- International
- Schools directory
- Resources Jobs Schools directory News Search


A* A Level Biology Essay- The Biological Significance of Carbohydrates
Subject: Biology
Age range: 16+
Resource type: Assessment and revision
Last updated
26 December 2020
- Share through email
- Share through twitter
- Share through linkedin
- Share through facebook
- Share through pinterest

This essay aims to explain the biological significance of Carbohydrates to plants and animals within biology. This essay uses external sources (cited) and closely aligns with the content taught in AQA A level Biology. It includes relevant examples and has been graded at A* Level.
Tes paid licence How can I reuse this?
Your rating is required to reflect your happiness.
It's good to leave some feedback.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resource to let us know if it violates our terms and conditions. Our customer service team will review your report and will be in touch.
Not quite what you were looking for? Search by keyword to find the right resource:
Carbohydrates
General characteristics.
- Carbohydrates are a major group of biologically important molecules .
- They are composed of carbon, hydrogen, and oxygen in a ratio of 1:2:1 respectively.
- They are a primary energy source for both plants and animals.
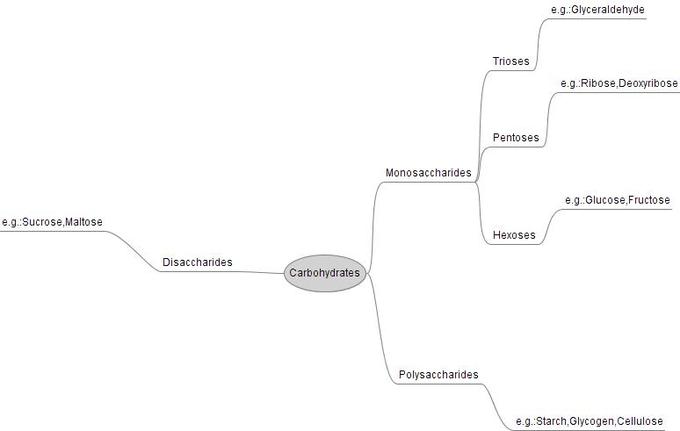
Monosaccharides
- Monosaccharides are the simplest form of carbohydrate.
- They are classified by the number of carbon atoms; triose , pentose , and hexose are examples.
- Glucose , a hexose, is a primary energy source for the body.
Disaccharides
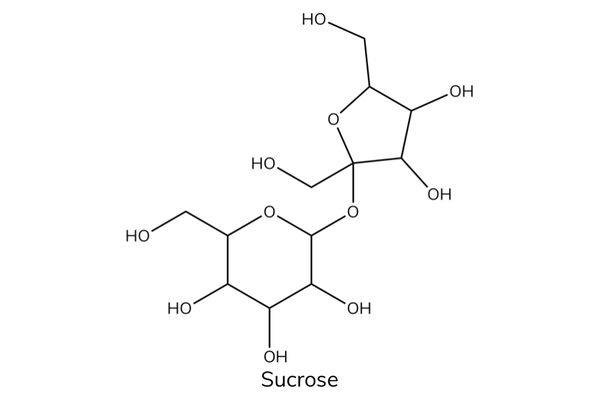
- Disaccharides are created when two monosaccharides combine in a condensation reaction , forming a glycosidic bond .
- Examples of disaccharides include sucrose , maltose , and lactose .
Polysaccharides
- When more than two monosacscharides combine, a polysaccharide is formed; this includes starch , glycogen , and cellulose .
- Starch and glycogen are used for energy storage in plants and animals respectively, while cellulose forms the structural framework of plant cell walls.
Structures of Carbohydrates
- Isomers : Different monosaccharides can have the same molecular formula but different structures, these are known as isomers .
- Ring Formations : In water, carbohydrates often form ring-shaped structures.
- Branched Chains : Polysaccharides can also exist as highly branched chains which allow for quicker mobilisation of energy reserves.
Functions of Carbohydrates
- Energy Supply : Carbohydrates are a key source of energy for many organisms, as they can be easily broken down in cellular respiration.
- Energy Storage : Complex carbohydrates like starch and glycogen serve as long-term energy storage molecules.
- Structural Roles : Some types of carbohydrates have structural roles, such as cellulose in plant cell walls or chitin in insect exoskeletons.
By understanding the characteristics and roles of carbohydrates, you’re equipped to analyse their function in various biological systems—and the processes that allow life as we know it to exist.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

3.3: Importance of Carbohydrates
- Last updated
- Save as PDF
- Page ID 51484

Learning Objectives
- Describe the benefits provided to organisms by carbohydrates
Benefits of Carbohydrates
Biological macromolecules are large molecules that are necessary for life and are built from smaller organic molecules. One major class of biological macromolecules are carbohydrates, which are further divided into three subtypes: monosaccharides, disaccharides, and polysaccharides. Carbohydrates are, in fact, an essential part of our diet; grains, fruits, and vegetables are all natural sources of carbohydrates. Importantly, carbohydrates provide energy to the body, particularly through glucose, a simple sugar that is a component of starch and an ingredient in many basic foods.
Carbohydrates in Nutrition
Carbohydrates have been a controversial topic within the diet world. People trying to lose weight often avoid carbs, and some diets completely forbid carbohydrate consumption, claiming that a low-carb diet helps people to lose weight faster. However, carbohydrates have been an important part of the human diet for thousands of years; artifacts from ancient civilizations show the presence of wheat, rice, and corn in our ancestors’ storage areas.
Carbohydrates should be supplemented with proteins, vitamins, and fats to be parts of a well-balanced diet. Calorie-wise, a gram of carbohydrate provides 4.3 Kcal. In comparison, fats provide 9 Kcal/g, a less desirable ratio. Carbohydrates contain soluble and insoluble elements; the insoluble part is known as fiber, which is mostly cellulose. Fiber has many uses; it promotes regular bowel movement by adding bulk, and it regulates the rate of consumption of blood glucose. Fiber also helps to remove excess cholesterol from the body. Fiber binds and attaches to the cholesterol in the small intestine and prevents the cholesterol particles from entering the bloodstream. Then cholesterol exits the body via the feces. Fiber-rich diets also have a protective role in reducing the occurrence of colon cancer. In addition, a meal containing whole grains and vegetables gives a feeling of fullness. As an immediate source of energy, glucose is broken down during the process of cellular respiration, which produces adenosine triphosphate (ATP), the energy currency of the cell. Without the consumption of carbohydrates, the availability of “instant energy” would be reduced. Eliminating carbohydrates from the diet is not the best way to lose weight. A low-calorie diet that is rich in whole grains, fruits, vegetables, and lean meat, together with plenty of exercise and plenty of water, is the more sensible way to lose weight.
Contributions and Attributions
- dehydration reaction. Provided by : Wiktionary. Located at : http://en.wiktionary.org/wiki/dehydration_reaction . License : CC BY-SA: Attribution-ShareAlike
- OpenStax College, Biology. October 16, 2013. Provided by : OpenStax CNX. Located at : http://cnx.org/content/m44400/latest...ol11448/latest . License : CC BY: Attribution
- isomer. Provided by : Wiktionary. Located at : en.wiktionary.org/wiki/isomer . License : CC BY-SA: Attribution-ShareAlike
- biopolymer. Provided by : Wiktionary. Located at : en.wiktionary.org/wiki/biopolymer . License : CC BY-SA: Attribution-ShareAlike
- OpenStax College, Carbohydrates. October 16, 2013. Provided by : OpenStax CNX. Located at : http://cnx.org/content/m44400/latest...e_03_02_07.jpg . License : CC BY: Attribution
- OpenStax College, Carbohydrates. October 16, 2013. Provided by : OpenStax CNX. Located at : http://cnx.org/content/m44400/latest...e_03_02_04.jpg . License : CC BY: Attribution
- OpenStax College, Carbohydrates. October 16, 2013. Provided by : OpenStax CNX. Located at : http://cnx.org/content/m44400/latest...e_03_02_01.jpg . License : CC BY: Attribution
- OpenStax College, Biology. October 22, 2013. Provided by : OpenStax CNX. Located at : http://cnx.org/content/m44400/latest...ol11448/latest . License : CC BY: Attribution
- ATP. Provided by : Wiktionary. Located at : en.wiktionary.org/wiki/ATP . License : CC BY-SA: Attribution-ShareAlike
- carbohydrate. Provided by : Wiktionary. Located at : en.wiktionary.org/wiki/carbohydrate . License : CC BY-SA: Attribution-ShareAlike
- glucose. Provided by : Wiktionary. Located at : en.wiktionary.org/wiki/glucose . License : CC BY-SA: Attribution-ShareAlike
- Wikimedia. Provided by : Wikimedia. Located at : www.wikimedia.org . License : Public Domain: No Known Copyright
- Carbohydrates provide energy to the body, particularly through glucose, a simple sugar that is found in many basic foods.
- Carbohydrates contain soluble and insoluble elements; the insoluble part is known as fiber, which promotes regular bowel movement, regulates the rate of consumption of blood glucose, and also helps to remove excess cholesterol from the body.
- As an immediate source of energy, glucose is broken down during the process of cellular respiration, which produces ATP, the energy currency of the cell.
- Since carbohydrates are an important part of the human nutrition, eliminating them from the diet is not the best way to lose weight.
- carbohydrate : A sugar, starch, or cellulose that is a food source of energy for an animal or plant; a saccharide.
- glucose : a simple monosaccharide (sugar) with a molecular formula of C6H12O6; it is a principal source of energy for cellular metabolism
- ATP : A nucleotide that occurs in muscle tissue, and is used as a source of energy in cellular reactions, and in the synthesis of nucleic acids. ATP is the abbreviation for adenosine triphosphate.
A-Level AQA Biology - Biological Molecules
Finish sign up, carbohydrates, monomers & polymers.
Monomers (mono meaning one, think monobrow!)
Small, single units act as the building blocks to create larger molecules.
Polymers (poly meaning more than two)
Made up of many monomers, usually thousands, chemically bonded together.

For monomers to bond together, a chemical reaction occurs, which is a condensation reaction. Condensation reactions involve the removal of water. This removal of water from monomers enables a chemical bond to form between the monomers. A hydrolysis reaction is the opposite of this - Hydro (water) lysis (to split). A water molecule is added between two bonded monomers (within a dimer or polymer) to break the chemical bond.

Carbohydrates are key biological molecules that store energy and can provide structural support to plant cells. Carbohydrates can be classified into three groups determined by how many units they are made of, as seen in the flow diagram below:

Larger carbohydrates, such as sucrose and starch, are made from monosaccharides. The monomers of carbohydrates are known as monosaccharides, and glucose, galactose, and fructose are three common examples. Monosaccharides are all sugars that are soluble in water. Their functions are either to provide energy or they are building blocks to create other molecules. All carbohydrates contain three elements: carbon, hydrogen, and oxygen (CHO). The general formula for a monosaccharide is C n H 2n O n , where n = the number of carbon atoms it contains.

Structure of Carbohydrates
Types of monosaccharides.
Carbohydrates are made of carbon, hydrogen and oxygen atoms. They are made from monosaccharides, which are simple sugars containing three to seven carbon atoms.
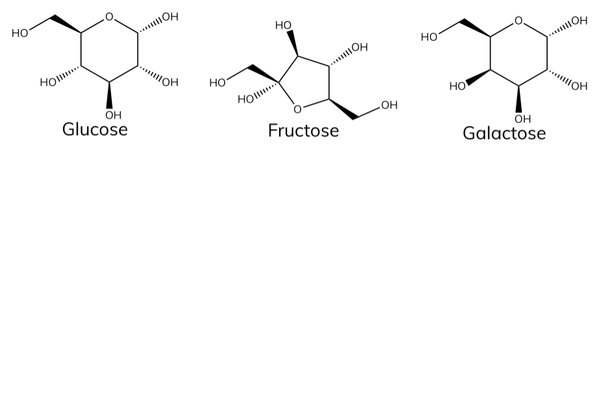
Examples of monosaccharides
- Galactose (found in milk).
- Fructose (found in fruit).
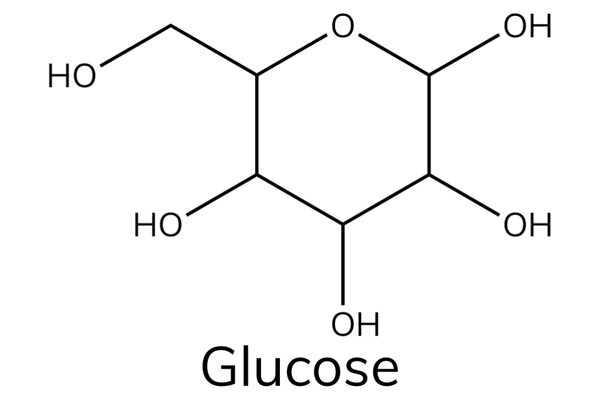
- Glucose is a hexose sugar that has the chemical formula C 6 H 12 O 6 .
- Glucose is an important source of energy in humans.
- During cellular respiration, the energy released from glucose helps to make adenosine triphosphate (ATP).
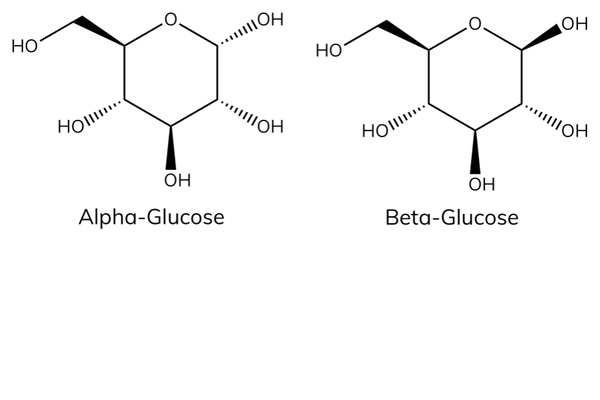
Alpha vs beta glucose
- Alpha- and beta-glucose are isomers. Isomers have the same molecular formula but a different arrangement of atoms in space.
- The carbon atoms are numbered from 1 – 6 and the OH (hydroxyl) groups are in a different orientation around C 1 .
Disaccharides and Polysaccharides
When two monosaccharides join via a condensation reaction, they form a disaccharide. When more than two disaccharides join together, they form a polysaccharide chain.
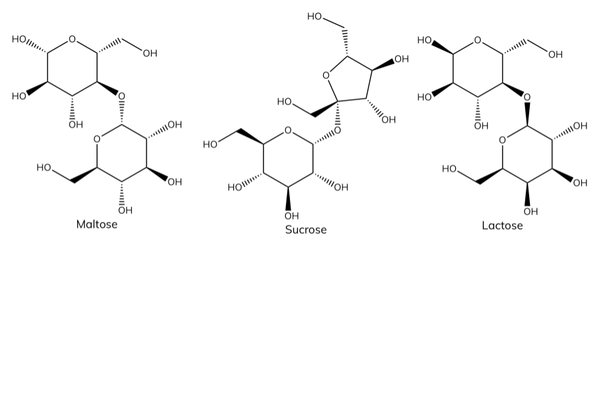
Examples of disaccharides
- Glucose + glucose → maltose.
- Glucose + fructose → sucrose.
- Glucose + galactose → lactose.
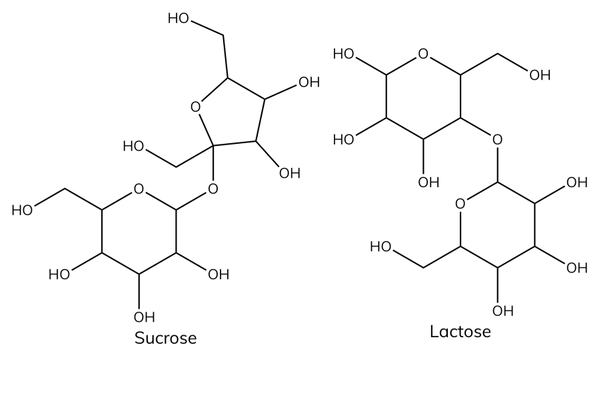
Functions of disaccharides
- Sucrose is common table sugar.
- Lactose intolerance is a common problem where the body is unable to digest lactose.
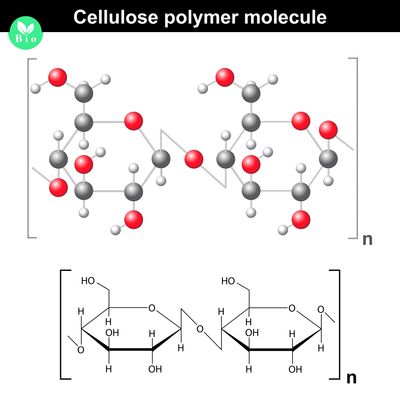
Polysaccharides
- Polysaccharides are made up of three or more monosaccharides joined together by glycosidic bonds.
- The chain may be branched or unbranched.
- The chain may contain different types of monosaccharides.
- Starch, glycogen, cellulose and chitin are examples of polysaccharides.
Benedict's Test for Sugars
Benedict’s solution (also known as Benedict's reagent or the Benedict’s test) can be used as a test for reducing and non-reducing sugars.


Reducing sugars
- E.g. Glucose, galactose and fructose.
- E.g. Lactose and maltose.
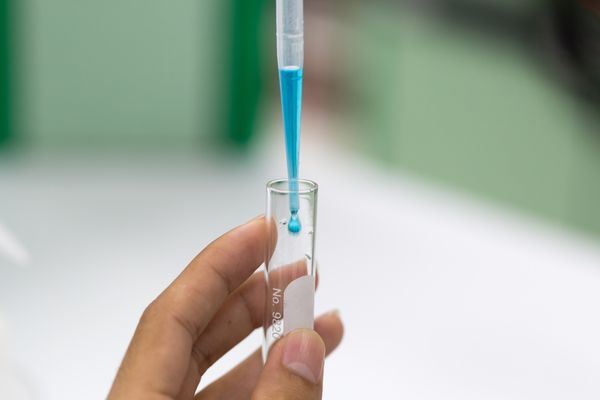
Test for reducing sugars
- Benedict's solution can be reduced by reducing sugars.
- Step 1: Place 2 ml of the substance in a boiling tube (substance must be in liquid form).
- Step 2: Add 10 drops of Benedict's solution.
- Step 3: Place in a boiling water bath for 3-5 minutes.

Results of the Benedict's test
- Blue solution → no reducing sugar.
- Green/yellow precipitate → traces of reducing sugar.
- Orange/red precipitate → moderate amounts of reducing sugar.
- Brick red precipitate → large amount of reducing sugar.
Non-reducing sugars
- Non-reducing sugars will show a negative result to the Benedict’s test. A second test is needed to determine if non-reducing sugar is present.
- Sucrose is a non-reducing sugar. It is a disaccharide made up of glucose and fructose joined by a glycosidic bond.

Test for non-reducing sugars
- Step 1: Boil in dilute HCl (to hydrolyse the non-reducing sugar).
- Step 2: Neutralise the solution by adding sodium hydrogen carbonate.
- The result will now be positive if a non-reducing sugar is present.
- If the solution remains blue, then no sugar is present.
1 Biological Molecules
1.1 Monomers & Polymers
1.1.1 Monomers & Polymers
1.1.2 Condensation & Hydrolysis Reactions
1.2 Carbohydrates
1.2.1 Structure of Carbohydrates
1.2.2 Types of Polysaccharides
1.2.3 End of Topic Test - Monomers, Polymers and Carbs
1.2.4 Exam-Style Question - Carbohydrates
1.2.5 A-A* (AO3/4) - Carbohydrates
1.3.1 Triglycerides & Phospholipids
1.3.2 Types of Fatty Acids
1.3.3 Testing for Lipids
1.3.4 Exam-Style Question - Fats
1.3.5 A-A* (AO3/4) - Lipids
1.4 Proteins
1.4.1 The Peptide Chain
1.4.2 Investigating Proteins
1.4.3 Primary & Secondary Protein Structure
1.4.4 Tertiary & Quaternary Protein Structure
1.4.5 Enzymes
1.4.6 Factors Affecting Enzyme Activity
1.4.7 Enzyme-Controlled Reactions
1.4.8 End of Topic Test - Lipids & Proteins
1.4.9 A-A* (AO3/4) - Enzymes
1.4.10 A-A* (AO3/4) - Proteins
1.5 Nucleic Acids
1.5.1 DNA & RNA
1.5.2 Polynucleotides
1.5.3 DNA Replication
1.5.4 Exam-Style Question - Nucleic Acids
1.5.5 A-A* (AO3/4) - Nucleic Acids
1.6.1 Structure of ATP
1.6.2 End of Topic Test - Nucleic Acids & ATP
1.7.1 Structure & Function of Water
1.7.2 A-A* (AO3/4) - Water
1.8 Inorganic Ions
1.8.1 Inorganic Ions
1.8.2 End of Topic Test - Water & Inorganic Ions
2.1 Cell Structure
2.1.1 Introduction to Cells
2.1.2 Eukaryotic Cells & Organelles
2.1.3 Eukaryotic Cells & Organelles 2
2.1.4 Prokaryotes
2.1.5 A-A* (AO3/4) - Organelles
2.1.6 Methods of Studying Cells
2.1.7 Microscopes
2.1.8 End of Topic Test - Cell Structure
2.1.9 Exam-Style Question - Cells
2.1.10 A-A* (AO3/4) - Cells
2.2 Mitosis & Cancer
2.2.1 Mitosis
2.2.2 Investigating Mitosis
2.2.3 Cancer
2.2.4 A-A* (AO3/4) - The Cell Cycle
2.3 Transport Across Cell Membrane
2.3.1 Cell Membrane Structure
2.3.2 A-A* (AO3/4) - Membrane Structure
2.3.3 Diffusion
2.3.4 Osmosis
2.3.5 Active Transport
2.3.6 End of Topic Test - Mitosis, Cancer & Transport
2.3.7 Exam-Style Question - Membranes
2.3.8 A-A* (AO3/4) - Membranes & Transport
2.3.9 A-A*- Mitosis, Cancer & Transport
2.4 Cell Recognition & the Immune System
2.4.1 Immune System
2.4.2 The Immune Response
2.4.3 Antibodies
2.4.4 Primary & Secondary Response
2.4.5 Vaccines
2.4.7 Ethical Issues
2.4.8 End of Topic Test - Immune System
2.4.9 Exam-Style Question - Immune System
2.4.10 A-A* (AO3/4) - Immune System
3 Substance Exchange
3.1 Surface Area to Volume Ratio
3.1.1 Size & Surface Area
3.1.2 A-A* (AO3/4) - Cell Size
3.2 Gas Exchange
3.2.1 Single-Celled Organisms
3.2.2 Multicellular Organisms
3.2.3 Control of Water Loss
3.2.4 Human Gas Exchange
3.2.5 Ventilation
3.2.6 Dissection
3.2.7 Measuring Gas Exchange
3.2.8 Lung Disease
3.2.9 Lung Disease Data
3.2.10 End of Topic Test - Gas Exchange
3.2.11 A-A* (AO3/4) - Gas Exchange
3.3 Digestion & Absorption
3.3.1 Overview of Digestion
3.3.2 Digestion in Mammals
3.3.3 Absorption
3.3.4 End of Topic Test - Substance Exchange & Digestion
3.3.5 A-A* (AO3/4) - Substance Ex & Digestion
3.4 Mass Transport
3.4.1 Haemoglobin
3.4.2 Oxygen Transport
3.4.3 The Circulatory System
3.4.4 The Heart
3.4.5 Blood Vessels
3.4.6 Cardiovascular Disease
3.4.7 Heart Dissection
3.4.8 Xylem
3.4.9 Phloem
3.4.10 Investigating Plant Transport
3.4.11 End of Topic Test - Mass Transport
3.4.12 A-A* (AO3/4) - Mass Transport
4 Genetic Information & Variation
4.1 DNA, Genes & Chromosomes
4.1.2 Genes
4.1.3 A-A* (AO3/4) - DNA
4.2 DNA & Protein Synthesis
4.2.1 Protein Synthesis
4.2.2 Transcription & Translation
4.2.3 End of Topic Test - DNA, Genes & Protein Synthesis
4.2.4 Exam-Style Question - Protein Synthesis
4.2.5 A-A* (AO3/4) - Coronavirus Translation
4.2.6 A-A* (AO3/4) - Transcription
4.2.7 A-A* (AO3/4) - Translation
4.3 Mutations & Meiosis
4.3.1 Mutations
4.3.2 Meiosis
4.3.3 A-A* (AO3/4) - Meiosis
4.3.4 Meiosis vs Mitosis
4.3.5 End of Topic Test - Mutations, Meiosis
4.3.6 A-A* (AO3/4) - DNA,Genes, CellDiv & ProtSynth
4.4 Genetic Diversity & Adaptation
4.4.1 Genetic Diversity
4.4.2 Natural Selection
4.4.3 A-A* (AO3/4) - Natural Selection
4.4.4 Adaptations
4.4.5 Investigating Natural Selection
4.4.6 End of Topic Test - Genetic Diversity & Adaptation
4.4.7 A-A* (AO3/4) - Genetic Diversity & Adaptation
4.5 Species & Taxonomy
4.5.1 Classification
4.5.2 DNA Technology
4.5.3 A-A* (AO3/4) - Species & Taxonomy
4.6 Biodiversity Within a Community
4.6.1 Biodiversity
4.6.2 Agriculture
4.6.3 End of Topic Test - Species,Taxonomy& Biodiversity
4.6.4 A-A* (AO3/4) - Species,Taxon&Biodiversity
4.7 Investigating Diversity
4.7.1 Genetic Diversity
4.7.2 Quantitative Investigation
5 Energy Transfers (A2 only)
5.1 Photosynthesis
5.1.1 Overview of Photosynthesis
5.1.2 Light-Dependent Reaction
5.1.3 Light-Independent Reaction
5.1.4 A-A* (AO3/4) - Photosynthesis Reactions
5.1.5 Limiting Factors
5.1.6 Photosynthesis Experiments
5.1.7 End of Topic Test - Photosynthesis
5.1.8 A-A* (AO3/4) - Photosynthesis
5.2 Respiration
5.2.1 Overview of Respiration
5.2.2 Anaerobic Respiration
5.2.3 A-A* (AO3/4) - Anaerobic Respiration
5.2.4 Aerobic Respiration
5.2.5 Respiration Experiments
5.2.6 End of Topic Test - Respiration
5.2.7 A-A* (AO3/4) - Respiration
5.3 Energy & Ecosystems
5.3.1 Biomass
5.3.2 Production & Productivity
5.3.3 Agricultural Practices
5.4 Nutrient Cycles
5.4.1 Nitrogen Cycle
5.4.2 Phosphorous Cycle
5.4.3 Fertilisers & Eutrophication
5.4.4 End of Topic Test - Nutrient Cycles
5.4.5 A-A* (AO3/4) - Energy,Ecosystems&NutrientCycles
6 Responding to Change (A2 only)
6.1 Nervous Communication
6.1.1 Survival
6.1.2 Plant Responses
6.1.3 Animal Responses
6.1.4 Reflexes
6.1.5 End of Topic Test - Reflexes, Responses & Survival
6.1.6 Receptors
6.1.7 The Human Retina
6.1.8 Control of Heart Rate
6.1.9 End of Topic Test - Receptors, Retina & Heart Rate
6.2 Nervous Coordination
6.2.1 Neurones
6.2.2 Action Potentials
6.2.3 Speed of Transmission
6.2.4 End of Topic Test - Neurones & Action Potentials
6.2.5 Synapses
6.2.6 Types of Synapse
6.2.7 Medical Application
6.2.8 End of Topic Test - Synapses
6.2.9 A-A* (AO3/4) - Nervous Comm&Coord
6.3 Muscle Contraction
6.3.1 Skeletal Muscle
6.3.2 Sliding Filament Theory
6.3.3 Contraction
6.3.4 Slow & Fast Twitch Fibres
6.3.5 End of Topic Test - Muscles
6.3.6 A-A* (AO3/4) - Muscle Contraction
6.4 Homeostasis
6.4.1 Overview of Homeostasis
6.4.2 Blood Glucose Concentration
6.4.3 Controlling Blood Glucose Concentration
6.4.4 End of Topic Test - Blood Glucose
6.4.5 Primary & Secondary Messengers
6.4.6 Diabetes Mellitus
6.4.7 Measuring Glucose Concentration
6.4.8 Osmoregulation
6.4.9 Controlling Blood Water Potential
6.4.11 End of Topic Test - Diabetes & Osmoregulation
6.4.12 A-A* (AO3/4) - Homeostasis
7 Genetics & Ecosystems (A2 only)
7.1 Genetics
7.1.1 Key Terms in Genetics
7.1.2 Inheritance
7.1.3 Linkage
7.1.4 Multiple Alleles & Epistasis
7.1.5 Chi-Squared Test
7.1.6 End of Topic Test - Genetics
7.1.7 A-A* (AO3/4) - Genetics
7.2 Populations
7.2.1 Populations
7.2.2 Hardy-Weinberg Principle
7.3 Evolution
7.3.1 Variation
7.3.2 Natural Selection & Evolution
7.3.3 End of Topic Test - Populations & Evolution
7.3.4 Types of Selection
7.3.5 Types of Selection Summary
7.3.6 Overview of Speciation
7.3.7 Causes of Speciation
7.3.8 Diversity
7.3.9 End of Topic Test - Selection & Speciation
7.3.10 A-A* (AO3/4) - Populations & Evolution
7.4 Populations in Ecosystems
7.4.1 Overview of Ecosystems
7.4.2 Niche
7.4.3 Population Size
7.4.4 Investigating Population Size
7.4.5 End of Topic Test - Ecosystems & Population Size
7.4.6 Succession
7.4.7 Conservation
7.4.8 End of Topic Test - Succession & Conservation
7.4.9 A-A* (AO3/4) - Ecosystems
8 The Control of Gene Expression (A2 only)
8.1 Mutation
8.1.1 Mutations
8.1.2 Effects of Mutations
8.1.3 Causes of Mutations
8.2 Gene Expression
8.2.1 Stem Cells
8.2.2 Stem Cells in Disease
8.2.3 End of Topic Test - Mutation & Gene Epression
8.2.4 A-A* (AO3/4) - Mutation & Stem Cells
8.2.5 Regulating Transcription
8.2.6 Epigenetics
8.2.7 Epigenetics & Disease
8.2.8 Regulating Translation
8.2.9 Experimental Data
8.2.10 End of Topic Test - Transcription & Translation
8.2.11 Tumours
8.2.12 Correlations & Causes
8.2.13 Prevention & Treatment
8.2.14 End of Topic Test - Cancer
8.2.15 A-A* (AO3/4) - Gene Expression & Cancer
8.3 Genome Projects
8.3.1 Using Genome Projects
8.4 Gene Technology
8.4.1 Recombinant DNA
8.4.2 Producing Fragments
8.4.3 Amplification
8.4.4 End of Topic Test - Genome Project & Amplification
8.4.5 Using Recombinant DNA
8.4.6 Medical Diagnosis
8.4.7 Genetic Fingerprinting
8.4.8 End of Topic Test - Gene Technologies
8.4.9 A-A* (AO3/4) - Gene Technology
Jump to other topics

Unlock your full potential with GoStudent tutoring
Affordable 1:1 tutoring from the comfort of your home
Tutors are matched to your specific learning needs
30+ school subjects covered
Condensation & Hydrolysis Reactions
Types of Polysaccharides
Carbohydrates
Structure of Carbohydrates
Monosaccharides All carbohydrates are formed from the elements carbon (C), hydrogen (H) and oxygen (O). The formula of a carbohydrate is always (CH 2 O) n . The n represents the number of times the basic CH 2 O unit is repeated, e.g. where n = 6 the molecular formula is C 6 H 12 O 6 . This is the formula shared by glucose and other simple sugars like fructose. These simple sugars are known as monosaccharides.
The molecular formula, C 6 H 12 O 6 , does not indicate how the atoms bond together. Bonded to the carbon atoms are a number of – H and – OH groups. Different positions of these groups on the carbon chain are responsible for different properties of the molecules. The structural formulae of α and β glucose are shown below.
Glucose is so small that it can pass through the villi and capillaries into our bloodstream. The molecules subsequently release energy as a result of respiration. Simple glucose molecules are capable of so much more. They can combine with others to form bigger molecules.
Disaccharides Each glucose unit is known as a monomer and is capable of linking others. This diagram shows two molecules of β glucose forming a disaccharide.
In your examinations look for different monosaccharides being given, like fructose or α glucose. You may be asked to show how they bond together. The principle will be exactly the same.
A condensation reaction means that as two carbohydrate molecules bond together a water molecule is produced. The link formed between the two glucose molecules is known as a glycosidic bond .
A glycosidic bond can also be broken down to release separate monomer units. This is the opposite of the reaction shown above. Instead of water being given off, a water molecule is needed to break each glycosidic bond. This is called hydrolysis because water is needed to split up the bigger molecule.
Polysaccharides Like disaccharides, they consist of monomer units linked by the glycosidic bond. However, instead of just two monomer units they can have many. Chains of these ‘sugar’ units are known as polymers . These larger molecules have important structural and storage roles.
Starch is a polymer of the sugar, glucose. The diagram below shows part of a starch molecule.
The table classifies carbohydrates
How useful are polysaccharides?
- Starch is stored in organisms as a future energy source, e.g. potato has a high starch content to supply energy for the buds to grow at a later stage.
- Glycogen is stored in the liver, which releases glucose for energy in times of low blood sugar.
Both starch and glycogen are insoluble which enables them to remain inside cells.
- Cellulose has long chains and branches which help form a tough protective layer around plant cells, the cell wall.
- Pectins are used alongside cellulose in the cell wall. They are polysaccharides which are bound together by calcium pectate. Pectins help cells to bind together.
Together the cellulose and pectins give exceptional mechanical strength. The cell wall is also permeable to a wide range of substances.


- TOP CATEGORIES
- AS and A Level
- University Degree
- International Baccalaureate
- Uncategorised
- 5 Star Essays
- Study Tools
- Study Guides
- Meet the Team
- Exchange, Transport & Reproduction
The Importance and Biological Functions of Carbohydrates.
This is a preview of the whole essay
Peer reviews, here's what a star student thought of this essay.
superwiseman
Quality of writing.
Other remarks - Another improvement would be to use scientifically common terms, so instead of saying “Carbon number one†write “C1†Good use of diagrams to visualise molecules Reading the essay back to yourself would help it flow better There are a lot of accurate and concise details, this shows good understanding. Ketosis was definitely undergraduate stuff, and it seems like you understood it. Putting the importance of dietary fibres into context of intestinal disease is great, this really answers the question. Having references to back up your points would make this an even better essay. Overall, this is a good essay, showing real understanding and extra knowledge.
Level of analysis
Organisation - Headings would help with the organisation of the essay, especially when you are listing all the different common sugars, having a heading such as “Examples of common sugars†would make it a lot clearer what you are trying to say. Instead of listing the carbohydrates and what they are made up, compare them together to say the similarities and differences, and give details. For example, “Cellulose and starch are both made up of d-glucose, but glucose in cellulose is linked by a β(1→4) linkage, whereas in starch it is α(1→4) linkage instead.†You mentioned the six main functions in the body, so headings for each of the headings would be appropriate.
Response to question
Good introduction - You mentioned where ribose and deoxyribose are found in the cell (RNA and DNA), so I would also add where glucose and fructose are found in the cell (namely in glycolysis pathways, in polymers, in glycoproteins etc)

Document Details
- Word Count 1253
- Page Count 5
- Level AS and A Level
- Subject Science
Related Essays

Carbohydrates play a large part in the lives of all living things; there ar...

The Role of Carbohydrates

The role of carbohydrates

The structure and function of carbohydrates.
A Level Biology
Instant Access to A Level Biology Revision
Sign up now to get access to the entire library of a level biology resources for all exam boards.
If you're ready to pass your A-Level Biology exams, become a member now to get complete access to our entire library of revision materials.
Join over 22,000 learners who have passed their exams thanks to us!
Sign up below to get instant access!
Or try a sample...
Not ready to purchase the revision kit yet? No problem. If you want to see what we offer before purchasing, we have a free membership with sample revision materials.
Signup as a free member below and you'll be brought back to this page to try the sample materials before you buy.
Carbohydrates – Introduction and Classification
Introduction, 1. monosaccharides, 2. disaccharides, 3. oligosaccharides, 4. polysaccharides, what are carbohydrates, what are 4 examples of carbohydrates, what are the 4 functions of carbohydrates, what are polysaccharides.
Carbohydrates are one of the four major classes of biomolecules along with proteins, nucleic acids, and lipids. Carbohydrates are compounds that contain at least three carbon atoms, a number of hydroxyl groups, and usually an aldehyde or ketone group. They may contain phosphate, amino, or sulfate groups. First, carbohydrates serve as energy stores, fuels, and metabolic intermediates. Second, ribose and deoxyribose sugars form part of the structural framework of RNA and DNA. Third, polysaccharides are structural elements in the cell walls of bacteria and plants.
Carbohydrates are also known as saccharides since many of those of relatively small molecular weight have a sweet taste, although this is not true of those with large molecules. They are widely distributed molecules in both plant and animal tissues. They are indispensable for living organisms, serving as skeletal structures in plants and also in insects and crustaceans. They also occur as food reserves in the storage organs of plants and in the liver and muscles of animals. In addition, they are an important source of energy required for the various metabolic activities of living organisms; the energy being derived as a result of their oxidation. They also serve to lubricate skeletal joints, to provide adhesion between cells and to confer biological specificity on the surface of animal calls.
Glucose is the most important carbohydrate; most dietary carbohydrate is absorbed into the bloodstream as glucose, and other sugars are converted into glucose in the liver. Glucose is the major metabolic fuel of mammals (except ruminants) and a universal fuel of the fetus. It is the precursor for synthesis of all the other carbohydrates in the body, including glycogen for storage; ribose and deoxyribose in nucleic acids; and galactose in lactose of milk, in glycolipids, and in combination with protein in glycoproteins and proteoglycans. Diseases associated with carbohydrate metabolism include diabetes mellitus, galactosemia, glycogen storage diseases, and lactose intolerance.
Read more about the Functions of Carbohydrates
Classification of Carbohydrates
Monosaccharides are colorless, crystalline solids that are freely soluble in water but insoluble in nonpolar solvents. Most have a sweet taste. The backbones of common monosaccharides are unbranched carbon chains in which all the carbon atoms are linked by single bonds. In this open-chain form, one of the carbon atoms is double-bonded to an oxygen atom to form a carbonyl group; each of the other carbon atoms has a hydroxyl group. If the carbonyl group is at an end of the carbon chain (that is, in an aldehyde group) the monosaccharide is an aldose ; if the carbonyl group is at any other position (in a ketone group) the monosaccharide is a ketose . The simplest monosaccharides are the two three-carbon trioses: glyceraldehyde, an aldotriose, and dihydroxyacetone, a ketotriose.
Monosaccharides with four, five, six, and seven carbon atoms in their backbones are called, respectively, tetroses, pentoses, hexoses, and heptoses. There are aldoses and ketoses of each of these chain lengths: aldotetroses and ketotetroses, aldopentoses and ketopentoses, and so on. The hexoses, which include the aldohexose D-glucose and the ketohexose D-fructose, are the most common monosaccharides in nature—the products of photosynthesis, and key intermediates in the central energy-yielding reaction sequence in most organisms. The aldopentoses D-ribose and 2-deoxy-D-ribose are components of nucleotides and nucleic acids. (Figure Below)
Disaccharides (such as maltose, lactose, and sucrose) consist of two monosaccharides joined covalently by an O-glycosidic bond, which is formed when a hydroxyl group of one sugar molecule, typically cyclic, reacts with the anomeric carbon of the other. This reaction represents the formation of an acetal from a hemiacetal (such as glucopyranose) and an alcohol (a hydroxyl group of the second sugar molecule), and the resulting compound is called a glycoside .
Glycosidic bonds are readily hydrolyzed by acid but resist cleavage by base. Thus disaccharides can be hydrolyzed to yield their free monosaccharide components by boiling with dilute acid. N-glycosyl bonds join the anomeric carbon of a sugar to a nitrogen atom in glycoproteins and nucleotides.
These are compound sugars that yield 2 to 10 molecules of the same or different monosaccharides on hydrolysis. Accordingly, an oligosaccharide yielding 2 molecules of monosaccharide on hydrolysis is designated as a dissaccharide, and the one yielding 3 molecules of monosaccharide as a trisaccharide and so on. The general formula of disaccharides is:
Cn(H2O)n – 1 and that of trisaccharides is Cn(H2O)n – 2 and so on.
A few examples are : Disaccharides – Sucrose, Lactose, Maltose, Cellobiose, Trehalose, Gentiobiose, Melibiose Trisaccharides – Rhamninose, Gentianose, Raffinose (= Melitose), Rabinose, Melezitose Tetrasaccharides – Stachyose, Scorodose Pentasaccharide – Verbascose
The molecular composition of the 3 legume oligosaccharides (viz., raffinose, stachyose and verbascose) is shown below : α-Galactose (1–6) + α-Glucose (1–2) + β-Fructose = Raffinose α-Galactose (1–6) + α-Galactose (1–6) + α-Glucose (1–2) + β-Fructose = Stachyose α-Galactose (1–6) + α-Galactose (1–6) + α-Galactose (1–6) + α-Glucose (1–2) + β-Fructose = Verbascose
These are also compound sugars and yield more than 10 molecules of monosaccharides on hydrolysis. These may be further classified depending on whether the monosaccharide molecules produced as a result of the hydrolysis of polysaccharides are of the same type (homopolysaccharides) or of different types (heteropolysaccharides).
Their general formula is (C6H10O5)x.
Some common examples are : Homopolysaccharides –Starch, Glycogen, Inulin, Cellulose, Pectin, Chitin Heteropolysaccharides – “Specific soluble sugar” of pneumococcus type III, Hyaluronic acid, Chondrotin
Frequently Asked Questions
These are the biological compounds that can be defined as hydrated carbon compounds. They are classified into monosaccharides, disaccharides, and polysaccharides.
These include glucose, fructose, lactose, and cellulose.
They act as a source of energy. They are involved in cell signaling. They act as structural components in the cell. They prevent protein breakdown for energy.
Polysaccharides are the polymers of carbohydrates. They are large molecules consisting of long chains of molecules that are made by combining hundreds or thousands of carbohydrate molecules.

COMMENTS
Biology is detailed and comprehensive A-level content, uses appropriate terminology, and is very well written and always clearly explained. No significant errors or irrelevant material. For top marks in the band, the answer shows evidence of reading beyond specification requirements. 16-20. Relational.
Study with Quizlet and memorize flashcards containing terms like The structure and function of carbohydrates, The importance of shapes fitting together in cells and organisms, Describe how the structures of different polymers are related to their functions and more. ... A LEVEL BIOLOGY: 25 Mark essays. 16 terms. joboyd12. Preview. Importance of ...
Importantly, carbohydrates provide energy to the body, particularly through glucose, a simple sugar that is a component of starch and an ingredient in many basic foods. Figure 3.2.1 3.2. 1: Carbohydrates: Carbohydrates are biological macromolecules that are further divided into three subtypes: monosaccharides, disaccharides, and polysaccharides.
3 Pages • Essays / Projects • Year Uploaded: 2021. This is a A* graded essay detailing the biological significance of Carbohydrates, with many relevant examples and wider reading with referenced sources. It perfectly aligns with the AQA Biology A Level and is perfect for use in the classroom as a summary activity or personal revision!
Carbohydrates also help with fat metabolism. If the body has enough energy for its immediate needs, it stores extra energy as fat. Carbohydrates are an important component of many industries like textile, paper, lacquers and breweries. Detoxification of physiological importance is carried out to some extent with carbohydrate derivatives.
Focusing on the essay performance on the 7402 biology specification only, we can see how the 2017-2019 essays have performed in isolation in Figure 2. The 7402 essay has shown performance that skews slightly to the higher end of the mark scale, which is understandable given the mean mark sits just above 50%.
The levels scheme states that more than two A-level topics need to be addressed to get higher than 10 marks. A minimum of four topics is required to get higher than 15 marks. A topic area is a numbered sub-section in the specification. For example, for the 2017 'diffusion' essay, gas exchange (3.3.2) was a topic area.
Join over 22,000 learners who have passed their exams thanks to us! Sign up below to get instant access! Undertanding the structure and function of carbohydrates is essential for A-level biology study. Keep reading to learn more about Mono-, di-, and polysaccharides as well as cellulose.
File previews. pdf, 76.42 KB. This essay aims to explain the biological significance of Carbohydrates to plants and animals within biology. This essay uses external sources (cited) and closely aligns with the content taught in AQA A level Biology. It includes relevant examples and has been graded at A* Level.
Carbohydrates Carbohydrates General Characteristics. Carbohydrates are a major group of biologically important molecules. They are composed of carbon, hydrogen, and oxygen in a ratio of 1:2:1 respectively. They are a primary energy source for both plants and animals. Monosaccharides. Monosaccharides are the simplest form of carbohydrate.
Importantly, carbohydrates provide energy to the body, particularly through glucose, a simple sugar that is a component of starch and an ingredient in many basic foods. Figure 3.3.1 3.3. 1: Carbohydrates: Carbohydrates are biological macromolecules that are further divided into three subtypes: monosaccharides, disaccharides, and polysaccharides.
Monosaccharides are all sugars that are soluble in water. Their functions are either to provide energy or they are building blocks to create other molecules. All carbohydrates contain three elements: carbon, hydrogen, and oxygen (CHO). The general formula for a monosaccharide is CnH2nOn, where n = the number of carbon atoms it contains.
Step 2: Neutralise the solution by adding sodium hydrogen carbonate. Step 3: Repeat the Benedict's test. The result will now be positive if a non-reducing sugar is present. If the solution remains blue, then no sugar is present. Carbohydrates are made of carbon, hydrogen and oxygen atoms. They are made from monosaccharides, which are simple ...
All carbohydrates are formed from the elements carbon (C), hydrogen (H) and oxygen (O). The formula of a carbohydrate is always (CH 2 O) n. The n represents the number of times the basic CH 2 O unit is repeated, e.g. where n = 6 the molecular formula is C 6 H 12 O 6. This is the formula shared by glucose and other simple sugars like fructose.
The Importance and Biological Functions of Carbohydrates. Carbohydrates have many functions. This essay will look at some of them and also what carbohydrates are constructed of. ... Level of analysis. Organisation - Headings would help with the organisation of the essay, especially when you are listing all the different common sugars, having a ...
quickly - something that is very important in animals. ‒ As a result of the branches, glycogen is very compact which makes it very good for energy storage. ‒ Glycogen does not affect the osmotic balance of cells - i.e. cause too much water to enter them. AQA A-Level Biology 3.1.2 Carbohydrates
AQA A level Biology Essay. The importance of responses to changes in the internal and external environment of an organism. Click the card to flip 👆. Control of heart rate (changes in pH and pressure) Control of blood glucose (glucagon and insulin) Osmoregulation (water potential changes) Action potentials/ pacinian corpuscles (stimulus)
The importance of ions in biology. Click the card to flip 👆. 1.) Na+ ions in cotransport of glucose. - importance: Na+ ion concentration gradient is what drives the movement of glucose into cell - utilising energy efficiently. 2.) Na+ ions in osmoregulation. - Loop of Henle - maintains Na+ ion gradient. - importance: ensures water can leave ...
This document contains the essay titles and mark schemes used in AQA A-level Biology examinations since 2007. The specifications these exam questions came from are no longer in use, but the marking method has largely remained unchanged. Further guidance on the marking method used with the essay can be found in Paper 3 Essay marking guidance.
Carbohydrates are compounds that contain at least three carbon atoms, a number of hydroxyl groups, and usually an aldehyde or ketone group. They may contain phosphate, amino, or sulfate groups. ... If you're ready to pass your A-Level Biology exams, become a member now to get complete access to our entire library of revision materials ...
The functions of enzymes a nd their import ance in or ganisms. Plan. -DNA replic ation-DNA polymer ase and DNA helicase.dna r eplication needs to ha ppen for mitosis. which is needed f or growth and r e pair of tissues and meiosis-pr oduction of g ametes. -tra nscription/tr an slation>pepti dyl trans f erase.
Energy can take many different forms, and is an essential component in the maintenance and continuity of life. One form the energy can take is light energy. Photosynthesis uses light energy in the synthesis of organic molecules, proteins, carbs and lipids. These are chemical energy stores. Light energy excites electrons in the photosystems and ...
Carbohydrates were the major source of total energy (56.61%), followed by fat (27.92%) and protein (15.46%). ... Feature papers represent the most advanced research with significant potential for high impact in the field. ... the primary source of energy was carbohydrates, which is an important contributor to the maternal gut microbiota during ...
The importance of bonds and bonding in organisms. Mass Transport in Plants. Click the card to flip 👆. - Cohesion Tension theory, the dipolar nature of water makes it "sticky" allowing water to form strong bonds between H2O molecules. - In this way the xylem can form long uninterrupted columns of water up the stems from the roots, utilizing ...