Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 08 April 2021
- Antoni Torres ORCID: orcid.org/0000-0002-8643-2167 1 , 2 , 3 , 4 ,
- Catia Cilloniz ORCID: orcid.org/0000-0002-4646-9838 1 , 2 , 3 , 4 ,
- Michael S. Niederman ORCID: orcid.org/0000-0003-0293-386X 5 ,
- Rosario Menéndez 6 ,
- James D. Chalmers 7 ,
- Richard G. Wunderink ORCID: orcid.org/0000-0002-8527-4195 8 &
- Tom van der Poll 9
Nature Reviews Disease Primers volume 7 , Article number: 25 ( 2021 ) Cite this article
207k Accesses
226 Citations
696 Altmetric
Metrics details
- Respiratory tract diseases
Pneumonia is a common acute respiratory infection that affects the alveoli and distal airways; it is a major health problem and associated with high morbidity and short-term and long-term mortality in all age groups worldwide. Pneumonia is broadly divided into community-acquired pneumonia or hospital-acquired pneumonia. A large variety of microorganisms can cause pneumonia, including bacteria, respiratory viruses and fungi, and there are great geographical variations in their prevalence. Pneumonia occurs more commonly in susceptible individuals, including children of <5 years of age and older adults with prior chronic conditions. Development of the disease largely depends on the host immune response, with pathogen characteristics having a less prominent role. Individuals with pneumonia often present with respiratory and systemic symptoms, and diagnosis is based on both clinical presentation and radiological findings. It is crucial to identify the causative pathogens, as delayed and inadequate antimicrobial therapy can lead to poor outcomes. New antibiotic and non-antibiotic therapies, in addition to rapid and accurate diagnostic tests that can detect pathogens and antibiotic resistance will improve the management of pneumonia.

Similar content being viewed by others
Clinical features for diagnosis of pneumonia among adults in primary care setting: A systematic and meta-review
Etiological and epidemiological features of acute respiratory infections in China
Respiratory viral infections in pragmatically selected adults in intensive care units
Introduction.
Pneumonia is a common acute respiratory infection that affects the alveoli and distal bronchial tree of the lungs. The disease is broadly divided into community-acquired pneumonia (CAP) or hospital-acquired pneumonia (HAP, which includes ventilation-associated pneumonia (VAP)) (Box 1 ). Aspiration pneumonia represents 5–15% of all cases of CAP; however, its prevalence amongst patients with HAP is not known 1 . The lack of robust diagnostic criteria for aspiration pneumonia may explain why the true burden of this type of pneumonia remains unknown 1 .
The causative microorganisms for CAP and HAP differ substantially. The most common causal microorganisms in CAP are Streptococcus pneumoniae , respiratory viruses, Haemophilus influenzae and other bacteria such as Mycoplasma pneumoniae and Legionella pneumophila . Conversely, the most frequent microorganisms in HAP are Staphylococcus aureus (including both methicillin-susceptible S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA)), Enterobacterales, non-fermenting gram-negative bacilli (for example, Pseudomonas aeruginosa ), and Acinetobacter spp. 2 , 3 . In health-care-associated pneumonia (HCAP), owing to patient risk factors, the microbial aetiology is more similar to that in HAP than to that in CAP. However, difficulties in standardizing risk factors for this population, coupled with the heterogeneity of post-hospital health care worldwide, suggest that the concept of HCAP has little usefulness, and indeed, HCAP was not included in recent guidelines for CAP and HAP 3 , 4 , 5 .
Differences in microbiology between CAP and HAP depend on whether pneumonia was acquired in the community or health care environment and on host risk factors, including abnormal gastric and oropharyngeal colonization. In addition, the aetiopathogenesis of CAP is different from that of HAP. In general, mild CAP is treated on an outpatient basis, moderately severe CAP in hospital wards, and severe CAP in intensive care units (ICUs) with or without mechanical ventilation 6 . The need for mechanical ventilation is used as a sub-classification of interest for prognosis and stratification in randomized clinical trials.
Both CAP 7 and HAP 4 can occur in either immunosuppressed or immunocompetent patients. To date, most research data have been based on studies of immunocompetent patients and, therefore, we rely on such sources in this Primer. However, CAP, HAP and VAP in immunosuppressed patients have attracted the attention of researchers, and more investigation is to come.
In this Primer, we cover and summarize the most important and recent updates related to epidemiology, pathophysiology, diagnostic screening, prevention, management, quality of life, and research perspectives. Additionally, owing to the profound impact of the coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome (SARS) coronavirus 2 (SARS-CoV-2), we summarize the main features of SARS-CoV-2 pneumonia (Box 2 ).
Box 1 Classifications of pneumonia
Community-acquired pneumonia (CAP)
Pneumonia acquired outside the hospital in individuals who have not been hospitalized during the month prior to symptom onset.
Hospital-acquired pneumonia (HAP)
Pneumonia acquired after at least 2 days of hospitalization and when no suspicion of disease incubation before hospital admission is present.
Ventilator-associated pneumonia (VAP)
HAP occurring >48 h after endotracheal intubation.
Aspiration pneumonia
Pneumonia occurring as a result of inhalation of contents from the stomach or mouth into the lungs. It is best considered as part of the continuum between CAP and HAP, and not as a distinct entity.
Health-care-associated pneumonia (HCAP)
Pneumonia acquired in non-hospital care institutions.
Box 2 COVID-19 features
Frequent symptoms
Shortness of breath
Less-common symptoms
Hyposmia (decreased sense of smell) and hypogeusia (decreased sense of taste)
Sore throat
Rhinorrhoea (runny nose)
Muscle pain
Diarrhoea and vomiting
Main complications
Acute respiratory distress syndrome (ARDS)
Sepsis and septic shock
Multiple organ failure
Secondary infection
Epidemiology
Global incidence.
Data from the 2019 Global Burden of Diseases (GBD) study 8 showed that lower respiratory tract infections (LRTIs) including pneumonia and bronchiolitis affected 489 million people globally. Children of <5 years of age and adults of >70 years of age are the populations most affected by pneumonia, according to the 2019 GBD study 8 . In 2019, there were 489 million incident cases of LRTI, and 11 million prevalent cases of LRTI. In the 2016 GBD study, the global incidence of LRTI was 155.4 episodes per 1,000 adults of >70 years of age and 107.7 episodes per 1,000 children of <5 years of age 9 . Finally, aspiration pneumonia contributes 5–15% of all cases of CAP and is associated with worse outcomes, especially in older patients with multiple comorbidities 10 , 11 . There is a lack of data about the incidence of aspiration pneumonia in patients with HAP 1 , 12 .
In the USA, the Etiology of Pneumonia in the Community (EPIC) study 13 found that the annual incidence of CAP was 2.4 cases per 1,000 adults, with the highest rates amongst adults of 65–79 years of age (6.3 cases per 1,000 individuals) and those of ≥80 years of age (16.4 cases per 1,000 people). In Europe, the annual incidence of CAP has been estimated at 1.07–1.2 cases per 1,000 people, increasing to 14 cases per 1,000 people amongst those of ≥65 years of age and with a preponderance in men 14 . Differences in epidemiology between the USA and Europe might be explained by the higher proportion of the adult population who received the pneumococcal vaccine in the USA (63.6% of adults of ≥65 years of age, compared with pneumococcal vaccination rates of 20% to 30% in most European countries 15 , 16 ); in addition, in 2015 in the USA, ~69% of adults of ≥65 years of age had received an influenza vaccine within the previous 12 months. Another possible contributing factor is the decreased rate of smoking in the USA: between 2005 and 2016, the percentage of smokers who quit increased from 51% to 59% 17 . Finally, marked differences between US and European health systems can influence epidemiological data.
The South American Andes region had the highest incidence of adults of >70 years of age with LRTIs (406.5 episodes per 1,000 people), while South Asia had the greatest number of LRTI episodes amongst adults of >70 years of age. Incidence per global region was 171.1 per 1,000 people in Central Europe, eastern Europe and central Asia; 234.4 per 1,000 people in Latin America and the Caribbean; 130.8 per 1,000 people in Southeast Asia, eastern Asia and Oceania; 246.6 per 1,000 people in North Africa and the Middle East; and 229.3 per 1,000 people in sub-Saharan Africa 9 .
According to the 2016 GBD study 9 , Oceania had the highest incidence of LRTI in children (171.5 per 1,000 children of <15 years of age), while South Asia had the greatest number of LRTI episodes amongst children of <5 years of age. Incidence per global region was: 107.1 per 1,000 children in Central Europe, eastern Europe, and central Asia; 94.9 per 1,000 children in Latin America and the Caribbean; 120.4 per 1,000 children in Southeast Asia, eastern Asia and Oceania; 133.2 per 1,000 children in North Africa and the Middle East; and 100.6 per 1,000 children in sub-Saharan Africa.
The epidemiology of pneumonia is constantly changing, owing to the development of molecular diagnostic tests, novel antimicrobial therapies and implementation of preventive measures. Since the beginning of the 21st century, pneumonia has been the most common cause of pandemic infections that have effects on its own epidemiology. In the 2009 influenza pandemic, the influenza virus A H1N1 infected ~200 million people and caused almost 250,000 deaths, with infectivity higher in children than in adults 18 . By contrast, in the current SARS-CoV-2 pandemic, 106 million people had been infected and >2 million had died worldwide by 9 February 2021. However, unlike the influenza virus A H1N1, SARS-CoV-2 affects adults more often than children 19 .
The annual incidence of HAP in adults ranges from 5 to 10 cases per 1,000 hospital admissions globally, whereas VAP affects 10–25% of all patients on mechanical ventilation 3 . HAP is the second most frequent hospital infection after urinary tract infection, and VAP is the most common cause of nosocomial infection and death in the ICU 3 , 4 . The incidence of HAP is highest amongst immunocompromised, post-surgical and older patients 20 . In the USA, the incidence of VAP is estimated to range from 2 to 6 cases per 1,000 ventilator-days 21 , and the incidence of non-ventilator-associated HAP is estimated to be 3.63 cases per 1,000 patient-days 22 . A 2018 systematic review and meta-analysis of studies of VAP in adults from 22 Asian countries found an overall incidence of 15.1 cases per 1,000 ventilator-days 23 . In 2015, data from the prospective French multicentre OUTCOMEREA database (1996–2012) indicated that the risk of VAP was ~1.5% per ventilator-day, decreasing to <0.5% per day after 14 days of mechanical ventilation 24 .
The 2019 GBD study 8 showed that LRTI was responsible for >2.49 million deaths, with mortality highest amongst patients of >70 years of age (1.23 million deaths). These data indicate that mortality due to LRTI is higher than mortality due to tuberculosis (1.18 million deaths) and HIV (864,000 deaths), making it the leading cause of infectious disease mortality worldwide. Indeed, data from a systematic review and meta-analysis on the global and regional burden of hospital admissions for pneumonia estimated that 1.1 million pneumonia-related hospital deaths occurred in 2015 amongst older adults 25 .
In 2016, the highest LRTI mortality rates amongst children of <5 years of age were in the Central African Republic (460 deaths per 100,000 children), Chad (425 deaths per 100,000) and Somalia (417 deaths per 100,000) 9 . Interestingly, data from the 2017 GBD study 26 showed that mortality due to LRTI decreased by 36.4% between 2007 and 2017 for children of <5 years of age, whereas it increased by an estimated 33.6% in adults of ≥70 years of age. LRTI-related deaths amongst children have substantially reduced as a result of the implementation of vaccines (against S. pneumoniae and H. influenzae ), antibiotic therapy, the continuous improvements in education, nutrition, water, sanitation and hygiene, and female empowerment. Nevertheless, in many areas the progress is slow; Nigeria, India, Pakistan, Ethiopia and the Democratic Republic of Congo are the five countries with the highest child mortality 27 .
Conversely, the increased mortality in adults of >70 years of age might be associated with the increasing longevity of the frail older population, chronic diseases, comorbidities 28 , multiple medication use and functional disability, especially in high-income countries. In low-income countries, the high mortality is associated with the effect of air pollution; smoke and alcohol consumption are the main risk factors for pneumonia in this age group.
Globally, amongst children and adults, mortality in those with CAP is related to the treatment setting: <1% in outpatient care, ~4–18% in hospital wards and up to 50% in the ICU 29 , 30 , 31 . However, in adults, age and comorbidities influence mortality. A study that investigated the effects of age and comorbidities on CAP mortality found a mortality of 5% in patients of <65 years of age, 8% amongst patients of 65–79 years and 14% amongst patients of ≥80 years of age 32 , and these rates increased to 20%, 42% and 43%, respectively, in patients with more than one comorbidity. On the basis of studies on long-term mortality across 1–10 years 33 , 34 , 35 , approximately one in three adults will die within one year of being hospitalized with CAP 36 . The estimated in-hospital mortality in patients with chronic obstructive pulmonary disorder (COPD) and CAP has been reported to be 6% during hospitalization and 12%, 24% and 33% within 30 days, 6 months and 1 year from discharge, respectively 37 . Interestingly, 30-day mortality amongst those with pneumococcal pneumonia remained fairly stable in a 20-year study 33 , and this was further confirmed in a review on the burden of pneumococcal CAP in Europe 38 .
Globally, HAP and VAP are considered the leading causes of death due to hospital-acquired infection 39 , 40 , 41 . The estimated global mortality due to HAP is 20–30%, whereas global mortality due to VAP is 20–50% 20 , 42 . Mortality due to VAP in the USA was ~13% 4 . By contrast, a prospective study in central Europe 43 indicated that 30-day mortality due to VAP was 30%. In a large French cohort of patients admitted to the ICU for >48 h, both non-ventilator-associated HAP and VAP were associated with an 82% and a 38% increase in the risk of 30-day mortality, respectively 44 . However, analysis of data from trials on antibiotic therapy for bacterial HAP and VAP to characterize all-cause mortality showed that mortality differed notably within and across studies; all-cause mortality at day 28 was 27.8% in bacterial HAP, 18% in bacterial VAP and 14.5% in non-ventilation-associated bacterial HAP 45 .
In a systematic review and meta-analysis 10 , aspiration pneumonia was significantly associated with increased in-hospital mortality (relative risk 3.62) and 30-day mortality (relative risk 3.57) in patients with CAP treated outside of the ICU. One of the largest studies in aspiration pneumonia demonstrated that mortality in patients with aspiration pneumonia (29%) was more than twice that in patients with CAP (12%) 11 .
Risk factors and differences in epidemiology
Children of <5 years of age 46 and older adults 13 , particularly those of of ≥65 years of age and with comorbidities 14 , 47 , have an increased risk of CAP (Table 1 ). In children, prematurity, malnutrition, household air pollution, ambient particulate matter or suboptimal breastfeeding are the main CAP-related risk factors 48 . In adults, respiratory disease (for example, COPD), diabetes mellitus, cardiovascular disease and chronic liver disease are the most frequent comorbidities that increase the risk of CAP 14 . Of note, men have a higher risk of CAP than women, which may be explained by differences in anatomy, and behavioural, socioeconomic and lifestyle factors 49 .
A US study on the incidence, outcomes and disease burden in >18,000 hospitalized patients with COPD 37 found that, during the 2-year study, 3,419 patients had pneumonia; the annual incidence for CAP was 93.6 cases per 1,000 in the COPD population. In patients without COPD, the incidence was 5.09 cases per 1,000. In the USA, 506,953 adults with COPD are estimated to be hospitalized every year due to pneumonia 37 .
Immunocompromised patients have a higher risk of CAP than the general population 7 , 14 . A secondary analysis of an international, multicentre study from 54 countries worldwide found that almost one in five patients hospitalized with CAP were not immunocompetent 7 . Amongst patients with CAP, 18% had one or more risk factors for immunodeficiency, with chronic steroid use (45%), haematological cancer (25%) and chemotherapy (22%) being the most frequent.
Several studies have also demonstrated an association between lifestyle factors and the risk of CAP, including smoking, high alcohol consumption, being underweight (owing to under-nutrition or underlying conditions that compromise the immune response), living conditions, such as a large household or regular contact with children, and others 14 . Smoking is associated with colonization by pathogenic bacteria and an increased risk of lung infection, especially by S. pneumoniae 50 . Consumption of 24 g, 60 g and 120 g of pure alcohol daily (one standard alcoholic beverage equals 10 ml or 8 g of pure alcohol, and it is the approximate amount of alcohol that the average adult can process in an hour) resulted in relative risks for CAP of 1.12, 1.33 and 1.76, respectively, compared with no consumption 51 . In addition, exposure to air pollution may increase the risk of pneumonia in the short and long term; a study in 345 hospitalized patients with CAP and 494 controls (patients who were admitted in the same period but for non-pneumonia reasons) demonstrated that long-term exposure (1–2 years) to high levels of air pollutants (particulate matter 2.5 μm and nitrogen dioxide) was associated with increased hospitalization in those of ≥65 years of age 52 .
Factors that increase the risk of HAP can be categorized into patient-related and treatment-related groups (Table 1 ). Oropharyngeal colonization is the main mechanism underlying HAP. However, much attention has been shifted to oropharyngeal colonization in critically ill patients (present at ICU admission or occurring during ICU stay) 53 . A study from Japan investigating oral colonization in residents in long-term care facilities found that 38% of these individuals were colonized with antibiotic-resistant pathogens, mainly Acinetobacter spp., Enterobacterales and Pseudomonas spp. The presence of these pathogens represents a potential risk for pneumonia 54 . Indeed, current international guidelines have suggested that previous colonization by antibiotic-resistant pathogens be considered when identifying patients with an increased risk of HAP due to such pathogens 3 , 4 .
Colonization and biofilm formation were present within 12 h of intubation and remained for >96 h in most patients 55 . Underpinning an important association between intubation and VAP pathogenesis, this study also showed that colonization in patients undergoing mechanical ventilation occurred in the oropharynx and stomach first, followed by the lower respiratory tract and, thereafter, the endotracheal tube 55 . Intubation and mechanical ventilation can increase the risk of developing VAP by 6–21-fold, with the highest risk within the first 5 days of intubation 53 . Endotracheal tubes enable the direct entry of bacteria into the lower respiratory tract, interfere with normal host defence mechanisms and serve as a reservoir for pathogenic microorganisms.
Multiple risk factors are related to aspiration pneumonia, each one increasing the chance of gastric contents reaching the lungs. The most frequent of these factors are impaired swallowing, decreased consciousness and an impaired cough reflex 1 (Table 1 ).
Microbial aetiology
Knowledge of pathogens associated with pneumonia is crucial to provide more targeted empiric antibiotic therapy, prevent the emergence of antimicrobial resistance through selection pressure and reduce health-care-associated costs.
The microbial aetiology of CAP differs by its severity at clinical presentation and by season 2 , 56 , 57 , 58 . However, the microbial aetiology of CAP is not detected in ~50% of patients; possible reasons include the failure to obtain a respiratory sample adequate for culture or before the initiation of antibiotic therapy and the inconsistent availability of newly improved molecular tests 59 . S. pneumoniae remains the most frequent pathogen in CAP, although a study in North America found that its incidence has decreased owing to the introduction of polysaccharide vaccines 60 and a reduced smoking rate 61 , 62 . No such decrease has been observed in Europe 2 , 63 , 64 , 65 (Fig. 1 ).
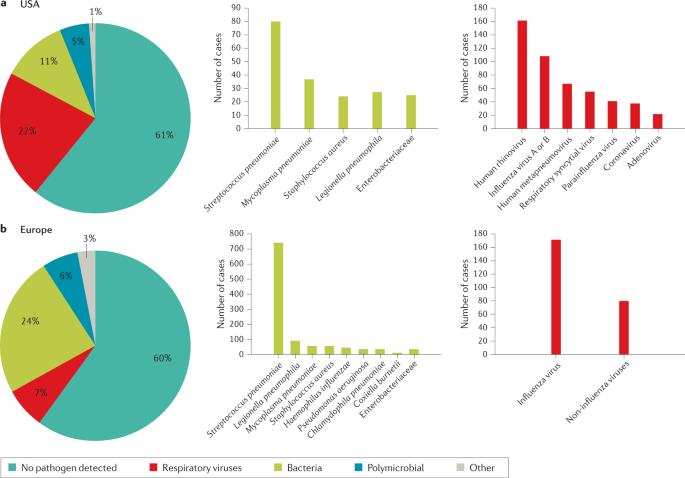
a | Aetiology of community-acquired pneumonia (CAP) in the adult population in the USA from 2010 to 2012 (from 2,488 cases) 9 . b | Aetiology of CAP in the adult population in Europe from 2003 to 2014 (from 3,854 cases) 6 . Possible reasons that may explain the challenge in identifying the aetiology of pneumonia include difficulty in obtaining samples from the lower respiratory tract, the effect of antibiotic use prior to sample collection and low sensitivity of some diagnostic tests.
In a small proportion of patients, CAP is caused by MRSA and antibiotic-resistant gram-negative bacteria (for example, P. aeruginosa and Klebsiella pneumoniae ) 2 , 66 . As antibiotic resistance complicates clinical management, clinicians need to recognize risk factors for these pathogens and initiate adequate empirical therapy in response (Box 3 ). The main risk factors for multidrug-resistant (MDR) pathogens in CAP include immunosuppression, previous antibiotic use, prior hospitalization, use of gastric acid-suppressing agents, tube feeding and non-ambulatory status 67 . Various scoring systems can help to determine the risk of infection by antibiotic-resistant pathogens.
The P. aeruginosa , extended-spectrum β-lactamase (ESBL)-positive Enterobacterales and MRSA (PES) score 68 is based on several risk factors, including age 40–65 years and male sex (one point each), age >65 years, previous antibiotic use, chronic respiratory disorder and impaired consciousness (two points each), and chronic renal failure (three points). The PES score has been validated in general wards, ICUs and a very old population (age ≥80 years). One study 69 demostrated that there is an 80% probability of detecting a PES pathogen with the PES score, demonstrating good accuracy of the score. In another study 70 , the accuracy of the PES score in patients of ≥80 years of age with CAP was ~64%, highlighting differences in clinical characteristics of this population who are more susceptible to infections, recurrent pneumonia and sepsis.
The drug resistance in pneumonia (DRIP) score 71 is based on both major and minor risk factors. Major risk factors (two points each) include previous antibiotic use, residence in a long-term care facility, tube feeding and prior infection by a drug-resistant pathogen (within the past year). Minor risk factors (one point each) include hospitalization within the previous 60 days, chronic pulmonary disease, poor functional status, gastric acid suppression, wound care and MRSA colonization (within the past year).
The use of new diagnostic molecular techniques has led to an increased interest in the role of respiratory viruses as potential aetiological agents in CAP. Recent studies have reported that respiratory viruses account for 7–36% of CAP cases with a defined microbial aetiology 13 , 72 , 73 . A recent study from China reported that in patients with viral CAP, influenza virus, non-influenza virus and mixed viral infections were the cause of CAP in 63%, 27% and 10% of patients, respectively (Fig. 2 ). The outcomes were similar between patients with CAP due to influenza virus and those with CAP due to non-influenza viruses, although in patients with CAP due to non-influenza viruses the incidence of complications was higher 74 . In another study, 3% of all patients with a diagnosis of CAP admitted to the emergency department had pure viral sepsis 75 . Viral sepsis was present in 19% of those admitted to ICU, and sepsis was present in 61% of all patients with viral CAP.
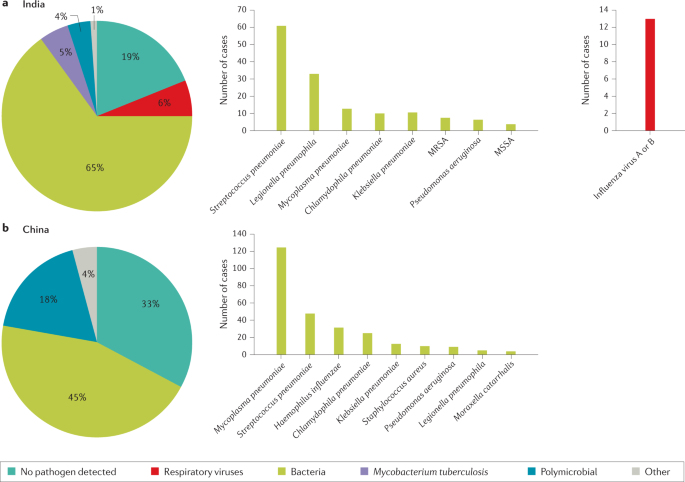
a | Aetiology of community-acquired pneumonia (CAP) in the adult population in India from 2013 to 2015 (from 225 cases) 54 . b | Aetiology of CAP in the adult population in China from 2004 to 2005 (from 593 cases) 55 . Possible reasons that may explain the challenge in identifying the aetiology of pneumonia include difficulty in obtaining samples from the lower respiratory tract, the effect of antibiotic use prior to sample collection and low sensitivity of some diagnostic tests. MRSA, methicillin-resistant Staphylococcus aureus ; MSSA, methicillin-susceptible Staphylococcus aureus .
Respiratory viruses are detected in more than half of children with CAP 76 . Respiratory viruses were the most frequent cause of pneumonia (66%) in children with an aetiological diagnosis in the USA, with respiratory syncytial virus, rhinovirus and metapneumovirus being the most common ones 76 . Bacterial pathogens were the cause of CAP in 8% of patients, with S. pneumoniae and S. aureus being the most common bacteria. Bacteria–virus co-infections were detected in 7% of patients.
Box 3 Pathogen-specific risk factors
Streptococcus pneumoniae : Dementia, seizure disorders, congestive heart failure, cerebrovascular disease, chronic obstructive pulmonary disease (COPD), HIV infection, overcrowded living conditions and smoking
Legionella pneumophila : Smoking, COPD, compromised immune system, travel to outbreak areas, residence in a health-care facility and proximity to cooling towers or whirlpool spas
MRSA : Previous MRSA infection or colonization, residence in a nursing home or long-term care facility and prior hospitalization within the previous 90 days
Pseudomonas aeruginosa : Pulmonary comorbidity
Enterobacterales: Residence in a nursing home
MRSA, methicillin-resistant Staphylococcus aureus
Data on microbial aetiology of HAP have mostly been obtained from patients with VAP. However, studies in patients with HAP or VAP with known microbial aetiology have shown that both HAP and VAP have similar microbial aetiology, with P. aeruginosa and S. aureus being the most frequent pathogens. Other pathogens such as Acinetobacter spp. and Stenotrophomonas spp. are more frequently reported in VAP 4 , 77 .
Antibiotic resistance is the main concern with HAP and VAP. Assessing risk factors for MDR organisms (resistant to at least one agent in three or more groups of antibiotics), extensively drug-resistant organisms (XDR; resistant to one or more agents in all but one or two antibiotic groups) and pandrug-resistant organisms (resistant to almost all groups of approved antibiotics) is central to managing patients with these pathogens 78 . In general, we can classify the risk into three categories: (1) local epidemiology (for example, ICU with high rates of MDR pathogens); (2) patient risk factors (including structural pulmonary diseases (for example, bronchiectasis), antibiotic use during the 90 days prior to HAP or VAP onset, hospitalization (2–5 days) during the 90 days prior to HAP or VAP onset, septic shock at VAP onset, acute respiratory distress syndrome (ARDS) preceding VAP, at least 5 days of hospitalization prior to VAP onset, and acute renal replacement therapy prior to VAP onset) 42 ; and (3) previous colonization or infection with MDR pathogens 42 . Anaerobes and gram-negative bacilli (for example, E. coli , K. pneumoniae and P. aeruginosa ) are the most frequent microorganisms found in aspiration pneumonia 1 .
Mechanisms/pathophysiology
From colonization to infection.
The mechanisms that drive LRTIs have become increasingly known. Most instances of bacterial pneumonia are caused by microorganisms that translocate from the nasopharynx to the lower respiratory tract 79 , 80 . Bacteria enter the nasopharynx after shedding from a colonized individual. Pathogens can spread between individuals via direct or indirect contact, droplets and aerosols 81 . Transmission success depends on many variables, including environmental conditions, gathering of people and host factors, such as the distribution of pattern recognition receptors in the epithelial cells of the airways 81 . Pathogen adherence to the upper airway epithelium is a crucial first step in colonization and subsequent infection. Once in the nasopharynx, bacteria escape from mucus and attach to the epithelium using multiple strategies to evade host clearance, including expression of host-mimicking or antigenically varying molecules 82 (that is, molecules that imitate the structure of host molecules or can vary their antigens to avoid recognition by host immune cells). Microorganisms gain entry to the lower airways through inhalation or, less frequently, by pleural seeding from blood. Selection of colonizing mutants that can evade immune clearance is considered to precede infection 79 . Infection occurs when host defences are impaired and/or there has been exposure to a highly virulent microorganism or a large inoculum. Several factors can facilitate the transition from colonization to infection, including preceding viral infection and chronic lung diseases. Other mechanisms involved in the increased susceptibility to infection include loss of barrier integrity and impaired host defences due to complex interactions amongst anatomical structures, microorganisms (and their virulence factors) and the host immune system 79 , 80 , 83 .
Of note, it has become clear that healthy lungs are not sterile; instead, they harbour a unique microbiota that includes ~100 different taxa 84 . The main genera in healthy lower airways are Prevotella , Streptococcus , Veillonella , Fusobacterium and Haemophilus 84 . The pathogenesis of pneumonia has been suggested to include a change in the lung microbiota, from a physiological, homeostatic state to dysbiosis, in association with a low microbial diversity and high microbial burden, and with corresponding immune responses 84 , 85 To further support this concept, longitudinal lung microbiota studies are required to document transitions from homeostatic to dysbiotic states during the development and resolution of pneumonia. An additional area of research lies in analysing the virome and mycobiome in airways and their influence on host defence against pneumonia. The mechanisms by which lung microbiota affect immunity in the airways have been partially elucidated. Bacteria present in the upper airways that potently stimulate nucleotide-binding oligomerization domain-containing (NOD)-like receptors ( Staphylococcus aureus and Staphylococcus epidermidis ) increase resistance to pneumonia through NOD2 and induction of release of granulocyte–macrophage colony-stimulating factor 86 .
Mechanisms of infection
A general mechanism of infection of the lower airways is difficult to define. The many different microorganisms that can cause pneumonia do not seem to express specific features. Even in specific populations (for example, young children, hospitalized patients, older individuals), a spectrum of pathogens, rather than a specific microorganism, can cause pneumonia. This finding has led to the assumptions that the development of pneumonia largely depends on the host response to the microbe in the airways, with pathogen characteristics playing a less prominent role 83 . Nonetheless, virulence factors expressed by microorganisms do contribute to the ability of specific pathogens to cause pneumonia 79 , 80 . For example, pneumolysin, a virulence factor expressed by S. pneumoniae , is a member of the cholesterol-dependent cytolysin family that can form large pores in (and thereby injure) eukaryotic cells with cholesterol-containing membranes 87 . S. aureus expresses several virulence factors, such as α-haemolysin (also known as α-toxin), a pore-forming toxin that causes cell death via activation of the inflammasome 88 . α-Haemolysin binds to the disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) and results in disruption of the barrier function of the respiratory epithelium 88 . Finally, toxins secreted by the type III secretion system are a key element in P. aeruginosa virulence in the lung. Genes encoding type III-secreted toxins are induced in P. aeruginosa upon contact with host cells, eliciting a plethora of effects, including cytotoxicity 89 .
Once an LRTI has occurred, the maintenance of lung homeostasis whilst in the presence of microbes depends on an adequate balance between two seemingly opposing processes, immune resistance and tissue resilience, that are largely mediated by the same cell types. Whilst immune resistance seeks to eliminate invading microbes, tissue resilience strives to prevent or resolve tissue damage caused by the immune response, the pathogen or both 83 . The organized actions of immune resistance and tissue resilience determine whether and how an LRTI progresses or resolves. Inadequate or unfitting immune responses can result in adverse outcomes, such as ARDS, defined as the acute onset of non-cardiogenic pulmonary oedema, hypoxaemia and the need for mechanical ventilation 90 , 91 . Unbalanced immune responses during pneumonia can also result in extrapulmonary complications, some of which can occur up to years after the respiratory illness (see below).
Immune resistance
Anatomical barriers present the first line of defence against pneumonia. Mucociliary clearance, mediated by mucous and liquid layers and cilia on the surface of respiratory epithelial cells, is considered the primary innate defence mechanism 92 . The respiratory epithelium produces a robust barrier composed of secretory products, surface glycocalyces and membranes, and intercellular junctional proteins linked to the actin cytoskeleton 92 . Cell-associated and secreted mucins form a polymeric glycoconjugate layer that can bind and transport pathogens from the airways 92 . The branching bronchial tree provides an additional defence mechanism by preventing particles of >3 µm in diameter from entering the lower airways 92 . If microbes do reach the lower respiratory tract, the host defence becomes shaped by an interplay between resident and recruited immune cells and mechanisms (Fig. 3 ).
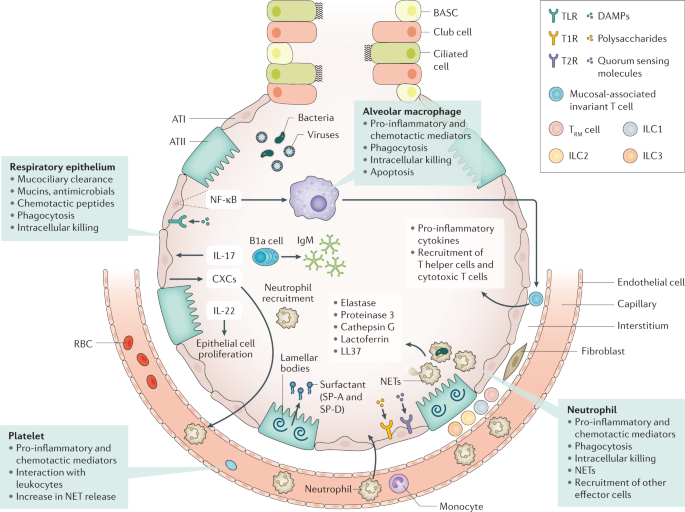
Immune resistance aims to eradicate microorganisms that invade the airways. Respiratory epithelial cells are covered by cell-associated and secreted mucins that form a layer of polymeric glycoconjugates that remove pathogens from the airways. The epithelium can also remove pathogens through phagocytosis and intracellular killing. The quiescent alveolar space contains many alveolar macrophages that, upon activation, can phagocytose and kill pathogens, which is improved by apoptosis. Innate lymphoid cells (ILCs) are tissue-resident cells populating the pulmonary mucosa. Together with natural killer cells, ILCs boost host defence during airway infection. Neutrophils migrate to the airways attracted by chemotactic proteins released by respiratory epithelial cells and alveolar macrophages; these chemotactic proteins also promote the recruitment of other leukocyte subsets. The lung contains a marginated pool of neutrophils tethered to the vasculature, enabling rapid neutrophil recruitment into tissue upon infection. Adequate pulmonary immunity entails neutrophil-mediated killing of invading microbes by several effector mechanisms, including the release of neutrophil extracellular traps (NETs). Platelets can form complexes with leukocytes, facilitating NET formation and the release of microbicidal agents. Resident memory T (T RM ) cells are generated after exposure to pathogens and reside in the quiescent lung. ATI, alveolar type I cell; ATII, alveolar type II cell; BASC, bronchioalveolar stem cell; CXCs, CXC chemokines; DAMPs, damage-associated molecular patterns; NF-κB, nuclear factor-κB; RBC, red blood cell; SP, surfactant protein; T1R, G-protein-coupled sweet taste receptor; T2R, G-protein-coupled bitter taste receptor; TLR, Toll-like receptor.
Innate immunity
Various innate immune cells reside in quiescent airways to provide the next line of defence against pathogens. Lung epithelial cells can be triggered through a variety of receptors that recognize not only pathogens but host-derived molecules as well, including damage-associated molecular patterns (released upon cell injury) and cytokines. Many pattern recognition receptors (for example, toll-like receptors) then induce nuclear factor ĸB, which is a major driver of protective immunity in the epithelium 93 , 94 . In the alveoli, surfactant proteins SP-A and SP-D produced by type II epithelial cells can directly inhibit microbes 95 . Recently, G-protein-coupled bitter taste receptors (T2R) and sweet taste receptors (T1R) were identified in respiratory epithelial cells 96 ; bacterial quorum-sensing molecules can trigger bitter taste receptors, whilst sugars can activate sweet receptors, and these interactions may then modify host defence mechanisms 97 . IL-17 and IL-22 mediate protection during pneumonia largely through epithelial cell activation 98 . IL-17 stimulates the epithelium to secrete antimicrobial proteins and CXC chemokines that trigger neutrophil recruitment. The protective properties of IL-22 are linked to its function in stimulating epithelial cell proliferation, which is indispensable for repair following injury 99 .
Alveolar macrophages (AMs), which reside on lower airway surfaces, have essential roles in both immune resistance and tissue resilience 100 . During homeostasis, they limit the effect of potentially noxious environmental stimuli through anti-inflammatory effects. The crucial role of AMs in immune resistance during pneumonia is illustrated by studies showing impairment of the host defence when AM function is disrupted 94 . Microbes can activate AMs via several pattern recognition receptors and nuclear factor ĸB, leading to the production of pro-inflammatory cytokines that orchestrate subsequent, innate immune responses necessary for resistance. In addition, stimulated by AM apoptosis, activated AMs can phagocytose and kill pathogens 101 . By contrast, AM death via non-apoptotic pathways, such as necroptosis, impairs antibacterial defence during pneumonia 102 . The complex role of necroptosis in the host response to bacterial infection is illustrated by reports linking necroptosis to exaggerated inflammation and impaired bacterial clearance during S. aureus pneumonia 103 , whereas it has a protective, anti-inflammatory effect associated with improved bacterial clearance during systemic S. aureus infection 104 . Local conditions may instruct AMs in providing the most suitable response.
Innate lymphoid cells (ILCs) serve as counterparts to T cells by regulating immune responses via the production of effector cytokines and by influencing functions of other innate and adaptive immune cells 105 . These cells are especially abundant on the mucosal surfaces of the lung. There are three major groups of ILCs, namely, ILC1, ILC2 and ILC3. ILC classification reflects these cells’ capacity to secrete types 1, 2 and 17 cytokines, respectively. Beneficial roles for ILC1s and ILC2s have been reported in viral pneumonia models 106 , 107 ; lung ILC3s have a protective role in pneumonia by secreting IL-17 and IL-22 (refs 108 , 109 ). Mucosal-associated invariant T cells are other innate-like T lymphocytes that are abundant in the lung mucosa 110 . These cells probably have a role in protective immunity during airway infection through a variety of mechanisms, including production of pro-inflammatory cytokines, macrophage activation and recruitment of effector helper and cytotoxic T cells 111 .
When resident cells are unable to eradicate invading pathogens, mechanisms are activated to attract additional effector cells to the site of infection. Neutrophils are the first and most profusely recruited cells in response to infection 112 . Primed neutrophils have a strongly increased capacity to phagocytose microbes and initiate a respiratory burst response 112 . In addition, neutrophil products, such as elastase, proteinase 3 (also known as myeloblastin), cathepsin G, lactoferrin and LL-37, exert potent antimicrobial activities 113 . Neutrophil extracellular traps, comprising decondensed chromatin fibres that carry histones and antimicrobial peptides, are also released to kill pathogens 113 . The crucial role of neutrophils in pulmonary immune resistance is illustrated by the increased susceptibility found in patients with neutropenia or neutrophil deficiencies and mouse pneumonia models, in which neutrophil depletion has been shown to exacerbate infection with several pathogens 112 . In addition to AMs, newly recruited inflammatory monocytes–macrophages are involved in immune resistance during pneumonia 114 . In mice, induction of K. pneumoniae -associated pneumonia has been found to lead to the recruitment of inflammatory monocytes to the lungs where they mediate the influx of protective ILCs producing IL-17 through the release of tumour necrosis factor 109 . Innate-like B1 B cells mainly reside in the pleural space. In response to infection, B1a B cells migrate to the lung parenchyma to produce polyreactive immunoglobulin M and contribute to protective immunity 115 . Platelets also provide immune resistance during pneumonia through various mechanisms, including platelet–bacteria interactions and complex formation with leukocytes. Other mechanisms include facilitating neutrophil extracellular trap formation and stimulating the release of microbicidal agents that can directly lyse bacteria 116 . Thrombocytopenia is associated with impaired antibacterial defence during murine pneumonia 117 , 118 .
Finally, several distant organs can affect immune resistance in the respiratory tract. For example, depletion of gut microbiota by broad-spectrum antibiotics has been shown to impair host defence during viral and bacterial pneumonia in mice 119 , 120 . This protective gut–lung axis has been hypothesized to be mediated, at least in part, by gut-derived microbial products that can improve host defence mechanisms in other tissue 121 . The existence of a liver–lung axis has been suggested in many studies; pneumonia elicits a robust acute-phase protein response in the liver, probably mediated by cytokines released into circulation, and distinct acute-phase proteins can improve antibacterial defence through several mechanisms, for example, by enhancing opsonophagocytosis (phagocytosis mediated by opsonins) and respiratory burst activity by immune cells and by limiting iron availability to bacteria.
Adaptive immunity
Previous encounters with respiratory pathogens shape memory defence mechanisms against pneumonia. Evidence highlights roles of innate immune cells (for example, epithelial cells and AMs) that had been modified by a prior infection to trigger epigenetic alterations in a so-called process of ‘trained immunity’ 122 . Trained immunity has received attention within the context of pneumonia in humans. The Bacille Calmette–Guérin vaccination induces trained immunity. When administered to older patients after hospital discharge, the vaccination increased time to first infection, and most of the protection was observed against respiratory tract infections of probable viral origin 123 . Humoral response to microbes improves host defence by producing pathogen-specific antibodies, as illustrated by the efficacy of vaccines in diminishing the risk of pneumonia.
The airways contain pools of memory cells that are assembled in tertiary lymphoid organs in the upper airways and in bronchus-associated lymphoid tissue in the lower airways. Together, these cells protect against infection through local and systemic antibody production 124 . The majority of CD4 + T cells and CD8 + T cells in the quiescent lung have a memory phenotype (hence they are named resident memory T (T RM ) cells) and are generated in response to exposure to respiratory pathogens 125 . In experimental mouse models, the lung is enriched with T RM cells specific for multiple viral and bacterial pathogens following a respiratory infection, and these cells contribute to future protective immunity. For example, lobar pneumococcal pneumonia in mice leads to the accumulation of CD4 + T RM cells in the infected lobe, but not in other areas of the lung. This T RM cell-populated lobe expresses better defence against reinfection by S. pneumoniae than other lobes 126 .
Tissue resilience
Tissue resilience is essential in controlling excessive inflammation whilst sustaining effective protection against microbes (Fig. 4 ). AMs contribute to tissue resilience by producing anti-inflammatory cytokines, such as IL-10 and IL-1 receptor antagonist, and through the phagocytosis of apoptotic leukocytes. This process is named efferocytosis and protects tissue in two manners: by preventing the release of pro-inflammatory and toxic contents from dying cells and by concurrently prompting the release of anti-inflammatory and repair factors, including transforming growth factor β1, prostaglandin E 2 , and pro-resolving lipid mediators 100 . Pro-resolving lipid mediators (resolvins, protectins and maresins) can mediate a large variety of immune responses in pneumonia, both increasing bacterial killing and promoting tissue repair 127 . Such mediators have been shown to have important protective roles in mouse models of bacterial pneumonia 128 , 129 .
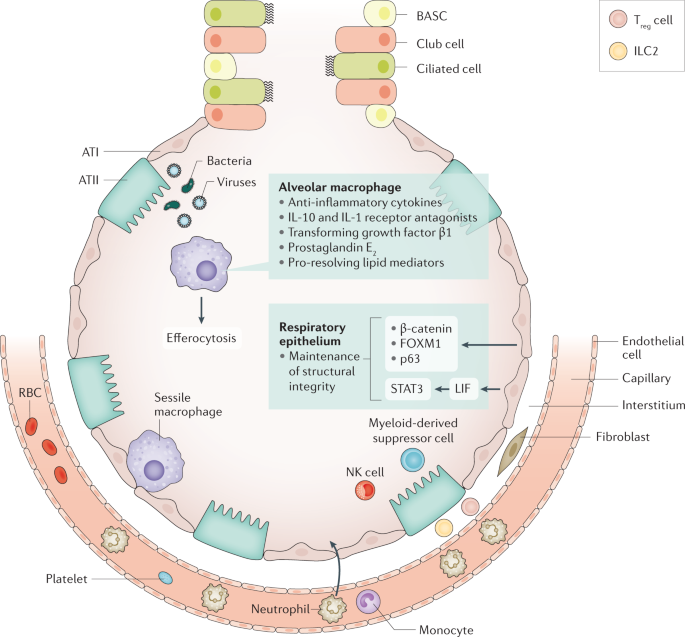
Tissue resilience controls excessive inflammation whilst safeguarding protection against pathogens. The respiratory epithelium is an important player in tissue resilience. Maintenance of the structural integrity of the epithelial barrier is a crucial factor here. Alveolar macrophages also have an important role, via release of anti-inflammatory mediators and efferocytosis (phagocytosis of apoptotic leukocytes). Sessile macrophages adhere to the epithelium, where they probably contribute to tissue resilience. Cell types recruited to the site of infection during pneumonia that are involved in tissue resilience include myeloid-derived suppressor cells, regulatory T (T reg ) cells, type 2 ILC2s and natural killer (NK) cells. ATI, alveolar type I cell; ATII, alveolar type II cell; BASC, bronchioalveolar stem cell; FOXM1, forkhead box protein M1; ILC, innate lymphoid cell; LIF, leukaemia inhibitory factor; RBC, red blood cell; STAT3, signal transducer and activator of transcription 3.
The structural integrity of the epithelial barrier in the respiratory tract is crucial to tissue resilience. Contributors to epithelial resilience include β-catenin (also known as catenin β1) 130 , forkhead box protein M1 (FOXM1) 131 , tumour protein 63 (p63) 132 and signal transducer and activator of transcription 3 (STAT3) 133 , 134 . Interestingly, a deficiency of STAT3 in airway epithelial cells results in exaggerated lung injury during experimental pneumonia 133 , 134 . Epithelial cell-derived leukaemia inhibitory factor (LIF) has been implicated as an important inducer of STAT3 in the respiratory epithelium, and inhibition of LIF has been shown to increase lung injury in pneumonia 135 . Several immune cells recruited to the site of infection during pneumonia are known to contribute to tissue resilience, including myeloid-derived suppressor cells 136 , regulatory T cells 137 , ILC2s 138 and natural killer cells 139 , 140 .
Lung pathology
With respect to the histopathology of bacterial pneumonia, four stages have classically been described: congestion, red hepatization, grey hepatization and resolution (Fig. 5 ). The term hepatization refers to an increased firmness of inflamed lung tissue that renders the tissue consistency similar to that attributed to hepatic tissue. In the early stages of bacterial pneumonia, lung tissue shows mild intra-alveolar oedema and congestion of the capillaries within the alveolar septa 141 . This stage is followed by inflammatory exudation with an accumulation in the alveolar spaces of neutrophils, red blood cells and fibrin, and a subsequent, gradual disintegration of red blood cells and neutrophils. The exudates are eventually transformed into intra-alveolar fibromyxoid moulds, consisting of macrophages and fibroblasts, and gradual resolution follows thereafter.
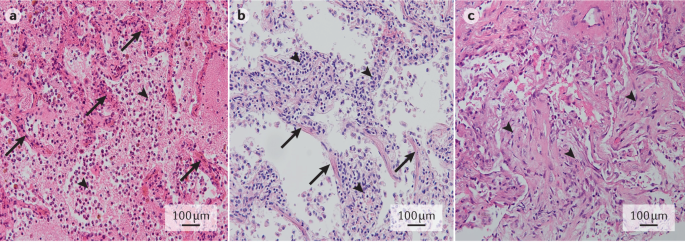
a | Early stage bacterial pneumonia, with congestion of septal capillaries (arrows) and intra-alveolar presence of oedema, neutrophils and a meshwork of fibrin strands (arrowheads). b | Early stage viral pneumonia, with interstitial lymphocytic infiltrates (arrowheads) and diffuse alveolar damage, as evidenced by the presence of hyaline membranes (arrows). c | Organizing pneumonia, with intra-alveolar fibroblast plugs (arrowheads) and few remnant fibrin deposits. Haematoxylin and eosin staining; original magnification ×20. Images in parts a – c courtesy of J.J.T.H. Roelofs, Amsterdam UMC, Netherlands.
Viral pneumonia is typically associated with interstitial inflammation and diffuse alveolar damage 142 . Interstitial inflammation involves the alveolar walls, which widen and usually contain an inflammatory infiltrate of lymphocytes, macrophages and plasma cells in some cases. Alveolar damage is characterized by pink hyaline membranes lining the alveolar septa that follow a pattern of organization and resolution similar to that of intra-alveolar inflammation in bacterial pneumonia.
In addition to these features, specific microorganisms may cause different histopathological changes such as granulomas, multinucleated giant cells or specific viral inclusions.
Extrapulmonary complications
Extrapulmonary complications are extremely common in patients with pneumonia, including those without sepsis. Such complications entail both acute and long-term adverse sequelae. Patients who have been hospitalized for pneumonia have higher rates of all-cause hospitalization and an increased mortality risk for 10 years after discharge 35 compared with matched patients hospitalized for other pneumonia-unrelated conditions.
Sepsis, defined as a life-threating organ dysfunction caused by a dysregulated host response to an infection 143 , is most often caused by pneumonia (up to half of all patients with sepsis) 144 . Conversely, of patients who are hospitalized with CAP 145 or HAP 146 , 36% and 48% have been reported to develop sepsis, respectively. Both pro-inflammatory and anti-inflammatory reactions characterize host response to sepsis, which varies strongly between individuals. Pro-inflammatory responses include the release of cytokines, activation of the complement and coagulation system (which could result in disseminated intravascular coagulation), and disruption of the normal barrier and anticoagulant function of the vascular endothelium. Anti-inflammatory responses can result in immune suppression, in part due to apoptotic loss of lymphoid cells 147 , 148 .
Cardiovascular disease
Pneumonia particularly affects the cardiovascular system, and its effects include depression of left ventricular function, myocarditis, arrhythmias, ischaemia and infarction 149 . Patients hospitalized for pneumonia have an increased short-term and long-term risk (up to ten years) of cardiovascular disease 150 . A meta-analysis of the incidence of cardiac events within 30 days of pneumonia diagnosis found new or worsening heart failure in 14% of all patients, new or worsening arrhythmias in 5% and acute coronary syndromes in 5% 151 . Approximately 90% of cardiac complications occur within 7 days of a pneumonia diagnosis, and more than half occur within the first 24 h 149 . In a multicentre study, one third of patients hospitalized for CAP experienced intrahospital cardiovascular events, mainly involving the heart, and such occurrence was associated with a fivefold increase in 30-day mortality. Independent risk factors for cardiovascular events were severity of pneumonia and pre-existing heart failure 152 . Additionally, hospitalization for pneumonia is associated with an increased risk of new-onset heart failure in the intermediate and long term, with a hazard ratio of 2 after 5 years 34 . In patients with pneumonia who require ICU treatment within 24 h of hospital admission, approximately half have diagnostic criteria for myocardial infarction 153 ; cardiac complications are the direct or main cause of death in 27% of patients hospitalized for pneumonia 154 . Notably, whilst the increased risk for myocardial infarction associated with pneumonia is proportional to disease severity, it is not restricted to patients with pneumonia-induced sepsis 155 . Even mild respiratory infection is associated with an increased risk of myocardial infarction for several months after the onset of infection 155 .
The mechanisms underlying an increased risk of cardiovascular disease after pneumonia are probably multifactorial. Hypoxaemia due to impaired gas exchange and ventilation–perfusion mismatching, as well as endothelial dysfunction causing vasoconstriction, may increase vulnerability to ischaemic events 149 . Systemic inflammation during pneumonia can increase inflammatory activity within coronary atherosclerotic plaques, rendering them prone to rupture 149 . The systemic host response during pneumonia also entails endothelial dysfunction and procoagulant changes, which can promote thrombus formation at the site of a ruptured coronary plaque 149 . Indeed, as reflected by elevated markers of coagulation activation in the circulation, the majority of patients admitted to hospital for pneumonia have a procoagulant phenotype 156 , 157 .
Patients with pneumonia and acute coronary syndromes show higher platelet-aggregating activity than patients with acute coronary syndromes without pneumonia 149 . Notably, the connection between pneumonia and cardiovascular disease is probably bidirectional. For example, pre-existing heart failure is a risk factor for pneumonia, perhaps partially related to impaired immune responses 149 . Preclinical investigations suggest that lung congestion can facilitate the growth of common respiratory pathogens in the airways 149 . With regard to long-term risk, investigations in mice predisposed to developing atherosclerosis 158 and post mortem examinations in humans 159 have suggested that infection can elicit pro-inflammatory responses in atherosclerotic lesions and result in increased vulnerability for coronary and cerebrovascular events. For example, acute lung inflammation induced by intratracheal administration of lipopolysaccharide in mice prone to atherosclerosis resulted in destabilization of atherosclerotic plaques; neutrophil depletion prevented this destabilization, suggesting a role for neutrophils in plaque weakness elicited by lung injury 160 . In addition, systemic inflammation and coagulation are sustained in many patients with pneumonia and have been associated with an increased risk of cardiovascular death 161 , 162 . Left ventricular dysfunction during pneumonia may be secondary to depressant activity of pro-inflammatory cytokines in circulation and/or altered vascular reactivity 149 .
Other complications
Additional extrapulmonary complications of pneumonia include a decline in cognition and functional status 163 , 164 . Pneumonia is associated with a 57% increase in the risk of dementia 164 . Encephalopathy associated with acute infectious disease has been studied in the context of sepsis 165 , 166 . Mechanisms involved include impaired circulation in the brain secondary to hypotension, a disturbed vasoreactivity, endothelial dysfunction and microvascular thrombosis, which can result in ischaemic and haemorrhagic lesions. The blood–brain barrier can be disturbed through increased activity of pro-inflammatory cytokines and reactive oxygen species produced at least in part by astrocytes. Activation of microglia can further contribute to neuronal damage in the brain 166 .
Approximately one fifth of patients hospitalized with pneumonia are readmitted to the hospital within 30 days; pneumonia, cardiovascular disease and (chronic obstructive) pulmonary disease are the most common diagnoses 167 . An increased susceptibility for infection after pneumonia may be related to a relatively immunocompromised state, as has been described in patients with sepsis 147 . Knowledge of immunological defects contributing to recurrent pneumonia (usually defined as a new episode of pneumonia within several months of the previous one, separated by at least a 1-month asymptomatic interval and/or radiographic clearing of the acute infiltrate) 168 is limited. A small study involving 39 patients suggested that immunoglobulin deficiency and an inability to react to polysaccharide antigens are associated with an increased incidence of recurrent pneumonia 169 . Further, a study in mice found a reduced capacity of AMs to phagocytose E. coli and S. aureus following recovery from primary pneumonia, a reduction mediated by signal-regulatory protein-α (also known as tyrosine–protein phosphatase non-receptor type substrate 1) and associated with an impaired host defence after secondary infection of the lower airways 170 .
Diagnosis, screening and prevention
The most common symptoms of pneumonia are cough, breathlessness, chest pain, sputum production and fatigue 171 , 172 . Symptoms are not a part of the initial severity assessment of patients, as the initial symptom burden does not influence outcome. Exceptions include delirium, which is associated with an increased risk of mortality 173 , and pleuritic chest pain, which is associated with an increased risk of para-pneumonic effusion and complicated (infected) para-pneumonic effusion 174 , 175 . Usually mild disease refers to patients with CAP who do not require hospitalization, moderate disease to those cared for in conventional hospital wards, and severe disease to those admitted to the ICU.
It is not possible to differentiate bacterial and viral pneumonia based on symptoms in adults or children, as patients report similar symptoms regardless of microbial aetiology 176 . A recent study found that artificial intelligence was also unable to differentiate microbial aetiology based on symptoms, clinical features and radiology 177 .
CAP is usually clinically suspected in the presence of acute (≤7 days) symptoms of LRTI, such as cough, expectoration, fever and dyspnoea, as well as the presence of new infiltrates on chest radiographs (CXRs) 178 . In older patients, symptoms are typically less evident, and fever can be absent in as many as 30% of patients 179 . Symptoms may also be less evident in patients treated with steroids, NSAIDs and antibiotics 6 . Other pulmonary diseases — most frequently pulmonary embolism and lung cancer — may present with fever and pulmonary infiltrates that can mimic CAP. Interstitial and systemic diseases can also mimic CAP. When diagnosing CAP, it is extremely important to review prior chest CXRs if available, as an additional means to help rule out the disease.
Although HAP is also suspected clinically, symptoms may be hidden by either other medications or the cause of admission. No studies exist about symptom duration in HAP before diagnosis; however, it is usually suspected when patients present with pyrexia (fever) and/or tachypnoea (rapid breathing). HAP diagnosis is believed to be usually delayed, which could explain the higher mortality observed in this population than in patients with VAP.
VAP is suspected when there are at least two of the following symptoms: fever or hypothermia, leukocytosis or leukopenia, and evidence of purulent secretions in an endotracheal tube or tracheostomy 4 . For VAP diagnosis, clinicians often rely on clinical parameters; radiological and laboratory parameters help initiate antimicrobial treatment. Scores have been proposed to facilitate diagnosis. For example, the clinical pulmonary infection score (CPIS) 180 is the most common one, and it is based on points assigned to various signs and symptoms of pneumonia. A CPIS score of >6 suggests VAP, although score sensitivity and specificity are not perfect. In fact, the FDA does not accept this score to diagnose VAP in randomized controlled trials studying antibiotics. In patients with VAP, fever and pulmonary infiltrates can present as atelectasis (collapse of parts of the lung), alveolar haemorrhage and pulmonary thromboembolism, amongst other conditions. In a landmark study using immediate post mortem lung histopathology and microbiology as a gold standard, the presence of two clinical criteria plus the presence of infiltrates on CXRs had a 70% sensitivity and 75% specificity in the diagnosis of VAP 181 .
Radiographic confirmation is essential for the diagnosis of pneumonia. CXRs provide important information about the site, extent and associated features of pneumonia (for example, the lobes involved and the presence of pleural effusion and cavitation) 5 (Fig. 6 ). CXRs have a sensitivity and specificity of 43.5% and 93%, respectively, for detecting pulmonary opacities 182 . In CAP, sensitivity and specificity of 66% and 77%, respectively, have been reported 183 using CT scans as the gold standard. The presence of either pleural fluid or multilobar pneumonia serve as indicators of severity 5 . In CAP, the development of pulmonary infiltrates that were not previously present on a simple posterior–anterior (PA) CXR is essential for CAP diagnosis. The standard CXR for CAP consists of a PA and lateral images; the use of lateral projection images increases diagnostic performance of PA images. In HAP, radiographic evidence of infiltrates is usually determined by CXR examination alone. In VAP, new infiltrates are usually detected by anterior–posterior projection in the supine position; however, in this situation, CXRs are insufficiently sensitive and specific.
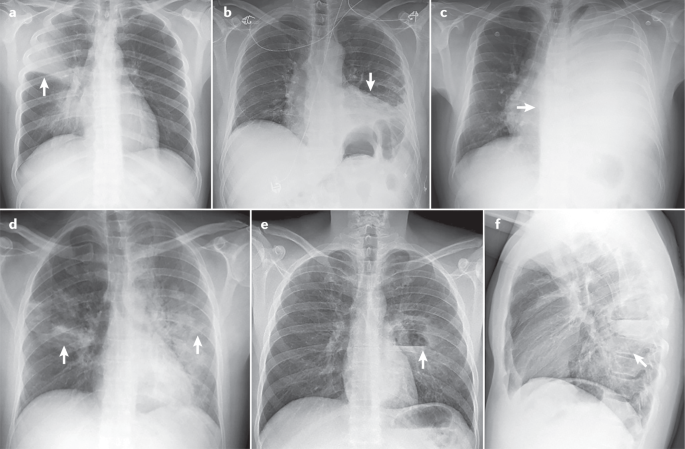
Pneumonia in upper right lobe (arrow) (part a ); pleural effusion on the left side (arrow) (part b ); massive pleural effusion in the left lung (arrow) (part c ); bilateral pneumonia (arrows) (part d ); lateral image showing left parahilar cavitation with air–fluid level in the lower left lobe (arrow) (part e ); front-to-back image in the same individual as in part e .
In studies in patients hospitalized with CAP, CT identified up to 35% of patients with CAP who had not initially been caught by CXRs 184 . In many patients with COVID-19, CT scans detect pulmonary infiltrates not observed on simple CXRs 185 . In patients with CAP, CT scans serve as a practical complement to CXRs in several cases: when radiographic findings are non-specific, when pulmonary complications such as empyema (pus in the pleural space) or cavitation are present, when there is suspicion of an underlying lesion such as lung carcinoma, and when recurrent pneumonia or non-resolving pneumonia is present 186 . Although this supporting role of CT scans is assumed to apply to patients with HAP as well, supporting evidence is lacking.
Ultrasonography
Lung ultrasonography is a non-invasive imaging method that is now frequently used in many emergency departments and ICUs. Advantages over CT include the absence of radiation exposure, ready use at the bedside and reasonable diagnostic sensitivity and specificity 187 . However, the technique has a steep learning curve, especially in mechanically ventilated patients. In a systematic review, lung ultrasonography was shown to have a sensitivity of 88% and a specificity of 89%, with a ~90% probability of diagnosing pneumonia 188 . Echographic diagnosis is more complex in patients with VAP, and only a few observational studies have been conducted to date 188 . The best of these studies have shown that such diagnosis had better accuracy than the CPIS score alone; the addition of direct Gram stain examination in quantitative cultures of endotracheal aspirates further improved accuracy 189 , 190 . On the basis of on these results, the ventilator-associated pneumonia lung ultrasound score (VPLUS) was developed, and has a sensitivity of 71% and a specificity of 69% for VAP diagnosis 190 .
Microbiology and laboratory tests
Recommendations for microbiological diagnosis in CAP vary according to disease severity (Table 2 ). Of note, microbiological diagnosis in CAP cannot be obtained in up to 50% of patients 5 . In patients with CAP who do not need hospital admission, obtaining samples such as sputum and pharyngeal swabs is optional or not recommended in recent guidelines 5 . In patients requiring hospitalization, obtaining good-quality sputum and blood samples, as well as pharyngeal swabs (for PCR), is recommended. Sputum is the most common respiratory sample in patients with CAP, and samples should be collected before antibiotic treatment. The sensitivity of Gram staining for a sputum sample is ~80% in patients with pneumococcal pneumonia and 78% in patients with pneumonia caused by Staphylococcus spp., and the specificity is 93–96% 191 , 192 . Most health care institutions perform viral PCR on pharyngeal swabs during the influenza season. In the COVID-19 pandemic, it is recommended that all patients admitted with CAP receive a PCR test for the detection of SARS-COV-2.
In patients requiring ICU admission, in addition to all tests mentioned above, bronchoscopic samples, such as bronchoalveolar lavage (BAL) in intubated patients, are not difficult to obtain and provide information on the lower respiratory tract microbiota. Urinary antigen detection tests for S. pneumoniae and L. pneumophila have good sensitivity and specificity, are not extremely expensive and are recommended in all hospitalized patients.
In patients with HAP or VAP, international guidelines 4 recommend obtaining distal respiratory samples for semiquantitative or quantitative cultures (Table 3 ). In patients with HAP, bronchoscopy is not easy to perform, and sputum samples are not often collected. In patients with VAP, distal respiratory samples are preferred. BAL (performed with or without concomitant bronchoscopy) is the sample that provides most information, as, in addition to cultures, cellularity analysis and PCR can be performed on the fluid. A recent meta-analysis showed that Gram staining of BAL performs well in detecting S. aureus 193 . Respiratory samples from patients with HAP or VAP have to be collected before the initiation of a new antibiotic treatment to avoid false-negative cultures. International guidelines 4 do not recommend using procalcitonin (PCT) for the initial diagnosis of HAP or VAP, as several studies have shown that it lacks diagnostic value 194 .
Since the 2000s, owing to multiple outbreaks, epidemics and pandemics caused by respiratory viruses in particular, several molecular tests have been developed, which have contributed to widened availability of molecular testing for the aetiological diagnosis of CAP. Molecular tests have several advantages, including detecting low levels of microbial genetic material, remaining unaffected by prior antibiotic therapy, and providing results within a clinically relevant time frame 195 . Molecular tests based on multiplex PCR have been developed to simultaneously detect and quantify multiple respiratory pathogens, as well as some genes related to antimicrobial resistance. Several commercial multiplex platforms are currently available for comprehensive molecular testing for respiratory pathogens that cause pneumonia (respiratory viruses, bacteria and fungi) and for the main resistance genes of the most common bacteria causing pneumonia 195 , 196 , 197 , 198 .
The WHO currently recommends COVID-19 diagnosis by molecular tests that detect SARS-CoV-2 RNA. SARS-CoV-2 viral sequences can be detected by real-time reverse transcriptase (RT-PCR) in nasopharyngeal swab samples 199 . The disadvantage of this method is that it requires specialized equipment and trained personnel. Additionally, two types of rapid tests are available for COVID-19 diagnosis. The direct SARS-CoV-2 antigen test detects viral components present during infection in samples such as nasopharyngeal secretions, and, therefore, can indicate whether an individual is currently carrying the virus. The indirect antibody test detects antibodies that can be found in serum as part of the immune response against the SARS-CoV-2; thus, it can yield false-negative results if performed before the antibody response has developed and cannot distinguish between past and current infections. These two tests are relatively simple to perform and interpret, requiring limited test operator training 199 .
Some biomarkers may be helpful in identifying which patients are likely to have bacterial pneumonia, in deciding whether antibiotic therapy should be administered, in determining prognosis and in facilitating decisions related to the site of care. However, biomarkers should only be used as an adjunctive tool when managing CAP, as no biomarker has proven full utility in predicting clinical outcomes in patients.
The most widely used biomarkers are acute phase reactants such as C-reactive protein (CRP) and PCT 200 . However, their serum kinetics differ: CRP levels increase after the first 3 days of infection (peak time from infection is 36–50 h), whereas PCT levels rise rapidly (peak time from infection is 12–24 h) in response to microbial toxins or host responses. These properties are useful in differentiating CAP from other non-infectious causes. CRP levels increase in response to any inflammation, and can be modified by the presence of corticosteroids and previous antibiotic therapy, whereas PCT is more specific in bacterial pneumonia. Viral infection-related cytokines attenuate induction of CRP and PCT; however, some elevation in their levels can occur when pneumonia is caused by atypical pathogens (for example, Mycoplasma spp., Chlamydia spp. and Legionella spp.) 201 .
Both CRP and PCT can assist in the clinical diagnosis of pneumonia, but CRP and PCT cannot be used in isolation as a basis for treatment decisions. A second test after 24–48 h is mandatory to monitor for any increases. Clinicians should also consider the pattern in the days preceding symptom onset in patients with CAP and whether a patient is taking medication that could have modified these values. For patients with radiographic CAP, PCT levels can be used with clinical assessment to identify those individuals from whom antibiotic therapy can be safely withheld. This assessment can be combined with a PCR test to identify viral infection, especially as new data show that viruses can frequently be a cause of CAP 13 , 75 . However, caution should be used when a mixed viral–bacterial infection is considered. The new American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA) CAP 5 guidelines do not recommend using PCT to determine the need for initial antibacterial therapy. The current recommendation is that empirical antibiotic therapy should be initiated in adults with clinically suspected and radiographically confirmed CAP, regardless of initial PCT level.
In studies in patients with HAP or VAP, in whom biomarkers had been monitored serially since before infection, steady increases or persistent elevations in CRP levels were shown to be associated with a high risk of VAP 202 . However, no such pattern was shown for PCT values (crude values or kinetics), with poor diagnostic accuracy for VAP 203 . Thus, a recent international consensus concluded that a combination of clinical assessment including PCT levels in well-defined antibiotic stewardship algorithms could improve diagnosis of bacterial infections and support antibiotic effectiveness 204 .
Prevention of CAP
Many factors increase the risk of CAP and can generally be divided into host factors (for example, age, and the presence of COPD and other chronic pulmonary diseases, diabetes mellitus and chronic heart failure), unhealthy habits (for example, smoking and excessive alcohol consumption) and medications (for example, immunosuppressive drugs, sedating medications such as opioids, and proton pump inhibitors within the first 3 months of administration 205 ). Prevention of CAP is crucial, especially in individuals with these risk factors. Available preventive measures include smoking and alcohol use cessation, improvements in dental hygiene, physical exercise, avoiding contact with children with respiratory infections, and pneumococcal and influenza vaccinations 14 . Implementing these measures in primary and specialized care could help reduce the burden of CAP. Presently, pneumococcal and influenza vaccination are the cornerstones of CAP prevention.
The 23-valent pneumococcal polysaccharide vaccine (PPV23) and the 13-valent pneumococcal conjugate vaccine (PCV13) are currently used in adults. Owing to the demonstrated effectiveness of PPV23 in preventing invasive pneumococcal disease (IPD) in people of ≥65 years of age, the use of the vaccine in this population is recommended in many countries 206 . However, PPV23 effectiveness in preventing non-IPD or CAP due to any cause is much less clear. The effectiveness of PPV23 has been reported to range from 25% to 63% in pneumococcal pneumonia 207 , 208 ; the effectiveness of PCV13 in preventing the first episode of CAP, non-bacteraemic and non-invasive CAP, and IPD due to serotypes contained in the vaccine amongst adults of ≥ 65 years of age has been reported to be 45.6%, 45% and 75%, respectively 209 . Efficacy persisted through the mean follow-up period of 4 years 209 . A post-hoc analysis based on data from the CAPITA trial showed that the effectiveness of PCV13 ranged from 43% to 50.0% for pneumococcal CAP, 36% to 49% for non-bacteraemic and non-invasive pneumococcal CAP, and 67% to 75% for pneumococcal IPD 210 . Of note, the most important measure in reducing pneumococcal CAP burden (bacteraemic and non-bacteraemic) in adults is conjugate vaccine programmes in children. Vaccination with pneumococcal conjugate vaccine in children substantially reduces disease in adults owing to the interruption of transmission and herd protection 211 , 212 .
Influenza vaccination can reduce the risk of complications of influenza, such as pneumonia, and is associated with a decrease in severity, hospitalization, ICU admission and mortality associated with influenza 213 , 214 . All age groups can be affected by influenza virus infection; however, older individuals, young children, pregnant women and those with underlying medical conditions have the highest risk of severe complications. In 2019, a study 75 found that viral sepsis was present in 19% of patients with CAP admitted to ICU and in 61% of patients with viral CAP; influenza virus was the main aetiology. More recently, a study 215 found influenza virus in 23% of patients with LRTI; 57% of these patients had radiographically confirmed CAP. The authors reported 35% vaccine effectiveness against influenza virus LRTI and 51% against influenza-associated CAP. These data demonstrate the importance of an annual influenza vaccination, especially in at-risk groups.
Prevention of HAP
HAP is the leading cause of death from hospital-acquired infection; however, only limited effort has been made in developing prevention strategies. HAP occurs owing to pharyngeal colonization with pathogenic organisms and, in the case of VAP, subsequent aspiration. Thus, oral care and precautions against aspiration may attenuate some of the risk. Although oral and/or digestive decontamination with antibiotics may also be effective, this approach could increase the risk of selecting resistant organisms. Other preventive measures, including isolation practices, remain theoretical or experimental. Indeed, most potential prevention strategies for HAP remain unproven 216 .
The individual measures included in prevention bundles can be divided into non-pharmacological and pharmacological categories. To date, most of our knowledge in HAP prevention is extrapolated from prevention strategies for VAP. An important concept in these strategies is that no single measure is deemed adequate to ensure prevention, with prevention bundles advocated instead. A prospective, interventional, multicentre study in Spain, the Pneumonia Zero project 217 , which included 181 ICUs and built on the experience from a previous study 218 , suggested VAP prevention via a bundle of mandatory and highly recommended measures. The mandatory measures were education and training of medical staff in airway management, hand hygiene with alcohol solutions, oral hygiene with an antiseptic (chlorhexidine), semirecumbent positioning and promotion of procedures and protocols that safely avoid or reduce duration of mechanical ventilation. The highly recommended measures were aspiration of subglottic secretions (removal of secretions that accumulate above the endotracheal tube cuff, in patients who were expected to be mechanically ventilated for >72 h), selective digestive decontamination (SDD)), and selective oropharyngeal decontamination (SOD) (prophylactic strategies to prevent or minimize infections in critically ill patients, based on the application of non-absorbable antibiotics in the oropharynx and gastrointestinal tract (SDD) or oropharynx (SOD) of patients). When implemented, these measures enabled a decrease in adjusted frequency of VAP from 9.83 to 4.34 per 1,000 ventilator-days over 21 months; similarly, the percentage of patients with VAP significantly decreased from 2.4% to 1.9%. In the ICUs with prolonged participation in the study (19–21 months), the incidence of VAP significantly decreased further to just 1.2%. Finally, significant decreases were observed in VAP recurrence rates (from 10.9% to 7.7%).
Non-pharmacological measures
Good hand hygiene using alcohol solution before airway management is firmly established as a fundamental component of clinical practice. Its inclusion in the VAP care bundle represents an opportunity to audit compliance with, and optimize the quality of, hand hygiene practices 217 , 219 .
Remaining in the supine position 220 , the use of gastric tubes and the presence of contents in the stomach contribute to the reflux of gastric contents, aspiration and VAP. Semirecumbent positioning at 30–60° may help to avoid these problems, as found in a 2016 meta-analysis 221 . The lateral Trendelenburg body position (the patient is positioned inclined with head down and feet elevated) has shown no substantial benefit, with research even showing an increase in the number of adverse events 222 . However, based on the results of a post-hoc analysis of the Gravity VAP trial, patients without pulmonary infiltrates at intubation and with no contraindications for the approach may benefit from this position for a short period 222 . The prone position is used to improve hypoxaemia in patients with severe ARDS 223 . This measure is frequently used in COVID-19-associated ARDS 224 , 225 . This approach might decrease the incidence of VAP, as it facilitates the drainage of secretions compared with a semirecumbent position 226 . Further confirmation is needed to assess the beneficial effect in reducing VAP in patients with COVID-19.
Endotracheal tubes also have an important role in the pathogenesis of VAP, and removing contaminated oropharyngeal secretions can reduce the risk of VAP. In a meta-analysis from 2016, evidence supported the use of endotracheal tubes with subglottic secretion drainage to decrease the rate of VAP 227 . Maintaining cuff pressure at >25 cmH 2 O may further prevent the leakage of bacterial pathogens into the lower respiratory tract 217 , and continuous cuff pressure regulation could be superior to intermittent control for preventing VAP 228 . Finally, the tube cuff shape and material may have a role in the aspiration of secretions; a randomized, multicentre trial showed that cuffs made of polyurethane or of a conical shape were not superior to conventional cylindrical polyvinyl chloride cuffs in preventing tracheal colonization and VAP 229 .
Pharmacological measures
Oral washing with chlorhexidine seems to be effective in preventing VAP; however, a recent meta-analysis 230 showed a trend for increased mortality in patients who received chlorhexidine. Consequently, recent international guidelines 3 did not recommend its use. It is plausible that this increased mortality could be due to direct lung toxicity from aspirated chlorhexidine.
Furthermore, the use of either SOD or SDD remains controversial, with most studies to date being performed in settings with low prevalence rates of MDR or XDR microorganisms. These studies have shown a decrease in both the incidence of VAP and overall mortality 231 . However, in a recent cluster randomized clinical trial performed in units with high rates of MDR or XDR pathogens, SOD and SDD were not effective in decreasing bacteraemia caused by those microorganisms 232 . SDD and SOD are not applied in many centres in the USA and in Europe, primarily for fear of inducing microbial resistance. Owing to the unclear balance between a potential reduction in pneumonia rate and a potential increase in mortality, the 2017 international guidelines 3 decided not to issue a recommendation on the use of chlorhexidine for SOD in patients requiring mechanical ventilation until more safety data becomes available. However, the guidelines did suggest the use of SOD — but not SDD — in settings with low rates of antibiotic-resistant bacteria and low antibiotic consumption. Although establishing a cut-off value for low and high resistance settings is a dilemma, the committee felt that a 5% threshold was reasonable.
Prevention of recurrent pneumonia
Recurrent pneumonia affects ~9% of patients hospitalized with CAP 233 , 234 . The main factors related to recurrent pneumonia are age ≥65 years, lack of pneumococcal vaccination, previous episode of pneumonia, COPD and corticoid therapy. S. pneumoniae is the most frequently identified pathogen in patients with recurrent pneumonia 233 , 234 . The main preventive measures for recurrent pneumonia are vaccination and adequate control of prior comorbidities, especially in an older population who have an increased risk of infection.
Antibiotics are the mainstay of therapy for pneumonia; however, the agents used depend on a variety of host and pathogen factors. Ideally, therapy should be pathogen-directed, even though a pathogen is often not identified. Nevertheless, as therapy must be started promptly, empirical therapy directed at the most likely aetiological pathogens is required. Because empirical therapy may be more broad-spectrum than definitive therapy, it is often necessary to narrow and target antibiotics once diagnostic testing results become available, usually after 48–72 h. Such a strategy is referred to as a ‘de-escalation’ of therapy 235 . Rapid comprehensive multiplex molecular methods have been cleared by the FDA and provide results within 2–4.5 h, prior to obtaining final diagnostic testing data. These methods include antibiotic resistance markers and facilitate identification of specific viruses and bacteria, thereby aiding in therapeutic choices and the escalation, de-escalation or cessation of antibiotics.
Considerations for therapeutic choices
Relevant host factors for choosing the type of empirical therapy are severity of illness, the presence of specific medical comorbidities and certain historical data. In detail, these include: chronic lung, heart or liver disease; diabetes mellitus; asplenia; alcohol use disorder; malignancy; malnutrition; recent hospitalization, antibiotic use or colonization by drug-resistant bacteria; the presence of risk factors for aspiration of gastric contents into the lungs (such as impaired swallowing, vomiting, altered consciousness and impaired cough reflex); and recent contact with a health care environment (for example, patients requiring haemodialysis) 236 . It is also important to know epidemiological data regarding individual patients. Seasonal viruses such as influenza viruses are worth examining during the autumn and winter. Contact with someone known to have an illness transmitted by an airborne route (for example, tuberculosis) is also relevant. Similarly, residence in an area with endemic mycoses is a risk for certain fungal pneumonias. Finally, an ICU with a high rate of drug-resistant pathogens poses a risk factor for VAP caused by such organisms 3 .
The site of pneumonia acquisition is also an important consideration, namely, in the community, hospital or ICU, or whilst on mechanical ventilation. Since the late 1990s, guidelines have been developed for patients with pneumonia in each of these settings; however, recent data suggest that patient risk factors, and not the site of infection, should be the main determinant for empirical antibiotic choice. Recently, a unified algorithm based on these risk factors has been proposed for all patients with pneumonia 236 .
In addition to choosing an antibiotic that is likely to target the aetiological pathogens, it is equally important to determine the right dose and route of administration, to ensure that the drug penetrates into the site of infection. In general, oral therapy is used in patients with less severe illness, whilst intravenous therapy is administered in patients with more serious illness. Aerosolized therapy can be used to boost drug delivery to infected lung tissue, especially if the chosen drugs penetrate into the lung poorly. When treating a critically ill patient with pneumonia and a MDR pathogen, it may be necessary to use high doses to ensure reaching bactericidal drug concentrations at the site of infection. Continuous or prolonged infusion may be needed in the case of β-lactam antibiotics to maximize the time during which the drug concentration exceeds the minimum inhibitory concentration (MIC) of the target organism. Other drugs, such as aminoglycosides, kill bacteria in a concentration-dependent fashion and are best administered at high dosages given once daily 237 . In young patients with pneumonia and sepsis, drug clearance by the kidney may be accelerated (augmented renal clearance), and dosing will need to be increased appropriately 238 . In those with renal impairment, dosing or the frequency of administration may need to be reduced and can be optimized by therapeutic drug monitoring, if available.
CAP therapy
Guidelines for CAP recommend empirical therapy based on the severity of illness and presence of risk factors for specific complex pathogens 5 , 53 , 239 (Table 4 ). In the past, patients with risk factors that included contact with a health care environment (haemodialysis, recent hospitalization, residence in a nursing home) were considered to have HCAP and were treated differently from patients with CAP. The new guidelines have eliminated HCAP as a category and recommended that these patients be treated as having CAP. Without forgoing consideration of the local frequency of penicillin and macrolide resistance, every patient with CAP should be treated for pneumococcus in most parts of the world. In addition, atypical pathogens may have a role, often as co-infecting agents; studies showed improved patient outcomes when macrolides or quinolones were added to β-lactam therapy in patients with CAP, particularly those with more severe illness 240 , suggesting a need to treat atypical pathogens in many patients with CAP. Patients with more severe illness may need empirical therapy for MRSA and/or P. aeruginosa , especially if colonization had occurred previously following influenza (in the case of MRSA) or after prior use of broad-spectrum antibiotics (for both pathogens) 241 .
Although in many patients CAP may have a viral aetiology, either as a single pathogen or as part of a mixed infection, antiviral therapy is not routinely recommended. However, for documented influenza-associated pneumonia, current guidelines recommend the use of an anti-influenza agent such as oseltamivir, regardless of illness duration 5 . Nonetheless, the benefit of these agents is greatest within the first 48 h of infection onset. Thus, in patients with a high suspicion of influenza, therapy should be started, whilst results from diagnostic testing are pending. Additionally, even with documented influenza, antibiotics should be used empirically to account for possible bacterial superinfection 5 .
Outpatients
For outpatients without comorbidities or risk factors for MDR pathogen infection, current guidelines recommend monotherapy with respiratory fluroquinolone or combination with amoxicillin–clavulanate or a cephalosporin and macrolide or doxycycline 5 . Regardless of the prevalence of resistance, good experience with macrolide monotherapy has been reported, suggesting that in vitro resistance is not always clinically relevant unless it is high-level resistance (resulting from a ribosomal mechanism) and not lower-level resistance (caused by efflux pumps) 242 . For example, in a Canadian study, patients with CAP who received macrolide therapy (usually as monotherapy) had lower mortality and hospitalization rates than those receiving alternative therapies 243 . For outpatients with comorbid illnesses, current guidelines recommend therapy with a β-lactam and macrolide combination or monotherapy with a respiratory fluoroquinolone, even though recent concerns about fluoroquinolone toxicity have limited their use 5 .
Hospitalized patients
In patients with CAP in hospital wards, therapy should be a β-lactam–macrolide combination or a quinolone (levofloxacin or moxifloxacin) alone (Table 4 ). In areas with a high prevalence of endemic tuberculosis, caution should be exercised with the use of a quinolone, as it can mask the presence of tuberculosis and select for drug-resistant tuberculosis. β-Lactams include ceftriaxone, ceftaroline and ampicillin–sulbactam, whilst macrolides should comprise azithromycin or clarithromycin; some recent data have shown more frequent cardiac complications with the use of erythromycin 244 . Many studies have shown that the addition of a macrolide to the β-lactam, particularly in those with moderately severe illness or with Legionella spp. infection, is associated with a lower mortality rate than β-lactam monotherapy 245 .
All ICU-admitted patients should receive a combination therapy of a β-lactam and either a macrolide or a quinolone. Admission to ICU should be guided by the presence of one of two major criteria (need for mechanical ventilation or septic shock requiring vasopressors) or three of nine minor criteria, as per the 2007 ATS/IDSA guidelines 239 . In this population, a macrolide is generally preferred, although some studies have shown that a quinolone may prove more effective if Legionella spp. infection is highly suspected or documented 246 . If the patient has risk factors for P. aeruginosa or MRSA infection, then treatment for such pathogens should be added.
HAP therapy
Patients can develop HAP in or outside the ICU and can be managed with or without mechanical ventilation, although as many as 30% of patients with HAP who are not initially ventilated will require mechanical ventilation 247 . In patients with a predicted mortality risk of <15% based on the presence or absence of septic shock, monotherapy is associated with lower mortality than combination therapy. In patients with a predicted mortality risk of >25%, combination therapy is associated with reduced mortality; the type of therapy has no effect on mortality in those with a predicted mortality risk of 15–25% 248 . MDR pathogen infection should be considered in patients with a history of prior antibiotic therapy or prolonged hospitalization in the previous 3 months, as well as patients hospitalized in an ICU with a >25% rate of MDR pathogen infections. Although empirical therapy can be guided by patient features, each ICU has its own unique bacteriology; thus, therapy should be guided by knowledge of the local antibiogram 3 , 249 .
Patients with a low mortality risk (estimated from published data in relation to the presence of sepsis and shock) and no MDR pathogen risk factors should receive monotherapy (Table 5 ). In patients with a mortality risk of >15% and/or risk factors for MDR pathogens but who are not in septic shock, monotherapy can be adequate (provided that the chosen antibiotic can target >90% of the gram-negative pathogens in the ICU). Although there is controversy in many hospitals about the need for combination therapy, two agents are often necessary to provide a >90% likelihood of appropriate therapy, especially in the high-risk population and in those with septic shock. The combination regimen should target P. aeruginosa and ESBL-producing Enterobacterales. In all patients with HAP, anti-MRSA therapy should be considered and, if necessary, administered with either vancomycin or linezolid. Depending on local epidemiology, some patients will be at risk of infection with Acinetobacter baumanii , carbapenem-producing Enterobacterales or Stenotrophomonas maltophilia , each one requiring a unique therapy approach. For VAP due to MDR pathogens, such as Acinetobacter baumanii , adjunctive inhaled antibiotics (amikacin or colistin) have been added to systemic therapy, with no proven mortality benefit; efficacy may vary with the type of aerosol delivery system used 250 .
The duration of HAP therapy is between 7 and 14 days, although most patients are successfully treated within only 7 days 251 . Although not all experts agree, the European guidelines list the following groups as exceptions to short duration therapy: patients with MDR pathogen infection, such as P. aeruginosa and Acinetobacte r spp.; those who received inappropriate therapy initially; those who are severely immunocompromised; and those receiving second-line antibiotic agents 217 , 252 , 253 . Current guidelines do not strongly endorse biomarkers such as PCT to guide therapy duration for HAP and VAP, although some randomized trial data do show efficacy for this approach 254 .
Therapy in immunocompromised patients
Immunocompromised patients can develop pneumonia due to the common community and nosocomial pathogens present in the setting as well as other pathogens related to a specific type of immune dysfunction and/or resistant bacteria, viruses, fungi and parasites. Common conditions that impair the immune system include malignancy, HIV infection with a CD4 + T cell count of <200 cells per mm 3 , and solid organ or stem cell transplantation. Therapies that cause immune suppression include prednisone, biological disease modifiers, and chemotherapeutic agents such as azathioprine, methotrexate and cyclophosphamide.
Although empirical therapy is often used, the range of possible pathogens in this population is so broad that aggressive diagnostic testing is necessary, including sampling of deep lower respiratory tract secretions with bronchoscopy in most patients 255 . In patients with HIV infection and a low CD4 + T cell count or with recent corticosteroid tapering, therapy should target common pathogens and Pneumocystis jirovecii 256 . Patients with severe neutropenia, steroid-induced immune suppression and those receiving biologic response modifiers (such as tumour necrosis factor inhibitors) can be infected with fungi such as Aspergillus spp. or Mucorales. Diagnostic testing in those with malignancy or drug-induced immune suppression should also consider other opportunistic pathogens, including cytomegalovirus, Varicella zoster virus, Nocardia spp., parasites such as Strongyloides stercoralis and Toxoplasma gondii , and Mycobacterium tuberculosis (for example, owing to a re-emergence of latent infection).
Aspiration pneumonia therapy
Patients with witnessed macro-aspiration of gastric or oral contents into the lung can develop chemical or bacterial pneumonitis, or simply have bland aspiration. If bacterial pneumonia occurs, patients should receive antibiotics aimed at common community or nosocomial pathogens that were likely to be colonizing the oral and gastric tract at the time of aspiration. In community aspiration, therapy is the same as in CAP unless the patient has poor dentition, which can make infection by anaerobic pathogens possible owing to favourable growth conditions for such microbes in the patient’s mouth. When patients with poor dentition have a lung infiltrate after a witnessed or clinically suspected aspiration event, therapy should be a β-lactam such as ampicillin–sulbactam or amoxicillin–clavulanate, or a quinolone, such as levofloxacin or moxifloxacin. Any of these drugs could also be used if dentition is normal; alternatively, ceftriaxone would be effective 1 . For those with nosocomial aspiration, therapy should be based on the presence of risk factors for MDR pathogens and aimed at common, local and drug-resistant organisms, similar to therapy in other forms of nosocomial pneumonia. There is no need to add specific anti-anaerobic coverage, as these organisms are uncommon in patients who aspirate whilst in hospital or chronic care facilities 257 .
Adjunctive therapy
In addition to antibiotics, patients with severe illness might benefit from adjunctive corticosteroid therapy. In general, this therapy should be restricted to those with severe CAP and a high inflammatory response 258 . In one trial, methylprednisolone was more effective than placebo, leading to less treatment failure (especially late failure) in a population with both severe CAP and elevated CRP levels in the serum 259 . However, before using corticosteroids, it is necessary to rule out influenza, as it may worsen with this line of therapy 260 . By contrast, studies in patients with COVID-19 and hypoxaemic respiratory failure have shown a benefit of corticosteroid therapy with dexamethasone 261 . Similarly, IgM-enriched immunoglobulin may be useful in patients with severe CAP, and high CRP levels and low IgM levels in the serum. In a randomized, double-blind, placebo-controlled trial, IgM-enriched immunoglobulin led to a reduction in mortality and an increase in ventilator-free days in this population, when compared with placebo 262 .
Another adjunctive and supportive therapy includes management of hypoxaemia with respiratory failure, which may necessitate mechanical ventilator support. However, some studies show that patients with CAP can be managed with either non-invasive ventilation or high-flow oxygen. Either modality can reduce the need for mechanical ventilation and, therefore, avoid some of the complications associated with endotracheal intubation and ventilation 263 .
Follow-up of patients after pneumonia
In some patients with CAP, pneumonia can be the start of an inexorable downhill course. In one study, the long-term mortality of patients of >65 years of age hospitalized with CAP far exceeded the in-hospital mortality (33.6% and 11%, respectively) 264 . In some studies, this long-term effect has been attributed to cardiac events that were initiated by acute lung infection 155 .
Pneumonia recurrence can occur in all forms of pneumonia. Recurrence should be classified on the basis of the site of infection. If re-infection occurs at the same site as the original infection, consideration should be given to local factors such as endobronchial obstruction (due to a tumour or foreign body), focal bronchiectasis, insufficient duration of therapy, or infection with a drug-resistant or inadequately treated pathogen. Recurrence elsewhere could be due to immune impairment (due to comorbid illness or certain medications), a non-infectious pulmonary process or recurrent aspiration.
Routine follow-up chest radiography after CAP is not generally recommended. However, if it is prescribed (to monitor resolution of a pleural effusion or infiltrate suggestive of a possible lung mass), it should be delayed for 4–6 weeks if the patient is responding well to therapy 5 . During follow-up, patients should be monitored for undiagnosed or ineffectively managed comorbid illness and encouraged to avoid cigarette smoking. Patients should also have up-to-date pneumococcal and influenza vaccinations. The 30-day readmission rate for patients with CAP has been found to vary from 16.8% to 20.1% 167 . Pneumonia itself was the cause of readmission in only 17.9–29.4% of patients; however, other common causes were exacerbations of congestive heart failure or COPD 167 . Patients with health-care-associated risk factors have a higher probability of readmission than patients with uncomplicated CAP 265 .
Quality of life
The effect of pneumonia is heavily influenced by both the origin of the disease (within the community or in health care environments) and its severity 266 . Most data regarding the effect on quality of life have been obtained in patients with CAP 171 . Antibiotic treatment starts to improve pneumonia symptoms rapidly; acute symptoms typically improve within 3–5 days in patients with mild CAP (outpatients) and 5–10 days in hospitalized patients with more severe CAP not requiring ICU admission; however, return to baseline levels of symptoms and function seems to take substantially longer 172 , 267 , 268 , 269 . In mild-to-moderate CAP, in most patients symptoms such as cough and breathlessness resolve within 14 days, although up to 6 months are required for full recovery 267 . Thus, the greatest burden seems to be a loss of function in the long term. Delayed recovery is associated with the number of comorbid conditions. In most cases, the presence of ongoing health impairment is largely related to a decompensation of underlying diseases rather than the ongoing acute symptoms of CAP 267 . A modelling study showed that in hospitalized patients with CAP, these acute symptoms reduced in intensity by ~50% within the first 3–5 days, and resolved in nearly all patients by day 28 (ref. 268 ). There does not seem to be a meaningful difference in symptom intensity or time to symptom resolution between viral and bacterial pneumonia 270 .
A French study in patients with pneumococcal pneumonia followed for 12 months after hospital discharge used the EQ-5D-3L questionnaire to evaluate health status 271 . Patients experienced a progressive improvement in quality of life after discharge, plateauing at six months. Importantly, quality of life either did not improve or deteriorated after discharge in 34% of patients; recovery was worse in old patients than in young patients. In a US study in patients with CAP, on average, patients were able to return to normal productivity in 3 weeks and missed 2 weeks of work 272 . Recovery was slowest in patients with comorbidities such as COPD, leading to recovery times of 2 months on average. Even after recovery, symptom scores in patients with CAP are worse than those in the general population, partially because CAP has a long-term effect on health. Another partial reason for these lower scores is the development of CAP in patients with high-risk comorbidities, which make these patients more symptomatic than the general population 273 . Lastly, long-term mortality is increased in patients with CAP compared with the general population 35 . LRTIs without radiographic infiltrates (non-CAP LRTIs) are associated with a similar impairment in quality of life to CAP 274 .
Studies comparing quality of life between patients with CAP and the general population have shown consistently worse quality of life up to 12 months after CAP. With a few exceptions, most of these studies used generic quality of life and productivity tools. A systematic review identified five CAP-specific, patient-reported outcome measures, of which the CAP symptom questionnaire (CAP-sym) was the most widely used 275 . This review concluded that most CAP-specific tools have thus far been evaluated in highly specific populations and may not be fully representative, and it recommends continuing to use generic tools until better tools are available.
Improved diagnostics
The key to a switch to pathogen-specific therapy is an accurate aetiological diagnosis, and the availability of rapid molecular diagnostic tests makes clinical trials and subsequent clinical use of these targeted therapies feasible. Most progress in diagnostics can be observed in two areas: rapid identification of pathogens in positive blood cultures and detection of respiratory viral pathogens. However, bacteraemia is uncommon in pneumonia and, therefore, the effect of these molecular assays on management is limited. By contrast, PCR diagnosis of respiratory viral infections has now become the standard of care. The greatest issue with these assays obtained from nasopharyngeal specimens is whether results reflect upper respiratory tract infections only or accurately detect the cause of pneumonia. In addition, negative nasopharyngeal samples have occurred in patients with positive concurrent bronchoalveolar samples for influenza and SARS-CoV-2 (ref. 276 ).
Several multiplex PCR platforms are available for clinical use for bacterial pneumonia, with approval based on comparison with standard diagnostic tools, specifically culture 277 , 278 . However, as culture itself is not a gold standard, the true operating characteristics of the tests remain unknown. One alternative is metagenomics sequencing to determine all microbiota present; clinically relevant platforms are available 279 , 280 . Generally, these molecular assays are more sensitive than culture, especially for fastidious microorganisms; nevertheless, none of the current multiplex assays detect all of the relevant pathogens and, therefore, cannot replace cultures. In addition, a limited ability to provide information on antibiotic susceptibility is a major weakness. Despite such limitations, substantial impact on antibiotic prescription is possible. Most evaluations to date comprise observational studies and analyses of the theoretical benefit if antibiotic decisions based on molecular assays were applied prospectively. Perhaps the best demonstration of such potential is to limit the use of vancomycin or linezolid for suspected MRSA pneumonia 197 . Multiple sensitive and specific gene targets for S. aureus identification are available, whilst the absence of the mecA gene detection essentially excludes methicillin resistance in that isolate; thus, a negative assay eliminates the need for MRSA coverage. However, the greatest hurdle for molecular assays is clinicians’ willingness to base antimicrobial treatment on results obtained from these novel diagnostic platforms; even a BAL assay with a 98% negative predictive value did not result in a decrease in empirical treatment of VAP 281 . Implementation trials are required to demonstrate the true benefit of more accurate diagnostics.
Improved diagnostic testing may enable a host of unanswered epidemiological matters surrounding pneumonia to be addressed. A leading question in the field of pneumonia is its cause in immunocompromised patients; only expert opinion guides treatment recommendations 256 . The COVID-19 pandemic also illustrates the probable high frequency of additional viral agents that may cause CAP of seemingly unknown aetiology 13 . The role of fungal superinfection of viral pneumonia also remains controversial owing to diagnostic uncertainty 282 .
Antibiotic therapy
For most of the ~75-year history of antibiotic treatment of pneumonia, the backbone of therapy has been a β-lactam 283 . The emergence of bacterial resistance to β-lactams has been tackled with two strategies: newer generations or types of β-lactams (penicillins, cephalosporins and carbapenems) 284 , 285 , 286 , 287 , 288 and combinations with β-lactamase inhibitors (BLIs). Ceftolozane is the newest β-lactam on the market; it has improved activity against P. aeruginosa compared with other cephalosporins 284 . Each BLI has slightly different activity against the variety of resistance mechanisms in Enterobacterales, including carbapenem-resistant and ESBL-producing Enterobacterales, which may affect local efficacy owing to geographical differences in resistance patterns 289 .
Each new drug had been intended to replace the prior generation, gain a large proportion of market share and, therefore, justify the large development costs for the pharmaceutical industry. However, the majority of infections, especially community-acquired 13 , remain susceptible to cheap generic antibiotics even today, and the probability of a new blockbuster drug that would garner a large market share is progressively in decline 290 . This and multiple other factors, including increased costs for registration trials, a regulatory environment and challenges in clinical trial design, have led many pharmaceutical firms to abandon antibiotic development, as it offers a poor return on investment 291 .
Nevertheless, the paradigm for antibiotic development has shifted and, since the 2000s, niche antibiotics, particularly for gram-negative pathogens, have progressively emerged, developed by small biotech companies. These niche antibiotics specifically address gaps in standard antibiotic treatment coverage, yet leverage high prices to compensate for a small market share. The future success of these niche antibiotics could be increased by the emergence of rapid diagnostic tests that can detect specific pathogens or specific resistance markers immediately.
New antibiotics
The first generation of niche antibiotics were new β-lactams or BLIs developed for individual MDR or XDR pathogens 292 . The greatest unmet need for pneumonia due to gram-negative pathogens is for treatment of carbapenem-resistant Acinetobacter spp.; the only agent in development specifically for Acinetobacter spp. is a combination of two BLIs 293 . Both BLIs also have intrinsic β-lactam activity but are being studied in combination with a carbapenem for serious Acinetobacter spp. infections, including pneumonia.
Agents specific for Pseudomonas spp. are also in development. Murepavadin is the first of a new class of antibiotics that inhibit the outer membrane assembly of P. aeruginosa ; other drugs targeting outer membrane assembly are in development, including phage-derived endolysins 294 . Small molecule inhibitors of the type-III secretion apparatus in P. aeruginosa , a crucial component of its pathogenesis, are also in development.
One exception to the niche drug approach is cefiderocol, an extremely broad-spectrum agent with activity against almost all MDR pathogens. Cefiderocol links ceftazidime and cefepime together, maintaining the β-lactam bactericidal mechanism whilst enhancing bacterial uptake 295 . Bacteria take up cefiderocol through iron channels, and this mechanism is extremely appealing, as many MDR gram-negative pathogens, including P. aeruginosa , Acinetobacter spp. and Stenotrophomonas spp., avidly take up iron, and a major component of the acute-phase host response is to sequester iron from pathogens. Cefiderocol was non-inferior to high-dose extended-infusion meropenem for HAP due to gram-negative pathogens 296 , but it was associated with a higher mortality than the best available therapy for pneumonia and bacteraemia, specifically due to carbapenem-resistant Acinetobacter spp. 292 .
Lefamulin is the first truly new antibiotic class since the oxazolidinone linezolid. The mechanism of action of lefamulin is via protein synthesis inhibition, and lefamulin is approved for the treatment of CAP based on equivalence to moxifloxacin 297 , 298 . This drug can be used as a single agent to target MRSA and other CAP pathogens resistant to macrolide, β-lactam and fluoroquinolone antibiotics, and possibly in cases of treatment failure and/or in patients with multiple drug allergies. Unfortunately, lefamulin does not have substantial activity against ESBL-producing gram-negative pathogens, which is an unmet need in CAP.
Non-antibiotic therapy
Monoclonal or polyclonal antibodies to specific MDR pathogens, including S. aureus and P. aeruginosa , are the ultimate narrow-spectrum agents, being both extremely safe and having the great advantage of not disturbing the commensal microbiota 299 , 300 . Antibodies against the P. aeruginosa type-III secretion apparatus, alginate and other unique targets have entered clinical trials. Several anti- S. aureus antibodies have also been developed 301 . The challenge for specific antibodies is whether they should be used for prevention or as adjuncts to antibiotic therapy. The lack of sensitive risk factors or predictive markers for pneumonia caused by a specific pathogen make prophylactic trials difficult and potential clinical use expensive; thus, development for preventive indications has been abandoned for several agents, and attention has shifted to adjunctive use, despite this being associated with loss of the microbiota-sparing effect with this strategy.
Case reports have been published on bacteriophage therapy as an alternative to antibiotics in patients with extremely difficult-to-treat pneumonia 302 . However, major logistic issues must be overcome before phage therapy becomes a legitimate option 303 : the individual patient’s isolate must be tested for susceptibility against a battery of bacteria-specific phages; a cocktail of at least three phages is usually needed, owing to the emergence of resistance to any single phage; and the availability of phages and susceptibility testing facilities remain extremely limited. Furthermore, the optimal delivery method, namely aerosolization, instillation or venous infusion, remains unclear. No large-scale clinical trials have been completed.
Lastly, the COVID-19 pandemic has generated a large number of studies of adjuvant treatments focusing on host response to SARS-CoV-2. It remains unclear whether any adjuvant treatments other than corticosteroids that may provide benefit in SARS-CoV-2 infection can be used for influenza or other serious viral pneumonias. However, the COVID-19 pandemic has clearly increased interest in both host-directed therapy and newer antivirals.
Mandell, L. A. & Niederman, M. S. Aspiration pneumonia. N. Engl. J. Med. 380 , 651–663 (2019). A review article about aspiration pneumonia, including new insights about microbial aetiology and antibiotic treatment .
CAS PubMed Google Scholar
Cillóniz, C. et al. Microbial aetiology of community-acquired pneumonia and its relation to severity. Thorax 66 , 340–346 (2011).
PubMed Google Scholar
Torres, A. et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur. Respir. J. 50 , 1700582 (2017). In these international European and Latin American guidelines, a panel of experts present recommendations about diagnosis, risk factor for antibiotic resistance and type and duration of treatment for HAP and VAP. PICO questions and GRADE methodology were used .
Kalil, A. C. et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 63 , e61–e111 (2016). These guidelines provide risk factors for suspected MDR or XDR microorganisms and recommendations for empirical treatments, use of biomarkers and duration of antibiotic administration .
PubMed PubMed Central Google Scholar
Metlay, J. P. et al. Diagnosis and treatment of adults with community-acquired pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 200 , e45–e67 (2019). These guidelines include new recommendations for microbiological diagnostic tests, in particular for empirical treatments in outside and in-hospital patients .
Prina, E., Ranzani, O. T. & Torres, A. Community-acquired pneumonia. Lancet 386 , 1097–1108 (2015).
Di Pasquale, M. F. et al. Prevalence and etiology of community-acquired pneumonia in immunocompromised patients. Clin. Infect. Dis. 68 , 1482–1493 (2019).
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396 , 1204–1222 (2020).
Google Scholar
GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 18 , 1191–1210 (2018).
Komiya, K. et al. Prognostic implications of aspiration pneumonia in patients with community acquired pneumonia: a systematic review with meta-analysis. Sci. Rep. 6 , 38097 (2016).
CAS PubMed PubMed Central Google Scholar
Lindenauer, P. K. et al. Variation in the diagnosis of aspiration pneumonia and association with hospital pneumonia outcomes. Ann. Am. Thorac. Soc. 15 , 562–569 (2018).
Neill, S. & Dean, N. Aspiration pneumonia and pneumonitis: a spectrum of infectious/noninfectious diseases affecting the lung. Curr. Opin. Infect. Dis. 32 , 152–157 (2019).
Jain, S. et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N. Engl. J. Med. 373 , 415–427 (2015).
Torres, A., Peetermans, W. E., Viegi, G. & Blasi, F. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax 68 , 1057–1065 (2013).
Norris, T., Vahratian, A. & Cohen, R. A. Vaccination coverage among adults aged 65 and over: United States, 2015. NCHS Data Brief No. 281 (CDC, 2017).
Fedson, D. S. et al. Pneumococcal polysaccharide vaccination for adults: new perspectives for Europe. Expert. Rev. Vaccines 10 , 1143–1167 (2011).
Jamal, A. et al. Current cigarette smoking among adults – United States, 2016. MMWR 67 , 53–59 (2018).
Louie, J. K. et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA 302 , 1896–1902 (2009).
Wu, C. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 180 , 934–943 (2020).
Barbier, F., Andremont, A., Wolff, M. & Bouadma, L. Hospital-acquired pneumonia and ventilator-associated pneumonia: recent advances in epidemiology and management. Curr. Opin. Pulm. Med. 19 , 216–228 (2013).
Rosenthal, V. D. et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004-2009. Am. J. Infect. Control. 40 , 396–407 (2012).
Giuliano, K. K., Baker, D. & Quinn, B. The epidemiology of nonventilator hospital-acquired pneumonia in the United States. Am. J. Infect. Control. 46 , 322–327 (2018).
Bonell, A. et al. A systematic review and meta-analysis of ventilator-associated pneumonia in adults in Asia: an analysis of national income level on incidence and etiology. Clin. Infect. Dis. 68 , 511–518 (2019).
Bouadma, L. et al. Ventilator-associated events: prevalence, outcome, and relationship with ventilator-associated pneumonia. Crit. Care Med. 43 , 1798–1806 (2015).
Shi, T. et al. Global and regional burden of hospital admissions for pneumonia in older adults: a systematic review and meta-analysis. J. Infect. Dis. 222 , S570–S576 (2020).
GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392 , 1736–1788 (2018).
JustActions. The Missing Piece. Why Continued Neglect of Pneumonia Threatens the Achivement of Health Goals (JustActions, 2018).
Nunes, B. P., Flores, T. R., Mielke, G. I., Thumé, E. & Facchini, L. A. Multimorbidity and mortality in older adults: a systematic review and meta-analysis. Arch. Gerontol. Geriatr. 67 , 130–138 (2016).
Arnold, F. W. et al. Mortality differences among hospitalized patients with community-acquired pneumonia in three world regions: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort Study. Respir. Med. 107 , 1101–1111 (2013).
Heo, J. Y. & Song, J. Y. Disease burden and etiologic distribution of community-acquired pneumonia in adults: evolving epidemiology in the era of pneumococcal conjugate vaccines. Infect. Chemother. 50 , 287–300 (2018).
Cillóniz, C. et al. Community-acquired pneumonia in outpatients: aetiology and outcomes. Eur. Respir. J. 40 , 931–938 (2012).
Luna, C. M. et al. The impact of age and comorbidities on the mortality of patients of different age groups admitted with community-acquired pneumonia. Ann. Am. Thorac. Soc. 13 , 1519–1526 (2016).
Cillóniz, C. et al. Twenty-year trend in mortality among hospitalized patients with pneumococcal community-acquired pneumonia. PLoS ONE 13 , e0200504 (2018).
Corrales-Medina, V. F. et al. Intermediate and long-term risk of new-onset heart failure after hospitalization for pneumonia in elderly adults. Am. Heart J. 170 , 306–312 (2015).
Eurich, D. T., Marrie, T. J., Minhas-Sandhu, J. K. & Majumdar, S. R. Ten-year mortality after community-acquired pneumonia. A prospective cohort. Am. J. Respir. Crit. Care Med. 192 , 597–604 (2015).
Ramirez, J. A. et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology and mortality. Clin. Infect. Dis. 65 , 1806–1812 (2017).
Bordon, J. et al. Hospitalization due to community-acquired pneumonia in patients with chronic obstructive pulmonary disease: incidence, epidemiology and outcomes. Clin. Microbiol. Infect. 26 , 220–226 (2020).
Torres, A. et al. Burden of pneumococcal community-acquired pneumonia in adults across Europe: a literature review. Respir. Med. 137 , 6–13 (2018).
Magill, S. S. et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 370 , 1198–1208 (2014).
Micek, S. T., Chew, B., Hampton, N. & Kollef, M. H. A case-control study assessing the impact of nonventilated hospital-acquired pneumonia on patient outcomes. Chest 150 , 1008–1014 (2016).
Melsen, W. G. et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 13 , 665–671 (2013).
Bassetti, M. et al. Risk stratification and treatment of ICU-acquired pneumonia caused by multidrug-resistant/extensively drug-resistant/pandrug-resistant bacteria. Curr. Opin. Crit. Care 24 , 385–393 (2018).
Herkel, T. et al. Epidemiology of hospital-acquired pneumonia: results of a Central European multicenter, prospective, observational study compared with data from the European region. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 160 , 448–455 (2016).
Ibn Saied, W. et al. A comparison of the mortality risk associated with ventilator-acquired bacterial pneumonia and nonventilator ICU-acquired bacterial pneumonia. Crit. Care Med. 47 , 345–352 (2019).
Talbot, G. H. et al. Evidence-based study design for hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. J. Infect. Dis. 219 , 1536–1544 (2019).
McAllister, D. A. et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob. Health 7 , e47–e57 (2019).
Weir, D. L., Majumdar, S. R., McAlister, F. A., Marrie, T. J. & Eurich, D. T. The impact of multimorbidity on short-term events in patients with community-acquired pneumonia: prospective cohort study. Clin. Microbiol. Infect. 21 , 264.e7–264.e13 (2015).
CAS Google Scholar
Bradley, J. S. et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 53 , e25–e76 (2011).
Barbagelata, E. et al. Gender differences in community-acquired pneumonia. Minerva Med. 111 , 153–165 (2020).
Mutepe, N. D. et al. Effects of cigarette smoke condensate on pneumococcal biofilm formation and pneumolysin. Eur. Respir. J. 41 , 392–395 (2013).
Samokhvalov, A. V., Irving, H. M. & Rehm, J. Alcohol consumption as a risk factor for pneumonia: a systematic review and meta-analysis. Epidemiol. Infect. 138 , 1789–1795 (2010).
Neupane, B. et al. Long-term exposure to ambient air pollution and risk of hospitalization with community-acquired pneumonia in older adults. Am. J. Respir. Crit. Care Med. 181 , 47–53 (2010).
American Thoracic Society & Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171 , 388–416 (2005).
Le, M. N.-T. et al. Oral colonisation by antimicrobial-resistant Gram-negative bacteria among long-term care facility residents: prevalence, risk factors, and molecular epidemiology. Antimicrob. Resist. Infect. Control. 9 , 45 (2020).
Feldman, C. et al. The presence and sequence of endotracheal tube colonization in patients undergoing mechanical ventilation. Eur. Respir. J. 13 , 546–551 (1999).
Cilloniz, C. et al. Seasonality of pathogens causing community-acquired pneumonia. Respirology 22 , 778–785 (2017).
Para, R. A., Fomda, B. A., Jan, R. A., Shah, S. & Koul, P. A. Microbial etiology in hospitalized North Indian adults with community-acquired pneumonia. Lung India 35 , 108–115 (2018).
Tao, L.-L. et al. Etiology and antimicrobial resistance of community-acquired pneumonia in adult patients in China. Chin. Med. J. 125 , 2967–2972 (2012).
Shoar, S. & Musher, D. M. Etiology of community-acquired pneumonia in adults: a systematic review. Pneumonia 12 , 11 (2020).
Moberley, S., Holden, J., Tatham, D. P. & Andrews, R. M. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst. Rev. 2013 , CD000422 (2013).
PubMed Central Google Scholar
Centers for Disease Control and Prevention (CDC). Current cigarette smoking among adults - United States, 2011. MMWR 61 , 889–894 (2012).
Luca, D. L. et al. Impact of pneumococcal vaccination on pneumonia hospitalizations and related costs in Ontario: a population-based ecological study. Clin. Infect. Dis. 66 , 541–547 (2017).
Johansson, N., Kalin, M., Tiveljung-Lindell, A., Giske, C. G. & Hedlund, J. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin. Infect. Dis. 50 , 202–209 (2010).
Rozenbaum, M. H. et al. The role of Streptococcus pneumoniae in community-acquired pneumonia among adults in Europe: a meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 32 , 305–316 (2013).
Huijts, S. M. et al. Diagnostic accuracy of a serotype-specific antigen test in community-acquired pneumonia. Eur. Respir. J. 42 , 1283–1290 (2013).
Aliberti, S. et al. Multidrug-resistant pathogens in hospitalised patients coming from the community with pneumonia: a European perspective. Thorax 68 , 997–999 (2013).
Shindo, Y. et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 188 , 985–995 (2013).
Prina, E. et al. Risk factors associated with potentially antibiotic-resistant pathogens in community-acquired pneumonia. Ann. Am. Thorac. Soc. 12 , 153–160 (2015).
Ceccato, A. et al. Validation of a prediction score for drug-resistant microorganisms in community-acquired pneumonia. Ann. Am. Thorac. Soc. 18 , 257–265 (2021).
Cilloniz, C. et al. Difficult to treat microorganisms in patients over 80 years with community-acquired pneumonia: the prevalence of PES pathogens. Eur. Respir. J. 56 , 2000773 (2020).
Webb, B. J. et al. Derivation and multicenter validation of the drug resistance in pneumonia clinical prediction score. Antimicrob. Agents Chemother. 60 , 2652–2663 (2016).
Karhu, J., Ala-Kokko, T. I., Vuorinen, T., Ohtonen, P. & Syrjälä, H. Lower respiratory tract virus findings in mechanically ventilated patients with severe community-acquired pneumonia. Clin. Infect. Dis. 59 , 62–70 (2014).
Wu, X. et al. Incidence of respiratory viral infections detected by PCR and real-time PCR in adult patients with community-acquired pneumonia: a meta-analysis. Respiration 89 , 343–352 (2015).
Zhou, F. et al. Disease severity and clinical outcomes of community acquired pneumonia caused by non-influenza respiratory viruses in adults: a multicenter prospective registry study from CAP-China Network. Eur. Respir. J. 54 , 1802406 (2019).
Cillóniz, C. et al. Pure viral sepsis secondary to community-acquired pneumonia in adults: risk and prognostic factors. J. Infect. Dis. 220 , 1166–1171 (2019).
Jain, S. et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N. Engl. J. Med. 372 , 835–845 (2015). This study is a prospective multicentre investigation of the CAP microbial aetiology in hospitalized patients. Very importantly, PCR tests for the detection of viral pathogens , Legionella spp. and Mycoplasma pneumoniae were systematically used in the diagnostic work-up. With this approach, viruses represented the first cause of CAP .
Weber, D. J. et al. Microbiology of ventilator-associated pneumonia compared with that of hospital-acquired pneumonia. Infect. Control. Hosp. Epidemiol. 28 , 825–831 (2007).
Magiorakos, A.-P. et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18 , 268–281 (2012).
Parker, D., Ahn, D., Cohen, T. & Prince, A. Innate immune signaling activated by MDR bacteria in the airway. Physiol. Rev. 96 , 19–53 (2016).
Grousd, J. A., Rich, H. E. & Alcorn, J. F. Host-pathogen interactions in gram-positive bacterial pneumonia. Clin. Microbiol. Rev. 32 , e00107-18 (2019).
Kutter, J. S., Spronken, M. I., Fraaij, P. L., Fouchier, R. A. & Herfst, S. Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 28 , 142–151 (2018).
Siegel, S. J. & Weiser, J. N. Mechanisms of bacterial colonization of the respiratory tract. Annu. Rev. Microbiol. 69 , 425–444 (2015).
Quinton, L. J., Walkey, A. J. & Mizgerd, J. P. Integrative physiology of pneumonia. Physiol. Rev. 98 , 1417–1464 (2018).
Dickson, R. P., Erb-Downward, J. R. & Huffnagle, G. B. Towards an ecology of the lung: new conceptual models of pulmonary microbiology and pneumonia pathogenesis. Lancet Respir. Med. 2 , 238–246 (2014). A review–opinion article about new insights into the aetiopathogenesis of pneumonia based on changes in the microbiota .
Pettigrew, M. M., Tanner, W. & Harris, A. D. The lung microbiome and pneumonia. J. Infect. Dis. https://doi.org/10.1093/infdis/jiaa702 (2020).
Article Google Scholar
Brown, R. L., Sequeira, R. P. & Clarke, T. B. The microbiota protects against respiratory infection via GM-CSF signaling. Nat. Commun. 8 , 1512 (2017).
Nishimoto, A. T., Rosch, J. W. & Tuomanen, E. I. Pneumolysin: pathogenesis and therapeutic target. Front. Microbiol. 11 , 1543 (2020).
von Hoven, G., Qin, Q., Neukirch, C., Husmann, M. & Hellmann, N. Staphylococcus aureus α-toxin: small pore, large consequences. Biol. Chem. 400 , 1261–1276 (2019).
Hauser, A. R. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat. Rev. Microbiol. 7 , 654–665 (2009).
Ferguson, N. D. et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 38 , 1573–1582 (2012).
Matthay, M. A. et al. Acute respiratory distress syndrome. Nat. Rev. Dis. Prim. 5 , 18 (2019).
Whitsett, J. A. & Alenghat, T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 16 , 27–35 (2015).
Cheng, D. et al. Airway epithelium controls lung inflammation and injury through the NF-κB pathway. J. Immunol. 178 , 6504–6513 (2007).
Quinton, L. J. et al. Functions and regulation of NF-κB RelA during pneumococcal pneumonia. J. Immunol. 178 , 1896–1903 (2007).
Han, S. & Mallampalli, R. K. The role of surfactant in lung disease and host defense against pulmonary infections. Ann. Am. Thorac. Soc. 12 , 765–774 (2015).
Carey, R. M. & Lee, R. J. Taste receptors in upper airway innate immunity. Nutrients 11 , 2017 (2019).
CAS PubMed Central Google Scholar
Lee, R. J. & Cohen, N. A. The emerging role of the bitter taste receptor T2R38 in upper respiratory infection and chronic rhinosinusitis. Am. J. Rhinol. Allergy 27 , 283–286 (2013).
McAleer, J. P. & Kolls, J. K. Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunol. Rev. 260 , 129–144 (2014).
Aujla, S. J. et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 14 , 275–281 (2008).
Allard, B., Panariti, A. & Martin, J. G. Alveolar macrophages in the resolution of inflammation, tissue repair, and tolerance to infection. Front. Immunol. 9 , 1777 (2018).
Preston, J. A. et al. Alveolar macrophage apoptosis-associated bacterial killing helps prevent murine pneumonia. Am. J. Respir. Crit. Care Med. 200 , 84–97 (2019).
González-Juarbe, N. et al. Pore-forming toxins induce macrophage necroptosis during acute bacterial pneumonia. PLoS Pathog. 11 , e1005337 (2015).
Kitur, K. et al. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog. 11 , e1004820 (2015).
Kitur, K. et al. Necroptosis promotes Staphylococcus aureus clearance by inhibiting excessive inflammatory signaling. Cell Rep. 16 , 2219–2230 (2016).
Panda, S. K. & Colonna, M. Innate lymphoid cells in mucosal immunity. Front. Immunol. 10 , 861 (2019).
Kaiko, G. E., Phipps, S., Angkasekwinai, P., Dong, C. & Foster, P. S. NK cell deficiency predisposes to viral-induced Th2-type allergic inflammation via epithelial-derived IL-25. J. Immunol. 185 , 4681–4690 (2010).
Jayaraman, A. et al. IL-15 complexes induce NK- and T-cell responses independent of type I IFN signaling during rhinovirus infection. Mucosal Immunol. 7 , 1151–1164 (2014).
Van Maele, L. et al. Activation of type 3 innate lymphoid cells and interleukin 22 secretion in the lungs during Streptococcus pneumoniae infection. J. Infect. Dis. 210 , 493–503 (2014).
Xiong, H. et al. Innate lymphocyte/ly6c(hi) monocyte crosstalk promotes Klebsiella pneumoniae clearance. Cell 165 , 679–689 (2016).
Hinks, T. S. C. et al. Steroid-induced deficiency of mucosal-associated invariant T cells in the chronic obstructive pulmonary disease lung. Implications for nontypeable Haemophilus influenzae infection. Am. J. Respir. Crit. Care Med. 194 , 1208–1218 (2016).
Meierovics, A. I. & Cowley, S. C. MAIT cells promote inflammatory monocyte differentiation into dendritic cells during pulmonary intracellular infection. J. Exp. Med. 213 , 2793–2809 (2016).
Liu, J. et al. Advanced role of neutrophils in common respiratory diseases. J. Immunol. Res. 2017 , 6710278 (2017).
Castanheira, F. V. S. & Kubes, P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 133 , 2178–2185 (2019).
Xiong, H. et al. Distinct contributions of neutrophils and CCR2+ monocytes to pulmonary clearance of different Klebsiella pneumoniae strains. Infect. Immun. 83 , 3418–3427 (2015).
Winter, C. et al. Important role for CC chemokine ligand 2-dependent lung mononuclear phagocyte recruitment to inhibit sepsis in mice infected with Streptococcus pneumoniae. J. Immunol. 182 , 4931–4937 (2009).
Weber, G. F. et al. Pleural innate response activator B cells protect against pneumonia via a GM-CSF-IgM axis. J. Exp. Med. 211 , 1243–1256 (2014).
de Stoppelaar, S. F. et al. Thrombocytopenia impairs host defense in gram-negative pneumonia-derived sepsis in mice. Blood 124 , 3781–3790 (2014).
van den Boogaard, F. E. et al. Thrombocytopenia impairs host defense during murine Streptococcus pneumoniae pneumonia. Crit. Care Med. 43 , e75–e83 (2015).
Ichinohe, T. et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl Acad. Sci. USA 108 , 5354–5359 (2011).
Schuijt, T. J. et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 65 , 575–583 (2016).
Haak, B. W. & Wiersinga, W. J. The role of the gut microbiota in sepsis. Lancet Gastroenterol. Hepatol. 2 , 135–143 (2017).
Netea, M. G., Schlitzer, A., Placek, K., Joosten, L. A. B. & Schultze, J. L. Innate and adaptive immune memory: an evolutionary continuum in the host’s response to pathogens. Cell Host Microbe 25 , 13–26 (2019).
Giamarellos-Bourboulis, E. J. et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell 183 , 315–323.e9 (2020).
Hwang, J. Y., Randall, T. D. & Silva-Sanchez, A. Inducible bronchus-associated lymphoid tissue: taming inflammation in the lung. Front. Immunol. 7 , 258 (2016).
Snyder, M. E. & Farber, D. L. Human lung tissue resident memory T cells in health and disease. Curr. Opin. Immunol. 59 , 101–108 (2019).
Smith, N. M. et al. Regionally compartmentalized resident memory T cells mediate naturally acquired protection against pneumococcal pneumonia. Mucosal Immunol. 11 , 220–235 (2018).
Serhan, C. N. & Levy, B. D. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 128 , 2657–2669 (2018).
Flitter, B. A. et al. Pseudomonas aeruginosa sabotages the generation of host proresolving lipid mediators. Proc. Natl Acad. Sci. USA 114 , 136–141 (2017).
Sham, H. P. et al. 15-epi-lipoxin A4, resolvin D2, and resolvin D3 induce NF-κB regulators in bacterial pneumonia. J. Immunol. 200 , 2757–2766 (2018).
Zemans, R. L. et al. Neutrophil transmigration triggers repair of the lung epithelium via β-catenin signaling. Proc. Natl Acad. Sci. USA 108 , 15990–15995 (2011).
Liu, Y. et al. FoxM1 mediates the progenitor function of type II epithelial cells in repairing alveolar injury induced by Pseudomonas aeruginosa. J. Exp. Med. 208 , 1473–1484 (2011).
Kumar, P. A. et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 147 , 525–538 (2011).
Matsuzaki, Y. et al. Stat3 is required for cytoprotection of the respiratory epithelium during adenoviral infection. J. Immunol. 177 , 527–537 (2006).
Quinton, L. J. et al. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am. J. Respir. Cell Mol. Biol. 38 , 699–706 (2008).
Quinton, L. J. et al. Leukemia inhibitory factor signaling is required for lung protection during pneumonia. J. Immunol. 188 , 6300–6308 (2012).
Poe, S. L. et al. STAT1-regulated lung MDSC-like cells produce IL-10 and efferocytose apoptotic neutrophils with relevance in resolution of bacterial pneumonia. Mucosal Immunol. 6 , 189–199 (2013).
D’Alessio, F. R. et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J. Clin. Invest. 119 , 2898–2913 (2009).
Monticelli, L. A. et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 12 , 1045–1054 (2011).
Laidlaw, B. J. et al. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity 41 , 633–645 (2014).
Xu, X. et al. Conventional NK cells can produce IL-22 and promote host defense in Klebsiella pneumoniae pneumonia. J. Immunol. 192 , 1778–1786 (2014).
Kradin, R. L. & Digumarthy, S. The pathology of pulmonary bacterial infection. Semin. Diagn. Pathol. 34 , 498–509 (2017).
Pritt, B. S. & Aubry, M. C. Histopathology of viral infections of the lung. Semin. Diagn. Pathol. 34 , 510–517 (2017).
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315 , 801–810 (2016).
Angus, D. C. & van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 369 , 840–851 (2013).
Dremsizov, T. et al. Severe sepsis in community-acquired pneumonia: when does it happen, and do systemic inflammatory response syndrome criteria help predict course? Chest 129 , 968–978 (2006).
Giuliano, K. K. & Baker, D. Sepsis in the context of nonventilator hospital-acquired pneumonia. Am. J. Crit. Care 29 , 9–14 (2020).
Hotchkiss, R. S. et al. Sepsis and septic shock. Nat. Rev. Dis. Prim. 2 , 16045 (2016).
van der Poll, T., van de Veerdonk, F. L., Scicluna, B. P. & Netea, M. G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 17 , 407–420 (2017).
Corrales-Medina, V. F., Musher, D. M., Shachkina, S. & Chirinos, J. A. Acute pneumonia and the cardiovascular system. Lancet 381 , 496–505 (2013).
Corrales-Medina, V. F. et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 313 , 264–274 (2015). The short-term and long-term risk of cardiovascular diseases after CAP hospitalization is shown in this capital study .
Corrales-Medina, V. F. et al. Cardiac complications in patients with community-acquired pneumonia: a systematic review and meta-analysis of observational studies. PLoS Med. 8 , e1001048 (2011).
Violi, F. et al. Cardiovascular complications and short-term mortality risk in community-acquired pneumonia. Clin. Infect. Dis. 64 , 1486–1493 (2017).
Ramirez, J. et al. Acute myocardial infarction in hospitalized patients with community-acquired pneumonia. Clin. Infect. Dis. 47 , 182–187 (2008).
Mortensen, E. M. et al. Causes of death for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study. Arch. Intern. Med. 162 , 1059–1064 (2002).
Musher, D. M., Abers, M. S. & Corrales-Medina, V. F. Acute infection and myocardial infarction. N. Engl. J. Med. 380 , 171–176 (2019). A review article showing the evidence of acute respiratory viral infection and the increased risk of myocardial infarction .
Milbrandt, E. B. et al. Prevalence and significance of coagulation abnormalities in community-acquired pneumonia. Mol. Med. 15 , 438–445 (2009).
van Vught, L. A. et al. Comparative analysis of the host response to community-acquired and hospital-acquired pneumonia in critically Ill patients. Am. J. Respir. Crit. Care Med. 194 , 1366–1374 (2016).
Naghavi, M. et al. Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E-deficient mice. Circulation 107 , 762–768 (2003).
Madjid, M., Vela, D., Khalili-Tabrizi, H., Casscells, S. W. & Litovsky, S. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries: clues to the triggering effect of acute infections on acute coronary syndromes. Tex. Heart Inst. J. 34 , 11–18 (2007).
Jaw, J. E. et al. Lung exposure to lipopolysaccharide causes atherosclerotic plaque destabilisation. Eur. Respir. J. 48 , 205–215 (2016).
Yende, S. et al. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am. J. Respir. Crit. Care Med. 177 , 1242–1247 (2008).
Yende, S. et al. Elevated hemostasis markers after pneumonia increases one-year risk of all-cause and cardiovascular deaths. PLoS ONE 6 , e22847 (2011).
Iwashyna, T. J., Ely, E. W., Smith, D. M. & Langa, K. M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304 , 1787–1794 (2010).
Shah, F. A. et al. Bidirectional relationship between cognitive function and pneumonia. Am. J. Respir. Crit. Care Med. 188 , 586–592 (2013).
Girard, T. D., Dittus, R. S. & Ely, E. W. Critical illness brain injury. Annu. Rev. Med. 67 , 497–513 (2016).
Chung, H.-Y., Wickel, J., Brunkhorst, F. M. & Geis, C. Sepsis-associated encephalopathy: from delirium to dementia? J. Clin. Med. 9 , 703 (2020).
Prescott, H. C., Sjoding, M. W. & Iwashyna, T. J. Diagnoses of early and late readmissions after hospitalization for pneumonia. A systematic review. Ann. Am. Thorac. Soc. 11 , 1091–1100 (2014).
Dang, T. T., Majumdar, S. R., Marrie, T. J. & Eurich, D. T. Recurrent pneumonia: a review with focus on clinical epidemiology and modifiable risk factors in elderly patients. Drugs Aging 32 , 13–19 (2015).
Ekdahl, K., Braconier, J. H. & Svanborg, C. Immunoglobulin deficiencies and impaired immune response to polysaccharide antigens in adult patients with recurrent community-acquired pneumonia. Scand. J. Infect. Dis. 29 , 401–407 (1997).
Roquilly, A. et al. Alveolar macrophages are epigenetically altered after inflammation, leading to long-term lung immunoparalysis. Nat. Immunol. 21 , 636–648 (2020).
Lamping, D. L. et al. The community-acquired pneumonia symptom questionnaire: a new, patient-based outcome measure to evaluate symptoms in patients with community-acquired pneumonia. Chest 122 , 920–929 (2002).
Metlay, J. P. et al. Measuring symptomatic and functional recovery in patients with community-acquired pneumonia. J. Gen. Intern. Med. 12 , 423–430 (1997).
Chalmers, J. D. et al. Severity assessment tools for predicting mortality in hospitalised patients with community-acquired pneumonia. Systematic review and meta-analysis. Thorax 65 , 878–883 (2010).
Chalmers, J. D. et al. Risk factors for complicated parapneumonic effusion and empyema on presentation to hospital with community-acquired pneumonia. Thorax 64 , 556–558 (2009).
Falguera, M. et al. Predictive factors, microbiology and outcome of patients with parapneumonic effusion. Eur. Respir. J. 38 , 1173–1179 (2011).
Bhuiyan, M. U. et al. Combination of clinical symptoms and blood biomarkers can improve discrimination between bacterial or viral community-acquired pneumonia in children. BMC Pulmonary Med. 19 , 71 (2019).
Lhommet, C. et al. Predicting the microbial cause of community-acquired pneumonia: can physicians or a data-driven method differentiate viral from bacterial pneumonia at patient presentation? BMC Pulmonary Med. 20 , 62 (2020).
Torres, A., & Cillóniz, C. Clinical Management of Bacterial Pneumonia (Springer, 2015).
Cilloniz, C., Ceccato, A., San Jose, A. & Torres, A. Clinical management of community acquired pneumonia in the elderly patient. Expert Rev. Respir. Med. 10 , 1211–1220 (2016).
Schurink, C. A. M. et al. Clinical pulmonary infection score for ventilator-associated pneumonia: accuracy and inter-observer variability. Intensive Care Med. 30 , 217–224 (2004).
Fàbregas, N. et al. Clinical diagnosis of ventilator associated pneumonia revisited: comparative validation using immediate post-mortem lung biopsies. Thorax 54 , 867–873 (1999). The most complete immediate post-mortem study of VAP to validate clinical diagnosis .
Self, W. H., Courtney, D. M., McNaughton, C. D., Wunderink, R. G. & Kline, J. A. High discordance of chest x-ray and computed tomography for detection of pulmonary opacities in ED patients: implications for diagnosing pneumonia. Am. J. Emerg. Med. 31 , 401–405 (2013).
Laursen, C. B. et al. Diagnostic performance of chest X-ray for the diagnosis of community acquired pneumonia in acute admitted patients with respiratory symptoms. Scand. J. Trauma. Resusc. Emerg. Med. 21 , A21 (2013).
Claessens, Y.-E. et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am. J. Respir. Crit. Care Med. 192 , 974–982 (2015).
Ding, X., Xu, J., Zhou, J., Long, Q. & Chest, C. T. findings of COVID-19 pneumonia by duration of symptoms. Eur. J. Radiol. 127 , 109009 (2020).
Franquet, T. Imaging of community-acquired pneumonia. J. Thorac. Imaging 33 , 282–294 (2018).
D’Amato, M. et al. Assessment of thoracic ultrasound in complementary diagnosis and in follow up of community-acquired pneumonia (CAP). BMC Med. Imaging 17 , 52 (2017).
Long, L., Zhao, H.-T., Zhang, Z.-Y., Wang, G.-Y. & Zhao, H.-L. Lung ultrasound for the diagnosis of pneumonia in adults: a meta-analysis. Medicine 96 , e5713 (2017).
Mongodi, S. et al. Lung ultrasound for early diagnosis of ventilator-associated pneumonia. Chest 149 , 969–980 (2016).
Bouhemad, B., Dransart-Rayé, O., Mojoli, F. & Mongodi, S. Lung ultrasound for diagnosis and monitoring of ventilator-associated pneumonia. Ann. Transl. Med. 6 , 418 (2018).
Musher, D. M., Montoya, R. & Wanahita, A. Diagnostic value of microscopic examination of Gram-stained sputum and sputum cultures in patients with bacteremic pneumococcal pneumonia. Clin. Infect. Dis. 39 , 165–169 (2004).
Fukuyama, H., Yamashiro, S., Kinjo, K., Tamaki, H. & Kishaba, T. Validation of sputum Gram stain for treatment of community-acquired pneumonia and healthcare-associated pneumonia: a prospective observational study. BMC Infect. Dis. 14 , 534 (2014).
Ranzani, O. T. et al. Diagnostic accuracy of Gram staining when predicting staphylococcal hospital-acquired pneumonia and ventilator-associated pneumonia: a systematic review and meta-analysis. Clin. Microbiol. Infect. 26 , 1456–1463 (2020).
Torres, A., Artigas, A. & Ferrer, R. Biomarkers in the ICU: less is more? No. Intensive Care Med. 47 , 97–100 (2021).
Torres, A., Lee, N., Cilloniz, C., Vila, J. & Van der Eerden, M. Laboratory diagnosis of pneumonia in the molecular age. Eur. Respir. J. 48 , 1764–1778 (2016). In-depth revision of available molecular diagnostic techniques for bacterial and viral pneumonia .
Schulte, B. et al. Detection of pneumonia associated pathogens using a prototype multiplexed pneumonia test in hospitalized patients with severe pneumonia. PLoS ONE 9 , e110566 (2014).
Paonessa, J. R. et al. Rapid detection of methicillin-resistant Staphylococcus aureus in BAL: a pilot randomized controlled trial. Chest 155 , 999–1007 (2019).
Gastli, N. et al. Multicentric evaluation of BioFire FilmArray Pneumonia Panel for rapid bacteriological documentation of pneumonia. Clin. Microbiol. Infect. https://doi.org/10.1016/j.cmi.2020.11.014 (2020).
Article PubMed Google Scholar
Centers for Disease Control and Prevention. Overview of Testing for SARS-CoV-2 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html (CDC, 2020).
Karakioulaki, M. & Stolz, D. The case of procalcitonin for lower respiratory tract infections. BRN Rev. 5 , 277–293 (2019).
Krüger, S. et al. Inflammatory parameters predict etiologic patterns but do not allow for individual prediction of etiology in patients with CAP: results from the German Competence Network CAPNETZ. Respir. Res. 10 , 65 (2009).
Ramirez, P. et al. Sequential measurements of procalcitonin levels in diagnosing ventilator-associated pneumonia. Eur. Respir. J. 31 , 356–362 (2008).
Luyt, C.-E. et al. Usefulness of procalcitonin for the diagnosis of ventilator-associated pneumonia. Intensive Care Med. 34 , 1434–1440 (2008).
Schuetz, P. et al. Procalcitonin (PCT)-guided antibiotic stewardship: an international experts consensus on optimized clinical use. Clin. Chem. Lab. Med. 57 , 1308–1318 (2019).
Liapikou, A., Cilloniz, C. & Torres, A. Drugs that increase the risk of community-acquired pneumonia: a narrative review. Expert Opin. Drug Saf. 17 , 991–1003 (2018).
Niederman, M. S. et al. Efficacy and effectiveness of a 23-valent polysaccharide vaccine against invasive and non-invasive pneumococcal disease and related outcomes: a review of available evidence. Expert Rev Vaccines https://doi.org/10.1080/14760584.2021.1880328 (2021).
Maruyama, T. et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ 340 , c1004 (2010).
Falkenhorst, G. et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine (PPV23) against pneumococcal disease in the elderly: systematic review and meta-analysis. PLoS ONE 12 , e0169368 (2017).
Bonten, M. J. M. et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N. Engl. J. Med. 372 , 1114–1125 (2015).
Patterson, S. et al. A post hoc assessment of duration of protection in CAPiTA (Community Acquired Pneumonia immunization Trial in Adults). Trials Vaccinol. 5 , 92–96 (2016).
Millar, E. V. et al. Indirect effect of 7-valent pneumococcal conjugate vaccine on pneumococcal colonization among unvaccinated household members. Clin. Infect. Dis. 47 , 989–996 (2008).
Hammitt, L. L. et al. Indirect effect of conjugate vaccine on adult carriage of Streptococcus pneumoniae: an explanation of trends in invasive pneumococcal disease. J. Infect. Dis. 193 , 1487–1494 (2006).
Chung, J. R. et al. Effects of influenza vaccination in the United States during the 2018-2019 influenza season. Clin. Infect. Dis. 71 , e368–e376 (2020).
Restivo, V. et al. Influenza vaccine effectiveness among high-risk groups: a systematic literature review and meta-analysis of case-control and cohort studies. Hum. Vaccin. Immunother. 14 , 724–735 (2018).
Chow, E. J. et al. Vaccine effectiveness against influenza-associated lower respiratory tract infections in hospitalized adults, Louisville, Kentucky, 2010-2013. Open. Forum Infect. Dis. 7 , ofaa262 (2020).
Lyons, P. G. & Kollef, M. H. Prevention of hospital-acquired pneumonia. Curr. Opin. Crit. Care 24 , 370–378 (2018).
Álvarez-Lerma, F. et al. Prevention of ventilator-associated pneumonia: the multimodal approach of the Spanish ICU “Pneumonia Zero” Program. Crit. Care Med. 46 , 181–188 (2018).
Palomar, M. et al. Impact of a national multimodal intervention to prevent catheter-related bloodstream infection in the ICU: the Spanish experience. Crit. Care Med. 41 , 2364–2372 (2013).
Ma, S. et al. A meta analysis of the effect of enhanced hand hygiene on the morbidity of ventilator-associated pneumonia. Zhonghua Wei Zhong Bing. Ji Jiu Yi Xue 26 , 304–308 (2014).
Drakulovic, M. B. et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet 354 , 1851–1858 (1999).
Wang, L. et al. Semi-recumbent position versus supine position for the prevention of ventilator-associated pneumonia in adults requiring mechanical ventilation. Cochrane Database Syst. Rev. 2016 , CD009946 (2016).
Li Bassi, G. et al. Randomized, multicenter trial of lateral Trendelenburg versus semirecumbent body position for the prevention of ventilator-associated pneumonia. Intensive Care Med. 43 , 1572–1584 (2017).
Guérin, C. et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 368 , 2159–2168 (2013).
Douglas, I. S. et al. Safety and outcomes of prolonged usual care prone position mechanical ventilation to treat acute coronavirus disease 2019 hypoxemic respiratory failure. Crit. Care Med. 49 , 490–502 (2021).
Shelhamer, M. C. et al. Prone positioning in moderate to severe acute respiratory distress syndrome due to COVID-19: a cohort study and analysis of physiology. J. Intensive Care Med. 36 , 241–252 (2021).
Sud, S., Sud, M., Friedrich, J. O. & Adhikari, N. K. J. Effect of mechanical ventilation in the prone position on clinical outcomes in patients with acute hypoxemic respiratory failure: a systematic review and meta-analysis. CMAJ 178 , 1153–1161 (2008).
Mao, Z. et al. Subglottic secretion suction for preventing ventilator-associated pneumonia: an updated meta-analysis and trial sequential analysis. Crit. Care 20 , 353 (2016).
Marjanovic, N. et al. Multicentre randomised controlled trial to investigate the usefulness of continuous pneumatic regulation of tracheal cuff pressure for reducing ventilator-associated pneumonia in mechanically ventilated severe trauma patients: the AGATE study protocol. BMJ Open 7 , e017003 (2017).
Philippart, F. et al. Randomized intubation with polyurethane or conical cuffs to prevent pneumonia in ventilated patients. Am. J. Respir. Crit. Care Med. 191 , 637–645 (2015).
Klompas, M., Speck, K., Howell, M. D., Greene, L. R. & Berenholtz, S. M. Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: systematic review and meta-analysis. JAMA Intern. Med. 174 , 751–761 (2014).
de Smet, A. M. G. A. et al. Decontamination of the digestive tract and oropharynx in ICU patients. N. Engl. J. Med. 360 , 20–31 (2009).
Wittekamp, B. H. et al. Decontamination strategies and bloodstream infections with antibiotic-resistant microorganisms in ventilated patients: a randomized clinical trial. JAMA 320 , 2087–2098 (2018).
Dang, T. T., Eurich, D. T., Weir, D. L., Marrie, T. J. & Majumdar, S. R. Rates and risk factors for recurrent pneumonia in patients hospitalized with community-acquired pneumonia: population-based prospective cohort study with 5 years of follow-up. Clin. Infect. Dis. 59 , 74–80 (2014).
Garcia-Vidal, C. et al. Aetiology of, and risk factors for, recurrent community-acquired pneumonia. Clin. Microbiol. Infect. 15 , 1033–1038 (2009).
Liu, P. et al. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established Antimicrobial Stewardship Program. BMC Infect. Dis. 16 , 751 (2016).
Maruyama, T. et al. A therapeutic strategy for all pneumonia patients: a 3-year prospective multicenter cohort study using risk factors for multidrug-resistant pathogens to select initial empiric therapy. Clin. Infect. Dis. 68 , 1080–1088 (2018).
Abdul-Aziz, M. H., Lipman, J. & Roberts, J. A. Antibiotic dosing for multidrug-resistant pathogen pneumonia. Curr. Opin. Infect. Dis. 30 , 231–239 (2017).
Tsai, D., Lipman, J. & Roberts, J. A. Pharmacokinetic/pharmacodynamic considerations for the optimization of antimicrobial delivery in the critically ill. Curr. Opin. Crit. Care 21 , 412–420 (2015).
Mandell, L. A. et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 44 (Suppl 2), S27–S72 (2007).
Sligl, W. I. et al. Macrolides and mortality in critically ill patients with community-acquired pneumonia: a systematic review and meta-analysis. Crit. Care Med. 42 , 420–432 (2014).
Torres, A. et al. Challenges in severe community-acquired pneumonia: a point-of-view review. Intensive Care Med. 45 , 159–171 (2019).
Niederman, M. S. Macrolide-resistant pneumococcus in community-acquired pneumonia. Is there still a role for macrolide therapy? Am. J. Respir. Crit. Care Med. 191 , 1216–1217 (2015).
Asadi, L. et al. Guideline adherence and macrolides reduced mortality in outpatients with pneumonia. Respir. Med. 106 , 451–458 (2012).
Postma, D. F. et al. Cardiac events after macrolides or fluoroquinolones in patients hospitalized for community-acquired pneumonia: post-hoc analysis of a cluster-randomized trial. BMC Infect. Dis. 19 , 17 (2019).
Garin, N. et al. β-Lactam monotherapy vs β-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: a randomized noninferiority trial. JAMA Intern. Med. 174 , 1894–1901 (2014).
Gershengorn, H. B., Keene, A., Dzierba, A. L. & Wunsch, H. The association of antibiotic treatment regimen and hospital mortality in patients hospitalized with Legionella pneumonia. Clin. Infect. Dis. 60 , e66–e79 (2015).
Niederman, M. S. Antibiotic treatment of hospital-acquired pneumonia: is it different from ventilator-associated pneumonia? Curr. Opin. Crit. Care 24 , 353–360 (2018).
Kumar, A., Safdar, N., Kethireddy, S. & Chateau, D. A survival benefit of combination antibiotic therapy for serious infections associated with sepsis and septic shock is contingent only on the risk of death: a meta-analytic/meta-regression study. Crit. Care Med. 38 , 1651–1664 (2010).
Martin-Loeches, I. et al. Potentially resistant microorganisms in intubated patients with hospital-acquired pneumonia: the interaction of ecology, shock and risk factors. Intensive Care Med. 39 , 672–681 (2013).
Niederman, M. S. et al. Inhaled amikacin adjunctive to intravenous standard-of-care antibiotics in mechanically ventilated patients with Gram-negative pneumonia (INHALE): a double-blind, randomised, placebo-controlled, phase 3, superiority trial. Lancet Infect. Dis. 20 , 330–340 (2020).
Chastre, J. et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA 290 , 2588–2598 (2003). A seminal article comparing 8 or 15 days of antibiotic treatment in VAP .
Garnacho-Montero, J. et al. Task force on management and prevention of Acinetobacter baumannii infections in the ICU. Intensive Care Med. 41 , 2057–2075 (2015).
Timsit, J.-F., Pilmis, B. & Zahar, J.-R. How should we treat hospital-acquired and ventilator-associated pneumonia caused by extended-spectrum β-lactamase-producing enterobacteriaceae? Semin. Respir. Crit. Care Med. 38 , 287–300 (2017).
de Jong, E. et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect. Dis. 16 , 819–827 (2016).
Sousa, D. et al. Community-acquired pneumonia in immunocompromised older patients: incidence, causative organisms and outcome. Clin. Microbiol. Infect. 19 , 187–192 (2013).
Ramirez, J. A. et al. Treatment of community-acquired pneumonia in immunocompromised adults: a consensus statement regarding initial strategies. Chest 158 , 1896–1911 (2020).
El-Solh, A. A. et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am. J. Respir. Crit. Care Med. 167 , 1650–1654 (2003).
Siemieniuk, R. A. C. et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann. Intern. Med. 163 , 519–528 (2015).
Torres, A. et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 313 , 677–686 (2015).
Rodrigo, C., Leonardi-Bee, J., Nguyen-Van-Tam, J. & Lim, W. S. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst. Rev. 3 , CD010406 (2016).
Recovery Collaborative Group. et al. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 384 , 693–704 (2021).
Welte, T. et al. Efficacy and safety of trimodulin, a novel polyclonal antibody preparation, in patients with severe community-acquired pneumonia: a randomized, placebo-controlled, double-blind, multicenter, phase II trial (CIGMA study). Intensive Care Med. 44 , 438–448 (2018).
Frat, J.-P. et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N. Engl. J. Med. 372 , 2185–2196 (2015).
Kaplan, V. et al. Pneumonia: still the old man’s friend? Arch. Intern. Med. 163 , 317–323 (2003).
Shorr, A. F. et al. Readmission following hospitalization for pneumonia: the impact of pneumonia type and its implication for hospitals. Clin. Infect. Dis. 57 , 362–367 (2013).
Chalmers, J. D. et al. Epidemiology, antibiotic therapy, and clinical outcomes in health care-associated pneumonia: a UK cohort study. Clin. Infect. Dis. 53 , 107–113 (2011).
El Moussaoui, R. et al. Long-term symptom recovery and health-related quality of life in patients with mild-to-moderate-severe community-acquired pneumonia. Chest 130 , 1165–1172 (2006).
Wootton, D. G. et al. A longitudinal modelling study estimates acute symptoms of community acquired pneumonia recover to baseline by 10 days. Eur. Respir. J. 49 , 1602170 (2017).
Marrie, T. J., Lau, C. Y., Wheeler, S. L., Wong, C. J. & Feagan, B. G. Predictors of symptom resolution in patients with community-acquired pneumonia. Clin. Infect. Dis. 31 , 1362–1367 (2000).
Almirall, J. et al. Epidemiology of community-acquired pneumonia in adults: a population-based study. Eur. Respir. J. 15 , 757–763 (2000).
Andrade, L. F. et al. Health related quality of life in patients with community-acquired pneumococcal pneumonia in France. Health Qual. Life Outcomes 16 , 28 (2018).
Wyrwich, K. W., Yu, H., Sato, R. & Powers, J. H. Observational longitudinal study of symptom burden and time for recovery from community-acquired pneumonia reported by older adults surveyed nationwide using the CAP Burden of Illness Questionnaire. Patient Relat. Outcome Meas. 6 , 215–223 (2015).
Carratala, J. et al. Outpatient care compared with hospitalization for community-acquired pneumonia: a randomized trial in low-risk patients. Ann. Intern. Med. 142 , 165–172 (2005).
Mangen, M.-J. J., Huijts, S. M., Bonten, M. J. M. & de Wit, G. A. The impact of community-acquired pneumonia on the health-related quality-of-life in elderly. BMC Infect. Dis. 17 , 208 (2017).
Lloyd, M., Callander, E., Karahalios, A., Desmond, L. & Karunajeewa, H. Patient-reported outcome measures in community-acquired pneumonia: a systematic review of application and content validity. BMJ Open. Respir. Res. 6 , e000398 (2019).
Gao, C. A. et al. Comparing nasopharyngeal and BAL SARS-CoV-2 assays in respiratory failure. Am. J. Respir. Crit. Care Med. 203 , 127–129 (2021).
Peiffer-Smadja, N. et al. Performance and impact of a multiplex PCR in ICU patients with ventilator-associated pneumonia or ventilated hospital-acquired pneumonia. Crit. Care 24 , 366 (2020).
Murphy, C. N. et al. Multicenter evaluation of the biofire filmarray pneumonia/pneumonia plus panel for detection and quantification of agents of lower respiratory tract infection. J. Clin. Microbiol. 58 , e00128-20 (2020).
Pendleton, K. M. et al. Rapid pathogen identification in bacterial pneumonia using real-time metagenomics. Am. J. Respir. Crit. Care Med. 196 , 1610–1612 (2017).
Chiu, C. Y. & Miller, S. A. Clinical metagenomics. Nat. Rev. Genet. 20 , 341–355 (2019).
Hellyer, T. P. et al. Biomarker-guided antibiotic stewardship in suspected ventilator-associated pneumonia (VAPrapid2): a randomised controlled trial and process evaluation. Lancet Respir. Med. 8 , 182–191 (2020).
Blot, S. I. et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am. J. Respir. Crit. Care Med. 186 , 56–64 (2012).
Bassetti, M., Welte, T. & Wunderink, R. G. Treatment of Gram-negative pneumonia in the critical care setting: is the beta-lactam antibiotic backbone broken beyond repair? Crit. Care 20 , 19 (2016).
Kollef, M. H. et al. Ceftolozane-tazobactam versus meropenem for treatment of nosocomial pneumonia (ASPECT-NP): a randomised, controlled, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 19 , 1299–1311 (2019). A randomized clinical trial comparing ceftolozane–tazobactam with meropenem in ventilated HAP and VAP. A post-hoc analysis in ventilated HAP demonstrated superiority of ceftolozane–tazobactam .
Kollef, M. H. et al. A randomized trial of 7-day doripenem versus 10-day imipenem-cilastatin for ventilator-associated pneumonia. Crit. Care 16 , R218 (2012).
File, T. M. et al. FOCUS 1: a randomized, double-blinded, multicentre, phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J. Antimicrob. Chemother. 66 , iii19–iii32 (2011).
Biedenbach, D. J., Kazmierczak, K., Bouchillon, S. K., Sahm, D. F. & Bradford, P. A. In vitro activity of aztreonam-avibactam against a global collection of Gram-negative pathogens from 2012 and 2013. Antimicrob. Agents Chemother. 59 , 4239–4248 (2015).
Awad, S. S. et al. A phase 3 randomized double-blind comparison of ceftobiprole medocaril versus ceftazidime plus linezolid for the treatment of hospital-acquired pneumonia. Clin. Infect. Dis. 59 , 51–61 (2014).
David, S. et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat. Microbiol. 4 , 1919–1929 (2019).
Watkins, R. R. & File, T. M. Lefamulin: a novel semisynthetic pleuromutilin antibiotic for community-acquired bacterial pneumonia. Clin. Infect. Dis. 71 , 2757–2762 (2020).
Spellberg, B., Bartlett, J., Wunderink, R. & Gilbert, D. N. Novel approaches are needed to develop tomorrow’s antibacterial therapies. Am. J. Respir. Crit. Care Med. 191 , 135–140 (2015).
Matteo Bassetti, R. E. et al. Efficacy and safety of cefiderocol for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): results of a phase 3 randomised, open-label, parallel-assigned, pathogen-focused study. Lancet 21 , 226–240 (2021).
Barnes, M. D. et al. Targeting multidrug-resistant Acinetobacter spp.: sulbactam and the diazabicyclooctenone β-lactamase inhibitor ETX2514 as a novel therapeutic agent. mBio 10 , e00159-19 (2019).
Lehman, K. M. & Grabowicz, M. Countering gram-negative antibiotic resistance: recent progress in disrupting the outer membrane with novel therapeutics. Antibiotics (Basel) 8 , 163 (2019).
Wu, J. Y., Srinivas, P. & Pogue, J. M. Cefiderocol: a novel agent for the management of multidrug-resistant gram-negative organisms. Infect. Dis. Ther. 9 , 17–40 (2020).
Wunderink, R. G. et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): a phase 3, randomised, double-blind, non-inferiority study. Lancet Infect. Dis. 21 , 213–225 (2020).
File, T. M. et al. Efficacy and safety of IV-to-oral lefamulin, a pleuromutilin antibiotic, for treatment of community-acquired bacterial pneumonia: the phase 3 LEAP 1 trial. Clin. Infect. Dis. 69 , 1856–1867 (2019).
Alexander, E. et al. Oral lefamulin vs moxifloxacin for early clinical response among adults with community-acquired bacterial pneumonia: the LEAP 2 randomized clinical trial. JAMA 322 , 1661–1671 (2019).
Que, Y.-A. et al. Assessment of panobacumab as adjunctive immunotherapy for the treatment of nosocomial Pseudomonas aeruginosa pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 33 , 1861–1867 (2014).
François, B. et al. Safety and pharmacokinetics of an anti-PcrV PEGylated monoclonal antibody fragment in mechanically ventilated patients colonized with Pseudomonas aeruginosa: a randomized, double-blind, placebo-controlled trial. Crit. Care Med. 40 , 2320–2326 (2012).
François, B. et al. Safety and tolerability of a single administration of AR-301, a human monoclonal antibody, in ICU patients with severe pneumonia caused by Staphylococcus aureus: first-in-human trial. Intensive Care Med. 44 , 1787–1796 (2018).
Maddocks, S. et al. Bacteriophage therapy of ventilator-associated pneumonia and empyema caused by Pseudomonas aeruginosa. Am. J. Respir. Crit. Care Med. 200 , 1179–1181 (2019).
Wunderink, R. G. Turning the phage on treatment of antimicrobial-resistant pneumonia. Am. J. Respir. Crit. Care Med. 200 , 1081–1082 (2019).
Sicot, N. et al. Methicillin resistance is not a predictor of severity in community-acquired Staphylococcus aureus necrotizing pneumonia – results of a prospective observational study. Clin. Microbiol. Infect. 19 , E142–E148 (2013).
Download references
Acknowledgements
A.T. is the recipient of ICREA award from Generalitat de Catalunya. C.C. is the recipient of the SEPAR fellowship 2018, a grant 2019 from the Fondo de Investigación Sanitaria (PI19/00207), and the SEPAR fellowship “Programa Mentor”. We thank J.J.T.H. Roelofs (Department of Pathology, Amsterdam UMC, Amsterdam, Netherlands) for his invaluable assistance with the section on lung pathology and in providing representative histopathology slides.
Author information
Authors and affiliations.
Department of Pneumology, Hospital Clinic of Barcelona, Barcelona, Spain
Antoni Torres & Catia Cilloniz
August Pi i Sunyer Biomedical Research Institute (IDIBAPS), Barcelona, Spain
University of Barcelona, Barcelona, Spain
Biomedical Research Networking Centers in Respiratory Diseases (CIBERES and CIBERESUCICOVID study), Barcelona, Spain
Division of Pulmonary and Critical Care, New York Presbyterian/Weill Cornell Medical Center, New York City, NY, USA
Michael S. Niederman
Department of Pneumology, Hospital Universitario y Politécnico La Fe, Valencia, Spain
Rosario Menéndez
Scottish Centre for Respiratory Research, University of Dundee, Ninewells Hospital and Medical School, Dundee, UK
James D. Chalmers
Division of Pulmonary and Critical Care, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
Richard G. Wunderink
Center of Experimental and Molecular Medicine, Division of Infectious Diseases, Amsterdam University Medical Centers, Location Academic Medical Center, University of Amsterdam, Amsterdam, Netherlands
Tom van der Poll
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (C.C. and A.T.); Epidemiology (C.C. and R.M.); Mechanisms/pathophysiology (T.v.d.P.); Diagnosis, screening and prevention (C.C. and A.T.); Management (M.S.N.); Quality of life (J.D.C.); Outlook (R.G.W); Overview of Primer (A.T. and C.C.).
Corresponding authors
Correspondence to Antoni Torres or Catia Cilloniz .
Ethics declarations
Competing interests.
A.T. has been a paid consultant to Pfizer, Jansen, and MSD, and a speaker for Pfizer and MSD. M.S.N. has received research grants from Shionogi, Bayer and Merck. He has been a paid consultant to Bayer, Merck, Paratek, Abbvie, Nabriva, and Thermo-Fisher. J.D.C. has received research funding from Astrazeneca, Boehringer-Ingelheim, Gilead Sciences, Glaxosmithkline, Insmed and Novartis; he has received consultancy fees from Chiesi, Grifols and Zambon. R.G.W. is a consultant to Merck, Shionogi, Polyphor, Microbiotix, bioMerieux, Curetis, KBP Biosciences, Idorsia and Accelerate. All other authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Disease Primers thanks Y. Arabi, C. Ginocchio, K. Klugman, M. Metersky, N. Suttorp and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Torres, A., Cilloniz, C., Niederman, M.S. et al. Pneumonia. Nat Rev Dis Primers 7 , 25 (2021). https://doi.org/10.1038/s41572-021-00259-0
Download citation
Accepted : 26 February 2021
Published : 08 April 2021
DOI : https://doi.org/10.1038/s41572-021-00259-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Risk factors and predicting nomogram for the clinical deterioration of non-severe community-acquired pneumonia.
- Cheng-bin Xu
- Shan-shan Su
BMC Pulmonary Medicine (2024)
Validating the accuracy of deep learning for the diagnosis of pneumonia on chest x-ray against a robust multimodal reference diagnosis: a post hoc analysis of two prospective studies
- Jeremy Hofmeister
- Nicolas Garin
- Virginie Prendki
European Radiology Experimental (2024)
Hospitalization, case fatality, comorbidities, and isolated pathogens of adult inpatients with pneumonia from 2013 to 2022: a real-world study in Guangzhou, China
- Zhufeng Wang
- Jinping Zheng
BMC Infectious Diseases (2024)
A methodical exploration of imaging modalities from dataset to detection through machine learning paradigms in prominent lung disease diagnosis: a review
- Sunil Kumar
- Harish Kumar
- Manoj Diwakar
BMC Medical Imaging (2024)
Prediction of new-onset atrial fibrillation with the C2HEST score in patients admitted with community-acquired pneumonia
- Daniele Pastori
- Danilo Menichelli
- Roberto Cangemi
Infection (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
CALCULATORS
Related pathways, related topics.
Contributor Disclosures
Please read the Disclaimer at the end of this page.
INTRODUCTION — Community-acquired pneumonia (CAP) is a leading cause of morbidity and mortality worldwide. The clinical presentation of CAP varies, ranging from mild pneumonia characterized by fever and productive cough to severe pneumonia characterized by respiratory distress and sepsis. Because of the wide spectrum of associated clinical features, CAP is a part of the differential diagnosis of nearly all respiratory illnesses.
This topic provides a broad overview of the epidemiology, microbiology, pathogenesis, clinical features, diagnosis, and management of CAP in immunocompetent adults. Detailed discussions of each of these issues are presented separately; links to these discussions are provided within the text below.
DEFINITIONS — Pneumonia is frequently categorized based on site of acquisition ( table 1 ).
● Community-acquired pneumonia (CAP) refers to an acute infection of the pulmonary parenchyma acquired outside of the hospital.
● Nosocomial pneumonia refers to an acute infection of the pulmonary parenchyma acquired in hospital settings and encompasses both hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP).
• HAP refers to pneumonia acquired ≥48 hours after hospital admission.
• VAP refers to pneumonia acquired ≥48 hours after endotracheal intubation.
Health care-associated pneumonia (HCAP; no longer used) referred to pneumonia acquired in health care facilities (eg, nursing homes, hemodialysis centers) or after recent hospitalization. The term HCAP was used to identify patients at risk for infection with multidrug-resistant pathogens. However, this categorization may have been overly sensitive, leading to increased, inappropriately broad antibiotic use and was thus retired. In general, patients previously classified as having HCAP should be treated similarly to those with CAP. (See "Epidemiology, pathogenesis, microbiology, and diagnosis of hospital-acquired and ventilator-associated pneumonia in adults" .)
EPIDEMIOLOGY
Incidence — CAP is one of the most common and morbid conditions encountered in clinical practice [ 1-3 ]. In the United States, CAP accounts for over 4.5 million outpatient and emergency room visits annually, corresponding to approximately 0.4 percent of all encounters [ 4 ]. CAP is the second most common cause of hospitalization and the most common infectious cause of death [ 5,6 ]. Approximately 650 adults are hospitalized with CAP every year per 100,000 population in the United States, corresponding to 1.5 million unique CAP hospitalizations each year [ 7 ]. Nearly 9 percent of patients hospitalized with CAP will be rehospitalized due to a new episode of CAP during the same year.
Risk factors
● Older age – The risk of CAP rises with age [ 7,8 ]. The annual incidence of hospitalization for CAP among adults ≥65 years old is approximately 2000 per 100,000 in the United States [ 7,9 ]. This figure is approximately three times higher than the general population and indicates that 2 percent of the older adult population will be hospitalized for CAP annually ( figure 1 ).
● Chronic comorbidities – The comorbidity that places patients at highest risk for CAP hospitalization is chronic obstructive pulmonary disease (COPD), with an annual incidence of 5832 per 100,000 in the United States [ 7 ]. Other comorbidities associated with an increased incidence of CAP include other forms of chronic lung disease (eg, bronchiectasis, asthma), chronic heart disease (particularly congestive heart failure), stroke, diabetes mellitus, malnutrition, and immunocompromising conditions ( figure 2 ) [ 7,10,11 ].
● Viral respiratory tract infection – Viral respiratory tract infections can lead to primary viral pneumonias and also predispose to secondary bacterial pneumonia. This is most pronounced for influenza virus infection. (See "Seasonal influenza in adults: Clinical manifestations and diagnosis", section on 'Pneumonia' .)
● Impaired airway protection – Conditions that increase risk of macroaspiration of stomach contents and/or microaspiration of upper airway secretions predispose to CAP, such as alteration in consciousness (eg, due to stroke, seizure, anesthesia, drug or alcohol use) or dysphagia due to esophageal lesions or dysmotility.
● Smoking and alcohol overuse – Smoking, alcohol overuse (eg, >80 g/day), and opioid use are key modifiable behavioral risk factors for CAP [ 7,10,12,13 ].
● Other lifestyle factors – Other factors that have been associated with an increased risk of CAP include crowded living conditions (eg, prisons, homeless shelters), residence in low-income settings, and exposure to environmental toxins (eg, solvents, paints, or gasoline) [ 7,10,11,14 ].
Combinations of risk factors, such as smoking, COPD, and congestive heart failure, are additive in terms of risk [ 15 ]. These risk factors and other predisposing conditions for the development of CAP are discussed separately. (See "Epidemiology, pathogenesis, and microbiology of community-acquired pneumonia in adults", section on 'Predisposing host conditions' .)
MICROBIOLOGY
Common causes — Streptococcus pneumoniae (pneumococcus) and respiratory viruses are the most frequently detected pathogens in patients with CAP [ 8,16 ]. However, in a large proportion of cases (up to 62 percent in some studies performed in hospital settings), no pathogen is detected despite extensive microbiologic evaluation [ 8,17,18 ].
The most commonly identified causes of CAP can be grouped into three categories:
● Typical bacteria
• S. pneumoniae (most common bacterial cause)
• Haemophilus influenzae
• Moraxella catarrhalis
• Staphylococcus aureus
• Group A streptococci
• Aerobic gram-negative bacteria (eg, Enterobacteriaceae such as Klebsiella spp or Escherichia coli )
• Microaerophilic bacteria and anaerobes (associated with aspiration)
● Atypical bacteria ("atypical" refers to the intrinsic resistance of these organisms to beta-lactams and their inability to be visualized on Gram stain or cultured using traditional techniques)
• Legionella spp
• Mycoplasma pneumoniae
• Chlamydia pneumoniae
• Chlamydia psittaci
• Coxiella burnetii
● Respiratory viruses
• Influenza A and B viruses
• Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
• Other coronaviruses (eg, CoV-229E, CoV-NL63, CoV-OC43, CoV-HKU1)
• Rhinoviruses
• Parainfluenza viruses
• Adenoviruses
• Respiratory syncytial virus
• Human metapneumovirus
• Human bocaviruses
The relative prevalence of these pathogens varies with geography, pneumococcal vaccination rates, host risk factors (eg, smoking), season, and pneumonia severity ( table 2 ).
Certain epidemiologic exposures also raise the likelihood of infection with a particular pathogen ( table 3 ). As examples, exposure to contaminated water is a risk factor for Legionella infection, exposure to birds raises the possibility of C. psittaci infection, travel or residence in the southwestern United States should raise suspicion for coccidioidomycosis, and poor dental hygiene may predispose patients with pneumonia caused by oral flora or anaerobes. In immunocompromised patients, the spectrum of possible pathogens also broadens to include fungi and parasites as well as less common bacterial and viral pathogens. (See "Epidemiology of pulmonary infections in immunocompromised patients" and "Approach to the immunocompromised patient with fever and pulmonary infiltrates" .)
While the list above details some of most common causes of CAP, >100 bacterial, viral, fungal, and parasitic causes have been reported. (See "Epidemiology, pathogenesis, and microbiology of community-acquired pneumonia in adults", section on 'Microbiology' .)
Important trends — Both the distribution of pathogens that cause CAP and our knowledge of these pathogens are evolving. Key observations that have changed our understanding of CAP and influenced our approach to management include:
● Decline in S. pneumoniae incidence – Although S. pneumoniae (pneumococcus) is the most commonly detected bacterial cause of CAP in most studies, the overall incidence of pneumococcal pneumonia is decreasing. This is in part due to widespread use of pneumococcal vaccination, which results in both a decline in the individual rates of pneumococcal pneumonia and herd immunity in the population. (See "Pneumococcal pneumonia in patients requiring hospitalization", section on 'Prevalence' .)
Because pneumococcal vaccination rates vary regionally, the prevalence of S. pneumoniae infection also varies. As an example, S. pneumoniae is estimated to cause approximately 30 percent of cases of CAP in Europe but only 10 to 15 percent in the United States, where the population pneumococcal vaccination rate is higher [ 8 ].
● The coronavirus disease 2019 (COVID-19) pandemic – SARS-CoV-2 is an important cause of CAP and is discussed in detail elsewhere. (See "COVID-19: Epidemiology, virology, and prevention" .)
● Increased recognition of other respiratory viruses – Respiratory viruses have been detected in approximately one-third of cases of CAP in adults when using molecular methods [ 8 ]. The extent to which respiratory viruses serve as single pathogens, cofactors in the development of bacterial CAP, or triggers for dysregulated host immune response has not been established.
● Low overall rate of pathogen detection – Despite extensive evaluation using molecular diagnostics and other microbiologic testing methods, a causal pathogen can be identified in only half of cases of CAP. This finding highlights that our understanding of CAP pathogenesis is incomplete. As molecular diagnostics become more advanced and use broadens, our knowledge is expected to grow.
● Discovery of the lung microbiome – Historically, the lung has been considered sterile. However, culture-independent techniques (ie, high throughput 16S ribosomal ribonucleic acid [rRNA] gene sequencing) have identified complex and diverse communities of microbes that reside within the alveoli [ 19-21 ]. This finding suggests that resident alveolar microbes play a role in the development of pneumonia, either by modulating the host immune response to infecting pathogens or through direct overgrowth of specific pathogens within the alveolar microbiome. (See 'Pathogenesis' below.)
Antimicrobial resistance — Knowledge of antimicrobial resistance patterns and risk factors for infection with antimicrobial-resistant pathogens help inform the selection of antibiotics for empiric CAP treatment ( table 4 ).
● S. pneumoniae may be resistant to one or more antibiotics commonly used for the empiric treatment of CAP.
• Macrolide resistance rates vary regionally but are generally high (>25 percent) in the United States, Asia, and southern Europe. Resistance rates tend to be lower in northern Europe. (See "Resistance of Streptococcus pneumoniae to the macrolides, azalides, and lincosamides" .)
• Estimates of doxycycline resistance are less certain and vary substantially worldwide. In the United States, rates tend to be less than 20 percent but may be rising. (See "Resistance of Streptococcus pneumoniae to the fluoroquinolones, doxycycline, and trimethoprim-sulfamethoxazole" .)
• Beta-lactam resistance rates also vary regionally but to a lesser extent than macrolide and doxycycline resistance. In the United States, <20 percent of isolates are resistant to penicillin and <1 percent to cephalosporins. (See "Resistance of Streptococcus pneumoniae to beta-lactam antibiotics" .)
• Fluoroquinolone resistance tends to be <2 percent in the United States but varies regionally and with specific risk factors such as recent antibiotic use or hospitalization. (See "Resistance of Streptococcus pneumoniae to the fluoroquinolones, doxycycline, and trimethoprim-sulfamethoxazole" .)
Because resistance rates vary even at local levels, clinicians should refer to local antibiograms to guide antibiotic selection when available. General epidemiologic data can be obtained through sources such as the OneHealthTrust (formerly the Center for Disease Dynamics, Economics & Policy [CDDEP]).
● Methicillin-resistant S. aureus (MRSA) is an uncommon cause of CAP. Risk factors for MRSA have two patterns: health care associated and community acquired. The strongest risk factors for MRSA pneumonia include known MRSA colonization or prior MRSA infection, particularly involving the respiratory tract. Gram-positive cocci on sputum Gram stain are also predictive of MRSA infection. Other factors that should raise suspicion for MRSA infection include recent antibiotic use (particularly receipt of intravenous antibiotics within the past three months), recent influenza-like illness, the presence of empyema, necrotizing/cavitary pneumonia, and immunosuppression ( table 4 ).
In contrast with health care-associated MRSA, community-acquired MRSA (CA-MRSA) infections tend to occur in younger healthy persons [ 22 ]. Risk factors for CA-MRSA infection include a history of MRSA skin lesions, participation in contact sports, injection drug use, crowded living conditions, and men who have sex with men. (See "Methicillin-resistant Staphylococcus aureus (MRSA) in adults: Epidemiology" .)
CAP caused by CA-MRSA can be severe and is associated with necrotizing and/or cavitary pneumonia, empyema, gross hemoptysis, septic shock, and respiratory failure. These features may be attributable to infection with toxin-producing CA-MRSA strains. In the United States, these strains tend to be methicillin resistant and belong to the USA300 clone. (See "Methicillin-resistant Staphylococcus aureus (MRSA): Microbiology and laboratory detection" .)
● Pseudomonas is also an uncommon cause of CAP and tends to occur more frequently in patients with known colonization or prior infection with Pseudomonas spp, recent hospitalization or antibiotic use, underlying structural lung disease (eg, cystic fibrosis or advanced chronic obstructive pulmonary disease [bronchiectasis]), and immunosuppression. Antibiotic resistance is common among pseudomonal strains, and empiric therapy with more than one agent that targets Pseudomonas is warranted for at-risk patients with moderate to severe CAP ( table 4 ). (See "Pseudomonas aeruginosa pneumonia" and 'Inpatient antibiotic therapy' below.)
PATHOGENESIS — Community-acquired pneumonia (CAP) pathogenesis Figure 3 Traditionally, CAP has been viewed as an infection of the lung parenchyma, primarily caused by bacterial or viral respiratory pathogens. In this model, respiratory pathogens are transmitted from person to person via droplets or, less commonly, via aerosol inhalation (eg, as with Legionella or Coxiella species). Following inhalation, the pathogen colonizes the nasopharynx and then reaches the lung alveoli via microaspiration. When the inoculum size is sufficient and/or host immune defenses are impaired, infection results. Replication of the pathogen, the production of virulence factors, and the host immune response lead to inflammation and damage of the lung parenchyma, resulting in pneumonia ( figure 3 ).
With the identification of the lung microbiome, that model has changed [ 19-21 ]. While the pathogenesis of pneumonia may still involve the introduction of respiratory pathogens into the alveoli, the infecting pathogen likely has to compete with resident microbes to replicate. In addition, resident microbes may also influence or modulate the host immune response to the infecting pathogen. If this is correct, an altered alveolar microbiome (alveolar dysbiosis) may be a predisposing factor for the development of pneumonia.
In some cases, CAP might also arise from uncontrolled replication of microbes that normally reside in the alveoli. The alveolar microbiome is similar to oral flora and is primarily comprised of anaerobic bacteria (eg, Prevotella and Veillonella ) and microaerophilic streptococci [ 19-21 ]. Hypothetically, exogenous insults such as a viral infection or smoke exposure might alter the composition of the alveolar microbiome and trigger overgrowth of certain microbes. Because organisms that compose the alveolar microbiome typically cannot be cultivated using standard cultures, this hypothesis might explain the low rate of pathogen detection among patients with CAP.
In any scenario, the host immune response to microbial replication within the alveoli plays an important role in determining disease severity. For some patients, a local inflammatory response within the lung predominates and may be sufficient for controlling infection. In others, a systemic response is necessary to control infection and to prevent spread or complications, such as bacteremia. In a minority, the systemic response can become dysregulated, leading to tissue injury, sepsis, acute respiratory distress syndrome, and/or multiorgan dysfunction.
The pathogenesis of CAP is discussed in greater detail separately. (See "Epidemiology, pathogenesis, and microbiology of community-acquired pneumonia in adults" .)
CLINICAL PRESENTATION — The clinical presentation of CAP varies widely, ranging from mild pneumonia characterized by fever, cough, and shortness of breath to severe pneumonia characterized by sepsis and respiratory distress. Symptom severity is directly related to the intensity of the local and systemic immune response in each patient.
● Pulmonary signs and symptoms – Cough (with or without sputum production), dyspnea, and pleuritic chest pain are among the most common symptoms associated with CAP. Signs of pneumonia on physical examination include tachypnea, increased work of breathing, and adventitious breath sounds, including rales/crackles and rhonchi. Tactile fremitus, egophony, and dullness to percussion also suggest pneumonia. These signs and symptoms result from the accumulation of white blood cells (WBCs), fluid, and proteins in the alveolar space. Hypoxemia can result from the subsequent impairment of alveolar gas exchange. On chest radiograph, accumulation of WBCs and fluid within the alveoli appears as pulmonary opacities ( image 1A-B ).
● Systemic signs and symptoms – The great majority of patients with CAP present with fever. Other systemic symptoms such as chills, fatigue, malaise, chest pain (which may be pleuritic), and anorexia are also common. Tachycardia, leukocytosis with a leftward shift, or leukopenia are also findings that are mediated by the systemic inflammatory response. Inflammatory markers, such as the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and procalcitonin may rise, though the latter is largely specific to bacterial infections. CAP is also the leading cause of sepsis; thus, the initial presentation may be characterized by hypotension, altered mental status, and other signs of organ dysfunction such as renal dysfunction, liver dysfunction, and/or thrombocytopenia [ 23 ].
Although certain signs and symptom such as fever, cough, tachycardia, and rales are common among patients with CAP, these features are ultimately nonspecific and are shared among many respiratory disorders (see 'Differential diagnosis' below). No individual symptom or constellation of symptoms is adequate for diagnosis without chest imaging. For example, the positive predictive value of the combination of fever, tachycardia, rales, and hypoxia (oxygen saturation <95 percent) among patients with respiratory complaints presenting to primary care was <60 percent when chest radiograph was used as a reference standard [ 24 ].
Signs and symptoms of pneumonia can also be subtle in patients with advanced age and/or impaired immune systems, and a higher degree of suspicion may be needed to make the diagnosis. As examples, older patients may present with mental status changes but lack fever or leukocytosis [ 25 ]. In immunocompromised patients, pulmonary infiltrates may not be detectable on chest radiographs but can be visualized with computed tomography.
The clinical and diagnostic features of CAP and sepsis are discussed in detail separately. (See "Clinical evaluation and diagnostic testing for community-acquired pneumonia in adults" and "Sepsis syndromes in adults: Epidemiology, definitions, clinical presentation, diagnosis, and prognosis", section on 'Clinical presentation' .)
Making the diagnosis — The diagnosis of CAP generally requires the demonstration of an infiltrate on chest imaging in a patient with a clinically compatible syndrome (eg, fever, dyspnea, cough, and sputum production) [ 26 ].
● For most patients with suspected CAP, we obtain posteroanterior and lateral chest radiographs. Radiographic findings consistent with the diagnosis of CAP include lobar consolidations ( image 1C ), interstitial infiltrates ( image 1D-E ), and/or cavitations ( image 2 ). Although certain radiographic features suggest certain causes of pneumonia (eg, lobar consolidations suggest infection with typical bacterial pathogens), radiographic appearance alone cannot reliably differentiate among etiologies.
● For selected patients in whom CAP is suspected based on clinical features despite a negative chest radiograph, we obtain computed tomography (CT) of the chest. These patients include immunocompromised patients, who may not mount strong inflammatory responses and thus have negative chest radiographs, as well as patients with known exposures to epidemic pathogens that cause pneumonia (eg, Legionella ). Because there is no direct evidence to suggest that CT scanning improves outcomes for most patients and cost is high, we do not routinely obtain CT scans when evaluating patients for CAP.
The combination of a compatible clinical syndrome and imaging findings consistent with pneumonia are sufficient to establish an initial clinical diagnosis of CAP. However, this combination of findings is nonspecific and is shared among many cardiopulmonary disorders. Thus, remaining attentive to the possibility of an alternate diagnosis as a patient's course evolves is important to care. (See 'Differential diagnosis' below.)
Defining severity and site of care — For patients with a working diagnosis of CAP, the next steps in management are defining the severity of illness and determining the most appropriate site of care. Determining the severity of illness is based on clinical judgement and can be supplemented by use of severity scores ( algorithm 1 ).
The most commonly used severity scores are the Pneumonia Severity Index (PSI) and CURB-65 [ 27,28 ]. We generally prefer the PSI, also known as the PORT score ( calculator 1 ), because it is the most accurate and its safety and effectiveness in guiding clinical decision-making have been validated [ 29-32 ]. However, the CURB-65 score is a reasonable alternative and is preferred by many clinicians because it is easier to use ( calculator 2 ).
The three levels of severity (mild, moderate, and severe) generally correspond to three levels of care:
● Ambulatory care – Most patients who are otherwise healthy with normal vital signs (apart from fever) and no concern for complication are considered to have mild pneumonia and can be managed in the ambulatory setting. These patients typically have PSI scores of I to II and CURB-65 scores of 0 (or a CURB-65 score of 1 if age >65 years).
● Hospital admission – Patients who have peripheral oxygen saturations <92 percent on room air (and a significant change from baseline) should be hospitalized. In addition, patients with PSI scores of ≥III and CURB-65 scores ≥1 (or CURB-65 score ≥2 if age >65 years) should also generally be hospitalized.
Because patients with early signs of sepsis, rapidly progressive illness, or suspected infections with aggressive pathogens are not well represented in severity scoring systems, these patients may also warrant hospitalization in order to closely monitor the response to treatment.
Practical concerns that may warrant hospital admission include an inability to take oral medications, cognitive or functional impairment, or other social issues that could impair medication adherence or ability to return to care for clinical worsening (eg, substance abuse, homelessness, or residence far from a medical facility).
● Intensive care unit (ICU) admission – Patients who meet either of the following major criteria have severe CAP and should be admitted to the ICU [ 26 ]:
• Respiratory failure requiring mechanical ventilation
• Sepsis requiring vasopressor support
Recognizing these two criteria for ICU admission is relatively straightforward. The challenge is to identify patients with severe CAP who have progressed to sepsis before the development of organ failure. For these patients, early ICU admission and administration of appropriate antibiotics improve outcomes. To help identify patients with severe CAP before development of organ failure, the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) suggest minor criteria [ 1,26 ].
The presence of three of these criteria warrants ICU admission:
• Altered mental status
• Hypotension requiring fluid support
• Temperature <36°C (96.8°F)
• Respiratory rate ≥30 breaths/minute
• Arterial oxygen tension to fraction of inspired oxygen (PaO 2 /FiO 2 ) ratio ≤250
• Blood urea nitrogen (BUN) ≥20 mg/dL (7 mmol/L)
• Leukocyte count <4000 cells/microL
• Platelet count <100,000/microL
• Multilobar infiltrates
Although several other scores for identifying patients with severe CAP and/or ICU admission have been developed, we generally use the ATS/IDSA major and minor criteria because they are well validated [ 33-35 ].
Detailed discussion on assessing severity and determining the site of care in patients with CAP is provided separately. (See "Community-acquired pneumonia in adults: Assessing severity and determining the appropriate site of care" .) (Related Pathway(s): Community-acquired pneumonia: Determining the appropriate site of care for adults .)
Triage of patients with known or suspected COVID-19 is also discussed elsewhere. (See "COVID-19: Evaluation of adults with acute illness in the outpatient setting", section on 'Disposition' .)
Microbiologic testing — The benefit of obtaining a microbiologic diagnosis should be balanced against the time and cost associated with an extensive evaluation in each patient.
Generally, we take a tiered approach to microbiologic evaluation based on CAP severity and the site of care ( table 5 ):
● Outpatients − For most patients with mild CAP being treated in the ambulatory setting, microbiologic testing is not needed (apart from testing for SARS-CoV-2 during the pandemic). Empiric antibiotic therapy is generally successful, and knowledge of the infecting pathogen does not usually improve outcomes.
● Patients with moderate CAP admitted to the general medicine ward − For most patients with moderate CAP admitted to the general medical ward, we obtain the following:
• Blood cultures
• Sputum Gram stain and culture
• Urinary antigen testing for S. pneumoniae
• Testing for Legionella spp (polymerase chain reaction [PCR] when available, urinary antigen test as an alternate)
• SARS-CoV-2 testing
During the pandemic, we test all patients for COVID-19. During respiratory virus season (eg, late fall to early spring in the northern hemisphere), we also test for other respiratory viruses (eg, influenza, adenovirus, parainfluenza, respiratory syncytial virus, and human metapneumovirus). When testing for influenza, PCR is preferred over rapid antigen testing. (See "Seasonal influenza in adults: Clinical manifestations and diagnosis" .)
For these patients, making a microbiologic diagnosis allows for directed therapy, which helps limit antibiotic overuse, prevent antimicrobial resistance, and reduce unnecessary complications, such as Clostridioides difficile infections.
● Patients with severe CAP (including ICU admission) − For most hospitalized patients with severe CAP, including those admitted to the ICU, we send blood cultures, sputum cultures, urinary streptococcal antigen, and Legionella testing. In addition, we obtain bronchoscopic specimens for microbiologic testing when feasible, weighing the benefits of obtaining a microbiologic diagnosis against the risks of the procedure (eg, need for intubation, bleeding, bronchospasm, pneumothorax) on a case-by-case basis. When pursuing bronchoscopy, we usually send specimens for aerobic culture, Legionella culture, fungal stain and culture, and testing for respiratory viruses.
The type of viral diagnostic tests used (eg, PCR, serology, culture) vary among institutions. In some cases, multiplex PCR panels that test for a wide array of viral and bacterial pathogens are used. While we generally favor using these tests for patients with severe pneumonia, we interpret results with caution as most multiplex assays have not been approved for use on lower respiratory tract specimens. In particular, the detection of single viral pathogen does not confirm the diagnosis of viral pneumonia because viruses can serve as cofactors in the pathogenesis of bacterial CAP or can be harbored asymptomatically.
In all cases, we modify this approach based on epidemiologic exposures, patient risk factors, and clinical features regardless of CAP severity or treatment setting ( table 3 ). As examples:
● For patients with known or probable exposures to epidemic pathogens such as Legionella or epidemic coronaviruses, we broaden our evaluation to include tests for these pathogens. (See "Clinical evaluation and diagnostic testing for community-acquired pneumonia in adults", section on 'Important pathogens' .)
● For patients with cavitary pneumonia, we may include testing for tuberculosis, fungal pathogens, and Nocardia .
● For immunocompromised patients, we broaden our differential to include opportunistic pathogens such as Pneumocystis jirovecii , fungal pathogens, parasites, and less common viral pathogens such as cytomegalovirus. The approach to diagnostic testing varies based on the type and degree of immunosuppression and other patient-specific factors. (See "Approach to the immunocompromised patient with fever and pulmonary infiltrates" and "Epidemiology of pulmonary infections in immunocompromised patients" .)
When defining the scope of our microbiologic evaluation, we also take the certainty of the diagnosis of CAP into consideration. Because a substantial portion of patients hospitalized with an initial clinical diagnosis of CAP are ultimately found to have alternate diagnoses [ 17 ], pursuing a comprehensive microbiologic evaluation can help reach the final diagnosis (eg, blood cultures obtained as part of the evaluation for CAP may help lead to a final diagnosis of endocarditis).
Detailed discussion on the microbiologic evaluation of CAP is provided separately. (See "Clinical evaluation and diagnostic testing for community-acquired pneumonia in adults" and "Sputum cultures for the evaluation of bacterial pneumonia" .)
The diagnosis of COVID-19 during the pandemic is also discussed in detail elsewhere. (See "COVID-19: Diagnosis" .)
DIFFERENTIAL DIAGNOSIS — CAP is a common working diagnosis and is frequently on the differential diagnosis of patients presenting with a pulmonary infiltrate and cough, patients with respiratory tract infections, and patients with sepsis. (See "Clinical evaluation and diagnostic testing for community-acquired pneumonia in adults", section on 'Differential diagnosis' .)
Noninfectious illnesses that mimic CAP or co-occur with CAP and present with pulmonary infiltrate and cough include:
• Congestive heart failure with pulmonary edema
• Pulmonary embolism
• Pulmonary hemorrhage
• Atelectasis
• Aspiration or chemical pneumonitis
• Drug reactions
• Lung cancer
• Collagen vascular diseases
• Vasculitis
• Acute exacerbation of bronchiectasis
• Interstitial lung diseases (eg, sarcoidosis, asbestosis, hypersensitivity pneumonitis, cryptogenic organizing pneumonia)
For patients with an initial clinical diagnosis of CAP who have rapidly resolving pulmonary infiltrates, alternate diagnoses should be investigated. Pulmonary infiltrates in CAP are primarily caused by the accumulation of white blood cells (WBCs) in the alveolar space and typically take weeks to resolve. A pulmonary infiltrate that resolves in one or two days may be caused by accumulation of fluid in the alveoli (ie, pulmonary edema) or a collapse of the alveoli (ie, atelectasis) but not due to accumulation of WBCs.
Respiratory illnesses that mimic CAP or co-occur with CAP include:
• Acute exacerbations of chronic obstructive pulmonary disease
• Influenza and other respiratory viral infections
• Acute bronchitis ( figure 4 )
• Asthma exacerbations
Febrile illness and/or sepsis can also be the presenting syndrome in patients with CAP; other common causes of these syndromes include urinary tract infections, intraabdominal infections, and endocarditis.
TREATMENT — For most patients with CAP and excluding COVID-19, the etiology is not known at the time of diagnosis, and antibiotic treatment is empiric, targeting the most likely pathogens. The pathogens most likely to cause CAP vary with severity of illness, local epidemiology, and patient risk factors for infection with drug-resistant organisms.
As an example, for most patients with mild CAP who are otherwise healthy and treated in the ambulatory setting, the range of potential pathogens is limited. By contrast, for patients with CAP severe enough to require hospitalization, potential pathogens are more diverse, and the initial treatment regimens are often broader. (Related Pathway(s): Community-acquired pneumonia: Empiric antibiotic selection for adults in the outpatient setting and Community-acquired pneumonia: Empiric antibiotic selection for adults admitted to a general medical ward and Community-acquired pneumonia: Empiric antibiotic selection for adults admitted to the intensive care unit .)
The management of COVID-19 is discussed in detail elsewhere. (See "COVID-19: Management in hospitalized adults" and "COVID-19: Management of adults with acute illness in the outpatient setting" .)
Outpatient antibiotic therapy — For all patients with CAP, empiric regimens are designed to target S. pneumoniae (the most common and virulent bacterial CAP pathogen) and atypical pathogens. Coverage is expanded for outpatients with comorbidities, smoking, and recent antibiotic use to include or better treat beta-lactamase-producing H. influenzae , M. catarrhalis , and methicillin-susceptible S. aureus . For those with structural lung disease, we further expand coverage to include Enterobacteriaceae, such as E. coli and Klebsiella spp ( algorithm 2 ).
Selection of the initial regimen depends on the adverse effect profiles of available agents, potential drug interactions, patient allergies, and other patient-specific factors.
● For most patients aged <65 years who are otherwise healthy and have not recently used antibiotics, we typically use oral amoxicillin (1 g three times daily) plus a macrolide (eg, azithromycin or clarithromycin ) or doxycycline . Generally, we prefer to use a macrolide over doxycycline.
This approach differs from the American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA), which recommend monotherapy with amoxicillin as first line and monotherapy with either doxycycline or a macrolide (if local resistance rates are <25 percent [eg, not in the United States]) as alternatives for this population [ 26 ]. The rationale for each approach is discussed separately. (See "Treatment of community-acquired pneumonia in adults in the outpatient setting", section on 'Empiric antibiotic treatment' .)
● For patients who have major comorbidities (eg, chronic heart, lung, kidney, or liver disease, diabetes mellitus, alcohol dependence, or immunosuppression), who are smokers, and/or who have used antibiotics within the past three months, we suggest oral amoxicillin-clavulanate (875 mg twice daily or extended release 2 g twice daily) plus either a macrolide (preferred) or doxycycline .
Alternatives to amoxicillin-based regimens include combination therapy with a cephalosporin plus a macrolide or doxycycline or monotherapy with lefamulin .
● For patients who can use cephalosporins, we use a third-generation cephalosporin (eg, cefpodoxime ) plus either a macrolide or doxycycline .
● For patients who cannot use any beta-lactam, we select a respiratory fluoroquinolone (eg, levofloxacin , moxifloxacin , gemifloxacin ) or lefamulin . For those with structural lung disease, we prefer a respiratory fluoroquinolone because its spectrum of activity includes Enterobacteriaceae.
In the absence of hepatic impairment or drug interactions, lefamulin is a potential alternative to fluoroquinolones for most others. However, clinical experience with this agent is limited. Use should be avoided in patients with moderate to severe hepatic dysfunction, known long QT syndrome, or in those taking QT-prolonging agents, pregnant and breastfeeding women, and women with reproductive potential not using contraception. There are drug interactions with CYP3A4 and P-gp inducers and substrates; in addition, lefamulin tablets are contraindicated with QT-prolonging CYP3A4 substrates. Refer to the drug interactions program included within UpToDate.
Omadacycline is another newer agent that is active against most CAP pathogens, including Enterobacteriaceae. It is a potential alternative for patients who cannot tolerate beta-lactams (or other agents) and want to avoid fluoroquinolones. (See "Treatment of community-acquired pneumonia in adults who require hospitalization", section on 'New antimicrobial agents' .)
Modifications to these regimens may be needed for antibiotic allergy, drug interactions, specific exposures, and other patient-specific factors. In particular, during influenza season, patients at high risk for poor outcomes from influenza may warrant antiviral therapy ( table 6 ).
We treat most patients for five days. However, we generally ensure that all patients are improving on therapy and are afebrile for at least 48 hours before stopping antibiotics. In general, extending the treatment course beyond seven days does not add benefit. Studies supporting this approach are discussed separately. (See "Treatment of community-acquired pneumonia in adults in the outpatient setting", section on 'Duration of therapy' .)
Detailed discussion on the treatment of CAP in the outpatient setting, including antibiotic efficacy data, is provided separately. (See "Treatment of community-acquired pneumonia in adults in the outpatient setting" .) (Related Pathway(s): Community-acquired pneumonia: Empiric antibiotic selection for adults in the outpatient setting .)
Inpatient antibiotic therapy
General medical ward — For patients with CAP admitted to the medical ward, empiric antibiotic regimens are designed to treat S. aureus , gram-negative enteric bacilli (eg, Klebsiella pneumoniae ) in addition to typical pathogens (eg, S. pneumoniae , H. influenzae , and M. catarrhalis ) and atypical pathogens (eg, Legionella pneumophilia , M. pneumoniae , and C. pneumoniae ).
We generally start antibiotic therapy as soon as we are confident that CAP is the appropriate working diagnosis and, ideally, within four hours of presentation. Delays in appropriate antibiotic treatment that exceed four hours have been associated with increased mortality [ 36 ].
The key factors in selecting an initial regimen for hospitalized patients with CAP are risk of infection with Pseudomonas and/or methicillin-resistant S. aureus (MRSA). The strongest risk factors for MRSA or Pseudomonas infection are known colonization or prior infection with these organisms, particularly from a respiratory tract specimen. Recent hospitalization (ie, within the past three months) with receipt of intravenous (IV) antibiotics is also a risk factor, particularly for pseudomonal infection. Suspicion for these pathogens should otherwise be based on local prevalence (when known), other patient-specific risk factors, and the overall clinical assessment ( algorithm 3 and table 4 ):
● For patients without suspicion for MRSA or Pseudomonas , we generally use one of two regimens: combination therapy with a beta-lactam plus a macrolide or monotherapy with a respiratory fluoroquinolone [ 26 ]. Because these two regimens have similar clinical efficacy, we select among them based on other factors (eg, antibiotic allergy, drug interactions). For patients who are unable to use either a macrolide or a fluoroquinolone, we use a beta-lactam plus doxycycline .
● For patients with known colonization or prior infection with Pseudomonas, recent hospitalization with IV antibiotic use, or other strong suspicion for pseudomonal infection , we typically use combination therapy with both an antipseudomonal beta-lactam (eg, piperacillin-tazobactam , cefepime , ceftazidime , meropenem , or imipenem ) plus an antipseudomonal fluoroquinolone (eg, ciprofloxacin or levofloxacin ). The selection of empiric regimens should also be informed by the susceptibility pattern for prior isolates.
● For patients with known colonization or prior infection with MRSA or other strong suspicion for MRSA infection , we add an agent with anti-MRSA activity, such as vancomycin or linezolid , to either of the above regimens. We generally prefer linezolid over vancomycin when community-acquired MRSA is suspected (eg, a young, otherwise healthy patient who plays contact sports presenting with necrotizing pneumonia) because of linezolid's ability to inhibit bacterial toxin production [ 37 ]. Ceftaroline is a potential alternative for the treatment of MRSA pneumonia but is not US Food and Drug Administration approved. (See "Treatment of community-acquired pneumonia in adults who require hospitalization", section on 'Community-acquired MRSA' .)
Modifications to initial empiric regimens may be needed for antibiotic allergy, potential drug interactions, current epidemics, specific exposures, resistance patterns of known colonizing organisms or organisms isolated during prior infections, and other patient-specific factors. In particular, antiviral treatment (eg, oseltamivir ) should be given as soon as possible for any hospitalized patient with known or suspected influenza. (See "Seasonal influenza in nonpregnant adults: Treatment" .)
Detailed discussion about antibiotic therapy, including use of new agents (eg, lefamulin , omadacycline ) for patients hospitalized to a general medical ward is provided separately. (See "Treatment of community-acquired pneumonia in adults who require hospitalization" .) (Related Pathway(s): Community-acquired pneumonia: Empiric antibiotic selection for adults admitted to a general medical ward .)
ICU admission
Antibiotic selection — For patients with CAP admitted to the intensive care unit (ICU), our approach to antibiotic selection is similar to that used for patients admitted to the general medical ward. However, because of the severity of illness in this population, we do not use monotherapy ( algorithm 4 ). In addition, we start antibiotic therapy within one hour of presentation for patients who are critically ill.
The spectrum of activity of the empiric regimen should be broadened in patients with risk factors for Pseudomonas infection or MRSA infection ( table 4 ).
● For most patients without suspicion for MRSA or Pseudomonas , we treat with a beta-lactam (eg, ceftriaxone , cefotaxime , ceftaroline , ampicillin-sulbactam , ertapenem ) plus a macrolide (eg, azithromycin or clarithromycin ) or a beta-lactam plus a respiratory fluoroquinolone (eg, levofloxacin or moxifloxacin ) [ 26 ].
For patients with penicillin hypersensitivity reactions, we select an appropriate agent (eg, later-generation cephalosporin, carbapenem, or a beta-lactam alternative) based on the type and severity of reaction ( algorithm 5 ). For patients who cannot use any beta-lactam (ie, penicillins, cephalosporins, and carbapenems), we typically use combination therapy with a respiratory fluoroquinolone and aztreonam .
● For patients with known colonization or prior infection with MRSA, recent hospitalization with IV antibiotic use, or other strong suspicion for MRSA infection , we add an agent with anti-MRSA activity, such as vancomycin or linezolid , to either of the above regimens [ 26 ].
● For patients with known colonization or prior infection with Pseudomonas , recent hospitalization with IV antibiotic use, or other strong suspicion for pseudomonal infection , we typically use combination therapy with both an antipseudomonal beta-lactam (eg, piperacillin-tazobactam , cefepime , ceftazidime , meropenem , or imipenem ) plus an antipseudomonal fluoroquinolone (eg, ciprofloxacin or levofloxacin ) for empiric treatment [ 26 ].
For patients with penicillin hypersensitivity reactions, we select an appropriate agent based on the type and severity of penicillin reaction ( algorithm 5 ) and prior pseudomonal susceptibility testing.
Modifications to initial empiric regimens may be needed for antibiotic allergy, potential drug interactions, current epidemics, specific exposures, resistance patterns of colonizing bacteria or bacteria isolated during prior infections, and other patient-specific factors. In particular, antiviral treatment (eg, oseltamivir ) should be given as soon as possible for any hospitalized patient with known or suspected influenza. (See "Seasonal influenza in nonpregnant adults: Treatment" .)
Detailed discussion about antibiotic treatment for patients with CAP admitted to the ICU and patients with sepsis and/or respiratory failure are provided separately. (See "Treatment of community-acquired pneumonia in adults who require hospitalization", section on 'Intensive care unit' and "Evaluation and management of suspected sepsis and septic shock in adults" .) (Related Pathway(s): Community-acquired pneumonia: Empiric antibiotic selection for adults admitted to the intensive care unit .)
Adjunctive glucocorticoids — The role of adjunctive glucocorticoid treatment for CAP is evolving. The rationale for use is to reduce the inflammatory response to pneumonia, which may in turn reduce progression to lung injury, ARDS, and mortality. Based on randomized trials, the greatest benefit is for patients with impending respiratory failure or those requiring mechanical ventilation, particularly when glucocorticoids are given early in the course.
● For most immunocompetent patients with respiratory failure due to CAP who require invasive or non-invasive mechanical ventilation or with significant hypoxemia (ie, PaO2:FIO2 ratio <300 with an FiO 2 requirement of ≥50 percent and use of either high flow nasal cannula or a nonrebreathing mask), we suggest continuous infusion of hydrocortisone 200 mg daily for 4 to 7 days followed by a taper. Because mortality benefit appears to be greatest with early initiation, hydrocortisone should ideally be started as soon as possible. The decision to taper glucocorticoids at day 4 or 7 is based on clinical response.
● Because glucocorticoid use may impair the immune control of influenza, tuberculosis, and fungal pathogens, we avoid hydrocortisone use in patients with CAP caused by these pathogens or for patients with concurrent acute viral hepatitis or active herpes viral infection, which may also be worsened with glucocorticoid use.
● For immunocompromised patients, we weigh the risks and benefits of use on an individual basis.
● While we do not treat CAP with adjunctive glucocorticoids in most other circumstances, we do not withhold glucocorticoids when they are indicated for other reasons, including:
• Refractory septic shock (see "Glucocorticoid therapy in septic shock in adults" )
• Acute exacerbations of COPD (see "COPD exacerbations: Management", section on 'Glucocorticoids in moderate to severe exacerbations' )
• COVID-19 (see "COVID-19: Management in hospitalized adults", section on 'Dexamethasone and other glucocorticoids' )
Additional detail on the use of glucocorticoids for CAP and review of the evidence are discussed separately. (See "Treatment of community-acquired pneumonia in adults who require hospitalization", section on 'Adjunctive glucocorticoids' .)
Disposition — Once a patient with CAP is hospitalized, further management will be dictated by the patient's response to initial empiric therapy. Clinical response should be assessed during daily rounds. While various criteria have been proposed to assess clinical response [ 38-40 ], we generally look for subjective improvement in cough, sputum production, dyspnea, and chest pain. Objectively, we assess for resolution of fever and normalization of heart rate, respiratory rate, oxygenation, and white blood cell count. Generally, patients demonstrate some clinical improvement within 48 to 72 hours ( table 7 ).
Antibiotic de-escalation — For patients in whom a causative pathogen has been identified, we tailor therapy to target the pathogen [ 41 ]. If coverage for MRSA was added empirically, and MRSA was not identified as a pathogen nor on a screening nasal swab and the patient is improving, we typically discontinue the anti-MRSA agent (eg, vancomycin ). However, for the majority of patients hospitalized with CAP, a causative pathogen is not identified. For these patients, we continue empiric treatment for the duration of therapy, provided that the patient is improving. Intravenous antibiotic regimens can be transitioned to oral regimens with a similar spectrum activity as the patient improves ( algorithm 6 ) [ 42,43 ].
Duration of therapy — We generally determine the duration of therapy based on the patient's clinical response to therapy.
For all patients, we treat until the patient has been afebrile and clinically stable for at least 48 hours and for a minimum of five days. Patients with mild infection generally require five to seven days of therapy. Patients with severe infection or chronic comorbidities generally require 7 to 10 days of therapy. Extended courses may be needed for immunocompromised patients, patients with infections caused by certain pathogens (eg, P. aeruginosa) , or those with complications. (See "Treatment of community-acquired pneumonia in adults who require hospitalization", section on 'Duration of therapy' .)
In accord with the ATS/IDSA, we do not use procalcitonin to help determine whether to start antibiotics [ 26 ]. However, we sometimes use procalcitonin thresholds as an adjunct to clinical judgment to help guide antibiotic discontinuation in clinically stable patients. We generally obtain a level at the time of diagnosis and repeat the level every one to two days in patients who are clinically stable. We determine the need for continued antibiotic therapy based on clinical improvement and serial procalcitonin levels ( algorithm 7 ). (See "Procalcitonin use in lower respiratory tract infections" .)
Discharge — Hospital discharge is appropriate when the patient is clinically stable, can take oral medication, has no other active medical problems, and has a safe environment for continued care. Patients do not need to be kept overnight for observation following the switch to oral therapy. Early discharge based on clinical stability and criteria for switching to oral therapy is encouraged to reduce risk associated with prolonged hospital stays and unnecessary cost.
Immunocompromised patients — The spectrum of potential pathogens expands considerably in immunocompromised patients to include invasive fungal infections, less common viral infections (eg, cytomegalovirus), and parasitic infections (eg, toxoplasmosis) [ 44 ].
The risk for specific infections varies with the type and degree of immunosuppression and whether the patient is taking prophylactic antimicrobials. As examples, prolonged neutropenia, T cell immunosuppression, and use of tumor necrosis factor-alpha inhibitors predispose to invasive fungal infections (eg, aspergillosis, mucormycosis) as well as mycobacterial infections. Advanced human immunodeficiency virus (HIV) infection (eg, CD4 cell count <200 cells/microL), prolonged glucocorticoid use (particularly when used with certain chemotherapeutics), and lymphopenia each should raise suspicion for pneumocystis pneumonia. Multiple infections may occur concurrently in this population, and the likelihood of disseminated infection is greater. Because signs and symptoms of infection can be subtle and nonspecific in immunocompromised patients, diagnosis can be challenging and invasive procedures are often required for microbiologic diagnosis. Broad-spectrum empiric therapy may be needed prior to obtaining a specific microbiologic diagnosis [ 45 ].
Because management is complex, drug interactions are common, adjustments in immunosuppressive regimens may be needed, and empiric treatment options (eg, amphotericin B) can be associated with significant toxicity, we generally involve a multidisciplinary team of specialists when caring for immunocompromised patients with pneumonia. (See "Epidemiology of pulmonary infections in immunocompromised patients" and "Approach to the immunocompromised patient with fever and pulmonary infiltrates" and "Tumor necrosis factor-alpha inhibitors: Bacterial, viral, and fungal infections" .)
FOLLOW-UP IMAGING — Follow-up imaging for immunocompetent adults who have recovered from community-acquired pneumonia Algorithm 8 Most patients with clinical resolution after treatment do not require a follow-up chest radiograph, as radiographic response lags behind clinical response. However, follow-up clinic visits are good opportunities to review the patient's risk for lung cancer based on age, smoking history, and recent imaging findings ( algorithm 8 ).
This approach is similar to that outlined by the ATS/IDSA, which recommend not obtaining a follow-up chest radiograph in patients whose symptoms have resolved within five to seven days [ 26 ]. (See "Treatment of community-acquired pneumonia in adults who require hospitalization", section on 'Follow-up chest radiograph' .)
COMPLICATIONS AND PROGNOSIS — While most patients with CAP will recover with appropriate antibiotic treatment, some will progress and/or develop complications despite appropriate therapy (ie, clinical failure) and some will remain symptomatic (ie, nonresolving pneumonia).
Clinical failure — Clear indicators of clinical failure include progression to sepsis and/or respiratory failure despite appropriate antibiotic treatment and respiratory support. Other indicators include an increase in subjective symptoms (eg, cough, dyspnea) usually in combination with objective criteria (eg, decline in oxygenation, persistent fever, or rising white blood cell). Various criteria have been proposed to define clinical failure but none widely adopted [ 46-48 ].
Reasons for clinical failure generally fall into these categories:
● Progression of the initial infection – For some patients, CAP can lead to overwhelming infection despite appropriate antibiotic treatment. In some, this indicates a dysregulated host immune response. In others, this may indicate that the infection has spread beyond the pulmonary parenchyma (eg, empyema, lung abscess, bacteremia, endocarditis).
Other possibilities include infection with a drug-resistant pathogen or an unusual pathogen not covered by the initial empiric antibiotic regimen. Alternatively, failure to respond to treatment may signify the presence of an immunodeficiency (eg, new diagnosis of HIV infection).
● Development of comorbid complications – Comorbid complications may be infectious or noninfectious. Nosocomial infections, particularly hospital-acquired pneumonia (HAP), are common causes of clinical failure. In addition to HAP, others include catheter-related bloodstream infections, urinary tract infections, and C. difficile infection [ 49 ].
Cardiovascular events are also common complications and include acute myocardial infarction, cardiac arrhythmias, congestive heart failure, pulmonary embolism, and stroke [ 50-52 ]. Older age, preexisting cardiovascular disease, severe pneumonia, and infection with certain pathogens (ie, S. pneumoniae and influenza) have each been associated with increased risk of cardiovascular events [ 50,53-55 ]. Recognition that cardiovascular events and other systemic complications can occur during the acute phase of CAP is also changing our view of CAP from an acute pulmonary process to an acute systemic disease. (See "Morbidity and mortality associated with community-acquired pneumonia in adults", section on 'Cardiac complications' .)
Because of these possibilities, we generally broaden our initial antibiotic regimen for patients who are progressing despite appropriate empiric treatment and evaluate for alternate diagnoses, less common or drug-resistant pathogens, and/or infectious and cardiovascular complications. (See 'Differential diagnosis' above and "Morbidity and mortality associated with community-acquired pneumonia in adults" .)
Nonresolving CAP — For some patients, initial symptoms will neither progress nor improve with at least seven days of appropriate empiric antibiotic treatment. We generally characterize these patients as having nonresolving pneumonia. Potential causes of nonresolving CAP include:
● Delayed clinical response – For some patients, particularly those with multiple comorbidities, severe pneumonia, bacteremia, and infection with certain pathogens (eg, S. pneumoniae ), treatment response may be slow. Eight or nine days of treatment may be needed before clinical improvement is evident.
● Loculated infection – Patients with complications such as lung abscess, empyema, or other closed space infections may fail to improve clinically despite appropriate antibiotic selection. Such infections may require drainage and/or prolonged antibiotic treatment. (See "Lung abscess in adults" and "Epidemiology, clinical presentation, and diagnostic evaluation of parapneumonic effusion and empyema in adults" .)
● Bronchial obstruction – Bronchial obstruction (eg, by a tumor) can cause a postobstructive pneumonia that may fail to respond or slowly respond to standard empiric antibiotic regimens for CAP.
● Pathogens that cause subacute/chronic CAP – Mycobacterium tuberculosis , nontuberculous mycobacteria (eg, Mycobacterium kansasii ), fungi (eg, Histoplasma capsulatum , Blastomyces dermatitidis ), or less common bacteria (eg, Nocardia spp, Actinomyces israelii ) can cause subacute or chronic pneumonia that may fail to respond or may incompletely respond to standard empiric antibiotic regimens for CAP.
● Incorrect initial diagnosis – Failure to improve despite seven days of treatment also raises the possibility of an alternate diagnosis (eg, malignancy or inflammatory lung disease). (See 'Differential diagnosis' above.)
Once a patient is characterized as having nonresolving CAP, a complete new physical examination, laboratory evaluation, imaging studies, and microbiologic workup will be necessary to define the etiology of nonresolving CAP [ 49 ]. Initiation of workup for nonresolving CAP should not be automatically associated with a change in initial empiric antibiotic therapy. (See "Nonresolving pneumonia" .)
Long-term complications and mortality — Although the majority of patient with CAP recover without complications, CAP is a severe illness and among the leading causes of mortality worldwide. Mortality can be directly attributable to CAP (eg, overwhelming sepsis or respiratory failure) or can result indirectly from cardiovascular events or other comorbid complications (eg, advanced chronic obstructive pulmonary disease [COPD]) [ 56 ].
Long-term complications resulting from pneumonia are increasingly recognized and there is a shift in the medical community to define pneumonia as a systemic illness that can lead to chronic disease [ 57 ]. While the precise incidence of long-term complications is not known, the more common long-term sequelae involve the respiratory tract and cardiovascular system [ 58 ].
In the United States, pneumonia (combined with influenza) is among the top 10 most common causes of death [ 5 ]. Thirty-day mortality rates vary with disease severity, ranging from less than 1 percent in ambulatory patients to approximately 20 to 25 percent in patients with severe CAP. In addition to disease severity, older age, comorbidities (eg, COPD, diabetes mellitus, cardiovascular disease), infection with certain pathogens (eg, S. pneumoniae ), and acute cardiac complications are each associated with increased short-term mortality [ 50,59,60 ].
CAP is also associated with increased long-term mortality [ 7,61-63 ]. In one population-based study evaluating 7449 patients hospitalized with CAP, mortality rates were 6.5 percent during hospitalization, 13 percent 30 days after hospitalization, 23 percent at six months after hospitalization, and 31 percent at one year after hospitalization [ 7 ]. During the same study year, an estimated 1,581,860 patients were hospitalized in the United States. Extrapolating mortality data to these patients, the number of deaths in the United States population will be 102,821 during hospitalization, 205,642 at 30 days, 370,156 at six months, and 484,050 at one year [ 7 ]. Causes of long-term mortality are primarily related to comorbidities and include malignancy, COPD, and cardiovascular disease [ 56 ].
Data associating CAP with long-term mortality indicate that CAP is not only a common cause of acute morbidity and mortality but also a disease with important chronic health outcomes.
PREVENTION — The three primary pillars for the prevention of CAP are [ 64-66 ]:
● Smoking cessation (when appropriate)
● Influenza vaccination for all patients
● Pneumococcal vaccination for at-risk patients
Each is discussed in detail separately. (See "Overview of smoking cessation management in adults" and "Seasonal influenza vaccination in adults" and "Pneumococcal vaccination in adults" .)
SOCIETY GUIDELINE LINKS — Links to society and government-sponsored guidelines from selected countries and regions around the world are provided separately. (See "Society guideline links: Community-acquired pneumonia in adults" .)
INFORMATION FOR PATIENTS — UpToDate offers two types of patient education materials, "The Basics" and "Beyond the Basics." The Basics patient education pieces are written in plain language, at the 5 th to 6 th grade reading level, and they answer the four or five key questions a patient might have about a given condition. These articles are best for patients who want a general overview and who prefer short, easy-to-read materials. Beyond the Basics patient education pieces are longer, more sophisticated, and more detailed. These articles are written at the 10 th to 12 th grade reading level and are best for patients who want in-depth information and are comfortable with some medical jargon.
Here are the patient education articles that are relevant to this topic. We encourage you to print or email these topics to your patients. (You can also locate patient education articles on a variety of subjects by searching on "patient info" and the keyword(s) of interest.)
● Basics topic (see "Patient education: Pneumonia in adults (The Basics)" )
● Beyond the Basics topic (see "Patient education: Pneumonia in adults (Beyond the Basics)" )
SUMMARY AND RECOMMENDATIONS
● Background – Community-acquired pneumonia (CAP) is a leading cause of morbidity and mortality worldwide. (See 'Incidence' above.)
● Risk factors – Risk factors include age ≥65 years, chronic comorbidities, concurrent or antecedent respiratory viral infections, impaired airway protection, smoking, alcohol abuse, and other lifestyle factors (eg, crowded living conditions). (See 'Risk factors' above.)
● Microbiology – The most commonly identified causes of CAP include respiratory viruses (particularly severe acute respiratory syndrome coronavirus 2 during the pandemic), typical bacteria (eg, Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis ) and atypical bacteria (eg, Legionella spp, Mycoplasma pneumoniae, Chlamydia pneumoniae ). Pseudomonas and methicillin-resistant Staphylococcus aureus (MRSA) are less common causes that predominantly occur in patients with specific risk factors. (See 'Microbiology' above and 'Pathogenesis' above.)
● Making the diagnosis – Diagnosis requires demonstration of an infiltrate on chest imaging in a patient with a clinically compatible syndrome (eg, fever, dyspnea, cough, and leukocytosis). For most patients, a posteroanterior and lateral chest radiograph is sufficient. Computed tomography scan is reserved for selected cases. (See 'Clinical presentation' above and 'Making the diagnosis' above.)
● Alternate and concurrent diagnoses – While the combination of a compatible clinical syndrome and an infiltrate on chest imaging are sufficient to establish an initial clinical diagnosis of CAP, these findings are nonspecific. Remaining attentive to the possibility of an alternate or concurrent diagnosis as a patient's course evolves is important to care. (See 'Differential diagnosis' above.)
● Determining severity of illness – For patients with a working diagnosis of CAP, the initial steps in management are defining the severity of illness and determining the most appropriate site of care ( algorithm 1 ). For most patients, we determine our approach to microbiologic testing based on this assessment ( table 5 ). (See 'Microbiologic testing' above.)
● Empiric antibiotic selection – The selection of an empiric antibiotic regimen is based on the severity of illness, site of care, and most likely pathogens. We generally start antibiotics as soon as we are confident that CAP is the appropriate working diagnosis and, ideally, within four hours of presentation for inpatients and within one hour of presentation for those who are critically ill (see 'Treatment' above):
• For most outpatients, we prefer to use combination therapy with a beta-lactam and either a macrolide (preferred) or doxycycline . Alternatives to beta-lactam-based regimens include monotherapy with either a fluoroquinolone or, alternatively, lefamulin or omadacycline (newer agents). Selection among these agents depends on patient comorbidities, drug interactions, allergies, and other intolerances. Clinical experience with lefamulin and omadacycline are limited; warnings and contraindications exist ( algorithm 2 ).
This approach differs from the American Thoracic Society/Infectious Diseases Society of America, which recommend monotherapy with amoxicillin as first line and monotherapy with either doxycycline or a macrolide (if local resistance rates are <25 percent [eg, not in the United States]) as alternatives for this population.
• For most inpatients admitted to the general medical ward, treatment options include either intravenous (IV) combination therapy with a beta-lactam plus a macrolide or doxycycline or monotherapy with a respiratory fluoroquinolone ( algorithm 3 ). These regimens should be expanded for patients with risk factors for Pseudomonas or MRSA ( table 4 ).
• For most patients admitted to the intensive care unit (ICU), treatment options include IV combination therapy with a beta-lactam plus either a macrolide or a respiratory fluoroquinolone ( algorithm 4 ). As with other hospitalized patients, regimens should be expanded for patients with risk factors for Pseudomonas or MRSA ( table 4 ).
● Adjunctive glucocorticoids – The benefit of adjunctive glucocorticoids appears greatest in patients with impending respiratory failure or requiring mechanical ventilation, particularly when they are given early in the course. Generally, we add hydrocortisone for most immunocompetent patients with respiratory failure due to CAP who require invasive or non-invasive mechanical ventilation or with significant hypoxemia (ie, PaO2:FIO2 ratio <300 with an FiO 2 requirement of ≥50 percent and use of either high flow nasal cannula or a nonrebreathing mask), unless there are reason to avoid their use (eg, infection with certain pathogen [influenza, fungi, tuberculosis, or immunocompromise]). (See 'Adjunctive glucocorticoids' above.)
● Directed antibiotic therapy – For patients in whom a causative pathogen has been identified, we tailor therapy to target the pathogen. (See 'Antibiotic de-escalation' above.)
● Duration of antibiotics – For all patients, we treat until the patient has been afebrile and clinically stable for at least 48 hours and for a minimum of five days. Patients with mild infection generally require five to seven days of therapy; those with severe infection or chronic comorbidities generally require 7 to 10 days of therapy. (See 'Duration of therapy' above.)
● Lack of response to antibiotics – Failure to respond to antibiotic treatment within 72 hours should prompt reconsideration of the diagnosis and empiric treatment regimen as well as an assessment for complications. (See 'Clinical failure' above and 'Nonresolving CAP' above.)
● Prevention – Key preventive measures include smoking cessation (when appropriate), influenza vaccination for the general population, and pneumococcal vaccination for at-risk populations. (See 'Prevention' above.)
ACKNOWLEDGMENT — UpToDate gratefully acknowledges John G Bartlett, MD (deceased), who contributed as Section Editor on earlier versions of this topic and was a founding Editor-in-Chief for UpToDate in Infectious Diseases.
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44 Suppl 2:S27.
- File TM. Community-acquired pneumonia. Lancet 2003; 362:1991.
- Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med 2014; 371:1619.
- National Ambulatory Medical Care Survey (NAMCS) and National Hospital Ambulatory Medical Care Survey (NHAMCS) 2009 - 2010. https://www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf (Accessed on June 06, 2018).
- Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: Final Data for 2013. Natl Vital Stat Rep 2016; 64:1.
- Pfuntner A, Wier LM, Stocks C. Most Frequent Conditions in U.S. Hospitals, 2011. HCUP Statistical Brief #162, Agency for Healthcare Research and Quality, Rockville, MD, September 2013.
- Ramirez JA, Wiemken TL, Peyrani P, et al. Adults Hospitalized With Pneumonia in the United States: Incidence, Epidemiology, and Mortality. Clin Infect Dis 2017; 65:1806.
- Jain S, Self WH, Wunderink RG, et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N Engl J Med 2015; 373:415.
- Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013; 369:155.
- Almirall J, Bolíbar I, Balanzó X, González CA. Risk factors for community-acquired pneumonia in adults: a population-based case-control study. Eur Respir J 1999; 13:349.
- Torres A, Peetermans WE, Viegi G, Blasi F. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax 2013; 68:1057.
- Bello S, Menéndez R, Antoni T, et al. Tobacco smoking increases the risk for death from pneumococcal pneumonia. Chest 2014; 146:1029.
- Wiese AD, Griffin MR, Schaffner W, et al. Opioid Analgesic Use and Risk for Invasive Pneumococcal Diseases: A Nested Case-Control Study. Ann Intern Med 2018; 168:396.
- Bain MR, Chalmers JW, Brewster DH. Routinely collected data in national and regional databases--an under-used resource. J Public Health Med 1997; 19:413.
- Curcio D, Cané A, Isturiz R. Redefining risk categories for pneumococcal disease in adults: critical analysis of the evidence. Int J Infect Dis 2015; 37:30.
- Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis 2010; 50:202.
- Musher DM, Roig IL, Cazares G, et al. Can an etiologic agent be identified in adults who are hospitalized for community-acquired pneumonia: results of a one-year study. J Infect 2013; 67:11.
- Musher DM, Abers MS, Bartlett JG. Evolving Understanding of the Causes of Pneumonia in Adults, With Special Attention to the Role of Pneumococcus. Clin Infect Dis 2017; 65:1736.
- Dickson RP, Erb-Downward JR, Martinez FJ, Huffnagle GB. The Microbiome and the Respiratory Tract. Annu Rev Physiol 2016; 78:481.
- Dickson RP, Erb-Downward JR, Huffnagle GB. Towards an ecology of the lung: new conceptual models of pulmonary microbiology and pneumonia pathogenesis. Lancet Respir Med 2014; 2:238.
- Faner R, Sibila O, Agustí A, et al. The microbiome in respiratory medicine: current challenges and future perspectives. Eur Respir J 2017; 49.
- Francis JS, Doherty MC, Lopatin U, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis 2005; 40:100.
- Annane D, Renault A, Brun-Buisson C, et al. Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. N Engl J Med 2018; 378:809.
- Moore M, Stuart B, Little P, et al. Predictors of pneumonia in lower respiratory tract infections: 3C prospective cough complication cohort study. Eur Respir J 2017; 50.
- Waterer GW, Kessler LA, Wunderink RG. Delayed administration of antibiotics and atypical presentation in community-acquired pneumonia. Chest 2006; 130:11.
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200:e45.
- Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336:243.
- Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58:377.
- Marrie TJ, Lau CY, Wheeler SL, et al. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. CAPITAL Study Investigators. Community-Acquired Pneumonia Intervention Trial Assessing Levofloxacin. JAMA 2000; 283:749.
- Yealy DM, Auble TE, Stone RA, et al. Effect of increasing the intensity of implementing pneumonia guidelines: a randomized, controlled trial. Ann Intern Med 2005; 143:881.
- Carratalà J, Fernández-Sabé N, Ortega L, et al. Outpatient care compared with hospitalization for community-acquired pneumonia: a randomized trial in low-risk patients. Ann Intern Med 2005; 142:165.
- Labarere J, Stone RA, Scott Obrosky D, et al. Factors associated with the hospitalization of low-risk patients with community-acquired pneumonia in a cluster-randomized trial. J Gen Intern Med 2006; 21:745.
- Liapikou A, Ferrer M, Polverino E, et al. Severe community-acquired pneumonia: validation of the Infectious Diseases Society of America/American Thoracic Society guidelines to predict an intensive care unit admission. Clin Infect Dis 2009; 48:377.
- Mandell LA. Severe community-acquired pneumonia (CAP) and the Infectious Diseases Society of America/American Thoracic Society CAP guidelines prediction rule: validated or not. Clin Infect Dis 2009; 48:386.
- Chalmers JD, Taylor JK, Mandal P, et al. Validation of the Infectious Diseases Society of America/American Thoratic Society minor criteria for intensive care unit admission in community-acquired pneumonia patients without major criteria or contraindications to intensive care unit care. Clin Infect Dis 2011; 53:503.
- Lim WS, Woodhead M, British Thoracic Society. British Thoracic Society adult community acquired pneumonia audit 2009/10. Thorax 2011; 66:548.
- Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 2012; 54:621.
- Aliberti S, Zanaboni AM, Wiemken T, et al. Criteria for clinical stability in hospitalised patients with community-acquired pneumonia. Eur Respir J 2013; 42:742.
- Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA 1998; 279:1452.
- Menéndez R, Torres A, Rodríguez de Castro F, et al. Reaching stability in community-acquired pneumonia: the effects of the severity of disease, treatment, and the characteristics of patients. Clin Infect Dis 2004; 39:1783.
- van der Eerden MM, Vlaspolder F, de Graaff CS, et al. Comparison between pathogen directed antibiotic treatment and empirical broad spectrum antibiotic treatment in patients with community acquired pneumonia: a prospective randomised study. Thorax 2005; 60:672.
- Ramirez JA, Srinath L, Ahkee S, et al. Early switch from intravenous to oral cephalosporins in the treatment of hospitalized patients with community-acquired pneumonia. Arch Intern Med 1995; 155:1273.
- Ramirez JA, Vargas S, Ritter GW, et al. Early switch from intravenous to oral antibiotics and early hospital discharge: a prospective observational study of 200 consecutive patients with community-acquired pneumonia. Arch Intern Med 1999; 159:2449.
- Di Pasquale MF, Sotgiu G, Gramegna A, et al. Prevalence and Etiology of Community-acquired Pneumonia in Immunocompromised Patients. Clin Infect Dis 2019; 68:1482.
- Ramirez JA, Musher DM, Evans SE, et al. Treatment of Community-Acquired Pneumonia in Immunocompromised Adults: A Consensus Statement Regarding Initial Strategies. Chest 2020; 158:1896.
- Menéndez R, Torres A, Zalacaín R, et al. Risk factors of treatment failure in community acquired pneumonia: implications for disease outcome. Thorax 2004; 59:960.
- Aliberti S, Amir A, Peyrani P, et al. Incidence, etiology, timing, and risk factors for clinical failure in hospitalized patients with community-acquired pneumonia. Chest 2008; 134:955.
- Rosón B, Carratalà J, Fernández-Sabé N, et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med 2004; 164:502.
- Arancibia F, Ewig S, Martinez JA, et al. Antimicrobial treatment failures in patients with community-acquired pneumonia: causes and prognostic implications. Am J Respir Crit Care Med 2000; 162:154.
- Violi F, Cangemi R, Falcone M, et al. Cardiovascular Complications and Short-term Mortality Risk in Community-Acquired Pneumonia. Clin Infect Dis 2017; 64:1486.
- Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Risk of heart failure after community acquired pneumonia: prospective controlled study with 10 years of follow-up. BMJ 2017; 356:j413.
- Ramirez J, Aliberti S, Mirsaeidi M, et al. Acute myocardial infarction in hospitalized patients with community-acquired pneumonia. Clin Infect Dis 2008; 47:182.
- Perry TW, Pugh MJ, Waterer GW, et al. Incidence of cardiovascular events after hospital admission for pneumonia. Am J Med 2011; 124:244.
- Warren-Gash C, Blackburn R, Whitaker H, et al. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J 2018; 51.
- Viasus D, Garcia-Vidal C, Manresa F, et al. Risk stratification and prognosis of acute cardiac events in hospitalized adults with community-acquired pneumonia. J Infect 2013; 66:27.
- Bruns AH, Oosterheert JJ, Cucciolillo MC, et al. Cause-specific long-term mortality rates in patients recovered from community-acquired pneumonia as compared with the general Dutch population. Clin Microbiol Infect 2011; 17:763.
- Dela Cruz CS, Wunderink RG, Christiani DC, et al. Future Research Directions in Pneumonia. NHLBI Working Group Report. Am J Respir Crit Care Med 2018; 198:256.
- Corrales-Medina VF, Alvarez KN, Weissfeld LA, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 2015; 313:264.
- Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA 1996; 275:134.
- Lepper PM, Ott S, Nüesch E, et al. Serum glucose levels for predicting death in patients admitted to hospital for community acquired pneumonia: prospective cohort study. BMJ 2012; 344:e3397.
- Peyrani P, Ramirez JA. One-year mortality in patients with community-acquired pneumonia. Univ Louisville J Respir Infect 2017; 1.
- Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-Year Mortality after Community-acquired Pneumonia. A Prospective Cohort. Am J Respir Crit Care Med 2015; 192:597.
- Mortensen EM, Kapoor WN, Chang CC, Fine MJ. Assessment of mortality after long-term follow-up of patients with community-acquired pneumonia. Clin Infect Dis 2003; 37:1617.
- Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and Control of Seasonal Influenza With Vaccines: Recommendations of the Advisory Committee on Immunization Practices-United States, 2017-18 Influenza Season. Am J Transplant 2017; 17:2970.
- Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014; 63:822.
- Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816.

JASON WOMACK, MD, AND JILL KROPA, MD
Am Fam Physician. 2022;105(6):625-630
Related Letter to the Editor: Recognizing Differing Evidence in the Literature
Author disclosure: No relevant financial relationships.
Community-acquired pneumonia (CAP) is a common condition with a hospitalization rate of about 2% in people 65 years or older and is associated with a 30-day mortality rate of 6% in hospitalized patients. In studies conducted before the COVID-19 pandemic, a bacterial pathogen was identified in 11% of patients, a viral pathogen in 23% of patients, and no organism in 62% of patients. Certain signs and symptoms can be helpful in diagnosing CAP and selecting imaging studies. Diagnosis is usually made with a combination of history, physical examination, and findings on chest radiography, lung ultrasonography, or computed tomography. Procalcitonin measurement is not recommended. CRB-65 (confusion, respiratory rate, blood pressure, 65 years of age) is a well-validated risk stratification tool in the primary care setting and does not require laboratory testing. For outpatients without comorbidities, treatment with amoxicillin, doxycycline, or a macrolide is recommended (the latter only in areas where pneumococcal resistance to macrolides is less than 25%). In outpatients with comorbidities and inpatients with nonsevere pneumonia, a combination of a beta-lactam or third-generation cephalosporin plus a macrolide, or monotherapy with a respiratory fluoroquinolone is recommended. Patients should be treated for methicillin-resistant Staphylococcus aureus or Pseudomonas infection only if they present with risk factors for those pathogens. All adults 65 years or older or those 19 to 64 with underlying conditions should receive the 20-valent pneumococcal conjugate vaccine alone or the 15-valent pneumococcal conjugate vaccine followed by 23-valent pneumococcal polysaccharide vaccine one year later. The 13-valent pneumococcal conjugate vaccine is no longer recommended for routine administration. The Centers for Disease Control and Prevention recommends vaccination against influenza and SARS-CoV-2 viruses for all adults.
Epidemiology
The annual incidence of CAP is 248 cases per 100,000 adults. However, this increases to 634 cases per 100,000 in adults 65 to 79 years of age and 16,430 cases per 100,000 in adults 80 years or older. 1
Hospitalization rates for CAP increase with advancing age. 1 – 3 A systematic review of population-based studies found that the rate was 1,830 per 100,000 adults 65 years or older and 199 per 100,000 adults younger than 65 years. 2
The hospitalization rate for CAP is nine times higher in people with comorbid chronic obstructive pulmonary disease. 3
Overall, the mortality rate for patients hospitalized with CAP is 6% at 30 days, even after initial clinical improvement. In hospitalized patients who do not improve initially or have unresolving pneumonia, the mortality rate is 34% at 30 days. 4
There are racial and ethnic disparities in the incidence of CAP. One population-based study found that the annual incidence of CAP was two to four times higher in Black adults than in White adults. 5
MICROBIOLOGY
A prospective, multicenter, population-based, active surveillance study sponsored by the Centers for Disease Control and Prevention analyzed radiograph and culture results from 2,488 inpatient adults to determine the incidence and microbiologic causes of CAP requiring hospitalization. An organism was not identified in 62% of these patients. A virus was present in 23% of patients and a bacterium in 11% of patients. 1
Another study examined the clinical and laboratory data of 323 inpatient adults with radiographically confirmed CAP and tested sputum and endotracheal aspirates to identify pathogens. 6 Samples were tested for 26 bacterial and viral pathogens using culture and polymerase chain reaction analysis. A pathogen was detected in 87% of patients; 56% had bacteria alone, 25% had a combination of bacteria and viruses, and 6% had viruses alone. 6 Among bacterial causes, the most common were Haemophilus influenzae (40%) and Streptococcus pneumoniae (36%). Mycoplasma and Legionella species were the most common atypical bacteria, and rhinovirus (13%) and influenza virus (7%) were the most common viral pathogens. 6
The incidence of Mycoplasma infection varies cyclically over years, and a species of Legionella is present in 3% of patients hospitalized for CAP. 7
SARS-CoV-2 infection has been a major cause of CAP during the pandemic, with data on prevalence continuing to change with emergence of disease variants and patient vaccination status; its contribution as a cause of CAP in the future is unclear. 8
False-negative results for viral pathogens are common in CAP. Samples from the lower respiratory tract have a greater diagnostic yield than nasopharyngeal or oropharyngeal samples, but obtaining lower respiratory tract samples is not usually feasible in the outpatient setting. 9
The differential diagnosis of CAP includes asthma or chronic obstructive pulmonary disease exacerbation, bronchitis, congestive heart failure, gastroesophageal reflux disease, lung cancer, and pulmonary embolism.
SIGNS AND SYMPTOMS
Patient-reported symptoms often include cough, subjective fever, chills, sputum production, and dyspnea.
A meta-analysis found that the following clinical signs and symptoms had the highest diagnostic odds ratios for pneumonia: physician's overall clinical impression (diagnostic odds ratio = 11.5), egophony (6.5), any abnormal vital sign (6.0), any abnormal lung finding (3.2), tachypnea (3.1), and measured fever (3.3). 10
A systematic review found that adults with an acute respiratory tract infection were unlikely to have CAP if they presented with normal vital signs and normal pulmonary examination findings (negative likelihood ratio = 0.1). 11
Fever is not always present in patients with bacteremia. 12
Clinicians should determine whether patients meet criteria for severe CAP ( Table 1 13 ) to inform diagnostic testing and antibiotic choice. 14
DIAGNOSTIC TESTING
The aforementioned physical examination findings with high diagnostic odds ratios for pneumonia can be helpful in determining the need for imaging. 10
Previous Infectious Diseases Society of America (IDSA) guidelines recommended chest radiography as the standard method for diagnosing CAP. 13
Updated IDSA guidelines are based on studies of patients with radiographically confirmed pneumonia despite acknowledging that chest radiography is not always used in the ambulatory setting. 14
Although chest radiography has value in the evaluation of CAP, its accuracy is limited. A study of more than 3,000 patients presenting to the emergency department found that chest radiography had a positive predictive value of only 26.9% for detection of pulmonary opacities when using computed tomography as the criterion standard, whereas the negative predictive value was 96.5%. 15
Computed tomography decreases the chance of a false-positive or false-negative diagnosis, but cost and availability make this modality less useful in the outpatient setting. 16
Procalcitonin measurement is not recommended by the IDSA and has not been found to reduce antibiotic use among patients admitted to the emergency department. 17
Diagnostic cultures and antigen testing should be obtained only in patients with severe CAP. 14
In patients with CAP who have had prior respiratory isolation of methicillin-resistant Staphylococcus aureus (MRSA) or who have had recent hospitalization and treatment with parenteral antibiotics and have locally validated risk factors for MRSA infection, microbiologic testing should be performed before escalating antibiotic treatment or to allow for future de-escalation if MRSA is not detected. Similarly, in patients with severe CAP who have had prior respiratory isolation of Pseudomonas species or who have had recent hospitalization and treatment with parenteral antibiotics and have locally validated risk factors for Pseudomonas infection, microbiologic testing should be performed before escalating antibiotic treatment or to allow for future de-escalation if a species of Pseudomonas is not detected. 14
Testing for Legionella species should be reserved for cases of severe CAP or in areas where a known outbreak of Legionella infection has occurred. 14
Microbiologic testing for influenza and SARS-CoV-2 should be considered if there is any clinical suspicion for these viruses.
Lung ultrasonography is an alternative imaging modality if the clinician has appropriate training and equipment. A meta-analysis using computed tomography as the criterion standard showed that ultrasonography was more accurate than chest radiography at diagnosing CAP. 18
INPATIENT VS. OUTPATIENT CARE
When determining if a patient should be treated for CAP as an inpatient or outpatient, the IDSA recommends using the Pneumonia Severity Index ( https://www.mdcalc.com/psi-port-score-pneumonia-severity-index-cap ) as an adjunct to clinical judgment. 14 However, its use may be limited because it requires more than 20 variables, including imaging and several blood tests.
The British Thoracic Society recommends using CURB-65 (confusion, urea nitrogen, respiratory rate, blood pressure, 65 years of age; https://www.mdcalc.com/curb-65-score-pneumonia-severity ) or CRB-65 for risk stratification. 19 The CRB-65 tool ( Table 2 20 ) is easier to use in the outpatient setting because it requires no laboratory testing and has been well validated in primary care settings. 21
EMPIRIC ANTIBIOTIC THERAPY
Antibiotics should be prescribed for outpatients if there is clinical suspicion for CAP without performing imaging studies, unless the diagnosis is in doubt. 22
For patients being treated in the outpatient setting, the British Thoracic Society recommends initiating antibiotics based on clinical suspicion without microbiologic testing. 22
Antibiotic therapy for outpatients is summarized in Table 3 . 14
In patients with severe CAP ( Table 1 13 ), combination therapy with a beta-lactam antibiotic plus a macrolide or a beta-lactam plus a respiratory fluoroquinolone is recommended. 14
In patients with severe CAP who have had prior respiratory isolation of MRSA or were recently hospitalized and treated with parenteral antibiotics and have locally validated risk factors for MRSA infection, vancomycin or linezolid (Zyvox) should be added to cover for MRSA infection. 14
In patients with severe CAP who have had prior respiratory isolation of a Pseudomonas species or who have had recent hospitalization and treatment with parenteral antibiotics and have locally validated risk factors for Pseudomonas infection, piperacillin/tazobactam (Zosyn), cefepime, ceftazidime (Fortaz), imipenem/cilastatin (Primaxin IV), meropenem (Merrem), or aztreonam (Azactam) should be added to cover for Pseudomonas infection. 14
When MRSA or Pseudomonas coverage is added, blood and sputum cultures should be obtained to allow for de-escalation of this coverage if the pathogen is ruled out. 14
Antibiotics should be continued for a minimum of five days, and discontinued after the patient improves and remains clinically stable. 14
The IDSA recommends treating adults with oseltamivir (Tamiflu) when influenza virus is isolated in the inpatient setting, regardless of the duration of illness before CAP diagnosis. In the outpatient setting, oseltamivir should be initiated regardless of duration of illness. 14
Research is ongoing to develop protocols and new drugs to treat pneumonia caused by SARS-CoV-2.
SYSTEMIC CORTICOSTEROIDS
The IDSA does not recommend corticosteroids for treatment of CAP in the inpatient or outpatient setting, regardless of illness severity. 14
A systematic review and meta-analysis of corticosteroid use in adults with CAP found that short-term use may reduce the risk of acute respiratory distress syndrome in severe CAP. However, all of the studies included in the analysis had significant limitations, and overall mortality was not improved with corticosteroid use. 23
Steroids may be used in patients with CAP if they are needed to treat a comorbid condition such as asthma, chronic obstructive pulmonary disease, or autoimmune disease.
Steroids may be used to treat CAP-related septic shock that is refractory to fluid resuscitation and vasopressor support. 14
Updated guidelines published in January 2022 recommend vaccinating adults 65 years or older or those 19 to 64 years with underlying conditions with 20-valent pneumococcal conjugate vaccine (PCV20; Prevnar 20) alone or the 15-valent pneumococcal conjugate vaccine (PCV15; Vaxneuvance) followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax 23) one year later. 24
PPSV23 decreases the relative risk of CAP by 13%. 25 The risk is reduced by 28% in adults 65 years or older and in younger adults with comorbid or immunocompromising conditions. 25 Updated guidelines are believed to provide broader coverage and be cost-effective.
The 13-valent pneumococcal conjugate vaccine (PCV13; Prevnar 13) is no longer recommended for routine use in immunocompetent adults 65 years or older.
Adults previously vaccinated with both PCV13 and PPSV23 do not require PCV20 or PCV15 vaccination at this time.
Adults vaccinated only with PPSV23 should receive a single dose of PCV15 or PCV20 one year after receiving PPSV23.
The Centers for Disease Control and Prevention recommends that all adults be immunized against influenza and SARS-CoV-2 viruses. 26
This article updates previous articles on this topic by Kaysin and Viera 27 ; Watkins and Lemonovich 28 ; Lutfiyya, et al. 29 ; and Thibodeau and Viera . 30
Data Sources: PubMed and OVID Medline searches were completed in Clinical Queries using the key term community-acquired pneumonia. The searches included randomized controlled trials, practice guidelines, and reviews. Essential Evidence Plus was also searched for data using the key terms acute lower respiratory tract infection, community-acquired pneumonia, and pneumonia. Search dates: March 2021 through January 2022.
- Jain S, Self WH, Wunderink RG, et al.; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415-427.
- McLaughlin JM, Khan FL, Thoburn EA, et al. Rate of hospitalization for community-acquired pneumonia among US adults: a systematic review. Vaccine. 2020;38(4):741-751.
- Ramirez JA, Wiemken TL, Peyrani P, et al.; University of Louisville Pneumonia Study Group. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812.
- Peyrani P, Arnold FW, Bordon J, et al. Incidence and mortality of adults hospitalized with community-acquired pneumonia according to clinical course. Chest. 2020;157(1):34-41.
- Burton DC, Flannery B, Bennett NM, et al.; Active Bacterial Core Surveillance/Emerging Infections Program Network. Socioeconomic and racial/ethnic disparities in the incidence of bacteremic pneumonia among US adults. Am J Public Health. 2010;100(10):1904-1911.
- Gadsby NJ, Russell CD, McHugh MP, et al. Comprehensive molecular testing for respiratory pathogens in community-acquired pneumonia. Clin Infect Dis. 2016;62(7):817-823.
- Marchello C, Dale AP, Thai TN, et al. Prevalence of atypical pathogens in patients with cough and community-acquired pneumonia: a meta-analysis. Ann Fam Med. 2016;14(6):552-566.
- Scobie HM, Johnson AG, Suthar AB, et al. Monitoring incidence of COVID-19 cases, hospitalizations, and deaths, by vaccination status – 13 U.S. jurisdictions, April 4–July 17, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(37):1284-1290.
- Burk M, El-Kersh K, Saad M, et al. Viral infection in community-acquired pneumonia: a systematic review and meta-analysis. Eur Respir Rev. 2016;25(140):178-188.
- Ebell MH, Chupp H, Cai X, et al. Accuracy of signs and symptoms for the diagnosis of community-acquired pneumonia: a meta-analysis. Acad Emerg Med. 2020;27(7):541-553.
- Marchello CS, Ebell MH, Dale AP, et al. Signs and symptoms that rule out community-acquired pneumonia in outpatient adults: a systematic review and meta-analysis. J Am Board Fam Med. 2019;32(2):234-247.
- Forstner C, Patchev V, Rohde G, et al.; CAPNETZ Study Group. Rate and predictors of bacteremia in afebrile community-acquired pneumonia. Chest. 2020;157(3):529-539.
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67.
- Self WH, Courtney DM, McNaughton CD, et al. High discordance of chest x-ray and computed tomography for detection of pulmonary opacities in ED patients: implications for diagnosing pneumonia. Am J Emerg Med. 2013;31(2):401-405.
- Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192(8):974-982.
- Montassier E, Javaudin F, Moustafa F, et al. Guideline-based clinical assessment versus procalcitonin-guided antibiotic use in pneumonia: a pragmatic randomized trial. Ann Emerg Med. 2019;74(4):580-591.
- Ye X, Xiao H, Chen B, et al. Accuracy of lung ultrasonography versus chest radiography for the diagnosis of adult community-acquired pneumonia: review of the literature and meta-analysis. PLoS One. 2015;10(6):e0130066.
- Lim WS, Smith DL, Wise MP, et al. British Thoracic Society community acquired pneumonia guideline and the NICE pneumonia guideline: how they fit together. Thorax. 2015;70(7):698-700.
Ebell MH. Community-acquired pneumonia: determining safe treatment in the outpatient setting. Am Fam Physician. 2019;99(12):768-769.
- Bauer TT, Ewig S, Marre R, et al.; CAPNETZ Study Group. CRB-65 predicts death from community-acquired pneumonia. J Intern Med. 2006;260(1):93-101.
- Lim WS, Baudouin SV, George RC, et al.; Pneumonia Guidelines Committee of the BTS Standards of Care Committee. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(suppl 3):1-55.
- Wan YD, Sun TW, Liu ZQ, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest. 2016;149(1):209-219.
- Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among U.S. adults: updated recommendations of the Advisory Committee on Immunization Practices - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):109-117.
- Diao WQ, Shen N, Yu PX, et al. Efficacy of 23-valent pneumococcal polysaccharide vaccine in preventing community-acquired pneumonia among immunocompetent adults: a systematic review and meta-analysis of randomized trials. Vaccine. 2016;34(13):1496-1503.
Centers for Disease Control and Prevention. Adult immunization schedule. Recommendations for ages 19 years or older, United States, 2022. Accessed April 11, 2022. https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html
Kaysin A, Viera AJ. Community-acquired pneumonia in adults: diagnosis and management [published correction appears in Am Fam Physician . 2017;95(7):414]. Am Fam Physician. 2016;94(9):698-706.
Watkins RR, Lemonovich TL. Diagnosis and management of community-acquired pneumonia in adults. Am Fam Physician. 2011;83(11):1299-1306.
- Lutfiyya MN, Henley E, Chang LF, et al. Diagnosis and treatment of community-acquired pneumonia. Am Fam Physician. 2006;73(3):442-450.
Thibodeau KP, Viera AJ. Atypical pathogens and challenges in community-acquired pneumonia. Am Fam Physician. 2004;69(7):1699-1706.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2022 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
- Patient Care & Health Information
- Diseases & Conditions
- Pneumonia and your lungs
Most pneumonia occurs when a breakdown in your body's natural defenses allows germs to invade and multiply within your lungs. To destroy the attacking organisms, white blood cells rapidly accumulate. Along with bacteria and fungi, they fill the air sacs within your lungs (alveoli). Breathing may be labored. A classic sign of bacterial pneumonia is a cough that produces thick, blood-tinged or yellowish-greenish sputum with pus.
Pneumonia is an infection that inflames the air sacs in one or both lungs. The air sacs may fill with fluid or pus (purulent material), causing cough with phlegm or pus, fever, chills, and difficulty breathing. A variety of organisms, including bacteria, viruses and fungi, can cause pneumonia.
Pneumonia can range in seriousness from mild to life-threatening. It is most serious for infants and young children, people older than age 65, and people with health problems or weakened immune systems.
Products & Services
- A Book: Living Medicine
The signs and symptoms of pneumonia vary from mild to severe, depending on factors such as the type of germ causing the infection, and your age and overall health. Mild signs and symptoms often are similar to those of a cold or flu, but they last longer.
Signs and symptoms of pneumonia may include:
- Chest pain when you breathe or cough
- Confusion or changes in mental awareness (in adults age 65 and older)
- Cough, which may produce phlegm
- Fever, sweating and shaking chills
- Lower than normal body temperature (in adults older than age 65 and people with weak immune systems)
- Nausea, vomiting or diarrhea
- Shortness of breath
Newborns and infants may not show any sign of the infection. Or they may vomit, have a fever and cough, appear restless or tired and without energy, or have difficulty breathing and eating.
When to see a doctor
See your doctor if you have difficulty breathing, chest pain, persistent fever of 102 F (39 C) or higher, or persistent cough, especially if you're coughing up pus.
It's especially important that people in these high-risk groups see a doctor:
- Adults older than age 65
- Children younger than age 2 with signs and symptoms
- People with an underlying health condition or weakened immune system
- People receiving chemotherapy or taking medication that suppresses the immune system
For some older adults and people with heart failure or chronic lung problems, pneumonia can quickly become a life-threatening condition.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Many germs can cause pneumonia. The most common are bacteria and viruses in the air we breathe. Your body usually prevents these germs from infecting your lungs. But sometimes these germs can overpower your immune system, even if your health is generally good.
Pneumonia is classified according to the types of germs that cause it and where you got the infection.
Community-acquired pneumonia
Community-acquired pneumonia is the most common type of pneumonia. It occurs outside of hospitals or other health care facilities. It may be caused by:
- Bacteria. The most common cause of bacterial pneumonia in the U.S. is Streptococcus pneumoniae. This type of pneumonia can occur on its own or after you've had a cold or the flu. It may affect one part (lobe) of the lung, a condition called lobar pneumonia.
- Bacteria-like organisms. Mycoplasma pneumoniae also can cause pneumonia. It typically produces milder symptoms than do other types of pneumonia. Walking pneumonia is an informal name given to this type of pneumonia, which typically isn't severe enough to require bed rest.
- Fungi. This type of pneumonia is most common in people with chronic health problems or weakened immune systems, and in people who have inhaled large doses of the organisms. The fungi that cause it can be found in soil or bird droppings and vary depending upon geographic location.
- Viruses, including COVID-19 . Some of the viruses that cause colds and the flu can cause pneumonia. Viruses are the most common cause of pneumonia in children younger than 5 years. Viral pneumonia is usually mild. But in some cases it can become very serious. Coronavirus 2019 (COVID-19) may cause pneumonia, which can become severe.
Hospital-acquired pneumonia
Some people catch pneumonia during a hospital stay for another illness. Hospital-acquired pneumonia can be serious because the bacteria causing it may be more resistant to antibiotics and because the people who get it are already sick. People who are on breathing machines (ventilators), often used in intensive care units, are at higher risk of this type of pneumonia.
Health care-acquired pneumonia
Health care-acquired pneumonia is a bacterial infection that occurs in people who live in long-term care facilities or who receive care in outpatient clinics, including kidney dialysis centers. Like hospital-acquired pneumonia, health care-acquired pneumonia can be caused by bacteria that are more resistant to antibiotics.
Aspiration pneumonia
Aspiration pneumonia occurs when you inhale food, drink, vomit or saliva into your lungs. Aspiration is more likely if something disturbs your normal gag reflex, such as a brain injury or swallowing problem, or excessive use of alcohol or drugs.
Risk factors
Pneumonia can affect anyone. But the two age groups at highest risk are:
- Children who are 2 years old or younger
- People who are age 65 or older
Other risk factors include:
- Being hospitalized. You're at greater risk of pneumonia if you're in a hospital intensive care unit, especially if you're on a machine that helps you breathe (a ventilator).
- Chronic disease. You're more likely to get pneumonia if you have asthma, chronic obstructive pulmonary disease ( COPD ) or heart disease.
- Smoking. Smoking damages your body's natural defenses against the bacteria and viruses that cause pneumonia.
- Weakened or suppressed immune system. People who have HIV / AIDS , who've had an organ transplant, or who receive chemotherapy or long-term steroids are at risk.
Complications
Even with treatment, some people with pneumonia, especially those in high-risk groups, may experience complications, including:
- Bacteria in the bloodstream (bacteremia). Bacteria that enter the bloodstream from your lungs can spread the infection to other organs, potentially causing organ failure.
- Difficulty breathing. If your pneumonia is severe or you have chronic underlying lung diseases, you may have trouble breathing in enough oxygen. You may need to be hospitalized and use a breathing machine (ventilator) while your lung heals.
- Fluid accumulation around the lungs (pleural effusion). Pneumonia may cause fluid to build up in the thin space between layers of tissue that line the lungs and chest cavity (pleura). If the fluid becomes infected, you may need to have it drained through a chest tube or removed with surgery.
- Lung abscess. An abscess occurs if pus forms in a cavity in the lung. An abscess is usually treated with antibiotics. Sometimes, surgery or drainage with a long needle or tube placed into the abscess is needed to remove the pus.
To help prevent pneumonia:
- Get vaccinated. Vaccines are available to prevent some types of pneumonia and the flu. Talk with your doctor about getting these shots. The vaccination guidelines have changed over time so make sure to review your vaccination status with your doctor even if you recall previously receiving a pneumonia vaccine.
- Make sure children get vaccinated. Doctors recommend a different pneumonia vaccine for children younger than age 2 and for children ages 2 to 5 years who are at particular risk of pneumococcal disease. Children who attend a group child care center should also get the vaccine. Doctors also recommend flu shots for children older than 6 months.
- Practice good hygiene. To protect yourself against respiratory infections that sometimes lead to pneumonia, wash your hands regularly or use an alcohol-based hand sanitizer.
- Don't smoke. Smoking damages your lungs' natural defenses against respiratory infections.
- Keep your immune system strong. Get enough sleep, exercise regularly and eat a healthy diet.
- Pneumonia. National Heart, Lung, and Blood Institute. http://www.nhlbi.nih.gov/health/health-topics/topics/pnu. Accessed April 15, 2016.
- AskMayoExpert. Community-acquired pneumonia (adult). Rochester, Minn.: Mayo Foundation for Medical Education and Research; 2014.
- Goldman L, et al., eds. Overview of pneumonia. In: Goldman-Cecil Medicine. 25th ed. Philadelphia, Pa.: Saunders Elsevier; 2016. http://www.clinicalkey.com. Accessed April 18, 2016.
- Schauner S, et al. Community-acquired pneumonia in children: A look at the IDSA guidelines. Journal of Family Practice. 2013;62:9.
- Attridge RT, et al. Health care-associated pneumonia: An evidence-based review. American Journal of Medicine. 2011;124:689.
- Hunter JD. Ventilator associated pneumonia. BMJ. 2012;344:e3325.
- Dockrell DH, et al. Pneumococcal pneumonia: Mechanisms of infection and resolution. Chest. 2012;142:482.
- Reynolds RH, et al. Pneumonia in the immunocompetent patient. British Journal of Radiology. 2010;83:998.
- Remington LT, et al. Community-acquired pneumonia. Current Opinion Pulmonary Medicine. 2014;20:215.
- Centers for Disease Control and Prevention. Adults: Protect yourself with pneumococcal vaccines. http://www.cdc.gov/features/adult-pneumococcal/. Accessed April 15, 2016.
- Marrie TJ, et al. Pneumococcal pneumonia in adults. http://www.uptodate.com/home. Accessed April 15, 2016.
- Barbara Woodward Lips Patient Education Center. Care following hospitalization for community-acquired pneumonia. Rochester, Minn.: Mayo Foundation for Medical Education and Research; 2013.
- AskMayoExpert. Community-acquired pneumonia (pediatric). Rochester, Minn.: Mayo Foundation for Medical Education and Research; 2014.
- Barson WJ. Community-acquired pneumonia in children: Outpatient treatment. http://www.uptodate.com/home. Accessed April 15, 2016.
- File TM. Treatment of community-acquired pneumonia in adults in the outpatient setting. http://www.uptodate.com/home. Accessed April 20, 2016.
- Chang CC, et al. Over-the-counter ( OTC ) medications to reduce cough as an adjunct to antibiotics for acute pneumonia in children and adults. Cochrane Database of Systematic Reviews. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD006088.pub4/full. Accessed April 20, 2016.
- Mycoplasma pneumoniae infection. Centers for Disease Control and Prevention. http://www.cdc.gov/pneumonia/atypical/mycoplasma/. Accessed April 20, 2016.
- Barson WJ. Community-acquired pneumonia in children: Clinical features and diagnosis. http://www.uptodate.com/home. Accessed April 20, 2016.
- Olson EJ (expert opinion). Mayo Clinic, Rochester, Minn. May 1, 2016.
- AskMayoExpert. COVID-19: Outpatient. Mayo Clinic; 2020.
- Chest X-ray showing pneumonia
- Walking pneumonia
Associated Procedures
- Chest X-rays
- Extracorporeal membrane oxygenation (ECMO)
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.

Introduction
Acknowledgements, financial disclosure and conflicts of interest, author contributions, risk factors for community-acquired pneumonia in adults: a systematic review of observational studies.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Jordi Almirall , Mateu Serra-Prat , Ignasi Bolíbar , Valentina Balasso; Risk Factors for Community-Acquired Pneumonia in Adults: A Systematic Review of Observational Studies. Respiration 23 August 2017; 94 (3): 299–311. https://doi.org/10.1159/000479089
Download citation file:
- Ris (Zotero)
- Reference Manager
We performed a systematic review of the literature to establish conclusive evidence of risk factors for community-acquired pneumonia (CAP). Observational studies (cross-sectional, case-control, and cohort studies) the primary outcome of which was to assess risk factors for CAP in both hospitalized and ambulatory adult patients with radiologically confirmed pneumonia were selected. The Newcastle-Ottawa Scale specific for cohort and case-control designs was used for quality assessment. Twenty-nine studies (20 case-control, 8 cohort, and 1 cross-sectional) were selected, with 44.8% of them focused on elderly subjects ≥65 years of age and 34.5% on mixed populations (participants' age >14 years). The median quality score was 7.44 (range 5-9). Age, smoking, environmental exposures, malnutrition, previous CAP, chronic bronchitis/chronic obstructive pulmonary disease, asthma, functional impairment, poor dental health, immunosuppressive therapy, oral steroids, and treatment with gastric acid-suppressive drugs were definitive risk factors for CAP. Some of these factors are modifiable. Regarding other factors (e.g., gender, overweight, alcohol use, recent respiratory tract infections, pneumococcal and influenza vaccination, inhalation therapy, swallowing disorders, renal and liver dysfunction, diabetes, and cancer) no definitive conclusion could be established. Prompt assessment and correction of modifiable risk factors could reduce morbidity and mortality among adult CAP patients, particularly among the elderly.
Community-acquired pneumonia (CAP) remains an important cause of morbidity and mortality in industrialized countries. In the general adult population, the annual incidence of CAP ranges between 1.6 and 13.4 cases per 1,000 inhabitants, 22-51% of whom require inpatient care, with a lethality of 3-24%. The mortality rate varies between 0.1 and 0.7 per 1,000 persons each year [ 1 ]. Despite considerable research, great improvement in medical care, and advances in antimicrobial therapy with the availability of active antibiotics against the known causative pathogens, mortality from CAP has not improved during the last decades. Targeted risk reduction interventions based on understanding and recognizing risk factors for CAP are of primary importance in reducing CAP-related death rates [ 2 ].
So far, only 1 systematic review analyzing risk factors for CAP has been published in the literature [ 3 ]. This study, however, was published in German and the search was limited to the MEDLINE database, including articles published between 1996 and December 2003. In the last years, new data have been published, sometimes corroborating previous evidence, sometimes with controversial results and in other occasions suggesting new possible risk factors that need to be further studied. These studies have different designs, methodological quality, and populations, have measured different risk factors, or have assessed the same risk factors in different ways. Therefore, they not always report conclusive and concordant results.
Because of this heterogeneity, there is a need to update all the evidence to reach a more definitive conclusion for the studied factors. Our systematic review not only updates the earlier review of Kohlhammer et al. [ 3 ] published in 2005, but also summarizes the scientific evidence from observational studies about risk factors for CAP in adults.
Study Selection
Observational studies in which the primary outcome was to assess risk factors for CAP in both hospitalized and ambulatory adult patients with radiologically confirmed pneumonia were selected for the study. Observational studies included cross-sectional, case-control, and cohort study designs. Exclusion criteria were as follows: (a) studies addressing other aspects of CAP, such as bacteremia, health care-acquired pneumonia, hospital-acquired pneumonia, and CAP caused by antibiotic-resistant pathogens; (b) studies focused on prognostic or treatment-related factors for CAP; and (c) studies of CAP carried out in specific populations, such as patients with diabetes mellitus, chronic obstructive pulmonary disease (COPD), or HIV infection.
The search was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [ 4 ]. The electronic databases PubMed MEDLINE (US National Library of Medicine, Bethesda, MD, USA) and EMBASE Elsevier were systematically searched until June 30, 2015. The search strategy is detailed in online supplementary Table 1 (for all online suppl. material, see www.karger.com/doi/10.1159/000479089 ). Reports published in English, Spanish, and French were considered. Potentially relevant articles were screened for eligibility independently in an unblinded standardized manner by the two reviewers (J.A. and V.B.), initially by abstract and then by full text if necessary, to determine whether they met the inclusion criteria. Disagreement between the reviewers was resolved by discussion with the rest of the researchers. The reference lists of the identified articles were manually searched for additional studies that may have been overlooked using the computer-assisted search strategy.
Data Extraction
We developed a data extraction sheet, pilot-tested it on 3 randomly selected included studies, and refined it accordingly. One author (J.A.) extracted the data from the included studies and a second author (M.S.-P.) checked the extracted data. Disagreements were resolved by discussion between the two review authors. The following information was extracted from each included paper: authors and year of publication; study design; characteristics of study participants (number, mean age, and gender); and risk factors for CAP. In order to reinforce the confidence of the predictive effect of risk factors for CAP, only those factors that were individually analyzed in at least 3 studies were considered. The number of at least 3 studies was arbitrarily decided on, because of the possibility of disagreement between only 2 studies. Risk factors were grouped into the broad categories of sociodemographic and lifestyle factors, comorbidities or clinical conditions, and therapeutic factors.
Quality Assessment
The quality of all included studies was evaluated using the Newcastle-Ottawa Scale (NOS) for nonrandomized studies. The NOS specific for cohort and case-control designs was used [ 5 ], with an overall quality score ranging from 0 (minimum) to 9 (maximum) stars. Discrepancies in quality assessment were discussed and resolved by three authors (J.A., I.B, and V.B.).
Data Synthesis and Analysis
The quality and concordance of the evidence was used to develop standardized statements for each risk factor. For each analysis in each individual study, the resulting effect of the risk factors was categorized as “significant risk factor,” “significant protective factor,” or “nonsignificant risk factor.” Only the results of multivariate adjusted models were taken into account. If a given category (significant risk factor, significant protective factor, or nonsignificant risk factor) was present in >66% of the studies and the other categories in ≤34% of the studies, and the given category had no clearly lower methodological quality, then standardized statements of “clear risk factor,” “clear protective factor,” or “no effect” were established; otherwise, a statement of “no definitive conclusion” was considered (Table 1 ). No clearly lower methodological quality was considered if the difference in median NOS scores between categories was <1 point.
Standardized definitions of the confidence of the predictive effect of risk factors for CAP
Whenever a factor was analyzed more than once in a study (e.g., it was included in ≥2 predictive models for CAP), it was counted in all the categories that applied. In these cases, the percentages of the “risk/protection/no effect” categories added up to >100%, and the standardized statement was extended as follows: if two categories had >66% of the studies and one category had a higher methodological quality than the other, then the statement was “possible risk factor/possible protective factor/possible no effect.” Each factor was classified according to its predictive profile (risk factor, protective factor, or no effect) and to the standardized consideration of the confidence that could be placed on that profile (clear, possible, or no definitive conclusion). To analyze and synthesize the evidence, for each risk factor we present the number of publications and the number of analyses in the three possible categories of effect, specifying the number of publications on elderly patients. We also present the median score (range) on the NOS, and, for those that are clearly risk or protective factors, the estimated effect independent from the multivariate models. It is expressed as the median (range) of all the adjusted odds ratios (OR), from the categories of clear risk/protective, and from those publications that presented risk factors in a homogeneous way to allow its synthesis (i.e., similar cutoff points or categorization and similar measure of the risk factor).
Search Results
The electronic search strategy identified a total of 2,731 records from electronic databases, 114 of which were retrieved for full paper evaluation. Of these 114 full papers, 36 met the eligibility criteria and were subjected to data extraction [ 2,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40 ]. However, these 36 articles referred to only 29 studies, since in 5 cases the same study or different aspects of the same study were published in more than 1 article [ 2,7,8,10,11,12,24,25,32,33,39,40 ]. Therefore, a total of 29 studies were included for qualitative synthesis (Fig. 1 ).

Flowchart of the studies included in this review. CAP, community-acquired pneumonia.
Characteristics of the Studies
Twenty studies (70%) had a case-control design, 8 (27.6%) had a cohort design, and 1 (3.4%) had a cross-sectional design (Table 2 ). The case-control and cross-sectional studies included 29,018 participants. The cohort studies were prospective in 6 cases and retrospective in 2, and overall they included more than 140,000 participants. The cohort studies were published between 1994 and 2015, with the longest follow-up being 16 years and 11 months. Most studies focused on elderly subjects ≥65 years of age (44.8%) or mixed populations where the participants' age was >14 years (34.5%), and a few studies (13.8%) considered only the age range between 18 and 60 years. The definitions used for cases and controls, as well as exposed and nonexposed subjects, in each of the included studies are detailed in online supplementary Table 2.
General characteristics of the 29 studies included in the systematic review of risk factors for CAP

The median quality score of the studies was 7.44 (range 5-9). The median quality score of the case-control and cross-sectional studies was 7.42, which was very similar to that of the cohort studies (7.5).
Evidence Synthesis
Sociodemographic and Lifestyle Factors
Clear risk factors for CAP were age, smoking, poor nutritional status, and environmental exposures to different substances (metals, dust, fumes, etc.) (Table 3 ). No definitive conclusions were drawn for the effect of male gender and alcohol use. No effect was demonstrated for overweight and passive smoking in the overall adult population.
Selected sociodemographic and lifestyle factors

Comorbidities or Clinical Conditions
As shown in Table 4 , functional impairment, COPD/chronic bronchitis, asthma, a history of CAP, and orodental and/or periodontal diseases were clear risk factors for CAP. Recent upper respiratory tract infections showed to be a possible risk factor. Chronic heart disease, dysphagia, cancer, chronic liver disease, and diabetes allowed no definitive conclusion, while chronic renal disease showed no effect.
Comorbidities or clinical conditions as risk factors for CAP

Therapeutic Factors
Immunosuppressive treatment, use of oral steroids, and proton pump inhibitors or H 2 blockers were classified as clear risk factors for CAP (Table 5 ). Treatment with antibiotics for any reason prior to admission for CAP and influenza vaccines had no effect, whereas the role of inhaled drugs remained in the “no definitive conclusion” category. Pneumococcal vaccination (polysaccharide 23V) was classified in the “possibly no effect” category.
Therapeutic risk factors for CAP

The results of other, less studied factors (i.e., individually analyzed in <3 studies) are detailed in Table 6 .
Other, less studied potential risk factors for CAP

This study shows conclusive evidence of age, smoking habit, environmental exposures, poor nutritional status, functional impairment, chronic bronchitis/COPD, asthma, previous CAP (in the past 1 or 2 years), poor oral health, immunosuppressive therapy, oral steroids, and treatment with proton pump inhibitors or H 2 antagonists as risk factors for CAP in adults. No effect was found for other factors, including overweight, passive smoking, influenza vaccine, use of antibiotics before CAP, and chronic renal disease. Also, the role of male gender, alcohol use, passive smoking in the older age subgroup, heart disease, dysphagia, cancer, chronic liver disease, diabetes, use of inhalers, and pneumococcal vaccine remained inconclusive. A large number of other potential risk factors for CAP (detailed in Table 6 ) need to be further studied.
Age is a well-known risk factor for CAP, especially in older people. In 2 studies carried out on subjects of all ages, significant results were only found for the oldest segment of the population [ 14,20 ], whereas in 1 study on young people [ 14 ], age was not a significant factor. These findings may suggest a nonlinear effect, with older age as a risk factor for CAP. Age may also modify or interact with other factors in elderly people. Active smoking has a direct and independent effect on the risk of pneumonia, but it may also act indirectly, causing COPD, which is a well-recognized risk factor for CAP. In relation to gender, the results of the studies are conflicting. In our systematic review, male gender was a significant risk factor in 4 analyses and a nonsignificant factor in 3 studies. Women are often less represented than men in COPD populations, and typically smoke substantially less than men. Only 3 studies [ 2,21,30 ] assessed the effect of passive smoking, 2 of which showed no significant effect [ 2,21 ]. However, the third study [ 30 ], of somewhat greater quality, observed a significant effect in elderly subjects, in whom the lung defense mechanisms might be insufficient to counteract the aggressive invasion of other people's cigarette smoke.
Poor nutritional status, which in the different studies encompassed hypoalbuminemia, hypoproteinemia, malnourishment, or a low nutritional score, was a strong predictor of CAP. Despite the lack of a standardized definition to identify frail older adults, functional impairment assessed according to different criteria was a clear risk factor for CAP.
Environmental substances are known to be involved in the pathogenesis of lung diseases (bronchitis, bronchiolitis, asthma, COPD, and lung cancer) and this review shows their effect on CAP. However, this effect may vary according to the different substances (metals, dust, fumes, etc.), settings (home, occupational environment), extent, and definitions of environmental exposures. The transmission, control, and treatment of pneumonias acquired at the workplace represent intriguing and challenging areas of concern to epidemiologists and clinicians.
We also found that chronic bronchitis, COPD, and asthma were definitive risk factors for CAP. In some studies [ 8,23 ], an effect of inhaled drugs on the risk of CAP was observed in COPD and asthma patients after adjusting for the effect of other respiratory diseases (and their concomitant treatments) and other nonrespiratory risk factors for CAP, including vaccines [ 8 ]. There are 8 studies assessing the effect of inhalation therapy on the risk of CAP. Four of them showed a significant effect of inhalers as a risk factor for CAP, while the other 4 studies, of similar methodological quality, obtained no significant and conclusive results. The lack of consistency among the studies may be due to differences in study design, type of the drug inhaled, number of puffs per day, or severity of the underlying disease (asthma or COPD) or differences in the use of spacers. Thus, a definitive conclusion regarding inhalation therapy has not yet been reached, and further research is required.
Likewise, in the observational studies, “possibly no effect” or “no effect” was obtained for the role of 23-valent pneumococcal polysaccharide and influenza vaccination, respectively, as a protective factor against CAP. The protective effect of vaccination should be better assessed in randomized clinical trials. The results of this systematic review agree with those of a meta-analysis of the efficacy of pneumococcal vaccination in adults, which also concluded that vaccination does not appear to be effective in preventing pneumonia [ 41,42 ]. However, meta-analyses have produced conflicting results for the efficacy of 23-valent pneumococcal polysaccharide vaccine [ 43,44 ]. A 13-valent pneumococcal conjugate vaccine (PCV13) is now available for the prevention of pneumonia and invasive pneumococcal disease caused by PCV13 serotypes in adults. In a randomized, double-blind, placebo-controlled trial involving 84,496 adults ≥65 years of age, PCV13 was effective in preventing vaccine-type pneumococcal, bacteremic, and nonbacteremic CAP and vaccine-type invasive pneumococcal disease, but not in preventing CAP from any cause [ 45 ]. The role of influenza vaccination as a protective factor against CAP is also unclear [ 46,47 ].
On the other hand, it has been argued that inappropriate antibiotic use increases bacterial resistance to common antibiotics and also alters the normal bacterial flora of the host [ 48 ]. Nonetheless, only 1 out of 3 reviewed studies found an association between a recent history of taking antibiotics and CAP [ 23 ]. Hence, the few available studies and their lower quality lead us to conclude that previous consumption of antibiotics has no effect on the risk of CAP. Ideally, it would be desirable to have more studies to answer this important question.
It has been reported that the use of gastric acid-suppressive therapy (proton pump inhibitors and H 2 receptor antagonists) increases the risk of CAP [ 49 ], particularly if treatment has been recently started [ 18,31 ]. It has been suggested that a reduction of gastric acid secretion facilitates pathogen colonization of the upper gastrointestinal tract and oral infections [ 49 ]. However, other studies do not support a pharmacological effect of gastric acid suppressors on the risk of CAP [ 2,50 ]. This review concludes that treatment with gastric acid-suppressive drugs is a risk factor for CAP. This finding is clinically relevant because acid-suppressive drugs are commonly prescribed for complaints of dyspepsia and gastroesophageal reflux disease and their effectiveness in the treatment of upper gastrointestinal symptoms is excellent. However, the use of these drugs may be reconsidered for patients with other risk factors for CAP, such as elderly subjects, persons with COPD or asthma, and immunocompromised persons.
Use of oral steroids and immunosuppressive therapy were also clear risk factors for CAP. The increased risk of infectious complications is an important safety concern when prescribing immunosuppressive therapy. Prevention of infection is a key management strategy, to which early recognition of other associated risk factors for CAP should be added.
Interestingly, poor oral health and orodental diseases increased the risk of CAP both in elderly subjects and in the general population. Dental plaque and dental prostheses favor colonization and may constitute a reservoir for respiratory pathogens [ 51 ]. Prevention of the accumulation of plaque and bacterial colonization, particularly in subjects with dental prostheses, dental caries, and periodontal disease, is an important practical consequence of our findings. Regular dental examinations and good oral hygiene should be recommended.
Other comorbidities, such as chronic heart disease, renal dysfunction, liver disease, diabetes, cancer, and dysphagia or swallowing disorders, had no effect, or no definitive conclusions could be drawn. Moreover, the effect of other potential risk factors for CAP identified in the present review should be confirmed in further studies. These include a large number of factors, such as the role of civil status, ethnicity, usual contact with children, deficient social support, sudden temperature changes at the workplace, impaired quality of life, physical activity, previous outpatient visits and hospital admissions, HTLV-1 (human T-lymphotropic virus 1) and HIV infections, epilepsy, stroke, thyroid dysfunction, connective tissue diseases, pulmonary tuberculosis, anemia, depression, dementia, and oxygen therapy, as well as treatment with amiodarone, benzodiazepines, N-acetylcysteine, atypical antipsychotics, aminophylline, narcotics, nonsteroidal anti-inflammatory drugs, motility agents, iron supplementation, vitamins, digoxin, and diuretics. All these factors have been inconsistently assessed in a few individual studies, and no definitive conclusions could be reached.
Observational studies are vulnerable to various sources of bias, and the evidence obtained by them is less robust than evidence derived from experimental studies. The present findings should be interpreted taking into account this limitation, but the clinical questions posed in this review could only be answered reasonably through observational designs. One of the main difficulties of this systematic review lies in the unavoidable heterogeneity of studies in terms of populations, risk factors analyzed, lengths of follow-up, and statistical methods, including differences in adjusting for confounding variables and covariates both within and between studies. By contrast, strengths of the present systematic review include the comprehensive literature search, the use of sound methodological review techniques, the use of a well-established tool (NOS) for quality assessment of the individual studies, the high quality scores obtained for the included studies, and the weighted conclusions based on the quality of the evidence.
The present results provide robust current evidence of the importance of age, smoking, malnutrition, environmental exposures, previous CAP, chronic bronchitis/COPD, asthma, functional impairment, poor dental health, immunosuppressive therapy, oral steroids, and gastric acid-suppressive drugs as definitive risk factors for CAP. Clinicians should be aware of these risk factors, because some of them are modifiable and amenable to effective interventions. Special attention should be devoted to oral hygiene, antacid drug reduction, functional impairment monitoring, and rehabilitation protocols. Implementation of the findings of the present systematic review into daily practice is clinically relevant, since early recognition and adequate management of those factors that are modifiable may reduce CAP-associated morbidity and mortality among adults, particularly elderly individuals.
The authors thank Centro Cochrane Iberoamericano for methodological support and Marta Pulido, MD, for editing the manuscript and editorial assistance. The fees for medical editing were paid by Fundació Privada Salut del Consorci Sanitari del Maresme.
No conflicts of interest are to be declared. No funding in the form of grants or other support was received for this study.
J.A. participated in the study design, selection of studies for inclusion in the review, assessment of the quality of articles, interpretation of the analysis, and writing of the manuscript. M.S.-P. carried out the search of the literature, selection of studies for inclusion in the review, selection of criteria for the analysis, assessment of the quality of articles, statistical analysis, interpretation of the data, and writing of the manuscript. I.B. participated in the study design, methodological assessment, selection of criteria for the analysis, assessment of the quality of articles, and writing of the manuscript. V.B. was involved in the methodological assessment, assessment of the quality of articles, analysis of the data, synthesis of the results, and critical review of the content of the manuscript. All authors read and approved the final manuscript.
Email alerts
Citing articles via, related articles.
- Online ISSN 1423-0356
- Print ISSN 0025-7931

INFORMATION
- Contact & Support
- Information & Downloads
- Rights & Permissions
- Terms & Conditions
- Catalogue & Pricing
- Policies & Information
- People & Organization
- Stay Up-to-Date
- Regional Offices
- Community Voice
SERVICES FOR
- Researchers
- Healthcare Professionals
- Patients & Supporters
- Health Sciences Industry
- Medical Societies
- Agents & Booksellers
Karger International
- S. Karger AG
- P.O Box, CH-4009 Basel (Switzerland)
- Allschwilerstrasse 10, CH-4055 Basel
- Tel: +41 61 306 11 11
- Fax: +41 61 306 12 34
- Contact: Front Office
- Experience Blog
- Privacy Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Open access
- Published: 08 June 2023
Incidence and risk factors of pneumococcal pneumonia in adults: a population-based study
- Olga Ochoa-Gondar 1 ,
- Verónica Torras-Vives 1 ,
- Cinta de Diego-Cabanes 1 , 2 ,
- Eva M. Satué-Gracia 1 , 2 ,
- Angel Vila-Rovira 1 ,
- María J. Forcadell-Perisa 1 ,
- Domingo Ribas-Seguí 1 ,
- Clara Rodríguez-Casado 3 &
- Angel Vila-Córcoles 1 , 2
BMC Pulmonary Medicine volume 23 , Article number: 200 ( 2023 ) Cite this article
1949 Accesses
2 Citations
7 Altmetric
Metrics details
Infection caused by Streptococcus pneumoniae, mainly invasive pneumococcal disease (IPD) and pneumococcal pneumonia (PP), are a major public health problem worldwide. This study investigated population-based incidence and risk of PP among Catalonian persons ≥ 50 years-old with and without specific underlying conditions/comorbidities, examining the influence of single and multi-comorbidities in the risk of suffering PP.
Population-based cohort study involving 2,059,645 persons ≥ 50 years-old in Catalonia, Spain, who were retrospectively followed between 01/01/2017-31/12/2018. The Catalonian information system for development of research in primary care (SIDIAP) was used to establish baseline characteristics of the cohort (comorbidities/underlying conditions), and PP cases were collected from discharge codes (ICD-10: J13) of the 68 referral Catalonian hospitals.
Global incidence rate (IR) was 90.7 PP cases per 100,000 person-years, with a 7.6% (272/3592) case-fatality rate (CFR). Maximum IRs emerged among persons with history of previous IPD or all-cause pneumonia, followed by haematological neoplasia (475.0), HIV-infection (423.7), renal disease (384.9), chronic respiratory disease (314.7), liver disease (232.5), heart disease (221.4), alcoholism (204.8), solid cancer (186.2) and diabetes (159.6). IRs were 42.1, 89.9, 201.1, 350.9, 594.3 and 761.2 in persons with 0, 1, 2, 3, 4 and ≥ 5 comorbidities, respectively. In multivariable analyses, HIV-infection (hazard ratio [HR]: 5.16; 95% CI: 3.57–7.46), prior all-cause pneumonia (HR: 3.96; 95% CI: 3.45–4.55), haematological neoplasia (HR: 2.71; 95% CI: 2.06–3.57), chronic respiratory disease (HR: 2.66; 95% CI: 2.47–2.86) and prior IPD (HR: 2.56; 95% CI: 2.03–3.24) were major predictors for PP.
Apart of increasing age and immunocompromising conditions (classically recognised as high-risk conditions), history of prior IPD/pneumonia, presence of chronic pulmonary/respiratory disease and/or co-existing multi-comorbidity (i.e., two or more underlying conditions) are major risk factors for PP in adults, with an excess risk near to immunocompromised subjects. Redefining risk categories for PP, including all the above-mentioned conditions into the high-risk category, could be necessary to improve prevention strategies in middle-aged and older adults.
Peer Review reports
Infections caused by Streptococcus Pneumoniae , mainly invasive pneumococcal disease (IPD) and pneumococcal pneumonia (PP), are a major cause of morbidity and mortality around the world. Young children, individuals with at-risk or immunocompromising conditions and elderly people support the greatest burden of pneumococcal disease, with higher incidence and mortality in these persons [ 1 ].
The incidence of IPD (which includes mainly bacteremic PP, but also pneumococcal meningitis, sepsis and non-focal bacteremias) is well documented, with an incidence of approximately 10–50 IPD cases per 100,000 person-years in developed countries. [ 1 , 2 , 3 , 4 ] However, the true incidence of PP (which includes bacteremic PP but mostly non-bacteremic PP) is not well known considering difficulties in characterizing non-bacteremic PP cases. [ 3 , 5 ] Indeed, there is very large difference between PP incidence rates (ranging from 68 to 7000 cases per 100,000 person-years) reported by different studies [ 6 ].
Regarding risk factors to suffer pneumococcal infections, it is well documented that some conditions such as anatomical or functional asplenia, immunocompromised status, presence of chronic illnesses (e.g., chronic pulmonary/respiratory disease, heart disease, diabetes mellitus), high-risk behaviours (e.g., alcoholism and/or smoking) and low socioeconomic status are well recognised risk factors for suffering IPD. [ 1 , 2 , 7 ] However, data on risk factors for suffering PP is scarce in the literature. On this concern, the existing population-based epidemiological data on PP must to be updated considering that the role of pneumococcus as causative pathogen of pneumonia could have decreased in recent years (after routine anti-pneumococcal vaccination use). [ 8 , 9 ] Of note, apart of the recognised role of the above mentioned underlying risk conditions, few is known about the possible effect of multiple concurrent risk conditions (multi-comorbidity) on the risk of suffering IPD/PP [ 10 ].
The present study aims to update population-based incidence and risk of hospitalisation from PP during 2017–2018 in the Catalonian general population ≥ 50 years-old examining the influence of different specific underlying medical conditions/comorbidities on the risk of suffering PP. We also assessed the role of concurrent multi-comorbidity on the risk of PP. Data assessing pneumococcal vaccination effectiveness in the same study cohort have been previously published. [ 11 , 12 ].
Design, setting and study population
This is a population-based retrospective cohort study involving 2,059,645 Catalonian middle-aged and older adults, who were all persons ≥ 50 years-old (birth day date before 01/01/1967) affiliated to the 274 Primary Care Centres (PCCs) managed by the Catalonian Health Institute (ICS, Institut Catala de la Salut ) around Catalonia (Spain).
In Catalonia (a Spanish region with 7.5 million people) there are 358 PCCs, of which 274 (76.5%) are managed by the ICS and 84 are managed by other providers. The analysed cohort (n = 2,059,645 persons ≥ 50 years) represented a 72.6% of the total 2,838,002 Catalonian inhabitants in this age strata according to census data on January 2017 [ 13 ]. In the study setting, pneumococal vaccines are routinely administered to the elderly (the 23-valent pneumococcal polysaccharide vaccine [PPsV23] publicly funded since the 2000s), high-risk individuals (PPsV23 and/or 13-valent pneumococcal conjugate vaccine [PCV13], publicly funded since 2012) and infants (universal free PCV13 publicly funded since 2016) [ 14 ].
Cohort members were followed since the study start date (01/01/2017) until the occurrence of any event, death, disenrollment from the PCC, or until the end of two-year follow-up (31/12/2018). The study was approved by the ethical committee of the Institution (Ethics Committee IDIAP Jordi Gol, file 20/065-PCV) and was conducted in accordance with the general principles for observational studies [ 15 ].
Data sources
The “Information System for the Development of Research of the Primary Care” (SIDIAP) of Catalonia, [ 16 , 17 ] which compiles administrative data and clinical information contained in the electronic PCC’s medical records (coded by the International Classification of Diseases, 10th Revision, ICD-10) was used to identify demographic characteristics, comorbidities and underlying medical conditions of cohort members at baseline.
To identify study events (hospitalisations from PP) occurred among cohort members across the study period, we used the national surveillance system for hospital discharge data (“ Conjunto Mínimo Básico de Datos ”, CMBD). The CMBD System, maintained by the Spanish Ministry of Health, includes 98% of Spanish hospitals, encompassing an estimated 99.5% of the Spanish population (covered in the National Health Care System by a compulsory health insurance) [ 18 ]. In the present study we used CMBD hospital discharge codes, coded according to the ICD-10 codes, reported during 2017 and 2018 from the 68 Catalonian hospitals. Methodology linking both SIDIAP and CMBD databases before analyses has been extensively described elsewhere [ 11 ].
Outcome definitions
Pneumococcal pneumonia (PP) was defined on the basis of hospital discharge codes reported by the CMBD in hospitalisations occurred among cohort members from January 1, 2017 to December 31, 2018 (ICD-10 code J13, any listed position). According institutional guidelines, [ 19 ] participating hospitals applied similar diagnoses checklist and treatment for patients with a clinical suspicion of pneumonia (which is established on the basis of an acute respiratory illness, with evidence of a new infiltrate in a chest radiograph), being blood/sputum cultures and urinary antigen testing used as conventional diagnostic techniques (performed according to the attending physician in each case). Code J13 is assigned on the basis of a laboratory-confirmed diagnosis (i.e., positive blood/sputum culture or urinary antigen test). Case-fatality was considered when the patient deceased (by any cause) within hospital stay. Deaths from any cause occurred among cohort members across study period were captured by administrative data (vital status), which is periodically updated in the SIDIAP database.
Covariables
Baseline covariables were age, sex, history of hospitalisation from IPD or all-cause pneumonia during previous two-years, pneumococcal vaccination (PCV13/PPsV23) and influenza vaccination status, presence of high-risk/immunocompromising conditions (asplenia, immunodeficiency, HIV infection, chronic severe renal disease, solid organ or haematological neoplasia and/or immunosuppressive treatment), and presence of at-risk conditions (chronic pulmonary/respiratory disease, chronic heart disease, diabetes mellitus, chronic liver disease, alcoholism and smoking). Criteria used to define underlying risk conditions are described in the Appendix.
Statistical analyses
Incidence rates (IRs) were calculated as person-years, considering that the numerator was the number of events and the denominator was the sum of the persons-time contributed to each cohort member during the study period. Only a first episode of hospitalisation from pneumonia during the study period was considered and, therefore, IRs do not include multiple events per person. Confidence intervals (CIs) for the IRs were calculated assuming a Poisson distribution for uncommon events. Chi-squared or Fisher’s tests, as appropriate, were used to calculate p-values in the comparison of categorical variables.
Cox regression analyses were used to calculate unadjusted and multivariable-adjusted hazards ratios (HRs) and evaluate the association between baseline conditions and the time to the first outcome during the study period [ 20 ]. We developed two regression analysis: in the first (model 1) specific comorbidities/risk conditions were considered individually; in the second (model 2) we considered multi-comorbidity (number of comorbidities). The final multivariable models were adjusted by all significant variables plus pneumococcal and influenza vaccine status (which were judged epidemiologically relevant variables). All results were expressed with 95% CIs. Statistical significance was set at p < 0.05 (two-tailed). Data was analysed by using IBM SPSS Statistics for Windows, version 24 (IBM Corp., Armonk, N.Y., USA).
Characteristics of the study cohort
The study cohort included 2,059,645 individuals, 951,011 (46.2%) men and 1,108,634 (53.8%) women, with a mean age of 66 years-old (Standard Deviation: 11.4) at baseline. By age groups, 1,040,009 (50.5%) cohort members were 50–64 years-old, 689,342 (33.5%) were 65–79 years-old and 330,294 (16%) were 80 years or older.
Overall, 1,055,206 (51.2%) cohort members were healthy subjects (persons without comorbidities/underlying risk conditions), 800,992 (38.9%) were at-risk subjects (immunocompetent persons with at least one at-risk condition) and 203,447 (9.9%) were high-risk subjects (one or more immunocompromising condition).
Considering multi-comorbidity, 668,608 (32.5%) cohort members had one comorbidity/risk condition alone, 244,543 (11.9%) had two, 70,688 (3.4%) had three, 16,851 (0.8%) had four and 3749 (0.2%) had five or more comorbidities/risk conditions. Table 1 shows baseline characteristics of cohort members (prevalence of specific comorbidities/underlying risk conditions and vaccinations’ history) according to age strata.
Time follow-up, number of events, incidence and case-fatality rates
Across study period, 83,440 (4.1%) cohort members died and 51,175 (2.5%) moved or were lost subjects. Overall, cohort members were observed for a total of 3,958,528 person-years.
An amount of 3592 cohort members had a first episode of hospitalisation from PP across study period (1997 [55.6%] men and 1595 [44.4%] women; 761 [21.2%] in 50–64 years old, 1313 [36.6%] in 65–79 years old and 1518 [42.3%] in 80 years or older). Considering baseline-risk strata, 865 PP cases (24.1%) occurred in healthy subjects, 1850 (51.5%) in at-risk persons and 877 (24.4%) in immunocompromised/high-risk persons.
Specifically, 1169 PP cases (32.5%) had a history of chronic pulmonary/respiratory disease, 1051 (29.3%) diabetes mellitus, 885 (24.6%) chronic heart disease, 604 (16.8%) were smokers, 435 (12.1%) had cancer (377 [10.5%] solid cancer and 58 [1.6%] haematological neoplasia), 427 (11.9%) had received immunosuppressive therapy in the previous 12 months, 251 (7.0%) had alcoholism, 177 (4.9%) liver disease, 139 (3.9%) severe renal disease, 31 (0.9%) HIV infection, and 5 (0.1%) other immunodeficiencies (Table 2 ).
With regard to multi-comorbidity, 865 (24.1%) Of the total 3592 PP cases had no comorbidities, 1156 (32.2%) had one comorbidity alone, 915 (25.5%) had two coexisting comorbidities, 441 (12.3%) had three, 169 (4.7%) had four and 46 (1.3%) had five or more comorbidities.
Global IR was 90.7 PP cases per 100,000 person-years (95% CI: 85.2–96.5). By sex, IRs were 109.7 in men and 74.6 in women. IRs substantially increased by age (37.3 in 50–64 years, 98.3 in 65–79 years and 259.8 in ≥ 80 years) and baseline-risk strata (42.0, 120.7 and 238.6 in healthy, at-risk and high-risk subjects, respectively).
The greatest incidence emerged among those cohort members with history of hospitalisation from IPD or all-cause pneumonia within previous two-years (2258.9 and 1223.4 per 100,000 person-years, respectively). Considering specific comorbidities, maximum IRs (per 100,000 person-years) appeared among persons with haematological neoplasia (475.0), followed by persons with HIV infection (423.7), severe renal disease (384.9), primary immunodeficiency (332.7), chronic pulmonary/respiratory disease (314.7), immunosuppressive treatment (286.6), liver disease (232.5), chronic heart disease (221.4), alcoholism (204.8), solid cancer (186.2), diabetes mellitus (159.6) and smoking (90.4) (Table 3 ).
IRs dramatically increased with the increasing number of baseline comorbidities, being 42.1, 89.9, 201.1, 350.9, 594.3 and 761.2 in persons with 0, 1, 2, 3, 4 and ≥ 5 comorbidities, respectively.
Overall case-fatality rate (CFR) was 7.6% (272/3592). By age groups, CFRs were 4.5% (34/761) in 50–64 years, 5.9% (77/1313) in 65–79 years and 10.6% (161/1518) in ≥ 80 years (p < 0.001). CFR did not significantly differ by gender, being 8% (160/1997) in men vs. 7% (112/1595) in women (p = 0.265). CFRs were greater among patients with haematological neoplasia (10.3%), severe renal disease (10.1%), chronic heart disease (9.3%) or solid neoplasia (8.5%) and were relatively lower among patients with diabetes mellitus (7.6%), alcoholism (6.4%), liver disease (6.2%), smoking (5.8%) and chronic pulmonary/respiratory disease (5.7%) (Table 4 ). CFR did not significantly vary by number of comorbidities, being 9.6%, 6.4%, 6.8%, 8.2%, 7.1% and 10.9% among patients with 0, 1, 2, 3, 4 and ≥ 5 comorbidities, respectively.
Risk for pneumococcal pneumonia
Table 5 shows Cox regression (model 1) evaluating the influence of different specific underlying conditions on the risk of hospitalisation from PP in the studied cohort. In the multivariable-adjusted analysis, HIV infection (HR: 5.16; 95% CI: 3.57–7.46), prior all-cause pneumonia (HR: 3.96; 95% CI: 3.45–4.55), haematological neoplasia (HR: 2.71; 95% CI: 2.06–3.57), chronic pulmonary/respiratory disease (HR: 2.66; 95% CI: 2.47–2.86) and history of prior IPD (HR: 2.56; 95% CI: 2.03–3.24) were the underlying conditions most strongly associated with an increasing risk of PP. In addition, age/years (HR: 1.06), sex male (HR: 1.33), alcoholism (HR: 1.84), severe liver disease (HR: 1.79), immunosuppressive treatment (HR: 1.76), smoking (HR: 1.58), chronic renal disease (HR: 1.42), chronic heart disease (HR: 1.31), diabetes mellitus (HR: 1.25) and solid cancer (HR: 1.18) were also associated with a statistically significant increased risk. To have received flu vaccine in prior autumn (HR: 1.02), PCV13 within the previous five years (HR: 1.24) and/or PPsV23 at any time (hr: 1.07) did not appear associated with a reduced risk.
Table 6 shows a supplementary Cox regression analysis (model 2) assessing the role of multimorbidity (number of baseline comorbidities) on the risk of suffering PP. As compared with persons without comorbidities, multimorbidity increased substantially the risk of HPP in our study population, with multivariable-adjusted HRs increasing from 1.76 (95% CI: 1.61–1.92) in persons with one comorbidity alone up to 3.10 (95% CI: 2.81–3.41) for two comorbidities, 4.66 (95% CI: 4.14–5.26) for three comorbidities, 7.07 (95% CI: 5.96–8.38) for four comorbidities and 8.70 (95% CI: 6.43–11.76) for five or more comorbidities.
In both models (Tables 5 and 6 ) increasing age, sex male and history of previous IPD or all-cause pneumonia appeared as statistically significant predictors of suffering PP. Vaccinations’ history did not appear associated with reduced risk.
This large population-based cohort study investigated population-based incidence and risk of hospitalisation from PP among middle-aged and older adults with and without specific underlying medical conditions (including theoretically preventive or predisposing conditions), examining the effect of specific/individual and concurrent multiple risk conditions in the risk of suffering PP. Importantly, the study was conducted throughout 2017–2018, early period after universal free (publicly funded) PCV13 approval for all infants on June 2016 in Catalonia (a setting where PPsV23 for elderly people and PCV13 for high-risk adults are implemented since 1999 and 2012 respectively) [ 14 ].
As main findings, our data shows that the burden of hospitalised PP among adults over 50 years in Catalonia during 2017–2018 was moderate overall (with a global IR of 90.7 cases per 100,000 person-years), while considerably larger IRs appeared in very elderly individuals (i.e., 80 years or older), persons with history of hospitalisation from IPD or all-cause pneumonia in previous two-years, patients with immunocompromising conditions or chronic pulmonary/respiratory diseases and those with multi-comorbidity (i.e., two or more baseline risk conditions). In the multivariable analyses (apart of increasing age and history of prior IPD or all-cause pneumonia) major underlying conditions associated with increasing risk of PP were HIV-infection (which increased 6.8 times the adjusted risk of PP) followed by haematological neoplasia and chronic pulmonary/respiratory disease (both increasing approximately 2.7 times the adjusted risk of PP). Other conditions associated with a significant increased multivariable-adjusted risk of PP (with a range of 1.16–1.84 times increasing risk) were sex male, solid cancer, immunosuppressive therapy, renal disease, liver disease, heart disease, diabetes mellitus, alcoholism and smoking. Multi-comorbidity increased substantially the risk of PP, with multivariable-adjusted HRs increasing since 1.76 in persons with one comorbidity alone up to 3.10, 4.66, 7.07 and 8.70 for persons with 2, 3, 4 and 5 or more comorbidities, respectively.
Global incidence in this study is in the low limit of IRs reported for PP among adults in European settings (where IRs between 16 and 3581 hospitalised PP cases per 100,000 person-years have been published [ 6 ]. CFR in the present study (7.6%) may be considered intermediate since CFRs around 5–10% are commonly described for PP cases in older adults. [ 4 , 5 , 6 ] Nevertheless, if we compare our results with data observed in the same population during 2015–2016 (82.8 hospitalised PP cases per 100,000 person), [ 11 , 21 ] we observe a little increase of PP burden despite increasing anti-pneumococcal vaccination coverage (especially in children) across this time period [ 22 ]. On this concern, public health impact of anti-pneumococcal vaccination programmes in adults remains a controversial issue at present. [ 12 , 23 , 24 , 25 , 26 , 27 ] Indeed, new extended-valency conjugate vaccines have been developed to replace the “old” PPsV23/PCV13 vaccines [ 28 ].
In the present study, the vast majority (75.9%) of PP cases occurred among those cohort members with one or more underlying risk condition (who represented only a half [51.2%] of the total study cohort). This finding fits with other data reported in North America and Europe where the percentage of hospitalised adult IPD/PP cases with underlying medical conditions reached approximately 60% among people 18–64 years-old and 80% among persons 65 years or older. [ 2 , 6 , 29 ].
Regarding multi-comorbidity, our data fits with data reported in a literature review where prevalence of multi-comorbidity in pneumonia patients aged 65 years or older ranged from 23 to 98% for two or more comorbidities and from 18 to 89% for three or more comorbidities [ 10 ]. Our data underlines the capital role of multiple concurrent underlying risk conditions on the risk of suffering pneumonia. [ 6 , 10 ].
As expected, immunocompromising conditions were related with a high excess risk of PP in the present study. Indeed, immunocompromised subjects, who were approximately 10% (9.9%) of the total study cohort, accounted for 24.4% of the total PP cases, supporting that immunocompromising conditions are major risk factors for pneumococcal disease (i.e., for both IPD/PP, not only for IPD). [ 2 , 6 , 7 ].
Cohort member classified as baseline at-risk subjects (38.9% of the total cohort) accounted for 51.5% of all PP cases in the study. Within at-risk subjects, the excess risk was highest for those with chronic pulmonary/respiratory disease, who were less than a 10% (9.7%) of the total study cohort but they suffered almost a third (32.5%) of the total PP cases and suffered an incidence/risk near to immunocompromised subjects. This result is in accordance with data reported in other studies and underlines the important role of chronic pulmonary/respiratory disease as predisposing factor to suffer pneumococcal infection. [ 2 , 6 , 7 , 10 ]
As major strengths in this study we note that it’s a population-based design and the large size of the study cohort (more than 2 million people ≥ 50 years-old which represented almost 75% of the overall Catalonian inhabitants in this age strata) [ 13 ].We also note the use of survival analysis methods to estimate accurately incidence and risk of PP adjusted for major underlying medical conditions (including single high-risk or at-risk conditions and coexisting multi-comorbidities. Prevalence of chronic illnesses among patients with IPD/PP have been reported in numerous hospital case-series studies, but population-based data on this concern is limited [ 6 ].
Regarding smoking, a possible underestimation risk for PP is likely considering that there are few smoking people over 64 years old but exists a lot ex-smoker in this age strata (and also some smokers have died before this age). Regarding pneumococcal and influenza vaccinations, it must also be noted that people with comorbidities have a higher probability of vaccination and this may underestimate the possible preventive role for both vaccines.
As limitation, we assumed that hospital discharge ICD diagnose coding was correct and assuming that validation of the diagnosis was not feasible because of the study design and sample size. Regarding pneumococcal and influenza vaccinations, it must also be noted that people with comorbidities have a higher probability of vaccination and this may underestimate the possible preventive role for both vaccines.
We note that definition criteria for PP may vary between different studies, but we also note that using ICD codes to define PP, despite recognised limitations, [ 30 ] has been commonly used in many epidemiological studies evaluating this concern [ 6 ]. Despite other limitations, mainly linked to retrospective design and absence of specific microbiological data, we highlights that our study provides uncommon population-based data on incidence and risk of PP among healthy, at-risk and high-risk adults in the present era of multiple-valent pneumococcal conjugate vaccines. Although, these data are scarce in the literature, they are greatly needed to interpret possible direct and indirect impact of currently implemented childhood and adult pneumococcal vaccination programs. [ 9 , 22 ] We underline that this study includes all diagnosed cases of PP, both confirmed (invasive/bacteremic) as well as presumptive cases (i.e., positive sputum culture or urinary antigen test with negative or not performed blood culture).
This is an important concern since bacteremic PP may represent only a little fraction (approximately 1/5) of the overall PP disease burden in adults. [ 3 , 5 , 6 , 9 ] Nonbacteremic PP represents the vast majority of pneumococcal disease in adults and, therefore, it must be included in the analyses if the overall spectrum of the disease needs to be assessed.
During 2017–2018 the overall burden of pneumococcal pneumonia requiring hospitalisation among middle-aged and older adults in Catalonia remained considerable, with an incidence of 90.7 hospitalised PP cases per 100,000 person-years and a global CFR of 7.6%. Besides increasing age and immunocompromising conditions, history of prior IPD/pneumonia, oldest age (i.e., 80 years or more), presence of chronic pulmonary disease and/or co-existing multi-comorbidity (i.e., two or more underlying risk conditions) are major independent risk factors for PP in adults (with an excess risk near to immunocompromised subjects). Redefining risk categories for PP, including all above-mentioned conditions into the high-risk category, could be necessary to improve prevention strategies in middle-aged and older adults.
Appendix. Criteria used to define comorbidities/underlying risk conditions in the study population
The following comorbidities and underlying risk conditions were stablished according to the presence of ICD-10 codes [International Classification of Diseases, 10th Revision] registered in the electronic primary care medical records of each cohort member at baseline:
Chronic pulmonary/respiratory disease : it included chronic bronchitis/emphysema (J41-J44), asthma (J45-J46) and/or other chronic pulmonary diseases (P27, E84, J47).
Chronic heart disease : it included congestive heart failure (I50), coronary artery disease (I20-I22, I25) and/or other chronic heart diseases (I05-I08, I11,I35-I37,I42, I51.7).
Diabetes mellitus (E10-E14).
Chronic liver disease : it included chronic viral hepatitis (B18), cirrhosis (K74) and/or alcoholic hepatitis (K70)).
Alcoholism (F10, G31.2, G62.1, G72.1, I42.6, K29.2, K70).
Smoking (F17).
Anatomic or functional asplenia (D57, D73, Q89).
Primary immunodeficiency (D80-D84).
HIV infection (B20-B24).
Chronic renal disease : it included nephrotic syndrome (N04, N39.1) and severe chronic renal failure (N18-N19 with glomerular filtration rate ≤ 30 ml/min).
Cancer : it included solid organ or haematological neoplasia (C00 to C97) diagnosed within previous 5 years.
Immunosuppressive therapy : it included long-term immunosuppressive medication and/or radiotherapy in the previous 12 months (coded according to specific SIDIAP codes).
Data Availability
These data have been obtained from the Catalonian Health Institute Information System for the Development of Research in Primary Care (SIDIAP). Interested authors might obtain SIDIAP data (previous ethics and scientific approval by the ethics and clinical research committee of the Primary Care Research Institute Jordi Gol/SIDIAP Jordi Gol) addressing purposes to the Institution. In accordance with current European and national law, the data used in this study is only available for the researchers participating in this study. Thus, we are not allowed to distribute or make publicly available the data to other parties. However, researchers from public institutions can request data from SIDIAP if they comply with certain requirements. Further information is available online ( https://www.sidiap.org/index.php/menu-solicitudesen/application-proccedure ) or by contacting SIDIAP ([email protected]), Clara Rodriguez-Casado ([email protected])).
Abbreviations
Case-Fatality Rate
Confidence Interval
Conjunto mínimo de base de datos , Spanish hospital discharge codes
Human Immunodeficience Virus
Hazard Ratio
International Classification of Diseases-10
Catalonian Health Institute, Institut Català de la Salut
Invasive pneumococcal disease
Incidence rate
Primary Care Centres (PCCs)
Pneumococcal Conjugate Vaccine
Pneumococcal Pneumonia
Polysaccharide Pneumococcal Vaccine
Catalonian Information System for Development of Research in Primary Care
- Ortqvist A, Hedlund J, Kalin M. Streptococcus pneumoniae: epidemiology, risk factors, and clinical features. Semin Respir Crit Care Med. 2005;26:563–74. https://doi.org/10.1055/s-2005-925523 .
Article PubMed Google Scholar
- Centers for Disease Control and Prevention. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 1997;46:1–24.
Google Scholar
- Fedson DS, Scott JA. The burden of pneumococcal disease among adults in developed and developing countries: what is and is not known. Vaccine. 1999;17(Suppl 1):11–8. https://doi.org/10.1016/s0264-410x(99)00122-x .
Article Google Scholar
Navarro-Torné A, Montuori EA, Kossyvaki V, Méndez C. Burden of pneumococcal disease among adults in Southern Europe (Spain, Portugal, Italy, and Greece): a systematic review and meta-analysis. Hum Vaccin Immunother. 2021;17(10):3670–86. https://doi.org/10.1080/21645515.2021.1923348 .
Article CAS PubMed PubMed Central Google Scholar
- Said MA, Johnson HL, Nonyane BA, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS ONE. 2013;8:e60273. https://doi.org/10.1371/journal.pone.0060273 .
- Torres A, Cillóniz C, Blasi F, et al. Burden of pneumococcal community-acquired pneumonia in adults across Europe: a literature review. Respir Med. 2018;137:6–13. https://doi.org/10.1016/j.rmed.2018.02.007 .
- Kyaw MH, Rose CE Jr, Fry AM, et al. The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis. 2005;192:377–86. https://doi.org/10.1086/431521 .
- Musher DM, Abers MS, Bartlett JG. Evolving understanding of the causes of pneumonia in adults, with special attention to the role of Pneumococcus. Clin Infect Dis. 2017;65(10):1736–44. https://doi.org/10.1093/cid/cix549 .
- WHO, World Health Organization. Considerations for pneumococcal vaccination in older adults. ; 2021 Jun 11 [accessed 2022 Mar 11]. Available at: https://www.who.int/publications/i/item/WER9623-217-228 .
- Curcio D, Cané A, Isturiz R. Redefining risk categories for pneumococcal disease in adults: critical analysis of the evidence. Int J Infect Dis 2015 Aug;37:30–5. doi: https://doi.org/10.1016/j.ijid.2015.05.003 .
Vila-Córcoles A, Ochoa-Gondar O, de Diego C, et al. Evaluating clinical effectiveness of 13-valent pneumococcal conjugate vaccination against pneumonia among middle-aged and older adults in Catalonia: results from the EPIVAC cohort study. BMC Infect Dis. 2018;18:196. https://doi.org/10.1186/s12879-018-3096-7 .
Vila-Córcoles A, Ochoa-Gondar O, de Diego-Cabanes C, Satué-Gracia EM, Torras-Vives V, Forcadell-Peris MJ, Ribas-Seguí D, Vila-Rovira A, Rodríguez-Casado C. Evaluating clinical effectiveness and impact of anti-pneumococcal vaccination in adults after universal childhood PCV13 implementation in Catalonia, 2017–2018. Vaccine X. 2023 Jan 21;13:100264. doi: https://doi.org/10.1016/j.jvacx.2023.100264 .
- Generalitat de Catalunya. IDESCAT. Population on 1 January. By age group. Catalunya: Statistical Institute of Catalonia. Annual indicators. Demography•Society. Population figures; 2021 [accessed 2022 May 11]. Available at: https://www.idescat.cat/indicadors/ ?id=anuals&n=10329&col=1〈=en
- Generalitat de Catalunya. Calendari de vacunacions sistematiques 2016. Barcelona (SP): Generalitat de Catalunya. Departament de Salut; 2017 [accessed 2022 Mar 25]. Available at: https://canalsalut.gencat.cat/web/.content/contingut_responsiu/salutAZ/V/vacunacions/documents/calendari_vacunacions.pdf .
- WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. World Medical Association. ; 2018 Jul 09 [accessed 2022 May 11]. Available at: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ .
- Information system for the development of research in primary care (SIDIAP data base). Catalonia: SIDIAP. ; 2022 [accessed 2022 May 11]. Available at: http://www.sidiap.org/ .
- García-Gil MM, Hermosilla E, Prieto-Alhambra D, et al. Construction and validation of a scoring system for the selection of high-quality data in a spanish population primary care database (SIDIAP). Inf Prim Care. 2011;19(3):135–45. https://doi.org/10.14236/jhi.v19i3.806 .
- Ministerio de Sanidad. Conjunto Mínimo Básico de Datos de Hospitalización (CMBD-H). Madrid: Ministerio de Sanidad. Portal estadístico. Área de inteligencia de gestión [accessed 2022 May 11]. Available at: https://pestadistico.inteligenciadegestion.mscbs.es/publicoSNS/N/rae-cmbd/cmbd-h .
Torres A, Barberán J, Falguera M, Menéndez R, Molina J, Olaechea P, Rodríguez A, Grupo de la Guía Multidisciplinar para el Manejo de la Neumonía Adquirida en la Comunidad. Multidisciplinary guidelines for the management of community-acquired pneumonia [in Spanish]. Med Clin (Barc). 2013;140(5):223e1–19. https://doi.org/10.1016/j.medcli.2012.09.034 . Spanish.
- Hosmer DW, Lemeshow S. Applied Survival Analysis. Regression modeling of time to Event Data. New York: John Wiley & Sons; 1999.
Vila-Corcoles A, Ochoa-Gondar O, Vila-Rovira A, et al. Incidence and risk of pneumococcal pneumonia in adults with distinct Underlying Medical Conditions: a Population-Based study. Lung. 2020 Jun;198(3):481–9. https://doi.org/10.1007/s00408-020-00349-y .
- Bonnave C, Mertens D, Peetermans W, Cobbaert K, Ghesquiere B, Deschodt M, Flamaing J. Adult vaccination for pneumococcal disease: a comparison of the national guidelines in Europe. Eur J Clin Microbiol Infect Dis. 2019;38(4):785–91. https://doi.org/10.1007/s10096-019-03485-3 .
Article CAS PubMed Google Scholar
- Berild JD, Winje BA, Vestrheim DF, Slotved HC, Valentiner-Branth P, Roth A, Storsäter J. A systematic review of studies published between 2016 and 2019 on the effectiveness and efficacy of pneumococcal vaccination on Pneumonia and Invasive Pneumococcal Disease in an Elderly Population. Pathogens. 2020;9(4):259. https://doi.org/10.3390/pathogens9040259 .
- Niederman MS, Folaranmi T, Buchwald UK, Musey L, Cripps AW, Johnson KD. Efficacy and effectiveness of a 23-valent polysaccharide vaccine against invasive and noninvasive pneumococcal disease and related outcomes: a review of available evidence. Expert Rev Vaccines. 2021;20(3):243–56. https://doi.org/10.1080/14760584.2021.1880328 .
- Leidner AJ, Murthy N, Chesson HW, Biggerstaff M, Stoecker C, Harris AM, Acosta A, Dooling K, Bridges CB. Cost-effectiveness of adult vaccinations: a systematic review. Vaccine. 2019;37(2):226–34. https://doi.org/10.1016/j.vaccine.2018.11.056 .
- Treskova M, Scholz SM, Kuhlmann A. Cost effectiveness of Elderly Pneumococcal Vaccination in Presence of Higher-Valent Pneumococcal Conjugate Childhood Vaccination: systematic literature review with focus on methods and assumptions. PharmacoEconomics. 2019;37(9):1093–127. https://doi.org/10.1007/s40273-019-00805-5 .
- Shao Y, Stoecker C. Cost-effectiveness of pneumococcal vaccines among adults over 50 years old in low- and middle-income countries: a systematic review. Expert Rev Vaccines. 2020;19(12):1141–51. https://doi.org/10.1080/14760584.2020.1874929 .
- Hurley D, Griffin C, Young M, et al. Safety, Tolerability, and immunogenicity of a 20-Valent Pneumococcal Conjugate Vaccine (PCV20) in adults 60 to 64 years of age. Clin Infect Dis. 2021;73:e1489–97. https://doi.org/10.1093/cid/ciaa1045 .
- Klemets P, Lyytikäinen O, Ruutu P, Ollgren J, Nuorti J. Invasive pneumococcal infections among persons with and without underlying medical conditions: implications for prevention strategies. BMC Infect Dis. 2008;8:96. https://doi.org/10.1186/1471-2334-8-96 .
- Guevara RE, Butler JC, Marston BJ, Plouffe JF, File TM Jr, Breiman RF. Accuracy of ICD-9-CM codes in detecting community-acquired pneumococcal pneumonia for incidence and vaccine efficacy studies. Am J Epidemiol. 1999;149(3):282–9. https://doi.org/10.1093/oxfordjournals.aje.a009804 .
Download references
Acknowledgements
All the authors thank to the IDIAP Jordi Gol Foundation (Barcelona) and the Hospital Joan XXIII Foundation (Tarragona) for the support received in publishing this paper.
This work was supported by a grant from the “Fondo de Investigación Sanitaria” of the ”Instituto de Salud Carlos III” (call 2020) for the “Acción Estratégica en Salud 2020/2030 (code file PI20/01223), cofinanced by the European Union through the “Fondo Europeo de Desarrollo Regional” (FEDER).
Author information
Authors and affiliations.
Primary Health Care Service “Camp de Tarragona”, Institut Català de la Salut, Tarragona, Spain
Olga Ochoa-Gondar, Verónica Torras-Vives, Cinta de Diego-Cabanes, Eva M. Satué-Gracia, Angel Vila-Rovira, María J. Forcadell-Perisa, Domingo Ribas-Seguí & Angel Vila-Córcoles
Unitat de Suport a la Recerca of Tarragona, Institut Universitari d’Investigació en Atenció Primària Jordi Gol (IDIAP Jordi Gol), Tarragona, Spain
Cinta de Diego-Cabanes, Eva M. Satué-Gracia & Angel Vila-Córcoles
Information System for the Improvement of Research in Primary Care (SIDIAP), Primary Care Research Institute Jordi Gol, Universitat Autonoma de Barcelona, Barcelona, Spain
Clara Rodríguez-Casado
You can also search for this author in PubMed Google Scholar
Contributions
OOG and AVC conceptualised and designed the study; OOG, VTV and AVC wrote and edited the manuscript; VTV, CDC, MFP and DRS assessed outcomes; CRC obtained data; ESG and AVR did statistical analyses; AVC coordinated the study. All authors have read and agreed to the final version of the manuscript. The two first listed authors contributed similarly to this manuscript.
Corresponding author
Correspondence to Cinta de Diego-Cabanes .
Ethics declarations
Conflict of interest.
The authors declare that they have no competing interests.
Ethics approval and consent to participate
All methods were performed in accordance with relevant guidelines and regulations. The study was approved by the Ethics Committee of the Institution (Ethics Committee IDIAP Jordi Gol, file 20/065-PCV) and was conducted in accordance with the general principles for observational studies. The need for informed consent was waived by the Ethics Committee of the Institution (Ethics Committee IDIAP Jordi Gol, file 20/065-PCV) due to the nature of data (pseudonymised) and according to the European General Regulation for Data Protection, RGDP, article 6.e,9.2.j and 89.
Consent for publication
Not applicable.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ochoa-Gondar, O., Torras-Vives, V., de Diego-Cabanes, C. et al. Incidence and risk factors of pneumococcal pneumonia in adults: a population-based study. BMC Pulm Med 23 , 200 (2023). https://doi.org/10.1186/s12890-023-02497-2
Download citation
Received : 22 March 2023
Accepted : 26 May 2023
Published : 08 June 2023
DOI : https://doi.org/10.1186/s12890-023-02497-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pneumococcal pneumonia
- Risk factors
- Multimorbidity
BMC Pulmonary Medicine
ISSN: 1471-2466
- Submission enquiries: [email protected]
- General enquiries: [email protected]
The Global Burden of Community-Acquired Pneumonia in Adults, Encompassing Invasive Pneumococcal Disease and the Prevalence of Its Associated Cardiovascular Events, with a Focus on Pneumolysin and Macrolide Antibiotics in Pathogenesis and Therapy
Affiliations.
- 1 Department of Immunology, Faculty of Health Sciences, University of Pretoria, Pretoria 0001, South Africa.
- 2 Department of Internal Medicine, Faculty of Health Sciences, University of the Witwatersrand Medical School, 7 York Road, Johannesburg 2193, South Africa.
- PMID: 37446214
- PMCID: PMC10341596
- DOI: 10.3390/ijms241311038
Despite innovative advances in anti-infective therapies and vaccine development technologies, community-acquired pneumonia (CAP) remains the most persistent cause of infection-related mortality globally. Confronting the ongoing threat posed by Streptococcus pneumoniae (the pneumococcus), the most common bacterial cause of CAP, particularly to the non-immune elderly, remains challenging due to the propensity of the elderly to develop invasive pneumococcal disease (IPD), together with the predilection of the pathogen for the heart. The resultant development of often fatal cardiovascular events (CVEs), particularly during the first seven days of acute infection, is now recognized as a relatively common complication of IPD. The current review represents an update on the prevalence and types of CVEs associated with acute bacterial CAP, particularly IPD. In addition, it is focused on recent insights into the involvement of the pneumococcal pore-forming toxin, pneumolysin (Ply), in subverting host immune defenses, particularly the protective functions of the alveolar macrophage during early-stage disease. This, in turn, enables extra-pulmonary dissemination of the pathogen, leading to cardiac invasion, cardiotoxicity and myocardial dysfunction. The review concludes with an overview of the current status of macrolide antibiotics in the treatment of bacterial CAP in general, as well as severe pneumococcal CAP, including a consideration of the mechanisms by which these agents inhibit the production of Ply by macrolide-resistant strains of the pathogen.
Keywords: Streptococcus pneumoniae; cardiovascular events; community-acquired pneumonia; dendritic cells; macrolides; macrophages; mannose receptor C-type1; platelets; pneumolysin; pro-inflammatory cytokines.
Publication types
- Anti-Bacterial Agents / pharmacology
- Anti-Bacterial Agents / therapeutic use
- Cardiovascular Diseases* / drug therapy
- Cardiovascular Diseases* / epidemiology
- Cardiovascular Diseases* / etiology
- Community-Acquired Infections* / complications
- Community-Acquired Infections* / drug therapy
- Community-Acquired Infections* / epidemiology
- Macrolides / therapeutic use
- Pneumococcal Infections* / drug therapy
- Pneumococcal Infections* / epidemiology
- Pneumonia, Pneumococcal* / complications
- Pneumonia, Pneumococcal* / drug therapy
- Pneumonia, Pneumococcal* / epidemiology
- Streptococcus pneumoniae
- plY protein, Streptococcus pneumoniae
- Anti-Bacterial Agents
Grants and funding
Decisions on eating and drinking in older adults admitted with pneumonia and referred for swallowing difficulties
- Brief Report
- Open access
- Published: 09 May 2024
Cite this article
You have full access to this open access article

- Yuki Yoshimatsu ORCID: orcid.org/0000-0003-0913-3507 1 , 2 ,
- Dharinee Hansjee ORCID: orcid.org/0000-0002-1137-9728 3 ,
- Marianne Markowski ORCID: orcid.org/0000-0003-4652-3168 4 ,
- Ryan Essex ORCID: orcid.org/0000-0003-3497-3137 4 &
- David G. Smithard ORCID: orcid.org/0000-0001-6863-3099 1 , 2
134 Accesses
19 Altmetric
Explore all metrics
Key summary points
We examined the frequency of different decisions, including eating and drinking with acknowledged risks (EDAR) in a single-institution retrospective study of older people with pneumonia and swallowing difficulties.
EDAR decisions were made in only a small fraction of patients (less than one fourth of patients on a modified diet). Most EDAR decisions were for end-of-life comfort care, and patients for EDAR had a significantly higher mortality despite the pneumonia recurrence rate not differing significantly.
The reasons underlying the relatively low frequency of EDAR decisions compared to modified diet needs to be investigated to maximise patient autonomy and comfort while minimising staff burden.
Older patients with pneumonia are commonly restricted from oral intake due to concerns towards aspiration. Eating and drinking with acknowledged risks (EDAR) is a shared decision-making process emphasising patient comfort. As part of our project to find the barriers and facilitators of EDAR, we aimed for this initial study to see how frequently EDAR was selected in practice.
We performed a retrospective cohort study at an acute hospital where EDAR was initially developed, of patients aged ≥ 75 years-old admitted with pneumonia and referred to speech and language therapy.
Out of 216 patients, EDAR decisions were made in 14.4%. The EDAR group had a higher 1-year mortality than the modified/normal diet groups ( p < 0.001). Pneumonia recurrence rate did not differ significantly between the groups ( p = 0.070).
EDAR decisions were comparatively less common and most were associated with end-of-life care. Underlying reasons for the low EDAR application rate must be investigated to maximise patient autonomy and comfort as intended by EDAR while minimising staff burden.
Avoid common mistakes on your manuscript.
Introduction
When a frail older adult is admitted to the hospital with pneumonia, the aetiology is frequently attributed to aspiration[ 1 , 2 ]. When aspiration is suggested, clinicians frequently restrict the patient from eating and drinking until assessed by a speech and language therapist (SLT). The SLT will advise on the patient’s ability to swallow safely. The management plan will vary from a normal diet (ND), through a modified diet (MD), or suggestion that the patient is too unsafe to eat and drink at all. Modified diet and nil-by-mouth (NBM) orders are associated with dehydration, malnutrition, oral health decline, poor quality of life and increased mortality[ 3 ].
For some patients, a better approach is to support them to eat and drink despite the risks; this is often termed “Risk Feeding” or “Eating and Drinking with Acknowledged Risks (EDAR).” EDAR is an alternative shared decision-making process that enables comfort, dignity, and autonomy for patients who prefer to continue oral intake, or where alternative management strategies such as tube feeding are inappropriate. In recent years, guidance has been developed by the Royal College of Speech and Language Therapists (RCSLT) to assist the decision-making process[ 4 ]. The recommended EDAR decision-making process includes a capacity assessment, a clinical evaluation of the swallow, establishing the goal of care, facilitating communication within the multidisciplinary team, and setting out an advance care plan where appropriate[ 4 ]. While the initial idea of EDAR may be suggested by the SLT, it is a patient-led decision. Capacity assessment forms part of the decision-making process, and the patient is always involved if they are capable. The Royal College of Physicians (RCP) has also published guidance on supporting people with eating and drinking difficulties[ 5 ].
However, questions have been raised regarding the risk management approach of the RCP guidance[ 6 ]. Moreover, despite guidance being available, in the clinical setting, supporting patients’ choices (or identifying patients who would benefit from EDAR even when their choice is unclear) and making these complex decisions remain a medical and ethical struggle. It is important to investigate how EDAR decisions are made in daily practice, to consider the next steps in further promoting it for appropriate patients.
We therefore conducted a retrospective study on how EDAR decisions are made in daily clinical practice in the management of older adults in hospital with a diagnosis of community-acquired pneumonia (CAP).
We performed a retrospective cohort study of older patients admitted with a diagnosis of pneumonia to Queen Elizabeth Hospital (Lewisham and Greenwich NHS Trust). Ethical approval was obtained from the Lewisham and Greenwich NHS Trust (Number 7211), and informed consent was waived due to the retrospective nature of the study.
We included patients aged 75 years-old and above admitted to the hospital with a diagnosis of CAP from 1st January 2021 to 31st December 2021 and were referred to an SLT for the assessment of suspected swallowing impairment. We excluded those who were admitted for COVID-19 pneumonitis, those who were admitted for more than once during the study period (only the first admission was included), those who did not have pneumonia according to the medical records, those who developed pneumonia after admission, and those admitted with a hospital acquired pneumonia.
We divided the patients into four groups according to the initial decisions made regarding their oral intake: the ND group, MD group, EDAR group, and NBM group. We compared the following between the four groups: patient backgrounds (age, Rockwood Clinical Frailty Scale (CFS)[ 7 ], initial diagnosis made by the consultant (aspiration pneumonia or non-aspiration pneumonia), pneumonia severity index (PSI)[ 8 ] and outcomes (in-hospital and 1-year mortality, pneumonia recurrence within 30 days). For the EDAR group, the reason for selecting EDAR was also extracted.
Statistical analyses
We used chi-square tests to compare outcomes and the one-way ANOVA test for continuous parametric variables (age, CFS and PSI). Analyses were performed using Microsoft Excel and online resources[ 9 ]. A p value < 0.05 was considered to be statistically significant for all analyses. Post hoc tests were performed where initial results indicated significant differences.
The initial list of 803 patients aged 75 years-old and above admitted with a diagnosis of CAP had a median age of 84 years-old (interquartile range 80–89) and a CFS score of 5 (4–6). 216 patients who underwent SLT assessment were included in the study (Fig. 1 ). Of these patients, 14.4% were considered appropriate for EDAR, 59.3% for MD, 19.9% for ND, and 6.5% for NBM. Demographic data and outcomes are summarised in Table 1 . Of the 31 patients who were eating and drinking with acknowledged risks, the reasons underlying the decisions were short life expectancy (58.1%), quality of life (38.7%), and refusing nasogastric tube feeding (3.2%). Only 19.4% of these patients were assessed as having the mental capacity to make these decisions. For those without capacity, attempts were made by the team to establish the wishes of the patient from significant others which forms part of the decision-making process. The EDAR decisions were mostly initiated by the SLT following a swallow assessment and then discussed with the doctor, patient (when having capacity), and family member. A shared decision making process was co-ordinated by SLT to ensure the patient’s views are included as part of the MDT decision.
Patient selection. CAP community-acquired pneumonia, HAP hospital-acquired pneumonia, SLT speech and language therapist
Patient background
The patients included in the study had a median age of 86 years-old (interquartile range: 81–91). As shown in Table 1 , significant differences among groups were indicated for frailty and being diagnosed with aspiration pneumonia. Post-hoc Tukey’s test revealed a statistically significant difference in CFS between the EDAR and ND groups (F(3212) = 4.14, p = 0.010) but not among any other groups. Post hoc comparison with Bonferroni correlation (adjusted alpha = 0.00625) indicated that an aspiration pneumonia diagnosis was significantly more common in the EDAR group than the ND group ( p < 0.001) but not among any other groups.
The EDAR and NBM groups showed a high short/long-term mortality, with half dying during the hospital stay and over 90% dying within a year. Bonferroni correlation (adjusted alpha = 0.00625) indicated that in-hospital mortality was significantly higher in the NBM group than in each of the three other groups ( p < 0.001), but there were no significant differences among other groups. One-year mortality was significantly higher in the EDAR group compared to the ND group ( p = 0.001) and MD group ( p = 0.001), and in the NBM group compared to the ND group ( p < 0.001) but not with any other groups. The pneumonia recurrence rate within 30 days did not differ significantly among the groups ( p = 0.070), as shown in Table 1 .
Our study revealed how EDAR decisions were not common in older patients diagnosed with pneumonia; EDAR decisions were made for one-fourth of patients compared to those offered MD alone. Reasons for this may include patient choice, physical condition, staff anxiety towards potentially contributing to risks of pneumonia and patient discomfort, staff members’ lack of awareness/understanding on EDAR, or staff members understanding but not wanting to support EDAR. Despite the setting being where EDAR was originally developed[ 10 ], there may still be a degree of insufficient awareness and understanding of EDAR. This was implied by the data that EDAR was chosen in more frail patients with higher severity of pneumonia, with the majority being chosen for end-of-life comfort care rather than a way to continue oral intake in patients with treatable pneumonia. This indicates a necessity for continuous education and training in the workplace. Choices and preferences, which form the foundation of EDAR decisions are not merely a part of terminal care but is also integral in the acute stages of disease. EDAR was established to enable patients the choice to continue oral intake regardless of disease stage, particularly where the patient refuses to accept modified food and liquids. It may be important at this stage to reconsider how and to whom to offer EDAR as a viable option.
The prognosis of older adults diagnosed with pneumonia (aspiration pneumonia in particular) is considerably poor[ 11 , 12 ], and multimodal multidisciplinary care is imperative[ 13 ]. It is important to have discussions regarding patients’ preference in eating and drinking and make a shared decision[ 14 ], rather than making assumptions about patient perception and paternalistically making a ‘safe’ decision[ 15 ]. Issues have been raised regarding the RCP guidance on EDAR, with concerns towards the risk management approach being standardised than an evidence-based informed consent approach[ 6 ]. With EDAR guidance being published, it is our responsibility as clinicians to ensure patients’ rights are protected, while also devoting attention towards the potential barriers such as staff anxiety and knowledge[ 16 ]. Adverse events such as pneumonia or choking may be another concern when considering EDAR. While our data shows that pneumonia recurrence within 30 days was not a significant concern, previous reports have shown increased readmissions with EDAR-linked conditions such as chest infections and reduced oral intake[ 17 ]. It is important to assess which patients are appropriate for EDAR, and monitor them throughout the course through to discharge where appropriate documentation of decisions is carried through into the community.
Eating and drinking is a basic right, and decisions for or against it are not straightforward. Clinicians have the responsibility to act under the basic ethical principles of medical ethics—autonomy, beneficence, non-maleficence and justice[ 18 ]. All individuals have the freedom to eat and drink as they wish ( autonomy ). However, as it could cause harm and discomfort to the patient, clinicians provide recommendations based on the evaluated risks ( non-maleficence ), and may recommend alternative methods of nutritional intake if deemed appropriate ( beneficence). These recommendations, however, do not always align with patient autonomy and bring forth dilemmas in the decision-making process. In addition, interventions related to dysphagia, including EDAR, are often inaccessible, leading to difficulties in maintaining equity across the community and globally ( justice ). These aspects support the importance of having guidance regarding decision-making in eating and drinking and increasing its awareness to provide a basis for all clinicians regardless of profession or setting, while additional case-based training is essential in the implementation and adaptation of EDAR and other methods in practice, as evidenced by clinical data. While EDAR is beneficial for some individuals, it is not always the best choice for individuals and caregivers, and the key lies in how to evaluate appropriate situations as a multidisciplinary team. The ethical balance between providing comfort and considering safety, or emphasising patient autonomy while being a responsible healthcare professional, is not a simple dilemma. Multidisciplinary team discussions with added expertise from stakeholders of other related specialties such as palliative care may be beneficial.
Strengths and limitations
Some limitations must be mentioned. This study was a single-centre, retrospective study where EDAR was originally developed, and results may not translate to situations in other regions or institutions. There is a well-established dissemination route on EDAR policy and practice through robust training programmes delivered to nurses and medical staff in the developing hospital. The likelihood therefore of EDAR being initiated and utilised appropriately at the developing hospital over other institutions is higher. However, this was a relatively large study in a 521-bed hospital. There have been no similar studies of EDAR in this population. This highlights the value of this study for the next steps. This will provide a basis for addressing the complex decision-making process surrounding EDAR and what can be done to make it easier for clinicians and patients.
EDAR decisions were made mostly as part of end-of-life care. EDAR should also be offered to appropriate patients in earlier disease stages, as comfort, dignity and autonomy are a priority regardless of disease stage. Underlying reasons for the low EDAR application rate must be investigated to maximise patient autonomy and comfort while minimising staff burden.
Data availability
All data are applicable in the paper.
Yoshimatsu Y, Smithard DG (2022) A paradigm shift in the diagnosis of aspiration pneumonia in older adults. J Clin Med. 11(17):5214
Article PubMed PubMed Central Google Scholar
Smithard DG, Yoshimatsu Y (2022) Pneumonia, aspiration pneumonia, or frailty-associated pneumonia? Geriatrics (Basel). 7(5):115
Maeda K, Koga T, Akagi J (2016) Tentative nil per os leads to poor outcomes in older adults with aspiration pneumonia. Clin Nutr 35(5):1147–1152
Article PubMed Google Scholar
D. Hansjee, N. Burch, L. Campbell, H. Crawford, Crowder R, D. Garrett, et al 2021 Eating and drinking With acknowledged risks: multidisciplinary team guidance for the shared decision-making process (adults) [Working Paper] London UK
Porter K, Burch N, Campbell C, Danbury C, Foster C, Gabe S, Goddard A, Harp K, Holdoway A, Hughes T, Le Ball K (2021) Supporting people who have eating and drinking difficulties. Clinical med 21(4):e344
Article Google Scholar
O’Keeffe ST, Murray A, Leslie P, Collins L, Lazenby-Paterson T, McCurtin A et al (2021) Aspiration, risk and risk feeding: a critique of the royal college of physicians guidance on care of people with eating and drinking difficulties. Adv Commun Swallowing. https://doi.org/10.3233/ACS-210031
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I et al (2005) A global clinical measure of fitness and frailty in elderly people. CMAJ 173(5):489–495
Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE et al (1997) A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 336(4):243–250
Article CAS PubMed Google Scholar
Social Science Statistics. https://www.socscistatistics.com/ .
Hansjee D (2018) An acute model of care to guide eating & drinking decisions in the frail elderly with dementia and dysphagia. Geriatrics (Basel). 3(4):65
Yoshimatsu Y, Thomas H, Thompson T, Smithard DG (2024) Prognostic factors of poor outcomes in pneumonia in older adults: aspiration or frailty? European Geriatric Medicine. 15(2):481–488
Yoshimatsu Y, Kragholm K, Clemmensen SZ, Melgaard D, Torp-Pedersen C, Smithard DG et al (2024) The predictive value of anticholinergic drug exposure and the outcome of pneumonia: a danish database study. Age Ageing. https://doi.org/10.1093/ageing/afae012
Yoshimatsu Y, Ohtake Y, Ukai M, Miyagami T, Morikawa T, Shimamura Y et al (2023) Diagnose, Treat, and SUPPORT clinical competencies in the management of older adults with aspiration pneumonia: a scoping review. Eur Geriatr Med 15(1):57–66
O’Keeffe ST, Leslie P, Lazenby-Paterson T, McCurtin A, Collins L, Murray A et al (2023) Informed or misinformed consent and use of modified texture diets in dysphagia. BMC Med Ethics 24(1):7
Leslie P, Lisiecka D (2020) Ethical issues in dysphagia management. Semin Speech Lang 41(3):257–265
Murray A, Mulkerrin S, O’Keeffe ST (2019) The perils of “risk feeding.” Age Ageing 48(4):478–481
Sommerville P, Hayton J, Soar N, Archer S, Fitzgerald A, Lang A et al (2022) Prognosis in dysphagic patients who are eating and drinking with acknowledged risk: results from the evaluation of the FORWARD project. Age Ageing. https://doi.org/10.1093/ageing/afac005
Beauchamp T, Childress J (2012) Principles of biomedical ethics, 7th edn. Oxford University Press, Inc, Oxford
Google Scholar
Download references
Funding received from Japanese Respiratory Society, 2021, Yuki Yoshimatsu, The Great Britain Sasakawa Foundation, B149, Yuki Yoshimatsu.
Author information
Authors and affiliations.
Elderly Care, Queen Elizabeth Hospital, Lewisham and Greenwich NHS Trust, Stadium Rd, London, SE18 4QH, UK
Yuki Yoshimatsu & David G. Smithard
Centre for Exercise Activity and Rehabilitation, School of Human Sciences, University of Greenwich, London, UK
Speech and Language Therapy, School of Health Sciences, University of Greenwich, London, UK
Dharinee Hansjee
The Institute for Lifecourse Development, University of Greenwich, London, UK
Marianne Markowski & Ryan Essex
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Yuki Yoshimatsu .
Ethics declarations
Conflict of interest.
The corresponding author is supported by The Japanese Respiratory Society Fellowship Grant. This study was funded by the Great Britain Sasakawa Foundation (Butterfield Award). The sponsors had no role in this study design, review process, writing of the manuscript, or decision to publish. The authors received no other financial support for the research, authorship and publication of this article. The authors declare that they have no other competing interests.
Ethical approval
Ethical approval was obtained from the Lewisham and Greenwich NHS Trust (Number 7211).
Informed consent
Informed consent was waived due to the retrospective nature of the study.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Yoshimatsu, Y., Hansjee, D., Markowski, M. et al. Decisions on eating and drinking in older adults admitted with pneumonia and referred for swallowing difficulties. Eur Geriatr Med (2024). https://doi.org/10.1007/s41999-024-00983-2
Download citation
Received : 15 February 2024
Accepted : 18 April 2024
Published : 09 May 2024
DOI : https://doi.org/10.1007/s41999-024-00983-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Aspiration pneumonia
- Risk feeding
- Comfort feeding
- Modified diet
- Find a journal
- Publish with us
- Track your research
ORIGINAL RESEARCH article
The positive impact of smoking on poor sleep quality is moderated by igf1 levels in cerebrospinal fluid: a case-control study among chinese adults.

- 1 Department of Anesthesiology, The Second Affiliated Hospital of Xiamen Medical College, Xiamen, China
- 2 School of Mental Health, Wenzhou Medical University, Wenzhou, Zhejiang, China
- 3 Department of Psychiatry, Yale University School of Medicine, New Haven, CT, United States
- 4 Zhejiang Provincial Clinical Research Center for Mental Disorders, The Affiliated Wenzhou Kangning Hospital, Wenzhou Medical University, Wenzhou, China
- 5 Department of Psychiatry, The Third Hospital of Quzhou, Quzhou, China
- 6 Psychosomatic Medicine Research Division, Inner Mongolia Medical University, Hohhot, China
- 7 Beijing Huilongguan Hospital, Peking University, Beijing, China
- 8 Infection Control Department, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China
- 9 Department of Infectious Diseases, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China
Objective: Previous research indicates associations between cigarette smoking, insulin-like growth factor-1 (IGF1), and sleep disturbances. This study aimed to examine the association between smoking and sleep quality and investigate the moderating role of IGF1.
Methods: This case-control study involved 146 Chinese adult males (53 active smokers and 93 non-smokers) from September 2014 to January 2016. Sleep quality and disturbances were evaluated using the Pittsburgh Sleep Quality Index (PSQI), which includes seven scales. Pearson correlation analysis and logistic regression analysis were utilized to examine the link between IGF1 levels in cerebrospinal fluid (CSF) and PSQI scores. The effect of IGF1 was assessed using the moderation effect and simple slope analysis, with adjustments made for potential confounders.
Results: Active smokers exhibited significantly higher global PSQI scores and lower IGF1 levels in CSF compared to non-smokers. A significant negative correlation was observed between IGF1 and PSQI scores (â = -0.28, P < 0.001), with a stronger association in non-smokers (Pearson r = -0.30) compared to smokers (Pearson r = -0.01). Smoking was associated with higher global PSQI scores (â = 0.282, P < 0.001), and this association was moderated by IGF1 levels in CSF (â = 0.145, P < 0.05), with a stronger effect at high IGF1 levels (Bsimple = 0.402, p < 0.001) compared to low IGF1 levels (Bsimple = 0.112, p = 0.268). Four subgroup analysis revealed similar results for sleep disturbances (Bsimple = 0.628, P < 0.001), with a marginal moderation effect observed on subjective sleep quality (Bsimple = 0.150, P = 0.070). However, independent associations rather than moderating effects were observed between IGF1 and sleep efficiency and daytime disturbance.
Conclusion: We provided evidence to demonstrate the moderation effect of IGF1 on the relationship between smoking and sleep in CSF among Chinese adult males.
1 Introduction
Poor sleep quality has become increasingly prevalent in various population over the past decades, affecting more than a quarter of the population worldwide ( 1 , 2 ). Existing epidemiological surveys have reported that approximately one-third of adults suffer from one or more sleep disorders during their aggregate lifetime ( 3 ). Of note, sleep disorder often coexists with physical health conditions and psychological comorbidities ( 4 , 5 ), which in turn exacerbate the symptoms that profoundly disturb sleep quality ( 6 ). Thus, sleep disorders have gained widespread concern from all walks of life as a major public health issue and a health management challenge.
Multiple factors such as unhealthy lifestyles (smoking, drinking, sedentary, diet), chronic diseases (mental illness, metabolic diseases) housing conditions, and socializing status may contribute to abnormal sleep patterns. Among these, cigarettes smoking has been shown to be detrimental to healthy sleep in several studies. A recently published meta-analysis indicated that smoking carried a higher risk of developing sleep-related issues than non-smoking ( 7 ), and sleep disorders similarly increased the difficulties for smoking cessation ( 8 ). Nevertheless, it has been reported that sleep disorders were still prevalent among smokers despite their intense quitting attempts ( 9 ). Indeed, large amounts of nicotine contained in cigarette smoke can readily penetrate the blood-brain barrier, rapidly distribute throughout the brain ( 10 ), and stimulate nicotinic receptors to release a series of neurotransmitters that independently or interactively regulate the sleep-wake cycle, thereby exacerbating sleep disorders and affecting overall sleep quality ( 11 , 12 ).
Moreover, sleep is associated with the optimal production and secretion of hormones, modulated by neuroendocrine signals ( 13 ). Recently, increasing interest has been devoted to exploring neurotrophic factors such as Insulin-like growth factor-1 (IGF1). Based on the previous studies, IGF1 is a hormone that plays a crucial role in the regulation of cell growth, differentiation, and metabolism ( 14 ). In population-based studies, high levels of peripheral IGF1 were found to be associated with better sleep quality ( 15 ). Epidemiological studies have shown that lower levels of IGF1 have been observed in individuals with chronic insomnia, while individuals with sleep extension have significantly higher levels of IGF1 concentrations in the blood compared to individuals with habitual sleep ( 15 , 16 ). IGF1 has been shown to have both neuroprotective and neurorestorative effects ( 17 ), and several studies have suggested that IGF1 may have a protective effect against the negative effects of smoking on sleep. For example, one study found that chronic nicotine exposure has been found to cause sleep disturbance in rats ( 18 ), and IGF1 supplementation can improve sleep quality in rats ( 19 ). However, there were inconsistent associations of tobacco exposure with IGF1, as well as differences in IGF1 levels between smokers and non-smokers ( 20 – 22 ). Some studies have not found the differences in the effect of smoking on sleep quality at different levels of IGF1, indicating that the relationship between these factors may be complex and multifaceted. Therefore, the aim of this study was to investigate the effect of IGF1 in cerebrospinal fluid (CSF) on the association between smoking and sleep quality.
2 Materials and methods
2.1 study population.
Considering the low proportion of female smokers in China (2.7%) ( 23 ), males were mainly recruited for the present study. The study design and population have been described in detail previously ( 24 ). Briefly, 191 subjects without fatal diseases who were scheduled for anterior cruciate ligament (ACL) reconstruction surgery were enrolled from September 2014 to January 2016 in this study. Information on sociodemographic data (age, marriage, and living) and lifestyles was obtained using interview questionnaire. Clinical information (personal and family history of diseases, history of substance abuse and dependence) was collected based on self-report and confirmed by family members. Physical examination (height and weight) was performed by a trained nurse, and body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. After excluding those with a family history of psychiatric diseases or systemic or central neurological diseases diagnosed by the Mini International Neuropsychiatric Interview, a total of 146 eligible adult males, comprising active smokers (n = 53) and non-smokers (n = 93), were recruited finally. None of the subjects had a history of alcohol abuse or psychiatric diseases identified by the Diagnostic and Statistical Manual of Mental Disorders (4th Edition). This study was conducted following the Declaration of Helsinki, approved by the Institutional Review Board of Inner Mongolian Medical University, and all the participants provided their written informed consent.
2.2 Biosamples collection and laboratory tests
The CSF biosamples were derived from lumbar puncture, the details of which have been elaborated in the previous literature ( 24 ). On the morning before ACL reconstruction surgery, a trained and licensed anesthesiologist performed the lumbar puncture operation on the subjects under local anesthesia (using 3 mL of 0.5% ropivacaine), thus collecting 5 mL CSF samples intrathecally. Each sample was distributed into 0.5mL-tubes and immediately stored in a -80°C refrigerator for determination within 24 hours. The entire procedure from hospitalization to surgery took no more than 2 days, during which the subjects were not required to quit smoking.
The levels of IGF1 in CSF were measured using atomic absorption spectrophotometry by professional laboratory technicians. The whole process of detection was in accordance with the principle of double-blind.
2.3 Definition of smoking
Non-smokers were defined as subjects who never smoked during their whole life without a history of substance abuse or dependence. Active smokers were those who smoked at least 10 cigarettes a day lasting for over one year. Otherwise, smokers in between–those who smoked less than 10 cigarettes/day–were excluded.
2.4 Assessment of PSQI
The Pittsburgh Sleep Quality Index (PSQI) is a recognized comprehensive measurement for subjective self-assessment of sleep quality and disturbances within an interval of the past month that was widely used in clinical practice and research, which can identify good and poor sleepers with high specificity, sensitivity and accuracy ( 25 ). Guided by the Chinese version of PSQI ( 26 ), all participants had to respond on a four-point Likert scale (from 0 to 3, indicating “no difficulty” to “severe difficulty”). Nineteen individual items were integrated into seven subscales: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction ( 25 ). The sum of these seven components generated a global score ranging from 0 to 21, with higher scores indicating poorer sleep quality and vice versa. Because fewer participants took sleeping medications, the other six subgroups were included in the subsequent analysis.
2.5 Statistical analysis
Categorical variables were described as number (percentage) and compared by Chi-square test. According to the normality distribution (mainly by the Shapiro-Wilk normality test), normally and skewed distributed continuous variables were presented as mean ± standard deviation and median (interquartile range), and the differences between groups were compared using independent t-test and Mann-Whitney U test, respectively. The correlation between the IGF1 levels and PSQI scores was examined by Pearson correlation analysis in the active smoking and non-smoking groups. We conducted the traditional linear regression model to investigate the interactive effect of IGF1 and cigarette dependence on PSQI score. Subsequently, a multivariate logistic regression model was employed to elucidate the associations between IGF1 levels in CSF and six subgroups of PSQI in all the subjects. Both linear regression and logistic regression models were adjusted for age (years, continuous), BMI (kg/m 2 , continuous), marriage (married/unmarried), and living (living alone/living with one roommate/living with family members). Furthermore, a moderation effect analysis and simple slope analysis were applied to assess the moderating effect of IGF1 on the relationship between smoking and PSQI scores and the significant components of PSQI. All statistical analyses were conducted using the R software (version 4.2.0, R Foundation for Statistical Computing). The moderation analysis was performed using the “Bruce R” package. All tests were two-tailed and a P < 0.05 was considered statistically significant.
3.1 Basic characteristics of study population
The basic characteristics of the 146 participants are presented in Table 1 , with a smoking rate of 36.3%. As demonstrated in Table 1 , active smokers were more likely to be older, unmarried, living alone, and have a higher BMI (all P < 0.05). Compared with non-smokers, the levels of IGF1 in CSF were significantly lower among active smokers (median levels of 33.0 ng/mL vs. 35.1 ng/mL, P < 0.001), while significantly higher in PSQI scores were observed (4.02 ± 2.27 vs. 2.60 ± 2.46, P < 0.001), particularly for sleep disturbance, sleep latency, and sleep quality. In addition, no differences were found for blood pressure and other components of PSQI between the two groups ( P > 0.05).
Table 1 Comparisons of descriptive characteristics between non-smokers and active smokers.
3.2 Correlation between IGF1 levels in CSF and PSQI scores and fractional latitude score in different groups
In the total population, the levels of IGF1 in CSF were negatively correlated with PSQI scores (β = -0.28, P < 0.001) ( Figure 1A ). However, the correlations differed across groups, where a significant negative correlation existed in non-smokers (Pearson r = -0.30, P < 0.001) but no correlation in active smokers Pearson r = -0.01, P = 0.703) ( Figure 1B ). Furthermore, the relationships between IGF1 levels (divided into two groups by the median) and six subgroups of PSQI were assessed by logistic regression model ( Figure 1C ). After adjusting for age, BMI, marriage and living, the levels IGF1 in CSF were inversely associated with sleep disturbances (OR = 0.268, 95% CI = 0.128-0.546, P = 0.001), as well as sleep quality, habitual efficiency, and daytime dysfunction (all P < 0.05).
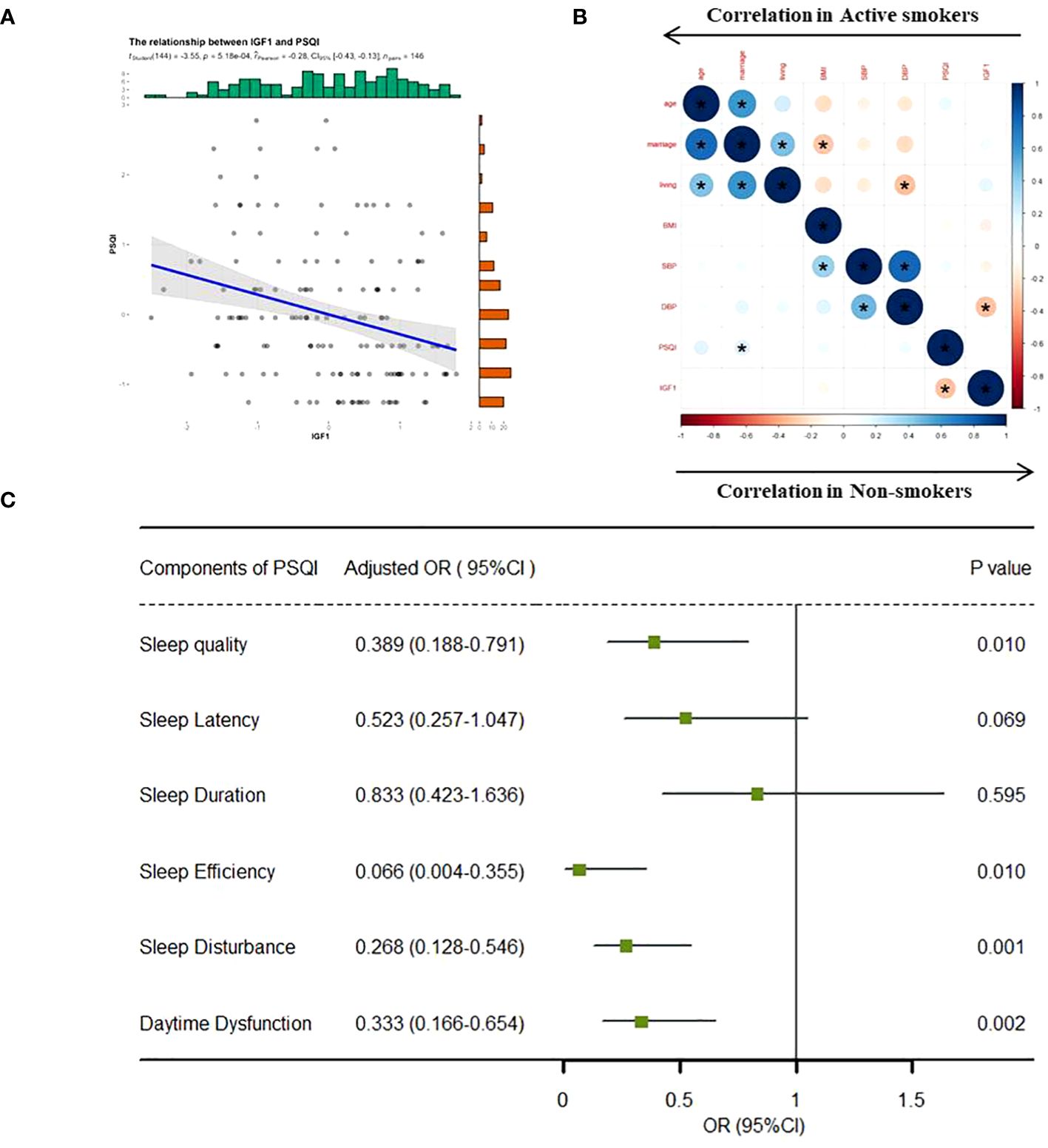
Figure 1 Correlation analysis between IGF1 and PSQI scores. IGF1, insulin-like growth factor-1; PSQI, Pittsburgh Sleep Quality Index; OR, odds ratio; CI, confidence interval. (A) Linear regression model was used to analyze the relationship between IGF1 levels and PSQI scores in all the groups. (B) Bivariate correlation matrix for the study variables in non-smokers and active smokers using pearson correlation analysis. * P < 0.05. (C) IGF1 (divided into two groups by the median level) and seven components of PSQI (yes/no) were included into the logistics regression model as dichotomous variables to assess the associations between the two. The linear regression and logistic regression models were adjusted for age, body mass index, marriage and living. Statistical significance ( P < 0.05) was denoted in boldface.
3.3 Moderation effect of IGF1
Based on the correlations in Figure 1 , we further estimated the moderating effect of IGF1 on the relationship between smoking and PSQI scores using moderation analysis ( Table 2 ). The first step of moderation analysis is to assess the association between independent and outcome variables. As shown in Model 1 ( Table 2 ), smoking was positively associated with PSQI scores after adjusting for potential confounders (β = 0.282, 95% CI = 0.135-0.428, R 2 = 0.109, P < 0.001). In the second step, moderating variables were introduced by including the interaction terms in the linear model. According to Model 3 ( Table 2 ), the positive impact of smoking on PSQI scores was moderated by IGF1 levels in CSF (β = 0.145, 95% CI = 0.004-0.285, R2 = 0.155, P < 0.001) ( Figure 2A ), with R2 increasing from 0.109 in Model 1 to 0.155 in model 3 and with F values increasing from 5.325 in Model 1 to 5.667 in Model 3 (ΔF = 0.342, p<0.05) ( Table 2 ). To elucidate the moderating role of IGF1 more clearly, we grouped all the subjects by the median level of IGF1 and performed simple slope analysis to investigate the impact of smoking on PSQI scores at different levels of IGF1 in CSF ( Figure 2B ). The positive predictive effect of smoking on PSQI scores was significantly increased in individuals with higher level of IGF1 (Bsimple = 0.402, P < 0.001), while it was weakened in those with a lower level of IGF1 (Bsimple = 0.112, P = 0.268). Moreover, we also assessed the moderating effect of IGF1 in four subgroups of PSQI which were significant in the logistic model ( Figure 1C ). Similar results were obtained in sleep disturbances (Bsimple of 0.628 and 0.200 in the groups with high and low IGF1 levels, P < 0.001, Figure 2C ), while a marginal moderating effect of IGF1 on sleep quality (Bsimple = 0.150, P = 0.070, Figure 3A ). However, there were independently negative associations rather than moderation between IGF1 and habitual efficiency (β = -0.14, P < 0.05) and daytime dysfunction (β = -0.24, P < 0.01, Figure 3B ).
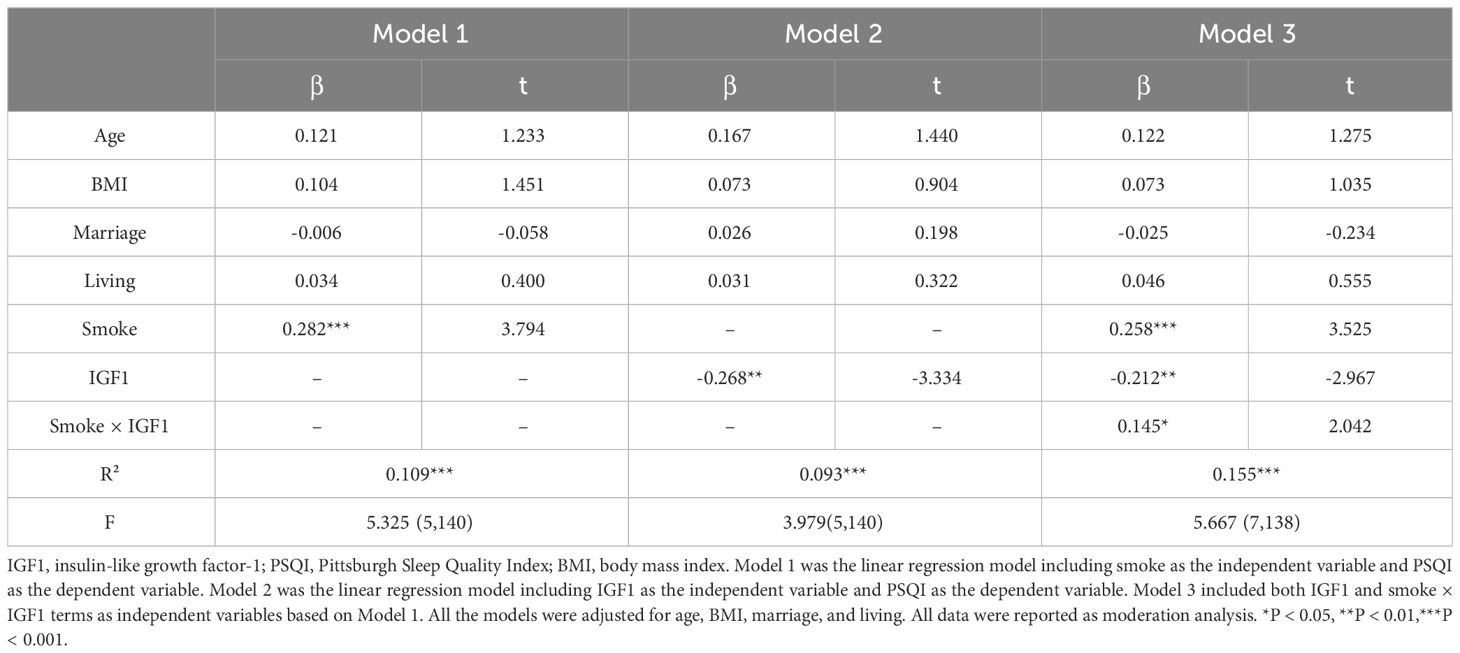
Table 2 Linear regression analysis for the moderation effect of IGF1 on the relationship between smoking and PSQI scores.
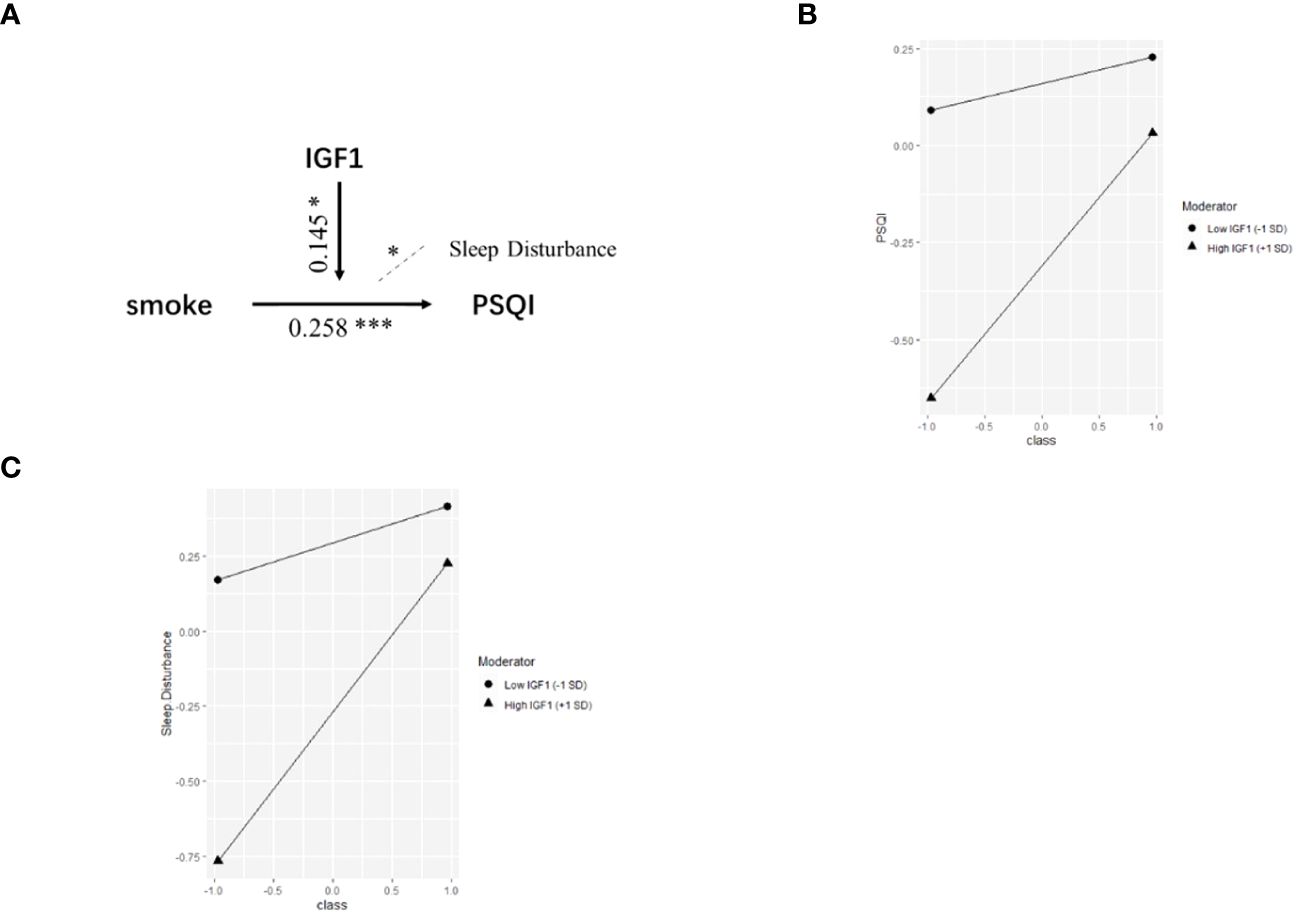
Figure 2 The moderation by IGF1 for the association of smoking with PSQI. IGF1, insulin-like growth factor-1; PSQI, Pittsburgh Sleep Quality Index. (A) Conceptual model of moderation analysis regarding IGF1 as moderator. (B, C) Simple slope analysis for the moderation effect of IGF1 on the relationship between smoking and PSQI scores (B) as well as sleep disturbance (C) . Class (-1) referred to non-smokers and Class (+1) referred to active smokers. The two lines represented the regression line of the association of smoking with PSQI scores (B) and sleep disturbance (C) when IGF1 was at low (circle) or high (triangle) levels. All data was included in the model as numerical variables and reported as moderation analysis. All the models were adjusted for age, BMI, marriage, and living. * P < 0.05, *** P < 0.001.
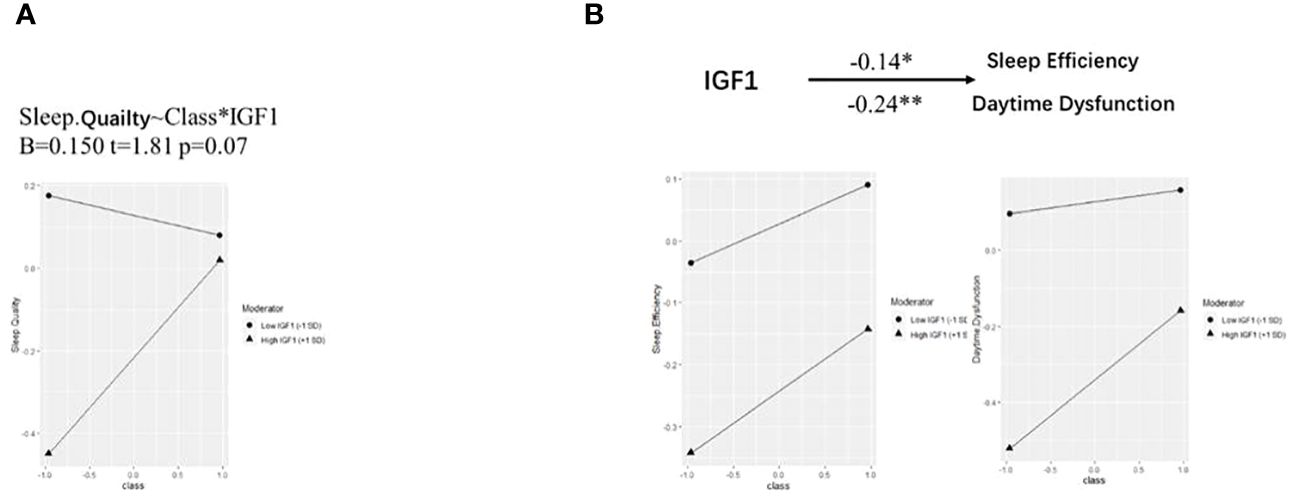
Figure 3 Simple slope analysis for the moderation effect of IGF-1 on PSQI components. IGF-1, insulin-like growth factor-1. (A, B) Simple slope analysis for the moderation effect of IGF-1 on the relationship between smoking and sleep quality (A) as well as sleep efficiency and daytime dysfunction (B) . Class (-1) referred to non-smokers and Class (+1) referred to active smokers. The two lines represented the regression line of the association of smoking with PSQI components when IGF-1 was at low (circle) or high (triangle) levels. All data was included in the model as numerical variables and reported as moderation analysis. All the models were adjusted for age, BMI, marriage, and living. * P < 0.05, ** P < 0.01.
4 Discussion
In the present study, we found global PSQI scores were significantly higher, while IGF1 levels in CSF were lower in active smokers than non-smokers. In addition, there was a significant negative correlation between CSF IGF1 level and global PSQI score, especially in non-smokers. Furthermore, smoking was positively associated with global PSQI scores (β = 0.282, P < 0.001), which was moderated by IGF1 levels in CSF (β = 0.145, P < 0.05). Our study revealed that IGF1 played a moderating role in the process of smoking-induced sleep disorders, which, to some extent, could provide new insights into the association between cigarette smoking and sleep disorders.
Cigarette smoking is one of the major known contributors to sleep disorders. It has been reported that the smoking rate among poorer sleepers is significantly higher than that of the general population ( 9 ). A study dating back to the 1990s showed that smoking was significantly positively associated with sleep disorders ( 27 ), which was subsequently supported by various studies in different populations from different regions ( 28 , 29 ). Sleep quality varies with the characteristics and intensity of smoking, a conclusion further supported by the present study. In addition. there are studies that support the possible interactions between smoking, sleep quality, and respiratory problems. For example, a study by Jang et al. found that smoking was associated with an increased risk of obstructive sleep apnea (OSA), a common respiratory disorder that can lead to poor sleep quality ( 30 ). Another study by Caliri et al. found that smoking was associated with increased inflammation and oxidative stress in the airways, which could contribute to the development of respiratory problems such as chronic obstructive pulmonary disease (COPD) ( 31 ). Moreover, some studies found that smoking was associated with poor sleep quality, and that the combination of smoking and poor sleep quality was associated with increased inflammation and oxidative stress, suggesting that these factors may interact to exacerbate respiratory problems ( 32 – 34 ). Overall, the existing literature suggests that there are complex interactions between smoking, sleep quality, and respiratory problems, and that these factors may influence each other in important ways. Further research is needed to fully understand these interactions and their clinical implications.
In the present study, IGF1 levels in CSF were lower in active smokers than non-smokers. Previous studies have shown that circulating IGF1 has the ability to reach the central nervous system through either the blood-CSF barrier or the blood-brain barrier ( 35 , 36 ). Thus, the lack of an increase in peripheral IGF1 can lead to a deficiency of IGF1 in the brain ( 36 ). Smoking has been shown to reduce the level of peripheral IGF1 ( 20 , 37 ) and destroy the blood-brain barrier ( 38 , 39 ), which can further explain our results.
The main finding of present study is that there was a significant negative correlation between the IGF1 level in CSF and PSQI score, especially in non-smokers. A low serum IGF1 level has been reported to be associated with sleep-related disease, and longer slow wave time could be associated with increased IGF1 levels ( 40 , 41 ). A recent case-control study in China indicated that serum IGF1 concentration was negatively associated with chronic insomnia, sleep disorders and anxiety scores ( 15 ). Behavioral symptoms of circadian rhythm imbalance and sleep-wake disorders were noted to be improved by increasing or releasing free IGF1 in serum ( 16 , 42 ). Similarly, animal experiments and epidemiological studies have revealed that sleep disorders might inhibit the IGF1 axis, with circulating IGF1 levels significantly declining after sustained sleep deprivation ( 43 , 44 ). The underlying mechanisms are complex. IGF1 is known for its neuroprotective properties, activating IGF1 receptor to initiate downstream phosphorylation cascades that regulate transcription, synaptic maturation, inhibits apoptosis, and promote growth, differentiation and metabolism of neuronal cells ( 35 ). Firstly, this relationship may be attributed to BDNF/IGF1 regulated neuronal plasticity changes, hypothesized to increase slow wave sleep activity ( 45 , 46 ). Moreover, IGF1 could facilitate the repair of neurons from hypoxia and improve sleep regulation ( 47 ). These studies suggest that IGF1 could improve sleep quality to some extent, which is similar to our results.
Moreover, our results imply that the level of IGF1 might differently influence the relationship between smoking and sleep quality. As mentioned, a high IGF1 level is associated with low PSQI scores in both non-smokers and all participants, indicating that IGF1, like cigarettes, leads to a direct effect on sleep quality. However, for participants with different CSF IGF1 levels, we not only found the independent effects from the two factors (β = 0.258***, t = 3.525 for smoking; β = -0.212**, t = -2.967 for IGF1). In addition, there was a complex interaction (β = 0.145*, t = 2.042). For participants with a low CSF IGF1 level, smoking did not activate sleep problems (β = 0.112, P = 0.268), but in those who with a high CSF IGF1 level, the sleep damage caused by smoking was greatly increased (β = 0.402, P < 0.001).
This finding is intriguing. Elevated IGF1 levels have the ability to regulate sleep, as mentioned above. Numerous studies have shown a positive correlation between IGF1 levels and sleep quality. Therefore, given the independent effects of smoking and IGF1, it is expected that elevated IGF1 levels will counteract the sleep disturbances induced by smoking. In the interaction model and its sub-dimensions, this effect is evident. Specifically, IGF1 exerts a significant protective effect on four dimensions of sleep disturbance: sleep quality, sleep efficiency, sleep disturbance and daytime dysfunction (sleep quality: OR = 0.389, p = 0.010; sleep efficiency: OR = 0.066, p = 0.010; daytime dysfunction: OR = 0.333, p = 0.002; sleep disturbance: OR = 0.268, p = 0.001). It is also noteworthy that an interaction similar to the PSQI results was only observed in the sleep disturbance dimension. Smoking and IGF1 had independent effects on sleep (see Figure 3 ) in the remaining dimensions.
It is likely that this effect is due to the activity of the orexin neurons. A study in 2020 clearly demonstrated that IGF1 in the central nervous system can directly influence the sleep-wake cycle of mice through the activation of orexin neurons. Orexin neurons significantly prolonged sleep duration in mice lacking IGF1 receptors, suggesting the involvement of IGF1 in wakefulness and maintenance via orexin neurons ( 48 ). In addition, previous studies have consistently shown a strong link between smoking and orexin expression. Exposure to smoke significantly increases orexin levels, thereby promoting wakefulness ( 49 – 51 ). Consequently, smokers with elevated levels of cerebrospinal fluid IGF1 may experience increased nicotine-induced stimulation of active orexin neurons, leading to this significant positive interaction.
To our knowledge, this is the first study to assess the role of IGF1 in CSF on smoking-induced sleep disorders (indicated by PSQI) in Chinese males. The effect of smoking on PSQI is moderated by different levels of IGF1 in CSF. Admittedly, there are several limitations in this study. First, causal inferences cannot be drawn from the case-control design, and a small sample size may restrict the statistical power to examine associations and moderations. Hence, evidence from prospective studies with larger sample size is warranted. Second, retrospective recall biases may occur using subjective sleep measurements and smoking assessments. Third, anterior cruciate ligament reconstructive surgery may be a potential confounder affecting smoking, sleep quality and biomarkers. Moreover, other potential confounding factor such as obstructive sleep apnea may affect our understanding of the relationship between smoking and sleep. Finally, only men were recruited due to the low smoking rate in women, resulting in limited applicability and generalizability.
5 Conclusion
The positive effect of smoking on PSQI scores and sleep disturbances were negatively moderated by the levels of IGF1 in cerebrospinal fluid in Chinese adult males. The results of this study have important clinical implications. Firstly, they highlight the importance of considering IGF1 levels in cerebrospinal fluid when assessing the relationship between smoking and sleep quality. Clinicians may need to monitor IGF1 levels in smokers who report poor sleep quality and consider interventions aimed at increasing IGF1 levels, such as exercise or nutritional supplements. Secondly, the findings suggest that targeting IGF1 may be a potential therapeutic strategy for improving sleep quality in smokers. Future studies are needed to explore the underlying mechanisms and to develop effective interventions. Overall, this study contributes to our understanding of the complex interplay between smoking, IGF1, and sleep quality. The findings have important implications for the development of targeted interventions to improve sleep quality in smokers and for the prevention of smoking-related sleep disturbances.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Inner Mongolian Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LS: Writing – original draft. YW: Writing – original draft, Formal Analysis. JL: Writing – review & editing, Resources. MM: Writing – review & editing, Resources. XL: Writing – review & editing. KZ: Writing – original draft, Resources. WH: Writing – review & editing. YK: Writing – review & editing, Resources. FW: Writing – review & editing, Resources. YL: Writing – review & editing, Supervision. YX: Writing – review & editing, Supervision. XJ: Writing – review & editing, Supervision.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Basic Public Welfare Research Project of Zhejiang Province (TGD23H030004), Wenzhou Basic Scientific Research Project (Y20220021), Health Science and Technology Project of Zhejiang Province (2022KY887).
Acknowledgments
We thank Prof. Li Chen for her statistic help.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Morin CM, Benca R. Chronic insomnia. Lancet . (2012) 379:1129–41. doi: 10.1016/S0140-6736(11)60750-2
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Soldatos CR, Allaert FA, Ohta T, Dikeos DG. How do individuals sleep around the world? Results from a single-day survey in ten countries. Sleep Med . (2005) 6:5–13. doi: 10.1016/j.sleep.2004.10.006
3. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev . (2002) 6:97–111. doi: 10.1053/smrv.2002.0186
4. Sutton EL. Insomnia. Ann Intern Med . (2021) 174:ITC33–48. doi: 10.7326/AITC202103160
5. Sun X, Liu B, Liu S, Wu DJH, Wang J, Qian Y, et al. Sleep disturbance and psychiatric disorders: A bidirectional Mendelian randomisation study. Epidemiol Psychiatr Sci . (2022) 31:e26. doi: 10.1017/S2045796021000810
6. Fang H, Tu S, Sheng J, Shao A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J Cell Mol Med . (2019) 23:2324–32. doi: 10.1111/jcmm.14170
7. Amiri S, Behnezhad S. Smoking and risk of sleep-related issues: A systematic review and meta-analysis of prospective studies. Can J Public Health . (2020) 111:775–86. doi: 10.17269/s41997-020-00308-3
8. Short NA, Mathes BM, Gibby B, Oglesby ME, Zvolensky MJ, Schmidt NB. Insomnia symptoms as a risk factor for cessation failure following smoking treatment. Addict Res Theory . (2017) 25:17–23. doi: 10.1080/16066359.2016.1190342
9. Liao Y, Xie L, Chen X, Kelly BC, Qi C, Pan C, et al. Sleep quality in cigarette smokers and nonsmokers: findings from the general population in central China. BMC Public Health . (2019) 19:808. doi: 10.1186/s12889-019-6929-4
10. Benowitz NL. Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addiction. N Engl J Med . (1988) 319:1318–30. doi: 10.1056/NEJM198811173192005
11. Jaehne A, Unbehaun T, Feige B, Cohrs S, Rodenbeck A, Schutz AL, et al. Sleep Changes in Smokers before, During and 3 Months after Nicotine Withdrawal. Addict Biol . (2015) 20:747–55. doi: 10.1111/adb.12151
12. Li H, Liu Y, Xing L, Yang X, Xu J, Ren Q, et al. Association of cigarette smoking with sleep disturbance and neurotransmitters in cerebrospinal fluid. Nat Sci Sleep . (2020) 12:801–8. doi: 10.2147/NSS.S272883
13. Chennaoui M, Léger D, Gomez-Merino D. Sleep and the Gh/Igf-1 axis: consequences and countermeasures of sleep loss/disorders. Sleep Med Rev . (2020) 49:101223. doi: 10.1016/j.smrv.2019.101223
14. Werner H. The Igf1 signaling pathway: from basic concepts to therapeutic opportunities. Int J Mol Sci . (2023) 24:14882. doi: 10.3390/ijms241914882
15. Zhang Y, Sun Q, Li H, Wang D, Wang Y, Wang Z. Lower serum insulin-like growth factor 1 concentrations in patients with chronic insomnia disorder. Front Psychiatry . (2023) 14:1102642. doi: 10.3389/fpsyt.2023.1102642
16. Chennaoui M, Arnal PJ, Drogou C, Sauvet F, Gomez-Merino D. Sleep extension increases Igf-I concentrations before and during sleep deprivation in healthy young men. Appl Physiol Nutr Metab . (2016) 41:963–70. doi: 10.1139/apnm-2016-0110
17. Huang YY, Wang HF, Wu BS, Ou YN, Ma LZ, Yang L, et al. Clinical laboratory tests and dementia incidence: A prospective cohort study. J Affect Disord . (2024) 351:1–7. doi: 10.1016/j.jad.2024.01.226
18. Salin-Pascual RJ, Moro-Lopez ML, Gonzalez-Sanchez H, Blanco-Centurion C. Changes in sleep after acute and repeated administration of nicotine in the rat. Psychopharmacol (Berl) . (1999) 145:133–8. doi: 10.1007/s002130051041
CrossRef Full Text | Google Scholar
19. Obál F Jr., Kapás L, Bodosi B, Krueger JM. Changes in sleep in response to intracerebral injection of insulin-like growth factor-1 (Ifg-1) in the rat. Sleep Res Online . (1998) 1:87–91.
PubMed Abstract | Google Scholar
20. Landin-Wilhelmsen K, Wilhelmsen L, Lappas G, Rosén T, Lindstedt G, Lundberg PA, et al. Serum insulin-like growth factor I in a random population sample of men and women: relation to age, sex, smoking habits, coffee consumption and physical activity, blood pressure and concentrations of plasma lipids, fibrinogen, parathyroid hormone and osteocalcin. Clin Endocrinol (Oxf) . (1994) 41:351–7. doi: 10.1111/j.1365-2265.1994.tb02556.x
21. Palmer RM, Wilson RF, Coward PY, Scott DA. Analysis of circulating insulin-like growth factor-1 (Igf-1) and Igf binding protein-3 (Igfbp-3) in tobacco smokers and non-smokers. Tob Induc Dis . (2002) 1:157–70. doi: 10.1186/1617-9625-1-2-157
22. Janssen JA, Stolk RP, Pols HA, Grobbee DE, Lamberts SW. Serum total Igf-I, free Igf-I, and Igfb-1 levels in an elderly population: relation to cardiovascular risk factors and disease. Arterioscler Thromb Vasc Biol . (1998) 18:277–82. doi: 10.1161/01.atv.18.2.277
23. Chinese Center for Disease Control and Prevention. 2015 Chinese Adults Tobacco Survey Report . Beijing (2015).
Google Scholar
24. Liu Y, Li H, Wang J, Xue Q, Yang X, Kang Y, et al. Association of cigarette smoking with cerebrospinal fluid biomarkers of neurodegeneration, neuroinflammation, and oxidation. JAMA Netw Open . (2020) 3:e2018777. doi: 10.1001/jamanetworkopen.2020.18777
25. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res . (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
26. Tsai PS, Wang SY, Wang MY, Su CT, Yang TT, Huang CJ, et al. Psychometric evaluation of the Chinese version of the Pittsburgh sleep quality index (Cpsqi) in primary insomnia and control subjects. Qual Life Res . (2005) 14:1943–52. doi: 10.1007/s11136-005-4346-x
27. Phillips BA, Danner FJ. Cigarette smoking and sleep disturbance. Arch Intern Med . (1995) 155:734–7. doi: 10.1001/archinte.155.7.734
28. Cohrs S, Rodenbeck A, Riemann D, Szagun B, Jaehne A, Brinkmeyer J, et al. Impaired sleep quality and sleep duration in smokers-results from the German multicenter study on nicotine dependence. Addict Biol . (2014) 19:486–96. doi: 10.1111/j.1369-1600.2012.00487.x
29. Bellatorre A, Choi K, Lewin D, Haynie D, Simons-Morton B. Relationships between smoking and sleep problems in black and white adolescents. Sleep . (2017) 40:zsw031. doi: 10.1093/sleep/zsw031
30. Jang YS, Nerobkova N, Hurh K, Park EC, Shin J. Association between smoking and obstructive sleep Apnea based on the stop-bang index. Sci Rep . (2023) 13:9085. doi: 10.1038/s41598-023-34956-5
31. Caliri AW, Tommasi S, Besaratinia A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat Res Rev Mutat Res . (2021) 787:108365. doi: 10.1016/j.mrrev.2021.108365
32. Purani H, Friedrichsen S, Allen AM. Sleep quality in cigarette smokers: associations with smoking-related outcomes and exercise. Addict Behav . (2019) 90:71–6. doi: 10.1016/j.addbeh.2018.10.023
33. Irwin MR. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol . (2019) 19:702–15. doi: 10.1038/s41577-019-0190-z
34. Htoo A, Talwar A, Feinsilver SH, Greenberg H. Smoking and sleep disorders. Med Clin North Am . (2004) 88:1575–91. doi: 10.1016/j.mcna.2004.07.003
35. Benarroch EE. Insulin-like growth factors in the brain and their potential clinical implications. Neurology . (2012) 79:2148–53. doi: 10.1212/WNL.0b013e3182752eef
36. Yan H, Mitschelen M, Bixler GV, Brucklacher RM, Farley JA, Han S, et al. Circulating Igf1 regulates hippocampal Igf1 levels and brain gene expression during adolescence. J Endocrinol . (2011) 211:27–37. doi: 10.1530/joe-11-0200
37. Fang F, Luo ZC, Dejemli A, Delvin E, Zhang J. Maternal smoking and metabolic health biomarkers in newborns. PloS One . (2015) 10:e0143660. doi: 10.1371/journal.pone.0143660
38. Randolph AC, Fukuda S, Ihara K, Enkhbaatar P, Micci MA. Blood-Brain Barrier Dysfunction after Smoke Inhalation Injury, with and without Skin Burn. Shock . (2019) 51:634–49. doi: 10.1097/shk.0000000000001196
39. Archie SR, Sifat AE, Zhang Y, Villalba H, Sharma S, Nozohouri S, et al. Maternal E-cigarette use can disrupt postnatal blood-brain barrier (Bbb) integrity and deteriorates motor, learning and memory function: influence of sex and age. Fluids Barriers CNS . (2023) 20:17. doi: 10.1186/s12987-023-00416-5
40. Galerneau LM, Borel AL, Chabre O, Sapene M, Stach B, Girey-Rannaud J, et al. The somatotropic axis in the sleep Apnea-obesity comorbid duo. Front Endocrinol (Lausanne) . (2020) 11:376. doi: 10.3389/fendo.2020.00376
41. Prinz PN, Moe KE, Dulberg EM, Larsen LH, Vitiello MV, Toivola B, et al. Higher plasma Igf-1 levels are associated with increased delta sleep in healthy older men. J Gerontol A Biol Sci Med Sci . (1995) 50:M222–6. doi: 10.1093/gerona/50a.4.m222
42. Kimura S, Toyoura M, Toyota Y, Takaoka Y. Serum concentrations of insulin-like growth factor-1 as a biomarker of improved circadian rhythm sleep-wake disorder in school-aged children. J Clin Sleep Med . (2020) 16:2073–8. doi: 10.5664/jcsm.8778
43. Chennaoui M, Drogou C, Sauvet F, Gomez-Merino D, Scofield DE, Nindl BC. Effect of acute sleep deprivation and recovery on insulin-like growth factor-I responses and inflammatory gene expression in healthy men. Eur Cytokine Netw . (2014) 25:52–7. doi: 10.1684/ecn.2014.0356
44. Everson CA, Crowley WR. Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats. Am J Physiol Endocrinol Metab . (2004) 286:E1060–70. doi: 10.1152/ajpendo.00553.2003
45. Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev . (2006) 10:49–62. doi: 10.1016/j.smrv.2005.05.002
46. Rusch HL, Guardado P, Baxter T, Mysliwiec V, Gill JM. Improved sleep quality is associated with reductions in depression and Ptsd arousal symptoms and increases in Igf-1 concentrations. J Clin Sleep Med . (2015) 11:615–23. doi: 10.5664/jcsm.4770
47. Mysliwiec V, Gill J, Matsangas P, Baxter T, Barr T, Roth BJ. Igf-1: A potential biomarker for efficacy of sleep improvement with automatic airway pressure therapy for obstructive sleep Apnea? Sleep Breath . (2015) 19:1221–8. doi: 10.1007/s11325-015-1142-x
48. Zegarra-Valdivia JA, Pignatelli J, Fernandez de Sevilla ME, Fernandez AM, Munive V, Martinez-RaChadell L, et al. Insulin-like growth factor I modulates sleep through hypothalamic orexin neurons. FASEB J . (2020) 34:15975–90. doi: 10.1096/fj.202001281RR
49. Liu ZB, Song NN, Geng WY, Jin WZ, Li L, Cao YX, et al. Orexin-a and respiration in a rat model of smoke-induced chronic obstructive pulmonary disease. Clin Exp Pharmacol Physiol . (2010) 37:963–8. doi: 10.1111/j.1440-1681.2010.05411.x
50. Hoyer D, Jacobson LH. Orexin in sleep, addiction and more: is the perfect insomnia drug at hand? Neuropeptides . (2013) 47:477–88. doi: 10.1016/j.npep.2013.10.009
51. De Luca R, Nardone S, Grace KP, Venner A, Cristofolini M, Bandaru SS, et al. Orexin neurons inhibit sleep to promote arousal. Nat Commun . (2022) 13:4163. doi: 10.1038/s41467-022-31591-y
Keywords: smoking, PSQI score, sleep disturbances, IGF1, moderation
Citation: Shan L, Wu Y, Lao J, Ma M, Luo X, Zheng K, Hu W, Kang Y, Wang F, Liu Y, Xu Y and Jin X (2024) The positive impact of smoking on poor sleep quality is moderated by IGF1 levels in cerebrospinal fluid: a case-control study among Chinese adults. Front. Psychiatry 15:1392732. doi: 10.3389/fpsyt.2024.1392732
Received: 28 February 2024; Accepted: 24 April 2024; Published: 10 May 2024.
Reviewed by:
Copyright © 2024 Shan, Wu, Lao, Ma, Luo, Zheng, Hu, Kang, Wang, Liu, Xu and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanlong Liu, [email protected] ; Xiaoya Jin, [email protected] ; Yali Xu, [email protected]
† These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Open access
- Published: 08 May 2024
Effect of digital game intervention on cognitive functions in older adults: a multiple baseline single case experimental design study
- Kyosuke Yorozuya 1 ,
- Yuta Kubo 1 ,
- Keisuke Fujii 2 ,
- Daiki Nakashima 3 ,
- Taiki Nagayasu 4 ,
- Hiroyuki Hayashi 1 ,
- Kazuya Sakai 1 &
- Keiji Amano 5 , 6
BMC Geriatrics volume 24 , Article number: 410 ( 2024 ) Cite this article
55 Accesses
Metrics details
Residents in nursing homes are prone to cognitive decline affecting memory, visuospatial cognition, and executive functions. Cognitive decline can lead to dementia, necessitating prioritized intervention.
The current study aimed to investigate whether an intervention using a digital game was effective for preserving and improving the cognitive function of residents in nursing homes. An intervention study was conducted using a single-case AB design with multiple baselines. The participants in the study were five older adults aged 65 and over who do not play digital games regularly. The study ran for 15 weeks, including a baseline (phase A) and an intervention phase (phase B). Phase A had five baselines (5 to 9 weeks) with random participant assignment. In phase B, participants engaged in a digital game (Space Invaders) individually. Cognitive function was assessed as the outcome, measured using the Brain Assessment (performed on a tablet through the Internet) at 16 measurement points. Four of five participants (two female and two male) were included in the analysis, using visual inspection and Bayesian statistics with multi-level modeling.
Visual inspection of the graphs revealed cognitive function score improvements after the intervention for most layers in terms of memory of numbers, memory of words, mental rotation test (visuospatial ability), and total scores in the Brain Assessment. These effects were also significant in the analysis by multi-level modeling.
Conclusions
The results suggest that the use of digital games may be effective for preserving and improving cognitive function among residents of nursing home.
Trial registration
This study was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN000048677; public title: Effect of a Digital Game Intervention for Cognitive Functions in Older People; registration date: August 30, 2022).
Peer Review reports
Introduction
In recent years, the number of people with dementia has reached 55 million globally, and is predicted to increase by approximately 10 million people each year, reaching 131.5 million by 2050; thus, developing solutions for dementia-related problems an important international challenge [ 1 , 2 ]. Cognitive decline causes behavioral and psychological symptoms of dementia, such as anxiety and wandering, leading to a decline in the quality of life of patients and caregivers. Therefore, measures against cognitive decline are needed to prevent dementia [ 2 , 3 ]. Residents of nursing homes may be particularly susceptible to cognitive decline because they tend to live in unchanging environments with fewer social activities and less stimulation compared with community-dwelling older adults [ 4 ]. Therefore, effective measures are required to maintain and improve cognitive function and prevent the onset of dementia in residents [ 5 ]. Specifically, decline in memory, visuospatial ability, and executive functions should be prioritized for intervention, because they reflect the decline in overall cognitive functioning, which can progress into dementia [ 6 , 7 , 8 ].
Previous studies have reported that stimulating specific cognitive functions through the implementation of digital games may help maintain cognitive function and prevent dementia among older adults [ 9 , 10 ]. Digital games are classified as entertainment, and often focus on fun, unlike serious games, which are task-oriented and focus on problem-solving and learning [ 11 ]. The operation of digital game controllers involves coordinated movements of the bilateral upper limbs, requiring activation of the cerebellum, which is also associated with procedural and episodic memory [ 10 ]. Digital games have the potential to promote the use of visuospatial abilities because of the nature of visuospatial information presented via screens [ 10 , 12 ]. In addition, the process of processing in-game information and developing operations in real time is likely to facilitate the use of executive functions such as judgment and working memory. Therefore, digital games, which can be easily set up in a facility and are expected to be fun and continuous, may be useful for the prevention of cognitive decline among older adults. However, previous studies have examined the effects of games on improving cognitive function in older adults using experimental designs such as randomized controlled trials. These studies often targeted community-dwelling older adults and utilized interventions involving serious games [ 10 , 13 ]. However, it has been reported that nursing home residents exhibit different characteristics to those of residents living in the community [ 14 , 15 ]. Additionally, serious games are typically categorized differently to digital games [ 11 ]. Thus, there appears to be a lack of research investigating the effects of digital games on the prevention of cognitive decline, including memory and visuospatial abilities, specifically among nursing home residents. Therefore, at the planning stage of the study, we deemed it necessary to mitigate uncertainty by assessing the feasibility and effectiveness of the research topic before conducting experiments requiring a larger number of participants, such as randomized controlled trials.
To develop effective interventions in situations in which the feasibility and effectiveness of the intervention are uncertain, single-case experimental designs are typically considered to be more appropriate than randomized controlled trial designs that require a large number of participants [ 12 ]. In addition, single-case experimental designs with multiple baselines are likely to be more useful for increasing the reliability of the intervention’s impact [ 16 ]. The purpose of the current study was to test whether digital games are effective for maintaining and improving global cognitive function, memory, visuospatial ability, and executive function in residents of nursing homes, using a multiple baseline single-case experimental design.
This study was conducted according to the Single-Case Reporting Guideline in Behavioural Interventions 2016 Checklist [ 17 ].
This study used a single-case AB design with multiple baselines, with phase A as the no-intervention phase and phase B as the intervention phase. This study was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN000048677; public title: Effect of a Digital Game Intervention for Cognitive Functions in Older People; registration date: August 30, 2022).
Setting and study population
In this study, five residents in one nursing home facility, were selected as participants between November 1, 2022, and March 31, 2023. Inclusion criteria were as follows: (1) 65 years of age or older (on the basis of previous studies, the older adults in this study were defined as individuals aged 65 years or older) [ 18 , 19 ], (2) Mini Mental State Examination-Japanese (MMSE-J) [ 20 , 21 ] score of 24 points or higher (maximum score: 30 points), (3) Ability to perform the test and tasks in the questionnaire. (4) Ability to operate a game controller without hand impairment, and (5) No regular habit of playing games (less than once a month), such as Nintendo Switch and smartphone-based games on a regular basis. Exclusion criteria were as follows: (1) severe behavioral disorders or medical requirements (e.g., a person whose sitting time is limited because of a disease such as heart failure, and who might be negatively impacted by the game intervention), (2) severe visual or hearing impairments, and (3) refusal to participate in the study.
Data collection
The minimum number of cases required for a single-case design with multiple baselines is three or more [ 16 ]. Several intervention studies with single-case designs that have been validated with three or four cases have previously been reported [ 12 , 22 ]. Therefore, we sought to include at least four cases in the current study, and the number of participants was set at 5 to take account of a potential dropout rate of 20%. The study collaborators collected information on basic characteristics such as age, gender, marital status (married, widowed, divorced, single), educational attainment (elementary school, junior high school, high school, university), length of stay, locomotion, and Barthel index from the facility records.
Digital game intervention
“Space Invaders” was used for the intervention in phase B of this study. Space Invaders is a two-dimensional (2D) platform digital game developed by Taito Corporation in 1978, which became popular worldwide ( https://spaceinvaders.jp/whats.html ). In Space Invaders, the aim is to shoot and eliminate the army of enemy aliens (Invaders) approaching from the top of the screen, using a beam cannon that can be moved left or right with the controller. When the invaders reach the player’s position at the bottom of the screen, the words “GAME OVER” are displayed, and the game ends. The game is completed when all invaders are eliminated before they reach the player’s position at the bottom of the screen, and the game proceeds to the next stage. The difficulty level increases as the game is cleared and progresses. If the “GAME OVER” message appears, the game can be restarted from the first stage. In the case of digital games that require complex controller-operation, older adults are likely to experience difficulty because of age-related loss of dexterity [ 23 ], which may interfere with continued implementation. Space Invaders can be easily operated with the left and right cross keys and a single right button, and the rules are easy to understand; it is classified as an entertainment game, which focuses on enjoyment, in contrast to task-oriented serious games that focus on the problem-solving and learning of the implementer [ 11 ]. In addition, because Space Invaders was a particularly popular game in the 1970s, we speculated that prior knowledge of the game among older adults (eliciting a sense of familiarity such as “I have seen it before” or “I know it”) might also contribute to continued engagement and enjoyment of the game. We speculated that this software would be suitable for this purpose because it is familiar to many older adults, has simple rules, and is linked to enjoyment and ongoing engagement. The game console used was the Nintendo switch and its dedicated family computer controller.
The duration and frequency of digital game playing ranged from 30 to 60 min per session, three times per week [ 10 ]. For the intervention environment, a private room for game playing was set up in the cooperating nursing home facility, and a staff member at the facility, who was a collaborator in the study, guided the participants to the room in which the game was played. After the induction, the participants played the game alone, during which time they had as little communication with others as possible. Digital games were played during the time in which participants would normally watch television or engage in hobbies or other activities. Instruction regarding the connection and operation of the TV monitor and Nintendo Switch (week 1 of phase B) was provided by staff. During the first week, all participants were able to understand and manipulate the rules of the game, and the staff confirmed that each participant was able to play the game alone to the same degree. The study period was 15 weeks (16 measurement points), with phases A and B divided into five layers (5 and 10 weeks, 6 and 9 weeks, 7 and 8 weeks, 8 and 7 weeks, and 9 and 6 weeks), and participants were randomly assigned to each layer. On the basis of previous studies with cognitive function as the outcome, the minimum duration of intervention in phase B was 6 weeks [ 24 ]. The phase A was defined as the period during which participants continued their usual lifestyle (Fig. 1 ).
Assigning participants to each layer. Participants 1, 2, 4, and 5 underwent the Brain Assessment once a week, for a total of 16 times, including the first time. The time required to conduct the Brain Assessment was 10 min each time (five tasks each requiring 2 min). Participants 1, 2, 4, and 5 underwent digital intervention for 27, 21, 18, and 30 sessions, respectively, for 30 to 60 min, three times a week during phase B. Participant 3 left the study midway through the study, so only underwent Brain Assessment a total of eight times, including the first time, and did not undergo the digital intervention
Outcome measure
The Brain Assessment instrument was used to assess specific and global cognitive functions. Brain Assessment consisted of five subsets, as follows. (1) Memory of numbers: participants were instructed to memorize numbers consisting of three to nine digits, and repeat them using the numeric keypad in ascending and descending order. (2) Memory of words: participants were instructed to memorize five target words. After a short distraction task, 10 words, half of which belong to the target word, were presented in turn. Participants reported whether each word was included in the target word. (3) Mental rotation test (MRT) to assess visuospatial ability: a target block diagram was displayed. One of the four rotated choices was different from the target. Participants chose which option was different. (4) N-back test to assess working memory: a series of numbers appeared, and participants performed the addition of the present and some antecedent numbers. (5) Judgment task: participants were instructed to identify changes in the presented Figs. 2 and 3 . Cognitive scores were calculated for each subset and total of each subset, allowing the state of the participant’s cognitive status to be assessed. Cognitive score is calculated as (raw score) – (mean of the raw score)/(standard deviation of the raw score) × 10 + 50. Higher cognitive score indicates higher cognitive function (by the same age and sex [mean and standard deviation are based on data from 5,000 subjects]). The validity and reliability of this measure have been confirmed [ 25 , 26 , 27 ]. The time required for each item was 2 min (a total of 10 min for five items), and the target age group was 40–90 years old. Each task is randomly assigned, and the results are not fed back, so the effect of learning through repetition is minimal. Each participant underwent the Brain Assessment individually using a tablet connected to the Internet. Assessments were conducted at the same time each week. During the study period, the survey was conducted approximately once a week from the time of participant selection to the end of follow-up (16 times in total). The total cognitive score in the Brain Assessment indicated global cognitive function, and was determined by the results of each cognitive score (five subsets) in the Brain Assessment. Therefore, the primary outcome of the intervention was assumed to be the cognitive score for each subset in the Brain Assessment, and the secondary outcome was assumed to be the total cognitive score in the Brain Assessment.
Progress of each Brain Assessment item (memory of numbers, memory of words, and MRT) score. The left side of the vertical line represents phase A, while the right side represents phase B. MRT, mental rotation test
Progress of each Brain Assessment item (N-back test, judgment, and total) score. The left side of the vertical line represents phase A, while the right side represents phase B
Statistical analysis
The single-case experimental design has a hierarchical structure in which the measurement period is tied to the study participants (the measurement period is nested by the study participants). In this study, we applied a two-level multilevel model with the measurement period as level 1 and the study participants as level 2 [ 28 , 29 ]. In addition, there were five participants in this study, and the number of data points was 16 points per participant, or 80 points for five participants. It was assumed that the sample size would not be large enough to take into account the possibility of drop-outs. Therefore, the analysis used Bayesian modeling, which can be used with small sample sizes [ 28 , 29 ].
In the modeling, the objective variable (each cognitive score of the Brain Assessment) was considered as a continuous variable, and phase was a dummy variable with phase A = 0 and phase B = 1. The parameters are denoted by \({mu}_{0}\) for the average baseline value and \({mu}_{1}\) for the average intervention effect. Variation among participants is indicated by \({sigma}_{0}\) , \({sigma}_{1}\) . The sum of \({mu}_{0}\) and \({mu}_{1}\) is the average value of phase B. The results are presented as expected a posteriori (EAP) and 95% Bayesian confidence interval (CI). In Bayesian statistics, if the 95% Bayesian CI of \({mu}_{1}\) , the parameter of the intervention effect, does not contain 0, 0 is judged to not be a possible value of the parameter, and the result is interpreted as significant [ 28 ].
Bayesian estimation and the Markov chain Monte Carlo (MCMC) method were used for parameter estimation, and MCMC sampling was conducted 18,000 times (chain = 3, burn-in = 1000). In the convergence judgment, MCMC was judged to have converged to a steady state when the potential scale reduction factor (PSRF) < 1.05 [ 30 ]. The statistical software R (version 4.0.5; R Foundation for Statistical Computing, Vienna, Austria) with the runjags (version 2.2.0–2) package and rjags (version 4–10) package were used for all statistical analyses.
Demographic characteristics
Of the five participants, two were female. The ages of participants 1–5 were 74, 86, 89, 90, and 65 years, respectively. MMSE-J scores for participants 1–5 were 24, 25, 26, 27, and 25, respectively (Table 1 ). All participants were independently ambulant, and all achieved a perfect score of 100 points on the Barthel Index, which serves as an indicator of activities of daily living (ADL). Participants 1, 2, 4, and 5 carried out the entire intervention, with a final implementation rate of 100% (Participant 1, 27/27; Participant 2, 21/21; Participant 4, 18/18; Participant 5, 30/30). Participant 3 was discharged and dropped out before the intervention.
No adverse events were observed in any of the participants throughout the study period. The results in Figs. 2 and 3 were determined through visual inspection [ 12 ]. Because participant 3 moved out at the 8-week mark and the intervention could not be completed, only phase A was assessed. In the layers of memory of numbers, memory of words, and total scores, cognitive scores appeared to gradually improve in all participants from the start of the intervention. In the MRT, cognitive scores appeared to gradually improve in Participants 2 and 5 from the start of the intervention. In the case of Participant 1, there was significant variability, and no apparent difference between phase A and phase B was observed. In Participant 4’s MRT performance, cognitive scores appeared to remain relatively stable throughout the study, but an improvement was observed towards the end. For the N-back test, Participant 5 exhibited an improvement in cognitive scores following the intervention, which appeared to be quite diverse. However, other participants displayed consistent fluctuations. In the judgment task, there was a minimal difference between phase A and phase B for all participants (Figs. 2 and 3 ).
Results of estimates for Brain Assessment using multilevel model
An analysis was conducted using a multi-level model with Bayesian statistics for the remaining four participants after excluding Participant 3, who dropped out. The MCMC results indicated that all posterior distributions converged to a steady state (PSRF < 1.05). Therefore, the posterior distribution was considered to be appropriate for use a probability distribution.
In the estimated results for the intervention effect, \({mu}_{1}\) , significant effects were observed in memory of numbers (EAP = 13.50, [95% Bayesian CI: 5.22, 21.40]), memory of words (EAP = 11.50, [95% Bayesian CI: 8.48, 14.43]), MRT (EAP = 11.57, [95% Bayesian CI: 2.70, 20.46]), and total score (EAP = 10.61, [95% Bayesian CI: 3.86, 16.98]) (Table 2 ). The N-back test and judgment task did not reveal significant intervention results.
The current study examined the effects of an intervention using a digital game (Space Invaders) on the cognitive function of five residents in nursing home. A single-case AB design with a multiple baseline approach was employed for this investigation. The results shown in Figs. 2 and 3 indicate a gradual improvement in cognitive scores for memory of numbers, memory of words, and total scores among all participants following the intervention. Most participants exhibited stable or improved performance after the intervention, suggesting that these changes may be attributed to the implementation of Space Invaders. In the analysis results, cognitive scores for memory of numbers, memory of words, and total scores were higher in phase B compared with those in phase A, indicating a significant intervention effect. This result supports the potential impact of playing Space Invaders observed through visual inspection. However, some participants showed a gradual improvement in cognitive scores even before the intervention, suggesting the involvement of residual variables other than playing the game. In the MRT, participant 1 exhibited substantial variability, indicating a lack of expected game-related effects. In contrast, other participants showed potential for improvement, and as a result, the analysis suggested a significant intervention effect.
The results for memory of numbers, memory of words, and the MRT, which reflect memory and visuospatial ability, appeared to support the hypothesis of this study [ 26 ]. We speculated that controller operation would promote cerebellar activity related to memory, and that processing information from the screen during the game would involve visuospatial cognitive abilities. These potential effects might be expected to occur in 2D platform digital games like Space Invaders [ 9 ]. Furthermore, the finding that simultaneous effects were observed for memory and visuospatial ability, both of which are considered to be crucial for the prevention of cognitive decline, suggests that the implementation of interventions involving Space Invaders may have potential for the prevention of cognitive decline [ 6 , 7 , 8 ]. Moreover, 2D platform digital games are easily recognizable in terms of controls and screen movements, potentially contributing to their consistent adherence (100% implementation rate). These aspects serve as advantages of this intervention and are likely to be factors contributing to its effectiveness. The significant improvement in total cognitive scores, reflecting global cognitive function, suggests that the use of 2D platform digital games like Space Invaders should be considered as a potential strategy in future efforts to prevent cognitive decline. Therefore, exploring the utilization of games like Space Invaders as a means of cognitive intervention for the prevention of cognitive decline should be encouraged.
Interventions related to executive functions, such as the N-back test and judgment task [ 31 , 32 ] did not reveal significant improvements. This might suggest the need for activities that involve three-dimensional (3D) environments or games that require predicting the surrounding space in a more 3D manner and strategizing [ 11 ]. A previous study using the Wii Fit® game console, a 3D platform, reported that after one session, the semantic memory and executive function of nursing home residents improved moderately [ 33 ]. A study examining the effects of the Wii Fit® game console on cognitive function in older adults living in a nursing home or attending a day care center reported improvements in global cognitive function [ 34 ]. Beyond the findings of the current study, it is crucial to continue researching the features of more effective games, including those with 3D platforms, competitive elements, or role-playing aspects.
The estimation results for each cognitive score in the Brain Assessment exhibited wide confidence intervals, suggesting that the dispersion of the estimated averages in the analysis was large. In this study, we targeted five participants, which was considered to be the minimum number of participants using a single-case design. However, one participant dropped out before the intervention, and the actual analysis was performed on four patients. Although the participants were selected using specific criteria, there was a large amount of variation in the cognitive scores of each participant, which may have affected the accuracy of the estimation. To further improve the accuracy of estimation in future studies, it will be necessary to secure more participants and verify the effectiveness of this method.
Although the current findings suggest several areas for further investigation, Space Invaders, as implemented in this study, demonstrated high practical utility, with a 100% implementation rate and no adverse events. Notably, the current findings indicated that residents of a nursing home facility were able to engage in this intervention independently.
This research paradigm was designed to examine the feasibility and effectiveness of a game-based intervention. Compared with the designs of some previous studies, such as randomized controlled trials, the ability of the current study design to confirm a potential effect on a population is relatively limited. However, to the best of our knowledge, no previous studies have specifically considered the effects of 2D digital games on preventing decline in cognitive functions such as memory and visuospatial abilities in nursing home residents. The current findings suggested the feasibility and effectiveness of an intervention using Space Invaders for preventing cognitive decline in nursing home residents, which may have implications for future research. In the future, higher-quality study designs should be used to confirm the current findings.
Limitations
However, some limitations of the current study should be considered. We found that some participants showed gradual improvements (covariation) in cognitive scores, even before the intervention. The Brain Assessment is considered to be less susceptible to learning effects because the task is presented with a choice of five versions, and no feedback regarding correct answers is provided to the test taker [ 26 ]. However, it is possible that the changes in the Brain Assessment in phase A in this study resulted from habituation caused by repeated administration of the test, and such a learning effect might be unavoidable. The current study design could not conclusively confirm the presence or impact of these residual variables. However, because the analysis also accounted for the effects of variables that are difficult to observe (such as learning effects) by incorporating random effects as level 2 for participant-specific variation, it is possible that the significant results for post-intervention cognitive score variation included intervention effects. In future research, it will be essential to develop more extended study plans that consider factors like seasonal variations and introduce interventions after ensuring the stability of fluctuations observed in the phase A. In addition, larger sample sizes will be needed to test the effects of digital game interventions.
In this study, the duration of the intervention varied among participants, and the possible influence of this variation on the observed effects cannot be excluded. In future studies, it will be important to standardize the intervention period for all participants and to unify the amount of intervention. Moreover, employing more robust research designs, such as randomized controlled trials, will be crucial for verifying the effects identified in the current study.
The Space Invaders intervention implemented in this study appears to have potential for enhancing memory of numbers, memory of words, MRT performance, and total cognitive scores for residents of nursing home. The high practicality of this intervention, which can be independently used by individuals and has no reported adverse events, further supports its utility in real-world settings. The effectiveness of this intervention should be tested in more detail in future, higher-quality research.
Data availability
All data generated or analysed during this study are included in the manuscript.
Abbreviations
Mini Mental State Examination-Japanese
two-dimensional
Mental rotation test
expected a posteriori
confidence interval
Markov chain Monte Carlo
potential scale reduction factor
activities of daily living
standard deviation
three-dimensional
Alzheimer’s Disease International: World Alzheimer Report. 2015. https://www.alzint.org/resource/world-alzheimer-report-2015/ . Accessed 1 Sept 2023.
World Health Organization. Dementia. https://www.who.int/news-room/fact-sheets/detail/dementia . Accessed 1 Sept 2023.
Abrahamson K, Clark D, Perkins A, Arling G. Does cognitive impairment influence quality of life among nursing home. Residents? Gerontologist. 2012;52:632–40. https://doi.org/10.1093/geront/gnr137 .
Article PubMed Google Scholar
Yorozuya K, Yamane S, Nobuhisa M, Owaki H, Suzuki T, Okahara H, et al. Bayesian analysis of the association between effective strategies of multimodal nonpharmacological intervention and characteristics of cognitive function in nursing home residents with cognitive impairment: a cross-sectional study. Med (Baltim). 2020;99(37):e22154. https://doi.org/10.1097/MD.0000000000022154 .
Article CAS Google Scholar
Yorozuya K, Kubo Y, Tomiyama N, Yamane S, Hanaoka H. A systematic review of multimodal non-pharmacological interventions for cognitive function in older people with dementia in nursing homes. Dement Geriatr Cogn Disord. 2019;48(1–2):1–16. https://doi.org/10.1159/000503445 .
Wiederman MW, Morgan CD. The neurobehavioral cognitive status exam (NCSE) with geriatric inpatients. Clin Gerontologist. 1995;15(4):35–47. https://doi.org/10.1300/J018V15N04_04 .
Article Google Scholar
Yu F, Kolanowski AM, Strumpf NE, Eslinger PJ. Improving cognition and function through exercise intervention in Alzheimer’s disease. JNS. 2006;38(4):358–65. https://doi.org/10.1111/j.1547-5069.2006.00127.x .
Tsuruoka Y, Takahashi M, Suzuki M, Sato K, Shirayama Y. Utility of the Neurobehavioral Cognitive Status Examination (COGNISTAT) in differentiating between depressive states in late–life depression and late–onset Alzheimer’s disease: a preliminary study. Ann Gen Psychiatry. 2016;15:1–8. https://doi.org/10.1186/s12991-016-0091-5 .
Kühn S, Gallinat J. Amount of lifetime video gaming is positively associated with entorhinal, hippocampal and occipital volume. Mol Psychiatry. 2014;19(7):842–7. https://doi.org/10.1038/mp.2013.100 .
West GL, Zendel BR, Konishi K, Benady-Chorney J, Bohbot VD, Peretz I, et al. Playing Super Mario 64 increases hippocampal grey matter in older adults. PLoS ONE. 2017;12(12):e0187779. https://doi.org/10.1371/journal.pone.0187779 .
Article CAS PubMed PubMed Central Google Scholar
Susi T, Johannesson M, Backlund P. Serious games: An overview. Technical Report HS-IKI-TR-07-001. Skövde: Institutionen för kommunikation och information. 2007;1–28.
Kanstrup M, Kontio E, Geranmayeh A, Lauri KO, Moulds ML, Holmes ME. A single case series using visuospatial task interference to reduce the number of visual intrusive memories of trauma with refugees. Clin Psychol Psychother. 2021;28(1):109–23. https://doi.org/10.1002/cpp.2489 .
Kleschnitzki JM, Beyer L, Beyer R, Großmann I. The effectiveness of a serious game (MemoreBox) for cognitive functioning among seniors in care facilities: Field study. JMIR Serious Games. 2022;10(2):e33169. https://doi.org/10.2196/33169 .
Article PubMed PubMed Central Google Scholar
Folkerts AK, Roheger M, Franklin J, Middelstädt J, Kalbe E. Cognitive interventions in patients with dementia living in long-term care facilities: systematic review and meta-analysis. Arch Gerontol Geriatr. 2017;73:204–21.
Wang G, Albayrak A, Van Der Cammen TJ. A systematic review of non-pharmacological interventions for BPSD in nursing home residents with dementia: from a perspective of ergonomics. Int Psychogeriatr. 2019;31(8):1137–49.
Alnahdi GH. Single-subject designs in special education: advantages and limitations. JORSEN. 2015;15(4):257–65. https://doi.org/10.1111/1471-3802.12039 .
Tate RL, Perdices M, Rosenkoetter U, Shadish W, Vohra S, Barlow DH, et al. The single-case reporting Guideline in BEhavioural interventions (SCRIBE) 2016 Statement. Neuropsychol Rehabil. 2017;27(1):1–15. https://doi.org/10.2522/ptj.2016.96.7.e1 .
Ouchi Y, Rakugi H, Arai H, Akishita M, Ito H, Toba K, et al. Redefining the elderly as aged 75 years and older: proposal from the Joint Committee of Japan Gerontological Society and the Japan Geriatrics Society. Geriatr Gerontol Int. 2017;17(7):1045–7. https://doi.org/10.1111/ggi.13118 .
Cuevas-Lara C, Izquierdo M, de Asteasu MLS, Ramírez-Vélez R, Zambom-Ferraresi F, Zambom-Ferraresi F. Impact of game-based interventions on health-related outcomes in hospitalized older patients: a systematic review. J Am Med Dir Assoc. 2021;22(2):364–e711. https://doi.org/10.1016/j.jamda.2020.07.027 .
Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. https://doi.org/10.1016/0022-3956(75)90026-6 .
Article CAS PubMed Google Scholar
Sugishita M. Mini Mental State Examination-Japanese (MMSE-J). Tokyo: Nihon Bunka Kagakusha Co., Ltd.; 2019.
Google Scholar
McGoldrick C, Crawford S, Evans JJ, MindMate. A single case experimental design study of a reminder system for people with dementia. Neuropsychol Rehabil. 2021;31(1):18–38. https://doi.org/10.1080/09602011.2019.1653936 .
Carmeli E, Patish H, Coleman R. The aging hand. J Gerontol Biol Sci Med Sci. 2003;58(2):146–52. https://doi.org/10.1093/gerona/58.2.m146 .
Amjad I, Toor H, Niazi IK, Pervaiz S, Jochumsen M, Shafique M, et al. Xbox 360 Kinect cognitive games improve slowness, complexity of EEG, and cognitive functions in subjects with mild cognitive impairment: a randomized control trial. Games Health J. 2019;8(2):144–52. https://doi.org/10.1089/g4h.2018.0029 .
Satoh M, Tabei K, Abe M, Kamikawa C, Fujita S, Ota Y. The correlation between a New Online Cognitive Test (the Brain Assessment) and widely used In-Person neuropsychological tests. Dement Geriatr Cogn Disord. 2021;50(5):473–81. https://doi.org/10.1159/000520521 .
Satoh M, Tabei K, Fujita S, Ota Y. Online Tool (Brain Assessment) for the detection of cognitive function changes during aging. Dement Geriatr Cogn Disord. 2021;50(1):85–95. https://doi.org/10.1159/000516564 .
Satoh M, Tabei K, Abe M, Kamikawa C, Fujita S, Ota Y. Shorter version of the Brain Assessment is suitable for longitudinal public cognitive evaluations. Dement Geriatr Cogn Disord. 2022;51(5):405–11. https://doi.org/10.1159/000526907 .
Rindskopf D. Nonlinear bayesian analysis for single case designs. J Sch Psychol. 2014;52(2):179–89. https://doi.org/10.1016/j.jsp.2013.12.003 .
Baek E, Beretvas SN, Van den Noortgate W, Ferron JM. Brief research report: bayesian versus REML estimations with noninformative priors in multilevel single-case data. J Experimental Educ. 2019;698–710. https://doi.org/10.1080/00220973.2018.1527280 .
Gelman A. Bayesian data analysis. 3rd ed. Oxford: Chapman and Hall/CRC; 2013.
Book Google Scholar
Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex frontal lobe tasks: a latent variable analysis. Cogn Psychol. 2000;41(1):49–100. https://doi.org/10.1006/cogp.1999.0734 .
Hinrichs KH, Hayek A, Kalmbach D, Gabel N, Bieliauskas LA. Cognitive reserve and executive function: Effect on judgment of health and safety. J Rehabil Res Dev. 2016;53(6):863–72. https://doi.org/10.1682/JRRD.2015.04.0073 .
Monteiro-Junior RS, Figueiredo LFS, Maciel-Pinheiro PT, Abud ELR, Braga AEMM, Barca ML, et al. Acute effects of exergames on cognitive function of institutionalized older persons: a single-blinded, randomized and controlled pilot study. Aging Clin Exp Res. 2017;29(3):387–94. https://doi.org/10.1007/s40520-016-0595-5
Jahouh M, González-Bernal JJ, González-Santos J, Fernández-Lázaro D, Soto-Cámara R, Mielgo-Ayuso J. Impact of an intervention with Wii video games on the autonomy of activities of daily living and psychologicalcognitive components in the institutionalized elderly. Int J Environ Res Public Health. 2021;18(4):1570. https://doi.org/10.3390/ijerph18041570
Download references
Acknowledgements
The authors would like to thank Seijoh University and Good Time Home Grand Hagi for support of the study. The authors would also like to thank all of the study participants who provided data. We thank Benjamin Knight, MSc., from Edanz ( https://jp.edanz.com/ac ) for editing a draft of this manuscript. We would like to express our sincere gratitude to the late Mr. Yoshihito Tsubouchi for many discussions regarding this study.
This work was supported by JSPS KAKENHI [Grant Number JP 23K16501] and Seijoh University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and affiliations.
Faculty of Rehabilitation and Care, Seijoh University, 2-172 Fukinodai, Tokai, 476-8588, Aichi, Japan
Kyosuke Yorozuya, Yuta Kubo, Hiroyuki Hayashi & Kazuya Sakai
Faculty of Health Science, Suzuka University of Medical Science, Suzuka, Mie, Japan
Keisuke Fujii
Faculty of Health Science, Naragakuen University, Nara, Nara, Japan
Daiki Nakashima
Good Time Club Grand Hagi, Hagi, Yamaguchi, Japan
Taiki Nagayasu
Faculty of Business Administration, Seijoh University, Tokai, Aichi, Japan
Keiji Amano
College of Image Arts and Sciences, Ritsumeikan University, Kyoto, Kyoto, Japan
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: K.Y, Y.K. Formal analysis: K.Y, Y.K, K.F, D.N. Funding acquisition: K.Y. Investigation: K.Y, T.N. Methodology: K.Y, Y.K, K.F, D.N, H.H, K.S, K.A. Project administration: K.Y, Y.K, T.N. Supervision: H.H, K.S, K.A. Writing - original draft: K.Y. Writing review & editing: Y.K, K.F, D.N, T.N, H.H, K.S, K.A.
Corresponding author
Correspondence to Kyosuke Yorozuya .
Ethics declarations
Ethical approval.
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Seijoh University (approval number: 2022C0006). The participants provided informed consent before participating in the study.
Consent for publication
Written informed consent was obtained from the participants for publication of this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Yorozuya, K., Kubo, Y., Fujii, K. et al. Effect of digital game intervention on cognitive functions in older adults: a multiple baseline single case experimental design study. BMC Geriatr 24 , 410 (2024). https://doi.org/10.1186/s12877-024-05011-3
Download citation
Received : 09 February 2024
Accepted : 25 April 2024
Published : 08 May 2024
DOI : https://doi.org/10.1186/s12877-024-05011-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Digital game
- Single-case design
- Cognitive functions
- Nursing home
- Bayesian analysis
BMC Geriatrics
ISSN: 1471-2318
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
An 81-year-old man presented with fever, cough, and shortness of breath. Within a few hours after presentation, chest pain and respiratory distress developed. A chest radiograph showed bilateral pa...
A 44-year-old woman presented with cough, dyspnea, and chest pain. On examination, she had tachycardia and hypotension. Evaluation revealed SARS-CoV-2 RNA in a nasopharyngeal swab, as well as eleva...
This case study exemplifies the potentially serious consequences of treatment failure following prescription of a macrolide for community-acquired bacterial pneumonia. ... Baudouin S. V., George R. C., et al. British Thoracic Society guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009; 64 ...
This was a retrospective case-control study of hospitalized adult (age ≥18 years) patients with pneumococcal pneumonia. The clinical outcomes of immunocompromised adults with pneumococcal pneumonia (cases) were compared to the clinical outcomes of non-immunocompromised adults with pneumococcal pneumonia (controls). Patients were eligible if ...
In a 2-year study done in the USA, the annual age-adjusted incidence was 649 patients hospitalised (admitted to hospital and treated there) with community-acquired pneumonia per 100 000 adults, corresponding to more than 1·5 million annual adult community-acquired pneumonia hospitalisations in the USA.
In the USA, the Etiology of Pneumonia in the Community (EPIC) study 13 found that the annual incidence of CAP was 2.4 cases per 1,000 adults, with the highest rates amongst adults of 65-79 years ...
Case Studies in Adult Intensive Care Medicine - April 2017. ... The pneumonia severity index: a decade after the initial derivation and validation. Clin Infect Dis 2008; 47 (Suppl 3): S133-9.CrossRef Google Scholar PubMed. 5 Lim, WS, Van der Eerden, MM, Laing, R et al.
Almirall J, Bolíbar I, Balanzó X, González CA. Risk factors for community-acquired pneumonia in adults: a population-based case-control study. Eur Respir J 1999; 13:349. Torres A, Peetermans WE, Viegi G, Blasi F. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax 2013; 68:1057.
INCIDENCE. The annual incidence of CAP is 248 cases per 100,000 adults. However, this increases to 634 cases per 100,000 in adults 65 to 79 years of age and 16,430 cases per 100,000 in adults 80 ...
studies of "elderly subjects" because the majority of pa-tients who develop CAP are older adults. Case reports, review articles, editorials and commentaries were also excluded. Data abstraction Date on the following variables were extracted from each article and listed in the study-designated spreadsheet
Chest pain when you breathe or cough. Confusion or changes in mental awareness (in adults age 65 and older) Cough, which may produce phlegm. Fatigue. Fever, sweating and shaking chills. Lower than normal body temperature (in adults older than age 65 and people with weak immune systems) Nausea, vomiting or diarrhea.
Abstract. We performed a systematic review of the literature to establish conclusive evidence of risk factors for community-acquired pneumonia (CAP). Observational studies (cross-sectional, case-control, and cohort studies) the primary outcome of which was to assess risk factors for CAP in both hospitalized and ambulatory adult patients with radiologically confirmed pneumonia were selected ...
BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009; 64: iii1 ... b In the case of patients taking steroid and who ... Oxygen saturations less than 92% are associated with major adverse events in outpatients with pneumonia: a population-based cohort study. Clin Infect Dis. 2011; 52: 325-331.
Abstract. Pneumonia is a potentially fatal infection and inflammation of the lower respiratory tract, namely the bronchioles and alveoli, caused by bacteria and viruses inhaled into the lungs. In older individuals all around the world, community-acquired pneumonia (CAP) is a common cause of hospitalisation and death.
In the United States, community-acquired pneumonia is one of the leading causes of hospitalization and death, with approximately 6 million cases reported each year. 1-6 The annual incidence of ...
Case presentation. A 19-year-old man was admitted to the acute medical unit overnight with 2 days' history of cough, fever and shortness of breath. He did not have any other symptoms or signs. He was treated as having community-acquired pneumonia with intravenous benzylpenicillin and clarithromycin. He was previously fit and healthy without ...
Background Infection caused by Streptococcus pneumoniae, mainly invasive pneumococcal disease (IPD) and pneumococcal pneumonia (PP), are a major public health problem worldwide. This study investigated population-based incidence and risk of PP among Catalonian persons ≥ 50 years-old with and without specific underlying conditions/comorbidities, examining the influence of single and multi ...
Background. Community-acquired pneumonia (CAP) is a common cause of morbidity and mortality worldwide. In Africa, CAP is associated with an in-hospital mortality of 6-15% among adults, as reported from hospital-based studies [1-3].Studies from high-income countries have identified several risk factors for CAP including smoking [4-7], age > 65 years [4, 8, 9], immunosuppression by any ...
The study aimed to investigate whether the clinical presentations and outcomes (length of stay, ICU admission, and 30-day mortality) of pneumococcal pneumonia in virologically suppressed patients who were HIV-infected on ART with a CD4+ T-cell count > 350 cells/mm3 are comparable to those seen in patients with HIV, using a case-control design.
Patient was managed with co-trimoxazole 48 hourly and prednisolone 25 mg BID.Case 2: A 70-year old male was admitted with high grade fever and SOB for 4 days. He was a known hypertensive, with a history of severe COVID-19 pneumonia 1.5 months back for which he was managed with IV tocilizumab and dexamethasone. His SpO2 was 63% and RR was 73 BPM.
Despite innovative advances in anti-infective therapies and vaccine development technologies, community-acquired pneumonia (CAP) remains the most persistent cause of infection-related mortality globally. Confronting the ongoing threat posed by <i>Streptococcus pneumoniae</i> (the pneumococcus), the …
Background: During and after the COVID-19 pandemic, there was a suspicion of varying rates of respiratory tract infections (RTIs), particularly pneumonia (PN). Methods: This research evaluated epidemiological indicators of community-acquired pneumonia (CAP) and hospital-acquired pneumonia (HAP) in the COVID-19 pandemic and post-pandemic period, including pathogens, ventilator-associated ...
Pneumonia Case Study Basic Adult Healthcare - NUR1211C. HS is 70 years of age and a male patient who is admitted to the medical-surgical unit with acute Community Acquired Pneumonia. He was diagnosed with paraseptal emphysema three years ago. The patient smoked cigarettes 1 pack per day for 55 years and quit three years ago.
Purpose Older patients with pneumonia are commonly restricted from oral intake due to concerns towards aspiration. Eating and drinking with acknowledged risks (EDAR) is a shared decision-making process emphasising patient comfort. As part of our project to find the barriers and facilitators of EDAR, we aimed for this initial study to see how frequently EDAR was selected in practice. Methods We ...
This study aimed to examine the association between smoking and sleep quality and investigate the moderating role of IGF1. Methods: This case-control study involved 146 Chinese adult males (53 active smokers and 93 non-smokers) from September 2014 to January 2016. Sleep quality and disturbances were evaluated using the Pittsburgh Sleep Quality ...
An intervention study was conducted using a single-case AB design with multiple baselines. The participants in the study were five older adults aged 65 and over who do not play digital games regularly. The study ran for 15 weeks, including a baseline (phase A) and an intervention phase (phase B). Phase A had five baselines (5 to 9 weeks) with ...