

Sample Consent Forms
Consent form templates.
These consent form templates have been posted for your reference. When completing and IRB submission in IRBIS, please fill in the application and use the consent form builder specific to your project. For more information, please find instructions here .
Summary of Changes to the Regulations for Informed Consent: Revised Common Rule Changes to Informed Consent and Waiver Requirements
Summary of Changes to Consent Documents:
- Informed Consent Documents – Version 2.0 Summary of Changes
- Informed Consent Documents – Version 2.1 Summary of Changes
- Informed Consent Documents – 10/26/2020 Summary of Changes
- Informed Consent Documents – 4/10/2023 Summary of Changes
Concise Summary examples can be found here .
Guidance on the use of plain language in consent forms:
- Clinical Research Glossary
- Webinar: The Promise of Plain Language: Launching a Glossary to Support Participant Understanding of Clinical Research – Recording & Slides
There are a few additional forms that are not provided online and may be accessed below. As needed, these should be completed and uploaded to your IRB application.
Foreign Language Consent Forms
COVID-19 Related Forms:
- Spanish-IRB-COVID Information Sheet
- Spanish COVID Consent Letter v2
- Spanish COVID Informational Sheet Translation Certificate
Informed Consent Short Form (for a single subject who may be illiterate, or otherwise unable to read the consent form — used when full consent form has to be read or translated for subject).
- Informed Consent Short Form Guidance
- Simplified Chinese
HIPAA Templates
- Sample HIPAA Authorization Template
- Sample HIPAA Authorization Template in Spanish ( Certification )

Consent, Assent, and Screening Templates
Biomedical Research Consent Templates
Minimal Risk Research Consent Templates (Expedited or Exempt)
These templates are appropriate for social, behavioral, and educational ("SBER") research that does not include any biomedical procedures.
- Study Information Sheet (no signature)
- Consent Form (includes signature)
- Consent Form for Federally Funded Research
- Parent Permission Form (parents provide permission for child to participate)
- Parent Consent Form (parents complete research procedures themselves)
- Oral Consent Script Outline
- Sample Debriefing Script
Biomedical Research Informed Consent Templates
- Biomedical Research Consent Template
- Consent Template for Expanded Access Research
- Right to Try Consent Template
- Humanitarian Use Device Consent Template
Child and Adolescent Assent Templates
- Child Assent Template (Age 7-12)
- Adolescent Assent Template for Non-Treatment Studies (Age 13-17)
Addendum Consent Templates
- Addendum Consent Template for Non-Treatment Studies (for new procedures, risks)
- Addendum Consent Template for Treatment Studies (for new procedures, risks)
Screening Scripts
- Screening Script for non-Treatment Studies
- Screening Script for Treatment Studies
Consent Standards and Sample Language
- Social, Behavioral & Educational ("SBER") Consent form Standards and Template Language
- Biomedical Research Consent Form Standards and Sample Language
Comprehension Tools
- PRISM Readability Tool Kit
- Self-Certification of Surrogate Decision Makers for Potential Research Subject's Participation in UC Research (For use only in studies the IRB has reviewed and explicitly approved for surrogate consent)
- Decision-Making Capacity Assessment Tool (for potential subjects who may have cognitive impairments)
Other References
- Research Participant Bill of Rights/Experimental Subjects Bill of Rights - available in 34 languages
- Conducting Risk-Benefit Assessments
- Obtaining and Documenting Informed Consent (v. 07-28-11)
- Requesting Waivers and Exceptions to Informed Consent (v. 07-28-11)
- Child Assent and Permission by Parents or Guardians (v. 09-06-11)
- The Use of Legally Authorized Representatives or Surrogate Consent (v. 06-21-10)
- Recruitment and Screening Methods and Materials (v. 09-05-11)
We appreciate your suggestions for improving templates and/or adding sample language to the standards documents. Please email OHRPPEQI@research.ucla.edu to provide your feedback.
As the nation’s largest public research university, the Office of the Vice President for Research (OVPR) aims to catalyze, support and safeguard U-M research and scholarship activity.
The Office of the Vice President for Research oversees a variety of interdisciplinary units that collaborate with faculty, staff, students and external partners to catalyze, support and safeguard research and scholarship activity.
ORSP manages pre-award and some post-award research activity for U-M. We review contracts for sponsored projects applying regulatory, statutory and organizational knowledge to balance the university's mission, the sponsor's objectives, and the investigator's intellectual pursuits.
Ethics and compliance in research covers a broad range of activity from general guidelines about conducting research responsibly to specific regulations governing a type of research (e.g., human subjects research, export controls, conflict of interest).
eResearch is U-M's site for electronic research administration. Access: Regulatory Management (for IRB or IBC rDNA applications); Proposal Management (eRPM) for the e-routing, approval, and submission of proposals (PAFs) and Unfunded Agreements (UFAs) to external entities); and Animal Management (for IACUC protocols and ULAM).
Sponsored Programs manages the post-award financial activities of U-M's research enterprise and other sponsored activities to ensure compliance with applicable federal, state, and local laws as well as sponsor regulations. The Office of Contract Administration (OCA) is also part of the Office of Finance - Sponsored Programs.
Ethics & Compliance
- eResearch IRB NextGen Project
- Class Assignments & IRB Approval
- Operations Manual (OM)
- Authorization Agreement Process
- ORCR Policies and Procedures
- Self-Assessment Tools
- Resources and Web Links
- Single IRB-of-Record (sIRB) Process
- Certificate of Confidentiality Process
- HRPP Education Resources
- How to Register a Clinical Trial
- Maintaining and Updating ClinicalTrial.gov Records
- How to Report Clinical Trial Results
- Research Study Participation - FAQ
- International Research
- Coordinated Services & Practices (CSP)
- Collaborative Research: IRB-HSBS sIRB Process
- Data Security Guidelines
- Research Incentive Guidelines
- Routine fMRI Study Guidelines
- IRB-HSBS Website Directory and Guidance
- Waivers of Informed Consent Guidelines
- IRB Review Process
- IRB Amendment Process
- Continuing Review Process
- Incident Reporting (AE/ORIO)
- IRB Repository Application
- IRB-HSBS Education
- Newsletter Archive
You are here
- Human Subjects
- IRB Health Sciences and Behavioral Sciences (HSBS)
Informed Consent Guidelines & Templates
U-m hrpp informed consent information.
See the HRPP Operations Manual, Part 3, Section III, 6 e .
The human subjects in your project must participate willingly , having been adequately informed about the research.
- If the human subjects are part of a vulnerable population (e.g., prisoners, cognitively impaired individuals, or children), special protections are required.
- If the human subjects are children , in most cases you must first obtain the permission of parents in addition to the consent of the children.
Contact the IRB Office for more information .
See the Waiver Guidelines for information about, and policies regarding, waivers for informed consent or informed consent documentation.

See the updated Basic Informed Consent Elements document for a list of 2018 Common Rule basic and additional elements.
Informed Consent Process
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent document containing the required information (i.e., elements of informed consent) and the presentation of that information to prospective participants.
In most cases, investigators are expected to obtain a signature from the participant on a written informed consent document (i.e., to document the consent to participate) unless the IRB has waived the consent requirement or documentation (signature) requirement .
- Projects which collect biospecimens for genetic analysis must obtain documented (signed) informed consent.
- It is an ethical best practice to include an informed consent process for most exempt research . IRB-HSBS reviews, as applicable, the IRB application for exempt research, but not the informed consent document itself. A suggested consent template for exempt research can be found below under the References and Resources section. A companion protocol template for exempt research may be found in the feature box, Related Information (top right).
Informed consent documents
An informed consent document is typically used to provide subjects with the information they need to make a decision to volunteer for a research study. Federal regulations ( 45 CFR 46.116 ) provide the framework for the type of information (i.e., the "elements") that must be included as part of the consent process. New with the revised 2018 Common Rule is the requirement that the consent document begin with a "concise and focused" presentation of key information that will help potential participants understand why they might or might not want to be a part of a research study.
Key Information Elements
The image below displays the five elements identified in the preamble to the revised Final Rule as suggested key information.
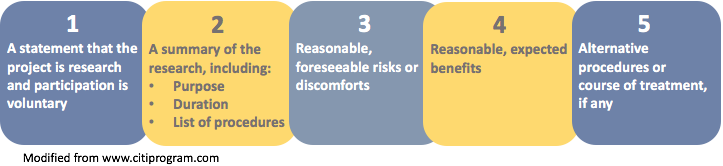
Note: Element number 5 (alternative procedures) applies primarily to clinical research.
General Information & Tips for Preparing a Consent Document
Reading level.
Informed consent documents should be written in plain language at a level appropriate to the subject population, generally at an 8th grade reading level . A best practice is to have a colleague or friend read the informed consent document for comprehension before submission with the IRB application. Always:
For guidance on using plain language, examples, and more, visit: http://www.plainlanguage.gov/
- Tailor the document to the subject population.
- Avoid technical jargon or overly complex terms.
- Use straightforward language that is understandable.
Writing tips
The informed consent document should succinctly describe the research as it has been presented in the IRB application.
- Use the second (you) or third person (he/she) to present the study details. Avoid use of the first person (I).
- Include a statement of agreement at the conclusion of the informed consent document.
- The consent doucment must be consistent with what is described in the IRB application.
Document Formating for Uploading into eResearch
- Remove "track changes" or inserted comments from the consent documentation prior to uploading the document into the IRB application (Section 10-1) for review.
- Use a consistent, clearly identified file naming convention for multiple consent/assent documents.
Informed Consent Templates
IRB-HSBS strongly recommends that investigators use one of the informed consent templates developed to include the required consent elements (per 45 CFR 46.116 ), as well as other required regulatory and institutional language. The templates listed below include the new consent elements outlined in the 2018 Common Rule.
References and Resources
Informed consent guidance.
PDF. Lists the basic and additional elements required for inclusion or to be included, as appropriate to the research, in the informed consent documentation, along with the citiation number [e.g., _0116(b)(1)] within the revised Common Rule. New elements associated with the 2018 Common Rule are indicated in bold text.
Informed Consent Templates (2018 Common Rule)
Strongly recommended for studies that involve the collection of biospecimens and/or genetic or genomic analysis, particularly federally sponsored clinical trials that are required to post a consent document on a public website. Last updated: 04/10/2024.
(Word) Blank template with 2018 revised Common Rule key information and other required informed consent elements represented as section headers; includes instructions and recommended language. It is strongly advised that you modify this template to draft a project-specific informed consent document for your study for IRB review and approval. Last updated: 04/10/2024
Other Templates
Informed Consent documents are not reviewed by the IRB for Exempt projects. However, researchers are ethically bound to conduct a consent process with subjects. This template is suggested for use with Exempt projects. Last updated 4/17/24
(Word) General outline to create and post a flyer seeking participation in a human subjects study. Includes instructions.
(Word) Two sample letters for site approval cooperation between U-M and other institutions, organizations, etc. Letters of cooperation must be on U-M letterhead and signed by an appropriate official. These letters are uploaded into the Performance Site section of the eResearch IRB application.
For use by U-M Dearborn faculty, staff, and students conducting non-exempt human subjects research using subject pools. Last updated 4/10/24
For use by U-M Dearborn faculty, staff, and students conducting exempt human subjects research using subject pools
Researchers who will conduct data collection that is subject to the General Data Protection Regulation (GDPR) must use this template in tandem with a general consent for participation template/document.
- Brief protocol for exempt research including data management and security questionnaire
Child Assent and Parental Permission
- Child assent ages 3-6
- Child assent 7-11
- Parent permission
- Child assent 12-14
IRB-Health Sciences and Behavioral Sciences (IRB-HSBS)
Phone: (734) 936-0933 Fax: (734) 936-1852 [email protected]
- Human Subjects Protections

- Find My GCO
- IACUC applications (Cayuse Animal Management System)
- IBC Applications (eMUA)
- IRB Applications (RASS-IRB) External
- Institutional Profile & DUNS
- Rates and budgets
- Report external interests (COI)
- Join List Servs
- Ask EHS External
- Research Development Services
- Cornell Data Services External
- Find Your Next Funding Opportunity
- Travel Registry External
- RASS (Formerly Form 10 and NFA) External
- International research activities External
- Register for Federal and Non-Federal Systems
- Disclose Foreign Collaborations and Support
- Web Financials (WebFin2) External
- PI Dashboard External
- Research metrics & executive dashboards
- Research Financials (formerly RA Dashboard) External
- Subawards in a Proposal
- Proposal Development, Review, and Submission
- Planning for Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Budgets, Costs, and Rates
- Collaborate with Weill Cornell Medicine
- Award Negotiation and Finalization
- Travel and International Activities
- Project Finances
- Project Modifications
- Research Project Staffing
- Get Confidential Info, Data, Equipment, or Materials
- Managing Subawards
- Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Project Closeout Financials
- Project Closeout
- End a Project Early
- Protecting an Invention, Creation, Discovery
- Entrepreneurial and Startup Company Resources
- Gateway to Partnership Program
- Engaging with Industry
- Responsible Conduct of Research (RCR)
- Export Controls
Research with Human Participants
- Research Security
- Work with Live Vertebrate Animals
- Research Safety
- Regulated Biological Materials in Research
- Financial Management
- Conflicts of Interest
- Search
IRB Informed Consent
UPDATED February 1, 2023
NIH Data Management and Sharing Policy Update: Learn more here . TRAINING UPDATE: Effective October 1, 2022, all Cornell study personnel involved in Exempt research protocols are now required to complete CITI training in human participant research ethics. See details here . For more information about Cornell IRB training requirements, visit the IRB Training webpage .
Informed consent is more than just a form; it is a process that takes place between researcher and participant, forming the basis of ethical research that respects the autonomy of research subjects.
Introduction to Informed Consent
Informed consent is one of the most important aspects of conducting ethical research with human participants. Respecting the autonomy of research participants, the investigator must provide complete and true information regarding the study. The "informed" in informed consent means the participant has all the information necessary to make a decision. To help new researchers develop a consent process that works, the IRB staff offers a brief video tutorial on the basics of informed consent.
The principle of informed consent applies to ALL types of research including surveys, interviews, and observations in which participants are identified, and other experiments, such as diet, drug and exercise studies.
Typically, a "consent form" documents that the informed consent process has taken place. It must contain all the required components of informed consent, as defined in 45 CFR 46.116 , and described below. The consent form must be written in language that is easy for the participant to understand. Avoid technical terms and complex sentences, regardless of your intended audience. When there are different groups or types of participants who may take part in a study, different consent documents may be required for different study populations.
Informed Consent Can Take Various Forms
- Signed informed consent is the gold standard, and what is generally required for research with human participants. This involves presentation of a written document to the prospective participant, including all elements of informed consent. The document is read and signed by the participant and kept as a record by the researcher.
Remember: if you choose a written informed consent process, the research will not be anonymous. Do not promise anonymity to prospective participants and then ask them to sign a consent form!
- Implied consent is the tacit indication that a person has knowingly agreed to participate in research by performing a research activity or task. This type of consent process is most common in internet-based research. Participants must still be presented with the consent information - the information a participants needs to know in order to make an educated decision about whether or not to participate. This type of process is be considered a waiver of documentation of informed consent, and must meet certain requirements to be applied to a given study (see IRB SOP #10 ).
- Oral consent occurs when the researcher reads a consent script, and the subject verbally indicates that they agree to participate. Subjects should be given the opportunity to ask questions and, in most cases, provided with an information sheet containing key information about the study, including contact information for the PI. See IRB SOP #10 for more details on how to implement and document an oral consent procedure.
- In research with children , a parent or guardian must typically give their permission (called "parental consent") to allow the child to participate. Also, if the child is mature enough, they will need to give their "assent" to participate. For detailed information, please read the IRB's guidance document on Research Involving Children .
Guidelines for Writing your Informed Consent Document
General advice:.
- Start with an IRB-approved consent form template .
- Headings for paragraphs are helpful and make the form easier to read.
- Use adequate white space so that the form is easy to read. Avoid fine print.
1. Provide a clear, concise explanation of the purposes of the research including the name of the study (the IRB can waive under certain specific circumstances if appropriate).
- Replace any technical terms with ordinary language, and use simple, clear phrasing. Avoid unnecessarily long or wordy paragraphs.
2. Explain what will be happening to the participant during the study and indicate participant's time commitment for each component.
- Be clear about what the participant will be asked to do, not vaguely say, "You might be asked to," state "You will be asked." The participant needs to understand what the experiment involves in order to decide whether they want to participate.
- Write in the second person "You" -- for example: "You are invited to participate in a research project..., " "You will be asked to..."
- Do not make coercive statements such as "you understand that ...," "you have been told that...
3. Describe the frequent and/or important risks, side effects or discomforts of the study procedures (e.g., even though it is not considered a risky procedure, a needle stick to draw blood creates discomfort).
- If it appears that there are no real risks to participation, state, "We do not anticipate any risks to you participating other than those encountered in daily life." See our Sample Consent Form .
4. Describe any direct or indirect benefit from participating.
- Learning about how experiments are conducted, receiving a gift, or earning extra credit for being a research participant are NOT direct benefits. Gifts and extra credit are considered compensation or incentives.
- Learning about how research is conducted, gaining some knowledge of a particular subject, or enjoying a game played during the research, may be indirect benefits.
5. State that the participant's involvement is voluntary, the participant may refuse to participate before the study begins, discontinue at any time, or skip any questions that may make him/her feel uncomfortable, with no penalty to him/her, and no effect on the compensation earned before withdrawing, or academic standing or record.
- If completing the entire study is required for compensation, make this clear. Likewise, if there are minimum requirements to be met to earn compensation, state this clearly.
- If partial compensation or pro-rated compensation will be given, please make clear how the amount of compensation will be determined.
6. State that the participant is allowed to ask questions concerning the study, both before agreeing to be involved and during the course of the study. See required contact information in #11 below.
7. describe how participant's confidentiality will be protected., 8. describe what will be done with the data once the study is completed, including identifiable information..
- Include any plans to share either identifiable or de-identified data with other researchers, or to publish in journals or present at conferences.
9. Indicate that recording devices, audio or visual, are being used (when applicable).
- If identifiable images or videos will be used for any purpose other than analysis of data, please make this clear and make sure the participant indicates permission for these uses (see below).
- Describe what will be done with the any video or audio tapes upon the completion of the study (destroyed, erased, archived, etc.), and when (after transcription,3 years, 5 years, etc.).
- If being audio/video recorded is required to participate in the study, please make this clear on the consent form. Whether this is required is up to the PI, but if so it should be clear for the participant.
- If recording is optional, provide a separate signature line on the consent form for the participant to agree to be video/audio taped or photographed. For example:
Please sign below if you are willing to have this interview recorded on tape (specify audio or video). You may still participate in this study if you are not willing to have the interview recorded. I am willing to have this interview recorded on tape: Signed: ______________ Date: __________
10. Indicate that the participant shall receive a copy of the signed and dated consent form.
11. provide the name(s) of the investigator(s) and contact information., 12. indicate that the participant may contact the institutional review board (irb) with any concerns or complaints..
- Include our email address ([email protected]), phone (607-255- 5138), and website .
- Concerns may also be reported anonymous through EthicsPoint online at www.hotline.cornell.edu or by calling toll free 1-866-293-3077. Ethicspoint is an independent organization that serves as a liaison between the University and the person bringing the complaint so that anonymity can be ensured.
13. Include at the bottom of the form: "This consent form will be kept by the researcher for at least three years beyond the end of the study and was approved by the IRB on [date]."
Remember, if the participant is under the age of 18, parental/guardian consent is required. This includes college and university students under the age of 18, unless the IRB approves a request for waiver of parental consent (at their discretion). If the participant is 7-17 years old, child assent is also required.
Research Involving Deception
- You will need to explain the deception and the reason for it to the participant as soon as the study is completed. Deception (including incomplete disclosure) is a technique that is sometimes used in social and behavioral research, in order to avoid response bias or for other valid scientific reasons. However, deception conflicts the subject's ability to make a fully informed decision about whether or not to participate in the research. To mitigate this concern, debriefing is a n essential part of the consent process whenever a study involves deception.
- Once a participant has been debriefed, it is almost always appropriate to offer them the opportunity to withdraw their consent to participate. A template for creating a debrief can be found here: Sample Debriefing Statement.
Cornell Guidance on Informed Consent
Informed Consent Options, Processes, and Documentation
Informed Consent - Research Involving Children
Informed Consent - Research Involving Normal, Healthy Participants
Informed Consent - Research Involving Cornell Students
Informed Consent - Research Involving Prisoners
Parent/Guardian Permission for Studies Involving Children
Guidance for Protocols Involving Oral Consent
Informed Consent 101 Video
IRB Consent Form Templates
About the irb committee, irb announcements & newsletters, irb covid-19 faqs, submit or manage your irb protocol, irb training, irb considerations: human participant data, data sets and internet research, irb biomedical research, irb considerations for international research, irb considerations for clinical trials.

Informed Consent Templates
As part of our continued efforts to improve the IRB application and review process, we have developed new ICF templates that address the issues identified by our stakeholders:
- Different templates have been created for different types of studies (SBER, Biomed, etc.).
- Guidance language has been added to each template to reduce confusion about which template to use, as well as how to complete sections.
- The Key Information section has been simplified.
- An Assent Template has been created, with guidance included to identify applicable populations.
- All language utilized is at the 8th grade reading level , and has been improved to promote clarity of instructions.
- The template has been shortened and simplified to facilitate completion.
- All signatures have been moved to the end of the document.
These new and improved ICF templates replace all previous versions posted to our site as of May 17th, 2023; any new intakes initiated within WRG-HS as of June 12th, 2023, must utilize these new templates.
- WCM Assent Template
- WCM Biomedical Informed Consent Template
- WCM Humanitarian Use Device Informed Consent Template
- WCM Informed Consent Addendum Template
- WCM Intermediate-Size Investigational Treatment Informed Consent Template
- WCM Pregnant Partner Non-Subject Informed Consent Template
- WCM Pregnant Partner Research Subject Informed Consent Template
- WCM Repository Informed Consent Template
- WCM SBER Informed Consent Template
- WCM Single Patient Investigational Treatment Informed Consent Template
Make sure your Informed Consent Form is a readable document!
See our Guidance Document on how to prepare a readable consent form .
See our list of Medical Terms in Lay Language for use in Informed Consent Forms.
Refer to the Program for Readability in Science & Medicine (PRISM) Readability Toolkit
Refer to the MRCT Research Glossary for easy-to-understand clinical research definitions.

Frequently Asked Questions (FAQs)
Will i have to start using these new consents today.
Any new studies initiated in WRG-HS starting 6/12, must use the new ICF templates.
Do I have to transition my existing study consent(s) to these new templates?
Any existing study using the previous consent templates can continue to use their IRB-approved consent. Any new studies initiated in WRG-HS starting 6/12, must use the new ICF templates.
Do these new ICF templates need to be used under a ceded/sIRB study with an external IRB as the IRB of record?
Studies with an external IRB are expected to use consent forms provided by the lead IRB. Once the externally reviewed ICF is provided to the WCM IRB, we will conduct a local context review to confirm any required language (i.e. research-related injury, NYS Genetic testing requirements, etc.). The new WCM ICF templates are available as a resource if needed, but not required for externally reviewed studies.
If I am updating my protocol and the change requires an update to the ICF, will I need to use the new ICF templates?
You can continue to use the same ICF.
Weill Cornell Medicine Human Research Protections 575 Lexington Avenue New York, NY 10022 Phone: (646) 962-8200
- Skip to navigation
- Skip to content
- UMB Shuttle
University of Maryland, Baltimore
About UMB History, highlights, administration, news, fast facts
- Accountability and Compliance
- Administration and Finance
- Center for Information Technology Services
- Communications and Public Affairs
- Community Engagement
- Equity, Diversity, and Inclusion
- External Relations
- Government Affairs
- Philanthropy
- Office of the President
- Office of the Provost
- Research and Development
- University Counsel
- Administrative Officers
- Boards of Visitors
- Faculty Senate
- Staff Senate
- Center for Health and Homeland Security
- Council for the Arts & Culture
- Interprofessional Education
- Leaders in Education: Academy of Presidential Scholars
- Middle States Self-Study
- President's Council for Women
- President's Symposium and White Paper Project
- For the Media
- Steering Committee Roster
- Logistics Committee Roster
- UMB Police and Public Safety
- Graduation Celebration 2024
- Founders Week
- UMB Holiday Craft Fair
Academics Schools, policies, registration, educational technology
- School of Dentistry
- Graduate School
- School of Medicine
- School of Nursing
- School of Pharmacy
- School of Social Work
- Carey School of Law
- Health Sciences and Human Services Library
- Thurgood Marshall Law Library
Admissions Admissions at UMB are managed by individual schools.
- Carey School of Law Admissions
- Graduate School Admissions
- School of Dentistry Admissions
- School of Medicine Admissions
- School of Nursing Admissions
- School of Pharmacy Admissions
- School of Social Work Admissions
- Tuition and Fees by School
- Student Insurance
- Academic Calendar
- Financial Assistance for Prospective Students
- Financial Assistance for Current Students
- Financial Assistance for Graduating Students
Research Offices, contracts, investigators, UMB research profile
- Organized Research Centers and Institutes
- UMB Institute for Clinical & Translational Research
- Sponsored Programs Administration
- Sponsored Projects Accounting and Compliance (SPAC)
- Kuali Research
- Clinical Trials and Corporate Contracts
- CICERO Log-in
- Conflict of Interest
Human Research Protections
- Environmental Health and Safety
- Export Compliance
- Effort Reporting
- Research Policies and Procedures
- Center for Innovative Biomedical Resources
- Baltimore Life Science Discovery Accelerator (UM-BILD)
- Find Funding
- File an Invention Disclosure
- Global Learning for Health Equity Network
- Manage Your Grant
- Research Computing
- UM Research HARBOR
- Center for Violence Prevention
- Office of Research and Development
- Center for Clinical Trials and Corporate Contracts
- Technology Transfer/UM Ventures
- Contact Research and Development
Services For students, faculty, and staff, international and on-campus
- Student Health Resources
- Educational Support and Disability Services
- Writing Center
- URecFit and Wellness
- Intercultural Leadership and Engagement
- Educational Technology
- Student Counseling Center
- UMB Scholars for Recovery
- UMB Student Affairs
- Human Resource Services
- Travel Services
- Strategic Sourcing and Acquisition Services
- Office of the Controller
- Office of the Ombuds
- Employee Assistance Program (EAP)
- Workplace Mediation Service
- Faculty Center for Teaching and Learning
- UMB Travel: Start Here
- International Students, Scholars, and Employees
- Center for Global Engagement
- International Travel SOS
- International Operations
- Parking and Transportation Services
- UMB shuttle
- SMC Campus Center Event Services
- Donaldson Brown Riverfront Event Center
- All-Gender Bathrooms
- Environmental Services
- Interprofessional Program for Academic Community Engagement
University Life Alerts, housing, dining, calendar, libraries, and recreation
- Emergency Reference Guide
- Campus Life Weekly with USGA
- Starting a New Universitywide Organization
- University Student Government Association
- Planned Closures
- Intramural Sports
- Safety Education
- About URecFit and Wellness
- How to Get Your One Card
- One Card Uses
- Lost One Card
- One Card Policies
- Photo Services
- One Card Forms
- One Card FAQs
- Office Hours and Directions
Give to UMB Sustain excellence and meet UMB's educational needs for today and tomorrow.

Thank You for Your Gift to UMB
The University of Maryland, Baltimore (UMB) is excited to share its new online giving page.
With enhanced searchability, a streamlined checkout process, and new ways to give such as Venmo, PayPal, Apple Pay, and Google Pay in addition to credit card, donors can support UMB quickly and securely.
- Ways to Give
- Where to Give
- Staying Connected: You and UMB
- The UMB Foundation
- Office of Philanthropy
- Maryland Charity Campaign

- OAC Services
- Research Compliance
- For Researchers
- Consent Form Templates
Human Research Protections Office
620 W. Lexington St. Second Floor Baltimore, MD 21201
P 410-706-5037
Office hours are 8:30 a.m.-4:30 p.m., Monday through Friday.
* The Human Research Protections Office is working remotely and is ready to assist our community during the COVID-19 pandemic. During this time, we request you contact us via email to promote a prompt response. With apologies, currently voicemail messages may be delayed in reaching us and cause a longer wait time for us to respond to your requests. We thank you for your patience and look forward to assisting you.
* Only the UMB IRB approved consent/assent templates (with the UMB logo) will be accepted when UMB IRB is the IRB of record
** Effective December 4th, 2023, the University of Maryland Baltimore Human Research Protections Program (HRPO) will adopt a revised version of the informed consent document (ICF) template. This announcement applies to new studies only that submit their application in CICERO after December 4th, 2023.
Consent and Assent Form Templates
- Informed Consent and HIPAA Authorization Form Template (Post 12-4-2023) : This template should be used as the consent document guide for all new research studies, including parental and LAR permission (consent) forms, submitted for IRB review on or after December 4th, 2023.
- Informed Consent and HIPAA Authorization Form Template (Pre 12-4-2023) : This template should be used as the consent document guide for all research studies, including parental and LAR permission (consent) forms, submitted for IRB review before December 4th, 2023. ( Informed Consent Concise Summary Examples 2019 )
- Assent Form Template : This template should be used to assent participants ages 13-17 and, if applicable to your study, cognitively impaired participants.
- HIPAA Authorization Form : All Non-VA uses.
- UM Dental School additional Consent Form Template : If you are conducting business through the School of Dentistry, add this paragraph.
- VA Informed Consent and HIPAA Authorization Form Template : This template should be used as a guide if your study is being conducted at the Baltimore VA. ( VA IC and HIPAA Guidelines )
- Revocation of HIPAA authorization (VA Form 10-10116)
- VA HIPAA Waiver Request (VA Form 10-0521)
Informed consent is required to be presented in language understandable to potential participants. The HSHSL Health Literacy guide contains a list of Plain Language resources .
The Health Sciences and Human Services Library offers a Research Consent Form Review service to improve consent form readability. Library staff will review your consent form and suggest changes that will help simplify the language, lower the reading grade level, and make the consent form more understandable to potential participants. * As a free service to all UMB faculty, researchers are strongly encouraged to use this service.
Click Here To Request a Research Consent Form Review
The following basic elements of informed consent are required to be disclosed in all research consent forms pursuant to 45 CFR 46(a-b):
Basic elements of informed consent.
- A statement that the study involves research, an explanation of the purposes of the research and the expected duration of the subject's participation, a description of the procedures to be followed, and identification of any procedures that are experimental.
- A description of any reasonably foreseeable risks or discomforts to the subject.
- A description of any benefits to the subject or to others that may reasonably be expected from the research.
- A disclosure of appropriate alternative procedures or courses of treatment, if any, that might be advantageous to the subject.
- A statement describing the extent, if any, to which confidentiality of records identifying the subject will be maintained, including the possibility that the Food and Drug Administration (FDA) may inspect the records if applicable.
- For research involving more than minimal risk, an explanation as to whether any compensation and an explanation as to whether any medical treatments are available if injury occurs and, if so, what they consist of, or where further information may be obtained.
- An explanation of whom to contact for answers to pertinent questions about the research and research subjects' rights, and whom to contact in the event of a research-related injury to the subject.
- A statement that participation is voluntary, refusal to participate will involve no penalty or loss of benefits to which the subject is otherwise entitled, and the subject may discontinue participation at any time without penalty or loss of benefits to which the subject is otherwise entitled.
Additional elements of informed consent. When appropriate, one or more of the following elements of information shall also be provided to each subject:
- A statement that the particular treatment or procedure may involve risks to the subject (or to the embryo or fetus, if the subject is or may become pregnant) that are currently unforeseeable.
- Anticipated circumstances under which the subject's participation may be terminated by the investigator without regard to the subject's consent.
- Any additional costs to the subject that may result from participation in the research.
- The consequences of a subject's decision to withdraw from the research and procedures for orderly termination of participation by the subject.
- A statement that significant new findings developed during the course of the research that may relate to the subject's willingness to continue participation will be provided to the subject.
- The approximate number of subjects involved in the study.
The University of Maryland, Baltimore is the founding campus of the University System of Maryland. 620 W. Lexington St., Baltimore, MD 21201 | 410-706-3100 © 2023-2024 University of Maryland, Baltimore. All rights reserved.
- IRB-SBS Home
- Contact IRB-SBS
- IRB SBS Staff Directory
- IRB SBS Board Members
- About the IRB-SBS
- CITI Training
- Education Events
- Virginia IRB Consortium
- IRB-SBS Learning Shots
- HRPP Education & Training
- Student Support
- Access iProtocol
- Getting Started
- iProtocol Question Guide
- iProtocol Management
- Protocol Review Process
- Certificate of Confidentiality
- Deception and/or Withholding Information from a Participant
- Ethnographic Research
- IRB-SBS 101
- IRB-SBS Glossary
- Participant Pools
- Paying Participants
- Research in an Educational Setting
- Research in an International Setting and/or Location
- Risk-Sensitive Populations
- Student Researchers and Faculty Sponsors
- Study Funding and the IRB
- Understanding Risk in Research
- Vulnerable Participants
- IRB-SBS PAM & Ed
- Federal Regulations
- Ethical Principals
- Partner Offices
- Determining Human Subjects Research
- Determining HSR or SBS

Consent Templates
The templates below were created to help you create the documents you will need to communicate to participants what they will do in the study. The documents you provide participants will range from recruitment materials to post-debrief consent forms, and you need to submit everything that you provide to a participant to our Board for review. For more information about the consent process see Consent .
- General Consent Template : This form covers all of the basic elements that are required for a consent document. Even if you don't plan to use this exact document, refer to it to ensure that you have all of the appropriate elements in place in your consent procedure.
- Electronic Consent Template : This form is modeled after the General Consent Template with some modifications that make it more appropriate for an online format. For more information about this template, see Electronic Consent .
- Parent Consent : If you are including minors in your study, you will need to provide a consent form for parents and an age appropriate assent form for minors. This form is a guide for creating a parent informed consent document. This form can also be used as a guide for surrogate consent procedures.
- Minor Assent (for ages 13-17) : This template provides the basic elements required for older minors to provide assent and could also be used as a model for higher functioning individuals with diminished mental capacity.
- Minor Assent (for ages 7-12) : This template provides the basic elements required for younger minors to provide assent and could also be used as a model for higher functioning individuals with diminished mental capacity. For children younger than 7, assent forms are not required but include information in the consent section regarding what you will say to them about the study (where appropriate).
- Capacity to Consent Template : For some participant populations, it may be necessary to determine if a participant is able to provide consent; if not, a surrogate can be used (you will also need a surrogate consent form and participant assent form, similar to the parent/child consent/assent forms).
- GDPR Informed Consent Addendum : If you are collecting data from citizens of the European Union or the United Kingdom, you will need to provide additional information to your participants, per the GDPR. For more information, see the Research in an International Setting and/or Location and International Research Data Source .
- Study Information Sheet : While many studies do not require researchers to collected signed consent forms, we generally require that participants receive a Study Information Sheet to provide them with information about the study. This information can be provided as a paper document at the beginning of a survey.
- Electronic Study Information Page : This template is similar to the Study Information Sheet with modification for an electronic delivery. For more information about this template, see Electronic Consent .
- Parent Notification Template : Typically used for studies in an educational setting (particularly where the study is exempt but parent notification is still required), this template is a guide for creating a notification letter to send home to parents.
- Oral Consent Card : Typically used in anthropology studies where the participant may be uncomfortable with a form and/or unable to use it, the Oral Consent Card provides all of the elements required for consent in a bullet format so that the researcher can refer to each point as he or she is obtaining consent from the participant.
- Oral Consent Template : This form is also used in situations where the participant is uncomfortable with a form and/or unable to use it. It is more suited to non-anthropology research (though anthropologists are welcome to refer to it as well).
- Sample Debriefing Form : A debriefing form is a summary of the study given to a participant in a deception study and/or a study that includes students from a participant pool. The purpose is to educate participants about the study and to provide them with resources, particularly if the study is upsetting.
- Advertising Flyer Template : Recruitment materials are part of the consent process and it is important that participants are accurately informed about the study throughout the process. You are not required to use this flyer template (it is a model appropriate for a flyer posted around campus), but it is important that you follow the guide provided in Recruitment .
- ResearchMatch Advertising Template : The NIH funds a free and secure recruitment tool called ResearchMatch that helps to connect researchers with volunteers that are interested in participating in studies. If you are interested in using ResearchMatch to help advertise for your study, complete this ResearchMatch Advertising Template and upload.
- Materials Release Form : The data you collect from your participants may be useful in other spheres, such as an educational tool and/or library archive. Using data in this manner is beyond the scope of the study and you should seek additional permission to use the participant's data in this way. This form allows a participant to declare how they would like their materials to be used by the researcher if the researcher wants to use the materials in situations beyond the study.
- Data Release Form : This form is similar to the Post-Debrief Consent Form; it is used when a participant has been recorded or photographed without their knowledge.
- Post-Debrief Consent Form : This form is used in a deception study after the deception is revealed to the participant. The participant is given an opportunity to decide if they still want to participate after the true purpose of the study is revealed.
- The title of protocol must match the title on all consent forms. The title must be relevant, appropriate, and easy to understand. Include the project title on all pages of the consent form.
- List the page numbers on all pages of the consent form in the standard format: Page 1.
- Delete all colored text from the final copy of your form. The colored text is for explanation purposes only.
- Make sure that the form matches the descriptions in the protocol and vice versa.
- Include all relevant information in the consent form rather than referring to previous verbal explanations. The consent form should provide a complete explanation of what the participant is agreeing to do in the study.
- Be aware of the needs of the participant. Avoid using jargon and acronyms that the participant may not understand; make sure the reading comprehension level is appropriate.
- Do not use statements that make implicit demands on participants to participate , e.g., "You will enjoy and benefit from participating in this study."
- Prepare the consent forms in the standard format provided in the template, with all headings addressed. Use the standard language provided on the template where appropriate.
- Please proofread the consent forms for grammar and spelling errors.
- Do not use language that revokes a participant’s legal rights . A consent form is not a legal document.
- Do not require the participants to sign consent to long statements written in first person , e.g., “I agree to participate in this research study. I understand that the risks are minimal and that I will receive no benefits. I know how to withdraw from this study. I will receive $X in payment for participating. I understand that if I withdraw from the study before my participation is complete, I will receive prorated payment according to the following schedule . . . I agree not to hold the researchers liable for any injuries resulting from participation in this study. . .” DO ask participants to sign consent to a simple agreement statement at the end of the consent form: “I agree to participate in the research study described above.”
Human Research Protection Program Office of Research Regulatory Support
Informed consent templates.
Informed consent templates have been developed to assist researchers in developing a consent form for a research study. The template is only a guide to help researchers and may be subject to change. Please note that IRB members may still request changes to your document.
The revised Common Rule implements January 21, 2019 for new studies. Several templates have been updated to incorporate the revised Common Rule requirements (noted below). Please use the updated templates. To view more detailed information about the revised Common Rule changes, please visit the Revised Common Rule webpage .
- HRP-502 - Template - Minimal Risk Consent Document (Word 28 KB) ( updated for revised Common Rule )
- HRP-507 - Template - Consent Document - Short Form (Word 17 KB) ( updated for revised Common Rule )
- HRP-517 - Template - Biomedical Consent Document (Word 36 KB) ( updated for revised Common Rule )
- HRP-518 - Template - Social Science - Behavioral Consent Document (Word 33 KB) ( updated for revised Common Rule )
- HRP-519 - Template - Genomics Consent Document (Word 43 KB) ( updated for revised Common Rule )
- HRP-520 - Template - Consent Document for Exempt Research (Word 30 KB)
- HRP-521 - Template - Assent Document (Word 17 KB)
- HRP-522 - Template - Parental Permission Document (Word 22 KB)
- HRP-523 - Template - Telephone Script Consent Document (24 KB) ( updated for revised Common Rule )
- HRP-524 - Template - Screening Consent Document (Word 26 KB)
- HRP-525 - Template - Consent Language when using Experimetrix (Word 17 KB)
- HRP-526 - Template - Consent Language when using Sona (Word 18 KB)
- HRP-543 - Template - MRI Screening Form Pregnancy (Word 18 KB)
More Information About the Revised Common Rule (2018 Requirements)
The revised Common Rule requires several changes to the informed consent document. Included below is guidance and help information on these requirements. To view more detailed information, please visit the Revised Common Rule Informed Consent Requirements webpage .
- Guidance for the New Informed Consent Requirement for a Concise and Focused Presentation of Key Information (80KB)
- About Research & Innovation
- Advanced Cardiovascular Care
- Health Equity
- Inflammation
- Maternal and Fetal Medicine
- Musculoskeletal Care
- Neuroscience
- Opioid & Pain
- Research Centers
- Departments
- About the Office of Research
- Funding & Proposal Development
- Regulatory Review & Compliance
- Research Project Management
- Cores & Resources
- Education & Training
- Peter Arvan Lab
- Antiphospholipid Syndrome Research Labs
- J. Michelle Kahlenberg Lab
- John Varga Lab (ScleroLab)
- Mulholland Lab
- Raghavendran Lab
- ALS Center of Excellence
- Institute for Heart & Brain Health
- Center for Basic & Translational Science
- Center for Bioethics and Social Sciences in Medicine
- Biomedical Research Core Facilities
- IT Route Map
- Clinical Research Route Map
- Commercialization Route Map
- Great Minds, Greater Discoveries
- Research Scouts
- Meet the ROMS Team
- ROMS Fellowship Application Information
- Working with a ROMS Fellow
- Pandemic Research Recovery
- Research Climate Council
- Support for Outstanding Research
- Research News Trivia
- Frequently Asked Questions (FAQ)
- Billing Calendar & Study Applications
- CRAO Frequently Asked Questions (FAQ)
- Pre-Onboarding Considerations
- Access Biospecimen
- Biospecimen Processing
- Biospecimen Inventory
- Regulatory & Governance
- Equipment & Instrumentation
- Central Biorepository Pricelist
- CBR Frequently Asked Questions (FAQ)
- Clinical Trials Support Units
- Clinical Research Coordinator Career Ladder
- Clinical Trials Management Systems: Accounts & Support
- Research Pharmacy
- Off-Campus Clinical Research
- Statistical Support for Large-Scale Clinical Trials
- MCRU Services
- MCRU Lab Study Team Guidelines
- MCRU Frequently Asked Questions (FAQ)
- Michigan Medicine Site Profile
- CTSO Frequently Asked Questions (FAQ)
- Self-Serve Data Tools
- Custom Data Request
- Data & Biospecimen Sharing
- Student Access to Patient Data
- FFMI fastPACE
- Frankel Cardiovascular Center Innovation Program
- Rogel Cancer Center Innovation Program
- Global Health Commercialization Competition
- Biomedical Innovation 101
- Certificate in Biomedical Innovation & Entrepreneurship
- Fast Forward Medical Innovation Fellowship Program
- Biotech Career Development Program
- Drug Discovery & Development Course
- Innovation Studio
- Oncology Drug Discovery & Development (3D) Workshop
- FFMI fastPACE Train-the-Trainer
- Intellectual Property in the Academic Setting
- Academic Drug Discovery
- Medical Device Regulation
- Idea to Impact Webinar Series
- 6 Tips for Customer Discovery
- Interacting with Industry
- Ask the Experts: A Biomedical Innovation Forum
- Business Development
- Michigan Biomedical Venture Fund
- R01 Boot Camp
- Facility & Resource Profile Templates
- BMRC Bridging Support Program for Biomedical Research
- Medical School Limited Submissions
- Budgeting & Costs
- NIH Specifics, Tips & Tricks
- Grant Routing Deadlines
- Proposal Review Checklists
- Department Contacts
- Authorized Signers
- Research Data Analytics
- Grant Processing Advisory Committee (GPAC)
- Post-Award Advisory Committee (PAAC)
- Grants Frequently Asked Questions (FAQ)
- Board Nominations Sought
- Informed Consent & Assent Templates
- IRBMED Seminar Series
- Information for IRB Members
- Information for Study Subjects
- SignNow Electronic Signature Service
- IRBMED Frequently Asked Questions (FAQ)
- IRBMED Contacts & Roster

Explore downloadable templates, including standard consent and assent templates, as well as several specialty templates, for use in specific types of research situations. You can search and bookmark any template in Research A-Z.
Obtaining the consent of a subject is a process that goes far beyond asking for a signature on a document. Potential participants must understand the nature of the study, the risks, discomforts, inconveniences, and potential benefits involved if they are to make an informed decision. We ensure respect for persons by providing information needed for thoughtful consent for a voluntary act.
- Tips for Preparing
NOTE: On April 15, 2024 IRBMED issued a revised Standard Template , as well as revised versions of the eligibility, survey, and blood draw Specialty Templates containing updates relating to the new HSIP compensation policy. Consent documents submitted on or after June 1 st must be created in the newly revised template. See Research A-Z for more information.
Informed consent is an ongoing process that begins with recruitment and continues through the completion of the study.
The first step is to provide potential subjects with general information about the nature of the study and why they might be suited to participate. The recruitment flyers, websites, and phone scripts are part of this process. All recruitment materials must be reviewed by an IRB prior to implementation.
Once someone indicates interest in joining a study, details should be presented by a study team member who is knowledgeable about both the study and informed consent.
- Provide information about the research in language the subject can understand. Encourage questions and query for understanding. Note that for children (see Guidance about children ) and for those who do not understand written English language documents, this requires additional steps (which require IRB approval). See the IRBMED's guidance for non-English speaking/reading subjects .
- Provide an explanation of the difference between treatment and research.
- Provide time for subjects to consider all options. The riskier the study, the more time that is usually required.
- Provide answers to all of the subject's questions before the decision is made.
- Provide documentation about the research (including calendars, instructions, etc.) that the subject can refer to later. A copy of the consent document must be provided to the subject (or the subject’s legally authorized representative) and the original signed consent document should be retained in the study records. Note that the regulations do not require the subject's copy to be a signed copy, although a photocopy with signature(s) is preferred. All materials provided to subjects need IRB approval.
- Provide on-going opportunities to re-affirm consent throughout the study. Remind the subject about important information. Provide new information as it becomes available. Ask the subjects if they still want to participate. Document these interactions.
At each study visit encourage subjects to ask questions and raise concerns. Thus, informed consent becomes an ongoing, interactive process, rather than a one-time information session. Following good clinical practice (GCP) these discussions should be recorded in the subjects' records.
Important note for UMHS studies: A copy of the complete (every page) signed consent form should be placed in the UM medical record of subjects, particularly when the research intervention may affect other treatment or care (in most cases this means scanning the document into MiChart; click here for instructions). However, doing so may not be appropriate in all cases (for example if identification of the subject as a study participant might put the subject at risk of criminal prosecution or harm to reputation).
Web Resources
- Office of Human Research Protections (OHRP)
- National Cancer Institute, 'A Guide to Understanding Informed Consent'
- Food and Drug Administration (FDA)
The informed consent document is an important tool in the informed consent process. The goal is to have a document, understandable to the subjects or their legal representatives, that explains what will happen to them as part of the study, when it happens, and why.
Target readability levels of consent forms should be between 6th and 8th grade. Examples of this level are found in the popular press, e.g. Newsweek or USA Today. Computer programs that analyze grade level, like the Flesh Kincaid method, are useful tools but cannot be relied upon in whole because informed consent documents often use words of a higher level (like the name of a procedure) that you will also explain in lay terms.
The sections below, Tips and Examples of protocol language ‘translated’ to subject-friendly language, are to assist you in achieving an understandable document. Also refer to the UM library system's plain language medical dictionary for more lay terms. Refer to the Education page for information about the IRBMED workshop, Informed Consent 201.
Special thanks to the Center for the Advancement of Clinical Research for contributions in the material below.
- Use one of the IRBMED provided templates (above).
- Review the IRBMED template instructions (above). Alternate text for many sections is provided in the instructions to tailor the document appropriately for the type of study being described. The federally required elements of consent are also provided.
- Use multiple consents tailored for each study group in unblinded studies where different subject groups undergo significantly different procedures or schedules.
- Use subheads within sections that require extensive detail.
- Use a logical order for the topic within each section. For example, in the risk section, begin with research related risks that are expected, likely, and serious. Conclude with rarely expected risks and convey the likelihood.
- Insert hard page breaks if the page breaks at a place that makes it hard for the reader to follow such as right after a subhead.
- Do not use text smaller than 12 point.
- Use at least 1.5 spacing between paragraphs.
- Discuss only one or two ideas per paragraph.
- Keep paragraphs short.
- Avoid compound sentences.
- Use shorter, simpler words whenever they can convey appropriate meaning.
- Define or explain medical terms, procedures, and technical or complex words.
- Use U.S. measurements for metric measurements, or both. State 2 teaspoons rather than 10 MLs.
- Use regular time not military time. For example, say 1 p.m. rather than 1300 hours.
- Do not use exculpatory language, that is, language that indicates somebody (the researchers or UM) is free from blame.
- Write in the ‘second person,’ For example, instead of "The patient will be asked some questions about her medical history, then she will have a small amount of blood drawn,” state, "You will be asked some questions about your medical history, then a small amount of your blood will be drawn."
- Do not imply ‘cut and paste’ protocol sections or include elaborate details of the procedures of the protocol.
- Do not include highly scientific objectives or hypotheses that are of little relevance to the participants. These may bias your results or confuse participants.
- Consider what the participant would want to know.
Examples of protocol language versus subject-friendly language text
IRBMED has full accreditation by they Association for the Accreditation of Human Research Protection Programs, Inc.
We transform lives through bold discovery, compassionate care and innovative education.
- Diversity, Equity & Inclusion
- News & Stories
- Find a Doctor
- Conditions & Treatments
- Patient & Visitor Guide
- Patient Portal
- Clinical Trials
- Research Labs
- Cores and Resources
- Programs & Admissions
- Our Community
- Departments, Centers & Offices
- About the Medical School
Global Footer Secondary Navigation

UCL Research Ethics
- Advice on writing an information sheet and consent form

Writing a Participant Information Sheet and Consent Form
Recruitment documents help people make informed choices about whether to participate in a research study. Find out how to write a Participant Information Sheet, example forms and further guidance.
Writing a Participant Information Sheet
Participant Information Sheets must be designed to assist participants to make informed choices. Potential recruits must be given sufficient information to allow them to decide whether or not they want to take part. The process of obtaining consent and the accompanying documentation must be approved by a research ethics committee and, where only verbal consent to research is contemplated include consideration of an appropriate process for witnessing the consent.
Researchers must take the steps necessary to ensure that all participants in the research understand the process in which they are to be engaged, including why their participation is necessary, how it will be used, and how and to whom it will be reported so that the prospective participant can make an informed decision about whether they really do want to take part.
It is highly recommended that the information provided is presented on headed paper and is accurate, clear and simple so that someone with a reading age of 8 would understand the contents (use short words, sentences and paragraphs). The information should be specific to the proposed research and appropriate for the social and cultural context in which is it being given. It is important to avoid technical terms, jargon and abbreviations, bias, coercion or any inappropriate inducements.
What should the Participant Information Sheet include:
- A friendly invitation to participate.
- A brief and simple explanation of the purposes of the research and a statement explaining how the participant was chosen and how many other participants will be involved in the study.
- A statement that participation is voluntary; refusal to participate will involve no penalty or loss of benefits to which the participant is otherwise entitled; and the participant may discontinue participation at any time without penalty or loss of benefits.
- A thorough explanation of the expected duration of participation in the research and the procedures to be followed.
- A description of any reasonably foreseeable risks or discomforts and any benefits to the participant. For research involving more than minimal risk, an explanation as to whether any compensation or any medical treatments are available if injury occurs and, if so, what they consist of, or where further information may be obtained.
- A statement describing the extent, if any, to which confidentiality of records identifying the participant will be maintained.
- It is considered good practice for researchers to debrief participants at the conclusion of the research and to provide them with copies of any reports or other publications arising from their participation.
- If appropriate, a statement indicating that the data might be used for additional or subsequent research.
- An explanation of who to contact for answers to pertinent questions about the research and the rights of the participant and who to contact in the event of a research-related injury to the participant.
- If applicable, a statement declaring that each researcher who may have access to children (aged under 18) or vulnerable adults has undergone a satisfactory criminal records check.
- Remember to thank your participant for considering taking part in the study and include a statement indicating that the research study has been approved by the UCL Research Ethics Committee.
Language and layout
It is highly recommended that the information provided is presented on headed paper and is accurate, clear, and simple. The information should be specific to the proposed research and appropriate for the social and cultural context in which is it being given. It is important to avoid technical terms, jargon, and abbreviations, bias, coercion, or any inappropriate inducements.
The following points should be considered when writing an information sheet:
- Use clear, non-technical language. We recommend that you refer to the Plain English Campaign
- Use appropriate language for the target audience. For example, consider the different ways needed to communicate with primary school children as opposed to their teachers, or people with expertise in the area of study as opposed to people with no such knowledge
- Divide the text into paragraphs for ease of reading
- Consider using sub-headings for clarity, such as questions and answers
- Make sure the font and font size are legible.
Ask someone else to review your information sheet before it is circulated.
- Template Participant Information Sheet (Word)
- Template Consent Form (Word)
- Guidance on obtaining consent from research participants online (for online and in-person study designs)
Authors: Dr Pippa Lally, Behavioural Science and Health, and Jack Hindley, Information Services Division, UCL
- Recording & Obtaining Consent
UCL Research Ethics Committee Guidance Note 2: Extract from Nuffield Council on Bioethics website
Page last updated: April 2023
- Privacy Policy

Home » Informed Consent in Research – Types, Templates and Examples
Informed Consent in Research – Types, Templates and Examples
Table of Contents

Informed Consent in Research
Informed consent is a process of communication between a researcher and a potential participant in which the researcher provides adequate information about the study, its risks and benefits, and the participant voluntarily agrees to participate. It is a cornerstone of ethical research involving human subjects and is intended to protect the rights and welfare of participants.
Types of Informed Consent in Research
There are different types of informed consent in research , which may vary depending on the nature of the study, the type of participants, and the context. Some of the common types of informed consent in research include:
Written Consent
This is the most common type of informed consent, where participants are provided with a written document that explains the study and its requirements. The document typically includes information about the purpose of the study, procedures involved, risks and benefits, confidentiality, and participant rights. Participants are asked to sign the document as an indication of their willingness to participate.
Oral Consent
In some cases, oral consent may be used when a written document is not practical or feasible. Oral consent involves explaining the study and its requirements to participants verbally and obtaining their consent. This method may be used for studies with illiterate or visually impaired participants or when conducting research remotely.
Implied Consent
Implied consent is used in studies where participants’ actions are taken as an indication of their willingness to participate. For example, a participant may be considered to have given implied consent if they show up for a scheduled appointment for the study.
Opt-out Consent
This method is used when participants are given the opportunity to decline participation in a study. Participants are provided with information about the study and are given the option to opt-out if they do not wish to participate. This method is commonly used in population-based studies or surveys.
Assent is used in studies involving minors or participants who are unable to provide informed consent due to cognitive impairment or disability. Assent involves obtaining the agreement of the participant to participate in the study, along with the consent of a legally authorized representative.
Informed Consent Format in Research
Here’s a basic format for informed consent that can be customized for specific research studies:
- Introduction : Begin by introducing yourself and the purpose of the study. Clearly state that participation is voluntary and that participants can withdraw at any time without penalty.
- Study Overview : Provide a brief overview of the study, including its purpose, methods, and expected outcomes.
- Procedures : Describe the procedures involved in the study in clear, concise language. Include information about the types of data that will be collected, how they will be collected, and how long the study will take.
- Risks and Benefits : Outline the potential risks and benefits of participating in the study. Be honest and upfront about any discomfort, inconvenience, or potential harm that may be involved, as well as any potential benefits.
- Confidentiality and Privacy : Explain how participant data will be collected, stored, and used, and what measures will be taken to ensure confidentiality and privacy.
- Voluntary Participation: Emphasize that participation is voluntary and that participants can withdraw at any time without penalty. Explain how to withdraw from the study and who to contact if participants have questions or concerns.
- Compensation and Incentives: If applicable, explain any compensation or incentives that will be offered to participants for their participation.
- Contact Information: Provide contact information for the researcher or a representative from the research team who can answer questions and address concerns.
- Signature : Ask participants to sign and date the consent form to indicate their voluntary agreement to participate in the study.
Informed Consent Templates in Research
Here is an example of an informed consent template that can be used in research studies:
Introduction
You are being invited to participate in a research study. Before you decide whether or not to participate, it is important for you to understand why the research is being done, what your participation will involve, and what risks and benefits may be associated with your participation.
Purpose of the Study
The purpose of this study is [insert purpose of study].
If you agree to participate, you will be asked to [insert procedures involved in the study].
Risks and Benefits
There are several potential risks and benefits associated with participation in this study. Some of the risks include [insert potential risks of participation]. Some of the benefits include [insert potential benefits of participation].
Confidentiality
Your participation in this study will be kept confidential to the extent allowed by law. All data collected during the study will be stored in a secure location and only accessed by authorized personnel. Your name and other identifying information will not be included in any reports or publications resulting from this study.
Voluntary Participation
Your participation in this study is completely voluntary. You have the right to withdraw from the study at any time without penalty. If you choose not to participate or if you withdraw from the study, there will be no negative consequences.
Contact Information
If you have any questions or concerns about the study, you can contact the investigator(s) at [insert contact information]. If you have questions about your rights as a research participant, you may contact [insert name of institutional review board and contact information].
Statement of Consent
By signing below, you acknowledge that you have read and understood the information provided in this consent form and that you freely and voluntarily consent to participate in this study.
Participant Signature: _____________________________________ Date: _____________
Investigator Signature: ____________________________________ Date: _____________
Examples of Informed Consent in Research
Here’s an example of informed consent in research:
Title : The Effects of Yoga on Stress and anxiety levels in college students
Introduction :
We are conducting a research study to investigate the effects of yoga on stress and anxiety levels in college students. We are inviting you to participate in this study.
If you agree to participate, you will be asked to attend four yoga classes per week for six weeks. Before and after the six-week period, you will be asked to complete surveys about your stress and anxiety levels. Additionally, we will measure your heart rate variability at the beginning and end of the six-week period.
Risks and Benefits:
There are no known risks associated with participating in this study. However, the benefits of practicing yoga may include decreased stress and anxiety levels, increased flexibility and strength, and improved overall well-being.
Confidentiality:
All information collected during this study will be kept strictly confidential. Your name will not be used in any reports or publications resulting from this study.
Voluntary Participation:
Participation in this study is completely voluntary. You are free to withdraw from the study at any time without penalty.
Contact Information:
If you have any questions or concerns about this study, you may contact the principal investigator at (phone number/email address).
By signing this form, I acknowledge that I have read and understood the above information and agree to participate in this study.
Participant Signature: ___________________________
Date: ___________________________
Researcher Signature: ___________________________
Importance of Informed Consent in Research
Here are some reasons why informed consent is important in research:
- Protection of participants’ rights : Informed consent ensures that participants understand the nature and purpose of the research, the risks and benefits of participating, and their rights as participants. It empowers them to make an informed decision about whether to participate or not.
- Ethical responsibility : Researchers have an ethical responsibility to respect the autonomy of participants and to protect them from harm. Informed consent is a crucial way to uphold these principles.
- Legality : Informed consent is a legal requirement in most countries. It is necessary to protect researchers from legal liability and to ensure that research is conducted in accordance with ethical standards.
- Trust : Informed consent helps build trust between researchers and participants. When participants understand the research process and their role in it, they are more likely to trust the researchers and the study.
- Quality of research : Informed consent ensures that participants are fully informed about the research and its purpose, which can lead to more accurate and reliable data. This, in turn, can improve the quality of research outcomes.
Purpose of Informed Consent in Research
Informed consent is a critical component of research ethics, and it serves several important purposes, including:
- Respect for autonomy: Informed consent respects an individual’s right to make decisions about their own health and well-being. It recognizes that individuals have the right to choose whether or not to participate in research, based on their own values, beliefs, and preferences.
- Protection of participants : Informed consent helps protect research participants from potential harm or risks that may arise from their involvement in a study. By providing participants with information about the study, its risks and benefits, and their rights, they are able to make an informed decision about whether to participate.
- Transparency: Informed consent promotes transparency in the research process. It ensures that participants are fully informed about the research, including its purpose, methods, and potential outcomes, which helps to build trust between researchers and participants.
- Legal and ethical requirements: Informed consent is a legal and ethical requirement in most research studies. It ensures that researchers obtain voluntary and informed agreement from participants to participate in the study, which helps to protect the rights and welfare of research participants.
Advantages of Informed Consent in Research
The advantages of informed consent in research are numerous, and some of the most significant benefits include:
- Protecting participants’ autonomy: Informed consent allows participants to exercise their right to self-determination and make decisions about whether to participate in a study or not. It also ensures that participants are fully informed about the risks, benefits, and implications of participating in the study.
- Promoting transparency and trust: Informed consent helps build trust between researchers and participants by providing clear and accurate information about the study’s purpose, procedures, and potential outcomes. This transparency promotes open communication and a positive research experience for all parties involved.
- Reducing the risk of harm: Informed consent ensures that participants are fully aware of any potential risks or side effects associated with the study. This knowledge enables them to make informed decisions about their participation and reduces the likelihood of harm or negative consequences.
- Ensuring ethical standards are met : Informed consent is a fundamental ethical requirement for conducting research involving human participants. By obtaining informed consent, researchers demonstrate their commitment to upholding ethical principles and standards in their research practices.
- Facilitating future research : Informed consent enables researchers to collect high-quality data that can be used for future research purposes. It also allows participants to make an informed decision about whether they are willing to participate in future studies.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

How to Cite Research Paper – All Formats and...

Data Collection – Methods Types and Examples

Delimitations in Research – Types, Examples and...

Research Paper Format – Types, Examples and...

Research Process – Steps, Examples and Tips

Research Design – Types, Methods and Examples
Forms & Consent Templates
Main navigation, consent templates.
The IRB recommends the use of the consent templates to help researchers meet the legal requirements for consent.
See the Informed Consent Process page for more information about the consent process .
Medical (SoM)
- School of Medicine (SoM)
- Lucile Packard Children's Hospital (LPCH)
- Stanford Hospital and Clinics (SHC)
- Veteran's Affairs (VA) Hospital
- Psychology fMRI studies
Social & Behavioral Research (Non-Medical)
- Engineering
- Humanities & Sciences
The IRB uses these checklists and forms to review protocols for compliance with regulations, policies and guidance:
Staff Checklists
- Exemption Eligibility
Protocol Checklists:
- Protocol - Medical
- Protocol - Expedited (initial review)
- Protocol - Chart Review (initial review)
- Protocol - Nonmedical
- Protocol - sIRB Checklist
- SCRO Renewal Review Checklist
- Research Involving VA Studies ; see Reviewing Veterans Affairs (VA) Research for additional requirements
- Exemption from IRB Review: Emergency Use of a Test Article
- Single Patient IND/IDE
Other Federal Agency Requirements:
- Dept. of Defense (DoD)
- Dept. of Education (ED)
- Dept. of Energy (DOE)
- Dept. of Justice (DOJ)
- Environmental Protection Agency (EPA)
Informed Consent:
- Informed Consent (medical: clinical studies)
- Informed Consent (medical: expedited/minimal risk)
- Informed Consent (nonmedical: surveys, social, behavioral, education research)
Continuing Review:
- Continuing Review - FULL/EXPEDITED
Reviewer Checklists :
- My UW-System
- Student Life
- Schools & Colleges
- Centers & Institutes
- Leadership Team
- For Faculty and Staff
- For Researchers
- Request Info
- Give to UWM
University of Wisconsin-Milwaukee
Human research protection program institutional review board, consent form templates.
Consent templates are provided as a convenience to our researchers. If you prefer to write your own consent document, you may do so, but be sure to include all required elements of informed consent .
Click here for guidance on informed consent from the Office of Human Research Protection (OHRP)
General Consent Form Templates
Standard Adult Informed Consent Form
Online Survey Consent Form – for studies collecting data via an online survey
Parental Permission and Child Assent Form Templates
Parent Consent Form – use in conjunction with the Child Assent form below
Child Assent Form – typically used for children ages ~6-12
Combined Parent Consent and Child Assent Form – Same as the Standard Adult Informed Consent; use with children ages ~12-17
ONLY for studies approved prior to August 2021: Waiver Request Form
If you’re using the new IRB Application Form in I-Manager, you do not need to submit the waiver as a separate document. The questions are included within the form.
This form should only be used when requesting a new waiver on existing studies.
Request to Waive, Waive Documentation of Consent, or Alter Consent – Complete page 1 if you will not be obtaining consent, or if you will be removing elements of consent from the consent form. – Complete page 2 if you’re not collecting a written signature on the consent form. You still also need to submit a consent script (see templates above).
Sample consent and permission forms
General consent form to participate in research (DOC)
Two stage project consent form (DOC)
Parent permission form for research with child (DOC)
Child assent form (DOC)
Multiple consent form including audio-recording and quotations (DOC)
Photo and video consent form (DOC)
Video-recording consent form (DOC)
Re-contact agreement form (DOC)
Post-debriefing consent form (DOC)

IMAGES
VIDEO
COMMENTS
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. General Consent Form Templates Social and Behavioral Research Projects (last updated 03/16/2023)
Plain Language Consent Template. Use this template for: Biomedical and cancer research. Social, behavioral, and educational research. One-time survey research. Simple blood draw research. Collection and/or storage or biological specimens for research (GWAS compliant) Last Updated October 2023. Companion Document.
Consent Template Exempt Research This consent form is an example, designed specifically for Exempt survey research and is provided purely as a service by the IRB. The IRB does not review or approve the content of exempt consent forms. The consent form should not include any mention of IRB approval and it should not include the standard IRB ...
Consent Templates and Guidance. The templates on this page are intended to help investigators construct documents that are as short as possible and written in plain language. The informed consent form (ICF) templates provided by the IRB comply with federal regulations. What if I only need to provide new study information to a limited number of ...
2023-04-10. Assent Form Ages 7-14. 2023-06-27. Consent Addendum for Unencrypted Communication. 2020-10-26. Information or Fact Sheet. 2023-04-10. The following documents are samples. IRBIS does NOT generate these documents with application-specific information.
IC Template - Standard v1.0 Page 1 of 9 version date 10.12.2018 . Standard Informed Consent Template for Research . Use this template if your research is . NOT Federally-sponsored. AND. participants are . adults. Black "we" and "our" (for researcher); not third person (e.g., "we will ask participants"). • Avoid Common Problems ...
These templates are appropriate for social, behavioral, and educational ("SBER") research that does not include any biomedical procedures. Study Information Sheet (no signature) Consent Form (includes signature) Consent Form for Federally Funded Research. Parent Permission Form (parents provide permission for child to participate)
The UW IRB provides the UW research community with a variety of consent templates that align with regulatory and policy requirements and best practices as described in our main Consent guidance and guidance on Designing the Consent Process. The first two templates, marked with an asterisk, are the templates most non-exempt studies will choose from.
If you are conducting a human subjects study at the University of Michigan, you need to follow the informed consent guidelines and templates provided by the Research Ethics & Compliance office. This webpage explains the meaning and purpose of informed consent, offers tips and examples for creating an effective consent document, and links to various templates and sample documents for different ...
Start with an IRB-approved consent form template. Headings for paragraphs are helpful and make the form easier to read. Use adequate white space so that the form is easy to read. Avoid fine print. 1. Provide a clear, concise explanation of the purposes of the research including the name of the study (the IRB can waive under certain specific ...
Informed Consent Templates. As part of our continued efforts to improve the IRB application and review process, we have developed new ICF templates that address the issues identified by our stakeholders: Different templates have been created for different types of studies (SBER, Biomed, etc.). Guidance language has been added to each template ...
Consent and Assent Form Templates. Informed Consent and HIPAA Authorization Form Template (Post 12-4-2023) DOCX: This template should be used as the consent document guide for all new research studies, including parental and LAR permission (consent) forms, submitted for IRB review on or after December 4th, 2023.
For multi-centre research studies, a common consent form will be taken as a minimum requirement to which additions may be made as dictated by local circumstances. In such cases, the common consent form ... To assist researchers, WHO has developed Informed Consent Form templates for various types of research studies.
Consent Templates. The templates below were created to help you create the documents you will need to communicate to participants what they will do in the study. The documents you provide participants will range from recruitment materials to post-debrief consent forms, and you need to submit everything that you provide to a participant to our ...
Informed consent templates have been developed to assist researchers in developing a consent form for a research study. The template is only a guide to help researchers and may be subject to change. Please note that IRB members may still request changes to your document. The revised Common Rule implements January 21, 2019 for new studies.
This is a template to assist researchers in the design of their informed consent form. You must adapt this template to the requirements of your particular study, using the notes and suggestions provided. Before using this template, check whether your organisation provides a template consent form and if so, incorporate their requirements into ...
Informed consent is an ongoing process that begins with recruitment and continues through the completion of the study. The first step is to provide potential subjects with general information about the nature of the study and why they might be suited to participate. The recruitment flyers, websites, and phone scripts are part of this process.
Template Consent Form (Word) Further Guidance. Guidance on obtaining consent from research participants online (for online and in-person study designs) Authors: Dr Pippa Lally, Behavioural Science and Health, and Jack Hindley, Information Services Division, UCL. Recording & Obtaining Consent;
If you have questions or are having trouble accessing these forms, please contact IRB Education ( email or call 650-724-7141). The consent/assent form should be in a language that is understandable to someone without a medical or scientific background. Please use the Microsoft Readability Statistics tool as needed when writing your consent form.
Informed Consent Templates in Research. Here is an example of an informed consent template that can be used in research studies: Title of Study: [Insert Title of Study] Investigator (s): [Insert Name (s) of Investigator (s)] Introduction. You are being invited to participate in a research study.
A step-by-step guide to filling out a general research informed consent form can be found below. Instructions - Use to fill in the blank template. How to Write. Step 1 - Download in PDF, Microsoft Word (.docx), or Open Document Text (.odt). Step 2 - The title of the research study being conducted must be included at the top of the consent ...
The IRB recommends the use of the consent templates to help researchers meet the legal requirements for consent. See the Informed Consent Process page for more information about the consent process . Medical (SoM) School of Medicine (SoM) Lucile Packard Children's Hospital (LPCH) Stanford Hospital and Clinics (SHC) Veteran's Affairs (VA) Hospital.
This form should only be used when requesting a new waiver on existing studies. - Complete page 1 if you will not be obtaining consent, or if you will be removing elements of consent from the consent form. - Complete page 2 if you're not collecting a written signature on the consent form. You still also need to submit a consent script ...
Cloned 2,070. A Research Consent Form is a document used to capture the consent of the participant in the research project. This document is important because it will protect both parties involved in any legal issues related to privacy and content management. This document can be used for any type of research like medical, clinical, scientific ...
The following is a sample consent form for a research project. It is a research project on faculty life on campus, carried out by the principle investigator (PI) of this project from the fake-named Century University. The interviewer (the investigator) should have the interviewee read this form carefully and ask any questions the interviewee ...
Participant Consent Form This template is designed primarily for those doing qualitative interviews with adults from non-vulnerable populations and dealing with non-sensitive topics. The form would be different in the case of focus groups or quantitative research. If conducting research with vulnerable populations and / or sensitive topics please
ЗГОДА НА ДОБРОВІЛЬНУ УЧАСТЬ ЯК СУБ'ЄКТА ДОСЛІДЖЕННЯ - КОРОТКА ФОРМА. Назва дослідження: _____
Sample consent and permission forms. General consent form to participate in research (DOC) Two stage project consent form (DOC) Parent permission form for research with child (DOC) Child assent form (DOC) Multiple consent form including audio-recording and quotations (DOC) Photo and video consent form (DOC)
Cloned 1,048. A Research Informed Consent Form is a consent acquisition form for persons who may avail participation in a research program. Researchers conduct a study that would require human participation. However, research and medical institutions who are conducting research must secure an informed consent from their participant.