
- The first peer-reviewed journal in the field of human gene therapy, providing all-inclusive coverage of the research, methods, and clinical developments that are driving today's explosion of gene therapy advances.
- Current Issue About this publication
Watch a video intro for this journal

Human Gene Therapy
Editor-in-Chief: Terence R. Flotte, MD Deputy Editor-in-Chief: Guangping Gao, PhD European Editor: Hildegard Büning, PhD Asian Editor : Yu-Quan Wei MD, PhD Deputy Editors: Mark Kay, MD, PhD, Thierry VandenDriessche, PhD, and Cheng Seng, PhD
Impact Factor: 4.2* *2022 Journal Citation Reports™ (Clarivate, 2023)
Citescore™: 7.0.

- View Aims & Scope
- Indexing/Abstracting
- Editorial Board
Announcements
Gene and Cell Therapy Connect — a free, comprehensive news source delivered bimonthly to readers. Sign up or View the archive
Scientific Abstracts from ESGCT 30th Annual Congress in Collaboration with SFTCG and NVGCT Human Gene Therapy is pleased to provide complimentary access to the scientific abstracts from the ESGCT 30th Annual Congress in Collaboration with SFTCG and NVGCT. The annual meeting took place on October 24-27, 2023 in Brussels, Belgium
Abstracts | Abstract Author Index
- Featured Content
- About This Publication
- Reprints & Permissions
- News Releases
- Sample Issue
Aims & Scope
Human Gene Therapy (HGT) is the premier, multidisciplinary journal covering all aspects of gene therapy. The Journal publishes important advances in DNA, RNA, cell and immune therapies, validating the latest advances in research and new technologies. Established in 1990, HGT provides a prestigious forum for publishing scientific and clinical research, including ethical, legal, regulatory, social, and commercial issues, which enables the advancement and progress of therapeutic procedures leading to improved patient outcomes, and ultimately, to curing diseases. HGT publishes 12 double issues per year, including sections on Methods (product testing and development) and Clinical Development (regulatory review, toxicology and commercial development). The journal also publishes a wide range of reviews, commentaries and editorials.
- Basic and clinical advances in gene therapy
- Delivery systems
- Cell therapy
- Immunotherapy
- Clinical genome editing
- Small nucleic acid therapeutics, including RNAi
- Clinical trials (including confirmatory or negative results)
- Improvements in vector developments
- Animal models
- Pre-clinical animal/in vitro studies to assess safety of gene and cell therapy products
- Clinical protocols
- Commercial development of gene and cell therapy products
Human Gene Therapy was the first journal devoted to cover the field of gene therapy.
Human Gene Therapy is under the editorial leadership of Editor-in-Chief Terence R. Flotte, MD, University of Massachusetts Medical School and other leading investigators. View the entire editorial board .
Audience: Geneticists, medical geneticists, molecular biologists, virologists, experimental researchers, and experimental medicine specialists, among others.
Human Gene Therapy and HGT Methods provide “Instant Online” publication 72 hours after acceptance
Indexing/Abstracting:
- PubMed/MEDLINE
- PubMed Central
- Web of Science: Science Citation Index Expanded™ (SCIE)
- Current Contents®/Life Sciences
- Biotechnology Citation Index®
- Biological Abstracts
- BIOSIS Citation Index™
- Journal Citation Reports/Science Edition
- EMBASE/Excerpta Medica
- Chemical Abstracts
- ProQuest databases
Society Affiliations
The Official Journal of nine international societies:
Special Issue: Inflammation Networks in Gene Therapy
More Special Issues...
Recommended Publications
Re:GEN Open
Nucleic Acid Therapeutics
The CRISPR Journal
Stem Cells and Development
Genetic Testing and Molecular Biomarkers
Recent Progress of Machine Learning in Gene Therapy
Affiliations.
- 1 Department of Computer Science, Pacific Lutheran University, Tacoma, WA, United States.
- 2 Department of Physics, Pacific Lutheran University, Tacoma, WA, United States.
- 3 Department of Mathematics, Pacific Lutheran University, Tacoma, WA, United States.
- 4 Department of Humanities, Pacific Lutheran University, Tacoma, WA, United States.
- 5 Division of Computing Software Systems, University of Washington-Bothell, Bothell, WA, United States.
- 6 Department of Computer Science, Saint Louis University, St. Louis, MO, United States.
- 7 School of Life Science and Technology and Center for Informational Biology, University of Electronic Science and Technology of China, Chengdu, China.
- PMID: 34161210
- DOI: 10.2174/1566523221666210622164133
With new developments in biomedical technology, it is now a viable therapeutic treatment to alter genes with techniques like CRISPR. At the same time, it is increasingly cheaper to perform whole genome sequencing, resulting in rapid advancement in gene therapy and editing in precision medicine. Understanding the current industry and academic applications of gene therapy provides an important backdrop to future scientific developments. Additionally, machine learning and artificial intelligence techniques allow for the reduction of time and money spent in the development of new gene therapy products and techniques. In this paper, we survey the current progress of gene therapy treatments for several diseases and explore machine learning applications in gene therapy. We also discuss the ethical implications of gene therapy and the use of machine learning in precision medicine. Machine learning and gene therapy are both topics gaining popularity in various publications, and we conclude that there is still room for continued research and application of machine learning techniques in the gene therapy field.
Keywords: CRISPR; Machine learning; cancer; cardiovascular disease; ethics; gene therapy; hemophilia; neurodegenerative disease.
Copyright© Bentham Science Publishers; For any queries, please email at [email protected].
- Artificial Intelligence*
- Genetic Therapy
- Machine Learning*
- Precision Medicine
Advertisement
Recent advances in gene therapy: genetic bullets to the root of the problem
- Review Article
- Published: 25 October 2022
- Volume 23 , pages 1107–1121, ( 2023 )
Cite this article

- Mohsen Danaeifar 1
4139 Accesses
8 Citations
2 Altmetric
Explore all metrics
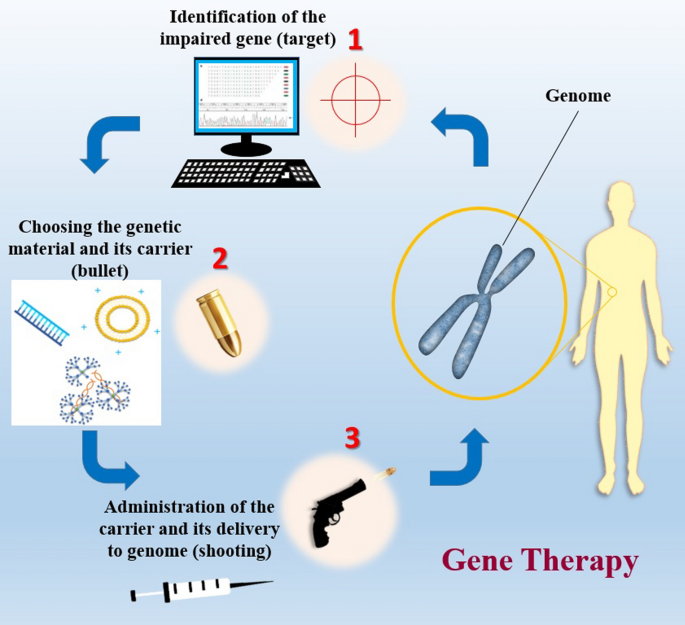
Genetics and molecular genetic techniques have changed many perspectives and paradigms in medicine. Using genetic methods, many diseases have been cured or alleviated. Gene therapy, in its simplest definition, is application of genetic materials and related techniques to treat various human diseases. Evaluation of the trends in the field of medicine and therapeutics clarifies that gene therapy has attracted a lot of attention due to its powerful potential to treat a number of diseases. There are various genetic materials that can be used in gene therapy such as DNA, single- and double-stranded RNA, siRNA and shRNA. The main gene editing techniques used for in vitro and in vivo gene modification are ZNF, TALEN and CRISPR-Cas9. The latter has increased hopes for more precise and efficient gene targeting as it requires two separate recognition sites which makes it more specific and can also cause rapid and sufficient cleavage within the target sequence. There must be carriers for delivering genes to the target tissue. The most commonly used carriers for this purpose are viral vectors such as adenoviruses, adeno-associated viruses and lentiviruses. Non-viral vectors consist of bacterial vectors, liposomes, dendrimers and nanoparticles.
Graphical abstract
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Special issue: Genome editing and gene therapy

Gene Therapy Approaches Toward Biomedical Breakthroughs
Non-viral vectors for gene-based therapy
Fauci AS. Infectious diseases: considerations for the 21st century. Clin Infect Dis. 2001;32(5):675–85.
CAS PubMed Google Scholar
Oti M, Brunner HG. The modular nature of genetic diseases. Clin Genet. 2007;71(1):1–11.
Mulligan RC. The basic science of gene therapy. Science. 1993;260(5110):926–32.
Yu M, Poeschla E, Wong-Staal F. Progress towards gene therapy for HIV infection. Gene Ther. 1994;1(1):13–26.
Kumar R, et al. BRAF mutations in metastatic melanoma: a possible association with clinical outcome. Clin Cancer Res. 2003;9(9):3362–8.
Tan J, et al. TERT promoter mutation determines apoptotic and therapeutic responses of BRAF-mutant cancers to BRAF and MEK inhibitors: Achilles Heel. Proc Natl Acad Sci. 2020;117(27):15846–51.
CAS PubMed PubMed Central Google Scholar
Da Vià MC, et al. CIC mutation as a molecular mechanism of acquired resistance to combined BRAF-MEK inhibition in extramedullary multiple myeloma with central nervous system involvement. Oncologist. 2020;25(2):112–8.
PubMed Google Scholar
Verma IM, et al. Gene therapy: promises, problems and prospects. In: Genes and resistance to disease. Springer; 2000. p. 147–57.
Google Scholar
Cavazzana-Calvo M, Thrasher A, Mavilio F. The future of gene therapy. Nature. 2004;427(6977):779–81.
Verma IM, Weitzman MD. Gene therapy: twenty-first century medicine. Annu Rev Biochem. 2005;74:711–38.
Meyer F, Finer M. Gene therapy: progress and challenges. Cell Mol Biol. 2001;47(8):1277–94.
Maria BL, et al. Topical review: gene therapy for neurologic disease: benchtop discoveries to bedside applications. 2. The bedside. J Child Neurol. 1997;12(2):77–84.
Brown BD, Lillicrap D. Dangerous liaisons: the role of “danger” signals in the immune response to gene therapy. Blood J Am Soc Hematol. 2002;100(4):1133–40.
CAS Google Scholar
Herzog RW, Cao O, Srivastava A. Two decades of clinical gene therapy–success is finally mounting. Discov Med. 2010;9(45):105.
PubMed PubMed Central Google Scholar
Robinson A. Idecabtagene Vicleucel (Abecma ® ). Oncol Times. 2021;43(10):21.
Jaklevic MC. CAR-T therapy is approved for Non-Hodgkin Lymphoma. JAMA. 2021;325(11):1032–1032.
Reach T. FDA approves first oncolytic virus therapy: imlygic for melanoma. Oncol Times. 2015;37:36.
Philippidis A. Kymriah, First CAR-T Cancer immunotherapy approved by FDA. Mary Ann Liebert, Inc., NY; 2017
Prado DA, Acosta-Acero M, Maldonado RS. Gene therapy beyond luxturna: a new horizon of the treatment for inherited retinal disease. Curr Opin Ophthalmol. 2020;31(3):147–54.
Voelker R. CAR-T therapy is approved for mantle cell lymphoma. JAMA. 2020;324(9):832–832.
Papadouli I, et al. EMA review of Axicabtagene Ciloleucel (Yescarta) for the treatment of diffuse large B-cell lymphoma. Oncologist. 2020;25(10):894.
Nuijten M. Pricing Zolgensma–the world’s most expensive drug. J Mark Access Health Policy. 2022;10(1):2022353.
Ferreira GN, et al. Downstream processing of plasmid DNA for gene therapy and DNA vaccine applications. Trends Biotechnol. 2000;18(9):380–8.
Prather KJ, et al. Industrial scale production of plasmid DNA for vaccine and gene therapy: plasmid design, production, and purification. Enzym Microb Technol. 2003;33(7):865–83.
Redon S, Reichenbach P, Lingner J. The non-coding RNA TERRA is a natural ligand and direct inhibitor of human telomerase. Nucleic Acids Res. 2010;38(17):5797–806.
Wahlestedt C. Targeting long non-coding RNA to therapeutically upregulate gene expression. Nat Rev Drug Discov. 2013;12(6):433–46.
Naveed A, et al. NEAT1 polyA-modulating antisense oligonucleotides reveal opposing functions for both long non-coding RNA isoforms in neuroblastoma. Cell Mol Life Sci. 2021;78(5):2213–30.
McManus MT, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nat Rev Genet. 2002;3(10):737–47.
Zeng Y, Yi R, Cullen BR. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc Natl Acad Sci. 2003;100(17):9779–84.
Chakraborty C. Potentiality of small interfering RNAs (siRNA) as recent therapeutic targets for gene-silencing. Curr Drug Targets. 2007;8(3):469–82.
Taxman DJ, et al. Short hairpin RNA (shRNA): design, delivery, and assessment of gene knockdown. In: RNA therapeutics. Springer; 2010. p. 139–56.
Jiang F, Doudna JA. CRISPR–Cas9 structures and mechanisms. Annu Rev Biophys. 2017;46:505–29.
Ran FA, et al. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–308.
Fonfara I, et al. Phylogeny of Cas9 determines functional exchangeability of dual-RNA and Cas9 among orthologous type II CRISPR-Cas systems. Nucleic Acids Res. 2014;42(4):2577–90.
Amitai G, Sorek R. CRISPR–Cas adaptation: insights into the mechanism of action. Nat Rev Microbiol. 2016;14(2):67–76.
Weber T, et al. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat Biotechnol. 2015;33(5):543–8.
Bachu R, Bergareche I, Chasin LA. CRISPR-Cas targeted plasmid integration into mammalian cells via non-homologous end joining. Biotechnol Bioeng. 2015;112(10):2154–62.
Cho SW, et al. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 2014;24(1):132–41.
Maikova A, et al. Protospacer-adjacent motif specificity during clostridioides difficile type ib crispr-cas interference and adaptation. MBio. 2021;12(4):e02136-21.
Wan H, et al. Probing the behaviour of Cas1-Cas2 upon protospacer binding in CRISPR-Cas systems using molecular dynamics simulations. Sci Rep. 2019;9(1):1–16.
Huang TK, et al. Efficient gene targeting in Nicotiana tabacum using CRISPR/SaCas9 and temperature tolerant LbCas12a. Plant Biotechnol J. 2021;19:1314.
Haapaniemi E, et al. CRISPR–Cas9 genome editing induces a p53-mediated DNA damage response. Nat Med. 2018;24(7):927–30.
Khalaf K, et al. CRISPR/Cas9 in cancer immunotherapy: animal models and human clinical trials. Genes. 2020;11(8):921.
Carroll D. Genome engineering with zinc-finger nucleases. Genetics. 2011;188(4):773–82.
Urnov FD, et al. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11(9):636–46.
Holt N, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010;28(8):839–47.
Pipe SW. Gene therapy for hemophilia. Pediatr Blood Cancer. 2018;65(2):e26865.
Hoban MD, et al. Delivery of genome editing reagents to hematopoietic stem/progenitor cells. Curr Protoc Stem Cell Biol. 2016;36(1):4.1-4.10.
Bedell VM, et al. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012;491(7422):114–8.
Xia E, et al. TALEN-mediated gene targeting for cystic fibrosis-gene therapy. Genes. 2019;10(1):39.
Dunbar CE, et al. Gene therapy comes of age. Science. 2018;359(6372):eaan4672.
Gardlík R, et al. Vectors and delivery systems in gene therapy. Med Sci Monit. 2005;11(4):110–21.
Maguire CA, et al. Gene therapy for the nervous system: challenges and new strategies. Neurotherapeutics. 2014;11(4):817–39.
Lundstrom K. Viral vectors in gene therapy. Diseases. 2018;6(2):42.
Robbins PD, Ghivizzani SC. Viral vectors for gene therapy. Pharmacol Ther. 1998;80(1):35–47.
Yi Y, Hahm SH, Lee KH. Retroviral gene therapy: safety issues and possible solutions. Curr Gene Ther. 2005;5(1):25–35.
Yi Y, Jong Noh M, Hee Lee K. Current advances in retroviral gene therapy. Curr Gene Ther. 2011;11(3):218–28.
Takeuchi Y. Gene therapy using retrovirus vectors: vector development and biosafety at clinical trials. Uirusu. 2015;65(1):27–36.
Palu G, et al. Progress with retroviral gene vectors. Rev Med Virol. 2000;10(3):185–202.
Cannon PM, et al. Murine leukemia virus-based Tat-inducible long terminal repeat replacement vectors: a new system for anti-human immunodeficiency virus gene therapy. J Virol. 1996;70(11):8234–40.
Suerth JD, et al. Self-inactivating alpharetroviral vectors with a split-packaging design. J Virol. 2010;84(13):6626–35.
Maier P, Von Kalle C, Laufs S. Retroviral vectors for gene therapy. Future Microbiol. 2010;5(10):1507–23.
Onodera M, et al. Gene therapy for severe combined immunodeficiency caused by adenosine deaminase deficiency: improved retroviral vectors for clinical trials. Acta Haematol. 1999;101(2):89–96.
Enquist IB, et al. Effective cell and gene therapy in a murine model of Gaucher disease. Proc Natl Acad Sci. 2006;103(37):13819–24.
Herrera-Carrillo E, Berkhout B. Bone marrow gene therapy for HIV/AIDS. Viruses. 2015;7(7):3910–36.
Narayan O, Clements JE. Biology and pathogenesis of lentiviruses. J Gen Virol. 1989;70(7):1617–39.
Naldini L. Lentiviruses as gene transfer agents for delivery to non-dividing cells. Curr Opin Biotechnol. 1998;9(5):457–63.
Escors D, Breckpot K. Lentiviral vectors in gene therapy: their current status and future potential. Arch Immunol Ther Exp. 2010;58(2):107–19.
Smith JG, et al. Adenovirus. Cell Entry Non-Enveloped Viruses. 2010; 195–224.
Ghosh SS, Gopinath P, Ramesh A. Adenoviral vectors. Appl Biochem Biotechnol. 2006;133(1):9–29.
Hartman ZC, Appledorn DM, Amalfitano A. Adenovirus vector induced innate immune responses: impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res. 2008;132(1–2):1–14.
McConnell MJ, Imperiale MJ. Biology of adenovirus and its use as a vector for gene therapy. Hum Gene Ther. 2004;15(11):1022–33.
Berns KI, Bohenzky RA. Adeno-associated viruses: an update. Adv Virus Res. 1987;32:243–306.
Gao G, et al. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc Natl Acad Sci. 2003;100(10):6081–6.
Berns K, Giraud C. Biology of adeno-associated virus. In: Adeno-Associated Virus (AAV) vectors in gene therapy. Springer; 1996. p. 1–23.
Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21(4):583–93.
Whitley RJ, Kimberlin DW, Roizman B. Herpes simplex viruses. In: Clinical infectious diseases. JSTOR; 1998. p. 541–53.
Andreansky SS, et al. The application of genetically engineered herpes simplex viruses to the treatment of experimental brain tumors. Proc Natl Acad Sci. 1996;93(21):11313–8.
Aurelian L. Herpes simplex viruses. In: Clinical virology manual. 4th ed. American Society of Microbiology; 2009. p. 424–53.
Roizman B. The function of herpes simplex virus genes: a primer for genetic engineering of novel vectors. Proc Natl Acad Sci. 1996;93(21):11307–12.
Chiocca EA. Oncolytic viruses. Nat Rev Cancer. 2002;2(12):938.
Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14(9):642–62.
Lawler SE, et al. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 2017;3(6):841–9.
Woller N, et al. Oncolytic viruses as anticancer vaccines. Front Oncol. 2014;4:188.
Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15(4):651–9.
Trager MH, Geskin LJ, Saenger YM. Oncolytic viruses for the treatment of metastatic melanoma. Curr Treat Options Oncol. 2020;21(4):1–16.
Vähä-Koskela MJ, Heikkilä JE, Hinkkanen AE. Oncolytic viruses in cancer therapy. Cancer Lett. 2007;254(2):178–216.
Roth JA, Cristiano RJ. Gene therapy for cancer: what have we done and where are we going? J Natl Cancer Inst. 1997;89(1):21–39.
Cross D, Burmester JK. Gene therapy for cancer treatment: past, present and future. Clin Med Res. 2006;4(3):218–27.
Amer MH. Gene therapy for cancer: present status and future perspective. Mol Cell Ther. 2014;2(1):1–19.
El-Aneed A. Current strategies in cancer gene therapy. Eur J Pharmacol. 2004;498(1–3):1–8.
Hambleton S. Chickenpox. Curr Opin Infect Dis. 2005;18(3):235–40.
Hacein-Bey-Abina S, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302(5644):415–9.
Bellini WJ, Rota JS, Rota PA. Virology of measles virus. J Infect Dis. 1994;170(Supp 1):S15–23.
Blechacz B, Russell SJ. Measles virus as an oncolytic vector platform. Curr Gene Ther. 2008;8(3):162–75.
Russell SJ, Peng KW. Measles virus for cancer therapy. In: Measles. Springer; 2009. p. 213–41.
Ganar K, et al. Newcastle disease virus: current status and our understanding. Virus Res. 2014;184:71–81.
Zhao H, Peeters BP. Recombinant Newcastle disease virus as a viral vector: effect of genomic location of foreign gene on gene expression and virus replication. J Gen Virol. 2003;84(4):781–8.
Burman B, Pesci G, Zamarin D. Newcastle disease virus at the forefront of cancer immunotherapy. Cancers. 2020;12(12):3552.
Barenholz Y. Liposome application: problems and prospects. Curr Opin Colloid Interface Sci. 2001;6(1):66–77.
Caracciolo G, Amenitsch H. Cationic liposome/DNA complexes: from structure to interactions with cellular membranes. Eur Biophys J. 2012;41(10):815–29.
Stewart MJ, et al. Gene transfer in vivo with DNA–liposome complexes: safety and acute toxicity in mice. Hum Gene Ther. 1992;3(3):267–75.
Masotti A, et al. Comparison of different commercially available cationic liposome–DNA lipoplexes: parameters influencing toxicity and transfection efficiency. Colloids Surf B. 2009;68(2):136–44.
Tseng W-C, Huang L. Liposome-based gene therapy. Pharm Sci Technol Today. 1998;1(5):206–13.
Bendas G. Immunoliposomes. BioDrugs. 2001;15(4):215–24.
Paszko E, Senge M. Immunoliposomes. Curr Med Chem. 2012;19(31):5239–77.
Zhang X-X, McIntosh TJ, Grinstaff MW. Functional lipids and lipoplexes for improved gene delivery. Biochimie. 2012;94(1):42–58.
de Ilarduya CT, Sun Y, Düzgüneş N. Gene delivery by lipoplexes and polyplexes. Eur J Pharm Sci. 2010;40(3):159–70.
Jewell CM, Lynn DM. Surface-mediated delivery of DNA: cationic polymers take charge. Curr Opin Colloid Interface Sci. 2008;13(6):395–402.
Hwang S, Davis M. Cationic polymers for gene delivery: designs for overcoming barriers to systemic administration. Curr Opin Mol Ther. 2001;3(2):183–91.
Vermeulen LM, et al. The proton sponge hypothesis: fable or fact? Eur J Pharm Biopharm. 2018;129:184–90.
Boas U, Heegaard PM. Dendrimers in drug research. Chem Soc Rev. 2004;33(1):43–63.
Navarro G, DeILarduya CT. Activated and non-activated PAMAM dendrimers for gene delivery in vitro and in vivo. Nanomed Nanotechnol Biol Med. 2009;5(3):287–97.
Mohanraj V, Chen Y. Nanoparticles—a review. Trop J Pharm Res. 2006;5(1):561–73.
Tian H, Chen J, Chen X. Nanoparticles for gene delivery. Small. 2013;9(12):2034–44.
Pissuwan D, Niidome T, Cortie MB. The forthcoming applications of gold nanoparticles in drug and gene delivery systems. J Control Release. 2011;149(1):65–71.
Baban CK, et al. Bacteria as vectors for gene therapy of cancer. Bioengineered bugs. 2010;1(6):385–94.
Lin D, et al. Bacterial-based cancer therapy: an emerging toolbox for targeted drug/gene delivery. Biomaterials. 2021;277:121124.
Xiang S, Fruehauf J, Li CJ. Short hairpin RNA–expressing bacteria elicit RNA interference in mammals. Nat Biotechnol. 2006;24(6):697–702.
Bernardes N, Chakrabarty AM, Fialho AM. Engineering of bacterial strains and their products for cancer therapy. Appl Microbiol Biotechnol. 2013;97(12):5189–99.
Wang X, Zhang H, Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019;2(2):141.
Nikolaou M, et al. The challenge of drug resistance in cancer treatment: a current overview. Clin Exp Metas. 2018;35(4):309–18.
Aleksakhina SN, Kashyap A, Imyanitov EN. Mechanisms of acquired tumor drug resistance. Biochim Biophys Acta Rev Cancer. 2019;1872:188310.
Dominiak A, et al. Communication in the cancer microenvironment as a target for therapeutic interventions. Cancers. 2020;12(5):1232.
Asiry S, et al. The cancer cell dissemination machinery as an immunosuppressive niche: a new obstacle towards the era of cancer immunotherapy. Front Immunol. 2021;12:654877.
Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature. 2019;575(7782):299–309.
Wu D, et al. Roles of tumor heterogeneity in the development of drug resistance: a call for precision therapy. Seminars in cancer biology. Elsevier; 2017.
Wang Y, et al. Nucleolin-targeted extracellular vesicles as a versatile platform for biologics delivery to breast cancer. Theranostics. 2017;7(5):1360.
Yao C, et al. Reducible self-assembling cationic polypeptide-based micelles mediate co-delivery of doxorubicin and microRNA-34a for androgen-independent prostate cancer therapy. J Control Release. 2016;232:203–14.
Wilhelm S, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1(5):1–12.
Krzyszczyk P, et al. The growing role of precision and personalized medicine for cancer treatment. Technology. 2018;6:79–100.
Barthélémy F, Wein N. Personalized gene and cell therapy for Duchenne muscular dystrophy. Neuromuscul Disord. 2018;28(10):803–24.
Morash M, et al. The role of next-generation sequencing in precision medicine: a review of outcomes in oncology. J Personal Med. 2018;8(3):30.
Siena S, et al. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann Oncol. 2018;29(5):1108–19.
De Boeck K, Amaral MD. Progress in therapies for cystic fibrosis. Lancet Respir Med. 2016;4(8):662–74.
Ichikawa H, et al. Actionable gene-based classification toward precision medicine in gastric cancer. Genome Med. 2017;9(1):1–12.
Chatzopoulou F, et al. Dissecting miRNA–gene networks to map clinical utility roads of pharmacogenomics-guided therapeutic decisions in cardiovascular precision medicine. Cells. 2022;11(4):607.
Download references
The authors have not disclosed any funding.
Author information
Authors and affiliations.
Department of Medical Biotechnology, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran
Mohsen Danaeifar
You can also search for this author in PubMed Google Scholar
Contributions
M.D. wrote the main manuscript text, prepared figures and tables, edited the manuscript and reviewed it.
Corresponding author
Correspondence to Mohsen Danaeifar .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Conflict of interest
The author declares that there is no potential conflict of interest related to this research and publication.
Human or animal rights
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Danaeifar, M. Recent advances in gene therapy: genetic bullets to the root of the problem. Clin Exp Med 23 , 1107–1121 (2023). https://doi.org/10.1007/s10238-022-00925-x
Download citation
Received : 08 April 2022
Accepted : 14 October 2022
Published : 25 October 2022
Issue Date : August 2023
DOI : https://doi.org/10.1007/s10238-022-00925-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Gene therapy
- Gene editing
- Find a journal
- Publish with us
- Track your research
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Vaccines, Blood & Biologics
- Science & Research (Biologics)
Safety and Effectiveness of Gene Therapy

Andrew P. Byrnes, Ph.D.
Office of Tissues and Advanced Therapies Division of Cellular and Gene Therapies Gene Transfer and Immunogenicity Branch
Chief, Gene Transfer and Immunogenicity Branch
BS, MS, Yale University, Department of Molecular Biophysics and Biochemistry
PhD, Oxford University, Department of Human Anatomy
Postdoctoral Fellow, Johns Hopkins University, School of Hygiene and Public Health
General Overview
Gene therapy holds great promise for treating cancer, inherited disorders, and other diseases. Gene therapy uses carriers called 'vectors' to deliver genes to tissues where they are needed. Researchers are currently investigating the safety and effectiveness of a variety of different gene therapy vectors in hundreds of clinical trials in the US.
We are studying one type of commonly-used gene therapy vector that is made from a disabled cold virus -- the adenovirus vector. While adenovirus vectors are very efficient at delivering genes, adenovirus vectors are not always easy to target to the correct tissue. In addition, adenovirus vectors can cause toxic effects that limit the amount of vector that doctors can give to patients. We are particularly interested in how to safely deliver large amounts of adenovirus vectors intravenously, with the goal of specifically targeting tumors and other tissues.
New adenovirus gene therapy vectors are tested in animals before human clinical trials begin, and it is important for both researchers and the FDA to know how well these animal studies can predict safety. Thus, another of our major goals is to develop animal models that reliably predict the safety and effectiveness of adenovirus vectors in humans.
Our studies will help us to understand the mechanisms for adenovirus vector targeting and toxicity, and the relevance of animal models to human outcomes. This new knowledge will enable researchers to design safer and more effective gene therapy vectors.
Scientific Overview
Adenovirus (Ad) vectors have shown considerable promise in animal models and are currently being used in numerous clinical trials, especially for the therapy of cancer. We are interested in improving the safety and efficacy of Ad vectors, especially when administered through the vascular system. Certain properties of Ad vectors make them hazardous to administer intravenously in large doses, and our laboratory is trying to understand and fix this problem.
One of our major areas of interest is the innate immune response to Ad vectors. These rapid responses can cause serious toxicity and may severely limit the doses of Ad vectors that are safe to use. In addition, we are also studying how cells in the liver such as Kupffer cells and hepatocytes recognize Ad, since the liver is the major site at which Ad vectors are cleared from the circulation. A better understanding of these mechanisms will help us to develop strategies to improve vector efficacy and reduce toxicity. We will also gain a better understanding of the advantages and disadvantages of using different animal species to predict the behavior of Ad vectors in humans, which is particularly relevant to the regulatory work of the FDA.
Recent work from our lab and others has shown that Ad vectors are heavily influenced by plasma proteins that rapidly opsonize the vectors after intravenous injection. We found that natural IgM antibodies bind to Ad vectors, activate complement, and reduce liver transduction. Intriguingly, the Ad hexon protein specifically binds to coagulation factor X (FX), and we found that recruitment of FX by Ad vectors protects them against neutralization by complement. These findings show that Ad vectors recruit a number of plasma proteins that interact in complex ways with each other and with cells, and that these host proteins ultimately help to determine whether the vector successfully reaches its target.
In the long run, a better fundamental understanding of Ad vector biology will facilitate the design of safer Ad vectors that are easier to target. Better animal models will be important for testing novel vectors for safety and efficacy.
Publications
- PLoS Pathog 2022 Sep 26;18(9):e1010859 Binding of adenovirus species C hexon to prothrombin and the influence of hexon on vector properties in vitro and in vivo. Tian J, Xu Z, Moitra R, Palmer DJ, Ng P, Byrnes AP
- FEBS Lett 2019 Dec;593(24):3449-60 Interaction of adenovirus with antibodies, complement and coagulation factors. Allen RJ, Byrnes AP
- PLoS One 2018 Feb 5;13(2):e0192353 Hexons from adenovirus serotypes 5 and 48 differentially protect adenovirus vectors from neutralization by mouse and human serum. Harmon AW, Moitra R, Xu Z, Byrnes AP
- Methods Mol Biol 2017;1643:187-96 Evaluating the impact of natural IgM on adenovirus Type 5 gene therapy vectors. Xu Z, Tian J, Harmon AW, Byrnes AP
- J Control Release 2016 Aug 10;235:379-92 Substitution of blood coagulation factor X-binding to Ad5 by position-specific PEGylation: Ppeventing vector clearance and preserving infectivity. Krutzke L, Prill JM, Engler T, Schmidt CQ, Xu Z, Byrnes AP, Simmet T, Kreppel F
- J Virol 2015 Mar;89(6):3412-6 Impact of natural IgM concentration on gene therapy with adenovirus type 5 vectors. Qiu Q, Xu Z, Tian J, Moitra R, Gunti S, Notkins AL, Byrnes AP

Study at Cambridge
About the university, research at cambridge.
- For Cambridge students
- For our researchers
- Business and enterprise
- Colleges and Departments
- Email and phone search
- Give to Cambridge
- Museums and collections
- Events and open days
- Fees and finance
- Postgraduate courses
- How to apply
- Fees and funding
- Postgraduate events
- International students
- Continuing education
- Executive and professional education
- Courses in education
- How the University and Colleges work
- Visiting the University
- Annual reports
- Equality and diversity
- A global university
- Public engagement
Baby born deaf can hear after breakthrough gene therapy
- Research home
- About research overview
- Animal research overview
- Overseeing animal research overview
- The Animal Welfare and Ethical Review Body
- Animal welfare and ethics
- Report on the allegations and matters raised in the BUAV report
- What types of animal do we use? overview
- Guinea pigs
- Equine species
- Naked mole-rats
- Non-human primates (marmosets)
- Other birds
- Non-technical summaries
- Animal Welfare Policy
- Alternatives to animal use
- Further information
- Funding Agency Committee Members
- Research integrity
- Horizons magazine
- Strategic Initiatives & Networks
- Nobel Prize
- Interdisciplinary Research Centres
- Open access
- Energy sector partnerships
- Podcasts overview
- S2 ep1: What is the future?
- S2 ep2: What did the future look like in the past?
- S2 ep3: What is the future of wellbeing?
- S2 ep4 What would a more just future look like?
- Research impact

A baby girl born deaf can hear unaided for the first time, after receiving gene therapy when she was 11 months old at Addenbrooke’s Hospital in Cambridge.
Gene therapy has been the future of otology and audiology for many years and I’m so excited that it is now finally here Manohar Bance
Opal Sandy from Oxfordshire is the first patient treated in a global gene therapy trial, which shows 'mind-blowing' results. She is the first British patient in the world and the youngest child to receive this type of treatment.
Opal was born completely deaf because of a rare genetic condition, auditory neuropathy, caused by the disruption of nerve impulses travelling from the inner ear to the brain.
Within four weeks of having the gene therapy infusion to her right ear, Opal responded to sound, even with the cochlear implant in her left ear switched off.
Clinicians noticed continuous improvement in Opal’s hearing in the weeks afterwards. At 24 weeks, they confirmed Opal had close to normal hearing levels for soft sounds, such as whispering, in her treated ear.
Now 18 months old, Opal can respond to her parents’ voices and can communicate words such as “Dada” and “bye-bye.”
Opal’s mother, Jo Sandy, said: “When Opal could first hear us clapping unaided it was mind-blowing - we were so happy when the clinical team confirmed at 24 weeks that her hearing was also picking up softer sounds and speech. The phrase ‘near normal’ hearing was used and everyone was so excited such amazing results had been achieved.”
Auditory neuropathy can be due to a variation in a single gene, known as the OTOF gene. The gene produces a protein called otoferlin, needed to allow the inner hair cells in the ear to communicate with the hearing nerve. Approximately 20,000 people across the UK, Germany, France, Spain, Italy and UK and are deaf due to a mutation in the OTOF gene.
The CHORD trial, which started in May 2023, aims to show whether gene therapy can provide hearing for children born with auditory neuropathy.
Professor Manohar Bance from the Department of Clinical Neurosciences at the University of Cambridge and an ear surgeon at Cambridge University Hospitals NHS Foundation Trust is chief investigator of the trial. He said:
“These results are spectacular and better than I expected. Gene therapy has been the future of otology and audiology for many years and I’m so excited that it is now finally here. This is hopefully the start of a new era for gene therapies for the inner ear and many types of hearing loss.”
Children with a variation in the OTOF gene often pass the newborn screening, as the hair cells are working, but they are not talking to the nerve. It means this hearing loss is not commonly detected until children are 2 or 3 years of age – when a delay in speech is likely to be noticed.
Professor Bance added: “We have a short time frame to intervene because of the rapid pace of brain development at this age. Delays in the diagnosis can also cause confusion for families as the many reasons for delayed speech and late intervention can impact a children’s development.”
“More than sixty years after the cochlear implant was first invented – the standard of care treatment for patients with OTOF related hearing loss – this trial shows gene therapy could provide a future alternative. It marks a new era in the treatment for deafness. It also supports the development of other gene therapies that may prove to make a difference in other genetic related hearing conditions, many of which are more common than auditory neuropathy.”
Mutations in the OTOF gene can be identified by standard NHS genetic testing. Opal was identified as being at risk as her older sister has the condition; this was confirmed by genetic test result when she was 3 weeks old.
Opal was given an infusion containing a harmless virus (AAV1). It delivers a working copy of the OTOF gene and is delivered via an injection in the cochlea during surgery under general anaesthesia. During surgery, while Opal was given the gene therapy in right ear, a cochlear implant was fitted in her left ear.
James Sandy, Opal’s father said: “It was our ultimate goal for Opal to hear all the speech sounds. It’s already making a difference to our day-to-day lives, like at bath-time or swimming, when Opal can’t wear her cochlear implant. We feel so proud to have contributed to such pivotal findings, which will hopefully help other children like Opal and their families in the future.”
Opal’s 24-week results, alongside other scientific data from the CHORD trial are being presented at the American Society of Gene and Cell Therapy (ASGC) in Baltimore, USA this week.
Dr Richard Brown, Consultant Paediatrician at CUH, who is an Investigator on the CHORD trial, said: “The development of genomic medicine and alternative treatments is vital for patients worldwide, and increasingly offers hope to children with previously incurable disorders. It is likely that in the long run such treatments require less follow up so may prove to be an attractive option, including within the developing world. Follow up appointments have shown effective results so far with no adverse reactions and it is exciting to see the results to date.
“Within the new planned Cambridge Children’s Hospital, we look forward to having a genomic centre of excellence which will support patients from across the region to access the testing they need, and the best treatment, at the right time.”
The CHORD trial has been funded by Regeneron. Patients are being enrolled in the study in the US, UK and Spain.
Patients in the first phase of the study receive a low dose to one ear. The second phase are expected to use a higher dose of gene therapy in one ear only, following proven safety of the starting dose. The third phase will look at gene therapy in both ears with the dose selected after ensuring the safety and effectiveness in parts 1 and 2. Follow up appointments will continue for five years for enrolled patients, which will show how patients adapt to understand speech in the longer term.
In Cambridge, the trial is supported by NIHR Cambridge Clinical Research Facility and NIHR Cambridge Biomedical Research Centre.
Adapted from a press release from CUH


Read this next

Study unpicks why childhood maltreatment continues to impact on mental and physical health into adulthood

New Cambridge-developed resources help students learn how maths can help tackle infectious diseases

School uniform policies linked to students getting less exercise, study finds

The Vice-Chancellor's Awards 2023 for Research Impact and Engagement
Media enquiries.
Baby Opal and mother Jo
Credit: Cambridge University Hospitals NHS Foundation Trust
Search research
Sign up to receive our weekly research email.
Our selection of the week's biggest Cambridge research news sent directly to your inbox. Enter your email address, confirm you're happy to receive our emails and then select 'Subscribe'.
I wish to receive a weekly Cambridge research news summary by email.
The University of Cambridge will use your email address to send you our weekly research news email. We are committed to protecting your personal information and being transparent about what information we hold. Please read our email privacy notice for details.
- Future therapeutics
- Manohar Bance
- School of Clinical Medicine
- Department of Clinical Neurosciences
- Cambridge University Hospitals NHS Foundation Trust
- Addenbrooke's Hospital
Related organisations
- NIHR Cambridge Biomedical Research Centre
Connect with us

© 2024 University of Cambridge
- Contact the University
- Accessibility statement
- Freedom of information
- Privacy policy and cookies
- Statement on Modern Slavery
- Terms and conditions
- University A-Z
- Undergraduate
- Postgraduate
- Cambridge University Press & Assessment
- Research news
- About research at Cambridge
- Spotlight on...
Eight imperatives for launching cell and gene therapies
Recent launches of new cell and gene therapies (CGTs) have yielded mixed results. In some cases, patient outcomes have been promising, with high rates of therapeutic success and transformed lives. In others, the desired therapeutic gains have been undercut by the difficulty of getting the right therapy to the right patients.
Few CGTs have reached the market over the past decade. However, judging from the pipeline of products in Phase III clinical trials, the number of approvals is likely to rise dramatically in the near future. In 2024 alone, up to 21 cell therapy launches and as many as 31 gene therapy launches—including more than 29 adeno-associated virus (AAV) therapies—are expected (Exhibit 1). 1 Evaluate Pharma August 2022 data, Evaluate Ltd.; US Food and Drug Administration.
Companies have worked hard to develop these exciting new products. 2 Emily Capra, Jeff Smith, and Guang Yang, “ Gene therapy coming of age: Opportunities and challenges to getting ahead ,” McKinsey, October 2, 2019. It behooves them to launch those products effectively so that the therapies can benefit as many patients as possible while ensuring a sufficient return on manufacturers’ investments—thereby encouraging continued research and development in CGT.
CGTs face steeper challenges at launch than traditional drugs do, potentially limiting their adoption and thus their potential to transform patients’ lives. In an environment in which the population of prospective patients for these therapies is small—and where patients frequently switch payers (at least in the United States)—the current payer system is not well suited to accommodate single-dose therapies for which long-term treatment efficacy, risk–benefit ratios, and safety remain uncertain.
Moreover, CGT patients face a highly complex and costly path to treatment, including long trips to widely spaced healthcare sites and frequent genetic testing and counseling. For healthcare providers, finding and training personnel at new sites requires significant investments of time and the development of new relationships with clinicians and administrators. Furthermore, payers can be reluctant to take on the increased financial risk inherent in treatments with higher one-time costs. Finally, companies themselves face considerable supply chain, manufacturing, and distribution challenges in the effort to make sure just-in-time doses are available when and where they are needed.
Would you like to learn more about our Life Sciences Practice ?
To overcome these obstacles, companies planning for future launches must rethink their go-to-market models, moving away from the models they’ve long used for traditional drug launches. This shift requires adequate preparation to ensure that potential patients, providers, and payers are in place; payment and risk-sharing mechanisms between pharmaceutical companies and providers have been established; and the therapy itself is readily available.
Drawing on our work across several recent CGT launches, as well as on interviews with experts and recent roundtables with experienced launchers, we have identified eight priorities that can significantly improve the likelihood that CGT launches will succeed. These priorities fall into three overarching categories: preparing the market, the product, and the company itself (Exhibit 2).
Preparing the market
The sheer novelty of CGTs means that companies cannot rely on past methods of launching new drugs. Instead, they should prepare patients, caregivers, payers, and healthcare systems for the complexity of new CGTs.
1. Identify appropriate sites of care
Traditional drug launches typically follow a prescriber-based approach, with treatment decisions for prescriptions made in a variety of local and community settings. Given the complexity and preparation needed for the typical CGT, the go-to-market model should shift to centralized sites of care—regional, specialized centers with the ability to make treatment recommendations to the right patients and to scale up CGT delivery to them.
Patients living within 60 miles of sites offering gene therapy are more than twice as likely to receive therapy, according to McKinsey analysis of data from Compile, a data provider for the healthcare industry. Locations should thus be carefully chosen to maximize patient concentration. Ensuring complete care models with clear roles and standardized practices can help sites manage the burden of preparing patients and providing care.
A well-functioning site-of-care model depends on three factors: site willingness, capabilities, and scalability. The first is a function of the attitude of site leaders (including both physicians and administrators) toward CGT and their perspectives on local demand for CGT. The second requires drug companies to carefully track the ability of prospective sites to run their first patients through its system; simple checklists for the infrastructure needed to provide therapy will allow companies to quickly determine a site’s suitability. The third requires that prospective sites have the capacity to meet patient and caregiver demand for CGTs (for more on these requirements, see sidebar “Equipping the front lines”).
Equipping the front lines
Site identification, certification , and preparation can take longer for cell and gene therapies (CGTs) than for traditional drugs, in part because these advanced treatments are a first for many potential locations. A large global pharma company recently engaged us to develop a site network strategy for an allogenic cell therapy with a short shelf life. A review of ten CGT launches helped us align on several site selection criteria. These included research and clinical experience, the ability to meet certification requirements, and familiarity with out-of-state Medicaid requirements (given the need for some patients to cross state lines to access care). With these filters, we identified a discrete set of potential treatment centers.
The CGT launches used for comparison also helped inform the sequence and timing of site preparation. After adapting to our unique launch context, we were able to estimate various time frames for the launch: approximately 12 months for site activation planning, six months for protocol review, three months for provider training dry runs, and three months for billing readiness. The outcome of our project was a comprehensive site network plan that included approximately 30 initial target sites, 30 expansion sites, and a clear road map for site certification and readiness.
2. Support patients and caregivers
Providing CGTs to patients typically requires a highly complex, often multiyear journey, so it is essential that both patients and their caregivers understand the process through well-designed patient services and associated infrastructure. Rather than offering standard information hotlines and patient-facing websites, some companies are providing personalized nurse educator programs and regular one-to-one patient outreach along with documentation to monitor the progress of each therapy and to ensure routine follow-up care. To support patients and caregivers with logistics and travel, some companies are now offering travel concierge services with full reimbursement of costs.
Identifying patients who would benefit most from CGT is also important given that CGTs are typically designed for rare diseases. Real-world evidence (RWE) collected both prior to and after launch—including insurance claims, lab data, diagnostic codes, and claims for other medications that potential patients are taking—can help providers discover likely patients and optimize their care. For example, by using natural-language processing and key words from electronic medical records and insurance claims, companies can identify pockets of potential patients and tailor physician outreach accordingly.
In general, each CGT patient has a unique set of needs. Some will require a full suite of services, while others may need only limited support, as with conventional therapies. The key lies in understanding each patient’s individual needs and delivering personalized support.
Each CGT patient has a unique set of needs. The key lies in understanding each patient’s individual needs and delivering personalized support.
3. Offer innovative payment structures
To enable access to and reimbursement for CGTs, companies are experimenting with novel outcomes-based pricing models. These models include the following:
- Outcomes-based payments. The payer covers a fraction of the full price up front and pays the remainder if the therapy achieves prespecified outcomes. One cell therapy company explored this method in the United States, arranging for payment only if a response was achieved after 30 days.
- Outcomes-based rebates. The payer pays the full price of the drug up front but receives a rebate if the drug fails to achieve prespecified outcomes within a predefined period. A US company providing a new gene therapy offered outcomes-based rebates to payers based on both short-term efficacy (30 to 90 days) and long-term durability (30 months).
- Outcomes-based annuity. The payer pays a fixed price, with payments spread over many installments, but only if the drug continues to meet certain prespecified outcomes. A US company launching a new gene therapy used this model to arrange outcomes-based payments for up to five years.

Gene therapy coming of age: Opportunities and challenges to getting ahead
Companies should consider engaging early with payers and sites of care to design the most suitable payment model. Enabling outcomes-based models usually requires involving intermediaries, such as pharmaceutical distributors, that can act as a risk-sharing vehicle, as well as writing a complex set of contracts. In the case of one gene therapy launch, the market access team began holding monthly meetings with payers three years before approval to educate them on the disease and the therapy. By engaging early with payers and providers on payment models, companies can get a head start on assessing the cost–benefit ratio of the CGT they plan to launch.
Preparing the product
A successful CGT launch will also depend on providing full support for the therapy itself by offering evidence-based information to providers and payers and delivering adequate supplies of the therapy.
4. Demonstrate long-term outcomes
RWE can address a range of unique challenges faced by CGT stakeholders. For instance, it can help provide payers with early visibility into the total health system costs related to a particular disease. This is especially important for CGTs because the disease burden is often not quantified for rare subpopulations indicated for CGT. Suitable price anchors and comparisons also may not exist due to a poor or missing standard of care. Some companies are using RWE to quantify the impact of specific diseases across mortality, morbidity, and financial measures, as well as to track long-term outcomes of patients using their therapy, thereby providing payers with the data needed to engage in outcomes-based contracts. Tactically, this can be achieved by identifying the drivers of each outcome measure and then using machine learning to extract the causal relationships between the disease and outcomes.
5. Optimize the supply chain
Given the uncertainties of the CGT supply chain, manufacturing capacity 3 Emily Capra, Andrea Gennari, Alberto Loche, and Carolin Temps, “ Viral-vector therapies at scale: Today’s challenges and future opportunities ,” McKinsey, March 29, 2022. should be matched carefully with demand so that patient-specific doses are delivered just in time to sites of care. To do so, companies should carry out dynamic, scenario-based demand forecasting, beginning as early as three years before the actual launch. If done well, a digital supply chain thread can provide track-and-trace data on critical information (such as the location and quality of cells extracted from patients for autologous cell therapies), demonstrate outcomes, provide timely information regarding when caregivers should bring patients in for treatment, and help automate key steps along the value chain.
Preparing the company
Companies should consider preparing themselves for successful CGT launches, reconsidering their go-to-market models across all potential markets, and updating their organizational models for efficiency and scale.
6. Rethink the go-to-market model
A team effort.
We recently supported an allogeneic cell therapy company in its effort to define its go-to-market model for a therapy that was indicated for an extremely rare disease found across a fragmented provider landscape. Success required seamless coordination across therapy education, cell selection, and reimbursement support. The process leading up to providing the therapy also required providers to transmit patient-specific information so the company could identify the exact version of the therapy for each patient.
Given this complexity, a traditional pharmaceutical field force would likely fall short. To design a model that met the unique needs of the product, we worked together to outline the activities required at each step of the patient, provider, and cell journeys. From there, we landed on priority roles, including a cell selection team, account executives, case managers, and a medical-information team. Governed by well-defined collaboration rules, these teams met the needs of all the stakeholders—patients, providers, and payers—while successfully protecting the personal information of each patient.
Across the patient journey, patients and caregivers face a more complex path with CGT than with traditional therapies. Companies looking to fully support patients along the journey will need to create new CGT-specific roles. For example, appointing CGT account coordinators who can serve as single representatives to site leaders can help build the necessary business relationships between the company and CGT site physicians and administrators. CGT coordinators can also ensure proper synchronization between apheresis—when the cells are extracted from patients—and when the cells are reinfused during therapy. Network coordinators can work with hospital networks and manage referrals to facilitate patient and data flow from one site of care to another as patients move. Across all field roles, tight cross-functional coordination is required to streamline the experience for patients, caregivers, and sites (see sidebar “A team effort”).
7. Enable and promote access across regions
When preparing to launch CGT internationally, companies should consider several areas. First, they need to prioritize markets carefully, considering several factors: the number of potential patients, how patients are treated, how treatments are reimbursed, and whether the infrastructure exists to provide care. This can include the availability of sites equipped to provide therapies and the sophistication of the cold chain for transport of therapies.
Second, companies should determine which model works best for which country: going direct, entering via a partnership, using licensing agreements, or some other arrangement. The model should consider the potential for clustering countries together and the dynamics of extraregional hubs. A regional headquarters with at-scale medical and commercial resources in Singapore, for example, could treat patients in other markets who can travel for treatment. Providers in neighboring countries, such as Malaysia and Thailand, could be supported remotely from the regional headquarters through education or virtual visits from sales representatives.
8. Reorganize for effectiveness and scale
Companies looking to meet the unique needs of CGT patients, caregivers, and payers at scale will likely have to rethink their operating models for maximum efficiency. When commercializing CGT within a larger established business, companies can choose between several models. At one end of the spectrum, companies can create an independent CGT unit for all relevant commercial functions across all markets; at the other end, they can fully integrate the CGT business into the central footprint, with dedicated CGT resources within each function.
Merging capabilities
Larger pharmaceutical companies are increasingly acquiring new cell and gene therapy (CGT) companies. We recently helped a global pharmaceutical company evolve its operating model after it acquired a gene therapy company with multiple products in development. The combined company faced the challenge of building a commercial model that was tailored to the unique needs of gene therapy while also leveraging the existing scale of the parent organization and presenting a unified voice to external stakeholders, including payers and regulators.
We started by defining the capabilities required to support the successful commercialization of CGT. From this foundation, we collectively designed an operating model that considered three key factors:
- Structure . A combined global and in-market organizational structure clarified which functions should be dedicated to gene therapies, including patient access and account support, and which could be shared with the parent company, such as market insights.
- Responsibilities . This includes identifying and adjusting the responsibilities of key groups in the core gene therapy functions. Setting the price of new therapies, for example, required a clear division of roles between the global pricing team and the in-market pricing team of the parent company and of the CGT.
- Interfaces . Solutions were designed to overcome potential friction points—such as the need to protect the resources of CGT-dedicated teams that report into parent company teams, as well as how to facilitate knowledge sharing between CGT and non-CGT groups.
The result was an aligned, fit-for-purpose model that supported the delivery of unique gene therapy capabilities and made strategic use of the parent company’s existing commercial footprint.
Companies that have recently acquired CGT assets have chosen a variety of models depending on circumstances. Some prefer not to integrate the new CGT asset at all to avoid disrupting ongoing efforts to launch it. If integrating the new asset—given that specialized CGT therapies often need distinct capabilities—many prefer to keep specific commercial functions (such as patient engagement, site operations, and market access) independent. In almost all examples, the model evolves over time, and learnings from CGT launches are integrated into parent organizations (see sidebar “Merging capabilities”).
With so many new CGTs in the pipeline and poised for commercialization, the imperative to enable their benefits as widely as possible is as urgent as ever. Successful launches are key to maximizing the benefits of CGTs for patients, for healthcare providers, and for the companies that develop and distribute these life-changing therapies. Ensuring that the market, the product, and the company itself are fully prepared for an effective launch will enable success. The time to resolve the challenges of commercializing CGT therapies is now.
Simon Alfano and Alex Gorham are associate partners in McKinsey’s Boston office, Alberto Loche is an associate partner in the Zurich office, and Pablo Salazar is a partner in the Stamford office.
The authors wish to thank Emily Capra, Yingkun Hou, Eric Koskins, Nils Peters, Arnold Scaglione, Jeff Smith, Michelle Suhendra, Lieven Van der Veken, and Guang Yang for their contributions to this article.
Explore a career with us
Related articles.

How could gene therapy change healthcare in the next ten years?

A call to action: Opportunities and challenges for CGTs in Europe
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- RESEARCH HIGHLIGHT
- 09 May 2024
CRISPR therapy restores some vision to people with blindness
The light-absorbing rods and cones in the retina (artificially coloured) deteriorate in people with Leber’s congenital amaurosis. Credit: Science Photo Library
A CRISPR-based gene-editing therapy led to improved vision in people with an inherited condition that causes blindness 1 .
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
doi: https://doi.org/10.1038/d41586-024-01285-0
Pierce, E. A. et al. N. Engl. J. Med . https://doi.org/10.1056/NEJMoa2309915 (2024).
Article Google Scholar
Download references
- Gene therapy

Targeting RNA opens therapeutic avenues for Timothy syndrome
News & Views 24 APR 24
Improving prime editing with an endogenous small RNA-binding protein
Article 03 APR 24
Stealthy stem cells to treat disease
Spotlight 28 FEB 24
Clinician Researcher/Group Leader in Cancer Cell Therapies
An excellent opportunity is available for a Group Leader with expertise in cellular therapies to join the Cancer Research program at QIMR Berghofer.
Herston, Brisbane (AU)
QIMR Berghofer
Faculty Positions at the Center for Machine Learning Research (CMLR), Peking University
CMLR's goal is to advance machine learning-related research across a wide range of disciplines.
Beijing, China
Center for Machine Learning Research (CMLR), Peking University
Faculty Positions at SUSTech Department of Biomedical Engineering
We seek outstanding applicants for full-time tenure-track/tenured faculty positions. Positions are available for both junior and senior-level.
Shenzhen, Guangdong, China
Southern University of Science and Technology (Biomedical Engineering)
Southeast University Future Technology Institute Recruitment Notice
Professor openings in mechanical engineering, control science and engineering, and integrating emerging interdisciplinary majors
Nanjing, Jiangsu (CN)
Southeast University
Staff Scientist
A Staff Scientist position is available in the laboratory of Drs. Elliot and Glassberg to study translational aspects of lung injury, repair and fibro
Maywood, Illinois
Loyola University Chicago - Department of Medicine
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
VIDEO
COMMENTS
Gene therapy is understood as the capacity for gene improvement by means of the correction of altered (mutated) genes or site-specific modifications that have therapeutic treatment as target. ... Currently, gene therapy is an area that exists predominantly in research laboratories, and its application is still experimental. Most trials are ...
Oncorine (rAd5-H101) It is the first replicative, oncolytic recombinant ad5 (rAd5-H101) approved to treat refractory nasopharyngeal cancer. Loss of p53 gene linked with drug resistance and survival rate reduction in non-small cell cancer patients. 50 Oncorine is an ad5 virus with a deletion in the E1B 55K gene.
Gene Therapy: A Comprehensive Review. Santosh R Patil 1), Ibrahim A. Al-Zoubi2), Raghuram PH 3), Neeta Misra 4), Nidhi Y adav 5), Mohammad Khursheed Alam6) ABSTRACT. Background: As gene therapy is ...
Metrics. Gene therapy is at an inflection point. Recent successes in genetic medicine have paved the path for a broader second wave of therapies and laid the foundation for next-generation ...
Twenty-four of these papers related specifically to cell therapy while 11 pertained to gene therapy. ... Studies involving the public generally reported support for cell and gene therapy research ...
At the forefront of medicine, Gene Therapy brings you the latest research into genetic and cell-based technologies to treat disease. It also publishes reviews and articles, which highlight the ...
Tragic Setbacks for Gene Therapy. Jesse Gelsinger, an 18-year-old with a mild form of the genetic disease ornithine transcarbamylase (OTC) deficiency, participated in a clinical trial which delivered a non-mutated OTC gene to the liver through a hepatic artery injection of the recombinant adenoviral vector housing the therapeutic gene.
Valoctocogene roxaparvovec (AAV5-hFVIII-SQ) is an AAV5-based gene-therapy vector that expresses a B-domain-deleted human factor VIII coding sequence from a hepatocyte-selective promoter. 8-10 ...
Gene therapy is the product of man's quest to eliminate diseases. Gene therapy has three facets namely, gene silencing using siRNA, shRNA and miRNA, gene replacement where the desired gene in the form of plasmids and viral vectors, are directly administered and finally gene editing based therapy where mutations are modified using specific nucleases such as zinc-finger nucleases (ZFNs ...
Interview with Dr. Katherine A. High on gene therapy for genetic disease. 15m 22s Download. Gene therapy has provided treatment options for diseases that are beyond the reach of traditional ...
Human Gene Therapy (HGT) is the premier, multidisciplinary journal covering all aspects of gene therapy. The Journal publishes important advances in DNA, RNA, cell and immune therapies, validating the latest advances in research and new technologies. Established in 1990, HGT provides a prestigious forum for publishing scientific and clinical ...
Read the latest Research articles from Gene Therapy. ... Paper (516) Research Article (897) Short Communication (214) Technical Note (1) Techniques (1) Viral Transfer Technology (80)
In this paper, we survey the current progress of gene therapy treatments for several diseases and explore machine learning applications in gene therapy. ... Machine learning and gene therapy are both topics gaining popularity in various publications, and we conclude that there is still room for continued research and application of machine ...
The following factors are taken into account when selecting a disease to be cured with gene therapy: disabling disorders that affect more than one percent of the population, lack of an effective treatment, and the high cost of current treatments [].The National Institutes of Health carried out the first successful gene transfer into the nucleus of a human cell in 1989 [].
Strategies of gene therapy. Initially, gene therapy mainly referred to the introduction of an exogenous copy of complementary DNA (cDNA) into targeted tissues or cells to correct or compensate for defective endogenous genes [].With the advance in recognition and technology, gene therapy has shifted to focus on manipulating the expression of a certain gene [] or modifying the genetic ...
Gene therapy aims to modify or manipulate gene expression or to alter the biological properties of living cells for therapeutic use, an innovative medical approach that currently sits at an inflection point (1, 2).After the first gene therapies developed in the 1990s, research and development (R&D) remained stagnant until the mid-2010s when the convergence of next-generation technologies ...
Abstract. Gene therapy is a contemporary therapeutic intervention with recent positive results and regulatory approvals either completed or expected in the next several years for various ...
M., et al. "Review article on gene therapy." Research Journal of . Pharmacology and Pharm acodynamics 4.2 (20 12): 77-83. ... Gene therapy, considered as treating genetically-caused diseases by ...
Gene therapy involves the introduction of new genes into cells, to restore or add gene expression, for the purpose of treating disease. Most commonly a mutated gene is replaced with DNA encoding a ...
Introduction. Gene therapy is a therapeutic strategy using genetic engineering techniques to treat various diseases. 1, 2) In the early 1960s, gene therapy first progressed with the development of recombinant DNA (rDNA) technology, 1) and was further developed using various genetic engineering tools, such as viral vectors. 3 - 5) More than ...
Gene therapy holds great promise for treating cancer, inherited disorders, and other diseases. Gene therapy uses carriers called 'vectors' to deliver genes to tissues where they are needed.
Opal was born completely deaf because of a rare genetic condition, auditory neuropathy, caused by the disruption of nerve impulses travelling from the inner ear to the brain. Within four weeks of having the gene therapy infusion to her right ear, Opal responded to sound, even with the cochlear implant in her left ear switched off.
Gene Therapy (Gene Ther) ISSN 1476-5462 (online) ISSN 0969-7128 (print) nature.com sitemap. About Nature Portfolio ... Research data; Language editing; Scientific editing; Nature Masterclasses;
These included research and clinical experience, the ability to meet certification requirements, and familiarity with out-of-state Medicaid requirements (given the need for some patients to cross state lines to access care). ... A US company providing a new gene therapy offered outcomes-based rebates to payers based on both short-term efficacy ...
Twenty-four of these papers related specifically to cell therapy while 11 pertained to gene therapy. Five of the included studies were conducted in the UK 12 - 16 , eight in the USA 17 - 24 , six in Canada 25 - 30 , two were conducted in Australia 31 , 32 and one each from Belgium 33 , China 34 , Germany 35 , Hungary 36 , Ireland 37 ...
Abstract. The discovery of the Cystic fibrosis (CF) gene in 1989 has paved the way for incredible progress in treating the disease such that the mean survival age of individuals living with CF is now ~58 years in Canada. Recent developments in gene targeting tools and new cell and animal models have re-ignited the search for a permanent genetic ...
Tom Edwards (left), from the Centre for Eye Research Australia, administers a viral-vector-based gene therapy. Credit: Mathew Lynn/CERA. In a landmark 1972 paper 1, physician Theodore Friedmann ...
A CRISPR-based gene-editing therapy led to improved vision in people with an inherited condition that causes blindness 1. Mutations in more than 20 genes can lead to Leber's congenital amaurosis ...