Wellness Begins
With A Breath

Spirometry, Oximetry and Mobile-Health
As a global medical device leader in spirometry, oximetry, and telemedicine, our innovative technology enables healthcare professionals and their patients to facilitate comprehensive personal health monitoring. Breathing well is essential for a good quality of life.
Our Products
The Smart One allows you to measure, monitor and track lung function no matter your location. Perfect for monitoring asthma, COPD, lung transplant care, cystic fibrosis, and other conditions.
Spirobank Smart
The Spirobank Smart is a personal-use spirometer that produces professional-level results. It is like having a portable PFT lab in your pocket!
All-in-one Spirometer with 7-inch Touchscreen, embedded printer and Oximetry (optional).
Spirobank II Smart
A versatile, clinical-grade spirometer complete with Oximetry (optional). Connect via Bluetooth with Tablet (iOS and Android) and PC (Windows and MacOS - also supports USB connection).
An easy-to-use, full function spirometer with minimal maintenance necessary.
Capable of performing spirometry, 6 min walk test, sleep study, and 24-hr oximetry.
Spirobank II Basic
Provides essential spirometry parameters, ideal for basic spirometry screening (e.g., General Practice, Occupational Medicine and Urgent Care).
FlowMIR™ Disposable Turbine
Compatible with all MIR professional spirometers for single-patient use. Factory calibrated and not affected by temperature, humidity or barometric pressure.
VBMax-FlowMIR Disposable Turbine
Compatible with all MIR professional spirometers for single-patient use. Includes viral/bacterial filter mouthpiece.
Reusable Turbine Flowmeter
Compatible with all MIR professional spirometers for multi-patient use. Requires cleaning and disinfection between patients.
Single Patient Reusable Turbine
Compatible with all MIR personal spirometers with a 1-year life span. No calibration needed.
MIR Spiro (Windows/Mac)
Complete and powerful desktop software solution for clinical Spirometry and Oximetry testing and results management.
MIR Spiro App (iOS/Android)
Allows Healthcare Professionals to connect the Spirobank II Smart to a tablet for portability and maximum ease-of-use.
MIR Spirobank App
Mobile App (iOS and Android), for real time at home spirometry and oximetry testing, directly on your Smartphone via Bluetooth Smart 4.0
Smart One App
Mobile App (iOS and Android), for real time spirometry and oximetry test, directly on your Smartphone/Tablet via Bluetooth Smart 4.0

Wellness Begins with a Breath
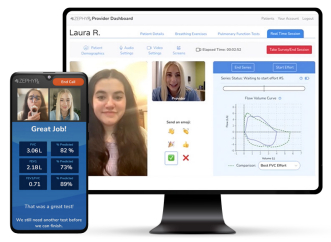
Introducing Live Video Exam
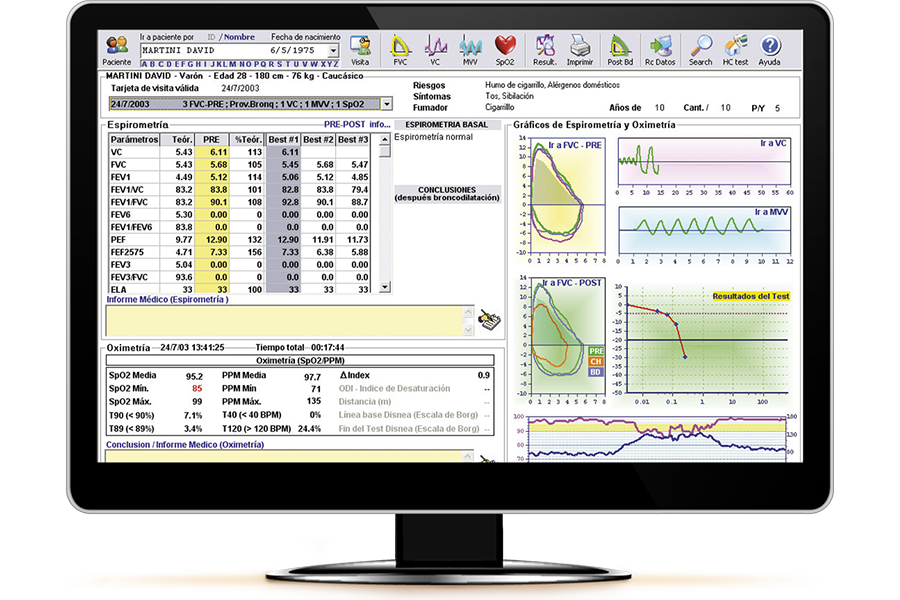
Quick Guide: MIR WinSpiroPRO
Visit us at these upcoming conferences!

Request More Information
We’re excited to share more information with you about MIR! Please fill out the below form to learn more.
- Documents Shipping / Returns Wish List Loyalty Discount
- --> Login or Sign Up

8:00am to 5:00pm CST
Talk to an Expert About New or Used Tuttnauer, Midmark, and Market Forge Autoclaves. Buy Medical Equipment for the Physician's Office and Other Related Industries.

- Medical Equipment
- Spirometers
- Medical International Research, Inc.
Spirolab Spirometer W/Bluetooth - Sp02 Optional- In Stock

- Create New Wish List
- Similar Products
Product Description
Medical international research/spirolab spirometer.
Documentation:
Fast and intuitive for modern professionals. Each function can be activated by a simple touch on the intuitive bar menu always present on the screen.
Suitable for children. Patented pediatric incentive directly on the screen to improve patient compliance during a Spirometry test.
- All in One Portable Desktop Spirometer
- FVC, FEV1, IVC, MVV, PRE/POST Bronchodilator comparison with a wide range of selectable parameters
- Long Life Rechargeable Battery
- Color Touchscreen 7 inch Display
- Direct Connection to External USB Printer
- Stores 10,000 Spirometry Tests
- Wireless Real Time Test on PC via Bluetooth
- Fast and Silent ted Printer with Customizable Printout Format
- New WinspiroPRO
- High performance PC software for spirometry
- Includes Carrying Case
Technical Specifications
- Power Supply: Rechargeable Battery and Mains Power
- Temperature Sensor: Semiconductor (0-45°C)
- Flow Sensor: Bi-directional Digital Turbine
- Flow Range: ±16L/s
- Volume Accuracy: ±3% or 50mL
- Flow Accuracy: ±5% or 200mL/s
- Dynamic Resistance:
- Connectivity: USB 2.0, Bluetooth 2.1
- Display: 7 inch Color Touch Screen LCD, 800 x 480 resolution
- Mouthpieces: Ø 30 mm (1.18 inch)
- Dimensions: 220 x 210 x 51 mm (8-5/8 x 8-1/4 x 2 in)
- Weight: 1450g (3.1 lbs.) - Battery pack included
- Spirometry Parameters: FVC, FEV1, FEV1/FVC, FEV1/VC, PEF, FEF25, FEF50, FEF75, FEF25–75, FEF75–85, Lung Age, Extrapolated Volume, FET, Time to PEF, FEV0.5, FEV0.5/FVC, FEV0.75, FEV0.75/FVC, FEV2, FEV2/FVC, FEV3, FEV3/FVC, FEV6, FEV1/ FEV6, FEV1/PEF, FEV1/ FEV0.5, FIVC, FIV1, FIV1/FIVC, PIF, FIF25, FIF50, FIF75, FEF50/FIF50, VC, IVC, IC, ERV, IRV, Rf, VE, VT, tI, tE, VT/tI, tE/tTOT, MVV (measured), MVV (calculated).
MIR Turbine Flowmeters (comply with ATS/ERS standards)
Spirometry testing requires maximum accuracy and hygiene. FlowMir is the answer to both requirements. Each turbine is factory calibrated with a computerized system and it is packaged individually in a clean room. 100% hygiene guaranteed! Option available: reusable turbine.
What's Included?
- Spirolab spirometer ( Sp02 is optional )
- Adult Sp02 probe if optional Sp02 is ordered
- Bluetooth Included: 1 WinspiroPRO CD
- Miniflowmeter with cable
- Power supply/battery charger
- 1 roll of thermal paper
- 1 nose clip
- 1 carrying case
Product Videos
Custom field, product reviews, write a review.

Medical Int'l Research
Recommended

Spirometer, Handheld MIR Spirobank II Smart Bluetooth

Schiller Cardiovit AT-102 ECG - Optional Spirometry - In Stock

Schiller Spiroscout Ultrasonic Spirometer

Hillrom/Welch Allyn Connex Cardio ECG/EKG W/optional Spirometry
Welch Allyn / Hillrom

Spirodoc Spirometer - PC Based With Oximetry SKU: 910610
- Medical International Research Manuals
- Medical Equipment
Medical International Research Spirolab Manuals

Medical International Research Spirolab User Manual (36 pages)
Table of contents.
- Table of Contents 2
1 Introduction
- User Type 4
- Ability and Experience Required 4
- Where the Device Is Used 4
- Individual Patient Factors that Can Affect Use of the Product 4
- Limitations of Use - Contraindications 4
- Risk of Cross Contamination 5
- Mouthpiece 6
- Sensor for Oximetry 6
- CE Mark for Medical Devices 8
- Electrical Safety Symbol 8
- USB Port Warning Label 8
- Spo2 Oximetry Port Warning Label 8
- WEEE Label 8
- Label Relating to the Method for Charging the Battery Pack 8
- FCC Certification Label 8
- Electrostatic Discharge Symbol 9
- Information on Protection against Ingress of Liquids 9
- Symbol for Devices that Include RF Transmitters 9
- Symbol for Reading the Operating Instructions 10
- Product Description 10
- Spirometer Specifications 11
- Oximeter Specifications 12
- Other Features 13
2 Using the Spirolab
- Turning the Spirolab on and off 13
- Saving Energy 14
- Main Screen 14
- Symbols and Icons 15
- Calibrating Turbines 17
- Entering New Patient Data 19
- Editing Patient Data 20
- How to Search the Archives 20
- Viewing Archived Data 20
- Displaying the Last Test Session 21
- Online Mode (Connected to a PC) 21
- FVC Test 22
- MVV Test 23
- Running POST Tests after the Administration of a Drug 23
- Interpreting Spirometry Results 24
- Instructions for Using the Sensor on Individual Adult Patients 27
3 Data Transmission
- Data Transmission Via Bluetooth 28
- Connecting with a PC Via USB Port 28
- Printing Data 29
- Up-Dating Internal Software 29
4 Maintenance
- Checking Correct Turbine Function 30
- Cleaning the Oximetry Sensor 30
- Replacing Wrap Sensor Adhesive Tape 30
- Recharging the Battery Pack 30
5 Trouble Shooting and Solutions
- Guarantee Conditions 34
Appendix 3 Information about the Correct Use of Device in an Electromagnetic Environment
Advertisement
Related Products
- Medical International Research spirodoc
- Medical International Research spirobank G
- Medical International Research Spirolab III
Medical International Research Categories
Upload manual.

You can adjust your Community Subscriptions in Settings
You can add Community Subscriptions in the search bar that says "Subscribe to more communities ..."
- All Categories
- Medical International Research - Spirolab III
Medical International Research Spirolab III Manuals / Documents
Medical international research - spirolab iii by medical international research.

Subscribe To eNews
© 2017 MedWrench, LLC. All Rights Reserved
- News & Trends
- Medical Technical Facilities
- Healthcare IT, Telemedicine
- Hospital software
- MIR - Medical International Research
Exhibitions
Medical software winspiropro® spirometry hospital analysis.

Characteristics
Description.

Meet this supplier at the following exhibition(s):
11-14 Apr 2024 Shanghai (China) Hall vide - Stand vide
11-14 Nov 2024 Düsseldorf (Germany)
Other MIR - Medical International Research products
- Analysis software
- Viewer software
- Windows software
- Reporting software
- Scheduling software
- Monitoring software
- Diagnostic software
- Measurement software
- Test software
- Printing software
- Communication software
- AI software
- Telehealth software
- Clinical iOS application
- Tablet PC iOS application
- ISO software
- Telemonitoring software
- Lung software
- MacOS software
The Peritoneal Dialysis Transfer Set Replacement Procedure
Affiliations.
- 1 Former Center Manager, Satellite Healthcare, San Jose, CA.
- 2 Director of Clinical Programs and Research, Satellite Healthcare, San Jose, CA.
- 3 Past President of ANNA.
- 4 member of the Nephrology Nursing Journal Editorial Board.
- 5 member of ANNA's Central Missouri Chapter.
- 6 Senior Research Director, Satellite Healthcare, San Jose, CA.
- 7 Adjunct Clinical Assistant Professor, Division of Nephrology, Department of Medicine, Stanford University School of Medicine Stanford University, Palo Alto, CA.
- 8 Senior Medical Director, Satellite Healthcare, San Jose, CA. and Division of Nephrology, Department of Medicine, Stanford University School of Medicine Stanford University, Palo Alto, CA.
- 9 Vice President of Home Services, Satellite Healthcare, San Jose, CA.
- 10 Director of Research, Satellite Healthcare, San Jose, CA; an Honorary Professor of Nursing, Faculty of Health, Deakin University. Melbourne, Australia.
- 11 International Member of ANNA.
- 12 Chief Medical Officer, Satellite Healthcare, San Jose, CA; and an Adjunct Lecturer, Division of Nephrology, Department of Medicine, Stanford University School of Medicine Stanford University, Palo Alto, CA.
- PMID: 32830940
Peritoneal dialysis transfer sets (extension lines) are replaced every six to nine months to minimize peritoneal dialysis catheter complications. The aim of this study was to compare a revised non-bag transfer set exchange procedure with the standard bag exchange procedure on nursing time, costs, and safety. Thirty-three people were randomized to two groups - a standard bag exchange procedure group (n = 16) and a non-bag transfer set exchange procedure group (n = 17). The standard bag exchange procedure took a median of 32 minutes (interquartile range [IQR] 25 to 38 minutes) compared to the non-bag transfer set exchange procedure of 6 minutes (IQR 4 to 8 minutes) (p Ò 0.0001). There was one episode of peritonitis in each group within the 72-hour follow-up period. The average cost of the non-bag transfer set exchange procedure was $24.54 lower, a 37% cost reduction. This study has shown the revised non-bag transfer set replacement procedure appears to be safe, consume less participant and staff time, and decreases costs.
Keywords: end stage kidney disease; home dialysis; nephrology nursing; peritoneal dialysis; transfer set.
Copyright© by the American Nephrology Nurses Association.
Publication types
- Randomized Controlled Trial
- Catheterization / adverse effects
- Costs and Cost Analysis
- Nursing Evaluation Research
- Peritoneal Dialysis / adverse effects
- Peritoneal Dialysis / economics
- Peritoneal Dialysis / methods*
- Peritoneal Dialysis / nursing*
- Peritonitis / etiology
- Peritonitis / prevention & control
- Treatment Outcome
- Workload / statistics & numerical data
- Clinical Studies

We are a medical device company based in Santa Clara, CA, dedicated to helping people with heart failure feel better and live longer.
Ancora Heart is dedicated to providing new treatment options for patients suffering from heart failure. Our team has a deep appreciation for the challenges faced by the estimated 26 million patients across the globe who are living with this chronic, progressive disease. Despite advances in heart failure treatment, high rehospitalization and mortality rates persist. Patients suffer from debilitating symptoms, exercise intolerance, poor quality of life, frequent hospitalization, and reduced life expectancy. In fact, up to 50 percent of people who develop heart failure die within five years of diagnosis. It is clear that patients need new options to treat symptoms and improve their quality of life, and we are committed to developing new solutions that help change the course of this challenging condition.
To address this challenge, we developed the AccuCinch ® Ventricular Restoration System, the only completely transcatheter device designed to treat the enlarged left ventricle, restore structure and function and bring relief to heart failure patients who remain symptomatic despite current guideline-directed medical care. The AccuCinch System procedure is minimally invasive and is designed to augment the existing care cardiologists provide their heart failure patients.
For patients who have heart failure with reduced ejection fraction (HFrEF, or pumping efficiency), in whom the disease has progressed beyond the ability of medications and pacemakers to manage symptoms, the AccuCinch System may provide a new treatment option by filling the gap between medication or pacemaker therapy and more invasive treatments, including left ventricular assist devices (LVADs) or heart transplant.
The AccuCinch System has been studied in multiple clinical trials in the United States and the European Union, and is currently enrolling patients in the CORCINCH-HF Study, a prospective, randomized, open-label, multicenter, international, clinical safety and efficacy investigation, which is designed to enroll 400 patients at up to 80 centers worldwide.
The study has a unique design allowing initial analysis of safety and clinical efficacy for PMA submission after the first 250 patients have reached six months of follow-up, and then a second analysis after the entire cohort has reached 12 months of follow-up. (ClinicalTrials.gov Identifier: NCT04331769 )
MEET OUR TEAM

PRESIDENT AND CEO
Jeffrey m. closs.
Mr. Closs brings proven leadership in medical devices, health policy, and technology to the Ancora Heart team. He has dedicated his career to working at the intersections of change to promote innovative, life-changing solutions. He has extensive experience founding new ventures (Benu), driving a successful IPO (Perclose), and having leadership roles at Fortune 500 companies (Fox Hollow, Edwards, Guidant, Bentley). Formerly a director at Nellix Endovascular, which was acquired by Endologix, and BioIQ, a health tech company, he is also an adjunct professor at Stanford University Center for Health Policy and the former president of the St. Raymond School Board. Mr. Closs holds an MBA from Emory University and a BA from the University of California, Los Angeles.

CHIEF TECHNICAL OFFICER
Russel sampson.
A champion for the development of minimally invasive surgical systems, Mr. Sampson brings a proven track record of invention (over 80 U.S. international patents) to his position at Ancora Heart. Leveraging his deep experience with CE and PMA approvals, commercialization, profitability and acquisition for companies such as Cytyc, Novacept, Abbott Laboratories, Space Systems/Loral and Ford Aerospace, he is responsible for our core technology, intellectual property and product development efforts. Mr. Sampson is the co-founder of Sierra Surgical Technologies (SST) and holds a BS in Mechanical Engineering (AMES) from the University of California, San Diego.

CHIEF COMMERCIAL OFFICER
Mr. Miles brings 25 years of experience scaling medical technology companies to his position as chief commercial officer (CCO) of Ancora Heart, including his most recent post as CCO of Terumo Aortic in Glasgow, Scotland. In that role, he was responsible for global sales and marketing, custom solutions, portfolio strategy and expansion into new markets, helping guide the company during a period of significant growth. Prior to his time at Terumo Aortic, Mr. Miles served as senior director of commercial strategy at Invuity, where he played a key role in driving adoption of their core product lines across key surgical specialties throughout the United States. He was instrumental in facilitating significant growth through the company’s eventual IPO in 2015. Previously, Mr. Miles held positions of increasing responsibility at Medtronic, serving as director of global marketing of the Endovascular Business Unit. He began his career at Eli Lilly and Company, serving various commercial leadership roles during his eight-year tenure. Mr. Miles received an MBA from Pepperdine University and a BS in health sciences from James Madison University.

VICE PRESIDENT OF BIOSTATISTICS AND CLINICAL DATA MANAGEMENT
Christine tjossem.
Ms. Tjossem brings a 35-year track record of successful growth and leadership in biostatistics and data management to her position at Ancora Heart. Her experience includes key roles establishing and managing biostatistical and data management efforts at some of the world’s leading clinical research and medical device organizations, including Medtronic, Boston Scientific, St. Jude and Berlin Heart. Ms. Tjossem began her career working in medical research for Mayo Clinic in Rochester, Minnesota. As Vice President of Biostatistics and Data Management, Ms. Tjossem is responsible for all facets of her team’s success in advising and implementing appropriate techniques and methods for statistical and data management areas. In addition to managing these line-of-business functions, Ms. Tjossem’s vast breadth and depth of experience allows her to make significant contributions to the company’s overall business, regulatory and clinical strategy. Christine received her Bachelor of Science degree in Statistics from Iowa State University.

VICE PRESIDENT AND GM, INT’L CLINICAL, REGULATORY AND THERAPY DEVELOPMENT
Michael zapien.
Drawing on his expertise in transitioning innovative medical devices from the developmental stage to commercial success at several emerging startups (Elixir, Nanostim), Mr. Zapien leads our clinical research. Previously the Director of Clinical and Medical Affairs at CardioDx, Inc., he also has clinical and commercial experience at Medi-Globe, Silk Road Medical, Abbott Vascular, and Guidant Corp. Mr. Zapien is a certified clinical researcher with a MS from the University of Maryland and a BS from San Francisco State University.

VICE PRESIDENT OF STRATEGIC INITIATIVES
Bart beasley.
A veteran of introducing early-stage and ground-breaking therapies to physicians, Mr. Beasley brings over 25 years of experience in healthcare marketing to Ancora Heart. By building partnerships with the clinical community, gathering first-hand insights towards procedure and product development, and enhancing clinical trial patient education and recruitment, he has helped drive the rapid growth of multiple revolutionary therapies and new therapeutic categories. Mr. Beasley served as Vice President of Marketing at Avinger, drove market development at Transcend Medical (acquired by Alcon), was Director of Marketing at Fox Hollow (acquired by ev3), and began his career at Perclose (acquired by Abbott). Mr. Beasley holds an MBA from IESE Business School in Barcelona, Spain and a BS in Economics from Santa Clara University.

VICE PRESIDENT OF REGULATORY AFFAIRS AND QUALITY ASSURANCE
Michael fritz.
Mr. Fritz comes to Ancora Heart with decades of medical device industry experience including strategic leadership, management, operational, and hands-on roles in regulatory affairs, clinical research, quality assurance, and engineering. His career includes roles with small start-ups (Synapse Biomedical and Cyberkinetics) and larger companies (Medtronic, Boston Scientific, and Guidant) developing class III implantable devices and class II minimally invasive devices in diverse fields including interventional cardiology, cardiac electrophysiology, neurology, pulmonary/respiratory, laparoscopic surgery, neurosurgery, rehabilitation medicine, and neuroprosthetics. Mr. Fritz holds an MS degree in software engineering from the University of St. Thomas and a bachelor of electrical engineering degree from the University of Minnesota.
Meet Our Board of Directors
Robert croce, andrew midler, beverly huss, jeffrey closs.
Bob Croce culminated 12 years as Company Group Chairman through a 36-year career at Johnson & Johnson. Beginning in 1968, Mr. Croce held a series of senior positions beginning as a Sales Representative for Ortho Pharmaceuticals where he was later named Vice President of Marketing. Prior to that, he served as Worldwide Franchise Chairman of Cordis and Ethicon Endo-Surgery. In 1985, Mr. Croce was named Vice President of Business Development at McNeil. He was later transferred to Ethicon, Inc., where he served as Group Vice President, and was later named President in 1990. In 1992, he was promoted to Worldwide President of Ethicon Endo-Surgery.
SAVITR CAPITAL
Savitr Capital was founded by Andrew Midler in 2008. Prior to Savitr Capital, Andrew founded Standard Pacific Capital in 1995. The investment management firm launched with $75 million of assets and grew to over $5 billion. Until 2005, Midler managed the firm’s Global Long/Short Equity Fund for Standard Pacific’s flagship product. He also supervised the firm’s credit opportunities, commodity, quantitative, and Japan long/short equity strategies. He served as the CIO and CEO of the firm from 1995 to 2007 before selling his majority ownership interest in the firm in December 2007. Prior to founding Standard Pacific, Midler was the Director of Equity Portfolio Management at CS First Boston, a global institutional money management firm. Previously, he managed a significant portion of the global hedge fund, Odyssey Partners. From 1986 through 1993, he worked at Fidelity Investments where he was Portfolio Manager of the Fidelity Convertible Securities Fund, Fidelity Equity Income II Fund and Fidelity Growth & Income Fund.
LCP VENTURES
Lew Pell is one of the most prolific and successful medical technology investors. Since 1975, Pell has been the lead investor and co-founder of 24 different medical device manufacturers, eight of which have been sold for a collective net gain of $1.6 billion. Pell’s founding of medical technology companies began in 1975, when he and Katsumi Oneda co-founded Machida America to sell flexible endoscopes. In 1979, he created Pentax Precision Instruments and sold it to Asahi Optical eleven years later. Over a span of 15 years, Pell co-founded various medical device companies including: American Endoscopy (acquired by Medtronic), Heart Technology (acquired by Boston Scientific), InStent (acquired by Medtronic), and Vision Sciences. He was also co-founder of Biosense (acquired by Johnson & Johnson), Influence, Inc. (acquired by American Medical Systems), and Disc-O-Tech (sold to Kyphon).
MADORRA, INC.
Beverly Huss is a medical device entrepreneur, board director and mentor who brings with her more than 20 years’ experience as a leader of both large corporations and start-up companies in the medical device space. She is currently Executive Chair of Madorra, Inc., a women’s health company focused on improving quality of life after menopause. Ms. Huss was co-founder and CEO of Pagonia Medical. Prior to founding Pagonia, she was president and CEO of Qool Therapeutics, Inc., a tissue preservation company. Previously she was co-founder and CEO of Vibrynt, Inc., a company that developed a novel minimally invasive therapeutic device for treatment of morbid obesity. Prior to this, Ms. Huss was with Santa Clara, California-based Guidant since 1986, fulfilling a variety of executive roles within the company’s various medical device divisions. She managed the worldwide Endovascular Solutions business as President and quadrupled worldwide revenues to $150 million for the carotid and peripheral vascular business in four years. In addition, she built Guidant’s coronary stent business to $1B with 90% gross margins.
PRESIDENT & CEO
Work with us.
It has come to our attention that an unknown entity is impersonating our organization and extending illegitimate job offers via email, purportedly in an attempt to defraud intended applicants.
Please be advised that these email solicitations appear to be part of a fake job scheme. For additional information, please see https://www.fbi.gov/contact-us/field-offices/elpaso/news/press-releases/fbi-warns-cyber-criminals-are-using-fake-job-listings-to-target-applicants-personally-identifiable-information .
Authorized Ancora Heart employees will reach out to potential candidates directly via telephone or email from ancoraheart.com but will never issue questionnaires or request personal information. Job offers will only be extended after a thorough interview process and multiple interactions with Ancora Heart staff members. When communicating, please be very cautious of fictitious email addresses.
Ancora Heart is looking for talented professionals to join our growing team. We value creativity, drive, accountability, and the ability to work collaboratively with one another. If you thrive in a fast-paced setting and are passionate about changing the way heart failure is treated, consider the following opportunities:
Clinical Research Associate (Device Accountability)
PURPOSE OF POSITION :
Support the Clinical Affairs team to ensure investigational device accountability such that the clinical trial is operating in accordance with the clinical protocol(s); Federal Regulations 21CFR821; any other applicable policies, regulations, procedures, and/or guidelines to ensure data integrity and Trial Master Files are maintained. Support the Clinical Operations team to ensure integrity of clinical and/or compliance data.
MAJOR DUTIES AND RESPONSIBILITIES:
- Verify, in conjunction with the Operations team, quantities, lot numbers, and expiration dates of outgoing shipments.
- Verify data in outgoing shipment manifests to include specific details regarding packing lists, device accountability log information, addresses, site specific information (i.e., contact name and number).
- In a timely manner, confirm receipt of product at the site and ensure the completion and collection of manifest documentation.
- Troubleshoot and resolve any issues that arise during transit, delivery and receipt of shipments.
- In conjunction with Site Management and Field Operations, ensure the completion of site training for the receipt and handling of investigative product.
- With Therapy Development and Field Operations teams, review and reconcile post-implant devices.
- Collect and review Device Use Forms and Device Accountability Logs prior to return shipments.
- Coordinate and expedite device returns including creation of FedEx labels. Ensure complete and accurate documentation.
- With the Operations staff, ensure the accuracy of return shipments, restocking, and tracking of devices.
- With Operations and Clinical Operations staff, create and maintain a system for the accurate tracking of devices returned and dispositioned (i.e., restocked, disposed, or recommissioned.)
- With Operations and Clinical Field Operations staff, fully review, reconcile, and file all documentation related to device accountability.
- Order, track and manage Clinical Trial materials.
- Manage in-house filing of critical materials.
- As needed, complete projects and tasks as assigned by the Clinical Affairs team.
- Work with Site Management to generate information and distribute EU and US study newsletters.
- Review, assess, create and update clinical quality and monitoring SOPs, monitoring plans, and other clinical affairs forms.
- Prepare Essential Document Tracker Status report as needed. Perform comprehensive eTMF audits.
- Work with Field and Clinical Operations teams to maintain accurate study documents.
- Complete projects and tasks in an aggressive manner consistent with corporate objectives. Keep supervisor informed of changes in work schedule and/or workload.
- Regularly recognize problems and recommend and implement methods of conduct of clinical study.
- Perform other duties as assigned.
- Support company goals and objectives, policies and procedures, and compliance with Ancora Heart SOPs, Good Clinical Practices, Good Manufacturing Practices and FDA regulations. Ensure compliance with Quality System Regulations and ISO 9001, EN 46001, and MDD requirements.
EDUCATION REQUIREMENTS :
- BS preferred in Life Sciences, Medicine, or other technical discipline.
EXPERIENCE REQUIREMENTS :
- Minimum five years related experience in health-related field in addition to working knowledge of FDA regulatory requirements.
OTHER QUALIFICATIONS :
- Must possess excellent written and verbal communication skills.
- Able to travel and work in the Santa Clara Office
- Must be willing and able to travel to work related meetings and seminars, as needed.
- May require the ability to deliver speeches and/or presentations effectively to a variety of audiences.
Clinical Safety Associate
PURPOSE OF JOB :
The Clinical Safety Associate assists the Safety Manager and is responsible for the oversight of the adverse events and product experiences reported within a clinical trial as directed by the Safety Manager. The Clinical Safety Associate assists with ensuring the appropriate reporting of the events to the necessary internal departments and applicable Authorities throughout the life of the trial.
The Clinical Safety Associate assists the Safety Manager who acts as a liaison between the internal departments as well as to external customers such as CROs, Clinical Events Committees and Data Safety Monitoring Boards associated to the trial.
MAJOR DUTIES AND RESPONSIBILITIES :
- Participate in the formulation of case report forms, specifically with regards to reporting and management of adverse events.
- Support preparation of safety section of clinical study reports, Clinical Expert reviews, clinical section of device reports, and other report documents as needed to support regulatory filings to US and International regulatory bodies.
- Review clinical safety databases for adverse events and device deficiencies and compose safety narrative reports.
- Assist sites with subject evaluation and protocol adherence for adverse event reporting timelines.
- Assist managing Clinical Events Committee (CEC), Data and Safety Monitoring Board (DSMB) and Safety outsource activities.
- Collaborate with team to resolve complex or unclear situations.
- Maintain a professional and credible image with clinical investigators, hospital staff and other customers, FDA, Notified Bodies, and other regulatory bodies, consultants, vendors, and co-workers.
- Maintain current knowledge of applicable clinical literature.
- Attend relevant meetings as appropriate.
- Support company goals and objectives, policies and procedures, and compliance with Good Clinical Practices and FDA regulations.
- Keep manager informed of changes in work schedule/and or workload.
Registered Nurse (Associate degree); Bachelor’s degree in a Scientific discipline, Life Sciences, Medicine, or other technical discipline.
- Experience of at least 5 years in the medical industry in addition to at least 2 years of Safety monitoring experience.
- Experience in conducting clinical trials.
- Clinical knowledge and understanding in medical record and medical diagnostic report review.
- Excellent internal and external customer service skills
- Excellent written and verbal communication skills
- Detail oriented and ability to problem solve
- Able to make evaluative judgements
- Able to work independently and in a team environment
- Able to develop and deliver presentations to a variety of audiences
- Able to provide technical advice, guidance, and support to others in your area of expertise
Manufacturing and Process Engineers
- Support the company’s transition from clinical stage to commercialization within manufacturing, process, and automation engineering. Evaluate and optimize existing processes, design & implement custom fixtures, tooling, automated equipment within and outside a cleanroom environment. Work closely with the R&D, Operations, and Quality Systems staffs to improve existing processes and perform process validations. This hands-on role requires accountability and the ability to work with minimal supervision.
- Design, document, assemble, and qualify production assembly / test / inspection fixtures and equipment.
- Specify, procure and implement off the shelf automated and semi-automated equipment
- Design, develop and implement custom equipment as-needed.
- Create verification or validation protocols, execute studies and/or tests, and author and release subject reports.
- Perform characterization studies and Design of Experiments (DoE).
- Author and perform validation/verification of processes/inspection methods for manufacturing (IQ, OQ, PQ).
- Write and maintain manufacturing documentation including material and component specifications, work instructions, BOMs, routers, Process FMEAs, DMR, protocols, reports, and DHF documents.
- Maintain compliance with Ancora Heart’s quality systems including all applicable SOPs, non-conforming materials disposition, corrective action investigations and closure, and Engineering Change Order (ECO).
- Train operators on new processes, tools, and equipment.
- Work as the liaison among R&D, Operations, and Quality Systems to ensure a smooth transition of design changes, tooling, and fixturing.
- Assure product and process quality by designing testing methods; testing sub-assemblies, finished product and process capabilities.
- Develop, qualify and implement processes to improve manufacturability.
- Complete projects and tasks in a timely manner consistent with corporate objectives. Keep manager informed of changes in work schedule and/or workload.
- Maintain compliance to QSR systems in coordination with the Document Control and Quality Assurance functions.
- Support company goals and objectives, policies and procedures.
- Minimum B.S. in Mechanical Engineering, Biomedical Engineering, Materials Engineering, or similar Engineering discipline.
- Minimum 5 years of related experience
- Knowledge of FDA, QSR and MDR regulations and relevant FDA Guidance and ISO standards.
- Strong background in IQ/OQ/PQ,
- PLC programming, operation
- Ability to work in a CER ISO 7 environment, engage with medical device assemblers to learn and understand current manufacturing challenges.
- Strong background in DFMEA, SHA, test methods, quality inspection methodology, report writing.
- Flexibility to perform a wide range of activities in parallel.
- Strong communication skills, sound judgement
- Willingness and ability to work independently and in teams
- Experienced with Mini-Tab and SolidWorks
- Skilled in all Microsoft Office products, including Excel, Word, and PowerPoint.
Ancora Heart offers a competitive compensation and benefits program as well as a progressive work environment. We are an equal opportunity employer. If you or someone you know might be interested in this position, please submit a resume and an introductory email to [email protected]
To Learn More
Our Affiliates
Our services, clinical trials, for patients.
- For Physicians
- For Sponsors
- GCRI Foundation
Welcome to GCRI for Personalized Oncology
M ission : Global Cancer Research Institute, also known as GCRI, is the first and only community-based dedicated Phase 1 to 4 Clinical Trial Unit in Hematology and Medical Oncology in Northern California. We offer patients access to cutting-edge, innovative new cancer drugs, some not available elsewhere. Our mission is to bring life-saving novel therapies to patients through extensive collaboration between academia, community physicians and biotechnology and pharmaceutical companies with one goal in mind - finding the next cancer cures and getting that to all patients as rapidly as possible.
We are dedicated to providing our patients with excellent, innovative, individualized, state-of-the-art medical care in a caring and nurturing environment, where each patient is treated as a whole. We believe that each patient should have peace of mind and the freedom to choose the best treatments available for their care. We will work together with you every step of the way to personalize your treatment and make your journey as simple and stress-free as possible while ensuring the best possible outcome and quality of life for you. Let us be your partner, your doctor, your nurse, your guide……every step of the way. We are here for you in every way.
Lynne A. Bui, MD
Founder and Chairman
Hematology/Oncology
How we can help you
We are partners with the following medical specialists throughout the Bay Area so we can provide the best care for you at your convenience
Global Cancer Research Institute provides cutting edge cancer
treatment and drug development for its valued patients
We offer patients phase I, II, and III cancer clinical trials at GCRI.
Information about appointments, refills, insurance, billing, and
frequently asked questions for GCRI patients
Support GCRI Foundation
GCRI Foundation, Inc. is a 501(c)(3) non-profit organization dedicated to
the funding of basic, translational, and clinical research for the advancement
of treatments for cancer patients and providing funding for educational
and supportive services for patients and their loved ones.
© www.gcrioncology.com | [email protected]
14911 National Ave #1 | Los Gatos , CA 95032 | P 408.384.9284 | F 408.847.6196
Our Team | Our Affiliates | Our Services | Clinical Trials | For Patients | For Physicians | For Sponsors | GCRI Foundation
Web View Mobile View
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 8, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
New review provides insights into the impact of osteoporosis and related medications on fracture healing
by International Osteoporosis Foundation
A new comprehensive review, authored by the International Osteoporosis Foundation (IOF) Committee of Scientific Advisors Fracture Working Group on behalf of the IOF and the International Society of Orthopaedic Surgery and Traumatology (SICOT), clarifies the evidence linking fracture healing with osteoporosis and the currently available medications used to treat osteoporosis.
Within clearly outlined chapters, " Impact of osteoporosis and osteoporosis medications on fracture healing. A narrative review " examines the biology of fracture healing , clinical and radiological evaluation of fracture healing, the impact of osteoporosis on fracture healing, the effect of osteoporosis medications on fracture healing, and important areas for further research. The findings are published in the journal Osteoporosis International .
Professor Manju Chandran, first author of the publication, stated, "A recent fracture significantly increases the likelihood of another fracture. Prompt osteoporosis treatment is imperative to reduce this risk. Concerns however linger among medical and surgical professionals about the potential adverse effects of osteoporosis medications on fracture healing.
"Furthermore, when patients undergoing osteoporosis treatment sustain fractures, clinicians may ask themselves whether to halt osteoporosis therapy during the healing process. Our review delves into the available evidence to provide some clarity and practical guidance that will be useful for the many clinicians and surgeons caring for patients with fragility fractures."
The following key points are highlighted:
- The assessment of fracture healing typically should involve a combination of clinical examination and radiographic imaging.
- While animal studies appear to support the view that osteoporosis negatively influences fracture healing, clinical studies in humans have yielded conflicting results.
- The effect of osteoporosis medications on fracture healing has been extensively studied, though most of the investigations have been in animal models and therefore may not fully translate to humans.
- Overall, there does not appear to be a negative effect of osteoporosis medications on fracture healing. Bisphosphonates can be safely started early after metaphyseal osteoporotic fractures without adversely affecting clinical outcomes.
- There is a suggestion of benefit for teriparatide in time to fracture healing. Data on the impact romosozumab on fracture healing is scant.
- Other factors such as aging, smoking, poor nutrition, and comorbidities such as diabetes and vitamin D deficiency may confound and contribute to delayed healing of osteoporotic fragility fractures.
- The benefit of treating osteoporosis and the urgent necessity to mitigate imminent fracture risk should take precedence over any theoretical risks of non-union or delayed union.
- More research is needed: new radiological and biological markers of fracture healing must be identified and clinical and basic science methodologies to assess fracture healing should be merged for greater understanding. Data on the impact of antiosteoporosis agents such as teriparatide, romosozumab on fracture healing deserve further attention.
Professor Nicholas Harvey, co-author and Chair of the IOF Committee of Scientific Advisors concluded, "Fortunately there appears to be no deleterious effect of osteoporosis medications on fracture healing. The evidence does not support a delay in the initiation of antiresorptive therapy following acute fragility fractures, nor is there reason for suspension of osteoporosis medication at the time of fracture if the person is already on treatment."
"Given the serious and potentially life-threatening consequences of secondary fractures, the benefit of treating osteoporosis and the urgent necessity to reduce imminent refracture risk after a fracture should be given prime consideration."
Explore further
Feedback to editors
Heart disease and depression may be genetically linked by inflammation
10 hours ago


Low cardiorespiratory fitness in youth associated with decreased work ability throughout adulthood, finds 45-year study
11 hours ago
Long COVID leaves telltale traces in the blood, finds new study
Preventive percutaneous coronary intervention for high-risk coronary plaques found to reduce cardiac events
Heart-on-a-chip model used to glean insights into COVID-19-induced heart inflammation
12 hours ago

Biomedical engineers use AI to build new tool for studying and diagnosing heart function

Everyday social interactions predict language development in infants

Research shows pregnancy accelerates biological aging in a healthy, young adult population

Researchers uncover underlying mechanism driving membranous nephropathy, a chronic kidney disease in children
13 hours ago

Researchers find more action is needed to prevent arthritis after knee reconstruction surgery
Related stories.

Women with osteoporosis want to know their fracture risk
Jan 1, 2024

New evidence-based guideline for the management of osteoporosis in men
Mar 25, 2024

Study reveals gaps in fracture risk communication and patients' preferences for visual representation
Nov 20, 2023
Cataracts linked to higher risks of osteoporosis and fracture
Oct 3, 2018
A new aid to osteoporosis and fracture risk patient discussion for primary care providers
Jun 1, 2022

Medicare spends more than $6 billion on secondary fractures
Oct 2, 2019
Recommended for you

Chronic kidney disease progresses faster in patients living in hot countries, new study finds
15 hours ago

Study finds treating heart attack patients with beta-blockers may be unnecessary

Study uncovers consequences of molnupiravir use to treat COVID-19
14 hours ago

Can a cup of tea keep COVID away? Study demonstrates that certain teas inactivate SARS-CoV-2 in saliva
17 hours ago

'Mini kidneys' reveal new insights into metabolic defects in polycystic kidney disease
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Anthropology
Anthropology doctoral student ebenezer adeyemi was awarded a t. anne cleary international dissertation research fellowship from the ui graduate college.
Anthropology doctoral student Ebenezer Adeyemi was awarded a T. Anne Cleary International Dissertation Research Fellowship from the UI Graduate College. Ebenezer’s research interests center around medical anthropology, infrastructure, the intersection of urban landscape and public health, African studies, and survival strategies in marginalized urban communities. For his ongoing doctoral research, Ebenezer is exploring strategies that residents of Makoko, a large informal settlement in Lagos State, Nigeria, use to access healthcare to treat malaria, the most prominent health issue in their community.
NOTICE: The University of Iowa Center for Advancement is an operational name for the State University of Iowa Foundation, an independent, Iowa nonprofit corporation organized as a 501(c)(3) tax-exempt, publicly supported charitable entity working to advance the University of Iowa. Please review its full disclosure statement.
- {{>productsMenu}} Products
- {{>trendsMenu}} News & Trends
- Catalogs >
- MIR - Medical International Research >
- SpiroConnect Winspiro Light®
- News & Trends
- Exhibitions
SpiroConnect Winspiro Light® 2 Pages

Catalog excerpts

Performing tool for direct integration in Electronic Medical Records (EMR) in hospital environment. Simple applications with essential functions for primary care and occupational medicine Winspiro Light® For direct integration into EMR The most innovative and simple solution to integrate spirometry and oximetry with Electronic Medical Record (EMR) or to a doctor office management program. Single EMR database system (Master). Winspiro Light® is an essential and efficient PC software, supplied with Minispir Light® for simplified spirometry. The software has a simple and intuitive interface with one main screen for all functions available with simple commands. Spirometry test carried out by SpiroConnect (Slave). Standardized communication system (HL7 or Exchange Protocol). Using SpiroConnect it is no longer necessary to write the same patient card twice on two different systems. Detailed documentation on request and online support for integrators. Winspiro Express® PDF spirometry reports generator Once a spirometry test has been carried out the results can be transferred via USB to the PC for printout and storage. Supplied with Spirobank USB.

Screenshot: summary of all tests carried out WinspiroPRO® Complete and powerful version for specialists and homecare Offers different communication protocols Supports Telemedicine projects WinspiroPRO® is a unique software standard with MIR products. Advanced features for oximetry management including nocturnal desaturation analysis and body position during sleep and 6-minute walk test. Pediatric incentive allows image selection and customization to get maximum patient compliance (exclusive MIR patent). WinspiroPRO® is compatible with Windows XP, VISTA, 7 and 8. The USB drivers are...
All MIR - Medical International Research catalogs and technical brochures

1 Pages

27 Pages

2 Pages

4 Pages

Archived catalogs

Related Searches
- Analysis software
- Visualization software
- Windows software
- Reporting software
- Hospital software
- Planning software
- Monitoring software
- Diagnostic software
- Antibacterial filter
- Measurement software
- Artificial ventilation filter
- Medical International Research spirometer
- Test software
- Printing software
- Computer-based spirometer
- Communication software
- Medical International Research hand-held spirometer
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group
share this!
April 8, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
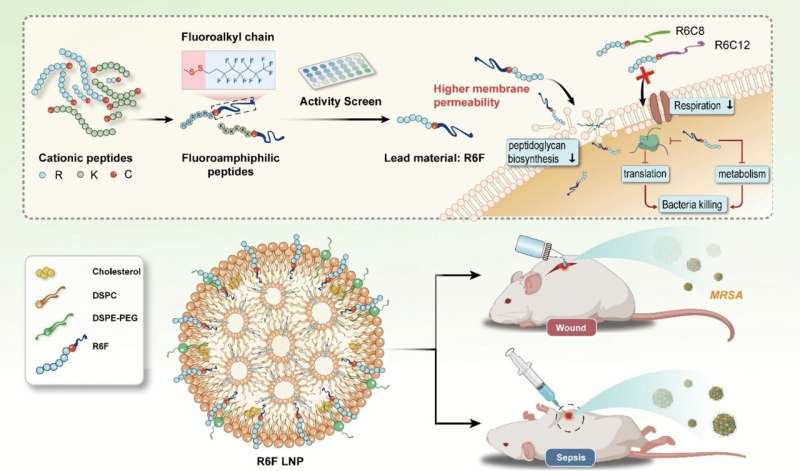
Fluorous lipopeptides act as highly effective antibiotics for multidrug-resistant pathogens
Multidrug-resistant bacterial infections that cannot be treated by any known antibiotics pose a serious global threat. Publishing in the journal Angewandte Chemie International Edition , a Chinese research team has now introduced a method for the development of novel antibiotics to fight resistant pathogens. The drugs are based on protein building blocks with fluorous lipid chains.
Antibiotics are often prescribed far too readily. In many countries they are distributed without prescriptions and administered in factory farming: prophylactically to prevent infections and enhance performance. As a result, resistance is on the rise—increasingly against reserve antibiotics as well. The development of innovative alternatives is essential.
It is possible to learn some lessons from the microbes themselves. Lipoproteins, small protein molecules with fatty acid chains , are widely used by bacteria in their battles against microbial competitors. A number of lipoproteins have already been approved for use as drugs.
The common factors among the active lipoproteins include a positive charge and an amphiphilic structure, meaning they have segments that repel fat and others that repel water. This allows them to bind to bacterial membranes and pierce through them to the interior.
The team led by Yiyun Cheng at East China Normal University in Shanghai aims to amplify this effect by replacing hydrogen atoms in the lipid chain with fluorine atoms. These make the lipid chain simultaneously water-repellant (hydrophobic) and fat-repellant (lipophobic). Their particularly low surface energy strengthens their binding to cell membranes while their lipophobicity disrupts the cohesion of the membrane.
The team synthesized a spectrum (substance library) of fluorous lipopeptides from fluorinated hydrocarbons and peptide chains. To link the two pieces, they used the amino acid cysteine, which binds them together via a disulfide bridge.
The researchers screened the molecules by testing their activity against methicillin-resistant Staphylococcus aureus (MRSA), a widespread, highly dangerous strain of bacteria that is resistant to nearly all antibiotics. The most effective compound they found was "R6F," a fluorous lipopeptide made of six arginine units and a lipid chain made of eight carbon and 13 fluorine atoms. To increase biocompatibility, the R6F was enclosed within phospholipid nanoparticles.
In mouse models, R6F nanoparticles were shown to be very effective against sepsis and chronic wound infections by MRSA. No toxic side effects were observed.
The nanoparticles seem to attack the bacteria in several ways: they inhibit the synthesis of important cell-wall components, promoting collapse of the walls; they also pierce the cell membrane and destabilize it; disrupt the respiratory chain and metabolism; and increase oxidative stress while simultaneously disrupting the antioxidant defense system of the bacteria.
In combination, these effects kill the bacteria—other bacteria as well as MRSA. No resistance appears to develop.
These insights provide starting points for the development of highly efficient fluorous peptide drugs to treat multi-drug resistant bacteria.
Journal information: Angewandte Chemie International Edition
Provided by Wiley
Explore further
Feedback to editors

Early medieval money mystery solved
8 hours ago

Finding new chemistry to capture double the carbon
11 hours ago

Americans are bad at recognizing conspiracy theories when they believe they're true, says study
12 hours ago

A total solar eclipse races across North America as clouds part along totality

New statistical-modeling workflow may help advance drug discovery and synthetic chemistry

Researchers develop better way to make painkiller from trees

Replacing plastics with alternatives is worse for greenhouse gas emissions in most cases, study finds
A targeted polymer to treat colorectal cancer liver metastases

When an antibiotic fails: Scientists are using AI to target 'sleeper' bacteria

Scientists discover new phage resistance mechanism in phage-bacterial arms race
Relevant physicsforums posts, what do large moles on the body indicate.
Mar 30, 2024
Avian flu - A new study led by a team from the University of Maryland
Mar 27, 2024
Are all biological catabolic reactions exergonic?
Mar 20, 2024
A First of Its Kind: A Calcium-based signal in the Human Brain
Mar 18, 2024
Biological culture and cultural biology
Mar 17, 2024
Potentially fatal dog parasite found in the Colorado River
Mar 15, 2024
More from Biology and Medical
Related Stories
New insights into how epilancin 15X kills bacteria
Feb 6, 2024
Steroid drugs used for HRT can combat E. coli and MRSA
Mar 13, 2024
Researchers introduce singlet oxygen battery for battling multidrug-resistant pathogens
Aug 16, 2023
Chemists develop a new class of antibiotics to fight resistant bacteria
Jun 1, 2023
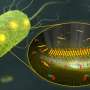
'Youngest' antibiotic kills bacteria via a new two-step mechanism
Aug 3, 2022
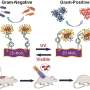
Nanomaterial with 'light switch' kills Gram-negative or Gram-positive bacteria
Dec 5, 2023
Recommended for you

New diagnostic tool achieves accuracy of PCR tests with faster and simpler nanopore system

New micromaterial releases nanoparticles that selectively destroy cancer cells
Apr 5, 2024

Improving infectious disease testing with gold nanoparticles
Apr 4, 2024
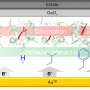
The role of interfacial amino acids in shaping bio-electronic communication between proteins
Apr 1, 2024

New method uses nanofibrils on magnetic microparticles to isolate HIV particles
Mar 28, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES
VIDEO
COMMENTS
MIR - Medical International Research is a global medical device leader in Spirometry, Oximetry and MedTech solutions. Present in more than 100 countries worldwide, MIR is making its most innovative technology available to healthcare professionals and their patients in the pneumology sector by pairing a series of new, reliable, and ready-to ...
Medical International Research (MIR) produces both professional and smartphone-compatible personal spirometers and oximeters for monitoring COPD, asthma and other lung disorders. ... Spirolab. All-in-one Spirometer with 7-inch Touchscreen, embedded printer and Oximetry (optional). More Details. Spirobank II Smart.
Spirolab is a complete desktop spirometer with an oximetry option. Equipped with a 7'' color touchscreen and an embedded thermal printer, Spirolab is the all-in-one spirometer for rapid and comprehensive reporting - without needing a computer! The long-lasting rechargeable battery and large internal memory make Spirolab the ideal device to take anywhere. ...
Spirolab User manual Contact the supplier or radio/TV technician for expert advice. The symbols defined may be found on the device ID label. 1.3.9 Electrostatic discharge symbol This symbol, required by the EN 60601-1-2 International Standard, is used near every connector that has been excluded from the electrostatic discharge test.
Medical International Research/Spirolab Spirometer. Documentation: Fast and intuitive for modern professionals. Each function can be activated by a simple touch on the intuitive bar menu always present on the screen. Suitable for children. Patented pediatric incentive directly on the screen to improve patient compliance during a Spirometry test.
Spirolab. Catalog excerpts. 7 inch Touchscreen All in one portable Desktop Spirometer with Oximetry option www.spirometry.com www.oximetry.com. Open the catalog to page 1. Fast and intuitive for modern professionals Each function can be activated by a simple touch on the intuitive bar menu always present on the screen. • 10,000 Spirometry ...
Find out all of the information about the MIR - Medical International Research product: electronic spirometer Spirolab®. Contact a supplier or the parent company directly to get a quote or to find out a price or your closest point of sale. ... Spirolab is an all-in-one desktop spirometer with oximetry option. With a 7'' color touchscreen ...
Spirometer. Spirolab III® is a multitasking and versatile spirometer ideal for accurate and early diagnosis of respiratory diseases (like COPD and Asthma). Fast, simple, durable diagnostic spirometer with large test memory capacity and built in printer. Easy to use for family doctors screening or pharmacy quick tests.
Connection to Bluetooth® Low Energy technology also available It comes additionally with: • SpO2 and pulse rate directly on the display of the device (including plethysmographic curve) • Medical-standard battery charger with interchangeable international plugs • Spirometry test with over 45 selectable parameters including PRE and POST test.
Spirolab Spirometers. Manufacturer: Medical International Research. Spirolab is a lightweight, portable, and desktop spirometer. It can be used in stand alone mode or connected to a PC via USB and features a 7in color LCD touchscreen. It has a built-in printer with customizable printout format.
Page 1 Spirolab III User's Manual Rev.0 Issued on 21/06/06 Approved on 21/06/06 SpirolabIII - User's Manual Code MIR 980067 REV 0 Page 1 / 47...; Page 2: Important Note Thank you for choosing a product from MIR Medical International Research The original packaging contains one of the following spirometers, complete with its standard accessories: PRODUCT without oximetry option CODE ...
We have 1 Medical International Research Spirolab manual available for free PDF download: User Manual . Medical International Research Spirolab User Manual (36 pages) Spirometer and oximeter. Brand: Medical ...
Medical International Research - Spirolab IIIby Medical International Research. Product Details. Forums. Documents. Videos.
Pocket-sized spirometer suitable from 3 to 93 years! On-screen Results Score Symptoms and Add Notes to each session Patient Incentive to improve compliance during test Apps Available for iOs and Android. Open the catalog to page 3. Technical Specifications • Dimensions: 49x109x21 mm (1,93x4,29x0,82 Inch) • Weight: 60.7 g (Oz. 2.14 ...
MIR 911080 Spirolab Desktop Spirometer. Availability: Non Stock Product. MIR - Medical International Research. Item #: MIR___911080 - MIR 911080 Spirolab: Spirometer + Bluetooth The standard packaging contains: 1 CD winspiro PRO PC software1 USB cable1 Mini flowmeter with cable1 power supply/battery charger1 roll of thermal paper1 nose clip1 carrying case1 CD with user manual Spirometry test ...
Find out all of the information about the MIR - Medical International Research product: medical software WinspiroPRO®. Contact a supplier or the parent company directly to get a quote or to find out a price or your closest point of sale. ... Spirodoc, Spirobank II, Spirotel, Minispir, Spirobank G, Spirolab III. Winspiro PRO NET® includes free ...
Contact information. For questions, please contact Dr. Abdelaziz by email at [email protected] or by phone at (216) 293-0282. Upvote. International Medical Education. Find the AMA's Observership Programs to help international medical graduates adapt to the practice of medicine in the United States.
There was one episode of peritonitis in each group within the 72-hour follow-up period. The average cost of the non-bag transfer set exchange procedure was $24.54 lower, a 37% cost reduction. This study has shown the revised non-bag transfer set replacement procedure appears to be safe, consume less participant and staff time, and decreases costs.
Pediatric incentive allows image selection and customization to get maximum patient compliance (exclusive MIR patent). WinspiroPRO® is compatible with Windows XP, VISTA, 7 and 8. The USB drivers are certified by Microsoft. Graphics and tables of all patient tests available on a single page. Bronchodilator reversibility test and bronchial ...
We are a medical device company based in Santa Clara, CA, dedicated to helping people with heart failure feel better and live longer. Ancora Heart is dedicated to providing new treatment options for patients suffering from heart failure. Our team has a deep appreciation for the challenges faced by the estimated 26 million patients across the ...
Global Cancer Research Institute. 14911 National Ave #1. Los Gatos , CA 95032. 408-384-9284. 408-847-6196. Second Location: 9460 No Name Uno Ste 230. Gilroy , CA 95020. 408-847-6191.
A new comprehensive review, authored by the International Osteoporosis Foundation (IOF) Committee of Scientific Advisors Fracture Working Group on behalf of the IOF and the International Society ...
Anthropology doctoral student Ebenezer Adeyemi was awarded a T. Anne Cleary International Dissertation Research Fellowship from the UI Graduate College. Ebenezer's research interests center around medical anthropology, infrastructure, the intersection of urban landscape and public health, African studies, and survival strategies in marginalized urban communities.
Performing tool for direct integration in Electronic Medical Records (EMR) in hospital environment. Simple applications with essential functions for primary care and occupational medicine Winspiro Light® For direct integration into EMR The most innovative and simple solution to integrate spirometry and oximetry with Electronic Medical Record (EMR) or to a doctor office management program.
Credit: Angewandte Chemie International Edition (2024). DOI: 10.1002/anie.202403140