- En español – ExME
- Em português – EME

An introduction to different types of study design
Posted on 6th April 2021 by Hadi Abbas

Study designs are the set of methods and procedures used to collect and analyze data in a study.
Broadly speaking, there are 2 types of study designs: descriptive studies and analytical studies.
Descriptive studies
- Describes specific characteristics in a population of interest
- The most common forms are case reports and case series
- In a case report, we discuss our experience with the patient’s symptoms, signs, diagnosis, and treatment
- In a case series, several patients with similar experiences are grouped.
Analytical Studies
Analytical studies are of 2 types: observational and experimental.
Observational studies are studies that we conduct without any intervention or experiment. In those studies, we purely observe the outcomes. On the other hand, in experimental studies, we conduct experiments and interventions.
Observational studies
Observational studies include many subtypes. Below, I will discuss the most common designs.
Cross-sectional study:
- This design is transverse where we take a specific sample at a specific time without any follow-up
- It allows us to calculate the frequency of disease ( p revalence ) or the frequency of a risk factor
- This design is easy to conduct
- For example – if we want to know the prevalence of migraine in a population, we can conduct a cross-sectional study whereby we take a sample from the population and calculate the number of patients with migraine headaches.
Cohort study:
- We conduct this study by comparing two samples from the population: one sample with a risk factor while the other lacks this risk factor
- It shows us the risk of developing the disease in individuals with the risk factor compared to those without the risk factor ( RR = relative risk )
- Prospective : we follow the individuals in the future to know who will develop the disease
- Retrospective : we look to the past to know who developed the disease (e.g. using medical records)
- This design is the strongest among the observational studies
- For example – to find out the relative risk of developing chronic obstructive pulmonary disease (COPD) among smokers, we take a sample including smokers and non-smokers. Then, we calculate the number of individuals with COPD among both.
Case-Control Study:
- We conduct this study by comparing 2 groups: one group with the disease (cases) and another group without the disease (controls)
- This design is always retrospective
- We aim to find out the odds of having a risk factor or an exposure if an individual has a specific disease (Odds ratio)
- Relatively easy to conduct
- For example – we want to study the odds of being a smoker among hypertensive patients compared to normotensive ones. To do so, we choose a group of patients diagnosed with hypertension and another group that serves as the control (normal blood pressure). Then we study their smoking history to find out if there is a correlation.
Experimental Studies
- Also known as interventional studies
- Can involve animals and humans
- Pre-clinical trials involve animals
- Clinical trials are experimental studies involving humans
- In clinical trials, we study the effect of an intervention compared to another intervention or placebo. As an example, I have listed the four phases of a drug trial:
I: We aim to assess the safety of the drug ( is it safe ? )
II: We aim to assess the efficacy of the drug ( does it work ? )
III: We want to know if this drug is better than the old treatment ( is it better ? )
IV: We follow-up to detect long-term side effects ( can it stay in the market ? )
- In randomized controlled trials, one group of participants receives the control, while the other receives the tested drug/intervention. Those studies are the best way to evaluate the efficacy of a treatment.
Finally, the figure below will help you with your understanding of different types of study designs.

References (pdf)
You may also be interested in the following blogs for further reading:
An introduction to randomized controlled trials
Case-control and cohort studies: a brief overview
Cohort studies: prospective and retrospective designs
Prevalence vs Incidence: what is the difference?
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
No Comments on An introduction to different types of study design
you are amazing one!! if I get you I’m working with you! I’m student from Ethiopian higher education. health sciences student
Very informative and easy understandable
You are my kind of doctor. Do not lose sight of your objective.
Wow very erll explained and easy to understand
I’m Khamisu Habibu community health officer student from Abubakar Tafawa Balewa university teaching hospital Bauchi, Nigeria, I really appreciate your write up and you have make it clear for the learner. thank you
well understood,thank you so much
Well understood…thanks
Simply explained. Thank You.
Thanks a lot for this nice informative article which help me to understand different study designs that I felt difficult before
That’s lovely to hear, Mona, thank you for letting the author know how useful this was. If there are any other particular topics you think would be useful to you, and are not already on the website, please do let us know.
it is very informative and useful.
thank you statistician
Fabulous to hear, thank you John.
Thanks for this information
Thanks so much for this information….I have clearly known the types of study design Thanks
That’s so good to hear, Mirembe, thank you for letting the author know.
Very helpful article!! U have simplified everything for easy understanding
I’m a health science major currently taking statistics for health care workers…this is a challenging class…thanks for the simified feedback.
That’s good to hear this has helped you. Hopefully you will find some of the other blogs useful too. If you see any topics that are missing from the website, please do let us know!
Hello. I liked your presentation, the fact that you ranked them clearly is very helpful to understand for people like me who is a novelist researcher. However, I was expecting to read much more about the Experimental studies. So please direct me if you already have or will one day. Thank you
Dear Ay. My sincere apologies for not responding to your comment sooner. You may find it useful to filter the blogs by the topic of ‘Study design and research methods’ – here is a link to that filter: https://s4be.cochrane.org/blog/topic/study-design/ This will cover more detail about experimental studies. Or have a look on our library page for further resources there – you’ll find that on the ‘Resources’ drop down from the home page.
However, if there are specific things you feel you would like to learn about experimental studies, that are missing from the website, it would be great if you could let me know too. Thank you, and best of luck. Emma
Great job Mr Hadi. I advise you to prepare and study for the Australian Medical Board Exams as soon as you finish your undergrad study in Lebanon. Good luck and hope we can meet sometime in the future. Regards ;)
You have give a good explaination of what am looking for. However, references am not sure of where to get them from.
Subscribe to our newsletter
You will receive our monthly newsletter and free access to Trip Premium.
Related Articles

Cluster Randomized Trials: Concepts
This blog summarizes the concepts of cluster randomization, and the logistical and statistical considerations while designing a cluster randomized controlled trial.

Expertise-based Randomized Controlled Trials
This blog summarizes the concepts of Expertise-based randomized controlled trials with a focus on the advantages and challenges associated with this type of study.

A well-designed cohort study can provide powerful results. This blog introduces prospective and retrospective cohort studies, discussing the advantages, disadvantages and use of these type of study designs.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- For Patients
- Clinical Trials: What Patients Need to Know
What Are the Different Types of Clinical Research?
Different types of clinical research are used depending on what the researchers are studying. Below are descriptions of some different kinds of clinical research.
Treatment Research generally involves an intervention such as medication, psychotherapy, new devices, or new approaches to surgery or radiation therapy.
Prevention Research looks for better ways to prevent disorders from developing or returning. Different kinds of prevention research may study medicines, vitamins, vaccines, minerals, or lifestyle changes.
Diagnostic Research refers to the practice of looking for better ways to identify a particular disorder or condition.
Screening Research aims to find the best ways to detect certain disorders or health conditions.
Quality of Life Research explores ways to improve comfort and the quality of life for individuals with a chronic illness.
Genetic studies aim to improve the prediction of disorders by identifying and understanding how genes and illnesses may be related. Research in this area may explore ways in which a person’s genes make him or her more or less likely to develop a disorder. This may lead to development of tailor-made treatments based on a patient’s genetic make-up.
Epidemiological studies seek to identify the patterns, causes, and control of disorders in groups of people.
An important note: some clinical research is “outpatient,” meaning that participants do not stay overnight at the hospital. Some is “inpatient,” meaning that participants will need to stay for at least one night in the hospital or research center. Be sure to ask the researchers what their study requires.
Phases of clinical trials: when clinical research is used to evaluate medications and devices Clinical trials are a kind of clinical research designed to evaluate and test new interventions such as psychotherapy or medications. Clinical trials are often conducted in four phases. The trials at each phase have a different purpose and help scientists answer different questions.
Phase I trials Researchers test an experimental drug or treatment in a small group of people for the first time. The researchers evaluate the treatment’s safety, determine a safe dosage range, and identify side effects.
Phase II trials The experimental drug or treatment is given to a larger group of people to see if it is effective and to further evaluate its safety.
Phase III trials The experimental study drug or treatment is given to large groups of people. Researchers confirm its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the experimental drug or treatment to be used safely.
Phase IV trials Post-marketing studies, which are conducted after a treatment is approved for use by the FDA, provide additional information including the treatment or drug’s risks, benefits, and best use.
Examples of other kinds of clinical research Many people believe that all clinical research involves testing of new medications or devices. This is not true, however. Some studies do not involve testing medications and a person’s regular medications may not need to be changed. Healthy volunteers are also needed so that researchers can compare their results to results of people with the illness being studied. Some examples of other kinds of research include the following:
A long-term study that involves psychological tests or brain scans
A genetic study that involves blood tests but no changes in medication
A study of family history that involves talking to family members to learn about people’s medical needs and history.
Evidence-Based Medicine: Types of Studies
- What is Evidence-Based Practice?
- Question Types and Corresponding Resources
- Types of Studies
- Practice Guidelines
- Step 3: Appraise This link opens in a new window
- Steps 4-5: Apply & Assess
Experimental vs. Observational Studies
An observational study is a study in which the investigator cannot control the assignment of treatment to subjects because the participants or conditions are not directly assigned by the researcher.
- Examines predetermined treatments, interventions, policies, and their effects
- Four main types: case series , case-control studies , cross-sectional studies , and cohort studies
In an experimental study , the investigators directly manipulate or assign participants to different interventions or environments
Experimental studies that involve humans are called clinical trials . They fall into two categories: those with controls, and those without controls.
- Controlled trials - studies in which the experimental drug or procedure is compared with another drug or procedure
- Uncontrolled trials - studies in which the investigators' experience with the experimental drug or procedure is described, but the treatment is not compared with another treatment
Definitions taken from: Dawson B, Trapp R.G. (2004). Chapter 2. Study Designs in Medical Research. In Dawson B, Trapp R.G. (Eds), Basic & Clinical Biostatistics, 4e . Retrieved September 15, 2014 from https://accessmedicine.mhmedical.com/book.aspx?bookid=2724
Levels of Evidence Pyramid
Levels of Evidence Pyramid created by Andy Puro, September 2014
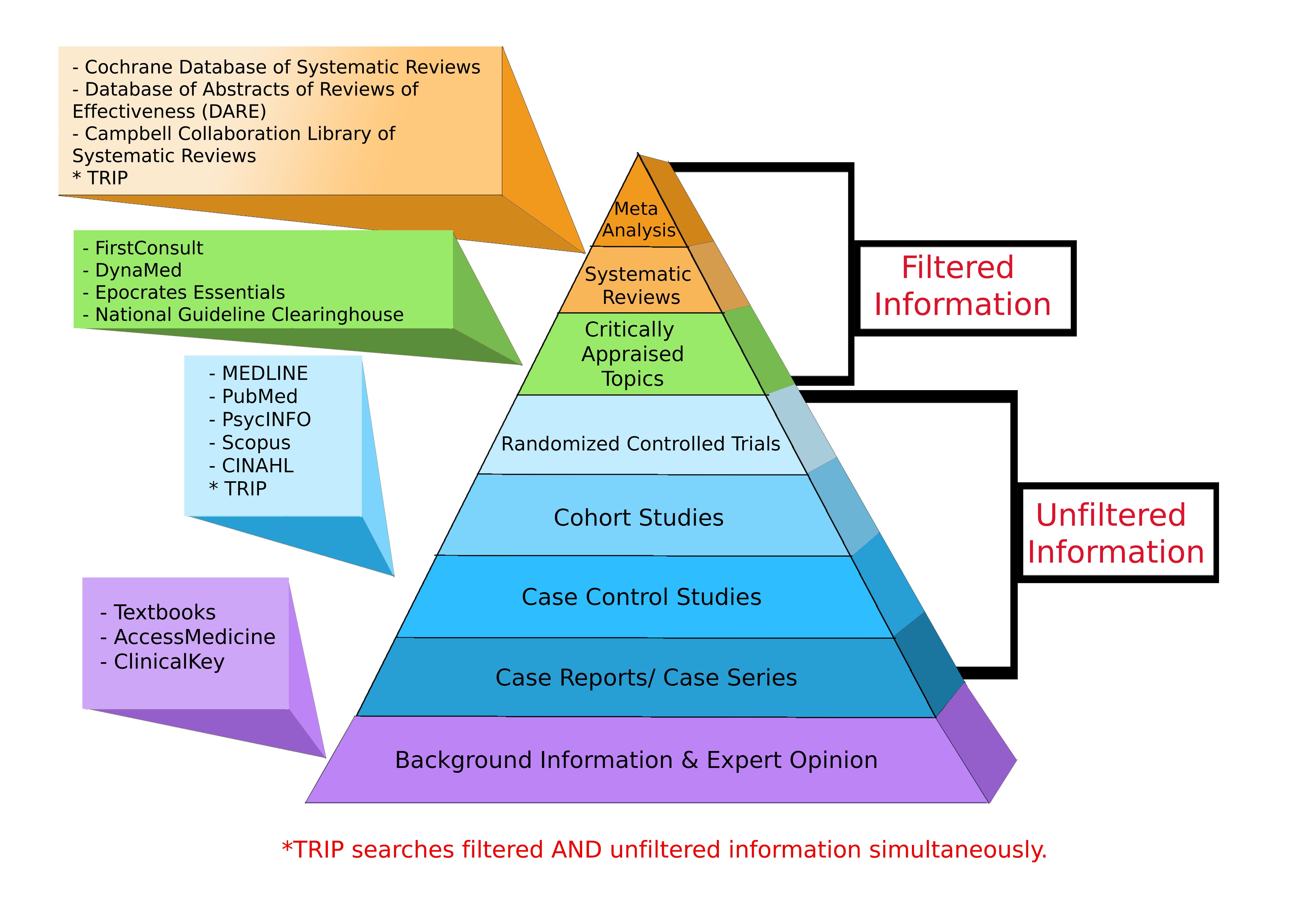
Additional Study Design Resources
Study Design 101 : Himmelfarb's tutorial on study types and how to find them
Study Designs (Centre for Evidence Based Medicine, University of Oxford)
Learn about Clinical Studies (ClinicalTrials.gov, National Institutes of Health)
Study Designs Guide (Deakin University)
- << Previous: Step 2: Acquire
- Next: Practice Guidelines >>

- Last Updated: Dec 6, 2023 7:59 AM
- URL: https://guides.himmelfarb.gwu.edu/ebm

- Himmelfarb Intranet
- Privacy Notice
- Terms of Use
- GW is committed to digital accessibility. If you experience a barrier that affects your ability to access content on this page, let us know via the Accessibility Feedback Form .
- Himmelfarb Health Sciences Library
- 2300 Eye St., NW, Washington, DC 20037
- Phone: (202) 994-2850
- [email protected]
- https://himmelfarb.gwu.edu

Types of Clinical Trials – [A Comprehensive Guide]
It is clinical trials that advance healthcare, serving as pivotal gateways to innovation. In addition to driving innovation, these meticulously designed studies also offer the chance to improve treatment outcomes. We’ll dive deep in the diverse landscape of clinical trials as we set out on this journey, shedding light on different types of clinical trials and therapeutic areas.
What are Clinical Trials?
Clinical trials are research studies conducted to evaluate the safety and efficacy of medical interventions, including treatments, drugs, devices, and therapeutic strategies, in humans. These trials are essential for determining whether a new intervention is safe, effective, and suitable for widespread use in patient populations.

5 Types of Clinical Trials:
Let’s unveil 5 most common types of clinical trials below:
Treatment Trials:
- Focus on testing new treatments, therapies, or interventions.
- Investigate the efficacy and safety of novel drugs, procedures, or combinations of treatments.
- Aim to identify more effective approaches for managing diseases or conditions.
Prevention Trials:
- Aim to prevent the onset or recurrence of diseases or health conditions.
- Evaluate interventions such as vaccines, medications, lifestyle modifications, or behavioral interventions.
Diagnostic Trials:
- Assess new diagnostic tools, tests, or procedures for identifying diseases or health conditions.
- Aim to improve early detection, accuracy, and efficiency in diagnosing medical conditions.
Screening Trials:
- Evaluate the effectiveness of screening methods for detecting diseases or health conditions in populations.
- Focus on early detection and intervention to improve treatment outcomes and prognosis.
Observational Trials:
- Observe and analyze participants over time to gather data on health outcomes, risk factors, or disease progression.
- Do not involve intervention or manipulation of variables, but rather focus on documenting natural history and patterns of diseases.
Different Types of Clinical Trial Studies:
Below-mentioned are the different types of Clinical trial studies that give you a clear picture of what comes under the roof of Clinical Trial Studies.
Randomized Controlled Trials (RCTs):
- Gold standard in clinical research .
- Participants are randomly assigned to different intervention groups to minimize bias and confounding factors.
- Rigorous design ensures reliable and robust evidence for assessing treatment effects.
Non-Randomized Trials:
- Participants are not randomly assigned to intervention groups.
- May lack the same level of control as RCTs but can provide valuable insights, particularly in real-world settings.
Cross-Over Trials:
- Participants receive different interventions sequentially, with a washout period in between.
- Designed to compare the effects of multiple treatments within the same group of participants.
Therapeutic Areas in Clinical Trials
Clinical trials span a wide range of therapeutic areas, reflecting the diverse landscape of medical research and healthcare needs. Some common therapeutic areas include:
- Cardiovascular diseases
- Infectious diseases
- Endocrinology
- Rare diseases
Ending Line:
Now that you know that the world of clinical trials is complex and dynamic, encompassing various types of studies and therapeutic areas. Each trial plays a crucial role in advancing medical knowledge, improving patient care, and shaping the future of healthcare.
Contact us to schedule a complimentary network consultation

Kerlo Research Inc 1100 Cornwall Rd Suite 220 Monmouth Junction, NJ 08852
© {{2024}} Kerlo Research Inc.
Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.
Search life-sciences literature (43,882,693 articles, preprints and more)
- Free full text
- Citations & impact
- Similar Articles
Types of study in medical research: part 3 of a series on evaluation of scientific publications.
Author information, affiliations.
Deutsches Arzteblatt International , 10 Apr 2009 , 106(15): 262-268 https://doi.org/10.3238/arztebl.2009.0262 PMID: 19547627 PMCID: PMC2689572
Free full text in Europe PMC
Abstract
Conclusions, free full text , types of study in medical research, bernd röhrig.
1 MDK Rheinland-Pfalz, Referat Rehabilitation/Biometrie, Alzey
Jean-Baptist du Prel
2 Zentrum für Präventive Pädiatrie, Zentrum für Kinder- und Jugendmedizin, Mainz
Daniel Wachtlin
3 Interdisziplinäres Zentrum Klinische Studien (IZKS), Fachbereich Medizin der Universität Mainz
Maria Blettner
4 Institut für Medizinische Biometrie, Epidemiologie und Informatik (IMBEI), Johannes Gutenberg Universität Mainz
The choice of study type is an important aspect of the design of medical studies. The study design and consequent study type are major determinants of a study’s scientific quality and clinical value.
This article describes the structured classification of studies into two types, primary and secondary, as well as a further subclassification of studies of primary type. This is done on the basis of a selective literature search concerning study types in medical research, in addition to the authors’ own experience.
Three main areas of medical research can be distinguished by study type: basic (experimental), clinical, and epidemiological research. Furthermore, clinical and epidemiological studies can be further subclassified as either interventional or noninterventional.
The study type that can best answer the particular research question at hand must be determined not only on a purely scientific basis, but also in view of the available financial resources, staffing, and practical feasibility (organization, medical prerequisites, number of patients, etc.).
The quality, reliability and possibility of publishing a study are decisively influenced by the selection of a proper study design. The study type is a component of the study design (see the article "Study Design in Medical Research") and must be specified before the study starts. The study type is determined by the question to be answered and decides how useful a scientific study is and how well it can be interpreted. If the wrong study type has been selected, this cannot be rectified once the study has started.
After an earlier publication dealing with aspects of study design, the present article deals with study types in primary and secondary research. The article focuses on study types in primary research. A special article will be devoted to study types in secondary research, such as meta-analyses and reviews. This article covers the classification of individual study types. The conception, implementation, advantages, disadvantages and possibilities of using the different study types are illustrated by examples. The article is based on a selective literature research on study types in medical research, as well as the authors’ own experience.
Classification of study types
In principle, medical research is classified into primary and secondary research. While secondary research summarizes available studies in the form of reviews and meta-analyses, the actual studies are performed in primary research. Three main areas are distinguished: basic medical research, clinical research, and epidemiological research. In individual cases, it may be difficult to classify individual studies to one of these three main categories or to the subcategories. In the interests of clarity and to avoid excessive length, the authors will dispense with discussing special areas of research, such as health services research, quality assurance, or clinical epidemiology. Figure 1 gives an overview of the different study types in medical research.

Classification of different study types
*1 , sometimes known as experimental research; *2 , analogous term: interventional; *3 , analogous term: noninterventional or nonexperimental
This scheme is intended to classify the study types as clearly as possible. In the interests of clarity, we have excluded clinical epidemiology — a subject which borders on both clinical and epidemiological research ( 3 ). The study types in this area can be found under clinical research and epidemiology.
Basic research
Basic medical research (otherwise known as experimental research) includes animal experiments, cell studies, biochemical, genetic and physiological investigations, and studies on the properties of drugs and materials. In almost all experiments, at least one independent variable is varied and the effects on the dependent variable are investigated. The procedure and the experimental design can be precisely specified and implemented ( 1 ). For example, the population, number of groups, case numbers, treatments and dosages can be exactly specified. It is also important that confounding factors should be specifically controlled or reduced. In experiments, specific hypotheses are investigated and causal statements are made. High internal validity (= unambiguity) is achieved by setting up standardized experimental conditions, with low variability in the units of observation (for example, cells, animals or materials). External validity is a more difficult issue. Laboratory conditions cannot always be directly transferred to normal clinical practice and processes in isolated cells or in animals are not equivalent to those in man (= generalizability) ( 2 ).
Basic research also includes the development and improvement of analytical procedures—such as analytical determination of enzymes, markers or genes—, imaging procedures—such as computed tomography or magnetic resonance imaging—, and gene sequencing—such as the link between eye color and specific gene sequences. The development of biometric procedures—such as statistical test procedures, modeling and statistical evaluation strategies—also belongs here.
Clinical studies
Clinical studies include both interventional (or experimental) studies and noninterventional (or observational) studies. A clinical drug study is an interventional clinical study, defined according to §4 Paragraph 23 of the Medicines Act [Arzneimittelgesetz; AMG] as "any study performed on man with the purpose of studying or demonstrating the clinical or pharmacological effects of drugs, to establish side effects, or to investigate absorption, distribution, metabolism or elimination, with the aim of providing clear evidence of the efficacy or safety of the drug."
Interventional studies also include studies on medical devices and studies in which surgical, physical or psychotherapeutic procedures are examined. In contrast to clinical studies, §4 Paragraph 23 of the AMG describes noninterventional studies as follows: "A noninterventional study is a study in the context of which knowledge from the treatment of persons with drugs in accordance with the instructions for use specified in their registration is analyzed using epidemiological methods. The diagnosis, treatment and monitoring are not performed according to a previously specified study protocol, but exclusively according to medical practice."
The aim of an interventional clinical study is to compare treatment procedures within a patient population, which should exhibit as few as possible internal differences, apart from the treatment ( 4 , e1 ). This is to be achieved by appropriate measures, particularly by random allocation of the patients to the groups, thus avoiding bias in the result. Possible therapies include a drug, an operation, the therapeutic use of a medical device such as a stent, or physiotherapy, acupuncture, psychosocial intervention, rehabilitation measures, training or diet. Vaccine studies also count as interventional studies in Germany and are performed as clinical studies according to the AMG.
Interventional clinical studies are subject to a variety of legal and ethical requirements, including the Medicines Act and the Law on Medical Devices. Studies with medical devices must be registered by the responsible authorities, who must also approve studies with drugs. Drug studies also require a favorable ruling from the responsible ethics committee. A study must be performed in accordance with the binding rules of Good Clinical Practice (GCP) ( 5 , e2 – e4 ). For clinical studies on persons capable of giving consent, it is absolutely essential that the patient should sign a declaration of consent (informed consent) ( e2 ). A control group is included in most clinical studies. This group receives another treatment regimen and/or placebo—a therapy without substantial efficacy. The selection of the control group must not only be ethically defensible, but also be suitable for answering the most important questions in the study ( e5 ).
Clinical studies should ideally include randomization, in which the patients are allocated by chance to the therapy arms. This procedure is performed with random numbers or computer algorithms ( 6 – 8 ). Randomization ensures that the patients will be allocated to the different groups in a balanced manner and that possible confounding factors—such as risk factors, comorbidities and genetic variabilities—will be distributed by chance between the groups (structural equivalence) ( 9 , 10 ). Randomization is intended to maximize homogeneity between the groups and prevent, for example, a specific therapy being reserved for patients with a particularly favorable prognosis (such as young patients in good physical condition) ( 11 ).
Blinding is another suitable method to avoid bias. A distinction is made between single and double blinding. With single blinding, the patient is unaware which treatment he is receiving, while, with double blinding, neither the patient nor the investigator knows which treatment is planned. Blinding the patient and investigator excludes possible subjective (even subconscious) influences on the evaluation of a specific therapy (e.g. drug administration versus placebo). Thus, double blinding ensures that the patient or therapy groups are both handled and observed in the same manner. The highest possible degree of blinding should always be selected. The study statistician should also remain blinded until the details of the evaluation have finally been specified.
A well designed clinical study must also include case number planning. This ensures that the assumed therapeutic effect can be recognized as such, with a previously specified statistical probability (statistical power) ( 4 , 6 , 12 ).
It is important for the performance of a clinical trial that it should be carefully planned and that the exact clinical details and methods should be specified in the study protocol ( 13 ). It is, however, also important that the implementation of the study according to the protocol, as well as data collection, must be monitored. For a first class study, data quality must be ensured by double data entry, programming plausibility tests, and evaluation by a biometrician. International recommendations for the reporting of randomized clinical studies can be found in the CONSORT statement (Consolidated Standards of Reporting Trials, www.consort-statement.org ) ( 14 ). Many journals make this an essential condition for publication.
For all the methodological reasons mentioned above and for ethical reasons, the randomized controlled and blinded clinical trial with case number planning is accepted as the gold standard for testing the efficacy and safety of therapies or drugs ( 4 , e1 , 15 ).
In contrast, noninterventional clinical studies (NIS) are patient-related observational studies, in which patients are given an individually specified therapy. The responsible physician specifies the therapy on the basis of the medical diagnosis and the patient’s wishes. NIS include noninterventional therapeutic studies, prognostic studies, observational drug studies, secondary data analyses, case series and single case analyses ( 13 , 16 ). Similarly to clinical studies, noninterventional therapy studies include comparison between therapies; however, the treatment is exclusively according to the physician’s discretion. The evaluation is often retrospective. Prognostic studies examine the influence of prognostic factors (such as tumor stage, functional state, or body mass index) on the further course of a disease. Diagnostic studies are another class of observational studies, in which either the quality of a diagnostic method is compared to an established method (ideally a gold standard), or an investigator is compared with one or several other investigators (inter-rater comparison) or with himself at different time points (intra-rater comparison) ( e1 ). If an event is very rare (such as a rare disease or an individual course of treatment), a single-case study, or a case series, are possibilities. A case series is a study on a larger patient group with a specific disease. For example, after the discovery of the AIDS virus, the Center for Disease Control (CDC) in the USA collected a case series of 1000 patients, in order to study frequent complications of this infection. The lack of a control group is a disadvantage of case series. For this reason, case series are primarily used for descriptive purposes ( 3 ).
Epidemiological studies
The main point of interest in epidemiological studies is to investigate the distribution and historical changes in the frequency of diseases and the causes for these. Analogously to clinical studies, a distinction is made between experimental and observational epidemiological studies ( 16 , 17 ).
Interventional studies are experimental in character and are further subdivided into field studies (sample from an area, such as a large region or a country) and group studies (sample from a specific group, such as a specific social or ethnic group). One example was the investigation of the iodine supplementation of cooking salt to prevent cretinism in a region with iodine deficiency. On the other hand, many interventions are unsuitable for randomized intervention studies, for ethical, social or political reasons, as the exposure may be harmful to the subjects ( 17 ).
Observational epidemiological studies can be further subdivided into cohort studies (follow-up studies), case control studies, cross-sectional studies (prevalence studies), and ecological studies (correlation studies or studies with aggregated data).
In contrast, studies with only descriptive evaluation are restricted to a simple depiction of the frequency (incidence and prevalence) and distribution of a disease within a population. The objective of the description may also be the regular recording of information (monitoring, surveillance). Registry data are also suited for the description of prevalence and incidence; for example, they are used for national health reports in Germany.
In the simplest case, cohort studies involve the observation of two healthy groups of subjects over time. One group is exposed to a specific substance (for example, workers in a chemical factory) and the other is not exposed. It is recorded prospectively (into the future) how often a specific disease (such as lung cancer) occurs in the two groups ( figure 2a ). The incidence for the occurrence of the disease can be determined for both groups. Moreover, the relative risk (quotient of the incidence rates) is a very important statistical parameter which can be calculated in cohort studies. For rare types of exposure, the general population can be used as controls ( e6 ). All evaluations naturally consider the age and gender distributions in the corresponding cohorts. The objective of cohort studies is to record detailed information on the exposure and on confounding factors, such as the duration of employment, the maximum and the cumulated exposure. One well known cohort study is the British Doctors Study, which prospectively examined the effect of smoking on mortality among British doctors over a period of decades ( e7 ). Cohort studies are well suited for detecting causal connections between exposure and the development of disease. On the other hand, cohort studies often demand a great deal of time, organization, and money. So-called historical cohort studies represent a special case. In this case, all data on exposure and effect (illness) are already available at the start of the study and are analyzed retrospectively. For example, studies of this sort are used to investigate occupational forms of cancer. They are usually cheaper ( 16 ).

Graphical depiction of a prospective cohort study (simplest case [2a]) and a retrospective case control study (2b)
In case control studies, cases are compared with controls. Cases are persons who fall ill from the disease in question. Controls are persons who are not ill, but are otherwise comparable to the cases. A retrospective analysis is performed to establish to what extent persons in the case and control groups were exposed ( figure 2b ). Possible exposure factors include smoking, nutrition and pollutant load. Care should be taken that the intensity and duration of the exposure is analyzed as carefully and in as detailed a manner as possible. If it is observed that ill people are more often exposed than healthy people, it may be concluded that there is a link between the illness and the risk factor. In case control studies, the most important statistical parameter is the odds ratio. Case control studies usually require less time and fewer resources than cohort studies ( 16 ). The disadvantage of case control studies is that the incidence rate (rate of new cases) cannot be calculated. There is also a great risk of bias from the selection of the study population ("selection bias") and from faulty recall ("recall bias") (see too the article "Avoiding Bias in Observational Studies"). Table 1 presents an overview of possible types of epidemiological study ( e8 ). Table 2 summarizes the advantages and disadvantages of observational studies ( 16 ).
1 = slight; 2 = moderate; 3 = high; N/A, not applicable.
*Individual cases may deviate from this pattern.
Selecting the correct study type is an important aspect of study design (see "Study Design in Medical Research" in volume 11/2009). However, the scientific questions can only be correctly answered if the study is planned and performed at a qualitatively high level ( e9 ). It is very important to consider or even eliminate possible interfering factors (or confounders), as otherwise the result cannot be adequately interpreted. Confounders are characteristics which influence the target parameters. Although this influence is not of primary interest, it can interfere with the connection between the target parameter and the factors that are of interest. The influence of confounders can be minimized or eliminated by standardizing the procedure, stratification ( 18 ), or adjustment ( 19 ).
The decision as to which study type is suitable to answer a specific primary research question must be based not only on scientific considerations, but also on issues related to resources (personnel and finances), hospital capacity, and practicability. Many epidemiological studies can only be implemented if there is access to registry data. The demands for planning, implementation, and statistical evaluation for observational studies should be just as high for observational studies as for experimental studies. There are particularly strict requirements, with legally based regulations (such as the Medicines Act and Good Clinical Practice), for the planning, implementation, and evaluation of clinical studies. A study protocol must be prepared for both interventional and noninterventional studies ( 6 , 13 ). The study protocol must contain information on the conditions, question to be answered (objective), the methods of measurement, the implementation, organization, study population, data management, case number planning, the biometric evaluation, and the clinical relevance of the question to be answered ( 13 ).
Important and justified ethical considerations may restrict studies with optimal scientific and statistical features. A randomized intervention study under strictly controlled conditions of the effect of exposure to harmful factors (such as smoking, radiation, or a fatty diet) is not possible and not permissible for ethical reasons. Observational studies are a possible alternative to interventional studies, even though observational studies are less reliable and less easy to control ( 17 ).
A medical study should always be published in a peer reviewed journal. Depending on the study type, there are recommendations and checklists for presenting the results. For example, these may include a description of the population, the procedure for missing values and confounders, and information on statistical parameters. Recommendations and guidelines are available for clinical studies ( 14 , 20 , e10 , e11 ), for diagnostic studies ( 21 , 22 , e12 ), and for epidemiological studies ( 23 , e13 ). Since 2004, the WHO has demanded that studies should be registered in a public registry, such as www.controlled-trials.com or www.clinicaltrials.gov . This demand is supported by the International Committee of Medical Journal Editors (ICMJE) ( 24 ), which specifies that the registration of the study before inclusion of the first subject is an essential condition for the publication of the study results ( e14 ).
When specifying the study type and study design for medical studies, it is essential to collaborate with an experienced biometrician. The quality and reliability of the study can be decisively improved if all important details are planned together ( 12 , 25 ).
Acknowledgments
Translated from the original German by Rodney A. Yeates, M.A., Ph.D.
Conflict of interest statement
The authors declare that there is no conflict of interest in the sense of the International Committee of Medical Journal Editors.
Full text links
Read article at publisher's site: https://doi.org/10.3238/arztebl.2009.0262
Citations & impact
Impact metrics, citations of article over time, alternative metrics.

Article citations
The role of ai in serious games and gamification for health: scoping review..
Tolks D , Schmidt JJ , Kuhn S
JMIR Serious Games , 12:e48258, 15 Jan 2024
Cited by: 0 articles | PMID: 38224472 | PMCID: PMC10825760
Hotspots and frontiers of the relationship between gastric cancer and depression: A bibliometric study.
Liu JY , Zheng JQ , Yin CL , Tang WP , Zhang JN
World J Gastroenterol , 29(46):6076-6088, 01 Dec 2023
Cited by: 0 articles | PMID: 38130743 | PMCID: PMC10731158
Factors associated with the effectiveness of immersive virtual therapy in alleviating depressive symptoms during sub-acute post-stroke rehabilitation: a gender comparison.
Juszko K , Kiper P , Wrzeciono A , Cieślik B , Gajda R , Szczepańska-Gieracha J
BMC Sports Sci Med Rehabil , 15(1):137, 20 Oct 2023
Cited by: 0 articles | PMID: 37864252 | PMCID: PMC10588095
The use of deep learning in medical imaging to improve spine care: A scoping review of current literature and clinical applications.
Constant C , Aubin CE , Kremers HM , Garcia DVV , Wyles CC , Rouzrokh P , Larson AN
N Am Spine Soc J , 15:100236, 19 Jun 2023
Cited by: 0 articles | PMID: 37599816 | PMCID: PMC10432249
The Cameroon Health Research and Evidence Database (CAMHRED): tools and methods for local evidence mapping.
Ongolo-Zogo C , El-Khechen H , Morfaw F , Djiadjeu P , Zani B , Darzi A , Nji PW , Nyambi A , Youta A , Zaman F , Youmbi CT , Siani IN , Mbuagbaw L
Health Res Policy Syst , 21(1):58, 19 Jun 2023
Cited by: 0 articles | PMID: 37337236 | PMCID: PMC10278273
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Study design in medical research: part 2 of a series on the evaluation of scientific publications.
Röhrig B , du Prel JB , Blettner M
Dtsch Arztebl Int , 106(11):184-189, 13 Mar 2009
Cited by: 18 articles | PMID: 19568374 | PMCID: PMC2695375
Review Free full text in Europe PMC
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K , Williams J , Qi YP , Gutman J , Yeung L , Mai C , Finkelstain J , Mehta S , Pons-Duran C , Menéndez C , Moraleda C , Rogers L , Daniels K , Green P
Cochrane Database Syst Rev , 2(2022), 01 Feb 2022
Cited by: 4 articles | PMID: 36321557 | PMCID: PMC8805585
Critical assessment of progress of medical sciences in Iran and Turkey: the way developing countries with limited resources should make effective contributions to the production of science.
Massarrat S , Kolahdoozan S
Arch Iran Med , 14(6):370-377, 01 Nov 2011
Cited by: 9 articles | PMID: 22039839
Suicidal Ideation
Harmer B , Lee S , Duong TVH , Saadabadi A
StatPearls Publishing, Treasure Island (FL) , 23 Dec 2020
Cited by: 0 articles | PMID: 33351435
Books & documents Free full text in Europe PMC
Scientific journals and their authors' financial interests: a pilot study.
Krimsky S , Rothenberg LS , Stott P , Kyle G
Psychother Psychosom , 67(4-5):194-201, 01 Jul 1998
Cited by: 29 articles | PMID: 9693346
Scientific basis of the OCRA method for risk assessment of biomechanical overload of upper limb, as preferred method in ISO standards on biomechanical risk factors.
Colombini D , Occhipinti E
Scand J Work Environ Health , 44(4):436-438, 01 Jul 2018
Cited by: 5 articles | PMID: 29961081
Public sector reforms and their impact on the level of corruption: A systematic review.
Mugellini G , Della Bella S , Colagrossi M , Isenring GL , Killias M
Campbell Syst Rev , 17(2):e1173, 24 May 2021
Cited by: 1 article | PMID: 37131927 | PMCID: PMC8356278
Systematic literature reviews and meta-analyses: part 6 of a series on evaluation of scientific publications.
Ressing M , Blettner M , Klug SJ
Dtsch Arztebl Int , 106(27):456-463, 03 Jul 2009
Cited by: 23 articles | PMID: 19652768 | PMCID: PMC2719096
Europe PMC is part of the ELIXIR infrastructure
We have a new app!
Take the Access library with you wherever you go—easy access to books, videos, images, podcasts, personalized features, and more.
Download the Access App here: iOS and Android . Learn more here!
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs

Chapter 4: Types of Studies in Clinical Research—Part II: Interventional Studies
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Introduction, different types of interventional studies.
- JUSTIFICATION FOR CLINICAL TRIALS
- RANDOMIZED CONTROLLED TRIALS
- TERMINOLOGY USED IN A RANDOMIZED CONTROLLED TRIAL
- HISTORY OF RANDOMIZED CONTROLLED TRIALS
- STEPS IN A RANDOMIZED CONTROLLED TRIAL
- ADVANTAGES OF RANDOMIZED CONTROLLED TRIALS
- SPECIAL ISSUES IN RANDOMIZED CONTROLLED TRIALS
- TYPES OF RANDOMIZED CONTROLLED TRIALS
- BEYOND CLINICAL TRIALS: APPROACH TO SECONDARY RESEARCH
- Full Chapter
- Supplementary Content
When you have completed this chapter, you will be able to understand:
What is an interventional study and its subtypes
What a randomized controlled trial is
The need for randomized controlled trials
How to plan and carry out a randomized controlled trial
The special characteristics of a randomized controlled trial
What the different types of randomized controlled trials are
The advantages and limitations of a randomized controlled trial as compared with other clinical research
Beyond clinical trials, the approach to secondary research such as systematic review and meta-analysis
In contrast to observational studies, in interventional studies the investigator tries to find a relation between an intervention and the outcome by exposing the participants to some kind of intervention, which can be a new drug, a surgical procedure, or a device, in order to evaluate it ( Box 4-1 ). These studies can be done in basic sciences, in the community, or in clinical settings. A randomized controlled trial (RCT) is a type of clinical trial in which participants are allocated at random to receive one of several clinical interventions. Although the term intervention usually refers to treatment, it can include any clinical maneuver offered to participants that may have an effect on their health status, such as preventive strategies, screening programs, diagnostic tests, interventional procedures, or educational and learning models. 1 The essence of an RCT is the process of randomization, through which each participant has an equal chance of being put either in the interventional or the control arm of the study. This prevents selection bias and increases the statistical power of the study.
Interventional studies try to find a relation between an intervention and the outcome by exposing participants to some kind of intervention in order to evaluate it. Although the term intervention usually refers to treatment, it can include any clinical maneuver offered to participants that may have an effect on their health status, such as preventive strategies, screening programs, diagnostic tests, interventional procedures, or educational and learning models. 1
Interventional studies can be done under various settings: in basic sciences, in the community, or in a clinical setup ( Table 4-1 ).
Sign in or create a free Access profile below to access even more exclusive content.
With an Access profile, you can save and manage favorites from your personal dashboard, complete case quizzes, review Q&A, and take these feature on the go with our Access app.
Pop-up div Successfully Displayed
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
Subscribe or renew today
Every print subscription comes with full digital access
Science News
How patient-led research could speed up medical innovation.
People with understudied chronic conditions are taking up science

Suffers of long COVID, ME/CFS and other chronic conditions are taking medical research into their own hands.
Share this:
By Betsy Ladyzhets
March 28, 2024 at 12:00 pm
Melissa Red Hoffman was “feeling really stuck” last summer. A 50-year-old surgeon in Asheville, N.C., Hoffman had been struggling with long COVID since getting infected with the coronavirus two and a half years earlier. “Deafening fatigue” was one of her worst symptoms, she says. “I feel tired behind my eyes from the moment I get up to the moment I go to sleep.” She managed to work part time, but much of her work had shifted to administrative tasks that she did from her couch.
“I was really at a point where I had tried so many different things myself, with so many different providers,” she says, “not really sure what the hell to do next.”
Then she found Remission Biome . It’s a research project started in early 2023 by Tamara Romanuk and Tess Falor, two people with myalgic encephalomyelitis/chronic fatigue syndrome, or ME/CFS, a chronic disease that shares symptoms with long COVID. Project participants have taken medical research into their own hands to determine whether and how changes to their gut bacteria can improve their health. After an initial test with three participants led to some symptom relief, Romanuk and Falor announced last July that they would recruit 50 people with ME/CFS, long COVID or both for a larger test of the project’s protocol.
Hoffman was one of 500 people who applied within 36 hours of the call for volunteers. By the fall, she and 49 other people, dubbed the “Renegade 50,” had joined the project.
Remission Biome’s protocol is a multistep process, which participants undertake in consultation with their physicians. Initial steps involve patients collecting samples of their guts, immune systems and other connected organ systems, either at home or at a health care provider’s office. After those samples are analyzed by a lab to get baseline data, participants take a regimen of over-the-counter supplements, such as probiotics to cultivate certain types of gut bacteria, and then a prescribed antibiotic. Next comes further testing to examine if and how the regimen altered the composition of the gut microbiome. Throughout the process, participants track their symptoms and learn about past research on the microbiome that informed the project, ensuring that they understand the rationale for every step.
Early in the testing process, Hoffman’s fatigue started to lift, she says. “That’s been exciting, just to feel a little bit of a change.”
Alleviating symptoms — which can include debilitating fatigue, trouble sleeping, intense allergic reactions and cognitive problems — motivates many members of the Renegade 50, who come from different countries, age groups and stages of illness. But participants also aim to collect and publish data that will give the broader scientific community more information about ME/CFS and long COVID, two complex, often fluctuating conditions.
Participant María Richardson, a 36-year-old former educator in Mexico City, has dealt with progressively worse ME/CFS symptoms since high school. She received her diagnosis in the United States in 2015, but when she moved back to her native Mexico, where knowledge of the condition is limited, trying to get care “was like starting from zero,” she says. Remission Biome helped her better understand her own symptoms and share scientific information with the ME/CFS community in Mexico, through the ME/CFS advocacy group Millions Missing Mexico .
Remission Biome is one effort in the growing movement of patient-led research, which seeks to investigate chronic conditions that have been under-researched by academic and clinical scientists yet impact many people’s lives.
“People who were ignored by the American health care system … often need to turn to each other in order to gather the data that gets the attention of the mainstream,” says health care researcher Susannah Fox, author of the new book Rebel Health: A Field Guide to the Patient-Led Revolution in Medical Care .
Compared with mainstream medical research that tends to focus on finding biological causes and disease cures, patient-led work is more often rooted in what’s immediately relevant to patients’ daily lives, like identifying symptom triggers or relievers. But the approach faces challenges — particularly a lack of funding and other research resources — as scientific institutions aren’t set up to support these projects.
Patient-researchers and their scientist collaborators say the patient-led approach has big potential to move chronic disease research forward, making it more informed, quicker and more poised to directly improve patients’ lives.
Projects like Remission Biome “are going to change how research into these chronic, multi-organ-system diseases is going to be done,” Hoffman says. The approach may someday become a standard part of more mainstream research.
Experimental design
Remission Biome is studying whether changes to the gut microbiome can improve the health of people with ME/CFS or long COVID. Participants follow a protocol that includes taking prebiotic and probiotic supplements and a course of antibiotics.
Initial safety checks and testing : Samples from several body systems are collected and analyzed to ensure it’s safe for a volunteer to participate and to collect baseline data.
Symptom tracking : Participants record their symptoms throughout the project.
Start supplements : Participants begin taking prebiotics and probiotics.
Antibiotic added : An antibiotic is added to the regimen for 14 days.
Post-antibiotic testing : Body systems are checked again to look for changes.
Result : Remission Biome members hope to experience some symptom relief, as well as publish study findings and inspire new research projects for professional scientists.
A history of patient activism and patient-led research
About 1.3 percent of adults in the United States have ME/CFS, according to the U.S. Centers for Disease Control and Prevention. Scientists first noticed the condition in the 1930s, but since then, it’s been hard to define and hasn’t attracted extensive research attention. Initial observations noted outbreaks characterized by fatigue, chronic pain and other symptoms now associated with ME/CFS, often occurring — but not always — after viral infections. Scientists started to link these mysterious outbreaks in the 1980s under the umbrella term chronic fatigue syndrome.
Progress on identifying the disease’s triggers has been slow, in part because of the wide variety of symptoms across many organ systems and in part due to relatively limited research funding. And some doctors have dismissed patients’ symptoms as all psychological — a factor that some experts connect to the disease’s higher burden on women.
Combined, these challenges have contributed to a lack of treatments for people with ME/CFS, despite the illness’s potentially devastating impact on patients. Long COVID — which 6.8 percent of U.S. adults currently have, according to data from the CDC and U.S. Census Bureau — raised the profile of ME/CFS during the pandemic because of the two conditions’ similarities ( SN: 3/4/24 ).
“Biomedical research has blind spots.” Susannah Fox
Remission Biome started thanks to a Twitter conversation in fall 2022. Falor and Romanuk realized they had both independently experienced what they call “remission events,” in which symptoms recede for a few hours or days after courses of antibiotics. These events led each of them to look into the possible connection between ME/CFS symptoms and the gut microbiome, an emerging area of study with many unanswered questions. The pair were also both working scientists before their symptoms became debilitating. Falor had worked as an aerospace engineer at NASA; Romanuk had been a biologist studying microbiomes.
The two scientists set out to replicate their remission events — and collect extensive data on how their microbiomes and bodily systems changed to better understand the underlying biology of these events. They started with a self-test in early 2023, which included taking a lengthy list of supplements chosen to either increase or decrease levels of specific bacteria with possible ME/CFS connections. In addition to Romanuk and Falor, Isabel Ramirez-Burnett, a 50-year-old engineer and health coach in Rhode Island who has lived with ME/CFS since childhood, participated in the experiment.
The testing “went even better than we could have expected,” Falor says, with two of the three participants experiencing remission events. So Remission Biome expanded to the Renegade 50 cohort and fundraised through a crowdfunding campaign, grants and sponsorships to support this larger project. The team also recruited the participants’ physicians, to help ensure safety, along with scientists to collaborate with the participants and other volunteer researchers working on the project. Scientists regularly attend research meetings hosted by Remission Biome, Falor says, which include presentations and discussions about new, relevant findings in other ME/CFS and long COVID research.
Theoharis Theoharides is one of those scientists. As director of the Center of Excellence for Neuroinflammation Research at Nova Southeastern University in Clearwater, Fla., he has decades of experience studying mast cell activation syndrome, a chronic condition characterized by intense allergic reactions that is often diagnosed alongside ME/CFS and long COVID. “They’re very bright, very dedicated,” Theoharides says of Falor and Romanuk. He has provided feedback on Remission Biome’s regimen of supplements and plans to help analyze microbiome and blood samples taken from the Renegade 50 participants to look at how immune system changes may connect to their gut bacteria.
Another collaborator is Tatyana Dobreva, cofounder and CEO of the San Francisco–based biotech start-up ImYoo , which operates remote clinical trials and other research. ImYoo is assisting Remission Biome with genetic analysis of patient blood samples. The Renegade 50 study is similar to other ImYoo projects studying conditions such as inflammatory bowel disease and sickle cell disease, in which participants tie symptom tracking to data from medical testing, Dobreva says.
Remission Biome adds to a long history of patients with complex and contested illnesses advocating for their communities, Fox says. “Every decade of the 20th century had an example of people who were either being ignored or who were being discriminated against” by scientists and doctors, and who “banded together to innovate or gather data,” she says. Examples include Black people with sickle cell disease in the 1970s and people with HIV/AIDS in the 1980s ( SN: 12/8/23 ). In some cases, this translated to patient-informed research, in which patients consult on scientific projects, informing everything from research questions to how results are disseminated.

In the 21st century, the internet aided patient-led projects, with patients actually doing research, as like-minded patients could more easily find each other, as happened with Romanuk and Falor, Fox says. In these projects, patients also closely follow scientific studies about their disease and may collaborate with academic experts to develop scientific frameworks, rather than self-experimenting individually.
ME/CFS patients have been particularly motivated to pursue their own research, says Emily Taylor, vice president of advocacy and engagement at the ME/CFS organization Solve M.E . One key motivator is “the failure of the medical establishment to provide any sort of support or treatment or quality of life improvements for this population,” she says. Previous ME/CFS research done without patient input, such as a now-debunked clinical trial examining exercise as a potential treatment, has led patients to push back with their own studies.
“There was a desperate need to validate the anecdotal stories of patients in a formalized way,” Taylor says.
In spring 2020, during the first months of the pandemic, patients whose symptoms persisted for weeks after the initial infection started documenting their complex symptoms in real time. The Patient-Led Research Collaborative , or PLRC, formed out of a long COVID support group, led by members who had scientific experience.
PLRC released its first report in May 2020 , documenting symptoms common among the group’s hundreds of members. “We saw a need to start collecting people’s experiences and really try to take things into our own hands,” says PLRC cofounder and long COVID patient Lisa McCorkell.
U.S. adults who have ME/CFS
U.S. adults who have long COVID
Patients are experts
Patient-led and patient-informed research can be a win-win for both patients and scientists, advocates say. For patients, this work is more likely to address questions that are meaningful to their daily lives, says Jaime Seltzer, director of science and medical outreach at the advocacy group #MEAction . In one pre-pandemic example, a patient group focused on polycystic kidney disease proposed potential treatments to scientists at the University of Cambridge, leading to clinical trials at a new patient-led research hub.
Patient leadership can also inspire people to participate in clinical trials, as the interest in joining Remission Biome demonstrates. And study designs informed by patient experience often prioritize accommodations for people with different levels of symptoms or access to care, meaning a more diverse group of patients may be able to participate. With a patient-led, “decentralized” approach to research, “we can reach more people in more diverse areas” who don’t live near medical facilities in big cities or aren’t able to travel for clinical trials, Dobreva says.
Connor, a member of the Renegade 50 who asked that only his first name be used to maintain medical privacy, “couldn’t participate in a traditional study,” says his wife, Nicole Bruno. Since a COVID-19 infection two and a half years ago, he has faced a severe case of both long COVID and ME/CFS, leaving him bedbound in a dark room.
“He could never go to a lab” or a doctor’s office to have samples collected, Bruno says. But with Remission Biome’s remote framework and individual support, he can be a patient-researcher. In addition to flexibility in locations, each member of the cohort is going through the testing protocol at their own pace, incorporating their microbiome test results, other diagnoses and input from their physicians. Flexibility also helps with logistical challenges; for example, test kits take longer to ship internationally.
For scientists, patient-led studies may move a field forward by highlighting key questions and hypotheses that might not emerge from traditional research. “Biomedical research has blind spots,” Fox says. McCorkell points to a paper from the PLRC , published in eClinicalMedicine in 2021, that expanded upon its 2020 survey work by describing 200 long COVID symptoms across 10 organ systems based on a detailed survey of about 3,800 people.
“It is still, to this day, one of the most cited papers in long COVID,” McCorkell says. Without this paper, she adds, other scientists might still be investigating “a small, limited set of symptoms” rather than the full scope of the condition. David Putrino, a long COVID clinician and director of rehabilitation innovation at Mount Sinai Health System in New York City, also points to the PLRC paper as an example of successful patient-led research that informed later studies.
Patient-led research “moves orders of magnitude faster than traditional modes of research,” Putrino says, because it focuses on the questions that are of greatest concern to patients, leading more quickly to impactful results. Patient-led groups may also be able to start new studies more quickly than institutions that have to, say, go through formal academic procedures, he says. In that way, this research is similar to how start-ups move faster than large corporations.
In addition, patients can help scientists design studies that are more likely to provide accurate results. For example, feedback from members of Remission Biome and other patient representatives helped David Esteban, a biologist at Vassar College in Poughkeepsie, N.Y., who was looking for people who had gotten COVID-19 but didn’t develop long COVID and could serve as control patients in a project funded by PLRC.
“Their perspective was, many people who recover from acute COVID go through a period where they feel better, but then get worse again,” he says. “I hadn’t really thought about that.” But that insight helped Esteban establish how long after a COVID-19 infection to wait before declaring a patient past the threshold for developing long COVID.
After studies are completed, patient teams may be more thoughtful about communicating results back to patient communities. In sharing a recent paper about managing ME/CFS that she coauthored with clinicians at the Mayo Clinic in Rochester, Minn., for instance, Seltzer anticipated questions that ME/CFS patients might have about the study. She explained up front that the paper was a concise review and could not include every relevant study, as patients would want to know “why I hadn’t mentioned their favorite paper,” she says. Such communication can “save a research group a lot of time and energy,” Seltzer says, and can encourage patients to bring the paper to their doctors so that the findings might inform their health care.
Groups like PLRC are working to build infrastructure to help scientists better engage with patients, including experienced patient-researchers and others who haven’t done scientific work before.
In January 2023, PLRC and the Council of Medical Specialty Societies introduced scorecards for academic teams interested in these collaborations. The scorecards can help teams evaluate success. “Our scorecards were developed with the intention of trying to change the baseline of what’s considered acceptable patient engagement,” McCorkell says, moving away from “tokenizing” engagement that she and other PLRC members have experienced. Taylor, at Solve M.E., would like to see the scorecards or a similar evaluation incorporated into traditional funding applications at scientific institutions.
ME/CFS funding
Of 73 diseases (dots) with research funding from the National Institutes of Health, ME/CFS is among those underfunded relative to its disease burden, the total number of healthy years lost due to premature death or disability from illness. The graph includes an estimate of ME/CFS burden with the arrival of COVID-19. The red line is expected funding levels based on burden.
NIH funding vs. disease burden, 2020

The challenges of patient-led research
Current institutional and financial support for patient-led research projects is limited. These projects typically are not eligible to apply for academic and government grants, leading them to seek money from nontraditional sources. Patient-researchers also don’t tend to have access to laboratory space, clinical tests and other research resources.
“We’re limited in the type of research that we can do,” McCorkell says. As a result, surveys and self-experimentation are the most common methods.
Internal capacity is another challenge: Chronically ill people tend to have limited energy to devote to projects; they must balance this work with managing their symptoms. Patients tend to be more ambitious than their available energy can support, Seltzer says. Sometimes a patient-researcher might have to take a break from a project to recover from a symptom flare-up. Projects like Remission Biome take these crashes into account when designing experiments and distributing tasks.
“If I disappear for a week,” it’s OK, says Katrin Boniface, a doctoral student studying the history of horses at the University of California, Riverside who had her own remission experience before joining the Renegade 50. But these constraints might frustrate academic or clinician collaborators who want patient-researchers to answer emails at all hours or pull together a last-minute grant proposal.
Nonpatient scientists might also be skeptical of results from patient-led research, as many in the scientific community haven’t yet recognized how lived experience can improve studies, Seltzer says. Although many patient-researchers have scientific backgrounds, they might not be experienced in biomedical research, leading to perceptions that they are underqualified and that their work is not rigorous or may even be biased.
Advocates like Seltzer argue that patient-researchers are more incentivized than anyone to make sure their results are accurate. “If we’re wrong, we and people like us suffer,” she says.
Taylor argues that data from patient-led research should be added to the types of evidence that regulatory agencies like the U.S. Food and Drug Administration consider for approving treatments. The FDA and the National Institutes of Health took one step in this direction earlier this year by soliciting data from long COVID patients and doctors about their experiences with treatments approved for other diseases.
“There was a desperate need to validate the anecdotal stories of patients in a formalized way.” Emily Taylor
But some scientists and doctors are concerned that patient-led projects might encourage some patients to self-experiment on their own without appropriate safety measures. This has been a big challenge for Remission Biome, especially after its members posted about remission events during the project’s first phase in early 2023. Initially, the plan was to openly share all aspects of the project, including protocols and results, says Ramirez-Burnett, one of the three early participants. “But then we realized that people were starting to pick pieces of the protocol in order to do it, which is not safe,” she says. “So we had to close that document.”
Now, when asked about the full protocol, as they often are on social media, Remission Biome participants typically encourage safety and emphasize that more testing is needed before it’s widely shared. In the future, Ramirez-Burnett hopes to educate more clinicians about the project so they can work with patients outside the Remission Biome infrastructure.
Patient-led projects may also struggle with logistics. This has been the case for Remission Biome. Its two founders split in December over disagreements about the project’s pace, its handling of safety aspects and how to incorporate the project as a formal business. As a result, Romanuk and the group parted ways.
The Renegade 50 test was put on hold until mid-March while Falor and other project members addressed this leadership change and set up as a nonprofit, she says. The team is also adding more safety steps and participant education on the antibiotic in the testing protocol because that antibiotic may have negative side effects for some people with ME/CFS. Falor expects the Renegade 50 phase will be completed later this year, after which the project will share preliminary results and begin setting up a cohort of 500 participants.
Tests and supplements for that next cohort will require more financial support, which Remission Biome will continue to raise from its GoFundMe campaign and grants. The project has also secured sponsorships from supplement and testing companies, such as the probiotics provider FitBiomics, to provide research supplies to participants. Financial support is especially important for participants living in places where it’s difficult to receive medical care for ME/CFS, says Richardson, the Renegade 50 member in Mexico. Many patients globally could benefit from this work, she says.
Remission Biome is also working toward scientific publications, based on data from the Renegade 50 cohort and from side projects. But the 50-person test might not lead to publishable results, says scientist-collaborator Theoharides. The microbiome is extremely complex, and, unlike a clinical trial, the Renegade 50 group does not include control patients not taking the treatments. But he hopes “the information that will come out of this study might actually give us some new directions.” One key advantage, he says, is that each participant is testing many supplements rather than focusing on one at a time; ME/CFS and long COVID are such complex diseases that it’s unlikely for a single treatment to work for all patients or have a lasting impact.
Esteban, the biologist at Vassar College, similarly hopes to examine how different antibiotics might work together to alleviate symptoms. “I’m already thinking about experiments that I could do,” he says, such as work in lab animals that would “start to explore some of the proposed mechanisms that might underlie the effects they’re seeing with the antibiotic treatments.”
While Remission Biome’s participants are excited to contribute to research, their most important goal is to provide “solutions for the ME/CFS community,” Ramirez-Burnett says. “So people don’t have to lose their jobs, lose their relationships, not get proper care.”
Among the three Renegade 50 participants who had completed the testing protocol as of January, one experienced a remission event, signifying a potential success, Falor says. Meanwhile, the project’s frequent meetings, Slack group, apps for shared symptom-tracking and other communication options could provide models for other patient-research efforts.
Remission Biome participants who have dealt with ME/CFS for a long time, like Richardson, feel particularly motivated to help find answers for the millions around the world newly struggling with long COVID. “People with mild long COVID sound like what I experienced 20 years ago,” Richardson says. She hopes that the lessons learned from Remission Biome and other projects like it can help prevent new long COVID patients from experiencing decades of symptoms.
More Stories from Science News on Health & Medicine

Bird flu has infected a person after spreading to cows. Here’s what to know

A new study has linked microplastics to heart attacks and strokes. Here’s what we know

Here’s what distorted faces can look like to people with prosopometamorphopsia

These are the chemicals that give teens pungent body odor

Brain fog is a debilitating symptom commonly reported by people with long COVID. Now, scientists have linked the symptom to leaky boundaries in the brain.
Long COVID brain fog may be due to damaged blood vessels in the brain

Don’t use unsterilized tap water to rinse your sinuses. It may carry brain-eating amoebas

The U.S. now has a drug for severe frostbite. How does it work?

Four years on, the COVID-19 pandemic has a long tail of grief
Subscribers, enter your e-mail address for full access to the Science News archives and digital editions.
Not a subscriber? Become one now .
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 2, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
One in five people with cancer participate in medical research studies
by Fred Hutchinson Cancer Center

Researchers from Fred Hutchinson Cancer Center, the American Cancer Society Cancer Action Network and peer institutions released new findings in the Journal of Clinical Oncology showing that when all types of cancer research studies are considered, at least one in five people with cancer, or 21.9%, participate in some form of clinical research.
The study evaluated all categories of cancer studies, such as treatment trials, biorepository studies and quality of life studies—the first time an estimate of participation in all types of cancer studies has been reported. Moreover, enrollment in cancer treatment trials was 7.1%, a notably higher participation rate than previous estimates of 2%–3%.
The study also found that enrollment in treatment trials was over five-fold higher at National Cancer Institute-designated cancer centers than at community sites (21.6% versus 4.1%), reflecting the impact that NCI funding for staff and infrastructure has on an institution's ability to offer trials and recruit patients.
Using deidentified accreditation data provided by the Commission on Cancer, the study updated decades-old estimates for participation in cancer research.
This expanded analysis includes more than 70% of people diagnosed with cancer in the U.S. each year who received care at a variety of clinical settings, from community hospitals and academic medical centers to NCI-designated comprehensive cancer centers. Additionally, the study reflects the broad spectrum of cancer research including different study types and those sponsored by industry, government and other sources.
"As we work to increase participation in cancer research studies and make them more accessible to patients, we need an inclusive, accurate assessment of current participation to inform these policies," said Joseph Unger, Ph.D., MS, a health services researcher and biostatistician at Fred Hutch and lead author of the study.
"While we knew that patients play a significant role in advancing all types of cancer research, now we better understand just how commonly people are participating in all types of cancer studies today."
While previous estimates of participation in cancer research studies were derived solely from government-sponsored trials, the study authors used patient data from a diverse range of trial sponsors and care settings for this analysis. Importantly, the data included settings such as community hospitals, where a majority of U.S. cancer patients receive care.
"We know that most patients with cancer will participate in a clinical trial if given the chance, and the level of enrollment we see at NCI-designated cancer centers shows what participation can be when patients are offered trials," stated Mark Fleury, Ph.D., a policy principle at ACS CAN and senior author of the study.
"These findings emphasize the need to offer more patients in community settings the chance to participate and that will require an investment in these sites that currently isn't there."
People with cancer enrolled in many different types of clinical studies. The study found the following participation rates in each type of clinical study: biorepository (12.9%), treatment (7.1%), registry (7.3%), genetic (3.6%), quality-of-life (2.8%), diagnostic (2.5%) and economic studies (2.4%).
Expanding the types of cancer clinical studies in this analysis demonstrates that there are a variety of ways people choose to participate in cancer research beyond the previous assessments, which were based only on participation in treatment studies.
"Cancer clinical research, in all of its forms, simply cannot be conducted without the contributions of people with cancer," emphasized Dr. Unger.
"These contributions are much more extensive than was previously recognized. Cancer clinical research is a true partnership between those with cancer and those who study and treat cancer."
Explore further
Feedback to editors

Oral vaccine for UTI is potential alternative to antibiotics, finds 9-year study
4 hours ago

Study: Epilepsy patients benefit from structured 'seizure action plans'
16 hours ago


Screening with a PSA test has a small impact on prostate cancer deaths but leads to overdiagnosis, finds study
20 hours ago

Clinical trial: First cardiac bioimplants for treatment of myocardial infarction using umbilical cord stem cells
Apr 5, 2024

Research team builds first tandem repeat expansions genetic reference maps
Human neuron model paves the way for new Alzheimer's therapies

A deep dive into the genetics of alcohol consumption

First atlas of the human ovary with cell-level resolution is a step toward artificial ovary
Pig hearts kept alive outside the body for more than 24 hours offers hope for many humans needing a transplant
New study suggests enhanced mitochondrial fusion fuels nerve cell function and plasticity
Related stories.
More than half of cancer patients willing to enroll in clinical trials
Oct 13, 2020

Study paves way for solutions to boost pediatric clinical trial enrollment
Feb 12, 2024

Better cancer trials could be around the corner
Mar 21, 2024

Study finds people of color more likely to participate in cancer clinical trials
Apr 28, 2021
Research shows structural barriers are the biggest reason for low participation in clinical trials
Feb 19, 2019

Expanded Medicaid coverage linked to higher participation in cancer clinical trials
Aug 17, 2023
Recommended for you

Radiation before mastectomy found to cut time delays for reconstructive surgery in breast cancer patients
The architectural role of p53: Early 3D chromatin remodeling to trigger cellular stress response

Molecular subtypes of advanced kidney cancer matter for treatment response

Diabetes drug shows promise against Parkinson's in clinical study
Apr 4, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- Clinical Trials
Exploring the Effectiveness of a Flipped Classroom Approach in Anatomy
- Print details
Tab Title Description
- Observational study — observes people and measures outcomes without affecting results.
- Interventional study (clinical trial) — studies new tests, treatments, drugs, surgical procedures or devices.
- Medical records research — uses historical information collected from medical records of large groups of people to study how diseases progress and which treatments and surgeries work best.
- Scottsdale/Phoenix, Arizona: 18-005942
About this study
The purpose of this study is to:
- Examine student performance on audience response questions, optional quizzes and exam questions on content taught with a flipped classroom versus lecture approach.
- Examine student perceptions of flipped classroom and lecture classroom instructional methods with a voluntary survey.
Participation eligibility
Participant eligibility includes age, gender, type and stage of disease, and previous treatments or health concerns. Guidelines differ from study to study, and identify who can or cannot participate. There is no guarantee that every individual who qualifies and wants to participate in a trial will be enrolled. Contact the study team to discuss study eligibility and potential participation.
Inclusion Criteria :
- Medical students enrolled in the 2018 anatomy course at Mayo Clinic School of Medicine, Arizona campus.
Exclusion Criteria:
Participating Mayo Clinic locations
Study statuses change often. Please contact the study team for the most up-to-date information regarding possible participation.
More information
- Publications
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Institutional Review Board
- Postdoctoral Fellowships
- Training Grant Programs
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- Science News
- Meetings and Events
- Social Media
- Press Resources
- Email Updates
- Innovation Speaker Series
Revolutionizing the Study of Mental Disorders
March 27, 2024 • Feature Story • 75th Anniversary
At a Glance:
- The Research Domain Criteria framework (RDoC) was created in 2010 by the National Institute of Mental Health.
- The framework encourages researchers to examine functional processes that are implemented by the brain on a continuum from normal to abnormal.
- This way of researching mental disorders can help overcome inherent limitations in using all-or-nothing diagnostic systems for research.
- Researchers worldwide have taken up the principles of RDoC.
- The framework continues to evolve and update as new information becomes available.
President George H. W. Bush proclaimed the 1990s “ The Decade of the Brain ,” urging the National Institutes of Health, the National Institute of Mental Health (NIMH), and others to raise awareness about the benefits of brain research.
“Over the years, our understanding of the brain—how it works, what goes wrong when it is injured or diseased—has increased dramatically. However, we still have much more to learn,” read the president’s proclamation. “The need for continued study of the brain is compelling: millions of Americans are affected each year by disorders of the brain…Today, these individuals and their families are justifiably hopeful, for a new era of discovery is dawning in brain research.”
Still, despite the explosion of new techniques and tools for studying the brain, such as functional magnetic resonance imaging (fMRI), many mental health researchers were growing frustrated that their field was not progressing as quickly as they had hoped.
For decades, researchers have studied mental disorders using diagnoses based on the Diagnostic and Statistical Manual of Mental Disorders (DSM)—a handbook that lists the symptoms of mental disorders and the criteria for diagnosing a person with a disorder. But, among many researchers, suspicion was growing that the system used to diagnose mental disorders may not be the best way to study them.
“There are many benefits to using the DSM in medical settings—it provides reliability and ease of diagnosis. It also provides a clear-cut diagnosis for patients, which can be necessary to request insurance-based coverage of healthcare or job- or school-based accommodations,” said Bruce Cuthbert, Ph.D., who headed the workgroup that developed NIMH’s Research Domain Criteria Initiative. “However, when used in research, this approach is not always ideal.”
Researchers would often test people with a specific diagnosed DSM disorder against those with a different disorder or with no disorder and see how the groups differed. However, different mental disorders can have similar symptoms, and people can be diagnosed with several different disorders simultaneously. In addition, a diagnosis using the DSM is all or none—patients either qualify for the disorder based on their number of symptoms, or they don’t. This black-and-white approach means there may be people who experience symptoms of a mental disorder but just miss the cutoff for diagnosis.
Dr. Cuthbert, who is now the senior member of the RDoC Unit which orchestrates RDoC work, stated that “Diagnostic systems are based on clinical signs and symptoms, but signs and symptoms can’t really tell us much about what is going on in the brain or the underlying causes of a disorder. With modern neuroscience, we were seeing that information on genetic, pathophysiological, and psychological causes of mental disorders did not line up well with the current diagnostic disorder categories, suggesting that there were central processes that relate to mental disorders that were not being reflected in DMS-based research.”
Road to evolution
Concerned about the limits of using the DSM for research, Dr. Cuthbert, a professor of clinical psychology at the University of Minnesota at the time, approached Dr. Thomas Insel (then NIMH director) during a conference in the autumn of 2008. Dr. Cuthbert recalled saying, “I think it’s really important that we start looking at dimensions of functions related to mental disorders such as fear, working memory, and reward systems because we know that these dimensions cut across various disorders. I think NIMH really needs to think about mental disorders in this new way.”
Dr. Cuthbert didn’t know it then, but he was suggesting something similar to ideas that NIMH was considering. Just months earlier, Dr. Insel had spearheaded the inclusion of a goal in NIMH’s 2008 Strategic Plan for Research to “develop, for research purposes, new ways of classifying mental disorders based on dimensions of observable behavior and neurobiological measures.”
Unaware of the new strategic goal, Dr. Cuthbert was surprised when Dr. Insel's senior advisor, Marlene Guzman, called a few weeks later to ask if he’d be interested in taking a sabbatical to help lead this new effort. Dr. Cuthbert soon transitioned into a full-time NIMH employee, joining the Institute at an exciting time to lead the development of what became known as the Research Domain Criteria (RDoC) Framework. The effort began in 2009 with the creation of an internal working group of interdisciplinary NIMH staff who identified core functional areas that could be used as examples of what research using this new conceptual framework looked like.
The workgroup members conceived a bold change in how investigators studied mental disorders.
“We wanted researchers to transition from looking at mental disorders as all or none diagnoses based on groups of symptoms. Instead, we wanted to encourage researchers to understand how basic core functions of the brain—like fear processing and reward processing—work at a biological and behavioral level and how these core functions contribute to mental disorders,” said Dr. Cuthbert.
This approach would incorporate biological and behavioral measures of mental disorders and examine processes that cut across and apply to all mental disorders. From Dr. Cuthbert’s standpoint, this could help remedy some of the frustrations mental health researchers were experiencing.
Around the same time the workgroup was sharing its plans and organizing the first steps, Sarah Morris, Ph.D., was a researcher focusing on schizophrenia at the University of Maryland School of Medicine in Baltimore. When she first read these papers, she wondered what this new approach would mean for her research, her grants, and her lab.
She also remembered feeling that this new approach reflected what she was seeing in her data.
“When I grouped my participants by those with and without schizophrenia, there was a lot of overlap, and there was a lot of variability across the board, and so it felt like RDoC provided the pathway forward to dissect that and sort it out,” said Dr. Morris.
Later that year, Dr. Morris joined NIMH and the RDoC workgroup, saying, “I was bumping up against a wall every day in my own work and in the data in front of me. And the idea that someone would give the field permission to try something new—that was super exciting.”
The five original RDoC domains of functioning were introduced to the broader scientific community in a series of articles published in 2010 .
To establish the new framework, the RDoC workgroup (including Drs. Cuthbert and Morris) began a series of workshops in 2011 to collect feedback from experts in various areas from the larger scientific community. Five workshops were held over the next two years, each with a different broad domain of functioning based upon prior basic behavioral neuroscience. The five domains were called:
- Negative valence (which included processes related to things like fear, threat, and loss)
- Positive valence (which included processes related to working for rewards and appreciating rewards)
- Cognitive processes
- Social processes
- Arousal and regulation processes (including arousal systems for the body and sleep).
At each workshop, experts defined several specific functions, termed constructs, that fell within the domain of interest. For instance, constructs in the cognitive processes domain included attention, memory, cognitive control, and others.
The result of these feedback sessions was a framework that described mental disorders as the interaction between different functional processes—processes that could occur on a continuum from normal to abnormal. Researchers could measure these functional processes in a variety of complementary ways—for example, by looking at genes associated with these processes, the brain circuits that implement these processes, tests or observations of behaviors that represent these functional processes, and what patients report about their concerns. Also included in the framework was an understanding that functional processes associated with mental disorders are impacted and altered by the environment and a person’s developmental stage.
Preserving momentum
Over time, the Framework continued evolving and adapting to the changing science. In 2018, a sixth functional area called sensorimotor processes was added to the Framework, and in 2019, a workshop was held to better incorporate developmental and environmental processes into the framework.;
Since its creation, the use of RDoC principles in mental health research has spread across the U.S. and the rest of the world. For example, the Psychiatric Ratings using Intermediate Stratified Markers project (PRISM) , which receives funding from the European Union’s Innovative Medicines Initiative, is seeking to link biological markers of social withdrawal with clinical diagnoses using RDoC-style principles. Similarly, the Roadmap for Mental Health Research in Europe (ROAMER) project by the European Commission sought to integrate mental health research across Europe using principles similar to those in the RDoC Framework.;
Dr. Morris, who has acceded to the Head of the RDoC Unit, commented: “The fact that investigators and science funders outside the United States are also pursuing similar approaches gives me confidence that we’ve been on the right pathway. I just think that this has got to be how nature works and that we are in better alignment with the basic fundamental processes that are of interest to understanding mental disorders.”
The RDoC framework will continue to adapt and change with emerging science to remain relevant as a resource for researchers now and in the future. For instance, NIMH continues to work toward the development and optimization of tools to assess RDoC constructs and supports data-driven efforts to measure function within and across domains.
“For the millions of people impacted by mental disorders, research means hope. The RDoC framework helps us study mental disorders in a different way and has already driven considerable change in the field over the past decade,” said Joshua A. Gordon, M.D., Ph.D., director of NIMH. “We hope this and other innovative approaches will continue to accelerate research progress, paving the way for prevention, recovery, and cure.”
Publications
Cuthbert, B. N., & Insel, T. R. (2013). Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Medicine , 11 , 126. https://doi.org/10.1186/1741-7015-11-126
Cuthbert B. N. (2014). Translating intermediate phenotypes to psychopathology: The NIMH Research Domain Criteria. Psychophysiology , 51 (12), 1205–1206. https://doi.org/10.1111/psyp.12342
Cuthbert, B., & Insel, T. (2010). The data of diagnosis: New approaches to psychiatric classification. Psychiatry , 73 (4), 311–314. https://doi.org/10.1521/psyc.2010.73.4.311
Cuthbert, B. N., & Kozak, M. J. (2013). Constructing constructs for psychopathology: The NIMH research domain criteria. Journal of Abnormal Psychology , 122 (3), 928–937. https://doi.org/10.1037/a0034028
Garvey, M. A., & Cuthbert, B. N. (2017). Developing a motor systems domain for the NIMH RDoC program. Schizophrenia Bulletin , 43 (5), 935–936. https://doi.org/10.1093/schbul/sbx095
Kozak, M. J., & Cuthbert, B. N. (2016). The NIMH Research Domain Criteria initiative: Background, issues, and pragmatics. Psychophysiology , 53 (3), 286–297. https://doi.org/10.1111/psyp.12518
Morris, S. E., & Cuthbert, B. N. (2012). Research Domain Criteria: Cognitive systems, neural circuits, and dimensions of behavior. Dialogues in Clinical Neuroscience , 14 (1), 29–37. https://doi.org/10.31887/DCNS.2012.14.1/smorris
Sanislow, C. A., Pine, D. S., Quinn, K. J., Kozak, M. J., Garvey, M. A., Heinssen, R. K., Wang, P. S., & Cuthbert, B. N. (2010). Developing constructs for psychopathology research: Research domain criteria. Journal of Abnormal Psychology , 119 (4), 631–639. https://doi.org/10.1037/a0020909
- Presidential Proclamation 6158 (The Decade of the Brain)
- Research Domain Criteria Initiative website
- Psychiatric Ratings using Intermediate Stratified Markers (PRISM)
- Open access
- Published: 05 April 2024
Unveilling the hidden skillset: exploring non-technical skills in surgical education across spanish medical universities
- Oves-Suarez B 1 ,
- García-Marín JA 2 ,
- Aguayo-Albasini JL 2 &
- Soria-Aledo V 3
BMC Medical Education volume 24 , Article number: 376 ( 2024 ) Cite this article
1 Altmetric
Metrics details
Non-Technical Skills (NTS) are cognitive, social, and personal resource skills that are crucial in complex and high-risk environments. The aims of our research are to determine the prevalence and content of NTS in the surgical rotation teaching guides of the Medicine Degree programs in Spanish Universities, to identify the most prevalent types and subtypes of NTS, and to analyze factors associated with the prevalence of surgical NTS in Medical Schools in Spain.
Descriptive observational cross-sectional study involving the identification and collection of competencies outlined in the surgical rotation teaching guides of Spanish Medical Schools. Information regarding university performance was obtained from the Foundation for Knowledge and Development Ranking webpage. The “Non-Technical Skills for Surgeons” (NOTSS) system was used to classify each competency in the teaching guides as NTS (categories and elements) and technical skills. Disagreements were resolved through group consensus.
A total of 1,846 competencies were analyzed in surgical rotations of the Medicine Degree programs across 40 Spanish Universities, with 99 competencies identified as surgical NTS, accounting for 5% of the total. The most frequently identified surgical NTS were “Decision Making” (46%), “Communication & Teamwork” (25%), and “Leadership” (19%). Additionally, several NOTSS were not identified in any institution. Public universities and those including a greater number of competencies had a higher rate of surgical NTS competencies, and we did not find a correlation between surgical NTS competencies and quality indices of University Centers.
Conclusions
There is a limited presence of surgical NTS in the educational plans of Spanish Universities.
Peer Review reports
Surgery stands as a pivotal and essential component of healthcare worldwide. Surgical safety is a global public health priority. In fact, 40–65% of events related to unsafe medical care occur within an operating theatre [ 1 , 5 ].
The current challenges in surgery (an overall change of the medical model from paternalistic to cooperative, work more focused on the team and less individual, etc.) differ from those of the past, rendering technical skills and manual dexterity alone inadequate to ensure comprehensive quality care [ 1 , 2 ]. Thus, the concept of Non-Technical Skills (NTS) has been introduced, encompassing cognitive, social, and personal resource skills crucial in complex and high-risk environments. In commercial aviation during the 1980s, once technological advancements mitigated common safety issues, it was realized that the “human factor” was the most frequent cause of aviation accidents. In 1981, United Airlines pilots became the first to receive training in key NTS under the “Crew Resource Management” (CRM) [ 3 ].
In 1997, James Reason, a psychologist from the University of Manchester, proposed the “Swiss cheese” model for risk analysis and management within organizations [ 4 ]. Presently, up to 60% of surgical adverse events result from NTS deficits [ 5 , 6 , 7 ]. NTS, categorized into “Situation Awareness,” “Communication & Teamwork,” “Decision Making,” and “Leadership,” are related to emotional intelligence and contribute to safe and efficient surgical performance [ 8 , 9 ]. A behavioral error in the operating theatre can lead to a serious adverse event. According to Gawande AA et al. [ 7 ] and Vioque SM et al. [ 10 ], 43% of surgical errors result from communication failures. NTS, both at the individual and team levels, are interrelated and constitute an essential complement to technical skills.
Publications such as “Crisis Management in Anesthesiology” by Gaba D et al. [ 11 ], and projects like “MedTeams” [ 12 ] and “TeamSTEPPS” [ 13 ] developed in the United States, have adapted CRM programs to the field of Medicine. However, it wasn’t until 2004 when Fletcher G et al. introduced an innovative system called “The Anaesthetists Non-Technical Skills” (ANTS), the first behavior marker system for NTS training and assessment, specifically in anaesthesia [ 14 ]. Two years later, Yule S et al. created another NTS taxonomy and system named “Non-Technical Skills for Surgeons” (NOTSS) for surgeons [ 15 ]. In 2010, Mitchell L et al. developed “Scrub Practitioners’ List of Intraoperative Non-Technical Skills” (SPLINTS) for instrument nurse practitioners [ 16 ]. Presently, NOTSS [ 15 ] is one of the most evidence-based and validated systems for individual assessment, and “Oxford Non-Technical Skills” (NOTECHS) for team assessment [ 17 ].
Nevertheless, little effort has been made to enhance formal training in surgical NTS during undergraduate studies, despite the clear importance of knowledge and implementation for effective surgical team performance. Investment in research and educational innovation by Scientific Societies and Universities is greatly needed in the field of surgery to train competent professionals adapted to new technologies in an ever-changing surgical landscape (robotic surgery, surgical artificial intelligence, etc.). Considering that university education forms the cornerstone of medical training, focusing on the initial step of the surgical education pyramid, medical students, is crucial. Mastery of surgical NTS will allow future physicians to flourish both professionally and personally, as these skills are applicable across all domains of human knowledge.
Currently, there is limited scientific literature concerning the learning of surgical NTS, and in Spain, there is no research evaluating the prevalence, content, or implementation of surgical NTS among medical students. Furthermore, no validated system exists for the periodic assessment of the level of surgical NTS training achieved by future doctors. Hence, the objectives of our research are to determine the prevalence and content of NTS in the teaching guides of surgical rotations within the Medicine Degree programs at Spanish universities, identify the most prevalent types and subtypes of NTS, and analyze the factors related to the prevalence of surgical NTS within our country’s Medical Schools.
Descriptive Cross-Sectional Observational Study through the identification and compilation of competencies outlined in the surgical rotation teaching guides of Medical Schools in Spain in the year 2022. The inclusion criteria were: Medical Schools located in Spanish territory with a curriculum that includes the subject “Surgical Rotation” and accessible teaching guides via web or email. Among the 49 Medical Schools in Spanish territory, teaching guides for surgery could be identified for 38 through their websites, while emails were sent to the remaining 11 secretariats, resulting in obtaining guides from only 2 institutions. The surgical rotation subject within the Medicine Degree program is included in all Medical Schools in Spain and provides students with the opportunity to apply theoretical concepts learned in the classroom in a real clinical setting. This helps them develop practical skills, gain experience in managing surgical patients, and explore various surgical specialties before making more informed decisions about their future medical careers.
Out of the 40 Medical Schools included in the study, 34 are public and 6 are private. Two categories of school size were considered, categorizing as small (< 200 incoming students) and large (≥ 200 incoming students). Information regarding university performance was obtained from the Foundation for Knowledge and Development Ranking (CYD) [ 18 ] website, collecting the following variables for all institutions: teaching and learning area (faculty qualifications, success rate, innovative teaching and assessment methods), research area (publications per faculty member, normalized impact of publications, highly cited publications), knowledge transfer area (private funds), international orientation area (foreign faculty, international publications, international research funds), regional development contribution area (regional publications, regional research funds), and employment rate area (Social Security affiliation rate after one year). High performance was understood as an indicator > 66th percentile, intermediate performance as 33rd ≤ indicator ≤ 66th percentile, and low performance as indicator < 33rd percentile.
The NOTSS [ 15 ] system was employed to classify each competency in the teaching guides as NTS (categories and elements) and technical skills (Table 1 ). Additionally, NOTSS elements were subdivided into three categories (1 item, 2 items, and ≥ 3 items NOTSS out of the total competencies, respectively). A database was designed in Excel (Microsoft) version 19.0 for data recording, where all collected data were archived, and identifying data of participating Medical Schools were protected and encrypted. Competencies were evaluated by a single evaluator who received training and guidance in NTS identification from experts in the NOTSS system at the Royal College of Surgeons of Edinburgh [ 15 ]. Disagreements were resolved through group consensus. An intraobserver reliability study was conducted in two periods of the study (first period in August 2022 and second period in October 2022). Intraobserver agreement analysis was assessed using Cohen’s weighted kappa test.
Descriptive analysis presented qualitative variables through frequency distribution of category percentages, while quantitative variables were assessed for normal distribution using the Kolmogorov-Smirnov test, and indicators of central tendency (mean or median) and dispersion (standard deviation or percentiles) were provided. In bivariate analysis, the Pearson’s Chi-Square test was used for qualitative variables, Spearman’s rank correlation for quantitative variables, and Student’s t-test or one-way ANOVA for mean comparison. Statistically significant differences were considered when p-value was less than 0.05. Statistical analysis was conducted using the Statistical Package for Social Sciences (SPSS) program (IBM) version 19.0.
This research was carried out in accordance with the publication standards for observational studies outlined in the STROBE Statement [ 19 ].
Among the 40 reviewed Medical Schools, a total of 1,846 competencies were analyzed within the surgical rotation subjects, identifying 99 surgical NTS, which accounts for 5% of the total competencies. Based on the total competencies required as stipulated in the curriculum of the subject (average score/university = 47.5), NTS exhibited an average of 2.3 per university.
The most frequent NOTSS categories were “Decision Making” (46%), “Communication & Teamwork” (25%), and “Leadership” (19%) (Fig. 1 : Distribution of Non-Technical Skills in Surgery in Spanish Universities). Furthermore, several NOTSS were not identified in any institution. Table 2 outlines the prevalence of each NOTSS element.
Distribution of non-technical skills in surgery in Spanish universities
Universities with a higher number of competencies in surgical subjects displayed more NTS in those subjects, showing statistical significance (rho = 0.668, p < 0.001) (Fig. 2 : Correlation between total competencies and NOTSS identified in surgical rotations of the Degree in Medicine from Spanish Universities). No significant relationships were found between surgical NTS competencies and other performance indices of the University Centers (Table 3 ).
Correlation between total competencies and NOTSS identified in surgical rotations of the degree in medicine from Spanish Universities
Medicine degrees offered by public Universities exhibited an average of 2.6 ± 3 surgical NTS competencies, whereas Medicine degrees offered by private Universities had an average of 0.8 ± 1, with the differences between these types of institutions being statistically significant ( p = 0.002).
Regarding the number of students, in terms of university size based on the number of available spots for enrolling in the Medicine Degree program at the institution, no significant differences were found ( p = 0.082). However, a slight trend was observed that Universities with ≥ 200 spots had a higher number of NOTSS in their curricula compared to universities with < 200 spots (3.2 ± 4 vs. 1.9 ± 2).
Regarding intraobserver agreement, the kappa index was 0.9 (95% CI 0.8-1.0).
The subject of “surgical rotation” within the Medicine Degree pertains to a practical training phase where medical students have the opportunity to rotate through different surgical specialties within a real clinical setting. The objective of this subject is to provide students with direct experience in the field of surgery, allowing them to acquire clinical skills and specific knowledge related to various surgical areas.
The outcomes of our study reveal the limited presence of surgical NTS within these subjects in Spanish Universities, figures that markedly differ from the total competencies that are evaluated to satisfactorily complete practical rotations in surgical specialties before graduation. Lee A et al. have affirmed this fact and underscored the necessity for NTS training during undergraduate studies within Medical Schools in Canada. Spanish Institutions exhibit a higher prevalence of NTS in their surgical rotations and differ in the order of the most prevalent NOTSS categories and elements identified compared to Canadian Institutions [ 20 ]. Since comprehensive figures on the prevalence of surgical NTS are not yet available, the trends in other countries remain unclear. What appears evident is that Medical students in both Spain and Canada are not receiving adequate training in surgical NTS, despite the evidence linking NTS to patient safety [ 21 , 22 , 23 ].
In our study, “Leadership” emerged as one of the least prevalent surgical NTS, with certain elements like “Coping with Pressure” not being identified in any institution. We believe that implementing specific leadership programmes for Medical students from the undergraduate level onwards could enhance the attitude of future professionals in critical situations.
The results of our study depict a greater prevalence of the NTS category “Communication & Teamwork” in surgical specialty rotations compared to other studies, despite communication failures being one of the foremost contributors to surgical errors today [ 7 , 10 , 24 ]. One possible explanation could be the challenge of imparting this skill to medical students.
Factors such as the type of university (public vs. private) have been associated with a higher number of NOTSS in the curricula of Spanish Medical Schools. This data has not been explored previously, and the rationale behind this observation is not straightforward to comprehend. While the observed differences in favour of public Institutions are statistically significant, it should be noted that the vast majority of Centers in Spain are public, and private Universities have a lesser tradition, which could explain these disparities; however, further insights from additional studies are warranted.
Surgical NTS, like any other skill, must be learned and can be honed through training [ 25 , 26 , 27 ]. Hence, an innovative teaching model is needed to enhance knowledge, interest, and bridge the “Learning Gap” of medical students concerning these matters. In this context, artificial intelligence or the metaverse could be novel and appealing educational tools for the youth due to their cutting-edge technology. Other strategies like case simulation, didactic courses, the GemaSim simulator, mentoring, or role-playing games have been extensively described in scientific literature as effective for acquiring NTS in risk-free environments [ 28 , 29 , 30 , 31 , 32 ].
One of the strengths of this study lies in the fact that the participating researchers possessed prior training in NTS and patient safety before designing the study. Additionally, the registration method assessed the technique and intraobserver variability in recording variables to ensure data reliability. Another strength of this research is that the team consisted of representation from medical students, senior surgeons, and teaching-research staff from the University, thus obtaining perspectives from all stakeholders involved in the medical training process.
Several methodological limitations in our study should be acknowledged. Firstly, the identification of competencies was carried out by a single observer; while we believe that observer training by experts and team resolution of doubts and conflicts have mitigated the potential impact of this limitation. Secondly, the NOTSS [ 15 ] system is not validated for application to medical students. However, we consider it a useful tool for the study’s objective. The third limitation stems from evaluating the competencies outlined in the teaching plan, but it doesn’t imply that teaching encompassing these behaviors and attitudes has not been imparted within theoretical or practical teachings of surgical subjects.
The most significant challenges in the future involve assessing the impact of implementing surgical NTS on patient safety-related outcomes and complications, and finally, securing the inclusion of surgical NTS training, refinement, and periodic evaluation as educational priorities by competent authorities and responsible bodies.
Our study examined 1,846 competencies in surgical rotation subjects within the Medicine Degree across 40 Spanish Universities, identifying 99 competencies falling within surgical NTS, which constitutes 5% of the total. The most frequently identified surgical NTS include “Decision Making,” “Communication & Teamwork,” and “Leadership.” Public Universities and those with a higher number of competencies exhibit a higher rate of surgical NTS competencies, and no correlation has been found between surgical NTS competencies and the quality indices of University Centers.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Non-Technical Skills
- Crew Resource Management
- Anaesthetists Non-Technical Skills
- Non-Technical Skills for Surgeons
- Scrub Practitioners’ List of Intraoperative Non-Technical Skills
- Oxford Non-Technical Skills
- Statistical Package for Social Sciences
McMullan M. Patients using the internet to obtain health information: how this affects the patient–health professional relationship. Patient Educ Couns. 2006;63:24–8. https://doi.org/10.1016/j.pec.2005.10.006 .
Article Google Scholar
Mayo WJ. Specialization in surgery. Arch Surg. 1925;10:264. https://doi.org/10.1001/archsurg.1925.01120100276011 .
Helmreich RL, Merritt AC, Wilhelm JA. The evolution of Crew Resource Management Training in Commercial Aviation. Int J Aviat Psychol. 1999;9(1):19–32. https://doi.org/10.1207/s15327108ijap0901_2 .
Reason JT, Brookfield. Vt., USA: Ashgate; 1997. 252. https://doi.org/10.4324/9781315543543 .
de Vries EN, Ramrattan MA, Smorenburg SM, Gouma DJ, Boermeester MA. The incidence and nature of in-hospital adverse events: a systematic review. Qual Saf Health Care. 2008;17:216–23. https://doi.org/10.1136/qshc.2007.023622 .
Gillespie BM, Harbeck E, Kang E, Steel C, Fairweather N, Panuwatwanich K, et al. Effects of a brief Team Training Program on Surgical teams’ nontechnical skills: an interrupted time-series study. J Patient Saf. 2021;17:e448–54. https://doi.org/10.1097/PTS.0000000000000361 .
Gawande AA, Zinner MJ, Studdert DM, Brennan TA. Analysis of errors reported by surgeons at three teaching hospitals. Surgery. 2003;133:614–21. https://doi.org/10.1067/msy.2003.169 .
Flin R, O ́Connor P, Crichton M. Safety at the Sharp end: a guide to non-technical skills. Burlington: Ashgate Publishing Company; 2008.
Google Scholar
Yule S, Flin R, Paterson-Brown S, Maran N, Rowley D. Development of a rating system for surgeons’ non-technical skills. Med Educ. 2006;40:1098–104. https://doi.org/10.1111/j.1365-2929.2006.02610 .
Vioque SM, Kim PK, McMaster J, Gallagher J, Allen SR, Holena DN, et al. Classifying errors in preventable and potentially preventable trauma deaths: a 9-year review using the Joint Commission’s standardized methodology. Am J Surg. 2014;208:187–94. https://doi.org/10.1016/j.amjsurg.2014.02.006 .
Gaba DM, Fish KJ, Howard SK. Crisis management in anesthesiology. New York: Churchill Livingstone; 1994. p. 294.
Risser DT, Rice MM, Salisbury ML, Simon R, Jay GD, Berns SD. The potential for Improved Teamwork to reduce medical errors in the Emergency Department. Ann Emerg Med. 1999;34:373–83. https://doi.org/10.1016/S0196-0644(99)70134-4 .
King HB, Battles J, Baker DP, Alonso A, Salas E, Webster J, et al. TeamSTEPPSTM: team strategies and tools to Enhance Performance and Patient Safety. In: Henriksen K, Battles JB, Keyes MA, Grady ML, editors. Advances in Patient Safety: new directions and alternative approaches. Performance and Tools. Volume 3. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008.
Flin R, Glavin R, Maran M, Patey R. Framework for Observing and Rating anaesthetics ́Non-Technical skills-anaesthetists ́Non-Technical skills (ANTS) System Handbook. 1st ed. Aberdeen: UniPrint, University of Aberdeen; 2012.
Yule S, Flin R, Paterson-Brown S, Maran N, Rowley D. The non-technical skills for surgeons (NOTSS) System Handbook. 1st ed. Aberdeen: UniPrint, University of Aberdeen; 2012.
Flin R, O ́Connor P, Crichton M. Scrub practitioners ́List of Intraoperativa non- technical skills (SPLINTS) System Handbook. 1st ed. Aberdeen: UniPrint, University of Aberdeen; 2014.
McMullan RD, Urwin R, Sunderland N, Westbrook J. Observational tools that quantify nontechnical skills in the operating room: a systematic review. J Surg Res. 2020;247:306–22.
Ranking CYD. https://www.rankingcyd.org; 2023 [accessed 26 March 2023].
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–8. https://doi.org/10.1136/bmj.39335.541782.AD .
Lee A, Finstad A, Gawad N, Boet S, Raiche I, Balaa F. Nontechnical skills (NTS) in the Undergraduate Surgical and Anesthesiology Curricula: are we adequately preparing medical students? J Surg Educ. 2021;78:502–11. https://doi.org/10.1016/j.jsurg.2020.08.001 .
Mazzocco K, Petitti DB, Fong KT, Bonacum D, Brookey J, Graham S, et al. Surgical team behaviors and patient outcomes. Am J Surg. 2009;197:678–85. https://doi.org/10.1016/j.amjsurg.2008.03.002 .
Armour Forse R, Bramble JD, McQuillan R. Team training can improve operating room performance. Surgery. 2011;150:771–8. https://doi.org/10.1016/j.surg.2011.07.076 .
Neily J, Mills PD, Young-Xu Y, Carney BT, West P, Berger DH, et al. Association between implementation of a medical team training program and surgical mortality. JAMA. 2010;304:1693–700. https://doi.org/10.1001/jama.2010.1506 .
Lingard L, Espin S, Whyte S, Regehr G, Baker GR, Reznick R, et al. Communication failures in the operating room: an observational classification of recurrent types and effects. Qual Saf Health Care. 2004;13:330–4. https://doi.org/10.1136/qshc.2003.008425 .
Chang Y, Lai C. Nontechnical skills for the surgery clerkship in the operating room based on adult learning principles in Taiwan. Kaohsiung J Med Sci. 2022;38:907–13. https://doi.org/10.1002/kjm2.12565 .
Savoldelli GL, Naik VN, Park J, Joo HS, Chow R, Hamstra SJ. Value of debriefing during simulated Crisis Management. Anesthesiology. 2006;106:279–85. https://doi.org/10.1097/00000542-200608000-00010 .
McCulloch P, Mishra A, Handa A, Dale T, Hirst G, Catchpole K. The effects of aviation-style non-technical skills training on technical performance and outcome in the operating theatre. Qual Saf Health Care. 2009;18:109–15. https://doi.org/10.1136/qshc.2009.032177 .
Phillips EC, Smith SE, Hamilton AL, Kerins J, Clarke B, Tallentire VR. Assessing medical students’ nontechnical skills using Immersive Simulation: what are the essential components? Simul Healthc J Soc Simul Healthc. 2021;16:98–104. https://doi.org/10.1097/SIH.0000000000000463 .
Lateef F. Simulation-based learning: just like the real thing. J Emerg Trauma Shock. 2010;3:348. https://doi.org/10.4103/0974-2700.70743 .
Kaiser D, Eberhart J, Butler C et al. Real Time Cockpit Resource Management (CRM) training, 2012. [consultado el 26 de marzo de 2023].
Nicolaides M, Cardillo L, Theodoulou I, Hanrahan J, Tsoulfas G, Athanasiou T, et al. Developing a novel framework for non-technical skills learning strategies for undergraduates: a systematic review. Ann Med Surg. 2018;36:29–40. https://doi.org/10.1016/j.amsu.2018.10.005 .
Yule S, Parker SH, Wilkinson J, McKinley A, MacDonald J, Neill A, et al. Coaching non-technical skills improves Surgical residents’ performance in a simulated operating room. J Surg Educ. 2015;72:1124–30. https://doi.org/10.1016/j.jsurg.2015.06.012 .
Download references
Acknowledgements
Not applicable.
No sources of funding have been used in these manuscript.
Author information
Authors and affiliations.
School of Medicine, University of Murcia, Murcia, Spain
Oves-Suarez B
Department of General and Digestive Surgery, University Hospital Morales Meseguer, Murcia, Spain
García-Marín JA & Aguayo-Albasini JL
Chief of Department of General and Digestive Surgery, University Hospital Morales Meseguer, Murcia, Spain
Soria-Aledo V
You can also search for this author in PubMed Google Scholar
Contributions
BOS designed the work, analyzed and interpreted the data and drafted the manuscript. JAGM and VSA designed the work, interpreted the data and revised it JAAA designed the work and revised the manuscript All authors have approved the manuscript.
Corresponding author
Correspondence to García-Marín JA .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
B, OS., JA, GM., JL, AA. et al. Unveilling the hidden skillset: exploring non-technical skills in surgical education across spanish medical universities. BMC Med Educ 24 , 376 (2024). https://doi.org/10.1186/s12909-024-05362-w
Download citation
Received : 26 August 2023
Accepted : 27 March 2024
Published : 05 April 2024
DOI : https://doi.org/10.1186/s12909-024-05362-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Medical education
- Patient safety
- Non-technical skills
- Human factors
BMC Medical Education
ISSN: 1472-6920
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Pediatr Investig
- v.3(4); 2019 Dec

Clinical research study designs: The essentials
Ambika g. chidambaram.
1 Children's Hospital of Philadelphia, Philadelphia Pennsylvania, USA
Maureen Josephson
In clinical research, our aim is to design a study which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods. The conclusions derived from a research study can either improve health care or result in inadvertent harm to patients. Hence, this requires a well‐designed clinical research study that rests on a strong foundation of a detailed methodology and governed by ethical clinical principles. The purpose of this review is to provide the readers an overview of the basic study designs and its applicability in clinical research.
Introduction
In clinical research, our aim is to design a study, which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods that can be translated to the “real world” setting. 1 Before choosing a study design, one must establish aims and objectives of the study, and choose an appropriate target population that is most representative of the population being studied. The conclusions derived from a research study can either improve health care or result in inadvertent harm to patients. Hence, this requires a well‐designed clinical research study that rests on a strong foundation of a detailed methodology and is governed by ethical principles. 2
From an epidemiological standpoint, there are two major types of clinical study designs, observational and experimental. 3 Observational studies are hypothesis‐generating studies, and they can be further divided into descriptive and analytic. Descriptive observational studies provide a description of the exposure and/or the outcome, and analytic observational studies provide a measurement of the association between the exposure and the outcome. Experimental studies, on the other hand, are hypothesis testing studies. It involves an intervention that tests the association between the exposure and outcome. Each study design is different, and so it would be important to choose a design that would most appropriately answer the question in mind and provide the most valuable information. We will be reviewing each study design in detail (Figure 1 ).

Overview of clinical research study designs
Observational study designs
Observational studies ask the following questions: what, who, where and when. There are many study designs that fall under the umbrella of descriptive study designs, and they include, case reports, case series, ecologic study, cross‐sectional study, cohort study and case‐control study (Figure 2 ).

Classification of observational study designs
Case reports and case series
Every now and then during clinical practice, we come across a case that is atypical or ‘out of the norm’ type of clinical presentation. This atypical presentation is usually described as case reports which provides a detailed and comprehensive description of the case. 4 It is one of the earliest forms of research and provides an opportunity for the investigator to describe the observations that make a case unique. There are no inferences obtained and therefore cannot be generalized to the population which is a limitation. Most often than not, a series of case reports make a case series which is an atypical presentation found in a group of patients. This in turn poses the question for a new disease entity and further queries the investigator to look into mechanistic investigative opportunities to further explore. However, in a case series, the cases are not compared to subjects without the manifestations and therefore it cannot determine which factors in the description are unique to the new disease entity.
Ecologic study
Ecological studies are observational studies that provide a description of population group characteristics. That is, it describes characteristics to all individuals within a group. For example, Prentice et al 5 measured incidence of breast cancer and per capita intake of dietary fat, and found a correlation that higher per capita intake of dietary fat was associated with an increased incidence of breast cancer. But the study does not conclude specifically which subjects with breast cancer had a higher dietary intake of fat. Thus, one of the limitations with ecologic study designs is that the characteristics are attributed to the whole group and so the individual characteristics are unknown.
Cross‐sectional study
Cross‐sectional studies are study designs used to evaluate an association between an exposure and outcome at the same time. It can be classified under either descriptive or analytic, and therefore depends on the question being answered by the investigator. Since, cross‐sectional studies are designed to collect information at the same point of time, this provides an opportunity to measure prevalence of the exposure or the outcome. For example, a cross‐sectional study design was adopted to estimate the global need for palliative care for children based on representative sample of countries from all regions of the world and all World Bank income groups. 6 The limitation of cross‐sectional study design is that temporal association cannot be established as the information is collected at the same point of time. If a study involves a questionnaire, then the investigator can ask questions to onset of symptoms or risk factors in relation to onset of disease. This would help in obtaining a temporal sequence between the exposure and outcome. 7
Case‐control study
Case‐control studies are study designs that compare two groups, such as the subjects with disease (cases) to the subjects without disease (controls), and to look for differences in risk factors. 8 This study is used to study risk factors or etiologies for a disease, especially if the disease is rare. Thus, case‐control studies can also be hypothesis testing studies and therefore can suggest a causal relationship but cannot prove. It is less expensive and less time‐consuming than cohort studies (described in section “Cohort study”). An example of a case‐control study was performed in Pakistan evaluating the risk factors for neonatal tetanus. They retrospectively reviewed a defined cohort for cases with and without neonatal tetanus. 9 They found a strong association of the application of ghee (clarified butter) as a risk factor for neonatal tetanus. Although this suggests a causal relationship, cause cannot be proven by this methodology (Figure 3 ).

Case‐control study design
One of the limitations of case‐control studies is that they cannot estimate prevalence of a disease accurately as a proportion of cases and controls are studied at a time. Case‐control studies are also prone to biases such as recall bias, as the subjects are providing information based on their memory. Hence, the subjects with disease are likely to remember the presence of risk factors compared to the subjects without disease.
One of the aspects that is often overlooked is the selection of cases and controls. It is important to select the cases and controls appropriately to obtain a meaningful and scientifically sound conclusion and this can be achieved by implementing matching. Matching is defined by Gordis et al as ‘the process of selecting the controls so that they are similar to the cases in certain characteristics such as age, race, sex, socioeconomic status and occupation’ 7 This would help identify risk factors or probable etiologies that are not due to differences between the cases and controls.
Cohort study
Cohort studies are study designs that compare two groups, such as the subjects with exposure/risk factor to the subjects without exposure/risk factor, for differences in incidence of outcome/disease. Most often, cohort study designs are used to study outcome(s) from a single exposure/risk factor. Thus, cohort studies can also be hypothesis testing studies and can infer and interpret a causal relationship between an exposure and a proposed outcome, but cannot establish it (Figure 4 ).

Cohort study design
Cohort studies can be classified as prospective and retrospective. 7 Prospective cohort studies follow subjects from presence of risk factors/exposure to development of disease/outcome. This could take up to years before development of disease/outcome, and therefore is time consuming and expensive. On the other hand, retrospective cohort studies identify a population with and without the risk factor/exposure based on past records and then assess if they had developed the disease/outcome at the time of study. Thus, the study design for prospective and retrospective cohort studies are similar as we are comparing populations with and without exposure/risk factor to development of outcome/disease.
Cohort studies are typically chosen as a study design when the suspected exposure is known and rare, and the incidence of disease/outcome in the exposure group is suspected to be high. The choice between prospective and retrospective cohort study design would depend on the accuracy and reliability of the past records regarding the exposure/risk factor.
Some of the biases observed with cohort studies include selection bias and information bias. Some individuals who have the exposure may refuse to participate in the study or would be lost to follow‐up, and in those instances, it becomes difficult to interpret the association between an exposure and outcome. Also, if the information is inaccurate when past records are used to evaluate for exposure status, then again, the association between the exposure and outcome becomes difficult to interpret.
Case‐control studies based within a defined cohort
Case‐control studies based within a defined cohort is a form of study design that combines some of the features of a cohort study design and a case‐control study design. When a defined cohort is embedded in a case‐control study design, all the baseline information collected before the onset of disease like interviews, surveys, blood or urine specimens, then the cohort is followed onset of disease. One of the advantages of following the above design is that it eliminates recall bias as the information regarding risk factors is collected before onset of disease. Case‐control studies based within a defined cohort can be further classified into two types: Nested case‐control study and Case‐cohort study.
Nested case‐control study
A nested case‐control study consists of defining a cohort with suspected risk factors and assigning a control within a cohort to the subject who develops the disease. 10 Over a period, cases and controls are identified and followed as per the investigator's protocol. Hence, the case and control are matched on calendar time and length of follow‐up. When this study design is implemented, it is possible for the control that was selected early in the study to develop the disease and become a case in the latter part of the study.
Case‐cohort Study
A case‐cohort study is similar to a nested case‐control study except that there is a defined sub‐cohort which forms the groups of individuals without the disease (control), and the cases are not matched on calendar time or length of follow‐up with the control. 11 With these modifications, it is possible to compare different disease groups with the same sub‐cohort group of controls and eliminates matching between the case and control. However, these differences will need to be accounted during analysis of results.
Experimental study design
The basic concept of experimental study design is to study the effect of an intervention. In this study design, the risk factor/exposure of interest/treatment is controlled by the investigator. Therefore, these are hypothesis testing studies and can provide the most convincing demonstration of evidence for causality. As a result, the design of the study requires meticulous planning and resources to provide an accurate result.
The experimental study design can be classified into 2 groups, that is, controlled (with comparison) and uncontrolled (without comparison). 1 In the group without controls, the outcome is directly attributed to the treatment received in one group. This fails to prove if the outcome was truly due to the intervention implemented or due to chance. This can be avoided if a controlled study design is chosen which includes a group that does not receive the intervention (control group) and a group that receives the intervention (intervention/experiment group), and therefore provide a more accurate and valid conclusion.
Experimental study designs can be divided into 3 broad categories: clinical trial, community trial, field trial. The specifics of each study design are explained below (Figure 5 ).

Experimental study designs
Clinical trial
Clinical trials are also known as therapeutic trials, which involve subjects with disease and are placed in different treatment groups. It is considered a gold standard approach for epidemiological research. One of the earliest clinical trial studies was performed by James Lind et al in 1747 on sailors with scurvy. 12 Lind divided twelve scorbutic sailors into six groups of two. Each group received the same diet, in addition to a quart of cider (group 1), twenty‐five drops of elixir of vitriol which is sulfuric acid (group 2), two spoonfuls of vinegar (group 3), half a pint of seawater (group 4), two oranges and one lemon (group 5), and a spicy paste plus a drink of barley water (group 6). The group who ate two oranges and one lemon had shown the most sudden and visible clinical effects and were taken back at the end of 6 days as being fit for duty. During Lind's time, this was not accepted but was shown to have similar results when repeated 47 years later in an entire fleet of ships. Based on the above results, in 1795 lemon juice was made a required part of the diet of sailors. Thus, clinical trials can be used to evaluate new therapies, such as new drug or new indication, new drug combination, new surgical procedure or device, new dosing schedule or mode of administration, or a new prevention therapy.
While designing a clinical trial, it is important to select the population that is best representative of the general population. Therefore, the results obtained from the study can be generalized to the population from which the sample population was selected. It is also as important to select appropriate endpoints while designing a trial. Endpoints need to be well‐defined, reproducible, clinically relevant and achievable. The types of endpoints include continuous, ordinal, rates and time‐to‐event, and it is typically classified as primary, secondary or tertiary. 2 An ideal endpoint is a purely clinical outcome, for example, cure/survival, and thus, the clinical trials will become very long and expensive trials. Therefore, surrogate endpoints are used that are biologically related to the ideal endpoint. Surrogate endpoints need to be reproducible, easily measured, related to the clinical outcome, affected by treatment and occurring earlier than clinical outcome. 2
Clinical trials are further divided into randomized clinical trial, non‐randomized clinical trial, cross‐over clinical trial and factorial clinical trial.
Randomized clinical trial
A randomized clinical trial is also known as parallel group randomized trials or randomized controlled trials. Randomized clinical trials involve randomizing subjects with similar characteristics to two groups (or multiple groups): the group that receives the intervention/experimental therapy and the other group that received the placebo (or standard of care). 13 This is typically performed by using a computer software, manually or by other methods. Hence, we can measure the outcomes and efficacy of the intervention/experimental therapy being studied without bias as subjects have been randomized to their respective groups with similar baseline characteristics. This type of study design is considered gold standard for epidemiological research. However, this study design is generally not applicable to rare and serious disease process as it would unethical to treat that group with a placebo. Please see section “Randomization” for detailed explanation regarding randomization and placebo.
Non‐randomized clinical trial
A non‐randomized clinical trial involves an approach to selecting controls without randomization. With this type of study design a pattern is usually adopted, such as, selection of subjects and controls on certain days of the week. Depending on the approach adopted, the selection of subjects becomes predictable and therefore, there is bias with regards to selection of subjects and controls that would question the validity of the results obtained.
Historically controlled studies can be considered as a subtype of non‐randomized clinical trial. In this study design subtype, the source of controls is usually adopted from the past, such as from medical records and published literature. 1 The advantages of this study design include being cost‐effective, time saving and easily accessible. However, since this design depends on already collected data from different sources, the information obtained may not be accurate, reliable, lack uniformity and/or completeness as well. Though historically controlled studies maybe easier to conduct, the disadvantages will need to be taken into account while designing a study.
Cross‐over clinical trial
In cross‐over clinical trial study design, there are two groups who undergoes the same intervention/experiment at different time periods of the study. That is, each group serves as a control while the other group is undergoing the intervention/experiment. 14 Depending on the intervention/experiment, a ‘washout’ period is recommended. This would help eliminate residuals effects of the intervention/experiment when the experiment group transitions to be the control group. Hence, the outcomes of the intervention/experiment will need to be reversible as this type of study design would not be possible if the subject is undergoing a surgical procedure.
Factorial trial
A factorial trial study design is adopted when the researcher wishes to test two different drugs with independent effects on the same population. Typically, the population is divided into 4 groups, the first with drug A, the second with drug B, the third with drug A and B, and the fourth with neither drug A nor drug B. The outcomes for drug A are compared to those on drug A, drug A and B and to those who were on drug B and neither drug A nor drug B. 15 The advantages of this study design that it saves time and helps to study two different drugs on the same study population at the same time. However, this study design would not be applicable if either of the drugs or interventions overlaps with each other on modes of action or effects, as the results obtained would not attribute to a particular drug or intervention.
Community trial
Community trials are also known as cluster‐randomized trials, involve groups of individuals with and without disease who are assigned to different intervention/experiment groups. Hence, groups of individuals from a certain area, such as a town or city, or a certain group such as school or college, will undergo the same intervention/experiment. 16 Hence, the results will be obtained at a larger scale; however, will not be able to account for inter‐individual and intra‐individual variability.
Field trial
Field trials are also known as preventive or prophylactic trials, and the subjects without the disease are placed in different preventive intervention groups. 16 One of the hypothetical examples for a field trial would be to randomly assign to groups of a healthy population and to provide an intervention to a group such as a vitamin and following through to measure certain outcomes. Hence, the subjects are monitored over a period of time for occurrence of a particular disease process.
Overview of methodologies used within a study design
Randomization.
Randomization is a well‐established methodology adopted in research to prevent bias due to subject selection, which may impact the result of the intervention/experiment being studied. It is one of the fundamental principles of an experimental study designs and ensures scientific validity. It provides a way to avoid predicting which subjects are assigned to a certain group and therefore, prevent bias on the final results due to subject selection. This also ensures comparability between groups as most baseline characteristics are similar prior to randomization and therefore helps to interpret the results regarding the intervention/experiment group without bias.
There are various ways to randomize and it can be as simple as a ‘flip of a coin’ to use computer software and statistical methods. To better describe randomization, there are three types of randomization: simple randomization, block randomization and stratified randomization.
Simple randomization
In simple randomization, the subjects are randomly allocated to experiment/intervention groups based on a constant probability. That is, if there are two groups A and B, the subject has a 0.5 probability of being allocated to either group. This can be performed in multiple ways, and one of which being as simple as a ‘flip of a coin’ to using random tables or numbers. 17 The advantage of using this methodology is that it eliminates selection bias. However, the disadvantage with this methodology is that an imbalance in the number allocated to each group as well as the prognostic factors between groups. Hence, it is more challenging in studies with a small sample size.
Block randomization
In block randomization, the subjects of similar characteristics are classified into blocks. The aim of block randomization is to balance the number of subjects allocated to each experiment/intervention group. For example, let's assume that there are four subjects in each block, and two of the four subjects in each block will be randomly allotted to each group. Therefore, there will be two subjects in one group and two subjects in the other group. 17 The disadvantage with this methodology is that there is still a component of predictability in the selection of subjects and the randomization of prognostic factors is not performed. However, it helps to control the balance between the experiment/intervention groups.
Stratified randomization
In stratified randomization, the subjects are defined based on certain strata, which are covariates. 18 For example, prognostic factors like age can be considered as a covariate, and then the specified population can be randomized within each age group related to an experiment/intervention group. The advantage with this methodology is that it enables comparability between experiment/intervention groups and thus makes result analysis more efficient. But, with this methodology the covariates will need to be measured and determined before the randomization process. The sample size will help determine the number of strata that would need to be chosen for a study.
Blinding is a methodology adopted in a study design to intentionally not provide information related to the allocation of the groups to the subject participants, investigators and/or data analysts. 19 The purpose of blinding is to decrease influence associated with the knowledge of being in a particular group on the study result. There are 3 forms of blinding: single‐blinded, double‐blinded and triple‐blinded. 1 In single‐blinded studies, otherwise called as open‐label studies, the subject participants are not revealed which group that they have been allocated to. However, the investigator and data analyst will be aware of the allocation of the groups. In double‐blinded studies, both the study participants and the investigator will be unaware of the group to which they were allocated to. Double‐blinded studies are typically used in clinical trials to test the safety and efficacy of the drugs. In triple‐blinded studies, the subject participants, investigators and data analysts will not be aware of the group allocation. Thus, triple‐blinded studies are more difficult and expensive to design but the results obtained will exclude confounding effects from knowledge of group allocation.
Blinding is especially important in studies where subjective response are considered as outcomes. This is because certain responses can be modified based on the knowledge of the experiment group that they are in. For example, a group allocated in the non‐intervention group may not feel better as they are not getting the treatment, or an investigator may pay more attention to the group receiving treatment, and thereby potentially affecting the final results. However, certain treatments cannot be blinded such as surgeries or if the treatment group requires an assessment of the effect of intervention such as quitting smoking.
Placebo is defined in the Merriam‐Webster dictionary as ‘an inert or innocuous substance used especially in controlled experiments testing the efficacy of another substance (such as drug)’. 20 A placebo is typically used in a clinical research study to evaluate the safety and efficacy of a drug/intervention. This is especially useful if the outcome measured is subjective. In clinical drug trials, a placebo is typically a drug that resembles the drug to be tested in certain characteristics such as color, size, shape and taste, but without the active substance. This helps to measure effects of just taking the drug, such as pain relief, compared to the drug with the active substance. If the effect is positive, for example, improvement in mood/pain, then it is called placebo effect. If the effect is negative, for example, worsening of mood/pain, then it is called nocebo effect. 21
The ethics of placebo‐controlled studies is complex and remains a debate in the medical research community. According to the Declaration of Helsinki on the use of placebo released in October 2013, “The benefits, risks, burdens and effectiveness of a new intervention must be tested against those of the best proven intervention(s), except in the following circumstances:
Where no proven intervention exists, the use of placebo, or no intervention, is acceptable; or
Where for compelling and scientifically sound methodological reasons the use of any intervention less effective than the best proven one, the use of placebo, or no intervention is necessary to determine the efficacy or safety of an intervention and the patients who receive any intervention less effective than the best proven one, placebo, or no intervention will not be subject to additional risks of serious or irreversible harm as a result of not receiving the best proven intervention.
Extreme care must be taken to avoid abuse of this option”. 22
Hence, while designing a research study, both the scientific validity and ethical aspects of the study will need to be thoroughly evaluated.
Bias has been defined as “any systematic error in the design, conduct or analysis of a study that results in a mistaken estimate of an exposure's effect on the risk of disease”. 23 There are multiple types of biases and so, in this review we will focus on the following types: selection bias, information bias and observer bias. Selection bias is when a systematic error is committed while selecting subjects for the study. Selection bias will affect the external validity of the study if the study subjects are not representative of the population being studied and therefore, the results of the study will not be generalizable. Selection bias will affect the internal validity of the study if the selection of study subjects in each group is influenced by certain factors, such as, based on the treatment of the group assigned. One of the ways to decrease selection bias is to select the study population that would representative of the population being studied, or to randomize (discussed in section “Randomization”).
Information bias is when a systematic error is committed while obtaining data from the study subjects. This can be in the form of recall bias when subject is required to remember certain events from the past. Typically, subjects with the disease tend to remember certain events compared to subjects without the disease. Observer bias is a systematic error when the study investigator is influenced by the certain characteristics of the group, that is, an investigator may pay closer attention to the group receiving the treatment versus the group not receiving the treatment. This may influence the results of the study. One of the ways to decrease observer bias is to use blinding (discussed in section “Blinding”).
Thus, while designing a study it is important to take measure to limit bias as much as possible so that the scientific validity of the study results is preserved to its maximum.
Overview of drug development in the United States of America
Now that we have reviewed the various clinical designs, clinical trials form a major part in development of a drug. In the United States, the Food and Drug Administration (FDA) plays an important role in getting a drug approved for clinical use. It includes a robust process that involves four different phases before a drug can be made available to the public. Phase I is conducted to determine a safe dose. The study subjects consist of normal volunteers and/or subjects with disease of interest, and the sample size is typically small and not more than 30 subjects. The primary endpoint consists of toxicity and adverse events. Phase II is conducted to evaluate of safety of dose selected in Phase I, to collect preliminary information on efficacy and to determine factors to plan a randomized controlled trial. The study subjects consist of subjects with disease of interest and the sample size is also small but more that Phase I (40–100 subjects). The primary endpoint is the measure of response. Phase III is conducted as a definitive trial to prove efficacy and establish safety of a drug. Phase III studies are randomized controlled trials and depending on the drug being studied, it can be placebo‐controlled, equivalence, superiority or non‐inferiority trials. The study subjects consist of subjects with disease of interest, and the sample size is typically large but no larger than 300 to 3000. Phase IV is performed after a drug is approved by the FDA and it is also called the post‐marketing clinical trial. This phase is conducted to evaluate new indications, to determine safety and efficacy in long‐term follow‐up and new dosing regimens. This phase helps to detect rare adverse events that would not be picked up during phase III studies and decrease in the delay in the release of the drug in the market. Hence, this phase depends heavily on voluntary reporting of side effects and/or adverse events by physicians, non‐physicians or drug companies. 2
We have discussed various clinical research study designs in this comprehensive review. Though there are various designs available, one must consider various ethical aspects of the study. Hence, each study will require thorough review of the protocol by the institutional review board before approval and implementation.
CONFLICT OF INTEREST
Chidambaram AG, Josephson M. Clinical research study designs: The essentials . Pediatr Invest . 2019; 3 :245‐252. 10.1002/ped4.12166 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Appointments at Mayo Clinic
- Consumer health
- Mindfulness exercises
See how mindfulness helps you live in the moment.
If you've heard of or read about mindfulness meditation — also known as mindfulness — you might be curious about how to practice it. Find out how to do mindfulness exercises and how they might benefit you.
What is mindfulness?
Mindfulness is a type of meditation in which you focus on being intensely aware of what you're sensing and feeling in the moment, without interpretation or judgment. Practicing mindfulness involves breathing methods, guided imagery, and other practices to relax the body and mind and help reduce stress.
Spending too much time planning, problem-solving, daydreaming, or thinking negative or random thoughts can be draining. It can also make you more likely to experience stress, anxiety and symptoms of depression. Practicing mindfulness exercises can help you direct your attention away from this kind of thinking and engage with the world around you.
What are the benefits of meditation?
Meditation has been studied in many clinical trials. The overall evidence supports the effectiveness of meditation for various conditions, including:
- High blood pressure (hypertension)
Preliminary research indicates that meditation can also help people with asthma and fibromyalgia.
Meditation can help you experience thoughts and emotions with greater balance and acceptance. Meditation also has been shown to:
- Improve attention
- Decrease job burnout
- Improve sleep
- Improve diabetes control
What are some examples of mindfulness exercises?
There are many simple ways to practice mindfulness. Some examples include:
- Pay attention. It's hard to slow down and notice things in a busy world. Try to take the time to experience your environment with all of your senses — touch, sound, sight, smell and taste. For example, when you eat a favorite food, take the time to smell, taste and truly enjoy it.
- Live in the moment. Try to intentionally bring an open, accepting and discerning attention to everything you do. Find joy in simple pleasures.
- Accept yourself. Treat yourself the way you would treat a good friend.
- Focus on your breathing. When you have negative thoughts, try to sit down, take a deep breath and close your eyes. Focus on your breath as it moves in and out of your body. Sitting and breathing for even just a minute can help.
You can also try more structured mindfulness exercises, such as:
- Body scan meditation. Lie on your back with your legs extended and arms at your sides, palms facing up. Focus your attention slowly and deliberately on each part of your body, in order, from toe to head or head to toe. Be aware of any sensations, emotions or thoughts associated with each part of your body.
- Sitting meditation. Sit comfortably with your back straight, feet flat on the floor and hands in your lap. Breathing through your nose, focus on your breath moving in and out of your body. If physical sensations or thoughts interrupt your meditation, note the experience and then return your focus to your breath.
- Walking meditation. Find a quiet place 10 to 20 feet in length, and begin to walk slowly. Focus on the experience of walking, being aware of the sensations of standing and the subtle movements that keep your balance. When you reach the end of your path, turn and continue walking, maintaining awareness of your sensations.
When and how often should I practice mindfulness exercises?
It depends on what kind of mindfulness exercise you plan to do.
Simple mindfulness exercises can be practiced anywhere and anytime. Research indicates that engaging your senses outdoors is especially beneficial.
For more structured mindfulness exercises, such as body scan meditation or sitting meditation, you'll need to set aside time when you can be in a quiet place without distractions or interruptions. You might choose to practice this type of exercise early in the morning before you begin your daily routine.
Aim to practice mindfulness every day for about six months. Over time, you might find that mindfulness becomes effortless. Think of it as a commitment to reconnecting with and nurturing yourself.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Bystritsky A. Complementary and alternative treatments for anxiety symptoms and disorders: Physical, cognitive, and spiritual interventions. https://uptodate.com/contents/search. Accessed June 14, 2018.
- Seaward BL. Meditation and mindfulness. In: Managing Stress: Principles and Strategies for Health and Well-being. 9th ed. Burlington, Mass.: Jones & Bartlett Learning; 2018.
- Shapiro SL, et al. The Art and Science of Mindfulness: Integrating Mindfulness into Psychology and the Helping Professions. 2nd ed. Washington, D.C.: American Psychological Association; 2017.
- Lymeus F, et al. Building mindfulness bottom-up: Meditation in natural settings supports open monitoring and attention restoration. Consciousness and Cognition. 2018;59:40.
- Blanck P, et al. Effects of mindfulness exercises as stand-alone interventions on symptoms of anxiety and depression: Systematic review and meta-analysis. Behaviour Research and Therapy. 2018;102:25.
- AskMayoExpert. Meditation. Rochester, Minn.: Mayo Foundation for Medical Education and Research; 2018.
- Khoury B, et al. Mindfulness-based stress reduction for healthy individuals: A meta-analysis. Journal of Psychosomatic Research. 2015;78:519.
- Practice mindfulness and relaxation. Springboard Beyond Cancer. https://survivorship.cancer.gov/springboard/stress-mood/practice-mindfulness. Accessed June 14, 2018.
Products and Services
- A Book: The Mayo Clinic Diet Bundle
- The Mayo Clinic Diet Online
- A Book: Mayo Clinic Guide to Pain Relief
- A very happy brain
- Alternative cancer treatments: 11 options to consider
- Candida cleanse diet
- Colloidal silver supplements
- Colon cleansing
- Detox foot pads
- Diabetes treatment: Can cinnamon lower blood sugar?
- Do infrared saunas have any health benefits?
- Prickly pear cactus
- Herbal supplements and heart drugs
- Kombucha tea
- Kratom: Unsafe and ineffective
- Kratom for opioid withdrawal
- Learn to reduce stress through mindful living
- Medical marijuana
- Meditation 2.0: A new way to meditate
- Natural remedies for depression: Are they effective?
- Tai Chi and Cardiac Rehab
- Valerian: A safe and effective herbal sleep aid?
- Alternative psoriasis treatments
- Do zinc supplements shorten colds?
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Healthy Lifestyle
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.

IMAGES
VIDEO
COMMENTS
Types of study design. Medical research is classified into primary and secondary research. Clinical/experimental studies are performed in primary research, whereas secondary research consolidates available studies as reviews, systematic reviews and meta-analyses. Three main areas in primary research are basic medical research, clinical research ...
The choice of study type will mainly depend on the research question being asked. There are various types of scientific studies such as experiments and comparative analyses, observational studies, surveys, or interviews. ... patients and doctors need reliable answers to a number of questions. Depending on the medical condition and patient's ...
We may approach this study by 2 longitudinal designs: Prospective: we follow the individuals in the future to know who will develop the disease. Retrospective: we look to the past to know who developed the disease (e.g. using medical records) This design is the strongest among the observational studies. For example - to find out the relative ...
Below are descriptions of some different kinds of clinical research. Treatment Research generally involves an intervention such as medication, psychotherapy, new devices, or new approaches to ...
Types of clinical research. There are many types of clinical research: Prevention studies look at ways to stop diseases from occurring or from recurring after successful treatment. Screening studies compare detection methods for common conditions. Diagnostic studies test methods for early identification of disease in those with symptoms.
Clinical research is the study of health and illness in people. There are two main types of clinical research: observational studies and clinical trials. Read and share this infographic (PDF, 317K) to learn why researchers do different kinds of clinical studies. Observational studies monitor people in normal settings.
Four main types: case series, case-control studies, cross-sectional studies, and cohort studies; In an experimental study, the investigators directly manipulate or assign participants to different interventions or environments. Experimental studies that involve humans are called clinical trials. They fall into two categories: those with ...
Primary research entails conducting studies and collecting raw data. Secondary research evaluates or synthesizes data collected during primary research. Primary medical research is categorized into three main fields: laboratorial, clinical, and epidemiological. Laboratory scientists analyze the fundamentals of diseases and treatments.
Cohort Design. Definition and Purpose. Often used in the medical sciences, but also found in the applied social sciences, a cohort study generally refers to a study conducted over a period of time involving members of a population which the subject or representative member comes from, and who are united by some commonality or similarity.
There are various types of clinical trials that are majorly grouped as analytical, observational, and experimental research. Clinical research can also be classified into non-directed data capture, directed data capture, and drug trials. Clinical research could be prospective or retrospective. It may also be a case-control study or a cohort study.
Clinical trials are research studies conducted to evaluate the safety and efficacy of medical interventions, including treatments, drugs, devices, and therapeutic strategies, in humans. These trials are essential for determining whether a new intervention is safe, effective, and suitable for widespread use in patient populations.
Type of studies in medical research can be broadly classified into primary and secondary studies. Primary studies are those that are actually performed by the investigators, while secondary studies summarize the results of different primary studies in the form of systematic reviews and meta-analyses without actually performing the studies. 1 Primary studies can be put into three groups based ...
Scientists may have many reasons for doing a clinical study, such as: • To explore the cause of a disease or a set of symptoms. • To test if a treatment will help with a symptom or condition. • To learn how a certain behavior affects people's health. Diferent types of clinical studies are used in diferent circumstances.
Search life-sciences literature (Over 39 million articles, preprints and more)
The structured classification of studies into two types, primary and secondary, as well as a further subclassification of studies of primary type are described, on the basis of a selective literature search concerning study types in medical research. BACKGROUND The choice of study type is an important aspect of the design of medical studies. The study design and consequent study type are major ...
Beyond clinical trials, the approach to secondary research such as systematic review and meta-analysis In contrast to observational studies, in interventional studies the investigator tries to find a relation between an intervention and the outcome by exposing the participants to some kind of intervention, which can be a new drug, a surgical ...
A history of patient activism and patient-led research. About 1.3 percent of adults in the United States have ME/CFS, according to the U.S. Centers for Disease Control and Prevention.
The study design used to answer a particular research question depends on the nature of the question and the availability of resources. In this article, which is the first part of a series on "study designs," we provide an overview of research study designs and their classification. The subsequent articles will focus on individual designs.
Expanding the types of cancer clinical studies in this analysis demonstrates that there are a variety of ways people choose to participate in cancer research beyond the previous assessments, which ...
Types include: Observational study — observes people and measures outcomes without affecting results. Interventional study (clinical trial) — studies new tests, treatments, drugs, surgical procedures or devices. Medical records research — uses historical information collected from medical records of large groups of people to study how ...
The Division of Intramural Research Programs (IRP) is the internal research division of the NIMH. Over 40 research groups conduct basic neuroscience research and clinical investigations of mental illnesses, brain function, and behavior at the NIH campus in Bethesda, Maryland. Learn more about research conducted at NIMH.
Background Non-Technical Skills (NTS) are cognitive, social, and personal resource skills that are crucial in complex and high-risk environments. The aims of our research are to determine the prevalence and content of NTS in the surgical rotation teaching guides of the Medicine Degree programs in Spanish Universities, to identify the most prevalent types and subtypes of NTS, and to analyze ...
Introduction. In clinical research, our aim is to design a study, which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods that can be translated to the "real world" setting. 1 Before choosing a study design, one must establish aims and objectives of the study, and choose an appropriate target population that is most representative of ...
The Sylvester Comprehensive Cancer Center (Sylvester) Biospecimen Shared Resource (BSSR) provides state-of-the-art, oncology-focused biorepository resources and services. The BSSR facilitates translational cancer research with specimen procurement and processing; quality control of specimens; secured specimen storage, retrieval, and distribution; and provision of relevant de-identified ...
Simple mindfulness exercises can be practiced anywhere and anytime. Research indicates that engaging your senses outdoors is especially beneficial. For more structured mindfulness exercises, such as body scan meditation or sitting meditation, you'll need to set aside time when you can be in a quiet place without distractions or interruptions.