- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

U.S. Food and Drug Administration
- Search
- Menu
- Tobacco Products
- Public Health Education
Health Effects of Tobacco Use
Tobacco use has serious effects on the health of users. In fact, tobacco use remains the leading preventable cause of disease and death in the United States, 1 leading to more than 480,000 deaths each year.
Different tobacco products, however, pose varying levels of health risk to users. Combustible products that burn tobacco, like a cigarette, are the most harmful to a user’s health, while noncombustible products, such as e-cigarettes, may be less harmful. However, no tobacco product is considered safe.
Health Effects of Smoking
Cigarettes are responsible for the vast majority of all tobacco-related disease and death in the U.S. Smokers are exposed to a toxic mix of over 7,000 chemicals when they inhale cigarette smoke, 2 the consequences of which can threaten their health in many ways.
Order Free Print Version | Download Infographic

If you or a loved one are among the 34 million U.S. adults who smoke cigarettes in this country 9 and want to quit , there are resources to help you on your journey to living a smoke-free life .
COVID-19 and Smoking
Am I at risk for serious complications from COVID-19 if I smoke cigarettes?
Subscribe to CTP Email Newsletters
Stay up to date on tobacco product news and announcements from FDA’s Center for Tobacco Products (CTP) by subscribing to our email newsletters.
Yes. Data show that when compared to never smokers, cigarette smoking increases the risk of more severe illness from COVID-19, which could result in hospitalization, the need for intensive care, or even death. Smoking cigarettes can cause inflammation and cell damage throughout the body, and can weaken your immune system, making it less able to fight off disease.
There’s never been a better time to quit smoking. If you need resources to help you quit smoking, the FDA has supportive tips and tools to help you get closer to quitting for good .
If I vape tobacco or nicotine am I at risk for complications from COVID-19?
E-cigarette use can expose the lungs to toxic chemicals, but whether those exposures increase the risk of COVID-19 or the severity of COVID-19 outcomes is not known. However, many e-cigarette users are current or former smokers, and cigarette smoking increases the risk of respiratory infections, including pneumonia.
In the Health Effects of Tobacco Use Section
Nicotine Is Why Tobacco Products Are Addictive

Keep Your Air Clear: How Tobacco Can Harm Your Lungs

How Smoking Affects Heart Health

What It’s Like to Quit Smoking

Quitting Smoking and Other Tobacco Public Health Resources
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: US Dept of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- U.S. Department of Health and Human Services (USDHHS). A Report of the Surgeon General: How Tobacco Smoke Causes Disease: What It Means to You (Consumer Booklet). Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010.
- U.S. Department of Health and Human Services. Smoking and Cardiovascular Disease (Fact Sheet). Atlanta, GA: US Dept of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- U.S. Department of Health and Human Services. Smoking and Cancer (Fact Sheet). Atlanta, GA: US Dept of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- U.S. Department of Health and Human Services. National Diabetes Statistics Report. Atlanta, GA: US Dept of Health and Human Services, Centers for Disease Control and Prevention; 2020.
- U.S. Department of Health and Human Services. Smoking and Diabetes (Fact Sheet). Atlanta, GA: US Dept of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- U.S. Department of Health and Human Services. Smoking and Respiratory Diseases (Fact Sheet). Atlanta, GA: US Dept of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- Smoking during pregnancy. Centers for Disease Control and Prevention website. www.cdc.gov/tobacco/basic_information/health_effects/pregnancy/. Updated January 8, 2014. Accessed April 17, 2015.
- Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco Product Use Among Adults — United States, 2019. MMWR Morb Mortal Wkly Rep 2020;69:1736–1742.
Subscribe to CTPConnect
Get regular updates on the health effects of tobacco, public health educational resources, and highlights on current tobacco issues and regulations.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 24 March 2022
Tobacco and nicotine use
- Bernard Le Foll 1 , 2 ,
- Megan E. Piper 3 , 4 ,
- Christie D. Fowler 5 ,
- Serena Tonstad 6 ,
- Laura Bierut 7 ,
- Lin Lu ORCID: orcid.org/0000-0003-0742-9072 8 , 9 ,
- Prabhat Jha 10 &
- Wayne D. Hall 11 , 12
Nature Reviews Disease Primers volume 8 , Article number: 19 ( 2022 ) Cite this article
32k Accesses
69 Citations
74 Altmetric
Metrics details
- Disease genetics
- Experimental models of disease
- Preventive medicine
Tobacco smoking is a major determinant of preventable morbidity and mortality worldwide. More than a billion people smoke, and without major increases in cessation, at least half will die prematurely from tobacco-related complications. In addition, people who smoke have a significant reduction in their quality of life. Neurobiological findings have identified the mechanisms by which nicotine in tobacco affects the brain reward system and causes addiction. These brain changes contribute to the maintenance of nicotine or tobacco use despite knowledge of its negative consequences, a hallmark of addiction. Effective approaches to screen, prevent and treat tobacco use can be widely implemented to limit tobacco’s effect on individuals and society. The effectiveness of psychosocial and pharmacological interventions in helping people quit smoking has been demonstrated. As the majority of people who smoke ultimately relapse, it is important to enhance the reach of available interventions and to continue to develop novel interventions. These efforts associated with innovative policy regulations (aimed at reducing nicotine content or eliminating tobacco products) have the potential to reduce the prevalence of tobacco and nicotine use and their enormous adverse impact on population health.
Similar content being viewed by others

Associations between classic psychedelics and nicotine dependence in a nationally representative sample
Use of electronic cigarettes and heated tobacco products during the covid-19 pandemic.
Smoking cessation behaviors and reasons for use of electronic cigarettes and heated tobacco products among Romanian adults
Introduction.
Tobacco is the second most commonly used psychoactive substance worldwide, with more than one billion smokers globally 1 . Although smoking prevalence has reduced in many high-income countries (HICs), tobacco use is still very prevalent in low-income and middle-income countries (LMICs). The majority of smokers are addicted to nicotine delivered by cigarettes (defined as tobacco dependence in the International Classification of Diseases, Tenth Revision (ICD-10) or tobacco use disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)). As a result of the neuro-adaptations and psychological mechanisms caused by repeated exposure to nicotine delivered rapidly by cigarettes, cessation can also lead to a well-characterized withdrawal syndrome, typically manifesting as irritability, anxiety, low mood, difficulty concentrating, increased appetite, insomnia and restlessness, that contributes to the difficulty in quitting tobacco use 2 , 3 , 4 .
Historically, tobacco was used in some cultures as part of traditional ceremonies, but its use was infrequent and not widely disseminated in the population. However, since the early twentieth century, the use of commercial cigarettes has increased dramatically 5 because of automated manufacturing practices that enable large-scale production of inexpensive products that are heavily promoted by media and advertising. Tobacco use became highly prevalent in the past century and was followed by substantial increases in the prevalence of tobacco-induced diseases decades later 5 . It took decades to establish the relationship between tobacco use and associated health effects 6 , 7 and to discover the addictive role of nicotine in maintaining tobacco smoking 8 , 9 , and also to educate people about these effects. It should be noted that the tobacco industry disputed this evidence to allow continuing tobacco sales 10 . The expansion of public health campaigns to reduce smoking has gradually decreased the use of tobacco in HICs, with marked increases in adult cessation, but less progress has been achieved in LMICs 1 .
Nicotine is the addictive compound in tobacco and is responsible for continued use of tobacco despite harms and a desire to quit, but nicotine is not directly responsible for the harmful effects of using tobacco products (Box 1 ). Other components in tobacco may modulate the addictive potential of tobacco (for example, flavours and non-nicotine compounds) 11 . The major harms related to tobacco use, which are well covered elsewhere 5 , are linked to a multitude of compounds present in tobacco smoke (such as carcinogens, toxicants, particulate matter and carbon monoxide). In adults, adverse health outcomes of tobacco use include cancer in virtually all peripheral organs exposed to tobacco smoke and chronic diseases such as eye disease, periodontal disease, cardiovascular diseases, chronic obstructive pulmonary disease, stroke, diabetes mellitus, rheumatoid arthritis and disorders affecting immune function 5 . Moreover, smoking during pregnancy can increase the risk of adverse reproductive effects, such as ectopic pregnancy, low birthweight and preterm birth 5 . Exposure to secondhand cigarette smoke in children has been linked to sudden infant death syndrome, impaired lung function and respiratory illnesses, in addition to cognitive and behavioural impairments 5 . The long-term developmental effects of nicotine are probably due to structural and functional changes in the brain during this early developmental period 12 , 13 .
Nicotine administered alone in various nicotine replacement formulations (such as patches, gum and lozenges) is safe and effective as an evidence-based smoking cessation aid. Novel forms of nicotine delivery systems have also emerged (called electronic nicotine delivery systems (ENDS) or e-cigarettes), which can potentially reduce the harmful effects of tobacco smoking for those who switch completely from combustible to e-cigarettes 14 , 15 .
This Primer focuses on the determinants of nicotine and tobacco use, and reviews the neurobiology of nicotine effects on the brain reward circuitry and the functioning of brain networks in ways that contribute to the difficulty in stopping smoking. This Primer also discusses how to prevent tobacco use, screen for smoking, and offer people who smoke tobacco psychosocial and pharmacological interventions to assist in quitting. Moreover, this Primer presents emerging pharmacological and novel brain interventions that could improve rates of successful smoking cessation, in addition to public health approaches that could be beneficial.
Box 1 Tobacco products
Conventional tobacco products include combustible products that produce inhaled smoke (most commonly cigarettes, bidis (small domestically manufactured cigarettes used in South Asia) or cigars) and those that deliver nicotine without using combustion (chewing or dipping tobacco and snuff). Newer alternative products that do not involve combustion include nicotine-containing e-cigarettes and heat-not-burn tobacco devices. Although non-combustion and alternative products may constitute a lesser risk than burned ones 14 , 15 , 194 , no form of tobacco is entirely risk-free.
Epidemiology
Prevalence and burden of disease.
The Global Burden of Disease Project (GBDP) estimated that around 1.14 billion people smoked in 2019, worldwide, increasing from just under a billion in 1990 (ref. 1 ). Of note, the prevalence of smoking decreased significantly between 1990 and 2019, but increases in the adult population meant that the total number of global smokers increased. One smoking-associated death occurs for approximately every 0.8–1.1 million cigarettes smoked 16 , suggesting that the estimated worldwide consumption of about 7.4 trillion cigarettes in 2019 has led to around 7 million deaths 1 .
In most populations, smoking prevalence is much higher among groups with lower levels of education or income 17 and among those with mental health disorders and other co-addictions 18 , 19 . Smoking is also more frequent among men than women (Figs 1 – 3 ). Sexual and/or gender minority individuals have disproportionately high rates of smoking and other addictions 17 , 20 . In addition, the prevalence of smoking varies substantially between regions and ethnicities; smoking rates are high in some regions of Asia, such as China and India, but are lower in North America and Australia. Of note, the prevalence of mental health disorders and other co-addictions is higher in individuals who smoke compared with non-smokers 18 , 19 , 21 . For example, the odds of smoking in people with any substance use disorder is more than five times higher than the odds in people without a substance use disorder 19 . Similarly, the odds of smoking in people with any psychiatric disorder is more than three times higher than the odds of smoking in those without a psychiatric diagnosis 22 . In a study in the USA, compared with a population of smokers with no psychiatric diagnosis, subjects with anxiety, depression and phobia showed an approximately twofold higher prevalence of smoking, and subjects with agoraphobia, mania or hypomania, psychosis and antisocial personality or conduct disorders showed at least a threefold higher prevalence of smoking 22 . Comorbid disorders are also associated with higher rates of smoking 22 , 23 .
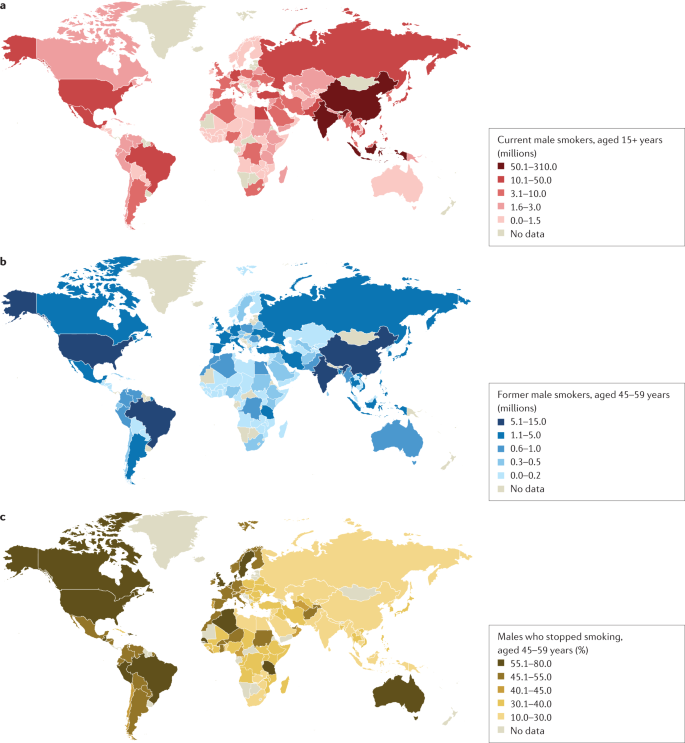
a | Number of current male smokers aged 15 years or older per country expressed in millions. b | Former male smokers aged 45–59 years per country expressed in millions. c | Former male smokers aged 45–59 years per country expressed as the percentage of smokers who stopped. The data shown are for male smokers for the period 2015–2019 from countries with direct smoking surveys. The prevalence of smoking among males is less variable than among females. Data from ref. 1 .
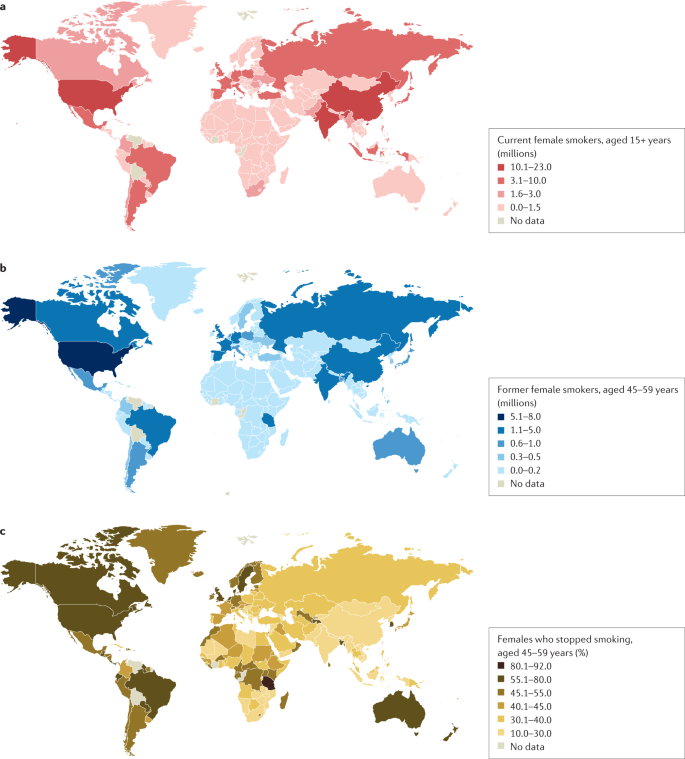
a | Number of current female smokers aged 15 years or older per country expressed in millions. b | Former female smokers aged 45–59 years per country expressed in millions. c | Former female smokers aged 45–59 years per country expressed as the percentage of smokers who stopped. The data shown are for female smokers for the period 2015–2019 from countries with direct smoking surveys. The prevalence of smoking among females is much lower in East and South Asia than in Latin America or Eastern Europe. Data from ref. 1 .
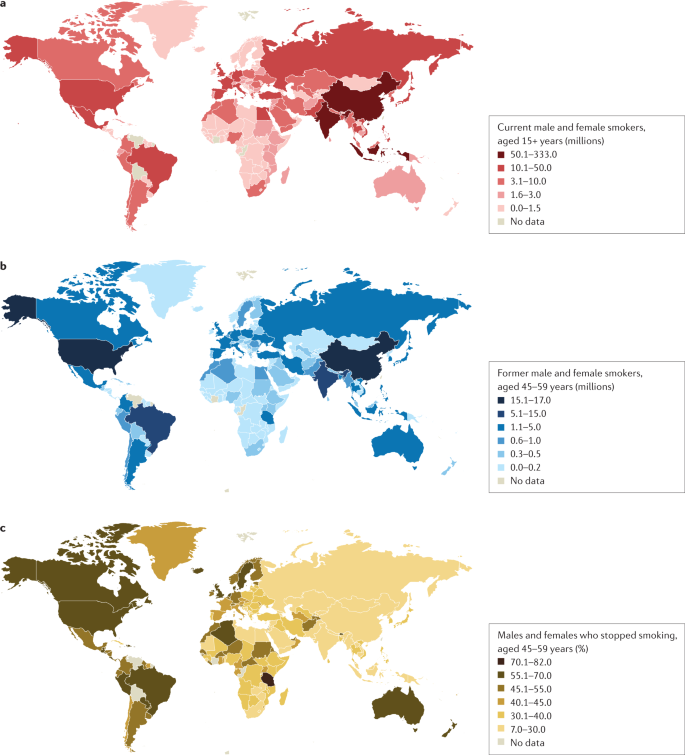
a | Number of current male and female smokers aged 15 years or older per country expressed in millions. b | Former male and female smokers aged 45–59 years per country expressed in millions. c | Former male and female smokers aged 45–59 years per country expressed as the percentage of smokers who stopped. The data shown are for the period 2015–2019 from countries with direct smoking surveys. Cessation rates are higher in high-income countries, but also notably high in Brazil. Cessation is far less common in South and East Asia and Russia and other Eastern European countries, and also low in South Africa. Data from ref. 1 .
Age at onset
Most smokers start smoking during adolescence, with almost 90% of smokers beginning between 15 and 25 years of age 24 . The prevalence of tobacco smoking among youths substantially declined in multiple HICs between 1990 and 2019 (ref. 25 ). More recently, the widespread uptake of ENDS in some regions such as Canada and the USA has raised concerns about the long-term effects of prolonged nicotine use among adolescents, including the possible notion that ENDS will increase the use of combustible smoking products 25 , 26 (although some studies have not found much aggregate effect at the population level) 27 .
Smoking that commences in early adolescence or young adulthood and persists throughout life has a more severe effect on health than smoking that starts later in life and/or that is not persistent 16 , 28 , 29 . Over 640 million adults under 30 years of age smoke in 22 jurisdictions alone (including 27 countries in the European Union where central efforts to reduce tobacco dependence might be possible) 30 . In those younger than 30 years of age, at least 320 million smoking-related deaths will occur unless they quit smoking 31 . The actual number of smoking-related deaths might be greater than one in two, and perhaps as high as two in three, long-term smokers 5 , 16 , 29 , 32 , 33 . At least half of these deaths are likely to occur in middle age (30–69 years) 16 , 29 , leading to a loss of two or more decades of life. People who smoke can expect to lose an average of at least a decade of life versus otherwise similar non-smokers 16 , 28 , 29 .
Direct epidemiological studies in several countries paired with model-based estimates have estimated that smoking tobacco accounted for 7.7 million deaths globally in 2020, of which 80% were in men and 87% were current smokers 1 . In HICs, the major causes of tobacco deaths are lung cancer, emphysema, heart attack, stroke, cancer of the upper aerodigestive areas and bladder cancer 28 , 29 . In some lower income countries, tuberculosis is an additional important cause of tobacco-related death 29 , 34 , which could be related to, for example, increased prevalence of infection, more severe tuberculosis/mortality and higher prevalence of treatment-resistant tuberculosis in smokers than in non-smokers in low-income countries 35 , 36 .
Despite substantial reductions in the prevalence of smoking, there were 34 million smokers in the USA, 7 million in the UK and 5 million in Canada in 2017 (ref. 16 ), and cigarette smoking remains the largest cause of premature death before 70 years of age in much of Europe and North America 1 , 16 , 28 , 29 . Smoking-associated diseases accounted for around 41 million deaths in the USA, UK and Canada from 1960 to 2020 (ref. 16 ). Moreover, as smoking-associated diseases are more prevalent among groups with lower levels of education and income, smoking accounts for at least half of the difference in overall mortality between these social groups 37 . Any reduction in smoking prevalence reduces the absolute mortality gap between these groups 38 .
Smoking cessation has become common in HICs with good tobacco control interventions. For example, in France, the number of ex-smokers is four times the number of current smokers among those aged 50 years or more 30 . By contrast, smoking cessation in LMICs remains uncommon before smokers develop tobacco-related diseases 39 . Smoking cessation greatly reduces the risks of smoking-related diseases. Indeed, smokers who quit smoking before 40 years of age avoid nearly all the increased mortality risks 31 , 33 . Moreover, individuals who quit smoking by 50 years of age reduce the risk of death from lung cancer by about two-thirds 40 . More modest hazards persist for deaths from lung cancer and emphysema 16 , 28 ; however, the risks among former smokers are an order of magnitude lower than among those who continue to smoke 33 .
Mechanisms/pathophysiology
Nicotine is the main psychoactive agent in tobacco and e-cigarettes. Nicotine acts as an agonist at nicotinic acetylcholine receptors (nAChRs), which are localized throughout the brain and peripheral nervous system 41 . nAChRs are pentameric ion channels that consist of varying combinations of α 2 –α 7 and β 2 –β 4 subunits, and for which acetylcholine (ACh) is the endogenous ligand 42 , 43 , 44 . When activated by nicotine binding, nAChR undergoes a conformational change that opens the internal pore, allowing an influx of sodium and calcium ions 45 . At postsynaptic membranes, nAChR activation can lead to action potential firing and downstream modulation of gene expression through calcium-mediated second messenger systems 46 . nAChRs are also localized to presynaptic membranes, where they modulate neurotransmitter release 47 . nAChRs become desensitized after activation, during which ligand binding will not open the channel 45 .
nAChRs with varying combinations of α-subunits and β-subunits have differences in nicotine binding affinity, efficacy and desensitization rate, and have differential expression depending on the brain region and cell type 48 , 49 , 50 . For instance, at nicotine concentrations found in human smokers, β 2 -containing nAChRs desensitize relatively quickly after activation, whereas α 7 -containing nAChRs have a slower desensitization profile 48 . Chronic nicotine exposure in experimental animal models or in humans induces an increase in cortical expression of α 4 β 2 -containing nAChRs 51 , 52 , 53 , 54 , 55 , but also increases the expression of β 3 and β 4 nAChR subunits in the medial habenula (MHb)–interpeduncular nucleus (IPN) pathway 56 , 57 . It is clear that both the brain localization and the type of nAChR are critical elements in mediating the various effects of nicotine, but other factors such as rate of nicotine delivery may also modulate addictive effects of nicotine 58 .
Neurocircuitry of nicotine addiction
Nicotine has both rewarding effects (such as a ‘buzz’ or ‘high’) and aversive effects (such as nausea and dizziness), with the net outcome dependent on dose and others factors such as interindividual sensitivity and presence of tolerance 59 . Thus, the addictive properties of nicotine involve integration of contrasting signals from multiple brain regions that process reward and aversion (Fig. 4 ).
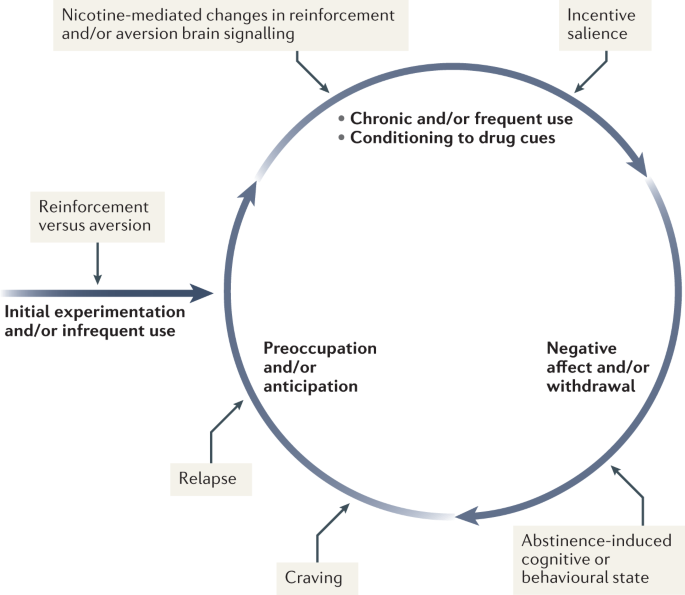
During initial use, nicotine exerts both reinforcing and aversive effects, which together determine the likelihood of continued use. As the individual transitions to more frequent patterns of chronic use, nicotine induces pharmacodynamic changes in brain circuits, which is thought to lead to a reduction in sensitivity to the aversive properties of the drug. Nicotine is also a powerful reinforcer that leads to the conditioning of secondary cues associated with the drug-taking experience (such as cigarette pack, sensory properties of cigarette smoke and feel of the cigarette in the hand or mouth), which serves to enhance the incentive salience of these environmental factors and drive further drug intake. When the individual enters into states of abstinence (such as daily during sleep at night or during quit attempts), withdrawal symptomology is experienced, which may include irritability, restlessness, learning or memory deficits, difficulty concentrating, anxiety and hunger. These negative affective and cognitive symptoms lead to an intensification of the individual’s preoccupation to obtain and use the tobacco/nicotine product, and subsequently such intense craving can lead to relapse.
The rewarding actions of nicotine have largely been attributed to the mesolimbic pathway, which consists of dopaminergic neurons in the ventral tegmental area (VTA) that project to the nucleus accumbens and prefrontal cortex 60 , 61 , 62 (Fig. 5 ). VTA integrating circuits and projection regions express several nAChR subtypes on dopaminergic, GABAergic, and glutamatergic neurons 63 , 64 . Ultimately, administration of nicotine increases dopamine levels through increased dopaminergic neuron firing in striatal and extrastriatal areas (such as the ventral pallidum) 65 (Fig. 6 ). This effect is involved in reward and is believed to be primarily mediated by the action of nicotine on α 4 -containing and β 2 -containing nAChRs in the VTA 66 , 67 .
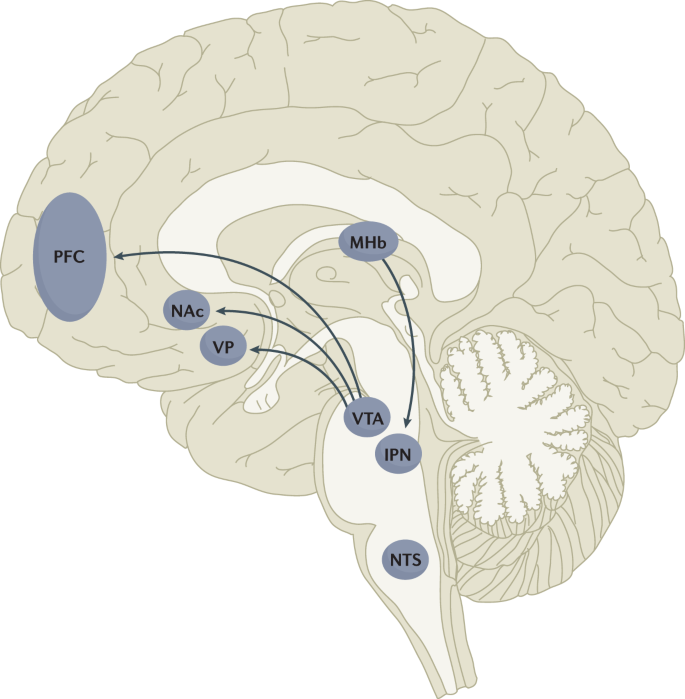
Multiple lines of research have demonstrated that nicotine reinforcement is mainly controlled by two brain pathways, which relay predominantly reward-related or aversion-related signals. The rewarding properties of nicotine that promote drug intake involve the mesolimbic dopamine projection from the ventral tegmental area (VTA) to the nucleus accumbens (NAc). By contrast, the aversive properties of nicotine that limit drug intake and mitigate withdrawal symptoms involve the fasciculus retroflexus projection from the medial habenula (MHb) to the interpeduncular nucleus (IPN). Additional brain regions have also been implicated in various aspects of nicotine dependence, such as the prefrontal cortex (PFC), ventral pallidum (VP), nucleus tractus solitarius (NTS) and insula (not shown here for clarity). All of these brain regions are directly or indirectly interconnected as integrative circuits to drive drug-seeking and drug-taking behaviours.
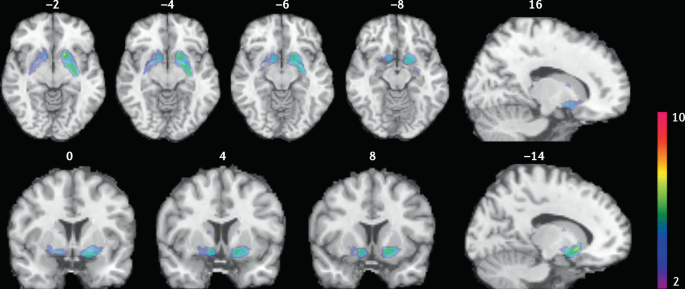
Smokers received brain PET scans with [ 11 C]PHNO, a dopamine D 2/3 PET tracer that has high sensitivity in detecting fluctuations of dopamine. PET scans were performed during abstinence or after smoking a cigarette. Reduced binding potential (BP ND ) was observed after smoking, indicating increased dopamine levels in the ventral striatum and in the area that corresponds to the ventral pallidum. The images show clusters with statistically significant decreases of [ 11 C]PHNO BP ND after smoking a cigarette versus abstinence condition. Those clusters have been superimposed on structural T1 MRI images of the brain. Reprinted from ref. 65 , Springer Nature Limited.
The aversive properties of nicotine are mediated by neurons in the MHb, which project to the IPN. Studies in rodents using genetic knockdown and knockout strategies demonstrated that the α 5 -containing, α 3 -containing and β 4 -containing nAChRs in the MHb–IPN pathway mediate the aversive properties of nicotine that limit drug intake, especially when animals are given the opportunity to consume higher nicotine doses 68 , 69 , 70 , 71 , 72 . In addition to nAChRs, other signalling factors acting on the MHb terminals in the IPN also regulate the actions of nicotine. For instance, under conditions of chronic nicotine exposure or with optogenetic activation of IPN neurons, a subtype of IPN neurons co-expressing Chrna5 (encoding the α 5 nAChR subunit) and Amigo1 (encoding adhesion molecule with immunoglobulin-like domain 1) release nitric oxide from the cell body that retrogradely inhibits MHb axon terminals 70 . In addition, nicotine activates α 5 -containing nAChR-expressing neurons that project from the nucleus tractus solitarius to the IPN, leading to release of glucagon-like peptide-1 that binds to GLP receptors on habenular axon terminals, which subsequently increases IPN neuron activation and decreases nicotine self-administration 73 . Taken together, these findings suggest a dynamic signalling process at MHb axonal terminals in the IPN, which regulates the addictive properties of nicotine and determines the amount of nicotine that is self-administered.
Nicotine withdrawal in animal models can be assessed by examining somatic signs (such as shaking, scratching, head nods and chewing) and affective signs (such as increased anxiety-related behaviours and conditioned place aversion). Interestingly, few nicotine withdrawal somatic signs are found in mice with genetic knockout of the α 2 , α 5 or β 4 nAChR subunits 74 , 75 . By contrast, β 2 nAChR-knockout mice have fewer anxiety-related behaviours during nicotine withdrawal, with no differences in somatic symptoms compared with wild-type mice 74 , 76 .
In addition to the VTA (mediating reward) and the MHb–IPN pathway (mediating aversion), other brain areas are involved in nicotine addiction (Fig. 5 ). In animals, the insular cortex controls nicotine taking and nicotine seeking 77 . Moreover, humans with lesions of the insular cortex can quit smoking easily without relapse 78 . This finding led to the development of a novel therapeutic intervention modulating insula function (see Management, below) 79 , 80 . Various brain areas (shell of nucleus accumbens, basolateral amygdala and prelimbic cortex) expressing cannabinoid CB 1 receptors are also critical in controlling rewarding effects and relapse 81 , 82 . The α 1 -adrenergic receptor expressed in the cortex also control these effects, probably through glutamatergic afferents to the nucleus accumbens 83 .
Individual differences in nicotine addiction risk
Vulnerability to nicotine dependence varies between individuals, and the reasons for these differences are multidimensional. Many social factors (such as education level and income) play a role 84 . Broad psychological and social factors also modulate this risk. For example, peer smoking status, knowledge on effect of tobacco, expectation on social acceptance, exposure to passive smoking modulate the risk of initiating tobacco use 85 , 86 .
Genetic factors have a role in smoking initiation, the development of nicotine addiction and the likelihood of smoking cessation. Indeed, heritability has been estimated to contribute to approximatively half of the variability in nicotine dependence 87 , 88 , 89 , 90 . Important advances in our understanding of such genetic contributions have evolved with large-scale genome-wide association studies of smokers and non-smokers. One of the most striking findings has been that allelic variation in the CHRNA5 – CHRNA3 – CHRNB4 gene cluster, which encodes α 5 , α 3 and β 4 nAChR subunits, correlates with an increased vulnerability for nicotine addiction, indicated by a higher likelihood of becoming dependent on nicotine and smoking a greater number of cigarettes per day 91 , 92 , 93 , 94 , 95 . The most significant effect has been found for a single-nucleotide polymorphism in CHRNA5 (rs16969968), which results in an amino acid change and reduced function of α 5 -containing nAChRs 92 .
Allelic variation in CYP2A6 (encoding the CYP2A6 enzyme, which metabolizes nicotine) has also been associated with differential vulnerability to nicotine dependence 96 , 97 , 98 . CYP2A6 is highly polymorphic, resulting in variable enzymatic activity 96 , 99 , 100 . Individuals with allelic variation that results in slow nicotine metabolism consume less nicotine per day, experience less-severe withdrawal symptoms and are more successful at quitting smoking than individuals with normal or fast metabolism 101 , 102 , 103 , 104 . Moreover, individuals with slow nicotine metabolism have lower dopaminergic receptor expression in the dopamine D2 regions of the associative striatum and sensorimotor striatum in PET studies 105 and take fewer puffs of nicotine-containing cigarettes (compared with de-nicotinized cigarettes) in a forced choice task 106 . Slower nicotine metabolism is thought to increase the duration of action of nicotine, allowing nicotine levels to accumulate over time, therefore enabling lower levels of intake to sustain activation of nAChRs 107 .
Large-scale genetic studies have identified hundreds of other genetic loci that influence smoking initiation, age of smoking initiation, cigarettes smoked per day and successful smoking cessation 108 . The strongest genetic contributions to smoking through the nicotinic receptors and nicotine metabolism are among the strongest genetic contributors to lung cancer 109 . Other genetic variations (such as those related to cannabinoid, dopamine receptors or other neurotransmitters) may affect certain phenotypes related to smoking (such as nicotine preference and cue-reactivity) 110 , 111 , 112 , 113 , 114 , 115 .
Diagnosis, screening and prevention
Screening for cigarette smoking.
Screening for cigarette smoking should happen at every doctor’s visit 116 . In this regard, a simple and direct question about a person’s tobacco use can provide an opportunity to offer information about its potential risks and treatments to assist in quitting. All smokers should be offered assistance in quitting because even low levels of smoking present a significant health risk 33 , 117 , 118 . Smoking status can be assessed by self-categorization or self-reported assessment of smoking behaviour (Table 1 ). In people who smoke, smoking frequency can be assessed 119 and a combined quantity frequency measure such as pack-year history (that is, average number of cigarettes smoked per day multiplied by the number of years, divided by 20), can be used to estimate cumulative risk of adverse health outcomes. The Association for the Treatment of Tobacco Use and Dependence recommends that all electronic health records should document smoking status using the self-report categories listed in Table 1 .
Owing to the advent of e-cigarettes and heat-not-burn products, and the popularity of little cigars in the US that mimic combustible cigarettes, people who use tobacco may use multiple products concurrently 120 , 121 . Thus, screening for other nicotine and tobacco product use is important in clinical practice. The self-categorization approach can also be used to describe the use of these other products.
Traditionally tobacco use has been classified according to whether the smoker meets criteria for nicotine dependence in one of the two main diagnostic classifications: the DSM 122 (tobacco use disorder) and the ICD (tobacco dependence) 123 . The diagnosis of tobacco use disorder according to DSM-5 criteria requires the presence of at least 2 of 11 symptoms that have produced marked clinical impairment or distress within a 12-month period (Box 2 ). Of note, these symptoms are similar for all substance use disorder diagnoses and may not all be relevant to tobacco use disorder (such as failure to complete life roles). In the ICD-10, codes allow the identification of specific tobacco products used (cigarettes, chewing tobacco and other tobacco products).
Dependence can also be assessed as a continuous construct associated with higher levels of use, greater withdrawal and reduced likelihood of quitting. The level of dependence can be assessed with the Fagerström Test for Nicotine Dependence, a short questionnaire comprising six questions 124 (Box 2 ). A score of ≥4 indicates moderate to high dependence. As very limited time may be available in clinical consultations, the Heaviness of Smoking Index (HSI) was developed, which comprises two questions on the number of cigarettes smoked per day and how soon after waking the first cigarette is smoked 125 . The HSI can guide dosing for nicotine replacement therapy (NRT).
Other measures of cigarette dependence have been developed but are not used in the clinical setting, such as the Cigarette Dependence Scale 126 , Hooked on Nicotine Checklist 127 , Nicotine Dependence Syndrome Scale 128 , the Wisconsin Inventory of Smoking Dependence Motives (Brief) 129 and the Penn State Cigarette Dependence Index 130 . However, in practice, these are not often used, as the most important aspect is to screen for smoking and encourage all smokers to quit smoking regardless of their dependence status.
Box 2 DSM-5 criteria for tobacco use disorder and items of the Fagerström Test for nicotine dependence
DSM-5 (ref. 122 )
Taxonomic and diagnostic tool for tobacco use disorder published by the American Psychiatric Association.
A problematic pattern of tobacco use leading to clinically significant impairment or distress as manifested by at least two of the following, occurring within a 12-month period.
Tobacco often used in larger amounts or over a longer period of time than intended
A persistent desire or unsuccessful efforts to reduce or control tobacco use
A great deal of time spent in activities necessary to obtain or use tobacco
Craving, or a strong desire or urge to use tobacco
Recurrent tobacco use resulting in a failure to fulfil major role obligations at work, school or home
Continued tobacco use despite having persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of tobacco (for example, arguments with others about tobacco use)
Important social, occupational or recreational activities given up or reduced because of tobacco use
Recurrent tobacco use in hazardous situations (such as smoking in bed)
Tobacco use continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by tobacco use
Tolerance, defined by either of the following.
A need for markedly increased amounts of tobacco to achieve the desired effect
A markedly diminished effect with continued use of the same amount of tobacco
Withdrawal, manifesting as either of the following.
Withdrawal syndrome for tobacco
Tobacco (or a closely related substance, such as nicotine) taken to relieve or avoid withdrawal symptoms
Fagerström Test for Nicotine Dependence 124
A standard instrument for assessing the intensity of physical addiction to nicotine.
How soon after you wake up do you smoke your first cigarette?
Within 5 min (scores 3 points)
5 to 30 min (scores 2 points)
31 to 60 min (scores 1 point)
After 60 min (scores 0 points)
Do you find it difficult not to smoke in places where you should not, such as in church or school, in a movie, at the library, on a bus, in court or in a hospital?
Yes (scores 1 point)
No (scores 0 points)
Which cigarette would you most hate to give up; which cigarette do you treasure the most?
The first one in the morning (scores 1 point)
Any other one (scores 0 points)
How many cigarettes do you smoke each day?
10 or fewer (scores 0 points)
11 to 20 (scores 1 point)
21 to 30 (scores 2 points)
31 or more (scores 3 points)
Do you smoke more during the first few hours after waking up than during the rest of the day?
Do you still smoke if you are so sick that you are in bed most of the day or if you have a cold or the flu and have trouble breathing?
A score of 7–10 points is classified as highly dependent; 4–6 points is classified as moderately dependent; <4 points is classified as minimally dependent.
DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.
Young people who do not start smoking cigarettes between 15 and 25 years of age have a very low risk of ever smoking 24 , 131 , 132 . This age group provides a critical opportunity to prevent cigarette smoking using effective, evidence-based strategies to prevent smoking initiation and reduce escalation from experimentation to regular use 131 , 132 , 133 , 134 , 135 .
Effective prevention of cigarette uptake requires a comprehensive package of cost-effective policies 134 , 136 , 137 to synergistically reduce the population prevalence of cigarette smoking 131 , 135 . These policies include high rates of tobacco taxation 30 , 134 , 137 , 138 , widespread and rigorously enforced smoke-free policies 139 , bans on tobacco advertising and promotions 140 , use of plain packaging and graphic warnings about the health risks of smoking 135 , 141 , mass media and peer-based education programmes to discourage smoking, and enforcement of laws against the sale of cigarettes to young people below the minimum legal purchase age 131 , 135 . These policies make cigarettes less available and affordable to young people. Moreover, these policies make it more difficult for young people to purchase cigarettes and make smoking a much less socially acceptable practice. Of note, these policies are typically mostly enacted in HICs, which may be related to the declining prevalence of smoking in these countries, compared with the prevalence in LMICs.
Pharmacotherapy
Three evidence-based classes of pharmacotherapy are available for smoking cessation: NRT (using nicotine-based patches, gum, lozenges, mini-lozenges, nasal sprays and inhalers), varenicline (a nAChR partial agonist), and bupropion (a noradrenaline/dopamine reuptake inhibitor that also inhibits nAChR function and is also used as an antidepressant). These FDA-approved and EMA-approved pharmacotherapies are cost-effective smoking cessation treatments that double or triple successful abstinence rates compared with no treatment or placebo controls 116 , 142 .
Combinations of pharmacotherapies are also effective for smoking cessation 116 , 142 . For example, combining NRTs (such as the steady-state nicotine patch and as-needed NRT such as gum or mini-lozenge) is more effective than a single form of NRT 116 , 142 , 143 . Combining NRT and varenicline is the most effective smoking cessation pharmacotherapy 116 , 142 , 143 . Combining FDA-approved pharmacotherapy with behavioural counselling further increases the likelihood of successful cessation 142 . Second-line pharmacotherapies (for example, nortriptyline) have some potential for smoking cessation, but their use is limited due to their tolerability profile.
All smokers should receive pharmacotherapy to help them quit smoking, except those in whom pharmacotherapy has insufficient evidence of effectiveness (among adolescents, smokeless tobacco users, pregnant women or light smokers) or those in whom pharmacotherapy is medically contraindicated 144 . Table 2 provides specific information regarding dosing and duration for each FDA-approved pharmacotherapy. Extended use of pharmacotherapy beyond the standard 12-week regimen after cessation is effective and should be considered 116 . Moreover, preloading pharmacotherapy (that is, initiating cessation medication in advance of a quit attempt), especially with the nicotine patch, is a promising treatment, although further studies are required to confirm efficacy.
Cytisine has been used for smoking cessation in Eastern Europe for a long time and is available in some countries (such as Canada) without prescription 145 . Cytisine is a partial agonist of nAChRs and its structure was the precursor for the development of varenicline 145 . Cytisine is at least as effective as some approved pharmacotherapies for smoking cessation, such as NRT 146 , 147 , 148 , and the role of cytisine in smoking cessation is likely to expand in the future, notably owing to its much lower cost than traditional pharmacotherapies. E-cigarettes also have the potential to be useful as smoking cessation devices 149 , 150 . The 2020 US Surgeon General’s Report concluded that there was insufficient evidence to promote cytisine or e-cigarettes as effective smoking cessation treatments, but in the UK its use is recommended for smoking cessation (see ref. 15 for regularly updated review).
Counselling and behavioural treatments
Psychosocial counselling significantly increases the likelihood of successful cessation, especially when combined with pharmacotherapy. Even a counselling session lasting only 3 minutes can help smokers quit 116 , although the 2008 US Public Health Service guidelines and the Preventive Services Task Force 151 each concluded that more intensive counselling (≥20 min per session) is more effective than less intensive counselling (<20 min per session). Higher smoking cessation rates are obtained by using behavioural change techniques that target associative and self-regulatory processes 152 . In addition, behavioural change techniques that will favour commitment, social reward and identity associated with changed behaviour seems associated with higher success rates 152 . Evidence-based counselling focuses on providing social support during treatment, building skills to cope with withdrawal and cessation, and problem-solving in challenging situations 116 , 153 . Effective counselling can be delivered by diverse providers (such as physicians, nurses, pharmacists, social workers, psychologists and certified tobacco treatment specialists) 116 .
Counselling can be delivered in a variety of modalities. In-person individual and group counselling are effective, as is telephone counselling (quit lines) 142 . Internet and text-based intervention seem to be effective in smoking cessation, especially when they are interactive and tailored to a smoker’s specific circumstances 142 . Over the past several years, the number of smoking cessation smartphone apps has increased, but there the evidence that the use of these apps significantly increases smoking cessation rates is not sufficient.
Contingency management (providing financial incentives for abstinence or engagement in treatment) has shown promising results 154 , 155 but its effects are not sustained once the contingencies are removed 155 , 156 . Other treatments such as hypnosis, acupuncture and laser treatment have not been shown to improve smoking cessation rates compared with placebo treatments 116 . Moreover, no solid evidence supports the use of conventional transcranial magnetic stimulation (TMS) for long-term smoking cessation 157 , 158 .
Although a variety of empirically supported smoking cessation interventions are available, more than two-thirds of adult smokers who made quit attempts in the USA during the past year did not use an evidence-based treatment and the rate is likely to be lower in many other countries 142 . This speaks to the need to increase awareness of, and access to, effective cessation aids among all smokers.
Brain stimulation
The insula (part of the frontal cortex) is a critical brain structure involved in cigarette craving and relapse 78 , 79 . The activity of the insula can be modulated using an innovative approach called deep insula/prefrontal cortex TMS (deep TMS), which is effective in helping people quit smoking 80 , 159 . This approach has now been approved by the FDA as an effective smoking cessation intervention 80 . However, although this intervention was developed and is effective for smoking cessation, the number of people with access to it is limited owing to the limited number of sites equipped and with trained personnel, and the cost of this intervention.
Quality of life
Generic instruments (such as the Short-Form (SF-36) Health Survey) can be used to evaluate quality of life (QOL) in smokers. People who smoke rate their QOL lower than people who do not smoke both before and after they become smokers 160 , 161 . QOL improves when smokers quit 162 . Mental health may also improve on quitting smoking 163 . Moreover, QOL is much poorer in smokers with tobacco-related diseases, such as chronic respiratory diseases and cancers, than in individuals without tobacco-related diseases 161 , 164 . The dimensions of QOL that show the largest decrements in people who smoke are those related to physical health, day-to-day activities and mental health such as depression 160 . Smoking also increases the risk of diabetes mellitus 165 , 166 , which is a major determinant of poor QOL for a wide range of conditions.
The high toll of premature death from cigarette smoking can obscure the fact that many of the diseases that cause these deaths also produce substantial disability in the years before death 1 . Indeed, death in smokers is typically preceded by several years of living with the serious disability and impairment of everyday activities caused by chronic respiratory disease, heart disease and cancer 2 . Smokers’ QOL in these years may also be adversely affected by the adverse effects of the medical treatments that they receive for these smoking-related diseases (such as major surgery and radiotherapy).
Expanding cessation worldwide
The major global challenge is to consider individual and population-based strategies that could increase the substantially low rates of adult cessation in most LMICs and indeed strategies to ensure that even in HICs, cessation continues to increase. In general, the most effective tools recommended by WHO to expand cessation are the same tools that can prevent smoking initiation, notably higher tobacco taxes, bans on advertising and promotion, prominent warning labels or plain packaging, bans on public smoking, and mass media and educational efforts 29 , 167 . The effective use of these policies, particularly taxation, lags behind in most LMICs compared with most HICs, with important exceptions such as Brazil 167 . Access to effective pharmacotherapies and counselling as well as support for co-existing mental health conditions would also be required to accelerate cessation in LMICs. This is particularly important as smokers living in LMICs often have no access to the full range of effective treatment options.
Regulating access to e-cigarettes
How e-cigarettes should be used is debated within the tobacco control field. In some countries (for example, the UK), the use of e-cigarettes as a cigarette smoking cessation aid and as a harm reduction strategy is supported, based on the idea that e-cigarette use will lead to much less exposure to toxic compounds than tobacco use, therefore reducing global harm. In other countries (for example, the USA), there is more concern with preventing the increased use of e-cigarettes by youths that may subsequently lead to smoking 25 , 26 . Regulating e-cigarettes in nuanced ways that enable smokers to access those products whilst preventing their uptake among youths is critical.
Regulating nicotine content in tobacco products
Reducing the nicotine content of cigarettes could potentially produce less addictive products that would allow a gradual reduction in the population prevalence of smoking. Some clinical studies have found no compensatory increase in smoking whilst providing access to low nicotine tobacco 168 . Future regulation may be implemented to gradually decrease the nicotine content of combustible tobacco and other nicotine products 169 , 170 , 171 .
Tobacco end games
Some individuals have proposed getting rid of commercial tobacco products this century or using the major economic disruption arising from the COVID-19 pandemic to accelerate the demise of the tobacco industry 172 , 173 . Some tobacco producers have even proposed this strategy as an internal goal, with the idea of switching to nicotine delivery systems that are less harmful ( Philip Morris International ). Some countries are moving towards such an objective; for example, in New Zealand, the goal that fewer than 5% of New Zealanders will be smokers in 2025 has been set (ref. 174 ). The tobacco end-game approach would overall be the best approach to reduce the burden of tobacco use on society, but it would require coordination of multiple countries and strong public and private consensus on the strategy to avoid a major expansion of the existing illicit market in tobacco products in some countries.
Innovative interventions
The COVID-19 pandemic has shown that large-scale investment in research can lead to rapid development of successful therapeutic interventions. By contrast, smoking cessation has been underfunded compared with the contribution that it makes to the global burden of disease. In addition, there is limited coordination between research teams and most studies are small-scale and often underpowered 79 . It is time to fund an ambitious, coordinated programme of research to test the most promising therapies based on an increased understanding of the neurobiological basis of smoking and nicotine addiction (Table 3 ). Many of those ideas have not yet been tested properly and this could be carried out by a coordinated programme of research at the international level.
GBD 2019 Tobacco Collaborators. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet 397 , 2337–2360 (2021). This study summarizes the burden of disease induced by tobacco worldwide .
Google Scholar
West, R. Tobacco smoking: health impact, prevalence, correlates and interventions. Psychol. Health 32 , 1018–1036 (2017).
PubMed PubMed Central Google Scholar
West, R. The multiple facets of cigarette addiction and what they mean for encouraging and helping smokers to stop. COPD 6 , 277–283 (2009).
PubMed Google Scholar
Fagerström, K. Determinants of tobacco use and renaming the FTND to the Fagerström test for cigarette dependence. Nicotine Tob. Res. 14 , 75–78 (2012).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General (Centers for Disease Control and Prevention, 2014).
Doll, R. & Hill, A. B. Smoking and carcinoma of the lung; preliminary report. Br. Med. J. 2 , 739–748 (1950).
CAS PubMed PubMed Central Google Scholar
Royal College of Physicians. Smoking and health. Summary of a report of the Royal College of Physicians of London on smoking in relation to cancer of the lung and other diseases (Pitman Medical Publishing, 1962).
Henningfield, J. E., Smith, T. T., Kleykamp, B. A., Fant, R. V. & Donny, E. C. Nicotine self-administration research: the legacy of Steven R. Goldberg and implications for regulation, health policy, and research. Psychopharmacology 233 , 3829–3848 (2016).
Le Foll, B. & Goldberg, S. R. Effects of nicotine in experimental animals and humans: an update on addictive properties. Hand. Exp. Pharmacol. https://doi.org/10.1007/978-3-540-69248-5_12 (2009).
Article Google Scholar
Proctor, R. N. The history of the discovery of the cigarette–lung cancer link: evidentiary traditions, corporate denial, global toll. Tob. Control. 21 , 87–91 (2012).
Hall, B. J. et al. Differential effects of non-nicotine tobacco constituent compounds on nicotine self-administration in rats. Pharmacol. Biochem. Behav. 120 , 103–108 (2014).
Musso, F. et al. Smoking impacts on prefrontal attentional network function in young adult brains. Psychopharmacology 191 , 159–169 (2007).
CAS PubMed Google Scholar
Goriounova, N. A. & Mansvelder, H. D. Short- and long-term consequences of nicotine exposure during adolescence for prefrontal cortex neuronal network function. Cold Spring Harb. Perspect. Med. 2 , a012120 (2012).
Fagerström, K. O. & Bridgman, K. Tobacco harm reduction: the need for new products that can compete with cigarettes. Addictive Behav. 39 , 507–511 (2014).
Hartmann-Boyce, J. et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 9 , CD010216 (2021).
Jha, P. The hazards of smoking and the benefits of cessation: a critical summation of the epidemiological evidence in high-income countries. eLife https://doi.org/10.7554/eLife.49979 (2020).
Article PubMed PubMed Central Google Scholar
Palipudi, K. M. et al. Social determinants of health and tobacco use in thirteen low and middle income countries: evidence from Global Adult Tobacco Survey. PLoS ONE 7 , e33466 (2012).
Goodwin, R. D., Pagura, J., Spiwak, R., Lemeshow, A. R. & Sareen, J. Predictors of persistent nicotine dependence among adults in the United States. Drug Alcohol. Depend. 118 , 127–133 (2011).
Weinberger, A. H. et al. Cigarette use is increasing among people with illicit substance use disorders in the United States, 2002-14: emerging disparities in vulnerable populations. Addiction 113 , 719–728 (2018).
Evans-Polce, R. J., Kcomt, L., Veliz, P. T., Boyd, C. J. & McCabe, S. E. Alcohol, tobacco, and comorbid psychiatric disorders and associations with sexual identity and stress-related correlates. Am. J. Psychiatry 177 , 1073–1081 (2020).
Hassan, A. N. & Le Foll, B. Survival probabilities and predictors of major depressive episode incidence among individuals with various types of substance use disorders. J. Clin. Psychiatry https://doi.org/10.4088/JCP.20m13637 (2021).
Article PubMed Google Scholar
Smith, P. H., Mazure, C. M. & McKee, S. A. Smoking and mental illness in the U.S. population. Tob. Control. 23 , e147–e153 (2014).
Bourgault, Z., Rubin-Kahana, D. S., Hassan, A. N., Sanches, M. & Le Foll, B. Multiple substance use disorders and self-reported cognitive function in U.S. adults: associations and sex-differences in a nationally representative sample. Front. Psychiatry https://doi.org/10.3389/fpsyt.2021.797578 (2022).
Reitsma, M. B. et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and initiation among young people in 204 countries and territories, 1990-2019. Lancet Public Health 6 , e472–e481 (2021).
Warner, K. E. How to think–not feel–about tobacco harm reduction. Nicotine Tob. Res. 21 , 1299–1309 (2019).
Soneji, S. et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 171 , 788–797 (2017).
Levy, D. T. et al. Examining the relationship of vaping to smoking initiation among US youth and young adults: a reality check. Tob. Control. 28 , 629–635 (2019).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking — 50 years of progress: a report of the Surgeon General (Centers for Disease Control and Prevention, 2014).
Jha, P. & Peto, R. Global effects of smoking, of quitting, and of taxing tobacco. N. Engl. J. Med. 370 , 60–68 (2014). This review covers the impact of tobacco, of quitting smoking and the importance of taxation to impact prevalence of smoking .
Jha, P. & Peto., R. in Tobacco Tax Reform: At the Crossroads of Health and Development . (eds Marquez, P. V. & Moreno-Dodson, B.) 55–72 (World Bank Group, 2017).
Jha, P. et al. 21st-century hazards of smoking and benefits of cessation in the United States. N. Engl. J. Med. 368 , 341–350 (2013).
Banks, E. et al. Tobacco smoking and all-cause mortality in a large Australian cohort study: findings from a mature epidemic with current low smoking prevalence. BMC Med. 13 , 38 (2015).
Pirie, K. et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 381 , 133–141 (2013).
Jha, P. et al. A nationally representative case-control study of smoking and death in India. N. Engl. J. Med. 358 , 1137–1147 (2008).
Chan, E. D. et al. Tobacco exposure and susceptibility to tuberculosis: is there a smoking gun? Tuberculosis 94 , 544–550 (2014).
Wang, M. G. et al. Association between tobacco smoking and drug-resistant tuberculosis. Infect. Drug Resist. 11 , 873–887 (2018).
Jha, P. et al. Social inequalities in male mortality, and in male mortality from smoking: indirect estimation from national death rates in England and Wales, Poland, and North America. Lancet 368 , 367–370 (2006).
Jha, P., Gelband, H, Irving, H. & Mishra, S. in Reducing Social Inequalities in Cancer: Evidence and Priorities for Research (eds Vaccarella, S et al.) 161–166 (IARC, 2018).
Jha, P. Expanding smoking cessation world-wide. Addiction 113 , 1392–1393 (2018).
Jha, P. Avoidable global cancer deaths and total deaths from smoking. Nat. Rev. Cancer 9 , 655–664 (2009).
Wittenberg, R. E., Wolfman, S. L., De Biasi, M. & Dani, J. A. Nicotinic acetylcholine receptors and nicotine addiction: a brief introduction. Neuropharmacology 177 , 108256 (2020).
Boulter, J. et al. Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc. Natl Acad. Sci. USA 84 , 7763–7767 (1987).
Couturier, S. et al. A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-BTX. Neuron 5 , 847–856 (1990).
Picciotto, M. R., Addy, N. A., Mineur, Y. S. & Brunzell, D. H. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog. Neurobiol. 84 , 329–342 (2008).
Changeux, J. P. Structural identification of the nicotinic receptor ion channel. Trends Neurosci. 41 , 67–70 (2018).
McKay, B. E., Placzek, A. N. & Dani, J. A. Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochem. Pharmacol. 74 , 1120–1133 (2007).
Wonnacott, S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 20 , 92–98 (1997).
Wooltorton, J. R., Pidoplichko, V. I., Broide, R. S. & Dani, J. A. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J. Neurosci. 23 , 3176–3185 (2003).
Gipson, C. D. & Fowler, C. D. Nicotinic receptors underlying nicotine dependence: evidence from transgenic mouse models. Curr. Top. Behav. Neurosci. 45 , 101–121 (2020).
Hamouda, A. K. et al. Potentiation of (α4)2(β2)3, but not (α4)3(β2)2, nicotinic acetylcholine receptors reduces nicotine self-administration and withdrawal symptoms. Neuropharmacology 190 , 108568 (2021).
Lallai, V. et al. Nicotine acts on cholinergic signaling mechanisms to directly modulate choroid plexus function. eNeuro https://doi.org/10.1523/ENEURO.0051-19.2019 (2019).
Benwell, M. E., Balfour, D. J. & Anderson, J. M. Evidence that tobacco smoking increases the density of (-)-[3H]nicotine binding sites in human brain. J. Neurochem. 50 , 1243–1247 (1988).
Perry, D. C., Davila-Garcia, M. I., Stockmeier, C. A. & Kellar, K. J. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J. Pharmacol. Exp. Ther. 289 , 1545–1552 (1999).
Marks, M. J. et al. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J. Neurosci. 12 , 2765–2784 (1992).
Le Foll, B. et al. Impact of short access nicotine self-administration on expression of α4β2* nicotinic acetylcholine receptors in non-human primates. Psychopharmacology 233 , 1829–1835 (2016).
Meyers, E. E., Loetz, E. C. & Marks, M. J. Differential expression of the beta4 neuronal nicotinic receptor subunit affects tolerance development and nicotinic binding sites following chronic nicotine treatment. Pharmacol. Biochem. Behav. 130 , 1–8 (2015).
Zhao-Shea, R., Liu, L., Pang, X., Gardner, P. D. & Tapper, A. R. Activation of GABAergic neurons in the interpeduncular nucleus triggers physical nicotine withdrawal symptoms. Curr. Biol. 23 , 2327–2335 (2013).
Jensen, K. P., Valentine, G., Gueorguieva, R. & Sofuoglu, M. Differential effects of nicotine delivery rate on subjective drug effects, urges to smoke, heart rate and blood pressure in tobacco smokers. Psychopharmacology 237 , 1359–1369 (2020).
Villanueva, H. F., James, J. R. & Rosecrans, J. A. Evidence of pharmacological tolerance to nicotine. NIDA Res. Monogr. 95 , 349–350 (1989).
Corrigall, W. A., Coen, K. M. & Adamson, K. L. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 653 , 278–284 (1994).
Nisell, M., Nomikos, G. G., Hertel, P., Panagis, G. & Svensson, T. H. Condition-independent sensitization of locomotor stimulation and mesocortical dopamine release following chronic nicotine treatment in the rat. Synapse 22 , 369–381 (1996).
Rice, M. E. & Cragg, S. J. Nicotine amplifies reward-related dopamine signals in striatum. Nat. Neurosci. 7 , 583–584 (2004).
Mameli-Engvall, M. et al. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron 50 , 911–921 (2006).
Picciotto, M. R., Higley, M. J. & Mineur, Y. S. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76 , 116–129 (2012).
Le Foll, B. et al. Elevation of dopamine induced by cigarette smoking: novel insights from a [11C]-+-PHNO PET study in humans. Neuropsychopharmacology 39 , 415–424 (2014). This brain imaging study identified the brain areas in which smoking elevates dopamine levels .
Maskos, U. et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436 , 103–107 (2005). This article discusses the implication of the β 2 - containing nAChRs in the VTA in mammalian cognitive function .
Picciotto, M. R. et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391 , 173–177 (1998). This article discusses the implication of the β 2 - containing nAChRs in addictive effects of nicotine .
Fowler, C. D., Lu, Q., Johnson, P. M., Marks, M. J. & Kenny, P. J. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471 , 597–601 (2011). This article discusses the implication of the α5 nicotinic receptor located in the MHb in a mechanism mediating the aversive effects of nicotine .
Elayouby, K. S. et al. α3* Nicotinic acetylcholine receptors in the habenula-interpeduncular nucleus circuit regulate nicotine intake. J. Neurosci. https://doi.org/10.1523/JNEUROSCI.0127-19.2020 (2020).
Ables, J. L. et al. Retrograde inhibition by a specific subset of interpeduncular α5 nicotinic neurons regulates nicotine preference. Proc. Natl Acad. Sci. USA 114 , 13012–13017 (2017).
Frahm, S. et al. Aversion to nicotine is regulated by the balanced activity of β4 and α5 nicotinic receptor subunits in the medial habenula. Neuron 70 , 522–535 (2011).
Jackson, K. J. et al. Role of α5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J. Pharmacol. Exp. Ther. 334 , 137–146 (2010).
Tuesta, L. M. et al. GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nat. Neurosci. 20 , 708–716 (2017).
Salas, R., Pieri, F. & De Biasi, M. Decreased signs of nicotine withdrawal in mice null for the β4 nicotinic acetylcholine receptor subunit. J. Neurosci. 24 , 10035–10039 (2004).
Salas, R., Sturm, R., Boulter, J. & De Biasi, M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J. Neurosci. 29 , 3014–3018 (2009).
Jackson, K. J., Martin, B. R., Changeux, J. P. & Damaj, M. I. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J. Pharmacol. Exp. Ther. 325 , 302–312 (2008).
Le Foll, B. et al. Translational strategies for therapeutic development in nicotine addiction: rethinking the conventional bench to bedside approach. Prog. Neuropsychopharmacol. Biol. Psychiatry 52 , 86–93 (2014).
Naqvi, N. H., Rudrauf, D., Damasio, H. & Bechara, A. Damage to the insula disrupts addiction to cigarette smoking. Science 315 , 531–534 (2007). This article discusses the implication of the insular cortex in tobacco addiction .
Ibrahim, C. et al. The insula: a brain stimulation target for the treatment of addiction. Front. Pharmacol. 10 , 720 (2019).
Zangen, A. et al. Repetitive transcranial magnetic stimulation for smoking cessation: a pivotal multicenter double-blind randomized controlled trial. World Psychiatry 20 , 397–404 (2021). This study validated the utility of deep insula/prefrontal cortex rTMS for smoking cessation .
Le Foll, B., Forget, B., Aubin, H. J. & Goldberg, S. R. Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: insights from pre-clinical and clinical studies. Addict. Biol. 13 , 239–252 (2008).
Kodas, E., Cohen, C., Louis, C. & Griebel, G. Cortico-limbic circuitry for conditioned nicotine-seeking behavior in rats involves endocannabinoid signaling. Psychopharmacology 194 , 161–171 (2007).
Forget, B. et al. Noradrenergic α1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology 35 , 1751–1760 (2010).
Garrett, B. E., Dube, S. R., Babb, S. & McAfee, T. Addressing the social determinants of health to reduce tobacco-related disparities. Nicotine Tob. Res. 17 , 892–897 (2015).
Polanska, K., Znyk, M. & Kaleta, D. Susceptibility to tobacco use and associated factors among youth in five central and eastern European countries. BMC Public Health 22 , 72 (2022).
Volkow, N. D. Personalizing the treatment of substance use disorders. Am. J. Psychiatry 177 , 113–116 (2020).
Li, M. D., Cheng, R., Ma, J. Z. & Swan, G. E. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction 98 , 23–31 (2003).
Carmelli, D., Swan, G. E., Robinette, D. & Fabsitz, R. Genetic influence on smoking–a study of male twins. N. Engl. J. Med. 327 , 829–833 (1992).
Broms, U., Silventoinen, K., Madden, P. A. F., Heath, A. C. & Kaprio, J. Genetic architecture of smoking behavior: a study of Finnish adult twins. Twin Res. Hum. Genet. 9 , 64–72 (2006).
Kendler, K. S., Thornton, L. M. & Pedersen, N. L. Tobacco consumption in Swedish twins reared apart and reared together. Arch. Gen. Psychiat 57 , 886–892 (2000).
Saccone, N. L. et al. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res. 69 , 6848–6856 (2009).
Bierut, L. J. et al. Variants in nicotinic receptors and risk for nicotine dependence. Am. J. Psychiatry 165 , 1163–1171 (2008). This study demonstrates that nAChR gene variants are important in nicotine addiction .
Bierut, L. J. et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum. Mol. Genet. 16 , 24–35 (2007).
Berrettini, W. et al. α-5/α-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol. Psychiatry 13 , 368–373 (2008).
Sherva, R. et al. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with ‘pleasurable buzz’ during early experimentation with smoking. Addiction 103 , 1544–1552 (2008).
Thorgeirsson, T. E. et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat. Genet. 42 , 448–453 (2010).
Ray, R., Tyndale, R. F. & Lerman, C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. J. Neurogenet. 23 , 252–261 (2009).
Bergen, A. W. et al. Drug metabolizing enzyme and transporter gene variation, nicotine metabolism, prospective abstinence, and cigarette consumption. PLoS ONE 10 , e0126113 (2015).
Mwenifumbo, J. C. et al. Identification of novel CYP2A6*1B variants: the CYP2A6*1B allele is associated with faster in vivo nicotine metabolism. Clin. Pharmacol. Ther. 83 , 115–121 (2008).
Raunio, H. & Rahnasto-Rilla, M. CYP2A6: genetics, structure, regulation, and function. Drug Metab. Drug Interact. 27 , 73–88 (2012).
CAS Google Scholar
Patterson, F. et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin. Pharmacol. Ther. 84 , 320–325 (2008).
Rodriguez, S. et al. Combined analysis of CHRNA5, CHRNA3 and CYP2A6 in relation to adolescent smoking behaviour. J. Psychopharmacol. 25 , 915–923 (2011).
Strasser, A. A., Malaiyandi, V., Hoffmann, E., Tyndale, R. F. & Lerman, C. An association of CYP2A6 genotype and smoking topography. Nicotine Tob. Res. 9 , 511–518 (2007).
Liakoni, E. et al. Effects of nicotine metabolic rate on withdrawal symptoms and response to cigarette smoking after abstinence. Clin. Pharmacol. Ther. 105 , 641–651 (2019).
Di Ciano, P. et al. Influence of nicotine metabolism ratio on [11C]-(+)-PHNO PET binding in tobacco smokers. Int. J. Neuropsychopharmacol. 21 , 503–512 (2018).
Butler, K. et al. Impact of Cyp2a6 activity on nicotine reinforcement and cue-reactivity in daily smokers. Nicotine Tob. Res. https://doi.org/10.1093/ntr/ntab064 (2021).
Benowitz, N. L., Swan, G. E., Jacob, P. 3rd, Lessov-Schlaggar, C. N. & Tyndale, R. F. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin. Pharmacol. Ther. 80 , 457–467 (2006).
Liu, M. et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat. Genet. 51 , 237–244 (2019).
McKay, J. D. et al. Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat. Genet. 49 , 1126–1132 (2017).
Chukwueke, C. C. et al. The CB1R rs2023239 receptor gene variant significantly affects the reinforcing effects of nicotine, but not cue reactivity, in human smokers. Brain Behav. 11 , e01982 (2021).
Ahrens, S. et al. Modulation of nicotine effects on selective attention by DRD2 and CHRNA4 gene polymorphisms. Psychopharmacology 232 , 2323–2331 (2015).
Harrell, P. T. et al. Dopaminergic genetic variation moderates the effect of nicotine on cigarette reward. Psychopharmacology 233 , 351–360 (2016).
Lerman, C. et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacology 31 , 231–242 (2006).
Le Foll, B., Gallo, A., Le Strat, Y., Lu, L. & Gorwood, P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav. Pharmacol. 20 , 1–17 (2009).
Chukwueke, C. C. et al. Exploring the role of the Ser9Gly (rs6280) dopamine D3 receptor polymorphism in nicotine reinforcement and cue-elicited craving. Sci. Rep. 10 , 4085 (2020).
The Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff A clinical practice guideline for treating tobacco use and dependence: 2008 update: a U.S. Public Health Service report. Am. J. Prev. Med. 35 , 158–176 (2008).
Hackshaw, A., Morris, J. K., Boniface, S., Tang, J. L. & Milenković, D. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ 360 , j5855 (2018).
Qin, W. et al. Light cigarette smoking increases risk of all-cause and cause-specific mortality: findings from the NHIS cohort study. Int. J. Env. Res. Public Health https://doi.org/10.3390/ijerph17145122 (2020).
Rodu, B. & Plurphanswat, N. Mortality among male smokers and smokeless tobacco users in the USA. Harm Reduct. J. 16 , 50 (2019).
Kasza, K. A. et al. Tobacco-product use by adults and youths in the United States in 2013 and 2014. N. Engl. J. Med. 376 , 342–353 (2017).
Richardson, A., Xiao, H. & Vallone, D. M. Primary and dual users of cigars and cigarettes: profiles, tobacco use patterns and relevance to policy. Nicotine Tob. Res. 14 , 927–932 (2012).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental disorders 5th edn (American Psychiatric Association, 2013).
World Health Organization. Tobacco fact sheet. WHO https://www.who.int/news-room/fact-sheets/detail/tobacco (2021).
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C. & Fagerström, K. O. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br. J. Addict. 86 , 1119–1127 (1991).
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C., Rickert, W. & Robinson, J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br. J. Addict. 84 , 791–799 (1989).
Etter, J. F., Le Houezec, J. & Perneger, T. V. A self-administered questionnaire to measure dependence on cigarettes: the cigarette dependence scale. Neuropsychopharmacology 28 , 359–370 (2003).
DiFranza, J. R. et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch. Pediatr. Adolesc. Med. 156 , 397–403 (2002).
Shiffman, S., Waters, A. & Hickcox, M. The Nicotine Dependence Syndrome Scale: a multidimensional measure of nicotine dependence. Nicotine Tob. Res. 6 , 327–348 (2004).
Smith, S. S. et al. Development of the Brief Wisconsin Inventory of Smoking Dependence Motives. Nicotine Tob. Res. 12 , 489–499 (2010).
Foulds, J. et al. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users. Nicotine Tob. Res. 17 , 186–192 (2015).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Preventing tobacco use among youth and young adults: a report of the Surgeon General (Centers for Disease Control and Prevention, 2012).
World Health Organization. Tobacco control to improve child health and development. Thematic brief (WHO, 2021).
Lantz, P. M. et al. Investing in youth tobacco control: a review of smoking prevention and control strategies. Tob. Control. 9 , 47–63 (2000).
Leão, T., Kunst, A. E. & Perelman, J. Cost-effectiveness of tobacco control policies and programmes targeting adolescents: a systematic review. Eur. J. Public Health 28 , 39–43 (2018).
Royal College of Physicians. Smoking and health 2021: a coming of age for tobacco control? (RCP, 2021).
Higashi, H. et al. Cost effectiveness of tobacco control policies in Vietnam: the case of population-level interventions. Appl. Health Econ. Health Policy 9 , 183–196 (2011).
Ranson, M. K., Jha, P., Chaloupka, F. J. & Nguyen, S. N. Global and regional estimates of the effectiveness and cost-effectiveness of price increases and other tobacco control policies. Nicotine Tob. Res. 4 , 311–319 (2002).
International Agency for Research on Cancer. I ARC Handbooks of Cancer Prevention: Tobacco control Vol. 14 (IARC, 2011).
Frazer, K. et al. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database Syst. Rev. 2 , CD005992 (2016).
Hoffman, S. J. & Tan, C. Overview of systematic reviews on the health-related effects of government tobacco control policies. BMC Public Health 15 , 744 (2015).
McNeill, A. et al. Tobacco packaging design for reducing tobacco use. Cochrane Database Syst. Rev. 4 , CD011244 (2017).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Smoking cessation: a report of the Surgeon General (Department of Health and Human Services, 2020).
Lindson, N. et al. Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev. 4 , CD013308 (2019).
Krist, A. H. et al. Interventions for tobacco smoking cessation in adults, including pregnant persons: US Preventive Services Task Force recommendation statement. JAMA 325 , 265–279 (2021).
Tutka, P. & Zatonski, W. Cytisine for the treatment of nicotine addiction: from a molecule to therapeutic efficacy. Pharmacol. Rep. 58 , 777–798 (2006).
Courtney, R. J. et al. Effect of cytisine vs varenicline on smoking cessation: a randomized clinical trial. JAMA 326 , 56–64 (2021).
Walker, N. et al. Cytisine versus nicotine for smoking cessation. N. Engl. J. Med. 371 , 2353–2362 (2014). This study validated the utility of cytisine for smoking cessation .
West, R. et al. Placebo-controlled trial of cytisine for smoking cessation. N. Engl. J. Med. 365 , 1193–1200 (2011).
Hajek, P. et al. E-cigarettes compared with nicotine replacement therapy within the UK Stop Smoking Services: the TEC RCT. Health Technol. Assess. 23 , 1–82 (2019).
Walker, N. et al. Nicotine patches used in combination with e-cigarettes (with and without nicotine) for smoking cessation: a pragmatic, randomised trial. Lancet Respir. Med. 8 , 54–64 (2020).
Siu, A. L., U.S. Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 163 , 622–634 (2015).
Black, N. et al. Behaviour change techniques associated with smoking cessation in intervention and comparator groups of randomized controlled trials: a systematic review and meta-regression. Addiction 115 , 2008–2020 (2020).
Center for Substance Abuse and Treatment. Detoxification and Substance Abuse Treatment (Center for Substance Abuse and Treatment, 2006).
Cahill, K., Hartmann-Boyce, J. & Perera, R. Incentives for smoking cessation. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD004307.pub5 (2015).
Secades-Villa, R., Aonso-Diego, G., García-Pérez, Á. & González-Roz, A. Effectiveness of contingency management for smoking cessation in substance users: a systematic review and meta-analysis. J. Consult. Clin. Psychol. 88 , 951–964 (2020).
Cahill, K. & Perera, R. Competitions and incentives for smoking cessation. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD004307.pub4 (2011).
Trojak, B. et al. Transcranial magnetic stimulation combined with nicotine replacement therapy for smoking cessation: a randomized controlled trial. Brain Stimul. 8 , 1168–1174 (2015).
Wing, V. C. et al. Brain stimulation methods to treat tobacco addiction. Brain Stimul. 6 , 221–230 (2013).
Dinur-Klein, L. et al. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol. Psychiatry 76 , 742–749 (2014).
Goldenberg, M., Danovitch, I. & IsHak, W. W. Quality of life and smoking. Am. J. Addict. 23 , 540–562 (2014).
Heikkinen, H., Jallinoja, P., Saarni, S. I. & Patja, K. The impact of smoking on health-related and overall quality of life: a general population survey in Finland. Nicotine Tob. Res. 10 , 1199–1207 (2008).
Moayeri, F., Hsueh, Y. A., Dunt, D. & Clarke, P. Smoking cessation and quality of life: insights from analysis of longitudinal Australian data, an application for economic evaluations. Value Health 24 , 724–732 (2021).
Taylor, G. M. et al. Smoking cessation for improving mental health. Cochrane Database Syst. Rev. 3 , CD013522 (2021).
López-Nicolás, Á., Trapero-Bertran, M. & Muñoz, C. Smoking, health-related quality of life and economic evaluation. Eur. J. Health Econ. 19 , 747–756 (2018).
Morris, A. Linking nicotine addiction and T2DM. Nat. Rev. Endocrinol. 16 , 6 (2020).
Willi, C., Bodenmann, P., Ghali, W. A., Faris, P. D. & Cornuz, J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. Jama 298 , 2654–2664 (2007).
World Health Organization. WHO report on the global tobacco epidemic (WHO, 2019).
Donny, E. C. et al. Randomized trial of reduced-nicotine standards for cigarettes. N. Engl. J. Med. 373 , 1340–1349 (2015). This study tested the impact of reducing the quantity of nicotine present in cigarettes on smoking .
Benowitz, N. L. & Henningfield, J. E. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N. Engl. J. Med. 331 , 123–125 (1994).
Benowitz, N. L. & Henningfield, J. E. Reducing the nicotine content to make cigarettes less addictive. Tob. Control. 22 , i14–i17 (2013).
Gottlieb, S. & Zeller, M. A nicotine-focused framework for public health. N. Engl. J. Med. 377 , 1111–1114 (2017).
Hall, W. & West, R. Thinking about the unthinkable: a de facto prohibition on smoked tobacco products. Addiction 103 , 873–874 (2008).
Ioannidis, J. P. A. & Jha, P. Does the COVID-19 pandemic provide an opportunity to eliminate the tobacco industry? Lancet Glob. Health 9 , e12–e13 (2021).
Smokefree. Smokefree 2025. Smokefree https://www.smokefree.org.nz/smokefree-in-action/smokefree-aotearoa-2025 (2021).
Morgan, C. J., Das, R. K., Joye, A., Curran, H. V. & Kamboj, S. K. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict. Behav. 38 , 2433–2436 (2013).
Elsaid, S., Kloiber, S. & Le Foll, B. Effects of cannabidiol (CBD) in neuropsychiatric disorders: a review of pre-clinical and clinical findings. Prog. Mol. Biol. Transl. Sci. 167 , 25–75 (2019).
Butler, K. & Le Foll, B. Novel therapeutic and drug development strategies for tobacco use disorder: endocannabinoid modulation. Expert Opin. Drug Discov. 15 , 1065–1080 (2020).
D’Souza, D. C. et al. Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: a double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trial. Lancet Psychiatry 6 , 35–45 (2019).
Robinson, J. D. et al. Pooled analysis of three randomized, double-blind, placebo controlled trials with rimonabant for smoking cessation. Addict. Biol. 23 , 291–303 (2018).
Gueye, A. B. et al. The CB1 neutral antagonist AM4113 retains the therapeutic efficacy of the inverse agonist rimonabant for nicotine dependence and weight loss with better psychiatric tolerability. Int. J. Neuropsychopharmacol. https://doi.org/10.1093/ijnp/pyw068 (2016).
Yammine, L. et al. Exenatide adjunct to nicotine patch facilitates smoking cessation and may reduce post-cessation weight gain: a pilot randomized controlled trial. Nicotine Tob. Res. 23 , 1682–1690 (2021).
Eren-Yazicioglu, C. Y., Yigit, A., Dogruoz, R. E. & Yapici-Eser, H. Can GLP-1 be a target for reward system related disorders? A qualitative synthesis and systematic review analysis of studies on palatable food, drugs of abuse, and alcohol. Front. Behav. Neurosci. 14 , 614884 (2020).
Vanderkam, P. et al. Effectiveness of drugs acting on adrenergic receptors in the treatment for tobacco or alcohol use disorders: systematic review and meta-analysis. Addiction 116 , 1011–1020 (2021).
Sokoloff, P. & Le Foll, B. The dopamine D3 receptor, a quarter century later. Eur. J. Neurosci. 45 , 2–19 (2017).
David, S. P., Lancaster, T., Stead, L. F., Evins, A. E. & Prochaska, J. J. Opioid antagonists for smoking cessation. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD003086.pub3 (2013).
Ray, L. A. et al. Efficacy of combining varenicline and naltrexone for smoking cessation and drinking reduction: a randomized clinical trial. Am. J. Psychiatry 178 , 818–828 (2021).
Mooney, M. E. et al. Bupropion and naltrexone for smoking cessation: a double-blind randomized placebo-controlled clinical trial. Clin. Pharmacol. Ther. 100 , 344–352 (2016).
Justinova, Z., Le Foll, B., Redhi, G. H., Markou, A. & Goldberg, S. R. Differential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on nicotine versus cocaine self-administration and relapse in squirrel monkeys. Psychopharmacology 233 , 1791–1800 (2016).
Le Foll, B., Wertheim, C. E. & Goldberg, S. R. Effects of baclofen on conditioned rewarding and discriminative stimulus effects of nicotine in rats. Neurosci. Lett. 443 , 236–240 (2008).
Franklin, T. R. et al. The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study. Drug Alcohol. Depend. 103 , 30–36 (2009).
Lotfy, N., Elsawah, H. & Hassan, M. Topiramate for smoking cessation: systematic review and meta-analysis. Tob. Prev. Cessat. 6 , 14 (2020).
Shanahan, W. R., Rose, J. E., Glicklich, A., Stubbe, S. & Sanchez-Kam, M. Lorcaserin for smoking cessation and associated weight gain: a randomized 12-week clinical trial. Nicotine Tob. Res. 19 , 944–951 (2017).
Higgins, G. A., Fletcher, P. J. & Shanahan, W. R. Lorcaserin: a review of its preclinical and clinical pharmacology and therapeutic potential. Pharmacol. Ther. 205 , 107417 (2020).
Stead, L. F. & Lancaster, T. Interventions to reduce harm from continued tobacco use. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD005231.pub2 (2007).
Download references
Acknowledgements
B.Le F. is supported by a clinician-scientist award from the Department of Family and Community Medicine at the University of Toronto and the Addiction Psychiatry Chair from the University of Toronto. The funding bodies had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication. The authors thank H. Fu (University of Toronto) for assistance with Figs 1–3.
Author information
Authors and affiliations.
Translational Addiction Research Laboratory, Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, University of Toronto, Toronto, Ontario, Canada
Bernard Le Foll
Departments of Family and Community Medicine, Psychiatry, Pharmacology and Toxicology, University of Toronto, Toronto, Ontario, Canada
Department of Medicine, University of Wisconsin, Madison, WI, USA
Megan E. Piper
University of Wisconsin Center for Tobacco Research and Intervention, Madison, WI, USA
Department of Neurobiology and Behaviour, University of California Irvine, Irvine, CA, USA
Christie D. Fowler
Section for Preventive Cardiology, Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway
Serena Tonstad
Department of Psychiatry, Washington University School of Medicine, St. Louis, MO, USA
Laura Bierut
Institute of Mental Health, Peking University Sixth Hospital, Peking University, Beijing, China
National Institute on Drug Dependence, Peking University Health Science Center, Beijing, China
Centre for Global Health Research, Unity Health Toronto, University of Toronto, Toronto, Ontario, Canada
- Prabhat Jha
National Centre for Youth Substance Use Research, The University of Queensland, St Lucia, Queensland, Australia
Wayne D. Hall
Queensland Alliance for Environmental Health Sciences, The University of Queensland, Woolloongabba, Queensland, Australia
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (B.Le F.); Epidemiology (P.J. and W.D.H.); Mechanisms/pathophysiology (C.D.F., L.B., L.L. and B.Le F.); Diagnosis, screening and prevention (P.J., M.E.P., S.T. and B.Le F.); Management (M.E.P., S.T., W.D.H., L.L. and B.Le F.); Quality of life (P.J. and W.D.H.); Outlook (all); Conclusions (all). All authors contributed substantially to the review and editing of the manuscript.
Corresponding author
Correspondence to Bernard Le Foll .
Ethics declarations
Competing interests.
B.Le F. has obtained funding from Pfizer (GRAND Awards, including salary support) for investigator-initiated projects. B.Le F. has received some in-kind donations of cannabis product from Aurora and medication donation from Pfizer and Bioprojet and was provided a coil for TMS study from Brainsway. B.Le F. has obtained industry funding from Canopy (through research grants handled by CAMH or the University of Toronto), Bioprojet, ACS, Indivior and Alkermes. B.Le F. has received in-kind donations of nabiximols from GW Pharma for past studies funded by CIHR and NIH. B.Le F. has been an advisor to Shinoghi. S.T. has received honoraria from Pfizer the manufacturer of varenicline for lectures and advice. All other authors declare no competing interests.
Peer review
Peer reviewer information.
Nature Reviews Disease Primers thanks Jamie Brown, Elisardo Iglesias and Ming Li for their contribution to the peer review of this work.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
CTRI website: https://d3futrf33lk36a.cloudfront.net/wp-content/uploads/sites/240/2019/04/Meds-Chart.pdf
Philip Morris International: https://www.pmi.com/
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Le Foll, B., Piper, M.E., Fowler, C.D. et al. Tobacco and nicotine use. Nat Rev Dis Primers 8 , 19 (2022). https://doi.org/10.1038/s41572-022-00346-w
Download citation
Accepted : 07 February 2022
Published : 24 March 2022
DOI : https://doi.org/10.1038/s41572-022-00346-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Integrated mrna- and mirna-sequencing analyses unveil the underlying mechanism of tobacco pollutant-induced developmental toxicity in zebrafish embryos.
- Jiasheng Chen
- Xiaoping Zhong
Journal of Translational Medicine (2024)
Reductions in smoking due to ratification of the Framework Convention for Tobacco Control in 171 countries
- Guillermo Paraje
- Mauricio Flores Muñoz
Nature Medicine (2024)
Impact of benzoic acid and 2,2’-methylenebis (6-tert-butyl-4-methylphenol) on the metabolome of flue-cured tobacco and rhizosphere microbial communities: implications for continuous cropping obstacles
- Qiulian Peng
- Jiayan Zhang
Plant and Soil (2024)
When smoke meets gut: deciphering the interactions between tobacco smoking and gut microbiota in disease development
- Guangyi Zeng
- Changtao Jiang
Science China Life Sciences (2024)
Effectiveness of health promotion intervention on the knowledge and selected practices related with oral cancer among a group of vulnerable youth in Sri Lanka
- Manori Dhanapriyanka
- Kanthi RDFC
- Prasanna Jayasekara
BMC Public Health (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Tobacco, Nicotine, and E-Cigarettes Research Report How can we prevent tobacco use?

The medical consequences of tobacco use—including secondhand exposure—make tobacco control and smoking prevention crucial parts of any public health strategy. Since the first Surgeon General’s Report on Smoking and Health in 1964, states and communities have made efforts to reduce initiation of smoking, decrease exposure to smoke, and increase cessation. Researchers estimate that these tobacco control efforts are associated with averting an estimated 8 million premature deaths and extending the average life expectancy of men by 2.3 years and of women by 1.6 years. 18 But there is a long way yet to go: roughly 5.6 million adolescents under age 18 are expected to die prematurely as a result of an illness related to smoking. 13
Prevention can take the form of policy-level measures, such as increased taxation of tobacco products; stricter laws (and enforcement of laws) regulating who can purchase tobacco products; how and where they can be purchased; where and when they can be used (i.e., smoke-free policies in restaurants, bars, and other public places); and restrictions on advertising and mandatory health warnings on packages. Over 100 studies have shown that higher taxes on cigarettes, for example, produce significant reductions in smoking, especially among youth and lower-income individuals. 217 Smoke-free workplace laws and restrictions on advertising have also shown benefits. 218
Prevention can also take place at the school or community level. Merely educating potential smokers about the health risks has not proven effective. 218 Successful evidence-based interventions aim to reduce or delay initiation of smoking, alcohol use, and illicit drug use, and otherwise improve outcomes for children and teens by reducing or mitigating modifiable risk factors and bolstering protective factors. Risk factors for smoking include having family members or peers who smoke, being in a lower socioeconomic status, living in a neighborhood with high density of tobacco outlets, not participating in team sports, being exposed to smoking in movies, and being sensation-seeking. 219 Although older teens are more likely to smoke than younger teens, the earlier a person starts smoking or using any addictive substance, the more likely they are to develop an addiction. Males are also more likely to take up smoking in adolescence than females.
Some evidence-based interventions show lasting effects on reducing smoking initiation. For instance, communities utilizing the intervention-delivery system, Communities that Care (CTC) for students aged 10 to14 show sustained reduction in male cigarette initiation up to 9 years after the end of the intervention. 220
Health Effects of Cigarette Smoking
Smoking and death, smoking and increased health risks, smoking and cardiovascular disease, smoking and respiratory disease, smoking and cancer, smoking and other health risks, quitting and reduced risks.
Cigarette smoking harms nearly every organ of the body, causes many diseases, and reduces the health of smokers in general. 1,2
Quitting smoking lowers your risk for smoking-related diseases and can add years to your life. 1,2
Cigarette smoking is the leading cause of preventable death in the United States. 1
- Cigarette smoking causes more than 480,000 deaths each year in the United States. This is nearly one in five deaths. 1,2,3
- Human immunodeficiency virus (HIV)
- Illegal drug use
- Alcohol use
- Motor vehicle injuries
- Firearm-related incidents
- More than 10 times as many U.S. citizens have died prematurely from cigarette smoking than have died in all the wars fought by the United States. 1
- Smoking causes about 90% (or 9 out of 10) of all lung cancer deaths. 1,2 More women die from lung cancer each year than from breast cancer. 5
- Smoking causes about 80% (or 8 out of 10) of all deaths from chronic obstructive pulmonary disease (COPD). 1
- Cigarette smoking increases risk for death from all causes in men and women. 1
- The risk of dying from cigarette smoking has increased over the last 50 years in the U.S. 1
Smokers are more likely than nonsmokers to develop heart disease, stroke, and lung cancer. 1
- For coronary heart disease by 2 to 4 times 1,6
- For stroke by 2 to 4 times 1
- Of men developing lung cancer by 25 times 1
- Of women developing lung cancer by 25.7 times 1
- Smoking causes diminished overall health, increased absenteeism from work, and increased health care utilization and cost. 1
Smokers are at greater risk for diseases that affect the heart and blood vessels (cardiovascular disease). 1,2
- Smoking causes stroke and coronary heart disease, which are among the leading causes of death in the United States. 1,3
- Even people who smoke fewer than five cigarettes a day can have early signs of cardiovascular disease. 1
- Smoking damages blood vessels and can make them thicken and grow narrower. This makes your heart beat faster and your blood pressure go up. Clots can also form. 1,2
- A clot blocks the blood flow to part of your brain;
- A blood vessel in or around your brain bursts. 1,2
- Blockages caused by smoking can also reduce blood flow to your legs and skin. 1,2
Smoking can cause lung disease by damaging your airways and the small air sacs (alveoli) found in your lungs. 1,2
- Lung diseases caused by smoking include COPD, which includes emphysema and chronic bronchitis. 1,2
- Cigarette smoking causes most cases of lung cancer. 1,2
- If you have asthma, tobacco smoke can trigger an attack or make an attack worse. 1,2
- Smokers are 12 to 13 times more likely to die from COPD than nonsmokers. 1
Smoking can cause cancer almost anywhere in your body: 1,2
- Blood (acute myeloid leukemia)
- Colon and rectum (colorectal)
- Kidney and ureter
- Oropharynx (includes parts of the throat, tongue, soft palate, and the tonsils)
- Trachea, bronchus, and lung
Smoking also increases the risk of dying from cancer and other diseases in cancer patients and survivors. 1
If nobody smoked, one of every three cancer deaths in the United States would not happen. 1,2
Smoking harms nearly every organ of the body and affects a person’s overall health. 1,2
- Preterm (early) delivery
- Stillbirth (death of the baby before birth)
- Low birth weight
- Sudden infant death syndrome (known as SIDS or crib death)
- Ectopic pregnancy
- Orofacial clefts in infants
- Smoking can also affect men’s sperm, which can reduce fertility and also increase risks for birth defects and miscarriage. 2
- Women past childbearing years who smoke have weaker bones than women who never smoked. They are also at greater risk for broken bones.
- Smoking affects the health of your teeth and gums and can cause tooth loss. 1
- Smoking can increase your risk for cataracts (clouding of the eye’s lens that makes it hard for you to see). It can also cause age-related macular degeneration (AMD). AMD is damage to a small spot near the center of the retina, the part of the eye needed for central vision. 1
- Smoking is a cause of type 2 diabetes mellitus and can make it harder to control. The risk of developing diabetes is 30–40% higher for active smokers than nonsmokers. 1,2
- Smoking causes general adverse effects on the body, including inflammation and decreased immune function. 1
- Smoking is a cause of rheumatoid arthritis. 1
- Quitting smoking is one of the most important actions people can take to improve their health. This is true regardless of their age or how long they have been smoking. Visit the Benefits of Quitting page for more information about how quitting smoking can improve your health.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General . Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014 [accessed 2017 Apr 20].
- U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: What It Means to You . Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010 [accessed 2017 Apr 20].
- Centers for Disease Control and Prevention. QuickStats: Number of Deaths from 10 Leading Causes—National Vital Statistics System, United States, 2010 . Morbidity and Mortality Weekly Report 2013:62(08);155. [accessed 2017 Apr 20].
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual Causes of Death in the United States . JAMA: Journal of the American Medical Association 2004;291(10):1238–45 [cited 2017 Apr 20].
- U.S. Department of Health and Human Services. Women and Smoking: A Report of the Surgeon General . Rockville (MD): U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General, 2001 [accessed 2017 Apr 20].
- U.S. Department of Health and Human Services. Reducing the Health Consequences of Smoking: 25 Years of Progress. A Report of the Surgeon General . Rockville (MD): U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 1989 [accessed 2017 Apr 20].
To receive email updates about Smoking & Tobacco Use, enter your email address:
- Tips From Former Smokers ®
- Division of Cancer Prevention and Control
- Lung Cancer
- National Comprehensive Cancer Control Program
- Division of Reproductive Health

Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Essay on Smoking
500 words essay on smoking.
One of the most common problems we are facing in today’s world which is killing people is smoking. A lot of people pick up this habit because of stress , personal issues and more. In fact, some even begin showing it off. When someone smokes a cigarette, they not only hurt themselves but everyone around them. It has many ill-effects on the human body which we will go through in the essay on smoking.

Ill-Effects of Smoking
Tobacco can have a disastrous impact on our health. Nonetheless, people consume it daily for a long period of time till it’s too late. Nearly one billion people in the whole world smoke. It is a shocking figure as that 1 billion puts millions of people at risk along with themselves.
Cigarettes have a major impact on the lungs. Around a third of all cancer cases happen due to smoking. For instance, it can affect breathing and causes shortness of breath and coughing. Further, it also increases the risk of respiratory tract infection which ultimately reduces the quality of life.
In addition to these serious health consequences, smoking impacts the well-being of a person as well. It alters the sense of smell and taste. Further, it also reduces the ability to perform physical exercises.
It also hampers your physical appearances like giving yellow teeth and aged skin. You also get a greater risk of depression or anxiety . Smoking also affects our relationship with our family, friends and colleagues.
Most importantly, it is also an expensive habit. In other words, it entails heavy financial costs. Even though some people don’t have money to get by, they waste it on cigarettes because of their addiction.
How to Quit Smoking?
There are many ways through which one can quit smoking. The first one is preparing for the day when you will quit. It is not easy to quit a habit abruptly, so set a date to give yourself time to prepare mentally.
Further, you can also use NRTs for your nicotine dependence. They can reduce your craving and withdrawal symptoms. NRTs like skin patches, chewing gums, lozenges, nasal spray and inhalers can help greatly.
Moreover, you can also consider non-nicotine medications. They require a prescription so it is essential to talk to your doctor to get access to it. Most importantly, seek behavioural support. To tackle your dependence on nicotine, it is essential to get counselling services, self-materials or more to get through this phase.
One can also try alternative therapies if they want to try them. There is no harm in trying as long as you are determined to quit smoking. For instance, filters, smoking deterrents, e-cigarettes, acupuncture, cold laser therapy, yoga and more can work for some people.
Always remember that you cannot quit smoking instantly as it will be bad for you as well. Try cutting down on it and then slowly and steadily give it up altogether.
Get the huge list of more than 500 Essay Topics and Ideas
Conclusion of the Essay on Smoking
Thus, if anyone is a slave to cigarettes, it is essential for them to understand that it is never too late to stop smoking. With the help and a good action plan, anyone can quit it for good. Moreover, the benefits will be evident within a few days of quitting.
FAQ of Essay on Smoking
Question 1: What are the effects of smoking?
Answer 1: Smoking has major effects like cancer, heart disease, stroke, lung diseases, diabetes, and more. It also increases the risk for tuberculosis, certain eye diseases, and problems with the immune system .
Question 2: Why should we avoid smoking?
Answer 2: We must avoid smoking as it can lengthen your life expectancy. Moreover, by not smoking, you decrease your risk of disease which includes lung cancer, throat cancer, heart disease, high blood pressure, and more.
Customize your course in 30 seconds
Which class are you in.

- Travelling Essay
- Picnic Essay
- Our Country Essay
- My Parents Essay
- Essay on Favourite Personality
- Essay on Memorable Day of My Life
- Essay on Knowledge is Power
- Essay on Gurpurab
- Essay on My Favourite Season
- Essay on Types of Sports
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Download the App


Essay on Harmful Effects of Smoking
Students are often asked to write an essay on Harmful Effects of Smoking in their schools and colleges. And if you’re also looking for the same, we have created 100-word, 250-word, and 500-word essays on the topic.
Let’s take a look…
100 Words Essay on Harmful Effects of Smoking
Introduction.
Smoking is a dangerous habit that poses significant health risks. It’s not only harmful to smokers, but also to those around them.
Health Risks
Smoking can cause lung cancer, heart disease, and stroke. It damages nearly every organ in the body, leading to premature death.
Secondhand Smoke
Non-smokers exposed to secondhand smoke face similar health risks. They can develop respiratory problems and increased risk of heart disease.
Impact on Environment
Cigarette butts litter the environment and release toxic chemicals into the soil and water, harming wildlife.
Smoking is harmful for everyone. It’s important to stay away from this deadly habit.
250 Words Essay on Harmful Effects of Smoking
Smoking is a widespread habit, yet it is one of the most detrimental practices to human health. Despite the awareness campaigns and statutory warnings, many continue to smoke, oblivious of the damaging effects it has on their health and wellbeing.
Physical Health Risks
Primarily, smoking causes numerous fatal diseases. It is the leading cause of lung cancer, accounting for about 85% of all cases. It also significantly increases the risk of heart diseases and stroke. The harmful chemicals in cigarettes damage blood vessels, leading to atherosclerosis, which can result in heart attack or stroke.
Impact on Respiratory System
Moreover, smoking adversely affects the respiratory system. It leads to chronic bronchitis, emphysema, and other lung diseases. The smoke and toxins inhaled damage the airways and alveoli, the tiny air sacs in the lungs, causing chronic obstructive pulmonary disease (COPD).
Effect on Mental Health
Smoking also influences mental health. Nicotine addiction can lead to increased stress, anxiety, and depression. The temporary relief from stress that smoking provides is often mistaken for a stress reliever, while it is actually exacerbating the problem.
In conclusion, smoking is a harmful habit that poses significant threats to physical and mental health. The myriad diseases it causes, coupled with its addictive nature, make it a dangerous lifestyle choice. It is imperative to raise awareness about these harmful effects and encourage cessation to safeguard public health.
500 Words Essay on Harmful Effects of Smoking
Smoking is a prevalent habit, often started out of curiosity, peer pressure, or stress management. However, its harmful effects are well-documented, impacting nearly every organ in the human body. Despite the widespread knowledge of its adverse effects, smoking continues to be a significant public health concern.
The Impact on Physical Health
One of the most severe consequences of smoking is its impact on physical health. Smokers are at a higher risk of developing a plethora of diseases, including lung cancer, heart disease, stroke, and chronic obstructive pulmonary disease (COPD). These conditions are often fatal, leading to premature death. The toxins in cigarette smoke damage the lining of the lungs, making smokers more susceptible to infections like pneumonia.
Detrimental Effects on Mental Health
Smoking doesn’t just harm the physical body; it also has a profound effect on mental health. Nicotine, the addictive substance in tobacco, alters the brain chemistry, leading to dependence. This dependence can exacerbate mental health conditions such as anxiety and depression. Furthermore, the stress of addiction and the struggle to quit smoking can also take a toll on mental well-being.
Smoking and Second-hand Smoke
The harmful effects of smoking are not confined to the smoker alone. Second-hand smoke, also known as passive smoking, is a significant concern. Non-smokers exposed to second-hand smoke inhale the same dangerous chemicals as smokers. This exposure increases their risk of developing heart disease, lung cancer, and other respiratory conditions.
Societal Impact
Smoking also has societal implications. The economic burden of smoking is substantial, with healthcare costs for smoking-related illnesses reaching astronomical levels. Additionally, the loss of productivity due to illness or premature death contributes to economic strain.
In conclusion, the harmful effects of smoking are far-reaching, affecting not only the smoker but also those around them and society at large. The physical and mental health implications, coupled with the economic burden, make it a significant public health issue. Despite the addictive nature of smoking, quitting is possible with the right support and resources, leading to improved health outcomes and quality of life. Understanding the full scope of smoking’s harmful effects is crucial in motivating smokers to quit and preventing non-smokers from starting.
That’s it! I hope the essay helped you.
If you’re looking for more, here are essays on other interesting topics:
- Essay on Effects of Smoking
- Essay on Dangers of Smoking
- Essay on Cyclone
Apart from these, you can look at all the essays by clicking here .
Happy studying!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Study Protocol
Effects of brief mindfulness training on smoking cue-reactivity in tobacco use disorder: Study protocol for a randomized controlled trial
Contributed equally to this work with: Linlin Cheng, Miaoling Luo
Roles Data curation, Methodology, Writing – original draft
Affiliations Medical School, Kunming University of Science and Technology, Kunming, China, Brain Science and Visual Cognition Research Center, Medical School of Kunming University of Science and Technology, Kunming, China
Roles Conceptualization, Data curation, Methodology, Writing – original draft
Roles Supervision
Affiliations Brain Science and Visual Cognition Research Center, Medical School of Kunming University of Science and Technology, Kunming, China, Students Counseling and Mental Health Center, Kunming University of Science and Technology, Kunming, China
Roles Funding acquisition, Supervision, Writing – review & editing
Roles Conceptualization, Formal analysis, Methodology, Supervision, Validation, Writing – review & editing
¶ ‡ QG and ZC also contributed equally to this work.
Affiliations Medical School, Kunming University of Science and Technology, Kunming, China, Brain Science and Visual Cognition Research Center, Medical School of Kunming University of Science and Technology, Kunming, China, Faculté de médecine, Université Paris-Saclay, Le Kremlin-Bicêtre, France
Roles Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing
* E-mail: [email protected]
- Linlin Cheng,
- Miaoling Luo,
- Jie Ge,
- Yu Fu,
- Quan Gan,
- Zhuangfei Chen

- Published: April 22, 2024
- https://doi.org/10.1371/journal.pone.0299797
- Peer Review
- Reader Comments
The prevalence of Tobacco Use Disorder (TUD) represents a significant and pressing global public health concern, with far-reaching and deleterious consequences for individuals, communities, and healthcare systems. The craving caused by smoking cue is an important trigger for relapse, fundamentally hindering the cessation of cigarette smoking. Mindfulness interventions focusing on cue-reactivity was effective for the treatment of related dependence. Brief mindfulness training (BMT) meets the short-term needs for intervention but the effects still need to be examined. The objective of the present study is to investigate the impact of BMT intervention on smoking cue-reactivity among Chinese college students with TUD, to uncover the dynamic models of brain function involved in this process.
A randomized control trial (RCT) based on electroencephalography (EEG) was designed. We aim to recruit 90 participants and randomly assign to the BMT and control group (CON) with 1:1 ratio. A brief mindfulness training will be administered to experimental group. After the intervention, data collection will be conducted in the follow-up stage with 5 timepoints of assessments. EEG data will be recorded during the smoking cue-reactivity task and ‘STOP’ brief mindfulness task. The primary outcomes include subjective reports of smoking craving, changes in EEG indicators, and mindfulness measures. The secondary outcomes will be daily smoking behaviours, affect and impulsivity, as well as indicators reflecting correlation between mindfulness and smoking cue-reactivity. To evaluate the impact of mindfulness training, a series of linear mixed-effects models will be employed. Specifically, within-group effects will be examined by analysing the longitudinal data. Additionally, the effect size for all statistical measurements will be reported, offering a comprehensive view of the observed effects.
The current study aims to assess the impact of brief mindfulness-based intervention on smoking cue-reactivity in TUD. It also expected to enhance our understanding of the underlying processes involved in brain function and explore potential EEG biomarkers at multiple time points.
Trial registration
Trial registration number: ChiCTR2300069363 , registered on 14 March 2023. Protocol Version 1.0., 10 April 2023.
Citation: Cheng L, Luo M, Ge J, Fu Y, Gan Q, Chen Z (2024) Effects of brief mindfulness training on smoking cue-reactivity in tobacco use disorder: Study protocol for a randomized controlled trial. PLoS ONE 19(4): e0299797. https://doi.org/10.1371/journal.pone.0299797
Editor: Lakshit Jain, University of Connecticut Health Center: UConn Health, UNITED STATES
Received: September 7, 2023; Accepted: January 26, 2024; Published: April 22, 2024
Copyright: © 2024 Cheng et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: No datasets were generated or analysed during the current study. All relevant data from this study will be made available upon study completion.
Funding: This study was supported by the National Natural Science Foundation of China (NSFC) (Nos. 32060196, 82360271 and 82201597) and Yunnan Ten Thousand Talents Plan Young and Elite Talents Project (YNWR-QNBJ-2018-027, YNWR-QNBJ-2018-056), and Innovation team of Stress and disorder in nervous system in Yunnan (202305AS350011).
Competing interests: This study was supported by the National Natural Science Foundation of China (NSFC), Yunnan Ten Thousand Talents Plan Young and Elite Talents Project and Innovation team of Stress and disorder in nervous system in Yunnan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The financial support was provided in forms of research materials. The authors have declared that no competing interests exist.
Introduction
Tobacco epidemic.
As a major determinant of preventable health consequences, Tobacco Use Disorder (TUD) is a problematic pattern of tobacco use that leads to significant health and economic burdens [ 1 ]. There are 1 billion people confronting the problem worldwide. Cigarette smoking can increase the incidence and mortality rates of various diseases [ 2 – 4 ], causing an annual death of over 8 million people worldwide [ 6 ]. The health costs and other economic losses caused by smoking had already reached $1.4 trillion annually a decade ago [ 7 ]. Since the WHO Framework Convention on Tobacco Control came into force, more and more countries have adopted effective measures to control smoking. From 2007 to 2021, the global average smoking prevalence has reduced from 22.8% to 17.0% [ 8 ].
In China, the population of individuals with TUD exceeds 300 million [ 9 ]. The smoking rate among people over 15 years reach to 26.6%, for male is up to 50.5% [ 8 ]. Due to the delay effect, it is projected that the disease burden for both individuals and the overall population of Chinese adult males with TUD will increase in the future. Additionally, those who begin smoking at a younger age are at a higher risk of morbidity and mortality from major diseases [ 10 ]. Without effective intervention, the number of tobacco related deaths is expected to rise to 2 million per year by 2030 and 3 million per year by 2050 [ 9 ].
In July 2019, the National Health Commission of China released the “Healthy China Action (2019–2030)” [ 11 ], which aims to decrease the smoking rate among individuals aged 15 and above to 20% by 2030.
Besides increasing individual awareness of the detrimental effects of smoking and taking steps to quit, it is also essential to establish smoke-free environments and implement practical measures at personal, family, societal, and governmental levels. This tobacco control initiative also emphasizes the promotion of effective short-term smoking cessation interventions, aiming to provide support to more individuals who are in the process of quitting smoking. Moreover, post-adolescence is a crucial transitional phase from substance misuse and experimentation to addiction, primarily due to the ongoing development of the brain during this period. Hence, this stage of ’pre-addiction’ is deemed a critical juncture for identifying brain circuits that could contribute to future challenges in life [ 12 ]. The necessity of strengthening smoking control during the young adult stage has been put on the agenda, and there is an urgent need to explore more targeted smoking cessation methods in this population.
Smoking craving
Craving is defined as a strong desire to pursue an addictive substance [ 12 ]. Smoking craving is the main symptom of tobacco dependence [ 14 ] and has been treated as an important indicator for smoking cessation treatment [ 15 ], and reflected by objective measures [ 16 ].
The acquisition and maintenance of nicotine addiction is a complex procedure. Individuals with TUD may experience an activation of their craving and consumption impulses in response to specific environmental contexts, situational cues, and stimuli [ 17 – 18 ]. When the brain fails to regulate reward impulses efficiently, cravings can disrupt one’s self-control and their ability to consider future consequences, thereby increasing their propensity to make impulsive decisions [ 20 , 21 ]. It is essential to note that the concept of cue-reactivity extends beyond the traditional stimulus-response framework. In the case of smoking, cue-reactivity encompasses not only the direct association between the cue and the smoking behavior but also the complex cognitive and emotional processes underlying craving and relapse. Simultaneously, the correlation among addictive cue stimuli is dynamic and can be influenced by interactions between multiple stimulus. Consequently, increased exposure and interventions are necessary during the process of extracting and reconsolidating addictive memories effectively [ 22 ]. Investigating cue-reactivity within the framework of TUD necessitates a comprehensive grasp of various elements. These include attentional bias, intensity of cravings, and subjective responses to cues, as well as the interaction of internal and external condition [ 22 , 24 ]. Moreover, due to the ubiquitous nature of addictive stimuli, simply suppressing cravings can lead to a greater rebound effect [ 25 ], which is a significant factor contributing to relapse and treatment resistance [ 26 ]. Therefore, some scholars argue that therapies solely targeting the addiction cycle are often challenging to succeed, highlighting the necessity for innovative treatment approaches.
Mindfulness intervention for TUD
Mindfulness can be defined as a conscious and open process of focusing on the present moment with curiosity [ 26 ]. The non-reactive, present-moment awareness associated with mindfulness can increase the likelihood of perceiving each moment as a unique and independent event, unaffected by past experiences. This can help individuals accept psychological and physiological discomfort, which conceptualized as a process of “interoceptive desensitization” [ 28 ] rather than relying on substances for relief. Furthermore, mindfulness can serve as a type of refined exposure since many clinical issues arise from inflexible efforts to avoid unpleasant internal experiences [ 28 ]. Compared to previous studies, incorporating mindfulness exposure may better capture the diverse influences of cue, emotion, and classical conditions on smoking behavior. By focusing on the downstream relationships between each factor and smoking behavior, it allows for a more comprehensive understanding of the variations observed [ 30 ]. As a result, mindfulness-based interventions have been explored within the context of treatment for TUD, with promising future in helping to prevent addiction and relapse [ 31 ]. It can directly affect the neurocircuitry of cravings, reducing addictive behaviour by decreasing subjective cravings, attention, and related physiological indicators of cue-reactivity to addictive substances [ 32 ]. At the same time, compared to active control with relaxation training [ 32 ], mindfulness can also help individuals manage negative emotions and physical sensations, allowing them to dissociate their emotional responses from their cravings for smoking [ 30 ].
However, due to the long-term intervention period of mindfulness, the demand for short-term practice is increasing. A few studies have confirmed that a single intervention of mindfulness and a 5-minute intervention can improve health outcomes [ 34 , 35 ]. Brief mindfulness intervention can reduce smokers’ response to craving [ 36 ], improve emotional health [ 30 ], increase 7-day point-prevalence abstinence [ 37 ], and reduce smoking frequency and the number of cigarettes [ 38 ]. Moreover, the participation in mindfulness exercises was related to the abstinence rate in the follow-up study. At the same time, it has been confirmed that BMT can reduce subjective reported negative emotions, smoking cravings, and cigarette use in natural situations [ 38 ], indicating the effectiveness of this method in smoking cessation treatment. Meanwhile, in terms of brain mechanisms, both traditional 8-week mindfulness [ 40 ] and short-term mindfulness can affect interoceptive processing, to be more targeted in regulating brain function in cue-reactivity [ 32 ], making it an ideal intervention strategy for cue-reactivity. Accordingly, exteroceptive processes also serve as targets for interventions of mindfulness training [ 41 ] for it acting a critical role in conditioned cue response in addiction. The balance between interoceptive and exteroceptive is also emphasized [ 41 ]. Among the non-clinical population, both short-term and high-dose mindfulness training programs demonstrate similar effects in alleviating psychological distress [ 43 ].
In line with prior research [ 43 ], our previous trial has also observed that standard 8-week mindfulness can lead to functional changes in brain areas involved in interoceptive processing such as the insula [ 45 ] as well as in the executive control system, including the cingulate gyrus, parietal lobe, and prefrontal lobe [ 46 , 47 ]. However, most EEG studies examining mindfulness have consistently found a positive correlation between improved emotional states and increased alpha power [ 48 ]. Additionally, internalized attention has been linked to heightened theta activity in the frontal midline [ 49 ].
In recent years, there has been increasing evidence suggesting that low-theta EEG coherence in the brains of smokers may serve as a potential biomarker for smoking cue reactivity and be able to predict addictive behaviors [ 50 ]. Mindfulness intervention was associated with lower P3 amplitude and reduced response inhibition [ 50 ]. Neurofeedback training also revealed deactivated EEG activity patterns (P300 amplitudes) associated with smoking cue reactivity [ 20 ]. The prefrontal-insula circuit could be a noninvasive imaging biomarker for monitoring treatment efficacy [ 51 ]. Microstate class C (duration) may indicate cue-induced cigarette craving, while class D (duration and contribution) could reflect the relationship between cue-elicited activation of the dorsal attention network and years of smoking [ 16 ].
Therefore, we hypothesize that brief mindfulness intervention can reduce smoking cue-reactivity in the population of TUD and can be reflected in subjective craving reports and changes in EEG functional indicators. To capture the diverse aspects of smoking craving, we will assess the EEG power spectrum, ERP components, microstate, and functional connectivity indicators before and after intervention. These will be analysed in combination with subjective craving and smoking behavior. By using source analysis and rely on the time resolution advantages of EEG, we can further explore the regulation effects of smoking cue-reactivity and related brain functional mechanisms of TUD following a brief mindfulness intervention.
This study aims to investigate the impact of a 7-day brief mindfulness practice under the treatment paradigm for substance dependence on smoking cue-reactivity through a RCT design, incorporating a follow-up of multi-session intensive training and online homework tracking of self-guided practices. Previous studies have shown that mindfulness, when compared with control group, does not reduce craving. However, it does influence individuals’ readiness and willingness to respond to cues, encouraging the use of mindfulness strategies to manage smoking urges and derive benefits from cue exposure paradigms [ 53 ].
On one hand, it may make up for the shortcoming of long mindfulness and promote acceptance in the population with TUD. On the other hand, the exploration of brain function mechanisms can also provide more targeted intervention strategies for smoking cession, which will be of great value for promoting the clinical application of brief mindfulness in tobacco addiction intervention. Therefore, the study has the potential to offer quantitative evidence supporting the decoupling of cue-reactivity and cravings in young adults with TUD.
Materials and methods
Study design and participants.
This study will adopt a randomized parallel control trial design, and about to recruit participants by combining online and offline advocation. Following previous mindfulness research methodologies, we will integrate relaxation training as an active control in this study to enhance its superiority design.
Participants who meet the experimental requirements will join the study by following a sequence that includes baseline data collection, intervention training, post-intervention data collection, and follow-up time points. After the completion of baseline data collection in two time points (-T1, T0; exp., EEG data in -T1), participants will be randomly assigned to the BMT and CON group respectively, to complete the corresponding training for a week. Except for the baseline, a total of 5 subsequent data collections are anticipated after intervention (T1) along with follow-up at four timepoints (T2-T5). During the follow-up period, participants will receive monthly intensive training sessions, as well as online homework tracking for self-guided practices every two weeks. The study sites will be the current living environment in the university for the participants.
The inclusions and exclusions
Inclusion criteria: (1) Right-handed. (2) Smoking 10 or more cigarettes per day. (3) Smoking history of at least 1 year. (4) Exhaled carbon monoxide (CO) 10-7ppm or lower before assessment (abstinent from smoking cigarette for 3 h prior to every visit). (5) Age between 18 and 40 years old. (6) Normal or corrected to normal vision. (7) Normal mental and physical health conditions (PHQ-9 [ 54 – 55 ] total score <20, GAD-7 [ 57 , 58 ] total score <11). (8) Meets the diagnostic criteria for tobacco use disorders in DSM-5 assessed by clinical structured interview [ 58 ].
Exclusion criteria: (1) Asthma, contact dermatitis, or allergies to silicone (exclude those allergic to materials undergoing EEG). (2) Recent use of corticosteroid medications within the past 3 months. (3) Practiced any meditation, yoga, tai chi, or qigong for more than 20 hours in the past year or lifetime, participated in meditation or yoga retreats, and attended any meditation courses. (4) Other situations not suitable for EEG (e.g., metal implants, severe head trauma and electrode allergy). (5) PHQ-9 total score ≥20. (6) GAD-7 total score ≥11. (7) Adhere to specific religious beliefs that and therefore unable to participate in meditation as required by the course. (8) Currently participating in similar trials or other neurophysiological studies. (9) On medication assisted treatment for tobacco use disorder before or during the study.
Handedness and vision criteria ensure task completion and minimize variability. Potential participants will also be screened by evaluating whether they met the aforementioned inclusion criteria and exclusion criteria after completing the demographic questionnaire and screening scales, concluding PHQ-9 and GAD-7, followed by written informed consent, as stipulated by the Declaration of Helsinki (2008).
Sample size and power calculation
In the current study, the primary indicator used to assess smoking urges will be self-reported subjective measures. Based on previous research conducted on similar populations [ 60 ], we have defined a reduction score of 4 on the Brief Questionnaire of Smoking Urges (QSU-B) as the expected decrease in smoking craving. Therefore, in the experimental group after the intervention, we anticipate observing a Δ QSU-B of 4. We intend to conduct multiple measurements on the participants before and after training, as well as during the online assessment period for the smoking cue-reactivity task, and anticipate achieving 80% power (1-beta = 0.8), at the significant level of 5% (alpha = 0.05, two sided). The Cohen’s d was estimated with by the formula of d = Δ QSU-B /SD estimate , where SD was 7.65 according to reference study, resulting in an estimated sample value of 34. For the estimation of the sample size of the experimental group, it is required to have no less than 34 people. Considering a natural dropout rate of 30%, the sample size for experimental group should be no less than 45 people. To ensure proper comparability, an additional 45 participants who match in demographic data and smoking severity will be assigned to the control group. Consequently, a total of 90 participants are required for the present study.
Recruitment
We will recruit participants by combining online and offline methods, including lectures, posters, and distributing flyers in the classroom, also through Qzone, WeChat Moments, campus BBS and school’s bulletin board. After filling in the registration form (collecting demographic characteristics information, i.e., age, gender, contact information, smoking years, average number of cigarettes smoked per day, etc.), the recruited participants will be informed of the general process of the study, and the participants need to sign the written informed consent according to their wish. After confirming their participation in the study, participants will be scheduled to fill out relevant trait scales, including the Smoking Addiction Scale (FTND), the Five Facet Mindfulness Questionnaire (FFMQ), the Smoking Desire Scale Simplified (TCQ-SF), the Edinburgh Handedness Inventory (EHI), and the Positive And Negative Affect Scale (PANAS) and so on.
Baseline assessment
The data collection in baseline will start from status-related scales. After filling out the scale, EEG data for pre-intervention will be recorded from all participants. Participants will be asked not to smoke for at least 3 hours before collecting EEG data. Upon arrival in the laboratory, participants will be required to report when the last cigarette smoked and exhale CO concentration need to be measured by using a Bedfont smoke detector. Only those with a CO value between 10-7ppm or lower before EEG data collection can be proceed to the EEG process, otherwise, they need to reschedule another time to come to the laboratory for EEG data collection. Data collection for all participants will take approximately two weeks.
The details are as follows: (1) Firstly, a time slot appointment form for collecting EEG will be sent to the group chats for participants. They will need to arrange a specific time for data collection according to their own schedule; (2) Notify participants who have already made appointments in advance of not smoking before collecting EEG data; (3) After the participants arrived at the laboratory, they need to record when their last cigarette smoked before starting EEG process, and accept CO testing; If the CO detection does not meet the standards, the current EEG collection cannot be performed and a new collection time needs to be scheduled. Only those who meet the EEG collection standards are required to collect baseline EEG data after filling out the scale.
Randomization and group allocation
Participants will be allocated into BMT and CON group. Each participant who meets the inclusion criteria will be assigned a unique number for this study. The statistician will then randomly assign the participants to the two groups in a 1:1 ratio, matched in demographic data and smoking severity (i.e., duration and daily consumption). Then retain the grouping information until the baseline data collection is completed, and the grouping results will be fed back to the researchers by the statistician before the intervention training begins. The random sequence generation website ( https://www.random.org/ ) will be used for the process.
Data collection
FTND, TCQ-SF, FFMQ, EHI, PHQ-9, GAD-7 and other scales as well as participant registration forms (for the collection of demographics characteristics information) will be produced, and released via WenJuanXing, a Chinese professional platform for online survey ( https://www.wjx.cn/ ). Online data collection will also be implemented on the platform to facilitate the unified management of the data. After collection is finished, the data will be downloaded for the preparation of analysis. EEG data acquisition will be conducted by using a 64-channel EEG acquisition system subsequently. To those participants failed to finish data collection, we will send a reminder through WhatsApp in time.
The overall process is shown in Figs 1 and 2 and S1 File . After completing the recruitment, scales will be distributed to all participants and combined with an exhale CO detection. For eligible participants, they will be required to sign the written informed consent according to participants’ will, and then randomly allocated into BMT and CON groups. The baseline data from all participants will be collected at two different time points within a week. A 7-day brief mindfulness intervention will be conducted immediately after the baseline assessment. Post-intervention (i.e., 1-week timepoint) data will be collected when the training is completed, and follow-up data in the other 4 timepoints will be collected sequentially.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
Note: -t1, 0, baseline; t1, post intervention; t2-t5, 1-month, 3-month, 6-month, 1-year follow-up.
https://doi.org/10.1371/journal.pone.0299797.g001
https://doi.org/10.1371/journal.pone.0299797.g002
Brief mindfulness intervention
A 7-day mindfulness training will be organized after baseline data collection, and guided by professional teachers from the school’s Students Counseling and Mental Health Center. The themes of brief mindfulness training in each day are listed in Table 1 . The audio guidance for exercise will be distributed by researchers after the end of each day’s training, lasting for one week. The BMT group need to carry out ‘STOP’ mindfulness exercises, while CON group carry out relaxation exercises in spare times as homework in each day for intervention. Participants can choose different time periods for multiple exercises throughout the day, and fill out both the daily smoking records and the daily practice records. They need to record the extra practice duration (in minutes) and evaluate their seriousness and compliance level (1–10 points) independently. The daily smoking behaviour (number of cigarettes smoked, total in 7 days) will also be collected by delivered forms.
https://doi.org/10.1371/journal.pone.0299797.t001
To address the concern that brief mindfulness training might not produce observable long-term effects due to limited exposure time, we have referred to the intervention settings in existing literature research [ 41 , 61 ]. After 7 days of intervention, a monthly intensive training session will be organized, lasting for one day (2 hours) each time. Following the training session, homework practicing time will be collected every two weeks, with follow-up evaluations of the intervention effect scheduled at the 1-month, 3-month, and 6-month timepoints. Group training is structured as collective activities, which enhance the effectiveness of the intervention. The brief mindfulness intervention with multiple sessions satisfies the current demand for shorter intervention duration among college students, while also providing the potential for accumulating sufficient exposure time.
Control group
Control group will be assigned to relaxation training, conducted at the same time each day as the experimental group during the 7-day period of intervention. The training will include watching documentaries of natural scenery without any other intervention, for which has been suggested a generic relaxation effect of video interventions on autonomic regulation [ 62 , 63 ]. The audio guidance and learning materials for both groups will be uploaded to the group chats separately, participants can practice at any time after the intervention period. The total number of meditation/relaxation sessions completed as homework for both groups will be recorded. The intensive training schedule during all follow-up periods is kept the same for both groups.
Post-intervention data collection
After the intervention training (T1), we will send a time slot appointment form for EEG collection to the participants’ group chats, and the participants need to arrange their own time for the appointment, following the same requirement of smoke cessation and EEG data collection procedure as in baseline. Follow-up data collection will be conducted for participants at consequent four time points, i.e., T2-T5 (1-month, 3-month, 6-month, and 1-year). EEG data and scale data will all be collected with the same setting in T1-T5 ( Fig 3 ).
Note: The upper portion of the figure illustrates the overall study design. The middle portion depicts the timeline for assessing scales and EEG at baseline, post-intervention, and follow-up timepoints. Brief mindfulness and relaxation training will be administered to both the intervention and control groups after recruitment. The baseline assessment (-T1, T0) will be completed before the intervention. Audio guidance/learning material will be provided for daily practice in the brief mindfulness training and control group, a monthly intensive training session will be organized in follow-up period. The lower portion of the figure represents the modules of psychological and behavioral assessments during the baseline and five follow-up sessions. The cue-reactivity task will be implemented using a customized two-choice oddball paradigm. INT: intervention, INST: intensive training, rs-EEG: resting-state EEG; BMT: brief mindfulness training; VAS: Visual analogue scale; SMT: state-mindfulness.
https://doi.org/10.1371/journal.pone.0299797.g003
‘STOP’ mindfulness practice
‘STOP’ stands for four mindful actions: ‘Stop’, ‘Take a breath’, ‘Observe’, ‘Proceed’. It is a helpful aid in becoming more mindful of our body, behaviour and emotion on a daily basis. The following is the adapted instruction of practicing STOP in confronting smoking cessation based on that developed by Liao [ 63 ]:
S = stop; Remind yourself to STOP. Whatever you are doing right the moment (e.g., looking for cigarettes or a lighter, ready to remove the cover of pack, hold the cigarette, putting cigarette into mouth etc.), pause for a minute.
T = take; Take a deep breath. The body will become relax and quiet in its natural breathing rhythm. Breath is the anchor of the mind and body, also present moment and awareness.
O = observe; Observe what is happening for you in this moment: except for breath, what is your thoughts, feelings and emotions (e.g., feel distracted, anxious or nervous). What do you notice in your body (e.g., feel tired or unease on any part of your body)? You can be aware of anything: floating and conflicting mind, urging for cigarettes, transferred attention, sensations or tension in your body, or the sound surrounding, and again back to the feeling of air come into your nasal when breath.
P = proceed; Proceed with whatever you were doing before you came to a STOP or something that you want to do in the moment (e.g., proceed with thinking of the cigarette, or stop thinking about it and take an alternative behaviour, or even merely watching it disappears).
The STOP practice is brief and easy to remember and practice. As a most popular technique, we recommend STOP as a daily exercise based on basic mindfulness course. It will also be used in the later on study procedure of cue-reactivity task. The practice of STOP may cultivate a space between stimulus and response, which could help avoid the mostly spontaneous circle of trigger-behaviour-reward [ 65 ]. The adoption of ‘STOP’ exercise also takes into account that for beginners, the consistency setting of mindfulness practice methods in daily practice and cue-reactivity tasks may be beneficial in reducing differences caused by mastery and guidance of the technique.
Cue-reactivity task
The experimental materials for this study are based on the image library proposed by Andrei Manoliu et al. [ 66 ]. The two-choice oddball paradigm will be adopted for it has the potential to offer dependable measurement indicators for assessing inhibitory behavior in individuals with addiction [ 67 ]. Moreover, the paradigm provides reaction time to understand the change of accuracy, which is absent in other tasks of tracking impulse inhibition (e.g., go or no-go task). The reaction time and accuracy may indicate an attentional bias or objective resistance towards smoking cues.
The task would involve presenting a series of stimuli, with cigarette-related pictures serving as the target (oddball) stimuli among a series of non-smoking-related pictures (standard stimuli). Standard stimuli and deviant stimuli account for 80% and 20%, respectively. The experimental paradigm consists of 5 blocks: rest (5 minutes), smoking cue reactivity (approx. 15 minutes), STOP mindfulness audio (5 minutes) for managing somatic experiences related to smoking urges, another smoking cue reactivity block (approx. 15 minutes), and rest (5 minutes). The task involves 200 trials per block (40 deviant stimuli, 160 standard stimuli), with randomized presentation. Participants will have sufficient rest periods between blocks. The total time for completing the task is estimated to be approximately 15 minutes. Participants would be instructed to respond to both standard and deviant stimuli. The handedness of the button press was counterbalanced across participants. Event-Related Potentials (ERPs) activities that purely reflect behavioural control effects by subtraction techniques. By manipulating the image types of deviant and standard stimuli, it is possible to investigate the impact of cigarette related cues on the reaction inhibition process of smokers [ 67 ]. This paradigm will be created and presented using E-prime 3.0.
The measures include smoking related indicators (craving, smoking behaviour, exhaled CO concentration, impulsivity), mindfulness level (state and trait mindfulness), affect (depression and anxiety), and records of daily practice for mindfulness. Relative spectrum power, microstate, phase synchronization indices and ERP components will be used to evaluate brain function (See Data analysis for details). The measurement tools and specific content are shown in Table 2 . The outcome level and timepoints for assessment is annotated. Other individual information such as demographic data and social support levels will be measured. Online questionnaire links will be sent to the participants through WhatsApp at each time-point for them to fill in on their own devices.
https://doi.org/10.1371/journal.pone.0299797.t002
Data management plan
The data collected from all participants will be uniformly anonymized before statistical analysis. Data encryption or other secure storage strategy will also be taken. Firstly, we will strictly follow the standard operating procedures for different data collection methods in collection procedure. Collection and recording will be carried out by specialized personnel. All collected information will not be shared with any third party. Secondly, the source data will be stored on data platform and backed up multiple times (e.g., stored on non-networked hard drives or mobile hard drives specifically used by data analysts for data analysis); timely download and backup of raw data; backup software and regularly detect vulnerabilities, and prevent virus intrusion. As to the data quality, we will conduct double person data verification by checking the range of data values.
Data will be analysed after the completion of all stages of data collection without interim data analysis. The data generated for this study will be available from the corresponding author to researchers who meet the criteria for access to confidential data after the completion of the study.
Any information and data obtained about participants throughout the entire study will be treated with the utmost confidentiality. Unless participants give explicit permission, no information that could identify them will be shared with individuals outside the research team. Public reports on the study results will not disclose the personal identities of the participants.
Given the limited timeframe and the low known risk to participants in the current study, the formation of a Data Monitoring Committee is deemed unnecessary. However, the research team will remain vigilant in monitoring the situation and will reassess the need for establishing such a committee if circumstances change.
Withdrawal from the program
Each participant can withdraw at any time during the study without giving any reason. Participants who voluntarily withdraw from the study will not be included in the analysis. The reason of withdraw will be recorded if the participants are willing to provide. It is important to emphasize that participant data may be used up to the point of withdrawal, unless individuals explicitly withdraw their consent for data usage.
Safety considerations
In current study, all participants who encounter any health-related issues at any time are encouraged to actively contact with the researchers. The contact information will be provided during the recruitment or via group chats. Complete safeguard measures will be conducted even though there are very few procedures with possible safety hazards during the study. Firstly, we will continuously collect the health status information for each participant to assure their safety during the intervention training process. Any discomfort symptoms for participants, will be measured and handled immediately. We will directly contact with campus clinics if the participants report of severe physical or mental withdrawal symptoms.
The collection of EEG data will be carried out in a shielded room to isolate external electromagnetic and sound interference. There will be video devices inside the shielded room to synchronize the states of the participants to the researchers’ terminal, to assure the safety when the participants in the room alone.
If, upon completion of the trial, it is determined that the stress level of any participant has reached the clinical threshold, active intervention will be offered by the psychological teachers at the on-campus psychological center. If required, participants will be referred to specialized hospitals for further intervention.
Ethics and dissemination
This study was approved by Medical Ethics Committee, Kunming University of Science and Technology (approval number: Kmust-MEC-2023-004); see S2 – S4 Files for registered and registering materials. Written informed consent will be obtained from each participant prior to participation in accordance with the Declaration of Helsinki and its later amendments or comparable ethical standards. The purpose, procedures and assessments, potential risks and benefits of the trial will be explained to all participants before recruitment. They will be informed that their participation in this research is total voluntary after fully understanding the study. Moreover, participation can withdrawal at any time without reasons. Any changes or deviations from the protocol will need to be reported to the Medical Ethics Committee at the Kunming University of Science and Technology. This can be done by submitting a hardcopy application for amendment of an approved project form. Any results from this trial will be propagated via social media and be published in peer reviewed journals and conference proceedings.

Data analysis
The current study aims to examine the efficacy of the intervention by using a two-arm parallel RCT design. For both the experimental and control groups, quantitative data, including scale measures and the EEG data, will be collected at baseline, post-intervention and four timepoints in follow-up procedure as mentioned above. All data will be analysed when collection is completed. The data analysis steps will also strictly follow the blind method principle. The R software ( https://www.r-project.org/ ) and SPSS (IBM SPSS Statistics for Windows, V20.0), MATLAB (MathWorks in Natick, Massachusetts, USA) and EEGlab ( https://sccn.ucsd.edu/eeglab ) will be used for data analysis. Descriptive statistics will be applied for demographic, psychological and behavioural data in baseline, i.e., two sample t-test and χ 2 test will be administered to continuous and categorical variables accordingly between BMT and CON group. For primary and secondary outcomes, repeated measures MANOVA will be performed to reveal the intervention effects while adjusting for individual factors and pre-intervention variables (e.g., trait mindfulness and smoking indices); within-group effects will be investigated through serial trend analysis from -T1 to T5. Data for all measures in different time points will be included in linear mixed-effects model analysis and growth curve model analysis to explore any factor associated with smoking cue-reactivity in different time-point for both groups. EEG data will be preprocessed using the Matlab software and the EEGLAB toolbox. The relative spectrum power for six EEG bands (delta: 1-4Hz, theta: 4-8Hz, alpha: 8-13Hz, low-beta: 13-20Hz, high-beta: 20-30Hz, and low-gamma: 30-48Hz) at both the single electrode level and regions of interest (ROI) level will be calculated. Microstate and phase synchronization analysis will be conducted. Functional changes involving subcortical brain regions will be investigated using source localization analysis.
For ERP data, ROIs comprising frontal, fronto-central, central and centro-parietal will be selected. The average wave amplitude of N2 (200-300ms) and P3 (350-550ms) components will be measured. ERPLAB toolbox will be used for all ERP component analysis. Additionally, the Fieldtrip toolkit will be utilized to calculate the phase lag index (PLI) [ 84 ] and phase locked value (PLV) [ 84 ]. A 2-second sliding window with a 50% overlap will be used to calculate the instantaneous phase difference for each sampling point. The frequency band of θ (4–8 Hz) α (8–13 Hz), low β (13–20 Hz) and high β (20–30 Hz) will be analysed. Both intra-band and cross-band coherence will be calculated. To avoid contamination from electromyographic signals, the γ frequency band will not be included in the analysis. The n*(n-1)/2 connection values will be calculated for each frequency band for all participants based on channel/data source. The individual matrices will be averaged and compared between groups to obtain the t-value matrix (see S4 File for more details).
Both repeated measurement analysis of variance and simple effect analysis will be conducted to EEG indices when considering the significance in professional field. Effect size will be measured using Cohen’s d or partial eta-squared values [ 85 ]. missing data resulting from drop-out will be addressed with multiple imputation [ 87 ]. To obtain characteristic brain functional change related to cravings, by correlation analysis of subjective cravings, behavior and EEG indicators.
The results of the aforementioned analysis will be reported following the CONSORT 2010 guidelines.
The status and timeline
The participants of this study have been recruited and have completed some data collection. All subsequent studies will be completed in April 2024. We expect that all data analysis will be completed by the end of May 2024, all results will be announced by the end of June 2024, and the article will be completed by August 2024. The publication plan will consist of several sections, including the research plan, analysis of panel data using various statistical methods, analysis of psychology-related data, and conclusion.
The aim of the current study is set to explore the intervention effect of BMT on smoking cue-reactivity in college students with TUD. Specifically, we expect to explore how participants’ responses to cues differ from their usual smoking behaviour by measuring their state mindfulness level, craving level, and EEG activity patterns before and after BMT. We ought to clarify the relationship between mindfulness improvement trajectory and subjective report of craving and to explore the intervention effect of BMT in reducing craving and smoking behaviour. Combined with EEG analysis, the RCT design will also be used to explore potential brain functional mechanisms. We will conduct EEG recordings during the cue-reactivity task to explore to what extent that EEG indicators may reflect this process and try to reveal the candidate dynamic models of brain function.
First and foremost, brief mindfulness usually defined in the duration of single and overall interventions, typically takes less than 20 minutes for a single training session, with a total intervention time of approximately one week, aimed at strengthening instantaneous attention and awareness [ 53 ]. However, compared with hospital patients, college student with TUD may have relatively weaker motivation to seek treatment, and the intervention effect of a single session may not reach the level of clinical patients. Therefore, on the basis of retaining the duration of a single intervention, we are planning to increase the duration of mindfulness exposure for participants through monthly intensive training and homework, to observe the long-term follow-up effect.
Additionally, the cue-reactivity paradigm is widely used as probe [ 88 ] for measuring addictive behavior, but there is significant heterogeneity in its application [ 88 , 89 ]. In this study, we will employ a publicly available addiction image library and followed a standard two-choice oddball paradigm design. Response time and accuracy will be measured for both standard and deviant stimuli. Repeated measures ANOVA will be conducted to assess the reproducibility of the results and provide a valuable reference.
Lastly, previous studies on addiction-related EEG activity encompassed various indicators including time domain, frequency domain, brain regions, brain networks and so on. In this study, our main focus will be on comparing phase synchronization indices, power spectrum, microstate and ERP components that may be associated with cue-reactivity. For example, we aim to analyze how energy changes in specific frequency bands relate to neural activity regulation during smoking cravings. Moreover, by examining attention, cognitive processing, subjective reports, and behavioral cues related to cravings, we can evaluate the effectiveness of mindfulness interventions objectively.
Strengths and limitations of this study
Although there are increasing researches on mindfulness among TUD population, most of them still limited to the adult population. By focusing the fragile also crucial period of brain maturation [ 90 ], our study is expected to yield several important findings.
Firstly, integrated analysis of behavioral and EEG records may provide new insights in investigating potential neurofunctional mechanisms of cue-reactivity in the young adults with TUD. The results can also enhance our understanding of pre-addiction brain changes, which will provide important supplements for different stages of addiction across all ages. The selection of subjective and objective indicators related to interventions are expected to provide clues for the development of targeted treatment strategies. Secondly, short-term mindfulness interventions align with the needs of the young people, the research findings of intervention setting could offer valuable guidance for practical applications. Moreover, The measurement of state mindfulness can track immediate effects, benefiting individuals in managing physical discomfort during cravings and mitigating stress and emotions triggered by daily life or cues.
However, there are still some limitations for the design. The primary consideration is as follows: 1) double blind method will not be used in this study, for the participants all from the same school, may inevitably discussing the experimental training they received. Moreover, the blind for experimental operators is also very challenging, as organization of the study need a lot of work in communication, would inevitably infiltrate by experimental related content. In order to mitigate the limitation (such as potential experimenter bias or participant expectations), the current study will involve a three-way evaluation that includes participants, psychological teachers, and experimental implementers, which will help partially address this issue. 2) the participants will be all in male, which limited the scope of analysis and generalization. As hormone indeed impacting brain functional status, the findings of brain mechanism research could restrict its generalizability across different genders. 3) biochemical indicators will not be measured with urinary cotinine, only through exhaled CO detection. As a result, false positives cannot be completely ruled out, and these measurements may not accurately reflect the actual level of nicotine metabolism. 4) no specialized behavioural measurement of intrinsic receptivity for mindfulness would be the Achilles heel for subjective research.
To address the challenges associated with double blinding and the absence of a data monitoring committee, the following procedures are necessary to uphold the integrity of blinding implementation: Impartial assessors will collect and evaluate data without prior knowledge of the study design to ensure unbiased evaluation. The data will be securely managed and analyzed using anonymized coding systems to maintain confidentiality. A clear separation will be maintained between the administrators of the intervention and the assessors of the outcomes to minimize bias. Robust standardized protocols will be implemented to promote consistency and minimize the risk of unblinding. Despite the challenges encountered, these measures are crucial for maintaining the integrity of the study. Furthermore, it is important to note that participants will not seek treatment. Although intensive training is about to be implemented, it is advisable to have lower expectations regarding training participation and effectiveness for this subgroup. However, it should be mentioned that the study will recruit volunteer who are not enrolled in psychological courses, which may indicate a certain level of enthusiasm among the participants. Therefore, different from smoking cessation plan, we will primarily focus on the intervention effect of BMT to cue-reactivity, and will emphasize brief mindfulness of STOP exercise training to facilitate the mastery of mindfulness for beginners. The audio guidance of STOP will also be used for online task of mindfulness in assessment to promote the homogeneity of research methods. The above factors are being of strength and weakness. Further studies with three-arm clinical design, adapting as full version of traditional training, or adding effective component of mindfulness would need to be adopt if significant results from such a brief intervention provision promise.
To summarize, we prospect to explore whether participants’ responses differed from their usual habitual behaviour of smoking after BMT and further assess the effects of mindfulness on response to urges. Possible biomarkers for this process and underlying mechanism in brain function will also be detected. The current study will present with reference value for studies in the similar population with higher smoking rates if the results of the current study proved to be effective.
Supporting information
S1 file. copy of the study protocol approved by the ethics committee..
https://doi.org/10.1371/journal.pone.0299797.s001
S2 File. SPIRIT checklist.
https://doi.org/10.1371/journal.pone.0299797.s002
S3 File. All items from the world health organization trial registration data set.
https://doi.org/10.1371/journal.pone.0299797.s003
S4 File. EEG preprocessing and analyses.
https://doi.org/10.1371/journal.pone.0299797.s004
Acknowledgments
The authors are grateful to the Students Counseling and Mental Health Center for the contributions to the implementation of the study.
- View Article
- PubMed/NCBI
- Google Scholar
- 3. Health NCfCDPaHPUOoSa. Reports of the Surgeon General. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014.
- 6. WHO. Tobacco Factsheet 2023 [updated 31 July 2019; cited 2023 15 August]. https://www.who.int/news-room/fact-sheets/detail/tobacco .
- 8. WHO. WHO report on the global tobacco epidemic, 2023: protect people from tobacco smoke 2023 [updated 31 July 2023; cited 2023 1 December]. https://www.who.int/publications/i/item/9789240077164 .
- 11. China NHCotPsRo. Healthy China action (2019–2030) [updated 15 July 2019; cited 2023 15 August]. https://www.gov.cn/xinwen/2019-07/15/content_5409694.htm .
- 62. Connolly C. Mental skills training. In: Williamon A, editor. Musical Excellence: Strategies and Techniques to Enhance Performance: Oxford University Press; 2004. p. 221–41.
Essay On Harmful Effects of Tobacco
Essay On Harmful Effects of Tobacco Chewing, Have you ever heard of tobacco smoking? Of course, yes. It is the most common thing to see people smoking tobacco cigarettes. It is not good to smoke, but people still are addicted to smoking. Today, we would be going to talk about tobacco like what is it, what are its effects, etc. So, start reading:

Essay on Tobacco
What is tobacco.

It is the most common name of some plants in the genus Nicotine belonging to the family Solanaceae. There are more than 70 species of tobacco, which are known in the world. But N. tabacum is the chief commercial crop. The more powerful variant N. Rustica is also utilized in some countries.
We have seen the use of tobacco cigarettes and other addictive products like pipes, cigars, and shishas. In these products, dried tobacco leaves are used for smoking. Tobacco is also being used as chewing the Tobacco, snuff, snus, and dipping Tobacco.
What does Tobacco contain?
Tobacco has the highly addictive stimulant alkaloid nicotine and many other harmful alkaloids. If we talk about the history of Tobacco, it has been used in America for many years, with some cultivation sites located in Mexico. People in the USA tribes grow and use Tobacco in a traditional way.
Historically, people belonging to the Northeast Woodlands cultures have brought Tobacco in pouches in the form of a readily accepted trade item. People used Tobacco smoking both ceremonially and socially like when they had to seal a peace treaty or any kind of trade agreement.
There are several native cultures, where Tobacco is considered a gift from the Creator, with the tobacco smoking in ceremonies carrying one’s prayers and thoughts to the creator.
What’s about Tobacco smoking?
Tobacco smoking is the method of burning Tobacco and consuming the smoke, which is developed throughout the process of smoking. The smoke produced through the use of Tobacco may be inhaled as is performed with cigarettes.
Some people like to release it from the mouth, as is generally performed with cigars and pipes. Tobacco was introduced to Eurasia by European colonists in the late 17 th century, where it followed common trade ways.
The practice encountered disparagement from its first import to the Western world forwards but surrounded itself in a certain section of many societies before becoming spread in the entire world upon the introduction of automated cigarette-rolling apparatus.
Smoking is the most common way of consuming Tobacco. The agricultural product is often combined with additives and then combusted. As a result, the smoke is then inhaled and the active substances are absorbed via the alveoli in the oral mucosa or lungs.
Several substances in cigarette smoking activate chemical reactions in nerve endings that increase alertness, heart rate, and reaction time. Endorphins and dopamine are released that give a sense of pleasure to humans.
Also Read: Nasha Mukti Par Essay in English
As of 2008-2010, Tobacco is consumed by about 49 percent of men and 11 percent of women having an age of 15 years or older in middle income and low-income nations such as India, Bangladesh, Mexico, China, Egypt, Philippines, Russia, Ukraine, Vietnam, and many others. People in these countries almost use Tobacco in the form of smoking.
Tobacco Essay in English
Health effects of tobacco.
Most people are addicted to Tobacco smoking. Today, the government has put many initiatives to control the use of Tobacco in smoking products. But still, people who are addicted have a hard time quitting smoking.
This is why it is important to understand the risk effects of Tobacco on health if anyone of us is an avid smoker. Using Tobacco for a long time can boost your risk for many health-related issues in human beings.
Tobacco is a plant and its leaves are chewed, smoked, or sniffed to feel a variety of effects. Due to the presence of the chemical substance known as nicotine, it is known to be an addictive substance.
Apart from nicotine, Tobacco smoking contains a number of other chemicals, which are 7000 in number and at least 70 of which are a reason for occurring cancer and other health problems. Tobacco, which is not burned is known as smokeless Tobacco.
There are around 30 chemicals in smokeless Tobacco that can develop cancer in humans, which also includes nicotine.
Health problems caused by smoking or smokeless Tobacco are mentioned below:
Heart and blood vessel problems:
- Blood clots in the legs that can reach the lungs
- Temporarily heightened blood pressure after Tobacco smoking
- Blood clots, as well as weakness in the walls of blood vessels in the human brain that arises stroke
- Poor blood supply to the legs
- Coronary artery disease, which includes heart attack and angina
- Problems faced by men with erections due to the reduced blood flow into the penile region
There are some other poor effects of Tobacco on the health of a human being. These are:
- Cancer can take place in various parts of the body such as lungs, larynx, mouth, throat, stomach, esophagus, kidney, cervix, colon, pancreas, nose and sinuses, rectum, and a lot more.
- Damage to sperm, which may create infertility in men.
- Poor wound healing after any kind of surgery
- Loss of sight because of an increased risk of macular degeneration
- Aging of the skin like wrinkles
- Tooth and gum diseases
- Lung-related problems like asthma, COPD, etc. that is not easy to control
- Reduced ability to taste and smell
- Problems in women during pregnancy like babies born at low birth weight, miscarriage, early labor, and cleft lip
Health effects of secondhand smoke
If someone is living around the smoke of others known as secondhand smoke, then he/she is having a higher risk for lung cancer, heart attack, heart disease, sudden and severe reactions (eye, throat, nose, and lower respiratory tract).
How to quit Tobacco smoking?
Like any form of addiction, quitting Tobacco is not an easy task, particularly if you are doing it on an individual basis. You can seek assistance from friends, family members, and coworkers.
We can engage in therapy sessions in a healthcare center. There are hospitals, community centers, health departments, and work sites, which can help us quit smoking.
This is an Essay On Harmful Effects of Tobacco Chewing, from this entire article, we cover information regarding essay on tobacco in English, anti tobacco essay in English. If found anything missing let us know by commenting below. For more info kindly visit us at wikiliv.com
Share this:
Leave a comment cancel reply.
Save my name, email, and website in this browser for the next time I comment.
Ad Blocker Detected!
Smoking: Causes and Effects Essay
Among numerous bad habits of modern society smoking seems to be of the greatest importance. Not only does it affect the person who smokes, but also those who are around him. Many people argue about the appropriate definition of smoking, whether it is a disease or just a bad habit. Considering the peculiarities of a habit and of a disease, smoking can be considered as a habit rather than a disease. Among signifiers of a bad habit, it should be pointed out that a bad habit can be controlled by willpower, it can be prevented, and it can be cured (Gilman and Zun 33). Smoking can be fought against with the help of all the points mentioned above. Thus, it is a bad habit which can be easily refused if an individual possessing it has a strong decision to quit. Moreover, it can be cured in many different ways, and it can be prevented by education and other social norms.
Considering the first element, which one of the most important out of the three, willpower is a key to get rid of such a bad habit as smoking, which is very difficult to give up. If a person has a strong determination to quit smoking, he will have to endure considerably a short period of time of physical discomfort. One of the most important part of quitting, is that that is doesn’t require medical help, that is to say, a person is not likely to suffer a procedure that is risky to health and life. In comparison to alcoholism or drug addiction, where medical help is essential to save life of a person who needs a certain amount of an alcohol or drug substance in has blood to survive, the lack of nicotine in blood produces just a physical discomfort that is not dangerous for health and can be handled with the help of willpower. Regarding the second aspect of a bad habit, prevention, smoking can be prevented in early childhood with the help of proper education and social norms (Brinkman et al 689). Many people start smoking when they are teenagers just to prove they are adults in companies. If the society was able to produce a negative impression of this bad habit, so that it doesn’t seem to be sign of being an adult, it would be easier to prevent many children from smoking (Albaum et al 11).
The last aspect of a bad habit is a cure for it. Smoking can be cured in many different ways. There are many different techniques, starting from a nicotine plaster and ending with special clinics and communities helping people to get rid of this problem. If a person wants to quit, he or she has various options to help him or her to solve this problem. To conclude, smoking is a bad habit that can be easily quitted. Although there is an addiction to smoking, the lack of nicotine is not dangerous to the life of a smoker and can be handled without medical intervention. The most important aspect of this bad habit, which actually makes a habit, is that it can be quitted with the help of willpower. Moreover, it can be prevented with alteration of attitude towards smoking and it can be cured in many different ways (Albaum et al 23).
Despite widespread public awareness of the multiple health risks associated with smoking, one out of every four girls under age 18 is a smoker and more than 25 million American women smoke. Whereas the last two decades have seen an overall decrease in smoking prevalence, the rate of smoking has declined much more slowly among women than among men. If current trends continue, smoking rates of women will overtake those of men by the year 2000. Smoking rates are highest, approaching 30%, among women of reproductive age (18–44 years). Rates of smoking are particularly high among young White women with a high school education or less and low income. Cessation rates are lower among African American women (30% have quit) compared to White women (43% have quit). Minority and young women who have low rates of self-initiated cessation are also underrepresented in formal smoking cessation programs (Gilman and Zun 87). A greater proportion of women than men are pre-contemplators, that is, not considering quitting smoking within 6 months and have lower self-confidence that they could quit if they were to try. The debate continues regarding whether or not women are less likely to be successful at quitting when they try than men, with some evidence suggesting that women are more likely than men to relapse and others indicating no gender differences). Regardless, rates of relapse are very high, both among self-quitters and those who participate in formal cessation programs (Albaum et al 24).
Interventions specifically designed for smokers have attempted to address the role of weight concerns as an inhibitor to cessation and long-term maintenance. A randomized trial tested nicotine gum or a behavioral weight control program each alone, or in combination as adjuncts to an intensive group cessation intervention for weight concerned women smokers. The intervention integrated accepted cognitive and behavioral coping strategies for quitting smoking, changing eating behaviors, and developing a walking program.
Works Cited
Albaum, G., Baker, K.G., Hozier, G.C., Rogers, R.D. Smoking Behavior, Information Sources, and Consumption Values of Teenagers: Implications for Public Policy and Other Intervention Failures. Journal of Consumer Affairs , 36 (1), 2002: 5-55.
Brinkman, M.C., Callahan, P.J., Gordon, S.M., Kenny, D.V., Wallace, L.A. Volatile Organic Compounds as Breath Biomarkers for Active and Passive Smoking. Environmental Health Perspectives, 110 (7), 2002, p. 689.
Gilman Sander L. and Xhou Zun. Smoke: A GlobalHistory of Smoking. Reaktion Books; illustrated edition edition, 2004.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2021, November 29). Smoking: Causes and Effects. https://ivypanda.com/essays/smoking-causes-and-effects/
"Smoking: Causes and Effects." IvyPanda , 29 Nov. 2021, ivypanda.com/essays/smoking-causes-and-effects/.
IvyPanda . (2021) 'Smoking: Causes and Effects'. 29 November.
IvyPanda . 2021. "Smoking: Causes and Effects." November 29, 2021. https://ivypanda.com/essays/smoking-causes-and-effects/.
1. IvyPanda . "Smoking: Causes and Effects." November 29, 2021. https://ivypanda.com/essays/smoking-causes-and-effects/.
Bibliography
IvyPanda . "Smoking: Causes and Effects." November 29, 2021. https://ivypanda.com/essays/smoking-causes-and-effects/.
- Free Will and Willpower: Is Consciousness Necessary?
- Francois Marie Arouet (Voltaire)
- Smoking Cessation for Ages 15-30
- Quitting Smoking: Strategies and Consequences
- Importance of Quitting Smoking
- Phineas Taylor Barnum - Biography Study
- Smoking Cessation Project Implementation
- Lifestyle Management While Quitting Smoking
- Is Alex in Burgess' "A Clockwork Orange" Cured?
- Advocating for Smoking Cessation: Health Professional Role
- The Relationship Between the High Rate of Urbanization in Africa and AIDS Spread
- California’s Growing Healthcare Crisis: An Existing Reality
- Environmental Health Problems and Health Inequity
- Public Health: Pertussis Cases in Wisconsin
- The Need for Health Care Reform in the USA
Home / Essay Samples / Health / Tobacco Use / Negative Effects of Tobacco Use
Negative Effects of Tobacco Use
- Category: Health
- Topic: Smoking , Tobacco , Tobacco Use
Pages: 1 (549 words)
- Downloads: -->
--> ⚠️ Remember: This essay was written and uploaded by an--> click here.
Found a great essay sample but want a unique one?
are ready to help you with your essay
You won’t be charged yet!
Assisted Suicide Essays
Drug Addiction Essays
Alcohol Abuse Essays
Smoking Essays
Underage Drinking Essays
Related Essays
We are glad that you like it, but you cannot copy from our website. Just insert your email and this sample will be sent to you.
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Your essay sample has been sent.
In fact, there is a way to get an original essay! Turn to our writers and order a plagiarism-free paper.
samplius.com uses cookies to offer you the best service possible.By continuing we’ll assume you board with our cookie policy .--> -->