
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Heart-Healthy Living
- High Blood Pressure
- Sickle Cell Disease
- Sleep Apnea
- Information & Resources on COVID-19
- The Heart Truth®
- Learn More Breathe Better®
- Blood Diseases and Disorders Education Program
- Publications and Resources
- Blood Disorders and Blood Safety
- Sleep Science and Sleep Disorders
- Lung Diseases
- Health Disparities and Inequities
- Heart and Vascular Diseases
- Precision Medicine Activities
- Obesity, Nutrition, and Physical Activity
- Population and Epidemiology Studies
- Women’s Health
- Research Topics
- Clinical Trials
- All Science A-Z
- Grants and Training Home
- Policies and Guidelines
- Funding Opportunities and Contacts
- Training and Career Development
- Email Alerts
- NHLBI in the Press
- Research Features
- Past Events
- Upcoming Events
- Mission and Strategic Vision
- Divisions, Offices and Centers
- Advisory Committees
- Budget and Legislative Information
- Jobs and Working at the NHLBI
- Contact and FAQs
- NIH Sleep Research Plan
- < Back To Total Artificial Heart
- How Does It Work?
- What Is Total Artificial Heart?
- Who Needs a Total Artificial Heart?
- What to Expect
- Living With

Total Artificial Heart How Does It Work?
Language switcher.
The total artificial heart replaces the lower chambers of the heart, called ventricles. Tubes called pneumatic drive lines run through holes in the stomach area to connect the total artificial heart pump to a machine outside the body called a driver. The total artificial heart then pumps blood through the major artery to the lungs and the rest of the body.
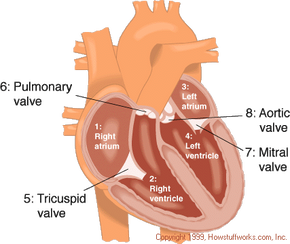
The total artificial heart has four mechanical valves that work like the heart’s own valves to manage blood flow. These valves connect the total artificial heart to the heart’s upper chambers, called the atria, and to the major arteries, which include the pulmonary artery and the aorta . Once the total artificial heart is connected, it acts like a healthy heart, allowing blood to flow normally through the body.
The driver powers and controls the total artificial heart. It is placed next to the bed when used in the hospital. When someone with a total artificial heart is outside the hospital, they use a portable driver that fits in a shoulder bag or backpack and weighs about 14 pounds. It can be recharged at home or in a car.

It may be helpful to understand how the heart works to understand how a total artificial heart works too.
Advertisement
How Artificial Hearts Work
- Share Content on Facebook
- Share Content on LinkedIn
- Share Content on Flipboard
- Share Content on Reddit
- Share Content via Email

Key Takeaways
- The AbioCor artificial could potentially the double life expectancies of patients with end-stage heart failure.
- Candidates for the AbioCor artificial heart must have end-stage heart failure, a life expectancy of less than 30 days, and no other viable treatment options.
- The first recipient of the AbioCor artificial heart was Robert Tools, who, despite battling an infection and needing a ventilator post-surgery, had his mechanical heart continue to function without problems.
Your heart is the engine inside your body that keeps everything running. Basically, the heart is a muscular pump that maintains oxygen and blood circulation through your lungs and body. In a day, your heart pumps about 2,000 gallons of blood. Like any engine, if the heart is not well taken care of it can break down and pump less efficiently, a condition called heart failure.
For a long time, the only option for many severe heart failure patients was a heart transplant. However, there are only slightly more than 2,000 heart transplants performed in the United States annually, meaning that tens of thousands of people die waiting for a donor heart. On July 2, 2001, heart failure patients were given new hope as surgeons at Jewish Hospital in Louisville, Kentucky, performed the first artificial heart transplant in nearly two decades. The AbioCor Implantable Replacement Heart was the first completely self-contained artificial heart and is expected to at least double the life expectancy of heart patients.
In this article, you will get an in-depth look at how this artificial heart works, how it's implanted into a patient's chest and who might be a candidate for receiving one of these mechanical hearts. We will also compare the AbioCor heart to the artificial hearts that have failed in the past.
A Hydraulic-driven Heart
The seven-hour surgery, the sickest of the sick.
The average adult human heart pumps blood at a rate of 60 to 100 beats per minute . If you've read How Your Heart Works , then you know that the heart contracts in two stages:
- In the first stage, the right and left atria contract at the same time, pumping blood to the right and left ventricles.
- In the second stage, the ventricles contract together to propel blood out of the heart.
The heart muscle then relaxes before the next heartbeat. This allows blood to fill up the heart again.
Patients with an implanted AbioCor heart still have atria that beat at the same time, but the artificial heart, which replaces both ventricles, can only force blood out one ventricle at a time . So, it alternately sends blood to the lungs and then to the body, instead of both at the same time as a natural heart does. The AbioCor can pump more than 10 liters per minute , which is enough for everyday activities.
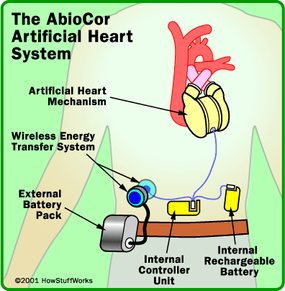
The AbioCor , developed by Abiomed, is a very sophisticated medical device, but the core mechanism of the device is the hydraulic pump that shuttles hydraulic fluid from side to side. To understand how it works, let's look at the various components of the system:
- Hydraulic pump - The basic idea with this device is similar to the hydraulic pumps used in heavy equipment . Force that is applied at one point is transmitted to another point using an incompressible fluid. A gear inside the pump spins at 10,000 revolutions per minute (rpm) to create pressure.
- Porting valve - This valve opens and closes to let the hydraulic fluid flow from one side of the artificial heart to the other. When the fluid moves to the right, blood gets pumped to the lungs through an artificial ventricle. When the fluid moves to the left, blood gets pumped to the rest of the body.
- Wireless energy-transfer system - Also called the Transcutaneous Energy Transfer (TET), this system consists of two coils, one internal and one external, that transmit power via magnetic force from an external battery across the skin without piercing the surface. The internal coil receives the power and sends it to the internal battery and controller device.
- Internal battery - A rechargeable battery is implanted inside the patient's abdomen. This gives a patient 30 to 40 minutes to perform certain activities, such as showering, while disconnected from the main battery pack.
- External battery - This battery is worn on a Velcro-belt pack around the patient's waist. Each rechargeable battery offers about four to five hours of power.
- Controller - This small electronic device is implanted in the patient's abdominal wall. It monitors and controls the pumping speed of the heart.
The AbioCor heart, which is composed of titanium and plastic, connects to four locations:
- Right atrium
- Left atrium
- Pulmonary artery
The entire system weighs about 2 pounds (0.9 kilograms). In the next section, you will learn how surgeons implanted the AbioCor heart during a seven-hour operation.
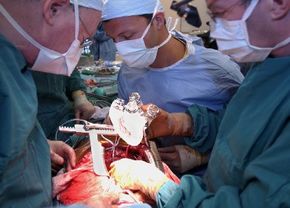
The surgery to implant a AbioCor artificial heart is extremely delicate. Not only are the surgeons cutting off and extracting the natural heart's right and left ventricles, but they are also placing a foreign object into the patient's chest. The patient must be placed on, and later removed from, a heart-lung machine . The surgery requires hundreds of stitches , to properly secure the heart to artificial ventricles. Grafts connect the AbioCor to remaining parts of the natural heart. Grafts are a kind of synthetic tissue used to connect the artificial device to the patient's natural tissue.
Due to the complexity of the surgery, there are lots of medical personnel on hand during the operation. The surgery on July 2, 2001, which was the first of its kind in the world, included a team of the two lead surgeons, 14 nurses, perfusionists, anesthesiologists and other support staff.
Here is the procedure, as described by University of Louisville surgeon Robert Dowling :
- Surgeons implant the energy-transfer coil in the abdomen.
- The breast bone is opened and the patient is placed on a heart-lung machine.
- Surgeons remove the right and left ventricles of the native heart. They leave in the right and left atria, the aorta and the pulmonary artery. This part of the surgery alone takes two to three hours.
- Atrial cuffs are sewn to the native heart's right and left atria.
- A plastic model is placed in the chest to determine the proper placement and fit of the heart in the patient.
- Grafts are cut to an appropriate length and sewn to the aorta and pulmonary artery.
- The AbioCor is placed in the chest. Surgeons use "quick connects" -- sort of like little snaps -- to connect the heart to the pulmonary artery, aorta and left and right atria.
- All of the air in the device is removed.
- The patient is taken off the heart-lung machine.
- The surgical team ensures that the heart is working properly.
Since this first transplant, more procedures have been done.
Originally, Abiomed officials cautioned against overly optimistic results; the most optimistic predictions were that a patient could live up to six months with the AbioCor heart. The device is designed to double life expectancy for patients who had only about 30 days to live before the operation.
Robert Tools , the patient who received the heart transplant on July 2, 2001, at Jewish Hospital in Louisville, has passed away. Ten other patients have also died, but the recipients of the AbioCor heart have lived an average of five months after their transplants.
In 1982, Dr. William Devries implanted the Jarvik-7 , the first device designed to be a completely permanent replacement heart. The surgery was done at the University of Utah, and the patient was Dr. Barney Clark , a dentist from Seattle. Clark lived for 112 days on the heart before succumbing to complications caused by the device.
The Jarvik-7 was an air-driven heart designed by Dr. Willem Kolff and Dr. Don Olsen. Unlike the self-contained AbioCor, the Jarvik-7 heart required several external wires, which protruded from the patient and connected to a large external unit. These protruding wires led to several infections in Clark.
Four more patients received the Jarvik-7 before it was discontinued due to complications, including stroke, mechanical failure and anatomical fit issues. It has since been enhanced and renamed, now called the CardioWest heart . It is used only in experimental situations and as an investigative bridge-to-transplantation device.
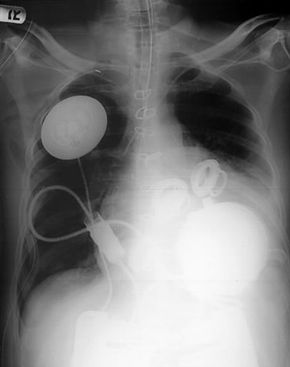
The U.S. Food and Drug Administration (FDA) has allowed Abiomed to perform 15 implants as part of a clinical trial. Twelve of the proposed 15 surgeries have been done. These surgeries have taken place at medical centers in Houston, Los Angeles, Boston and Philadelphia. The FDA has been reviewing the results of these transplants on a case-by-case basis to determine the future of the AbioCor device. If the $70,000 to $100,000 device is successful, meaning it can prolong the life of a patient without complications, it could be approved for use in more heart centers around the United States. In fact, several news agencies report that Abiomed will seek approval from the U.S. Food and Drug Administration this year to sell the AbioCor heart. The device will be used to treat patients with end-stage heart failure under what is known as a "Humanitarian Device Exemption."
Laman Gray said that initial candidates for the heart will be the "sickest of the sick." FDA and Abiomed officials have laid a few parameters for who can become one of the initial recipients of the artificial heart. The patient must fit the following profile:
- Have end-stage heart failure
- Have a life-expectancy of less than 30 days
- Is not a candidate for a natural heart transplant
- Have no other viable treatment option
Another requirement is that the grapefruit-sized device must fit inside the patient's chest. To determine the fit of the device, the patient must undergo a CAT scan and chest X-ray . Then, using a computer-aided design (CAD) program, the natural heart is virtually removed and the AbioCor heart is virtually placed in the patient's chest. If the computer program shows that the device will fit, doctors can proceed with the operation to implant the artificial heart.
For nearly two months following the surgery, hospital and AbioMed officials kept the name of the first recipient a secret. However, on August 21, 2001, it was revealed that Robert Tools , a Kentucky resident and former telephone company employee, was the history-making patient. Although Tools has battled an infection and needed a ventilator following his surgery, his doctors report that the mechanical heart continues to function without problems.

Here is what was known about Tools prior to his surgery:
- He suffered from class IV heart failure.
- He had severe bi-ventricular failure.
- He was turned down by a heart transplant center.
- He had undergone previous coronary bypass surgery.
- He had multiple heart attacks .
- He had diabetes .
Currently, there are between 2 million and 3 million Americans living with heart failure, and 400,000 new cases are diagnosed annually. Heart failure causes 39,000 deaths per year , according to the National Heart, Lung, and Blood Institute (NHLBI). Most people diagnosed with heart failure can expect to live about five years, and will usually need to have a heart transplant to extend their life.
In the United States alone, 2,143 heart transplants were performed in 2003. However, thousands of potential recipients die annually while waiting for a heart transplant. Doctors still encourage the public to become organ donors, but the AbioCor may save many of those who don't have the option of a natural transplant or of waiting for an available heart.
For more information on the AbioCor and related topics, check out the links on the next page.
Lots More Information
Related howstuffworks links.
- How Your Heart Works
- How Congestive Heart Failure Works
- How Heart Attacks Work
Other Great Links
- Jewish Hospital, Louisville, KY
- American Heart Association
- Yahoo! - Artificial Heart Full Coverage
Please copy/paste the following text to properly cite this HowStuffWorks.com article:
Find anything you save across the site in your account
How to Build an Artificial Heart
By Joshua Rothman

Daniel Timms started working on his artificial heart in 2001, when he was twenty-two years old. A graduate student in biomedical engineering, he was living with his parents in Brisbane, Australia. He was searching for a dissertation topic when his father, Gary, who was fifty, suffered a massive heart attack. At first, the problem seemed to be a faulty valve; soon they learned that Gary’s entire heart was failing. Heart failure is a progressive condition—a person can live for years while his heart slowly gives out. There was a narrow window of time. A course of study had presented itself.
Gary was a plumber, and Timms’s mother, Karen, was a high-school science assistant. Theirs was a tinkering, experimenting household; as a kid, Timms and his father had spent countless afternoons in the back yard building an elaborate system of fountains, ponds, and waterfalls. It was only natural that he and his dad would work together on a heart. They bought tubes, pipes, and valves at the hardware store and, in their garage, constructed a crude approximation of the circulatory system. Timms started reading about the history of the artificial heart. The first human implantation had been done in 1969, by a surgeon named Denton Cooley, of the Texas Heart Institute, in Houston. The patient, Haskell Karp, had been sustained for sixty-four hours—a great success, considering that his heart had been cut out of his chest. Engineers felt sure that, within a few years, they’d have the problem licked.
From there, however, the story became uncertain, even contentious. It was hard to design a small, implantable device that could beat thirty-five million times annually, pumping two thousand gallons of blood each day, for years on end. In the following decades, patients survived for days, months, even years on various kinds of artificial hearts, but their quality of life was often poor. They were connected by tubes to large machines; they frequently suffered from strokes and infections; their new hearts were too big or had parts that wore down. Every year, heart disease killed millions around the world. Only a few thousand transplantable hearts were available. And yet, Timms learned, existing artificial hearts could be used only temporarily, to “bridge” patients to transplants that might never come. There was no such thing as a permanent artificial heart.
Reviewing the designs, Timms saw that many had taken shape in the nineteen-sixties, seventies, and eighties. He thought that improving them substantially should be straightforward. In the past, most artificial hearts had been made of flexible plastic; he’d create one from durable titanium. Their pumps had often been driven pneumatically, by air pushed into the body through tubes; he’d use an electromagnetic motor. Most crucially, where traditional artificial hearts had been “pulsatile”—they squeezed blood rhythmically out of artificial ventricles—his would move blood in a continuous flow: instead of beating, it would whoosh . In a lab notebook, he sketched a possible heart. Blood would flow into a small chamber with a spinning metal disk at its center; the disk, like a propeller, would push blood outward into the lungs and the rest of the body. It was a clever, parsimonious design that, instead of seeking to emulate the biological heart, completely reimagined it. Beneath the sketch, he wrote, “Fuck yeah!”
In their garage, he and his dad built a prototype. Made of clear plastic, it successfully moved water through their mock circulatory system, in which tiny beads served as blood cells. But there was a problem—a spot beneath the rotating disk where the currents stalled and the beads got stuck. This eddy was dangerous; blood cells that hang around together tend to coagulate, creating clots that can cause strokes. Over Skype, Timms talked with a researcher in Japan who worked on the magnetic-levitation systems used in high-speed trains. They decided that stronger magnets could be used to suspend the disk away from the walls of the heart, so that blood could flow around it more easily. This “maglev” approach would also eliminate wear and tear: none of the parts would touch.
Timms was still a graduate student when he finagled a meeting with some cardiologists at the Brisbane hospital where his father was receiving treatment. He pulled the plastic pump out of his backpack and explained how a heart based on his design would function. One doctor, incredulous, walked out of the meeting. Another secured Timms a small stipend and a room in the basement. By 2004, while Gary was recovering from valve-replacement surgery upstairs, Timms was working on prototypes downstairs. Soon he used one to keep a sheep alive for a couple of hours. Like the artificial-heart engineers of the past, he anticipated that further progress would come quickly.
Today, more than a decade and a half later, Timms’s company, Bivacor, has an engineering office in Cerritos, a suburb of Los Angeles. About a dozen engineers work in a building surrounded by palm trees and flowering hedges. Last year, before the pandemic, Wilson Xie, a twenty-three-year-old biomechanical engineer, stood over a lab bench, using zip ties to attach the newest version of the Bivacor heart to a mock circulatory system. The system, known as “the loop,” was a vast improvement on the one Timms and his father had built; made of plastic tubes and about four feet tall, it resembled a model roller coaster. Filled with sugar water mixed to the viscosity of human blood, it used valves to simulate different circulatory circumstances: high pressure, low pressure, standing up, sprinting. The heart attached to it was solid and steampunk, made of black and gold titanium. Four openings were designed to lead to the aorta, the vena cava, the pulmonary artery, and the pulmonary vein; a cable connected it to a black dictionary-size control box. The cable would pass through the skin of the abdomen; users would need to carry the box with them at all times.
As Xie adjusted the valves of the loop, air was pumped out with a hiss. Nicholas Greatrex, an Australian electrical engineer, entered a command on a computer, and current began flowing to the heart’s electromagnets. Water began to course through the loop, moving with a low, vibrating hum.
The Bivacor and human hearts work on entirely different principles. A human heart has two distinct sides. Blood first loops from the smaller, right side to the lungs and back, so that its oxygen can be replenished; it then crosses over to the larger, stronger left side, which pumps it forcefully into the body. The Bivacor heart is one combined chamber. It sends blood in two directions using its spinning disk, or “rotor,” which has two differently contoured sides, each shaped to create the appropriate level of blood pressure. Where the heart of a healthy adult beats anywhere between sixty and a hundred times a minute, the Bivacor spins at between sixteen hundred and twenty-four hundred r.p.m.
Take the pulse of someone using such a heart, and you’d feel only a steady pressure, as in a garden hose. But some heart surgeons and cardiologists are uncomfortable with the idea of a pulseless heart. Tapping the computer keyboard, Greatrex instructed the rotor to oscillate its speed. “By accelerating and decelerating the rotor, we can create an artificial pulse,” he said. I reached out and touched one of the loop’s white rubber hoses. Uncannily, it was warm; beneath my fingers, it began to pulse with a familiar human rhythm.
“Blood pressure of a hundred over seventy,” Greatrex said triumphantly, touching his own wrist. “A doctor might look at that and say, ‘You’re doing pretty well!’ ” According to the Centers for Disease Control and Prevention, an estimated 6.2 million Americans suffer from some form of heart failure, often feeling weak, out of breath, and unsteady. Having this kind of artificial heart would turn back time.
Bivacor is in a transitional stage. It has never sold a product and is still run entirely on venture capital, angel investment, and government grants. Its hearts have been implanted in sheep and calves, which have survived for months, occasionally jogging on treadmills; it’s preparing to submit an application to the Food and Drug Administration for permission to perform human implantations. To cross the animal-human threshold is to enter a harsh regulatory environment. In the early days of artificial-heart research, a team could implant a device in a dying person on an emergency basis—as a last-ditch effort to save his life—and see how it functioned. Ethicists were uneasy, but progress was swift. Today, such experimentation is prohibited: a heart’s design must be locked in place and approved before a clinical trial can begin; the trial may take years, and, if it reveals that the heart isn’t good enough, the process must start again. Bivacor is currently deciding which features will be included in the clinical trial of its heart. A wrong decision would likely sink the company; almost certainly, there wouldn’t be a second attempt on the summit.
Timms, trim and sandy-haired, is now forty-two. Since his days in Brisbane, he has devoted almost all of his working life to the heart, moving to Japan, Germany, Taiwan, and Houston to work with particular surgeons or engineers. Quiet and alert, he is the opposite of a TED talker: he prefers not to tell people what he does for a living, lest the ensuing conversation tempt him to hype a project that has blown every deadline. Wearing jeans, running shoes, and a rumpled dress shirt unbuttoned to the third button, he led me into a back room where half a dozen prototype hearts had been operating continuously for as long as sixteen months. “It’s really important to show that they never, ever stop,” he said, above the hum of moving water. Timms himself looked as if he hadn’t slept properly in a couple of decades.
On our way out of the lab, we passed a conference room where an engineer was discussing, on video chat, how the Bivacor could be tested before implantation: “Your thumb goes on the left inlet port, and you’re going to very gently push,” she said. In Timms’s office, the furniture might have belonged in a home study. (It was a gift from an early investor—the owner of a Houston furniture store.) A pressed shirt haunted a hanger on the wall, and a road bike leaned in one corner.
From his creaky desk chair, Timms recalled driving his father to the hospital, in 2006. Gary’s valve-replacement surgery had helped him regain heart function, but only temporarily. “He’d got a clot on his mechanical valve,” Timms said. “It was backing the blood flow up into the left side of his heart and into his lungs.” Timms mimed the buildup with his hands, tracing a path from his left breast to his sternum and up his neck—the blood piling up, like water struggling to navigate a drain. “That’s when you get edema,” he said. “You cough up blood because it’s transferring across the pulmonary membrane.”

Link copied
Two weeks later, Timms was in Germany, meeting with pump engineers, when he learned that his father had taken a decisive turn for the worse. He flew home immediately, but arrived too late for a final conversation with his dad. “He was in I.C.U., with the trach and everything,” Timms said. “His dying made me even more resolute. It was, like, That’s it. We’re gonna do it, at any cost.”
I asked Timms if, two decades ago, he’d actually believed that he might invent an artificial heart in time to save his father.
He swivelled back and forth, nodding. “At that stage, I was, like, if there was a device that could be implanted for him, then maybe he could stay around for another five or ten years—for the time when I got married and had kids. He could experience that. That was the philosophy then. Just another five or ten years of life.” He laughed. “That still hasn’t happened,” he said, referring to marriage and children. He gestured around his office. “I’ve been stuck on this .”
Before the heart was replaceable, it was untouchable—a forbidding marker of the surgical frontier. Nineteenth-century physicians thought that, when it came to surgery , the heart was “the limit set by nature.” In the first half of the twentieth century, anesthesia made surgeons braver; they began darting in to mend arteries and valves while the heart was still beating. They tried cooling patients to hypothermic levels, then operating on their hearts quickly, while they were stopped. It wasn’t until the nineteen-fifties, with advances in the heart-lung machine, that open-heart surgery became routine. Blood travels out of the body and into the machine, bypassing the heart and lungs, and giving surgeons access to a still and bloodless heart, which they can treat almost like an ordinary muscle.
Early heart-lung machines were desk-size, and could be used safely only for short intervals; still, they made an artificial heart seem both desirable and feasible. So did several other converging trends. More people were living into their sixties and seventies, when hearts start to deteriorate; by mid-century, as many as forty per cent of American deaths were caused by heart disease. These statistics were of grave concern to policymakers. In 1948, Congress—a group of aging men—passed the National Heart Act, initiating a decades-long expansion in federal funding for cardiological research.
It was the Apollo era, and the artificial heart seemed like an inner moon shot to rival the outer one. In 1964, the National Institutes of Health launched the Artificial Heart Program, a multimillion-dollar engineering effort that aimed to put hearts into patients by the end of the decade. Structured like a NASA project, it awarded grants and contracts to teams of engineers who competed to develop the best valve, pump, or power source; a few groups experimented, unsuccessfully, with nuclear-powered hearts. Time and Life devoted covers to the work. As the medical historian Shelley McKellar writes, in “ Artificial Hearts: The Allure and Ambivalence of a Controversial Medical Technology ,” the high expectations for artificial-organ implants “did not necessarily reflect contemporary surgical reality.”
The true difficulty of the challenge quickly became apparent. At Maimonides hospital, in Brooklyn, Adrian Kantrowitz, a surgeon-inventor who had helped perfect the pacemaker and the heart-lung machine, began working on pump designs. He landed on a clever approach: instead of replacing the heart, he would install a pump just outside it, compensating for the failing heart and perhaps giving it time to heal. Kantrowitz’s prototype pumps were tested on dogs, and by 1966 he was ready to implant them in people. But the first human patient to receive one died after bleeding extensively; the second—a sixty-three-year-old bedridden diabetic woman who’d had two heart attacks—survived for twelve days but died after a series of strokes.
When Kantrowitz retrieved his pump and opened it up, he found clots. He had encountered an obstacle that would become known as “hemocompatibility.” Subjected to too much force or pressure, blood cells can tear apart; caught in eddies or crevices, they can stick together; on textured surfaces, they can catch and form tangled beds that narrow passages. Kantrowitz’s devices mangled the blood they pumped, and, as the “blood damage” mounted, the consequences spread.
Meanwhile, at Baylor College of Medicine, in Houston, Michael DeBakey and Denton Cooley, widely regarded as the world’s best heart surgeons, navigated a different set of complexities. DeBakey and Cooley began as partners, performing vast numbers of heart surgeries at a prodigious pace. Then, as the journalist Mimi Swartz explains in “ Ticker: The Quest to Create an Artificial Heart ”—a sweeping account of the effort, from DeBakey to Bivacor—the two men fell out. Cooley left DeBakey’s practice in 1960, and later founded the Texas Heart Institute, just down the road. DeBakey, meanwhile, hired Domingo Liotta, a pioneering Argentine heart surgeon, to work on artificial hearts. By 1969, Liotta had begun implanting prototypes in calves. The results were discouraging—of seven animals, four died on the operating table—and DeBakey thought they weren’t yet ready for use in humans. But Cooley was eager to move the work forward; he had patients waiting for donor hearts not just at his hospital but in nearby motels. Without informing DeBakey, he hired Liotta to moonlight at the Texas Heart Institute, with an eye to using his heart there.
Cooley began looking among his patients for a candidate. Haskell Karp, a forty-seven-year-old printing estimator from Skokie, Illinois, had been hospitalized thirteen times for heart trouble; he was routinely so out of breath that he struggled to brush his hair. Cooley would see whether Karp’s heart could be surgically repaired, but Karp and his wife agreed that, if it couldn’t be, Cooley could implant Liotta’s prototype, in the hope that a donor heart would later become available. “Mr. Karp was wheeled into the surgical ward,” Cooley later wrote, in a memoir. “He was pale, sweaty, and breathing with difficulty. His blood pressure had fallen to half its normal level.” Halfway through the operation, it became obvious that his heart was unfixable.
In its place, Cooley installed an air-powered device connected by hoses, which ran through Karp’s side, to a refrigerator-size console. The heart’s ventricles were made of rubbery plastic, with a bendable polyester lining; when air was driven between the lining and the plastic, the ventricles contracted and the heart pumped. The device kept Karp alive for sixty-four hours, until the transplanted heart of Barbara Ewan, a forty-year-old mother of three, could replace it. Still, Karp died thirty-two hours later, of pneumonia and kidney failure—consequences of the advanced heart disease that had made him a candidate for the risky procedure in the first place. Cooley regarded the operation as a success. But DeBakey, incensed by what he saw as the theft of his artificial heart, doubted whether his former partner had acted ethically. There were a series of investigations, and Cooley was censured by the American College of Surgeons. Observers disagreed about whether the surgery was heroic or reckless, but, either way, a new difficulty had emerged: by the time people were willing to try an artificial heart, they were so sick that they were almost beyond saving.
Willem Kolff, the Dutch-born internist who had invented dialysis, in the nineteen-forties, was undeterred. He aimed to create not just a bridge to transplantation but a heart so good that it could be used permanently. In Kolff’s lab, at the University of Utah, a physician-engineer named Clifford Kwan-Gett created a ventricle gentle enough to avoid blood damage. Robert Jarvik, a gifted biomedical engineer who joined the Utah team while he was in medical school, relentlessly refined the design and the manufacturing process, giving the heart space-saving and more hemocompatible lines. When Jarvik arrived, in 1971, the group’s prototype could sustain a calf for just ten days. But progress was steady; within a decade, a calf named Alfred Lord Tennyson lived for two hundred and sixty-eight days on what was by then called the Jarvik-5 artificial heart.
In December, 1982, the heart surgeon William DeVries implanted an upgraded version of the heart—the Jarvik-7—in Barney Clark, a sixty-one-year-old dentist. Clark’s heart had been functioning at about a sixth of its normal capacity; he felt so bad that, upon meeting some calves and sheep living with Jarvik hearts, he said, “I believe they feel a lot better than I feel at this time.” The surgery drew international attention, often centered on the personalities of the participants: DeVries, accomplished and “Lincolnesque”; Jarvik, young and handsome; and Clark, a charismatic Everyman who had flown combat missions in the Second World War. Television networks broadcast video of the seven-and-a-half-hour surgery; afterward, reporters attended daily press briefings held in the university’s cafeteria.
Clark lived for a hundred and twelve days, with tubes connecting him to a four-hundred-pound pump and control console. He was, by turns, in decline and recovering, miserable and optimistic; briefly, he stood, and even used an exercise bike, but more often he was in bed and short of breath, drawing air through a respirator mask. One of his mechanical valves had to be replaced in a follow-up surgery; he suffered from nosebleeds, seizures, kidney failure, and pneumonia. Speaking above the chugging sound of the heart’s pneumatic pump, not long before he died of sepsis and organ failure, Clark said, “It has been a pleasure to be able to help people.”
The F.D.A. had given DeVries permission to implant seven artificial hearts, and he moved forward. In 1984, DeVries installed a slightly improved Jarvik-7 in William Schroeder, a fifty-two-year-old former Army munitions inspector. Before the surgery, Schroeder asked for the last rites; he ended up living for six hundred and twenty days, moving out of the hospital into an apartment, and occasionally using a new, portable pumping unit, with three hours of battery life, to go untethered in the hallways or on drives with his son. On the phone with Ronald Reagan, Schroeder complained in jest about the tardiness of his Social Security check; reporters, feeling his chest, marvelled at his heartbeat, which seemed more powerful than a normal man’s. Still, Schroeder suffered from a variety of ailments, including strokes, one of them massive. After he succumbed to chronic infections and lung problems, he was buried with a gravestone featuring a drawing of two overlaid hearts—a human one and the Jarvik-7.
The hearts were getting better, as were the surgical techniques. But incremental improvements left the essential contours of the technology unchanged. DeVries did a few more implantations, with varying degrees of success; in Sweden, a man who had been given a Jarvik-7 did extraordinarily well, going for extended walks and eating at his favorite restaurants. Still, he died after seven and a half months, sparking a legal debate about whether he’d been alive in the first place. (According to Swedish law at the time, he’d died when his heart stopped.) The attitudes of doctors, patients, and reporters began to cool. Confidence in the idea of permanent heart replacement started fading, and funders wondered if the money wouldn’t be better spent elsewhere. What was the point of a short-term artificial heart? Were surgeons trying to save their patients, or just experimenting on them? Were the days of life gained worth having?
The first artificial-heart engineers had achieved a narrow kind of success. Their devices could sustain patients for long periods, but not permanently; they made heart failure survivable, but with a quality of life that seemed too low. A qualified wonder; a mixed blessing. “They did it,” Timms said, in his office, as we discussed this history. “But nobody wanted it.” A sobering conclusion for someone trying to do it again.
A couple of decades ago, at the beginning of my senior year of college, I found myself living next to two charming women: Suz from Montana and Jess from New Jersey. We became friends, and I soon learned Jess’s story. During her senior year of high school, she had a massive heart attack. After receiving the last rites, she was saved through the implantation of an experimental heart pump—a “ventricular assist device,” or VAD , called the HeartMate. The device, loosely descended from the ones that Adrian Kantrowitz had pioneered in the nineteen-sixties, was not an artificial heart; it replaced the function of the left side only, leaving the right side in place. Jess had attended prom and performed a lead role in her high school’s production of “How to Succeed in Business Without Really Trying” while connected through a wire to a battery in a handbag. She also learned to walk using a prosthesis, because a complication after the heart attack had required the amputation of her left leg above the knee. A few days before her high-school graduation, she received the transplanted heart of a teen-age girl who’d died after a car crash. Not long afterward, she developed non-Hodgkin’s lymphoma, likely resulting from the immunosuppressive drugs she took to prevent rejection. When I met Jess, all this was behind her; she was battery-free, cancer-free, getting a college degree.
We stayed friends after college. Jess worked in health care, as an organ-donation advocate. Her trick was to be both sweet and steely. She travelled the world, beat cancer twice, went to concerts, ate a lot of dessert, had boyfriends, earned promotions. Generally, she acted like a young person, not like a living miracle. It was only by observing the ease with which she navigated any medical setting—bonding with nurses, sending e-mails from her hospital bed—that I grasped the degree to which she lived provisionally, and with courage.
Curious to meet some of the people behind the HeartMate, I travelled to the Texas Heart Institute, in Houston. Situated near St. Luke’s hospital, within the city-like Texas Medical Center—the world’s largest medical complex, with ten million patient visits a year—T.H.I. is the Vatican of cardiology; it contains a substantial museum dedicated to the history of heart surgery and pump devices. Not far from where Denton Cooley had implanted the first artificial heart, more than five decades ago, I sat in a windowless conference room with two surgeons, O. H. (Bud) Frazier and Billy Cohn. Cohn, intense in a black button-down and jeans, was fifty-nine; Frazier, laconic in a sports coat, trousers, and tortoiseshell glasses, was seventy-nine. Both men wore cowboy boots. Between them, they had implanted more than a thousand “mechanical circulatory support” devices. Today, the vast majority of patients receive VAD s, which usually assist or replace the left side of the heart—an LVAD . But Cohn and Frazier, like Timms, are among the small group of researchers who are still working to develop a complete, permanent heart replacement. In 2011, they implanted two HeartMate IIs simultaneously—one for the left side, one for the right—into a fifty-five-year-old man whose heart had failed completely and been removed; the VAD s amounted to an artificial heart that kept him alive for five weeks.
Frazier’s career began, as Swartz recounts, during the golden age of the artificial heart and extended into its wilderness period. In 1963, he arrived at Baylor College of Medicine and studied under Michael DeBakey; he joined Cooley at T.H.I. in the seventies, and was still there in the eighties, when the discovery of cyclosporine, an immunosuppressive drug, made heart transplantation dependably survivable. Convinced of the value of heart pumps as bridge devices, he began working in a basement lab stocked with pigs, sheep, cows, and goats; over decades, he partnered with engineers to test and refine almost every heart pump currently in existence, including the original HeartMate. (Earlier, in Cerritos, I’d watched a video of a Bivacor-powered calf strolling on a treadmill in Frazier’s lab; Frazier and Cohn are advisers to the company.)
“Here’s this guy, O. H. Frazier,” Cohn said, pointing to a photograph on his laptop of Frazier in bloodstained scrubs, taken long ago. “Total rock star.” Frazier chuckled.
Cohn, radiating messianic energy, described how, in 1986, Frazier had been the first surgeon to use the HeartMate successfully, as part of a clinical trial that lasted until 1993. After it was approved by the F.D.A., in 1994, around four thousand people received one worldwide. It was doughnut-shaped, with a mechanical “pusher-plate” pump, and one of its core innovations was the use of specially textured plastic and titanium on which blood cells could grow a smooth, biological surface. Early versions were powered by air, delivered through a hose; later models, like the one Jess received, were motor-driven. The device had a life span of a year and a half at most, but that was enough for patients who came into the hospital blue-lipped and close to death. “You put ’em on the breathing machine, you made a big cut, you put this pump in their abdomen, you hook it up, and, at the end of the operation, the lips turn pink.” The challenge was, and remains, a lack of transplantable hearts: “At a year and a half, it would break, and you’d better find ’em a transplant in that period of time, or they would all die.”
To address this problem, Frazier began working with a Massachusetts-based heart-pump company, Abiomed, to design a next-generation artificial heart, the AbioCor. Devised in the early nineteen-nineties, the heart was traditional in some ways (it had two chambers, like a real heart) and futuristic in others. There were no air hoses or electrical cables leaving the body; completely self-contained, the AbioCor used hydraulic fluid, which could be recirculated, to squeeze its ventricles. It was powered by a battery that could be recharged wirelessly, through the skin. In theory, you could swim with it.
“Super, super ambitious,” Cohn said, pulling up a diagram. “They spent a quarter of a billion dollars developing this. Several hundred animals, half of ’em done here, by Bud and his team.” In 2001 and 2002, the heart was installed in fourteen patients. That’s when the ambitious plans began to falter. “By nine months, all of them except four had died from either complications or device failure,” Cohn recounted.
The F.D.A. gave Abiomed permission to implant sixty more devices, but it was clear that the heart would need to be updated, and then approved all over again—a lengthy process for which no one had the fortitude. “Abiomed threw in the towel,” Cohn said. “They were, like, ‘This is too hard!’ ” One problem was that the heart was so large that it fit only in the chests of the biggest male patients.
“You know, your heart beats a hundred thousand times a day,” Frazier drawled.
“Thirty-five million times a year,” Cohn said.
“So, looking back on it, it’s amazing it lasted as long as it did,” Frazier said.

Throughout the eighties and nineties, even as he helped with the HeartMate and AbioCor, Frazier argued that engineers should shift from pulsatile pump designs to ones based on the more mechanically straightforward principle of “continuous flow”—the strategy that Bivacor later adopted. Some researchers argued that the circulatory system might benefit from the pulse; there’s evidence that blood-vessel walls expand in response to a quickening beat. But Frazier had come to believe that, whatever the benefits of pulsation, they were outweighed by the virtues of durability and simplicity. He began working on two continuous-flow designs in parallel—one with a cardiologist named Richard Wampler, the other with Robert Jarvik—implanting them in animals, taking them out, disassembling them, and analyzing how they’d performed. By the two-thousands, the designs had come into use as the Jarvik 2000 and HeartMate II, respectively.
On his laptop, Cohn pulled up a diagram of the HeartMate II. Essentially, it’s a narrow pipe filled by a corkscrew; as the screw turns between two bearings, it acts like a stationary propeller, pushing blood continuously out from the heart and into the aorta above it. (In farming, the same design—a so-called Archimedes’ screw—is used to pump water for irrigation.)
Cohn pointed to the screw: “One moving part, suspended by ruby bearings. People said, ‘Well, you can’t have bearings in the blood.’ It turns out you can! There’s enough blood washing over them that they stay cool and clean. One of these on a bench will pump forever.” Clots are still a problem, as is infection. Still, more than a thousand people each year now receive HeartMate IIs or similar devices, living with them as they inch their way up the transplant list; a HeartMate II kept Dick Cheney alive, with a fainter pulse, from 2010 to 2012, until he could receive a transplant.
In the summer of 2019, I got an e-mail from Jess. “I recently celebrated twenty years with my heart transplant,” she wrote to a group of us. “But heart transplants don’t last as long as native hearts.” I hadn’t known this; I’d assumed that her transplant was permanent. In fact, her borrowed heart was giving out. She’d been short of breath and, one night, had almost collapsed while walking home to her apartment. Now she was back in the hospital, waiting for a second heart. “It could be weeks, or months, or (less likely) tomorrow,” she wrote. “Please send good vibes.”
I visited Jess in the critical-care unit, where we talked about restaurants, careers, and television shows. We looked at a few photos of my son, who was around a year old. I was about to visit again when she died.
“She did great,” Cohn said. “Many heart-transplant recipients are dead in ten years.”
“Recently, I went to a birthday party for a guy I transplanted thirty years ago,” Frazier said. “But those are rare, rare, rare. Only about five per cent of transplant recipients make it to thirty years.” The artificial pumps on the market are considered bridge therapies, and heart transplants “destination” therapies; but, if you live long enough, the transplants, too, are merely bridges.
I asked Frazier and Cohn how they felt about all the people who had died while, or after, using their devices—whether they lingered in the mind, and how.
“Martyrs,” Cohn said. “They were clinging to life. The technology may not have been there, but it sure beat drawing their last breath. Many of them spent years with their loved ones, doing the stuff they enjoyed. Some went to the I.C.U., were gravely ill for six weeks, and then died, when in retrospect they would’ve been better off if we’d just let ’em die. But you don’t know! It’s a statistics game, and they were willing to go for it, for a couple more days of life. And it advanced the field, every time.”
“I had a lot of experience working with leukemic children when I was a medical student,” Frazier said, quietly. “They all died. In fact, the doctors at Texas Children’s wanted to stop the work.”
“Because you were torturing them with these poisons,” Cohn said.
“They looked awful,” Frazier said. “Their bellies swelled, and they lost their hair, and it scared the other children. But they kept plugging away with it. And I think that helped me. Because the first twenty-two people of the seventy that we put the first LVADs into all died.”
It was getting late. Frazier led me through the deserted office area, along a series of twisty, silent corridors, and finally down an elevator to the basement. We entered his lab—the vast lair where he’d spent most of his working life. We passed through a veterinary operating theatre and a pathology lab, where deceased animals and failed pumps could be disassembled and analyzed.
“We’ve got pigs in here,” Frazier said, opening a door. There was an animal smell, and a large pink pig lumbered into view, snuffling.
“Pigs have a heart that’s most like the human heart,” he said, closing the door. He gestured down the corridor: “Goats. I don’t like to do goats. They’re too smart!” He laughed. “They look up at you.”
We went deeper into the lab. In a carpeted conference room, a display case contained a few dozen artificial hearts and heart pumps—the history of the field, more or less. “The one in the middle is the AbioCor,” Frazier said, indicating a heart-shaped twist of metal and plastic. “This is the old Jarvik-7”: two yellow-beige ventricles with tubes running out. “That’s a HeartMate II”: a gray metal cylinder with white tubing at either end, like something you’d find under a sink. Framed on a wall, an issue of Life , from September, 1981, declared, “ The Artificial Heart Is Here .”
Frazier pointed to a big metal pump, and to a white bit of tubing protruding from it—a “long inlet,” he said. Until it was corrected, it had doomed the device to failure. Small differences, iteratively tweaked, their effects uncovered only after death. It was invention in slow motion.
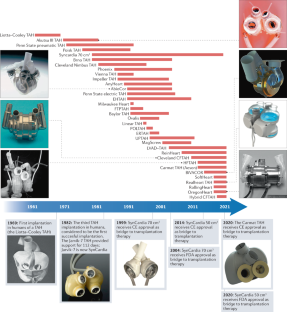
The AbioCor was cancelled. The Bivacor is years away. Today, the only company manufacturing and selling artificial hearts that are actually implanted in people is SynCardia Systems, of Tucson, Arizona. The company was formed as a rescue mission. Symbion, the Utah company that Robert Jarvik helped found, had lost F.D.A. approval for the Jarvik-7 heart in 1990, because of quality-control issues; its heart technology was acquired by another firm, which ran a decade-long clinical trial with an improved version of the heart, only to exhaust its funding in 2001. For a time, it seemed that the technology would vanish from the earth. But two heart surgeons and a biomedical engineer scraped together the venture capital to buy the rights to the system; they rebranded the heart as the SynCardia Total Artificial Heart, or T.A.H. The company, now based in a handful of buildings surrounding a sandy parking lot, sells somewhere north of a hundred hearts a year, all descended from the old-style, air-powered Jarvik-7. Although SynCardia has succeeded in building a network of surgeons capable of installing its heart, the company is only tenuously in business. A few years ago, it declared Chapter 11, and was bought by new investors. It coped with the coronavirus pandemic, which has led to the cancellation of surgeries around the country, by manufacturing hand sanitizer.
With Karen Stamm, SynCardia’s director of program management, and Matt Schuster, an engineer, I watched through a window as a technician in a clean room assembled one of the hearts. “The key to being able to build the artificial heart is the material we use,” Schuster said. “Segmented polyurethane solution. You’ll hear us call it ‘spuzz’—S.P.U.S.” Stamm laughed. “We actually manufacture spus here on campus,” Schuster continued. “It’s our own proprietary mix. It comes out of our manufacturing equipment almost like a sap, or a thick honey.” Using a dental pick, the technician carefully manipulated layers of the molded honey. A translucent something shifting over another translucent something. The assembly process takes two and a half weeks.
We walked through a lab dedicated to “explant analysis”—“If we get a heart back, we’ll take it apart and inspect it,” Schuster said—and into another room filled with a few dozen water tanks on shelves. Inside each tank was a heart, beating; next to the tanks were the air pumps, or “drive units.” The sound in the room was deafening: a fast, loud whump-whump, whump-whump, and within that a mechanical clacking, like a typewriter. The sounds cycled twice a second—an industrial rhythm, as though we were in a factory for the manufacture of circulation. “This is where we run our long-term studies,” Stamm shouted, above the din. On one side of the room were the fifty-c.c. hearts, used by smaller patients; on the other, the seventy-c.c. models, used by larger ones. “There’s the driver, which is the mechanical sound,” she said, pointing to a lunchbox-like mechanical pump that was connected by an air tube to a heart inside a tank. “Then you hear the clack-clack—that’s actually the valve inside the heart.”
The drive unit has been a focus of innovation for SynCardia. Its heart can be driven by one of two units, the first the size of a mini-fridge, the second the size of a toaster—both much smaller than the ones DeVries’s patients used. The drivers need to be serviced after a few months; when a warning light comes on, a caregiver unplugs the drive line and reattaches it to another unit as swiftly as possible, lest the user’s heart skip a beat. As I watched, the water in the tanks rippled slightly, in rhythm. It takes a lot of whump to push five or six litres of blood through the body every minute.
“What does this actually sound like in a person?” I asked.
“It’s much quieter,” Stamm said. “But you can hear it. I’ve heard stories where patients say that, if they open their mouths, other people can hear the clicking.” She told me that some patients couldn’t tolerate the noise at first. But then, she said, “they couldn’t sleep without the sound of the ca-chunk, ca-chunk .”
We continued through a warehouse area, where a dozen or so hearts were kept on shelves, ready to ship; surgical kits, containing the materials required to install them, were boxed in a separate stack. Then we traversed the parking lot to another building, where a group of engineers waited with safety glasses in a high-ceilinged lab space. One of them handed me a small piece of hourglass-shaped plastic: the SPUS . See-through but slightly milky, slick but grippy on my fingertips, it was almost surreally stretchy—I pulled on its ends, drawing the neck of the hourglass to several times its initial length, and it effortlessly returned to its original shape.
Through a doorway I spied a giant, well-worn machine, perhaps a dozen feet tall, combining aspects of an oil derrick and a KitchenAid. “The SPUS reactor,” Troy Villazon, SynCardia’s production manager, said. “It’s from the early sixties.” SynCardia had acquired the machine in the early twenty-tens, to insure a steady supply. “The machinery itself has gone through the whole history of this material,” Villazon said. For a while, we stood speculating about whether this very machine had been used in the creation of the Jarvik hearts. “It very well might have,” Schuster said.
I stopped in front of a whiteboard where four photographs of SynCardia patients had been arranged above the usual hand-drawn schematics. A Black man in a hospital bed, holding a shopping bag; a balding white man on a golf course, with a slender air hose running out from under his shirt and toward his clubs; a blond man, perhaps in his teens, carrying a backpack; and a young brother and sister sitting together. “We like to keep some motivation up on the wall,” Villazon said. The boy, nine years old, was the youngest person ever to receive a SynCardia heart. The longest-surviving SynCardia patient has been using the heart for almost seven years—an achievement that, in the nineteen-eighties, might have made the cover of Life.
One of the biggest problems that SynCardia faces is obsolescence. The Jarvik-7, on which the SynCardia heart is based, was designed nearly forty years ago; the company’s initial F.D.A. approvals are decades old. Today, changing any single part of the heart—a bolt, a valve, a resistor—can require a new approval process. As suppliers go out of business or update their offerings, SynCardia engineers must hunt down, test, and then win approval for replacement components. They live in fear of a fatal malfunction in the SPUS reactor: constructing and securing approval for a new one could take a year, leaving potential new patients without hearts. Maintaining a legacy device is expensive. “Even if you’re not improving it or changing it, just to keep manufacturing that same thing—people don’t realize,” Schuster said. “I’ve worked in aerospace, and I can tell you, it’s often easier to make massive aerospace-defense changes than it is to change something on an artificial heart.” Listening, I imagined the focus with which users of the heart must track SynCardia’s ups and downs.
In the United States, there are fewer than twenty hospitals at which surgeons have been trained to install the heart. “It’s a narrow market,” Don Webber, the C.E.O. of the company, told me. He took out his phone and pulled up a spreadsheet that listed all the candidate patients for the heart at that moment. “We have a daily sheet that comes out,” he explained. “We’ll get a phone call, or a text or an e-mail, that says, ‘We may have a patient.’ ” On the phone’s screen, rows of patients scrolled by, color-coded.

SynCardia faces the same problem that Cooley encountered in the nineteen-sixties: you have to be very sick to consider cutting your heart out of your chest, but if you wait too long, and get too sick, you are beyond saving. “There are cases that sit there,” Webber said, alarm in his voice. “You see it on the list this week, you see it on the list at the end of the week, you see it on next week’s list. They’re just waiting, and waiting, and waiting.” The longer a patient waits, the less likely she is to survive the implantation of the artificial heart and any subsequent transplant. “It’s not a clean decision,” Webber said. “You have several people that are on that team”—surgeons, cardiologists, hospitalists, all of whom have to agree.
Business scholars use many different metaphors to describe invention and innovation. They say that technologies can progress in continuous and discontinuous ways; that new products must climb an “adoption curve,” or leap across a chasm of usability. Nobody wanted mobile phones until they got so small that everyone wanted one. Electric cars have seemed impractical, but hybrid engines, by giving drivers a taste of the technology, have speeded their adoption.
The artificial heart faces a unique challenge. Only those confronting imminent death are willing to use today’s models. And yet nearly six hundred and sixty thousand Americans die of heart disease annually—a pandemic-level death toll about which we feel little sense of emergency. An increasing number of us live with diseased hearts and suffer the consequences. To realize their full potential, artificial hearts must get good enough that people actually want to use them; they must be preferable not to death but to a failing heart, the way hip replacements are preferable to failing hips. Meanwhile, until they achieve wider adoption, they will remain a niche product—and so be unavailable to many people who need them. For another moment, Webber scrolled his list; I wondered whether Jess should’ve been on it. Then he put the phone away.
The engineers at SynCardia are charged with sustaining a legacy technology, but they also understand that it needs to advance. Before I left Tucson, Villazon told me about a next-generation heart that SynCardia was developing. The heart would use a new, battery-driven pump engine, which could be housed entirely within the patient; like the AbioCor, it would be wireless, without an external driver. At the same time, it would pump blood using the company’s preëxisting, SPUS -based ventricles, which had already been approved by the F.D.A. By hooking this new engine up to its old car—a hybrid model—SynCardia hoped to develop and market the heart quickly, and to bring its existing customers along. The new heart could be a reliable, permanent implantation, Villazon said. It might be used by people a little further from the precipice.
I’m no Bud Frazier, but I’ve seen a lot of artificial hearts, and Villazon’s design struck me as both simple and ingenious. And yet SynCardia’s engineers were busy—manufacturing, selling, and updating the current heart, saving more than a hundred lives a year. They were struggling to find time to get the new heart up and running. They’d 3-D-printed a few prototypes and shopped the specs around; they were talking with investors.
The Bivacor team, in Cerritos, is blissfully unencumbered by the technological past. When I visited, everyone was going out for Thai—a weekly team lunch. It was a large group for the restaurant, but a small one for the design of an artificial heart. Timms sat at one end of the table, near Nicholas Greatrex, the electrical engineer.
“Now that you’re getting closer to putting a device in a person, how does it feel?” I asked. “Is it exciting, or freaky, or what?”
“The closer you get to it going into a human, the more you think about everything that can go wrong, and what you can do,” Matthias Kleinheyer, a bearded engineer, said. “Even if I had no doubt that the system is performing the way it’s supposed to, it would still be very scary.” Kleinheyer is in charge of the heart’s backup systems; there are backups to the backups to the backups.
“Nick wants to go and live with the person who gets the first heart,” Timms said.
“Yeah,” Greatrex said.
“In case something goes wrong, we could fix it right away,” Timms said.
I pictured Timms, two decades younger, tinkering in the garage with his dad. Once the company shipped its heart—to a human implantation, a clinical trial, and eventually the market—its design would have to be set in stone. The process of approval was at odds with the process of improvement.
“If I could, I’d just keep working on it and working on it and working on it,” Greatrex said. “I’d never implant it.” People laughed, but he wasn’t exactly joking.
If patients in need of artificial hearts can wait too long before taking the leap, the engineers who design them face a parallel decision. Implant too soon, and the device may be immature; chase perfection, and it may never leave the lab. Back at the office, I talked with Timms about the question of wireless hearts. Investors have offered to give the company more money if it will design a wireless, rechargeable heart right away; gritting his teeth, Timms has decided to refuse the funding, saving wireless charging for version 2.0. “We’d rather keep the money to make sure that the device works properly in the body,” he said. “If we do the test flight, and we try to do too many things at once, we’re going to crash.” It was, he thought, the most consequential design decision the team had made; if it prevents broader adoption of the heart, it could bring the whole effort to an end. “I hate the drive line so much,” Timms said. “I mean, that thing has to go.” But not yet.
In the lab, Greatrex walked me through a technical innovation of which the team was especially proud. The human circulatory system is housed within a body that is constantly adjusting its configuration in space. As a result of the body’s movements and exertions, the rate of blood flow changes. Lie down and it generally decreases; stand and it speeds up. Run or jump and it spikes, in order to feed oxygen to the muscles. All this movement poses a challenge for Bivacor’s maglev rotor. As the body moves and stops, and as blood flow surges and ebbs, the disk can find itself pushed toward the walls. Ideally, the rotor would resist the currents—floating and spinning as if weightless, holding the position no matter the circumstances.
On a whiteboard, Greatrex outlined the elaborate magnet-control systems that the heart uses to sense and adjust to the forces around it. Timms himself had worked out the math that made the adjustments possible—a difficult problem in fluid dynamics. The engineering depended on digital technologies that had been unavailable to previous generations of designers.
Greatrex handed me one of the rotors: a coin-shaped object, a few inches across, made of burnished gold-colored titanium. It was a source of regret for the team, he said, that, in the final production version, the titanium would be a more practical gray. I hefted it. On one side, eight metal tines clustered, Stonehenge-like, in the center. On the other, eight curving, windswept triangles studded the edges, as though sails, or shark fins, were performing a circumnavigation. An intricate pattern of whorls filled the middle of the disk—machining marks, or waves on the sea.
“I think if you showed that to a bunch of people no one would figure out that it’s part of an artificial heart,” Greatrex said.
I turned it in the light and snapped a photo. It was a strangely evocative object—a beautiful one. It wasn’t biological, but it didn’t seem entirely mechanical, either. It had the idiosyncratic, refined particularity of something that had evolved. In a way, it had. ♦
This article has been updated to more fully describe the scope of Mimi Swartz’s “Ticker: The Quest to Create an Artificial Heart.”

By signing up, you agree to our User Agreement and Privacy Policy & Cookie Statement . This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 06 June 2022
The ongoing quest for the first total artificial heart as destination therapy
- Annemijn Vis ORCID: orcid.org/0000-0002-2343-5686 1 , 2 ,
- Maziar Arfaee ORCID: orcid.org/0000-0002-4459-5582 1 , 2 ,
- Husain Khambati 1 , 2 ,
- Mark S. Slaughter 3 ,
- Jan F. Gummert 4 ,
- Johannes T. B. Overvelde ORCID: orcid.org/0000-0003-0253-0147 5 , 6 &
- Jolanda Kluin ORCID: orcid.org/0000-0002-5942-4727 1 , 2
Nature Reviews Cardiology volume 19 , pages 813–828 ( 2022 ) Cite this article
2458 Accesses
12 Citations
13 Altmetric
Metrics details
- Cardiac device therapy
- Heart failure
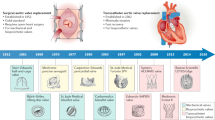
Many patients with end-stage heart disease die because of the scarcity of donor hearts. A total artificial heart (TAH), an implantable machine that replaces the heart, has so far been successfully used in over 1,700 patients as a temporary life-saving technology for bridging to heart transplantation. However, after more than six decades of research on TAHs, a TAH that is suitable for destination therapy is not yet available. High complication rates, bulky devices, poor durability, poor biocompatibility and low patient quality of life are some of the major drawbacks of current TAH devices that must be addressed before TAHs can be used as a destination therapy. Quickly emerging innovations in battery technology, wireless energy transmission, biocompatible materials and soft robotics are providing a promising opportunity for TAH development and might help to solve the drawbacks of current TAHs. In this Review, we describe the milestones in the history of TAH research and reflect on lessons learned during TAH development. We summarize the differences in the working mechanisms of these devices, discuss the next generation of TAHs and highlight emerging technologies that will promote TAH development in the coming decade. Finally, we present current challenges and future perspectives for the field.
After decades of research on total artificial hearts, only two devices are clinically available as a bridge to transplantation therapy; a total artificial heart suitable for destination therapy has not yet been developed.
Currently available total artificial hearts have major drawbacks, including bulkiness, limited durability, poor biocompatibility, high complication rates and low quality of life for the recipients.
We are on the verge of an era in total artificial heart development in which rapidly evolving technologies from different fields will lead to new approaches in total artificial heart design and development.
More powerful and more compact batteries and transcutaneous energy transfer systems will omit the need for percutaneous cables and will improve the quality of life of the recipients of a total artificial heart.
With the rise of soft robotic technologies and smart biomaterials, completely soft total artificial hearts might soon be developed and are likely to have fewer biocompatibility issues than current devices.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Temporary mechanical circulatory support devices: practical considerations for all stakeholders
Benjamin S. Salter, Caroline R. Gross, … Anuradha Lala
Next-generation tissue-engineered heart valves with repair, remodelling and regeneration capacity
Emanuela S. Fioretta, Sarah E. Motta, … Maximilian Y. Emmert

A nonrandomized open-label phase 2 trial of nonischemic heart preservation for human heart transplantation
Johan Nilsson, Victoria Jernryd, … Stig Steen
Metra, M. & Teerlink, J. R. Heart failure. Lancet 390 , 1981–1995 (2017).
Article PubMed Google Scholar
Heidenreich, P. A. et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ. Heart Fail. 6 , 606–619 (2013).
Article CAS PubMed PubMed Central Google Scholar
Colvin, M. et al. OPTN/SRTR 2018 annual data report: heart. Am. J. Transpl. 20 , 340–426 (2020).
Article Google Scholar
Molina, E. J. et al. The Society of Thoracic Surgeons Intermacs 2020 annual report. Ann. Thorac. Surg. 111 , 778–792 (2021).
Han, J. J., Acker, M. A. & Atluri, P. Left ventricular assist devices. Circulation 138 , 2841–2851 (2018).
Gurvits, G. E. & Fradkov, E. Bleeding with the artificial heart: gastrointestinal hemorrhage in CF-LVAD patients. World J. Gastroenterol. 23 , 3945–3953 (2017).
Article PubMed PubMed Central Google Scholar
Galand, V. et al. Predictors and clinical impact of late ventricular arrhythmias in patients with continuous-flow left ventricular assist devices. JACC Clin. Electrophysiol. 4 , 1166–1175 (2018).
Ross, D. W. et al. Left ventricular assist devices and the kidney. Clin. J. Am. Soc. Nephrol. 13 , 348–355 (2018).
Aissaoui, N. et al. Understanding left ventricular assist devices. Blood Purif. 46 , 292–300 (2018).
National Heart Lung and Blood Institute. What Is Total Artificial Heart? Total Artificial Heart https://www.nhlbi.nih.gov/health-topics/total-artificial-heart (NIH, 2020).
Cooley, D. A. et al. Orthotopic cardiac prosthesis for two-staged cardiac replacement. Am. J. Cardiol. 24 , 723–730 (1969).
Article CAS PubMed Google Scholar
US National Library of Medicine. SynCardia 70cc TAH-t for Destination Therapy (DT) (RA-540). ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02232659 (2021).
Akutsu, T. & Kolff, W. J. Permanent substitutes for valves and hearts. ASAIO J. 4 , 230–234 (1958).
Google Scholar
Houston, C. S., Akutsu, T. & Kolff, W. J. Pendulum type of artificial heart within the chest: preliminiary report. Am. Heart J. 59 , 723–730 (1960).
Seidel, W., Akutsu, T., Mirkovitch, V., Brown, F. & Kolff, W. J. Air-driven artificial hearts inside the chest. Trans. Am. Soc. Artif. Intern. Organs 7 , 378–387 (1961).
CAS PubMed Google Scholar
Liotta, D. et al. Artificial heart in the chest: preliminary report. Trans. Am. Soc. Artif. Intern. Organs 7 , 318–322 (1961).
Atsumi, K. et al. Artificial heart incorporated in the chest. Trans. Am. Soc. Artif. Intern. Organs 9 , 292–298 (1963).
Pierce, W. S. et al. Total heart replacement by a single intrathoracic blood pump. J. Surg. Res. 5 , 387–394 (1965).
Nosé, Y., Tretbar, L. L., SenGupta, A., Topaz, S. R. & Kolff, W. J. An artificial heart inside the chest. J. Thorac. Cardiovasc. Surg. 50 , 792–799 (1965).
Cohn, W. E., Timms, D. L. & Frazier, O. H. Total artificial hearts: past, present, and future. Nat. Rev. Cardiol. 12 , 609–617 (2015).
Curran, W. J. Law-medicine notes. The first mechanical heart transplant: informed consent and experimentation. N. Engl. J. Med. 291 , 1015–1016 (1974).
Morris, D. T. & Couves, C. M. Experiences with a sac-type artificial heart. Can. Med. Assoc. J. 105 , 483–487 (1971).
CAS PubMed PubMed Central Google Scholar
Kwan-Gett, C. S., Van Kampen, K. R., Kawai, J., Eastwood, N. & Kolff, W. J. Results of total artificial heart implantation in calves. J. Thorac. Cardiovasc. Surg. 62 , 880–889 (1971).
Kawai, J. et al. Implantation of a total artificial heart in calves under hypothermia with 10 day survival. J. Thorac. Cardiovasc. Surg. 64 , 45–60 (1972).
Hastings, W. L. et al. A retrospective study of nine calves surviving five months on the pneumatic total artificial heart. Trans. Am. Soc. Artif. Intern. Organs 27 , 71–76 (1981).
Fukumasu, H., Iwaya, F., Olsen, D. B., Lawson, J. H. & Kolff, W. J. Surgical implantation of the Jarvik-5 total artificial heart in a calf. Trans. Am. Soc. Artif. Intern. Organs 25 , 232–238 (1979).
Akutsu, T., Takagi, H. & Takano, H. Total artificial hearts with built-in valves. Trans. Am. Soc. Artif. Intern. Organs 16 , 392–397 (1970).
Honda, T. et al. One 25 day survivor with total artificial heart. J. Thorac. Cardiovasc. Surg. 69 , 92–101 (1975).
Nakazono, M. et al. A case report of 17 days survival with an implanted artificial heart in a calf. Jpn Heart J. 15 , 485–497 (1974).
Kasai, S. et al. Survival for 145 days with a total artificial heart. J. Thorac. Cardiovasc. Surg. 73 , 637–646 (1977).
Kennedy, J. H. et al. Development of an orthotopic cardiac prosthesis. J. Thorac. Cardiovasc. Surg. 65 , 673–683 (1973).
Backman, D. K., Donovan, F. M., Sandquist, G., Kessler, T. & Kolff, W. J. The design and evaluation of ventricles for the aec artificial heart nuclear power source. ASAIO J. 19 , 542–552 (1973).
Article CAS Google Scholar
Smith, L. et al. Development on the implantation of a total nuclear-powered artificial heart system. ASAIO J. 20 , 732–735 (1974).
Urzua, J., Sudilovsky, O., Panke, T., Kiraly, R. J. & Nosé, Y. Preliminary report: anatomic constraints for the implantation of an artificial heart. J. Surg. Res. 17 , 262–268 (1974).
Cooley, D. A., Akutsu, T., Norman, J. C., Serrato, M. A. & Frazier, O. H. Total artificial heart in two-staged cardiac transplantation. Cardiovasc. Dis. 8 , 305–319 (1981).
PubMed PubMed Central Google Scholar
DeVries, W. C. et al. Clinical use of the total artificial heart. N. Engl. J. Med. 310 , 273–278 (1984).
Vasků, J. & Urbánek, P. Constructional and functional characteristics of recent total artificial heart models TNS Brno VII, VIII, and IX. Artif. Organs 19 , 535–543 (1995).
Davis, P. K., Pae, W. E. Jr & Pierce, W. S. Toward an implantable artificial heart. Experimental and clinical experience at The Pennsylvania State University. Invest. Radiol. 24 , 81–87 (1989).
Hsu, C. H. Fuzzy logic automatic control of the Phoenix-7 total artificial heart. J. Artif. Organs 7 , 69–76 (2004).
Hsu, C. H. In vivo and clinical study of Phoenix-7 total artificial heart. Biomed. Eng. Appl. Basis Commun. 13 , 133–139 (2001).
Shumakov, V. I. et al. Use of an ellipsoid artificial heart. Artif. Organs 11 , 16–19 (1987).
Shumakov, V. I. et al. New design of an orthotopic fluorosiloxane rubber heart prosthesis. Biomed. Eng. 10 , 223–224 (1976).
Nawrat, Z. & Malota, Z. The analysis of driving mode influence on energy dissipation in pneumatic artificial heart chambers. Artif. Organs 22 , 898–904 (1998).
Rokitansky, A. et al. The new small Viennese total artificial heart: experimental and first clinical experiences. Artif. Organs 15 , 129–135 (1991).
Homma, A. et al. Development of an electrohydraulic total artificial heart system: improvement of pump unit. Electron. Commun. Jpn. 93 , 34–46 (2010).
Ford, B. J. A new generation of cardiology: the AbioCor implantable replacement heart. Air Med. J. 22 , 26–30 (2003).
Dowling, R. D. et al. The AbioCor implantable replacement heart. Ann. Thorac. Surg. 75 , S93–S99 (2003).
Smith, P. A., Cohn, W. E. & Frazier, O. H. in Mechanical Circulatory and Respiratory Support (eds Gregory, S. D., Stevens, M. C. & Fraser, J. F.) Ch. 7, 221–244 (Academic, 2018).
Spiliopoulos, S., Dimitriou, A. M., Guersoy, D., Koerfer, R. & Tenderich, G. Expanding applicability of total artificial heart therapy: the 50-cc SynCardia total artificial heart. Ann. Thorac. Surg. 100 , e55–e57 (2015).
Cohrs, N. H. et al. A soft total artificial heart-first concept evaluation on a hybrid mock circulation. Artif. Organs 41 , 948–958 (2017).
Stepanenko, A. & Kaufmann, F. A novel total artificial heart: search for haemocompatibility. Lancet 386 , 1517–1519 (2015).
CARMAT. Artificial hearts: devices from France’s Carmat to go on sale in Europe. YouTube https://www.youtube.com/watch?v=j9T2HnSfVME (2021).
US Food and Drug Administration. SynCardia Temporary Cardio West Total Artificial Heart (TAH-T). AccessData https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?ID=P030011 (2004).
EC Design-Examination Certificate. SynCardia temporary Total Artificial Heart (TAH-t) and external drivers. SynCardia http://syncardia.com/wp-content/uploads/2018/02/CE-665479-Design-Examination-26-May-2017.pdf (2017).
SynCardia. SynCardia 70cc total artificial heart. SynCardia https://syncardia.com/clinicians/our-products/see-all-our-products/ (2021).
US National Library of Medicine. Carmat TAH early feasibility study. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04117295 (2022).
Guex, L. G. et al. Increased longevity and pumping performance of an injection molded soft total artificial heart. Soft Robot. 23 , 23 (2020).
Mihaylov, D., Verkerke, G. J. & Rakhorst, G. Mechanical circulatory support systems — a review. Technol. Health Care 8 , 251–266 (2000).
Min, B. G. et al. A moving-actuator type electromechanical total artificial heart — Part II: circular type and animal experiment. IEEE Trans. Biomed. Eng. 37 , 1195–1200 (1990).
Min, B. G. et al. A moving-actuator type electromechanical total artificial heart — Part I: linear type and mock circulation experiments. IEEE Trans. Biomed. Eng. 37 , 1186–1194 (1990).
Ahn, J. M., Kang, D. W., Kim, H. C. & Min, B. G. In vivo performance evaluation of a transcutaneous energy and information transmission system for the total artificial heart. ASAIO J. 39 , M208–M212 (1993).
Ohashi, Y., de Andrade, A. & Nosé, Y. Hemolysis in an electromechanical driven pulsatile total artificial heart. Artif. Organs 27 , 1089–1093 (2003).
Takatani, S. et al. One piece ultracompact totally implantable electromechanical total artificial heart for permanent use. ASAIO J. 48 , 538–545 (2002).
Irié, H. et al. Initial in vivo tests of an electrohydraulic actuated total artificial heart. ASAIO J. 38 , M497–M500 (1992).
Fukamachi, K. et al. Anatomic fitting studies of a total artificial heart in heart transplant recipients. Critical dimensions and prediction of fit. ASAIO J. 42 , M337–M342 (1996).
Sueshiro, M., Fukunaga, S., Hirai, S., Sueda, T. & Matsuura, Y. Eccentric roller type total artificial heart designed for implantation. Artif. Organs 22 , 451–457 (1998).
Kobayashi, M. et al. In vitro evaluation of linear motor-driven total artificial heart. Artif. Organs 20 , 1320–1324 (1996).
Yamada, H., Yamaguchi, M., Kobayashi, K., Matsuura, Y. & Takano, H. Development and test of a linear motor-driven total artificial heart. IEEE Eng. Med. Biol. Mag. 14 , 84–90 (1995).
Weber, S. et al. MagScrew TAH: an update. ASAIO J. 51 , xxxvi–xlvi (2005).
Gao, H. et al. In vitro assessment of the Milwaukee heart and right to left balance. ASAIO J. 38 , M722–M725 (1992).
Sauer, I. M., Frank, J., Spiegelberg, A. & Bücherl, E. S. Ovalis TAH: development and in vitro testing of a new electromechanical energy converter for a total artificial heart. ASAIO J. 46 , 744–748 (2000).
Mehta, S. M. et al. Testing of a 50 cc stroke volume completely implantable artificial heart: expanding chronic mechanical circulatory support to women, adolescents, and small stature men. ASAIO J. 46 , 779–782 (2000).
Szabó, Z. et al. Scandinavian real heart (SRH) 11 implantation as total artificial heart (TAH)-experimental update. J. Clin. Exp. Cardiol. 9 , 2 (2018).
Sonntag, S. J. et al. Virtual implantations to transition from porcine to bovine animal models for a total artificial heart. Artif. Organs 44 , 384–393 (2020).
Pelletier, B. et al. System overview of the fully implantable destination therapy — ReinHeart total artificial heart. Eur. J. Cardiothorac. Surg. 47 , 80–86 (2015).
Tozzi, P. et al. An original valveless artificial heart providing pulsatile flow tested in mock circulatory loops. Int. J. Artif. Organs 40 , 683–689 (2017).
Kirklin, J. K. et al. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J. Heart Lung Transpl. 36 , 1080–1086 (2017).
Potapov, E. V., Kaufmann, F., Müller, M., Mulzer, J. & Falk, V. Longest ongoing support (13 years) with magnetically levitated left ventricular assist device. ASAIO J. 66 , e121–e122 (2020).
Yang, M. in Technology and Therapy Management 99–100 (Springer, 2020).
Healy, A. H. et al. Physiologic effects of continuous-flow left ventricular assist devices. J. Surg. Res. 202 , 363–371 (2016).
Bhimaraj, A., Uribe, C. & Suarez, E. E. Physiological impact of continuous flow on end-organ function: clinical implications in the current era of left ventricular assist devices. Methodist. Debakey Cardiovasc. J. 11 , 12–17 (2015).
Isoyama, T. et al. New version of flow-transformed pulsatile total artificial heart with no electrical switching valve. Artif. Organs 19 , 694–696 (1995).
Abe, Y. et al. Results of animal experiments with the fourth model of the undulation pump total artificial heart. Artif. Organs 35 , 781–790 (2011).
Qian, K. X., Ru, W. M., Zeng, P. & Yuan, H. Y. A novel impeller TAH using magnetic bearings for load reduction. J. Med. Eng. Technol. 26 , 214–216 (2002).
Feng, J. et al. New continuous-flow total artificial heart and vascular permeability. J. Surg. Res. 199 , 296–305 (2015).
Lebreton, G., Mastroianni, C., Amour, J. & Leprince, P. Implantation of two HVADs used as a total artificial heart: a new approach. Ann. Thorac. Surg. 107 , e165–e167 (2019).
Mulvihill, M. S. et al. Usefulness of two centrifugal ventricular assist devices in a total artificial heart configuration: a preliminary report. J. Heart Lung Transpl. 36 , 1266–1268 (2017).
Cohn, W. E. et al. Eight-year experience with a continuous-flow total artificial heart in calves. ASAIO J. 60 , 25–30 (2014).
Pirk, J. et al. Total artificial heart support with two continuous-flow ventricular assist devices in a patient with an infiltrating cardiac sarcoma. ASAIO J. 59 , 178–180 (2013).
Frazier, O. H. & Cohn, W. E. Continuous-flow total heart replacement device implanted in a 55-year-old man with end-stage heart failure and severe amyloidosis. Tex. Heart Inst. J. 39 , 542–546 (2012).
Strueber, M., Schmitto, J. D., Kutschka, I. & Haverich, A. Placement of two implantable centrifugal pumps to serve as a total artificial heart after cardiectomy. J. Thorac. Cardiovasc. Surg. 143 , 507–509 (2012).
Frazier, O. H., Cohn, W. E., Tuzun, E., Winkler, J. A. & Gregoric, I. D. Continuous-flow total artificial heart supports long-term survival of a calf. Tex. Heart Inst. J. 36 , 568–574 (2009).
Baldwin, A. C., Gemmato, C. J., Cohn, W. E. & Frazier, O. H. Feasibility of long-term continuous flow total heart replacement in calves. Int. J. Artif. Organs 45 , 44–51 (2021).
Daneshmand, M. A., Bishawi, M., Milano, C. A. & Schroder, J. N. The HeartMate 6. ASAIO J. 66 , e46–e49 (2020).
Kleinheyer, M. et al. Rapid speed modulation of a rotary total artificial heart impeller. Artif. Organs 40 , 824–833 (2016).
Fukamachi, K. et al. Generating pulsatility by pump speed modulation with continuous-flow total artificial heart in awake calves. J. Artif. Organs 20 , 381–385 (2017).
Miyamoto, T. et al. Analysis of Cleveland clinic continuous-flow total artificial heart performance using the virtual mock loop: comparison with an in vivo study. Artif. Organs 44 , 375–383 (2020).
Abe, Y. et al. Animal experiments of the helical flow total artificial heart. Artif. Organs 39 , 670–680 (2015).
Fox, C. et al. Hybrid continuous-flow total artificial heart. Artif. Organs 42 , 500–509 (2018).
Glynn, J. et al. The OregonHeart total artificial heart: design and performance on a mock circulatory loop. Artif. Organs 41 , 904–910 (2017).
Jurney, P. L. et al. Characterization of a pulsatile rotary total artificial heart. Artif. Organs 45 , 135–142 (2021).
Franklin, D. L., Van Citters, R. L. & Rushmer, R. F. Balance between right and left ventricular output. Circ. Res. 10 , 17–26 (1962).
Ley, S., Kreitner, K. F., Morgenstern, I., Thelen, M. & Kauczor, H. U. Bronchopulmonary shunts in patients with chronic thromboembolic pulmonary hypertension: evaluation with helical CT and MR imaging. Am. J. Roentgenol. 179 , 1209–1215 (2002).
Baile, E. M., Ling, H., Heyworth, J. R., Hogg, J. C. & Pare, P. D. Bronchopulmonary anastomotic and noncoronary collateral blood flow in humans during cardiopulmonary bypass. Chest 87 , 749–754 (1985).
Bhunia, S. K. & Kung, R. T. Indirect bronchial shunt flow measurements in AbioCor implantable replacement heart recipients. ASAIO J. 50 , 211–214 (2004).
Nestler, F. et al. Investigation of the inherent left-right flow balancing of rotary total artificial hearts by means of a resistance box. Artif. Organs 44 , 584–593 (2020).
Latrémouille, C. et al. A bioprosthetic total artificial heart for end-stage heart failure: results from a pilot study. J. Heart Lung Transpl. 37 , 33–37 (2018).
Slepian, M. J. et al. The Syncardia™ total artificial heart: in vivo, in vitro, and computational modeling studies. J. Biomech. 46 , 266–275 (2013).
Crosby, J. R. et al. Physiological characterization of the SynCardia total artificial heart in a mock circulation system. ASAIO J. 61 , 274–281 (2015).
Diedrich, M. et al. Experimental investigation of right–left flow balance concepts for a total artificial heart. Artif. Organs 45 , 364–372 (2021).
Horvath, D. et al. Mechanism of self-regulation and in vivo performance of the cleveland clinic continuous-flow total artificial heart. Artif. Organs 41 , 411–417 (2017).
Kung, R. T. et al. Progress in the development of the ABIOMED total artificial heart. ASAIO J. 41 , M245–M248 (1995).
Harasaki, H. et al. Progress in Cleveland Clinic — Nimbus total artificial heart development. ASAIO J. 40 , M494–M498 (1994).
Kim, H. C., Khanwilkar, P. S., Bearnson, G. B. & Olsen, D. B. Development of a microcontroller-based automatic control system for the electrohydraulic total artificial heart. IEEE Trans. Biomed. Eng. 44 , 77–89 (1997).
Snyder, A. J. et al. An electrically powered total artificial heart. Over 1 year survival in the calf. ASAIO J. 38 , M707–M712 (1992).
Abe, Y. et al. Third model of the undulation pump total artificial heart. ASAIO J. 49 , 123–127 (2003).
Dowling, R. D. et al. Initial experience with the AbioCor implantable replacement heart at the University of Louisville. ASAIO J. 46 , 579–581 (2000).
Latrémouille, C. et al. Animal studies with the Carmat bioprosthetic total artificial heart. Eur. J. Cardiothorac. Surg. 47 , e172–e179 (2015).
Vasků, J. et al. A comparative study of a group of eight calves, surviving longer than 1 month with the total artificial heart. Artif. Organs 7 , 470–478 (1983).
Vasků, J., Urbánek, P., Dostál, M. & Vasků, J. The applicability of experimental experience with the total artificial heart to its clinical use. Int. J. Artif. Organs 15 , 307–311 (1992).
Copeland, J. G. et al. The total artificial heart as a bridge to transplantation. A report of two cases. JAMA 256 , 2991–2995 (1986).
Shumakov, V. et al. Clinical indications for the use of the “Poisk-IOM” total artificial heart: the experience of 13 implantations in humans. Artif. Organs 15 , 372–375 (1991).
Wei, J. et al. Successful use of Phoenix-7 total artificial heart. Transpl. Proc. 30 , 3403–3404 (1998).
Trubel, W. et al. Clinical total artificial heart bridging: Viennese strategy and experiences. Artif. Organs 13 , 470–475 (1989).
Frazier, O. H. et al. The total artificial heart: where we stand. Cardiology 101 , 117–121 (2004).
Alaeddine, M., Ploutz, M., Arabia, F. A. & Velez, D. A. Implantation of total artificial heart in a 10-year-old after support with a temporary perventricular assist device. J. Thorac. Cardiovasc. Surg. 159 , e227–e229 (2020).
SynCardia. Turkish man becomes world’s longest supported syncardia temporary total artificial heart patient. SynCardia https://syncardia.com/news/turkish-man-becomes-worlds-longest-supported-syncardia-temporary-total-artificial-heart-patient/ (2017).
David, C. H. et al. A heart transplant after total artificial heart support: initial and long-term results. Eur. J. Cardiothorac. Surg. 58 , 1175–1181 (2020).
Carrier, M. et al. Outcomes after heart transplantation and total artificial heart implantation: a multicenter study. J. Heart Lung Transplant. 28 , 28 (2020).
Hulman, M., Artemiou, P., Hudec, V., Olejarova, I. & Goncalvesova, E. SynCardia, total artificial heart, as a bridge to transplant. Bratisl. Lek. Listy 120 , 325–330 (2019).
Nguyen, A. et al. Experience with the SynCardia total artificial heart in a Canadian centre. Can. J. Surg. 60 , 375–379 (2017).
Kirsch, M. E. et al. SynCardia temporary total artificial heart as bridge to transplantation: current results at La Pitié hospital. Ann. Thorac. Surg. 95 , 1640–1646 (2013).
Copeland, J. G. et al. Experience with more than 100 total artificial heart implants. J. Thorac. Cardiovasc. Surg. 143 , 727–734 (2012).
Roussel, J. C. et al. CardioWest (Jarvik) total artificial heart: a single-center experience with 42 patients. Ann. Thorac. Surg. 87 , 124–130 (2009).
El-Banayosy, A. et al. CardioWest total artificial heart: bad Oeynhausen experience. Ann. Thorac. Surg. 80 , 548–552 (2005).
Thanavaro, K. L., Tang, D. G., Kasirajan, V. & Shah, K. B. Clinical indications for implantation of the total artificial heart. ASAIO J. 60 , 594–596 (2014).
Carmat. Carmat outlines commercial and development plan for its total artificial heart. Carmat https://www.carmatsa.com/carmat-content/uploads/2021/01/pr_carmat_conference_06-01-21.pdf (2021).
Netuka, I. et al. Initial bridge to transplant experience with a bioprosthetic autoregulated artificial heart. J. Heart Lung Transpl. 39 , 1491–1493 (2020).
Unthan, K. et al. Design and evaluation of a fully implantable control unit for blood pumps. Biomed. Res. Int. 2015 , 257848 (2015).
Kluin, J. et al. In situ heart valve tissue engineering using a bioresorbable elastomeric implant — from material design to 12 months follow-up in sheep. Biomaterials 125 , 101–117 (2017).
Zilla, P., Deutsch, M., Bezuidenhout, D., Davies, N. H. & Pennel, T. Progressive reinvention or destination lost? Half a century of cardiovascular tissue engineering. Front. Cardiovasc. Med. 7 , 159 (2020).
Chen, Z. et al. Device-induced platelet dysfunction in mechanically assisted circulation increases the risks of thrombosis and bleeding. Artif. Organs 43 , 745–755 (2019).
Zaiser, A. S. et al. Adverse events of percutaneous microaxial left ventricular assist devices-a retrospective, single-centre cohort study. J. Clin. Med. 10 , 3710 (2021).
HybridHeart. The development of a soft biocompatible artificial heart. HybridHeart https://www.hybridheart.eu/ (2021).
Kohll, A. X. et al. Long-term performance of a pneumatically actuated soft pump manufactured by rubber compression molding. Soft Robot. 6 , 206–213 (2019).
Roche, E. T. et al. Soft robotic sleeve supports heart function. Sci. Transl. Med. 9 , eaaf3925 (2017).
Banerjee, H., Tse, Z. T. H. & Ren, H. Soft robotics with compliance and adaptation for biomedical applications and forthcoming challenges. Int. J. Robot. Autom. 33 , 69–80 (2018).
Greatrex, N., Kleinheyer, M., Nestler, F. & Timms, D. The Maglev heart. IEEE Spectr. 56 , 22–29 (2019).
Parnis, S. M. et al. Chronic in vivo evaluation of an electrohydraulic total artificial heart. ASAIO J. 40 , M489–M493 (1994).
Dowling, R. D., Etoch, S. W., Stevens, K. A., Johnson, A. C. & Gray, L. A. Jr Current status of the AbioCor implantable replacement heart. Ann. Thorac. Surg. 71 , S147–S149 (2001).
Takatani, S. et al. Totally implantable total artificial heart and ventricular assist device with multipurpose miniature electromechanical energy system. Artif. Organs 18 , 80–92 (1994).
Takatani, S. et al. Left and right pump output control in one-piece electromechanical total artificial heart. Artif. Organs 17 , 176–184 (1993).
Cohn, W. E. et al. Pulsatile outflow in cows supported long-term with the BiVACOR rotary TAH. J. Heart Lung Transplant. 36 , S14 (2017).
Dostál, M. et al. Hematological and biochemical studies in calves living over 100 days with the polymethylmethacrylate total artificial heart TNS Brno II. Int. J. Artif. Organs 9 , 39–48 (1986).
Vasků, J. et al. Recent efforts in artificial heart research in Czechoslovakia. ASAIO Trans. 35 , 805–811 (1989).
PubMed Google Scholar
Smadja, D. M. et al. The Carmat bioprosthetic total artificial heart is associated with early hemostatic recovery and no acquired von Willebrand syndrome in calves. J. Cardiothorac. Vasc. Anesth. 31 , 1595–1602 (2017).
Karimov, J. H. et al. First report of 90-day support of two calves with a continuous-flow total artificial heart. J. Thorac. Cardiovasc. Surg. 150 , 687–693.e681 (2015).
Taenaka, Y. et al. Development and evaluation of components for a totally implantable artificial heart system. ASAIO J. 40 , M314–M318 (1994).
Taenaka, Y. et al. An electrohydraulic total artificial heart with a separately placed actuator. ASAIO Trans. 36 , M242–M245 (1990).
Tatsumi, E. et al. A blood pump with an interatrial shunt for use as an electrohydraulic total artificial heart. ASAIO J. 38 , M425–M430 (1992).
Tatsumi, E. et al. The National Cardiovascular Center electrohydraulic total artificial heart and ventricular assist device systems: current status of development. ASAIO J. 49 , 243–249 (2003).
Tatsumi, E. et al. Current status of development and in vivo evaluation of the National Cardiovascular Center electrohydraulic total artificial heart system. J. Artif. Organs 3 , 62–69 (2000).
Doi, K. et al. In vivo studies of the MagScrew total artificial heart in calves. ASAIO J. 48 , 222–225 (2002).
Kuroda, H. et al. Postoperative pulmonary complications in calves after implantation of an electric total artificial heart. ASAIO J. 44 , M613–M618 (1998).
Pierce, W. S. et al. An electric artificial heart for clinical use. Ann. Surg. 212 , 339–343 (1990).
Snyder, A. J. et al. In vivo testing of a completely implanted total artificial heart system. ASAIO J. 39 , M177–M184 (1993).
Shaffer, L. J. et al. Total artificial heart implantation in calves with pump on an angled port design. Trans. Am. Soc. Artif. Intern. Organs 25 , 254–259 (1979).
Abe, Y. et al. Development of mechanical circulatory support devices at the University of Tokyo. J. Artif. Organs 10 , 60–70 (2007).
Mochizuki, S. et al. Results of animal experiments using an undulation pump total artificial heart: analysis of 10 day and 19 day survival. ASAIO J. 46 , 500–504 (2000).
Wampler, R. et al. Performance of a novel shuttling total artificial heart on a on a mock circulatory loop. J. Heart Lung Transplant. 36 , S56–S57 (2017).
Cooley, D. The total artificial heart. Nat. Med. 9 , 108–111 (2003).
Emmanuel, S. et al. Anatomical human fitting of the BiVACOR total artificial heart. Artif. Organs 46 , 50–56 (2022).
Mohacsi, P. & Leprince, P. The Carmat total artificial heart. Eur. J. Cardiothorac. Surg. 46 , 933–934 (2014).
Pieper, I. L. et al. Evaluation of the novel total artificial heart Realheart in a pilot human fitting study. Artif. Organs 44 , 174–177 (2020).
Yu, L. S. et al. A compact and noise free electrohydraulic total artificial heart. ASAIO J. 39 , M386–M391 (1993).
Rosenberg, G. et al. Dynamic in vitro and in vivo performance of a permanent total artificial heart. Artif. Organs 22 , 87–94 (1998).
Marieb, E. N. & Hoehn, K. in The Heart 679–681 (Pearson, 2013).
Download references
Acknowledgements
The authors are grateful to L. C. van Laake (AMOLF, Netherlands), D. Zrinscak (Scuola Superiore Sant’Anna, Italy) and A. Henseler (evos GmbH, Germany) for their contributions and discussions; D. van Urk (Amsterdam UMC, The Netherlands) for assisting with screening and selecting articles; T. Azami (Amsterdam UMC, The Netherlands) and C. M. van de Beek (Amsterdam UMC, The Netherlands) for assistance with data extraction; C. E. J. M. Limpens (Amsterdam UMC, The Netherlands) and A. Malekzadeh (Amsterdam UMC, The Netherlands) for their help with the literature search; and ReinHeart TAH GmbH, Germany for providing additional data upon request. The authors’ work is part of the HybridHeart project and is funded by the European Union Horizon 2020 research and innovation programme under grant agreement no. 767195.
Author information
Authors and affiliations.
Cardiothoracic Surgery, Amsterdam UMC location University of Amsterdam, Amsterdam, The Netherlands
Annemijn Vis, Maziar Arfaee, Husain Khambati & Jolanda Kluin
Heart Failure and Arrhythmias, Amsterdam Cardiovascular Sciences, Amsterdam, The Netherlands
Department of Cardiovascular and Thoracic Surgery, University of Louisville, Louisville, KY, USA
Mark S. Slaughter
Department of Thoracic and Cardiovascular Surgery, Heart and Diabetes Center NRW, Ruhr-University Bochum, Bad Oeynhausen, Germany
Jan F. Gummert
Autonomous Matter Department, AMOLF, Amsterdam, The Netherlands
Johannes T. B. Overvelde
Institute for Complex Molecular Systems and Department of Mechanical Engineering, Eindhoven University of Technology, Eindhoven, The Netherlands
You can also search for this author in PubMed Google Scholar
Contributions
A.V. and M.A. did the major literature search and wrote the first draft. All of the authors contributed to the discussion of content and reviewed and edited the manuscript before submission.
Corresponding author
Correspondence to Jolanda Kluin .
Ethics declarations
Competing interests.
A.V., M.A., H.K., J.T.B.O. and J.K. are part of the HybridHeart consortium. The other authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Cardiology thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Percutaneous cable that transmits electrical power from an external driver to the internally implanted device such as a TAH or LVAD.
A wireless power delivery system that uses magnetic fields to transfer power across the skin without the need for direct electrical connectivity.
The physiological passage of oxygenated blood from the aorta to the bronchial circulation. This blood returns directly to the left atrium, thereby bypassing the right side of the heart.
This law states that the stroke volume of the heart increases in response to an increase in the volume of blood in the ventricles before contraction (the end diastolic volume), when all other factors remain constant.
The filling pressure of the ventricle at the end of diastole, which is determined by the atrial pressure.
The stationary part of a rotary machine or device.
Type of FDA approval that allows the investigational device to be used in a clinical study in order to collect safety and efficacy data.
The amount of pressure that the ventricle needs to exert to eject the blood during ventricular contraction.
The modification of a material to add a biological function, such as replace or repair, while at the same time being accepted by the host organism.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Vis, A., Arfaee, M., Khambati, H. et al. The ongoing quest for the first total artificial heart as destination therapy. Nat Rev Cardiol 19 , 813–828 (2022). https://doi.org/10.1038/s41569-022-00723-8
Download citation
Accepted : 09 May 2022
Published : 06 June 2022
Issue Date : December 2022
DOI : https://doi.org/10.1038/s41569-022-00723-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Stem cell therapy, artificial heart or xenotransplantation: what will be new “regenerative” strategies in heart failure during the next decade.
- David M. Smadja
Stem Cell Reviews and Reports (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

- My presentations
Auth with social network:
Download presentation
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you!
Presentation is loading. Please wait.
Artificial Heart Design & Development
Published by Mark Blair Modified over 8 years ago
Similar presentations
Presentation on theme: "Artificial Heart Design & Development"— Presentation transcript:

Presented By : Mahgul Gulzai ( ). Introduction History Types of Artificial Heart Why do we need Artificial Heart Artificial Heart Surgery.

The man-made “bridges” to continued life What and why? man-made devices that act as an organ within the body extends life of patients with organ failures.

Replaces the heart. Commonly used in heart transplants operations. Goal: Permanently replace the heart.
APPLICATIONS AND ADVANCEMENTS IN BIOMECHANICAL ENGINEERING A sub-field of Bio-medical Engineering Riyad Mohammed.

Artificial Heart Valves By: Brian Volpe. What is it? An artificial heart valve is a mechanism that mimics the function of a human heart valve It’s used.

Copyright © 2006 Pearson Education, Inc. publishing as Benjamin Cummings PowerPoint® Lectures Lectures by Chris Romero, Front Range Community College The.

Cardiovascular Disease Figures

Building A Better Heart Second Chance: artificial heart

Vathana Ngeth Biomedical Engineering University of Rhode Island Heart Valves for Congenital Heart Disease Patients.

Skipping the Beat The “Beatless” Heart Tyler BlakeProfessor Ying Sun University of Rhode Island BME 281.

Arteriosclerosis By: Timothy Granter & Megan Heath.

Artificial Heart Valves

Arrhythmia Treatment …which you researched! Coronary Artery Bypass Surgery Angioplasty Valve Replacement Aneurysm Repair Transplant.

Biomedical Engineering By Jacob Carpenter and Brandon Lurie.
CRICOS: 00116K Biomedical Engineer: Design products and procedures that solve medical problems. These include artificial organs, prostheses, instrumentation,

Biomedical Engineering Overview

The Artificial Heart: A Design Example BIOE 1000 October 18, 2001.

By Casey Schuler Advanced Physiology Deoxygenated blood enters right atrium from superior and inferior vena cava Blood is pushed into the right.

DMD Conference McNamara Alumni Center April 10, 2013 Bin He, Ph.D. Director, Institute for Engineering in Medicine.
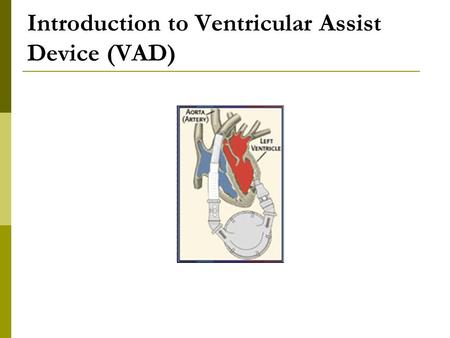
Introduction to Ventricular Assist Device (VAD)
About project
© 2024 SlidePlayer.com Inc. All rights reserved.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Committee to Evaluate the Artificial Heart Program of the National Heart, Lung, and Blood Institute; Hogness JR, VanAntwerp M, editors. The Artificial Heart: Prototypes, Policies, and Patients. Washington (DC): National Academies Press (US); 1991.

The Artificial Heart: Prototypes, Policies, and Patients.
- Hardcopy Version at National Academies Press
10 Conclusions and Recommendations
A S THE PRECEDING CHAPTERS HAVE RELATED , the artificial heart program of the National Heart, Lung, and Blood Institute (NHLBI) is nearing one of its goals, that of developing a fully implantable device that offers indefinite maintenance of cardiac function to individuals suffering from end-stage heart disease. Surgeons will soon implant the first long-term ventricular assist device ( VAD ) that needs no skin-penetrating air hose or wire. It is therefore time, despite many uncertainties, to begin examining how appropriately these devices will be used in health care. Because of their life-or-death implications and high cost, their use poses profound economic, ethical, and clinical questions.
At the request of NHLBI, an Institute of Medicine (IOM) study committee examined the agency's artificial heart program and the devices being developed for long-term use. 1 This document is the committee's report.
Nine questions set forth below, formulated in 1989 by another IOM committee, have guided this evaluation. A number of closely related issues have also been addressed, including government and private-sector roles in research and in the development of new technologies.
To succeed in improving patient care, the goals of the NHLBI artificial heart program and the outputs of its R&D efforts must be compatible with the U.S. health care system, particularly because:
- on a per-patient basis, the mechanical circulatory support systems (MCSSs) being developed will be among the most costly therapeutic devices to date;
- MCSSs may be coming into general use as the health care system is under growing scrutiny for the quality and cost of care and patients' access to it; and
- the heart, an organ that has special cultural symbolism and life-sustaining importance, is involved.
- FOCUS OF THIS STUDY
A 1989 IOM planning committee formulated nine questions to be addressed in this evaluation although, as seen in the preceding chapters, the committee's deliberations went beyond answering the questions. The committee's responses to the questions are very briefly summarized here.
What are the nature and magnitude of the target populations for which MCSSs may be applied? Once a fully implantable MCSS is established to be clinically useful for long-term cardiac support, many more patients will receive one than the current annual volume of 2,000 heart transplant recipients. The number of potential MCSS candidates in the primary group (those most urgently in need of cardiac support) is between 35,000 and 70,000 annually, but practical limits on the growth of this technology's use will hold patient volumes below this range, for perhaps 10 years. In the 2010-2020 period, if device and transplantation outcomes are then similar, potential use may grow substantially beyond this range.
Most of the devices implanted during the first decade of MCSS use will be VADs. As many as 10,000 to 20,000 of each year's primary group of patients have impairment of both of the heart's ventricles, however, and will be candidates to receive a total artificial heart ( TAH ) after these devices become available, likely between 2005 and 2010.
What are the alternative technologies for preventing and treating end-stage heart disease that may affect the need for MCSSs? For the foreseeable future, transplantation will remain the treatment of choice for end-stage heart disease. Current forms of treatment other than transplantation offer most patients only limited benefits, such as relieving symptoms but not significantly prolonging life. Early in the next century, advances in drug therapy may at least be able to delay the onset of end-stage disease for some patients, and perhaps prevent it.
What is the potential for MCSSs to prevent and treat end-stage heart disease, and what are the current technological and other barriers to their development? Almost three decades of research and development have overcome many technological barriers to developing a fully implantable long-term device and have identified areas susceptible to solution by further technological development. Forthcoming clinical trials may clarify the relative importance of those areas needing additional research. Areas that have already been identified for further R&D include potential thromboembolic problems, through-the-skin power transmission, valves, and biomaterials.
What is the clinical effectiveness of MCSSs? The experience to date with temporary-use devices provides reasonable assurance that one or more long-term MCSSs can be proven clinically effective for specific patient groups, probably before the turn of the century. As R&D efforts yield more technological advances, device performance and effectiveness levels (e.g., survival probability) are likely to improve steadily.
What are the projected costs of research and development of MCSSs? NHLBI currently spends about $7 million each year through the contracts that provide most of the support for MCSS development and trials. Another $8 million is spent annually for investigator-initiated grants related to cardiovascular technology. The level of past funding of MCSS contracts has been constraining for MCSS developers; the annual total may need to be slightly greater if NHLBI accepts the committee's recommendations to continue R&D with TAHs, as well as with more than one model of VAD , for an interim period. Not including the costs of the Novacor VAD trial, for which funds are already committed, approximately $2 million per year will be needed for additional VAD development for at least the next two fiscal years, and thereafter a possible additional amount to support further clinical trials. For TAH development, the total amount needed will depend upon findings during the recommended extensions of current contracts. The extensions themselves will likely cost a total of $3 to $6 million per year for a 2- or 3-year period, perhaps to be followed by a total cost of $30 to $90 million (over 10 years) for the two final stages of R&D, as discussed further in Chapter 6 . (All of the foregoing amounts are expressed in 1991 dollars.) A number of NHLBI decisions in future years will have a substantial impact on these funding requirements.
The devices themselves will, in the initial phase of use, cost about $50,000 for a VAD and $100,000 for a TAH , expressed in 1991 dollars. The hospital and physician care required to implant either type will cost about $100,000 in addition. Later costs will depend heavily on the extent to which care is required for complications or for repair or replacement of the device.
What is the cost-effectiveness or cost-benefit of research and development of the various MCSSs, and how does this compare with what is known about the cost-effectiveness or cost-benefit of the research and development of alternative technologies for preventing and treating end-stage heart disease? The cost-effectiveness of long-term TAH use depends on the extent to which a device benefits patients, in particular the quality-adjusted life years ( QALYs ) gained in comparison with outcomes of conventional medical treatment. The TAH's estimated incremental cost-effectiveness, $105,000 per added QALY (in 1991 dollars), is substantially less favorable than for heart transplantation and most other forms of heart disease treatment. If technological developments lead to improved TAHs with life-year gains that approach those of transplantation, cost-effectiveness will improve.
What should the roles of government and industry be with respect to research and development of MCSSs? The NHLBI artificial heart program is unusual among health care R&D in the extent of its government support, in that the government has funded virtually all work to date, including the device development and testing costs usually borne by industry for similar technologies. As discussed later in this chapter and in Chapter 9 , existing circumstances warrant continued NHLBI funding of both VAD and TAH research for at least a two- to three-year period. Further, NHLBI may wish to pursue formal cost-sharing arrangements with industry for some future efforts.
Should decisions concerning further investment in the artificial heart program depend on whether the current cost-effectiveness or cost-benefit findings indicate the technology is acceptable or unacceptable, or are there additional factors that should be taken into account? Cost-effectiveness is one of several factors relevant to the committee's recommendations to NHLBI concerning the artificial heart program. Other factors argue for NHLBI to continue its involvement, at least for an interim period. NHLBI's participation, for instance, may influence the manner in which clinical trials are conducted; also important is studying the quality of life of patients during clinical trials, which might well not occur without NHLBI support. Further, it is important that NHLBI participate in activities such as developing clinical practice guidelines for MCSSs and monitoring posttrial device performance.
How can these findings be used to support decisions on allocation of research funds for artificial heart technologies? All of the topics considered by the committee are relevant to the decisions that NHLBI must make. Because of the risk of unforeseen technological problems, if clinical trials are conducted with only one VAD model, NHLBI funding of other scientifically meritorious VAD development efforts should continue for a period of at least two to three years. NHLBI support for the development of TAHs should also continue for the same interim period until the early results of long-term VAD trials are available, because of the potential future benefit to patients from TAHs, and because information from those trials will help in future decisions about TAH development. NHLBI decisions about allocating R&D funds should be aided by the use of cost-effectiveness analysis ( CEA ) and other explicit criteria, as described in Chapter 3 and Chapter 6 .
Other topics. The committee has also reviewed a number of other areas related to MCSS development and use. The recommendations in this chapter cover such topics as promoting the appropriateness of MCSS use, deciding about patient access to this technology, and avoiding a possible adverse impact of industry support for academic R&D on open, collegial communication among researchers.
- PROMOTING APPROPRIATE USE OF MECHANICAL CIRCULATORY SUPPORT DEVICES
The committee is very concerned about possible inappropriate MCSS use, similar to concerns expressed over the years about other new technologies. The proper response is not to terminate federal support for R&D in this area. Rather, the decade or more that will be needed to complete MCSS development offers an unusual opportunity for all interested parties to deal positively with this concern.
Substantial variations in rates of use of many technologies, as well as in other physician practice patterns, indicate that either overuse or underuse occurs regularly; both possibilities raise questions about the effects of such patterns on the quality of patient care. Further, the overuse of technology continues to be blamed for much of the steady increase in the cost of health care. Many regulatory and educational measures that once seemed promising as means of achieving appropriate technology diffusion and use have proven insufficient for the task. With MCSSs, crucial activities can occur before these devices go into general use, to improve the likelihood that they will be applied appropriately.
The 1990s provide an opportunity to improve the prospects for appropriate MCSS use, once their development and clinical trials are completed, and activities with that goal should be undertaken. The committee recommends continued federal support for MCSS development, based on an assumption that this will be done. Among the relevant activities that can begin immediately are the development of provisional clinical practice guidelines (indications for use) 2 and of guidelines for the institutional resources and staff expertise necessary for appropriate MCSS use, as well as the initiation of selective coverage programs (e.g., third-party payers approving coverage for MCSS use only when the device is implanted at designated institutions).
Participants in developing and implementing measures to enhance the appropriateness of MCSS use should include: the researchers developing these devices; cardiothoracic surgeons, cardiologists, and other physicians; allied health professionals; representatives of the Medicare program and other third-party payers; and others such as health services researchers, economists, and ethicists. Advice and consultation should be sought from patients who have experienced MCSS use and from family members and others who have cared for them. Further, because of their in-depth knowledge of this technology, NHLBI personnel should be intimately involved in these activities, in particular to design evaluative studies of MCSS use.
Efforts to enhance the appropriateness of MCSS use will fit well with current trends to develop clinical practice guidelines and to tighten the granting of practitioner privileges to use particular technologies. Additional studies of these devices' long-term outcomes will be an important basis for the efforts to enhance appropriateness of use that should continue indefinitely. Because of this technology's cost and visibility and because clinical studies and technology assessments of MCSS use will be important in these and other efforts, the Public Health Service's Agency for Health Care Policy and Research (AHCPR) might give such studies a special priority. Considering the foregoing, the committee recommends the following:
- MCSS developers, physicians, and others should work together during the 1990s to establish clinical practice guidelines and other measures to promote the likelihood of appropriate MCSS use.
- Recognizing the cost of this technology, adequate federal and private research funds should be devoted to clinical studies and technology assessments of MCSS use.
- Staff of the NHLBI artificial heart program should participate in these appropriateness-enhancing activities and work with AHCPR to develop support for the needed studies.
- ACCESS BY ALL TO CIRCULATORY SUPPORT DEVICES?
The health care community and state and federal governments may or may not take action in the years ahead to assure equitable access, either to health care generally or to specific forms of treatment, for the 30 million or more people in the United States who have no insurance or are inadequately insured. The committee has not considered itself charged to break new ground on the issue of access to health care; see discussions of access to care such as that of the President's Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research (1983). The committee would, however, be reluctant to recommend continued federal support for MCSS development, if it believed these devices would be available only to those with personal resources or adequate insurance coverage.
It is also beyond the scope of this committee's charge to decide, or even to recommend, whether an MCSS should be part of a basic level of health care that is applicable nationwide, or whether the federal government or states should provide universal access to MCSSs or to an overall basic level of care. Nonetheless, as with the appropriateness issue, the nation's health care policymakers could and should use the emergence of this new technology during the 1990s as an opportunity to face the access issue squarely and examine it in depth before MCSSs come into general use. The time is ripe for the United States to make clear decisions about access to health care, including costly new technologies. A conscious policy decision about MCSS access would also minimize future claims that products of government-sponsored research should automatically become a public entitlement.
Access via Third-Party Payers
As an alternative to universal access either to MCSSs for a clinically defined population or to a basic level of services, partial but more uniform access could be achieved via third-party payers. Many state legislatures have mandated specific benefits to be provided by all private health insurers in their states; such measures, although sometimes controversial, have proven to be more feasible at the state than the federal level.
If no legislative mandate for MCSS coverage exists, third-party payers have a strong incentive to deny coverage under even the most generous benefit packages, because the high per-patient cost will result in a very high aggregate cost if all those who need MCSSs are able to obtain them. Medicare and state Medicaid programs are likely to be more reluctant, even, than private insurers to approve MCSS coverage.
The nature of the health care gains provided by an MCSS presents a strong counter-argument. If these devices are proven clinically effective, it will be difficult for either private or public third-party payers to assert that they are properly a “second tier” technology that can be denied to identified patients. There remains, however, a possibility that long-term MCSSs will, in fact, become available only to those who are able to pay for them through special insurance coverage or with private funds.
Clinically Limited Access
At least in the early stages of MCSS diffusion, it will be imperative to limit access on clinical grounds, much as the number of heart transplantation candidates is limited by stringent approval criteria for placement on the transplant waiting list. If provisional indications for long-term MCSS use can appropriately be restricted on specific clinical grounds to a relatively small group of candidates, third-party payers may be more comfortable in approving such a limited degree of MCSS coverage and payment.
This approach must recognize, however, that narrow indications for use are likely to be temporary, considering the evolution of indications for technologies such as percutaneous transluminal coronary angioplasty, as additional clinical experience was gained. Limited access to heart transplantation is warranted by the small number of available donors; with a manufactured device, no such curbs will occur on the supply side. Furthermore, those developing any restrictive indications for use and the clinicians who apply them to individual patients must take great care that they do not favor a particular demographic group (e.g., middle-class white males) disproportionately to disease incidence.
Limiting Access by Inadequate Payments
As discussed in Chapter 9 , the Medicare program, in particular, sometimes approves coverage of a new technology but then establishes a payment rate for its use that is substantially below the cost of providing the service. Paying 50 or 75 percent of costs is an unacceptable way to ration access to technology.
Medicare and other third-party payers should determine coverage policies on clinical and cost-effectiveness grounds. If coverage is approved for a particular clinically defined group of patients, a payment rate should be established that adequately compensates providers for the costs of the service, both direct and indirect.
As discussed more fully in Chapter 8 , the committee has four recommendations concerning access:
- Long-term MCSSs should become an example of a specific technology that, once it qualifies clinically, must be explicitly included or excluded from basic health care coverage as defined for particular insurance programs.
- Legislators and policymakers at state and federal levels should begin now to decide about equitable access to MCSSs and other technologies, establishing commissions or other broadly based groups to aid in making those decisions.
- Below-cost payment rates should not be used to restrict access.
- If access decisions are made on a state-by-state rather than national basis, an adequately funded organization or mechanism should be established to provide information and assistance to such state decision-making processes.
- CLINICAL AND COST-EFFECTIVENESS
It is clear to the committee that the years of substantial federal commitments to artificial heart R&D will soon lead to clinically useful long-term devices that are adequate to prolong life and restore function for thousands of end-stage heart disease patients, as discussed more fully in Chapter 2 . Clinical trials will first establish whether these devices are efficacious. Later clinical use and patient follow-up studies will be needed to identify more details of their effectiveness, as these terms are differentiated (see Chapter 3 ).
Given the health care environment that now exists and will continue for the foreseeable future, scrutiny of the care that is delivered will become increasingly stringent. Attention to the assessment and assurance of quality in the provision of care is at an all-time high. Costs are receiving an equal degree of review but, in its consideration of the future role of MCSSs, the committee is particularly concerned that increases in aggregate health care costs resulting from MCSS use are matched by patient-care gains.
Clinical Effectiveness
It is premature to judge precisely when a clinically effective long-term VAD will be approved for general marketing, but such an approval appears likely to occur before the end of the century. A fully implantable TAH will likely follow about 10 years later, depending on future NHLBI decisions about funding of TAH development.
The review mechanisms of NHLBI and the Food and Drug Administration (FDA), coupled with the efforts of those developing the devices and conducting their clinical trials, will provide reasonable assurance that devices coming into general use are efficacious, based on evidence from the clinical trials. Data will need to be obtained from a registry and from follow-up studies to establish their long-term clinical effectiveness. Continuing attention to assessing patient gains from implanting these devices will be important as their volume of use grows.
Quality of Life
No comprehensive assessment of a technology's clinical effectiveness can be complete without carefully examining its impact on patients' quality of life, because therapeutic interventions may have similar physiological effectiveness but still differ in terms of quality-of-life outcomes of patients. Quality-of-life measures can be especially important in assessing the outcomes of patients receiving therapies that carry high risks of negative consequences.
NHLBI is, perhaps, preeminent among others of the National Institutes of Health (NIH) in focusing attention on this essential component of patient outcomes; the committee is gratified to note NHLBI's broad quality-of-life initiative, not only in the artificial heart program but in other aspects of its research. Assessment of patients' postimplantation quality of life is a component of the current NHLBI-sponsored VAD clinical trial.
Irrespective of the degree and direction of change in any one or more quality-of-life domains (e.g., social functioning might improve and self-care might deteriorate for a patient, over time), three general health state outcomes are possible from a treatment. The determination of whether improvement, deterioration, or “no change” has occurred in health status is subjective, depending on the weights and values that are assigned by the individual making the trade-offs among the various states described. The results or products of trade-offs can be converted to quantitative values, namely preference weights (utilities), for particular health states; in the committee's cost-effectiveness analysis ( CEA ), these weights form the basis for calculating quality-adjusted life years ( QALYs ). All NHLBI-sponsored research and clinical trials that include cost-benefit analyses or CEAs should include preference-weighted or utility measures.
Further work is needed in quality-of-life instrument development and validation to determine which domains are important to particular patient populations and, within domains, which components are sensitive to burdens perceived by patients. In addition to the more traditional core set of domains used in assessing health states, the study committee identified three health-related quality-of-life domains of potential significance to patients considering or having received an MCSS —machine dependence and societal reaction, the meaning and purpose of life, and spiritual well-being.
To the extent that policymakers judge it to be in the public interest to use public resources to develop MCSSs, those decisions should include commitments to support comprehensive assessments of health-related quality of life in patients receiving MCSSs during clinical trials and even thereafter. A core set of quality-of-life domains, similar to those used for this study's utility measures, should be assessed in all MCSS clinical trials. More research is needed to identify and understand support systems and selected other determinants of health-related quality of life that might be helpful in identifying those patients, among the groups meeting certain clinical conditions, who are more likely to benefit from an MCSS.
Cost-Effectiveness
The cost of health care and its clinical effectiveness, including quality-of-life gains, can be related to one another and assessed in various ways; the most highly quantified are CEA and cost-benefit analysis. As described in Chapter 6 , the committee has used CEA methods to examine both TAH clinical effectiveness and the funding of TAH development. In clinical studies, CEA yields a cost-effectiveness (C/E) ratio that expresses the incremental cost of each QALY gained from using the technology studied, compared with one or more alternate forms of treatment.
The committee's CEA examined the projected cost-effectiveness of TAH use and heart transplantation, comparing both with conventional medical treatment for end-stage heart disease as of 2010, the earliest that TAHs are likely to be in routine use. This required making a number of estimates of such aspects of TAH use as device failure rates and the probability of various TAH-related clinical complications, as well as lengths of hospital stays and outcomes of such events. The estimates developed for the CEA may well be more conservative than occurs when a current technology is examined, because of uncertainty about the outcome of use of these devices 20 years in the future.
From the committee's analysis, the estimated relative cost-effectiveness of using a TAH instead of medical treatment is about $105,000 per added QALY (in 1991 dollars), a C/E ratio that is considerably less favorable than those for other generally acceptable forms of heart disease treatment, such as $32,000 per QALY for heart transplantation and $34,000 per QALY for coronary artery bypass surgery for two-vessel disease with severe angina. The C/E ratio for TAH use is also less favorable than C/E ratios for other costly interventions (e.g., renal dialysis).
The committee's CEA examined only TAH use, the primary focus of the study. Because of the lower device cost and the fact that VAD failures may cause death less often than do TAH failures, the C/E ratio for long-term VADs may be somewhat more favorable than for TAHs. Further, results of the Novacor VAD clinical trial, other VAD use during the 1990s, and continuing technological gains will likely, over time, form a basis for improved C/E ratios for both types of MCSS .
Cost-Effectiveness in Research and Development Decisions
Cost-effectiveness analysis can also be applied to the consideration of research and development options. The committee used CEA to examine alternative levels of R&D investment during the next phase of TAH development. Based on assumptions of the impact of increased funding on the length of time required to complete R&D and on the future selling price of TAHs, this CEA reveals that increased R&D funding may be beneficial, assuming that the basic $105,000-per-QALY C/E ratio of TAH use is considered acceptable. If performed with care, CEA can make a useful contribution to decision making about R&D activities.
- MAKING IMPORTANT DECISIONS
The committee has reviewed the ways in which NHLBI decides about the allocation of extramural research funds. It has also examined how decisions in the regulatory environment influence the entry of new technologies into the health care system. The committee believes there is room for improvement in both of these areas.
Allocation of Research Funds
The traditional peer-review process, as applied by study sections in their review of research grant applications, remains the best means of assessing the relative scientific merit of specific R&D proposals. Peer review through such mechanisms as the NHLBI Cardiology Advisory Committee can also assist in making some allocation-of-funds decisions.
For many other types of decisions about the allocation of funds among R&D program alternatives, peer review is less useful. In making these decisions, NHLBI essentially uses subjective professional judgment by its executive and administrative personnel. In this respect, it is no different from many other government and private-sector organizations that support academic and industrial research.
The committee has suggested in Chapter 3 and Chapter 6 several approaches that can serve as aids to decision making about the allocation of R&D funds at various levels within NHLBI and other organizations. Two of them involve cost-effectiveness analysis; the committee also developed 18 specific criteria, ranked as to their importance in R&D allocation decisions, that NHLBI can use as a starting point in developing explicit criteria for particular allocation decisions.
The committee recommends that, in addition to its appropriate reliance on the peer-review process, NHLBI consider adopting explicit criteria and more systematic methods such as cost-effectiveness analysis to aid it in allocating research funds among and within R&D programs at various levels.
Regulatory Decisions About Technologies
Several agencies and organizations must consider and approve new medical devices, drugs, and sometimes surgical procedures before they can be widely used. These actions by FDA, the Medicare program, and numerous other third-party payers are the regulatory counterparts to the development of clinical practice guidelines and other measures that help to improve the appropriateness of technology use.
Food and Drug Administration
One important proposition is that FDA, in making decisions about VAD and TAH clinical trials and posttrial applications, apply principles that are consistent with those applied by NHLBI in formulating the particular R&D program, as discussed in Chapter 9 . The current level of cooperation between these two agencies of the Public Health Service is gratifying, and experience is likely to improve the consistency of their actions.
The committee recommends that NHLBI and FDA work together to assure that the design and conduct of MCSS clinical trials reflect agreed-upon principles that ensure safe and effective patient care and that facilitate the product approval process.
Third-Party Payers
The high visibility and cost of implanting MCSSs are likely to lead to individual coverage and payment decisions by a number of third-party payers. Additionally, as mentioned in the preceding discussion of access, this technology's high cost (both individually and in the aggregate) may predispose third parties toward denying coverage or establishing payment rates at below-cost levels in order to discourage MCSS use.
Relatively few third-party payers have explicit criteria or standards for their coverage decisions, particularly ones that can be applied to an emerging technology for which data are limited. Clinical practice guidelines may eventually be able to guide third parties in their actions concerning MCSSs, both in developing coverage and payment policies and in acting on pretreatment authorization requests. Indeed, these are likely to be important applications of clinical practice guidelines that are developed for use of these devices.
The committee recommends that third-party payers take into account the clinical evidence derived from MCSS trials and these devices' cost-effectiveness when establishing coverage policies that recognize both the technology's cost and clinical benefit to patients on a provisional basis, subject to later review and modification.
Consistent with the recommendation concerning institutional qualifications for MCSS implantations, the committee encourages third-party payers to consider implementing selective coverage programs as a part of their coverage decisions. In this manner, procedures will be performed only by surgeons and at hospitals that can demonstrate the necessary skills, experience, resources, and record of favorable outcomes. To the extent possible, such programs might be undertaken on a cooperative basis or, as a minimum, using consistent criteria.
- CONTINUING DEVELOPMENT OF TOTAL ARTIFICIAL HEARTS
The committee's principal charge is to advise NHLBI about continuing to fund TAH development. Considerations favoring the continuation of this support include:
- the substantial progress achieved over the artificial heart program's 27 years;
- the number of end-stage heart disease patients—probably at least 10,000 annually—for whom no other form of treatment appears able to prevent disability and death; and
- the likelihood, as explained elsewhere, that this development effort will not move forward without government support.
Offsetting these arguments is the current great uncertainty about virtually every aspect of this technology's likely status early in the next century. We have no hard information about either the clinical effectiveness of these devices or their cost. It is premature, with so little information, to move—with a new five-year phase of R&D—in the direction of routine use of a technology with the potential to add billions of dollars to the nation's health care costs.
Clinically effective performance, including an adequate patient quality of life, will be the principal factor in establishing clinical indications for the use of both TAHs and VADs. Further, clinical effectiveness and cost will be the principal factors in decisions by third-party payers and government policymakers about MCSS access; decisions about indications for use, third-party coverage, and access to MCSSs will combine to determine how many patients receive a device each year.
Based on reasonable but possibly conservative estimates of TAH performance, clinical effectiveness, and costs as of 2010, the committee's analysis found the TAH's C/E ratio to be $105,000 per QALY gained, in 1991 dollars, when compared with conventional medical treatment of end-stage heart disease. This ratio is substantially less favorable than that for heart transplantation and other generally accepted forms of treatment for heart disease, as well as for other catastrophic diseases.
A C/E ratio of this magnitude could be interpreted as reason to suspend or terminate all work on MCSS development, on the theory that the health care system cannot afford such a costly technology regardless of its potential to prevent death and disability for many thousands of patients each year. The committee considered this question carefully and is not recommending blindly moving ahead with a major commitment to the next five-year phase of TAH development.
The committee believes, instead, that the TAH 's currently estimated C/E ratio is a reason to exercise great caution in making further commitments. At this stage of development, considerable uncertainty exists about these devices' eventual clinical effectiveness, some of which may be resolved in the next two to five years as results of the Novacor VAD trial and additional temporary MCSS use become available. The committee thus recommends that NHLBI continue its support of TAH development for a two- to three-year period only, with funding thereafter to depend on information developed during the next few years.
In addition to the financial commitment already made to the Novacor VAD trial, the committee estimates that it would cost between $5 and $10 million per year between 1993 and 1995 to continue development of TAHs as well as development of at least one additional approach to VAD design, a subject discussed further in the next section. (The precise figure would depend on peer-review evaluation of the developers' proposals to continue R&D and on NHLBI administrative decisions about the funding needed under each contract.) Such a level of expenditure is consistent with the current level of spending on targeted, contractual MCSS development and, in the committee's view, could be achieved with little or no effect on other R&D such as the investigator-initiated (R01) grants also supported by the artificial heart program.
Another consideration in the recommendation to continue R&D support for an interim period is the strong likelihood that, if TAH funding is suspended when the current contracts expire, at least some of the four developers will not be able to obtain private financing to continue their R&D work and the development teams will disband. If findings from VAD clinical trials during the 1990s yield revised performance estimates substantially better than those we can make at present but development teams have been disbanded, it would take years to resume TAH development and regain the current momentum.
NHLBI and other organizations regularly commit funds to R&D ventures with varying certainties of outcome; no reason exists to demand guaranteed success from the artificial heart program. Uncertainty admittedly exists about the ultimate TAH C/E ratio and its consequences, but moving ahead cautiously with additional TAH development during 1993-1995 offers the possibility of substantial benefits to patients who might otherwise die if TAH development is delayed by a suspension of funding. Because the needed $5 to $10 million per year is a relatively small amount, the committee believes the possible gain in future patient benefits outweighs the opposing uncertainty.
A final consideration relates directly to the TAH 's C/E ratio. It is a reason for exercising caution in making long-term R&D commitments but should not be viewed as an immediate, absolute reason to suspend this R&D support. Society may well be willing to pay $105,000 per added QALY for life-sustaining therapy. If it is, spending a relatively modest amount in the next few years is worthwhile to provide options for patients who may need TAHs early in the next century. The committee itself has no basis on which to place a maximum value on extending life and no authoritative entity exists to answer such questions. If some such entity were to decide that even $60,000 or $70,000 per QALY is too great a cost, then it would be reasonable to suspend both TAH development and R&D on new approaches to VADs until the current VAD development efforts yield MCSS clinical effectiveness data that are sufficiently improved to reduce the C/E ratio below $60,000.
The committee thus concludes that continuing NHLBI support of TAH development, for a limited period of two to three years beyond the current contracts' expiration in September 1993, is in the public interest because of its possible future benefit to patients and the additional information to be developed during that time. The developers whose work is to receive continued funding should be chosen through peer review of the scientific merit of progress to date and their proposed continuing efforts.
Between now and the end of the interim period of R&D funding, the developers should also be requested to specifically study and report to NHLBI on the possible impact of various levels of future support on (1) the duration of the preclinical or device readiness testing phase and thus device availability, and (2) the ultimate selling price of the device, once it goes into manufacture. The committee understands that somewhat larger contract budgets than have been customary could have an impact in both of these respects; the CEA it performed to test the effects of such scenarios established that more funding for certain stages of R&D might be a beneficial investment.
During this same period, NHLBI and the various interested sectors of the health care community also should begin to take action on this committee's general recommendations about appropriateness of use and access to care. It is important that this work, which is generic to both TAHs and VADs, proceed immediately, because the development of long-term VADs is likely to be completed several years sooner than that of TAHs.
Considerations for the Next Decision Point
Based on the information developed between 1991 and 1995, NHLBI will then have to make a decision about the next phase of TAH development. Information from long-term VAD clinical trials and continuing use of temporary MCSSs will be critical in confirming or revising the various estimates used in our CEA . Clinical findings about a single VAD's ability to sustain cardiac function despite biventricular failure also will be helpful in determining the extent of the need for long-term TAH use; the need may be small enough to be met by transplantation, within the available supply of donor organs. As additional preparation for the next decision point on TAH development, NHLBI also should examine systematically the likely impact on future MCSS demand of several of the alternative forms of treatment discussed in this report. What are the prospects, and timetable, for the development of drugs that effectively prevent or delay end-stage disease? To what extent are cardiomyoplasty or other less drastic alternative forms of long-term cardiac support likely to reduce the need for costly implantable devices? What are the prospects for immunologic gains making human xenografts (heterografts) of animal hearts possible, and how will this affect the need for MCSSs? All are relevant questions.
Another possible consideration for NHLBI involves international activity concerning MCSS development. Decisions about continuing to develop TAHs or VADs may have international-trade or balance-of-payments implications, but the extent to which NHLBI should consider such implications in its decisions about R&D programs is debatable. The committee believes that potential benefits to patients should be the primary basis for federal support of health care R&D, with MCSSs or other technologies. If successful development of MCSSs leads to substantial exporting of these devices and thus a balance-of-trade gain, so much the better.
By assessing all of this information against explicit criteria such as those recommended in Chapter 3 , NHLBI will be able to expand on this committee's work and make a sound decision about the next full-scale stage of TAH development. If the decision is made to proceed, the information reported by TAH developers about the impact of increased R&D funding can be used to fix the funding level that is most appropriate and, as well, can be reflected in contractual provisions for the next phase of R&D so as to provide incentives for the developers to accomplish those objectives.
Both the committee's recommendations and any NHLBI action consistent with them should be understood by everyone involved not to imply a long-term commitment to TAH development. If clinical performance estimates do not improve based on information developed during the interim period, NHLBI's proper course in 1994 or 1995 may well be to suspend all support for TAH development until further VAD experience has been gained. A much broader range of sensitivity analyses than this study's resources allowed will also be helpful in testing the impact of the estimates developed for NHLBI's next decision.
The committee concludes that NHLBI should continue to support scientifically meritorious TAH development for a two- to three-year period until preliminary results of the Novacor VAD trial (and perhaps other trials) are known. Based on the important assumption that the broader concerns previously discussed—appropriate use and access—are being addressed concurrently, the committee has a three-part recommendation:
- NHLBI should continue to support the four current TAH developers, to the extent warranted by the scientific merit of their work, for a two- to three-year period following the current contract expirations in September 1993 and at a level sufficient to ensure that research teams are not disbanded.
- TAH R&D should be reconsidered in 1994 or 1995, taking into account the VAD experience and information about other potential long-term TAH benefits generated in the interim.
- NHLBI should decide at that time whether to move ahead with funding for the next phase of TAH development, after further peer review and a decision-making process that takes into account a then-current cost-effectiveness projection and other relevant criteria.
By proceeding in this manner, it may be possible to devote some funds to other uses in the intervening years, additional potential long-term savings and gains can be explored in detail, and the TAH designs that ultimately result can reflect lessons learned from early use of long-term VADs. The committee's cost-effectiveness example, analyzing the impact of increased levels of R&D funding, will be useful to NHLBI in its 1994-1995 TAH decision, as will be the explicit decision-making criteria suggested in Chapter 3 .
- OTHER RECOMMENDATIONS
The preceding recommendations and discussions fulfill the committee's principal obligation to NHLBI, responding to the nine questions set forth in the 1989 report of the IOM planning committee and making recommendations concerning future TAH development. The committee believes that several additional conclusions and recommendations about aspects of MCSS development are also appropriate.
Future Development of Ventricular Assist Devices
The need for a fully implantable, long-term VAD is substantial, and at least one long-term VAD appears reasonably certain to be approved for routine clinical use during the 1990s. Although it will be perhaps 2010 before the technology's use will diffuse sufficiently to meet the need, an annual primary pool of between 35,000 and 70,000 candidates to receive either a VAD or TAH now exists. If continuing development efforts result in a device whose performance approaches transplantation outcomes (now about a 70 percent 5-year survival rate), the size of the candidate pool will increase by perhaps another 200,000 per year, as of about 2020.
Taking full advantage of the potential of VADs is thus a vital goal. In this regard, the committee is concerned about the possibility that development of long-term VADs other than Novacor's may not continue. If NHLBI ends its contractual funding of other VAD developers, the economic uncertainties might preclude private funding of their R&D work and they may suspend it. Therefore, a risk exists in relying on a single approach to VAD development. Further, lower device prices may also result, if more than one VAD is available. (This discussion specifically does not refer to VAD development under investigator-initiated [R01] grants; consideration and funding of such grants should continue via the usual peer-review process, under any circumstances.)
All current models of VADs were essentially designed in the late 1970s; they do not reflect either more recent R&D results or possible new approaches to VAD design that have not yet reached the prototype stage. Knowledge and a range of approaches gained during the 1980s, but not yet applied, may prevent unnecessary delays in developing a clinically useful VAD, particularly if the Novacor VAD encounters unanticipated problems during the forthcoming trial. An end to federal R&D support may also adversely affect the continuity of research by causing the loss of key research personnel to other fields.
Yet another reason for continuing NHLBI involvement in additional VAD research is that it enables the agency to ensure that clinical trials are properly designed and conducted. FDA review of a developer's investigational device exemption application is theoretically sufficient to ensure that all aspects of a trial are properly designed and conducted, including periodic postimplantation assessments of patients' physiologic status and health-related quality of life. In practice, however, the committee recalls the criticisms of past artificial heart trials at Salt Lake City, Louisville, Phoenix, and other locations as having important shortcomings; by continuing its R&D involvement NHLBI can exert strong influence over all aspects of VAD trials.
Finally, because of the relatively large potential volume of long-term VAD use, private-sector interest in this technology will likely increase after one VAD has gone into general use and has received third-party coverage approvals. Commitment of substantial private resources to long-term VADs at their current stage of R&D appears unlikely, however, because of the length of time consumed in the development and regulatory processes and the uncertainty about when regulatory approvals will be granted and general use begin.
The committee therefore believes that ending contractual support for all but one VAD developer carries substantial risks that NHLBI may wish to avoid and concludes that continued support of other scientifically meritorious VAD development is desirable, for at least a two- to three-year period. This could be done either by implementing a new peer-reviewed initiative such as a request for proposals to design a state-of-the-art implantable VAD or by continuing to support, at least temporarily, one or more VAD developers whose approaches differ from the current Novacor model.
After preliminary postdischarge follow-up results for the early patients in the forthcoming VAD trial are available, perhaps in 1993, NHLBI will be able to decide with greater certainty about the need for continuing support of VAD development.
The committee therefore recommends that, in addition to the Novacor clinical trial, NHLBI consider the continuation of targeted R&D support for other VAD development for at least a period ending in late 1993 or 1994, with further support to be considered at that time.
Clinical Trials and Patient Follow-up
The committee has several concerns in the area of MCSS clinical trials and ongoing patient follow-up.
Clinical Trials
In order to determine the continuing role of MCSSs, three types of data should be routinely collected in clinical trials: (1) physiologic data (e.g., cardiovascular, renal, neurologic, hematologic), obtained periodically throughout the trial period; (2) health-related quality-of-life data, using core sets of test questions that tap both generic and condition- or treatment-specific domains; and (3) data about the clinical outcome and cost of treatment so that reports of the trial can include cost-effectiveness analysis. Funding of all trials, including that of the Novacor VAD , should be adequate to ensure that all aspects of device use, and their implications, can be addressed by those conducting the trial. Trial design and implementation should also ensure that study populations include adequate representation of women and minority populations.
It is important that MCSS trials, whether supported by public or private funds, make provision for each patient to provide a fully informed consent, an advance directive concerning termination of treatment, and legal designation of a proxy; see the review of these ethical safeguards in Chapter 8 .
The committee thus recommends that all NHLBI-supported and private MCSS clinical trials include adequate funding for the collection of physiologic, health-related quality-of-life, and cost-effectiveness data and, as well, include provisions protecting patients' ethical rights, such as a requirement for written advance directives and, if allowed by state law, designation of a proxy decision maker. Trial protocols should ensure adequate representation of women and minorities and should provide for comparability with other studies by using, for instance, a standard core set of quality-of-life domains and assessment methods.
Postmarketing Surveillance
All patients receiving a long-term MCSS should be followed for the remainder of their lives, at least to the extent of obtaining rudimentary survival information on a periodic basis. An MCSS implantation is not a simple, time-limited treatment episode. This type of lifetime follow-up can best be accomplished through a registry that receives information from clinicians who implant devices and patients who receive them. If public and quasi-public funds, the latter provided via health insurers, are to support the development and use of this costly technology, special measures are warranted to oversee its long-term clinical effectiveness.
The committee recommends that long-term MCSS patients and their physicians provide lifelong follow-up information to a registry, and that hospitals, stimulated by requirements of third-party payers and their payments for this component of care, fund such a registry as an integral part of providing the care.
Posttrial Follow-up
The two-year follow-up period that is typical of clinical trials of implanted devices is not long enough to reveal all problems that may occur with MCSS use. In addition to any registry that is established, an adequate sample of patients receiving MCSSs during clinical trials should be followed periodically after the trial period ends, to ascertain and study details of posttrial patient management and outcomes. This could be accomplished by various means. For subjects in clinical trials sponsored by NHLBI, that agency should fund follow-up studies of either all patients or a sample. If such a study is to be undertaken by a device developer, such as to fulfill FDA postmarketing surveillance requirements, provisions should be included to ensure that results of the study will be published promptly, regardless of outcome.
For patients receiving an MCSS after it is FDA-approved, AHCPR should fund one or more long-term follow-up studies of an adequate sample of patients, to be performed by academic or independent researchers. In all instances, funding should be sufficient for the study to gather and report data about patients' physiologic status, health-related quality of life, and treatment costs.
The committee therefore recommends that NHLBI and AHCPR support long-term follow-up studies of an adequate sample of all patients receiving MCSSs and fund such studies adequately.

Fostering Collaborative Interdisciplinary Research
As previously mentioned, the artificial heart program, with its targeted, largely developmental R&D effort, is unusual not only within NHLBI but also in NIH as a whole. The policies and mechanisms by which the artificial heart program operates were originally developed to review, approve, and manage traditional investigator-initiated, nontargeted research by basic scientists, not the development of complex devices and support of their extensive technical evaluation, redesign, preclinical testing, and clinical trials.
Additionally, although traditional scientific peer review is as important to MCSS research as it is to any other field, considering artificial heart program proposals in the existing peer-review committees puts this truly interdisciplinary research involving biomedical engineers, clinicians, and others at a serious competitive disadvantage, as discussed in Chapter 9 . Adequate peer review of MCSS R&D requires the involvement of biomedical engineers and professionals with other relevant expertise (e.g., quality-of-life assessment, cost-effectiveness analysis).
The committee recommends that all proposed grants and contracts for MCSS development be peer-reviewed by a group or groups including appropriate numbers of biomedical engineers, clinicians, and professionals from other relevant disciplines.
The organizational structure within which the artificial heart program operates, designed as it is to administer grants to individual investigators, is not as flexible as would be ideal to make collaborative grants that involve both academe and industry. The committee believes that collaborative multidisciplinary research, structured to encourage industry to share in the R&D costs, is an important aspect of government support of private-sector research, consistent with the examples of R&D support by other government agencies (e.g., Department of Defense, National Science Foundation).
The committee recommends that NHLBI amplify its attention to alternative policies and structures for programs such as the artificial heart program that allow greater flexibility in operating modes and that might serve as models for future efforts to encourage collaborative R&D on a cost-sharing basis, considering the successful experience of other agencies.
Patients' Quality of Life and Treatment Preferences
In many sectors of health care, patients' health-related quality of life has not received adequate attention. For a device such as an MCSS , which patients and their families must learn to live with for an indefinite period, as discussed in Chapter 5 ,
the committee recommends that physicians and hospitals involved in implanting long-term MCSSs develop support programs to enhance the quality of life of MCSS patients and their families during the period of hospitalization and after discharge.
Attention to explicit patient preferences is a growing aspect of medical care. Fully informed patient preferences, or those of a properly designated surrogate decision maker, should be accorded great weight in treatment decisions about implanting an MCSS in the first place as well as withdrawing support at a later time. Thus, as recommended above, protocols for MCSS clinical trials should provide for advance directives and the naming of surrogate decision makers by prospective patients.
Research Needs
Heart failure research.
Whatever NHLBI's decisions about continuing to support MCSS development, that support should not be at the expense of basic and clinical research concerning medical treatment of end-stage heart disease. The borderline cost-effectiveness of TAH use, as well as the costliness and other logistical problems of transplantation, support giving a high priority to research that may ultimately prevent heart failure or delay its onset. The committee therefore concludes that NHLBI should not use the development of MCSSs as a reason to reduce its level of funding of basic heart disease research. Instead,
the committee recommends that NHLBI continue to support, at an adequate level, scientifically meritorious research into the mechanisms of heart failure and approaches to the prevention and medical treatment of end-stage heart disease.
Epidemiological Research
The committee's projections of MCSS use required reliance on numerous sources of epidemiological data about end-stage heart disease, none of which was completely satisfactory. Little is known from an epidemiological perspective about the course of this disease, as discussed in Chapter 4 . Better basic data will make future projections of MCSS use more accurate. The committee therefore applauds NHLBI for supporting the longitudinal studies recently begun; further,
the committee recommends that, in addition to current longitudinal studies of heart disease in persons over age 65, NHLBI undertake as a high priority, and possibly with the involvement of the National Center for Health Statistics, the development of additional epidemiological data about the natural history of end-stage heart disease in patients of all ages, with special attention to the inclusion of women and minority populations.
Communication Among MCSS Researchers
Medical device manufacturers and other private sources of financing are increasingly becoming involved in the support of academic MCSS research and development. The committee encourages this, but it is also concerned that such involvement may have an adverse effect on the open, collegial communication among researchers that has been a valuable aspect of MCSS development to date.
The committee recommends that, when undertaking relationships with the private sector, academic researchers work cooperatively to minimize restrictions on their freedom of communication with other researchers, and that their universities develop policies consistent with this goal. It further recommends that this topic be explicitly and thoroughly discussed at a meeting involving all researchers and developers in the field.
- HOW THIS REPORT SHOULD BE USED
This study, following six panels that conducted similar evaluations over the past 22 years, has posed a special challenge for the committee: namely, to review all aspects of a relatively small, but nonetheless important and highly visible, segment of federal support of biomedical research and to make projections and recommendations about the future of this unique technology. Faced with the absence of key data in numerous areas, the committee has made estimates that it considers reasonable based on the current evidence.
This study has been able to add to the knowledge base of previous NHLBI studies of the artificial heart program because the technologies involved are so much nearer to clinical use than they were in earlier years. It has also reviewed some aspects of the NHLBI research funding process, a topic not discussed in previous studies.
Most important, any one of the comprehensive studies of the artificial heart that have been performed could serve as a model of the type of study that should become an integral part of the U.S. health care system. Technologies have gone into widespread use with little understanding of their clinical, quality-of-life, ethical, and economic prospects. By and large, patients have been fortunate; most new technologies prove to have clinical value.
Yet technology continues to be blamed for much of the steady rise in health care costs. Comprehensive studies—whether to assess new technologies, guide their diffusion, or measure their impact on costs—have been few in number. It is neither simple nor inexpensive to study complex new technologies, let alone to develop measures that assess and improve the appropriateness of their use.
The committee expresses the hope that leaders in key components of the U.S. health care system, as well as policymakers in the administration, Congress, and state capitals, will consider the recommendations of this study, particularly those that are broadly applicable. The research, development, and diffusion of future technologies require attention to methods and oversight mechanisms that reflect the full range of considerations discussed here. These conclusions and recommendations can, if applied both to MCSSs and to other new technologies, improve the ways in which the nation's health care providers and regulators deal with them, as well as the ways in which those who support health care R&D manage the innovative research that yields so many health care gains.
- President's Commission for the Study of Ethical Problems in Medicine and Bio-medical and Behavioral Research . 1983. Securing Access to Care. Vol. 1. Report on the Ethical Implications of Differences in the Availability of Health Services. Washington, D.C.: U.S. Government Printing Office.
This discussion and the report as a whole do not consider devices that are intended only for temporary cardiac support, as they were not a focus of the study.
In this report, the term “clinical practice guidelines” should be construed as including the closely analogous medical review criteria that will be developed from such guidelines and used by third-party payers in acting on preauthorization and payment requests, as well as in posttreatment reviews of care such as those performed by peer review organizations.
- Cite this Page Institute of Medicine (US) Committee to Evaluate the Artificial Heart Program of the National Heart, Lung, and Blood Institute; Hogness JR, VanAntwerp M, editors. The Artificial Heart: Prototypes, Policies, and Patients. Washington (DC): National Academies Press (US); 1991. 10, Conclusions and Recommendations.
- PDF version of this title (2.9M)
- Disable Glossary Links
In this Page
Other titles in this collection.
- The National Academies Collection: Reports funded by National Institutes of Health
Recent Activity
- Conclusions and Recommendations - The Artificial Heart Conclusions and Recommendations - The Artificial Heart
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Artificial Pacemaker: The Heart
Aug 28, 2014
210 likes | 597 Views
Artificial Pacemaker: The Heart. Kaitlin Abbate. The Healthy Heart. Natural Pacemaker: Sinoatrial Node (SA node) Located in the right atrium Sends electric impulses from atriums to ventricles which causes them to contract. Contractions are what allows blood to flow
Share Presentation
- health howstuffworks
- heart block
- abnormal heart rhythms
- london cardiac institute

Presentation Transcript
Artificial Pacemaker:The Heart Kaitlin Abbate
The Healthy Heart • Natural Pacemaker: Sinoatrial Node (SA node) • Located in the right atrium • Sends electric impulses from atriums to ventricles which causes them to contract
Contractions are what allows blood to flow What happens when SA node does not work properly? Blood cannot flow like it should, so we need a substitute…
The Artificial Pacemaker! • A small device placed in the chest to control abnormal heart rhythms • Uses a computer memory to generate electronic signals at the right pace • Doctors pre-determine the “right pace” and it can be adjusted at any time
Who needs a pacemaker? • Atrial Fibrillation: heart beats very rapidly and chaotically • Not life threatening itself but may lead to stroke or chest pain • Bradycardia: heart beats too slowly • Caused by age or heart block • Heart Blockthe electrical signal is slowed or disrupted as it moves through chambers of heart • Caused by a heart attack or muscular dystrophy
Anyone else? Yes! • Syncope: “the common faint” • Heart rate sometimes becomes very slow • Heart Failure: heart cannot supply enough blood to meet body’s needs • Caused by heart disease, hypertension, cardiomyopathy
What does a pacemaker look like? Composed of TWO main parts Generator (battery) Leads (wires)
The Generator • Acts as a very small computer • Sends out sparks of electricity that trigger the heart to beat • Also records heart’s electrical activity and heart rhythm so doctors can better adjust the pacemaker. • This way, each pacemaker works according to individual needs
The Leads • Wires threaded through veins and attached to heart muscle, carrying the impulses • Single Chamber: one lead in ventricle OR atria • Dual Chamber: one lead in ventricle AND atria • Biventricular: one lead in atria and one lead in EACH ventricle
Single Chambered Dual Chambered
The Surgery Overview • Requires MINOR surgery • Takes 2-3 hours • Generally, NOT put to sleep • However, most children are for obvious reasons • Stay in hospital overnight • Return to normal activities a few days after surgery
The Actual Procedure • Inject local numbing medication • Put needle in a large vein in shoulder • Thread the leads of pacemaker through the needle and into correct places in heart muscle • Cut a small hole into skin of chest and place pacemaker battery just under skin • Connect the wires to the battery and test • Sew ‘em up!
Located just under the skin…
Life Post-Pacemaker • Electrical Devices can interfere! • Cell phones, ipods • Microwaves • Metal detectors • Electrical generators • However, only long periods of contact really have an impact • Also, this danger is lowered with newer devices
Limited Physical Activity? • Not really! One can pretty much participate in sports, and even strenuous exercises • Still, ask your doctor though, cases may vary • However, full contact sports should always be avoided • do not want to disrupt the battery or move any wires • BAD
Pacemaker Maintenance • Pacemakers do not last forever • Battery actually last between 5-15 years with 6-8 years being average • Wires usually last a little longer • All depends on how active the pacemaker is • Regular check-ups with doctor are a must to make sure the battery/wires are up to par
Pacemakers for Pets! • Used for the same reasons and same types • Usually less expensive • Pacemakers used are actually ones that power source is below acceptable limits for people • Implanted generally the same way • Except most wires inserted through jugular vein rather than shoulder vein simply because bodies differ • Animals that have been able to accept pacemakers so far: • Dogs, cats • Cows, bulls • Horses • Sheep, goats
References/Picture Sources • Artificial Pacemaker -." Wikipedia, the Free Encyclopedia. Wikipedia, 20 Mar. 2010. Web. 21 Mar. 2010. <http://en.wikipedia.org/wiki/Artificial_pacemaker>. • "HowStuffWorks "Chambers and Valves"" Howstuffworks "Health" Discovery, 1998. Web. 24 Mar. 2010. <http://health.howstuffworks.com/heart2.htm>. • "Living with a Pacemaker." National Heart, Lung and Blood Institute. Web. 25 Mar. 2010. <http://www.nhlbi.nih.gov/health/dci/Diseases/pace/pace_lifestyle.html>. • "Pacemakers." Pets With Pacemakers - Info and Support for Pet Pacemaker Recipients. Web. 25 Mar. 2010. <http://www.petswithpacemakers.org/index.php?option=com_content&view=section&layout=blog&id=12&Itemid=119>. • "Pacemakers." Welcome to the London Cardiac Institute. London Cardiac Institute. Web. 22 Mar. 2010. <http://londoncardiac.ca/pages/pacer.htm>. • "Pacemakers and Abnormal Heart Rhythms." WebMD - Better Information. Better Health. Web. 25 Mar. 2010. <http://www.webmd.com/heart-disease/abnormal-rhythyms-pacemaker>. • <http://biomed.brown.edu/Courses/BI108/BI108_2008_Groups/group10/img/pacemaker/2.jpg> • <http://www.blogcdn.com/www.switched.com/media/2008/08/799205_96399001.jpg> • <http://upload.wikimedia.org/wikipedia/commons/8/82/St_Jude_Medical_pacemaker_in_hand.jpg> • <http://www.southwestlondoncardiacnetwork.nhs.uk/Images/pacemaker_cartoon.jpg>
- More by User

What part of the heart is referred to as the pacemaker of the heart? ... Electro-cardiogram. Hypertrophic Cardiomyopathy. Autopsy Reveals Cause of Collier Death. By ...
614 views • 35 slides

The Artificial Heart: A Design Example
The Artificial Heart: A Design Example. BIOE 1000 October 18, 2001. The Human Heart. Heart has four chambers Right chambers pump blood to lungs to receive oxygen Left chambers pump oxygenated blood from lungs to rest of the body. The Human Heart. Right and left atria receive blood
703 views • 17 slides

Pacemakers and Implantable Cardioverter Defibrillators
Temporary and Permanent Cardiac Pacing. IntroductionTemporary pacing : Indications, TechniquePermananent Pacing : Pacemaker Nomenclature Indications Selection of Pacing Mode Pacing for Hemodynamic Improvement Pacemaker Implantation, Complications Pacemaker Troubleshooting Implantable Cardioverter Defibrillator .
1.64k views • 49 slides

ENTC 4350. Pacemakers. A pacemaker is a prosthetic device for the heart, first conceived in 1932 by Albert S. Hymen, an American cardiologist. In 1952 the pacemaker was used clinically by Paul M. Zoll as an external device. .
988 views • 68 slides

Chapter 18. The Heart. Heart Overview. Heart anatomy Cardiac muscle cells Heart chambers, valves and vessels Conducting system EKG Cardiac cycle Contractile and pacemaker cells Cardiodynamics Cardiac disorders. Organization of the Cardiovascular System. Figure 20–1. Heart.
1.94k views • 117 slides

Pacemaker Implantation
Pacemaker Implantation. Pacemaker Implantation. Pre-implantation Clinical assessment of patient Age Symptons Underlying cardiac rhythm Underlying medical state Size Evaluation of daily living activities Future needs. Pacemaker Implantation. Temporary pacing Access - Subclavian
4.53k views • 15 slides

Regulating Mechanisms
Regulating Mechanisms. Homeostasis. Homeostasis. This is the regulation of the body’s internal environment within tolerable limits despite changes in the external environment. Negative Feedback Control. Control of Heart Rate. The pacemaker initiates each heart beat.
390 views • 29 slides

The heart is is myogenic
Outline the control of the heart beat in terms of myogenic muscle contraction, the role of the pacemaker, nerves, the medulla of the brain and adrenalin (epinephrine). . The heart is is myogenic.
424 views • 16 slides

Guarding The Heart
Guarding The Heart. Heart idioms. At heart Break one’s heart By heart Change of heart Do one’s h. good Eat one’s h. out Have a heart Have h. in…mouth Heart and soul. Heart in right place In heart of hearts Lose one’s heart Near one’s heart Set heart at rest Take heart
755 views • 16 slides

Trends In Healthcare :
Trends In Healthcare :. The Use Of Artificial Heart Devices To Treat Heart Disease Presented By: Justine Lighthall. Objectives. Describe different device types of devices Define the types of therapy the Artificial Heart Device is used for.
382 views • 27 slides

Roger A. Freedman, M.D.
Roger A. Freedman, M.D. Anatomy of the cardiac conduction system Relationship between intracardiac events and ECG Bradyarrhythmias Pacemakers. THE CONDUCTION SYSTEM. Heart Beat Anatomy. The Heart’s ‘Natural Pacemaker’ - 60-100 BPM at rest. SINUS NODE. Sinus Node (SA Node).
833 views • 59 slides

Cardiovascular and Cerebrovascular Diseases
Cardiovascular and Cerebrovascular Diseases. Chapter 12. Physiology of the Cardiovascular System. Cardiovascular system Heart Blood vessels Lymphatics. Conduction System. Specialized conducting tissue Sinoatrial node The pacemaker Initiates electrical contraction in the heart
651 views • 33 slides

Artificial life and artificial intelligence
Artificial life and artificial intelligence. Today’s agenda. Complex Adaptive Systems Artificial intelligence Artificial life Genetic algorithms Foundation Genetic operators GeneIPD. Complexity theory.
732 views • 40 slides

The Bjork-Shiley Heart Valve
The Bjork-Shiley Heart Valve. By Daniel Wec. Brief Overview. the disc to open and close to enable the blood to flow through. The Bjork-Shiley Heart Valve is an artificial heart valve used to deal with defective heart valves or heart valvular diseases.
279 views • 7 slides

The Human Heart
The Human Heart. Lubb-Dubb. The Heart. Made of muscle tissue Heart wall is made of cardiac muscle Contains pacemaker Contains nervous tissue responding to stimuli (leading to increase or decrease in heart rate) Protection of heart Ribs, sternum, spine
338 views • 13 slides

THE HUMAN HEART
THE HUMAN HEART. A JOURNEY THROUGH THE HUMAN HEART Presentation by Samantha Cameron. TABLE OF CONTENTS. The heart itself. Heart rate. Heart valves. Sounds of the heart. Heart at work. Listen to your heart. The heart to the lungs. Techno tools. The heart to the body.
1.31k views • 14 slides

The Streamliner Artificial Heart
The Streamliner Artificial Heart. Brad Paden University of California, Santa Barbara & LaunchPoint Technologies LLC. Outline. LVAD’s for artificial heart assist Background Next generation devices Design & Prototypes Actuators Sensor Control Commercialization.
785 views • 55 slides

BASICS OF PACEMAKER
BASICS OF PACEMAKER. DN. HISTORY. 1958 – Senning and Elmqvist Asynchronous (VVI) pacemaker implanted by thoracotomy and functioned for 3 hours Arne Larsson First pacemaker patient
4.61k views • 120 slides

Pacemaker troubleshooting-single chamber pacemakers
Pacemaker troubleshooting-single chamber pacemakers. Reasons for evaluation. Patient symptomatic Palpitation Syncope presyncope Pacemaker malfunction suspected ECG Telemetry Ambulatory ECG Routine pacemaker follow up. Patient details. Indication for pacing Implant operative note
1.11k views • 63 slides
My 2-year-old daughter needed a pacemaker. That spurred me to engineer a virtual heart, and now customized 3D simulations are saving lives

My daughter was two when she got her first pacemaker. She needed it because of a congenital condition that had reversed the lower chambers of her heart. That resulted in electrical problems—making her heart less efficient at circulating blood and significantly shortening her expected lifespan.
There was little guidance for the doctors managing her condition as it was so rare that there was no way to predict how she would react to treatments. Hearts are complex organs with many moving parts, and each person’s is unique. There are countless ways for unsuspecting problems to arise, especially in children. That means there’s often not enough information available to make data-driven decisions about unusual disorders.
As an engineer, I knew there had to be a solution. I’d seen how advanced three-dimensional models—known as “virtual twins”—help airplane and automotive manufacturers test new, complex machines in digital simulations. I thought, the heart is designed like a machine. It pumps blood in a regular pattern. It runs on electricity. Maybe I could help.
That realization led me to start the Living Heart Project more than a decade ago. While it’s possible to capture the specifics of a person’s heart with medical imaging tools like CT and MRI scans, that imaging is still incomplete.
So we gathered a team of experts to get a better picture. Together, our coalition of engineers and physicians developed a way to create simulations of living people’s hearts—first creating a generic “average” heart, and more recently modeling the hearts of specific individuals, accurate down to the smallest, most singular details.
My daughter’s condition was one in a million, but her predicament was not. Heart disease is consistently the leading U.S. cause of death and accounts for larger medical costs than any other condition. By 2035, the American Heart Association projects that 45% of Americans will have some form of heart disease.
Despite being so commonplace, heart disease is difficult to diagnose because the symptoms are similar to those of other diseases like respiratory ailments. Patients with heart failure are misdiagnosed at a rate of 16% in hospitals and nearly 70% when general practitioners refer patients to specialists, according to a study in the Journal of Cardiac Failure .
Seeing inside the heart
But with a virtual twin involved, the diagnostic picture changes. The twin can be displayed on a screen or in virtual reality as a three-dimensional model that can be enlarged, rotated, and examined by a team of experts. Doctors can “see” inside a patient’s heart , pumping under different conditions to locate problems. They can hear the heart beating and watch the blood and oxygen flow—all without cutting into the patient.
Because virtual twins include the necessary features of each patient’s heart, they allow doctors to design complex reconstructions and even test drugs and devices such as coronary stents safely on the twin. In short, they help determine the best course of treatment by using simulations.
In one case, Dr. David Hoganson at Boston Children’s Hospital encountered a toddler with a perplexing condition: Oxygen in the blood was flowing from the heart to only one of their lungs. Using a virtual twin of the heart, Hoganson and his team identified the problem, designed and tested a surgical procedure, and used it to save the child’s life . They’ve since done hundreds more surgeries using virtual twins.
Virtual twins are also valuable teaching tools, helping students learn about the heart and other organs. They let patients and their families see what’s happening up close, promoting understanding and hope during an otherwise frightening experience. And thanks to artificial intelligence and cloud services, insights from one doctor can inform the work of others across the world.
Today, the possibilities continue to grow. We’re beginning to understand how to simulate other organs, such as lungs and brains . One day, we’ll be able to create virtual twins of our entire bodies, transforming the way that we manage our health. We’re already working with the FDA to build virtual human subjects for early clinical trials, helping deliver treatments to people who need them faster than ever.
From verifying the best location for medical devices to understanding how a new drug will interact with living tissue, a virtual twin enables researchers to conduct testing in days that used to require years of patient trials.
When I started this work, people wondered if I would trust my daughter’s life to this new technology. When she was first diagnosed, doctors had little confidence in their options to keep her heart working. They thought her heart would last just 30 years. She’s now 34, and not slowing down. She’s a pediatric neuroscientist at a top program in the country, where she works to discover treatments for children with brain disorders. Her future is still uncertain, but with a personalized health care plan and her virtual twin, she has the best chance ever.
Virtual twin technology has already improved the lives of countless patients. In the years to come, it will change the world.
Steve Levine, Ph.D., is senior director of virtual human modeling at Dassault Systèmes and founder and executive director of the Living Heart Project.
More must-read commentary published by Fortune :
- Snowflake CEO : Hallucinations are the bane of AI-driven insights. Here’s what search can teach us about trustworthy responses
- We analyzed 46 years of consumer sentiment data–and found that today’s ‘vibecession’ is just men starting to feel as bad about the economy as women historically have
- 90% of homebuyers have historically opted to work with a real estate agent or broker. Here’s why that’s unlikely to change, according to the National Association of Realtors
- Intel CEO : ‘Our goal is to have at least 50% of the world’s advanced semiconductors produced in the U.S. and Europe by the end of the decade’
The opinions expressed in Fortune.com commentary pieces are solely the views of their authors and do not necessarily reflect the opinions and beliefs of Fortune .
Latest in Commentary
- 0 minutes ago

The U.S. and China are leading the world in AI innovation–but the U.K. can punch above its weight. Here’s how

Dow CEO: My company is a major plastic producer. We must end plastic pollution

I’m a 24-year-old small business owner. Here’s why I’m suing the SEC

Norfolk Southern is getting back on track–but activist investor Ancora is trying to derail it in a vicious proxy fight

Healing the workplace means letting ex-employees tell their side of the story without fear of retribution. It’s time to stop signing them into silence

America is mirroring China’s tech protectionism and reversing decades of free trade doctrine. Here’s why that’s bad news for U.S. leadership
Most popular.

Apple loses its spot as the world’s top seller of smartphones after tough China competition from brands like Xiaomi and Huawei

The Marriage Pact, a Stanford economics project that has expanded to nearly 90 colleges, is disrupting dating apps by not focusing on looks

Fashion giant Shein has been slapped with yet another lawsuit alleging copyright infringement, data scraping, and AI to steal art: ‘It’s somewhat shocking that they’ve been able to get away with it’

America is debating whether to raise the retirement age—but boomers are already working well into their sixties and seventies

Gen Z favorite Dr. Martens is struggling as its CEO steps down—and it might be because the shoes last too long

Some ex-TikTok employees say the social media service worked closely with its China-based parent despite claims of independence
Artificial Heart Presentation Template
Download this template as well as our other 21,747 templates for only $99.

Slide: 1 / 20
Artificial Heart Presentation Template Special Features
Make your next PowerPoint, Google Slides, or Keynote presentation more effective with our professionally designed Artificial Heart template. Improve the way you present seminars, webinars and lectures. Make more exciting sales presentations, trade show displays and product promotions, or use them in any educational setting for more impact and greater retention.
Artificial Heart presentation template includes built-in layouts and stunning backgrounds to make your presentation a winner. The attention we pay to the finest detail make this presentation template truly world-class.
We take pride in employing features most companies skip because it's "too much work." This Artificial Heart presentation theme is carefully considered and implemented to maximize the beauty, consistency, clarity and -- most importantly -- audience impact of your presentation.
Intuitive and Easy To Use
Enhanced colors, stunning imagery, professional typography, make life easy, 17 professional pre-made slides, make life even easier.
All our presentation templates are saved in POT, PPT, and PPTX formats to make them compatible with your presentation software like Office for Mac, Keynote, Google Docs, OpenOffice, and others. Some MS PowerPoint features may not be supported by your presentation software. Please read more about Microsoft PowerPoint compatibility in your software documentation.
- All Themes (16676)
- Abstract/Textures (2449)
- Agriculture (338)
- America (206)
- Animals and Pets (344)
- Animated (32)
- Art & Entertainment (802)
- Business (1951)
- Business Concepts (3754)
- Careers/Industry (2977)
- Cars and Transportation (482)
- Computers (284)
- Construction (976)
- Consulting (2852)
- Education & Training (2803)
- Financial/Accounting (903)
- Flags/International (297)
- Food & Beverage (754)
- General (12382)
- Global (704)
- Health and Recreation (306)
- Holiday/Special Occasion (1167)
- Legal (219)
- Medical (1236)
- Military (137)
- Nature & Environment (1665)
- People (1868)
- Politics and Government (234)
- Real Estate (283)
- Religious/Spiritual (480)
- Sports (732)
- Technology and Science (2159)
- Telecommunication (497)
- Utilities/Industrial (407)
With the all-inclusive price of $99 you are only paying $.01 per template!
Join our 81,532 happy customers and get complete access to our 21,747 templates, 100% satisfaction guaranteed or your money back.
sign up today
- Preferences

The Artificial Heart: A Design Example - PowerPoint PPT Presentation

The Artificial Heart: A Design Example
Right chambers pump blood to lungs to receive oxygen ... 1967: dr. christian barnard transplants a donor heart into a 59 year old man (he ... – powerpoint ppt presentation.
- October 18, 2001
- Heart has four chambers
- Right chambers pump blood to lungs to receive oxygen
- Left chambers pump oxygenated blood from lungs to rest of the body
- Right and left atria receive blood
- Right and left ventricles pump blood
- Valves produce one-way blood flow from atria ? ventricles ? arteries
- Energy to pump blood comes from nutrients and oxygen in blood
- The blood supply to the heart is provided by coronary arteries
- Heart attack blockage of coronary artery damages portion of heart muscle
- Congestive heart failure gradual weakening of heart
- Millions suffer from heart disease
- Many cases are treatable with lifestyle changes, drugs and/or surgery
- Surviving patients suffering from most severe cases need new hearts!
- 100,000 Americans/year suffering from severe heart disease need new hearts
- Only 2,000 patients receive heart transplants
- Conclusion many patients die waiting for a new heart!
- A suitable alternative to donor hearts could prolong thousands of lives
- WWII first open heart surgeries
- 1953 heart-lung machine successfully used during heart surgery
- 1958 Drs. Willem Kolff and Tetsuzo Akutsu sustain a dog for 90 minutes with a PVC artificial heart
- 1967 Dr. Christian Barnard transplants a donor heart into a 59 year old man (he survived 18 days)
- 1969 Dr. Denton Cooley uses an artificial heart to sustain a patient waiting for a donor (survived 3 days)
- 1972 Cyclosporine introduced to suppress immune responses of transplant recipients
- 1982 Dr. William DeVries implants the Jarvik-7 artificial heart into Dr. Barney Clark (he survived 112 days)
- Immune response rejects transplant or side effects due to immune suppression
- Infection due to tubes and wires passing through skin
- Formation of clots
- Damage to red blood cells
- Lack of pulsatile blood flow?
- Identify the problem or need to address
- Specify details/criteria of an adequate solution to your problem
- Implement various solutions that meet the criteria you specified
- Test to determine which solution is most viable
- Further testing to refine the solution you chose
- Process is iterative
- You need to repeat various steps after testing
- Make design changes based on test results
- Failed designs
- Design didnt meet criteria
- Could be due to inappropriate criteria
- Must fit into chest cavity and connect to atria, pulmonary artery and aorta quickly
- Provide an adequate blood flow (8 10 liters/min)
- Send deoxygenated blood to the lungs and oxygenated blood to the body
- Operate continuously for an indefinite period of time
- Provide adequate warning if something is wrong or if it is going to fail
- Should increase/decrease blood flow based on patient activity level
- Should not evoke an immune response
- No wires or tubes that penetrate the skin
- Should not produce blood clots
- Should not damage red blood cells
- Ideally should have pulsatile blood flow
- Many others we havent thought of!
- Implanted into 59 year old Robert Tools on July 2, 2001 at Jewish Hospital in Louisville KY (96 days)
- Patient is able to walk around, organs are functioning normally, undergoing daily rehabilitation for eventual release
- Hydraulic pump forces blood to lungs and body
- Power is provided by an internal rechargeable battery
- Battery is recharged by coils on surface and below skin
- Internal controller monitors system and controls pump speed
- Implant controller, battery and coil
- Connect patient to heart-lung machine
- Cut away ventricles
- Sew grafts onto atria and arteries
- Connect implants to grafts
- Remove patient from heart-lung machine
- Grapefruit size, weighs 2 lbs, requires a 7 hour surgery for implantation
- Can provide up to 8 liters/min of blood to the lungs and body
- Has two chambers for pumping deoxygenated blood to the lungs and oxygenated blood to the body
- Wireless energy transfer system allows for continuous operation
- Internal controller monitors operation
- Internal controller increases/decreases blood flow based on blood oxygen levels
- Materials are inert to the immune system
- Completely contained within the chest no wires or tubing through skin!
- Made of special materials and special pump design to prevent clots and RBC damage
- Pumping alternates between chambers, creating a pulsatile blood flow
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics , the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.

IMAGES
VIDEO
COMMENTS
5. VENTRICULAR ARTIFICIAL HEART Each pump is small enough to be implanted into the void that was left behind from the extraction. Both pumps receive power from a large external console. The console pushes air through the tubing. Air enters inside the pump and is expelled through a series of thin flexible diaphragms. 5 The jarvik-7 design incorporates two heart pumps that are connected to a ...
Once the total artificial heart is connected, it acts like a healthy heart, allowing blood to flow normally through the body. The driver powers and controls the total artificial heart. It is placed next to the bed when used in the hospital. When someone with a total artificial heart is outside the hospital, they use a portable driver that fits ...
An artificial heart is an artificial organ device that replaces the heart.Artificial hearts are typically used to bridge the time to complete heart transplantation surgery, but research is ongoing to develop a device that could permanently replace the heart in the case that a heart transplant (from a deceased human or, experimentally, from a deceased genetically engineered pig) is unavailable ...
Porting valve - This valve opens and closes to let the hydraulic fluid flow from one side of the artificial heart to the other. When the fluid moves to the right, blood gets pumped to the lungs through an artificial ventricle. When the fluid moves to the left, blood gets pumped to the rest of the body.
1969: The First Artificial Heart Implant [Slide 4 & 5] On April 4, 1969, Texas Heart Institute cardiac surgeon Denton Cooley replaced the failing heart of 47-year-old Haskell Karp with a total artificial heart. Karp's heart was greatly enlarged due to advanced coronary arterial occlusive disease and myocardial fibrosis, and there was complete
Artificial heart. Sep 2, 2012 •. 8 likes • 10,419 views. BalaSuresh AsaiThambi. Health & Medicine. 1 of 17. Download Now. Download to read offline. Artificial heart - Download as a PDF or view online for free.
As Xie adjusted the valves of the loop, air was pumped out with a hiss. Nicholas Greatrex, an Australian electrical engineer, entered a command on a computer, and current began flowing to the ...
Many patients with end-stage heart disease die because of the scarcity of donor hearts. A total artificial heart (TAH), an implantable machine that replaces the heart, has so far been successfully ...
The total artificial heart (TAH) is a form of mechanical circulatory support in which the patient's native ventricles and valves are explanted and replaced by a pneumatically powered artificial heart. Currently, the TAH is approved for use in end-stage biventricular heart failure as a bridge to heart transplantation. However, with an ...
Artificial Hearts. What is an Artificial Heart?. Total Artificial Heart - device that replaces at least the lower two chambers (ventricles) of the heart and fully takes over cardiac function Slideshow 3042869 by hedy. ... During download, if you can't get a presentation, the file might be deleted by the publisher. E N D . Presentation Transcript.
Presentation on theme: "Artificial Heart Design & Development"— Presentation transcript: 3 Introduction Cardiovascular disease is the leading global cause of death, accounting for 17.3 million deaths per year, a number that is expected to grow to more than 23.6 million by 2030. About 2,150 Americans die each day from these diseases, one every ...
AS THE PRECEDING CHAPTERS HAVE RELATED, the artificial heart program of the National Heart, Lung, and Blood Institute (NHLBI) is nearing one of its goals, that of developing a fully implantable device that offers indefinite maintenance of cardiac function to individuals suffering from end-stage heart disease. Surgeons will soon implant the first long-term ventricular assist device (VAD) that ...
The Artificial Heart VD October 30, 2007 Chemistry 51. Abstract Researched the advantages of a man-made artificial heart.. Introduction • An artificial heart is a medical instrument that is was created in the 1950's. • The estimated cost is $150,000 per implanted artificial heart, which can increase the U.S. medical bill by anywhere from $2.5 to $3 million (Jarvik & Callahan, 1986).
2. Total Artificial Heart (TAH): one available option when long-term support of both ventricles is required. The population of patients with end-stage heart failure has increased over the years, and the availability of donor organs has not be Sufficient. End-stage heart failure represents a highly morbid condition for the patient with limited ...
The Artificial Heart: A Design Example. The Artificial Heart: A Design Example. BIOE 1000 October 18, 2001. The Human Heart. Heart has four chambers Right chambers pump blood to lungs to receive oxygen Left chambers pump oxygenated blood from lungs to rest of the body. The Human Heart. Right and left atria receive blood. 703 views • 17 slides
Patients with heart failure are misdiagnosed at a rate of 16% in hospitals and nearly 70% when general practitioners refer patients ... And thanks to artificial intelligence and cloud services ...
Artificial Heart presentation template includes built-in layouts and stunning backgrounds to make your presentation a winner. The attention we pay to the finest detail make this presentation template truly world-class. We take pride in employing features most companies skip because it's "too much work." This Artificial Heart presentation theme ...
Artificial Organs and Bionic Implants Market - The artificial organs and bionic implants market is on a remarkable trajectory of growth, with a significant increase in its value expected in the coming years. In 2022, the market was valued at a substantial $44.4 billion, and experts predict that it will continue to expand, reaching a projected value of $92.1 billion by 2032.
A basic understanding of AI's strengths, applica-tions and limitations is now essential for researchers and clinical cardiologists. In this context, AI refers to a collec-tion of computational concepts that can be summarised as a machine's ability to generalise learning in order to efficiently achieve complex tasks autonomously.
Participate and Experience. oral abstract and poster presentations. 125 Abstract Categories. across 26 basic, clinical and population programming communities . Global Opportunity to present your accepted abstracts. to an audience of your peers and thought leaders. Accepted Abstracts. published in. Circulation,