies are jaundiced at 1 month.
Types of Jaundice
Physiological jaundice.
Jaundice in a healthy baby, born at term, is normal and may result from:
- Increased red blood cell breakdown: in utero the fetus has a high concentration of Hb (to maximise oxygen exchange and delivery to the fetus) that breaks down releasing bilirubin as high Hb is no longer needed
- Immature liver not able to process high bilirubin concentrations
Starts at day 2-3, peaks day 5 and usually resolved by day 10. The baby remains well and does not require any intervention beyond routine neonatal care.
Physiological jaundice can progress to pathological jaundice if the baby is premature or there is increased red cell breakdown e.g. Extensive bruising or cephalohaematoma following instrumental delivery.
Pathological jaundice
Jaundice which requires treatment or further investigation.
- Onset less than 24 hours
- ?previous siblings treated for jaundice/family history/maternal rhesus status
- Maternal blood group (type O most likely to produce enough IgG antibodies to cause haemolysis)
- Requires investigation and treatment
- Onset after 24 hours
- likely dehydrated ?breast fed baby establishing feeding
- increased haemolysis due to bruising/cephalohaematoma
- Unwell neonate: jaundice as a sign of congenital or post-natal infection
- Metabolic: Hypothyroid/pituitarism, galactosaemia
- Breast milk jaundice: well baby, resolves between 1.5-4 months
- GI: biliary atresia, choledhocal cyst
Risk factors and history
Risk factors for pathological hyperbilirubinaemia: to be asked in history
- Prematurity, low birth weight, small for dates
- Previous sibling required phototherapy
- Exclusively breast fed
- Jaundice <24 hours
- Infant of diabetic mother
Clinical Presentation
- Colour: All babies should be checked for jaundice with the naked eye in bright, natural light (if possible). Examine the sclera, gums and blanche the skin. Do not rely on your visual inspection to estimate bilirubin levels, only to determine the presence or absence of jaundice.
- Drowsy: difficult to rouse, not waking for feeds, very short feeds
- Neurologically: altered muscle tone, seizures-needs immediate attention
- Other: signs of infection , poor urine output, abdominal mass/organomegaly, stool remains black/not changing colour
Investigations
- Transcutaneous bilirubinometer (TCB) can be used in >35/40 gestation and >24 hours old for first measurement. TCB can be used for all subsequent measurements, providing the level remains <250 µmol/L and the child has not required treatment
- Serum bilirubin to be measured if <35/40 gestation, <24 hours old or TCB >250 µmol/L
- Infants that are not jaundice to the naked eye do not need routine bilirubin checking.
- Total and Conjugated Bilirubin is important if suspected; liver or biliary disorder, metabolic disorder, congenital infection or prolonged jaundice. Do not subtract conjugated from total to make management decisions for hyperbilirubinaemia.
Further investigations
- Serum bilirubin for all subsequent levels
- Blood group (Mother and Baby) and DCT
- FBC for haemoglobin and haematocrit
- U&Es if excessive weight loss/dehydrated
- Infection screen if unwell or <24 hours including Microbiological cultures if infection suspected: blood, urine, CSF. Consider TORCH screen.
- Glucose-6-phosphate dehydrogenase especially if Mediterranean or African origin
- LFTs if suspected hepatobiliary disorder
Phototherapy
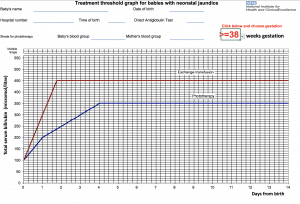
Figure 1 – NICE treatment threshold graph [3]
- Above: If level is on or above the phototherapy line for their gestation and age (in days) phototherapy should be initiated and bilirubin monitored
- >50µmol/L below, clinically well with no risk factors for neonatal jaundice do not routinely repeat level
- <50µmol/L below, clinically well repeat level within 18 hours (risk factors present) to 24 hours (no risk factors present)
- Repeat bilirubin 4-6 hours post initiation to ensure not still rising, 6-12 hourly once level is stable or reducing.
- NB. Maximum skin coverage, eye protection for babies, breaks for breastfeeding/nappy changes/cuddles to be coordinated to maximise phototherapy
- Stop phototherapy once level >50µmol/L below treatment line on the threshold graphs
- Check for rebound of hyperbilirubinaemia 12-18 hours after stopping phototherapy
Fluid intake
Do not give additional fluids with phototherapy unless indicated and if possible expressed maternal milk is preferred. If phototherapy intensified or feeding poorly consider NGT feeding or IV fluids.
Give consideration to underlying cause i.e. infection, biliary obstruction
Exchange Transfusion
This is the simultaneous exchange of the baby’s blood (hyperbilirubinaemic) with donated blood or plasma (normal levels of bilirubin) to prevent further bilirubin increase and decrease circulating levels of bilirubin.
Performed via umbilical artery or vein and is indicated when there are clinical features and signs of acute bilirubin encephalopathy or the level/rate of rise (>8.5µmol/L/hour) of bilirubin indicates necessity based on threshold graphs. This will require admission to an intensive care bed.
IV Immunglobulin
IVIG can be used as adjunct to intensified phototherapy in rhesus haemolytic disease or ABO haemolytic disease.
Complications
Kernicterus , billirubin-induced brain dysfunction, can result from neonatal jaundice. Bilirubin is neurotoxic and at high levels can accumulate in the CNS gray matter causing irreversible neurological damage . Depending on level of exposure, effects can range from clinically undetectable damage to severe brain damage.
Depends on underlying cause but if correctly and promptly treated prognosis is excellent.
Always refer to local trust guidelines.
1st Author: Dr Phil Jordan
Senior Reviewer: Dr Umberto Piaggio
Found an error? Is our article missing some key information? Make the changes yourself here!
Once you've finished editing, click 'Submit for Review', and your changes will be reviewed by our team before publishing on the site.
We use cookies to improve your experience on our site and to show you relevant advertising. To find out more, read our privacy policy .
Privacy Overview
Neonatal Hyperbilirubinemia
(jaundice in neonates).
- Pathophysiology |
- Evaluation |
- Treatment |
- Key Points |
- More Information |
Jaundice is a yellow discoloration of the skin and eyes caused by hyperbilirubinemia (elevated serum bilirubin concentration). The serum bilirubin level required to cause jaundice varies with skin tone and body region, but jaundice usually becomes visible on the sclera at a level of 2 to 3 mg/dL (34 to 51 micromol/L) and on the face at about 4 to 5 mg/dL (68 to 86 micromol/L). With increasing bilirubin levels, jaundice seems to advance in a head-to-foot direction, appearing at the umbilicus at about 15 mg/dL (257 micromol/L) and at the feet at about 20 mg/dL (342 micromol/L). Slightly more than half of all neonates become visibly jaundiced in the first week of life. Almost all hyperbilirubinemia in the immediate neonatal period is unconjugated, which is termed indirect bilirubin, based on older laboratory measurement methods; conjugated bilirubin is termed direct bilirubin. For further discussions of cholestasis and disorders of bilirubin excretion in the neonatal period see neonatal cholestasis .
Consequences of hyperbilirubinemia
Hyperbilirubinemia may be harmless or harmful depending on its cause and the degree of elevation. Some causes of jaundice are intrinsically dangerous whatever the bilirubin level. But hyperbilirubinemia of any etiology is a concern once the level is high enough. The threshold for concern varies by
Degree of prematurity
Health status
Among healthy term infants, the threshold for concern typically is considered to be a level > 18 mg/dL ( > 308 micromol/L); see figure Risk of hyperbilirubinemia in neonates ( 1 ). However, infants who are premature , small for gestational age , and/or ill (eg, with sepsis , hypothermia , or hypoxia) are at greater risk and intervention may be done at lower levels. In such infants, although risk increases with increasing hyperbilirubinemia, there is no level of hyperbilirubinemia that is considered safe; treatment is given based on age and clinical factors. There are now suggested operational thresholds to initiate phototherapy based on gestational age.
Neurotoxicity is the major consequence of neonatal hyperbilirubinemia. An acute encephalopathy can be followed by a variety of neurologic impairments, including cerebral palsy and sensorimotor deficits; cognition is usually spared. Kernicterus
When serum bilirubin concentration is markedly elevated
Risk of hyperbilirubinemia in neonates
General reference.
1. Maisels MJ, Bhutani VK, Bogen D, et al : Hyperbilirubinemia in the newborn infant ≥ 35 weeks gestation: An update with clarifications. Pediatrics 124(4):1193–1198, 2009. doi: 10.1542/peds.2009-0329
Pathophysiology of Neonatal Hyperbilirubinemia
Bilirubin metabolism ).
Mechanisms of hyperbilirubinemia
Hyperbilirubinemia can be caused by one or more of the following processes:
Increased production
Decreased hepatic uptake
Decreased conjugation
Impaired excretion
Impaired bile flow ( cholestasis )
Increased enterohepatic circulation
Etiology of Neonatal Hyperbilirubinemia
Classification.
There are several ways to classify and discuss causes of hyperbilirubinemia. Because transient jaundice is common among healthy neonates (unlike adults, in whom jaundice always signifies a disorder), hyperbilirubinemia can be classified as physiologic or pathologic. It can be classified by whether the hyperbilirubinemia is unconjugated, conjugated, or both. It also can be classified by mechanism ( see Table: Causes of Neonatal Hyperbilirubinemia ).
Most cases involve unconjugated hyperbilirubinemia. Some of the most common causes of neonatal jaundice include
Physiologic hyperbilirubinemia
Breastfeeding jaundice
Breast milk jaundice
Pathologic hyperbilirubinemia due to hemolytic disease
Liver dysfunction (eg, caused by parenteral alimentation causing cholestasis, neonatal sepsis, neonatal hepatitis) may cause a conjugated or mixed hyperbilirubinemia.
Physiologic hyperbilirubinemia occurs in almost all neonates. Shorter neonatal red blood cell life span increases bilirubin production, deficient conjugation due to the deficiency of uridine diphosphate-glucuronosyltransferase (UGT) decreases clearance, and low bacterial levels in the intestine combined with increased hydrolysis of conjugated bilirubin increase enterohepatic circulation. Bilirubin levels can rise up to 18 mg/dL (308 micromol/L) by 3 to 4 days of life (7 days in Asian infants) and fall thereafter.
Breastfeeding jaundice develops in one sixth of breastfed infants during the first week of life. Breastfeeding increases enterohepatic circulation of bilirubin in some infants who have decreased milk intake and who also have dehydration or low caloric intake. The increased enterohepatic circulation also may result from reduced intestinal bacteria that convert bilirubin to nonresorbed metabolites.
Breast milk jaundice is different from breastfeeding jaundice. It develops after the first 5 to 7 days of life and peaks at about 2 weeks. It is thought to be caused by an increased concentration of beta-glucuronidase in breast milk, causing an increase in the deconjugation and reabsorption of bilirubin.
Pathologic hyperbilirubinemia in term infants is diagnosed if
Jaundice appears in the first 24 hours, after the first week of life, or lasts > 2 weeks
Total serum bilirubin rises by > 5 mg/dL/day (> 86 micromol/L/day)
Total serum bilirubin is > 18 mg/dL (> 308 micromol/L/day)
Infant shows symptoms or signs of a serious illness
Some of the most common pathologic causes are
Immune and nonimmune hemolytic anemia
Hematoma resorption
Hypothyroidism
Evaluation of Neonatal Hyperbilirubinemia
History of present illness should note age of onset and duration of jaundice. Important associated symptoms include lethargy and poor feeding (suggesting possible kernicterus), which may progress to stupor, hypotonia, or seizures and eventually to hypertonia. Patterns of feeding can be suggestive of possible breastfeeding failure or underfeeding. Therefore, history should include what the infant is being fed, how much and how frequently, urine and stool production (possible breastfeeding failure or underfeeding), how well the infant is latching on to the breast or taking the nipple of the bottle, whether the mother feels that her milk has come in, and whether the infant is swallowing during feedings and seems satiated after feedings.
Review of systems should seek symptoms of causes, including respiratory distress, fever, and irritability or lethargy (sepsis); hypotonia and poor feeding (hypothyroidism, metabolic disorder); and repeated episodes of vomiting (intestinal obstruction).
Past medical history should focus on maternal infections (toxoplasmosis, other pathogens, rubella, cytomegalovirus, and herpes simplex [TORCH] infections), disorders that can cause early hyperbilirubinemia (maternal diabetes), maternal Rh factor and blood group (maternofetal blood group incompatibility), and a history of a prolonged or difficult birth (hematoma or forceps trauma).
Family history should note known inherited disorders that can cause jaundice, including glucose-6-phosphate dehydrogenase (G6PD) deficiency, or other red cell enzyme deficiencies, thalassemias, and spherocytosis, and also any history of siblings who have had jaundice.
Physical examination
Overall clinical appearance and vital signs are reviewed.
The skin is inspected for extent of jaundice. Gentle pressure on the skin can help reveal the presence of jaundice.
The physical examination should focus on signs of causative disorders.
The general appearance is inspected for plethora (maternofetal transfusion), macrosomia (maternal diabetes), and lethargy or extreme irritability (sepsis or infection) and for any dysmorphic features such as macroglossia (hypothyroidism) and flat nasal bridge or bilateral epicanthal folds (Down syndrome).
For the head and neck examination, any bruising and swelling of the scalp consistent with a cephalohematoma are noted. Lungs are examined for crackles (rales), rhonchi, and decreased breath sounds (pneumonia). The abdomen is examined for distention, mass (hepatosplenomegaly), or pain (intestinal obstruction). Neurologic examination should focus on signs of hypotonia or weakness (metabolic disorder, hypothyroidism, sepsis).
The following findings are of particular concern:
Jaundice in the first day of life
Total serum bilirubin > 18 mg/dL (> 308 micromol/L)
Rate of rise of total serum bilirubin > 0.2 mg/dL/hour ( > 3.4 micromol/L/hour) or > 5 mg/dL/day (> 86 micromol/L/day)
Conjugated bilirubin concentration > 1 mg/dL ( > 17 micromol/L) if total serum bilirubin is < 5 mg/dL ( > 20% of total serum bilirubin (suggests neonatal cholestasis)
Jaundice after 2 weeks of age
Lethargy, irritability, respiratory distress
Interpretation of findings
Evaluation should focus on distinguishing physiologic from pathologic jaundice. History, physical examination, and timing can help, but typically total serum bilirubin and conjugated serum bilirubin levels are measured.
Jaundice that develops in the first 24 to 48 hours, or that persists > 2 weeks, is most likely pathologic. Jaundice that does not become evident until after 2 to 3 days is more consistent with physiologic, breastfeeding, or breast milk jaundice. An exception is undersecretion of bilirubin due to metabolic factors (eg, Crigler-Najjar syndrome, hypothyroidism, drugs), which may take 2 to 3 days to become evident. In such cases, bilirubin typically peaks in the first week, accumulates at a rate of < 5 mg/dL/day (
Diagnosis of hyperbilirubinemia is suspected by the infant’s color and is confirmed by measurement of serum bilirubin. Noninvasive techniques for measuring bilirubin in infants, including transcutaneous and digital photography–based techniques, are being used increasingly and correlate well with serum bilirubin measurements. Risk of hyperbilirubinemia is based on age-specific total serum bilirubin levels.
A bilirubin concentration > 10 mg/dL ( > 171 micromol/L) in preterm infants or > 18 mg/dL ( > 308 micromol/L) in term infants warrants additional testing, including hematocrit, blood smear, reticulocyte count, direct Coombs test, total serum bilirubin and direct serum bilirubin concentrations, and blood type and Rh group of the infant and mother.
Other tests, such as blood, urine, and cerebrospinal fluid cultures to detect sepsis and measurement of red blood cell enzyme levels to detect unusual causes of hemolysis, may be indicated by the history and physical examination. Such tests also may be indicated for any neonates with an initial bilirubin level > 25 mg/dL ( > 428 micromol/L).
Treatment of Neonatal Hyperbilirubinemia
Treatment of hyperbilirubinemia is directed at the underlying disorder. In addition, treatment for hyperbilirubinemia itself may be necessary.
Physiologic jaundice usually is not clinically significant and resolves within 1 week. Frequent formula feedings can reduce the incidence and severity of hyperbilirubinemia by increasing gastrointestinal motility and frequency of stools, thereby minimizing the enterohepatic circulation of bilirubin. The type of formula does not seem important in increasing bilirubin excretion.
Breastfeeding jaundice may be prevented or reduced by increasing the frequency of feedings. If the bilirubin level continues to increase > 18 mg/dL ( >
Definitive treatment of hyperbilirubinemia involves
Phototherapy
Exchange transfusion.
This treatment remains the standard of care, most commonly using fluorescent white light. (Blue light, wavelength 425 to 475 nm, is most effective for intensive phototherapy.) Phototherapy is the use of light to photoisomerize unconjugated bilirubin into forms that are more water-soluble and can be excreted rapidly by the liver and kidney without glucuronidation. It provides definitive treatment of neonatal hyperbilirubinemia and prevention of kernicterus. (See also the American Academy of Pediatrics' technical report on using phototherapy to prevent severe neonatal hyperbilirubinemia in neonates who are ≥ 35 weeks gestation.)
For neonates born at ≥ 35 weeks gestation, phototherapy is an option when unconjugated bilirubin is > 12 mg/dL ( > 205.2 micromol/L) and may be indicated when unconjugated bilirubin is > 15 mg/dL (257 micromol/L) at 25 to 48 hours, 18 mg/dL (308 micromol/L) at 49 to 72 hours, and 20 mg/dL (342 micromol/L) at > 72 hours ( see figure Risk of hyperbilirubinemia in neonates ). Phototherapy is not indicated for conjugated hyperbilirubinemia.
For neonates born at see Table: Suggested Thresholds* for Starting Phototherapy or Exchange Transfusion in Infants 35 Weeks Gestation ).
Suggested Thresholds* for Starting Phototherapy or Exchange Transfusion in Infants 35 Weeks Gestation
Because visible jaundice may disappear during phototherapy even though serum bilirubin remains elevated, skin color cannot be used to evaluate jaundice severity. Blood taken for bilirubin determinations should be shielded from bright light, because bilirubin in the collection tubes may rapidly photo-oxidize.
This treatment can rapidly remove bilirubin from circulation and is indicated for severe hyperbilirubinemia, which most often occurs with immune-mediated hemolysis. Small amounts of blood are withdrawn and replaced through an umbilical vein catheter, or other access as available, to remove partially hemolyzed and antibody-coated red blood cells (RBCs) as well as circulating immunoglobulins. The blood is replaced with uncoated donor RBCs that do not have the RBC membrane antigen that binds the circulating antibodies. That is, type O blood is used if the neonate is sensitized to AB antigens and Rh-negative blood is used if the neonate is sensitized to Rh antigen. Because adult donor RBCs have more ABO antigen sites than fetal cells, type-specific transfusion will intensify the hemolysis. Only unconjugated hyperbilirubinemia can cause kernicterus, so if conjugated bilirubin is elevated, the level of unconjugated rather than total bilirubin is used to determine the need for exchange transfusion.
For term infants, specific indications are serum bilirubin ≥ 20 mg/dL ( ≥ 342 micromol/L) at 24 to 48 hours or ≥ 25 mg/dL ( ≥ 428 micromol/L) at > 48 hours and failure of phototherapy to result in a 1- to 2-mg/dL (17- to 34-micromol/L) decrease within 4 to 6 hours of initiation or at the first clinical signs of kernicterus regardless of bilirubin levels. If the serum bilirubin level is > 25 mg/dL ( ≥ 428 micromol/L) when the neonate is initially examined, preparation for an exchange transfusion should be made in case intensive phototherapy fails to lower the bilirubin level.
Thresholds have been suggested for neonates born at Suggested Thresholds* for Starting Phototherapy or Exchange Transfusion in Infants 35 Weeks Gestation ). Previously, some clinicians used criteria based solely on patient weight, but these criteria have been replaced by the more specific guidelines described above.
Most often, 160 mL/kg (twice the infant’s total blood volume) of packed RBCs is exchanged over 2 to 4 hours; an alternative is to give 2 successive exchanges of 80 mL/kg each over 1 to 2 hours. To do an exchange, a volume of blood is withdrawn and then immediately replaced by transfused blood. The volume of each can vary depending on the infant's size, but volumes are typically near 20 mL for the average term infant. This procedure is repeated until the total desired volume is exchanged. For critically ill or premature infants, aliquots of 5 to 10 mL are used to avoid sudden major changes in blood volume. The goal is to reduce bilirubin by nearly 50%, with the knowledge that hyperbilirubinemia may rebound to about 60% of pretransfusion level within 1 to 2 hours. It is also customary to lower the target level by 1 to 2 mg/dL (17 to 34 micromol/L) in conditions that increase the risk of kernicterus (eg, fasting, sepsis, acidosis). Exchange transfusions may need to be repeated if bilirubin levels remain high. Finally, there are risks and complications with the procedure, and the success of phototherapy has reduced the frequency of exchange transfusion.
Neonatal jaundice is caused by increased bilirubin production, decreased bilirubin clearance, or increased enterohepatic circulation.
Some jaundice is normal in neonates.
Risk varies with postnatal age, total serum bilirubin value, prematurity, and health of the neonate.
Treatment depends on cause and degree of bilirubin elevation; the more preterm the infant, the lower the threshold level for treatment.
Definitive treatments include phototherapy and exchange transfusion.
More Information
The following is an English-language resource that may be useful. Please note that THE MANUAL is not responsible for the content of this resource.
American Academy of Pediatrics technical report: Using phototherapy to prevent severe neonatal hyperbilirubinemia in neonates who are ≥ 35 weeks gestation

- Cookie Preferences

Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.

SARAH K. MOERSCHEL, MD, LAUREN B. CIANCIARUSO, DO, AND LLOYD R. TRACY, MD
A more recent article on neonatal hyperbilirubinemia is available.
Am Fam Physician. 2008;77(9):1255-1262
Author disclosure: Nothing to disclose.
Kernicterus and neurologic sequelae caused by severe neonatal hyperbilirubinemia are preventable conditions. A structured and practical approach to the identification and care of infants with jaundice can facilitate prevention, thus decreasing rates of morbidity and mortality. Primary prevention includes ensuring adequate feeding, with breastfed infants having eight to 12 feedings per 24 hours. Secondary prevention is achieved by vigilant monitoring of neonatal jaundice, identifying infants at risk of severe hyperbilirubinemia, and ensuring timely outpatient follow-up within 24 to 72 hours of discharge. Total serum bilirubin or transcutaneous bilirubin levels should be routinely monitored in all newborns, and these measurements must be plotted on a nomogram according to the infant's age in hours. The resultant low-, intermediate-, or high-risk zones, in addition to the infant's risk factors, can guide timing of postdischarge follow-up. Another nomogram that consists of age in hours, risk factors, and total bilirubin levels can provide guidance on when to initiate phototherapy. If the infant requires phototherapy or if the bilirubin level is increasing rapidly, further work-up is indicated.
Although jaundice is present in most newborns and is usually benign, it is imperative to carefully monitor newborns to identify those at risk of developing bilirubin-induced neurologic dysfunction. Acute bilirubin encephalopathy is caused by the toxic effects of unconjugated bilirubin on the central nervous system. Symptoms include lethargy, high-pitched cry, and poor feeding in a jaundiced infant. If acute bilirubin encephalopathy is untreated, it may progress rapidly to advanced manifestations, such as opisthotonus and seizures. 1 Kernicterus is the chronic, permanent clinical sequelae of bilirubin toxicity; it is characterized by severe athetoid cerebral palsy, paralysis of upward gaze, hearing loss, and intellectual impairment, 2 and it is preventable. The approach to preventing this condition has changed over time.
Throughout the 1950s, exchange transfusion was the primary treatment for hyperbilirubinemia. 3 It was not until the late 1960s that phototherapy became widespread in the United States. 4 In the 1980s and 1990s, there was a resurgence of kernicterus in the United States and abroad, which has been attributed in part to early hospital discharge, the influence of managed care, and an increase in the number of breastfed infants, with a proportional increase in breastfeeding inadequacy in the first week of life. 5
The American Academy of Pediatrics (AAP) developed an evidence-based clinical practice guideline for use by all health care professionals who care for newborns in hospital and outpatient settings. 2 The primary goal of the guideline, as well as this article, is to increase awareness and educate health care professionals to reduce the incidence of severe hyperbilirubinemia and to prevent bilirubin encephalopathy.
Bilirubin Metabolism
Bilirubin is produced by the catabolism of hemoglobin. Compared with older children and adults, newborns have a high rate of hemoglobin catabolism and bilirubin production because of their elevated hematocrit and red blood cell volume per body weight, and their shorter life span of red blood cells (70 to 90 days). Although bilirubin production is elevated in newborns, conjugation and clearance of bilirubin can be slow. Immaturity of hepatic glucuronosyltransferase and inadequate milk intake can cause delayed clearance of bilirubin.
Within the reticuloendothelial system, heme is broken down into biliverdin and carbon monoxide. Biliverdin is reduced to bilirubin by biliverdin reductase. At this initial stage, bilirubin is lipid soluble and unconjugated (indirect-reacting). Unconjugated bilirubin binds to albumin. If the albumin-binding sites are saturated, or if unconjugated bilirubin is displaced from the binding sites by medications (e.g., sulfisoxazole [Gantrisin], streptomycin, vitamin K), free bilirubin can cross the blood-brain barrier. Free, unconjugated bilirubin is toxic to the central nervous system.
When unconjugated bilirubin reaches the liver, it is conjugated by glucuronosyltransferase to bilirubin diglucuronide (conjugated or direct-reacting), which is water soluble and easily excreted by the liver and biliary tract. In the intestine, some bilirubin may be converted back to its unconjugated form by a glucuronidase and reabsorbed by the intestine. Breast milk increases bilirubin reabsorption through this enterohepatic absorption. 6
Primary Prevention: Preventing Jaundice
Physicians should promote and support breastfeeding, advising eight to 12 feedings per day for the first several days of life. 7 , 8 Formula-fed, full-term infants should consume 150 kcal per kg per day, which is equivalent to approximately 1 to 2 oz every two to three hours in the first week of life. Routine supplementation with water or dextrose water is not recommended in breastfeeding infants because it will not prevent hyperbilirubinemia or decrease total serum bilirubin levels. 9
Secondary Prevention: Assessing At-Risk Infants
The key to secondary prevention is vigilance on the part of the health care team. All hospitalized newborns should be routinely monitored by nursing staff and physicians for the development of jaundice every eight to 12 hours, including at the time that vital signs are taken. 2 Measurement and interpretation of the predischarge bilirubin level can help determine the timing of outpatient follow-up evaluations. Although jaundice in newborns can usually be detected by blanching the skin with digital pressure and is usually initially visible in the face with caudal progression, visual estimation of bilirubin levels is largely inaccurate and unreliable. 10 Transcutaneous bilirubin (TcB) measurement, which is noninvasive, is equivalent to total serum bilirubin (TSB) measurement. 11 – 16 Table 1 2 addresses the primary and secondary prevention of neonatal hyperbilirubinemia, and Figure 1 2 provides an algorithm for the management of jaundice in the newborn.
INTERPRETING BILIRUBIN LEVELS
Bilirubin levels should be interpreted based on the infant's age in hours. 2 Figure 2 shows a nomogram for plotting TSB and TcB levels according to the infant's age in hours, with resultant low-, intermediate-, and high-risk zones. 2 If the TSB or TcB level falls in the low-risk zone, the physician can conclude that the infant is likely at a very low risk for developing severe hyperbilirubinemia. If the level falls in the high-risk zone, the risk for severe hyperbilirubinemia is high, thus even more vigilance and closer follow-up of the infant is warranted. 2 These zones can help dictate the need for and timing of subsequent bilirubin measurements and timing of post-discharge follow-up.
Determining intervention based on age in days is inaccurate and can lead to serious oversights. For example, Baby A has a TSB level of 10 mg per dL (171 μmol per L) at 25 hours of age (high-risk). Baby B has the same TSB level at 47 hours of age (low-intermediate risk). Although both infants are one day old, Baby A is at higher risk of severe hyperbilirubinemia than Baby B and should have a repeat TSB measurement in six to 12 hours. Baby B can be reevaluated safely in 48 hours.
DISCHARGE RISK ASSESSMENT AND FOLLOW-UP
In addition to using the time-based nomogram, the physician must be aware of the risk factors most often associated with the development of severe hyperbilirubinemia, as listed in Table 2 . 2 An infant who was delivered at less than 38 weeks' gestation and who is breastfeeding exclusively is at higher risk of developing severe hyperbilirubinemia than a formula-fed infant who was delivered at 40 weeks' gestation. 2 The combination of risk factor awareness, a screening predischarge TcB or TSB level plotted on a nomogram, and clinical judgment can guide the physician in determining the timing of discharge and follow-up evaluations.
Newborns should be examined within 24 to 72 hours of hospital discharge to assess for jaundice and general well-being. 2 An infant should be seen by the age of 72 hours if discharged before 24 hours; by the age of 96 hours if discharged between 24 and 47.9 hours; and by the age of 120 hours if discharged between 48 and 72 hours. 2 Earlier follow-up (within 24 to 48 hours) should be instituted for infants with more risk factors for severe hyperbilirubinemia, shorter hospital stays, or predischarge bilirubin levels in the high-intermediate or high-risk zones. For example, Baby C is a breastfed infant delivered at 36 weeks' gestation who has a predischarge bilirubin level in the low-intermediate range. Therefore, he has two major risk factors for severe hyperbilirubinemia and should be seen in the primary care office within 24 hours of hospital discharge. Baby D, who has the same predischarge bilirubin level as Baby C, is a formula-fed infant delivered at 39 weeks' gestation. Therefore, Baby D has no risk factors and can be seen 48 hours after discharge.
Outpatient evaluation should include follow-up on weight, intake, voiding, and stooling. A TSB or TcB level should be obtained in the outpatient setting if jaundice is increasing or if the clinical assessment is unclear as to the severity of jaundice. Because visual estimation of jaundice is often inaccurate, liberal testing of TcB and TSB levels is a safer approach. 2
HOSPITAL PROTOCOLS
All newborn nurseries need to establish a protocol for identifying and evaluating hyperbilirubinemia. Some institutions with such a protocol report a reduced proportion of neonates with hyperbilirubinemia, its complications, and subsequent hospitalizations. 17 , 18 One study showed that the combined use of the AAP nomogram and a predischarge risk-factor assessment has a stronger predictive value than the nomogram alone, especially in infants with elevated TSB levels. 19
Protocols should specify circumstances in which nurses can obtain bilirubin measurements. Some hospitals perform universal screening with TcB or TSB measurement on every newborn. If universal screening is not performed, bilirubin measurement should be performed on every newborn with jaundice in the first 24 hours after birth, when jaundice appears excessive for age, and when the degree of jaundice is unclear. 2
Routine discharge counseling should include an explanation of monitoring for jaundice; this should ideally be provided in verbal and written formats. Printer-friendly patient information handouts about infant jaundice are available in English and Spanish at https://familydoctor.org/familydoctor/en/diseases-conditions/jaundice.html .
Evaluation of Elevated Bilirubin Levels
The differential diagnosis of neonatal hyperbilirubinemia is broad. Table 3 6 lists the most common causes; however, the point at which intervention is recommended is based on percentiles for the infant's age in hours, regardless of the cause. Laboratory evaluation may vary based on certain indications in the infant ( Table 4 2 ).
A healthy, full-term (i.e., completion of 36 weeks' gestation) infant with a mildly elevated bilirubin level does not require any laboratory studies beyond TSB measurement. An infant with physical examination findings that explain the level of jaundice (e.g., large hematoma) does not require further work-up, although the infant may require ongoing monitoring. Other laboratory studies should be considered if the infant requires phototherapy ( Figure 3 2 ) or if the TSB level is increasing rapidly. ABO incompatibility and glucose-6-phosphate dehydrogenase (G6PD) deficiency are the most common causes of hemolytic anemia. If these conditions are present, phototherapy and exchange transfusion may be considered at lower TSB levels because these conditions can cause predictably severe hyperbilirubinemia. A complete blood count should be done to evaluate for anemia resulting from hemolysis.
Blood type and Coombs' testing should be performed in all infants who are receiving phototherapy or whose bilirubin level is increasing rapidly. 2 In infants with isoimmune hemolysis (ABO incompatibility), the Coombs' test will be positive because the infant's red blood cells are coated with maternal antibodies. These cells will be hemolyzed, putting the infant at risk for severe hyperbilirubinemia. Although most tests have poor sensitivity and specificity for hemolysis, it is possible to measure the rate of heme catabolism and bilirubin production by measuring the end-tidal carbon monoxide level (corrected for ambient levels) because carbon monoxide is a byproduct of heme catabolism. Elevated end-tidal carbon monoxide levels may prompt the physician to suspect ongoing hemolysis and to be prepared for rapidly increasing bilirubin levels. 2
Screening for G6PD deficiency should be considered in infants with severe jaundice who are from high-risk populations, such as persons of African, Mediterranean, Middle Eastern, or Southeast Asian descent. 2 G6PD deficiency occurs in 11 to 13 percent of African Americans, and G6PD was the cause of kernicterus in 26 out of 125 patients (21 percent), according to the kernicterus registry. 20 G6PD testing is part of the newborn screening programs in Pennsylvania and the District of Columbia. 21
A direct bilirubin level should be obtained for ill-appearing infants with jaundice or those with jaundice after three weeks of age. Levels of more than 20 percent of the TSB level are considered elevated. Because an elevated direct bilirubin level can be an early sign of a urinary tract infection, a culture should be obtained for urinalysis. 2 , 22 A sepsis evaluation should be considered in ill-appearing infants. Elevated direct bilirubin levels can also indicate cholestasis, especially in infants who have jaundice after three weeks of age. Screening for hypothyroidism and galactosemia and evaluation for cholestasis is indicated if the infant has prolonged jaundice with no known cause. 2
Treatment of Hyperbilirubinemia
For infants with mild jaundice (i.e., when the bilirubin level is not approaching the threshold for phototherapy), increasing the frequency of feedings is indicated. Breast-fed infants should continue breastfeeding, whether or not they require phototherapy. Interruption of breastfeeding and substitution of formula can reduce bilirubin levels, but optimal breastfeeding (eight to 12 times per day) increases removal of bilirubin through the gastrointestinal tract and ensures continued breastfeeding. Infants with inadequate oral intake, excessive weight loss (more than 12 percent of birth weight), or dehydration should receive supplemental breast milk or formula; supplementation with water or dextrose water is not recommended. Intravenous fluids should be given if feeding is unsuccessful and the infant is dehydrated. 2
The physician should consider TSB measurements, the infant's age in hours, and the presence of risk factors to determine when to initiate phototherapy ( Figure 3 2 ) and exchange transfusion ( Figure 4 2 ) . Home phototherapy can be considered for infants at risk of reaching the threshold for intensive phototherapy. 2 Intensive phototherapy should be initiated when the TSB level exceeds the threshold in the AAP phototherapy nomogram, based on age and risk factors. Every hospital that cares for newborns should be able to provide intensive phototherapy (i.e., irradiance in the blue-green spectrum [430 to 490 nm] of at least 30 μW per cm 2 per nm, delivered to as much of the infant's surface area as possible). Frequency of TSB monitoring during intensive phototherapy is determined by previous measurements ( Table 5 2 ) .
Exchange transfusion is recommended when the TSB level exceeds the threshold in the AAP exchange transfusion nomogram ( Figure 4 2 ) , based on age and risk factors, or if the TSB level is greater than 25 mg per dL (428 μmol per L). The infant should be transferred to a neonatal intensive care unit for immediate intensive phototherapy and consideration of exchange transfusion. Exchange transfusion should be performed immediately in any infant with jaundice and signs of acute bilirubin encephalopathy. Initial symptoms include poor feeding, hypotonia, and lethargy. Worsening bilirubin encephalopathy is characterized by irritability and hypertonia, at times alternating with lethargy. Symptoms of severe bilirubin encephalopathy include hypertonia, arching, retrocollis, opisthotonos, fever, and high-pitched cry. Only trained personnel in a neonatal intensive care unit should perform exchange transfusion. Administration of intravenous gamma globulin is an alternative in infants with isoimmune hemolytic disease (dose: 0.5 to 1 g per kg over two hours, may repeat after 12 hours, if necessary). 2
Bhutani VK, Johnson LH, Keren R. Treating acute bilirubin encephalopathy—before it's too late. Contemp Pediatr. 2005;22(5):57-74.
American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation [published correction appears in Pediatrics . 2004;114(4):1138]. Pediatrics. 2004;114(1):297-316.
Watchko JF. Vigintiphobia revisited. Pediatrics. 2005;115(6):1747-1753.
Bryla DA. Randomized, controlled trial of phototherapy for neonatal hyperbilirubinemia. Development, design, and sample composition. Pediatrics. 1985;75(2 pt 2):387-392.
Joint Commission on Accreditation of Healthcare Organizations. Kernicterus threatens healthy newborns. http://www.jointcommission.org/SentinelEvents/SentinelEventAlert/sea_18.htm. Accessed November 8, 2007.
Gowen CW Jr. Anemia and hyperbilirubinemia. In: Kliegman R. Nelson Essentials of Pediatrics . 5th ed. Philadelphia, Pa.: Elsevier Saunders; 2006:313–322.
Yamauchi Y, Yamanouchi I. Breast-feeding frequency during the first 24 hours after birth in full-term neonates. Pediatrics. 1990;86(2):171-175.
de Carvalho M, Klaus MH, Merkatz RB. Frequency of breast-feeding and serum bilirubin concentration. Am J Dis Child. 1982;136(8):737-738.
de Carvalho M, Hall M, Harvey D. Effects of water supplementation on physiological jaundice in breast-fed babies. Arch Dis Child. 1981;56(7):568-569.
Moyer VA, Ahn C, Sneed S. Accuracy of clinical judgment in neonatal jaundice. Arch Pediatr Adolesc Med. 2000;154(4):391-394.
Bhutani VK, Gourley GR, Adler S, Kreamer B, Dalin C, Johnson LH. Noninvasive measurement of total serum bilirubin in a multiracial pre-discharge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics. 2000;106(2):E17.
Yasuda S, Itoh S, Isobe K, et al. New transcutaneous jaundice device with two optical paths. J Perinat Med. 2003;31(1):81-88.
Maisels MJ, Ostrea EJ, Touch S, et al. Evaluation of a new transcutaneous bilirubinometer. Pediatrics. 2004;113(6):1628-1635.
Ebbesen F, Rasmussen LM, Wimberley PD. A new transcutaneous bilirubinometer, BiliCheck, used in the neonatal intensive care unit and the maternity ward. Acta Paediatr. 2002;91(2):203-211.
Rubaltelli FF, Gourley GR, Loskamp N, et al. Transcutaneous bilirubin measurement: a multicenter evaluation of a new device. Pediatrics. 2001;107(6):1264-1271.
Agency for Healthcare Research and Quality. Management of neonatal hyperbilirubinemia. Rockville, Md. United States Department of Health and Human Services, Agency for Healthcare Research and Quality; 2003. AHRQ Publication No. 03–E011. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat1a.chapter.22160. Accessed November 8, 2007.
Bhutani VK, Johnson LH, Schwoebel A, Gennaro S. A systems approach for neonatal hyperbilirubinemia in term and near-term newborns. J Obstet Gynecol Neonatal Nurs. 2006;35(4):444-455.
Eggert LD, Wiedmeier SE, Wilson J, Christensen RD. The effect of instituting a prehospital-discharge newborn bilirubin screening program in an 18-hospital health system. Pediatrics. 2006;117(5):e855-e862.
Newman TB, Liljestrand P, Escobar GJ. Combining clinical risk factors with serum bilirubin levels to predict hyperbilirubinemia in newborns. Arch Pediatr Adolesc Med. 2005;159(2):113-119.
Bhutani VK, Johnson LH, Jeffrey Maisels M, et al. Kernicterus: epidemiological strategies for its prevention through systems-based approaches. J Perinatol. 2004;24(10):650-662.
National Newborn Screening and Genetics Resource Center. National newborn screening status report. Austin, Tex.: National Newborn Screening and Genetics Resource Center; 2007. http://genesr-us.uthscsa.edu/nbsdisorders.pdf. Accessed November 8, 2007.
Garcia FJ, Nager AL. Jaundice as an early diagnostic sign of urinary tract infection in infancy. Pediatrics. 2002;109(5):846-851.
Continue Reading
More in afp, more in pubmed.
Copyright © 2008 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- A guide to neonatal...
A guide to neonatal jaundice
- Related content
- Peer review
- Ben Green , foundation year one doctor 1 ,
- Lydia Burland , foundation year two doctor 2 ,
- Chris Smith , consultant paediatrician 1
- 1 Leeds General Infirmary, Leeds Teaching Hospitals Trust, Leeds LS1 3EX, UK
- 2 Dewsbury and District Hospital, Dewsbury, West Yorkshire WF13 4HS, UK
What should the junior doctor know?
Neonatal jaundice or hyperbilirubinaemia, is one of the most commonly observed conditions in the newborn infant. It specifically refers to the distinct yellow discolouration of sclera and skin, resulting from the accumulation of bilirubin. Although neonatal jaundice can be the result of serious underlying pathology, it is more typically a normal transitional phenomenon that resolves spontaneously. Around 60% of infants born at term will develop this condition. The risk is inversely proportional to gestational age, with those born before 37 weeks having an 80% risk during the first week of life. 1 Treatment might be indicated if the concentration of bilirubin is initially high, if it continues to rise, or if specific pathology is identified; the aim being to prevent complications of hyperbilirubinaemia such as kernicterus, which left untreated can lead to lifelong neurological disability or even death.
Neonatal jaundice is commonly seen in paediatrics, and all doctors working in this specialty should have an up to date working knowledge of this topic. While the junior doctor would not be expected to instigate management, having an awareness of the common causes, as well as knowing how to identify those at risk and those requiring further intervention, is of vital importance.
What are the causes of neonatal jaundice?
Neonatal jaundice can be broadly categorised into physiological and pathological jaundice. Physiological jaundice is by far the most common cause and is easily recognisable, typically appearing two to three days after birth in an otherwise well infant and normally resolving by 2 weeks of age. Physiological jaundice results through two distinct mechanisms—either through relative polycythaemia causing a rapid breakdown of fetal erythrocytes after birth and exposure to normal environmental oxygen concentrations, or through the reduced ability of the neonatal liver to excrete raised concentrations of bilirubin. 2
Pathological causes fall into three distinct groups: those resulting in early jaundice, prolonged jaundice, and conjugated jaundice (fig 1). ⇓ Some causes, however, can fall into more than one category—notably infection, which is an important cause of jaundice and must not be missed. Signs of infection can often be non-specific in neonates; features such as lethargy, poor feeding, and hypothermia or pyrexia, however, can point to an underlying infection and should be further investigated. It is additionally important to understand basic terminology regarding bilirubin and how it is measured clinically. Box 1 provides basic definitions.
Box 1: Definitions of types of bilirubin
Unconjugated: The unconjugated (indirect) bilirubin refers to insoluble bilirubin that is free in the plasma. It is classified as indirect as the concentration can be calculated only by subtracting direct from total bilirubin
Conjugated: The conjugated (direct) bilirubin refers to soluble bilirubin that is bound to glucuronic acid. This is excreted in the urine and undergoes enterohepatic circulation
Total: Total bilirubin measures total bilirubin concentration—that is, conjugated and unconjugated fractions
Split: Split bilirubin refers to the individual concentration of conjugated and unconjugated bilirubin

Fig 1 Causes of early, prolonged, and conjugated neonatal jaundice 1 3 4
- Download figure
- Open in new tab
- Download powerpoint
Early jaundice begins within the first 24 hours of life and should prompt investigation as the cause is always pathological. The most common causes include haematoma or bruising associated with delivery and congenital or postnatal infection. Less common causes include red cell haemolysis, which is most commonly caused by glucose 6 phosphate dehydrogenase (G6PD) deficiency; this can cause a particularly aggressive form of jaundice and is common in those of African, Eastern Mediterranean, or South East Asian descent. More rarely haemolysis is caused by ABO and rhesus incompatibility, as well as inborn errors of bilirubin metabolism. 4 “Breast feeding jaundice” can also cause an early jaundice within the first few days of life because of inadequate production or intake of breast milk; this is distinct from “breast milk jaundice.” When a diagnosis is being considered, it is important to take into account relevant risk factors, including a detailed history of pregnancy: factors including maternal blood group, maternal infections, and factors related to delivery such as prolonged rupture of membranes (PROM) are all important and can help determine a cause—for instance, PROM increases the chances of early jaundice being linked to infection.
Prolonged jaundice is defined as that lasting more than 14 days in the term infant or more than 21 days in a preterm infant, defined as less than 37 weeks’ gestational age. 1 Again full investigation is warranted, although the most common cause is breast milk jaundice. While the exact mechanism of breast milk jaundice is yet to be elucidated, it is largely benign and self limiting, usually occurring four to seven days after birth and resolving completely by week 12. 5 It is important to realise that breast milk jaundice is a diagnosis of exclusion, and that while it is beneficial to offer parents reassurance, the diagnosis does not exclude the need for further treatment if bilirubin concentrations are above the threshold for treatment. Other notable causes of prolonged jaundice include infection, thyroid disorders, metabolic disorders, and hepatobiliary disease.
Conjugated jaundice is most commonly caused by parenteral nutrition but can otherwise be caused by liver disease secondary to conditions such as infection and hepatitis and surgical causes such as biliary/duodenal atresia involving the ampulla of Vater and choledochal cysts. 6 An important clinical pattern involves conjugated hyperbilirubinaemia in conjunction with pale stools and dark urine—that is, an obstructive presentation that can indicate either biliary or duodenal atresia. Obstructive jaundice is another commonly tested clinical pattern that is important to recognise, not least because surgical correction is time critical.
How can I identify those who are at risk?
Identification of infants at risk can help to enable early diagnosis and timely intervention. As such, doctors should have a sound awareness of known risk factors, which will help to facilitate the recognition of those neonates requiring additional care. Several factors are recognised to increase the risk of neonatal jaundice, including race, with an increased risk in those of American Indian, South East Asian, and Indian descent; conversely, there is a decreased risk in black infants. Other risk factors include the use of vacuum devices during delivery as this increases the risk of haematoma or extensive bruising after birth. 7
Certain additional risk factors are associated with severe jaundice and therefore more commonly require intervention. Factors including exclusive breast feeding, previous jaundice in a sibling requiring phototherapy, gestational age under 38 weeks, and visible jaundice occurring within the first 24 hours of birth can all contribute to a state of severe hyperbilirubinaemia. 1 While many of these factors can be clinically apparent, this does not exclude the need for a thorough history to establish subjective risk factors.
How should I investigate a neonate with jaundice?
Early recognition has been shown to improve outcomes. While identification of an infant with jaundice might seem simple, variation in skin tone and other factors can, in some cases, make identification difficult. Figure 2 summarises several recommendations, as suggested by the National Institute for Health and Care Excellence (NICE) ⇓ .

Fig 2 Identification and investigation of neonatal jaundice. Timings refer to the onset of jaundice. Light grey boxes indicate actions, while darker grey boxes indicate investigations. HCP=healthcare professional; SBR=split bilirubin; FBC=full blood count; LFTs=liver function tests; DCT=direct Coomb’s test; TORCH=toxoplasmosis, others (including but not limited to syphilis), rubella, cytomegalovirus, herpes simplex 1
Investigation of infants presenting with jaundice depends on their age at presentation, whether the infant is clinically well, and the length of time for which jaundice has been present. The most useful initial investigation consists of measurement of total and split bilirubin concentrations, and this should ideally be requested in all infants who merit clinical concern. Although local guidance varies, figure 2 ⇑ summarises the important investigations depending on age at presentation: investigations vary slightly according to whether jaundice presents within 24 hours, after 24 hours, or after two weeks. It should be noted that testing of the maternal blood type can also be helpful in identifying a cause—such as ABO or rhesus incompatibility. All of the above mentioned investigations could be realistically requested by an astute house officer.
In patients with conjugated jaundice or signs of cholestatic disease, imaging studies such as ultrasongraphy is typically preferred to rule out surgical causes. Further investigations such as HIDA (hepatobiliary iminodiacetic acid) scanning is reserved for particularly complex cases. Surgical management ultimately depends on the underlying cause.
How do I manage a neonate with jaundice?
The management of neonatal jaundice is highly specialised and therefore the role of the junior doctor is somewhat limited; an awareness of available treatments, however, means that junior doctors are competent to explain procedures to parents/relatives (box 2). Treatment generally aims to resolve the underlying cause in addition to correcting hyperbilirubinaemia. This is achieved by two main methods: phototherapy and exchange transfusion. The indications for treatment depend on the serum bilirubin concentration as well as the age of the infant (fig 3 ⇓ ); it is important to note that treatment boundaries vary significantly depending on exact gestation. 8
Box 2: Talking to the parents: clarifying the role of the junior doctor
A commonly overlooked part of effective management is ensuring that the parents are fully informed. As a junior doctor, it is possible that you will be asked to talk to concerned parents of a neonate with jaundice. While this article obviously can’t cover every eventuality, we have tried to establish some key points on which to base a consultation.
Introduce yourself and check the identity of the parents/relatives
Explain in basic terms why the baby is jaundiced—that is, early break down of red blood cells and immaturity of liver enzymes
Explain what is being done and whether or not the baby requires treatment
Elicit and respond to parental ideas, concerns, and expectations
Offer reassurance whenever appropriate to do so
Most importantly, don’t be afraid to admit when you don’t know something, if this is the case

Fig 3 Treatment protocol for neonatal jaundice. As serum bilirubin concentration increases, the likelihood of phototherapy and exchange transfusion being required also increases. This is also partly dependent on the age of the infant, with younger infants being more likely to require intervention for any given concentration
The goal of phototherapy is to reduce the concentration of circulating unconjugated bilirubin by using visible light in the blue spectrum. The absorbed light energy causes a change in the structure of the bilirubin molecules, resulting in a more hydrophilic lower molecular weight product that can be excreted in bile and urine without the need for conjugation. 9
For most infants with a bilirubin concentration on or above the threshold for phototherapy treatment, single phototherapy treatment is appropriate. If the infant is preterm, the bilirubin concentrations are rising rapidly, or the concentration is close to the threshold for exchange transfusion fibreoptic phototherapy or multiple light therapy might be more appropriate. 1 During phototherapy parents should be encouraged to interact with the infant, with short breaks for feeds and changing if single light therapy is in use; multiple light therapy, however, should not be interrupted.
For a small group of infants who present with severe hyperbilirubinaemia, for whom multiple light phototherapy is not successful or who show signs of acute bilirubin encephalopathy, urgent exchange transfusion is necessary. Features of acute bilirubin encephalopathy can include lethargy, irritability, abnormal posture, apnoea, and convulsions. Exchange transfusion aims to modify the blood’s composition by removing small volumes of blood containing excess bilirubin and replacing it with prewarmed blood or plasma, thereby maintaining overall volume. Typical exchange transfusion causes a reduction of about 50% in serum bilirubin concentration, but the result can be transient and therefore close monitoring is essential after transfusion.
While it is important to correct hyperbilirubinaemia, the underlying cause must also be dealt with. Physiological and breast milk jaundice can require phototherapy, though they are self limiting and don’t normally require further management. Pathological causes are treated in the same manner but might additionally require further specific interventions. Surgical management is indicated only in conditions that cause bile outflow obstruction, such as biliary and duodenal atresia.
What is the prognosis for neonates with jaundice?
With modern diagnostic techniques and the widespread availability of treatment, the prognosis after neonatal jaundice is generally excellent. To minimise the risk of complications, infants should be regularly monitored. This can be easily achieved through the use of transcutaneous bilirubin meters in babies over 35 weeks, though a simple venous or capillary blood sample might be required if concentrations are high. Serum bilirubin concentrations should be rechecked four to six hours after initiation of phototherapy, and after six to 12 hours if the concentrations have begun to fall. Multiple light therapy should be considered if the concentrations are static or are continuing to rise. Once concentrations have fallen to >50 µmol/L below the treatment threshold, phototherapy can be stopped, with repeat concentrations being measured 12-18 hours later to check for rebound hyperbilirubinaemia. 1
The main complication arising from hyperbilirubinaemia is kernicterus, which can result from unrecognised or untreated hyperbilirubinaemia. This is a syndrome of acute neurological dysfunction resulting from deposition of bilirubin in the brain, and, although extremely rare, 10 long term sequelae can include permanent brain damage and cerebral palsy. Kernicterus tends to occur in three distinct phases, and it is therefore vital that junior doctors recognise the signs and symptoms present during the first stage, when treatment is still likely to be effective. Typical features including hypotonia progressing to opisthotonos (a state of spastic hyperextension), high pitched cry, apathy, and poor feeding should raise concern regarding the possibility of kernicterus. 11 Infants at particularly high risk include those with blood disorders such as ABO and/or rhesus incompatibility; such infants should be closely monitored for complications. 12 Although the features described are relatively non-specific, it is recommended that you consult a senior colleague immediately if you identify these changes, either in an infant with jaundice or an infant who is known to be at high risk.
Originally published as: Student BMJ 2014;22:g2836
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
- ↵ National Institute for Health and Care Excellence. Neonatal jaundice [CG98]. National Institute for Health and Care Excellence, 2010. http://guidance.nice.org.uk/CG98 .
- ↵ Avery GB, MacDonald MG, Seshia MMK, Mullett MD. Avery’s neonatology: pathophysiology and management of the newborn. Lippincott and Wilkins, 2005.
- ↵ Tasker RC, McClure RJ, Acerini CL. Oxford handbook of paediatrics. Oxford University Press, 2008.
- ↵ Porter ML, Dennis BL. Hyperbilirubinemia in the term newborn. Am Fam Physician 2002 ; 65 : 599 -606. OpenUrl PubMed Web of Science
- ↵ Preer GL, Philipp BL. Understanding and managing breast milk jaundice. Arch Dis Child Fetal Neonatal Ed 2011 ; 96 : 461 -6. OpenUrl CrossRef
- ↵ Davis AR, Rosenthal P, Escobar GJ, Newman TB. Interpreting conjugated bilirubin levels in newborns. J Pediatr 2011 ; 158 : 562 -5. OpenUrl CrossRef PubMed Web of Science
- ↵ Huang MJ, Kua KE, Teng HC, Tang KS, Weng HW, Huang CS. Risk factors for severe hyperbilirubinemia in neonates. Pediatr Res 2004 ; 56 : 682 -9. OpenUrl CrossRef PubMed Web of Science
- ↵ Rennie JM, Sehgal A, De A, Kendall GS, Cole TJ. Range of UK practice regarding thresholds for phototherapy and exchange transfusion in neonatal hyperbilirubinaemia. Arch Dis Child Fetal Neonatal Ed 2009 ; 94 : 323 -7. OpenUrl
- ↵ Maisels MJ, McDonagh AF. Phototherapy for neonatal jaundice. N Engl J Med 2008 ; 358 : 920 -8. OpenUrl CrossRef PubMed Web of Science
- ↵ Manning D, Todd P, Maxwell M, Platt MJ. Prospective surveillance study of severe hyperbilirubinaemia in the newborn in the UK and Ireland. Arch Dis Child Fetal Neonatal Ed 2007 ; 92 : 342 -6. OpenUrl CrossRef Web of Science
- ↵ Johnson LH, Bhutani VK, Brown AK. System-based approach to management of neonatal jaundice and prevention of kernicterus. J Pediatr 2002 ; 140 : 396 -403. OpenUrl CrossRef PubMed Web of Science
- ↵ Gamaleldin R, Iskander I, Seoud I, Aboraya H, Aravkin A, Sampson PD. Risk factors for neurotoxicity in newborns with severe neonatal hyperbilirubinemia. Pediatrics 2011 ; 128 : e925 -31. OpenUrl Abstract / FREE Full Text

The Royal Children's Hospital Melbourne
- My RCH Portal

- Health Professionals
- Patients and Families
- Departments and Services
- Health Professionals
- Departments and Services
- Patients and Families
- Research
Clinical Practice Guidelines
- About Clinical Practice Guidelines
- Nursing Guidelines
- Paediatric Improvement Collaborative
- Parent resources
- Retrieval services
- CPG Committee Calendar
- CPG information
- Other resources
- CPG feedback
In this section
Jaundice in early infancy

Recognition of the seriously unwell neonate and young infant
- If significant jaundice is clinically suspected, a serum bilirubin level should be performed as visual estimation of jaundice is unreliable
- The majority of jaundice in well infants is physiological, and does not require investigation and management
- Features suggestive of pathological jaundice include: onset <24 hours old, unwell baby, elevated conjugated bilirubin component, prolonged jaundice, pale stool. These require prompt investigation and management
- Jaundice (or hyperbilirubinaemia) occurs in approximately 60% of full term and 80% of pre-term babies within the first week of life
- Hyperbilirubinaemia occurs when there is an imbalance between bilirubin production, conjugation and elimination
- Kernicterus is a rare complication of neonatal unconjugated hyperbilirubinaemia that can lead to major long-term neurological sequelae
- Jaundice within the first 24 hours
- Unwell/febrile child
- Dark urine and pale stools (biliary obstruction)
- Significant weight loss >10% within the first week of life
- Cephalohaematoma or significant bruising
Examination
- General tone
- Neurological exam
- Hydration status: capillary refill time, heart rate, mucous membranes
- Plethora
- Bruising/ cephalohaematoma
- Hepatosplenomegaly
- Pattern and degree of jaundice

Investigations
- Total serum bilirubin (SBR): unconjugated (indirect) and conjugated (direct), then FBE and Coombs depending on clinical presentation
- Transcutaneous bilirubinometers (TCB) can be used as a screening tool to assess bilirubin levels from 24 hours – 2 weeks of age in near-term infants.
- Needs confirmation with serum bilirubin if within 50 micromol of treatment threshold
- Reliability of TCB decreases after phototherapy commenced
- SBR should always be used to check rebound levels
- TCB/SBR should be plotted on an appropriate gestation-based chart/nomogram in order to determine need for treatment
- Refer to local state guideline (see additional notes section below)
1) Assessment & treatment of jaundice:
- Severity of jaundice is judged based on a newborn’s age and gestation, as well as clinical presentation, hydration status, and other risk factors
- Please refer to local charts (see additional notes section below)
Phototherapy
- Refer to local protocol regarding intensity of lights required (including biliblanket use) and monitoring
- Ongoing close monitoring of weight, hydration, and bilirubin levels should be performed during treatment as per local protocol, with serial checks of SBR to ensure resolution of hyperbilirubinaemia
Exchange transfusion
- Should only be performed in, or in conjunction with, a Neonatal Intensive Care Unit
- Contact local paediatric retrieval service for support
2) Treatment of the cause
Consider consultation with local paediatric team when
- Child is unwell
- Cause of jaundice is unclear
- Conjugated bilirubin is >10% of total level
Consider transfer when
- Jaundice level rising despite adequate treatment offered at your local centre
- Patient needs exchange transfusion
For emergency advice and paediatric or neonatal ICU transfers, see Retrieval Services
Consider discharge when
- Causes requiring further treatment or investigation have been excluded
- Baby is clinically well and feeding well
Discharge advice
- Sunlight exposure is not recommended as a treatment for jaundice
- Arrange early follow-up with maternal and child health nurse and/or GP to ensure adequate oral intake, especially if:
- <7 days old
- exclusively breastfeeding or still establishing adequate oral feeds
- bilirubin level is borderline for requiring treatment
- Re-check bilirubin in 24–48 hours if borderline level or still rising
- Parents should be advised to seek medical review if:
- jaundice is present for 2–3 weeks and cause has not previously been established
- parents believe jaundice is worsening or there is any other cause for clinical concern
Parent information
Jaundice in Newborns – Children’s Health Queensland Jaundice and Your Newborn Baby – The Royal Women’s Hospital What is jaundice and phototherapy – The Royal Women’s Hospital Phototherapy at home
Additional notes
NSW Jaundice Identification and Management in Neonates >32 Weeks Gestation
Queensland Neonatal Jaundice Nomograms for jaundice management for all weight/gestations
Victoria Jaundice in neonates – Safer Care Victoria
Last updated October 2020
Reference List
- Nice Guidelines 2016, Jaundice in newborn babies under 28 days , National Institute for Health and Care Excellence, viewed August 2020 < http://www.nice.org.uk/nicemedia/live/12986/48678/48678.pdf >
- Safer Care Victoria 2020, Jaundice in neonates , Victorian Agency for Health Information, viewed August 2020 < https://www.bettersafercare.vic.gov.au/resources/clinical-guidance/maternity-and-newborn/jaundice-in-neonates >
- Queensland Clinical Guidelines 2019, Neonatal Jaundice , Queensland Health, viewed August 2020 < https://www.health.qld.gov.au/__data/assets/pdf_file/0018/142038/g-jaundice.pdf >
- Virtual Grand Rounds
- Meetings Calendar
- Education Universe
- Journal CME & MOC
- Board Prep/Self-Assessment Tests
- Claim CME & MOC
- Educating Your Colleagues & Patients
- Training Program Resources
- Exhibitors & Sponsors
- Endorsed Courses
Journals & Publications
- ACG Case Reports Journal
- Evidence-Based GI
- ACG Magazine
- Benefits & Resources
- My ACG/Log in
- Renew/Pay Dues
- Membership Directory
- Advanced Practice Providers
- Apply for FACG
- Join a Committee
- GI Circles: Connect with Colleagues
- Contact Your ACG Governor
Patients & Families
- Find a Gastroenterologist
- GI Health & Disease Resources
ACG Institute
- About the Institute
- The Center for Leadership, Ethics & Equity
- Research Grants & Awards
- Programs & Publications
- Emerging Leaders Program
- Early Career Leadership Program
- Advanced Leadership Development Program
- Clinical Research Leadership Program
- Edgar Achkar Visiting Professorships
- Visiting Scholar in Equity, Diversity, & Ethical Care
- Courses & Events
- Find a Mentor
- Research, Training Grants, & Publishing
- Question of the Week Competition
- GI Jeopardy Competition
- Program Director Resources
- GI Fellowship Program Information
- Health Equity Research Award
- Established Investigator Bridge Funding Award
- ACG/ASGE Epidemiologic Research Award in Gastrointestinal Endoscopy
- Junior Faculty Development Award
- Clinical Research Awards
- Clinical Research Award Pilot Projects
- Resident Clinical Research Award
- Medical Student Research Award
- Grant Recipients
Media & Press
- Media Statements
- Annual Meeting Press Information
- Join Press List
Find/Post A Job
Practice management.
- Prior Authorization Tools & Templates
- ACG Wellness Central
- GI OnDEMAND
- Coding & Reimbursement
- ASC Quality Reporting
- GIQuIC Registry
- FDA Information
- Making Sense of MACRA
Public Policy
- This Week in Washington DC
- Contact Policy Makers
- Legislative Action Center
- Legislative & Public Policy Council
- State Society Information
- Board of Trustees
- Board of Governors
- International Affiliate Societies
- Public Statements of Support
Neonatal Jaundice
- Gastrointestinal (GI) Health and Disease
- Recursos en Español
- Video and Audio Podcasts
- What is a Gastroenterologist (GI Doctor)?
- Gastrointestinal (GI) Brochures
Locate an ACG member gastroenterologist in your area.
Find an ACG member gastroenterologist with a specialized interest in liver disease.
- Neonatal Jaundice Overview
Neonatal jaundice describes a condition in which an infant’s skin appears yellow within the first few days of life. The yellowish appearance is a sign of an increased blood pigment called Bilirubin , which then settles in the skin. In many cases this is a normal process and occurs in about 2/3 of all healthy newborns. However, it may at times be a sign of a problem with the baby’s feeding, level of hydration or red blood cells lifespan. Other rare causes such as metabolism disorders, gland malfunction or liver disease can also present with jaundice. Only the health care provider can determine if the infant’s jaundice is normal and may order a blood test to help with diagnosis. In some cases, a specialist in liver disease or blood disorders may be called in to help take care of the newborn. Treatment can be very simple from increasing the baby’s water intake and modifying the feeding to very complex treatment. The choice of treatment is made according to the severity of the jaundice, the cause for the increase of bilirubin or the type of bilirubin.
The first symptom is yellow appearance of the skin and the eyes. The infant’s skin may appear yellow as early as the 1st or 2nd day of life. The jaundice starts around the head and the face then progresses to the shoulders, arms and the rest of the body including the legs and feet. The appearance may become more yellow when the baby is 3 to 4 days old and then slowly gets better. This is called “physiologic” or normal neonatal jaundice. Most infants have this pattern so no testing is needed.
At times, the yellow appearance may occur earlier (shortly after birth), last longer than 5-6 days or may be much more pronounced. A consultation with your health care provider is then needed to determine if testing is indicated.
Along with the skin becoming more yellow, the color of the baby’s urine can change from very light yellow or very dark brown. In the same manner, the color of the baby’s stool can vary from a yellow mustard color (normal) to light beige. These 2 color changes in the urine or the stool can indicate that the jaundice is due to different pigments. Although very rare in the first days of life, the presence of a very dark urine or light beige stool should be evaluated by a doctor immediately.
The yellow appearance comes from the accumulation of a yellow pigment called bilirubin in the skin. Right after birth, the infant body has to break down the red blood cells used while in the womb and make new ones now that the baby breathes the ambient air. The red color of the blood comes from a protein called hemoglobin, which carries the oxygen. As cells are being broken down, the hemoglobin gets modified in the liver and becomes bilirubin. Because the infant’s liver is so young and immature, it cannot keep up with all the produced bilirubin, which then leaks into the blood stream and settles in the skin.
- Risk Factors
A variety of conditions or diseases may present with an increase of the amount of pigment (bilirubin) produced. Poor feeding due to decreased breast feeding or the amount of breast milk can contribute to the increase in bilirubin. The same can happen with formula if the infant is not able to drink a sufficient amount. Maternal diabetes, exposure to some medications such as sulfa drugs or being underweight can also cause an increased bilirubin level.
Other conditions may be more serious:
- Increased production of bilirubin: In certain diseases, the red blood cells of the baby are destroyed at a faster rate than normal (this is called hemolysis). An example of such a disease is when the baby’s and mother’s blood type are different and not compatible. When this occurs, the mother’s immune system reacts and will form antibodies that attack the baby’s red blood cells. Babies also become anemic (low number of red blood cells) due to rapid destruction (hemolysis).
- Birth trauma: When vacuum extractors or forceps are used to deliver the baby a very large bruise over the scalp or the head may occur. This very large bruise will be re-absorbed. The old blood from the bruise will break down to make more bilirubin, which needs to be cleared by the liver. Some also may leak into the blood stream.
- Infection: Babies with infections may not be able to process bilirubin normally resulting in increased levels in the blood. This can occur with infection in the urine, blood, liver or other organs.
- Problems with bilirubin metabolism : In very rare cases the baby’s liver is not able to change the bilirubin into a form that can easily be removed from the body. This occurs in a condition called Crigler-Najjar syndrome. This is a very rare disease; the level of bilirubin increases very rapidly within hours. Immediate attention by a newborn specialist is then needed.
- Problems with digesting galactose : Rarely, babies cannot normally break down the sugar in breast milk (lactose) or in regular formulas made using the protein from cow’s milk. The sugar of the milk (lactose) is broken down into 2 smaller sugars called glucose and galactose. Rarely, the baby’s liver cannot process galactose. This is called galactosemia. This disease can present with jaundice in the newborn period and is associated with other severe symptoms (such as lethargy, vomiting, irritability and possibly convulsions). Galactosemia is often detected by a blood test (heel prick) before discharge from the nursery as part of the mandatory state screening for newborn diseases. Galactosemia is treated with strict dietary avoidance of galactose. This is not the same as being lactose intolerant and the two conditions should not be confused.
- Screening/Diagnosis
If the doctor is concerned about the severity of the jaundice, a blood test called a serum total bilirubin level is performed using a very small amount of blood. Other tests such as a transcutaneous (through the skin) test may be used to determine the bilirubin level in certain hospitals. This test is less accurate and needs to be confirmed with blood testing.
If the result is high, your doctor will order a blood test that will measure the different types of Bilirubin pigments, which make up the total bilirubin:
- Unconjugated or indirect bilirubin : This pigment is increased mostly in infants with neonatal jaundice. It is the bilirubin associated with normal destruction of older red blood cells. This is called physiologic jaundice. The baby’s urine is usually light yellow and the stool color is mustard yellow or darker. In some cases, the level of indirect bilirubin can go very high. Then, a neonatal specialist or blood specialist may be called in to help care for the newborn. Doctors are concerned if the bilirubin levels are more than 20-25 mg/dl (deciliter) and will start treatment to prevent the bilirubin from getting to this level. A level of indirect bilirubin at or above 20-25 mg/dl may cause irritation in some areas of the brain. This is called acute encephalopathy (inflammation of the brain). If the bilirubin remains very high, above 25 mg/dl, babies can be at risk for significant brain damage. This very rare condition is called kernicterus. Because of that risk, the doctor will start testing early for the bilirubin level and repeat the test often to identify the trend and start treatment rapidly.
- Conjugated or Direct bilirubin : The previous pigment (indirect or unconjugated bilirubin) is packaged in the liver into a form ready for removal into the bile and the gallbladder. This pigment is called conjugated (packaged) or direct bilirubin. For a variety of reasons, the liver cannot get rid of it, the direct bilirubin leaks back into the blood and also settles in the skin. At times the urine of the baby can be dark “coca cola” color and the stool can be light beige. The symptoms can be very different from those of normal neonatal jaundice. Babies can be very irritable, fussy, may have fever or they can have no symptoms. In addition to blood work to look for infections, other testing may be performed. A specialist in liver disease in children, called a pediatric gastroenterologist, may be called in consultation to help sort out the diagnosis. Liver disease is diagnosed with additional blood tests; the specialist may order an ultrasound or other specialized testing. These may lead to a procedure called a liver biopsy, where a small sample of liver tissue is taken for examination under a microscope.
The treatment varies based on the cause of the jaundice and the bilirubin level. For the purpose of this information we will discuss only the treatment for the elevation of the unconjugated or indirect bilirubin pigment. We will not discuss the jaundice associated with liver disease or the rare diseases causing an elevation of the pigment called conjugated or direct bilirubin .
Usually in normal physiologic neonatal jaundice, the process will be self-limiting and the baby does not need to be treated. The unconjugated bilirubin is broken down just with some exposure to the indirect sunlight. This is by far the vast majority. The baby may be able to be discharged home from the nursery within 48 hours of life without problems. The baby will need to be followed up by the pediatrician to ensure that the bilirubin level is going down and the baby’s weight is appropriate. This is especially true with breast-fed babies.
If the unconjugated or indirect bilirubin level remains high or is increasing, the baby may need further treatment to decrease the bilirubin level. Treatments might include:
- Some babies have a high indirect bilirubin level associated with breast feeding. Holding breast feeding and supplementing with infant formula for 48 hours may in some cases decrease the bilirubin in babies with “breast feeding jaundice.” A small amount of breast fed babies may continue to have elevated indirect bilirubin after 10-14 days. Once again holding breast feeding for 2 or 3 days may be sufficient and breast feeding can resume when the level of indirect bilirubin is lower. Breast feeding is by far the best option for neonates and should not be stopped completely because of a mild elevation of the unconjugated or indirect bilirubin . Families should speak with their physician or health care provider to determine if stopping breast feeding is appropriate and for instructions on how to proceed.
- Phototherapy is a treatment that allows the bilirubin under the skin to be broken down by a special light that illuminates the baby’s body. These lights are usually blue-green. They are placed about 4 inches above the baby. The more skin that is exposed to the lights, the better they work to break down a larger amount of unconjugated or indirect bilirubin . The lights do not prevent the baby from drinking formula or being breast-fed. The baby can be safely removed from the phototherapy at feeding times without decreasing the efficacy of the treatment. There are generally no significant risks with phototherapy. The eyes of the baby will have shields to prevent the light from harming the baby’s eyes and retina. There are no risks to the genitals of the baby. As long as the level of bilirubin is not very high, the phototherapy treatment can be done at home with a special blanket called a “bili” blanket. The physicians will then arrange for blood tests to be done regularly to ensure that the treatment is working. Most insurance will pay for this treatment at home.
- For a few babies, the level of unconjugated or indirect bilirubin is so high (greater than 20-25 mg/dl) that physicians are concerned about brain damage. The level has to be brought down very fast using a technique called exchange transfusion. An exchange transfusion is performed in the neonatal intensive care unit. The baby’s blood is exchanged and replaced very slowly and carefully with a donor’s blood. This allows for the indirect bilirubin to be removed faster, which will decrease the risk of further complications. This treatment is reserved for the most serious cases at risk for developing kernicterus (a condition where the indirect bilirubin is stored in areas of the brain and causes abnormal movements and seizures.)
- Author(s) and Publication Date(s)
Daniel L. Preud’Homme, MD, University of South Alabama, Mobile, AL – Published August 2006. Updated December 2012.
Return to Top
Got any suggestions?
We want to hear from you! Send us a message and help improve Slidesgo
Top searches
Trending searches
infertility
30 templates

linguistics
89 templates
15 templates

28 templates

public health
35 templates

holy spirit
38 templates
Neonatal Jaundice Disease
Neonatal jaundice disease presentation, free google slides theme, powerpoint template, and canva presentation template.
We know that being a mom or a dad is hard and scary, but when it comes to Jaundice, we recommend you not to panic. If your newborn’s eyes turn a bit yellow, it could be Jaundice, a condition that is common in babies and that is usually non-threatening. It happens because your kids’ body is still not efficient enough to handle all the bilirubin it creates. Speak about it with this template for health professionals!
Features of this template
- 100% editable and easy to modify
- 28 different slides to impress your audience
- Contains easy-to-edit graphics such as graphs, maps, tables, timelines and mockups
- Includes 500+ icons and Flaticon’s extension for customizing your slides
- Designed to be used in Google Slides, Canva, and Microsoft PowerPoint
- 16:9 widescreen format suitable for all types of screens
- Includes information about fonts, colors, and credits of the resources used
How can I use the template?
Am I free to use the templates?
How to attribute?
Combines with:
This template can be combined with this other one to create the perfect presentation:

Attribution required If you are a free user, you must attribute Slidesgo by keeping the slide where the credits appear. How to attribute?
Related posts on our blog.

How to Add, Duplicate, Move, Delete or Hide Slides in Google Slides

How to Change Layouts in PowerPoint

How to Change the Slide Size in Google Slides
Related presentations.

Premium template
Unlock this template and gain unlimited access

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Am J Trop Med Hyg
- v.107(2); 2022 Aug

Neonatal Jaundice: Knowledge and Practices of Healthcare Providers and Trainees in Southwest Nigeria
Eta barclay.
1 Department of Hospital Medicine, Children’s Minnesota, Minneapolis, Minnesota;
Ifelayo Ojo
2 Department of Pediatrics, Hennepin Healthcare, Minneapolis, Minnesota;
3 Department of Pediatrics, University of Minnesota, Minneapolis, Minnesota;
4 U.S. Centers for Disease Control, Atlanta, Georgia;
Abayomi Oyenuga
5 Department of Internal Medicine, University of Minnesota, Minneapolis, Minnesota;
Katherine Satrom
Mosunmoluwa oyenuga.
6 Department of Internal Medicine, SSM Health St. Mary’s Hospital, St. Louis, Missouri;
Tina Slusher
7 Department of Pediatrics, Bowen University Teaching Hospital, Ogbomosho, Nigeria
Daniel Gbadero
Associated data.
Supplemental materials
Severe neonatal jaundice (SNNJ) is a leading cause of neonatal morbidity and mortality in low- and middle-income countries (LMICs). Risk mitigation and management modalities for SNNJ have led to marked reduction in complications in high-income countries but not in LMICs likely in part due to knowledge gaps among healthcare providers. This study, a cross-sectional study conducted in Ogbomosho, Nigeria, aimed to identify SNNJ knowledge and practices among Nigerian healthcare providers/trainees. Healthcare providers/trainees completed a structured questionnaire. Healthcare providers/trainees included are nurse midwives (33.4%), nurses (18.6%), nursing students (15.2%), traditional birth attendants (TBAs) (12.7%), physicians (10.2%), and medical students (9.9%). Most physicians were aware of the common causes of SNNJ; however, knowledge deficits in other groups were notable. Despite most providers endorsing that glucose-6-phosphate dehydrogenase deficiency can cause SNNJ (91% of physicians, 60% of nurses, 71% of midwives, 81% of medical students, 43% of nursing students, 7% of TBAs), very few providers recognized that it is common, ranging from 3% in nurses up to a high of 47% among medical students. Gaps in provider knowledge regarding preventative measures and sequela were also noted. These data identified significant knowledge gaps regarding the etiology of SNNJ among healthcare providers/trainees, which can lead to missed opportunities in effective prevention and treatment. These deficits must be addressed if we are to eliminate tragic and preventable complications from SNNJ in Nigeria and other LMICs.
INTRODUCTION
From 1990 to 2018, child mortality decreased by 59%, from 93 to 39 deaths per 1,000 live births worldwide. 1 However, the gains in child survival in low- and middle-income countries (LMICs) have not resulted in a commensurate reduction in neonatal mortality, which currently represents nearly half of all deaths in children under 5 years old. 1 , 2 Severe neonatal jaundice/hyperbilirubinemia (SNNJ) has been demonstrated to be a leading cause of both neonatal morbidity and mortality, especially in LMICs. 3 , 4 Despite risk factors, treatment, and preventive measures noted in numerous references, many neonatal practices leading to SNNJ continue, leading experts to identify knowledge gaps in understanding and practices regarding SNNJ in healthcare providers. 5 – 8
Jaundice occurs in most neonates, and usually resolves without intervention in the majority of cases. 9 About 10% require treatment, which includes phototherapy and, in severe cases, exchange transfusions. 4 , 10 – 12 Severe cases of neonatal jaundice, including those developing acute bilirubin encephalopathy (ABE), or jaundice-related death, disproportionately affect neonates in LMICs, especially in the African region. 13 , 14 These severe jaundice-related clinical outcomes occur in 668/10,000 live births in the African region compared with 4/10,000 live births in the Americas and Europe. 4 Morbidity and mortality due to SNNJ are directly correlated with knowledge of causes, preventative strategies, time of diagnosis, and prompt initiation of treatment at appropriate thresholds. It is not possible or desirable to eliminate the majority of risk factors such as exclusive breastfeeding, blood group incompatibility, inheritable hemolytic disease (glucose-6-phosphate dehydrogenase [G6PD] deficiency), and delayed cord clamping. Rather these risks are assessed for each neonate by healthcare providers as they counsel families and manage patients. 9
Appropriate evaluation and country-wide management protocols do not exist in many LMICs including Nigeria. 15 , 16 This situation is often further compounded by difficulties measuring total and unconjugated serum bilirubin. 17 , 18 Additionally, there is rarely an expectation that neonates be examined for SNNJ by a healthcare provider after hospital discharge. 19 , 20 This remains the standard of care despite the knowledge that neonatal jaundice peaks on day 3–4 of life, while most mothers and neonate dyads are discharged from the hospital by the second day after delivery. Together, these factors have led to a wide range of unsafe practices such as those noted by Iliyasu et al. in their recent article (exposure to iatrogenic agents in the face of high rates of G6PD deficiency (G6PDD), exposure to unfiltered sunlight, raw paw-paw also known as papaya, and giving glucose water) 21 contributing to an increased incidence of SNNJ often leading to lifelong complications known as kernicterus spectrum disorder (KSD), 14 , 22 , 23 which includes hearing loss, choreoathetoid cerebral palsy, language processing disorders, or death.
Maternal knowledge has recently been shown to be extremely important in the prevention of ABE/kernicterus. 20 , 24 Data on the SNNJ knowledge of healthcare providers who ultimately inform these mothers in LMICs are extremely limited. 6 , 7 Quality medical education is important at all levels from trainee through the graduate, hence, the ongoing attempts of training programs to improve and evaluate the effectiveness of their programs. 25 , 26 Several studies have demonstrated improvement in neonatal outcomes as a result of training traditional birth attendants (TBAs). 27 , 28 Of note modern TBAs in Nigeria and specifically in Ogbomosho, Nigeria, have some formal health education and training but the extent of training on neonatal jaundice was not known. The goal of this study was to evaluate the knowledge of healthcare providers and trainees with regard to the prehospital evaluation and management of SNNJ in a semirural area of southwestern Nigeria. Understanding the knowledge and practice gaps of both trainees and providers will provide the basis for appropriately tailored education for each level of trainee and provider.
Study design and dataset.
This cross-sectional study was conducted in Ogbomosho, Nigeria, from November 2016 to January 2017. The study was conducted at various health facilities, and respondents completed questionnaires at the health facilities where they worked. This was a convenience sample of health providers and trainees, we did not attempt to obtain a representative randomized sample from the health facilities surveyed nor track nonresponders. Traditional birth attendants were questioned during their meeting at a church using an interpreter (author D. G.). Respondents either completed the paper questionnaire themselves or had it read to them with their responses recorded by the research team if illiterate. The questionnaire included questions related to the respondent’s knowledge and approach to the management of SNNJ.
Sample population.
A total of 357 healthcare providers and trainees aged 17–73 years were recruited to complete the questionnaire. Respondents included house officers/resident physicians, nurses, nurse midwives, TBAs, community health extension workers (CHEWs), and medical and nursing students. Respondents were informed of the goals of the study. Participation was strictly voluntary and no incentives were offered. As a result, those who responded to the study were considered to have provided implied verbal consent. The data were deidentified with respect to the healthcare facility location for confidentiality. We excluded responses from participants who were CHEWs due to limited numbers, participants who did not list a professional designation, and those who identified themselves as “Other.” The study was deemed exempt by the Hennepin Healthcare Research Institute as no personal health information was collected and approved by the Bowen University Teaching Hospital IRB.
Data analysis.
Data were entered using REDCap 29 and exported to STATA. Data analysis was performed using STATA 15 and SAS 9.4 (SAS Institute Inc., Cary, NC). Descriptive analysis was performed and results for continuous variables were expressed as mean and SD, and categorical variables were reported as frequencies and percentages.
Our sample was composed of 323 healthcare providers and trainees: nurse midwives 108 (33.4%), nurses 60 (18.6%), nursing students 49 (15.2%), TBAs 41 (12.7%), physicians 33 (10.2%), and medical students 32 (9.9%). The average age of the respondents was 33.9 years ±11.4 ( N = 307) and the average duration of practice was 8 years (range 4–16 years, N = 212) (see Table Table1 1 ).
Demographics and other characteristics of healthcare practitioners and trainees
N = number; Q1 = 25 th percentile; Q3 = 75 th percentile; TBA = traditional birth attendants.
In this sample, most respondents had seen a case of neonatal jaundice (87%); across professional designations, 100% of physicians, 98.2% of nurse midwives, 96.7% of nurses, 79.6% of nursing students, 71.9% of medical students, and 53.7% of TBAs. The leading causes of SNNJ noted in this questionnaire were sepsis, G6PDD, and ABO incompatibility among most groups. For details by type of healthcare provider see Table Table2 2 .
Common causes of NNJ and the ability of healthcare practitioners to identify these as risk factors
G6PD = glucose-6-phosphate dehydrogenase; N = number; NNJ = neonatal jaundice; TBA = traditional birth attendants.
Responses to questions specifically regarding G6PDD and practices known/suspected to cause hemolysis in neonates are shown in Table Table3. 3 . About 21.6% of our total sample responded that G6PDD was common in Nigerians. By professional designation, the percentage of respondents who chose this response was highest among medical students (46.9%) and lowest among nurses (3.3%). On the importance of G6PDD as a cause of SNNJ, 59.7% of respondents indicated that G6PDD “can be a cause of very serious jaundice in newborns.” The percentage of those who chose this response varied by professional designation: 90.9% of physicians, 81.3% of medical students, 71.3% of nurse midwives, 60% of nurses, 42.9% of nursing students, and 7.3% of TBAs selected this response.
Knowledge and practice related to G6PD deficiency and SNNJ among healthcare practitioners and trainees
G6PD = glucose-6-phosphate dehydrogenase; N = number; RBCs = red blood cells; SNNJ = severe neonatal jaundice; TBA = traditional birth attendants; WBCs = white blood cells.
When questioned about cord care, only 9.3% (30/323) reported recommending chlorohexidine, whereas 82% (265/323) recommended methylated spirit; with the other nine healthcare providers recommending mentholated products (0.9%, 3/323), eucalyptus oil (1.6%, 5/323), and herbs (0.3%, 1/323). Regarding care of the newborn’s skin, 5.0% (16/323) recommended mentholated products, 68.7% (222/323) recommended baby lotion, and 20.7% (67/323) recommended other products. In total, 18 (5.6%) healthcare workers recommended using mentholated products on newborns while an additional 5 (1.6%) recommended using eucalyptus oil. Furthermore, 5.5% (17/311) recommended storing cloth in naphthalene. The majority 63.2% (199/315) recommended washing clothes stored in naphthalene, whereas 32.1% (101/315) did not recommend washing, and 4.8% (15/315) did not know what to do. Table Table3 3 contains more details of the knowledge and practice domains by professional designation.
Overall, 95% of respondents were aware of one or more complications of SNNJ. In the total sample, the percentages for the responses to the specific complications we assessed were death (65.6%), seizures (56.4%), choreoathetoid palsy (46.1%), deafness (38.4%), language processing disorders (25.7%), and dental enamel dysplasia (8.7%). The top two most identified complications of SNNJ by professional designation were seizures (97%) and deafness (81.8%) among physicians; seizures (63.3%) and death (53.3%) among nurses; death (67.6%) and seizures (66.7%) among nurse midwives; death (80.5%) and seizures (4.9%) among TBAs; seizures (84.4%) and choreoathetoid cerebral palsy (71.9%) among medical students; also death (55.1%) and deafness (22.5%) among nursing students. The distribution of the percentages of respondents choosing other possible complications is presented in Table Table4 4 .
Knowledge of the complications of SNNJ among healthcare practitioners and trainees
N = number; SNNJ = severe neonatal jaundice; TBA = traditional birth attendants.
Finally, responses to questions regarding knowledge about prehospital management strategies for SNNJ are presented in Tables Tables5 5 and and6. 6 . The majority of respondents ranging from 63.3% to 93.9% in all categories stated that they would seek help from a hospital or refer to a hospital if a baby had SNNJ. Many stated that the appropriate time for sunlight phototherapy was early morning including 100% of the physicians and 81.3% of the nurse midwives.
Management strategies of SNNJ among healthcare practitioners and trainees
Knowledge of timing of sunlight phototherapy for management of SNNJ among healthcare practitioners and trainees who selected sunlight phototherapy as a management strategy
Our study assessed the knowledge of common causes, prehospital management strategies, and potential complications of SNNJ among different groups of healthcare providers/trainees. It was important to assess each group of practitioners separately as they have different training and practice independently in disparate settings. Traditional birth attendants are individuals who assist with the birth of babies, in a home or religious setting (churches, in our study population). These individuals are usually women who have traditionally received their education not by formal training but through apprenticeship with other TBAs. However, as noted earlier, many TBAs now have formal training programs covering some aspects of newborn care. 27 – 30 They were included in the study as they are important members of the healthcare delivery system in Nigeria, especially in more rural areas, and have been shown to be involved in about 20% of births. 30 Additionally, as noted earlier, studies have shown that training TBAs improves neonatal outcomes. 27 , 28 Nurses were included because they often practice independently from physicians in LMICs including Nigeria. Some differences in knowledge that we noted were not unexpected given differences in training requirements between the different groups. A management guideline for jaundice in LMICs suggests that primary prevention, prompt detection and monitoring, as well as appropriate treatment will decrease the morbidity and mortality associated with SNNJ. 15
Similar to the prior study of community health workers where almost half of the respondents correctly defined jaundice and 75% knew the correct physical examination, 9 most of the participants in our study reported that they had seen a case of SNNJ. However, about 46% of TBAs reported that they had not seen a case of SNNJ, despite 90% of these workers indicating that they examine patients for jaundice all or most of the time. This difference between physical examination and jaundice identification is surprising given both the prevalence of SNNJ and that over half of the births in Nigeria occur out of hospital 31 and are conducted by healthcare workers who are not physicians, including TBAs. This may indicate the need to reinforce education on accurate identification of SNNJ particularly in nonhospital settings. Despite having highly educated healthcare providers in Nigeria and other LMICs, knowledge gaps remain among many of the providers 8 , 32 – 34 as we have demonstrated in this study. Efforts are ongoing to improve both the quality of the educational material widely available and to assure distribution of this material. Two of the authors (D. G. and T.S.) have been involved in developing and distributing teaching material to help fill this gap. Additionally, the Stop Kernicterus International (SKI) in Nigeria and a subgroup of SKIN, Stop Kernicterus in Northern Nigeria, [which includes one of the authors (TMS)] have developed teaching modules for various healthcare providers 35 initially through a Saving Lives at Birth Grant. They have subsequently worked together with SKI 35 to provide much of this material and make it freely available around the world, allowing other countries to translate and use the material in their teaching. More efforts are needed if we are to eliminate kernicterus/KSD worldwide. In addition to strengthening and modifying curriculum where needed, efforts to ensure widespread distribution of this knowledge are needed. Some of these efforts should include reinforcements of training through seminars, workshops at both medical institutions, and more public venues such as houses of worship, and use of diverse media for public education geared to each level of healthcare provider from TBA through physician.
In Nigeria, the leading causes of SNNJ are G6PDD, prematurity/low birthweight, infection, and ABO incompatibility. 36 – 38 Rhesus disease, a risk factor of hemolysis, is less common in Nigeria but can cause SNNJ when it does occur due to the lack of availability and affordability of anti-D immune globulin. 39 – 41 Though the majority of our respondents had some knowledge of these pathologies, there were still significant knowledge gaps noted in all groups. About 27% of the physicians did not correctly identify ABO incompatibility as being a cause of SNNJ, and less than half of other groups did, except for medical students, as shown in Table Table2. 2 . This was unexpected as ABO incompatibility is well known to be a common cause of jaundice. Another significant difference in practice between types of healthcare providers was the finding that 85% of physicians and 95% of nurse midwives indicated that they either administered anti-D to Rh-negative mothers or referred these mothers to other facilities for anti-D. Only 12% of TBAs referred mothers for treatment of anti-D. These results highlight missed opportunities to recognize and manage Rh incompatibility as a risk factor for SNNJ. 42
Studies have shown the burden of G6PDD in males to be about 12–22% in many populations of African descent, with a much lower but significant percentage of heterozygote females. 43 , 44 Given this, the knowledge of G6PDD incidence and its estimated contribution to SNNJ in our respondents was relatively low. Thirty-nine percent of nurse midwives, 42% of nurses, and 38% of TBAs did not correctly identify G6PDD as a cause of SNNJ. Although it is reassuring that 94% of physicians correctly identified this as a cause of SNNJ, only 11% of them thought it was common in Nigerians. Also, only half of the physicians correctly identified it as an inherited abnormality of red blood cells that may protect from more severe forms of malaria. 45 , 46 It is concerning that over 60% of TBAs had no knowledge of G6PDD. Though management of G6PDD may be considered too technical for these primary care workers given their level of training, 7 interventions to educate them about G6PDD as a cause of SNNJ will be helpful in ensuring close supervision or early referral of neonates who may be at greater risk of developing SNNJ.
Mentholated products and naphthalene have long been known to be hemolytic agents in neonates with G6PDD. 47 While many healthcare providers may not recommend the use of these products, many still lack the knowledge that these products can cause jaundice in neonates with G6PDD and thus may not advocate against the use of or educate about potentially dangerous products. In a study by Adebami, 32 he notes that 61.7% of primary healthcare workers and 30.9% of secondary healthcare workers did not know that drugs or substances could cause jaundice in some neonates. Additionally, products/drugs that could cause hemolysis in neonates are still being studied. Eucalyptus oil has been suspected to cause hemolysis in these same neonates and has been reported in lay literature as a cause of hemolysis in G6PDD individuals. 48 A recent laboratory study has shown both eucalyptus oil and methylated spirits can cause hemolysis in G6PDD zebra fish. 49 Surprisingly, very few respondents indicated that they used or recommended the use of substances previously shown to cause oxidative stress and hemolysis in G6PDD patients, such as mentholated products and naphthalene balls or camphor. 5 , 49 This is different from previous studies that have shown the use of these products to be relatively widespread. 5 This reported change in practice may be due to the public education campaign to decrease the use of these products in all neonates or simply reluctance to admit to the use of products not approved by “Western” medicine.
Neonatal sepsis is another well-known cause of SNNJ. 50 Although 100% of physicians recognized this connection, knowledge among other practitioners was relatively low with only 50% of nurses, 27% of nurse midwives, and 7% of TBAs identifying sepsis/infection as a cause of SNNJ. Lack of identification of the causality link between sepsis and jaundice may mean that jaundiced infants are not being investigated for sepsis, or that infants with sepsis may not be managed for hyperbilirubinemia. Both of these outcomes could result in increased neonatal morbidity and mortality.
Severe neonatal jaundice is also common in preterm/low-birthweight infants in Nigeria, 51 , 52 though only 50% of healthcare providers were aware of this. Caring appropriately for preterm/low-birth-weight infants requires that their caretakers recognize and manage SNNJ to prevent poor neurodevelopmental outcomes in this vulnerable population. 53
The respondents were also questioned regarding their management practices of SNNJ. 13% of nurses and 17% of nurse midwives indicated that they gave neonates sugar water or herbs for the management of SNNJ. This is consistent with other studies that have shown that these alternative practices are relatively common among primary health workers including 75% of physicians in one study. 6 , 7 , 34 Surprisingly, no TBAs indicated that this was their practice. The reason for this practice difference among the primary health workers is unclear. A large portion of respondents indicated that they exposed jaundiced neonates to (presumably unfiltered) sunlight, historically noted to decrease unconjugated serum bilirubin. 54 As noted in the study by Slusher et al., early morning sunlight is much less effective with much lower irradiance levels than sunlight during the midday. 55 Even though it is not recommended in most high-income countries due to the side effects such as hyperthermia and exposure to ultraviolet radiation, as well as the readily available effective phototherapy, it remains relatively common in LMICs. 54 , 56 , 57 Filtered sunlight phototherapy (FSPT), a therapy that could be used without electricity or high-cost commercial devices, has been shown to be safe and effective in two studies in Nigeria but still needs further study before widespread implementation. 55 , 58 Most healthcare practitioners did indicate that they referred affected neonates to the hospital for treatment of SNNJ. This is also reassuring, although the availability and effectiveness of phototherapy at these referral centers was not assessed in this study.
The morbidity and mortality from SNNJ are related to its significant neurotoxicity in neonates who are not adequately treated. 59 As a whole, the respondents demonstrated good understanding of the complications of SNNJ, though TBAs only indicated death as a complication, with none selecting other neurotoxic effects such as deafness, seizures, and language processing disorders. This represents yet another point for potential intervention to ensure that the mothers who are being cared for by these practitioners are receiving adequate anticipatory guidance.
Regarding the responses of trainees, notably, only 53% of medical students and 12% of nursing students recognized ABO incompatibility as a cause of SNNJ, only 10% of nursing students recognized the significance of Rh incompatibility in SNNJ, and 23% of them indicated no knowledge of G6PDD. Although originally intended to be a study focused on healthcare practitioners, data on the responses of trainees are important so as to address any gaps in training. These identified opportunities should be targets or points of emphasis for educational intervention to ensure that these trainees become competent practitioners.
Limitations of this study include that the responses were self-reported, therefore actual practices could not be verified. Responses were solicited from a nonrandomized sample, which may limit the external validity of the study. Also, as this was a convenience sample, it is possible that the respondents were those who were more comfortable with their knowledge of SNNJ compared with the nonrespondents. However, our study reflects similar deficiencies in knowledge and practice found by prior studies, where cluster and individual randomization were performed. 6 , 9 Additionally, the study region is the site of ongoing clinical trials on the effectiveness of filtered sunlight in the treatment of hyperbilirubinemia. 58 As a result, the participants may be more knowledgeable than healthcare practitioners and trainees in other similar geographical regions. We also failed to record the year of study for nursing students. Thus, nursing students early in their education may have been taught this material after their participation in our study. Finally, because many of the healthcare providers enrolled in the study would not be responsible for treating with phototherapy or exchanging blood transfusions or evaluating admissions for the degree of ABE using the modified bilirubin-induced neurological score (BIND_M), 60 we elected not to include these questions in this study.
In conclusion, this study highlights knowledge and practice gaps pertaining to neonatal jaundice among all groups of healthcare students and practitioners in our study population. Moving forward, it is important to tailor the educational packages accompanying country-specific guidelines to the appropriate level of training and practice for each learner category and cadre of healthcare providers. Additionally, it will be important to include strong public health campaigns to reach mothers and their families as well as all healthcare providers on the appropriate recognition, referral, and treatment practices to prevent SNNJ.
Addressing these deficits through targeted and widespread education is one of the pieces needed to effectively relegate ABE and KSD to medical history books worldwide.
Supplemental Material
Acknowledgments.
We are grateful to all the health workers and healthcare trainees who participated in this study. We also thank Mr. John Onasunmibo for his assistance with data collection. This research was supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, grant UL1TR002494. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health’s National Center for Advancing Translational Sciences.
Note: Supplemental Appendix appears at www.ajtmh.org .
Neonatal Jaundice
May 01, 2012
750 likes | 5.75k Views
Neonatal Jaundice. Visible form of bilirubinemia Adult sclera >2mg / dl Newborn skin >5 mg / dl Occurs in 60% of term and 80% of preterm neonates However, significant jaundice occurs in 6 % of term babies . Bilirubin metabolism. Hb → globin + haem 1g Hb = 34mg bilirubin.
Share Presentation
- direct bilirubin
- kg bilirubin ligandin
- ideal o cells
- glucuronidase bacteria
- poor feeding

Presentation Transcript
Neonatal Jaundice • Visible form of bilirubinemia • Adult sclera >2mg / dl • Newborn skin >5 mg / dl • Occurs in 60% of term and 80% of preterm neonates • However, significant jaundice occurs in 6 % of term babies
Bilirubin metabolism Hb → globin + haem 1g Hb = 34mg bilirubin Non – heme source 1 mg / kg Bilirubin Ligandin (Y - acceptor) Intestine Bilirubin glucuronidase Bil glucuronide Bil glucuronide β glucuronidase bacteria Bilirubin Stercobilin
Clinical assessment of jaundice Area of body Bilirubin levels mg/dl Face 4-8 Upper trunk 5-12 Lower trunk & thighs 8-16 Arms and lower legs 11-18 Palms & soles > 15
Physiological jaundice Characteristics • Appears after 24 hours • Maximum intensity by 4th-5th day in term & 7th day in preterm • Serum level less than 15 mg / dl • Clinically not detectable after 14 days • Disappears without any treatment Note: Baby should, however, be watched for worsening jaundice
Why does physiological jaundice develop? • Increased bilirubin load • Defective uptake from plasma • Defective conjugation • Decreased excretion • Increased entero-hepatic circulation
15 10 5 Bilirubin level mg/dl Term Preterm 1 2 3 4 5 6 10 11 12 13 14 Age in Days Course of physiological jaundice
Pathological jaundice • Appears within 24 hours of age • Increase of bilirubin > 5 mg / dl / day • Serum bilirubin > 15 mg / dl • Jaundice persisting after 14 days • Stool clay / white colored and urine staining clothes yellow • Direct bilirubin> 2 mg / dl
Causes of jaundice Appearing within 24 hours of age • Hemolytic disease of NB : Rh, ABO • Infections: TORCH, malaria, bacterial • G6PD deficiency Appearing between 24-72 hours of life • Physiological • Sepsis • Polycythemia • Concealed hemorrhage • Intraventricular hemorrhage • Increased entero-hepatic circulation
Causes of jaundice After 72 hours of age • Sepsis • Cephalhaematoma • Neonatal hepatitis • Extra-hepatic biliary atresia • Breast milk jaundice • Metabolic disorders
Risk factors for jaundice JAUNDICE • J - jaundice within first 24 hrs of life • A - a sibling who was jaundiced as neonate • U - unrecognized hemolysis • N – non-optimal sucking/nursing • D - deficiency of G6PD • I - infection • C – cephalhematoma /bruising • E - East Asian/North Indian
Common causes in India • Physiological • Blood group incompatibility • G6PD deficiency • Bruising and cephalhaematoma • Intrauterine and postnatal infections • Breast milk jaundice
Approach to jaundiced baby • Ascertain birth weight, gestation and postnatal age • Assess clinical condition (well or ill) • Decide whether jaundice is physiological or pathological • Look for evidence of kernicterus* in deeply jaundiced NB *Lethargy and poor feeding, poor or absent Moro's, opisthotonus or convulsions
Workup • Maternal & perinatal history • Physical examination • Laboratory tests (must in all)* • Total & direct bilirubin* • Blood group and Rh for mother and baby* • Hematocrit, retic count and peripheral smear* • Sepsis screen • Liver and thyroid function • TORCH titers, liver scan when conjugated hyperbilirubinemia
Management • Rationale: reduce level of serum bilirubin and prevent bilirubin toxicity • Prevention of hyperbilirubinemia: early feeds, adequate hydration • Reduction of bilirubin levels: phototherapy, exchange transfusion, drugs
Principle of phototherapy Native bilirubin Photo isomers of bilirubin Insoluble Soluble 450-460nm of light
Phototherapy equipment • White light tubes 6-8*/ 4 blue light tubes • Cradle or incubator • Eye shades *May use 150 W halogen bulb
Babies under phototherapy Baby under conventional phototherapy Baby under triple unit intense phototherapy
Phototherapy Technique • Perform hand wash • Place baby naked in cradle or incubator • Fix eye shades • Keep baby at least 45 cm from lights, if using closer monitor temperature of baby • Start phototherapy
Phototherapy • Frequent extra breast feeding every 2 hourly • Turn baby after each feed • Temperature record 2 to 4 hourly • Weight record- daily • Monitor urine frequency • Monitor bilirubin level
Side effects of phototherapy • Increased insensible water loss • Loose stools • Skin rash • Bronze baby syndrome • Hyperthermia • Upsets maternal baby interaction • May result in hypocalcemia
Choice of blood for exchangeblood transfusion • ABO incompatibility • Use O blood of same Rh type, ideal O cells suspended in AB plasma • Rh isoimmunization • Emergency 0 -ve blood Ideal 0 -ve suspended in AB plasma or baby's blood group but Rh -ve • Other situations • Baby's blood group
Maisel’s chart
Prolonged indirect jaundice Causes • Crigler Najjar syndrome • Breast milk jaundice • Hypothyroidism • Pyloric stenosis • Ongoing hemolysis, malaria
Conjugated hyperbilirubinemia Suspect • High colored urine • White or clay colored stool Caution • Always refer to hospital for investigations so that biliary atresia or metabolic disorders can be diagnosed and managed early
Conjugated hyperbilirubinemia Causes • Idiopathic neonatal hepatitis • Infections -Hepatitis B, TORCH, sepsis • Biliary atresia, choledochal cyst • Metabolic -Galactosemia, tyrosinemia, hypothyroidism • Total parenteral nutrition
- More by User

NEONATAL JAUNDICE
NEONATAL JAUNDICE. Yousef K. Abu-Osba MD Neonatal Intensive Care Unit Jordan Hospital, Amman, Jordan. CAUSES OF PHYSIOLOGIC HYPERBILIRUBINEMIA - 1. Definition: Full term infant 6-8 mg/l00 ml by 3 days Premature infant 10-15 fig/100 ml by 5 days
2.2k views • 31 slides

NEONATAL JAUNDICE. Y. K. Abu-Osba MD Neonatal Intensive Care Unit Jordan Hospital, Amman, Jordan. TRANSPORT OF BILIRUBIN IN PLASMA. Unconjugated bilirubin binds to albumin in a 2:1 molar ratio. Organic anions take % of binding sites.
1.77k views • 30 slides

Neonatology Neonatal Jaundice
Neonatology Neonatal Jaundice. Contents. Billirubin metabolism in normal neonates Special problems in neonates The diseases in relation with Neonatal Jaundice Dangerous of the Hyperbillirubinemia. Normal Billirubin Metabolism in neonates. ineffective erythropoiesis. liver.
1.11k views • 32 slides

Definition . JAUNDICE: yellowish discoloration of skin, sclera and mucus membraneResults from accumulation of un conjugated bilirubin pigment in the skin occur in 60% of term infant and 80% of preterm infant.. Bilirubin production . Bilirubin is a product of heme catabolism. Approximately 80 to 90%
728 views • 17 slides

NEONATAL JAUNDICE. Outline. Introduction Definition Epidemiology Bilirubin metabolism Aetiopathogenesis /Types Clinical features Evaluation of a jaundiced neonate Management Complications Surgical/ C holestatic jaundice. Introduction.
1.94k views • 104 slides

Neonatal jaundice
Neonatal jaundice. BILIRUBIN METABOLISM. 1-Bilirubin production. 2-Transport in blood. 3-Hepatocellular uptake. 4-Intracellular transport in hepatocytes. 5-Conjugation with glucuronic acid. 6-Secretion into bile ducts. 7- Intestinal metabolism. 8- Renal excretion of bilirubin
3.61k views • 63 slides

NEONATAL JAUNDICE IN MALAYSIA
characteristic. The word 'Jaundice' is derived from the French word 'Jaune' meaning yellow.Jaundice is the yellowish discolouration of the skin caused by an increase of bilirubin in the blood.Neonatal jaundice is jaundice that occurs within the first month of baby's life.It is a very common and occurs in 50% of babies in the first week of life.
1.73k views • 15 slides

Neonatal Jaundice Hyperbilirubinemia
Neonatal Jaundice Hyperbilirubinemia. Fred Hill, MA, RRT. Neonatal Jaundice. What is Bilirubin?. End product of catabolism of iron protoporphyrin (heme) Primarily from circulating hemoglobin 75% from erythrocytes 25% from heme in liver enzymes. Why in Newborns?.
546 views • 8 slides

Neonatal Jaundice. By Dr Swati Prashant,MD Paediatrics www.paediatrics4all.com [email protected] Index Medical college,Indore,MP,India. JAUNDICE. Jaundice is the commonest abnormal finding during the initial wks .of life .
981 views • 32 slides

Neonatal Jaundice. Carrie Phillipi, MD, PhD. Newborn with Jaundice. Neonatal Jaundice Definitions. Physiologic Pathologic Indirect (unconjugated) Direct (conjugated) Breast feeding jaundice Breast milk jaundice. Production of Bilirubin. Conjugation of Bilirubin.
1.2k views • 19 slides

Neonatal Jaundice. Ruben Bromiker Department of Neonatology Shaare Zedek Medical Center. Physiologic Jaundice. Healthy infants up to 12mg% in 3rd day; in premature, 5th day. No hemolysis or bleedings No underlying metabolic disease. Mechanism. Production: Volemia,
1.15k views • 40 slides

Neonatal Jaundice. Neonatal Ward Dr. Ziyu Hua. Classification of neonatal jaundice. Physiological jaundice. Pathological jaundice. Etiology of physiological jaundice. In the first few days after birth, haemoglobulin concentration falls rapidly.
8.29k views • 18 slides

Neonatal Jaundice. Promoting multiprofessional education and development in Scottish maternity care. Neonatal Jaundice. Definition = Total serum bilirubin (SBR) > 85 µ mol/L. Why is it important?. Common Worrying for parents and / or staff Condition and treatment
1.6k views • 27 slides

NEONATAL JAUNDICE. BY DR BLESSING OFEJIRO OKPERI B.Med.Sc.(Hons), MBBS, FWACP (Paed) SENIOR LECTURER / CONSULTANT PAEDIATRICIAN H.O.D, DEPT OF PAEDIATRICS, DELSU & DELSUTH . MEDICAL DIRECTOR
1.6k views • 12 slides

Neonatal Jaundice. Dr. Kalpana Malla MD Pediatrics Manipal Teaching Hospital. Incidence Term—60% Preterm—80% Bilirubin Source – Hb – 75% Non Hb – 25% ( Myoglobin). Normal Physiology.
2.29k views • 81 slides

NEONATAL JAUNDICE IN MALAYSIA. Nur Anis Amira binti Mat Isa Norhidayah binti Danial. characteristic. The word 'Jaundice' is derived from the French word ' Jaune ' meaning yellow. Jaundice is the yellowish discolouration of the skin caused by an increase of bilirubin in the blood.
1.03k views • 15 slides

Neonatal Jaundice SGD
Neonatal Jaundice SGD. Dr Saffiullah AP Paeds. By the end of this discussion you should be able to; 1.Make a differential diagnosis of common and significant causes of jaundice in neonates
544 views • 16 slides
Neonatal Jaundice. Visible form of bilirubinemia Adult sclera >2mg / dl Newborn skin >5 mg / dl Occurs in 60% of term and 80% of preterm neonates However, significant jaundice occurs in 6 % of term babies. Bilirubin metabolism. Hb → globin + haem 1g Hb = 34mg bilirubin.
471 views • 25 slides

Neonatal jaundice (N.Hyperbilirubinemia)
Neonatal jaundice (N.Hyperbilirubinemia). Bilirubin metabolism:. RBCs destructions Hb which catabolized in RES by hemeoxygenase enzyme. Biliverdin + Co Unconjucated bilirubin (indirect, lipid soluble) by bilverdin reductase enzyme. Bind to the albumin in the circulation
436 views • 20 slides

NEONATAL JAUNDICE. Dr.mirzarahimi. CAUSES OF PHYSIOLOGIC HYPERBILIRUBINEMIA - 1. Definition: Full term infant 6-8 mg/l00 ml by 3 days Premature infant 10-15 fig/100 ml by 5 days An increased bilirubin load : more than 8.5 mg/kg/day Increased RBCs volume/kg
712 views • 31 slides

IMAGES
VIDEO
COMMENTS
Introduction. Jaundice is the yellow colouring of skin and sclera caused by the accumulation of bilirubin in the skin and mucous membranes. Neonatal jaundice occurs in 60% of term infants and 80% of preterm infants [1] and is caused by hyperbilirubinaemia that is unconjugated (divided into physiological or pathological) or conjugated (always ...
Neonatal jaundice or neonatal hyperbilirubinemia results from elevated total serum bilirubin (TSB) and clinically manifests as yellowish discoloration of the skin, sclera, and mucous membrane. The term jaundice derives from the French word "jaune," which means yellow. It is the most commonly encountered medical problem in the first two weeks of life and a common cause of readmission to the ...
Aug 1, 2017 •. 948 likes • 346,355 views. Sayed Ahmed. approach to neonatal jaundice , Healthcare. 1 of 64. Download Now. Download to read offline. Neonatal jaundice - 2017 - Download as a PDF or view online for free.
Neonatal Jaundice Guide. This document provides an overview of neonatal jaundice, including its epidemiology, pathophysiology, etiology, clinical presentation, management, and complications. Key points include: - Neonatal jaundice is common, occurring in 50-80% of newborns, and is usually harmless. It is caused by elevated bilirubin levels in ...
Jaundice is a yellow discoloration of the skin and eyes caused by hyperbilirubinemia (elevated serum bilirubin concentration). The serum bilirubin level required to cause jaundice varies with skin tone and body region, but jaundice usually becomes visible on the sclera at a level of 2 to 3 mg/dL (34 to 51 micromol/L) and on the face at about 4 to 5 mg/dL (68 to 86 micromol/L).
Presentation and duration of neonatal jaundice. Note the following: Typically, neonatal jaundice presents on the second or third day of life. Jaundice that is visible during the first 24 hours of life is likely to be nonphysiologic; further evaluation is suggested. Infants who present with jaundice after 3-4 days of life may also require closer ...
This document provides information on neonatal jaundice, including definitions, causes, pathophysiology, assessment, diagnosis, signs and symptoms, complications, and management. The key points are: - Neonatal jaundice is the yellow discoloration of skin and mucous membranes due to high bilirubin levels in newborns.
Continue Reading. Kernicterus and neurologic sequelae caused by severe neonatal hyperbilirubinemia are preventable conditions. A structured and practical approach to the identification and care of ...
What should the junior doctor know? Neonatal jaundice or hyperbilirubinaemia, is one of the most commonly observed conditions in the newborn infant. It specifically refers to the distinct yellow discolouration of sclera and skin, resulting from the accumulation of bilirubin. Although neonatal jaundice can be the result of serious underlying pathology, it is more typically a normal transitional ...
Neonatal jaundice, also called icterus, is the yellowish pigmentation of the skin and sclera that appears in newborns, when total bilirubin levels rise above the 95th percentile for age, which is usually around 2 mg/dL. Bilirubin is produced when the body breaks down red blood cells, and it is normally excreted from the body through the liver ...
1) Assessment & treatment of jaundice: Severity of jaundice is judged based on a newborn's age and gestation, as well as clinical presentation, hydration status, and other risk factors; Please refer to local charts (see additional notes section below) Phototherapy
1.2. Care for all babies. A randomised controlled trial (RCT) by Mishra et al. (2009) investigated whether transcutaneous bilirubinometry reduced the need to test for total serum bilirubin (TSB) compared with visual evaluation of neonatal jaundice. Infants of gestational age of 35 weeks or more were enrolled; the exclusion criteria were Rhesus haemolytic disease, neonatal intensive care needs ...
Neonatal Jaundice Overview. Neonatal jaundice describes a condition in which an infant's skin appears yellow within the first few days of life. The yellowish appearance is a sign of an increased blood pigment called Bilirubin, which then settles in the skin. In many cases this is a normal process and occurs in about 2/3 of all healthy newborns.
Neonatal jaundice (NNJ) is a preventable cause of neonatal morbidity and mortality. Improving mothers' knowledge will help with early recognition of NNJ, prompt and appropriate intervention. ... The mean age at presentation was 5 days [range 3 - 7 days]. Out of these 348 neonates, 27 (7.8%) exhibited sign of bilirubin encephalopathy at ...
Classification of neonatal jaundice Physiological jaundice Pathological jaundice. Etiology of physiological jaundice In the first few days after birth, haemoglobulin concentration falls rapidly. Red cell life span of newborn infants is 70 days which is much shorter than that of adults (120 days). Hepatic bilirubin metabolism is less efficiency.
Neonatal jaundice is a condition in newborns marked by high levels of bilirubin in the blood, causing yellowing of the skin and whites of the eyes. Bilirubin levels are often higher in neonates due to increased red blood cell breakdown, liver immaturity, and bacterial colonization. Without treatment, hyperbilirubinemia can cause permanent brain ...
NEONATAL JAUNDICE PPT - Free download as Powerpoint Presentation (.ppt / .pptx), PDF File (.pdf), Text File (.txt) or view presentation slides online. Neonatal hyperbilirubinemia
Background. Jaundice is the most common condition that requires medical attention and hospital readmission in newborns. [ 89] The yellow coloration of the skin and sclera in newborns with jaundice is the result of accumulation of unconjugated bilirubin. In most infants, unconjugated hyperbilirubinemia reflects a normal transitional phenomenon.
Free Google Slides theme, PowerPoint template, and Canva presentation template. We know that being a mom or a dad is hard and scary, but when it comes to Jaundice, we recommend you not to panic. If your newborn's eyes turn a bit yellow, it could be Jaundice, a condition that is common in babies and that is usually non-threatening.
Suggested Medical Management ofConsequences of Persistent Cholestasis - 2. NEONATAL JAUNDICE. Yousef K. Abu-Osba MD Neonatal Intensive Care Unit Jordan Hospital, Amman, Jordan. CAUSES OF PHYSIOLOGIC HYPERBILIRUBINEMIA - 1. Definition: Full term infant 6-8 mg/l00 ml by 3 days Premature infant 10-15 fig/100 ml by 5 days Slideshow 152867 by Anita.
Severe neonatal jaundice (SNNJ) is a leading cause of neonatal morbidity and mortality in low- and middle-income countries (LMICs). Risk mitigation and management modalities for SNNJ have led to marked reduction in complications in high-income countries but not in LMICs likely in part due to knowledge gaps among healthcare providers.
Presentation Transcript. Neonatal jaundice. BILIRUBIN METABOLISM 1-Bilirubin production. 2-Transport in blood. 3-Hepatocellular uptake. 4-Intracellular transport in hepatocytes. 5-Conjugation with glucuronic acid. 6-Secretion into bile ducts. 7- Intestinal metabolism. 8- Renal excretion of bilirubin 9- Renal excretion of urobilinogen.
Presentation Transcript. Neonatal Jaundice • Visible form of bilirubinemia • Adult sclera >2mg / dl • Newborn skin >5 mg / dl • Occurs in 60% of term and 80% of preterm neonates • However, significant jaundice occurs in 6 % of term babies. Bilirubin metabolism Hb → globin + haem 1g Hb = 34mg bilirubin Non - heme source 1 mg / kg ...