Graduate Opportunities
ICON's Graduate programs are designed to provide recent university graduates with opportunities for professional development and career growth.
These programs often include mentorship, training, and hands-on experience in various departments of the company. Graduate programs represent a crucial step towards advancing your career and gaining a deeper understanding of your field of study. Through rigorous coursework, research opportunities, and specialized training, graduate programs equip students & graduates with the knowledge and skills necessary to succeed in their chosen profession.
Whether you're looking to deepen your expertise in a particular subject or make a career change altogether, a graduate program can help you achieve your goals and take your career to the next level.

Biostatistician Academy - UK
The Biostatistician Academy is formatted to provide comprehensive training for university graduates who have their Master’s Degree in Statistics or Biostatistics. In this graduate programme, participants undergo a comprehensive group training programme offering a combination of classroom-based and practical learning which develops SAS programming and builds upon the formal Master’s program. Once individuals graduate from the academy, they become eligible for our bridge to lead program, so the learning and support continues. We pride ourselves on our amazing company culture, where we work as one team to achieve industry excellence.
Statistical Programming Academy - China, India, UK
The Statistical Programming Academies offer new graduates in Math and Life Sciences the opportunity to start their career in Statistical Programming with a comprehensive group training programme offering a combination of classroom-based and practical learning that develops SAS programming skills and develops end to end programming abilities (including SDTM, ADaM and TFLs). Once individuals graduate from the academy, they become eligible for our bridge to lead program, so the learning and support continues. We have been running this academy for many years and now have some of our academy hires in Director level roles. We pride ourselves on our amazing company culture, where we work as one team to achieve industry excellence.
Clinical Trial Assistant Academy - India
The Clinical Trial Assistant Academy is a 16-17 week programme comprised of 5 core modules which are aimed at enhancing the skills and competencies required to understand the foundations of Clinical Trials within the IPH division. The program is delivered via blended learning and includes facilitator-led sessions, self-directed learning, discussion- based group sessions, and applied learning group projects and presentations. Whilst participating in the Academy, individuals are also exposed to real life application of learning through the support to study teams as Clinical Trial Assistants. Continuous mentoring and coaching throughout the program ensures we appropriately equip all candidates with every opportunity for success. All candidates who successfully graduate from the Clinical Academy, become eligible for promotion to the CRA Academy.
Site Management Associate Academy - Canada, China, Japan, Mexico, UK, US
ICON Biotech hires new graduates with educational backgrounds in life or health sciences, research, or healthcare/clinical experience into Site Management Administrator/ and Site Management Associate (SMA) roles. In SMA roles, they gain the required experience to continue their career growth within the organisation, frequently advancing to Study Start-Up Lead, then Site Activation Manager, or Clinical Research Associate (CRA), then Clinical Trial Manager (CTM). The SMA performs investigative site communication, essential document collection and review, clinical status tracking, in-house site management, and data review activities in accordance with the project protocol, client and ICON standard operating procedures (SOPs), and all applicable guidelines and regulatory requirements.
Project Associate Pathways - Canada, Japan, Mexico, UK, US
ICON Biotech hires new graduates in Project Associate roles where they support studies across all regions. New Hires complete a 90-day Onboarding Progression Plan (OPP) covering company policies/processes and core competencies. In the following 12 months, the leadership team works with each Project Associate to create an individualized career development plan. To date, we have identified 39 career pathways for Project Associates at ICON.
Clinical Data Academy - India, Mexico
The Clinical Data Management Academy offer new graduates the opportunity to start their career in Clinical Data Coordinator with a comprehensive group training programme offering a combination of classroom-based and practical learning that develops data management skills. Once individuals graduate from the academy, they become eligible for our bridge to lead program, so the learning and support continues. We have been running this academy for many years. .
Heading here for accessibility
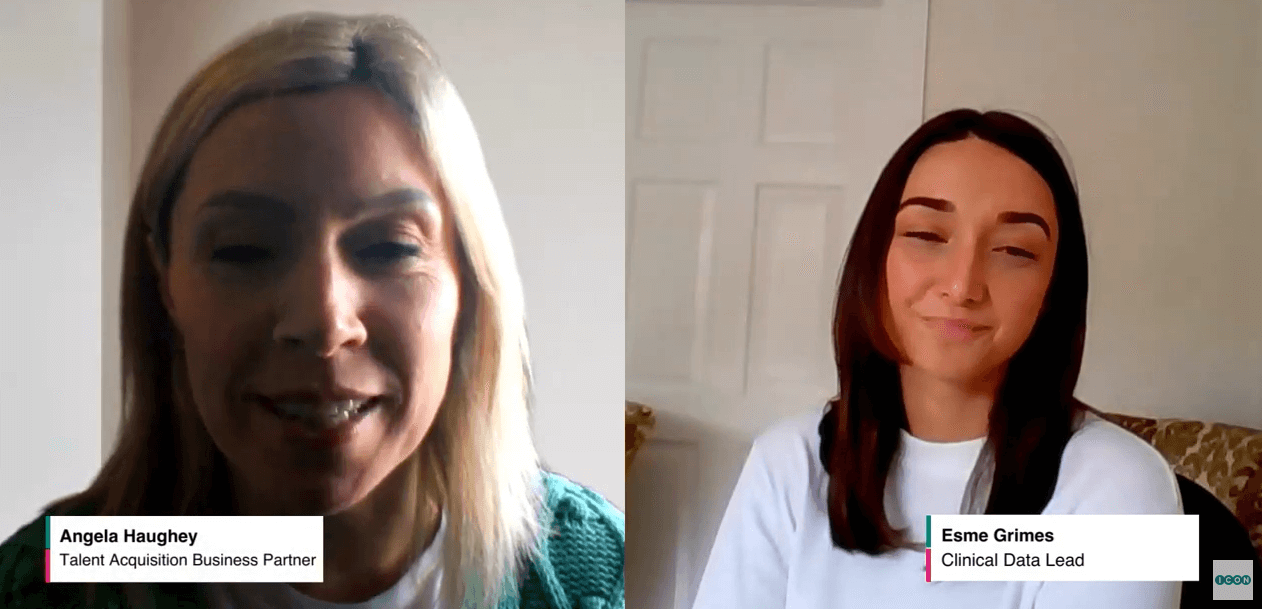
Clinical Data Academy
Angela Haughey, Talent Acquisition Business Partner, speaks with Esme Grimes, Clinical Data Lead to hear more about her ICON journey having gone through the Clinical Data Academy.

The thing I love the most about my role is how every day is different and the experience gained through this is extremely valuable. I feel that in my 6 months as a Project Associate I have learned so much already. Another thing I love about the role is how supportive every single person I have worked with has been, ranging from management, other PAs in the office and across the world to the study teams I work with each day.
Project Associate

My role offers me the opportunity to gain experience in various areas of the business, allowing me to explore and understand different functions within the organization. This exposure has helped me identify my strengths, interests, and areas I enjoy. This valuable experience will undoubtedly guide my future career decisions and enable me to pursue a path aligned with my passions and strengths.

I first started as an Assistant Clinical Data Coordinator in 2010 after completing my graduation in Biotechnology and post graduate diploma. It is such a pleasure to work in the Data Management department, with excellent guidance and opportunities to learn and grow. I am now a Group Lead in this department. ICON is a great place; the employees are welcoming and very supportive. With steady career progression and a lot more to learn, I will always recommend ICON to the applicants looking for a career in the CRO industry. There is always something challenging happening and every day is filled with new learning opportunities.
Data Management Group Leader

“ICON have provided me various opportunities which have helped me to expand my knowledge and skills. I have been able to learn from some of the best in the industry, and I feel confident in my abilities to contribute in important projects and company’s growth.”
Clinical Data Coordinator II
Roles in this area
US, Blue Bell (ICON)
Full Service - Symphony Health
Remote Working
Office Based
Business Area
ICON Full Service & Corporate Support
Job Categories
Description
** This internship is based in our Blue Bell, Pennsylvania, office. Remote / Work from Home is not an option. **At ICON, it’s our people that set us apart. Our diverse teams enable us to become a bett
Expiry date

Mexico, Benito Juarez (PRA)
Clinical Monitoring
Real World Solutions
Mexico City
Benito Juarez
At ICON, it’s our people that set us apart. Our diverse teams enable us to become a better partner to our customers and help us to fulfil our mission to advance and improve patients’ lives.Our ‘Own It

US, Wilmington (PRA)
At ICON, it’s our people that set us apart. Our diverse teams enable us to become a better partner to our customers and help us to fulfil our mission to advance and improve patients’ lives. Our ‘Ow

Study Start Up
At ICON, it’s our people that set us apart. Our diverse teams enable us to become a better partner to our customers and help us to fulfil our mission to advance and improve patients’ lives. Our ‘Own I

US, Cary, NC

Bulgaria, Sofia

A better career. A better world. A better you.
Browse popular job categories below or search all jobs above
- About AstraZeneca
Life at AstraZeneca
- Our Locations
- Information Technology
- BioPharmaceuticals
- BioPharmaceuticals R&D
- International
- Enabling functions
- Oncology R&D
- Early Talent
Inclusion & Diversity
- Application Hints & Tips
Keyword Search
City, State, or ZIP
Discover your career with us
Want to start your career at the cutting-edge of medical innovation? To unleash creative ideas that stand out in a global market leader? And work with some of the brightest minds in the industry who will push you to be your best? All while building the personal and professional skills you need to lead our exciting, dynamic evolution? Join us and impact millions of lives, together.
Early Talent programmes
Read about and apply to our programmes using the links below.
Business Apprenticeships UK
Locations: Cambridge and Macclesfield, UK
Our business teams support the research and development of life-changing medicines for our patients every step of the way and help turn our most ambitious goals into reality. Functions such as Finance, Human Resources, Project Management and Supply Chain play a part in supporting our people and our processes. So, whether you have a passion you want to pursue or are still figuring it out, there’s endless opportunity to experience new things and learn from different areas of the business to discover what truly inspires and motivates you.
Digital, Technology Solutions, and Data Apprenticeships UK
In the AstraZeneca Information Technology (IT) organisation, we are passionate about driving technological innovation, continuously improving our IT environment to ensure our internal teams can do their best work and successfully deliver our business goals. Here, you’ll work with world-renowned talent and leaders on real projects to gain hands-on experience whilst also working towards your qualification.
Operations Apprenticeships UK
Locations: Macclesfield and Speke UK
Global Operations at AstraZeneca facilitates innovation and connection between our science laboratories and our patients. We provide a vital platform for our business to play a key role in the development, manufacturing, testing and delivery of our medicines across the globe. Here, you’ll learn and earn in a supportive team, guided by expert managers, mentors and buddies who will help you develop the skills you seek, while pushing and encouraging you to get the most out of your time with us.
Scientific Apprenticeships UK
We are a scientifically led business and our purpose is all about pushing the boundaries of what science can do. Our science apprenticeships involve working in our laboratories and being part of our journey to provide life changing medicines to patients all over the world – transforming lives for the better.
Year 10 work experience UK
Locations: Virtual Programme, UK
At AstraZeneca we recognise that access to work experience is important for students to gain valuable insights into different industries and to help them with their professional and personal development.
We are delighted that AstraZeneca will be hosting virtual work experience opportunities for Year 10 students in the UK during June and July 2024. Please see below for an overview of the week and to book your place, please click on the link below:
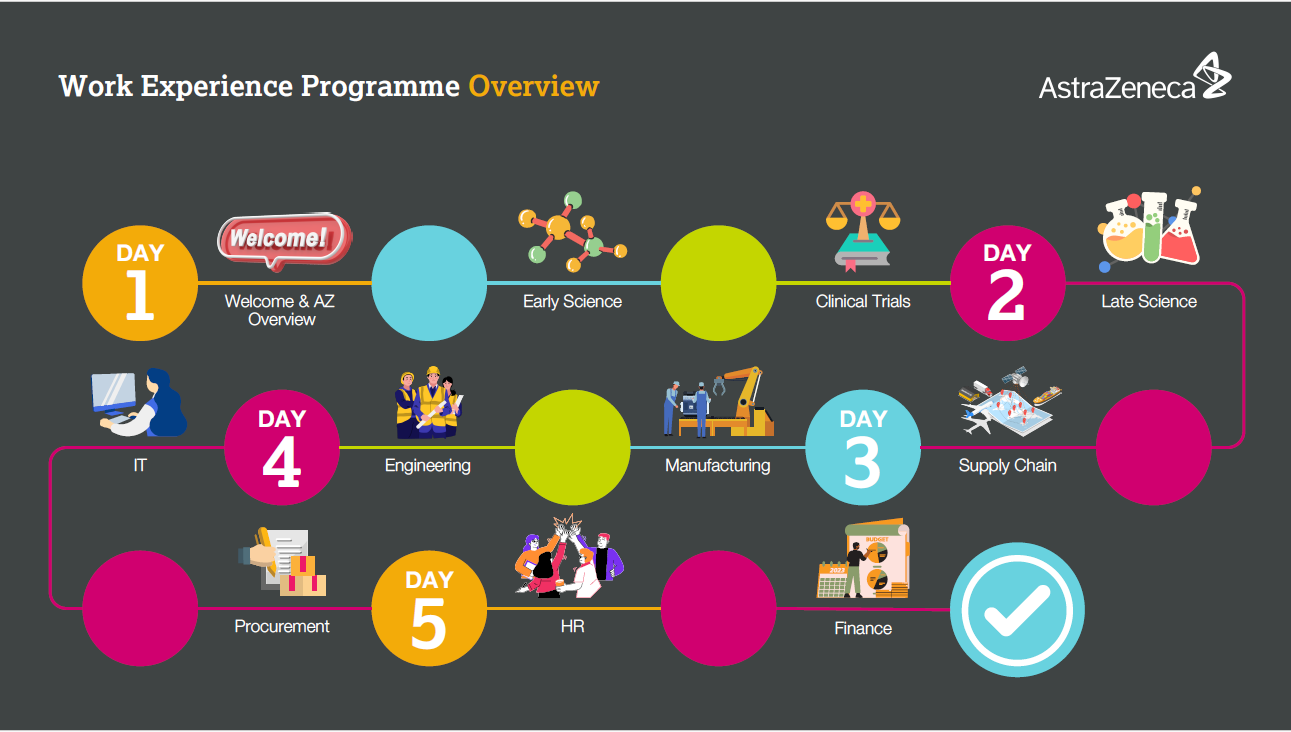
Year 12 work experience UK
Locations: Macclesfield, UK
At AstraZeneca we recognise that access to work experience is important for students to gain valuable insights into different industries and to help them with their professional and personal development Please see below for available opportunities:
Pharmaceutical Technology & Development
Macclesfield Campus, Cheshire
Week of 8th or 15th July 2024
Application link coming soon
Supply Chain
W/c 15th July 2024
Internships (UK)
AstraZeneca is a place where we all help and empower each other. Our supervisors are committed to providing you with all the guidance and support you need to build your skills, confidence and capabilities. Our internships are for those who want to gain an insight into a particular sector or role within our global organisation. Varying in length and aim, our internships are the perfect opportunity to set yourself up for a successful career.
Leaders in Finance Tomorrow (US)
The Leaders in Finance Tomorrow (LIFT) is an AstraZeneca US internship program that provides the best and the brightest students with a unique opportunity to gain meaningful finance and accounting experience within our industry-leading business. As a young professional, you’ll be treated as such – given all the trust and empowerment you need to take on new challenges, but with all the help and guidance you need to succeed.
Pharmaceutical Technology & Development Undergraduate Industrial Placement Studentship (UK)
Our Pharmaceutical Technology & Development Undergraduate Industrial Placement Studentship (PT&D UIPS) introduces you to the world of ground-breaking drug development, placing you in highly dedicated teams committed to delivering impactful new medicines to patients. Here, you’ll gain an insight into what it’s like to work in a big biopharma, with exposure to people who truly care about your personal career development.
Research & Development Undergraduate Industrial Placement Studentship (UK & Sweden)
The Research & Development Undergraduate Industrial Placement Studentship (R&D UIPS) introduces undergrads to the world of drug discovery, embedding students within highly dedicated teams driven to create impactful new medicines. Working on projects with real patient implications, you could have the opportunity to contribute to making a meaningful, life-changing impact for millions of people around the world.
Student Workers & Internships (US)
Join AstraZeneca’s student workers or interns, and you’ll find a biopharmaceutical environment that’s full of unique opportunities and exciting challenges. Here, you’ll have the opportunity to pursue your areas of interest whilst equally developing a broad skillset and knowledge base to get the best out of your experience. You’ll be given meaningful projects from day one, and you’ll enjoy plenty of chances to network within your chosen business area, too.
Work Placement Student Programme (Sweden)
The Work Placement Student Programme provides the opportunity for you to undertake a 12-month placement in our Gothenburg site in Sweden that will introduce you to the world of innovative drug discovery pushing the boundaries of science to deliver life-changing medicines. You’ll get to do meaningful work in a pioneering research and development driven organisation.
Biometrics Graduate Programme
Locations: Canada, Poland, Spain, Sweden, UK, US
Collaborating cross-functionally with experts in a stimulating, fast-paced environment, you’ll have all the freedom, empowerment and support to add value in study design, data analytics and interpretation of our innovative portfolio of medicines. Applying your knowledge to accelerate your career and make an impact. Because here, you’re truly valued. Encouraged to speak up, ask questions, get stuck in and contribute in a meaningful way. Using your skills, passion and desire to learn, drive your development and achieve your personal and professional objectives.
Biopharmaceutical Development Graduate Programme
Locations: Cambridge, UK & Gaithersburg, US
Designed to help you transition from academia to industry, you’ll progress through different programme rotations – gaining valuable experience and insight across various functions within Biopharmaceutical Development to really push and expand your skills and knowledge. Working and collaborating on challenging technical projects across a range of therapeutic areas, you’ll find continuous opportunities to learn, develop and make a real impact. All whilst fuelling your curiosity, building lasting relationships, and working towards your personal and professional goals.
Data Science & Artificial Intelligence Graduate Programme
Locations: Cambridge, UK; Boston & Gaithersburg, US; Gothenburg, Sweden
We’re passionate about what the power of Data Science and AI can do. Right now, we’re on a journey to become a data-led enterprise that disrupts the industry and sets the standard. So, if you’re an innovative thinker with a passion for Data Science and AI, there’s never been a better time to be part of our Graduate Programme. Through the challenging, developmental placements, you’ll work closely with peers, mentors and experts to develop a broad set of skills You’ll work collaboratively, embracing new ideas and different perspectives. And you’ll bring creative, critical thinking to turn data and insights into actions that help develop life-changing medicines.
Finance Graduate Development Programme
The pace is fast and the levels of responsibility are high, but we know you have what it takes. Here, you’ll bring your ambition, skills and knowledge to enhance our business and drive progress. Leveraging data and new technology to provide insights and predictions to address business challenges and improve our organisational performance. Working closely and collaboratively with international peers and leaders, you’ll develop a deep and broad understanding of finance and the critical role it plays in supporting business performance and outcomes across our global enterprise. Grow your skills and gain the experience needed to join our next generation of finance leaders.
Local Graduate Programmes
In addition to our Global Graduate Programmes AstraZeneca has a number of local Graduate/Management Trainee Programmes available. Search by country on our global careers website, or follow the links to explore careers on your local AstraZeneca website.
Operations Graduate Programmes
Locations: China, France, Japan, Sweden, UK, US
We’re looking for open-minded, forward-thinking individuals eager to drive innovative change and influence our future direction of travel. Take the initiative, own it and run with it to make a difference for patients and our business. All while strengthening your capabilities and gaining a valuable insight into our broad business. Whether you’re looking to pursue a particular passion or are still figuring it out, the diverse experiences and transitions in the Operations Graduate Programmes will help you shape your future, no matter which path you follow.
Patient Safety Graduate Programme
Designed to help you deliver the next generation of life-changing medicines by progressing through our range of exciting placements in early and late stage drug development. Learning how Patient Safety supports clinical drug development and helps ensure the safety of our patients. You’ll grow, develop and get the opportunity to make real contributions to our projects.
Join our Patient Safety graduate programme and enjoy a high level of emphasis on your personal development and career progression, helping make a difference to our medicine, patients and society!
Pharmaceutical Technology & Development Graduate Programme
Locations: Macclesfield, UK & Gothenburg, Sweden
Tackle new scientific challenges. Seek opportunities to learn and grow in a highly supportive team. Collaborate and build close connections with peers and experts across the business. Join our PT&D graduate programme and enjoy a high level of emphasis on your personal development and career progression. Work on a variety of projects aligned to your experience and career ambitions. Build a broad skill base that will enable you to strengthen both your managerial and technical capabilities. And accelerate your learning.
Precision Medicine Academy
Precision Medicine is at the heart of what science can do at AstraZeneca. It combines the expertise of specialists from across the organisation, unlocking new potential approaches that put patients first, setting us apart from our competitors. Through advances in diagnostic testing, we are now able to identify how unique individuals respond to treatments, and match them with medicines that deliver the best outcome for them. Since 2014, we've been ranked among the top pharmaceutical companies for precision medicine approvals - proof of our world-class practices.
R&D Graduate Programme: Early Phase Drug Discovery
We’re looking for the next wave of scientists to make an impact and influence the agenda. Join our R&D programme and bring your skills, drive and curiosity to our teams and projects. Thinking innovatively and taking the initiative to help drive our drug design and development. Completing novel and cutting-edge research in state-of-the-art laboratories and developing the knowledge and confidence to become the scientist you aspire to be.
Technology Graduate Leadership Programme
Locations: UK, US, Sweden, Mexico & India
Our IT team is at the forefront of discovering and driving digital health and workplace automation to ensure our teams can continue to develop life-changing medicines. As a member of our Technology Graduate Leadership Programme, you’ll join our next wave of technology leaders. Use your skills and influence to deliver technology solutions. Improve the way we operate, serve customers and enable our teams to do their best work. And enjoy being recognised for your knowledge, experience and ideas. Together, we’ll drive our technology capabilities and make meaningful impact for our business and patients.
UK Commercial Graduate
At AstraZeneca UK, we bring life-changing medicines to patients across England, Scotland, Wales and Northern Ireland. As a member of the Association of the British Pharmaceutical Industry (ABPI), all of our sales, marketing and communications activities are subject to the ABPI Code of Practice.
Being based in the heart of London, at Pancras Square, offers us the strongest possible platform to transform the lives of patients in the UK, as the country’s leading biopharmaceutical company. It will bring AstraZeneca UK closer to our customers, partners and stakeholders in the UK’s healthcare environment, as well as foster greater collaboration with the wider AstraZeneca footprint.
Clinical Research Associate (CRA) Graduate Programme
Location: Wilmington, DE
Designed to prepare you for a career as a Clinical Research Associate, you’ll progress through different programme rotations, gaining valuable experience across functions within Site Management and Monitoring and building multidisciplinary skills. Supported by experts in their field, you’ll receive mentoring and training, bringing limitless opportunities to learn, grow, and develop your strengths in different ways.
Commercial Leadership Development Programme (MBA)
On this rotational programme, you’ll have the ability to learn and grow across global functions, markets and our broad pipeline of products. Expanding your knowledge and developing a global mindset to take you to the next level. Collaborating with bright, diverse minds across the business. There’s never been a better time to be part of our journey – at the cutting edge of innovation, impacting millions of people around the world. Explore our transformational opportunities and accelerate your development.
AstraZeneca's Graduate Programmes are open to Masters level applicants. Applicants with an MBA should consider our Commercial Leadership Development Programme.
Search "AstraZeneca" on www.findaphd.com to explore any PhD studentships we are currently offering in partnership with our academic collaborators.
Postdoc Programme
Locations: UK, US and Sweden
Our Postdoc Programme is for self-motivated individuals looking to take on interesting, high-impact projects in a collaborative, challenging and reputable environment. As a respected specialist, you’ll have the freedom and autonomy to contribute known skills, and the support to rapidly learn new approaches to follow the science, innovate and make an impact. Leveraging scientific development to springboard your career whilst making a meaningful difference to patients, science, and our business. Collaborating and sharing knowledge cross functionally, together with independently leading projects in your field of interest.
R&D Postdoctoral Challenge
The R&D Postdoctoral Challenge invites final year MD and/or PhD students and Postdoctoral researchers to propose innovative ideas to transform the treatment of some of the world's most complex diseases. This exciting opportunity offers a fully funded postdoctoral position, where you will join our vibrant scientific community to conduct pioneering research within one of AstraZeneca's core disease areas or expert functions. You will have access to the expertise, compounds, novel tools and technologies, and mentoring support you need to turn your ideas into reality.
Why choose AstraZeneca?
Starting your career with us means following the science, boldly disrupting norms and supporting one another in shaping the future. Collaborate with leaders and teams across a wide range of disciplines and explore your passions. Discover your career purpose with us. And build strong bonds that will last a lifetime.
We discover, design, develop and deliver life-changing medicines. Our teams deliver innovations across all areas of our business – from diabetes care to genetics. With career paths spanning the medical lifecycle, you could be joining us in areas like Clinical Development, Data Science and AI, Finance, Manufacturing or Supply. Explore our opportunities and kickstart the career that could take you from emerging professional to renowned subject matter expert.

Our Early Talent Awards
People are our top priority – our patients, colleagues and communities. Regardless of what programme you’re in, we bring diverse talent from all over the world together. We enable international perspectives and combine our strengths and knowledge to multiply our impact. That’s why we’ve won multiple awards as a trusted Biopharmaceuticals employer. These awards show us that we’re on the right track and they’re an incentive to work even harder on nurturing our culture and making it ever richer and more diverse.

Writing the perfect CV
Recall experiences in which you've demonstrated the behaviours listed in the job description
Highlight your contact information and make sure your voicemail message is clear
Make pertinent information like education, professional experience, leadership roles, team participation and volunteer experience easy to find
Format your CV so it's readable and concise. One page is preferable but two makes sense if you’ve got a lot of experience
Use descriptive language to present your experiences
Have evidence and examples to support your statements
Wordiness. Use short sentences. Treat your CV like an advertisement
Excessive use of borders, italics and fancy fonts. They don't upload cleanly into online systems
Overly small font size. Stick to 10-12 point
Too much bold type. Only enbold major achievements
Navigating the Application Process
Discover detailed insights and get on an AstraZeneca recruiter's short list with our application hints and tips.

We’ll keep you up-to-date
Sign up to be the first to receive job updates.
Email Address
Confirm Email
We are AstraZeneca, one of the world’s most forward-thinking and connected BioPharmaceutical companies. Explore our world.
At AstraZeneca, our purpose is to help patients all over the world by delivering life-changing medicines as one collaborative team.
The success of AstraZeneca is founded on innovation, creativity and diversity. Discover what this means for you.

Great culture, great work assignments, supportive management. Rotation opportunity within the company. They value inclusion and diversity.
Programs in Clinical Research
1 year | full-time, 2 years | part-time, boston university medical campus.
Boston University’s programs in Clinical Research aim to meet the needs of health professionals engaged in the full-spectrum of patient-oriented research. We offer both master’s degree and certificate programs to individuals seeking careers in clinical research in industry or academic settings.
Clinical Research program offerings:
- Master of Science in Clinical Research (MSCR)
- Online Graduate Certificate
- Dual Degree in Medical Sciences and Clinical Research
- Dual Degree in Medicine and Clinical Research
About the MS in Clinical Research Program (MSCR)
Located in Boston’s South End at the Boston University Chobanian & Avedisian School of Medicine, the Master of Science in Clinical Research Program (MSCR) has been in existence since 2001 and is ranked #1 by College Choice among universities that offer an MS in Clinical Research. As an entity of BU CAMED, students are provided with opportunities and are exposed to resources that are part of the Chobanian & Avedisian School of Medicine , the School of Public Health , the Goldman School of Dental Medicine , Boston Medical Center , two VA Administrations, and BioSquare. All of this offers students endless opportunities for personal, academic and professional development.
The Master’s in Clinical Research program, which is a STEM program, teaches students the scientific fundamentals of human research. Courses in our curriculum provide an in-depth look at all of the key elements in clinical research, including: trial design, trial management, biostatistics, ethical issues, and clinical research regulations. Other courses cover how basic science discoveries translate into clinical research and new therapies. The total course requirement is 32 credit hours: 22 hours are required curriculum, including practicum and research credits; 10 hours are elective courses. Students will also complete a research “practicum”; a hands-on involvement in a clinical research project under a scientific mentor. The final requirement for the degree is to conduct clinical research, write, and present a capstone project.
Who is this program designed for?
The Master’s in Clinical Research program is designed for anyone interested in a career in clinical research. This includes those with MD or PhD degrees interested in becoming independent principal investigators, as well as those with bachelors or masters degrees who seek advancement in a research career in industry or academic settings.
Online Graduate Clinical Research Certificate
If you are not ready to commit to our Master’s program, we also offer an Online Graduate Certificate in Clinical Research. This certificate program involves 4 courses across two semesters, and can be completed in one academic year.
Learning Objectives
Upon completion of the Master’s in Clinical Research program, students are expected to:
- Demonstrate the ability to design and conduct clinical research, analyze results and answer a research question.
- Demonstrate the ability to read and critique the clinical research literature.
- Present clinical research findings (from literature or their own research) to peers.
Our Mission
Inspire, Instruct, Innovate … The MS in Clinical Research program is dedicated to the discovery, development, and application of knowledge as it pertains to all areas of clinical research. Our mission is to foster an engaging and effective educational environment that promotes the pursuit of outstanding teaching and learning through formal classroom and practical training. With established collaborative relationships with pharmaceutical, biotech, and academic institutions, students are provided with unique opportunities to pursue clinical research in areas that are of personal and professional interest.
We hope that the information you receive about our program encourages you to pursue your graduate degree in clinical research with us. If you are interested, click here to get your application started.
If you would like to visit our campus or have any questions about our program, please contact Stacey Hess Pino at [email protected] . We look forward to meeting and/or speaking with you!
Janice Weinberg, ScD Director, MS in Clinical Research; P rofessor, Department of Biostatistics
Stacey Hess Pino, MS, MS Assistant Director, MS in Clinical Research; Director, Online Graduate Certificate Program in Clinical Research; Assistant Professor, Department of Medical Sciences & Education

- Recommendations
- Notifications
- My Favorites
Favorites, recommendations, and notifications are only available for UCLA Graduate Students at this time.
Access features exclusively for UCLA students and staff.
As a student, you can:
- Add funding awards to your favorites list
- Get notified of upcoming deadlines and events
- Receive personalized recommendations for funding awards
We're Sorry
You've signed in with a UCLA undergraduate student account.
UCLA Graduate Programs

Graduate Program: Clinical Research
UCLA's Graduate Program in Clinical Research offers the following degree(s):
Master of Science (M.S.)
With questions not answered here or on the program’s site (above), please contact the program directly.
Clinical Research Graduate Program at UCLA David Geffen School of Medicine 5303 Life Sciences Box 951766 Los Angeles, CA 90095-1766
Visit the Computational Medicine Department’s faculty roster
COURSE DESCRIPTIONS
Visit the registrar's site for the Computational Medicine Department’s course descriptions
- Admission Requirements
- Program Statistics
(310) 825-6312
MAJOR CODE: CLINICAL RESEARCH
Boston University Academics
Boston University
- Campus Life
- Schools & Colleges
- Degree Programs
- Search Academics
- MS in Clinical Research
For more information, please visit the Graduate Medical Sciences website .
The Master of Science in Clinical Research is a rigorous program that meets the needs of health professionals engaged in the full spectrum of patient-oriented research. This flexible degree program is designed for a variety of professionals, including physicians who will plan and oversee translational research and clinical trials; research nurses; study coordinators; managers in clinical research and site management organizations (CROs and SROs); and professionals in the pharmaceutical, biotechnology, and medical device industries.
Learning Objectives
Upon completion of the MS in Clinical Research , students are expected to:
- Demonstrate the ability to design and conduct clinical research, analyze results, and answer a research question.
- Demonstrate the ability to read and critique the clinical research literature.
- Present clinical research findings (from literature or their own research) to peers.
Our Mission
Inspire, Instruct, Innovate. The MS in Clinical Research program is dedicated to the discovery, development, and application of knowledge as it pertains to all areas of clinical research. Our mission is to foster an engaging and effective educational environment that promotes the pursuit of outstanding teaching and learning through formal classroom and practical training. With established collaborative relationships with pharmaceutical, biotech, and academic institutions, students are provided with unique opportunities to pursue clinical research in areas that are of personal and professional interest.
We hope that the information you receive about our program encourages you to pursue your graduate degree in clinical research with us. If you are interested, click here to get your application started. We accept applications for both September and January start dates.
Degree Requirements
The program consists of three components:
- Minimum of 32 graduate credits; 22 required and 10 elective
- Clinical research practicum; hands-on involvement in a clinical research project
- Capstone Project; clinical research project resulting in a written research paper
Master of Science in Clinical Research degree candidates are required to complete all of the following:
A minimum of 32 credits at the graduate level across four semesters. These must include the following 22 credits of required coursework:
- GMS CI 631 Management of Clinical Trials (4 cr), spring semester
- GMS CI 640 OL Regulatory and Compliance Issues (4 cr), spring semester
- GMS CI 670 Biostatistics with Computing (4 cr), fall semester
- GMS CI 675 Designing Clinical Research Studies (4 cr), fall semester
- GMS CI 790 Seminar in Clinical Research (2 cr), spring semester
- GMS CI 794/795 Practicum in Clinical Research (2 cr), fall or spring semester
- GMS CI 804/805 Research (2 cr), fall or spring semester
A minimum of 10 credits in elective coursework: A wide variety of courses offered in Graduate Medical Sciences will count toward elective credit. A minimum of 10 credits must be taken as electives or directed study. Up to 4 credits across two semesters may be taken as the practicum or for research. Students who have completed one or more of the required courses before matriculation may acquire “advanced standing” for that requirement. “Advanced standing” means that the student may waive the requirement but would need to replace the course requirement credits by taking an elective course(s). The student would not need to retake the course requirement. To waive a course requirement, students must speak to their academic advisor and complete/submit a “Petition for Approval of Advanced Standing.” No transfer credits from other BU departments or institutions will be accepted.
Completion of a minimum of 240 hours of a practicum in clinical research is required for the degree. The goal of the practicum component is to provide the student hands-on exposure to clinical research. The student will work with a mentor and will be actively involved in the development, execution, and evaluation of a clinical research project or projects. During the practicum, it is expected that the student will be exposed to some or all of the following: clinical research planning, protocol preparation, interaction with Institutional Review Boards, regulatory requirements, selection of subjects/patients for the clinical trial, study monitoring, and data analysis. The practicum may be completed with a mentor who is actively conducting clinical research studies within a clinical research or hospital setting. It may also be performed under the direction of a clinical research professional within a drug, device, or biotechnology company, a clinical research organization (CRO), or site management organization (SMO) actively involved in clinical trials.
Capstone Project
Students in the MSCR program are required to complete a capstone project that provides a culminating experience and applies the principles and methods learned in the coursework to a real-life clinical study.
The goal of the capstone project is to demonstrate the student’s understanding of the clinical research process from both a theoretical and a practical point of view. Students conduct their capstone research in a wide variety of settings, including academic medical centers and local drug or device companies.
Students generally identify their capstone mentor and develop their capstone proposal while they are completing their coursework or practicum. The capstone project must involve the analysis and interpretation of data. Students are encouraged but are not required to conduct primary data collection. Once the final draft is approved, the student gives a short oral presentation on their capstone project to the readers, capstone mentor, MSCR students and faculty members, and any other interested parties. The purpose of the oral presentation is to demonstrate the student’s ability to (1) describe clearly the capstone topic, methods, and results, (2) demonstrate their understanding of study design and analytic principles and methods, and (3) place their research into a clinical context.
Additional Information
Students are required to abide by the rules and regulations of Graduate Medical Sciences:
- Credit toward a degree will only be obtained from a passing grade (A to B–).
- Grades of I and C+ or lower are interpreted as failures. A student receiving such grades in a total of 8 credit hours may be terminated. A student receiving a failing grade will not be permitted to take a makeup examination.
- A degree candidate, after completing all departmental course requirements, must register each regular semester as a continuing student and pay the continuing student fee until all remaining degree requirements are completed.
Please visit the Graduate Medical Sciences website at bumc.bu.edu/gms to view detailed administrative policies and procedures.
Study Options
The MSCR program can be completed on either a part-time or full-time basis depending on the student’s goal. Most of the courses take place in the late afternoons or early evenings to accommodate those who work during the day.
The program is designed so that a full-time student may complete their coursework in one academic year, including summer. Practicum and capstone components of the program should begin near completion of the coursework and the time frame for finishing them will be determined on a student-by-student basis by the program director or assistant director. A full-time student is enrolled in 12–18 credits per academic semester (fall and spring).
Part-time students must register for at least 4 but not more than 11 credits each academic semester until all course requirements are fulfilled.
Continuing Student
Students who have completed all departmental course requirements (32 credits) must register each subsequent semester as continuing students until all requirements for the degree have been completed.
Nondegree Option
A number of individuals with an accredited bachelor’s degree or its international equivalent, who are uncertain as to whether they want to enroll in MSCR, have the option of taking a few of the MSCR courses as a nondegree student, and may then make their decision about completing the MSCR application process. We allow the transfer toward the MSCR degree of up to 2 MSCR courses (8 credits) taken as a nondegree student. Applicants must submit a copy of the Application for Admission, indicating the specific objectives of the studies/courses sought. In addition, the applicant must submit (with the application) the nonrefundable application fee. Nondegree applicants are not eligible for University sources of financial aid or aid that requires matriculation in a degree program.
Related Bulletin Pages
- Graduate Medical Sciences Courses
- Abbreviations and Symbols
Beyond the Bulletin
- GMS Admissions
- Financial Assistance
- The Vesalius Certificate
- Anatomy & Neurobiology
- Behavioral Neuroscience
- Biochemistry
- MD/PhD in Bioinformatics
- Biomedical Forensic Sciences
- Biomedical Research Technologies
- Biomedical Sciences (PiBS)
- Dual MS in Medical Sciences and Clinical Research
- MD/MS in Clinical Research
- Hybrid Graduate Certificate Program in Clinical Research
- Online Graduate Certificate Program in Clinical Research
- Forensic Anthropology
- Genetic Counseling
- Genetics & Genomics
- Health Care Emergency Management
- Health Professions Education
- Medical Anthropology & Cross-Cultural Practice
- Medical Sciences
- Mental Health Counseling & Behavioral Medicine Program
- Microbiology
- Molecular & Translational Medicine
- Neuroscience
- Nutrition & Metabolism
- Oral Biology
- Oral Health Sciences
- Pathology & Laboratory Medicine
- Pharmacology & Experimental Therapeutics
- Physician Assistant
- Physiology or Biophysics
- Departments
- BU Medical Campus Library
- Graduate Medical Sciences Student Organization (GMSSO)
Terms of Use
Note that this information may change at any time. Read the full terms of use .
related websites
- Graduate Medical Sciences
Accreditation
Boston University is accredited by the New England Commission of Higher Education (NECHE).

- © Copyright
- Mobile Version
College of Graduate Studies
- Coronavirus Updates
- Adult Patient Care
- Children's Health
- Education at MUSC
- Hollings Cancer Center
- Research at MUSC
- Master's
- Clinical Research
Maritza Dawkins-Holnes
Program Coordinator [email protected] 843-876-2216
Daniel T. Lackland, DrPH, FACE, FAHA
Director, Masters of Science in Clinical Research Program [email protected] 843-876-1141
Master of Science in Clinical Research
Program overview.
The Master of Science in Clinical Research Program (MSCR) provides training in clinical research for healthcare professionals whose careers in academic medicine would require successful and consistent extramural funding as principal investigators on grants, implement clinical research projects and produce clinical science publications. The program prepares healthcare professionals for development and opportunities to further their skills in the clinical research environment and workforce.
The MSCR program is a component of the educational core of the South Carolina Clinical and Translational Research Institute (SCTR) and is compliant with core competencies recommended by the National Center for the Advancement of Translational Sciences. The degree is granted through the College of Graduates Studies.
Course Descriptions Curriculum Learning Objectives

Start Your Application
Begin your online application to the Medical University of South Carolina.
Important Links
- Getting Ready to Apply
- Cost to Apply
- Test Score Submission
- Required Transcript Policy
- Check Application Progress
Description
The Master of Science in Clinical Research (MSCR) Program provides training in clinical research for health care professionals whose careers in academic medicine would require successful and consistent extramural funding as principal investigators on grants, implement clinical research projects and produce clinical science publications.
Application Process
All applications to the College of Graduate Studies are submitted online. If you are unable to apply online, a petition to the Office of the Dean with an explanation must be submitted for special accommodation.
In addition to the application itself the applicant must complete supplemental instruction materials specific to the program to which they are applying. These forms are provided via email once an application has been submitted (and are listed under Application Materials through program links above).
Program Information:
Ms in clinical research part-time, ms in clinical research full-time, ms in clinical research online.
The Master of Science in Clinical Research (MSCR) Program provides additional training in clinical research for professionals whose careers in academic medicine would require successfully and consistent extramural funding as principal investigators on grants and for investigator industry initiated clinical research projects. The program prepares physicians, healthcare professionals and investigators at all career and training stages for development and opportunities in the clinical research environment.
Requirements
Candidates in this program will primarily be senior residents, fellows, or junior faculty. While this program specifcally targets individuals at MUSC, faculty and staff at other institutions previously trained in the health professions and other qualified professionals in healthcare who wish to develop their clinical research skills, may also enroll. Senior residents, fellows, junior faculty, or health professionals must possess:
- a doctoral degree from an accredited school in the health/clinical sciences (e.g., M.D., Pharm. D., O. D., D. M. D., Ph. D.);
- A bachelor's or a master's degree in the health/clinical sciences from an accredited institution of higher education (e.g., BSN, MHS)
Application Materials
- Academic transcripts from the institution of the highest degree, as well as any professional certifications that indicate the applicant has either
- a doctoral degree from an accredited institution in the health/clinical sciences (e.g., M.D., PharmD, O.D., D.M.D., Ph.D.)
- a graduate or bachelor's degree in the healthcare or clinical sciences from an accredited institution of higher education (e.g., BSN, MHS)
a. Future professional goals b. Your purpose for applying to the graduate program c. Area of research interest and qualifications d. Why you chose this institution's graduate certificate program e.An acknowledgement, upon acceptance, that you will commit to all scheduled classes
- Letters of Support stating the department's guarantee the applicant will have protected time to commit to the program: a. For residents – from the Residency Director b. For fellows – from the Fellowship Director c. For faculty – from the Division or Department Chairperson
Please Note: For your application to be complete a transcript of your highest degree earned, and letter of support must be submitted.
International Students
Foreign nationals are urged to submit complete information as early as possible. In addition to other official entrance credentials, applicants whose native language is not English must submit the official results of the Test of English as a Foreign Language (TOEFL or IELTS score). The TOEFL or IELTS score must not be more than two years old. Final consideration cannot be granted to an international application for admission until the official scores are received by the Office of Enrollment Management.
If an international applicant has graduated or will graduate from an institution that uses English as the language of instruction, the TOEFL may be waived upon request.
Back to Program List
The one year, three semester program is designed to increase the number of well-trained clinical researchers who will assume leadership roles in the design, conduct, and oversight of future multidisciplinary clinical investigations critical to the mission of the National Institute of Health (NIH). The program is designed for students who are currently pursuing doctoral degrees at MUSC.
For admission, a candidate must possess one of the following: Bachelor or master's degree in the clinical or health sciences from a U.S. accredited institution of higher education (e.g. BSN, MHS). International applicants must have a degree equivalent to a U.S. four-year baccalaureate degree from an officially recognized institution and a letter of recommendation or support letter. Learn more about admission requirements for international applicants.
Complete the applications and submit admission materials online. If you are unable to apply you must petition the College of Graduate Studies Office of the Dean for special accommodations with an explanation of why you are unable to apply online. In addition to the application, supplemental instruction materials specific to each program is also uploaded. These forms are provided via email once an application has been submitted and are listed above under Application Materials. Official transcripts are sent directly to the Office of Enrollment Management.
All applications to the College of Graduate Studies must be submitted online. If you are unable to apply online you must petition the Office of the Dean for special accommodation explaining why you are unable to apply online. In addition to the application must complete supplemental instruction materials specific to the program to which they are applying. These forms are provided via email once an application has been submitted and are listed above under Application Materials.
The online M.S. Clinical Research program provides additional training in clinical research to professionals whose careers in academic medicine would require successfully and consistent extramural funding as principal investigators on grants and industry initiated clinical research projects.
While this program target MUSC residents, fellows, and faculty. It is also recommended for clinical and healthcare professionals in and outside of the state who wish to develop their clinical research skills. You must possess:
- a doctoral degree from an accredited institution in the healthcare or clinical sciences (e.g., M.D., Pharm.D., O.D., D.M.D., Ph.D.);
- a graduate or bachelor's degree in the healthcare or clinical sciences from an accredited institution of higher education (e.g., BSN, MHS.)
Distance Education Information
MUSC has been approved by South Carolina to participate in the National Council for State Authorization Reciprocity Agreements. NC-SARA is a voluntary, regional approach to state oversight of postsecondary distance education. Currently, MUSC accepts applications to the Master of Science in Clinical Research – Online from students who live in all states.
To learn more please see the Office of Institutional Effectiveness' State Authorization page .
- Academic transcripts from the institution of the highest degree, as well as any professional certifications that indicate the applicant has either a doctoral degree from an accredited school in the health/clinical sciences (e.g., M.D., PharmD, O.D., D.M.D., Ph.D.) or a bachelor’s or master’s degree in the health or clinical sciences from an accredited institution of higher education (e.g., BSN, MHS) *and* a professional designation (e.g., RN, CT, PT, OTR).
a. Future goals b. Area of clinical research interest c. Why you chose the MSCR program d. Name of your research mentor (if you have one identified) – not having a mentor will not affect your eligibility
Letters of Support stating the department’s guarantee that the residents, fellows, or faculty will have protected time to attend the program classes:
a. For residents – from the residency director b. For fellows – from the fellowship director c. For faculty – from the division or department chair
Test scores (SAT, ACT, GRE, MAT, etc.) are not required
Please Note: For your application to be complete a transcript of your highest degree earned and letter of support must be received by the application deadline.
All applications to the College of Graduate Studies must be submitted online. If you are unable to apply online you must petition the Office of the Dean for special accommodation explaining why you are unable to apply online.
In addition to the application itself the applicant must complete supplemental instruction materials specific to the program to which they are applying. These forms are provided via email once an application has been submitted (and are listed above under Application Materials).
Use of Marijuana and/or CBD Products
Marijuana is a Schedule 1 drug and is illegal to purchase in South Carolina. Apart from a narrow and limited scope of codified/documented medical exceptions, it is illegal for individuals to use marijuana/tetrahydrocannabinol (THC) in South Carolina. Although cannabidiol (CBD) products may be purchased and used in South Carolina, please be aware that CBD products may contain higher levels of THC than represented on packaging and use of CBD products can result in a positive drug screen for THC/marijuana. Be aware that current drug testing methods cannot accurately ascertain the origins of THC metabolites (i.e., whether from marijuana or CBD products). Your academic program has the authority to conduct random and/or scheduled drug testing; if your test result is reported as positive for THC metabolites (even if you only used a CBD product), your ability to be accepted into the program, progress in the program, and/or successfully complete the program may be negatively impacted.
Scholarship Eligibility
MUSC offers scholarships for which you may be eligible. Some are awarded based on academic achievement; others are awarded based on community service, for example. However, the majority of scholarships awarded at MUSC are based on financial need. This means those scholarships are only awarded to students who need some financial assistance to cover the cost of tuition and fees. If you would like to be considered for a financial need-based scholarship, you must have an up-to-date Free Application for Federal Student Aid (FAFSA) on file. Make sure you list Medical University of South Carolina on your FAFSA form, along with MUSC’s code: 003438. We encourage you to submit the FAFSA as early as possible. It is recommended to submit the FAFSA in January if you plan to enroll in the Fall.
Application Deadlines
Ms in clinical research part-time deadlines, ms in clinical research full-time deadlines, ms in clinical research online deadlines.
Together we are beating cancer
About cancer
Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
Cancers in general
- Clinical trials
Causes of cancer
Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
Get involved
- Make a donation
By cancer type
- Leave a legacy gift
- Donate in Memory
Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay For Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our We Are campaign
Our research
- Brain tumours
- Skin cancer
- All cancer types
By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
Funding for researchers
Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
Applying for funding
- Start your application online
- How to make a successful application
- Funding committees
- Successful applicant case studies
How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
Shop online
- Wedding favours
- Cancer Care
- Flower Shop
Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
Cancer news
- Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
ABOUT CANCER
GET INVOLVED
NEWS & RESOURCES
FUNDING & RESEARCH
You are here


Early careers at Cancer Research UK

Join us to beat cancer
We are at the forefront of the global fight against cancer, bringing together millions of people who share our determination. But we need to go much further.
We’re looking for people who can work collaboratively and focus on what matters to help us to get to where we want to be.
Whether you’re a graduate, undergraduate, school leaver or looking for a career change, we have programmes that will help develop your skills at the UK’s leading cancer charity. Our schemes offer insight into working for a charity in a supportive environment, all while playing a vital role in the fight against cancer.
Our early career programmes are unique as no previous experience or qualifications are required. This is because we are committed to breaking down barriers and creating an inclusive and diverse workforce, which we believe will help us beat cancer.
Paid internships
Build experience and gain skills that will last a lifetime with our internships. We’re passionate about your personal development and we have opportunities across the charity throughout the year.
Length of programme: 12 weeks
For: anyone looking to gain work experience within the charity sector.
Student placements
Gain invaluable experience with our placements within the Centre for Drug Development or lab-based Cancer Research Horizons. We provide students with an interest in scientific or clinical research the unique opportunity to develop key skills in a world-class clinical research or lab environment.
Length of programme: 12 months
For: life sciences or chemistry students who want to gain experience in their placement year.
Accelerate emerging talent scheme
Develop your talents, strengths and experience with our emerging talent scheme, Accelerate. Gain valuable cross-organisational insight as you rotate through placements across the charity while earning a competitive salary in a supportive environment.
Length of programme: 2 years
For: high-potential individuals who want to develop their career in the charity sector.
Apprenticeships
Learn and study for a qualification whilst working in a role that contributes to our mission of supporting people affected by cancer.
Length of programme: Various
For: anyone wanting to start their career in a new profession, or to progress their career in their current profession, whilst getting an insight into what it’s like working for one of the UK’s leading charities.
All our programmes offer a competitive salary and a range of benefits. Our Accelerate trainees earn £26,000 in your first year with an increase to £27,750 in your second year , whilst our Interns earn the London Living Wage .
Each and every one of our employees contributes to our progress. And every ounce of effort you put in will be supporting our work to beat cancer. We think that’s impressive. In return, we make sure you are supported by high-quality tools, policies and processes to help you to do your job well. Our work – from funding cutting-edge research to developing public policy – will change the world. It’s exciting to be part of our team.
Our benefits include:

Flexibility
We want our people to have time away from work to support their different commitments, needs and interests. We encourage a flexible working culture, including options such as home-working, reduced or flexi-hours, job shares, job-splits, compressed and core hours. We offer a high level of flexibility for the vast majority of our office-based roles, meaning that you’ll only be required to work from a specific location for 1 or 2 days a week on average.

Development
We offer a wide range of career and personal development opportunities, including induction, core business skills, leadership and management development, talent programmes, accelerated progression opportunities for those with leadership potential, secondments and much more.

Annual leave
Working with us, you’ll be encouraged to take your 25 days holiday a year, plus bank holidays, and an extra day for Christmas Eve. We provide flexibility on religious holidays meaning you can choose to use the 5 UK religious bank holidays at another time for any reason including religious festivals, caring, wellbeing or other interests.

Cancer Research UK staff enjoy great benefits in and around Stratford with deals at local shops, bars and restaurants. Our staff have access to a wide range of discounts, benefits and cashback deals to support you with your financial wellbeing, through our Rewards platform. We offer an online savings and discounts portal covering many widely recognised retailers and brands from electronics, groceries, home and leisure, holidays and more.

My journey at Cancer Research UK has been incredible. I've grown, learned many skills, and discovered aspects of myself. Working alongside diverse individuals has allowed me to tap into a wealth of knowledge and witness skills in others that I aspire to learn. Everyone is incredibly supportive, and always ready to offer assistance. It's been a fulfilling experience for me so far.
Vivian Boamah, Marketing, Fundraising and Engagement

Join an inclusive team
Beating cancer means beating it for everyone. Our vision is to create a charity where everyone feels like they belong, benefits from and participates in, the work we do.
This will help us make faster progress: it is well known that more diverse organisations perform better, and we will enable our community of cancer researchers to thrive.
We actively encourage applications from people of all backgrounds and cultures, in particular those from ethnic minority backgrounds who are currently under-represented, and believe that a diverse workforce will help us to beat cancer sooner.
We want to see every candidate performing at their best throughout the job application process, interview process and whilst at work. We therefore ask you to inform us of any questions or concerns you have about how we can support you with a disability or health condition or any adjustments you might need to enable this to happen.
Together we will beat cancer.
What to expect from working at Cancer Research UK
At Cancer Research UK, we want you to get involved with all we have to offer. Outside of your day-to-day role, you will have the chance to:
- Get involved in exciting staff fundraising activities
- Join our expansive range of Equality, Diversity and Inclusion networks
- Benefit from our comprehensive wellbeing services
Please contact us if you have any questions or should you require any adjustment due to a disability or a long-term health condition.

Clinical Research
Study design, biostatistics, data management, scientific communication and the ethical, legal and regulatory issues in clinical research..
The 12-credit certificate in Clinical Research program provides training in the core competencies of clinical research including study design, biostatistics, data management, scientific communication and the ethical, legal and regulatory issues in clinical research. Clinicians and scientists who wish to work in clinical research often need additional didactic training in order to gain analytic skills that are not covered in their health professional or graduate education.

Details, Dates & Deadlines
Program details, class format, program location.
University of Maryland, Baltimore Campus
Program Length
Credits to complete, cost/credit hour.
In State: 738 Out of State: 1353
- Online application
- $75 application fee
- Official transcripts
- 300–500 word essay
- Proof of English language proficiency
- CV or resume
Dates & Deadlines
Priority Deadline: March 1 Fall Deadline: May 15
Program Contacts
Jon Shinnick Coordinator 410.706.8492 [email protected]
Dr. Jennifer Albrecht Program Director 410.706.0071 [email protected]
Your path to success starts here
- Request Information
- Register for an Info Session
620 W. Lexington St. Baltimore, MD 21201 (410) 706-3100
University of Maryland Graduate School. All Rights Reserved.
- Privacy Policy
- Web Accessibility

Clinical Research
Online full-time programs.
Online full-time programs are offered as either Daytime, or a combination of Evenings and Saturdays. Check your program Dates and Times to see what the program commitment will be.
Find out more about Full-Time Online programs
Humber is proud to have the highest graduate employment and employer satisfaction rate of the GTA colleges based on Colleges Ontario’s key performance indicators for college graduates in 2022-2023.
Program Overview
Humber’s Clinical Research graduate certificate program prepares graduates with the transferable skills needed to build successful careers in a variety of clinical trial management and research sectors. This program focuses on developing the concepts, skills and techniques required to work in the clinical research field. You will gain knowledge and skills in the planning and management of clinical research including practices related to the organization, execution and monitoring of clinical trials. You will learn clinical trial protocol development as part of the principles and processes of clinical trial design.
There is an emphasis on maintaining good clinical practice (GCP) as presented by the International Conference on Harmonization (ICH), along with the importance of data collection, analysis, recording and auditing - all to ensure that clinical trial data are credible and accurate. You will become familiar with the many regulations and guidelines established to ensure trials are conducted ethically and in ways that respect the rights of clinical trial participants, while ensuring the execution of robust scientific research.
Teamwork and communication skills are reinforced throughout the program, and you will acquire the necessary technological skills to assist with data management specific to the field.
You will also benefit from:
- a unique integrated approach
- acquiring a recognized skillset applicable to a wide variety of employment opportunities
- integrative project work that links applied and academic fields
- learning to adapt to the changing field and staying current
- simulation trials mimicking real work experiences
Program Delivery: Courses are scheduled over two 14-week semesters, and are asynchronous, self-directed online modules, with set dates for evaluations. A field experience placement occurs in Semester 3 and is in-person.
This program is not available to international applicants.
At Humber, courses are delivered in a variety of formats:
In-Person - An in-person course is delivered fully on campus.
Online Asynchronous (A) - An online asynchronous course has no fixed class schedule and allows students to engage with the course at different times according to their needs. Faculty provide modules, which are completed independently by the students according to established deadlines.
Online Synchronous (S) - An online synchronous course is delivered fully online and requires faculty and students to participate in real-time according to a fixed schedule. Classes are scheduled for a specific day and time.
Hybrid - A hybrid course is a combination of in-person and online classes and follows a set schedule. Students must be available to attend in-person classes at scheduled times during the semester.
The chart below outlines the delivery options available for each course in this program, by campus. For some academic terms, there may be more than one delivery option available. You’ll be able to select your preferred options when building your course schedule during open enrolment. Preferences for course delivery will be considered on a first come, first served basis. Some Humber programs are also delivered fully online, where all courses are delivered online.
International students: the impact of studying from outside of Canada on Post-Graduation Work Permit (PGWP) eligibility differs significantly based on when you start your program. Please review the PGWP eligibility before choosing your program and course delivery.
Work-Integrated Learning
Work-integrated learning .
Following two online academic semesters, students complete a three-month (450 hours) field experience that provides opportunities to apply and integrate theoretical knowledge and skills into real-world work settings. Field experiences are conducted in a wide range of organizations such as pharmaceutical, natural health products (NHP), medical device and biotechnology companies, as well as contract research organizations, hospitals, government agencies/departments and scientific institutions.
During Semester 3, students complete their learning at field experience sites, and assessments are carried out by assigned supervisors at the site of field experience. While Humber does assist students in finding their field experience by working with industry partners to identify openings, students are responsible for finding their own field experience that is aligned with the learning outcomes of our program.
Work-Integrated Learning (WIL) at Humber
Work-integrated learning.
Work-integrated learning opportunities prepare you for your future career. You will apply what you’ve learned in class and in real-world environments through a wide range of academic, community and industry partnerships. These work-integrated learning opportunities may include field experiences, professional practicums and co-operative education.
Field Experience
A field experience offers students an opportunity to engage in intensive experiences related to their field of study or career goals to build their skills, knowledge and abilities. Field experiences may be paid or unpaid.
Professional Practicum
Programs requiring a professional practicum offer practice-based experience or work hours for a professional license or certification. Students work under the direct supervision of an experienced professional. Placements are unpaid.
Co-operative Education
Students in co-op programs gain experience through paid work terms in their field of study that become progressively more complex as their skill level increases.
Optional Co-operative Education
Students in co-op programs gain experience through paid work terms in their field of study that become progressively more complex as their skill level increases. The co-op portion of this program is optional.
If you would like to learn more about work-integrated learning at Humber, visit WIL AT HUMBER

Watch the video to learn what is work-integrated learning.
An Education in High Demand
The medical, pharmaceutical, and natural products industry is facing constant development. As a society focused on health and wellness much attention is being given to the development of new interventions, supplements and drugs as well as improvement of health care. Comprehensive training combined with work experience and previous degrees make Humber students highly marketable.

Clinical Trials
Clinical research involves human studies where products are tested in multiple phases to assess their safety and efficacy. Clinical research is a necessary step (ethical and regulatory) in the development of new therapeutic/diagnostic products. Industry has the responsibility to follow stringent international, federal, and provincial regulations when planning and implementing preclinical and clinical studies; along with the development and manufacturing of medical products.
Responsibilities
Clinical Research Professionals are responsible for conducting clinical trials, which includes planning, designing. and monitoring clinical experimentation and later, analyzing data to draw conclusions about the treatments. Furthermore, they are responsible for carrying out clinical trials in compliance with Good Clinical Practices, clinical trial protocols, international and local regulations. When working in the industry, interactions with medical personnel (nurses and physicians) and plenty of travelling are a regular part of life for a Clinical Research Professional. When employed by a clinic or hospital the Clinical Researchers are responsible for conducting studies at that facility as well as interacting with sponsoring companies regarding the planning and execution of clinical trials.

The Humber Advantage
Industry recognition.
Every year our program receives excellent feedback from the industry regarding the professional preparation of the graduates and the content of the program; our graduates are an important resource for the health care industry. This is an outcome of our ongoing efforts to ensure that students have solid science, medical, and clinical research knowledge.
Superior Program Design
The Clinical Research Graduate Certificate program at Humber is designed, developed and created so that it can be delivered online with high efficiency, during the first two semesters of the program. Subsequently, theoretical learning is extensively applied through hands-on experiences during the third semester, including a 450-hour internship placement.
The fundamental skills are taught, developed, and reinforced throughout the one-year comprehensive program. Work ready graduates are developed in this program to confidently transition into the workforce.
The Humber Experience
The right fit.
If you are science-oriented, self-regulated, have collaborative interpersonal skills and enjoy managing and working in variety of laboratory and research settings, the Clinical Research program and a career in this field may be for you.
Testimonials
Interested in Clinical Research at Humber College yet want to know more before enrolling?
Your Career
Our graduates typically pursue careers in research settings such as pharmaceutical, medical device and biotechnology industries, hospitals, and research institutes. Their work may help lead to the development of new treatments and therapeutic approaches to enhance the quality of life.
Related Programs

Regulatory Affairs
Credential: Ontario Graduate Certificate Length: 3 semesters

Health Sector Regulatory Compliance
Program Availability
Humber is a publicly-funded institution and does not have a public-private partnership. International students graduating from Humber or Humber’s International Graduate School (IGS) are eligible to apply for a Post-Graduation Work Permit .
International Students in Canada who apply for May 2024 start could be eligible for a $1,000 Scholarship*. Apply now
Please note the new International Admissions Process and Provincial Attestation Letters. Read the update
International Students Out of Canada can Apply through Humber International
Recruitment Events
Open House Book a Tour
Can't make it to campus?
Experience Humber Virtually
Program Delivery Types
Block-based: Students select a pre-set weekly schedule of courses that best meets their needs. Block-Based schedules may include in-person, hybrid and online courses.
Course-based: Students create their own schedule of courses from among in-person, hybrid and online options.
Condensed Week - Courses requiring students to come to campus are scheduled over 2-3 days per week. Online courses are scheduled on other days.
Online - Courses are scheduled only online and may be delivered asynchronously, where students study independently or synchronously, where students attend the online class on a specified time and day.
Twilight - In-person, online synchronous and hybrid courses are generally scheduled after 3:00pm.
Twilight-Online: Online synchronous courses are generally scheduled after 3:00 pm.

IPE Blackboard Site
Wed, December 20, 2023
The Faculty of Health Sciences & Wellness is launching a new tutorial Blackboard site entirely dedicated to IPE!

Navigating Health Care
Thu, September 28, 2023
Humber’s North Campus was proud to host The Central West Navigation Conference on Tues Sep 26.

Humber Launching Student Led Hearing Clinic
Mon, August 21, 2023
A multi-service clinic servicing the Humber community and area.
No news at this time.
Every attempt is made to ensure that information contained on this website is current and accurate. Humber reserves the right to correct any error or omission, modify or cancel any course, program, fee, timetable or campus location at any time without prior notice or liability to users or any other Person.
- Close Menu ×
- How To Apply How To Apply
- Admission Requirements Admission Requirements
- Equipment & Device Requirements Equipment & Device Requirements
- Fees & Financial Aid Fees & Financial Aid
- Contact Us Contact Us
- Apply Now APPLY NOW
Admissions Questions
General enquiries.
Call 416-675-3111 or email [email protected] . If you have already applied, be sure to check your application status on myhumber.ca .
Domestic Applicants Enquiries
Domestic applicants can book a one-on-one advising appointment with an admissions representative.
International Applicants Enquiries
Contact the International Centre for information about full-time programs (including the International Graduate School), how to apply and to follow up on your submitted application.
Program-Specific Questions
Speak to the Program Co-ordinator about the course curriculum, projects and career options.
Aparna Bhan, program manager [email protected]
Campus Information
Book a campus tour to take a closer look at what it's like to be a student at Humber.
Want More Info?
Find out more about the student experience and everything that Humber has to offer Future Students .
Sign-up now for more info on Humber, including programs, special events and more!
How To Become An Apprentice
Becoming an apprentice.
Find an employer willing to sponsor you as an apprentice.
Contact the Ministry of Labour, Immigration, Training and Skills Development to register as an apprentice.
Work with your employer approximately one year before attending Humber.
View Instructions
Ontario Youth Apprenticeship Program (OYAP)
If you’re in high school – grade 11 or 12 – you can earn co-op education credits through work placements in some skilled trades.
Visit OYAP
How to Apply
Domestic students.
Applications to Humber are made through ontariocolleges.ca . Be sure to submit your application by the equal consideration deadline of February 1. You may apply after February 1, however, post-February 1 applications will be considered on a first-come, first-served basis depending on the availability of the space in the program.
To check program availability refer to the Campus/Availability listing on Humber’s program pages, search by availability , or ontariocolleges.ca .
To see where you are in the admissions process, visit the Admissions Road Map .
International Students
If you’re an international student, you can apply directly to Humber via our International Centre .
Need Advice?
Program advising appointments.
Get help narrowing down your program options or book a one-on-one pre-enrolment advising appointment with one of our Recruitment Officers.
Transfer & Pathway Advising
Book a virtual appointment with a Student Mobility Advisor learn more about getting Transfer Credit(s) for previous post-secondary experience, Prior Learning Assessment and Recognition (PLAR), and Pathways options.
Admission Requirements
Admission selection is based on the academic criteria indicated. Meeting minimum eligibility requirements does not guarantee admission.
Admission selection is based on the following three requirements:
To be eligible for admission, you must possess the following:
- A Bachelor of Science degree, majoring in health science, pharmacy, some area in life sciences or a related field.
Mature Applicants
Diplomas and certificates.
An applicant is considered a mature applicant if they have not completed secondary school or other postsecondary school, and will be 19 or older as of the first day of classes. Humber will invite you for testing to demonstrate that you meet all listed course requirements.
An applicant is considered a mature applicant if they have not completed secondary school or attended postsecondary studies, and will be 21 or older as of the first day of classes. Mature applicants for degree programs will be required to meet course requirements at the U/M level or equivalent.
College Transfer Applicants
An applicant is considered a college transfer applicant if they have completed some or all of a college-level credential. Humber may use a combination of secondary school and/or college courses and grades to determine program eligibility.
An applicant is considered a college transfer applicant if they have completed some or all of a college-level credential. Humber may use a combination of secondary school and/or college courses and grades to determine program eligibility. Applicants must have an overall minimum grade point average (GPA) of 65 per cent in the program. Applicants are required to disclose and provide academic transcripts for all course work completed at the postsecondary level.
University Transfer Applicants
An applicant is considered a university transfer applicant if they have completed some or all of a university-level credential. Humber may use a combination of secondary school and/or university courses and grades to determine program eligibility.
An applicant is considered a university transfer applicant if they have completed some or all of a university-level credential. Humber may use a combination of secondary school and/or university courses and grades to determine program eligibility. Applicants are required to disclose and provide academic transcripts for all course work completed at the postsecondary level.
English Language Proficiency
All applicants whose first language is not English must meet Humber’s English Language Proficiency Policy .
International Credit Evaluation
Canadian citizens or permanent residents with international education are required to provide a credential evaluation. Note, for international High school education course by course evaluations, ICAS must be used. For international post-secondary education, a WES evaluation must be provided. In situations where you expect to apply for transfer credit, it is recommended that a course by course WES evaluation is completed.
Please note: A WES course by course evaluation is required for this program.
International Academic Equivalency
Admission equivalencies for Humber depend on your country of study. Please enter your location or choose detect my location to see the requirements for your country below.
Applying with an International Baccalaureate (IB)
Post-Admission Requirements
Once you have been accepted, and have confirmed your offer, you may need to complete a further set of requirements related to your program (Post-Admission Requirements).
Equipment & Device Requirements
Fees & financial aid.
The 2024/2025 fee for three semesters is:
- domestic: $7,501.08
- international: N/A
Fees are subject to change.
Fees by Semester
Domestic Fees by Semester
International fees by semester.
*Plus Mandatory Health Insurance fee once per academic year: Fall start - $420 Winter start - $280 Summer start - $140
Financial Aid, Scholarships and Bursaries
Understand the costs associated with coming to Humber and explore resources available from first year to your final year on Student Fees and Financial Resources .
Scholarships
Humber scholarships.
Find out more about scholarships and bursaries that you may be eligible for, visit Student Scholarships . International students can visit International Student Scholarships .
Humber Bursaries
Bursaries are available for Certificate, Diploma and Degree programs primarily based on financial need, visit Humber Bursaries.
External Awards, Bursaries & Scholarships
Find out more information about external scholarships and bursaries, visit External Awards.
Indigenous Student Awards, Bursaries & Scholarships
Humber offers a variety of bursaries and scholarships for Indigenous students, visit Indigenous Student Awards.
Explore Opportunities through Humber Pathways
Humber Pathways include:
- Opportunities to build on your college education and complete your diploma or degree at Humber.
- Degree and graduate study opportunities at other institutions in Ontario, Canada and abroad.
Additional information will be made available to students from their program before the beginning of the Winter term. Courses with in-person requirements will likely also have online components. The delivery mode of some courses is still being determined. Humber may need to change plans for in-person learning, subject to government and public health directives and/or additional health and safety considerations.
You can find a complete list of programs with downloads including program and course details at Current Student Resources
Students in programs marked as online/in-person will have a combination of those two types of delivery. Additional information will be made available to students from their program in the first week of June. Courses with in-person requirements will likely also have online components. The delivery mode of some courses is still being determined. Humber may need to change plans for in-person learning, subject to government and public health directives and/or additional health and safety considerations.
Learning Outcomes:
Upon successful completion of the program, a graduate will:
- Perform the duties of a clinical research professional, as part of a project team, at all phases of the product/treatment development and post-market processes.
- Consider political, social, and economic factors when making decisions related to clinical research practices in order to plan responses for potentially challenging and complex outcomes.
- Analyze clinical research processes and products from multiple perspectives to identify potential impacts on industry.
- Synthesize scientific, regulatory, and business information from various sources to prepare effective clinical research documents.
- Maintain ethical, legal, regulatory, and professional standards associated with clinical research.
- Create a clinical development plan for a novel therapeutic product.
- Evaluate clinical research practices according to recognized Quality Assurance Process.
- Integrate effective technology and record-keeping practices within all stages of clinical research and post marketing processes to ensure compliance with research approvals and professional and ethical standards of practice.
- Adhere to the principles and practices of specific Standard Operating Procedures to prepare and manage documentation and data in compliance with approved protocols.
- Prepare and critique submissions for clinical trials and marketing approvals that meet regulatory and industry requirements.
- Apply critical analysis, problem solving, and project management skills to recognize and respond to complex clinical research challenges.
- Engage in knowledge translation to contribute to the advancement of the health care industry.
- Collaborate with study participants, research teams, and regulatory and business professionals to contribute to high quality clinical research processes.
You are now leaving this website. By continuing, you will be directed to a site intended only for residents of the United States and Canada. We are called MSD everywhere, except in the United States and Canada where we are known as Merck & Co Inc, Rahway, NJ USA.
You are now leaving this website. By continuing, you will be directed to a site intended only for residents of the United States and Canada. We are called MSD everywhere, except in the United States and Canada where we are known as Merck & Co., Inc., Rahway, NJ, USA.

Clinical Research
A patient-first focus and the freedom to chase something big, let us tell you about the important work we're doing in clinical research, meet the talent, we asked, and here are the standout reasons people are joining and staying with our clinical research teams.
- Real world impact on patients
- We are a top investor in biopharma R&D
- Scientific freedom combined with global-scale security
- Dedication to being the leading research-driven biopharmaceutical
- A wide, robust pipeline of game-changing products
- State-of-the-art labs
- The flexibility to work from home
Inside views from people on our team
The best thing that happens in my day is happy employees, happy patients. i’m ready to inspire myself and my team to be one of the top 10 countries in the world to support global development activities., betül erdoğan, msd is commited to developing and engaging with people. diversity and inclusion are very strong pillars in the company., mercedes navarro, key roles in clinical research, clinical research associate, biostatistician, clinical research associate manager, clinical research manager, what obsesses and pushes us to get up everyday, our future is looking bright, uk discovery centre, lorem ipsum, join our talent community today, receive updates on career opportunities tailored just for you., connect with us on social, beware of hiring scams. review our applicant notice ..
Faculty of Medicine & Health Sciences
Main navigation.
- Agricultural & Environmental Sciences
- Bachelor of Arts & Science
- Continuing Studies
- Dental Med. & Oral Health Sci.
- Engineering
- Environment
- Interfaculty Studies
- Medicine & Health Sciences
- Physical & Occupational Therapy
- Study Abroad & Field Studies
- Summer Studies
- All Courses
- All Programs
- University Regulations & Resources
- Important Dates
- The Faculty
- Undergraduate
- Professional
- Search Courses
- Search Programs
- Dean's Welcome
- Graduate and Postdoctoral Studies
- Graduate Studies at a Glance
Program Requirements
- Graduate Admissions and Application Procedures
- Fellowships, Awards, and Assistantships
- Postdoctoral Research
- Graduate Studies Guidelines and Policies
- Graduate Student Services and Information
- Information on Research Policies and Guidelines, Patents, Postdocs, Associates, Trainees
- Biomedical Sciences
- Communication Sciences and Disorders
- Population and Global Health
Graduate Diploma (Gr. Dip.) Clinical Research (30 credits)
The objectives of this program are to give students exposure to both theoretical and practical issues relevant to the conception and conduct of a clinical research study, and to put these principles into practice by participating in an ongoing clinical trial. The training provided qualifies students to manage and design clinical research studies in both academic and industrial settings.
Required Courses (24 credits)
Offered by: Medicine ( Faculty of Medicine and Health Sciences )
Administered by: Graduate Studies
Experimental Medicine : Intensive day-long workshop discussing Industrial/Academic/Governmental interactions in the design, testing and approval of drugs.
Terms: Winter 2024
Instructors: Jean-Claude, Bertrand; Cournoyer, Denis (Winter)
Experimental Medicine : Intensive day-long workshop discussing the role of the physician in drug testing.
Terms: Fall 2023
Instructors: Jean-Claude, Bertrand; Di Battista, Giovanni (John); Mihalcioiu, Catalin Liviu D (Fall)
Experimental Medicine : Intensive day-long workshop discussing the pharmacoeconomics of drug design and testing.
Instructors: Jean-Claude, Bertrand; Djiana, Rose; Gilfix, Brian; Thibeault, Denis (Fall)
Experimental Medicine : Intensive day-long workshop discussing a topical subject or recent advance relevant to clinical research and the conduct of clinical trials.
Instructors: Jean-Claude, Bertrand; Routy, Jean-Pierre (Winter)
Instructors: Jean-Claude, Bertrand (Winter)
Experimental Medicine : Six-step program: 1. Identification of the problem; 2. Experimental design; 3. Protocol development; 4. Execution of the protocol; 5. Data analysis; 6. Generation of final report with active "clerkship" participation in each component with team leaders and experts designated for each stage.
Terms: Fall 2023, Winter 2024, Summer 2024
Instructors: Jean-Claude, Bertrand (Fall) Jean-Claude, Bertrand (Winter) Jean-Claude, Bertrand (Summer)
Complementary Courses (6 credits)
Six credits at the 500 level or higher chosen from: Experimental Medicine (EXMD), Pharmacology and Therapeutics (PHAR), Epidemiology and Biostatistics (EPIB). With prior approval from the Division's Student Affairs Coordinator, courses at the 500 level or higher, from other Allied Health Sciences departments may be accepted.
Department and University Information

Faculty Links
- Medicine website
Non-Credit Certificate Program in Clinical Trials Management and Regulatory Compliance
Accelerate your career in the field of clinical research with hands-on training in every step of the clinical trials process.

Upcoming Events

Freelancing in Medical Writing and Editing
Apr 9, 2024 • Online
At a Glance
The online Clinical Trials Management and Regulatory Compliance certificate program, designed and delivered by experts in clinical research, gives you the skills and knowledge you need to jumpstart your career in the growing clinical trials field.
Become a Better Clinical Researcher
In six core courses built around real-world clinical trials, you will master the procedure cycle and administration of the entire clinical trials process, learn to navigate stakeholder interests, and implement up-to-the-minute regulatory compliance practices and ethical standards. You will finish the program with the ability to initiate clinical research studies, apply monitoring methods, and write exemplary documents and reports.
Designed For
Designed for early or mid-career professionals who want to work in regulatory compliance, medical writing, site management, or data analysis in the pharmaceutical industry, at a clinical research organization, or with an academic institution.

You Value Your Career, We Value Your Time.
Staying up-to-date on your career skills doesn’t have to take a lot of time. Our Clinical Trials certificate program is competitively priced and takes as little as nine months.
Learn with clinical research experts
Scientists and pharmaceutical industry executives, consultants and project managers, our instructors know every angle of the clinical trials process. In live classes informed by their considerable professional experience, Clinical Trials certificate instructors give feedback, support, and expert insight into the field.
The UChicago edge
Our professional courses take innovative learning approaches that uphold the University of Chicago’s distinct brand of academic excellence while driving career advancement.
- Synchronous class sessions engage students with instructors and peers.
- Content-specific and networking webinars foster extracurricular training and allow students to make valuable professional connections.
- Professional development services include resume review, access to exclusive job listings, and more.
- Program administrators support students throughout the certificate and beyond, from individual advising sessions to alumni services.
Career benefits
The global clinical trials market has been projected to grow to $84.43 billion dollars by 2030. The field is thriving. Key drivers like the globalization of clinical trials and new, personalized treatments continue to impact market growth, while demand for skilled professionals widens the job market: the need for clinical trials professionals will continue to outpace that for similar roles. To learn how our Clinical Trials management certificate can boost your career, please visit our career benefits page for more information.
The estimated total pay for a Clinical Research Associate is $71,868 per year, according to Glassdoor .
Triple your medical writing skills
The University of Chicago Professional Education offers certificate programs in Clinical Trials, Medical Writing and Editing , and now Regulatory Writing . Our programs feature a blended learning model comprising of live synchronous sessions, real-world case studies, and writing exercises that work to elevate your medical writing skills. These part-time programs are tailored to develop your skillset so you can apply to your career immediately.
Explore how you can become an expert medical writer in three leading areas:
- Clinical Trials Management and Regulatory Compliance : Learn to use real-world clinical trials to reinforce your foundational knowledge and boost your career in clinical research.
- Medical Writing and Editing : This program will provide the foundation for mastering the fundamentals and best practices of medical writing, editing, and communication.
- Regulatory Writing : Building on the strengths of our Medical Writing and Editing program, Regulatory Writing courses will provide students with high-demand, professionally valuable skills to write submissions to the FDA and other regulatory bodies.

Clinical trials management offers a real spectrum of career paths. Students who complete the certificate have options spanning the pharmaceutical industry, clinical research organizations, and academic institutions, where they can consider careers in medical writing, site management, regulatory, and more.
Offered by The University of Chicago's Professional Education
Ready to Take Your Next Step?
Of interest, non-credit certificate program in regulatory writing.
Gain in-demand medical writing skills that will help elevate your career in healthcare or medical...

From Healthcare Technician to Clinical Researcher
Accelerate your career in clinical trials.

A Global Perspective on the Pharmaceutical Industry
- Leading in Healthcare Through Project Management
- Global Clinical Research: The Process from Start to Finish
- New Frontier in Patient Recruitment

IMAGES
VIDEO
COMMENTS
Essential Requirements. To be eligible for the program, you'll need: A bachelor's or master's degree in life sciences or a clinical research field. No more than two years' post-graduate work experience. Strong organizational, communication, and collaboration skills. A passion to work in pharmaceuticals, with a real desire to make a ...
In this graduate programme, participants undergo a comprehensive group training programme offering a combination of classroom-based and practical learning which develops SAS programming and builds upon the formal Master's program. ... then Site Activation Manager, or Clinical Research Associate (CRA), then Clinical Trial Manager (CTM). The ...
The program not only offers graduates insights into how their skills contribute to reimagining medicine, but helps to define the areas of work most of interest to them. Recruitment start: Q1-Q2. Chemical and Analytical Development (CHAD) Program. Gain unique insight into chemical research and development and drug substance supply.
Research and Development (R&D) Graduate programme. Locations: Cambridge, UK; Boston & Gaithersburg, US; Gothenburg, Sweden Salary: Competitive Our 2024 intake recruitment advertising dates are: US & UK: Applications for our 2024 intake are now closed.Recruitment for our 2025 intake will commence in autumn 2024.
Clinical Research Associate (CRA) Graduate Programme Location: Wilmington, DE Designed to prepare you for a career as a Clinical Research Associate, you'll progress through different programme rotations, gaining valuable experience across functions within Site Management and Monitoring and building multidisciplinary skills.
The MSCR MD/MS Program leads to a Master of Science in Clinical Research graduate degree and is designed to develop physician scientists interested in clinical research or biomedical informatics to: Design and conduct clinical research (clinical trials and observational studies) Successfully compete for funding (e.g., foundation grants, NIH K23 ...
The Master of Science in Clinical Research is a rigorous program that meets the needs of individuals engaged in the full spectrum of clinical research. Our mission is to provide you with a high-quality education and a personalized, hands-on research experience. Our curriculum prepares you to enter the workforce as competently trained clinical ...
The Master's in Clinical Research program, which is a STEM program, teaches students the scientific fundamentals of human research. Courses in our curriculum provide an in-depth look at all of the key elements in clinical research, including: trial design, trial management, biostatistics, ethical issues, and clinical research regulations.
The Clinical Research Graduate Group is an interdisciplinary graduate group in clinical and translational research offering a Master of Advanced Study (M.A.S.) in Clinical Research degree. The Clinical Research degree program provides a solid clinical/translational research foundation for junior faculty, clinical and postdoctoral fellows ...
What You'll Earn. You'll earn a Stanford Graduate Certificate in Epidemiology and Clinical Research when you successfully earn a grade of B (3.0) or better in each course in the program.. With each successful completion of a course in this program, you'll receive a Stanford University transcript and academic credit, which may be applied to a relevant graduate degree program that accepts ...
Clinical Research Graduate Scheme. Clinical Professionals train the most talented Life Sciences University Graduates within the UK and deploys them into "first to industry" clinical roles. The Clinical Professionals Group launched their graduate training Academy for UK graduates looking to work in clinical trials research and development in ...
The Master of Science in Clinical Research is designed for physicians and other health care professionals. It provides rigorous training in the... Search. How to Apply to UCLA Grad School. ... Clinical Research Graduate Program at UCLA David Geffen School of Medicine 5303 Life Sciences Box 951766 Los Angeles, CA 90095-1766.
The Foundations of Clinical Research program is rooted in the belief that clinical research training is critical to professional development in health care. Clinical research training not only creates potential independent investigators but also enables clinicians to advance their careers through a greater understanding of research evidence.Designed to provide learners with the foundational ...
The Master of Science in Clinical Research is a rigorous program that meets the needs of health professionals engaged in the full spectrum of patient-oriented research. This flexible degree program is designed for a variety of professionals, including physicians who will plan and oversee translational research and clinical trials; research nurses; study coordinators; managers in clinical ...
Daniel T. Lackland, DrPH, FACE, FAHA. Director, Masters of Science in Clinical Research Program. [email protected]. 843-876-1141. The MS in Clinical Research program trains individuals to become principal investigators, med school faculty, and team leaders.
Program Description. The Graduate Diploma (Gr. Dip.) in Clinical Research offered by the Division of Experimental Medicine in the Faculty of Medicine & Health Sciences is a course-based program that emphasizes practical and hands-on learning opportunities. Our comprehensive and innovative program offers a specialized and practical curriculum ...
Through our Clinical Research Education Program, students learn to harness and translate the promise of scientific discovery into meaningful and clinically impactful solutions. The Graduate School of Biomedical Sciences at the Icahn School of Medicine at Mount Sinai offers a number of clinical research training opportunities.
Gain invaluable experience with our placements within the Centre for Drug Development or lab-based Cancer Research Horizons. We provide students with an interest in scientific or clinical research the unique opportunity to develop key skills in a world-class clinical research or lab environment. Length of programme: 12 months
The 12-credit certificate in Clinical Research program provides training in the core competencies of clinical research including study design, biostatistics, data management, scientific communication and the ethical, legal and regulatory issues in clinical research. ... need additional didactic training in order to gain analytic skills that are ...
The Clinical Research Graduate Certificate program at Humber is designed, developed and created so that it can be delivered online with high efficiency, during the first two semesters of the program. Subsequently, theoretical learning is extensively applied through hands-on experiences during the third semester, including a 450-hour internship ...
Our Clinical Research area is filled with passionate, purpose-driven professionals dedicated to our mission of saving and improving lives. Join our team that is dedicated to driving and supporting programs that are consistently aiming to innovate in the field, fueled by a relentless passion to invent for life.
Program Requirements. The objectives of this program are to give students exposure to both theoretical and practical issues relevant to the conception and conduct of a clinical research study, and to put these principles into practice by participating in an ongoing clinical trial. The training provided qualifies students to manage and design ...
MSD's Clinical Research Graduate Programme runs for 24 months and will have the incumbent become part of the organization's Global Clinical Trial Organization SA. This is a developmental role that aims to equip and develop the successful candidate by gaining key skills in the R&D industry. Duties and responsibilities.
Become a Better Clinical Researcher In six core courses built around real-world clinical trials, you will master the procedure cycle and administration of the entire clinical trials process, learn to navigate stakeholder interests, and implement up-to-the-minute regulatory compliance practices and ethical standards. You will finish the program with the ability to initiate clinical research ...
Completed post-graduate physician associate course (either PG Diploma or MSc) Education: Master's (required) Work Location: In person. Application deadline: 19/04/2024. Apply to Clinical Research Associate Graduate jobs now hiring on Indeed.com, the worlds largest job site.