- Patient Care & Health Information
- Diseases & Conditions
- Type 2 diabetes
Type 2 diabetes is a condition that happens because of a problem in the way the body regulates and uses sugar as a fuel. That sugar also is called glucose. This long-term condition results in too much sugar circulating in the blood. Eventually, high blood sugar levels can lead to disorders of the circulatory, nervous and immune systems.
In type 2 diabetes, there are primarily two problems. The pancreas does not produce enough insulin — a hormone that regulates the movement of sugar into the cells. And cells respond poorly to insulin and take in less sugar.
Type 2 diabetes used to be known as adult-onset diabetes, but both type 1 and type 2 diabetes can begin during childhood and adulthood. Type 2 is more common in older adults. But the increase in the number of children with obesity has led to more cases of type 2 diabetes in younger people.
There's no cure for type 2 diabetes. Losing weight, eating well and exercising can help manage the disease. If diet and exercise aren't enough to control blood sugar, diabetes medications or insulin therapy may be recommended.

Products & Services
- A Book: The Essential Diabetes Book
- A Book: The Mayo Clinic Diabetes Diet
- Assortment of Health Products from Mayo Clinic Store
Symptoms of type 2 diabetes often develop slowly. In fact, you can be living with type 2 diabetes for years and not know it. When symptoms are present, they may include:
- Increased thirst.
- Frequent urination.
- Increased hunger.
- Unintended weight loss.
- Blurred vision.
- Slow-healing sores.
- Frequent infections.
- Numbness or tingling in the hands or feet.
- Areas of darkened skin, usually in the armpits and neck.
When to see a doctor
See your health care provider if you notice any symptoms of type 2 diabetes.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Type 2 diabetes is mainly the result of two problems:
- Cells in muscle, fat and the liver become resistant to insulin As a result, the cells don't take in enough sugar.
- The pancreas can't make enough insulin to keep blood sugar levels within a healthy range.
Exactly why this happens is not known. Being overweight and inactive are key contributing factors.
How insulin works
Insulin is a hormone that comes from the pancreas — a gland located behind and below the stomach. Insulin controls how the body uses sugar in the following ways:
- Sugar in the bloodstream triggers the pancreas to release insulin.
- Insulin circulates in the bloodstream, enabling sugar to enter the cells.
- The amount of sugar in the bloodstream drops.
- In response to this drop, the pancreas releases less insulin.
The role of glucose
Glucose — a sugar — is a main source of energy for the cells that make up muscles and other tissues. The use and regulation of glucose includes the following:
- Glucose comes from two major sources: food and the liver.
- Glucose is absorbed into the bloodstream, where it enters cells with the help of insulin.
- The liver stores and makes glucose.
- When glucose levels are low, the liver breaks down stored glycogen into glucose to keep the body's glucose level within a healthy range.
In type 2 diabetes, this process doesn't work well. Instead of moving into the cells, sugar builds up in the blood. As blood sugar levels rise, the pancreas releases more insulin. Eventually the cells in the pancreas that make insulin become damaged and can't make enough insulin to meet the body's needs.
Risk factors
Factors that may increase the risk of type 2 diabetes include:
- Weight. Being overweight or obese is a main risk.
- Fat distribution. Storing fat mainly in the abdomen — rather than the hips and thighs — indicates a greater risk. The risk of type 2 diabetes is higher in men with a waist circumference above 40 inches (101.6 centimeters) and in women with a waist measurement above 35 inches (88.9 centimeters).
- Inactivity. The less active a person is, the greater the risk. Physical activity helps control weight, uses up glucose as energy and makes cells more sensitive to insulin.
- Family history. An individual's risk of type 2 diabetes increases if a parent or sibling has type 2 diabetes.
- Race and ethnicity. Although it's unclear why, people of certain races and ethnicities — including Black, Hispanic, Native American and Asian people, and Pacific Islanders — are more likely to develop type 2 diabetes than white people are.
- Blood lipid levels. An increased risk is associated with low levels of high-density lipoprotein (HDL) cholesterol — the "good" cholesterol — and high levels of triglycerides.
- Age. The risk of type 2 diabetes increases with age, especially after age 35.
- Prediabetes. Prediabetes is a condition in which the blood sugar level is higher than normal, but not high enough to be classified as diabetes. Left untreated, prediabetes often progresses to type 2 diabetes.
- Pregnancy-related risks. The risk of developing type 2 diabetes is higher in people who had gestational diabetes when they were pregnant and in those who gave birth to a baby weighing more than 9 pounds (4 kilograms).
- Polycystic ovary syndrome. Having polycystic ovary syndrome — a condition characterized by irregular menstrual periods, excess hair growth and obesity — increases the risk of diabetes.
Complications
Type 2 diabetes affects many major organs, including the heart, blood vessels, nerves, eyes and kidneys. Also, factors that increase the risk of diabetes are risk factors for other serious diseases. Managing diabetes and controlling blood sugar can lower the risk for these complications and other medical conditions, including:
- Heart and blood vessel disease. Diabetes is associated with an increased risk of heart disease, stroke, high blood pressure and narrowing of blood vessels, a condition called atherosclerosis.
- Nerve damage in limbs. This condition is called neuropathy. High blood sugar over time can damage or destroy nerves. That may result in tingling, numbness, burning, pain or eventual loss of feeling that usually begins at the tips of the toes or fingers and gradually spreads upward.
- Other nerve damage. Damage to nerves of the heart can contribute to irregular heart rhythms. Nerve damage in the digestive system can cause problems with nausea, vomiting, diarrhea or constipation. Nerve damage also may cause erectile dysfunction.
- Kidney disease. Diabetes may lead to chronic kidney disease or end-stage kidney disease that can't be reversed. That may require dialysis or a kidney transplant.
- Eye damage. Diabetes increases the risk of serious eye diseases, such as cataracts and glaucoma, and may damage the blood vessels of the retina, potentially leading to blindness.
- Skin conditions. Diabetes may raise the risk of some skin problems, including bacterial and fungal infections.
- Slow healing. Left untreated, cuts and blisters can become serious infections, which may heal poorly. Severe damage might require toe, foot or leg amputation.
- Hearing impairment. Hearing problems are more common in people with diabetes.
- Sleep apnea. Obstructive sleep apnea is common in people living with type 2 diabetes. Obesity may be the main contributing factor to both conditions.
- Dementia. Type 2 diabetes seems to increase the risk of Alzheimer's disease and other disorders that cause dementia. Poor control of blood sugar is linked to a more rapid decline in memory and other thinking skills.
Healthy lifestyle choices can help prevent type 2 diabetes. If you've received a diagnosis of prediabetes, lifestyle changes may slow or stop the progression to diabetes.
A healthy lifestyle includes:
- Eating healthy foods. Choose foods lower in fat and calories and higher in fiber. Focus on fruits, vegetables and whole grains.
- Getting active. Aim for 150 or more minutes a week of moderate to vigorous aerobic activity, such as a brisk walk, bicycling, running or swimming.
- Losing weight. If you are overweight, losing a modest amount of weight and keeping it off may delay the progression from prediabetes to type 2 diabetes. If you have prediabetes, losing 7% to 10% of your body weight may reduce the risk of diabetes.
- Avoiding long stretches of inactivity. Sitting still for long periods of time can increase the risk of type 2 diabetes. Try to get up every 30 minutes and move around for at least a few minutes.
For people with prediabetes, metformin (Fortamet, Glumetza, others), a diabetes medication, may be prescribed to reduce the risk of type 2 diabetes. This is usually prescribed for older adults who are obese and unable to lower blood sugar levels with lifestyle changes.
More Information
- Diabetes prevention: 5 tips for taking control
- Professional Practice Committee: Standards of Medical Care in Diabetes — 2020. Diabetes Care. 2020; doi:10.2337/dc20-Sppc.
- Diabetes mellitus. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/diabetes-mellitus-dm. Accessed Dec. 7, 2020.
- Melmed S, et al. Williams Textbook of Endocrinology. 14th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Dec. 3, 2020.
- Diabetes overview. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/all-content. Accessed Dec. 4, 2020.
- AskMayoExpert. Type 2 diabetes. Mayo Clinic; 2018.
- Feldman M, et al., eds. Surgical and endoscopic treatment of obesity. In: Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 11th ed. Elsevier; 2021. https://www.clinicalkey.com. Accessed Oct. 20, 2020.
- Hypersmolar hyperglycemic state (HHS). Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/hyperosmolar-hyperglycemic-state-hhs. Accessed Dec. 11, 2020.
- Diabetic ketoacidosis (DKA). Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/diabetic-ketoacidosis-dka. Accessed Dec. 11, 2020.
- Hypoglycemia. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/hypoglycemia. Accessed Dec. 11, 2020.
- 6 things to know about diabetes and dietary supplements. National Center for Complementary and Integrative Health. https://www.nccih.nih.gov/health/tips/things-to-know-about-type-diabetes-and-dietary-supplements. Accessed Dec. 11, 2020.
- Type 2 diabetes and dietary supplements: What the science says. National Center for Complementary and Integrative Health. https://www.nccih.nih.gov/health/providers/digest/type-2-diabetes-and-dietary-supplements-science. Accessed Dec. 11, 2020.
- Preventing diabetes problems. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/all-content. Accessed Dec. 3, 2020.
- Schillie S, et al. Prevention of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recommendations and Reports. 2018; doi:10.15585/mmwr.rr6701a1.
- Caffeine: Does it affect blood sugar?
- GLP-1 agonists: Diabetes drugs and weight loss
- Hyperinsulinemia: Is it diabetes?
- Medications for type 2 diabetes
Associated Procedures
- Bariatric surgery
- Endoscopic sleeve gastroplasty
- Gastric bypass (Roux-en-Y)
- Glucose tolerance test
News from Mayo Clinic
- Mayo study uses electronic health record data to assess metformin failure risk, optimize care Feb. 10, 2023, 02:30 p.m. CDT
- Mayo Clinic Minute: Strategies to break the heart disease and diabetes link Nov. 28, 2022, 05:15 p.m. CDT
- Mayo Clinic Q and A: Diabetes risk in Hispanic people Oct. 20, 2022, 12:15 p.m. CDT
- The importance of diagnosing, treating diabetes in the Hispanic population in the US Sept. 28, 2022, 04:00 p.m. CDT
- Mayo Clinic Minute: Managing Type 2 diabetes Sept. 28, 2022, 02:30 p.m. CDT
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED PATHWAYS
Related topics.
INTRODUCTION
This topic will review the clinical presentation, diagnosis, and initial evaluation of diabetes in nonpregnant adults. Screening for and prevention of diabetes, the etiologic classification of diabetes mellitus, the treatment of diabetes, as well as diabetes during pregnancy are discussed separately.
● (See "Screening for type 2 diabetes mellitus" .)
● (See "Prevention of type 2 diabetes mellitus" and "Type 1 diabetes mellitus: Prevention and disease-modifying therapy" .)
● (See "Classification of diabetes mellitus and genetic diabetic syndromes" .)
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Diabetes Overview
What Is Diabetes?
- Español
Diabetes is a disease that occurs when your blood glucose, also called blood sugar, is too high. Glucose is your body’s main source of energy. Your body can make glucose, but glucose also comes from the food you eat.
Insulin is a hormone made by the pancreas that helps glucose get into your cells to be used for energy. If you have diabetes, your body doesn’t make enough—or any—insulin, or doesn’t use insulin properly. Glucose then stays in your blood and doesn’t reach your cells.
Diabetes raises the risk for damage to the eyes, kidneys, nerves, and heart. Diabetes is also linked to some types of cancer. Taking steps to prevent or manage diabetes may lower your risk of developing diabetes health problems.

What are the different types of diabetes?
The most common types of diabetes are type 1, type 2, and gestational diabetes.
Type 1 diabetes
If you have type 1 diabetes , your body makes little or no insulin. Your immune system attacks and destroys the cells in your pancreas that make insulin. Type 1 diabetes is usually diagnosed in children and young adults, although it can appear at any age. People with type 1 diabetes need to take insulin every day to stay alive.
Type 2 diabetes
If you have type 2 diabetes , the cells in your body don’t use insulin properly. The pancreas may be making insulin but is not making enough insulin to keep your blood glucose level in the normal range. Type 2 diabetes is the most common type of diabetes. You are more likely to develop type 2 diabetes if you have risk factors , such as overweight or obesity , and a family history of the disease. You can develop type 2 diabetes at any age, even during childhood.
You can help delay or prevent type 2 diabetes by knowing the risk factors and taking steps toward a healthier lifestyle, such as losing weight or preventing weight gain.
Gestational diabetes
Gestational diabetes is a type of diabetes that develops during pregnancy. Most of the time, this type of diabetes goes away after the baby is born. However, if you’ve had gestational diabetes, you have a higher chance of developing type 2 diabetes later in life. Sometimes diabetes diagnosed during pregnancy is type 2 diabetes.
Prediabetes
People with prediabetes have blood glucose levels that are higher than normal but not high enough to be diagnosed with type 2 diabetes. If you have prediabetes, you have a higher risk of developing type 2 diabetes in the future. You also have a higher risk for heart disease than people with normal glucose levels.
Other types of diabetes
A less common type of diabetes, called monogenic diabetes , is caused by a change in a single gene . Diabetes can also come from having surgery to remove the pancreas, or from damage to the pancreas due to conditions such as cystic fibrosis or pancreatitis .
How common are diabetes and prediabetes?
More than 133 million Americans have diabetes or prediabetes. 1
As of 2019, 37.3 million people—or 11.3% of the U.S. population—had diabetes. 1 More than 1 in 4 people over the age of 65 had diabetes. Nearly 1 in 4 adults with diabetes didn’t know they had the disease. 2
About 90% to 95% of diabetes cases are type 2 diabetes. 3
In 2019, 96 million adults—38% of U.S. adults—had prediabetes. 4
What other health problems can people with diabetes develop?
Over time, high blood glucose can damage your heart , kidneys , feet , and eyes . If you have diabetes, you can take steps to lower your chances of developing diabetes health problems by taking steps to improve your health and learning how to manage the disease . Managing your blood glucose, blood pressure, and cholesterol levels can help prevent future health problems.

This content is provided as a service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health. NIDDK translates and disseminates research findings to increase knowledge and understanding about health and disease among patients, health professionals, and the public. Content produced by NIDDK is carefully reviewed by NIDDK scientists and other experts.
NIDDK would like to thank: Daniel Bessesen, M.D., University of Colorado; Domenico Accili, M.D., Columbia University
Diabetes Basics

- Previous Article
- Next Article
Classification
Diagnostic tests for diabetes, type 1 diabetes, prediabetes and type 2 diabetes, cystic fibrosis–related diabetes, posttransplantation diabetes mellitus, monogenic diabetes syndromes, pancreatic diabetes or diabetes in the context of disease of the exocrine pancreas, gestational diabetes mellitus, 2. classification and diagnosis of diabetes: standards of medical care in diabetes—2021.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
American Diabetes Association; 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021 . Diabetes Care 1 January 2021; 44 (Supplement_1): S15–S33. https://doi.org/10.2337/dc21-S002
Download citation file:
- Ris (Zotero)
- Reference Manager
The American Diabetes Association (ADA) “Standards of Medical Care in Diabetes” includes the ADA's current clinical practice recommendations and is intended to provide the components of diabetes care, general treatment goals and guidelines, and tools to evaluate quality of care. Members of the ADA Professional Practice Committee, a multidisciplinary expert committee ( https://doi.org/10.2337/dc21-SPPC ), are responsible for updating the Standards of Care annually, or more frequently as warranted. For a detailed description of ADA standards, statements, and reports, as well as the evidence-grading system for ADA's clinical practice recommendations, please refer to the Standards of Care Introduction ( https://doi.org/10.2337/dc21-SINT ). Readers who wish to comment on the Standards of Care are invited to do so at professional.diabetes.org/SOC .
Diabetes can be classified into the following general categories:
Type 1 diabetes (due to autoimmune β-cell destruction, usually leading to absolute insulin deficiency, including latent autoimmune diabetes of adulthood)
Type 2 diabetes (due to a progressive loss of adequate β-cell insulin secretion frequently on the background of insulin resistance)
Specific types of diabetes due to other causes, e.g., monogenic diabetes syndromes (such as neonatal diabetes and maturity-onset diabetes of the young), diseases of the exocrine pancreas (such as cystic fibrosis and pancreatitis), and drug- or chemical-induced diabetes (such as with glucocorticoid use, in the treatment of HIV/AIDS, or after organ transplantation)
Gestational diabetes mellitus (diabetes diagnosed in the second or third trimester of pregnancy that was not clearly overt diabetes prior to gestation)
This section reviews most common forms of diabetes but is not comprehensive. For additional information, see the American Diabetes Association (ADA) position statement “Diagnosis and Classification of Diabetes Mellitus” ( 1 ).
Type 1 diabetes and type 2 diabetes are heterogeneous diseases in which clinical presentation and disease progression may vary considerably. Classification is important for determining therapy, but some individuals cannot be clearly classified as having type 1 or type 2 diabetes at the time of diagnosis. The traditional paradigms of type 2 diabetes occurring only in adults and type 1 diabetes only in children are no longer accurate, as both diseases occur in both age-groups. Children with type 1 diabetes typically present with the hallmark symptoms of polyuria/polydipsia, and approximately one-third present with diabetic ketoacidosis (DKA) ( 2 ). The onset of type 1 diabetes may be more variable in adults; they may not present with the classic symptoms seen in children and may experience temporary remission from the need for insulin ( 3 – 5 ). Occasionally, patients with type 2 diabetes may present with DKA ( 6 ), particularly ethnic and racial minorities ( 7 ). It is important for the provider to realize that classification of diabetes type is not always straightforward at presentation and that misdiagnosis is common (e.g., adults with type 1 diabetes misdiagnosed as having type 2 diabetes; individuals with maturity-onset diabetes of the young [MODY] misdiagnosed as having type 1 diabetes, etc.). Although difficulties in distinguishing diabetes type may occur in all age-groups at onset, the diagnosis becomes more obvious over time in people with β-cell deficiency.
In both type 1 and type 2 diabetes, various genetic and environmental factors can result in the progressive loss of β-cell mass and/or function that manifests clinically as hyperglycemia. Once hyperglycemia occurs, patients with all forms of diabetes are at risk for developing the same chronic complications, although rates of progression may differ. The identification of individualized therapies for diabetes in the future will require better characterization of the many paths to β-cell demise or dysfunction ( 8 ). Across the globe many groups are working on combining clinical, pathophysiological, and genetic characteristics to more precisely define the subsets of diabetes currently clustered into the type 1 diabetes versus type 2 diabetes nomenclature with the goal of optimizing treatment approaches. Many of these studies show great promise and may soon be incorporated into the diabetes classification system ( 9 ).
Characterization of the underlying pathophysiology is more precisely developed in type 1 diabetes than in type 2 diabetes. It is now clear from studies of first-degree relatives of patients with type 1 diabetes that the persistent presence of two or more islet autoantibodies is a near certain predictor of clinical hyperglycemia and diabetes. The rate of progression is dependent on the age at first detection of autoantibody, number of autoantibodies, autoantibody specificity, and autoantibody titer. Glucose and A1C levels rise well before the clinical onset of diabetes, making diagnosis feasible well before the onset of DKA. Three distinct stages of type 1 diabetes can be identified ( Table 2.1 ) and serve as a framework for future research and regulatory decision-making ( 8 , 10 ). There is debate as to whether slowly progressive autoimmune diabetes with an adult onset should be termed latent autoimmune diabetes in adults (LADA) or type 1 diabetes. The clinical priority is awareness that slow autoimmune β-cell destruction can occur in adults leading to a long duration of marginal insulin secretory capacity. For the purpose of this classification, all forms of diabetes mediated by autoimmune β-cell destruction are included under the rubric of type 1 diabetes. Use of the term LADA is common and acceptable in clinical practice and has the practical impact of heightening awareness of a population of adults likely to develop overt autoimmune β-cell destruction ( 11 ), thus accelerating insulin initiation prior to deterioration of glucose control or development of DKA ( 4 , 12 ).
Staging of type 1 diabetes ( 8 , 10 )
FPG, fasting plasma glucose; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; 2-h PG, 2-h plasma glucose.
The paths to β-cell demise and dysfunction are less well defined in type 2 diabetes, but deficient β-cell insulin secretion, frequently in the setting of insulin resistance, appears to be the common denominator. Type 2 diabetes is associated with insulin secretory defects related to inflammation and metabolic stress among other contributors, including genetic factors. Future classification schemes for diabetes will likely focus on the pathophysiology of the underlying β-cell dysfunction ( 8 , 9 , 13 – 15 ).
Diabetes may be diagnosed based on plasma glucose criteria, either the fasting plasma glucose (FPG) value or the 2-h plasma glucose (2-h PG) value during a 75-g oral glucose tolerance test (OGTT), or A1C criteria ( 16 ) ( Table 2.2 ).
Criteria for the diagnosis of diabetes
DCCT, Diabetes Control and Complications Trial; FPG, fasting plasma glucose; OGTT, oral glucose tolerance test; WHO, World Health Organization; 2-h PG, 2-h plasma glucose.
In the absence of unequivocal hyperglycemia, diagnosis requires two abnormal test results from the same sample or in two separate test samples.
Generally, FPG, 2-h PG during 75-g OGTT, and A1C are equally appropriate for diagnostic screening. It should be noted that the tests do not necessarily detect diabetes in the same individuals. The efficacy of interventions for primary prevention of type 2 diabetes ( 17 , 18 ) has mainly been demonstrated among individuals who have impaired glucose tolerance (IGT) with or without elevated fasting glucose, not for individuals with isolated impaired fasting glucose (IFG) or for those with prediabetes defined by A1C criteria.
The same tests may be used to screen for and diagnose diabetes and to detect individuals with prediabetes ( Table 2.2 and Table 2.5 ) ( 19 ). Diabetes may be identified anywhere along the spectrum of clinical scenarios—in seemingly low-risk individuals who happen to have glucose testing, in individuals tested based on diabetes risk assessment, and in symptomatic patients.
Fasting and 2-Hour Plasma Glucose
The FPG and 2-h PG may be used to diagnose diabetes ( Table 2.2 ). The concordance between the FPG and 2-h PG tests is imperfect, as is the concordance between A1C and either glucose-based test. Compared with FPG and A1C cut points, the 2-h PG value diagnoses more people with prediabetes and diabetes ( 20 ). In people in whom there is discordance between A1C values and glucose values, FPG and 2-h PG are more accurate ( 21 ).
Recommendations
2.1 To avoid misdiagnosis or missed diagnosis, the A1C test should be performed using a method that is certified by the NGSP and standardized to the Diabetes Control and Complications Trial (DCCT) assay. B
2.2 Marked discordance between measured A1C and plasma glucose levels should raise the possibility of A1C assay interference and consideration of using an assay without interference or plasma blood glucose criteria to diagnose diabetes. B
2.3 In conditions associated with an altered relationship between A1C and glycemia, such as hemoglobinopathies including sickle cell disease, pregnancy (second and third trimesters and the postpartum period), glucose-6-phosphate dehydrogenase deficiency, HIV, hemodialysis, recent blood loss or transfusion, or erythropoietin therapy, only plasma blood glucose criteria should be used to diagnose diabetes. (See other conditions altering the relationship of a1c and glycemia below for more information.) B
The A1C test should be performed using a method that is certified by the NGSP ( www.ngsp.org ) and standardized or traceable to the Diabetes Control and Complications Trial (DCCT) reference assay. Although point-of-care A1C assays may be NGSP certified and cleared by the U.S. Food and Drug Administration (FDA) for use in monitoring glycemic control in people with diabetes in both Clinical Laboratory Improvement Amendments (CLIA)-regulated and CLIA-waived settings, only those point-of-care A1C assays that are also cleared by the FDA for use in the diagnosis of diabetes should be used for this purpose, and only in the clinical settings for which they are cleared. As discussed in Section 6 “Glycemic Targets” ( https://doi.org/10.2337/dc21-S006 ), point-of-care A1C assays may be more generally applied for assessment of glycemic control in the clinic.
A1C has several advantages compared with FPG and OGTT, including greater convenience (fasting not required), greater preanalytical stability, and less day-to-day perturbations during stress, changes in diet, or illness. However, these advantages may be offset by the lower sensitivity of A1C at the designated cut point, greater cost, limited availability of A1C testing in certain regions of the developing world, and the imperfect correlation between A1C and average glucose in certain individuals. The A1C test, with a diagnostic threshold of ≥6.5% (48 mmol/mol), diagnoses only 30% of the diabetes cases identified collectively using A1C, FPG, or 2-h PG, according to National Health and Nutrition Examination Survey (NHANES) data ( 22 ).
When using A1C to diagnose diabetes, it is important to recognize that A1C is an indirect measure of average blood glucose levels and to take other factors into consideration that may impact hemoglobin glycation independently of glycemia, such as hemodialysis, pregnancy, HIV treatment ( 23 , 24 ), age, race/ethnicity, pregnancy status, genetic background, and anemia/hemoglobinopathies. (See other conditions altering the relationship of a1c and glycemia below for more information.)
The epidemiologic studies that formed the basis for recommending A1C to diagnose diabetes included only adult populations ( 22 ). However, recent ADA clinical guidance concluded that A1C, FPG, or 2-h PG can be used to test for prediabetes or type 2 diabetes in children and adolescents (see screening and testing for prediabetes and type 2 diabetes in children and adolescents below for additional information) ( 25 ).
Race/Ethnicity/Hemoglobinopathies
Hemoglobin variants can interfere with the measurement of A1C, although most assays in use in the U.S. are unaffected by the most common variants. Marked discrepancies between measured A1C and plasma glucose levels should prompt consideration that the A1C assay may not be reliable for that individual. For patients with a hemoglobin variant but normal red blood cell turnover, such as those with the sickle cell trait, an A1C assay without interference from hemoglobin variants should be used. An updated list of A1C assays with interferences is available at www.ngsp.org/interf.asp .
African Americans heterozygous for the common hemoglobin variant HbS may have, for any given level of mean glycemia, lower A1C by about 0.3% compared with those without the trait ( 26 ). Another genetic variant, X-linked glucose-6-phosphate dehydrogenase G202A, carried by 11% of African Americans, was associated with a decrease in A1C of about 0.8% in homozygous men and 0.7% in homozygous women compared with those without the variant ( 27 ).
Even in the absence of hemoglobin variants, A1C levels may vary with race/ethnicity independently of glycemia ( 28 – 30 ). For example, African Americans may have higher A1C levels than non-Hispanic Whites with similar fasting and postglucose load glucose levels ( 31 ). Though conflicting data exists, African Americans may also have higher levels of fructosamine and glycated albumin and lower levels of 1,5-anhydroglucitol, suggesting that their glycemic burden (particularly postprandially) may be higher ( 32 , 33 ). Similarly, A1C levels may be higher for a given mean glucose concentration when measured with continuous glucose monitoring ( 34 ). Despite these and other reported differences, the association of A1C with risk for complications appears to be similar in African Americans and non-Hispanic Whites ( 35 , 36 ).
Other Conditions Altering the Relationship of A1C and Glycemia
In conditions associated with increased red blood cell turnover, such as sickle cell disease, pregnancy (second and third trimesters), glucose-6-phosphate dehydrogenase deficiency ( 37 , 38 ), hemodialysis, recent blood loss or transfusion, or erythropoietin therapy, only plasma blood glucose criteria should be used to diagnose diabetes ( 39 ). A1C is less reliable than blood glucose measurement in other conditions such as the postpartum state ( 40 – 42 ), HIV treated with certain protease inhibitors (PIs) and nucleoside reverse transcriptase inhibitors (NRTIs) ( 23 ), and iron-deficient anemia ( 43 ).
Confirming the Diagnosis
Unless there is a clear clinical diagnosis (e.g., patient in a hyperglycemic crisis or with classic symptoms of hyperglycemia and a random plasma glucose ≥200 mg/dL [11.1 mmol/L]), diagnosis requires two abnormal test results, either from the same sample ( 44 ) or in two separate test samples. If using two separate test samples, it is recommended that the second test, which may either be a repeat of the initial test or a different test, be performed without delay. For example, if the A1C is 7.0% (53 mmol/mol) and a repeat result is 6.8% (51 mmol/mol), the diagnosis of diabetes is confirmed. If two different tests (such as A1C and FPG) are both above the diagnostic threshold when analyzed from the same sample or in two different test samples, this also confirms the diagnosis. On the other hand, if a patient has discordant results from two different tests, then the test result that is above the diagnostic cut point should be repeated, with careful consideration of the possibility of A1C assay interference. The diagnosis is made on the basis of the confirmed test. For example, if a patient meets the diabetes criterion of the A1C (two results ≥6.5% [48 mmol/mol]) but not FPG (<126 mg/dL [7.0 mmol/L]), that person should nevertheless be considered to have diabetes.
Each of the tests has preanalytic and analytic variability, so it is possible that a test yielding an abnormal result (i.e., above the diagnostic threshold), when repeated, will produce a value below the diagnostic cut point. This scenario is likely for FPG and 2-h PG if the glucose samples remain at room temperature and are not centrifuged promptly. Because of the potential for preanalytic variability, it is critical that samples for plasma glucose be spun and separated immediately after they are drawn. If patients have test results near the margins of the diagnostic threshold, the health care professional should discuss signs and symptoms with the patient and repeat the test in 3 – 6 months.
In a patient with classic symptoms, measurement of plasma glucose is sufficient to diagnose diabetes (symptoms of hyperglycemia or hyperglycemic crisis plus a random plasma glucose ≥200 mg/dL [11.1 mmol/L]). In these cases, knowing the plasma glucose level is critical because, in addition to confirming that symptoms are due to diabetes, it will inform management decisions. Some providers may also want to know the A1C to determine the chronicity of the hyperglycemia. The criteria to diagnose diabetes are listed in Table 2.2 .
2.4 Screening for type 1 diabetes risk with a panel of islet autoantibodies is currently recommended in the setting of a research trial or can be offered as an option for first-degree family members of a proband with type 1 diabetes. B
2.5 Persistence of autoantibodies is a risk factor for clinical diabetes and may serve as an indication for intervention in the setting of a clinical trial. B
Immune-Mediated Diabetes
This form, previously called “insulin-dependent diabetes” or “juvenile-onset diabetes,” accounts for 5 – 10% of diabetes and is due to cellular-mediated autoimmune destruction of the pancreatic β-cells. Autoimmune markers include islet cell autoantibodies and autoantibodies to GAD (GAD65), insulin, the tyrosine phosphatases IA-2 and IA-2β, and zinc transporter 8 (ZnT8). Numerous clinical studies are being conducted to test various methods of preventing type 1 diabetes in those with evidence of islet autoimmunity ( www.clinicaltrials.gov and www.trialnet.org/our-research/prevention-studies ) ( 12 , 45 – 49 ). Stage 1 of type 1 diabetes is defined by the presence of two or more of these autoimmune markers. The disease has strong HLA associations, with linkage to the DQA and DQB genes. These HLA-DR/DQ alleles can be either predisposing or protective ( Table 2.1 ). There are important genetic considerations, as most of the mutations that cause diabetes are dominantly inherited. The importance of genetic testing is in the genetic counseling that follows. Some mutations are associated with other conditions, which then may prompt additional screenings.
The rate of β-cell destruction is quite variable, being rapid in some individuals (mainly infants and children) and slow in others (mainly adults) ( 50 ). Children and adolescents may present with DKA as the first manifestation of the disease. Others have modest fasting hyperglycemia that can rapidly change to severe hyperglycemia and/or DKA with infection or other stress. Adults may retain sufficient β-cell function to prevent DKA for many years; such individuals may have remission or decreased insulin needs for months or years and eventually become dependent on insulin for survival and are at risk for DKA ( 3 – 5 , 51 , 52 ). At this latter stage of the disease, there is little or no insulin secretion, as manifested by low or undetectable levels of plasma C-peptide. Immune-mediated diabetes is the most common form of diabetes in childhood and adolescence, but it can occur at any age, even in the 8th and 9th decades of life.
Autoimmune destruction of β-cells has multiple genetic predispositions and is also related to environmental factors that are still poorly defined. Although patients are not typically obese when they present with type 1 diabetes, obesity is increasingly common in the general population, and there is evidence that it may also be a risk factor for type 1 diabetes. As such, obesity should not preclude the diagnosis. People with type 1 diabetes are also prone to other autoimmune disorders such as Hashimoto thyroiditis, Graves disease, celiac disease, Addison disease, vitiligo, autoimmune hepatitis, myasthenia gravis, and pernicious anemia (see Section 4 “Comprehensive Medical Evaluation and Assessment of Comorbidities,” https://doi.org/10.2337/dc21-S004 ).
Idiopathic Type 1 Diabetes
Some forms of type 1 diabetes have no known etiologies. These patients have permanent insulinopenia and are prone to DKA but have no evidence of β-cell autoimmunity. However, only a minority of patients with type 1 diabetes fall into this category. Individuals with autoantibody-negative type 1 diabetes of African or Asian ancestry may suffer from episodic DKA and exhibit varying degrees of insulin deficiency between episodes (possibly ketosis-prone diabetes). This form of diabetes is strongly inherited and is not HLA associated. An absolute requirement for insulin replacement therapy in affected patients may be intermittent. Future research is needed to determine the cause of β-cell destruction in this rare clinical scenario.
Screening for Type 1 Diabetes Risk
The incidence and prevalence of type 1 diabetes is increasing ( 53 ). Patients with type 1 diabetes often present with acute symptoms of diabetes and markedly elevated blood glucose levels, and approximately one-third are diagnosed with life-threatening DKA ( 2 ). Multiple studies indicate that measuring islet autoantibodies in individuals genetically at risk for type 1 diabetes (e.g., relatives of those with type 1 diabetes or individuals from the general population with type 1 diabetes–associated genetic factors) identifies individuals who may develop type 1 diabetes ( 10 ). Such testing, coupled with education about diabetes symptoms and close follow-up, may enable earlier identification of type 1 diabetes onset. A study reported the risk of progression to type 1 diabetes from the time of seroconversion to autoantibody positivity in three pediatric cohorts from Finland, Germany, and the U.S. Of the 585 children who developed more than two autoantibodies, nearly 70% developed type 1 diabetes within 10 years and 84% within 15 years ( 45 ). These findings are highly significant because while the German group was recruited from offspring of parents with type 1 diabetes, the Finnish and American groups were recruited from the general population. Remarkably, the findings in all three groups were the same, suggesting that the same sequence of events led to clinical disease in both “sporadic” and familial cases of type 1 diabetes. Indeed, the risk of type 1 diabetes increases as the number of relevant autoantibodies detected increases ( 48 , 54 , 55 ). In The Environmental Determinants of Diabetes in the Young (TEDDY) study, type 1 diabetes developed in 21% of 363 subjects with at least one autoantibody at 3 years of age ( 56 ).
There is currently a lack of accepted and clinically validated screening programs outside of the research setting; thus, widespread clinical testing of asymptomatic low-risk individuals is not currently recommended due to lack of approved therapeutic interventions. However, one should consider referring relatives of those with type 1 diabetes for islet autoantibody testing for risk assessment in the setting of a clinical research study (see www.trialnet.org ). Individuals who test positive should be counseled about the risk of developing diabetes, diabetes symptoms, and DKA prevention. Numerous clinical studies are being conducted to test various methods of preventing and treating stage 2 type 1 diabetes in those with evidence of autoimmunity with promising results (see www.clinicaltrials.gov and www.trialnet.org ).
2.6 Screening for prediabetes and type 2 diabetes with an informal assessment of risk factors or validated tools should be considered in asymptomatic adults. B
2.7 Testing for prediabetes and/or type 2 diabetes in asymptomatic people should be considered in adults of any age with overweight or obesity (BMI ≥25 kg/m 2 or ≥23 kg/m 2 in Asian Americans) and who have one or more additional risk factors for diabetes ( Table 2.3 ). B
2.8 Testing for prediabetes and/or type 2 diabetes should be considered in women with overweight or obesity planning pregnancy and/or who have one or more additional risk factor for diabetes ( Table 2.3 ). C
2.9 For all people, testing should begin at age 45 years. B
2.10 If tests are normal, repeat testing carried out at a minimum of 3-year intervals is reasonable, sooner with symptoms. C
2.11 To test for prediabetes and type 2 diabetes, fasting plasma glucose, 2-h plasma glucose during 75-g oral glucose tolerance test, and A1C are equally appropriate ( Table 2.2 and Table 2.5 ). B
2.12 In patients with prediabetes and type 2 diabetes, identify and treat other cardiovascular disease risk factors. A
2.13 Risk-based screening for prediabetes and/or type 2 diabetes should be considered after the onset of puberty or after 10 years of age, whichever occurs earlier, in children and adolescents with overweight (BMI ≥85th percentile) or obesity (BMI ≥95th percentile) and who have one or more risk factor for diabetes. (See Table 2.4 for evidence grading of risk factors.) B
2.14 Patients with HIV should be screened for diabetes and prediabetes with a fasting glucose test before starting antiretroviral therapy, at the time of switching antiretroviral therapy, and 3−6 months after starting or switching antiretroviral therapy. If initial screening results are normal, fasting glucose should be checked annually. E
Criteria for testing for diabetes or prediabetes in asymptomatic adults
CVD, cardiovascular disease; GDM, gestational diabetes mellitus; IFG, impaired fasting glucose; IGT, impaired glucose tolerance.
Risk-based screening for type 2 diabetes or prediabetes in asymptomatic children and adolescents in a clinical setting ( 202 )
GDM, gestational diabetes mellitus.
After the onset of puberty or after 10 years of age, whichever occurs earlier. If tests are normal, repeat testing at a minimum of 3-year intervals (or more frequently if BMI is increasing or risk factor profile deteriorating) is recommended. Reports of type 2 diabetes before age 10 years exist, and this can be considered with numerous risk factors.
Prediabetes
“Prediabetes” is the term used for individuals whose glucose levels do not meet the criteria for diabetes but are too high to be considered normal ( 35 , 36 ). Patients with prediabetes are defined by the presence of IFG and/or IGT and/or A1C 5.7 – 6.4% (39 – 47 mmol/mol) ( Table 2.5 ). Prediabetes should not be viewed as a clinical entity in its own right but rather as an increased risk for diabetes and cardiovascular disease (CVD). Criteria for testing for diabetes or prediabetes in asymptomatic adults is outlined in Table 2.3 . Prediabetes is associated with obesity (especially abdominal or visceral obesity), dyslipidemia with high triglycerides and/or low HDL cholesterol, and hypertension.
Criteria defining prediabetes *
FPG, fasting plasma glucose; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; OGTT, oral glucose tolerance test; 2-h PG, 2-h plasma glucose.
For all three tests, risk is continuous, extending below the lower limit of the range and becoming disproportionately greater at the higher end of the range.
IFG is defined as FPG levels from 100 to 125 mg/dL (from 5.6 to 6.9 mmol/L) ( 57 , 58 ) and IGT as 2-h PG during 75-g OGTT levels from 140 to 199 mg/dL (from 7.8 to 11.0 mmol/L) ( 59 ). It should be noted that the World Health Organization (WHO) and numerous other diabetes organizations define the IFG cutoff at 110 mg/dL (6.1 mmol/L).
As with the glucose measures, several prospective studies that used A1C to predict the progression to diabetes as defined by A1C criteria demonstrated a strong, continuous association between A1C and subsequent diabetes. In a systematic review of 44,203 individuals from 16 cohort studies with a follow-up interval averaging 5.6 years (range 2.8 – 12 years), those with A1C between 5.5% and 6.0% (between 37 and 42 mmol/mol) had a substantially increased risk of diabetes (5-year incidence from 9% to 25%). Those with an A1C range of 6.0–6.5% (42 – 48 mmol/mol) had a 5-year risk of developing diabetes between 25% and 50% and a relative risk 20 times higher compared with A1C of 5.0% (31 mmol/mol) ( 60 ). In a community-based study of African American and non-Hispanic White adults without diabetes, baseline A1C was a stronger predictor of subsequent diabetes and cardiovascular events than fasting glucose ( 61 ). Other analyses suggest that A1C of 5.7% (39 mmol/mol) or higher is associated with a diabetes risk similar to that of the high-risk participants in the Diabetes Prevention Program (DPP) ( 62 ), and A1C at baseline was a strong predictor of the development of glucose-defined diabetes during the DPP and its follow-up ( 63 ). Hence, it is reasonable to consider an A1C range of 5.7 – 6.4% (39 – 47 mmol/mol) as identifying individuals with prediabetes. Similar to those with IFG and/or IGT, individuals with A1C of 5.7 – 6.4% (39 – 47 mmol/mol) should be informed of their increased risk for diabetes and CVD and counseled about effective strategies to lower their risks (see Section 3 “Prevention or Delay of Type 2 Diabetes,” https://doi.org/10.2337/dc21-S003 ). Similar to glucose measurements, the continuum of risk is curvilinear, so as A1C rises, the diabetes risk rises disproportionately ( 60 ). Aggressive interventions and vigilant follow-up should be pursued for those considered at very high risk (e.g., those with A1C >6.0% [42 mmol/mol]).
Table 2.5 summarizes the categories of prediabetes and Table 2.3 the criteria for prediabetes testing. The ADA diabetes risk test is an additional option for assessment to determine the appropriateness of testing for diabetes or prediabetes in asymptomatic adults ( Fig. 2.1 ) ( diabetes.org/socrisktest ). For additional background regarding risk factors and screening for prediabetes, see screening and testing for prediabetes and type 2 diabetes in asymptomatic adults and also screening and testing for prediabetes and type 2 diabetes in children and adolescents below.

ADA risk test ( diabetes.org/socrisktest ).
Type 2 Diabetes
Type 2 diabetes, previously referred to as “noninsulin-dependent diabetes” or “adult-onset diabetes,” accounts for 90 – 95% of all diabetes. This form encompasses individuals who have relative (rather than absolute) insulin deficiency and have peripheral insulin resistance. At least initially, and often throughout their lifetime, these individuals may not need insulin treatment to survive.
There are various causes of type 2 diabetes. Although the specific etiologies are not known, autoimmune destruction of β-cells does not occur, and patients do not have any of the other known causes of diabetes. Most, but not all, patients with type 2 diabetes have overweight or obesity. Excess weight itself causes some degree of insulin resistance. Patients who do not have obesity or overweight by traditional weight criteria may have an increased percentage of body fat distributed predominantly in the abdominal region.
DKA seldom occurs spontaneously in type 2 diabetes; when seen, it usually arises in association with the stress of another illness such as infection, myocardial infarction, or with the use of certain drugs (e.g., corticosteroids, atypical antipsychotics, and sodium–glucose cotransporter 2 inhibitors) ( 64 , 65 ). Type 2 diabetes frequently goes undiagnosed for many years because hyperglycemia develops gradually and, at earlier stages, is often not severe enough for the patient to notice the classic diabetes symptoms caused by hyperglycemia. Nevertheless, even undiagnosed patients are at increased risk of developing macrovascular and microvascular complications.
Patients with type 2 diabetes may have insulin levels that appear normal or elevated, yet the failure to normalize blood glucose reflects a relative defect in glucose-stimulated insulin secretion. Thus, insulin secretion is defective in these patients and insufficient to compensate for insulin resistance. Insulin resistance may improve with weight reduction, exercise, and/or pharmacologic treatment of hyperglycemia but is seldom restored to normal. Recent interventions with intensive diet and exercise or surgical weight loss have led to diabetes remission ( 66 – 72 ) (see Section 8 “Obesity Management for the Treatment of Type 2 Diabetes,” https://doi.org/10.2337/dc21-S008 ).
The risk of developing type 2 diabetes increases with age, obesity, and lack of physical activity. It occurs more frequently in women with prior gestational diabetes mellitus (GDM), with hypertension or dyslipidemia, with polycystic ovary syndrome, and in certain racial/ethnic subgroups (African American, American Indian, Hispanic/Latino, and Asian American). It is often associated with a strong genetic predisposition or family history in first-degree relatives (more so than type 1 diabetes). However, the genetics of type 2 diabetes is poorly understood and under intense investigation in this era of precision medicine ( 13 ). In adults without traditional risk factors for type 2 diabetes and/or younger age, consider islet autoantibody testing (e.g., GAD65 autoantibodies) to exclude the diagnosis of type 1 diabetes.
Screening and Testing for Prediabetes and Type 2 Diabetes in Asymptomatic Adults
Screening for prediabetes and type 2 diabetes risk through an informal assessment of risk factors ( Table 2.3 ) or with an assessment tool, such as the ADA risk test ( Fig. 2.1 ) (online at diabetes.org/socrisktest ), is recommended to guide providers on whether performing a diagnostic test ( Table 2.2 ) is appropriate. Prediabetes and type 2 diabetes meet criteria for conditions in which early detection via screening is appropriate. Both conditions are common and impose significant clinical and public health burdens. There is often a long presymptomatic phase before the diagnosis of type 2 diabetes. Simple tests to detect preclinical disease are readily available. The duration of glycemic burden is a strong predictor of adverse outcomes. There are effective interventions that prevent progression from prediabetes to diabetes (see Section 3 “Prevention or Delay of Type 2 Diabetes,” https://doi.org/10.2337/dc21-S003 ) and reduce the risk of diabetes complications ( 73 ) (see Section 10 “Cardiovascular Disease and Risk Management,” https://doi.org/10.2337/dc21-S010 , and Section 11 “Microvascular Complications and Foot Care,” https://doi.org/10.2337/dc21-S011 ). In the most recent National Institutes of Health (NIH) Diabetes Prevention Program Outcomes Study (DPPOS) report, prevention of progression from prediabetes to diabetes ( 74 ) resulted in lower rates of developing retinopathy and nephropathy ( 75 ). Similar impact on diabetes complications was reported with screening, diagnosis, and comprehensive risk factor management in the U.K. Clinical Practice Research Datalink database ( 73 ). In that report, progression from prediabetes to diabetes augmented risk of complications.
Approximately one-quarter of people with diabetes in the U.S. and nearly half of Asian and Hispanic Americans with diabetes are undiagnosed ( 57 , 58 ). Although screening of asymptomatic individuals to identify those with prediabetes or diabetes might seem reasonable, rigorous clinical trials to prove the effectiveness of such screening have not been conducted and are unlikely to occur. Based on a population estimate, diabetes in women of childbearing age is underdiagnosed ( 76 ). Employing a probabilistic model, Peterson et al. ( 77 ) demonstrated cost and health benefits of preconception screening.
A large European randomized controlled trial compared the impact of screening for diabetes and intensive multifactorial intervention with that of screening and routine care ( 78 ). General practice patients between the ages of 40 and 69 years were screened for diabetes and randomly assigned by practice to intensive treatment of multiple risk factors or routine diabetes care. After 5.3 years of follow-up, CVD risk factors were modestly but significantly improved with intensive treatment compared with routine care, but the incidence of first CVD events or mortality was not significantly different between the groups ( 59 ). The excellent care provided to patients in the routine care group and the lack of an unscreened control arm limited the authors' ability to determine whether screening and early treatment improved outcomes compared with no screening and later treatment after clinical diagnoses. Computer simulation modeling studies suggest that major benefits are likely to accrue from the early diagnosis and treatment of hyperglycemia and cardiovascular risk factors in type 2 diabetes ( 79 ); moreover, screening, beginning at age 30 or 45 years and independent of risk factors, may be cost-effective (<$11,000 per quality-adjusted life year gained—2010 modeling data) ( 80 ). Cost-effectiveness of screening has been reinforced in cohort studies ( 81 , 82 ).
Additional considerations regarding testing for type 2 diabetes and prediabetes in asymptomatic patients include the following.
Age is a major risk factor for diabetes. Testing should begin at no later than age 45 years for all patients. Screening should be considered in adults of any age with overweight or obesity and one or more risk factors for diabetes.
BMI and Ethnicity
In general, BMI ≥25 kg/m 2 is a risk factor for diabetes. However, data suggest that the BMI cut point should be lower for the Asian American population ( 83 , 84 ). The BMI cut points fall consistently between 23 and 24 kg/m 2 (sensitivity of 80%) for nearly all Asian American subgroups (with levels slightly lower for Japanese Americans). This makes a rounded cut point of 23 kg/m 2 practical. An argument can be made to push the BMI cut point to lower than 23 kg/m 2 in favor of increased sensitivity; however, this would lead to an unacceptably low specificity (13.1%). Data from WHO also suggests that a BMI of ≥23 kg/m 2 should be used to define increased risk in Asian Americans ( 85 ). The finding that one-third to one-half of diabetes in Asian Americans is undiagnosed suggests that testing is not occurring at lower BMI thresholds ( 86 , 87 ).
Evidence also suggests that other populations may benefit from lower BMI cut points. For example, in a large multiethnic cohort study, for an equivalent incidence rate of diabetes, a BMI of 30 kg/m 2 in non-Hispanic Whites was equivalent to a BMI of 26 kg/m 2 in African Americans ( 88 ).
Medications
Certain medications, such as glucocorticoids, thiazide diuretics, some HIV medications ( 23 ), and atypical antipsychotics ( 66 ), are known to increase the risk of diabetes and should be considered when deciding whether to screen.
Individuals with HIV are at higher risk for developing prediabetes and diabetes on antiretroviral (ARV) therapies, so a screening protocol is recommended ( 89 ). The A1C test may underestimate glycemia in people with HIV; it is not recommended for diagnosis and may present challenges for monitoring ( 24 ). In those with prediabetes, weight loss through healthy nutrition and physical activity may reduce the progression toward diabetes. Among patients with HIV and diabetes, preventive health care using an approach used in patients without HIV is critical to reduce the risks of microvascular and macrovascular complications. Diabetes risk is increased with certain PIs and NRTIs. New-onset diabetes is estimated to occur in more than 5% of patients infected with HIV on PIs, whereas more than 15% may have prediabetes ( 90 ). PIs are associated with insulin resistance and may also lead to apoptosis of pancreatic β-cells. NRTIs also affect fat distribution (both lipohypertrophy and lipoatrophy), which is associated with insulin resistance. For patients with HIV and ARV-associated hyperglycemia, it may be appropriate to consider discontinuing the problematic ARV agents if safe and effective alternatives are available ( 91 ). Before making ARV substitutions, carefully consider the possible effect on HIV virological control and the potential adverse effects of new ARV agents. In some cases, antihyperglycemic agents may still be necessary.
Testing Interval
The appropriate interval between screening tests is not known ( 92 ). The rationale for the 3-year interval is that with this interval, the number of false-positive tests that require confirmatory testing will be reduced and individuals with false-negative tests will be retested before substantial time elapses and complications develop ( 92 ). In especially high-risk individuals, particularly with weight gain, shorter intervals between screening may be useful.
Community Screening
Ideally, testing should be carried out within a health care setting because of the need for follow-up and treatment. Community screening outside a health care setting is generally not recommended because people with positive tests may not seek, or have access to, appropriate follow-up testing and care. However, in specific situations where an adequate referral system is established beforehand for positive tests, community screening may be considered. Community testing may also be poorly targeted; i.e., it may fail to reach the groups most at risk and inappropriately test those at very low risk or even those who have already been diagnosed ( 93 ).
Screening in Dental Practices
Because periodontal disease is associated with diabetes, the utility of screening in a dental setting and referral to primary care as a means to improve the diagnosis of prediabetes and diabetes has been explored ( 94 – 96 ), with one study estimating that 30% of patients ≥30 years of age seen in general dental practices had dysglycemia ( 96 , 97 ). A similar study in 1,150 dental patients >40 years old in India reported 20.69% and 14.60% meeting criteria for prediabetes and diabetes using random blood glucose. Further research is needed to demonstrate the feasibility, effectiveness, and cost-effectiveness of screening in this setting.
Screening and Testing for Prediabetes and Type 2 Diabetes in Children and Adolescents
In the last decade, the incidence and prevalence of type 2 diabetes in children and adolescents has increased dramatically, especially in racial and ethnic minority populations ( 53 ). See Table 2.4 for recommendations on risk-based screening for type 2 diabetes or prediabetes in asymptomatic children and adolescents in a clinical setting ( 25 ). See Table 2.2 and Table 2.5 for the criteria for the diagnosis of diabetes and prediabetes, respectively, which apply to children, adolescents, and adults. See Section 13 “Children and Adolescents” ( https://doi.org/10.2337/dc21-S013 ) for additional information on type 2 diabetes in children and adolescents.
Some studies question the validity of A1C in the pediatric population, especially among certain ethnicities, and suggest OGTT or FPG as more suitable diagnostic tests ( 98 ). However, many of these studies do not recognize that diabetes diagnostic criteria are based on long-term health outcomes, and validations are not currently available in the pediatric population ( 99 ). The ADA acknowledges the limited data supporting A1C for diagnosing type 2 diabetes in children and adolescents. Although A1C is not recommended for diagnosis of diabetes in children with cystic fibrosis or symptoms suggestive of acute onset of type 1 diabetes and only A1C assays without interference are appropriate for children with hemoglobinopathies, the ADA continues to recommend A1C for diagnosis of type 2 diabetes in this cohort to decrease barriers to screening ( 100 , 101 ).
2.15 Annual screening for cystic fibrosis–related diabetes (CFRD) with an oral glucose tolerance test should begin by age 10 years in all patients with cystic fibrosis not previously diagnosed with CFRD. B
2.16 A1C is not recommended as a screening test for cystic fibrosis–related diabetes. B
2.17 Patients with cystic fibrosis–related diabetes should be treated with insulin to attain individualized glycemic goals. A
2.18 Beginning 5 years after the diagnosis of cystic fibrosis–related diabetes, annual monitoring for complications of diabetes is recommended. E
Cystic fibrosis–related diabetes (CFRD) is the most common comorbidity in people with cystic fibrosis, occurring in about 20% of adolescents and 40 – 50% of adults ( 102 ). Diabetes in this population, compared with individuals with type 1 or type 2 diabetes, is associated with worse nutritional status, more severe inflammatory lung disease, and greater mortality. Insulin insufficiency is the primary defect in CFRD. Genetically determined β-cell function and insulin resistance associated with infection and inflammation may also contribute to the development of CFRD. Milder abnormalities of glucose tolerance are even more common and occur at earlier ages than CFRD. Whether individuals with IGT should be treated with insulin replacement has not currently been determined. Although screening for diabetes before the age of 10 years can identify risk for progression to CFRD in those with abnormal glucose tolerance, no benefit has been established with respect to weight, height, BMI, or lung function. OGTT is the recommended screening test; however, recent publications suggest that an A1C cut point threshold of 5.5% (5.8% in a second study) would detect more than 90% of cases and reduce patient screening burden ( 103 , 104 ). Ongoing studies are underway to validate this approach. Regardless of age, weight loss or failure of expected weight gain is a risk for CFRD and should prompt screening ( 103 , 104 ). The Cystic Fibrosis Foundation Patient Registry ( 105 ) evaluated 3,553 cystic fibrosis patients and diagnosed 445 (13%) with CFRD. Early diagnosis and treatment of CFRD was associated with preservation of lung function. The European Cystic Fibrosis Society Patient Registry reported an increase in CFRD with age (increased 10% per decade), genotype, decreased lung function, and female sex ( 106 , 107 ). Continuous glucose monitoring or HOMA of β-cell function ( 108 ) may be more sensitive than OGTT to detect risk for progression to CFRD; however, evidence linking these results to long-term outcomes is lacking, and these tests are not recommended for screening outside of the research setting ( 109 ).
CFRD mortality has significantly decreased over time, and the gap in mortality between cystic fibrosis patients with and without diabetes has considerably narrowed ( 110 ). There are limited clinical trial data on therapy for CFRD. The largest study compared three regimens: premeal insulin aspart, repaglinide, or oral placebo in cystic fibrosis patients with diabetes or abnormal glucose tolerance. Participants all had weight loss in the year preceding treatment; however, in the insulin-treated group, this pattern was reversed, and patients gained 0.39 (± 0.21) BMI units (P = 0.02). The repaglinide-treated group had initial weight gain, but this was not sustained by 6 months. The placebo group continued to lose weight ( 110 ). Insulin remains the most widely used therapy for CFRD ( 111 ). The primary rationale for the use of insulin in patients with CFRD is to induce an anabolic state while promoting macronutrient retention and weight gain.
Additional resources for the clinical management of CFRD can be found in the position statement “Clinical Care Guidelines for Cystic Fibrosis–Related Diabetes: A Position Statement of the American Diabetes Association and a Clinical Practice Guideline of the Cystic Fibrosis Foundation, Endorsed by the Pediatric Endocrine Society” ( 112 ) and in the International Society for Pediatric and Adolescent Diabetes's 2014 clinical practice consensus guidelines ( 102 ).
2.19 Patients should be screened after organ transplantation for hyperglycemia, with a formal diagnosis of posttransplantation diabetes mellitus being best made once a patient is stable on an immunosuppressive regimen and in the absence of an acute infection. B
2.20 The oral glucose tolerance test is the preferred test to make a diagnosis of posttransplantation diabetes mellitus. B
2.21 Immunosuppressive regimens shown to provide the best outcomes for patient and graft survival should be used, irrespective of posttransplantation diabetes mellitus risk. E
Several terms are used in the literature to describe the presence of diabetes following organ transplantation ( 113 ). “New-onset diabetes after transplantation” (NODAT) is one such designation that describes individuals who develop new-onset diabetes following transplant. NODAT excludes patients with pretransplant diabetes that was undiagnosed as well as posttransplant hyperglycemia that resolves by the time of discharge ( 114 ). Another term, “posttransplantation diabetes mellitus” (PTDM) ( 114 , 115 ), describes the presence of diabetes in the posttransplant setting irrespective of the timing of diabetes onset.
Hyperglycemia is very common during the early posttransplant period, with ∼90% of kidney allograft recipients exhibiting hyperglycemia in the first few weeks following transplant ( 114 – 117 ). In most cases, such stress- or steroid-induced hyperglycemia resolves by the time of discharge ( 117 , 118 ). Although the use of immunosuppressive therapies is a major contributor to the development of PTDM, the risks of transplant rejection outweigh the risks of PTDM and the role of the diabetes care provider is to treat hyperglycemia appropriately regardless of the type of immunosuppression ( 114 ). Risk factors for PTDM include both general diabetes risks (such as age, family history of diabetes, etc.) as well as transplant-specific factors, such as use of immunosuppressant agents ( 119 ). Whereas posttransplantation hyperglycemia is an important risk factor for subsequent PTDM, a formal diagnosis of PTDM is optimally made once the patient is stable on maintenance immunosuppression and in the absence of acute infection ( 117 – 120 ). In a recent study of 152 heart transplant recipients, 38% had PTDM at 1 year. Risk factors for PTDM included elevated BMI, discharge from the hospital on insulin, and glucose values in the 24 h prior to hospital discharge ( 121 ). In an Iranian cohort, 19% had PTDM after heart and lung transplant ( 122 ). The OGTT is considered the gold standard test for the diagnosis of PTDM (1 year posttransplant) ( 114 , 115 , 123 , 124 ). However, screening patients using fasting glucose and/or A1C can identify high-risk patients requiring further assessment and may reduce the number of overall OGTTs required.
Few randomized controlled studies have reported on the short- and long-term use of antihyperglycemic agents in the setting of PTDM ( 119 , 125 , 126 ). Most studies have reported that transplant patients with hyperglycemia and PTDM after transplantation have higher rates of rejection, infection, and rehospitalization ( 117 , 119 , 127 ). Insulin therapy is the agent of choice for the management of hyperglycemia, PTDM, and preexisting diabetes and diabetes in the hospital setting. After discharge, patients with preexisting diabetes could go back on their pretransplant regimen if they were in good control before transplantation. Those with previously poor control or with persistent hyperglycemia should continue insulin with frequent home self-monitoring of blood glucose to determine when insulin dose reductions may be needed and when it may be appropriate to switch to noninsulin agents.
No studies to date have established which noninsulin agents are safest or most efficacious in PTDM. The choice of agent is usually made based on the side effect profile of the medication and possible interactions with the patient's immunosuppression regimen ( 119 ). Drug dose adjustments may be required because of decreases in the glomerular filtration rate, a relatively common complication in transplant patients. A small short-term pilot study reported that metformin was safe to use in renal transplant recipients ( 128 ), but its safety has not been determined in other types of organ transplant. Thiazolidinediones have been used successfully in patients with liver and kidney transplants, but side effects include fluid retention, heart failure, and osteopenia ( 129 , 130 ). Dipeptidyl peptidase 4 inhibitors do not interact with immunosuppressant drugs and have demonstrated safety in small clinical trials ( 131 , 132 ). Well-designed intervention trials examining the efficacy and safety of these and other antihyperglycemic agents in patients with PTDM are needed.
2.22 All children diagnosed with diabetes in the first 6 months of life should have immediate genetic testing for neonatal diabetes. A
2.23 Children and those diagnosed in early adulthood who have diabetes not characteristic of type 1 or type 2 diabetes that occurs in successive generations (suggestive of an autosomal dominant pattern of inheritance) should have genetic testing for maturity-onset diabetes of the young. A
2.24 In both instances, consultation with a center specializing in diabetes genetics is recommended to understand the significance of these mutations and how best to approach further evaluation, treatment, and genetic counseling. E
Monogenic defects that cause β-cell dysfunction, such as neonatal diabetes and MODY, represent a small fraction of patients with diabetes (<5%). Table 2.6 describes the most common causes of monogenic diabetes. For a comprehensive list of causes, see Genetic Diagnosis of Endocrine Disorders ( 133 ).
Most common causes of monogenic diabetes ( 133 )
AD, autosomal dominant; AR, autosomal recessive; IUGR, intrauterine growth restriction; OGTT, oral glucose tolerance test; UPD6, uniparental disomy of chromosome 6; 2-h PG, 2-h plasma glucose.
Neonatal Diabetes
Diabetes occurring under 6 months of age is termed “neonatal” or “congenital” diabetes, and about 80 – 85% of cases can be found to have an underlying monogenic cause ( 134 – 137 ). Neonatal diabetes occurs much less often after 6 months of age, whereas autoimmune type 1 diabetes rarely occurs before 6 months of age. Neonatal diabetes can either be transient or permanent. Transient diabetes is most often due to overexpression of genes on chromosome 6q24, is recurrent in about half of cases, and may be treatable with medications other than insulin. Permanent neonatal diabetes is most commonly due to autosomal dominant mutations in the genes encoding the Kir6.2 subunit ( KCNJ11 ) and SUR1 subunit ( ABCC8 ) of the β-cell K ATP channel. A recent report details a de novo mutation in EIF2B1 affecting eIF2 signaling associated with permanent neonatal diabetes and hepatic dysfunction, similar to Wolcott-Rallison syndrome but with few severe comorbidities ( 138 ). Correct diagnosis has critical implications because most patients with K ATP -related neonatal diabetes will exhibit improved glycemic control when treated with high-dose oral sulfonylureas instead of insulin. Insulin gene ( INS ) mutations are the second most common cause of permanent neonatal diabetes, and, while intensive insulin management is currently the preferred treatment strategy, there are important genetic counseling considerations, as most of the mutations that cause diabetes are dominantly inherited.

Maturity-Onset Diabetes of the Young
MODY is frequently characterized by onset of hyperglycemia at an early age (classically before age 25 years, although diagnosis may occur at older ages). MODY is characterized by impaired insulin secretion with minimal or no defects in insulin action (in the absence of coexistent obesity). It is inherited in an autosomal dominant pattern with abnormalities in at least 13 genes on different chromosomes identified to date. The most commonly reported forms are GCK-MODY (MODY2), HNF1A-MODY (MODY3), and HNF4A-MODY (MODY1).
For individuals with MODY, the treatment implications are considerable and warrant genetic testing ( 139 , 140 ). Clinically, patients with GCK-MODY exhibit mild, stable fasting hyperglycemia and do not require antihyperglycemic therapy except sometimes during pregnancy. Patients with HNF1A- or HNF4A-MODY usually respond well to low doses of sulfonylureas, which are considered first-line therapy. Mutations or deletions in HNF1B are associated with renal cysts and uterine malformations (renal cysts and diabetes [RCAD] syndrome). Other extremely rare forms of MODY have been reported to involve other transcription factor genes including PDX1 ( IPF1 ) and NEUROD1 .
Diagnosis of Monogenic Diabetes
A diagnosis of one of the three most common forms of MODY, including GCK-MODY, HNF1A-MODY, and HNF4A-MODY, allows for more cost-effective therapy (no therapy for GCK-MODY; sulfonylureas as first-line therapy for HNF1A-MODY and HNF4A-MODY). Additionally, diagnosis can lead to identification of other affected family members. Genetic screening is increasingly available and cost-effective ( 138 , 140 ).
A diagnosis of MODY should be considered in individuals who have atypical diabetes and multiple family members with diabetes not characteristic of type 1 or type 2 diabetes, although admittedly “atypical diabetes” is becoming increasingly difficult to precisely define in the absence of a definitive set of tests for either type of diabetes ( 135 – 137 , 139 – 145 ). In most cases, the presence of autoantibodies for type 1 diabetes precludes further testing for monogenic diabetes, but the presence of autoantibodies in patients with monogenic diabetes has been reported ( 146 ). Individuals in whom monogenic diabetes is suspected should be referred to a specialist for further evaluation if available, and consultation is available from several centers. Readily available commercial genetic testing following the criteria listed below now enables a cost-effective ( 147 ), often cost-saving, genetic diagnosis that is increasingly supported by health insurance. A biomarker screening pathway such as the combination of urinary C-peptide/creatinine ratio and antibody screening may aid in determining who should get genetic testing for MODY ( 148 ). It is critical to correctly diagnose one of the monogenic forms of diabetes because these patients may be incorrectly diagnosed with type 1 or type 2 diabetes, leading to suboptimal, even potentially harmful, treatment regimens and delays in diagnosing other family members ( 149 ). The correct diagnosis is especially critical for those with GCK-MODY mutations where multiple studies have shown that no complications ensue in the absence of glucose-lowering therapy ( 150 ). Genetic counseling is recommended to ensure that affected individuals understand the patterns of inheritance and the importance of a correct diagnosis.
The diagnosis of monogenic diabetes should be considered in children and adults diagnosed with diabetes in early adulthood with the following findings:
Diabetes diagnosed within the first 6 months of life (with occasional cases presenting later, mostly INS and ABCC8 mutations) ( 134 , 151 )
Diabetes without typical features of type 1 or type 2 diabetes (negative diabetes-associated autoantibodies, nonobese, lacking other metabolic features, especially with strong family history of diabetes)
Stable, mild fasting hyperglycemia (100 – 150 mg/dL [5.5 – 8.5 mmol/L]), stable A1C between 5.6% and 7.6% (between 38 and 60 mmol/mol), especially if nonobese
Pancreatic diabetes includes both structural and functional loss of glucose-normalizing insulin secretion in the context of exocrine pancreatic dysfunction and is commonly misdiagnosed as type 2 diabetes. Hyperglycemia due to general pancreatic dysfunction has been called “type 3c diabetes” and, more recently, diabetes in the context of disease of the exocrine pancreas has been termed pancreoprivic diabetes ( 1 ). The diverse set of etiologies includes pancreatitis (acute and chronic), trauma or pancreatectomy, neoplasia, cystic fibrosis (addressed elsewhere in this chapter), hemochromatosis, fibrocalculous pancreatopathy, rare genetic disorders ( 152 ), and idiopathic forms ( 1 ), which is the preferred terminology. A distinguishing feature is concurrent pancreatic exocrine insufficiency (according to the monoclonal fecal elastase 1 test or direct function tests), pathological pancreatic imaging (endoscopic ultrasound, MRI, computed tomography), and absence of type 1 diabetes–associated autoimmunity ( 153 – 157 ). There is loss of both insulin and glucagon secretion and often higher-than-expected insulin requirements. Risk for microvascular complications is similar to other forms of diabetes. In the context of pancreatectomy, islet autotransplantation can be done to retain insulin secretion ( 158 , 159 ). In some cases, autotransplant can lead to insulin independence. In others, it may decrease insulin requirements ( 160 ).
2.25 Test for undiagnosed prediabetes and diabetes at the first prenatal visit in those with risk factors using standard diagnostic criteria. B
2.26 Test for gestational diabetes mellitus at 24 – 28 weeks of gestation in pregnant women not previously found to have diabetes. A
2.27 Test women with gestational diabetes mellitus for prediabetes or diabetes at 4 – 12 weeks postpartum, using the 75-g oral glucose tolerance test and clinically appropriate nonpregnancy diagnostic criteria. B
2.28 Women with a history of gestational diabetes mellitus should have lifelong screening for the development of diabetes or prediabetes at least every 3 years. B
2.29 Women with a history of gestational diabetes mellitus found to have prediabetes should receive intensive lifestyle interventions and/or metformin to prevent diabetes. A
For many years, GDM was defined as any degree of glucose intolerance that was first recognized during pregnancy ( 60 ), regardless of the degree of hyperglycemia. This definition facilitated a uniform strategy for detection and classification of GDM, but this definition has serious limitations ( 161 ). First, the best available evidence reveals that many, perhaps most, cases of GDM represent preexisting hyperglycemia that is detected by routine screening in pregnancy, as routine screening is not widely performed in nonpregnant women of reproductive age. It is the severity of hyperglycemia that is clinically important with regard to both short- and long-term maternal and fetal risks. Universal preconception and/or first trimester screening is hampered by lack of data and consensus regarding appropriate diagnostic thresholds and outcomes and cost-effectiveness ( 162 , 163 ). A compelling argument for further work in this area is the fact that hyperglycemia that would be diagnostic of diabetes outside of pregnancy and is present at the time of conception is associated with an increased risk of congenital malformations that is not seen with lower glucose levels ( 164 , 165 ).
The ongoing epidemic of obesity and diabetes has led to more type 2 diabetes in women of reproductive age, with an increase in the number of pregnant women with undiagnosed type 2 diabetes in early pregnancy ( 166 – 169 ). Because of the number of pregnant women with undiagnosed type 2 diabetes, it is reasonable to test women with risk factors for type 2 diabetes ( 170 ) ( Table 2.3 ) at their initial prenatal visit, using standard diagnostic criteria ( Table 2.2 ). Women found to have diabetes by the standard diagnostic criteria used outside of pregnancy should be classified as having diabetes complicating pregnancy (most often type 2 diabetes, rarely type 1 diabetes or monogenic diabetes) and managed accordingly. Women who meet the lower glycemic criteria for GDM should be diagnosed with that condition and managed accordingly. Other women should be rescreened for GDM between 24 and 28 weeks of gestation (see Section 14 “Management of Diabetes in Pregnancy,” https://doi.org/10.2337/dc21-S014 ). The International Association of the Diabetes and Pregnancy Study Groups (IADPSG) GDM diagnostic criteria for the 75-g OGTT as well as the GDM screening and diagnostic criteria used in the two-step approach were not derived from data in the first half of pregnancy, so the diagnosis of GDM in early pregnancy by either FPG or OGTT values is not evidence based ( 171 ) and further work is needed.
GDM is often indicative of underlying β-cell dysfunction ( 172 ), which confers marked increased risk for later development of diabetes, generally but not always type 2 diabetes, in the mother after delivery ( 173 , 174 ). As effective prevention interventions are available ( 175 , 176 ), women diagnosed with GDM should receive lifelong screening for prediabetes to allow interventions to reduce diabetes risk and for type 2 diabetes to allow treatment at the earliest possible time ( 177 ).
GDM carries risks for the mother, fetus, and neonate. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study ( 178 ), a large-scale multinational cohort study completed by more than 23,000 pregnant women, demonstrated that risk of adverse maternal, fetal, and neonatal outcomes continuously increased as a function of maternal glycemia at 24 – 28 weeks of gestation, even within ranges previously considered normal for pregnancy. For most complications, there was no threshold for risk. These results have led to careful reconsideration of the diagnostic criteria for GDM.
GDM diagnosis ( Table 2.7 ) can be accomplished with either of two strategies:
The “one-step” 75-g OGTT derived from the IADPSG criteria, or
The older “two-step” approach with a 50-g (nonfasting) screen followed by a 100-g OGTT for those who screen positive, based on the work of Carpenter and Coustan's interpretation of the older OʼSullivan ( 179 ) criteria.
Screening for and diagnosis of GDM
GDM, gestational diabetes mellitus; GLT, glucose load test; OGTT, oral glucose tolerance test.
American College of Obstetricians and Gynecologists notes that one elevated value can be used for diagnosis ( 189 ).
Different diagnostic criteria will identify different degrees of maternal hyperglycemia and maternal/fetal risk, leading some experts to debate, and disagree on, optimal strategies for the diagnosis of GDM.
One-Step Strategy
The IADPSG defined diagnostic cut points for GDM as the average fasting, 1-h, and 2-h PG values during a 75-g OGTT in women at 24 – 28 weeks of gestation who participated in the HAPO study at which odds for adverse outcomes reached 1.75 times the estimated odds of these outcomes at the mean fasting, 1-h, and 2-h PG levels of the study population. This one-step strategy was anticipated to significantly increase the incidence of GDM (from 5 – 6% to 15–20%), primarily because only one abnormal value, not two, became sufficient to make the diagnosis ( 180 ). Many regional studies have investigated the impact of adopting the IADPSG criteria on prevalence and have seen a roughly one- to threefold increase ( 181 ). The anticipated increase in the incidence of GDM could have a substantial impact on costs and medical infrastructure needs and has the potential to “medicalize” pregnancies previously categorized as normal. A recent follow-up study of women participating in a blinded study of pregnancy OGTTs found that 11 years after their pregnancies, women who would have been diagnosed with GDM by the one-step approach, as compared with those without, were at 3.4-fold higher risk of developing prediabetes and type 2 diabetes and had children with a higher risk of obesity and increased body fat, suggesting that the larger group of women identified by the one-step approach would benefit from increased screening for diabetes and prediabetes that would accompany a history of GDM ( 182 , 183 ). The ADA recommends the IADPSG diagnostic criteria with the intent of optimizing gestational outcomes because these criteria are the only ones based on pregnancy outcomes rather than end points such as prediction of subsequent maternal diabetes.
The expected benefits of using IADPSG to the offspring are inferred from intervention trials that focused on women with lower levels of hyperglycemia than identified using older GDM diagnostic criteria. Those trials found modest benefits including reduced rates of large-for-gestational-age births and preeclampsia ( 184 , 185 ). It is important to note that 80 – 90% of women being treated for mild GDM in these two randomized controlled trials could be managed with lifestyle therapy alone. The OGTT glucose cutoffs in these two trials overlapped with the thresholds recommended by the IADPSG, and in one trial ( 185 ), the 2-h PG threshold (140 mg/dL [7.8 mmol/L]) was lower than the cutoff recommended by the IADPSG (153 mg/dL [8.5 mmol/L]). No randomized controlled trials of treating versus not treating GDM diagnosed by the IADPSG criteria but not the Carpenter-Coustan criteria have been published to date. Data are also lacking on how the treatment of lower levels of hyperglycemia affects a mother's future risk for the development of type 2 diabetes and her offspring's risk for obesity, diabetes, and other metabolic disorders. Additional well-designed clinical studies are needed to determine the optimal intensity of monitoring and treatment of women with GDM diagnosed by the one-step strategy ( 186 , 187 ).
Two-Step Strategy
In 2013, the NIH convened a consensus development conference to consider diagnostic criteria for diagnosing GDM ( 188 ). The 15-member panel had representatives from obstetrics and gynecology, maternal-fetal medicine, pediatrics, diabetes research, biostatistics, and other related fields. The panel recommended a two-step approach to screening that used a 1-h 50-g glucose load test (GLT) followed by a 3-h 100-g OGTT for those who screened positive. The American College of Obstetricians and Gynecologists (ACOG) recommends any of the commonly used thresholds of 130, 135, or 140 mg/dL for the 1-h 50-g GLT ( 189 ). A systematic review for the U.S. Preventive Services Task Force compared GLT cutoffs of 130 mg/dL (7.2 mmol/L) and 140 mg/dL (7.8 mmol/L) ( 190 ). The higher cutoff yielded sensitivity of 70–88% and specificity of 69 – 89%, while the lower cutoff was 88 – 99% sensitive and 66 – 77% specific. Data regarding a cutoff of 135 mg/dL are limited. As for other screening tests, choice of a cutoff is based upon the trade-off between sensitivity and specificity. The use of A1C at 24–28 weeks of gestation as a screening test for GDM does not function as well as the GLT ( 191 ).
Key factors cited by the NIH panel in their decision-making process were the lack of clinical trial data demonstrating the benefits of the one-step strategy and the potential negative consequences of identifying a large group of women with GDM, including medicalization of pregnancy with increased health care utilization and costs. Moreover, screening with a 50-g GLT does not require fasting and is therefore easier to accomplish for many women. Treatment of higher-threshold maternal hyperglycemia, as identified by the two-step approach, reduces rates of neonatal macrosomia, large-for-gestational-age births ( 192 ), and shoulder dystocia, without increasing small-for-gestational-age births. ACOG currently supports the two-step approach but notes that one elevated value, as opposed to two, may be used for the diagnosis of GDM ( 189 ). If this approach is implemented, the incidence of GDM by the two-step strategy will likely increase markedly. ACOG recommends either of two sets of diagnostic thresholds for the 3-h 100-g OGTT—Carpenter-Coustan or National Diabetes Data Group ( 193 , 194 ). Each is based on different mathematical conversions of the original recommended thresholds by O'Sullivan ( 179 ), which used whole blood and nonenzymatic methods for glucose determination. A secondary analysis of data from a randomized clinical trial of identification and treatment of mild GDM ( 195 ) demonstrated that treatment was similarly beneficial in patients meeting only the lower thresholds per Carpenter-Coustan ( 193 ) and in those meeting only the higher thresholds per National Diabetes Data Group ( 194 ). If the two-step approach is used, it would appear advantageous to use the Carpenter-Coustan lower diagnostic thresholds as shown in step 2 in Table 2.7 .
Future Considerations
The conflicting recommendations from expert groups underscore the fact that there are data to support each strategy. A cost-benefit estimation comparing the two strategies concluded that the one-step approach is cost-effective only if patients with GDM receive postdelivery counseling and care to prevent type 2 diabetes ( 196 ). The decision of which strategy to implement must therefore be made based on the relative values placed on factors that have yet to be measured (e.g., willingness to change practice based on correlation studies rather than intervention trial results, available infrastructure, and importance of cost considerations).
As the IADPSG criteria (“one-step strategy”) have been adopted internationally, further evidence has emerged to support improved pregnancy outcomes with cost savings ( 197 ), and IADPSG may be the preferred approach. Data comparing population-wide outcomes with one-step versus two-step approaches have been inconsistent to date ( 198 , 199 ). In addition, pregnancies complicated by GDM per the IADPSG criteria, but not recognized as such, have outcomes comparable to pregnancies with diagnosed GDM by the more stringent two-step criteria ( 200 , 201 ). There remains strong consensus that establishing a uniform approach to diagnosing GDM will benefit patients, caregivers, and policy makers. Longer-term outcome studies are currently underway.
Suggested citation: American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2021 . Diabetes Care 2021;44(Suppl. 1):S15−S33
Email alerts
- Addendum. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021 . Diabetes Care 2021;44(Suppl. 1):S15–S33
- Online ISSN 1935-5548
- Print ISSN 0149-5992
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 23 July 2015
Type 2 diabetes mellitus
- Ralph A. DeFronzo 1 ,
- Ele Ferrannini 2 ,
- Leif Groop 3 ,
- Robert R. Henry 4 ,
- William H. Herman 5 ,
- Jens Juul Holst 6 ,
- Frank B. Hu 7 ,
- C. Ronald Kahn 8 ,
- Itamar Raz 9 ,
- Gerald I. Shulman 10 ,
- Donald C. Simonson 11 ,
- Marcia A. Testa 12 &
- Ram Weiss 13
Nature Reviews Disease Primers volume 1 , Article number: 15019 ( 2015 ) Cite this article
47k Accesses
1084 Citations
125 Altmetric
Metrics details
- Diabetes complications
- Type 2 diabetes
Type 2 diabetes mellitus (T2DM) is an expanding global health problem, closely linked to the epidemic of obesity. Individuals with T2DM are at high risk for both microvascular complications (including retinopathy, nephropathy and neuropathy) and macrovascular complications (such as cardiovascular comorbidities), owing to hyperglycaemia and individual components of the insulin resistance (metabolic) syndrome. Environmental factors (for example, obesity, an unhealthy diet and physical inactivity) and genetic factors contribute to the multiple pathophysiological disturbances that are responsible for impaired glucose homeostasis in T2DM. Insulin resistance and impaired insulin secretion remain the core defects in T2DM, but at least six other pathophysiological abnormalities contribute to the dysregulation of glucose metabolism. The multiple pathogenetic disturbances present in T2DM dictate that multiple antidiabetic agents, used in combination, will be required to maintain normoglycaemia. The treatment must not only be effective and safe but also improve the quality of life. Several novel medications are in development, but the greatest need is for agents that enhance insulin sensitivity, halt the progressive pancreatic β-cell failure that is characteristic of T2DM and prevent or reverse the microvascular complications. For an illustrated summary of this Primer, visit: http://go.nature.com/V2eGfN
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 1 digital issues and online access to articles
92,52 € per year
only 92,52 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
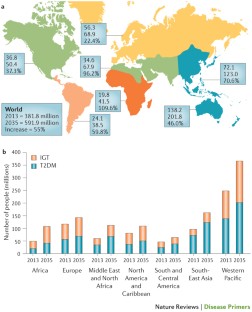
Similar content being viewed by others
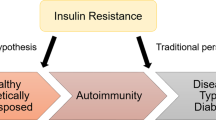
Double or hybrid diabetes: A systematic review on disease prevalence, characteristics and risk factors
Novel therapies with precision mechanisms for type 2 diabetes mellitus
Heterogeneity and endotypes in type 1 diabetes mellitus
DeFronzo, R. A. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58 , 773–795 (2009). A classic review of the aetiology of T2DM, with a therapeutic approach based on its pathophysiology.
Article CAS PubMed PubMed Central Google Scholar
Abdul-Ghani, M. A., Tripathy, D. & DeFronzo, R. A. Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 29 , 1130–1139 (2006).
Article CAS PubMed Google Scholar
Gerstein, H. C. et al . Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res. Clin. Pract. 78 , 305–312 (2007).
Article PubMed Google Scholar
Hawa, M. I. et al . Adult-onset autoimmune diabetes in Europe is prevalent with a broad clinical phenotype: action LADA 7. Diabetes Care 36 , 908–913 (2013).
Article PubMed PubMed Central Google Scholar
Gardner, D. S. & Tai, E. S. Clinical features and treatment of maturity onset diabetes of the young (MODY). Diabetes. Metab. Syndr. Obes. 5 , 101–108 (2012).
American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 37 , S14–S80 (2014). A comprehensive overview of the standards of medical care published by the ADA.
Article Google Scholar
DeFronzo, R. A. & Abdul-Ghani, M. A. Preservation of β-cell function: the key to diabetes prevention. J. Clin. Endocrinol. Metab. 96 , 2354–2366 (2011).
Ferrannini, E., Gastaldelli, A. & Iozzo, P. Pathophysiology of prediabetes. Med. Clin. North Am. 95 , 327–339 (2011).
Garvey, W. T. et al . Prevention of type 2 diabetes in subjects with prediabetes and metabolic syndrome treated with phentermine and topiramate extended release. Diabetes Care 37 , 912–921 (2014).
Nathan, D. M. et al . Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 30 , 753–759 (2007).
DeFronzo, R. A. et al . Pioglitazone for diabetes prevention in impaired glucose tolerance. N. Engl. J. Med. 364 , 1104–1115 (2011). A large prospective study demonstrating the efficacy of thiazolidinediones in preventing the progression of IGT to T2DM.
Zinman, B. et al . Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): a double-blind randomised controlled study. Lancet 376 , 103–111 (2010).
Dansinger, M. L., Tatsioni, A., Wong, J. B., Chung, M. & Balk, E. M. Meta-analysis: the effect of dietary counseling for weight loss. Ann. Intern. Med. 147 , 41–50 (2007).
Purcell, K. et al . The effect of rate of weight loss on long-term weight management: a randomised controlled trial. Lancet Diabetes Endocrinol. 2 , 954–962 (2014).
Ali, M. K., Echouffo-Tcheugui, J. & Williamson, D. F. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff. (Millwood) 31 , 67–75 (2012).
Tuomilehto, J. et al . Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 344 , 1343–1350 (2001).
Inzucchi, S. E. et al . Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35 , 1364–1379 (2012). ADA position statement on the treatment of T2DM, advocating a stepped care approach starting with metformin.
American Association of Clinical Endocrinologists. AACE Comprehensive Diabetes Algorithm 2013 Consensus Statement. Endocr. Pract. Suppl. 1 , 1–87 (2015). AACE position statement on the treatment of T2DM, advocating initial monotherapy or combination therapy based upon the starting HbA1c, and recommending various antidiabetic medications as initial therapy.
Google Scholar
Pozzilli, P. et al . The A1C and ABCD of glycaemia management in type 2 diabetes: a physician's personalized approach. Diabetes Metab. Res. Rev. 26 , 239–244 (2010). The first published report by key opinion leaders recommending individualized therapy based on the age and body weight of patients, the presence or absence of complications, and duration and aetiology of disease.
International Diabetes Federation. IDF Diabetes Atlas 6th Edition. IDF [online] , (2013).
Hu, F. B. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 34 , 1249–1257 (2011). An important study emphasizing the role of diet, physical activity and genes — beyond obesity — in the diabetes epidemic that is engulfing Asian countries as they are exposed to westernization.
Chan, J. C. et al . Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 301 , 2129–2140 (2009).
Ley, S. H., Hamdy, O., Mohan, V. & Hu, F. B. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 383 , 1999–2007 (2014).
Grøntved, A., Rimm, E. B., Willett, W. C., Andersen, L. B. & Hu, F. B. A prospective study of weight training and risk of type 2 diabetes mellitus in men. Arch. Intern. Med. 172 , 1306–1312 (2012).
Grøntved, A. & Hu, F. B. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA 305 , 2448–2455 (2011).
Cappuccio, F. P., D'Elia, L., Strazzullo, P. & Miller, M. A. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 33 , 414–420 (2009).
Pan, A., Schernhammer, E. S., Sun, Q. & Hu, F. B. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 8 , e1001141 (2011).
Barnett, A. H., Eff, C., Leslie, R. D. & Pyke, D. A. Diabetes in identical twins. A study of 200 pairs. Diabetologia 20 , 87–93 (1981).
Wang, Y. C., McPherson, K., Marsh, T., Gortmaker, S. L. & Brown, M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 378 , 815–825 (2011).
Wang, X. et al . Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 36 , 166–175 (2013).
Li, S., Shin, H. J., Ding, E. L. & van Dam, R. M. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 302 , 179–188 (2009).
Ding, E. L. et al . Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N. Engl. J. Med. 361 , 1152–1163 (2009).
Wang, T. J. et al . Metabolite profiles and the risk of developing diabetes. Nat. Med. 17 , 448–453 (2011).
Esteve, E., Ricart, W. & Fernández-Real, J.-M. Gut microbiota interactions with obesity, insulin resistance and type 2 diabetes: did gut microbiote co-evolve with insulin resistance? Curr. Opin. Clin. Nutr. Metab. Care 14 , 483–490 (2011).
Hu, F. B. et al . Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 345 , 790–797 (2001).
Schellenberg, E. S., Dryden, D. M., Vandermeer, B., Ha, C. & Korownyk, C. Lifestyle interventions for patients with and at risk for type 2 diabetes. Ann. Intern. Med. 159 , 543–551 (2013). A comprehensive review of the effectiveness of lifestyle intervention in the treatment of T2DM, emphasizing that, although initially successful, most subjects with diabetes regain the majority of lost weight over the subsequent 3–5 years.
DeFronzo, R. A. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 53 , 1270–1287 (2010). A comprehensive review describing the role of excess tissue lipid deposition in the development of insulin resistance, β-cell failure and atherosclerotic cardiovascular disease: that is, lipotoxicity.
Hemminki, K., Li, X., Sundquist, K. & Sundquist, J. Familial risks for type 2 diabetes in Sweden. Diabetes Care 33 , 293–297 (2010).
Groop, L. et al . Metabolic consequences of a family history of NIDDM (the Botnia study): evidence for sex-specific parental effects. Diabetes 45 , 1585–1593 (1996).
Lyssenko, V. et al . Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes 54 , 166–174 (2005).
Grant, S. F. et al . Variant of transcription factor 7-like 2 ( TCF7L2 ) gene confers risk of type 2 diabetes. Nat. Genet. 38 , 320–323 (2006).
Lyssenko, V. et al . Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J. Clin. Invest. 117 , 2155–2163 (2007).
Sladek, R. et al . A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445 , 881–885 (2007).
Saxena, R. et al . Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316 , 1331–1336 (2007).
Morris, A. P. et al . Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat. Genet. 44 , 981–990 (2012).
Flannick, J. et al . Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat. Genet. 46 , 357–363 (2014).
Lyssenko, V. et al . Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat. Genet. 41 , 82–88 (2009).
Rosengren, A. H. et al . Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science 327 , 217–220 (2010).
Tang, Y. et al . Genotype-based treatment of type 2 diabetes with an α2A-adrenergic receptor antagonist. Sci. Transl Med. 6 , 257ra139 (2014). These paper provides an example in which a genetic finding in an animal model of diabetes has been translated into a drug target in humans, the ADRA2A gene.
De Jesus, D. F. & Kulkarni, R. N. Epigenetic modifiers of islet function and mass. Trends Endocrinol. Metab. 25 , 628–636 (2014).
Ozcan, S. Minireview: microRNA function in pancreatic β cells. Mol. Endocrinol. 28 , 1922–1933 (2014).
Lyssenko, V. et al . Clinical risk factors, DNA variants, and the development of type 2 diabetes. N. Engl. J. Med. 359 , 2220–2232 (2008). This paper presents a genetic explanation for the development of T2DM.
Travers, M. E. et al . Insights into the molecular mechanism for type 2 diabetes susceptibility at the KCNQ1 locus from temporal changes in imprinting status in human islets. Diabetes 62 , 987–992 (2013).
Gulli, G., Ferrannini, E., Stern, M., Haffner, S. & DeFronzo, R. A. The metabolic profile of NIDDM is fully established in glucose-tolerant offspring of two Mexican-American NIDDM parents. Diabetes 41 , 1575–1586 (1992).
Martin, B. C. et al . Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet 340 , 925–929 (1992).
Ferrannini, E. & Mari, A. β-cell function in type 2 diabetes. Metabolism 63 , 1217–1227 (2014).
Kahn, S. E., Cooper, M. E. & Del Prato, S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 383 , 1068–1083 (2014).
Muller, D. C., Elahi, D., Tobin, J. D. & Andres, R. Insulin response during the oral glucose tolerance test: the role of age, sex, body fat and the pattern of fat distribution. Aging (Milano) 8 , 13–21 (1996).
CAS Google Scholar
Nauck, M. A., Vardarli, I., Deacon, C. F., Holst, J. J. & Meier, J. J. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia 54 , 10–18 (2011).
Madsbad, S. The role of glucagon-like peptide-1 impairment in obesity and potential therapeutic implications. Diabetes Obes. Metab. 16 , 9–21 (2014).
Bays, H., Mandarino, L. & DeFronzo, R. A. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J. Clin. Endocrinol. Metab. 89 , 463–478 (2004).
Perry, R. J., Samuel, V. T., Petersen, K. F. & Shulman, G. I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 510 , 84–91 (2014). An excellent review of the specific lipid varieties and the molecular events through which they cause insulin resistance in the liver.
Bensellam, M., Laybutt, D. R. & Jonas, J.-C. The molecular mechanisms of pancreatic β-cell glucotoxicity: recent findings and future research directions. Mol. Cell. Endocrinol. 364 , 1–27 (2012).
Ritzel, R. A., Meier, J. J., Lin, C.-Y., Veldhuis, J. D. & Butler, P. C. Human islet amyloid polypeptide oligomers disrupt cell coupling, induce apoptosis, and impair insulin secretion in isolated human islets. Diabetes 56 , 65–71 (2007).
Collins, S., Pi, J. & Yehuda-Shnaidman, E. Uncoupling and reactive oxygen species (ROS) — a double-edged sword for β-cell function? “Moderation in all things”. Best Pract. Res. Clin. Endocrinol. Metab. 26 , 753–758 (2012).
Cabrera, O. et al . The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl Acad. Sci. USA 103 , 2334–2339 (2006).
Hodson, D. J. et al . Lipotoxicity disrupts incretin-regulated human β cell connectivity. J. Clin. Invest. 123 , 4182–4194 (2013).
Brandhorst, H., Brandhorst, D., Brendel, M. D., Hering, B. J. & Bretzel, R. G. Assessment of intracellular insulin content during all steps of human islet isolation procedure. Cell Transplant. 7 , 489–495 (1998).
Rahier, J., Guiot, Y., Goebbels, R. M., Sempoux, C. & Henquin, J. C. Pancreatic β-cell mass in European subjects with type 2 diabetes. Diabetes Obes. Metab. 10 (Suppl. 4), 32–42 (2008). A post-mortem study demonstrating a decline in β-cell mass with preservation of α-cell mass in individuals with T2DM.
Marselli, L. et al . Are we overestimating the loss of beta cells in type 2 diabetes? Diabetologia 57 , 362–365 (2014).
Marchetti, P. et al . The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia 50 , 2486–2494 (2007).
Marchetti, P. & Masini, M. Autophagy and the pancreatic beta-cell in human type 2 diabetes. Autophagy 5 , 1055–1056 (2009).
Gupta, D. & Leahy, J. L. Islet amyloid and type 2 diabetes: overproduction or inadequate clearance and detoxification? J. Clin. Invest. 124 , 3292–3294 (2014).
Mezza, T. et al . Insulin resistance alters islet morphology in nondiabetic humans. Diabetes 63 , 994–1007 (2014). This work in human islets describes the impact of insulin resistance on the relative proportion of α-cells and β-cells, and the functional consequences — in terms of insulin and glucagon secretion — of this chronic adaptation.
Deng, S. et al . Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes 53 , 624–632 (2004).
Igoillo-Esteve, M. et al . Palmitate induces a pro-inflammatory response in human pancreatic islets that mimics CCL2 expression by beta cells in type 2 diabetes. Diabetologia 53 , 1395–1405 (2010).
Giacca, A., Xiao, C., Oprescu, A. I., Carpentier, A. C. & Lewis, G. F. Lipid-induced pancreatic β-cell dysfunction: focus on in vivo studies. Am. J. Physiol. Endocrinol. Metab. 300 , E255–E262 (2010).
Halban, P. A. et al . β-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. J. Clin. Endocrinol. Metab. 99 , 1983–1992 (2014).
Ferrannini, E. et al . Natural history and physiological determinants of changes in glucose tolerance in a non-diabetic population: the RISC Study. Diabetologia 54 , 1507–1516 (2011). This longitudinal study of non-diabetic subjects identifies baseline insulin resistance and β-cell dysfunction as predictors of future dysglycaemia.
Michaliszyn, S. F. et al . β-cell function, incretin effect, and incretin hormones in obese youth along the span of glucose tolerance from normal to prediabetes to type 2 diabetes. Diabetes 63 , 3846–3855 (2014).
Mari, A. et al . Mechanisms of the incretin effect in subjects with normal glucose tolerance and patients with type 2 diabetes. PLoS ONE 8 , e73154 (2013).
Holst, J. J., Knop, F. K., Vilsbøll, T., Krarup, T. & Madsbad, S. Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes. Diabetes Care 34 , S251–S257 (2011).
Camastra, S. et al . Long-term effects of bariatric surgery on meal disposal and β-cell function in diabetic and nondiabetic patients. Diabetes 62 , 3709–3717 (2013).
Ferrannini, E. The stunned β cell: a brief history. Cell Metab. 11 , 349–352 (2010).
Shulman, G. I. et al . Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N. Engl. J. Med. 322 , 223–228 (1990). This study demonstrated that defects in insulin-stimulated muscle glycogen synthesis was the major factor responsible for whole-body insulin resistance in patients with T2DM.
Groop, L. C. et al . Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J. Clin. Invest. 84 , 205–213 (1989).
Guilherme, A., Virbasius, J. V., Puri, V. & Czech, M. P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9 , 367–377 (2008).
Gerich, J. E., Meyer, C., Woerle, H. J. & Stumvoll, M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care 24 , 382–391 (2001).
Honka, H. et al . Validation of [ 18 F]fluorodeoxyglucose and positron emission tomography (PET) for the measurement of intestinal metabolism in pigs, and evidence of intestinal insulin resistance in patients with morbid obesity. Diabetologia 56 , 893–900 (2013).
Meijer, R. I. et al . Insulin-induced microvascular recruitment in skin and muscle are related and both are associated with whole-body glucose uptake. Microcirculation 19 , 494–500 (2012).
Blázquez, E., Velázquez, E., Hurtado-Carneiro, V. & Ruiz-Albusac, J. M. Insulin in the brain: its pathophysiological implications for states related with central insulin resistance, type 2 diabetes and Alzheimer's disease. Front. Endocrinol. (Lausanne) 5 , 161 (2014).
Kleinridders, A., Ferris, H. A., Cai, W. & Kahn, C. R. Insulin action in brain regulates systemic metabolism and brain function. Diabetes 63 , 2232–2243 (2014).
Kulkarni, R. N. et al . Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96 , 329–339 (1999). An insightful study documenting that β-cell-specific insulin receptor knockout results in markedly impaired insulin secretion and overt diabetes, thereby providing a unifying mechanism whereby insulin resistance explains both the defects in insulin-stimulated tissue glucose uptake and decreased insulin secretion.
Oliveira, J. M., Rebuffat, S. A., Gasa, R. & Gomis, R. Targeting type 2 diabetes: lessons from a knockout model of insulin receptor substrate 2. Can. J. Physiol. Pharmacol. 92 , 613–620 (2014).
Samuel, V. T. & Shulman, G. I. Mechanisms for insulin resistance: common threads and missing links. Cell 148 , 852–871 (2012). An excellent review of the molecular mechanism responsible for insulin resistance in T2DM and obesity.
Magnusson, I., Rothman, D. L., Katz, L. D., Shulman, R. G. & Shulman, G. I. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J. Clin. Invest. 90 , 1323–1327 (1992). This study demonstrated that increased rates of hepatic glucose production in patients with poorly controlled T2DM could entirely be attributed to increased rates of gluconeogenesis.
Matsuda, M. et al . Glucagon dose-response curve for hepatic glucose production and glucose disposal in type 2 diabetic patients and normal individuals. Metabolism 51 , 1111–1119 (2002).
Samuel, V. T. et al . Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with type 2 diabetes. Proc. Natl Acad. Sci. USA 106 , 12121–12126 (2009).
Baron, A. D., Schaeffer, L., Shragg, P. & Kolterman, O. G. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes 36 , 274–283 (1987).
DeFronzo, R. A., Ferrannini, E., Hendler, R., Wahren, J. & Felig, P. Influence of hyperinsulinemia, hyperglycemia, and the route of glucose administration on splanchnic glucose exchange. Proc. Natl Acad. Sci. USA 75 , 5173–5177 (1978).
Ferrannini, E. et al . The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism 37 , 79–85 (1988).
DeFronzo, R. A. et al . Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 36 , 3169–3176 (2013).
Barrett, E. J., Wang, H., Upchurch, C. T. & Liu, Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am. J. Physiol. Endocrinol. Metab. 301 , E252–E263 (2011).
Baron, A. D. Hemodynamic actions of insulin. Am. J. Physiol. 267 , E187–E202 (1994).
CAS PubMed Google Scholar
Krüger, M. et al . Dissection of the insulin signaling pathway via quantitative phosphoproteomics. Proc. Natl Acad. Sci. USA 105 , 2451–2456 (2008).
Cusi, K. et al . Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J. Clin. Invest. 105 , 311–320 (2000). The first study in humans with T2DM to demonstrate impaired insulin signal transduction through the IRS1–PI3K pathway in muscle, with normal to increased insulin signalling through the MAPK pathway.
Krook, A. et al . Characterization of signal transduction and glucose transport in skeletal muscle from type 2 diabetic patients. Diabetes 49 , 284–292 (2000).
Copps, K. D. & White, M. F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 55 , 2565–2582 (2012).
Bouzakri, K. et al . IRS-1 serine phosphorylation and insulin resistance in skeletal muscle from pancreas transplant recipients. Diabetes 55 , 785–791 (2006).
Hiratani, K. et al . Roles of mTOR and JNK in serine phosphorylation, translocation, and degradation of IRS-1. Biochem. Biophys. Res. Commun. 335 , 836–842 (2005).
Krssak, M. et al . Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 42 , 113–116 (1999).
Petersen, K. F. et al . Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J. Clin. Invest. 109 , 1345–1350 (2002).
Petersen, K. F. et al . Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 54 , 603–608 (2005).
Lara-Castro, C. & Garvey, W. T. Intracellular lipid accumulation in liver and muscle and the insulin resistance syndrome. Endocrinol. Metab. Clin. North Am. 37 , 841–856 (2008).
Yu, C. et al . Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 277 , 50230–50236 (2002).
Bezy, O. et al . PKCδ regulates hepatic insulin sensitivity and hepatosteatosis in mice and humans. J. Clin. Invest. 121 , 2504–2517 (2011).
Samuel, V. T. et al . Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 279 , 32345–32353 (2004).
Samuel, V. T. et al . Inhibition of protein kinase Cε prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J. Clin. Invest. 117 , 739–745 (2007).
Choi, C. S. et al . Suppression of diacylglycerol acyltransferase-2 ( DGAT2 ), but not DGAT1 , with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J. Biol. Chem. 282 , 22678–22688 (2007).
Morino, K. et al . Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J. Clin. Invest. 115 , 3587–3593 (2005).
Szendroedi, J. et al . Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc. Natl Acad. Sci. USA 111 , 9597–9602 (2014).
Larsen, P. J. & Tennagels, N. On ceramides, other sphingolipids and impaired glucose homeostasis. Mol. Metab. 3 , 252–260 (2014).
Turpin, S. M. et al . Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 20 , 678–686 (2014).
Cantley, J. L. et al . CGI-58 knockdown sequesters diacylglycerols in lipid droplets/ER-preventing diacylglycerol-mediated hepatic insulin resistance. Proc. Natl Acad. Sci. USA 110 , 1869–1874 (2013).
Patti, M.-E. & Corvera, S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr. Rev. 31 , 364–395 (2010). Mitochondrial dysfunction as a causative factor in the development of insulin resistance in T2DM is reviewed.
Ritov, V. B. et al . Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54 , 8–14 (2005).
Petersen, K. F. et al . Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300 , 1140–1142 (2003).
Petersen, K. F., Dufour, S., Befroy, D., Garcia, R. & Shulman, G. I. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N. Engl. J. Med. 350 , 664–671 (2004).
Mogensen, M. et al . Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 56 , 1592–1599 (2007).
Petersen, K. F., Dufour, S. & Shulman, G. I. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med. 2 , e233 (2005).
Wang, C.-H., Wang, C.-C., Huang, H.-C. & Wei, Y.-H. Mitochondrial dysfunction leads to impairment of insulin sensitivity and adiponectin secretion in adipocytes. FEBS J. 280 , 1039–1050 (2013).
Rains, J. L. & Jain, S. K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 50 , 567–575 (2011).
Morino, K. et al . Regulation of mitochondrial biogenesis by lipoprotein lipase in muscle of insulin-resistant offspring of parents with type 2 diabetes. Diabetes 61 , 877–887 (2012).
Romeo, G. R., Lee, J. & Shoelson, S. E. Metabolic syndrome, insulin resistance, and roles of inflammation — mechanisms and therapeutic targets. Arterioscler. Thromb. Vasc. Biol. 32 , 1771–1776 (2012).
Arkan, M. C. et al . IKK-β links inflammation to obesity-induced insulin resistance. Nat. Med. 11 , 191–198 (2005).
De Alvaro, C., Teruel, T., Hernandez, R. & Lorenzo, M. Tumor necrosis factor α produces insulin resistance in skeletal muscle by activation of inhibitor κB kinase in a p38 MAPK-dependent manner. J. Biol. Chem. 279 , 17070–17078 (2004).
Howard, J. K. & Flier, J. S. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol. Metab. 17 , 365–371 (2006).
Lebrun, P. & Van Obberghen, E. SOCS proteins causing trouble in insulin action. Acta Physiol. (Oxf.) 192 , 29–36 (2008).
Article CAS Google Scholar
Uysal, K. T., Wiesbrock, S. M. & Hotamisligil, G. S. Functional analysis of tumor necrosis factor (TNF) receptors in TNF-α-mediated insulin resistance in genetic obesity. Endocrinology 139 , 4832–4838 (1998).
Ofei, F., Hurel, S., Newkirk, J., Sopwith, M. & Taylor, R. Effects of an engineered human anti-TNF-α antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes 45 , 881–885 (1996).
Kim, J. K. et al . Prevention of fat-induced insulin resistance by salicylate. J. Clin. Invest. 108 , 437–446 (2001).
Yuan, M. et al . Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of IKK β. Science 293 , 1673–1677 (2001).
Goldfine, A. B. et al . The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann. Intern. Med. 152 , 346–357 (2010).
Lumeng, C. N. & Saltiel, A. R. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121 , 2111–2117 (2011).
Nishimura, S. et al . CD8 + effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15 , 914–920 (2009).
Feuerer, M. et al . Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15 , 930–939 (2009).
Bertola, A. et al . Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes 61 , 2238–2247 (2012).
Cai, D. et al . Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 11 , 183–190 (2005).
Perry, R. J. et al . Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160 , 745–758 (2015).
Mori, M. A. et al . A systems biology approach identifies inflammatory abnormalities between mouse strains prior to development of metabolic disease. Diabetes 59 , 2960–2971 (2010).
Mauer, J. et al . Myeloid cell-restricted insulin receptor deficiency protects against obesity-induced inflammation and systemic insulin resistance. PLoS Genet. 6 , e1000938 (2010).
Shi, H. et al . TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116 , 3015–3025 (2006).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8 , 519–529 (2007).
Boden, G. et al . Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 57 , 2438–2444 (2008).
Eizirik, D. L., Cardozo, A. K. & Cnop, M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 29 , 42–61 (2008). A comprehensive review of ER stress and the UPR in the development of insulin resistance and obesity.
Gregor, M. F. et al . Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 58 , 693–700 (2009).
Ozawa, K. et al . The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes 54 , 657–663 (2005).
Herschkovitz, A. et al . Common inhibitory serine sites phosphorylated by IRS-1 kinases, triggered by insulin and inducers of insulin resistance. J. Biol. Chem. 282 , 18018–18027 (2007).
Boden, G. Endoplasmic reticulum stress: another link between obesity and insulin resistance/inflammation? Diabetes 58 , 518–519 (2009).
Sengupta, S., Peterson, T. R. & Sabatini, D. M. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell 40 , 310–322 (2010).
Shah, O. J., Wang, Z. & Hunter, T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr. Biol. 14 , 1650–1656 (2004).
Ozcan, U. et al . Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol. Cell 29 , 541–551 (2008).
Park, S. W. et al . The regulatory subunits of PI3K, p85α and p85β, interact with XBP-1 and increase its nuclear translocation. Nat. Med. 16 , 429–437 (2010).
Stratton, I. M. et al . Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321 , 405–412 (2000). A seminal UK Prospective Diabetes Study study unequivocally demonstrating that improved glycaemic control reduced the incidence of microvascular, and to a lesser extent, macrovascular complications in patients with T2DM.
Holman, R. R., Paul, S. K., Bethel, M. A., Matthews, D. R. & Neil, H. A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 359 , 1577–1589 (2008). A long-term follow-up of the UK Prospective Diabetes Study showing that early intensive glycaemic control has a persistent impact on preventing both microvascular and macrovascular complications long after initiation of the intensified antidiabetic regimen has been discontinued: that is, the ‘legacy effect’.
Brownlee, M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54 , 1615–1625 (2005). A lucid discussion of the molecular pathways involved in the development of diabetic microvascular complications.
Giacco, F. & Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 107 , 1058–1070 (2010).
Coutinho, M., Gerstein, H. C., Wang, Y. & Yusuf, S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 22 , 233–240 (1999).
Taskinen, M.-R. & Borén, J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis 239 , 483–495 (2015). An up-to-date review of the pathogenesis of diabetic dyslipidaemia and its treatment.
Isomaa, B. et al . Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24 , 683–689 (2001).
Adler, A. I. et al . Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 321 , 412–419 (2000).
Williams, B. Treating hypertension in patients with diabetes: when to start and how low to go? JAMA 313 , 573–574 (2015). The optimal blood pressure goal in hypertensive patients with T2DM is discussed in light of the controversial results observed in the blood pressure arm of the ACCORD trial.
Lastra, G., Syed, S., Kurukulasuriya, L. R., Manrique, C. & Sowers, J. R. Type 2 diabetes mellitus and hypertension: an update. Endocrinol. Metab. Clin. North Am. 43 , 103–122 (2014).
International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32 , 1327–1334 (2009).
[No authors listed.] Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 20 , 1183–1197 (1997). A reference publication by the ADA on the diagnosis and classification of diabetes mellitus.
Herman, W. H. Diabetes epidemiology: guiding clinical and public health practice: the Kelly West Award Lecture, 2006. Diabetes Care 30 , 1912–1919 (2007). A landmark lecture providing a comprehensive overview of the epidemiology of T2DM and the public health implications for diabetes prevention.
DECODE Study Group, European Diabetes Epidemiology Group. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care 26 , 688–696 (2003).
Engelgau, M. M., Narayan, K. M. & Herman, W. H. Screening for type 2 diabetes. Diabetes Care 23 , 1563–1580 (2000).
LeFevre, M. L. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 161 , 587–593 (2014).
Lindström, J. & Tuomilehto, J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 26 , 725–731 (2003).
Tabaei, B. P. & Herman, W. H. A multivariate logistic regression equation to screen for diabetes: development and validation. Diabetes Care 25 , 1999–2003 (2002).
World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. report of a WHO consultation. Part 1: diagnosis and classification of diabetes mellitus (WHO, 1999).
Pan, X. R. et al . Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT Diabetes Study. Diabetes Care 20 , 537–544 (1997).
Knowler, W. C. et al . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 346 , 393–403 (2002).
Ramachandran, A. et al . The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 49 , 289–297 (2006).
Chiasson, J.-L. et al . Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 359 , 2072–2077 (2002).
Kawamori, R. et al . Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet 373 , 1607–1614 (2009).
Knowler, W. C. et al . Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes 54 , 1150–1156 (2005).
Gerstein, H. C. et al . Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 368 , 1096–1105 (2006).
Li, G. et al . The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 371 , 1783–1789 (2008).
Lindström, J. et al . Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 368 , 1673–1679 (2006).
Knowler, W. C. et al . 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 374 , 1677–1686 (2009). Long-term follow-up of body weight regain and diabetes incidence in patients with IGT in the Diabetes Prevention Program treated with lifestyle heavy, lifestyle light and metformin, showing that gradual weight regain is the norm and that 40–50% of patients with IGT develop diabetes despite successful weight loss.
DeFronzo, R. A., Eldor, R. & Abdul-Ghani, M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care 36 , S127–S138 (2013). A rational approach to the treatment of T2DM is presented based on its pathophysiology.
Raz, I. et al . Personalized management of hyperglycemia in type 2 diabetes: reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care 36 , 1779–1788 (2013).
Nakagami, T., Kawahara, R., Hori, S. & Omori, Y. Glycemic control and prevention of retinopathy in Japanese NIDDM patients. A 10-year follow-up study. Diabetes Care 20 , 621–622 (1997).
Lim, E. L. et al . Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 54 , 2506–2514 (2011).
Jazet, I. M. et al . Loss of 50% of excess weight using a very low energy diet improves insulin-stimulated glucose disposal and skeletal muscle insulin signalling in obese insulin-treated type 2 diabetic patients. Diabetologia 51 , 309–319 (2008).
Abdul-Ghani, M. A. et al . Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes (EDICT): a randomized trial. Diabetes Obes. Metab. 17 , 268–275 (2015). This prospective randomized trial using a combination of antidiabetic agents proven to reverse known pathophysiological abnormalities in T2DM demonstrated superiority of glycaemic control compared with the stepped approach of metformin followed by a sulfonylurea and then basal insulin recommended by most national diabetes organizations.
Harrison, L. B., Adams-Huet, B., Raskin, P. & Lingvay, I. β-cell function preservation after 3.5 years of intensive diabetes therapy. Diabetes Care 35 , 1406–1412 (2012).
Gram, J. et al . Pharmacological treatment of the pathogenetic defects in type 2 diabetes: the randomized multicenter South Danish Diabetes Study. Diabetes Care 34 , 27–33 (2011).
DeFronzo, R. A. et al . Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care 38 , 384–393 (2015).
Weng, J. et al . Effect of intensive insulin therapy on β-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 371 , 1753–1760 (2008).
Hu, Y. et al . Short-term intensive therapy in newly diagnosed type 2 diabetes partially restores both insulin sensitivity and β-cell function in subjects with long-term remission. Diabetes Care 34 , 1848–1853 (2011). One of several recent studies demonstrating that intensive insulin therapy to correct the decompensated metabolic state in newly diagnosed patients with T2DM can lead to durable glycaemic control without or with a marked reduction in antidiabetic medications.
Xiang, A. H. et al . Effect of pioglitazone on pancreatic β-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes 55 , 517–522 (2006).
Astrup, A. et al . Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int. J. Obes. (Lond.) 36 , 843–854 (2012).
Cusi, K., Consoli, A. & DeFronzo, R. A. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 81 , 4059–4067 (1996).
Turner, R. C., Cull, C. A., Frighi, V. & Holman, R. R. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 281 , 2005–2012 (1999). A landmark UK Prospective Diabetes Study documenting the need for progressive add-on therapies in newly diagnosed patients with T2DM receiving initial therapy with metformin or with a sulfonylurea.
Brown, J. B., Conner, C. & Nichols, G. A. Secondary failure of metformin monotherapy in clinical practice. Diabetes Care 33 , 501–506 (2010).
Kahn, S. E. et al . Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 355 , 2427–2443 (2006). A 5-year ADOPT study demonstrating long-term durable HbA1c reduction with rosiglitazone compared with a progressive rise in HbA1c observed with metformin and sulfonylureas, and a more rapid deterioration of glycaemic control with sulfonylureas compared with metformin.
Madiraju, A. K. et al . Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510 , 542–546 (2014).
Ferrannini, E. The target of metformin in type 2 diabetes. N. Engl. J. Med. 371 , 1547–1548 (2014).
[No authors listed.] Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352 , 854–865 (1998).
Maedler, K. et al . Sulfonylurea induced β-cell apoptosis in cultured human islets. J. Clin. Endocrinol. Metab. 90 , 501–506 (2005).
Roumie, C. L. et al . Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann. Intern. Med. 157 , 601–610 (2012).
Simpson, S. H., Majumdar, S. R., Tsuyuki, R. T., Eurich, D. T. & Johnson, J. A. Dose–response relation between sulfonylurea drugs and mortality in type 2 diabetes mellitus: a population-based cohort study. CMAJ 174 , 169–174 (2006).
Simpson, S. H. et al . Mortality risk among sulfonylureas: a systematic review and network meta-analysis. Lancet Diabetes Endocrinol. 3 , 43–51 (2015). A review of the published literature that examines the relationship between sulfonylurea therapy and the development of adverse cardiovascular events.
Yki-Järvinen, H. Thiazolidinediones. N. Engl. J. Med. 351 , 1106–1118 (2004).
Eldor, R., DeFronzo, R. A. & Abdul-Ghani, M. In vivo actions of peroxisome proliferator-activated receptors: glycemic control, insulin sensitivity, and insulin secretion. Diabetes Care 36 , S162–S174 (2013). An exhaustive review of the mechanism of action, efficacy and side-effect profile of the thiazolidinedione class of antidiabetic medications.
Miyazaki, Y., He, H., Mandarino, L. J. & DeFronzo, R. A. Rosiglitazone improves downstream insulin receptor signaling in type 2 diabetic patients. Diabetes 52 , 1943–1950 (2003).
Gastaldelli, A. et al . Thiazolidinediones improve β-cell function in type 2 diabetic patients. Am. J. Physiol. Endocrinol. Metab. 292 , E871–E883 (2007).
DeFronzo, R. A. et al . Prevention of diabetes with pioglitazone in ACT NOW: physiologic correlates. Diabetes 62 , 3920–3926 (2013).
Kahn, S. E. et al . Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT. Diabetes 60 , 1552–1560 (2011).
Dormandy, J. A. et al . Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366 , 1279–1289 (2005). A large prospective study (PROactive) demonstrating that pioglitazone significantly reduced the second principal end point of myocardial infarction, stroke and cardiovascular death; the primary end point did not reach statistical significance because of the inclusion of peripheral arterial disease and leg revascularization, which is known to be refractory to medical intervention, including statin therapy.
Aronoff, S. et al . Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care 23 , 1605–1611 (2000).
Erdmann, E., Song, E., Spanheimer, R., van Troostenburg de Bruyn, A.-R. & Perez, A. Observational follow-up of the PROactive study: a 6-year update. Diabetes Obes. Metab. 16 , 63–74 (2014).
[No authors listed.] Takeda announces completion of the post-marketing commitment to submit data to the FDA, the EMA and the PMDA for pioglitazone containing medicines including ACTOS. Takeda [online] , (2014).
Levin, D. et al . Pioglitazone and bladder cancer risk: a multipopulation pooled, cumulative exposure analysis. Diabetologia 58 , 493–504 (2015).
Kjems, L. L., Holst, J. J., Vølund, A. & Madsbad, S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on β-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 52 , 380–386 (2003).
Vilsbøll, T., Krarup, T., Madsbad, S. & Holst, J. J. Defective amplification of the late phase insulin response to glucose by GIP in obese Type II diabetic patients. Diabetologia 45 , 1111–1119 (2002).
Aroda, V. R. et al . Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin. Ther. 34 , 1247–1258.e22 (2012).
Deacon, C. F. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes. Metab. 13 , 7–18 (2011).
Balas, B. et al . The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 92 , 1249–1255 (2007).
Drucker, D. J. Incretin action in the pancreas: potential promise, possible perils, and pathological pitfalls. Diabetes 62 , 3316–3323 (2013). A comprehensive review of the effect of incretin hormones on pancreatic hormone secretion and pathology by one of the world's leading authorities.
White, W. B. et al . Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 369 , 1327–1335 (2013).
Scirica, B. M. et al . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 369 , 1317–1326 (2013).
Cervera, A. et al . Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 294 , E846–E852 (2008).
Bunck, M. C. et al . Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care 34 , 2041–2047 (2011). A landmark 3-year prospective study demonstrating the marked and durable improvement in β-cell function using the combined hyperglycaemic and euglycaemic insulin clamp techniques following exenatide treatment in patients with T2DM.
Klonoff, D. C. et al . Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr. Med. Res. Opin. 24 , 275–286 (2008).
Schwartz, S. & Kohl, B. A. Type 2 diabetes mellitus and the cardiometabolic syndrome: impact of incretin-based therapies. Diabetes Metab. Syndr. Obes. 3 , 227–242 (2010).
Eng, C., Kramer, C. K., Zinman, B. & Retnakaran, R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet 384 , 2228–2234 (2014).
Egan, A. G. et al . Pancreatic safety of incretin-based drugs — FDA and EMA assessment. N. Engl. J. Med. 370 , 794–797 (2014).
Van de Laar, F. A. et al . Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2 , CD003639 (2005).
Esposito, K. et al . Dipeptidyl peptidase-4 inhibitors and HbA1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 13 , 594–603 (2011).
Richter, B., Bandeira-Echtler, E., Bergerhoff, K. & Lerch, C. L. Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2 , CD006739 (2008).
Abdul-Ghani, M. A., Norton, L. & DeFronzo, R. A. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr. Rev. 32 , 515–531 (2011). An excellent review of the mechanism of action, efficacy and safety of the recently approved SGLT2 inhibitor class of antidiabetic medications.
Wright, E. M., Loo, D. D. & Hirayama, B. A. Biology of human sodium glucose transporters. Physiol. Rev. 91 , 733–794 (2011).
Merovci, A. et al . Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J. Clin. Invest. 124 , 509–514 (2014).
Ferrannini, E. et al . Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Invest. 124 , 499–508 (2014).
Abdul-Ghani, M. A., DeFronzo, R. A. & Norton, L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30–50% of filtered glucose load in humans. Diabetes 62 , 3324–3328 (2013).
Cherney, D. Z. I. et al . Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129 , 587–597 (2014).
Holman, R. R. et al . Three-year efficacy of complex insulin regimens in type 2 diabetes. N. Engl. J. Med. 361 , 1736–1747 (2009). A comparison of the efficacy and side-effect profile of commonly used complex insulin regimens for the treatment of patients with T2DM.
Gough, S. C. L. et al . Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2 , 885–893 (2014).
Wilding, J. P. et al . Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann. Intern. Med. 156 , 405–415 (2012).
Anderson, M., Powell, J., Campbell, K. M. & Taylor, J. R. Optimal management of type 2 diabetes in patients with increased risk of hypoglycemia. Diabetes Metab. Syndr. Obes. 7 , 85–94 (2014).
PubMed PubMed Central Google Scholar
Schopman, J. E. et al . The incidence of mild and severe hypoglycaemia in patients with type 2 diabetes mellitus treated with sulfonylureas: a systematic review and meta-analysis. Diabetes Metab. Res. Rev. 30 , 11–22 (2014).
Desouza, C., Salazar, H., Cheong, B., Murgo, J. & Fonseca, V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care 26 , 1485–1489 (2003).
Gerstein, H. C. et al . Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 358 , 2545–2559 (2008). The ORIGIN trial demonstrated that physiological insulin replacement doses (30–40 units per day) in newly diagnosed patients with T2DM could control HbA1c without an increased risk of cardiovascular events; however, the risk of hypoglycaemia was significantly increased, and the study did not examine the effect of higher doses of insulin, which are usually required to normalize glycaemia in more long-standing diabetes, on cardiovascular risk or other potential side effects of insulin therapy.
Cushman, W. C. et al . Effects of intensive blood-pressure control in type 2 diabetes mellitus. N. Engl. J. Med. 362 , 1575–1585 (2010).
James, P. A. et al . 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311 , 507–520 (2014).
Emdin, C. et al . Association of cardiovascular trial registration with positive study findings: Epidemiological Study of Randomized Trials (ESORT). JAMA Intern. Med. 175 , 304–307 (2015).
Testa, M. A. & Simonson, D. C. Health economic benefits and quality of life during improved glycemic control in patients with type 2 diabetes mellitus: a randomized, controlled, double-blind trial. JAMA 280 , 1490–1496 (1998). This was the first randomized trial to demonstrate that better glucose control improves QOL, cognitive function and general perceived health, and reduces symptom distress and absenteeism from work.
Testa, M. A. & Simonson, D. C. Assesment of quality-of-life outcomes. N. Engl. J. Med. 334 , 835–840 (1996).
Testa, M. A., Simonson, D. C. & Turner, R. R. Valuing quality of life and improvements in glycemic control in people with type 2 diabetes. Diabetes Care 21 , C44–C52 (1998).
Bode, B. W. et al . Patient-reported outcomes following treatment with the human GLP-1 analogue liraglutide or glimepiride in monotherapy: results from a randomized controlled trial in patients with type 2 diabetes. Diabetes Obes. Metab. 12 , 604–612 (2010).
Testa, M. A. et al . Comparative effectiveness of basal-bolus versus premix analog insulin on glycemic variability and patient-centered outcomes during insulin intensification in type 1 and type 2 diabetes: a randomized, controlled, crossover trial. J. Clin. Endocrinol. Metab. 97 , 3504–3514 (2012). This randomized trial demonstrated that patient satisfaction with treatment was more positively affected by improved QOL, reduced glucose variability and better glycaemic control with a basal-bolus regimen than negatively affected by the burden of additional injections.
Cotter, A. P., Durant, N., Agne, A. A. & Cherrington, A. L. Internet interventions to support lifestyle modification for diabetes management: a systematic review of the evidence. J. Diabetes Complications 28 , 243–251 (2014).
Rose, M. et al . The PROMIS Physical Function item bank was calibrated to a standardized metric and shown to improve measurement efficiency. J. Clin. Epidemiol. 67 , 516–526 (2014).
DeFronzo, R. A. & Triplitt, C. Novel agents for T2DM. Diabetes Spectr. 27 , 100–112 (2014). This article presents a more detailed review of novel antidiabetic agents that currently are being investigated in animals and humans for the treatment of T2DM.
Wong, A. K., Howie, J., Petrie, J. R. & Lang, C. C. AMP-activated protein kinase pathway: a potential therapeutic target in cardiometabolic disease. Clin. Sci. (Lond.) 116 , 607–620 (2009).
Agrawal, N. K. & Kant, S. Targeting inflammation in diabetes: newer therapeutic options. World J. Diabetes 5 , 697–710 (2014). Inflammation in insulin target tissues and β-cells is a now well-established pathogenetic abnormality T2DM. This article reviews the mechanism by which inflammation contributes to glucose intolerance in T2DM and potential interventions to suppress inflammation and improve insulin sensitivity and β-cell function.
Poy, M. N. et al . miR-375 maintains normal pancreatic α- and β-cell mass. Proc. Natl Acad. Sci. USA 106 , 5813–5818 (2009).
Burant, C. F. et al . TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 379 , 1403–1411 (2012).
Assmann, A., Hinault, C. & Kulkarni, R. N. Growth factor control of pancreatic islet regeneration and function. Pediatr. Diabetes 10 , 14–32 (2009).
Vasavada, R. C. et al . Protein kinase C-ζ activation markedly enhances β-cell proliferation: an essential role in growth factor mediated β-cell mitogenesis. Diabetes 56 , 2732–2743 (2007).
Wiederkehr, A. & Wollheim, C. B. Mitochondrial signals drive insulin secretion in the pancreatic β-cell. Mol. Cell. Endocrinol. 353 , 128–137 (2012).
Wang, C. et al . Deficiency of APPL1 in mice impairs glucose-stimulated insulin secretion through inhibition of pancreatic beta cell mitochondrial function. Diabetologia 56 , 1999–2009 (2013).
Sivitz, W. I. & Yorek, M. A. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid. Redox Signal. 12 , 537–577 (2010).
Li, N., Stojanovski, S. & Maechler, P. Mitochondrial hormesis in pancreatic β cells: does uncoupling protein 2 play a role? Oxid. Med. Cell. Longev. 2012 , 740849 (2012).
Aquilano, K., Baldelli, S., Pagliei, B. & Ciriolo, M. R. Extranuclear localization of SIRT1 and PGC-1α: an insight into possible roles in diseases associated with mitochondrial dysfunction. Curr. Mol. Med. 13 , 140–154 (2013).
Matschinsky, F. M. et al . Glucokinase activators for diabetes therapy: May 2010 status report. Diabetes Care 34 , S236–S243 (2011).
Engel, S. S. Glycemic and lipid effects of the short-acting glucagon receptor antagonist MK-3577 in patients with type 2 diabetes. Diabetes Abstr. 61 , A266 (2012).
Gumbiner, B. Pronounced glucose (G) reduction in poorly controlled T2DM with MB07803, a novel fructose-1, 6-biphosphatase inhibitor (FBPasel) with reduced potential for acid-base disturbance versus the 1st generation FBPasel CS-917. Diabetes Abstr. 58 , LB4 (2009).
Kumashiro, N. et al . Targeting pyruvate carboxylase reduces gluconeogenesis and adiposity and improves insulin resistance. Diabetes 62 , 2183–2194 (2013).
Stark, R. et al . A role for mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) in the regulation of hepatic gluconeogenesis. J. Biol. Chem. 289 , 7257–7263 (2014).
Harlan, D. M., Kenyon, N. S., Korsgren, O. & Roep, B. O. Current advances and travails in islet transplantation. Diabetes 58 , 2175–2184 (2009).
Motté, E. et al . Composition and function of macroencapsulated human embryonic stem cell-derived implants: comparison with clinical human islet cell grafts. Am. J. Physiol. Endocrinol. Metab. 307 , E838–E846 (2014).
Pagliuca, F. W. et al . Generation of functional human pancreatic β cells in vitro . Cell 159 , 428–439 (2014).
Blum, B. et al . Reversal of β cell de-differentiation by a small molecule inhibitor of the TGFβ pathway. eLife 3 , e02809 (2014).
Pickup, J. C. Banting Memorial Lecture 2014* Technology and diabetes care: appropriate and personalized. Diabet. Med. 32 , 3–13 (2015).
Peyser, T., Dassau, E., Breton, M. & Skyler, J. S. The artificial pancreas: current status and future prospects in the management of diabetes. Ann. NY Acad. Sci. 1311 , 102–123 (2014). This article presents an up-to-to-date status report on progress with the artificial pancreas (closed-loop system).
Klonoff, D. C. Afrezza inhaled insulin: the fastest-acting FDA-approved insulin on the market has favorable properties. J. Diabetes Sci. Technol. 8 , 1071–1073 (2014).
Eldor, R., Arbit, E., Corcos, A. & Kidron, M. Glucose-reducing effect of the ORMD-0801 oral insulin preparation in patients with uncontrolled type 1 diabetes: a pilot study. PLoS ONE 8 , e59524 (2013).
DeFronzo, R. A. Dissociation between metformin plasma exposure and its glucose-lowering effect: a novel gut-mediated mechanism of action. Diabetes 62 , a281 (2013).
DePaoli, A. M., Higgins, L. S., Henry, R. R., Mantzoros, C. & Dunn, F. L. Can a selective PPARγ modulator improve glycemic control in patients with type 2 diabetes with fewer side effects compared with pioglitazone? Diabetes Care 37 , 1918–1923 (2014).
Colca, J. R., Tanis, S. P., McDonald, W. G. & Kletzien, R. F. Insulin sensitizers in 2013: new insights for the development of novel therapeutic agents to treat metabolic diseases. Expert Opin. Investig. Drugs 23 , 1–7 (2014).
Suh, J. M. et al . Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature 513 , 436–439 (2014).
Gaich, G. et al . The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 18 , 333–340 (2013).
Jeoung, N. H. & Harris, R. A. Role of pyruvate dehydrogenase kinase 4 in regulation of blood glucose levels. Korean Diabetes J. 34 , 274–283 (2010).
Povel, C. M. et al . Metabolic syndrome model definitions predicting type 2 diabetes and cardiovascular disease. Diabetes Care 36 , 362–368 (2013).
Pacini, G., Mari, A., Fouqueray, P., Bolze, S. & Roden, M. Imeglimin increases glucose-dependent insulin secretion and improves β-cell function in patients with type 2 diabetes. Diabetes Obes. Metab. 17 , 541–545 (2015).
Birch, A. M., Buckett, L. K. & Turnbull, A. V. DGAT1 inhibitors as anti-obesity and anti-diabetic agents. Curr. Opin. Drug Discov. Devel. 13 , 489–496 (2010).
Liu, L. et al . Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J. Clin. Invest. 117 , 1679–1689 (2007).
Harrima, G., Greenwood, J. & Bhar, S. Acetyl-CoA carboxylase inhibition by NDI-630 inhibits fatty acid synthesis stimulates fatty acid oxidative, reduces body weight, improvise insulin sensitivity, and modulates dyslipidemia in rats. Diabetes Abstr. 62 , A161 (2013).
Tao, H., Zhang, Y., Zeng, X., Shulman, G. I. & Jin, S. Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat. Med. 20 , 1263–1269 (2014).
Perry, R. J. et al . Reversal of hypertriglyceridemia, fatty liver disease, and insulin resistance by a liver-targeted mitochondrial uncoupler. Cell Metab. 18 , 740–748 (2013).
Garvey, W. T. et al . Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am. J. Clin. Nutr. 95 , 297–308 (2012).
Carlsson, L. M. et al . Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N. Engl. J. Med. 367 , 695–704 (2012). The effectiveness and safety of bariatric surgery in the treatment of obesity and T2DM is reviewed in this longest ongoing study on surgical intervention.
Neuschwander-Tetri, B. A. et al . Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385 , 956–965 (2014).
Out, C., Groen, A. K. & Brufau, G. Bile acid sequestrants: more than simple resins. Curr. Opin. Lipidol. 23 , 43–55 (2012).
Cellitti, S. A novel GLP-1-FGF21 fusion protein for the treatment of diabetes and obesity. Keystone Symp. Obes. (2014).
Thareja, S., Aggarwal, S., Bhardwaj, T. R. & Kumar, M. Protein tyrosine phosphatase 1B inhibitors: a molecular level legitimate approach for the management of diabetes mellitus. Med. Res. Rev. 32 , 459–517 (2012).
Chakraborty, C., Doss, C. G., Bandyopadhyay, S. & Agoramoorthy, G. Influence of miRNA in insulin signaling pathway and insulin resistance: micro-molecules with a major role in type-2 diabetes. Wiley Interdiscip. Rev. RNA 5 , 697–712 (2014).
Tilg, H. & Moschen, A. R. Microbiota and diabetes: an evolving relationship. Gut 63 , 1513–1521 (2014).
Patel, S. R., Hakim, D., Mason, J. & Hakim, N. The duodenal–jejunal bypass sleeve (EndoBarrier Gastrointestinal Liner) for weight loss and treatment of type 2 diabetes. Surg. Obes. Relat. Dis. 9 , 482–484 (2013).
Bhatt, M. P., Lim, Y.-C. & Ha, K.-S. C-peptide replacement therapy as an emerging strategy for preventing diabetic vasculopathy. Cardiovasc. Res. 104 , 234–244 (2014).
Bhat, M., Pouliot, M., Couture, R. & Vaucher, E. The kallikrein–kinin system in diabetic retinopathy. Prog. Drug Res. 69 , 111–143 (2014).
PubMed Google Scholar
Hajhosseiny, R. et al . Have we reached the limits for the treatment of diabetic nephropathy? Expert Opin. Investig. Drugs 23 , 511–522 (2014).
Williams, M. E. et al . Effects of pyridoxamine in combined phase 2 studies of patients with type 1 and type 2 diabetes and overt nephropathy. Am. J. Nephrol. 27 , 605–614 (2007).
De Zeeuw, D. et al . The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J. Am. Soc. Nephrol. 25 , 1083–1093 (2014).
Boussageon, R. et al . Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ 343 , d4169 (2011).
Colditz, G. A., Willett, W. C., Rotnitzky, A. & Manson, J. E. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann. Intern. Med. 122 , 481–486 (1995).
Chan, J. M., Rimm, E. B., Colditz, G. A., Stampfer, M. J. & Willett, W. C. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 17 , 961–969 (1994).
Download references
Acknowledgements
The authors acknowledge grants from: the South Texas Veterans Healthcare System to R.A.D.; the National Institutes of Health (grants R01DK24092 to R.A.D.; DK58845 and P30 DK46200 to F.B.H.; R01 DK-040936, R01 DK-049230, R24 DK-085836, UL1 RR-045935, R01 DK-082659 and R24 DK085610 to G.I.S.; P30 DK036836 to C.R.K. Novo Nordisk Foundation for Basic Metabolic Research and the University of Copenhagen to G.I.S. and C.R.K.; DVA-Merit Review grant and VA San Diego Healthcare System to R.H.; National Institute for Diabetes and Digestive and Kidney Disease (grant P30DK092926) to W.H.; the Swedish Research Council (grants 2010–3490 and 2008–6589) and European Council (grants GA269045) to L.G.; Italian Ministry of University & Research (MIUR 2010329EKE) to E.F.; the Patient-Centered Outcomes Research Institute (PCORI) Program Award (CE1304-6756) to D.C.S. and M.A.T.; NovoNordisk Foundation to the NNF Center for Basic Metabolic Research to J.H. W.H. acknowledges the Michigan Center for Diabetes Translational Research and I.R. thanks R. Sprung for editorial assistance.
Author information
Authors and affiliations.
Diabetes Division, Department of Medicine, University of Texas Health Science Center, South Texas Veterans Health Care System and Texas Diabetes Institute, 701 S. Zarzamoro, San Antonio, 78207, Texas, USA
Ralph A. DeFronzo
CNR Institute of Clinical Physiology, Pisa, Italy
Ele Ferrannini
Department of Clinical Science Malmoe, Diabetes & Endocrinology, Lund University Diabetes Centre, Lund, Sweden
University of California, San Diego, Section of Diabetes, Endocrinology & Metabolism, Center for Metabolic Research, VA San Diego Healthcare System, San Diego, California, USA
Robert R. Henry
University of Michigan, Ann Arbor, Michigan, USA
William H. Herman
University of Copenhagen, Kobenhavn, Denmark
Jens Juul Holst
Department of Nutrition, Harvard T.H. Chan School of Public Health and Department of Epidemiology, Harvard T.H. Chan School of Public Health and Channing Division of Network Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, USA
Frank B. Hu
Harvard Medical School and Joslin Diabetes Center, Boston, Massachusetts, USA
C. Ronald Kahn
Division of Internal Medicine, Diabetes Unit, Hadassah Hebrew University Hospital, Jerusalem, Israel
Howard Hughes Medical Institute and the Departments of Internal Medicine and Cellular & Molecular Physiology, Yale University School of Medicine, New Haven, Connecticut, USA
Gerald I. Shulman
Division of Endocrinology, Diabetes and Hypertension, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, USA
Donald C. Simonson
Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA
Marcia A. Testa
Department of Human Metabolism and Nutrition, Braun School of Public Health, Hebrew University, Jerusalem, Israel
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (R.R.H.); Epidemiology (F.B.H.); Mechanisms/pathophysiology (L.C.G., C.R.K., E.F., G.I.S. and R.A.D.); Diagnosis, screening and prevention (W.H.H.); Management (R.A.D.); Quality of life (D.C.S. and M.A.T.); Outlook (I.R., J.J.H. and R.W.); overview of Primer (R.A.D.).
Corresponding author
Correspondence to Ralph A. DeFronzo .
Ethics declarations
Competing interests.
The authors declare the following potential COI: (1) R.A.D.: Research Grant Support - AstraZeneca, Bristol Myers Squibb, Janssen; Speaker's Bureau - AstraZeneca, Novo Nordisk, Advisory Board/Consultant - AstraZeneca, Janssen, Novo Nordisk, Boehringer Ingelheim, Lexicon, Intarcia; (2) E.F.: Research Grant Support - Boehringer Ingelheim, Eli Lilly; Consultant/Speaker Bureau-Boehringer Ingelheim, Eli Lilly, Sanofi, Novo Nordisk, Janssen, AstraZeneca, Takeda, Medtronic, Intarcia; (3) C.R.K. serves as a consultant for Medimmune, Merck, Five Prime Therapeutics, CohBar, Antriabio, and Catabasis; (4) L.G. has no conflict of interest; (5) R.H. has received grant support from Hitachi, Janssen, Eli Lilly, Sanofi-Aventis and Viacyte and is a consultant/advisory board member for Alere, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Clin Met, Eisai, Elcelyx, Gilead, Intarcia, Isis, Janssen, Merck, Novo Nordisk, Sanofi-Aventis, and Vivus; (6) W.H.H. has no conflict of interest; (7) J.J.H. has received grant support from Novartis and Merck and is a consultant/advisory board member for Glaxo, Smith, Kline, Novo Nordisk, and Zealand Pharmaceuticals; (8) M.A.T. has no conflict of interest; (9) R.W. serves as a consultant for Medtronics and Kamada and is on the speaker's bureau for Medtronics and Novo Nordisk; (10) F.H. has received research support from California Walnut Commission and Metegenics; (11) G.I.S. serves on scientific advisory boards for Merck and Novartis and he has received research grant support from Gilead Pharmaceuticals; (12) D.C.S. has no conflict of interest; (13) I.R. – Advisory Board: Novo Nordisk, Astra Zeneca/BMS, MSD, Eli Lilly, Sanofi, Medscape Cardiology; Consultant: Astra Zeneca/BMS, Insuline; Speaker's Bureau: Eli Lilly, Novo Nordisk, Astra Zeneca/BMS, J&J, Sanofi, MSD, Novartis, Teva; Shareholder: Insuline, Labstyle.
PowerPoint slides
Powerpoint slide for fig. 1, powerpoint slide for fig. 2, powerpoint slide for fig. 3, powerpoint slide for fig. 4, powerpoint slide for fig. 5, powerpoint slide for fig. 6, powerpoint slide for fig. 7, powerpoint slide for fig. 8, rights and permissions.
Reprints and permissions
About this article
Cite this article.
DeFronzo, R., Ferrannini, E., Groop, L. et al. Type 2 diabetes mellitus. Nat Rev Dis Primers 1 , 15019 (2015). https://doi.org/10.1038/nrdp.2015.19
Download citation
Published : 23 July 2015
DOI : https://doi.org/10.1038/nrdp.2015.19
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Prevalence of thyroid dysfunction and associated factors among adult type 2 diabetes mellitus patients, 2000–2022: a systematic review and meta-analysis.
- Rishan Hadgu
- Abebaw Worede
- Sintayehu Ambachew
Systematic Reviews (2024)
The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003–2018
- Xuanyang Wang
Cardiovascular Diabetology (2024)
Deep learning enables the quantification of browning capacity of human adipose samples
- Pingping Shen
Journal of Big Data (2024)
Targeting aging and age-related diseases with vaccines
- Guang-Hui Liu
Nature Aging (2024)
Long-term health outcomes associated with hydration status
- Natalia I. Dmitrieva
- Manfred Boehm
- Sofia Enhörning
Nature Reviews Nephrology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Talk to us about diabetes
0345 123 2399
customer support
Type 2 diabetes
Type 2 diabetes is high blood sugar levels due to your body not making enough of a hormone called insulin, or the insulin it makes not working properly — known as insulin resistance.
High blood sugar levels over time can cause other health problems like heart attacks and strokes, as well as problems with your eyes, kidneys, and feet. These are called diabetes complications .
So treatment includes regular health checks and getting support to be active, eat healthily , and maintain a healthy weight. You may need to take medication including insulin and check your blood sugars regularly too.
Type 2 diabetes can go undiagnosed for years if you don’t have symptoms or your symptoms are missed.
It doesn't just affect people living with overweight or obesity, although this is one of the risk factors, along with ethnicity .
There are many reasons type 2 diabetes develops but it mostly affects people over 25 often with a family history.
Many people can do things to try and prevent type 2 diabetes.
There’s no cure but some people with type 2 diabetes can put their diabetes into remission .
Read our stories about people living with diabetes .
What are the differences between type 1 and type 2 diabetes?

How to prevent type 2 diabetes

Find out the symptoms of type 2 diabetes

What causes type 2 diabetes?

Type 2 diabetes treatments

Type 2 medicine

Type 2 diabetes remission

Ethnicity and type 2 diabetes

Young people and type 2 diabetes

Get a copy of Your Guide to Type 2 diabetes
Share this page.
© The British Diabetic Association operating as Diabetes UK, a charity registered in England and Wales (no. 215199) and in Scotland (no. SC039136). A company limited by guarantee registered in England and Wales with (no.00339181) and registered office at Wells Lawrence House, 126 Back Church Lane London E1 1FH

What is type 2 diabetes? - Type 2 diabetes
- Type 2 diabetes is a common condition that causes the level of sugar (glucose) in the blood to become too high.
- It can cause symptoms like excessive thirst, needing to pee a lot and tiredness. Many people have no symptoms.
- It increases your risk of getting serious problems with your eyes, feet, heart and nerves.
- It's a long-term condition that can affect your everyday life. You may need to change your diet, take medicines and have regular check-ups.
- It's caused by problems with a chemical in the body (hormone) called insulin. It's often linked to being overweight or inactive, or having a family history of type 2 diabetes.
Page last reviewed: 22 December 2023 Next review due: 22 December 2026

Diabetes Symptoms Differ In Men And Women; Telltale Signs To Watch Out
New Delhi: Diabetes is among the most widely suffered health conditions across the world. According to the World Health Organization, at least 537 million adults have been living with diabetes and the number is only expected to rise to 643 million by 2030 and 783 million by 2045.
This condition causes your body to have trouble managing the sugar levels in your blood because it either is not able to produce insulin or cannot use it properly. As a result, your blood sugar levels become too high, which can lead to various health problems over time.
Experts say the symptoms of diabetes - especially type-2 in men and women are very different from each other. However, type-2 diabetes gets more complicated and dangerous for women, bringing in a greater risk for fatalities.
Related News | Can Long Cycling/Bike Riding Affect Your Urologic Health? Expert Opinion What Complications Can Arise From Heart Failure?
How is type-2 diabetes different in men and women.
Visceral fat is more metabolically active than subcutaneous fat – and so it produces hormones that can affect a person’s health. Having more visceral fat is linked to having a higher risk of metabolic syndrome, including type-2 diabetes.
Need To Poop Urgently All The Time? Could Be A Sign Of This Deadly Heart Ailment
Experts say women suffering from type-2 diabetes are likely to be more metabolically healthier than men and have normal blood sugar levels without hypertension or elevated cholesterol.
Signs that are different in men and women
Loss of muscle mass in men.
Also known as muscle atrophy, the condition adversely affects muscle function and can slowly reduce the capacity to perform daily activities and quality of life and ultimately may increase the risk of premature fatality. 1
If you have muscle atrophy in your limbs, you may feel tingling, numbness, or weakness in your arms and legs. If you have atrophied muscles in your face or throat, your facial muscles also begin to feel weak, and you may even find it difficult to speak or swallow.
Genital thrush in men
Excess sugar in the blood gets passed in the urine which has lots of yeast and grows on the penis of a man with diabetes. Symptoms of genital thrush include:
- Swelling and redness around the penis
- A lumpy, white appearance on the skin of the penis
- Soreness and discomfort during sex
Erectile dysfunction
Genital yeast infections in women.
Experts say the infection, also known as vaginal candidiasis affects up to 3 out of 4 women at some point in their lifetimes.
The condition happens when your blood sugar levels spike to dangerous levels, increasing sugar to cause yeast to overgrow, particularly in the vaginal area. Your body may develop a yeast infection in response. Some types of candidiasis can lead to serious health complications if left untreated.
General symptoms of diabetes
- Increased hunger
- Increased thirst
- Weight loss
- Frequent urination
- Blurry vision
- Extreme fatigue and tiredness
- Sores that do not heal


IMAGES
VIDEO
COMMENTS
Type 2 diabetes used to be known as adult-onset diabetes, but both type 1 and type 2 diabetes can begin during childhood and adulthood. Type 2 is more common in older adults. But the increase in the number of children with obesity has led to more cases of type 2 diabetes in younger people. There's no cure for type 2 diabetes.
DM is broadly classified into three types by etiology and clinical presentation, type 1 diabetes, type 2 diabetes, and gestational diabetes (GDM). Some other less common types of diabetes include monogenic diabetes and secondary diabetes. Type 1 Diabetes Mellitus (T1DM) Type 1 diabetes mellitus (T1DM) accounts for 5% to 10% of DM and is ...
The Dawn phenomenon, defined as a blood glucose increase of over 20 mg/dL occurring at the end of the night, appears to be common in type 2 diabetes. In a study of 248 noninsulin-treated patients with type 2 diabetes who underwent continuous glucose monitoring for 2 consecutive days, approximately half were found to have the dawn phenomenon.
Type 2 diabetes, the most common type of diabetes, is a disease that occurs when your blood glucose, also called blood sugar, is too high. Blood glucose is your main source of energy and comes mainly from the food you eat. Insulin, a hormone made by the pancreas, helps glucose get into your cells to be used for energy.
Insulin is a hormone made by your pancreas that acts like a key to let blood sugar into the cells in your body for use as energy. If you have type 2 diabetes, cells don't respond normally to insulin; this is called insulin resistance. Your pancreas makes more insulin to try to get cells to respond. Eventually your pancreas can't keep up ...
This topic will review the clinical presentation, diagnosis, and initial evaluation of diabetes in nonpregnant adults. Screening for and prevention of diabetes, the etiologic classification of diabetes mellitus, the treatment of diabetes, as well as diabetes during pregnancy are discussed separately. (See "Screening for type 2 diabetes mellitus" .)
How common is Type 2 diabetes? Type 2 diabetes is very common. More than 37 million people in the U.S. have diabetes (about 1 in 10 people), and about 90% to 95% of them have T2D. Researchers estimate that T2D affects about 6.3% of the world's population. T2D most commonly affects adults over 45, but people younger than 45 can have it as well ...
Type 2 diabetes is the most common type of diabetes. You are more likely to develop type 2 diabetes if you have risk factors, such as overweight or obesity, and a family history of the disease. You can develop type 2 diabetes at any age, even during childhood. You can help delay or prevent type 2 diabetes by knowing the risk factors and taking ...
Type 2 diabetes is the most common form, representing 90% to 95% of all diabetes cases. About 537 million adults across the world have diabetes. Experts predict this number will rise to 643 million by 2030 and 783 million by 2045.
Type 2 diabetes. Type 2 diabetes affects how your body uses sugar (glucose) for energy. It stops the body from using insulin properly, which can lead to high levels of blood sugar if not treated. Over time, type 2 diabetes can cause serious damage to the body, especially nerves and blood vessels. Type 2 diabetes is often preventable.
Diabetes follows a progressive pattern with complex pathogenesis and varied presentation.[1,2] Hyperglycemia and its associated carbohydrate, fat, and protein metabolic dysfunctions affect multiple organs of the body and disrupt their normal functioning. ... rather than the most common T2DM. This type of diabetes is often described as "Latent ...
Diabetes Basics. Diabetes is a chronic (long-lasting) disease that affects how your body turns food into energy. There are three main types of diabetes: type 1, type 2, and gestational diabetes (diabetes while pregnant). More than 133 million Americans are living with diabetes (37.3 million) or prediabetes (96 million).
The designations employed and the presentation of the material in ... The most common type of diabetes mellitus is type 2 diabetes (T2DM). The majority of people with T2DM are overweight or obese, which either causes or aggravates HEARTS D Diagnosis and management of Type 2 diabetes 9.
1. Introduction. Type 2 Diabetes Mellitus (T2DM) is one of the most common metabolic disorders worldwide and its development is primarily caused by a combination of two main factors: defective insulin secretion by pancreatic β-cells and the inability of insulin-sensitive tissues to respond to insulin [].Insulin release and action have to precisely meet the metabolic demand; hence, the ...
It is important for the provider to realize that classification of diabetes type is not always straightforward at presentation and that misdiagnosis is common (e.g., adults with type 1 diabetes misdiagnosed as having type 2 diabetes; individuals with maturity-onset diabetes of the young [MODY] misdiagnosed as having type 1 diabetes, etc ...
Clinical Presentation of Type 2 Diabetes. Type 2 DM may present to the clinician in one of 4 ways: • Classical symptoms; • ... Foot problems are a major cause of morbidity in type 2 DM and one of the most common reasons for hospital admissions in diabetes mellitus. The diabetic foot represents a spectrum of disorders ranging from vascular ...
Type 2 diabetes mellitus (T2DM) is an expanding global health problem, closely linked to the epidemic of obesity. Individuals with T2DM are at high risk for both microvascular complications ...
Type 2 diabetes is high blood sugar levels due to your body not making enough of a hormone called insulin, or the insulin it makes not working properly — known as insulin resistance. High blood sugar levels over time can cause other health problems like heart attacks and strokes, as well as problems with your eyes, kidneys, and feet. These are called diabetes complications.
Type 2 diabetes is a common condition that causes the level of sugar (glucose) in the blood to become too high. It can cause symptoms like excessive thirst, needing to pee a lot and tiredness. Many people have no symptoms. It increases your risk of getting serious problems with your eyes, feet, heart and nerves.
Type 2 diabetes mellitus (T2DM) is the most common form of diabetes. With this form of the condition, the body does not respond as expected to the hormone insulin.
Which choice best describes the most common presentation of a patient with Type 2 diabetes? Insidious onset of hyperglycemia with weight gain Most patients with type 2 diabetes mellitus are asymptomatic at presentation. They are identified because of screening and identification of risk factors. Hence, diabetes usually has an insidious onset ...
After an average follow-up of 7 years, the scientists identified seven food additive emulsifiers associated with an increased risk for type 2 diabetes. These are: tripotassium phosphate (E340 ...
The condition is often called latent autoimmune diabetes of adults, or LADA for short. Patients with it can be misdiagnosed with Type 2 diabetes and spend months or years trying to manage the ...
Background Gestational diabetes mellitus (GDM) is one of the most common metabolic disorders during pregnancy and is associated with adverse outcomes in both mothers and their children. After delivery, women who experience GDM are also at higher risk of both subsequent GDM and type 2 diabetes mellitus (T2DM) than those who do not. Therefore, healthcare providers and public health practitioners ...
Autoimmune polyglandular syndromes (APS) are a rare group of polyendocrine conditions that include multiple glandular deficiencies associated with other autoimmune diseases. There are three types of APS, of which APS type 2 (APS-2) is the most common. APS-2 is defined by the presence of Addison's disease associated with autoimmune thyroid disease and/or diabetes mellitus type 1.
Type-2 diabetes is among the most common types of diabetes that millions suffer from across the world. In this condition, body cells become less responsive to insulin. Even though many symptoms ...
This is called hypoglycemia, very low blood sugar. In fact, if it gets too low, the person can pass out. So, very quickly, the brain says, pancreas, stop the quick, release glucagon. Now, glucagon is the other hormone that the pancreas makes and it's designed to get the blood sugar level up again.