
- Andhra Pradesh
- Chhattisgarh
- West Bengal
- Madhya Pradesh
- Maharashtra
- Jammu & Kashmir
- NCERT Books 2022-23
- NCERT Solutions
- NCERT Notes
- NCERT Exemplar Books
- NCERT Exemplar Solution
- States UT Book
- School Kits & Lab Manual
- NCERT Books 2021-22
- NCERT Books 2020-21
- NCERT Book 2019-2020
- NCERT Book 2015-2016
- RD Sharma Solution
- TS Grewal Solution
- DK Goel Solution
- TR Jain Solution
- Selina Solution
- Frank Solution
- ML Aggarwal Solution
- Lakhmir Singh and Manjit Kaur Solution
- I.E.Irodov solutions
- ICSE - Goyal Brothers Park
- ICSE - Dorothy M. Noronhe
- Sandeep Garg Textbook Solution
- Micheal Vaz Solution
- S.S. Krotov Solution
- Evergreen Science
- KC Sinha Solution
- ICSE - ISC Jayanti Sengupta, Oxford
- ICSE Focus on History
- ICSE GeoGraphy Voyage
- ICSE Hindi Solution
- ICSE Treasure Trove Solution
- Thomas & Finney Solution
- SL Loney Solution
- SB Mathur Solution
- P Bahadur Solution
- Narendra Awasthi Solution
- MS Chauhan Solution
- LA Sena Solution
- Integral Calculus Amit Agarwal Solution
- IA Maron Solution
- Hall & Knight Solution
- Errorless Solution
- Pradeep's KL Gogia Solution
- OP Tandon Solutions
- Sample Papers
- Previous Year Question Paper
- Value Based Questions
- CBSE Syllabus
- CBSE MCQs PDF
- Assertion & Reason
- New Revision Notes
- Revision Notes
- HOTS Question
- Marks Wise Question
- Toppers Answer Sheets
- Exam Paper Aalysis
- Concept Map
- CBSE Text Book
- Additional Practice Questions
- Vocational Book
- CBSE - Concept
- KVS NCERT CBSE Worksheets
- Formula Class Wise
- Formula Chapter Wise
- JEE Crash Course
- JEE Previous Year Paper
- Important Info
- JEE Mock Test
- JEE Sample Papers
- SRM-JEEE Mock Test
- VITEEE Mock Test
- BITSAT Mock Test
- Manipal Engineering Mock Test
- AP EAMCET Previous Year Paper
- COMEDK Previous Year Paper
- GUJCET Previous Year Paper
- KCET Previous Year Paper
- KEAM Previous Year Paper
- Manipal Previous Year Paper
- MHT CET Previous Year Paper
- WBJEE Previous Year Paper
- AMU Previous Year Paper
- TS EAMCET Previous Year Paper
- SRM-JEEE Previous Year Paper
- VITEEE Previous Year Paper
- BITSAT Previous Year Paper
- UPSEE Previous Year Paper
- CGPET Previous Year Paper
- CUSAT Previous Year Paper
- AEEE Previous Year Paper
- Crash Course
- Previous Year Paper
- NCERT Based Short Notes
- NCERT Based Tests
- NEET Sample Paper
- Previous Year Papers
- Quantitative Aptitude
- Numerical Aptitude Data Interpretation
- General Knowledge
- Mathematics
- Agriculture
- Accountancy
- Business Studies
- Political science
- Enviromental Studies
- Mass Media Communication
- Teaching Aptitude
- NAVODAYA VIDYALAYA
- SAINIK SCHOOL (AISSEE)
- Mechanical Engineering
- Electrical Engineering
- Electronics & Communication Engineering
- Civil Engineering
- Computer Science Engineering
- CBSE Board News
- Scholarship Olympiad
- School Admissions
- Entrance Exams
- All Board Updates
- Miscellaneous
- State Wise Books
- Engineering Exam
- STATE WISE BOOKS
- ENGINEERING EXAM
- SCHOLARSHIP OLYMPIAD
- STATE BOOKS

CBSE Board Class 12 Chemistry Case Based Questions With Solutions
CBSE Board Class 12 Chemistry Case Based Questions With Solutions to prepare for the upcoming term 1 board exams are given here. It is extremely helpful since the expert team has crafted this by following the Syllabus of class 12th. Case study questions are also known as passage based problems because it includes the paragraph from which 5 or more problems are curated.
To solve these types of problems students need to have a thorough understanding of all the basics and fundamental concepts. Also, a student who is good in problem solving skills can easily answer such questions.
Those who are preparing for their CBSE Class 12 board exam term 1 can use it to practice the questions on a daily basis. Also, the experts have given step by step solutions to all those problems so that students can cope up with the given problem very easily.
CBSE Class 12 Chemistry Case Based Questions, Assertion & Reason, MCQs
CBSE Class 12 Chemistry includes many chemical reactions, chemical bondings, theories, experiments, discoveries, etc. All of these types of things should be well versed by the students to answer the Case study, Multiple Choice Questions, Assertion and Reason. So, practicing the given CBSE Class 12 Chemistry Case Study PDF will be very handy in boosting the basic knowledge and preparing for the final papers.
We have provided the complete set of PDFs so that candidates can prepare for the board examination very easily. Before attempting the given PDF candidates are suggested here to complete their syllabus for the term 1 exam. Doing so will aid in self-assessment and give a sense of board exam preparation.
Class 12 Chemistry Case Study Questions For Term 1 Exam
Class 12 Chemistry Case Study Questions for Term 1 exam includes The Solid State, The P block elements, Haloalkanes and Haloarenes, Biomolecules, etc. Questions for all these chapters are given in the PDF file that are available here for free to download.
Term 1 exam is about to be held in November-December this year. So, students should start their board exam preparation early. For that purpose we have provided the complete chapter wise case based questions, assertion and reason and a lot of Objective type problems for the practice purposes.
CBSE 12 Case Based Question With Answers
CBSE Case Based Questions with Answers are designed and developed for the term 1 exam which is developed by the subject matter experts. The answers are provided to help the learners to solve the problem if they are stuck at some questions and don’t know how to tackle that.
Not only answers are given but step by step explanations are given too. By taking help of those explanations students can easily get a good grasp over the concepts.
To Download case study based questions class 12 chemistry PDF visit here or download from Selfstudys.com.
To solve Class 12 Chemistry Assertion and Reason Questions read the given statement and then reason. Now, you need to verify both the assertion and reason. If both are given correctly then you have to check whether the given reason supports the statement or not.

CBSE Board Class 12 Computer Science Answer Key 2024 and Question Papers, Download PDF All SETs

CBSE Class 12 Computer Science Exam 2024 : Important MCQs with Answers For Last Minute Revision

CBSE Board Class 12 History Answer Key 2024 and Question Papers, Download PDF All SETs

CBSE Board 12th History Exam 2024 : Chapterwise Most Important Question with Answers

CBSE Board Class 12 Business Studies Answer Key 2024 and Question Papers, Download PDF All SETs

CBSE Class 12th Exam 2024: Business Studies Important MCQs & Assertion-Reason Questions

- NCERT Solutions for Class 12 Maths
- NCERT Solutions for Class 10 Maths
- CBSE Syllabus 2023-24
- Social Media Channels
- Login Customize Your Notification Preferences

- Second click on the toggle icon

Provide prime members with unlimited access to all study materials in PDF format.
Allow prime members to attempt MCQ tests multiple times to enhance their learning and understanding.
Provide prime users with access to exclusive PDF study materials that are not available to regular users.


Class 12 Chemistry Case Study Questions PDF Download
- Post author: studyrate
- Post published:
- Post category: Class 12 / 12 board
- Post comments: 0 Comments
Looking for Class 12 Chemistry Case Study Based Questions in PDF format? This comprehensive article provides expert insights, engaging content, and answers to frequently asked questions to help you excel in your studies. Download the PDF now and boost your chemistry knowledge!
Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th.

You need to improve your preparation for the Class 12 Chemistry Case Study Questions exams if you want to achieve a 95+% on the boards. You may find case study questions from every chapter that will be covered in the CBSE Class 12 Chemistry Board Exams in this post.
Table of Contents
Case Study-Based Questions for Class 12 Chemistry
Welcome to the world of Class 12 Chemistry Case Study Questions! As a student pursuing Chemistry in the 12th grade, you have already shown dedication and commitment to the subject. However, dealing with case study-based questions can be both intriguing and challenging. In this article, we will explore the nuances of such questions and offer valuable guidance to excel in your exams.
Class 12 Physics Case Study Questions Class 12 Chemistry Case Study Questions Class 12 Biology Case Study Questions Class 12 Maths Case Study Questions
Importance of Class 12 Chemistry Case Study-Based Questions
Class 12 Chemistry case study-based questions play a vital role in your overall understanding of the subject. They enable you to:
- Apply Theoretical Knowledge : Case studies allow you to apply the concepts you have learned in real-life situations, bridging the gap between theory and practical application.
- Develop Analytical Skills : By critically analyzing case scenarios, you enhance your analytical abilities, which are essential in various professional fields.
- Enhance Problem-Solving Abilities : Tackling case study-based questions hones your problem-solving skills, preparing you to face challenges with confidence.
- Gain Deeper Insights : Exploring different case studies exposes you to a wide range of chemical reactions and phenomena, broadening your understanding of Chemistry.
Tips to Excel in Class 12 Chemistry Case Study Questions
- Thoroughly Understand the Concepts: Before attempting case study questions, ensure you have a strong grasp of the underlying concepts and theories.
- Analyze the Scenario Carefully: Take your time to read and comprehend the given case study. Pay attention to every detail to identify the key points.
- Relate to Real-Life Scenarios: Try to connect the case study with real-life situations, as this will make the problem-solving process more intuitive.
- Practice Regularly: Practice a wide variety of case study questions to familiarize yourself with different scenarios and improve your problem-solving skills.
- Collaborate with Peers: Engage in group discussions and brainstorming sessions with your peers. This will provide diverse perspectives and enhance your critical thinking.
Best Books for Class 12 Chemistry
Strictly in accordance with the new term-by-term curriculum for the Class 12 Chemistry Case Study Questions exams to be held in the academic session 2024, including the new board-introduced multiple-choice question types, Stand-Alone MCQs, and MCQs based on Assertion-Reason Case-based MCQs. Included are inquiries from the official CBSE Question Bank that was released in April 2024. What changes have been made to the book: strictly in accordance with the term-by-term syllabus for the board exams that will be held during the 2024 academic year? Chapter- and topic-specific multiple-choice questions based on the unique assessment plan for the Class 12 Chemistry Case Study Questions Board Examination.

Chemistry Syllabus for 2024
Unit II: Solutions (15 Periods)
Types of solutions, expression of concentration of solutions of solids in liquids, solubility of gases in liquids, solid solutions, Raoult’s law, colligative properties – relative lowering of vapour pressure, elevation of boiling point, depression of freezing point, osmotic pressure, determination of molecular masses using colligative properties, abnormal molecular mass, Van’t Hoff factor.
Unit III: Electrochemistry (18 Periods)
Redox reactions, EMF of a cell, standard electrode potential, Nernst equation and its application to chemical cells, Relation between Gibbs energy change and EMF of a cell, conductance in electrolytic solutions, specific and molar conductivity, variations of conductivity with concentration, Kohlrausch’s Law, electrolysis and law of electrolysis (elementary idea), dry cell-electrolytic cells and Galvanic cells, lead accumulator, fuel cells, corrosion.
Unit IV: Chemical Kinetics (15 Periods)
Rate of a reaction (Average and instantaneous), factors affecting rate of reaction: concentration, temperature, catalyst; order and molecularity of a reaction, rate law and specific rate constant, integrated rate equations and half-life (only for zero and first order reactions), concept of collision theory (elementary idea, no mathematical treatment), activation energy, Arrhenius equation.
Unit VIII: d and f Block Elements (18 Periods)
General introduction, electronic configuration, occurrence and characteristics of transition metals, general trends in properties of the first-row transition metals – metallic character, ionization enthalpy, oxidation states, ionic radii, colour, catalytic property, magnetic properties, interstitial compounds, alloy formation, preparation and properties of K 2 Cr 2 O 7 and KMnO 4 .
Lanthanoids – Electronic configuration, oxidation states, chemical reactivity and lanthanoid contraction and its consequences.
Actinoids – Electronic configuration, oxidation states and comparison with lanthanoids.
Unit IX: Coordination Compounds (18 Periods)
Coordination compounds – Introduction, ligands, coordination number, colour, magnetic properties and shapes, IUPAC nomenclature of mononuclear coordination compounds. Bonding, Werner’s theory, VBT, and CFT; structure and stereoisomerism, the importance of coordination compounds (in qualitative analysis, extraction of metals and biological system).
Unit X: Haloalkanes and Haloarenes (15 Periods)
Haloalkanes: Nomenclature, nature of C–X bond, physical and chemical properties, optical rotation mechanism of substitution reactions.
Haloarenes: Nature of C–X bond, substitution reactions (Directive influence of halogen in monosubstituted compounds only). Uses and environmental effects of – dichloromethane, trichloromethane, tetrachloromethane, iodoform, freons, DDT.
Unit XI: Alcohols, Phenols and Ethers (14 Periods)
Alcohols: Nomenclature, methods of preparation, physical and chemical properties (of primary alcohols only), identification of primary, secondary and tertiary alcohols, mechanism of dehydration, uses with special reference to methanol and ethanol.
Phenols: Nomenclature, methods of preparation, physical and chemical properties, acidic nature of phenol, electrophilic substitution reactions, uses of phenols.
Ethers: Nomenclature, methods of preparation, physical and chemical properties, uses.
Unit XII: Aldehydes, Ketones and Carboxylic Acids (15 Periods)
Aldehydes and Ketones: Nomenclature, nature of carbonyl group, methods of preparation, physical and chemical properties, mechanism of nucleophilic addition, reactivity of alpha hydrogen in aldehydes, uses.
Carboxylic Acids: Nomenclature, acidic nature, methods of preparation, physical and chemical properties; uses.
Unit XIII: Amines (14 Periods)
Amines: Nomenclature, classification, structure, methods of preparation, physical and chemical properties, uses, identification of primary, secondary and tertiary amines
Diazonium salts: Preparation, chemical reactions and importance in synthetic organic chemistry.
Unit XIV: Biomolecules (18 Periods)
Carbohydrates – Classification (aldoses and ketoses), monosaccharides (glucose and fructose), D-L configuration oligosaccharides (sucrose, lactose, maltose), polysaccharides (starch, cellulose, glycogen); Importance of carbohydrates.
Proteins – Elementary idea of – amino acids, peptide bond, polypeptides, proteins, structure of proteins – primary, secondary, tertiary structure and quaternary structures (qualitative idea only), denaturation of proteins; enzymes.
Hormones – Elementary idea excluding structure.
Vitamins – Classification and functions.
Nucleic Acids: DNA and RNA.
FAQ on Class 12 Chemistry Case Study Questions
Q: can i rely solely on class 12 chemistry case study based questions exam preparation.
Yes, case study-based questions are an essential part of your preparation. However, it is advisable to supplement them with other study materials and revision of theoretical concepts for comprehensive preparation.
Q: How often should I practice Class 12 Chemistry Case Study Based Questions?
Frequent practice is crucial for mastering case study-based questions. Set aside dedicated practice sessions and gradually increase the difficulty level of the questions.
Q: Can I discuss case study questions with my teachers?
Absolutely! Engaging with your teachers regarding case study questions will provide valuable insights and clarifications.

You Might Also Like
Class 12 chemistry case study questions chapter 11 alcohols, phenols, and ethers, memories of childhood summary class 12 english pdf, class 12 maths: case study of chapter 13 probability pdf download, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .

The Topper Combo Flashcards
- Contains the Latest NCERT in just 350 flashcards.
- Colourful and Interactive
- Summarised Important reactions according to the latest PYQs of NEET(UG) and JEE
No thanks, I’m not interested!
CBSE Expert
CBSE Class 12 Chemistry Case Study Questions PDF
Case studies play a pivotal role in CBSE Class 12 Chemistry, as they enable students to apply theoretical knowledge to real-life scenarios. CBSE Class 12 Chemistry Case Study Questions PDF section introduces the significance of case studies in enhancing analytical skills and understanding complex chemical reactions.
Case studies challenge students to think critically, analyze experimental data, and devise problem-solving strategies. They provide a deeper understanding of chemical principles and their practical applications, fostering a holistic learning experience. Familiarize yourself with the structure of case study questions to streamline your preparation. Each case study presents a unique chemical problem, encouraging students to identify relevant concepts and devise accurate solutions.
Table of Contents
Class 12 Chemistry Case Study Questions
CBSE Class 12 Chemistry question paper will have case study questions too. These case-based questions will be objective type in nature. So, Class 12 Chemistry students must prepare themselves for such questions. First of all, you should study NCERT Textbooks line by line, and then you should practice as many questions as possible.

Chapter-wise Solved Case Study Questions for Class 12 Chemistry
Class 12 students should go through important Case Study problems for Chemistry before the exams. This will help them to understand the type of Case Study questions that can be asked in Grade 12 Chemistry examinations. Our expert faculty for standard 12 Chemistry have designed these questions based on the trend of questions that have been asked in last year’s exams. The solutions have been designed in a manner to help the grade 12 students understand the concepts and also easy-to-learn solutions.
Tips to Excel in CBSE Class 12 Chemistry Examinations
Excel in your Chemistry exams with these practical tips.
A. Regular Practice with Case Studies
Consistent practice with case study questions enhances your ability to tackle complex problems. Dedicate time to solving various case studies to build confidence.
B. Understanding Analytical Skills
Develop strong analytical skills to approach case studies logically. Break down complex problems into simpler components and analyze them step-by-step.
C. Time Management Strategies
Allocate sufficient time for each case study during the exam. Practice time management in mock tests to complete the paper within the stipulated time.
Best Books for Class 12 Chemistry
Strictly as per the new term-wise syllabus for Board Examinations to be held in the academic session 2024 for class 12 Multiple Choice Questions based on new typologies introduced by the board- Stand-Alone MCQs, MCQs based on Assertion-Reason Case-based MCQs. Include Questions from CBSE official Question Bank released in April 2024 Answer key with Explanations What are the updates in the book: Strictly as per the Term wise syllabus for Board Examinations to be held in the academic session 2024. Chapter-wise -Topic-wise Multiple choice questions based on the special scheme of assessment for Board Examination for Class 12th Chemistry.

Mastering CBSE Class 12 Chemistry case study questions is crucial for excelling in the exams. Embrace case studies as a valuable learning tool, and with practice, you’ll ace your Chemistry exams with confidence.
Benefits of Utilizing the CBSE Class 12 Chemistry Case Study PDF
- Enhanced Learning Experience : The case study PDF offers practical examples and scenarios, making the learning process engaging and relatable for students.
- Application of Theoretical Concepts : It enables students to apply theoretical knowledge to practical situations, honing their problem-solving and analytical skills.
- Real-World Relevance : By connecting classroom learning to real-life applications, students can grasp the practical significance of chemistry in various industries.
- Critical Thinking Development : Analyzing case studies encourages students to think critically and make informed decisions based on chemical principles.
- Exam Preparation : Exposure to case studies aids in better preparation for chemistry examinations by providing a comprehensive understanding of the subject.
The CBSE Class 12 Chemistry case study PDF brings a refreshing perspective to the world of education. By intertwining theoretical knowledge with practical applications, it equips students to face real-world challenges with confidence. The diverse case studies provide invaluable insights, encouraging students to explore chemistry beyond the classroom and make a positive impact on society.
What is the CBSE Class 12 Chemistry case study PDF?
The CBSE Class 12 Chemistry case study PDF is a curated document by CBSE, presenting real-life applications of chemistry concepts for students to understand the subject’s practical relevance.
How does the case study PDF benefit students?
The case study PDF enhances the learning experience, fosters critical thinking, promotes application-based learning, and prepares students for examinations.
Are the case studies diverse in content?
Yes, the case studies cover various branches of chemistry, including organic, inorganic, physical, environmental, and analytical chemistry.
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Download India's best Exam Preparation App Now.
Key Features
- Revision Notes
- Important Questions
- Previous Years Questions
- Case-Based Questions
- Assertion and Reason Questions
No thanks, I’m not interested!
CBSE NCERT Solutions
NCERT and CBSE Solutions for free
Case Study Class 12 Chemistry With Questions Answers
In Coming Exams, CBSE will ask two Case Study Questions in the CBSE class 12 Chemistry questions paper. Each theme will have five questions and students will have a choice to attempt any four of them. Here are some example questions Based On Case Study Problems:
Question-1 Read the passage given below and answer any four out of the following questions: Ammonia is present in small quantities in air and soil where it is formed by the decay of nitrogenous organic matter e.g., urea. On a large scale, ammonia is manufactured by Haber’s process. In accordance with Le Chatelier’s principle, high pressure would favour the formation of ammonia. Ammonia is a colourless gas with a pungent odour. Its freezing and boiling points are 198.4 and 239.7 K respectively. In the solid and liquid states, it is associated with hydrogen bonds as in the case of water and that accounts for its higher melting and boiling points than expected on the basis of its molecular mass. Ammonia gas is highly soluble in water. Its aqueous solution is weakly basic due to the formation of OH– ions. The presence of a lone pair of electrons on the nitrogen atom of the ammonia molecule makes it a Lewis base.

The following questions are multiple-choice questions. Choose the most appropriate choice 1. On a small scale, ammonia is obtained from ammonium salts which decompose when treated with 1.caustic soda 2.calcium chloride 3.sodium hydroxide 4.sodium chloride
2.The optimum conditions for the production of ammonia are a pressure of 1. 200*105 Pa 2. 400*105 Pa 3. 100*105 Pa 4. 300*105 Pa
3. The catalyst which is used in the preparation of NH3 by Haber’s process 1. Mg2O3 + K2O 2. Al2O3 + K2O 3. NaO3 + K2O 4. None of these 4. The ammonium molecule has: 1. five bond pair and two lone pair 2. four lone pair and one bond pair 3. three bond pair and one lone pair 4. three bond pair and two lone pair 5. A compound reacts with ammonia to form deep colour solution, identify the compound 1. Au2+ 2. Cu2+ 3. Al3+
Questions-2 Read the passage and answer any four out of the following questions: Colloidal particles always carry an electric charge. The nature of this charge is the same on all the particles in a given colloidal solution and may be either positive or negative. The charge on the sol particles is due to one or more reasons, viz., due to electron capture by sol particles during electrodispersion of metals. When two or more ions are present in the dispersion medium, preferential adsorption of the ion common to the colloidal particle usually takes place. When silver nitrate solution is added to the potassium iodide solution, the precipitated silver iodide adsorbs iodide ions from the dispersion medium, and negatively charged colloidal solution results. acquired a positive or a negative charge by selective adsorption on the surface of a colloidal particle The combination of the two layers of opposite charges around the colloidal particle is called Helmholtz electrical double layer. The presence of equal and similar charges on colloidal particles is largely responsible for providing stability to the colloidal solution. In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
1. Assertion and reason both are correct statements and reason is correct explanation for assertion 2. Assertion and reason both are correct statements but reason is not correct explanation for assertion 3. Assertion is correct statement and reason is wrong statement 4. Assertion is wrong statement but reason is correct statement
1. Assertion: The presence of equal and similar charges on colloidal particles is largely responsible in providing stability to the colloidal solution. Reason: The repulsive forces between charged particles having the same charge prevent them from aggregating and provide stability.
2. Assertion: The first layer is mobile in Helmholtz electrical double layer. Reason: The potential difference between the fixed layer and the diffused layer of opposite charges is called zeta potential.
3. Assertion: The sol particle in the colloid has a charge. Reason: The charge in sol is due to electron capture by sol particles during the electrodispersion of metals.
4. Assertion: Methylene blue sol is a negatively charged sol. Reason: When KI solution is added to AgNO3 solution, positively charged sol formed.
5. Assertion: If FeCl3 is added to an excess of hot water, a positively charged sol of hydrated ferric oxide is formed. Reason: When ferric chloride is added to NaOH a negatively charged sol is obtained with adsorption of OH- ions.
Question-3 .Read the passage and answer the following questions: The crystal field theory (CFT) is an electrostatic model which considers the metal-ligand bond to be ionically arising purely from electrostatic interactions between the metal ion and the ligand. Ligands are treated as point charges in case of anions or point dipoles in case of neutral molecules. The five d orbitals in an isolated gaseous metal atom/ion have the same energy, i.e., they are degenerate. In an octahedral coordination entity with six ligands surrounding the metal atom/ion, there will be repulsion between the electrons in metal d orbitals and the electrons (or negative charges) of the ligands. This splitting of the degenerate levels due to the presence of ligands in a definite geometry is termed crystal field splitting and the energy separation is denoted by Δ0. The colour in the coordination compounds can be readily explained in terms of the crystal field theory. In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices. 1.Assertion and reason both are correct statements and reason is correct explanation for assertion. 2. Assertion and reason both are correct statements but reason is not correct explanation for assertion. 3. Assertion is correct statement but reason is wrong statement. 4. Assertion is wrong statement but reason is correct statement.
1. Assertion: The dx2-y2 and dz2 orbitals which point towards the axes along the direction of the ligand will experience more repulsion. Reason: The dxy, dyz and dxz orbitals which are directed between the axes will be lowered in energy.
2. Assertion: The complex [Ti(H2O)6]3+, which is red in colour. Reason: The crystal field theory attributes the colour of the coordination compounds to d-d transition of the electron.
3. Assertion: Ligands for which Δ0Δ0 < P are known as weak field ligands and form high spin complexes. Reason: If Δ0 > P, then the fourth electron enters one of the eg orbitals giving the configuration t2g3 eg1.
4. Assertion: In tetrahedral coordination entity formation, the d orbital splitting is inverted and is smaller as compared to the octahedral field splitting. Reason: Spectrochemical series is based on the absorption of light by complexes with different ligands.
5. Assertion: The crystal field model is successful in explaining the formation, structures, colour and magnetic properties of coordination compounds. Reason: The anionic ligands are found at the low end of the spectrochemical series.
Answer Key:
1. (b) Assertion and reason both are correct statements and reason is not correct explanation for assertion. 2. (c) Assertion is correct statement but reason is wrong statement. 3. (a) Assertion and reason both are correct statements and reason is correct explanation for assertion. 4. (a) Assertion and reason both are correct statements and reason is correct explanation for assertion. 5. (d) Assertion is wrong statement but reason is correct statement.
Question-4 Read the passage and answer any four out of the following question Alfred Werner (1866-1919), a Swiss chemist was the first to formulate his ideas about the structures of coordination compounds. Werner proposed the concept of a primary valence and a secondary valence for a metal. The coordination entity constitutes a central metal atom or ion bonded to a fixed number of ions or molecules. In a coordination entity, the atom/ion to which a fixed number of ions/groups are bound in a definite geometrical arrangement around it is called the central atom or ion. The ions or molecules bound to the central atom/ion in the coordination entity are called ligands. Ligands may be simple ions such as Cl-, small molecules such as H2O or NH3, larger molecules such as H2NCH2CH2NH2 or N(CH2CH2NH2)3 or even macromolecules, such as protein. Ligands are unidentate, bidentate and polydentate. The coordination number (CN) of a metal ion in a complex is the number of ligand donor atoms to which the metal is directly bonded.
In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
1. Assertion and reason both are correct statements and reason is correct explanation for assertion 2. Assertion and reason both are correct statements but reason is not correct explanation for assertion 3. Assertion is correct statement but reason is wrong statement 4. Assertion is wrong statement but reason is correct statement
1. Assertion: Binary compounds such as CrCl3, have a primary valence of 3. Reason: Coordinate compound metals show only one type of linkage that is primary linkage.
2. Assertion: CoCl3(NH3)3 is a coordination entity in which the cobalt ion is surrounded by three ammonia molecules and three chloride ion. Reason: The central atom/ion in the coordination entities: [NiCl2(H2O)4] is Ni2+.
3. Assertion: H2NCH2CH2NH2 (ethane-1,2-diamine) ligand is said to be didentate. Reason: Didentate ligands are bind through two donor atoms.
4. Assertion: The complex ions, [PtCl6]2- the coordination number of Pt is 4. Reason: Ligand which can ligate through two different atoms is called ambidentate ligand.
5. Assertion: EDTA can bind through two nitrogen and four oxygen atoms to a central metal ion. Reason: The number of ligating groups attach to an atom is called the denticity of the ligand.
1. (c) Assertion is correct statement but reason is wrong statement 2. (b) Assertion and reason both are correct statements but reason is not correct explanation for assertion 3. (a) Assertion and reason both are correct statements and reason is correct explanation for assertion 4. (d) Assertion is wrong statement but reason is correct statement 5. (b) Assertion and reason both are correct statements but reason is not correct explanation for assertion
Question-5 Read the passage given below and answer any four out of the following questions: Nitrogen differs from the rest of the members of group 15 due to its smaller size, high electronegativity, high ionisation enthalpy, and non-availability of d orbitals. Nitrogen has a unique ability to form pπ-pπ multiple bonds with itself. Nitrogen exists as a diatomic molecule with a triple bond one s and two p between the two atoms. Phosphorus, arsenic and antimony from single bonds as P–P, As–As and Sb–Sb while bismuth forms metallic bonds in an elemental state. Dinitrogen is produced commercially by the liquefaction and fractional distillation of air. Liquid dinitrogen (b.p. 77.2 K) distils out first leaving behind liquid oxygen (b.p. 90 K). In the laboratory, dinitrogen is prepared by treating an aqueous solution of ammonium chloride with sodium nitrite. Dinitrogen is a colourless, odourless, tasteless and non-toxic gas. It has two stable isotopes 14N and 15N. It has very low solubility in water. The main use of dinitrogen is in the manufacture of ammonia and other industrial chemicals containing nitrogen.
The following questions are multiple-choice questions. choose the most appropriate answer
1. N–N bond is weaker than the single P–P bond because 1. high interelectronic repulsion of the bonding electrons 2. high interelectronic repulsion of the non-bonding electrons 3. no repulsion between bonding electrons 4. no repulsion between non-bonding electrons 2. Very pure nitrogen can be obtained by the 1. thermal decomposition of sodium 2. thermal decomposition of barium azide 3. thermal decomposition of ammonium dichromate 4. both (a) and (b) 3. Dinitrogen is rather inert at room temperature because of 1. low bond enthalpy of N≡≡N bond 2. high bond enthalpy of N≡≡N bond 3. low freezing point 4. low boiling point 4. Dinitrogen combines with dioxygen only at very high temperature (at about 2000 K) to form 1. nitric oxide 2. nitrate 3. nitrites 4. nitric acid 5. Liquid dinitrogen is used as a refrigerant to 1. preserve biological materials 2. preserve food items 3. in cryosurgery 4. all of these
1. (b) high interelectronic repulsion of the non-bonding electrons 2. (d) both (a) and (b) 3. (b) high bond enthalpy of N≡≡N bond 4. (a) nitric oxide 5. (d) all of these
Question-6 Read the following passage and answer any four out of the following questions: Transition metal oxides are generally formed by the reaction of metals with oxygen at high temperatures. The highest oxidation number in the oxides coincides with the group number. In vanadium, there is a gradual change from the basic V2O3 to less basic V2O4 and to amphoteric V2O5. V2O4 dissolves in acids to give VO2+ salts. Potassium dichromate is a very important chemical used in the leather industry and as an oxidant for the preparation of many azo compounds. Dichromates are generally prepared from chromate. Sodium dichromate is more soluble than potassium dichromate. The latter is, therefore, prepared by treating the solution of sodium dichromate with potassium chloride. Sodium and potassium dichromates are strong oxidising agents; sodium salt has a greater solubility in water and is extensively used as an oxidising agent in organic chemistry. Potassium dichromate is used as a primary standard in volumetric analysis.
The following questions are multiple-choice questions. Choose the most appropriate answer.
1. All transition metal reacts with oxygen to form MO oxide except 1. scandium 2. vanadium 3. cupper 4. zinc 2. As the oxidation number of a metal increases, ionic character 1. increases 2. decreases 3. remain the same 4. none of these 3. The shape of chromate ion is 1. tetrahedral 2. pyramidal 3. square planer 4. triangular 4. Dichromates are generally prepared from chromate, which in turn are obtained by the fusion of 1. FeCr2O 2. FeCr2O 4 3. Na2CrO 4 4. Na2Cr2O 7 5. The oxo cations stabilise VIV 1. VO 2. VO 4+ 3. VO 2+ 4. all of these
Related Posts

CBSE Class 10 English The Making of a Scientist Summary

Desktop Publishing Class 11 Computer Science Notes and Questions

Print Culture and the Modern World Class 10 Social Science Notes and Questions

- Book Solutions
- State Boards
CBSE Class 12 Chemistry Case study Questions & Answers For Chapter 1, 2, 4, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16
Understudies can discover the chapter astute vital questions for course 12th Chemistry within the table underneath. These imperative questions incorporate questions that are regularly inquired in a long time. Moreover, arrangements are to give for these questions, with extraordinary accentuation on ease-of-study. Tap on the joins underneath to begin investigating.
Below we posted all the Case Study Questions & Answers for Class 12 Chemistry all Chapters –
CBSE Class 12 Case Study Question for Chemistry
Case study 1.
(1) Read the passage given below and answer the questions that follow.
Are there nuclear reactions going on in our bodies?
There are nuclear reactions constantly occurring in our bodies, but there are very few of them compared to the chemical reactions, and they do not affect our bodies much. All of the physical processes that take place to keep a human body running are chemical processes. Nuclear reactions can lead to chemical damage, which the body may notice and try to fix. The nuclear reaction occurring in our bodies is radioactive decay. This is the change of a less stable nucleus to a more stable nucleus. Every atom has either a stable nucleus or an unstable nucleus, depending on how big it is and on the ratio of protons to neutrons. The ratio of neutrons to protons in a stable nucleus is thus around 1:1 for small nuclei (Z < 20). Nuclei with too many neutrons, too few neutrons, or that are simply too big are unstable. They eventually transform to a stable form through radioactive decay. Wherever there are atoms with unstable nuclei (radioactive atoms), there are nuclear reactions occurring naturally. The interesting thing is that there are small amounts of radioactive atoms everywhere: in your chair, in the ground, in the food you eat, and yes, in your body.
The most common natural radioactive isotopes in humans are carbon-14 and potassium-40. Chemically, these isotopes behave exactly like stable carbon and potassium. For this reason, the body uses carbon-14 and potassium-40 just like it does normal carbon and potassium; building them into the different parts of the cells, without knowing that they are radioactive. In time, carbon-14 atoms decay to stable nitrogen atoms and potassium-40 atoms decay to stable calcium atoms. Chemicals in the body that relied on having a carbon-14 atom or potassium-40 atom in a certain spot will suddenly have a nitrogen or calcium atom. Such a change damages the chemical. Normally, such changes are so rare, that the body can repair the damage or filter away the damaged chemicals. The natural occurrence of carbon-14 decay in the body is the core principle behind carbon dating. As long as a person is alive and still eating, every carbon-14 atom that decays into a nitrogen atom is replaced on average with a new carbon-14 atom. But once a person dies, he stops replacing the decaying carbon-14 atoms. Slowly the carbon-14 atoms decay to nitrogen without being replaced, so that there is less and less carbon-14 in a dead body. The rate at which carbon-14 decays is constant and follows first order kinetics. It has a half – life of nearly 6000 years, so by measuring the relative amount of carbon-14 in a bone, archeologists can calculate when the person died. All living organisms consume carbon, so carbon dating can be used to date any living organism, and any object made from a living organism. Bones, wood, leather, and even paper can be accurately dated, as long as they first existed within the last 60,000 years. This is all because of the fact that nuclear reactions naturally occur in living organisms. (source: The textbook Chemistry: The Practical Science by Paul B. Kelter, Michael D. Mosher and Andrew Scott states)
(a) Why is Carbon -14 radioactive while Carbon -12 not? (Atomic number of Carbon: 6)
Ans: Ratio of neutrons to protons is 2.3: 1 which is not the stable ratio of 1:1
(b) Researchers have uncovered the youngest known dinosaur bone, dating around 65 million years ago. How was the age of this fossil estimated?
Ans: Age of fossils can be estimated by C-14 decay. All living organisms have C-14 which decays without being replaced back once the organism dies.
(c) Which are the two most common radioactive decays happening in human body?
Ans: carbon-14 atoms decay to stable nitrogen atoms and potassium-40 atoms decay to stable calcium.
(d) Suppose an organism has 20 g of Carbon -14 at its time of death. Approximately how much Carbon -14 remains after 10,320 years? (Given antilog 0.517 = 3.289)
Ans: t = 2.303/ k log (Co/Ct)
Co = 20 g Ct = ?
t = 10320 years k = 0.693/6000 (half-life given in passage)
substituting in equation:
10320 = 2.303 / (0.693/6000) log 20/ Ct
0.517 = log 20 / Ct anlilog (0.517) = 20/Ct
3.289 = 20/Ct
Ct = 6.17 g
(d) Approximately how old is a fossil with 12 g of Carbon -14 if it initially possessed 32 g of Carbon -14? (Given log 2.667 = 0.4260)
Co = 32 g Ct = 12
t = ? k = 0.693/6000 (half life given in passage) substituting in equation:
t = 2.303 / (0.693/6000) log 32/ 12
t = 2.303 x 60000 /0.693 log 2.667
t = 2.303x6000x0.4260 /0.693
= 8494 years
Key questions for 12th review Biology are outlined agreeing to the CBSE NCERT program. All address sorts are accessible within the PDF, from one-word to one-line answers, brief reply sorts to five point long reply sorts. Hence, understudies can plan for exams and indeed clarify their concepts through them. On the off chance that they refer to these questions, it’ll get ready their minds to pick up a competitive advantage. Understudies will gotten to be commonplace with question patterns and the sorts of questions that will show up on exams.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
We have a strong team of experienced Teachers who are here to solve all your exam preparation doubts

RS Aggarwal Class 8 Math First Chapter Rational Numbers Exercise 1A Solution
Factors promoting growth of nationalism foundation of the indian national congress class 10 icse chapter 2 complete notes pdf, the first war of independence 1857 class 10 icse complete notes pdf, justify the statement “industrialisation and urbanisation go hand in hand” in details.
Sign in to your account
Username or Email Address
Remember Me
myCBSEguide
- Case Study Questions Class...

Case Study Questions Class 12 Chemistry
Table of Contents
myCBSEguide App
Download the app to get CBSE Sample Papers 2023-24, NCERT Solutions (Revised), Most Important Questions, Previous Year Question Bank, Mock Tests, and Detailed Notes.
CBSE will ask Case Study Questions class 12 Chemistry in session 2020-21. These will be the first two questions in the board exam question paper. The first question will have 5 MCQs out of which students will attempt any 4 questions. The second question will carry 5 Assertion & Reason type questions with the choice to attempt any four.
Case Study Questions
As you know, CBSE will hold exams in May-June this year. There is already a reduction of 30% in the syllabus. Now, the case study questions have been added. So, this year the question paper is going to be a bit easier. Although it is easy yet these case study questions need special attention and regular practice.
We have added around 10 sample questions based on the latest pattern in myCBSEguide App. These all questions include two case study questions.
Class 12 Chemistry Question Bank
If you go through the previous year question papers, you will analyze that many questions are repeated word by word and many others are almost similar. So, it is always recommended to check all questions asked in previous years. This will not only help you to get an idea about the question pattern but also help you to understand the difficulty level of the questions.
myCBSEguide App has the previous year’s question bank. These questions are arranged chapter-wise. If you are preparing a particular chapter, you will get all questions asked from that chapter in the last 10 years.
Case Study Questions Examples
Here are two examples of case study questions. To get more such questions download the myCBSEguide App and browse Sample Papers there.
Read the passage given below and answer any four out of the following questions: Ammonia is present in small quantities in air and soil where it is formed by the decay of nitrogenous organic matter e.g., urea. On a large scale, ammonia is manufactured by Haber’s process. In accordance with Le Chatelier’s principle, high pressure would favour the formation of ammonia. Ammonia is a colourless gas with a pungent odour. Its freezing and boiling points are 198.4 and 239.7 K respectively. In the solid and liquid states, it is associated through hydrogen bonds as in the case of water and that accounts for its higher melting and boiling points than expected on the basis of its molecular mass. Ammonia gas is highly soluble in water. Its aqueous solution is weakly basic due to the formation of OH– ions. The presence of a lone pair of electrons on the nitrogen atom of the ammonia molecule makes it a Lewis base.
- caustic soda
- calcium chloride
- sodium hydroxide
- sodium chloride
- 200 10 5 Pa
- 400 10 5 Pa
- 100 10 5 Pa
- 300 10 5 Pa
- Mg 2 O 3 + K 2 O
- Al 2 O 3 + K 2 O
- NaO 3 + K 2 O
- None of these
- five bond pair and two lone pair
- four lone pair and one bond pair
- three bond pair and one lone pair
- three bond pair and two lone pair
Read the passage and answer any four out of the following questions: Colloidal particles always carry an electric charge. The nature of this charge is the same on all the particles in a given colloidal solution and may be either positive or negative. The charge on the sol particles is due to one or more reasons, viz., due to electron capture by sol particles during electrodispersion of metals. When two or more ions are present in the dispersion medium, preferential adsorption of the ion common to the colloidal particle usually takes place. When silver nitrate solution is added to the potassium iodide solution, the precipitated silver iodide adsorbs iodide ions from the dispersion medium, and negatively charged colloidal solution results. acquired a positive or a negative charge by selective adsorption on the surface of a colloidal particle The combination of the two layers of opposite charges around the colloidal particle is called Helmholtz electrical double layer. The presence of equal and similar charges on colloidal particles is largely responsible for providing stability to the colloidal solution.
In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
- Assertion and reason both are correct statements and reason is correct explanation for assertion
- Assertion and reason both are correct statements but reason is not correct explanation for assertion
- Assertion is correct statement and reason is wrong statement
- Assertion is wrong statement but reason is correct statement
- Assertion: The presence of equal and similar charges on colloidal particles is largely responsible in providing stability to the colloidal solution. Reason: The repulsive forces between charged particles having the same charge prevent them from aggregating and provide stability.
- Assertion: The first layer is mobile in Helmholtz electrical double layer. Reason: The potential difference between the fixed layer and the diffused layer of opposite charges is called zeta potential.
- Assertion: The sol particle in colloid has a charge. Reason: The charge in sol is due to electron capture by sol particles during the electrodispersion of metals.
- Assertion: Methylene blue sol is a negatively charged sol. Reason: When KI solution is added to AgNO 3 solution, positively charged sol formed.
- Assertion: If FeCl3 is added to an excess of hot water, a positively charged sol of hydrated ferric oxide is formed. Reason: When ferric chloride is added to NaOH a negatively charged sol is obtained with adsorption of OH- ions.
Test Generator
Create question paper PDF and online tests with your own name & logo in minutes.
Question Bank, Mock Tests, Exam Papers, NCERT Solutions, Sample Papers, Notes
Related Posts
- Competency Based Learning in CBSE Schools
- Class 11 Physical Education Case Study Questions
- Class 11 Sociology Case Study Questions
- Class 12 Applied Mathematics Case Study Questions
- Class 11 Applied Mathematics Case Study Questions
- Class 11 Mathematics Case Study Questions
- Class 11 Biology Case Study Questions
- Class 12 Physical Education Case Study Questions
1 thought on “Case Study Questions Class 12 Chemistry”
Answer for questions
Leave a Comment
Save my name, email, and website in this browser for the next time I comment.
Talk to our experts
1800-120-456-456
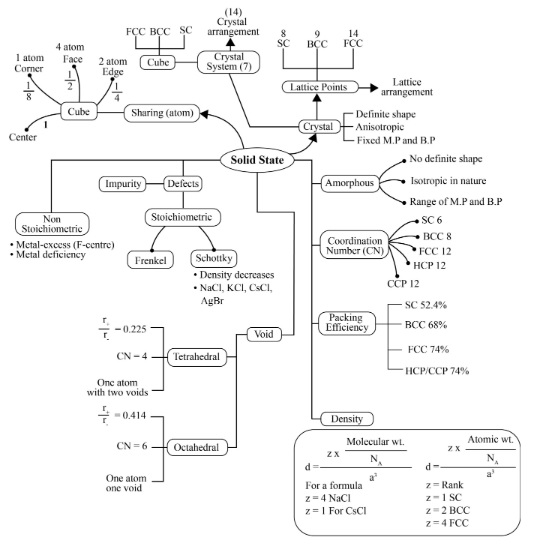
NCERT Solutions for Class 12 Chemistry Chapter 1 - The Solid State
- NCERT Solutions

Solid states deal with arrangements of particles in solids that result in several types of structure in solids. These differences in the structural unit also result in different properties of each Solid-State. Chemistry Class 12 NCERT Solution chapter tries to clarify the concepts on general characteristics of Solid-State, the difference between amorphous crystalline solids, helps students to learn about the nature of binding forces in matter. The experts at Vedantu have prepared the NCERT Solutions for chapter 1 Chemistry class 12 to provide greater insight into the topics covered in the chapter.
The Solid State Chapter at a Glance - Class 12 NCERT Solutions
Solid State
Access NCERT Solutions for Class 12 Chemistry Chapter 1- Solid State
Properties of solid as discussed in class 12 chemistry chapter 1.
From Class 12 Chemistry Chapter 1, we learn that anything that can occupy a space and has a mass is known as matter, which can be in any of the three states i.e., solid, liquid and gas.
In a solid state of matter, the constituent particles are packed very closely to each other resulting in strong interactions. These strong interactions between the particles are the reasons why any solid object has a certain shape, mass and volume.
Here a few properties of solid that we have learned from Class 12 Chemistry Chapter 1:
All solid objects have a definite volume, shape and mass due the presence of strong intermolecular forces between the constituent particles.
The constituent particles present in a solid object can be either atoms, molecules or ions.
Solids are rigid in nature and are incompressible. It is due to the tightly packed particles and strong intermolecular forces between the constituent particles.
The distance between the constituent particles (atoms, molecules or ions) are short and these particles can only oscillate around their mean positions.
Classification of Solids
Solids can be classified into two types based on the arrangement of their particles:
1. Crystalline Solids: These solids have a definite arrangement of their particles in a long-range order throughout the three-dimensional crystal. Diamond, Quartz and Sodium Chloride are some examples of crystalline solids. The crystalline solids are again classified into four types based on their chemical bonding:
Ionic solids
Metallic solids
Molecular Solids
Covalent solids
2. Amorphous Solids: These solids are irregular in shape and don't have a definite geometry. They are rigid and compressible in nature and can be molded into different shapes under a particular temperature. Few examples of amorphous solids are plastic, glass, rubber, etc.
NCERT Exercise
1. Define the Term ‘Amorphous’ and Give a Few Examples of Amorphous Solids.
Ans: Amorphous solids are the solids that have their constituent particles of irregular shape and have short range order. These solids are isotropic in nature and melt over a range of temperatures. Therefore, amorphous solids are sometimes called pseudo solids or super cooled liquids. They do not have definite heat of fusion. When cut with a sharp-edged tool, they cut into two pieces with irregular surfaces. Examples of amorphous solids include glass, rubber, and plastic.
2. What Makes a Glass Different From a Solid Such As Quartz? Under What Conditions Could Quartz Be Converted Into Glass?
Ans: The arrangement of the constituent particles makes glass different from quartz. In glass, the constituent particles have short range order, but in quartz, the constituent particles have both long range and short range orders. Quartz can be converted into glass by heating and then cooling it rapidly.
3. Classify Each of the Following Solids As Ionic, Metallic, Molecular, Network (Covalent) or Amorphous.
Tetra phosphorus decoxide (${{\text{P}}_{\text{4}}}{{\text{O}}_{\text{10}}}$)
Ammonium phosphate ${{\text{(N}{{\text{H}}_{\text{4}}}\text{)}}_{\text{3}}}\text{P}{{\text{O}}_{\text{4}}}$
${{\text{I}}_{\text{2}}}$
${{\text{P}}_{\text{4}}}$
Plastic
Graphite
Brass
LiBr
Si
Ans: The given solids are classified as follows:
Ionic (ii) Ammonium phosphate${{\left( N{{H}_{4}} \right)}_{3}}P{{O}_{4}}$ , (x)LiBr
Metallic (viii) Brass, (ix) Rb
Molecular Tetra phosphoursdecoxide$\left( {{P}_{4}}{{O}_{10}} \right)$ , (iv) ${{I}_{2}}$ (v) ${{P}_{4}}$
Covalent (network) (iii) SiC, (vii) Graphite, (xi) Si
Amorphous (vi) Plastic
Ⅰ. What is Meant by the Term Coordination Number?
Ans: The number of nearest neighbors of any constituent particle present in the crystal lattice is called its coordination number.
Ⅱ. What is the Coordination Number of Atoms:
(a) In a Cubic Close-Packed Structure?
Ans: The coordination number of atoms in a cubic close-packed structure is12.
(b) In a Body-Centred Cubic Structure?
Ans: The coordination number of atoms in a body-centred cubic structure is 8.
5. How Can You Determine the Atomic Mass of an Unknown Metal if You Know Its Density and the Dimension of Its Unit Cell? Explain.
Ans: By knowing the density of an unknown metal and the dimension of its unit cell, the atomic mass of the metal can be determined.
Let ‘a’ be the edge length of a unit cell of a crystal, ‘d’ be the density of the metal, ‘m’ be the atomic mass of the metal and ‘z’ be the number of atoms in the unit cell.
Now, density of the unit cell = $\frac{Mass\,of\,the\,unit\,cell}{Volume\,of\,the\,unit\,cell}$ = $\Rightarrow d=\frac{zm}{{{a}^{2}}}...(i)$
[Since mass of the unit cell = Number of atoms in the unit cell $\times $Atomic mass]
(Volume of the unit cell = ${{\left( Edged\text{ }length\text{ }of\text{ }the\text{ }cubic\text{ }unit\text{ }cell \right)}^{3}}$
From equation (i) we have:
\[M=\frac{d{{a}^{3}}}{z}...(ii)\]
Now, mass of the metal (m) = $\frac{atomic\,mass\,(M)}{Avogadro's\,number\,({{N}_{A}})}$
Therefore, \[M=\frac{d{{a}^{3}}{{N}_{A}}}{z}...(iii)\]
Thus, if the edge lengths are different (say a, b and c), then equation (iii) becomes:
\[M=\frac{d(abc){{N}_{A}}}{z}...(iv)\]
Hence, from equation (iii) and (iv), we can determine the atomic mass of the unknown metal.
6. ‘Stability of crystal is reflected in the magnitude of its melting point’. Comment. Collect melting points of solid water, ethyl alcohol, diethyl ether and methane from a data book. What can you say about the intermolecular forces between these molecules?
Ans: Higher the melting point, greater are the intermolecular forces of attraction between the atoms of a molecule and greater is the stability of that molecule. A substance with higher melting point is more stable than a substance with lower melting point.
The melting points of the given substances (in Kelvin) are:
Solid water = 273 K
Ethyl alcohol =158.8 K
Diethyl ether = 156.85 K
Methane = 89.34 K
Now, on observing the values of the melting points, it can be said that among the given substances, the intermolecular force in solid water is the strongest and that in methane is the weakest.
7. How You Distinguish Between the Following Pairs of Terms:
1). Hexagonal Close-Packing and Cubic Close-Packing?
Ans: A 2-d hexagonal close-packing contains two types of triangular voids (a and b) as shown in figure 1. Let us call this 2-D structure as layer A. Now, particles are kept in the voids present in layer A (it can be easily observed from figures 2 and 3 that only one of the voids will be occupied in the process, i.e., either a or b). Let us call the particles or spheres present in the voids of layer A as layer B. Now, two types of voids are present in layer B (c and d). Unlike the voids present in layer A, the two types of voids present in layer B are not similar. Void C is surrounded by 4 spheres and is called the tetrahedral void. Void d is surrounded by 6 spheres and is called the octahedral void.
(Image will be uploaded soon-1)
(Image will be uploaded soon-2) (Image will be uploaded soon-3)
Now the next layer can be placed over layer B in two ways.
Case 1: When the third layer (layer C) is placed over the second one (layer B) in such a manner that the spheres of layer C occupy the tetrahedral voids c. In this case we get hexagonal close packing. This is shown in figure 4. In figure 4.1, layer B is present over the voids a and layer C is present over the voids x. In figure 4.2, layer B is present over the voids b and layer C is present over the voids c. It can be observed from the figure that in this arrangement, the spheres are present. in layer C are present directly above the spheres of layer A. Hence, we can say that the layers in hexagonal close-packing are arranged in an ABAB…….. pattern.
(Image will be uploaded soon-1) (Image will be uploaded soon-2)
Case 2: When the third layer (layer C) is placed over layer B in such a manner that the spheres of layer C occupy the octahedral voids d. in this case we get cubic close-packing. In figure 5.1, layer B is present over the voids a and layer C is present over the voids d. In figure 5.2, layer B is present over the voids b and layer C is present over the voids d. It can be observed from the figure that the arrangement of particles in layer C is completely different from that in layers A or
B. When the fourth layer is kept over the third layer, the arrangement of particles in this layer is similar to that in layer A. Hence, we can say that the layers in cubic close packing are arranged in an ABCABC………. Pattern.
The side views of hcp and ccp are given in figures 6.1 and 6.2 respectively.
2).Crystal Lattice and Unit Cell?
Ans: The diagrammatic representation of the constituent particles (atoms, ions, or molecules) present in a crystal in a regular three-dimensional arrangement is called a crystal lattice.
A unit cell is the smallest three-dimensional portion of a crystal lattice. When repeated again and again in different directions, it generates the entire crystal lattice.
3). Tetrahedral Void and Octahedral Void?
Ans: A void surrounded by 4 spheres is called a tetrahedral void and a void surrounded by 6 spheres is called an octahedral void. Figure 1 represents a tetrahedral void and figure 2 represents an octahedral void.
8. How Many Lattice Points are There in One Unit Cell of Each of the Following Lattices?
1. Face-Centered Cubic
Ans: There are 14 (8 from the corners + 6 from the faces) lattice points in face-centered cubic.
2. Face-Centered Tetragonal
Ans: There are 14 (8 from the corners + 6 from the faces) lattice points in face-centered tetragonal.
3. Body-Centered
Ans: There are 9 (1 from the centre +8 from the corners) lattice points in body-centered cubic.
9. Explain
(ⅰ) The Basis of Similarities and Differences Between Metallic and Ionic Crystals.
Ans: The basis of similarities between metallic and ionic crystals is that both these crystal types are held by the electrostatic force of attraction. In metallic crystals, the electrostatic force acts between the positive ions and the electrons. In ionic crystals, it acts between the oppositely charged ions. Hence, both have high melting points.
The basis of differences between metallic and ionic crystals is that in metallic crystals, the electrons are free to move and so, metallic crystals can conduct electricity. However, in ionic crystals, the ions are not free to move. As a result they cannot conduct electricity. However, in molten state or in aqueous solution, they do conduct electricity.
(ⅱ) Ionic Solids are Hard and Brittle.
Ans: The constituent particles of ionic crystals are ions. These ions are held together in three dimensional arrangements by the electrostatic force of attraction. Since the electrostatic force of attraction is very strong, the charged ions are held in fixed positions. This is the reason why ionic crystals are hard and brittle.
10. Calculate the Efficiency of Packing in Case of Metal Crystal For
(ⅰ) Simple Cubic
Ans: In a simple cubic lattice, the particles are located only at the corners of the cube and touch each other along the edge.
(Image will be uploaded soon)
Let the edge length of the cube be ‘a’ and the radius of each of each particle be r.
So, we can write:
a = 2r
Now, volume of the cubic unit cell = \[{{a}^{3}}\]
\[={{(2r)}^{3}}\]
\[=8{{r}^{3}}\]
We know that the number of particles per unit cell is 1.
Therefore, volume of the occupied unit cell =$\frac{4}{3}\pi {{r}^{3}}$
Hence, packing efficiency = \[\frac{volume\,of\,one\,particle}{volume\,of\,cubic\,unit\,cell}\times 100%\]
\[=\frac{\frac{4}{3}\pi {{r}^{3}}}{8{{r}^{3}}}\times 100%\]
\[=\frac{1}{6}\pi \times 100%\]
\[=\frac{1}{6}\times \frac{22}{7}\times 100%\]
\[=52.4%\]
Therefore, packing efficiency of simple cubic crystals is 52.4%.
(ⅱ) Body-Centered Cubic
Ans: It can be observed from the above figure that the atom at the centre is in contact with the other two atoms diagonally arranged.
From $\Delta $FED, we have:
\[{{b}^{2}}={{a}^{2}}+{{a}^{2}}\]
\[\Rightarrow {{b}^{2}}=2{{a}^{2}}\]
\[\Rightarrow b=\sqrt{2a}\]
Again, from $\Delta $AFD, we have:
\[{{c}^{2}}={{a}^{2}}+{{b}^{2}}\]
\[\Rightarrow {{c}^{2}}={{a}^{2}}+2{{a}^{2}}\] (Since ${{b}^{2}}=2{{a}^{2}}$)
\[\Rightarrow {{c}^{2}}=3{{a}^{2}}\]
\[\Rightarrow {{c}^{2}}=\sqrt{3a}\]
Let the radius of the atom be r.
Length of the body diagonal, c = 4n
\[\Rightarrow \sqrt{3a}=4r\]
\[\Rightarrow a=\frac{4r}{\sqrt{3}}\]
\[r=\frac{\sqrt{3a}}{4}\]
Volume of the cube, ${{a}^{3}}={{\left( \frac{4r}{\sqrt{3}} \right)}^{3}}$
A body-centered cubic lattice contains 2 atoms.
So, volume of the occupied cubic lattice = \[2\pi \frac{4}{3}{{r}^{3}}\]
\[=\frac{8}{3}{{r}^{3}}\]
Therefore, packing efficiency =$\frac{Volume\,occupied\,by\,two\,spheres\,in\,the\,unit\,cell}{Total\,volume\,of\,the\,unit\,cell}\times 100%$
\[=\frac{\frac{8}{3}\pi {{r}^{3}}}{{{\left( \frac{4}{\sqrt{3}}r \right)}^{3}}}\times 100%\]
\[=\frac{\frac{8}{3}\pi {{r}^{3}}}{\frac{64}{3\sqrt{3}}{{r}^{3}}}\times 100%\]
\[=68%\]
Therefore, packing efficiency in the body – centered is 68%.
(ⅲ) Face-centered cubic
Let the edge length of the unit cell be ‘a’ and the length of the face diagonal AC be b.
From$\Delta $ABC, we have:
\[A{{C}^{2}}=B{{C}^{2}}+A{{B}^{2}}\]
\[\Rightarrow {{b}^{2}}={{a}^{2}}+{{a}^{2}}\]
\[\Rightarrow {{b}^{2}}=2{{a}^{2}}\]
Let r be the radius of the atom.
Now, from the figure, it can be observed that:
b = 4r
\[\Rightarrow \sqrt{2a}=4r\]
\[\Rightarrow a=2\sqrt{2r}\]
Now, volume of the cube, ${{a}^{3}}$ = ${{\left( 2\sqrt{2}r \right)}^{3}}$
We know that the number of atoms per unit cell is 4.
So, volume of the occupied unit cell = $4\pi \frac{4}{3}{{r}^{3}}$
Therefore, packing efficiency = $\frac{Volume\,occupied\,by\,four\,spheres\,in\,the\,unit\,cell}{Total\,volume\,of\,the\,unit\,cell}\times 100%$
\[==\frac{4\pi \frac{4}{3}{{r}^{3}}}{{{\left( 2\sqrt{2} \right)}^{3}}}\times 100%\]
\[==\frac{\frac{16}{3}\pi {{r}^{3}}}{16\sqrt{2{{r}^{3}}}}\times 100%\]
\[=74%\]
Therefore, packing efficiency in face centered is 74%.
11. Silver Crystallises in the Fcc Lattice. If Edge Length of the Cell is $\text{4}\text{.07 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-8}}}\text{cm}$ and density is $\text{10}\text{.5 g c}{{\text{m}}^{\text{-3}}}$ , Calculate the Atomic Mass of Silver.
Ans: It is given that the edge length, a = $4.07\text{ }\times \text{ }{{10}^{-8}}cm$and density is d = $10.5\text{ }g\text{ }c{{m}^{-3}}$
As the lattice is fcc type, the number of atoms per unit cell, z = 4
We also know that, ${{N}_{A}}=6.022\times {{10}^{23}}\text{ }mo{{l}^{-1}}$
Using the relation:
\[d=\frac{zM}{{{a}^{3}}{{N}_{A}}}\]
\[\Rightarrow M=\frac{d{{a}^{3}}{{N}_{A}}}{z}\]
\[=\frac{10.5gc{{m}^{-3}}\times {{(4.077\times {{10}^{-8}}cm)}^{3}}\times 6.022\times {{10}^{23}}mo{{l}^{-1}}}{4}\]
\[=107.13\,gmo{{l}^{-1}}\]
Therefore, atomic mass of silver = 107.13u
12. A Cubic Solid Is Made of Two Elements P and Q. Atoms of Q Are at the Corners of the Cube and P at the Body-Centre. What Is the Formula of the Compound? What Are the Coordination Numbers of P and Q?
Ans: It is given that the atoms of Q are present at the corners of the cube.
Therefore, number of atoms of Q in one unit cell = $8\text{ }\times \text{ }\left( 1/8 \right)\text{ }=\text{ }1$
It is also given that the atoms of P are present at the body-centre.
Therefore, number of atoms of P in one unit cell = 1
This means that the ratio of the number of P atoms to the number of Q atoms, P:Q = 1:1
Hence, the formula of the compound is PQ
The coordination number of both P and Q is 8.
13. Niobium Crystallises in Body-Centered Cubic Structure. If Density is 8.55 $\text{gc}{{\text{m}}^{\text{-3}}}$, Calculate the Atomic Radius of Niobium Using Its Atomic Mass 93 U.
Ans: It is given that the density of niobium, d = 8.55 $gc{{m}^{-3}}$
Atomic mass, M = 93$gmo{{l}^{-1}}$
As the lattice is bcc type, the number of atoms per unit cell, z = 2
\[\Rightarrow {{a}^{3}}=\frac{zM}{d{{N}_{A}}}\]
\[=\frac{2\times 93gmo{{l}^{-1}}}{8.55gc{{m}^{-3}}\times 6.022\times {{10}^{23}}mo{{l}^{-1}}}\]
\[=3.612\times {{10}^{-23}}\,c{{m}^{3}}\]
So, a = $3.612\times {{10}^{-23}}\,c{{m}^{3}}$
For body-centred cubic unit cell:
\[r=\frac{\sqrt{3}}{4}a\]
\[=\frac{\sqrt{3}}{4}\times 3.306\times {{10}^{-8}}cm\]
\[=\text{ }1.432\text{ }\times \text{ }{{10}^{-8}}cm\]
\[=\text{ }14.32\text{ }\times \text{ }{{10}^{-9}}cm\]
\[r=\text{ }14.32\text{ }nm\]
14. If the Radius of the Octahedral Void Is R and Radius of the Atoms in Close Packing is R, Derive Relation Between R and R
A sphere with centre O, is fitted into the octahedral void as shown in the above figure. It can be observed from the figure that $\Delta $POQ is right-angled as$\angle POQ=90{}^\circ $
Now, applying Pythagoras theorem, we can write:
\[P{{Q}^{2}}\text{ }=P{{O}^{2}}\text{ }+\text{ }O{{Q}^{2}}\]
\[\Rightarrow ~{{\left( 2R \right)}^{2}}={{\left( R+r \right)}^{2}}+{{\left( R+r \right)}^{2}}\]
\[\Rightarrow ~{{\left( 2R \right)}^{2}}\text{ }=2{{\left( R+r \right)}^{2}}\]
\[\Rightarrow ~2{{R}^{2}}={{\left( R+r \right)}^{2}}\]
\[\Rightarrow ~\sqrt{2}R=R+r\]
\[\Rightarrow r=\sqrt{2}R-R\]
\[\Rightarrow r=\left( \sqrt{2}-1 \right)R\]
\[\Rightarrow r=0.414R\]
Therefore, the relation between r and R is r = 0.414R.
15. Copper Crystallises into a fcc Lattice with Edge Length$\text{3}\text{.61 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-8}}}\text{cm}$ . Show that the Calculated Density is in Agreement with its Measured Value of $\text{8}\text{.92 g c}{{\text{m}}^{\text{-3}}}$.
Ans: Edge length, a = $3.61\times {{10}^{-8}}cm$
As the lattice is fcc type, the number of atoms per unit cell, z = 4
Atomic mass, M = 63.5 $g\text{ }mo{{l}^{-1}}$
We also know that,${{N}_{A}}=6.022\times {{10}^{23}}\text{ }mo{{l}^{-1}}$
\[=\frac{4\times 63.5gmo{{l}^{-1}}}{(3.61\times {{10}^{-8}}cm)\times 6.022\times {{10}^{23}}mo{{l}^{-1}}}\]
\[=8.97\,g\,c{{m}^{-3}}\]
The measured value of density is given as $8.92\text{ }g\text{ }c{{m}^{-3}}$ . Hence, the calculated density $8.97\text{ }g\text{ }c{{m}^{-3}}$ is in agreement with its measured value.
16. Analysis shows that nickel oxide has the formula$\text{N}{{\text{i}}_{\text{0}\text{.98}}}{{\text{O}}_{\text{1}\text{.00}}}$ . What fractions of nickel exist as $\text{N}{{\text{i}}^{\text{2+}}}$ and $\text{N}{{\text{i}}^{\text{3+}}}$ions?
Ans: The formula of nickel oxide is $N{{i}_{0.98}}{{O}_{1.00}}$
Therefore, the ratio of the number of Ni atoms to the number of O atoms,
Ni : O = 0.98 : 1.00 = 98 : 100
Now, total charge on 100$~{{O}^{2-}}$ ions = 100 $\times $(-2)
= -200
Let the number of $N{{i}^{2+}}$ions be x.
So, the number of $N{{i}^{3+}}$ ions is 98 - x.
Now, total charge on $N{{i}^{2+}}$ions = x (+2) = +2x
And, total charge on $N{{i}^{3+}}$ions (98 - x) (+3)
= 294 – 3x
Since, the compound is neutral, we can write:
\[2x+\left( 294-3x \right)+\left( -200 \right)=0\]
\[\Rightarrow x\text{ }+\text{ }94\text{ }=\text{ }0\]
\[\Rightarrow x\text{ }=\text{ }94\]
Therefore, number of $N{{i}^{2+}}$ ions = 94
And, number of $N{{i}^{3+}}$ions = 98 - 94 = 4
Hence, fraction of nickel that exists as $N{{i}^{2+}}\frac{94}{98}$= 0.959
17. What is a Semiconductor? Describe the Two Main Types of Semiconductors and Contrast Their Conduction Mechanism.
Ans: Semiconductors are substances having conductance in the intermediate range ${{10}^{-6}}$ to ${{10}^{4}}$ $oh{{m}^{-1}}{{m}^{-1}}$.
The two main types of semiconductors are:
(ⅰ) n-Type Semiconductor
(ⅱ) P-Type semiconductor
n-Type Semiconductor : The semiconductor whose increased conductivity is a result of negatively-charged electrons is called an n-type semiconductor. When the crystal of a group 14 element such as Si or Ge is doped with a group 15 element such as P or As, an n-type semiconductor is generated.
Si and Ge have four valence electrons each. In their crystals, each atom forms four covalent bonds. On the other hand, P and As contain five valence electrons each. When Si or Ge is doped with P or As, the latter occupies some of the lattice sites in the crystal. Four out of five electrons are used in the formation of four covalent bonds with four neighbouring Si or Ge atoms. The remaining fifth electron becomes delocalised and increases the conductivity of the doped Si or
p-type semiconductor: The semiconductor whose increased conductivity is a result of an electron hole is called a p-type semiconductor. When a crystal of group 14 elements such as Si or Ge is doped with a group 13 element such as B, Al, or Ga (Which contains only three valence electrons), a p-type of semiconductor is generated.
When a crystal of Si is doped with B, the three electrons of B are used in the formation of three covalent bonds and an electron hole is created. An electron from the neighboring atom can come and fill this electron hole, but in doing so, it would leave an electron hole at its original position.
The process appears as if the electron hole has moved in the direction opposite to that of the electron that filled it. Therefore, when an electric field is applied, electrons will move toward the positively-charged plate through electron holes. However, it will appear as if the electron holes are positively-charged and are moving toward the negatively-charged plate.
18. Non-Stoichiometric Cuprous Oxide, $\text{C}{{\text{u}}_{\text{2}}}\text{O}$can be prepared in the laboratory. In this Oxide, Copper to Oxygen Ratio is Slightly Less than 2:1 Can You Account for the Fact that this Substance is a p-type Semiconductor?
Ans: In the cuprous oxide$\left( C{{u}_{2}}O \right)$ prepared in the laboratory, copper to oxygen ratio is slightly less
than 2:1. This means that the number of $C{{u}^{+}}$ ions is slightly less than twice the number of ${{O}^{2-}}$ions. This is because some $C{{u}^{+}}$ ions have been replaced by $C{{u}^{2+}}$ions. Every $C{{u}^{2+}}$ion replaces two $C{{u}^{+}}$ions, thereby creating holes. As a result, the substance conducts electricity with the help of these positive holes. Hence, the substance is a p-type semiconductor.
19. Ferric Oxide Crystallises in a Hexagonal Close-Packed Array of Oxide Ions With Two Out of Every Three Octahedral Holes Occupied by Ferric Ions. Derive the Formula of the Ferric Oxide.
Ans: Let the number of oxide (${{O}^{2-}}$) ions be x.
So, number of octahedral voids = x
It is given that two out of every three octahedral holes are occupied by ferric ions.
So, number of ferric $\left( F{{e}^{3+}} \right)$ ions = $\frac{2}{3}x$
Therefore, ratio of number of $F{{e}^{3+}}$ions to the number of ${{O}^{2-}}$ions,
$F{{e}^{3+}}$: ${{O}^{2-}}$= $\frac{2}{3}x$ : x
= $\frac{2}{3}$ : 1
Hence, the formula of the ferric oxide is $F{{e}_{2}}{{O}_{3}}$.
20. Classify Each of the Following As Being Either a p-Type or an n-Type Semiconductor.
(ⅰ) Ge Doped with In
Ans: Ge (a group 14 element) is doped with In (a group 13 element). Therefore, a hole will be created and the semiconductor generated will be a p-type semiconductor.
(ⅱ) B Doped with Si
Ans: B (a group 13 element) is doped with Si (a group 14 element). So, there will be an extra electron and the semiconductor generated will be an n-type semiconductor.
21. Gold (Atomic Radius = 0.144 nm) Crystallises in a Face-Centred Unit Cell. What is the Length of a Side of the Cell?
Ans: For a face-centred unit cell:
\[a=2\sqrt{2}r\]
It is given that the atomic radius, r = 0.144 nm
So $a=2\sqrt{2}\times 0.144\,nm$
= 0.407nm
Hence, length of a side of the cell = 0.407 nm
22. In Terms of Band Theory, What is the Difference:
(ⅰ) Between a Conductor and an Insulator
Ans: The valence band of a conductor is partially-filled or it overlaps with a higher energy, unoccupied conduction band. On the other hand, in the case of an insulator, the valence band is fully – filled and there is a large gap between the valence band and the conduction band.
(Image will be uploaded soon)
(ⅱ) Between a Conductor and a Semiconductor
Ans: In the case of a conductor, the valence band is partially-filled or it overlaps with a higher energy, unoccupied conduction band, so, the electrons can flow easily under an applied electric field. On the other hand, the valence band of a semiconductor is filled and there is a small gap between the valence band and the next higher conduction band. Therefore, some electrons can jump from the valence band to the conduction band and conduct electricity.
23. Explain the Following Terms With Suitable Examples:
(ⅰ) Schottky Defect:
Ans: Schottky defect is basically a vacancy defect shown by ionic solids. In this defect, an equal number of cations and anions are missing to maintain electrical neutrality. It decreases the density of a substance. Significant number of Schottky defects are present in ionic solids. For example, in NaCl, there are approximately 106 Schottky pairs per $c{{m}^{3}}$ at room temperature. Ionic substances containing similar sized cations and anions show this type of defect. For example: NaCl, KCl, CsCl, AgBr, etc
(ⅱ) Frenkel Defect:
Ans: Ionic solids containing large differences in the sizes of ions show this type of defect. When the smaller ion (usually cation) is dislocated from its normal site to an interstitial site, Frenkel defect is created. It creates a vacancy defect as well as an interstitial defect. It is also known as a dislocation defect. Ionic solids such as AgCl, AgBr, AgI and ZnS show this type defect.
(ⅲ) Interstitials:
Ans: Interstitial defect is shown by non-ionic solids. This type of defect is created when some constituent particles (atoms or molecules) occupy an interstitial site of the crystal. The density of a substance increases because of this defect.
(ⅳ) F-Centres:
Ans: When the anionic sites of a crystal are occupied by unpaired electrons, the ionic sites are called F-centres. These unpaired electrons impart colour to the crystals. For example, when crystals of NaCl are heated in an atmosphere of sodium vapour, the sodium atoms are deposited on the surface of the crystal. The Cl ions diffuse from the crystal to its surface and combine with Na atoms, forming NaCl. During this process, the Na atoms on the surface of the crystal lose electrons. These released electrons diffuse into the crystal and occupy the vacant anionic sites, creating F-Centres.
24. Aluminium Crystallises in a Cubic Close-Packed Structure. Its Metallic Radius is 125 PM.
(ⅰ) What is the Length of the Side of the Unit Cell?
Ans: For cubic close-packed structure:
\[\Rightarrow 2\sqrt{2}=125\,pm\]
\[=\text{ }353.55\text{ }pm\]
Hence, the length of the side of the unit cell is 354 pm (approximately).
(ⅱ) How Many Unit Cells are There in 1.00 $c{{m}^{3}}$ of Aluminium?
Ans: Volume of one unit cell = {{\left( 354\text{ }pm \right)}^{3}}
\[=\text{ }4.4\text{ }\times {{10}^{7}}\text{ }p{{m}^{3}}\]
\[=\text{ }4.4\text{ }\times {{10}^{7}}\text{ }\times {{10}^{-30}}c{{m}^{3}}\]
\[~~=\text{ }4.4\text{ }\times {{10}^{-23}}c{{m}^{3}}\]
Therefore, number of unit cells in 1.00$c{{m}^{3}}$= $\frac{1.00c{{m}^{3}}}{4.4\times {{10}^{-23}}c{{m}^{-3}}}=2.27\times {{10}^{22}}$
25. If NaCl is Doped with $\text{1}{{\text{0}}^{\text{-3}}}\text{mol }\!\!%\!\!\text{ }$ of$\text{SrC}{{\text{l}}_{\text{2}}}$, What is the Concentration of Cation Vacancies?
Ans: It is given that NaCl is doped with ${{10}^{-3}}mol\text{ }%\]of \[SrC{{l}_{2}}$.
This means that 100 mol of NaCl is doped with ${{10}^{-3}}mol\text{ }$ of $SrC{{l}_{2}}$.
Therefore, 1 mol of NaCl is doped with $\frac{{{10}^{-3}}}{100}mol$ of $SrC{{l}_{2}}$
= ${{10}^{-5}}mol$ of $SrC{{l}_{2}}$
Cation vacancies produced by one $S{{r}^{2+}}$ion = 1
Concentration of the cation vacancies produced by ${{10}^{-5}}mol$of $S{{r}^{2+}}$ions = ${{10}^{-5}}\times 6.022\times {{10}^{23}}$
= $6.022\times {{10}^{18}}mo{{l}^{-1}}$
Hence, the concentration of cation vacancies created by $SrC{{l}_{2}}$is $6.022\times {{10}^{18}}$per mol of NaCl.
26. Explain the Following With Suitable Examples:
(ⅰ) Ferromagnetism:
Ans: The substances that are strongly attracted by a magnetic field are called ferromagnetic substances. Ferromagnetic substances can be permanently magnetized even in the absence of a magnetic field. Some examples of ferromagnetic substances are iron, cobalt, nickel, gadolinium. In solid state, the metal ions of ferromagnetic substances are grouped together into small regions called domains and each domain acts as a tiny magnet. In an unmagnetised piece of a ferromagnetic substance, the domains are randomly-oriented and so, their magnetic moments get cancelled. However, when the substance is placed in a magnetic field, all the domains get oriented in the direction of the magnetic field. As a result, a strong magnetic effect is produced. This ordering of domains persists even after the removal of the magnetic field. Thus, the ferromagnetic substance becomes a permanent magnet. Schematic alignment of magnetic moments in ferromagnetic substances
(ⅱ) Paramagnetism:
Ans: The substances that are attracted by a magnetic field are called paramagnetic substances. Some examples of paramagnetic substances are${{O}_{2}},C{{u}^{2+}},F{{e}^{3+}}$and$C{{r}^{3+}}$. Paramagnetic substances get magnetised in a magnetic field in the same direction, but lose magnetism when the magnetic field is removed. To undergo paramagnetism, a substance must have one or more unpaired electrons. This is because the unpaired electrons are attracted by a magnetic field, thereby causing paramagnetism.
(ⅲ) Ferrimagnetism:
Ans: The substances in which the magnetic moments of the domains are aligned in parallel and antiparallel directions, in unequal numbers, are said to have ferrimagnetism. Examples include $F{{e}_{3}}{{O}_{4}}$ (magnetite), ferrites such as $MgF{{e}_{2}}{{O}_{4}}\] and\[ZnF{{e}_{2}}{{O}_{4}}$. Ferrimagnetic substances are weakly attracted by a magnetic field as compared to ferromagnetic substances. On heating, these substances become paramagnetic.
Schematic alignment of magnetic moments in ferromagnetic substances.
(ⅳ) Antiferromagnetism:
Ans: Antiferromagnetic substances have domain structures similar to ferromagnetic substances, but are oppositely oriented. The oppositely- oriented domains cancel out each other’s magnetic moments.
Schematic alignment of magnetic moments in antiferromagnetic substances.
(ⅴ) 12-16 and 13-15 Group Compounds:
Ans: The 12 – 16 group compounds are prepared by combining group 12 and group 16 elements and the 13-15 group compounds are prepared by combining group 13 and group 15 elements. These compounds are prepared to stimulate average valence of four as in Ge or Si. Indium(III) antimonide(InSb), aluminium phosphide(AlP), and gallium arsenide(GaAs) are typical compounds of groups 13-15. GaAs semiconductors have a very fast response time and have revolutionised the designing of semiconductor devices. Examples of group 12-16 compounds include zinc sulphide(ZnS), cadmium sulphide(CdS), Cadmium selenide(CdSe) and mercury(II)telluride(HgTe). The bonds in these compounds are not perfectly covalent. The ionic character of the bonds depends on the electronegativities of the two elements.
Intext questions
1. Why are Solids Rigid?
Ans: The intermolecular forces of attraction that are present in solids are very strong. The constituent particles of solids cannot move from their positions i.e., they have fixed positions. However, they can oscillate about their mean positions. This is the reason solids are rigid.
2. Why Do Solids Have a Definite Volume?
Ans: The intermolecular forces of attraction that are present in solids are very strong. The constituent particles of solids have fixed positions i.e., they are rigid. Hence, solids have a definite volume.
3. Classify the Following As Amorphous or Crystalline Solids: Polyurethane, Naphthalene, Benzoic Acid, Teflon, Potassium Nitrate, Cellophane, Polyvinyl Chloride, Fiberglass, Copper.
Ans: Amorphous solids Polyurethane, teflon, cellophane, polyvinyl chloride, fiberglass.
Crystalline solids
Naphthalene, benzoic acid potassium nitrate, copper.
4. Why is Glass Considered a Super Cooled Liquid?
Ans: Similar to liquids, glass has a tendency to flow, though very slowly. Therefore, glass is considered as a super cooled liquid. This is the reason that glass windows and doors are slightly thicker at the bottom than at the top.
5. Refractive Index of a Solid Is Observed To Have the Same Value Along All Directions. Comment on the Nature of This Solid. Would It Show Cleavage Property?
Ans: As isotropic solid has the same value of physical properties when measured along different directions. Therefore, the given solid, having the same value of refractive index along all directions, is isotropic in nature. Hence, the solid is an amorphous solid. When an amorphous solid is cut with a sharp edged tool, it cuts into two pieces with irregular surfaces.
6. Classify the Following Solids in Different Categories Based on the Nature of Intermolecular Forces Operating in Them: Potassium Sulphate, Tin, Benzene, Urea, Ammonia, Water, Zinc Sulphide, Graphite, Rubidium, Argon, Silicon Carbide.
Ans: Potassium sulphate $\to $ ionic solid
Tin Metallic $\to $ solid
Benzene $\to $ Molecular (non-polar) solid
Urea $\to $ Polar molecular solid
Ammonia $\to $ Polar molecular solid
Water $\to $ Hydrogen bonded molecular solid
Zinc sulphide$\to $ Ionic solid
Graphite $\to $ Covalent or network solid
Rubidium$\to $ Metallic solid
Argon$\to $ Non-polar molecular solid
Silicon carbide$\to $ Covalent or network solid
7. Solid A is a Very Hard Electrical Insulator in Solid as Well as in Molten State and Melts at Extremely High Temperature. What Type of Solid is It?
Ans: The given properties are the properties of a covalent or network solid. Therefore, the given solid is a covalent or network solid. Examples of such solids include diamond (C) and quartz ($Si{{O}_{2}}$).
8. Ionic Solids Conduct Electricity in Molten State but Not in Solid State. Explain.
Ans: In ionic compounds, electricity is conducted by ions. In solid state, ions are held together by strong electrostatic forces and are not free to move about within the solid. Hence, in molten state or in solution form, the ions are free to move and can conduct electricity.
9. What Type of Solids Are Electrical Conductors, Malleable and Ductile?
Ans: Metallic solids are electrical conductors, malleable, and ductile.
10. Give the Significance of a Lattice Point.
Ans: The significance of a lattice point is that each lattice point represents one constituent particle of a solid which may be an atom, a molecule (group of atom), or an ion.
11. Name the Parameters That Characterize a Unit Cell.
Ans: The six parameters that characterize a unit cell are as follows.
(ⅰ) Its dimensions along the three edges, a, b, and c. These edges may or may not be equal.
(ⅱ) Angles between the edges. These are the angles $\alpha $(between edges b and c), $\beta $(between edges a and c), and $\gamma $(between edges a and b).
12. Distinguish between
(ⅰ) Hexagonal and Monoclinic Unit Cells
Ans: Hexagonal unit cell :
For a hexagonal unit cell
a = b$\ne $c
and $\alpha =\beta ={{90}^{o}}$
$\gamma $= ${{120}^{o}}$
Monoclinic unit cell:
For a monoclinic cell,
a $\ne $b$\ne $c
and$\alpha $ =$\gamma $ = 90
$\beta $ $\ne $90
(ⅱ) Face-Centered and End-Centered Unit Cell
Ans : Face – centered unit cell
In a face-centered unit cell, the constituent particles are present at the corners and one at the centre of each face.
End-centered unit cell
An end-centered unit cell contains particles at the corners and one at the centre of any two opposite faces.
13. Explain How Much Portion of an Atom Located at (I) Corner and (II) Body-Centre of a Cubic Unit Cell Is Part of Its Neighbouring Unit Cell.
(ⅰ) An atom located at the corner of a cubic unit cell is shared by eight adjacent unit cells. Therefore,1/8th portion of the atom is shared by one unit cell.
(ⅱ) An atom located at the body centre of a cubic unit cell is not shared by its neighbouring unit cell. Therefore, the atom belongs only to the unit cell in which it is present, that is its contribution to the unit cell is 1.
14. What is the Two Dimensional Coordination Number of a Molecule in a Square Close Packed Layer?
Ans: In a square close-packed layer, the molecule is in contact with four of its neighbours. Therefore, the two-dimensional coordination number of a molecule in a square close packed layer is 4.
15. A Compound Forms a Hexagonal Close-Packed Structure. What is the Total Number of Voids in 0.5 Mol of It? How Many of These Are Tetrahedral Voids?
Ans: Number of close-packed particles $~=0.5\text{ }\times 6.022\times {{10}^{23}}=3.011\times {{10}^{23}}$ Therefore, number of octahedral voids $=3.011\times {{10}^{23}}$
And, number of tetrahedral voids $=2\times 3.011\times {{10}^{23}}=6.022\times {{10}^{23}}$
Therefore, total number of voids $3.011\times {{10}^{23}}+6.022\times {{10}^{23}}=9.033\times {{10}^{23}}$
16. A Compound is Formed by Two Elements M and N. the Element N Forms CCP and Atoms of M Occupy $\mathbf{1}/\mathbf{3rd}$of tetrahedral voids. What is the Formula of the Compound?
Ans: The ccp lattice is formed by the atoms of the element N.
Here, the number of tetrahedral voids generated is equal to twice the number of atoms of the element N.
According to the question, the atoms of element M occupy 1/3rd of the tetrahedral voids. Therefore, the number of atoms of M is equal to $2~\times 1/3\text{ }=\text{ }2/{{3}^{rd}}$ of the number of atoms of N. Therefore, ratio of the number of atoms of M to that of N is M : N = (2/3):1 = 2:3 Thus, the formula of the compound is${{M}_{2}}{{N}_{3}}$ .
17. Which of the Following Lattices Has the Highest Packing Efficiency (I) Simple Cubic (II) Body Centred Cubic and (III) Hexagonal Close-Packed Lattice?
Ans: Hexagonal close-packed lattice has the highest packing efficiency of 74%. The packing efficiencies of simple cubic and body-centred cubic lattices are 52.4% and 68% respectively.
18. An element with molar mass $\text{2}\text{.7 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-2}}}\text{kg mo}{{\text{l}}^{\text{-1}}}$ forms cubic unit cell with edge length 405 pm. If its density is $\text{2}\text{.7 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{3}}}\text{kg }{{\text{m}}^{\text{-3}}}$what is the nature of the cubic unit cell?
Ans: It is given that density of the element, d = $2.7\text{ }\times {{10}^{3}}kg\text{ }{{m}^{-3}}$
Molar mass, M = $2.7\text{ }\times {{10}^{-2}}kg\text{ }mo{{l}^{-1}}$
Edge length, a = 405 pm =$~405\text{ }\times {{10}^{-12}}m\text{ }=\text{ }4.05\text{ }\times {{10}^{-10}}m$
It is known that Avogadro’s number,${{N}_{A}}=6.022\times {{10}^{23}}\text{ }mo{{l}^{-1}}$
\[d=\frac{zM}{{{a}^{3}}{{N}_{A}}}\]
\[z=\frac{d{{a}^{3}}{{N}_{A}}}{M}\]
\[\frac{2.7\times {{10}^{3}}kg{{m}^{-3}}\times {{(4.05\times {{10}^{-10}}m)}^{3}}\times 6.022\times {{10}^{23}}mo{{l}^{-1}}}{2.7\times {{10}^{-2}}kgmo{{l}^{-1}}}\]
\[=4.004\simeq 4\]
This implies that four atoms of the element are present per unit cell. Hence, the unit cell is face centered cubic (fcc) or cubic close-packed (ccp).
19. What Type of Defect Can Arise When a Solid is Heated? Which Physical Property is Affected by It and in What Way?
Ans: When a solid is heated, vacancy defects can arise. A solid crystal is said to have vacancy defects when some of the lattice sites are vacant. Vacancy defect leads to a decrease in the density of the solid.
20. What type of Stoichiometric Defect is Shown by:
(ⅰ) (ZnS
Ans: ZnS shows Frenkel defect.
Ans: AgBr shows Frenkel defect as well as Schottky defect.
21. Explain How Vacancies are Introduced in an Ionic Solid When a Cation of Higher Valence is Added as an Impurity in It.
Ans: When a cation of higher valence is added to an ionic solid as an impurity to it, the cation of higher valence replaces more than one cation of lower valence so as to keep the crystal electrically neutral. As a result, some sites become vacant. For example, when $S{{r}^{2+}}$ is added to NaCl, each $S{{r}^{2+}}$ ion replaces two $N{{a}^{+}}$ions. However, one $S{{r}^{2+}}$ion occupies the site of one $N{{a}^{+}}$ion and the other site remains vacant. Hence, vacancies are introduced.
22. Ionic Solids, Which Have Anionic Vacancies Due to Metal Excess Defect, Develop Colour. Explain With the Help of a Suitable Example.
Ans: The colour develops because of the presence of electrons in the anionic sites. These electrons absorb energy from the visible part of radiation and get excited. For example, when crystals of NaCl are heated in an atmosphere of sodium vapours, the sodium atoms get deposited on the surface of the crystal and the chloride ions from the crystal diffuse to the surface to form NaCl with the deposited Na atoms. During this process, the Na atoms on the surface lose electrons to form $N{{a}^{+}}$ ions and the released electrons diffuse into the crystal to occupy the vacant anionic sites. These electrons get excited by absorbing energy from the visible light and impart yellow colour to the crystals.
22. A Group 14 Element is To Be Converted Into N-Type Semiconductor by Doping It With a Suitable Impurity. To Which Group Should This Impurity Belong?
Ans: An n-type semiconductor conducts because of the presence of extra electrons. Therefore a group 14 element can be converted to n-type semiconductor by doping it with a group 15 element.
22. What Type of Substances Would Make Better Permanent Magnets, Ferromagnetic or Ferrimagnetic. Justify Your Solution.
Ans: Ferromagnetic substances would make better permanent magnets. In solid state, the metal ions of ferromagnetic substances are grouped together into small regions. These regions are called domains and each domain acts as a tiny magnet. In an unmagnified piece of a ferromagnetic substance, the domains are randomly oriented. As a result, the magnetic moments of the domains get cancelled. However, when the substance is placed in a magnetic field, all the domains get oriented in the direction of the magnetic field and a strong magnetic effect is produced. The ordering of the domains persists even after the removal of the magnetic field. Thus the ferromagnetic substance becomes a permanent magnet.
Topics of Class 12 Chemistry Chapter 1
The subtopics included under NCERT Class 12 Chapter 1 are given below.
Important Points
Solids have a definite shape, volume and mass because of the short distance between the fixed position of particles and strong interactions between them.
Characteristics Properties of the Solid State are as follows.
They have definite mass, volume and shape.
Intermolecular distances are short.
Intermolecular forces are strong.
Their constituent particles (atoms, molecules or ions) have fixed positions and can only oscillate about their mean positions.
They are incompressible and rigid.
The classification of solid is given below.
Solid State Chemistry Class 12 NCERT PDF Download
Chemistry class 12 NCERT Solution chapter 1 can be easily accessed through free PDF download. The PDF comes in handy for all students preparing for their Class 12 Chemistry board exam. They can download the PDF for free from the website and mobile application of Vedantu. These NCERT Solutions are prepared in such a way that facilitates an easy learning process for students.
NCERT Solutions for Class 12 Chemistry
Chapter 1 - The Solid State
Chapter 2 - Solutions
Chapter 3 - Electrochemistry
Chapter 4 - Chemical Kinetics
Chapter 5 - Surface Chemistry
Chapter 6 - General Principles and Processes of Isolation of Elements
Chapter 7 - The p-Block Elements
Chapter 8 - The d and f Block Elements
Chapter 9 - Coordination Compounds
Chapter 10 - Haloalkanes and Haloarenes
Chapter 11 - Alcohols, Phenols and Ethers
Chapter 12 - Aldehydes, Ketones and Carboxylic Acids
Chapter 13 - Amines
Chapter 14 - Biomolecules
Chapter 15 - Polymers
Chapter 16 - Chemistry in Everyday life
NCERT Solution for CBSE Class 12 Chemistry Chapter 1
Solid State Chemistry Class 12 Solution for Chapter 1: Question 1
The answers will provide the students with a detailed understanding of amorphous solids. The Solution also provides examples of solids whose constituent particles are of irregular shapes. This will help students to understand the topics easily.
Class 12th Chemistry Chapter 1 Solution: Question 2
The Solution helps the students to understand how the arrangement of constituent particles makes a glass different from another solid such as quartz. By learning this, students will also understand how quartz can be converted into glass by heating and cooling rapidly.
Solid-State Chemistry Class 12 NCERT Solutions: Question 3 and 4
Through these two answers, students will be able to classify solid as ionic, metallic, molecular, covalent or amorphous. Students will also learn the meaning of coordination number in both cubic close-packed structure and body centred cubic structure.
Solid-State Class 12 NCERT Solution for Question 5 and Question 6
The Solution to question 5 gives a detailed analysis of how to determine the atomic mass of an unknown metal if the density and dimension of its unit cell are given. Students also get a step by step explanation in the Solution of question 6 of how the stability of crystal is reflected on the melting point.
Chapter 1 Chemistry class 12 Solution to question 7
The Solution comes with detailed diagrams and figures. It helps students to understand the difference between:
Hexagonal close-packing and cubic close packing.
Crystal lattice and Unit Cell.
Tetrahedral void and Octahedral void.
Through the detailed diagrammatic representation, students will be able to distinguish between the different solid states easily.
Solutions to the Solid-State Class 12: In-Text Question
The Solution to this question will help the student to understand the intermolecular force of attraction that causes rigidity in solids. After solving this question, students will understand why the constituent particles of a solid have a fixed position.
The Solution to this question helps students to understand elaborately how solids have a definite volume. By going through the elaborate explanation, students will learn how the intermolecular force of attraction results in a fixed volume for solids.
The Solution to this question will help the students to understand the classification between amorphous and crystalline solid. After solving these questions, students will easily classify the solids into two categories.
Solid-State Chemistry Class 12 NCERT Solution: Question 4 and 5
The Solution to both these questions comes with a detailed explanation. With theoretical reasons, it explains why glass is called supercooled and discusses the refractive index of solids having the same value across all directions. Students will have a detailed understanding of solid states.
The marks distribution for Chemistry class 12 chapter 1 is given in the table below.
Solid-State Chemistry Chapter 1 Marks Distribution
Though a minimum mark is assigned to this chapter, yet it is crucial for understanding several other concepts of chemistry. Chemistry Class 12 NCERT Solutions Chapter 1 will help the students to easily understand the concepts in detail.
Term I CBSE Class 12 Chemistry Syllabus Course Structure 2021-22
Term ii cbse class 12 chemistry syllabus course structure 2021-22, advantages of ncert class 12 chemistry chapter 1 solution.
The benefits of referring to the Solid-State chemistry class 12 Solutions are as follows.
The simple approach of these NCERT Solutions makes it easier for students to understand all the topics covered in this chapter easily.
Also, the diagrams, charts, and other illustrations are provided in these NCERT Solutions for better understanding.
Since these NCERT Solutions are prepared by the subject matter experts at Vedantu, in close reference to the CBSE guidelines, students can rely upon them for their board exam preparation.
Every question is solved in a step by step manner and is followed by elaborate explanations to facilitate a deeper understanding of the concepts.

FAQs on NCERT Solutions for Class 12 Chemistry Chapter 1 - The Solid State
1. How can you Prepare the Topic of Solid-States for the 12th Board Exams?
Preparation for the topic of Solid-States, ch 1 Chemistry class 12 requires a detailed understanding of its physical properties. Topics such as the difference between amorphous and crystalline solids must be understood in detail.
Students should also learn about the difference between Hexagonal close packing and Cubic close packing, Crystal lattice and unit cell, Tetrahedral void, and Octahedral void. The NCERT Solutions are a great help in this regard.
These Solutions of CBSE class 12 Chemistry Chapter 1 will help the students easily understand the topics. The diagrams and step by step calculations in NCERT Solutions are immensely student-friendly and helps them to get a grip on the crucial concepts.
2. What Topics are Covered in the NCERT Solutions Chemistry Chapter 1?
The NCERT Solutions for class 12 Chemistry ch 1 Solid-State covers the following topics.
(1) General characteristics of solids,
(2) Amorphous and crystalline solids,
(3) Classification of crystalline solids,
(4) Ionic solids,
(5) Metallic solids,
(6) Covalent and network solids,
(7) Primitive and centered unit cells,
(8) Primitive cubic unit cell,
(9) Body centred cubic unit cell,
(10) Face centred cubic unit cell.
Apart from this, NCERT Solution class 12 Chemistry Chapter 1 also deals with topics related to the formulas of compounds and number of voids filled, packing efficiency in HCP and CCP structures, an imperfection in solid, electrical properties, conduction of electricity and semiconductors, a calculation involving unit cell dimension, Magnetic properties, and conduction of electricity in metals.
3. How is the NCERT Class 12 Chemistry Chapter 1 PDF Helpful?
The NCERT class 12 Chemistry chapter 1 is extremely helpful for the students preparing for the boards. The simple language, detailed explanations of chapters, use of illustrative diagrams, and step by step method of calculation makes the learning process easy for the students. Also, class 12 Chemistry ch 1 NCERT Solutions are prepared by the subject matter experts of Chemistry, keeping in view the CBSE syllabus and marks distribution for Chemistry paper. All key topics and essential questions related to solid states are covered in these NCERT Solutions. All the important topics such as general characteristics of solids, amorphous and crystalline solids, classification of crystalline solids, ionic solids, metallic solids are delved in detail with adequate examples to help the students understand the concepts better.
4. How do I prepare for Chapter 1 of Class 12 Chemistry?
The preparation for Chapter 1 of Class 12 Chemistry is to be done in a very systematic method. One needs to clearly understand some basic concepts and theories like that of amorphous and crystalline substances. Another important concept to be understood is the geometric arrangement of crystals.
NCERT Solutions for Chapter 1 of Class 12 Chemistry is the best resource to understand this Chapter in the best possible way. These solutions are available free of cost on the Vedantu website and the Vedantu app.
5. Define the term amorphous solids. Give a few examples, according to Chapter 1 of Class 12 Chemistry.
These types of solids have an irregular arrangement of constituent particles. The building constituents like atoms, molecules, and ions are arranged in a haphazard manner. These solids have a short-range order. Also, their melting point is not high. They are isotropic. When these solids are cut into two pieces with a sharp tool, they have irregular surfaces. Amorphous solids do not have a definite heat of fusion. They are also called supercooled liquids or pseudo solids.
Examples- quartz glass, rubber, etc.
6. What are crystalline solids, according to Chapter 1 of Class 12 Chemistry?
Crystalline solids have a regular arrangement of constituent particles like atoms, molecules, and ions throughout the three-dimensional network. They are built up by large unit cells. They have definite characteristics and geometrical shapes. They melt at a high temperature. Crystalline solids are anisotropic. They have a long-range order. These solids are known as true solids. Crystalline solids have a definite and characteristic heat of fusion. When these solids are cut with a sharp tool, they split into two pieces, which are both smooth and plain.
Example- quartz, sodium chloride, etc.
7. Explain Schottky defect, according to Chapter 1 of Class 12 Chemistry.
This is a stoichiometric defect that arises due to missing an equal number of anions and cations from the lattice. This defect is commonly seen in ionic compounds with a high coordination number. Both the anions and cations are similar in size. The density of the crystal decreases due to this stoichiometric defect. It also conducts electricity due to its smaller extent.
Examples- potassium chloride, sodium chloride, potassium bromide, etc.
8. Explain the Frenkel defect, according to Chapter 1 of Class 12 Chemistry.
Frenkel defect is a stoichiometric interstitial defect that arises when some ions occupy interstitial sites in the lattice. These ions leave the lattice sites vacant and occupy new spaces. This defect is commonly found in ionic crystals where the cation is smaller than the anion. This defect does not change the density of the lattice. Due to a small extent, the electrical conductivity increases. There is no change in the crystal's overall composition (chemical) due to the Frenkel defect.
Example- silver bromide, zinc sulphide, etc.
NCERT Solutions Class 12 Chemistry
NCERT Solutions for Class 12 Chemistry Chapter 1 Solutions
NCERT Solutions for Class 12 Chemistry Chapter 1 Solutions in Hindi Medium and English Medium. Get here Exercises Questions and Intext Questions to view online or download in PDF format. These solutions are updated for new academic year 2024-25 for all boards using NCERT Books.
Class 12th Chemistry Chapter 1 Solutions Answers
- Class 12 Chemistry Chapter 1 Exercises
- Class 12 Chemistry Chapter 1 Intext
- Class 12 Chemistry Chapter 1 in Hindi
- Class 12 Chemistry Chapter 1 NCERT Book
- Class 12 Chemistry Chapter 1 Revision Book
- Class 12 Chemistry Chapter 1 Study Materials
- Class 12 Chemistry Chapter 1 Assignment 1
- Class 12 Chemistry Chapter 1 Assignment 2
- Class 12 Chemistry Chapter 1 Assignment 3
- Class 12 Chemistry Chapter 1 Assignment 4
- Class 12 Chemistry Chapter 1 Level 1 Test 1
- Class 12 Chemistry Chapter 1 Level 2 Test 1
- Class 12 Chemistry Chapter 1 Level 3 Test 1
- Class 12 Chemistry NCERT Solutions
- Class 12 all Subjects NCERT Solutions

NCERT Solutions for Class 12 Chemistry Chapter 1 in PDF format to free download is given below updated for new academic session 2024-25. Download NCERT Books and offline as well as online apps based on latest CBSE Syllabus.
Download App for Class 12

1. Which of the following units is useful in relating concentration of solution with its vapour pressure? (i) mole fraction (ii) parts per million (iii) mass percentage (iv) molality 2. Maximum amount of a solid solute that can be dissolved in a specified amount of a given liquid solvent does not depend upon ____________. (i) Temperature (ii) Nature of solute (iii) Pressure (iv) Nature of solvent 3. An unripe mango placed in a concentrated salt solution to prepare pickle, shrivels because _____________. (i) it gains water due to osmosis. (ii) it loses water due to reverse osmosis. (iii) it gains water due to reverse osmosis. (iv) it loses water due to osmosis. 4. Low concentration of oxygen in the blood and tissues of people living at high altitude is due to ____________. (i) low temperature (ii) low atmospheric pressure (iii) high atmospheric pressure (iv) both low temperature and high atmospheric pressure
5. The value of Henry’s constant KH is _____________. (i) greater for gases with higher solubility. (ii) greater for gases with lower solubility. (iii) constant for all gases. (iv) not related to the solubility of gases. 6. At a given temperature, osmotic pressure of a concentrated solution of a substance _____________. (i) is higher than that at a dilute solution. (ii) is lower than that of a dilute solution. (iii) is same as that of a dilute solution. (iv) cannot be compared with osmotic pressure of dilute solution. 7. Considering the formation, breaking and strength of hydrogen bond, predict which of the following mixtures will show a positive deviation from Raoult’s law? (i) Methanol and acetone. (ii) Chloroform and acetone. (iii) Nitric acid and water. (iv) Phenol and aniline.

8. Which of the following statements is false? (i) Units of atmospheric pressure and osmotic pressure are the same. (ii) In reverse osmosis, solvent molecules move through a semipermeable membrane from a region of lower concentration of solute to a region of higher concentration. (iii) The value of molal depression constant depends on nature of solvent. (iv) Relative lowering of vapour pressure, is a dimensionless quantity. 9. Colligative properties depend on ____________. (i) the nature of the solute particles dissolved in solution. (ii) the number of solute particles in solution. (iii) the physical properties of the solute particles dissolved in solution. (iv) the nature of solvent particles. 10. On dissolving sugar in water at room temperature solution feels cool to touch. Under which of the following cases dissolution of sugar will be most rapid? (i) Sugar crystals in cold water. (ii) Sugar crystals in hot water. (iii) Powdered sugar in cold water. (iv) Powdered sugar in hot water.
1 (i) 2 (iii) 3 (iv) 4 (ii) 5 (ii) 6 (i) 7 (i) 8 (ii) 9 (ii) 10 (iv).
NCERT Books for all classes and subjects, Offline Apps 2024-25 based on new CBSE Syllabus. Ask your doubts related to NIOS or CBSE Board and share your knowledge with your friends and other users through Discussion Forum.

Copyright 2024 by Tiwari Academy | A step towards Free Education

- New QB365-SLMS
- NEET Materials
- JEE Materials
- Banking first yr Materials
- TNPSC Materials
- DIPLOMA COURSE Materials
- 5th Standard Materials
- 12th Standard Materials
- 11th Standard Materials
- 10th Standard Materials
- 9th Standard Materials
- 8th Standard Materials
- 7th Standard Materials
- 6th Standard Materials
- 12th Standard CBSE Materials
- 11th Standard CBSE Materials
- 10th Standard CBSE Materials
- 9th Standard CBSE Materials
- 8th Standard CBSE Materials
- 7th Standard CBSE Materials
- 6th Standard CBSE Materials
- Tamilnadu Stateboard
- Scholarship Exams
- Scholarships

CBSE 12th Standard Chemistry Subject The d- and f- Block Elements Chapter Case Study Questions With Solution 2021
By QB365 on 21 May, 2021
QB365 Provides the updated CASE Study Questions for Class 12 , and also provide the detail solution for each and every case study questions . Case study questions are latest updated question pattern from NCERT, QB365 will helps to get more marks in Exams
QB365 - Question Bank Software
Cbse 12th standard chemistry subject the d- and f- block elements case study questions with solution 2021.
12th Standard CBSE
Final Semester - June 2015
Read the passage given below and answer the following questions: The f-block elements are those in which the differentiating electron enters the (n -2) forbital. There are two series of f-block elements corresponding to filling of 4f and 5f-orbitals. The series of 4f- orbitals is called lanthanides. Lanthanides show different oxidation states depending upon stability of f 0 , f 7 and f 14 configurations, though the most common oxidation states is +3. There is a regular decrease in size of lanthanides ions with increase in atomic number which is known as lanthanide contraction. The following questions are multiple choice questions. Choose the most appropriate answer: (i) The atomic numbers of three lanthanide elements X, Y and 2 are 65, 68 and 70 respectively, their Ln 3+ electronic configuration is
(ii) Lanthanide contraction is observed in
(iii) Name a member of the lanthanoid series which is well known to exhibit +4 oxidation state.
(iv) Identify the incorrect statement among the following.
Read the passage given below and answer the following questions: The transition elements have incompletely filled d-subshells in their ground state or in any of their oxidation states. The transition elements occupy position in between s- and p-blocks in groups 3-12 of the Periodic table. Starting from fourth period, transition elements consists of four complete series : Sc to Zn, Y to Cd and La, Hf to Hg and Ac, Rf to Cn. In general, the electronic configuration of outer orbitals of these elements is (n - 1) d 1-10 ns 1-2 . The electronic configurations of outer orbitals of Zn, Cd, Hg and Cn are represented by the general formula (n - 1)d 10 n 2 . All the transition elements have typical metallic properties such as high tensile strength, ductility, malleability. Except mercury, which is liquid at room temperature, other transition elements have typical metallic structures. The transition metals and their compounds also exhibit catalytic property and paramagnetic behaviour. Transition metal also forms alloys. An alloy is a blend of metals prepared by mixing the components. Alloys may be homogeneous solid solutions in which the atoms of one metal are distributed randomly among the atoms of the other. The following questions are multiple choice questions. Choose the most appropriate answer : (i) Which of the following characteristics of transition metals is associated with higher catalytic activity?
(ii) Transition elements form alloys easily because they have
(iii) The electronic configuration of tantalum (Ta) is
(iv) Which one of the following outer orbital configurations may exhibit the largest number of oxidation states?
Read the passage given below and answer the following questions: The unique behaviour of Cu, having a positive E o accounts for its inability to liberate H 2 from acids. Only oxidising acids (nitric and hot concentrated sulphuric acid) react with Cu, the acids being reduced. The stability of the half-filled (d 5 ) subshell in Mn 2+ and the completely filled (d 10 ) configuration in Zn 2+ are related to their E o (M 3+ /M 2+ ) values. The low value for Sc reflects the stability of Sc 3+ which has a noble gas configuration. The comparatively high value for Mn shows that Mn 2+ (d 5 ) is particularly stable, whereas a comparatively low value for Fe shows the extra stability of Fe 3+ (d 5 ). The comparatively low value for V is related to the stability of V 2+ (half-filled t 2g level). The following questions are multiple choice questions.Choose the most appropriate answer : (i) Standard reduction electrode potential of Zn 2+ /Zn is - 0.76 V. This means
(ii) E o values for the couples Cr 3+ /Cr 2+ and Mn 3+ /Mn 2+ are -0.41 and +1.51 volts respectively. These values suggest that
(iii) The reduction potential values of M, Nand O are +2.46, -1.13 and -3.13 Y respectively. Which of the following order is correct regarding their reducing property?
(iv) Which of the following statements are true? (i) Mn 2+ compounds are more stable than Fe 2+ towards oxidation to +3 state. (ii) Titanium and copper both in the first series of transition metals exhibits +1 oxidation state most frequently. (iii) Cu + ion is stable in aqueous solutions. (iv) The E 0 value for the Mn 3+ /Mn 2+ couple is much more positive than that for Cr 3+ /Cr 2+ or Fe 3+ /Fe 2+ .
Read the passage given below and answer the following questions: Transition metal oxides are compounds formed by the reaction of metals with oxygen at high temperature. The highest oxidation number in the oxides coincides with the group number. In vanadium, there is a gradual change from the basic V 2 O 3 to less basic V 2 O 4 and to amphoteric V 2 O 5・ V 2 O 4 dissolves in acids to give VO 2+ salts. Transition metal oxides are commonly utilized for their catalytic activity and semiconductive properties. Transition metal oxides are also frequently used as pigments in paints and plastic. Most notably titatnium dioxide. One of the earliest application of transition metal oxides to chemical industry involved the use of vanadium oxide for catalytic oxidation of sulfur dioxide to sulphuric acid. Since then, many other applications have emerged, which include benzene oxidation to maleic anhydride on vandium oxides; cyclohexane oxidation to adipic acid on cobalt oxides. An important property of the catalyst material used in these processes is the ability of transition metals to change their oxidation state under a given chemical potential of reductants and oxidants. The following questions are multiple choice questions. Choose the most appropriate answer: (i) Which oxide of vanadium is most likely to be basic and ionic ?
(ii) Vanadyl ion is
(iii) The oxidation state of vanadium in V 2 O 5 is
(iv) Identify the oxidising agent in the following reaction.
Read the passage given below and answer the following questions: Transition elements are elements that have partially filled d-orbitals. The configuration of these elements corresponds to (n - 1)d 1-10 ns 1-2 . It is important to note that the elements mercury, cadmium and zinc (Ire not considered transition elements because of their electronic configurations, which corresponds to (n - 1)d 1-10 ns 2 . Some general properties of transition elements are : These elements can form coloured compounds and ions due to d-d transition; These elements exhibit many oxidation states; A large variety of ligands can bind themselves to these elements, due to this, a wide variety of stable complexes formed by these ions. The boiling and melting point of these elements are high. These elements have a large ratio of charge to the radius. In these questions (i-iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices. (a) Assertion and reason both are correct statements and reason is correct explanation for assertion. (b) Assertion and reason both are correct statements but reason is not correct explanation for assertion. (c) Assertion is correct statement but reason is wrong statement. (d) Assertion is wrong statement but reason is correct statement. (i) Assertion: Tungsten has very high melting point. Reason: Tungsten is a covalent compound. (ii) Assertion: Zn, Cd and Hg are normally not considered transition metals. Reason: d-Orbitals in Zn, Cd and Hg elements are completely filled, hence these metals do not show the general characteristics properties of the transition elements. (iii) Assertion: Copper metal gets readily corroded in acidic aqueous solution such as HCI and dil. H 2 SO 4 Reason: Free energy change for this process is positive. (iv) Assertion: Tailing of mercury occurs on passing ozone through it. Reason: Due to oxidation of mercury.
*****************************************
Cbse 12th standard chemistry subject the d- and f- block elements case study questions with solution 2021 answer keys.
(i) (a): Terbium (65), 4f 8 ; Dysprosium (Dy), 4f 9 ; Ytterbium (Yb), 4f 13 . (ii) (a) (iii) (a) (iv) (b): The almost identical radii of Zr (160 pm) and Hf(159 pm), a consequence of lanthanoid contraction.
(i) (b): The transition metals and their compounds are known for their catalytic activity. This activity is ascribed to their ability to adopt multiple oxidation states to form complexes. (ii) (c) : Because of similar radii and other characteristics of transition metals, alloys are readily formed by these metals. (iii) (c) (iv) (b): Greater the number of valence electrons, more will be the number of oxidation states exhibited by the element.
(i) (a) (ii) (a): Lesser and negative reduction potential indicates that Cr 2+ is a reducing agent. Higher and positive reduction potential indicates that Mn 3+ is a stronger oxidizing agent. (iii) (a) : The electrode which has more reduction potential is a good oxidizing agent and has least reducing power. (iv) (b): (i) It is because Mn 2+ has 3d 5 electronic configuration which has extra stability. (ii) Not titanium but copper, because with + 1 oxidation state an extra stable configuration, 3d 10 results. (iii) It is not stable as it undergoes disproportionation; 2Cu + (aq) ➝Cu 2+ (aq) + Cu(s). The E o value for this is favourable. (iv) Much larger third ionisation energy ofMn (where the required change is d 5 to d 4 ) is mainly responsible for this.
(i) (a): Oxide of V in lowest oxidation state, i.e., VO is basic and ionic in character. (ii) (a): Vanadyl ion is VO 2+ where V is in +4 oxidation state. (iii) (c) (iv) (a)
(i) (c) : Tungsten is a transition element and is very hard due to high metallic bonding. (ii) (a) (iii) (d): Non-oxidising acids (HCI and dil. H 2 SO 4 ) do not have any effect on copper. However they dissolve the metal in presence of air. As it is a non-spontaneous process so, \(\Delta\) G cannot be -ve. (iv) (a): When mercury is exposed to ozone it gets superficially oxidised and loses its meniscus and sticks to the glass.
Related 12th Standard CBSE Chemistry Materials
12th standard cbse syllabus & materials, cbse 12th physics wave optics chapter case study question with answers, cbse 12th physics ray optics and optical instruments chapter case study question with answers, cbse 12th physics nuclei chapter case study question with answers, cbse 12th physics moving charges and magnetism chapter case study question with answers, cbse 12th physics electromagnetic induction chapter case study question with answers, cbse 12th physics atoms chapter case study question with answers, 12th physics alternating current chapter case study question with answers cbse, 12th maths vector algebra chapter case study question with answers cbse, 12th maths three dimensional geometry chapter case study question with answers cbse, 12th maths probability chapter case study question with answers cbse, 12th maths linear programming chapter case study question with answers cbse, 12th maths differential equations chapter case study question with answers cbse, 12th maths continuity and differentiability chapter case study question with answers cbse, 12th maths application of integrals chapter case study question with answers cbse, class 12th economics - non-competitive markets case study questions and answers 2022 - 2023.

Class VI to XII
Tn state board / cbse, 3000+ q&a's per subject, score high marks.

12th Standard CBSE Study Materials

12th Standard CBSE Subjects

Case Study Questions Haloalkanes and Haloarenes Class 12 Chemistry
Case Study Questions Haloalkanes and Haloarenes Class 12 Chemistry
1. Read the passage given below and answer the following questions: Nucleophilic substitution reactions are of two types; substitution nucleophilic bimolecular (S N 2) and substitution nucleophilic unimolecular (S N 1) depending on molecules taking part in determining the rate of reaction. The reactivity of alkyl halide towards S N 1 and S N 2 reactions depends on various factors such as steric hindrance, stability of intermediate or transition state, and polarity of the solvent. S N 2 reaction mechanism is favoured mostly by primary alkyl halide or transition state and polarity of the solvent, S N 2 reaction mechanism is favoured mostly by primary alkyl halide then secondary and then tertiary. This order is reversed in the case of S N 1 reactions. (i) Which of the following is most reactive towards nucleophilic substitution reaction? (a) C 6 H 5 Cl (b) CH 2 = CHCl (c) ClCH 2 CH = CH 2 (d) CH 3 CH = CHCl
(ii) Isopropyl chloride undergoes hydrolysis by (a) S N 1 mechanism (b) S N 2 mechanism (c) S N 1 and S N 2 mechanism (d) Neither S N 1 nor S N 2 mechanism
(iii) Tertiary alkyl halides are practically inert to substitution by S N 2 mechanism because of (a) Insolubility (b) Instability (c) Inductive effect (d) Steric Hindrance
(iv) Which of the following is the correct order of decreasing S N 2 reactivity? (a) RCH 2 X > R 2 CHX > R 3 CX (b) R 3 CX > R 2 CHX >RCH 2 X (c) R 2 CHX > R 3 CX > RCH 2 X (d) RCH 2 X > R 3 CX > R 2 CHX
(v) An organic molecule necessarily shows optical activity if it- a) Contains asymmetric carbon atoms b) Is non-polar c) Is non-superimposable on its mirror image d) Is superimposable on its mirror image.
2. Read the passage given below and answer the following questions: The replacement of hydrogen atom in a hydrocarbon, aliphatic or aromatic results in the formation of haloalkanes and haloarenes respectively. Haloalkanes contain a halogen atom attached to sp 3 hybridized carbon atom of an alkyl group whereas haloarenes contain a halogen atom attached to sp 2 hybridized carbon atom of an aryl group. Haloalkanes and haloarenes may be classified on the basis of the number of halogen atoms in their structures as mono, di, or poly halogen compounds and also on the basis of the state of hybridization of the carbon atom to which the halogen atom is bonded. (i) Which of the following halide is 2°? (a) Isopropyl chloride (b) Isobutyl chloride (c) n-propyl chloride (d) n-butyl chloride
(ii) Which of the following is a Gem-dibromide is: (a) CH 3 CH(Br)CH 2 (Br) (b) CH 3 CBr 2 CH 3 (c) CH 2 (Br)CH 2 CH 2 (d) CH 2 BrCH 2 Br
(iii) IUPAC name of (CH 3 ) 3 CCl is: (a) 3-Chlorobutane (b) 2-Chloro-2-methylpropane (c) t-butyl chloride (d) n-butyl chloride
(iv) Which of the following is a primary halide? (a) Isopropyl iodide (b) Secondary butyl iodide (c) Tertiarybutyl bromide (d) Neohexyl chloride
(v) Which one of the following is not an allylic halide? (a) 4-Bromopent-2-ene (b) 3-Bromo-2-methylbut-1-ene (c) 1-Bromobut-2-ene (d) 4-Bromobut-1-ene
3. Read the passage given below and answer the following questions: Alkyl halides are prepared by the free radical halogenation of alkanes, addition of halogen acids to alkenes, replacement of -OH group of alcohols with halogens using phosphorus halides, thionyl chloride, or halogen acids. Aryl halides are prepared by electrophilic substitution to arene. Fluorine and iodides are best prepared by the halogen exchange method. These compounds find wide applications in industry as well as in day-to-day life. These compounds are generally used as solvents and as starting materials for the synthesis of a large number of organic compounds. (i) The best method for the conversion of an alcohol into an alkyl chloride is by treating the alcohol with (a) PCl 5 (b) Dry HCl in the presence of anhydrous ZnCl 2 (c) SOCl 2 in presence of pyridine (d) None of these
(ii) The catalyst used in the preparation of an alkyl chloride by the action of dry HCl on alcohol is (a) anhydrous AlCl 3 (b) FeCl 3 (c) anhydrous ZnCl 2 (d) Cu
(iii) An alkyl halide reacts with metallic sodium in dry ether. The reaction is known as: (a) Frankland’s reaction (b) Sandmeyer’s reaction (c) Wurtz reaction (d) Kolbe’s reaction
(iv) Fluorobenzene (C 6 H 5 F) can be synthesized in the laboratory (a) By direct fluorination of benzene with F 2 gas (b) By reacting bromobenzene with NaF solution (c) By heating phenol with HF and KF (d) From aniline by diazotization followed by heating the diazonium salt with HBF 4
Related Posts

CBSE Sample Paper Session 2022-23 (Chemistry) PDF

Haloalkanes and Haloarenes Class 12 – Questions & Answers

CBSE Important Questions Electrochemistry
Save my name, email, and website in this browser for the next time I comment.
Type above and press Enter to search. Press Esc to cancel.

Serving Quality Education
Case Study Question 1 on Solutions – Chapter 2 CBSE Class 12 Chemistry
- May 14, 2022
- Chemistry , CBSE , Class 12
Case-based Questions
Read the given passages and answer the questions that follow :
The spontaneous flow of the solvent through a semipermeable membrane from a pure solvent to a solution or from a dilute solution to a concentrated solution is called osmosis. The phenomenon of osmosis can be demonstrated by taking two eggs of the same size. In an egg, the membrane below the shell and around the egg material is semi- permeable. The outer hard shell can be removed by putting the egg in dilute hydrochloric acid. After removing the hard shell, one egg is placed in distilled water and the other in a saturated salt solution. After some time, the egg placed in distilled water swells-up while the egg placed in salt solution shrinks.
The external pressure applied to stop the osmosis is termed as osmotic pressure (a Colligative property). Reverse osmosis takes place when the applied external pressure becomes larger than the osmotic pressure.
1. What do you expect to happen when red blood corpuscles (RBC’s) are placed in 0.5% NaCl solution?
Ans. RBC’s are isotonic with 0.9% NaCl solution, so they will swell and may even burst when placed in 0.5% NaCl solution.
2. Which one of the following will have higher osmotic pressure in 1 M KCl or 1 M urea solution?
Ans. 1 M KCl will have higher osmotic pressure because its dissociates to give K + and Cl – ions while urea does not dissociate into ions in the solution.
3. Name one SPM which can be used in the process of reverse osmosis.
Ans. Cellulose acetate placed on a suitable support.
4. What are isotonic solutions?
Ans. Solutions having equal osmotic pressure are called isotonic solutions.
5. Write van’t Hoff equation for dilute solution.
Ans. p V = nRT ,
Where, p = Osmotic pressure, n = Number of moles, V = Volume of solution in litre, R = Gas constant ,
T = Temperature
Related Posts
Ncert solutions class 11 maths chapter 9 exercise 9.2 straight lines.
- January 13, 2024
Handwritten Notes of Chapter 2, Physics, Electrical Potential and Capacitance
- August 20, 2023
EMF, Chapter 6, Worksheet- 1 (2023-24)
- July 9, 2023
Leave a Reply Cancel Reply
Your email address will not be published. Required fields are marked *
Add Comment *
Save my name, email, and website in this browser for the next time I comment.
Post Comment

Gurukul of Excellence
Classes for Physics, Chemistry and Mathematics by IITians
Join our Telegram Channel for Free PDF Download
Case Study Questions for Class 12 Chemistry Chapter 2 Solutions
- Last modified on: 4 days ago
- Reading Time: 5 Minutes

Table of Contents
There is Case Study Questions in class 12 Chemistry in session 2020-21. For the first time, the board has introduced the case study questions in the board exam. The first two questions in the board exam question paper will be based on Case Study and Assertion & Reason. The first question will have 5 MCQs out of which students will have to attempt any 4 questions. The second question will carry 5 Assertion & Reason type questions with the choice to attempt any four. Here are the questions based on case study.
Case Study Question 1:
Read the passage given below and answer the following questions:
The properties of the solutions which depend only on the number of solute particles but not on the nature of the solute are called colligative properties. Relative lowering in vapour pressure is also an example ofcolligative properties.
For an experiment, sugar solution is prepared for which lowering in vapour pressure was found to be 0.061 mm of Hg. (Vapour pressure of water at 20 0 C is 17.5 mm of Hg)
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Relative lowering of vapour pressure for the given solution is (a) 0.00348 (b) 0.061 (c) 0.122 (d) 1.75
(ii) The vapour pressure (mm of Hg) of solution will be (a) 17.5 (b) 0.61 (c) 17.439 (d) 0.00348
(iii) Mole fraction of sugar in the solution is (a) 0.00348 (b) 0.9965 (c) 0.061 (d) 1.75
If weight of sugar taken is 5 g in 108 g of water then molar mass of sugar will be (a) 358 (b) 120 (c) 240 (d) 400
(iv) The vapour pressure (mm of Hg) of water at 293K when 25g of glucose is dissolved in 450 g of water is (a) 17.2 (b) 17.4 (c) 17.120 (d) 17.02
Related Posts
Download cbse books.
Exam Special Series:
- Sample Question Paper for CBSE Class 10 Science (for 2024)
- Sample Question Paper for CBSE Class 10 Maths (for 2024)
- CBSE Most Repeated Questions for Class 10 Science Board Exams
- CBSE Important Diagram Based Questions Class 10 Physics Board Exams
- CBSE Important Numericals Class 10 Physics Board Exams
- CBSE Practical Based Questions for Class 10 Science Board Exams
- CBSE Important “Differentiate Between” Based Questions Class 10 Social Science
- Sample Question Papers for CBSE Class 12 Physics (for 2024)
- Sample Question Papers for CBSE Class 12 Chemistry (for 2024)
- Sample Question Papers for CBSE Class 12 Maths (for 2024)
- Sample Question Papers for CBSE Class 12 Biology (for 2024)
- CBSE Important Diagrams & Graphs Asked in Board Exams Class 12 Physics
- Master Organic Conversions CBSE Class 12 Chemistry Board Exams
- CBSE Important Numericals Class 12 Physics Board Exams
- CBSE Important Definitions Class 12 Physics Board Exams
- CBSE Important Laws & Principles Class 12 Physics Board Exams
- 10 Years CBSE Class 12 Chemistry Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Physics Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Maths Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Biology Previous Year-Wise Solved Papers (2023-2024)
- ICSE Important Numericals Class 10 Physics BOARD Exams (215 Numericals)
- ICSE Important Figure Based Questions Class 10 Physics BOARD Exams (230 Questions)
- ICSE Mole Concept and Stoichiometry Numericals Class 10 Chemistry (65 Numericals)
- ICSE Reasoning Based Questions Class 10 Chemistry BOARD Exams (150 Qs)
- ICSE Important Functions and Locations Based Questions Class 10 Biology
- ICSE Reasoning Based Questions Class 10 Biology BOARD Exams (100 Qs)
✨ Join our Online JEE Test Series for 499/- Only (Web + App) for 1 Year
✨ Join our Online NEET Test Series for 499/- Only for 1 Year
7 thoughts on “ Case Study Questions for Class 12 Chemistry Chapter 2 Solutions ”
Why answers are not given ???
Answers uploaded
can you share the pictures of solutions too??
Sure! Please wait for some time.
where are sols.,
Click on answer, it will expand.
can you share solutions of these also
Leave a Reply Cancel reply
Join our Online Test Series for CBSE, ICSE, JEE, NEET and Other Exams

Editable Study Materials for Your Institute - CBSE, ICSE, State Boards (Maharashtra & Karnataka), JEE, NEET, FOUNDATION, OLYMPIADS, PPTs
Discover more from Gurukul of Excellence
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading

IMAGES
VIDEO
COMMENTS
Class 12 Chemistry Case Study Questions for Term 1 exam includes The Solid State, The P block elements, Haloalkanes and Haloarenes, Biomolecules, etc. Questions for all these chapters are given in the PDF file that are available here for free to download. Term 1 exam is about to be held in November-December this year.
Here, we have provided case-based/passage-based questions for Class 12 Chemistry Chapter 1 The Solid State. Case Study/Passage-Based Questions. Case Study 1: In a hexagonal system of crystals, a frequently encountered arrangement of atoms is described as a hexagonal prism. Here, the top and bottom of the cell are regular hexagons and three ...
There is Case Study Questions in class 12 Chemistry in session 2020-21. The first two questions in the board exam question paper will be based on Case Study and Assertion & Reason. The first question will have 5 MCQs out of which students will have to attempt any 4 questions. The second question will carry 5 Assertion & Reason type questions ...
4. (a) Read the passage given below and answer the following questions: At 298 K, the vapour pressure of pure benzene, C 6 H 6 is 0.256 bar and the vapour pressure of pure toluene. C 6 H 5 CH 3 is 0.0925 bar. Two mixtures were prepared as follows: (i) 7.8 g of C 6 H 6 + 9.2 g of toluene.
Download the PDF now and boost your chemistry knowledge! Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th. You need to improve your preparation for the Class 12 Chemistry Case Study Questions exams if you want to achieve a 95+% on the boards.
This will help them to understand the type of Case Study questions that can be asked in Grade 12 Chemistry examinations. Our expert faculty for standard 12 Chemistry have designed these questions based on the trend of questions that have been asked in last year's exams. The solutions have been designed in a manner to help the grade 12 ...
In Coming Exams, CBSE will ask two Case Study Questions in the CBSE class 12 Chemistry questions paper. Each theme will have five questions and students will have a choice to attempt any four of them. Here are some example questions Based On Case Study Problems: Question-1 Read the passage given below and answer any four out of the following ...
CBSE Class 12 Case Study Questions and Answers for Chemistry all Chapter 1 to 16. Case Based Factual Passage Questions & Answer. 100% FREE Exercise & Practice for CBSE, NCERT and ICSE ... Surface Chemistry: Chapter - VI: Isolation of Elements: Chapter -VII: p-Block Elements: Chapter - VIII: d- and f-Block Elements: Chapter - IX ...
Install Now. CBSE will ask Case Study Questions class 12 Chemistry in session 2020-21. These will be the first two questions in the board exam question paper. The first question will have 5 MCQs out of which students will attempt any 4 questions. The second question will carry 5 Assertion & Reason type questions with the choice to attempt any four.
Topics and Subtopics in NCERT Solutions for Class 12 Chemistry Chapter 1 The Solid State: Section Name: Topic Name: 1: The Solid State: 1.1: ... 1.10 Calculate the efficiency of packing in case of a metal crystal for (i) simple cubic, (ii) body ... = 10-5 × 6.022 × 10 23 = 6.022 × 10 18 mol-1. Question 26. Explain the following with suitable ...
CBSE 12th Standard Chemistry Subject Solution Case Study Questions 2021. 12th Standard CBSE. Reg.No. : Chemistry. Time : 00:30:00 Hrs. Total Marks : 20. Read the passage given below and answer the following questions: The solubility of gases increases with increase of pressure. William Henry made a systematic investigation of the solubility of ...
CBSE Class 12 Chemistry Chapter-1 Important Questions - Free PDF Download. Important questions of chemistry class 12 chapter 1 are prepared by Vedantu experts. These questions are prepared for the students to get a clear conception of the topics. These also help the students in revision and practice for their upcoming tests.
Solid State Chemistry Class 12 Solution for Chapter 1: Question 1. The answers will provide the students with a detailed understanding of amorphous solids. The Solution also provides examples of solids whose constituent particles are of irregular shapes. This will help students to understand the topics easily. Class 12th Chemistry Chapter 1 ...
on September 2, 2023, 5:13 AM. NCERT Solutions for Class 12 Chemistry Chapter 1 Solutions in Hindi Medium and English Medium. Get here Exercises Questions and Intext Questions to view online or download in PDF format. These solutions are updated for new academic year 2024-25 for all boards using NCERT Books.
The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. NCERT solutions for Mathematics Chemistry Class 12 CBSE, Karnataka Board PUC 1 (The Solid State) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application ...
By Dr. Vikas Jasrotia December 9, 2020 No Comments. Case-Based Questions Chemistry. Solid State Passage - 1. Read the given passage and answer the questions that follow: A lattice can be generated by repeating a small portion called the unit cell. A crystal structure is made of atoms. In other words, the structure is an ordered array of atoms ...
QB365 Provides the updated CASE Study Questions for Class 12 , and also provide the detail solution for each and every case study questions . Case study questions are latest updated question pattern from NCERT, QB365 will helps to get more marks in Exams ... 2021 Chemistry chapter wise case study questions. ...
May 18, 2021 Physics Gurukul Leave a Comment on Case Study Questions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids. ... March 31, 2021 May 6, 2021 Physics Gurukul Leave a Comment on Case Study Questions for Class 12 Chemistry Chapter 8 The d- and f-Block Elements.
d) Is superimposable on its mirror image. Case Study Questions Haloalkanes and Haloarenes Class 12 Chemistry. 2. Read the passage given below and answer the following questions: The replacement of hydrogen atom in a hydrocarbon, aliphatic or aromatic results in the formation of haloalkanes and haloarenes respectively.
Case Study Questions for Class 9 Science Chapter 1 Matter in Our Surroundings. Here we are providing case study questions for class 9 science chapter 12 sound. Students are suggested to go through each and every case study questions for better understanding of the chapter. Case Study/Passage Based Questions: Question 1:
Ans. pV = nRT, Where, p = Osmotic pressure, n = Number of moles, V = Volume of solution in litre, R = Gas constant, T = Temperature. Case Study Question 1 on Solutions - Chapter 2 CBSE Class 12 Chemistry Case-based Questions Read the given passages and answer the questions that follow: The spontaneous flow of the solvent through a ...
Good morning!! Welcome!! Comment to let us know you are with us today
Case Study Question 1: Read the passage given below and answer the following questions: The properties of the solutions which depend only on the number of solute particles but not on the nature of the solute are called colligative properties. Relative lowering in vapour pressure is also an example ofcolligative properties.