- Study protocol
- Open access
- Published: 29 October 2019

A multistage mixed methods study protocol to evaluate the implementation and impact of a reconfiguration of acute medicine in Ireland’s hospitals
- E. Hurley ORCID: orcid.org/0000-0001-6776-1224 1 ,
- S. McHugh 2 ,
- J. Browne 2 ,
- L. Vaughan 3 &
- C. Normand 1
BMC Health Services Research volume 19 , Article number: 766 ( 2019 ) Cite this article
3319 Accesses
2 Citations
4 Altmetric
Metrics details
To address deficits in the delivery of acute services in Ireland, the National Acute Medicine Programme (NAMP) was established in 2010 to optimise the management of acutely ill medical patients in the hospital setting, and to ensure their supported discharge to primary and community-based care. NAMP aims to reduce inappropriate hospital admissions, reduce length of hospital stay and ensure patients receive timely treatment in the most appropriate setting. It does so primarily via the development of Acute Medical Assessment Units (AMAUs) for the rapid assessment and management of medical patients presenting to hospitals, as well as streamlining the care of those admitted for further care. This study will examine the impact of this programme on patient care and identify the factors influencing its implementation and operation.
We will use a multistage mixed methods evaluation with an explanatory sequential design. Firstly, we will develop a logic model to describe the programme’s outcomes, its components and the mechanisms of change by which it expects to achieve these outcomes. Then we will assess implementation by measuring utilisation of the Units and comparing the organisational functions implemented to that recommended by the NAMP model of care. Using comparative case study research, we will identify the factors which have influenced the programme’s implementation and its operation using the Consolidated Framework for Implementation Research to guide data collection and analysis. This will be followed by an estimation of the impact of the programme on reducing overnight emergency admissions for potentially avoidable medical conditions, and reducing length of hospital stay of acute medical patients. Lastly, data from each stage will be integrated to examine how the programme’s outcomes can be explained by the level of implementation.
This formative evaluation will enable us to examine whether the NAMP is improving patient care and importantly draw conclusions on how it is doing so. It will identify the factors that contribute to how well the programme is being implemented in the real-world. Lessons learnt will be instrumental in sustaining this programme as well as planning, implementing, and assessing other transformative programmes, especially in the acute care setting.
Peer Review reports
Ireland, as with other jurisdictions [ 1 , 2 ], has seen a significant reduction in its acute beds with a 13% reduction in in-patient beds between 2007 and 2012 [ 3 ], and has a large unmet demand for long term care beds [ 3 , 4 ]. This situation, along with continued growth in demand for emergency services, is resulting in patients waiting longer in overcrowded Emergency Departments (EDs) [ 5 , 6 , 7 , 8 , 9 ], and often receiving suboptimal care on trolleys and wards which are not fit for purpose [ 10 , 11 ]. In view of this increased demand and reduced capacity, hospitals are finding innovative ways to make better use of existing bed stock by implementing interventions to reduce avoidable admissions, reduce variations in length of stay and improve the safe discharge of patients [ 1 ]. The development of the discipline of Acute Medicine and the introduction of Acute Medical Units (AMUs) is seen as one such approach to manage the rates of increase [ 12 ]. An AMU is defined as ‘... a dedicated facility within a hospital that acts as the focus for acute medical care for patients who have presented as medical emergencies to hospital or who have developed an acute medical illness while in hospital’ [ 13 ]. These Units are also known in other jurisdictions as Acute Medical Assessment Units (AMAUs), Medical Assessment Units (MAUs), Acute Assessment Units (AAUs), Medical Assessment and Planning Units (MAPUs), and Admission and Planning Units (APUs). While there is wide variation in how these Units are designed and operated, it is recommended that they are co-located on the same floor with other acute and emergency services, and are staffed by acute medicine physicians or specialist consultants with an interest in acute medicine. It is expected that the presence of a senior decision maker expedites the clinical decision making process and improves patient care by facilitating timely review of each patient as they arrive in the Unit [ 14 ]. This model of acute care delivery has been adopted in the UK, Australia and New Zealand [ 15 , 16 ], and more recently the Netherlands [ 17 ]. The majority of medical patients presenting to hospitals as emergencies in the UK are now assessed and treated in AMUs, either directly, or after triage in an Emergency Department [ 18 ], and these Units are considered essential for improving the quality of care for patients presenting to hospitals with complex medical conditions [ 19 , 20 ].
The Irish National Acute Medicine Programme
The National Acute Medicine Programme (NAMP) was introduced in Ireland in 2010 to provide a framework for the delivery of acute medical services and to address deficits in the care of acutely ill medical patients presenting as emergencies to Irish hospitals [ 21 ]. Central to the programme was the development of AMUs in all major hospitals, and similar functioning, but smaller AMAUs in smaller hospitals. [A note on terminology used in this study: a fully functioning AMU consists of an AMAU with an associated short stay ward (SSW) for patients whose length of hospital stay is not expected to be greater than 48 h. For consistency, we will refer to Units in the Irish setting as AMAUs, and identify those with an SSW]. As with the UK model, the purpose of these Units is to facilitate the streaming of medical patients either directly from GPs or from ED at triage, into a designated assessment area where they will be rapidly assessed and diagnosed by a senior decision maker (a consultant physician or a registrar/specialist registrar) within a 1 h target and the decision made within a 6 h target to discharge home, admit to an adjacent short stay unit (up to a 48 h stay), or admit to an in-patient ward [ 21 , 22 ].
To assist with the implementation of this service reconfiguration, the National Acute Medicine Programme ‘categorised’ Irish hospitals into 4 generic hospital models, from the smaller Model 1 community/district hospitals to the largest Model 4 hospitals. The type of AMAU at each hospital was determined by the hospital’s model [see Additional file 1 ]. The Programme recognised that Units should be designed firstly around function, such as identifying and clarifying their role in the hospital’s acute services and specifying the patient groups to be assessed there, rather than form (e.g., physical layout and structure) and sites were given the flexibility to adapt the Units to suit local needs and resources [ 21 ]. This approach has been highlighted in Australia as being of significant importance in the performance of AMUs [ 23 ]. In addition to the establishment of these Units, the National Acute Medicine Programme identified four medical patient pathways - from ambulatory care through to care for complex patients requiring longer hospital stays - and recommended specific practice changes in each pathway [ 24 , 25 ] [see Additional file 2 ].
While hospitals were not mandated to adopt this new framework for acute medical care, they were actively encouraged to do so. In 2010, when the NAMP model of care was published, there were eight acute public hospitals with an AMAU. Implementation started over the course of 2012 and 2013, with the last Unit opening in 2014. Currently there are 30 hospitals with an AMAU, representing over 88% of acute public hospitals. Seven out of nine model 4 hospitals, 16 of 17 model 3 hospitals and seven of eight model 2 hospitals have an operating AMAU [ 22 ].
Understanding successful implementation of AMUs
Effectiveness of amus in improving patient outcomes.
There is mixed evidence on the effectiveness of AMUs in improving patient care. Two recent reviews have expanded upon the initial systematic review conducted by Scott et al. in 2009 [ 26 ] and conclude that hospital length of stay, in-hospital mortality and 28-day readmission rates are reduced when AMUs are introduced into hospitals. However, the included studies were of moderate quality; the majority presented aggregate results (unadjusted for potential confounders), and relied on historical controls and ignored secular trends [ 17 , 27 ]. A more recent systematic review by NICE assessed whether admission or assessment through an AMU (compared with direct admission to a general medical ward) increased hospital discharges, improved patient outcomes and hospital resource usage, and found that there is mixed evidence for the benefit of admission through an AMU [ 28 ]. With stricter inclusion and exclusion criteria, their review was limited to just three observational studies [ 29 , 30 , 31 ], which they classed as very low quality. Recognising the continuing growth in the area of Acute Medicine and the fact that over 90% of hospitals in the UK now have an AMU, the NICE committee felt that ongoing assessment of AMUs was crucial, especially in terms of adherence to standards and quality indicators, and called for higher quality research on the impact of AMUs, including measuring improvements in patient flow and reduced length of hospital stay [ 28 ].
Components of AMUs related to better outcomes
The heterogeneity of the AMU models studied in these effectiveness reviews, in terms of Unit organisation, consultant work patterns, ward round frequency, policies on length of stay, and admission criteria, and the fact that most studies have examined a single site, makes it hard to deduce which elements of the AMU are associated with better patient outcomes [ 17 , 27 ]. The Society of Acute Medicine in the UK called for research to describe what features of an AMU contribute most to improved patients outcomes [ 20 ]. In response to this, Reid et al., conducted a second systematic review - this time to examine the evidence base on how best to deliver care in AMUs. They found limited evidence and a significant knowledge gap on the topic. The one component with consistent evidence of improved patient outcomes is the presence of a consultant for a sustained period [ 18 ]. This has been associated with a reduction of potentially avoidable admissions to hospital [ 8 ], reductions in mortality and 28-day readmission rates [ 32 ], and reduced length of hospital stay [ 14 ]. Hence, consultant presence is deemed a core component of AMUs worldwide [ 13 , 15 , 20 , 33 , 34 ] and the Royal College of Physicians in the UK have published recommendations on how to provide this consultant cover [ 35 ]. Vaughan et al., synthesised the literature on the benefits of a multidisciplinary team (MDT) in the acute medical setting on patient experience and clinical outcomes [ 36 ]. They found that there is a consistent, albeit methodologically flawed, body of evidence that supports MDT working in this setting. They highlight that the recent shift toward individualised care plans for patients, and the introduction of care bundles with specific interventions, necessitates a MDT approach to care. Whilst these care bundles and comprehensive care plans have not been extensively studied in the AMU setting, the literature supports the contentions that they are highly adaptable and promote MDT working, while certain components appear highly suitable for transfer into the AMU context [ 36 ].
Determinants of successful implementation of AMUs
There is a significant gap in the acute medicine literature concerning the factors influencing the implementation of these AMUs. To date, there are no published studies which have qualitatively examined the barriers and enablers to the establishment and embedding of these Units. The London Quality Standards programme which aimed to improve the quality of acute and emergency care, set out the minimum quality of care that patients with medical illnesses should expect when admitted to hospital. An evaluation of its implementation identified many barriers and enablers to adherence to standards in acute care [ 37 ], and it is likely that many of these will be of relevance to this study, given the similarity of programme objectives.
As other jurisdictions consider the expansion of AMUs [ 17 ], evaluating the recent, large scale, country-wide, implementation of Units into Irish hospitals provides an excellent opportunity to highlight the factors (contextual and others) which can facilitate or impede the implementation and impact of these Units on patient care.
Approach to evaluation and conceptual frameworks
We will use a mixed methods approach (a multistage evaluation with an embedded explanatory sequential design) to examine whether the programme is achieving its desired outcomes, and how these outcomes are affected by the context within which the programme is operating [ 38 ].
The UK Medical Research Council (MRC) guidance on process evaluation of complex interventions will serve as the overarching framework [ 39 , 40 ]. This framework recognizes that to inform policy and practice, we need to understand not only whether interventions work but how they were implemented, their causal mechanisms, and how effects differ from one context to another [ 39 ]. Programmes are frequently deemed to be ineffective, simply because they have not been implemented as planned [ 41 , 42 ]. Therefore, evaluating how well a programme has been implemented is essential to understanding and interpreting an impact evaluation.
Damshroder’s ‘Consolidated Framework for Implementation Research’ (CFIR) [ 43 ] and Proctor’s ‘ Conceptual Model of Implementation research’ [ 44 ] will be used to understand the determinants of implementation and how they influence outcomes. The CFIR provides a comprehensive taxonomy of constructs that are likely to influence the implementation of complex programmes [ 43 ]. When using the CFIR in post-implementation evaluation studies, a focus on outcomes is essential and the meaningful use of the CFIR in this regard involves linking CFIR constructs (i.e., the determinants of implementation) to outcomes (both implementation & programme outcomes) [ 43 , 45 ]. A recent systematic review by Kirk et al., categorising the empirical use of the framework, found a dearth of studies linking determinants of successful implementation to such outcomes [ 46 ]. Proctor et al., provide a model for distinguishing between implementation outcomes (e.g., adoption, reach and fidelity) and programme outcomes (e.g., service level outcomes - efficiency, effectiveness; patient level outcomes - satisfaction, quality-of-life) and highlights that a programme will not be effective if it is not implemented well [ 44 ]. We will use these frameworks to examine the hypothesized relationships depicted in Fig. 1 .
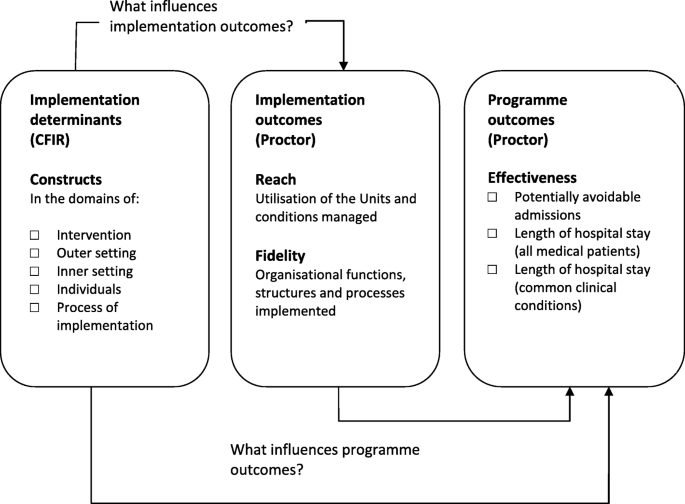
Conceptual approach to the evaluation of the National Acute Medicine Programme. Combining Damshroder’s ‘ Consolidated Framework for Implementation Research’ (CFIR) [ 43 ] and Proctor’s ‘Conceptual Model of Implementation research’ [ 44 ]
This study aims to evaluate the impact of Ireland’s National Acute Medicine Programme and identify the factors influencing its implementation and operation.
Study objectives
Elicit the programme’s theories, and ‘mechanisms of change’ necessary to achieve the desired outcomes
Assess how the programme has been implemented across hospitals by measuring utilisation of the Units and documenting which organisational functions (i.e., structures, resources and processes) have been put in place to support the programme
Identify the factors (contextual and others) which have influenced the implementation of the programme and its outcomes
Determine whether the programme is achieving its outcomes and measure how well variation across sites can be ‘attributed’ to the level of implementation.
Study design
This multistage mixed methods study uses an explanatory sequential design whereby qualitative research will be undertaken to explain quantitative findings (see Table 1 and Fig. 2 ). In Stage 1, documentary analysis and data from expert interviews with the national programme team will be used to develop the programme’s logic model, specifying the underlying programme theory. In Stage 2, implementation effectiveness will be examined by using routine hospital administrative data to assess utilisation of the Units, and by conducting surveys to assess the organisational functions (i.e., structures, resources and processes) put in place at each site to support the Units. In Stage 3, comparative case study work will be conducted at eight sites to explore in detail the factors that have influenced the programme’s implementation and its ability to achieve desired outcomes. In Stage 4, routine administrative hospital data will be analysed again, this time to examine the impact of the programme in reducing length of stay of medical patients. In Stage 5, data from Stages1 to 4 will be integrated to examine how variation in programme outcomes across sites is explained by the level of implementation, and components implemented.
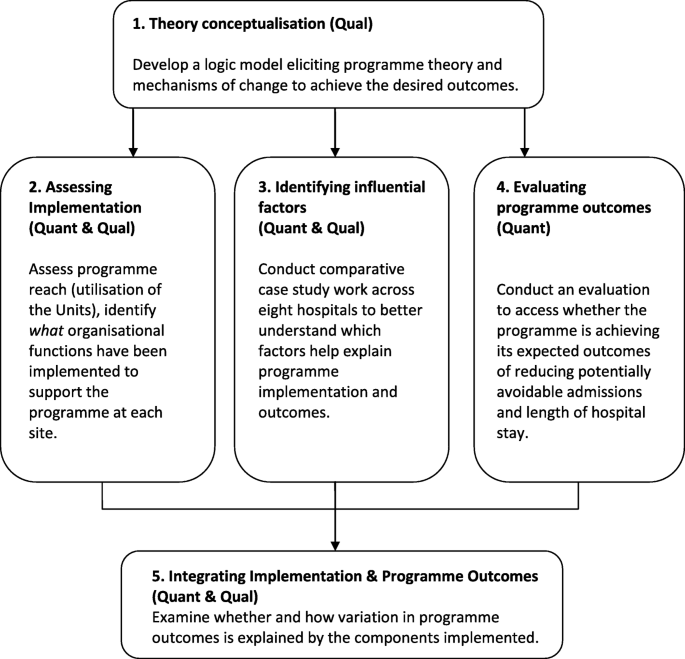
A multistage evaluation of the National Acute Medicine Programme
Stage 1: Theory conceptualization
As suggested by the UK Medical Research Council guidance on process evaluation, unearthing the programme’s theory and depicting same in a logic model is a crucial first step in evaluating a complex intervention [ 40 ]. A logic model can be used to present both process and impact theory and is a replica of what the programme is intended to be which can then be analysed [ 40 , 41 ]. It can be used for identifying the programme’s functions, activities and outputs to assess fidelity, and to understand how the programme interacts with the organisation’s structures and functions.
Data collection and analysis
Following the guidance of Rossi et al. [ 41 ], a stepwise approach to eliciting the programme theory was taken, and this stage is completed. Rossi advises that to describe the theory embodied in an existing programme’s structure and operation, it is necessary that the evaluator work with stakeholders to draw out the theory that is represented in their actions and assumptions. Therefore, a logic model outlining the programme’s theory was developed by a combination of documentary review, key informant interviews, and in-person meetings with the NAMP team. Documents were reviewed to identify the underlying programme theory, the core components of the programme, the expected outcomes and the mechanisms as to how the programme expects to achieve these [ 47 ]. These included the national plan ‘Report of the National Acute Medicine Programme (2010), ‘standards’ and ‘guidance’ for AMUs in other jurisdictions [ 13 , 15 , 48 ] and published literature on their operation [ 18 ] and impact on patient care [ 17 , 27 , 28 ]. Key informant interviews were conducted with the NAMP team - physicians, nurses and allied health professionals with expertise in acute medicine, and programme managers - (past and present NAMP members, n = 6) and an initial group meeting (current members, n = 6) held to understand programme processes and how they could be influenced by the system into which they were introduced. A first draft of the model was developed in the format recommended by the Kellogg Foundation with emphasis not only on the programme’s outcomes and components but the mechanisms by which it expects to achieve these outcomes [ 49 ] and revised through face-to-face discussions with the national team [see Fig. 3 ].
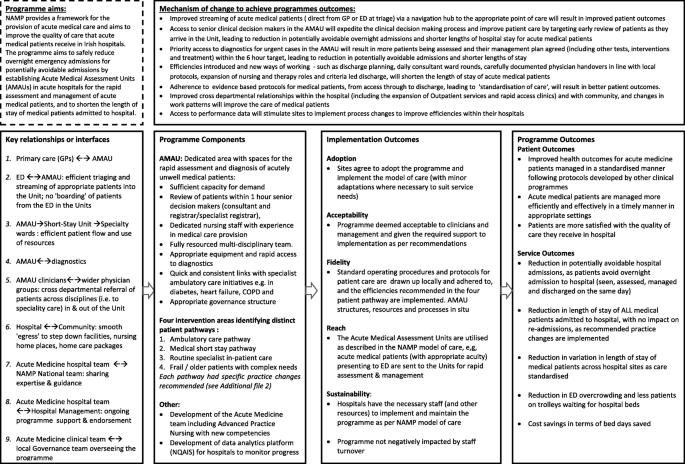
Logic model of the National Acute Medicine Programme
Stage 2. Assessing programme implementation
Studies in the UK and Australasia have shown considerable variation across hospitals in terms of compliance with recommendations on how care should be delivered in AMUs [ 50 , 51 , 52 ]. We are interested in examining whether the NAMP model of care has been implemented as designed. In our study, programme implementation will be assessed in terms of (i) ‘service utilisation’ (programme reach), defined by Rossi as ‘...the extent to which the intended targets actually receive programme services, and (ii) the ‘organisational functions implemented’ again, defined by Rossi as ‘whether the programme’s actual activities and arrangements sufficiently approximate the intended ones ’ [ 41 ].
Measuring the programme’s organisational functions focuses on how well the programme is organising its efforts and using its resources to accomplish the essential programme tasks [ 41 ]. The logic model will be used to develop a survey to collect data on the structure of the Unit (e,g, location, bed capacity, opening hours), resources (e.g., priority access to diagnostics, medical and nursing staff and workforce patterns), processes and procedures (e.g., mode of access to the Unit, referral pathway from ED/GP, patient profiles to be seen, escalation policy, return policy,) and changes in acute care throughout the hospital (e.g., improved patient streaming, integrated discharge planning, use of a common screening tool). Surveys will be completed at each site by the AMAU lead physician or Clinical Nurse Manager (CNM).
Service utilisation (programme reach) will be measured by examining the proportion of ‘emergency medical patients’ streamed through each Unit for 2017 and the case-mix and characteristics of these patients.
Stage 3. Identifying factors that influence programme implementation and outcomes
To identify the factors which have influenced the implementation of the programme and its outcomes, we will conduct comparative case study work, using the approach by Yin which is suited to the complex nature of health services research, and allows for in-depth data gathering on organisational processes and programme impact [ 53 , 54 ]. The purpose of this stage of the evaluation is to understand the factors that are influencing the level of utilisation of the Units in terms of the proportion of acute medical in-patients that are streamed through the AMAU, but also the factors that are influencing the programme’s ability to achieve its desired outcomes. Experience with the intervention, including participants’ perception of it and its compatibility with the hospital system will be explored in detail during this stage.
Selecting the sites for comparative case study research
Cases will be purposively sampled based on the level of utilisation identified during Stage 2, with four ‘high’ and four ‘low’ implementation sites selected. Sampling cases at either end of the implementation spectrum will allow us identify the factors that contribute to or hinder ‘successful’ implementation. This approach has been taken by Damshroder & Lowery in their study assessing implementation determinants for their propensity to distinguish between sites with high versus low implementation effectiveness [ 18 ].
Data collection at sites
We will conduct semi-structured interviews with health professionals (AMAU lead physician, clinical nurse manager for the AMAU, Assistant Director of Nursing for patient flow) to elicit information on the determinants of implementation [see Additional file 3 for list of CFIR constructs].
Analysing and interpreting the case studies
Data will be managed using NVivo 12. Qualitative data (interviews and documents) will be analysed using the Framework method which comprises five stages (familiarisation; identifying a thematic framework; indexing; charting; mapping and interpretation) [ 55 , 56 ]. The CFIR framework will be applied as pre-defined deductive codes, however open coding will also be used to identify factors that do not fit within the definitions of CFIR constructs [ 57 ]. A case memo will be created for each of the eight sites, and constructs rated to reflect the magnitude of their influence on implementation, using the approach recommended by the authors of the CFIR framework [ 45 ]. A matrix will then be created listing the ratings for each construct for each site, and cross case comparison made between high and low implementation sites to identify patterns in ratings of the constructs that distinguish between high and low implementation effectiveness—i.e., constructs that were qualitatively correlated with implementation effectiveness [ 45 ].
Stage 4. Evaluating programme impact
Outcomes were identified during the development of the logic model, which involved an examination of how AMUs are evaluated elsewhere. Programme effectiveness will be assessed by examining changes over time in (i) rates of potentially avoidable admissions (ii) lengths of hospital stay of medical patients (iii) lengths of hospital stay of potentially avoidable medical conditions.
Programme outcome measures will be derived from Hospital In-Patient Enquiry (HIPE) which is an administrative database of all public hospital admissions in Ireland, including episodes of care in the AMAU. Programme impact will be estimated by comparing monthly data from 2009 to 2017, using interrupted time series regression (ITS) and ARIMA (autoregressive integrated moving average), accounting for secular and seasonal trends, and using the proportion of patients treated in the Units as a time varying covariate. Models will be run for individual AMAUs and for all hospitals combined. Several sensitivity analyses will be conducted including using length of stay truncated at 30 days, to account for the deficiencies in community services which can skew the average LOS.
Stage 5. Integration of programme outcomes and programme implementation
To explain the variation in implementation and programme outcomes between sites, we will then construct a joint display table presenting the data for each site - the constructs that were identified as influential and the outcomes achieved - and examine patterns and inconsistencies across and between cases [ 58 , 59 ] In this manner, the constructs which influenced implementation (Stage 3), the level of implementation (Stage 2) and the programme outcomes achieved (Stage 5), will be presented for each case in line with our conceptual framework from Fig. 1 .
This protocol outlines a mixed methods study to evaluate whether the reconfiguration of acute medical care in hospitals, is effective in everyday practice. The study will examine the variation in implementation and effectiveness of Acute Medical Units from a national perspective, and be the first to comprehensively assess the factors that contribute to how well these Units are implemented and how well they perform. This work is timely as other jurisdictions consider the wide-scale introduction of Acute Medical Units [ 17 ]. It addresses the call for high quality research (including qualitative studies) to describe which features of Acute Medicine contribute most to its success [ 20 ]. The work of Reid et al., highlights the lack of research into the active ingredients of the AMU that contribute to its success and the clear gap in knowledge of how best to deliver care in the AMU [ 18 , 27 , 52 , 60 ]. By examining variation in service and patient level outcomes in parallel to the organisational functions (e.g., structures, resources and processes of care), this study will assess the association between implementation of the AMU and outcomes achieved. The comparative case study will identify which components and processes contribute to improved outcomes and importantly, help decipher the factors that have influenced the successful establishment and operation of these Units. The results of this study will inform further refinement of the national programme and contribute to the design of more effective AMAUs.
Our study has some limitations. The fragmented health IT infrastructure in Irish hospitals, and the lack of a Unique Health Identifier, means we are unable to examine the trajectory of care received by patients streamed through the Units, and the impact on outcomes such as 30-day mortality, health services utilisation and quality of life. For this reason, we are examining the efficiency, effectiveness and timeliness of care [ 44 ] which are seen as ‘proximal’ outcomes upstream on the pathway to improved health outcomes [ 61 ]. We will examine changes in potentially avoidable admissions and lengths of hospital stay; the most common outcomes examined in previous studies evaluating the effectiveness of Acute Medical Units. We are also limited in our ability to examine indicators of performance. For example, we are unable to track - for all hospitals participating in the programme- the patient journey from ED through to the AMAU and the length of time spent on this pathway, which is an important indicator of the timeliness of patient care. Work is underway to address these IT shortcomings with the introduction of an Acute Floor Information System (AFIS), which will facilitate tracking of the patient journey and enhance the collection and reporting of these key performance indicators. A second limitation is the inherent risk of confounding that presents in observational studies of this nature. We have tried to minimise these risks by the use of robust statistical techniques such as interrupted time series analysis [ 62 ] at the individual hospital level, and the triangulation of various data sources to elicit a greater understanding of how the programme is resulting in improved outcomes.
This study has many strengths; most notably its explanatory sequential design which strengthens the validity of our findings on what influences implementation and how implementation leads to better outcomes. According to Creswell, combining statistical trends (quantitative date) with personal experiences (qualitative data), provides a better understanding of the research problem than either form of data alone [ 59 ]. Additionally, because complex interventions such as NAMP, tend to be highly context-specific in their effects, generalising the results of effect estimation for policy and practice requires more nuanced analyses of why these effects occur [ 63 ].
Second, programme and implementation theory will be used throughout; from the creation of the logic model which provides a blueprint of the programme to be analysed [ 40 ], to the use of the Consolidated Framework for Implementation Research [ 43 ] to guide data collection, measurement, coding, analysis and reporting of the findings of the comparative site work.
Third, we will use robust statistical methods to evaluate the performance and impact of these Units. Recent reviews of the literature on the effectiveness of these Units have highlighted the shortcomings in the research to date with many studies reporting effect estimates that have not taken into consideration potential biases (such as selection bias), confounding and underlying secular and seasonal trends [ 17 , 27 , 28 ]. We endeavour to minimise the influence of these on the effect estimates by using interrupted time series analysis and applying ARIMA modelling to estimate programme impact, adjusting for autocorrelation and seasonality [ 64 , 65 , 66 ].
Finally, the access to and collaboration with the national programme is a key strength of the study, as it facilities the co-development of the programme theory from the outset.
We expect the findings of this evaluation to be of interest to a wide audience given the growing need to demonstrate effectiveness of complex interventions. Findings on the mechanisms and contexts that optimise the implementation of this complex multi-faceted intervention will be useful to those developing and implementing other change programmes, especially given the growing realisation that failure to deliver effective services is largely attributable to the lack of knowledge on how best to implement and sustain these changes. This is a formative evaluation and the National team tasked with implementing and overseeing the programme are keenly interested in knowing what the determinants of a successful AMU are and how this information can be used to support sites struggling to reach full potential. Additionally, those in jurisdictions where the discipline of acute medicine is better developed and AMUs are well established, as well those in countries contemplating expansion of their AMUs, will be interested in the challenges that implementers face, and how context ‘interacts’ with and ‘shapes’ the programme being implemented.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author.
Abbreviations
- Acute medicine
Acute Medical Assessment Unit
Acute Medical Unit
Consolidated Framework for Implementation Research
Emergency Department
General Practitioner
Health Service Executive
National Acute Medicine Programmme
Short Stay Ward
Ewbank, L., Thompson, J., McKenna, H., NHS hospital bed numbers: past, present, future., The King’s Fund, Editor. 2017, The King's Fund: https://www.kingsfund.org.uk/publications/nhs-hospital-bed-numbers .
Google Scholar
OECD, editor. Health care activities: Hospital beds, in Health at a glance, OECD, Editor: OECD; 2015. http://www.oecd-ilibrary.org/sites/health_glance-2015-en/06/03/index.html?itemId=/content/chapter/health_glance-2015-32-en&mimeType=text/html . Accessed 12 Dec 2018.
Wren M-A, Keegan C, Walsh B, Bergin A, Eighan J, et al. Projections of demand for healthcare in Ireland, 2015–2030. First report from the hippocrates model, The Economic and Social Research Institute (ESRI), Editor. Dublin: ESRI; 2017.
Book Google Scholar
Smyth B, et al. Planning for Health: Trends and Priorities to Inform Health Service Planning 2017. Report from the Health Service Executive. Dublin: Health Service Executive; 2017.
Pallin DJ, et al. Population aging and emergency departments: visits will not increase , Lengths-Of-Stay And Hospitalizations Will. Health Affairs. 2013;32(7):1306–12.
Article PubMed Google Scholar
Ham C. Emergency department pressures need to be tackled through integrated urgent and emergency care. BMJ. 2015;350:h322.
O'Cathain A, et al. A system-wide approach to explaining variation in potentially avoidable emergency admissions: national ecological study. BMJ Qual Saf. 2014;23(1):47–55.
Pinkney J, et al. How can frontline expertise and new models of care best contribute to safely reducing avoidable acute admissions? A mixed-methods study of four acute hospitals. Health Serv Deliv Res. 2016;4(3).
Article Google Scholar
Karakusevic S. Understanding patient flow in hospitals, Nuffield Trust, Editor. Nuffield Trust: London; 2016.
Carter EJ, Pouch SM, Larson EL. The relationship between emergency department crowding and patient outcomes: a systematic review. J Nurs Scholarsh. 2014;46(2):106–15.
Hoot NR, Aronsky D. Systematic Review of Emergency Department Crowding: Causes, Effects, and Solutions. Ann Emerg Med. 2008;52(2):126–136.e1.
Article PubMed PubMed Central Google Scholar
Mason S, et al. Innovations to reduce demand and crowding in emergency care; a review study. Scand J Trauma Resusc Emerg Med. 2014;22:55.
Royal College of Physicians, Acute medical care. The right person, in the right setting - first time. Report of the Acute Medicine Task Force. London: Royal College of Physicians; 2007.
McNeill G, et al. What is the effect of a consultant presence in an acute medical unit? Clin Med (Lond). 2009;9(3):214–8.
ACI Acute Care Taskforce Medical Assessment Unit Working Group. NSW Medical Assessment Unit Model of care. Sydney: NSW Agency for Clinical Innovation; 2014.
Internal Medicine Society of Australia and New Zealand, Standards for Medical Assessment and Planning Units in Public and Private Hospitals. 2006.
van Galen LS, et al. Acute medical units: the way to go? A literature review. Eur J Intern Med. 2017;39:24–31.
Reid LEM, et al. How is it best to deliver care in acute medical units? A systematic review. QJM. 2017. https://doi.org/10.1093/qjmed/hcx161 .
Schipper EM. Acute medical units, more capacity without increasing resources. Eur J Intern Med. 2017;39:e13.
Holland M, et al. Acute medical units, definitely the way to go. Eur J Intern Med. 2017;39:e10–1.
Royal College of Physicians of Ireland, et al. Report of the National Acute Medicine Programme. Dublin: National Acute Medicine Programme; 2010.
O'Reilly O, et al. National Acute Medicine Programme--improving the care of all medical patients in Ireland. J Hosp Med. 2015;10(12):794–8.
Jenkins P, Barton L, McNeil G. Contrasts in acute medicine: a comparison of the British and Australian systems for managing emergency medical patients. MJA. 2010;193(4):227–8.
PubMed Google Scholar
Royal College of Physicians of Ireland and Health Service Executive, National Acute Medicine Programme. 2012.
Royal College of Physicians of Ireland and Health Service Executive, National Acute Medicine Programme. Key elements of the programme to deliver the best patient outcomes. 2012.
Scott I, Vaughan L, Bell D. Effectiveness of acute medical units in hospitals: a systematic review. Int J Qual Health Care. 2009;21(6):397–407.
Reid LE, et al. The effectiveness and variation of acute medical units: a systematic review. Int J Qual Health Care. 2016;28(4):433–46.
National Institute for Health and Care Excellence. Chapter 24 Assessment through acute medical units. In: Emergency and acute medical care in over 16s: service delivery and organisation. DRAFT NICE guideline for consultation; 2017.
Li JY, et al. Outcomes of establishing an acute assessment unit in the general medical service of a tertiary teaching hospital. Med J Aust. 2010;192(7):384–7.
Rooney T, et al. Impact of an acute medical admission unit on hospital mortality: a 5-year prospective study. Qjm. 2008;101(6):457–65.
Article CAS PubMed Google Scholar
Coary R, et al. Does admission via an acute medical unit influence hospital mortality? 12 years’ experience in a large Dublin hospital. Acute Med. 2014;13(4):152–8.
CAS PubMed Google Scholar
Bell D, et al. Consultant input in acute medical admissions and patient outcomes in hospitals in England: a multivariate analysis. PLoS One. 2013;8(4):e61476.
Article CAS PubMed PubMed Central Google Scholar
McGovern E. Acute medical assessment units: a literature review. Health Services Executive: Dublin; 2013.
Royal College of Physicians: Designing Services. Acute internal medicine services: Acute medical unit. 2017; Available from: http://www.rcpmedicalcare.org.uk/designing-services/specialties/acute-internal-medicine/services-delivered/acute-medical-unit/ . Cited 2017 12/7/2017.
Royal College of Physicians, Delivering a 12-hour, 7-day consultant presence on the acute medical unit. Appendix 1: Example calculation of numbers of patient contacts per day., in Acute care toolkit 4 . 2012.
Vaughan L, Mitchell A. Multi-disciplinary teams for better patient experience and clinical outcomes: the context of providing a multi-professional approach, in RCPE UK Consensus Conference on Acute medicine. London: Journal of the Royal College of Physicians of Edinburgh; 2013. p. 23–9.
Vaughan LM, Machaqueiro S, Gaskins M, Imison C. The London Quality Standards: A case study in changing clinical care. London: Research report, N. Trust, Editor; 2017.
Bamberger M, Rao V, Woolcock M. In: Tashakkori A, Teddlie C, editors. Editors Using mixed-methods in monitoring and evaluation: Experiences from international development, in Sage Handbook of Mixed- Methods in Social and Behavioral Research. Thousand Oaks: Sage Publications Inc; 2010. p. 613–42.
Moore GF, et al. Process evaluation of complex interventions. UK Medical Research Council (MRC) guidance, MRC population health science research network, editor. London: UK Medical Research Council; 2015.
Moore GF, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ. 2015;350:h1258.
Rossi P, Lipsey M, Freeman H. Evaluation: a systematic approach. 7th ed. ed. Thousand Oaks: SAGE Publications, Inc; 2004.
Dobson D, Cook TJ. Avoiding type III error in program evaluation: results from a field experiment. Eval Porgram Plan. 1980;3(4):269–76.
Damschroder LJ, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50.
Proctor E, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Admin Pol Ment Health. 2011;38(2):65–76.
Damschroder LJ, Lowery JC. Evaluation of a large-scale weight management program using the consolidated framework for implementation research (CFIR). Implement Sci. 2013;8:51.
Kirk MA, et al. A systematic review of the use of the consolidated framework for implementation research. Implement Sci. 2016;11(1):72.
McHugh S, et al. Evaluating the implementation of a national clinical programme for diabetes to standardise and improve services: a realist evaluation protocol. Implement Sci. 2016;11(1):107.
NHS West Midlands Quality Review Service, Quality Standards for Acute Medical Units (AMUs). Version 2. 2012.
W.K Kellogg Foundation. Logic Model Development Guide: Using Logic Models to Bring Together Planning, Evaluation, and Action. Michigan: W. K Kellogg Foundation; 2004.
Ward D, et al. Acute medical care. The right person, in the right setting--first time: how does practice match the report recommendations? Clin Med (Lond). 2009;9(6):553–6.
McNeill GBS, et al. Optimizing care for acute medical patients: the Australasian medical assessment unit survey. Intern Med J. 2011;41(1a):19–26.
Reid L, Rae R. Structures in AMUs - Qualitative and Quantitative, in Edinburgh International Conference of Medicine. Past, Present & Future. Edinburgh: Health Service Executive; 2016.
Yin RK. Enhancing the quality of case studies in health services research. Health Serv Res. 1999;34(5 Pt 2):1209–24.
CAS PubMed PubMed Central Google Scholar
Fulop N, et al. Implementing changes to hospital services: factors influencing the process and ‘results’ of reconfiguration. Health Policy. 2012;104:128–35.
Gale NK, et al. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol. 2013;13:117.
Crowe S, et al. The case study approach. BMC Med Res Methodol. 2011;11(1):100.
Desveaux L, et al. Improving the appropriateness of antipsychotic prescribing in nursing homes: a mixed-methods process evaluation of an academic detailing intervention. Implement Sci. 2017;12(1):71.
O’Cathain A, Murphy E, Nicholl J. Three techniques for integrating data in mixed methods studies. BMJ. 2010;341:c4587.
Creswell JW. A concise introduction to mixed methods research; 2015.
Reid L, Lone NI, Morrison ZJ, Weir CJ, Jones MC. An Examination of Acute Medical Units in Scottish Hospitals, in IMSANZ 17. Wellngton: Health Service Executive; 2017.
Glasgow RE, Brownson RC, Kessler RE. Thinking about health-related outcomes: what do we need evidence about? Clin Transl Sci. 2013;6(4):286–91.
Wagner A, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309.
Gillies C, Freemantle N, Grieve R, Sekhon J, Forder J. Advancing quantitative methods for the evaluation of complex interventions. In Raine R, Fitzpatrick R, Barratt H, Bevan G, black N, Boaden R, et al. challenges, solutions and future directions in the evaluation of service innovations in health care and public health. Health Serv Deliv Res. 2016;4(16):37–54.
Gilmour S, et al. Using intervention time series analyses to assess the effects of imperfectly identifiable natural events: a general method and example. BMC Med Res Methodol. 2006;6:16.
Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46(1):348–55.
Nelson BK. Time series analysis using autoregressive integrated moving average (ARIMA) models. Acad Emerg Med. 1998;5(7):739–44.
Download references
Acknowledgments
We would like to acknowledge the valuable assistance of the NAMP team in drawing up the logic model and their patience in answering the many questions the primary researcher had in relation to understanding the programme’s theories, its mechanisms of change, and the programme’s components, as well as facilitating access to the sites. The team however, have not had input into the design or conduct of the evaluation. Additionally, we are very grateful to the Health Intelligence Unit of the HSE who developed the NQAIS Clinical tool, and were instrumental in the identification of measures to assess programme impact.
The Irish Health Services Executive (HSE), commissioned this evaluation which was peer reviewed and awarded funding (Project ref. 205377/14065). However, they had no role in the study design, data collection, analysis, interpretation of results or writing the manuscript.
Author information
Authors and affiliations.
Centre for Health Policy and Management, Trinity College Dublin, Dublin, Ireland
E. Hurley & C. Normand
School of Public Health, University College Cork, Cork, Ireland
S. McHugh & J. Browne
Nuffield Trust, London, UK
You can also search for this author in PubMed Google Scholar
Contributions
EH designed the study and wrote the initial draft of the paper. SMH, JB, LV and CN contributed to the study design and revised the manuscript for publication. All authors have given final approval of the version to be published and agree to be accountable for all aspects of the work.
Corresponding author
Correspondence to E. Hurley .
Ethics declarations
Ethics approval and consent to participate.
Ethical approval was granted by the Health Policy and Management and Centre for Global Health (HPM/CGH) Research Ethics Committee, Trinity College Dublin, Ireland in June 2017 (25/2017/01). Each participant in the study will be asked for written informed consent, and their data will be anonymised for analysis and reporting. Anonymity will be assured at each case study site and all participants will be given a unique ID number. Any potentially identifiable information will be removed prior to reporting and publishing the findings.
Consent for publication
Not applicable.
Competing interests
EH, the primary author, receives an educational grant from the HSE. However, this agency does not have input into the design of the study or the analysis, and interpretation of data, or the writing of this manuscript. SMH, JB, LV and CN have no competing interests to declare.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1..
Description of AMAUs to be established by hospital model. Word document describing the Units.
Additional file 2.
The four patient pathways specified by NAMP and the practice changes recommended. Word document (table format) describing the four patient pathways.
Additional file 3.
Description of the five CFIR domains and constructs within each domain. Word document (table format) describing the CFIR constructs.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Hurley, E., McHugh, S., Browne, J. et al. A multistage mixed methods study protocol to evaluate the implementation and impact of a reconfiguration of acute medicine in Ireland’s hospitals. BMC Health Serv Res 19 , 766 (2019). https://doi.org/10.1186/s12913-019-4629-5
Download citation
Received : 26 March 2019
Accepted : 11 October 2019
Published : 29 October 2019
DOI : https://doi.org/10.1186/s12913-019-4629-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Acute medical unit
- System reconfiguration
- Programme implementation
- Mixed methods
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]

First Year in a Multilingual University pp 31–53 Cite as
Methodology: Multiple-Case Qualitative Study
- Feng Ding 2
- First Online: 01 July 2021
231 Accesses
This longitudinal multiple-case study is qualitative in nature, with data collection lasting for more than a year. As this study set out to understand the experiences, feelings, emotions, conceptions, and understanding of students about their experiences, a qualitative research approach was adopted to “identify issues from the perspective of” the participants, and to study them in a natural setting (Hennink et al., 2011, p. 9).
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Apple Newspaper, 28/04/2013, http://hk.apple.nextmedia.com/news/art/20130428/18242744
Source: http://life.dayoo.com/edu/113887/201301/22/113887_28581159.htm
University of Hong Kong Public Poll, 2012. http://hkupop.hku.hk/english/release/release937.html
Buddy writer, 2010-1011 http://web02.carmelss.edu.hk/buddingwriters/index.php?option=com_content&view=article&id=971:importing-students-from-the-mainland-brings-more-harm-than-good-to-hong-kong&catid=109&Itemid=66
Bazeley, P., & Richards, L. (2000). The NVivo qualitative project book . Sage.
Book Google Scholar
Bogdan, R. C., & Biklen, S. K. (2007). Qualitative research for education. An introduction to theory and methods . Allyn and Bacon.
Google Scholar
Chee, W. C. (2012). Envisioned belonging: Cultural differences and ethnicities in Hong Kong schooling. Asian Anthropology (1683478X), 11 (1), 89–105.
Article Google Scholar
Creswell, J. W. (2007). Qualitative inquiry and research design: Choosing among five approaches . Sage.
Denzin, N. K., & Lincoln, Y. S. (2000). Handbook of qualitative research . Sage Publications.
Ding, F., & Stapleton, P. (2015). Self-emergent peer support using online social networking during cross-border transition. Australasian Journal of Educational Technology, 31 (6), 671–684.
Dörnyei, Z. (2007). Research methods in applied linguistics: Quantitative, qualitative, and mixed methodologies . Oxford University Press.
Evans, S. (2011). Hong Kong English and the professional world. World Englishes, 30 (3), 293–316.
Gao, X. (2010). Strategic language learning: The roles of agency and context . Multilingual Matters.
Hennink, M., Hutter, I., & Bailey, A. (2011). Qualitative research methods . Sage.
Miles, M. B., & Huberman, A. M. (1994). Qualitative data analysis: An expanded sourcebook (2nd ed.). Sage.
Ministry of Education. (2007). College English curriculum requirements . Foreign Language Teaching and Research Press.
Patton, M. (1990). Qualitative evaluation and research methods . Sage Publications.
Patton, M. Q. (2002). Qualitative research and evaluation methods . Sage Publications.
Reason, R., Terenzini, P., & Domingo, R. (2006). First things first: Developing academic competence in the first year of college. Research in Higher Education, 47 (2), 149–175.
Stake, R. E. (2006). Multiple case study analysis . Guilford Press.
Strauss, A. L., & Corbin, J. M. (1998). Basics of qualitative research: Techniques and procedures for developing grounded theory (2nd ed.). Sage Publications.
Thaler, R. H., & Sunstein, C. R. (2009). Nudge: Improving decisions about health, wealth, and happiness . Penguin Books.
Willis, J. (2007). Qualitative research methods in education and educational technology . Sage Publications.
Xie, C. X. (2010). Mainland Chinese students’ adjustment to studying and living in Hong Kong . Unpublished doctoral dissertation of the University of Leicester.
Yin, R. (1994). Case study research: Design and methods . Sage.
Yin, R. (2003). Case study research: Design and methods . Sage.
Download references
Author information
Authors and affiliations.
Guangdong University of Foreign Studies, Guangzhou, Guangdong, China
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter.
Ding, F. (2021). Methodology: Multiple-Case Qualitative Study. In: First Year in a Multilingual University. Springer, Singapore. https://doi.org/10.1007/978-981-16-0796-7_3
Download citation
DOI : https://doi.org/10.1007/978-981-16-0796-7_3
Published : 01 July 2021
Publisher Name : Springer, Singapore
Print ISBN : 978-981-16-0795-0
Online ISBN : 978-981-16-0796-7
eBook Packages : Education Education (R0)

Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Qualitative case study data analysis: an example from practice
Affiliation.
- 1 School of Nursing and Midwifery, National University of Ireland, Galway, Republic of Ireland.
- PMID: 25976531
- DOI: 10.7748/nr.22.5.8.e1307
Aim: To illustrate an approach to data analysis in qualitative case study methodology.
Background: There is often little detail in case study research about how data were analysed. However, it is important that comprehensive analysis procedures are used because there are often large sets of data from multiple sources of evidence. Furthermore, the ability to describe in detail how the analysis was conducted ensures rigour in reporting qualitative research.
Data sources: The research example used is a multiple case study that explored the role of the clinical skills laboratory in preparing students for the real world of practice. Data analysis was conducted using a framework guided by the four stages of analysis outlined by Morse ( 1994 ): comprehending, synthesising, theorising and recontextualising. The specific strategies for analysis in these stages centred on the work of Miles and Huberman ( 1994 ), which has been successfully used in case study research. The data were managed using NVivo software.
Review methods: Literature examining qualitative data analysis was reviewed and strategies illustrated by the case study example provided. Discussion Each stage of the analysis framework is described with illustration from the research example for the purpose of highlighting the benefits of a systematic approach to handling large data sets from multiple sources.
Conclusion: By providing an example of how each stage of the analysis was conducted, it is hoped that researchers will be able to consider the benefits of such an approach to their own case study analysis.
Implications for research/practice: This paper illustrates specific strategies that can be employed when conducting data analysis in case study research and other qualitative research designs.
Keywords: Case study data analysis; case study research methodology; clinical skills research; qualitative case study methodology; qualitative data analysis; qualitative research.
- Case-Control Studies*
- Data Interpretation, Statistical*
- Nursing Research / methods*
- Qualitative Research*
- Research Design
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Impacts of COVID-19 on clinical research in the UK: A multi-method qualitative case study
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliations School of Population Health and Environmental Sciences, King’s College London, United Kingdom, National Institute for Health Research Biomedical Research Centre at Guy’s and St. Thomas’ NHS Foundation Trust and King’s College London, United Kingdom
Roles Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Writing – original draft, Writing – review & editing
Roles Conceptualization, Funding acquisition, Writing – review & editing
- David Wyatt,
- Rachel Faulkner-Gurstein,
- Hannah Cowan,
- Charles D. A. Wolfe

- Published: August 31, 2021
- https://doi.org/10.1371/journal.pone.0256871
- Peer Review
- Reader Comments
Clinical research has been central to the global response to COVID-19, and the United Kingdom (UK), with its research system embedded within the National Health Service (NHS), has been singled out globally for the scale and speed of its COVID-19 research response. This paper explores the impacts of COVID-19 on clinical research in an NHS Trust and how the embedded research system was adapted and repurposed to support the COVID-19 response.
Methods and findings
Using a multi-method qualitative case study of a research-intensive NHS Trust in London UK, we collected data through a questionnaire (n = 170) and semi-structured interviews (n = 24) with research staff working in four areas: research governance; research leadership; research delivery; and patient and public involvement. We also observed key NHS Trust research prioritisation meetings (40 hours) and PPI activity (4.5 hours) and analysed documents produced by the Trust and national organisation relating to COVID-19 research. Data were analysed for a descriptive account of the Trust’s COVID-19 research response and research staff’s experiences. Data were then analysed thematically. Our analysis identifies three core themes: centralisation; pace of work; and new (temporary) work practices. By centralising research prioritisation at both national and Trust levels, halting non-COVID-19 research and redeploying research staff, an increased pace in the setup and delivery of COVID-19-related research was possible. National and Trust-level responses also led to widescale changes in working practices by adapting protocols and developing local processes to maintain and deliver research. These were effective practical solutions borne out of necessity and point to how the research system was able to adapt to the requirements of the pandemic.
The Trust and national COVID-19 response entailed a rapid large-scale reorganisation of research staff, research infrastructures and research priorities. The Trust’s local processes that enabled them to enact national policy prioritising COVID-19 research worked well, especially in managing finite resources, and also demonstrate the importance and adaptability of the research workforce. Such findings are useful as we consider how to adapt our healthcare delivery and research practices both at the national and global level for the future. However, as the pandemic continues, research leaders and policymakers must also take into account the short and long term impact of COVID-19 prioritisation on non-COVID-19 health research and the toll of the emergency response on research staff.
Citation: Wyatt D, Faulkner-Gurstein R, Cowan H, Wolfe CDA (2021) Impacts of COVID-19 on clinical research in the UK: A multi-method qualitative case study. PLoS ONE 16(8): e0256871. https://doi.org/10.1371/journal.pone.0256871
Editor: Quinn Grundy, University of Toronto, CANADA
Received: April 14, 2021; Accepted: August 17, 2021; Published: August 31, 2021
Copyright: © 2021 Wyatt et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: Data from this study take the form of interview transcripts, Hospital Trust and national documents, and observations of closed meetings. These data cannot be shared publicly, but extracts from interviews are presented within the body of the paper that make up the "minimal dataset."
Funding: DW, RFG, HC and CADW are all funded by the National Institute for Health Research ( http://nihr.ac.uk/ ) Biomedical Research Centre at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London (Grant number IS‐BRC‐1215‐20006). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Competing interests: No
Introduction
Clinical research is a core part of the global response to COVID-19. The United Kingdom (UK), with its research system embedded within the National Health Service (NHS), has been singled out by commentators globally for the scale and speed of its COVID-19 research response, particularly in terms of trial recruitment [ 1 – 3 ]. Reporting from within the UK context, Darzi et al. suggest that participating in clinical trials should be part of the clinical pathway for all COVID-19 patients [ 4 ]. To date, 95 nationally prioritised COVID-19 research projects, labelled Urgent Public Health studies, have commenced [ 5 ]. These and a large number of other COVID-19 studies have rapidly been set up and rolled out across UK hospitals. Supporting and facilitating such research has been made possible by the widespread reorganisation of the NHS’ existing embedded research infrastructure. This reorganisation was initiated by the UK’s Department Health and Social Care (DHSC), which on 16 th March 2020 stated that all National Institute for Health Research (NIHR) funded staff should “prioritise nationally-sponsored COVID-19 research activity” [ 6 ]. They later clarified, stating “the NIHR Clinical Research Network is pausing the site set up of any new or ongoing studies at NHS and social care sites that are not nationally prioritised COVID-19 studies [ 6 ].” Such decisions were said to “enable our research workforce to focus on delivering the nationally prioritised COVID-19 studies or enable redeployment to frontline care where necessary [ 6 ].” To date, reports have focused on the outputs of this research, such as the outcomes of vaccine studies or results of treatment trials, and on frontline clinical staffing, healthcare provision and resource strains faced by hospitals and health care systems at national and global levels [ 7 – 12 ]. As yet, there has been no analysis of the organisation of the research response and the broader impact of the reorganisation of hospitals and research facilities that has allowed clinical research and emergency care work to take place during the pandemic.
In this paper we provide a detailed exploration of how the embedded research infrastructure in one NHS Trust in South London. Throughout this paper, we e use the pseudonym South London Acute Trust (SLAT) to avoid direct identification. This Trust was repurposed to support the completion of COVID-19 research and delivery of frontline care. SLAT is one of the UK’s most research-active Trusts, annually recruiting over 19,000 patients to more than 550 studies. Between February and December 2020, SLAT opened over 80 COVID-19 studies, with more than 18 of these classed as Urgent Public Health studies, recruiting over 7,000 participants. Within this context, we ask: what have been the impacts of COVID-19 on SLAT’s clinical research system, and how has the embedded research system been adapted and repurposed to support the COVID-19 response?
Prior to the pandemic, the process of setting up and managing a clinical research study within a UK NHS Trust involved multiple steps and several actors. Decisions on whether or not to open specific studies rested primarily with the relevant clinical directorate who would vet the study for its appropriateness, scientific merit and feasibility. Other processes were centralised by the Trust’s Research and Development (R&D) governance office, like the sponsorship review (that is, deciding whether the Trust will take responsibility for the study and study compliance) or assisting researchers to gain approvals from national regulatory bodies like the Medicines and Healthcare products Regulatory Agency (MHRA) and the Health Research Authority (HRA). With approvals in place, R&D would then assess whether sufficient resources were available to support the study (the capacity and capability review). Completing this process was often both onerous and time consuming. As a result of the COVID-19 pandemic, substantial parts of this process were reconfigured, as we detail below.
This is a case study of how the embedded research infrastructure at one NHS Trust was repurposed to support the delivery of frontline care and COVID-19 research. The case study method allowed us to track how the research system was adapting in real time, and enabled an in-depth look at the processes and mechanisms that have underpinned operational changes [ 13 ]. As an instrumental case study, one that focuses on socially, historically and politically situated issues, we use a single site to examine issues that are also faced by other hospital Trusts [ 14 ]. We employed an online questionnaire of research-involved staff, document analysis of emails and official national and Trust documents, observations of planning meetings and semi-structured interviews. Data were collected from individuals working in four levels of the research infrastructure: (1) central research oversight and governance (including R&D leads and research governance staff); (2) principal investigators (PIs); (3) the research delivery workforce (including research nurses, clinical research practitioners, data analysts and research managers); and (4) Patient and Public Involvement (PPI) managers and PPI representatives. Triangulating these four data sources and four levels allowed us to consider the representativeness of our data across the case. Redeployment figures and wider workforce information were provided through a request to SLAT’s research management office.
Sampling and data collection
Data were collected by DW, RFG and HC over a period of six months, from May to October 2020. In the first stage of research, an online questionnaire was disseminated to all research-involved staff at SLAT (approx. 700) on 18 th May 2020 via pre-existing mailing lists. The questionnaire closed on 10 th June 2020 with 170 responses, yielding a response rate of approximately 24%. Whilst 24% would be an inadequate response rate for statistical analysis [ 15 ], it was not intended as a validated survey, but rather a method to gain a broad understanding of staff’s experiences of the COVID-19 research response, with most questions open-ended. We received completed questionnaires from nearly a quarter of research staff during the pandemic. The questionnaire also enabled us to identify and recruit a maximum variation sample of staff involved in the research response across the four groups to interview. Interviews allowed us to explore in more depth some of the recurring themes first identified in the questionnaire.
Interview participants were also recruited using purposive and snowball sampling with an aim to maximise the representation of a variety of experiences across the case [ 16 ]. Key staff within SLAT were identified based on searching the Trust’s website, reviewing staff lists and by speaking to senior personnel for guidance. Interviews were conducted digitally on Microsoft Teams and were recorded and transcribed verbatim. Interviews focused on participants’ work prior to the pandemic, how this work has changed as a result of COVID-19, and the short and long term impacts of COVID-19 on health research more broadly.
Additionally, we obtained permission to observe the regular research prioritisation meetings convened by the Trust’s Director of R&D. These meetings took place over Microsoft Teams once or twice a week and were attended by an average of 10 senior clinical, research and research delivery leaders per session. We attended the meetings as non-participant observers, taking notes and recording proceedings. Recordings were transcribed verbatim. We also analysed all documents that were produced or circulated in connection to the prioritisation meetings. These included email discussions about specific projects, national directives, Trust protocols as well as the applications submitted by investigators to the prioritisation committee.
Lastly, we attended the handful of PPI meetings that were held by the few active PPI groups during this period. We participated in discussions about specific research projects and heard participants’ experiences of PPI during the pandemic. PPI is a core part of the pre-COVID-19 research and research design process [ 17 ]. It was therefore important that changes to PPI were considered within our study. We were also able to present our research and get feedback from groups about our aims. PPI meetings were not recorded, but detailed notes were taken during each session.
Conducting qualitative research during the COVID-19 pandemic has required us to adapt data collection methods to accommodate restrictions on face-to-face meetings and access to the hospital. Studies note that while video conferencing has many benefits, issues such as the familiarity of participants with online platforms and access to technology and high-speed internet can be barriers to the successful use of these technologies in interviewing [ 18 , 19 ]. We experienced only a handful of technical problems in our interviews. In all but two instances, interviews were conducted with cameras on so that we could observe non-verbal communication [ 20 ].
Our data were managed and analysed through NVivo 12 using a two stage process [ 21 ]. In the first stage, we analysed the data for a descriptive and narrative account, paying attention to the contours of the emerging response to COVID-19, including national and Trust decision-making and action [ 22 ]. In the second stage we used thematic analysis to develop an analytic account based on emerging themes [ 21 , 23 ]. Data were coded for key themes independently by DW, RFG and HC iteratively throughout the data collection process. Codes and core themes were then discussed and verified across the researchers. As part of our analysis process, we also presented initial findings to research staff at SLAT and at another NHS Trust. These methods of challenging our analysis both internally and externally were crucial for ensuring we reflected on our own influences on the data and the data’s utility beyond our specific case [ 24 ].
Ethics approval for the study was granted by North East—Newcastle & North Tyneside 2 REC (reference: 20/NE/0138).
We completed 24 interviews, lasting from 24 to 105 minutes (mean average of 52 minutes), observed approximately 40 hours of research prioritisation meetings and 4.5 hours of PPI meetings, and received 170 responses to the questionnaire. In the results that follow our interview participants are divided into four groups. We identify participants using a letter to denote group and number for interview within this group:—G-n (Governance/R&D staff), R-n (Research leaders/PIs), D-n (Research delivery staff), P-n (PPI managers). 3 participants sit in more than one of these groups due to their multiple roles within the Trust. These participants were interviewed using questions from interview guides for all relevant groups. Questionnaire participants are identified as Q-n, followed by a brief description of their role. See Tables 1 and 2 for a breakdown of participants.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0256871.t001
https://doi.org/10.1371/journal.pone.0256871.t002
Centralisation: Prioritising COVID-19 research and redeploying research staff
Centralisation within the research apparatus occurred across two levels.
National decision-making.
At the outset of the pandemic, DHSC took steps to assert central control over national research priorities in order to coordinate the national response to COVID-19. This included the shut down or partial shutdown of the normal functioning of the research system. A document circulated throughout the NHS on the 13 th March 2020, which included information from 25 separate Trusts, announced that elements of the UK’s national R&D infrastructure, including the UK Clinical Research Facilities (CRF) and NIHR Clinical Research Network (NIHR CRN) Coordinating Centre were “joining up working to ensure consistency of approach” and that “currently UK NIHR/RC and EU research funding bodies are in the process of selecting research that will be prioritised for approval and delivery across the NHS during the pandemic.” On 16 th March 2020 a directive from the DHSC and the Chief Medical Officer (CMO) ordered the suspension of all non-COVID-19-related research and the reorientation of research capacity towards the effort to develop COVID-19 treatments and vaccines [ 6 ]. Only those studies funded by the NIHR and where “discontinuing them will have significant detrimental effects on the ongoing care of individual participants involved in those studies” were allowed to continue [ 6 ]—in short, those studies where research was the standard of care, for example, with experimental cancer treatments. Decisions on which studies met this threshold were decided at the Trust level. Table 3 documents the scale of the pause in the normal research pipeline at SLAT. Participant G-2 saw this DHSC and CMO directive as an effective way to focus research resources:
I think the really helpful bit was the sort of diktat from Chris Whitty and Louise Wood at DH [Department of Health and Social Care] to say, “Stop everything that’s not COVID.” […] So, to actually have something centrally that said, “No, you’re not actually allowed to do that because we’ve got to focus on the COVID stuff,” was very helpful because people just stopped asking–which was great. And we were freed up to change processes as we needed to.
https://doi.org/10.1371/journal.pone.0256871.t003
Following this directive, a new system of badging certain studies as of Urgent Public Health (UPH) was established, run by DHSC and the CMO. All clinical studies including COVID-19 treatment and vaccine trials that hoped to recruit patients within NHS sites were required to apply for UPH status. An Urgent Public Health Group was convened, chaired by Nick Lemoine, the medical director of the NIHR CRN. The group was responsible for deciding which protocols to label UPH, based on evaluations of scientific merit, feasibility and greatest potential patient benefit [ 25 , 26 ]. Of the 1600 research protocols received by the CMO from March 2020 to February 2021, only 83 were considered national priorities [ 5 , 27 ]. Once a study had received UPH badging, hospital sites like SLAT were required to open them, if resources were available.
This centrally-organised prioritisation of COVID-19-related research removed the authority of individual Trusts and directorates to shape their own research portfolios. This was an unprecedented move by the DHSC, but allowed resources to be concentrated on studies deemed to have the greatest potential impact.
Trust-level decision-making.
In order to enact the DHSC mandate to prioritise COVID-19 research, SLAT created a Trust-level prioritisation process. Twice-weekly prioritisation meetings commenced early April 2020 and were attended by research governance managers, research delivery managers and senior clinicians as well as representatives from the local Clinical Research Network and partner hospitals within the network. The aim of the prioritisation meetings was to protect resources and ensure capacity to undertake UPH-badged research. However, it also ensured effective, timely communication with PIs, helped identify local PIs for new COVID-19 studies led elsewhere, and managed the pause and restart of all non-COVID research. A proforma was introduced to facilitate and standardise prioritisation decision-making. Investigators were asked to provide information summarising their projects, resource requirements and whether they had received UPH badging. Proformas were reviewed during these meetings. By the end of February 2021, this group had reviewed 170 research projects using these proformas across 68 meetings, approving over 80 studies for local setup.
During the first wave of the pandemic, prioritisation group meetings focused mainly on how to open UPH-badged studies, as all other new research had been halted. One important exception was COVID-19 studies that require little or no NHS resource and took place within a single NHS site. These studies were also discussed in these prioritisation group meetings, often with a focus placed on clinical and academic merit. Most of the studies that fitted these criteria and were approved by the prioritisation group involved university researchers analysing patient data collected and pooled in the COVID patient ‘data lake’. This enabled the Trust to maintain research activity in areas not explicitly identified as urgent public health. The research reported in this article was approved through this process.
The joined up approach between national and local decision-making however did cause confusion and frustration. The process of determining whether or not a study would be badged UPH and thus allowed to proceed was initially opaque to Trust researchers and R&D, and the national UPH review process often took weeks from application submission to outcome. Furthermore, the decision to grant a study UPH was and remains out of the hands of the sites that are tasked with delivering this research, even when internally questions were raised about the appropriateness, feasibility or scientific merit of the study. Some researchers designing studies to address key issues in relation to COVID-19 struggled to negotiate the system:
In terms of national COVID studies, we tried to get a number of studies up and going, focusing on older patients. And ran into quite a lot of obstacles and barriers. [..P]eople weren’t certain whether this was research or whether it was quality improvement, audit-type, survey-type work. And that was pretty frustrating, not being able to get clear answers on that from the senior team within R&D. And access to data was very difficult. So, despite lots of conversations about why we really needed to be focusing on older patients, the majority of people with COVID, the biggest impact being in care homes, it was quite frustrating getting hold of people who could actually sign off on studies that we would have like to have done (R-7).
At the Trust level, the prioritisation of research was also important because of the reduction in available research delivery staff. As Table 4 documents, the clinical research delivery workforce, which totalled 165 on 14 th April 2020, was reduced by 79% or 131 staff members during the peak of the first wave due to redeployment to frontline care. A further 52 non-clinical research staff were redeployed to support other Trust activity. With such a reduction of staff, the ability to maintain even those studies which had not been halted was not certain and indeed many studies required changes and protocol deviations as a result. A key point of discussion in all prioritisation meetings was the resourcing requirements of proposed studies and how these requirements might be managed alongside existing commitments. In tandem with these discussions, work was done by the research delivery manager to create a central register of research delivery staff within the Trust. The push to centralise oversite of research delivery staff was initially driven by the requirement to rapidly redeploy staff including nurses and clinical trials practitioners to support the Trust’s emergency response but it was also crucial to the prioritisation group’s understanding of the availability of research resources. Prior to the pandemic, there was no central list of all research delivery staff at the Trust, as D-2 discusses:
A benefit was actually establishing who all the staff are. The systems we have in R&D which relate to where staff sit within the Trust system depends on where they’re funded from. And because research teams have lots of mixed types of funding, some of the staff are visible to me through the systems and some aren’t. So, the only way for me to know who all the staff were, was to manually myself, physically ask. There was no system anywhere that listed who the research staff are.
https://doi.org/10.1371/journal.pone.0256871.t004
In addition to being redeployed to the clinical frontline, research staff were also pulled from across the Trust’s many directorates to form a new dedicated COVID-19 research delivery team. This team became responsible for the rapid set up and roll out of COVID studies of national and international importance, like the Oxford AstraZeneca vaccine trial, among others. Centralising oversight and management of the previously dispersed research delivery workforce enabled SLAT’s research system to react quickly and flexibly to the rapidly evolving clinical demands and research requirements of the pandemic.
While research activity was centrally coordinated within SLAT, R&D were initially left out of Trust emergency planning. An organogram produced by the Trust to represent its emergency response plan did not include R&D or any element of the research system, and a briefing document prepared by SLAT R&D for the Trust’s Gold Tactical Command Unit dated 14 th April 2020 noted this absence, and that there was also no “obvious place in the structure for R&D to naturally sit.” Participant G-3 reflected on what was perceived initially as a failure to consider the role of research:
I think […] the Trust essentially, corporately, hadn’t involved the R&D department in what they were thinking. […] We didn’t have a tactical subgroup where everybody else, every other area in the Trust had a tactical subgroup. […] There was nothing in place. You know, we’ve all voiced this, certainly in meetings at the senior management level–is that, and the words used were, “R&D has been forgotten.” We were forgotten. So, what the Trust had set up and which is, I think, probably a policy or a set of actions that they have for crisis management […] was very militarily organised. […] And we didn’t slot in, nor were we invited on to any of those tactical groups. And didn’t have representation on gold or silver command either. So we were left out of that whole process. […] We had to make real efforts to reach out and offer up. We felt that obligation and we did that.
By late April 2020, R&D were fully integrated into the Trust’s Gold Tactical Command Unit. By this time, however, the prioritisation process had been implemented and oversight of research delivery staff had been centralised, facilitating redeployment to frontline care and COVID-19 research. While the research system contributed staff and other resources to the Trust’s emergency response, it did so at its own initiation.
Pace of work: Shifting gears for the COVID-19 response
One of the most striking aspects of the research infrastructure’s response to the pandemic was the sheer pace of activity and change. The sociological literature on pace suggests that demands for faster productivity are common, and indeed this demand can be seen in the health services literature which often criticises clinical research for not moving fast enough [ 28 – 31 ]. However, the sociological literature also notes the importance of considering where things slow down or even halt [ 28 , 32 ]. In this section we document how pace appeared in participants’ accounts, acknowledging both areas where there were rapid increases in the speed of research work as well as how research work slowed down in other areas.
Increasing pace: Redeployment, research set up and research completion.
Particularly within the first wave, it was the “reserve army” (D-3) of the research delivery workforce who were required to act at speed. As per Table 4 , staff were quickly released from research duties and redeployed to the frontlines to help deliver care. In addition, all NIHR funded staff with clinical training who were not completing COVID-19 research were asked to prioritise frontline care if their employer asked [ 6 ]. Within two weeks, more research delivery staff were redeployed to COVID-19 research teams. Staff were called up one day and told to “come in on the next day” (D-8), and managers were told “they’re going tomorrow. This is their last day with you” (D-4).
As pace of redeployment accelerated, so too did the speed of research. The pace with which researchers demanded studies be delivered and set up was “ten times quicker than normal […] as if someone’s taken a time warp machine to it” (R-2). Those already working in the research infrastructure were aware that research was vital to the pandemic response and, as one participant (D-1) explained:
we needed to start the research while we’re right in the middle of the surge in numbers. And so […] you have studies that come, they need to be set up tomorrow, recruit the first patient by the end of the week.
Such shifts in normal timeframes for work were facilitated in part through centralisation, as noted above. “The real step change,” research manager G-4 suggested, “was having a Prioritisation Group and having [the] team agree a fast-track way of doing things.” Alongside streamlined approval and set-up processes, wider research infrastructures and research practices were adapting at great speed:
I was amazed that, for example, by the end of March, there were–I counted them– 13 granting agencies that, some way or another, had calls on urgent COVID-19 research (R-4).
As a result of these rapid research projects, new knowledge was being produced at an unprecedented rate, as one participant succinctly put it, “science doesn’t usually change that quickly” (D-9). This speed was met with enthusiasm by PIs and research delivery staff alike, but also caused some nervousness. Some were concerned, for example, that PPI had “dropped off the radar” (G-3), whilst others were wary of publication prior to peer review:
the […] thing which is a challenge is that we’re pre-printing research, we’re putting pre-prints out when we’re submitting to journals, because–and we’re rushing to get the pre-prints out. […] And I guess that’s good. But it is also a bit of a–a stresser because […] maybe we haven’t quite got the message right yet (R-1).
Others warned that the pace of research during the first wave of the pandemic came at a human cost. Some researchers had vastly increased workloads, “going at max […] for 5 months” (R-1), where in some cases “there’s not been a single day when [they’ve] not been working in the laboratory including all Sundays and Saturdays, Easter and so on” (R-4). Whilst some enjoyed this fast-paced moment, for those closer to the frontline it has caused anxiety. As one participant (G-5) explained, “we’ve been fire-fighting”, and at least one member of staff, another explained, “can’t come near the hospital. She has panic attacks” (D-3). Whilst it has already been documented that critical care staff’s mental health has suffered in the pandemic, these participants suggest there may also be concern for the staff involved in the research response [ 33 ].
Seeing what is possible within the exceptional circumstances of a global pandemic led some researchers and PPI managers to question the normal slower pace of regulatory approvals and assert, “if you can do it during COVID-19, you can do it any other time” (R-6). The often slow processes such as ethical approvals, data sharing guidelines, funding applications, and study set-up was a common comparator to what has been possible during the COVID-19 pandemic. Yet, as G-1 explained: “The reason [research processes have] been quicker is just because there’s been less studies.” This is evident in SLAT’s own R&D data. Table 5 documents the difference in study numbers and timeframes from initial sponsorship review to final capacity and capability approval (allowing the site set up and recruitment to commence) across 3 financial years. While some approval processes were adapted, generally research governance requirements, both internal to the Trust and at the regulators the MHRA and the Health Research Authority, remained the same. The quick approval processes were possible because no new non-COVID-19 studies were reviewed, COVID-19 studies were processed as quickly as possible and almost all non-COVID-19 related research was halted.
https://doi.org/10.1371/journal.pone.0256871.t005
Slowing or halting non-COVID-19 research.
For some investigators, the halting of non-COVID-19 research led to a slower pace where researchers could play catch up. “People have just been writing up their papers” (R-3), and this period of time “gave […] the opportunity, freed up time” (R-6) to apply for grants. Whilst many tried to set up studies so they were ready to go when restrictions were lifted, they also found that “regulatory bodies have been slower” (R-6) due to their focus on COVID-19. It was apparent that these researchers had more time to engage in PPI whilst putting these grants together–one PPI manager working in cancer (P-3) suggested “PPI activity has probably increased” during the pandemic. Whilst many researchers were understanding of the need to halt research, others found it devastating for patients and the reputation of UK research. These researchers (R-3 and R-6) pointed to other international contexts where they saw standard research continuing. Researcher R-6 was surprised “with the UK being such a […] clinical trials powerhouse”, that decision-makers didn’t “do everything it could to retain that reputation even through the COVID-19 crisis.”
On 21 st May 2020 the DHSC and NIHR circulated a framework for restarting new and paused non-COVID-19 research. Stratifying research studies into three levels of priority, this framework made no distinction between commercial and non-commercial research. Using this framework, the Trust implemented its operational Restart Plan the week commencing 1 st June 2020. Recommendations on which research studies were important or urgent to restart within each directorate was managed a directorate level, with the Prioritisation Group acting as the Trust-level decision making body for the restart plan. The Prioritisation Group continued to meet weekly to approve restart plans for research projects. By mid-summer restart was well underway but the pace of resuming all these studies could not match the pace that research stopped, and researchers were concerned that they “haven’t really been able to pick up our trial recruitment in between [waves], because recovery has been so slow” (R-5). The time of “let’s get back to normal quickly because COVID’s over”, participant R-2 explained soon turned to “actually, let’s not rush back into things because we don’t know what’s coming.” At this point the centralisation of research infrastructures hindered speed rather than aided it–one research governance manager (G-4) suggested that “we need to respect the decision-making of the research managers and matron and the R&D leads now”, but instead studies were “number 507 in the queue”, and having to “wait another week for this prioritisation meeting” whilst “people are really scared about their finances […] frightened about not finishing […] patients are waiting.”
Adopting new and virtual working practices
The response to COVID-19 pandemic has resulted in broad shifts in working patterns across the labour market, and will likely lead to longer term transformations to work practices stemming from these temporary changes [ 34 – 36 ]. In health, research highlights the accelerated adoption of digital and virtual working practices as a result of COVID-19, such as the use of telemedicine in secondary care [ 37 – 39 ]. The implementation of new working practices, taking advantage of digital technologies for communication and the adaptation of existing processes so that they can be completed (at least in part) during the pandemic are also crucial elements of the research response to COVID-19, particularly for facilitating the continuation of research.
Reducing patient visits.
Clinical research is a highly regulated domain, with strict oversight on practices and procedures, and reporting requirements overseen by multiple regulators. While research setup and governance processes became more centralised, the successful conduct of research during the pandemic required a degree of flexibility and creative adaptation. The move to more remote or virtual ways of completing, supporting, regulating, and facilitating research relied on the speedy adoption of new technologies and ways of working.
On 12 th March 2020, the MHRA issued guidance to sites and investigators “regarding protocol compliance during exceptional circumstances” [ 40 ]. The guidance stated that the MHRA recognised “the difficult current situation” and advised on how to manage trials during the pandemic [ 40 ]. The MHRA also noted in this guidance and on the MHRA Inspectorate website that a redistribution of human resources during the pandemic:
may mean certain oversight duties, such as monitoring and quality assurance activities might need to be reassessed and alternative proportionate mechanisms of oversight introduced (such as phone calls, video calls) to ensure ongoing subject safety and well-being. We would advise a brief risk assessment and documentation of the impact of this [ 40 ].
While this guidance came before the formal research shutdown, it remained important, especially for the small amount of research which was allowed to continue because it was the best or only treatment option left available for patients. However, research practices and trial protocols needed to be adapted, particularly as there were restrictions on who could physically visit hospital sites, as G-5 highlights:
If a protocol says that a participant will have a visit at week 1, week 2, week 3 and week 4 and those are protocol visits–it’s unacceptable not to do those visits. They are protocol deviations. However, during the real surge of the pandemic, those visits couldn’t be done. They couldn’t come in and have an MRI scan, and ECG and bloods taken. What they did have was someone contacting them by telephone or by Skype or other formats, media format–to say, “How are you doing? Are you okay? Is there anything you need to report? Keep in touch” (G-5).
Through delaying or adapting follow-up appointment requirements so they could be completed over the telephone or through videoconferencing, many studies were able to maintain some level of continuity. For these research participating patients, other parts of the research process needed sensitive negotiation, as one PI explains in relation to changes in the format of patient consultations:
Some [participants] were actually a bit reluctant and felt a bit fobbed off to be called at home [when] they were due a face-to-face consultation. We had to be a bit careful about that, particularly if we were discontinuing treatment or discharging people from our care. That almost always went badly if we tried to do it remotely. And if we were having a really definitive conversation like that, it was worth–we found, in the end, patients coming up. Other patients were reluctant to come and readily accepted our advice that rather than coming for a CT scan, we just do a chest x-ray when we next saw them. So, there is a difference of approach, which is personal–not particular to their circumstance (R-5).
Balancing the need for face-to-face consultations and the protection offered by telephone or video consultations required thoughtful, individualised decision-making. For other studies however, digital consultation was simply not possible, which lead to investment in supporting people to attend the hospital:
A few studies have been done remotely, but the one that I have taken on, patients really have to come in. So, we had to do a lot of logistic development there, like bringing them in by car, paying for whatever is necessary just to make sure that they continue coming in (D-6).
Working from home.
Another crucial step in facilitating research and frontline care was asking large numbers of staff to complete their work from home. For some participants, working from home lead to greater productivity, but for many others it meant the blurring of home and work lives. Numerous factors impacted on participants’ experiences, from juggling work alongside home schooling and caring responsibilities, to feelings of isolation, through to more practical issues, such as having a space to work at home, having sufficient internet bandwidth and having stable access to Trust systems (see Box 1 ).
Box 1. Indicative questionnaire responses to: What, if any, challenges have you had to face working from home?.
https://doi.org/10.1371/journal.pone.0256871.t006
While research staff were transitioning to working from home, research spaces were transformed to facilitate frontline care. By April, two of the four Clinical Research Facilities (CRFs) in the Trust were repurposed to deliver frontline care and training space for frontline staff. The remaining two CRFs were refocused on supporting COVID-19 research. The vacant R&D department’s office spaces were also used by Trust staff to facilitate socially-distanced meetings and computer work for those who needed to be onsite. Careful repurposing of offices and clinical space provided the Trust with additional, flexible physical space to assist in the emergency response to the pandemic.
Digitalising research processes.
Research work still occurred within the normal parameters of how health research is conducted in the NHS. These practices were, however, done differently to adapt to COVID-19 social distancing measures.
Firstly, researchers initially had to find a workaround for consent to research in COVID-19 wards. Because of infection control protocols no materials, including paper consent forms, could be removed from COVID positive wards. As there were no protocols in place to gain consent digitally, staff developed a local workaround, as D-1 explains:
we managed to get some […] work phones so that we could take a picture of the consent [form]. So, the consent [form] was held up to the window [in the COVID ward], the team outside could take a picture of the consent form and send it directly through on the Pando app, because [Pando] could have patient details. So, it could then be turned into a PDF and printed and put in the patient file.
Another example of a slow but necessary digital solution was with site monitoring. Site monitoring allows commercial companies and other trial sponsors to visit research sites to assess the quality of the data and ensure study protocols are being followed. Despite MHRA instruction that this “should not add extra burden to trial sites” [ 40 ] and that monitors could not be justified as an extra body in the building, these activities are crucial not just for validating data but for hospitals to be able to bill sponsors for the completed research. Workarounds were further limited because of data protection regulations that prevent the digital transfer of patient data or remote access to Trust systems by external individuals. Where site monitors would usually work alone on site, it became a long and arduous process:
a member of the research team within the Trust sits at a screen and shared that screen through Microsoft Teams with the external person. So, no data is held, no recordings are being done, no data is transferred. But it’s very, very labour-intensive. (G-5)
Whilst workarounds were quickly found for some research practices, others took longer. Despite the fact that Patient and Public Involvement in research (PPI) is a core element of contemporary UK health research [ 17 ], there was initially “zero PPI” (G-1). Rather PPI group managers focused on care work: “putting them in touch with local services that could do things like pick up prescriptions for them, get shopping, get the food boxes delivered” (P-1). It was only with time that not only did researchers planning non-COVID research begin to engage more than usual with their PPI groups, but that funders and regulators demanded that PPI should still be prioritised even in emergency research [ 41 , 42 ].
While researchers voiced concerns about the equity of shifting online and assumptions about who will and will not engage with online PPI, this did not appear to be a problem in practice:
There’s often a sort of an ageism about who can–it’s like kind of what you were just saying about older people can’t do PPI. Well, bollocks. I mean actually they’ve been as responsive to this pandemic as anybody else. The rates of use of, you know, technology, has like skyrocketed in the over 65s, because of their need to talk to their grandchildren etc. So, you know, they are adaptive (R-1).
R-1’s experience was echoed by PPI representatives. Reflecting on the move online, these representatives noted some disadvantages, such as the absence of many social aspects of attending PPI meetings, and video fatigue. But participants were generally positive about the potential of virtual PPI for involving those who cannot always travel long distances due to their illnesses, those who work full-time but could attend an hour session online in their lunch break, and representatives in different countries.
In short, the process of realigning and digitalising research practices was not simply one that sped up research and productivity, but it involved a set of necessary, labour-intensive workarounds. It did, however, also bring about possibilities for long term positive effects, such as diversifying involvement in PPI groups.
COVID-19 has brought to the fore the critical importance of the UK’s clinical research infrastructure which has over the past 15 years become increasingly embedded within the NHS. It has enabled NHS hospitals to deliver research of global importance at an unprecedented pace while simultaneously providing critical care for record numbers of acutely ill patients. We provide an analysis of how this was possible through an in-depth case study of the transformations and reconfigurations of the research system at one research-intensive Trust. Our data show that a large-scale reorganisation of research staff, research infrastructures and research priorities took place during the first few weeks and months of the pandemic. We have documented many of the changes in organisational structure, national policy and everyday working practices that facilitated the Trust’s response to COVID-19. These rapid changes have brought about new ways of working, and new perspectives on the role of research which may have far reaching consequences for the future of the clinical research system in the UK.
The pandemic occasioned a large-scale mobilisation of research staff as a “reserve army.” Research staff were crucial in supporting the care-function of NHS hospitals during the first wave of the pandemic. At the same time, the embedded research system helped to streamline, facilitate and deliver rapid COVID-19 research.
Our study documented some of the challenges that the research system has faced in seeking to operate in a COVID-safe manner. At the same time, our participants described instances of improvisation in order to adapt protocols to the COVID-19 environment. Research staff developed effective practical solutions borne out of necessity, rather than the result of prior planning. This points to the resourcefulness of research staff, but also highlights the ways in which the research system was initially largely absent from existing emergency planning within the health system.
Our research was conducted while the Trust we were studying enacted national COVID-19 policy, responded to local care needs and supported clinical research during a global pandemic. This allowed us to observe these events unfolding while gathering data in a COVID-safe manner. But the pandemic created limitations as well, especially impacting the range of methods we were able to use. While working digitally did give us a first-hand experience of how a large proportion of the decision-making infrastructure had to move online, it limited our access to frontline care and everyday research activity.
There are also limitations of looking at a research active Trust like SLAT. While research is increasingly becoming a routine component of all NHS settings, SLATs size and existing research portfolio meant there was a large amount of resource available to redeploy towards COVID-19 care and research delivery. This picture may not be representative of all NHS Trusts, particularly those that are smaller, where less research takes place. Such resource, particularly in the form of biomedical research infrastructures embedded within NHS Trusts, have provided what Roope et al. label ‘option value’ in research, additional capacity to support public good, which in normal times may appear an inefficient use of resource [ 43 ]. Roope et al. highlight that, in comparison to funded, individual research studies, funding research infrastructures allows greater flexibility and speed of response when emergencies arise, such as the COVID-19 pandemic. While the research workforce, funds and infrastructures were used to support other research prior to COVID-19 (as opposed to being excess capacity), the ability of such resource to be reallocated to COVID-19 at such pace underpinned much of the UK’s success in its research response and much of the work described in this paper. It is important to acknowledge, however, that research capacity is distributed unevenly throughout the NHS, and resources such as Clinical Research Facilities and Biomedical Research Centres tend to be situated in major teaching hospitals and trauma centres rather than geographically more localised hospitals. More research is needed to understand how this unequal distribution of resources affected outcomes of care and research during the pandemic.
In documenting how the pace of research work changed dramatically during the pandemic, both in terms of increasing the speed of certain activities and decreasing the speed of others, our paper also contributes to broader discussions of pace in clinical research. In particular, the key question—how do we most effectively streamline the research pipeline, from bench to bedside? Hanney et al. highlight the potential to overlap parts of the translational research pathway to speed up the process, and some of the barriers to this, such as ethical approvals and resourcing issues [ 30 , 31 ]. Many of these issues were removed during the pandemic because of the targeting of resources towards COVID-19 research. On a more practical level, however, our analysis suggests some ways that the research system may be adapted in the future. The potential offered by digital communications to facilitate certain research and PPI activities have led some clinical researchers to question the necessity for research participants and patients to always attend hospital sites for consultations. Trust-level research prioritisation has proved positive in managing finite local resources as effectively as possible, enabling a more holistic view of the research portfolio at a local level as well as take into account national priorities. At the same time, it is clear that the new technologies and new ways of working that were developed to cope with the crisis are not automatically more efficient, and there is a danger that some key steps such as adequate PPI might be overlooked when research pace is increased. Further research and planning will be needed to develop suitable governance processes to facilitate research activities both when on a crisis footing, and in more routine practice. Wider investment in networked digital applications and hardware (such as Trust compliant laptop computers) is needed to facilitate better working from home.
Our study suggests a number of additional lessons for future national emergency planning and policy. Research infrastructure must be better included in advanced planning, both in terms of the personnel, equipment and other resources that can be made available for redeployment as well as the direct impact that research can make. The capacity to develop new treatments and vaccines should be treated as a strategic asset that is a central part of any emergency response. This has been recognised at the national level, and internationally [ 1 – 3 ], but our data suggest that it has not fully translated into Trust-level operations. Planning for future emergencies should include protocols for the rapid establishment of strategic research prioritisation and redeployment of research infrastructure and capacity. Our data also show that throughout the pandemic, there remained a demand for public input in research, which should be included in future emergency planning. Public input is vital in clinical research, especially in an emergency response which requires publics to respond to clinical-expert advice, and planners should recognise it as such.
Future emergency planning must, however, take into account the exhaustion and stress faced by research staff who suddenly found themselves on the front line of a national mobilisation. Research staff experienced the same well-documented stresses experienced by other NHS workers [ 33 , 44 ]. Emergency planning should acknowledge this human cost and find ways to mitigate such costs and provide support for staff as a national priority.
At a global level, the UK response and its specific organisation, as described within this case study Trust, demonstrates some of the benefits of embedding research infrastructures within a national health provider, and how this set up not only enabled a coherent national response, but also provided staff resource to facilitate such research at great speed as well as support the delivery of frontline care. As we look to the future, how we integrate healthcare and research at more national and global levels are important areas for further research and discussion.
Acknowledgments
We are grateful to Christopher McKevitt and Nina Fudge for providing astute comments on drafts of this paper and to our participants who shared their experiences and time with us during this period of unprecedented strain on the NHS.
- View Article
- PubMed/NCBI
- Google Scholar
- 5. National Institute for Health Research. Urgent Public Health COVID-19 Studies 2021 [27/07/2021]. Available from: https://www.nihr.ac.uk/covid-studies/ .
- 6. National Institute for Health Research. DHSC issues guidance on the impact of COVID-19 on research funded or supported by NIHR 2020 [01/05/2020]. Available from: https://www.nihr.ac.uk/news/dhsc-issues-guidance-on-the-impact-on-covid-19-on-research-funded-or-supported-by-nihr/24469 .
- 13. Yin R K. Case study research: Design and methods. 3rd Edition ed. London: Sage; 2003.
- 14. Stake RE. The art of case study research. London: Sage; 1995.
- 16. Quinn Patton M. Qualitative research and evaluation methods. 3rd Edition ed. London: Sage; 2002.
- 17. National Institute for Health Research. National Standards for Public Involvement in Research. 2019.
- 25. National Institute for Health Research. Urgent Public Health Designation Guidance Notes 2020 [03/03/2021]. Available from: https://www.nihr.ac.uk/documents/urgent-public-health-designation-guidance-notes/24992 .
- 26. National Institute for Health Research. Prioritised support for urgent COVID-19 research 2020 [03/03/2021]. Available from: https://www.nihr.ac.uk/covid-19/prioritised-support-for-urgent-covid-19-research.htm .
- 27. Pharmaphorum. Coordinating and delivering research in the pandemic: the UK approach 2021 [01/03/2021]. Available from: https://pharmaphorum.com/webinars/uk-covid-coronavirus-research-nihr/ .
- 28. Sharma S. In the meantime: Temporality and cultural politics. Durham: Duke University Press; 2014.
- 32. Baraitser L. Enduring time. London: Bloomsbury Publishing; 2017.
- 40. MHRA Inspectorate. Advice for Management of Clinical trials in relation to Coronavirus 2020 [03/03/2021]. Available from: https://mhrainspectorate.blog.gov.uk/2020/03/12/advice-for-management-of-clinical-trials-in-relation-to-coronavirus/ .
- 41. National Institute for Health Research. NIHR reaffirms its support for patient and public involvement, engagement and participation during the COVID-19 pandemic 2020 [11/03/2021]. Available from: https://www.nihr.ac.uk/news/nihr-reaffirms-its-support-for-patient-and-public-involvement-engagement-and-participation-during-the-covid-19-pandemic/24641 .
- 42. Health Research Authority. Public involvement in a pandemic: lessons from the UK COVID-19 public involvement matching service. 2021.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMC Med Res Methodol

The case study approach
Sarah crowe.
1 Division of Primary Care, The University of Nottingham, Nottingham, UK
Kathrin Cresswell
2 Centre for Population Health Sciences, The University of Edinburgh, Edinburgh, UK
Ann Robertson
3 School of Health in Social Science, The University of Edinburgh, Edinburgh, UK
Anthony Avery
Aziz sheikh.
The case study approach allows in-depth, multi-faceted explorations of complex issues in their real-life settings. The value of the case study approach is well recognised in the fields of business, law and policy, but somewhat less so in health services research. Based on our experiences of conducting several health-related case studies, we reflect on the different types of case study design, the specific research questions this approach can help answer, the data sources that tend to be used, and the particular advantages and disadvantages of employing this methodological approach. The paper concludes with key pointers to aid those designing and appraising proposals for conducting case study research, and a checklist to help readers assess the quality of case study reports.
Introduction
The case study approach is particularly useful to employ when there is a need to obtain an in-depth appreciation of an issue, event or phenomenon of interest, in its natural real-life context. Our aim in writing this piece is to provide insights into when to consider employing this approach and an overview of key methodological considerations in relation to the design, planning, analysis, interpretation and reporting of case studies.
The illustrative 'grand round', 'case report' and 'case series' have a long tradition in clinical practice and research. Presenting detailed critiques, typically of one or more patients, aims to provide insights into aspects of the clinical case and, in doing so, illustrate broader lessons that may be learnt. In research, the conceptually-related case study approach can be used, for example, to describe in detail a patient's episode of care, explore professional attitudes to and experiences of a new policy initiative or service development or more generally to 'investigate contemporary phenomena within its real-life context' [ 1 ]. Based on our experiences of conducting a range of case studies, we reflect on when to consider using this approach, discuss the key steps involved and illustrate, with examples, some of the practical challenges of attaining an in-depth understanding of a 'case' as an integrated whole. In keeping with previously published work, we acknowledge the importance of theory to underpin the design, selection, conduct and interpretation of case studies[ 2 ]. In so doing, we make passing reference to the different epistemological approaches used in case study research by key theoreticians and methodologists in this field of enquiry.
This paper is structured around the following main questions: What is a case study? What are case studies used for? How are case studies conducted? What are the potential pitfalls and how can these be avoided? We draw in particular on four of our own recently published examples of case studies (see Tables Tables1, 1 , ,2, 2 , ,3 3 and and4) 4 ) and those of others to illustrate our discussion[ 3 - 7 ].
Example of a case study investigating the reasons for differences in recruitment rates of minority ethnic people in asthma research[ 3 ]
Example of a case study investigating the process of planning and implementing a service in Primary Care Organisations[ 4 ]
Example of a case study investigating the introduction of the electronic health records[ 5 ]
Example of a case study investigating the formal and informal ways students learn about patient safety[ 6 ]
What is a case study?
A case study is a research approach that is used to generate an in-depth, multi-faceted understanding of a complex issue in its real-life context. It is an established research design that is used extensively in a wide variety of disciplines, particularly in the social sciences. A case study can be defined in a variety of ways (Table (Table5), 5 ), the central tenet being the need to explore an event or phenomenon in depth and in its natural context. It is for this reason sometimes referred to as a "naturalistic" design; this is in contrast to an "experimental" design (such as a randomised controlled trial) in which the investigator seeks to exert control over and manipulate the variable(s) of interest.
Definitions of a case study
Stake's work has been particularly influential in defining the case study approach to scientific enquiry. He has helpfully characterised three main types of case study: intrinsic , instrumental and collective [ 8 ]. An intrinsic case study is typically undertaken to learn about a unique phenomenon. The researcher should define the uniqueness of the phenomenon, which distinguishes it from all others. In contrast, the instrumental case study uses a particular case (some of which may be better than others) to gain a broader appreciation of an issue or phenomenon. The collective case study involves studying multiple cases simultaneously or sequentially in an attempt to generate a still broader appreciation of a particular issue.
These are however not necessarily mutually exclusive categories. In the first of our examples (Table (Table1), 1 ), we undertook an intrinsic case study to investigate the issue of recruitment of minority ethnic people into the specific context of asthma research studies, but it developed into a instrumental case study through seeking to understand the issue of recruitment of these marginalised populations more generally, generating a number of the findings that are potentially transferable to other disease contexts[ 3 ]. In contrast, the other three examples (see Tables Tables2, 2 , ,3 3 and and4) 4 ) employed collective case study designs to study the introduction of workforce reconfiguration in primary care, the implementation of electronic health records into hospitals, and to understand the ways in which healthcare students learn about patient safety considerations[ 4 - 6 ]. Although our study focusing on the introduction of General Practitioners with Specialist Interests (Table (Table2) 2 ) was explicitly collective in design (four contrasting primary care organisations were studied), is was also instrumental in that this particular professional group was studied as an exemplar of the more general phenomenon of workforce redesign[ 4 ].
What are case studies used for?
According to Yin, case studies can be used to explain, describe or explore events or phenomena in the everyday contexts in which they occur[ 1 ]. These can, for example, help to understand and explain causal links and pathways resulting from a new policy initiative or service development (see Tables Tables2 2 and and3, 3 , for example)[ 1 ]. In contrast to experimental designs, which seek to test a specific hypothesis through deliberately manipulating the environment (like, for example, in a randomised controlled trial giving a new drug to randomly selected individuals and then comparing outcomes with controls),[ 9 ] the case study approach lends itself well to capturing information on more explanatory ' how ', 'what' and ' why ' questions, such as ' how is the intervention being implemented and received on the ground?'. The case study approach can offer additional insights into what gaps exist in its delivery or why one implementation strategy might be chosen over another. This in turn can help develop or refine theory, as shown in our study of the teaching of patient safety in undergraduate curricula (Table (Table4 4 )[ 6 , 10 ]. Key questions to consider when selecting the most appropriate study design are whether it is desirable or indeed possible to undertake a formal experimental investigation in which individuals and/or organisations are allocated to an intervention or control arm? Or whether the wish is to obtain a more naturalistic understanding of an issue? The former is ideally studied using a controlled experimental design, whereas the latter is more appropriately studied using a case study design.
Case studies may be approached in different ways depending on the epistemological standpoint of the researcher, that is, whether they take a critical (questioning one's own and others' assumptions), interpretivist (trying to understand individual and shared social meanings) or positivist approach (orientating towards the criteria of natural sciences, such as focusing on generalisability considerations) (Table (Table6). 6 ). Whilst such a schema can be conceptually helpful, it may be appropriate to draw on more than one approach in any case study, particularly in the context of conducting health services research. Doolin has, for example, noted that in the context of undertaking interpretative case studies, researchers can usefully draw on a critical, reflective perspective which seeks to take into account the wider social and political environment that has shaped the case[ 11 ].
Example of epistemological approaches that may be used in case study research
How are case studies conducted?
Here, we focus on the main stages of research activity when planning and undertaking a case study; the crucial stages are: defining the case; selecting the case(s); collecting and analysing the data; interpreting data; and reporting the findings.
Defining the case
Carefully formulated research question(s), informed by the existing literature and a prior appreciation of the theoretical issues and setting(s), are all important in appropriately and succinctly defining the case[ 8 , 12 ]. Crucially, each case should have a pre-defined boundary which clarifies the nature and time period covered by the case study (i.e. its scope, beginning and end), the relevant social group, organisation or geographical area of interest to the investigator, the types of evidence to be collected, and the priorities for data collection and analysis (see Table Table7 7 )[ 1 ]. A theory driven approach to defining the case may help generate knowledge that is potentially transferable to a range of clinical contexts and behaviours; using theory is also likely to result in a more informed appreciation of, for example, how and why interventions have succeeded or failed[ 13 ].
Example of a checklist for rating a case study proposal[ 8 ]
For example, in our evaluation of the introduction of electronic health records in English hospitals (Table (Table3), 3 ), we defined our cases as the NHS Trusts that were receiving the new technology[ 5 ]. Our focus was on how the technology was being implemented. However, if the primary research interest had been on the social and organisational dimensions of implementation, we might have defined our case differently as a grouping of healthcare professionals (e.g. doctors and/or nurses). The precise beginning and end of the case may however prove difficult to define. Pursuing this same example, when does the process of implementation and adoption of an electronic health record system really begin or end? Such judgements will inevitably be influenced by a range of factors, including the research question, theory of interest, the scope and richness of the gathered data and the resources available to the research team.
Selecting the case(s)
The decision on how to select the case(s) to study is a very important one that merits some reflection. In an intrinsic case study, the case is selected on its own merits[ 8 ]. The case is selected not because it is representative of other cases, but because of its uniqueness, which is of genuine interest to the researchers. This was, for example, the case in our study of the recruitment of minority ethnic participants into asthma research (Table (Table1) 1 ) as our earlier work had demonstrated the marginalisation of minority ethnic people with asthma, despite evidence of disproportionate asthma morbidity[ 14 , 15 ]. In another example of an intrinsic case study, Hellstrom et al.[ 16 ] studied an elderly married couple living with dementia to explore how dementia had impacted on their understanding of home, their everyday life and their relationships.
For an instrumental case study, selecting a "typical" case can work well[ 8 ]. In contrast to the intrinsic case study, the particular case which is chosen is of less importance than selecting a case that allows the researcher to investigate an issue or phenomenon. For example, in order to gain an understanding of doctors' responses to health policy initiatives, Som undertook an instrumental case study interviewing clinicians who had a range of responsibilities for clinical governance in one NHS acute hospital trust[ 17 ]. Sampling a "deviant" or "atypical" case may however prove even more informative, potentially enabling the researcher to identify causal processes, generate hypotheses and develop theory.
In collective or multiple case studies, a number of cases are carefully selected. This offers the advantage of allowing comparisons to be made across several cases and/or replication. Choosing a "typical" case may enable the findings to be generalised to theory (i.e. analytical generalisation) or to test theory by replicating the findings in a second or even a third case (i.e. replication logic)[ 1 ]. Yin suggests two or three literal replications (i.e. predicting similar results) if the theory is straightforward and five or more if the theory is more subtle. However, critics might argue that selecting 'cases' in this way is insufficiently reflexive and ill-suited to the complexities of contemporary healthcare organisations.
The selected case study site(s) should allow the research team access to the group of individuals, the organisation, the processes or whatever else constitutes the chosen unit of analysis for the study. Access is therefore a central consideration; the researcher needs to come to know the case study site(s) well and to work cooperatively with them. Selected cases need to be not only interesting but also hospitable to the inquiry [ 8 ] if they are to be informative and answer the research question(s). Case study sites may also be pre-selected for the researcher, with decisions being influenced by key stakeholders. For example, our selection of case study sites in the evaluation of the implementation and adoption of electronic health record systems (see Table Table3) 3 ) was heavily influenced by NHS Connecting for Health, the government agency that was responsible for overseeing the National Programme for Information Technology (NPfIT)[ 5 ]. This prominent stakeholder had already selected the NHS sites (through a competitive bidding process) to be early adopters of the electronic health record systems and had negotiated contracts that detailed the deployment timelines.
It is also important to consider in advance the likely burden and risks associated with participation for those who (or the site(s) which) comprise the case study. Of particular importance is the obligation for the researcher to think through the ethical implications of the study (e.g. the risk of inadvertently breaching anonymity or confidentiality) and to ensure that potential participants/participating sites are provided with sufficient information to make an informed choice about joining the study. The outcome of providing this information might be that the emotive burden associated with participation, or the organisational disruption associated with supporting the fieldwork, is considered so high that the individuals or sites decide against participation.
In our example of evaluating implementations of electronic health record systems, given the restricted number of early adopter sites available to us, we sought purposively to select a diverse range of implementation cases among those that were available[ 5 ]. We chose a mixture of teaching, non-teaching and Foundation Trust hospitals, and examples of each of the three electronic health record systems procured centrally by the NPfIT. At one recruited site, it quickly became apparent that access was problematic because of competing demands on that organisation. Recognising the importance of full access and co-operative working for generating rich data, the research team decided not to pursue work at that site and instead to focus on other recruited sites.
Collecting the data
In order to develop a thorough understanding of the case, the case study approach usually involves the collection of multiple sources of evidence, using a range of quantitative (e.g. questionnaires, audits and analysis of routinely collected healthcare data) and more commonly qualitative techniques (e.g. interviews, focus groups and observations). The use of multiple sources of data (data triangulation) has been advocated as a way of increasing the internal validity of a study (i.e. the extent to which the method is appropriate to answer the research question)[ 8 , 18 - 21 ]. An underlying assumption is that data collected in different ways should lead to similar conclusions, and approaching the same issue from different angles can help develop a holistic picture of the phenomenon (Table (Table2 2 )[ 4 ].
Brazier and colleagues used a mixed-methods case study approach to investigate the impact of a cancer care programme[ 22 ]. Here, quantitative measures were collected with questionnaires before, and five months after, the start of the intervention which did not yield any statistically significant results. Qualitative interviews with patients however helped provide an insight into potentially beneficial process-related aspects of the programme, such as greater, perceived patient involvement in care. The authors reported how this case study approach provided a number of contextual factors likely to influence the effectiveness of the intervention and which were not likely to have been obtained from quantitative methods alone.
In collective or multiple case studies, data collection needs to be flexible enough to allow a detailed description of each individual case to be developed (e.g. the nature of different cancer care programmes), before considering the emerging similarities and differences in cross-case comparisons (e.g. to explore why one programme is more effective than another). It is important that data sources from different cases are, where possible, broadly comparable for this purpose even though they may vary in nature and depth.
Analysing, interpreting and reporting case studies
Making sense and offering a coherent interpretation of the typically disparate sources of data (whether qualitative alone or together with quantitative) is far from straightforward. Repeated reviewing and sorting of the voluminous and detail-rich data are integral to the process of analysis. In collective case studies, it is helpful to analyse data relating to the individual component cases first, before making comparisons across cases. Attention needs to be paid to variations within each case and, where relevant, the relationship between different causes, effects and outcomes[ 23 ]. Data will need to be organised and coded to allow the key issues, both derived from the literature and emerging from the dataset, to be easily retrieved at a later stage. An initial coding frame can help capture these issues and can be applied systematically to the whole dataset with the aid of a qualitative data analysis software package.
The Framework approach is a practical approach, comprising of five stages (familiarisation; identifying a thematic framework; indexing; charting; mapping and interpretation) , to managing and analysing large datasets particularly if time is limited, as was the case in our study of recruitment of South Asians into asthma research (Table (Table1 1 )[ 3 , 24 ]. Theoretical frameworks may also play an important role in integrating different sources of data and examining emerging themes. For example, we drew on a socio-technical framework to help explain the connections between different elements - technology; people; and the organisational settings within which they worked - in our study of the introduction of electronic health record systems (Table (Table3 3 )[ 5 ]. Our study of patient safety in undergraduate curricula drew on an evaluation-based approach to design and analysis, which emphasised the importance of the academic, organisational and practice contexts through which students learn (Table (Table4 4 )[ 6 ].
Case study findings can have implications both for theory development and theory testing. They may establish, strengthen or weaken historical explanations of a case and, in certain circumstances, allow theoretical (as opposed to statistical) generalisation beyond the particular cases studied[ 12 ]. These theoretical lenses should not, however, constitute a strait-jacket and the cases should not be "forced to fit" the particular theoretical framework that is being employed.
When reporting findings, it is important to provide the reader with enough contextual information to understand the processes that were followed and how the conclusions were reached. In a collective case study, researchers may choose to present the findings from individual cases separately before amalgamating across cases. Care must be taken to ensure the anonymity of both case sites and individual participants (if agreed in advance) by allocating appropriate codes or withholding descriptors. In the example given in Table Table3, 3 , we decided against providing detailed information on the NHS sites and individual participants in order to avoid the risk of inadvertent disclosure of identities[ 5 , 25 ].
What are the potential pitfalls and how can these be avoided?
The case study approach is, as with all research, not without its limitations. When investigating the formal and informal ways undergraduate students learn about patient safety (Table (Table4), 4 ), for example, we rapidly accumulated a large quantity of data. The volume of data, together with the time restrictions in place, impacted on the depth of analysis that was possible within the available resources. This highlights a more general point of the importance of avoiding the temptation to collect as much data as possible; adequate time also needs to be set aside for data analysis and interpretation of what are often highly complex datasets.
Case study research has sometimes been criticised for lacking scientific rigour and providing little basis for generalisation (i.e. producing findings that may be transferable to other settings)[ 1 ]. There are several ways to address these concerns, including: the use of theoretical sampling (i.e. drawing on a particular conceptual framework); respondent validation (i.e. participants checking emerging findings and the researcher's interpretation, and providing an opinion as to whether they feel these are accurate); and transparency throughout the research process (see Table Table8 8 )[ 8 , 18 - 21 , 23 , 26 ]. Transparency can be achieved by describing in detail the steps involved in case selection, data collection, the reasons for the particular methods chosen, and the researcher's background and level of involvement (i.e. being explicit about how the researcher has influenced data collection and interpretation). Seeking potential, alternative explanations, and being explicit about how interpretations and conclusions were reached, help readers to judge the trustworthiness of the case study report. Stake provides a critique checklist for a case study report (Table (Table9 9 )[ 8 ].
Potential pitfalls and mitigating actions when undertaking case study research
Stake's checklist for assessing the quality of a case study report[ 8 ]
Conclusions
The case study approach allows, amongst other things, critical events, interventions, policy developments and programme-based service reforms to be studied in detail in a real-life context. It should therefore be considered when an experimental design is either inappropriate to answer the research questions posed or impossible to undertake. Considering the frequency with which implementations of innovations are now taking place in healthcare settings and how well the case study approach lends itself to in-depth, complex health service research, we believe this approach should be more widely considered by researchers. Though inherently challenging, the research case study can, if carefully conceptualised and thoughtfully undertaken and reported, yield powerful insights into many important aspects of health and healthcare delivery.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AS conceived this article. SC, KC and AR wrote this paper with GH, AA and AS all commenting on various drafts. SC and AS are guarantors.
Pre-publication history
The pre-publication history for this paper can be accessed here:
http://www.biomedcentral.com/1471-2288/11/100/prepub
Acknowledgements
We are grateful to the participants and colleagues who contributed to the individual case studies that we have drawn on. This work received no direct funding, but it has been informed by projects funded by Asthma UK, the NHS Service Delivery Organisation, NHS Connecting for Health Evaluation Programme, and Patient Safety Research Portfolio. We would also like to thank the expert reviewers for their insightful and constructive feedback. Our thanks are also due to Dr. Allison Worth who commented on an earlier draft of this manuscript.
- Yin RK. Case study research, design and method. 4. London: Sage Publications Ltd.; 2009. [ Google Scholar ]
- Keen J, Packwood T. Qualitative research; case study evaluation. BMJ. 1995; 311 :444–446. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sheikh A, Halani L, Bhopal R, Netuveli G, Partridge M, Car J. et al. Facilitating the Recruitment of Minority Ethnic People into Research: Qualitative Case Study of South Asians and Asthma. PLoS Med. 2009; 6 (10):1–11. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pinnock H, Huby G, Powell A, Kielmann T, Price D, Williams S, The process of planning, development and implementation of a General Practitioner with a Special Interest service in Primary Care Organisations in England and Wales: a comparative prospective case study. Report for the National Co-ordinating Centre for NHS Service Delivery and Organisation R&D (NCCSDO) 2008. http://www.sdo.nihr.ac.uk/files/project/99-final-report.pdf
- Robertson A, Cresswell K, Takian A, Petrakaki D, Crowe S, Cornford T. et al. Prospective evaluation of the implementation and adoption of NHS Connecting for Health's national electronic health record in secondary care in England: interim findings. BMJ. 2010; 41 :c4564. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pearson P, Steven A, Howe A, Sheikh A, Ashcroft D, Smith P. the Patient Safety Education Study Group. Learning about patient safety: organisational context and culture in the education of healthcare professionals. J Health Serv Res Policy. 2010; 15 :4–10. doi: 10.1258/jhsrp.2009.009052. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- van Harten WH, Casparie TF, Fisscher OA. The evaluation of the introduction of a quality management system: a process-oriented case study in a large rehabilitation hospital. Health Policy. 2002; 60 (1):17–37. doi: 10.1016/S0168-8510(01)00187-7. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stake RE. The art of case study research. London: Sage Publications Ltd.; 1995. [ Google Scholar ]
- Sheikh A, Smeeth L, Ashcroft R. Randomised controlled trials in primary care: scope and application. Br J Gen Pract. 2002; 52 (482):746–51. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- King G, Keohane R, Verba S. Designing Social Inquiry. Princeton: Princeton University Press; 1996. [ Google Scholar ]
- Doolin B. Information technology as disciplinary technology: being critical in interpretative research on information systems. Journal of Information Technology. 1998; 13 :301–311. doi: 10.1057/jit.1998.8. [ CrossRef ] [ Google Scholar ]
- George AL, Bennett A. Case studies and theory development in the social sciences. Cambridge, MA: MIT Press; 2005. [ Google Scholar ]
- Eccles M. the Improved Clinical Effectiveness through Behavioural Research Group (ICEBeRG) Designing theoretically-informed implementation interventions. Implementation Science. 2006; 1 :1–8. doi: 10.1186/1748-5908-1-1. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Netuveli G, Hurwitz B, Levy M, Fletcher M, Barnes G, Durham SR, Sheikh A. Ethnic variations in UK asthma frequency, morbidity, and health-service use: a systematic review and meta-analysis. Lancet. 2005; 365 (9456):312–7. [ PubMed ] [ Google Scholar ]
- Sheikh A, Panesar SS, Lasserson T, Netuveli G. Recruitment of ethnic minorities to asthma studies. Thorax. 2004; 59 (7):634. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hellström I, Nolan M, Lundh U. 'We do things together': A case study of 'couplehood' in dementia. Dementia. 2005; 4 :7–22. doi: 10.1177/1471301205049188. [ CrossRef ] [ Google Scholar ]
- Som CV. Nothing seems to have changed, nothing seems to be changing and perhaps nothing will change in the NHS: doctors' response to clinical governance. International Journal of Public Sector Management. 2005; 18 :463–477. doi: 10.1108/09513550510608903. [ CrossRef ] [ Google Scholar ]
- Lincoln Y, Guba E. Naturalistic inquiry. Newbury Park: Sage Publications; 1985. [ Google Scholar ]
- Barbour RS. Checklists for improving rigour in qualitative research: a case of the tail wagging the dog? BMJ. 2001; 322 :1115–1117. doi: 10.1136/bmj.322.7294.1115. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mays N, Pope C. Qualitative research in health care: Assessing quality in qualitative research. BMJ. 2000; 320 :50–52. doi: 10.1136/bmj.320.7226.50. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mason J. Qualitative researching. London: Sage; 2002. [ Google Scholar ]
- Brazier A, Cooke K, Moravan V. Using Mixed Methods for Evaluating an Integrative Approach to Cancer Care: A Case Study. Integr Cancer Ther. 2008; 7 :5–17. doi: 10.1177/1534735407313395. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Miles MB, Huberman M. Qualitative data analysis: an expanded sourcebook. 2. CA: Sage Publications Inc.; 1994. [ Google Scholar ]
- Pope C, Ziebland S, Mays N. Analysing qualitative data. Qualitative research in health care. BMJ. 2000; 320 :114–116. doi: 10.1136/bmj.320.7227.114. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cresswell KM, Worth A, Sheikh A. Actor-Network Theory and its role in understanding the implementation of information technology developments in healthcare. BMC Med Inform Decis Mak. 2010; 10 (1):67. doi: 10.1186/1472-6947-10-67. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Malterud K. Qualitative research: standards, challenges, and guidelines. Lancet. 2001; 358 :483–488. doi: 10.1016/S0140-6736(01)05627-6. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yin R. Case study research: design and methods. 2. Thousand Oaks, CA: Sage Publishing; 1994. [ Google Scholar ]
- Yin R. Enhancing the quality of case studies in health services research. Health Serv Res. 1999; 34 :1209–1224. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Green J, Thorogood N. Qualitative methods for health research. 2. Los Angeles: Sage; 2009. [ Google Scholar ]
- Howcroft D, Trauth E. Handbook of Critical Information Systems Research, Theory and Application. Cheltenham, UK: Northampton, MA, USA: Edward Elgar; 2005. [ Google Scholar ]
- Blakie N. Approaches to Social Enquiry. Cambridge: Polity Press; 1993. [ Google Scholar ]
- Doolin B. Power and resistance in the implementation of a medical management information system. Info Systems J. 2004; 14 :343–362. doi: 10.1111/j.1365-2575.2004.00176.x. [ CrossRef ] [ Google Scholar ]
- Bloomfield BP, Best A. Management consultants: systems development, power and the translation of problems. Sociological Review. 1992; 40 :533–560. [ Google Scholar ]
- Shanks G, Parr A. Proceedings of the European Conference on Information Systems. Naples; 2003. Positivist, single case study research in information systems: A critical analysis. [ Google Scholar ]

- Adolescent and Young Adult Cancer
- Bile Duct Cancer
- Bladder Cancer
- Brain Cancer
- Breast Cancer
- Cervical Cancer
- Childhood Cancer
- Colorectal Cancer
- Endometrial Cancer
- Esophageal Cancer
- Head and Neck Cancer
- Kidney Cancer
- Liver Cancer
- Lung Cancer
- Mouth Cancer
- Mesothelioma
- Multiple Myeloma
- Neuroendocrine Tumors
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer/Melanoma
- Stomach Cancer
- Testicular Cancer
- Throat Cancer
- Thyroid Cancer
- Prevention and Screening
- Diagnosis and Treatment
- Research and Clinical Trials
- Survivorship

Request an appointment at Mayo Clinic

Multiple myeloma: Its evolution, treatment and the quest to catch it early
Share this:.
By Nicole Brudos Ferrara
Multiple myeloma is a cancer of a type of white blood cell called a plasma cell in the bone marrow. When multiple myeloma develops in the bone marrow, cancerous plasma cells multiply, crowding out healthy cells.
"Over time, people develop abnormalities or mutations in their plasma cells. Those mutations cause plasma cells to become cancerous," says Joselle Cook, M.B.B.S., a Mayo Clinic hematologist specializing in multiple myeloma and other plasma cell disorders. "Older age is a risk factor. Multiple myeloma is commonly diagnosed in people in their 60s and 70s. We also know that Black people develop myeloma about 10 years earlier than white people, and two to three times more frequently."
Having a family history of multiple myeloma may also increase the risk of the disease.
An estimated 35,780 new cases of multiple myeloma will be diagnosed in the United States in 2024. While multiple myeloma is a serious condition, people with the disease are living longer because treatments have advanced. "The prognosis has changed remarkably over the last few years," says Dr. Cook.
Read on for an overview of how multiple myeloma evolves, how healthcare professionals treat it, and the quest to find a screening test to diagnose the disease before it can damage the body.
The evolution of multiple myeloma
Cancerous plasma cells — myeloma cells — make proteins that cause the symptoms and complications of multiple myeloma. "When the plasma cells develop mutations and produce a monoclonal protein, this group of conditions is called monoclonal gammopathies," says Dr. Cook.
The earliest phase of monoclonal gammopathies is monoclonal gammopathy of undetermined significance (MGUS) , which doesn't cause symptoms. When a person has MGUS, monoclonal protein, or M-protein, is found in their blood at a level too low to damage the body. "If we detect M-protein in a person's blood, and we aren't concerned about organ damage, we monitor them," says Dr. Cook.
If cancerous plasma cells continue to multiply and produce M-proteins, MGUS evolves into smoldering multiple myeloma. People still don't have symptoms at this phase but have a higher M-protein level in their blood and urine.
People with smoldering multiple myeloma are classified based on their risk of progressing to multiple myeloma: low risk, intermediate risk or high risk. "We may treat some people with high-risk smoldering multiple myeloma, typically as part of a clinical trial, but for the most part, we actively monitor people without treating them," says Dr. Cook.
Multiple myeloma might be suspected when blood tests conducted for another reason raise red flags. "We may see that the protein is quite elevated or that a patient has a lower blood count than usual or an abnormality in their kidney numbers," she says. This prompts a care team to order blood tests to detect M-protein and assess blood chemistry and kidney function, urine tests to detect proteins, imaging tests to identify bone problems, and a bone marrow biopsy to look for myeloma cells.
People with multiple myeloma can experience a variety of symptoms or none. This can make diagnosing the disease a challenge. When a healthcare professional suspects multiple myeloma, they frequently check for specific signs and symptoms. "People with multiple myeloma may have anemia . If the M-proteins deposit in the kidneys and cause them to fail, they will have kidney abnormalities. They may have bone pain and high blood calcium levels caused by bone destruction," says Dr. Cook.
Multiple myeloma treatment options
Treatment for multiple myeloma typically starts with a combination of medications called induction chemotherapy. "Treatment involves plasma-cell directed therapy," says Dr. Cook. "It's usually a combination of three or four drugs: A steroid, an immunomodulating agent (a drug that stimulates the immune system to fight cancer), an antibody ( anti-CD38 ) that targets a surface marker on the cancerous plasma cell, and a proteasome inhibitor that targets the cell's protein manufacturing."
Your care team will also decide if you are a candidate for a bone marrow transplant. "This decision depends on factors like fitness and age — it's a soft cut-off, but generally, we don't transplant patients over 75. There are exceptions, though," says Dr. Cook.
The drugs your care team uses in your induction chemotherapy will depend on your overall health and whether you are a candidate for a bone marrow transplant.
A bone marrow transplant — a stem cell transplant — is a procedure that infuses healthy blood-forming stem cells into your body to regenerate the bone marrow's ability to produce blood cells. Stem cell transplants can pose risks, and some people can have serious complications.
If eligible, people with multiple myeloma typically have a stem cell transplant after about four to six months of induction chemotherapy. Before the transplant, they receive a high dose of a different type of chemotherapy called conditioning chemotherapy.
Dr. Cook uses a garden metaphor to explain how conditioning and stem cell transplants work to treat multiple myeloma. "In myeloma, your bone marrow is akin to a garden overgrown with weeds (the myeloma). You use strong weed killers (the conditioning chemotherapy) to eliminate those weeds, but then the garden is barren. You need to plant seeds to allow the garden to grow. That's what a stem cell transplant does. The reinfusion of stem cells is like planting seeds so your bone marrow can recover faster."
Dr. Cook says other promising treatment options exist if you cannot undergo a bone marrow transplant. "For people who relapse after several types of treatment, CAR-T cell therapy , where people's T cells are engineered to recognize and kill a myeloma cell, offers great response rates and good survival. And we've seen success with new drugs called bispecific antibodies — specially designed antibodies that redirect a patient's T cells (immune cells) to kill myeloma cells," she says.
Radiation therapy may also be an option to treat areas of the body affected by myeloma that are painful or causing other problems, says Dr. Cook.
Research aimed at catching multiple myeloma early
"Being diagnosed early is important because you want to avoid organ damage, renal impairment and bone destruction. If we can detect and diagnose multiple myeloma early, we can prevent that damage," says Dr. Cook.
"MGUS and multiple myeloma are detected most often. It's less common to detect smoldering multiple myeloma," says Dr. Cook. She says researchers are exploring screening options — primarily blood tests — to identify MGUS and smoldering myeloma.
Dr. Cook is working with colleagues on a clinical trial in Rochester, Minnesota, to screen people of East African descent. Other clinical trials are studying people with MGUS and those in higher-risk populations. "There are screening studies focused on Black people and those who have first-degree relatives with myeloma or MGUS," she says.
Dr. Cook is confident that the outlook for people diagnosed with multiple myeloma will continue to improve. "There are so many treatment options being developed," she says. "The field is just forging ahead."
Learn more about multiple myeloma and find a clinical trial at Mayo Clinic.
Join the Blood Cancers and Disorders Support Group on Mayo Clinic Connect , an online community moderated by Mayo Clinic for patients and caregivers.
Also, read these articles:
- " CAR-T cell researchers at Mayo Clinic optimistic about future of treating blood cancers ."
- " Monoclonal antibody drugs for cancer: How they work "
- " Advances in treating multiple myeloma help extend quality of life for patients "
- " Investigating dual CAR-T cell therapy for multiple myeloma "
- " Is a cancer clinical trial right for me? "
- " Multiple myeloma: New, better treatments are improving outcomes "
- " What is multiple myeloma? "
Related Posts

After receiving CAR-T cell therapy at Mayo Clinic, Welsh-born John Cadwallader achieved remission and found new hope. He now receives care in the U.K. and is monitored by Mayo in the U.S. and London.

Mayo Clinic's Advanced Care at Home program helped David Elder recover at home from a bone marrow transplant to treat his multiple myeloma.

Learn about CAR-T cell therapy and research at Mayo Clinic to reduce its side effects and expand its use beyond blood cancers.

IMAGES
VIDEO
COMMENTS
Case Studies are a qualitative design in which the researcher explores in depth a program, event, activity, process, or one or more individuals. ... Multiple-case study design however has a distinct advantage over a single case study design. Multiple-case studies are generally considered more compelling and robust, and worthy of undertaking ...
In this study, each participant is a case. The multiple-case approach was used to understand the complexity of the experience of nine mainland Chinese students in their school-university and cross-border transitions, their prob-lems, and reasons behind the complexity. As this qualitative inquiry intends to obtain a "situated or contextual ...
Abstract. The aims of multisite qualitative research, originally developed within the case study tradition, are to produce findings that are reflective of context, while also holding broader applicability across settings. Such knowledge is ideal for informing health and social interventions by overcoming the limitations of research developed ...
Design: A multi-stage qualitative case study using the Fundamentals of Care framework as the overarching theoretical and explanatory mechanism. Methods: Repeated reflective interviews were conducted with five adult patients over a 6-month period in 2013 at a university hospital in Sweden. The interviews (n = 14) were analysed using directed ...
Qualitative case study methodology (QCSM) is a useful research approach that has grown in popularity within the social ... single or multiple case studies, and by their purpose (Stake, 1995; Yin, 2014). Yin (2014) describes three types of case studies exploratory (exam- ... Stage 3. Study Selection Three reviewers screened the titles and ...
This study is a multiple qualitative case study, based on multiple data sources (Baxter & Jack, 2008), as this improves the credibility and reliability of the result (Yin, 2003). The primary data ...
Case studies are designed to suit the case and research question and published case studies demonstrate wide diversity in study design. There are two popular case study approaches in qualitative research. The first, proposed by Stake ( 1995) and Merriam ( 2009 ), is situated in a social constructivist paradigm, whereas the second, by Yin ( 2012 ...
MMCSR. "A mixed methods case study design is a type of mixed methods study in which the quantitative and qualitative data collection, results, and integration are used to provide in-depth evidence for a case(s) or develop cases for comparative analysis" (Creswell & Plano Clarke, 2018, p.116).
The authors have recently conducted an in-depth case study in the Information and Communication Technology (ICT) industry of New Zealand. A multiple case studies approach was adopted that spanned over 2 years, as it is difficult to investigate all the aspects of a phenomenon in a single case study (Cruzes, Dybå, Runeson, & Höst, 2015). The ...
Study design. This multistage mixed methods study uses an explanatory sequential design whereby qualitative research will be undertaken to explain quantitative findings (see Table 1 and Fig. 2). In Stage 1, documentary analysis and data from expert interviews with the national programme team will be used to develop the programme's logic model ...
The major advantage of multiple case research lies in cross-case analysis. A multiple case research design shifts the focus from understanding a single case to the differences and similarities between cases. Thus, it is not just conducting more (second, third, etc.) case studies. Rather, it is the next step in developing a theory about factors ...
Qualitative Case Study Guidelines Saša Baškarada ... The planning stage focuses on identifying the research questions or other rationale for doing a case study, deciding to use the case study method (compared with other methods), ... causality and usually involves multi-site, multi-method assessments; and cumulative—this , , ,
A multi-stage qualitative case study using the Fundamentals of Care framework as the overarching theoretical and explanatory mechanism. Methods. Repeated reflective interviews were conducted with five adult patients over a 6-month period in 2013 at a university hospital in Sweden. The interviews (n = 14) were analysed using directed content ...
Abstract. This chapter focuses on what Creswell (2015) has termed multimethod research. Multimethod research is research that uses multiple forms of qualitative data (e.g., interviews and ...
In this study, each participant is a case. The multiple-case approach was used to understand the complexity of the experience of nine mainland Chinese students in their school-university and cross-border transitions, their problems, and reasons behind the complexity. As this qualitative inquiry intends to obtain a "situated or contextual ...
This stage of analysis in multiple case narrative was found to be distinctive and distinct from grounded theory and other qualitative research approaches in a number of ways, including the collective analysis of the categories generated from all the cases and the identification of patterns and causal relationships between them using a ...
Consistent with multistage sampling in qualitative research, the design is somewhat iterative in nature in the sense that information gained from analysis of data collected at the first stage influences the nature of the data collected, and the way they are collected, at subsequent stages (Denzen, 1978). Furthermore, many of these adaptive ...
First is to provide a step-by-step guideline to research students for conducting case study. Second, an analysis of authors' multiple case studies is presented in order to provide an application of step-by-step guideline. This article has been divided into two sections. First section discusses a checklist with four phases that are vital for ...
Specifically, we present two lessons from the multi-stage sampling approach adopted to select the four case-study villages, which first prioritizedkey-informant observations regarding intervention ...
Furthermore, the ability to describe in detail how the analysis was conducted ensures rigour in reporting qualitative research. Data sources: The research example used is a multiple case study that explored the role of the clinical skills laboratory in preparing students for the real world of practice. Data analysis was conducted using a ...
Methods and findings. Using a multi-method qualitative case study of a research-intensive NHS Trust in London UK, we collected data through a questionnaire (n = 170) and semi-structured interviews (n = 24) with research staff working in four areas: research governance; research leadership; research delivery; and patient and public involvement.
A case study is a research approach that is used to generate an in-depth, multi-faceted understanding of a complex issue in its real-life context. It is an established research design that is used extensively in a wide variety of disciplines, particularly in the social sciences. A case study can be defined in a variety of ways (Table 5 ), the ...
2013-01-01. This qualitative multiple case study examined diversity perceptions of California community college senior leaders and sought to provide insights into how a senior leader's view of diversity concepts influences their actions in succession planning and selection of leaders and faculty. An in-depth qualitative analysis of participant ...
Research aimed at catching multiple myeloma early "Being diagnosed early is important because you want to avoid organ damage, renal impairment and bone destruction. If we can detect and diagnose multiple myeloma early, we can prevent that damage," says Dr. Cook. "MGUS and multiple myeloma are detected most often.