Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character

Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

- Winter 2024 | VOL. 36, NO. 1 CURRENT ISSUE pp.A5-81
The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Case Study 1: A 55-Year-Old Woman With Progressive Cognitive, Perceptual, and Motor Impairments
- Scott M. McGinnis , M.D. ,
- Andrew M. Stern , M.D., Ph.D. ,
- Jared K. Woods , M.D., Ph.D. ,
- Matthew Torre , M.D. ,
- Mel B. Feany , M.D., Ph.D. ,
- Michael B. Miller , M.D., Ph.D. ,
- David A. Silbersweig , M.D. ,
- Seth A. Gale , M.D. ,
- Kirk R. Daffner , M.D.
Search for more papers by this author
CASE PRESENTATION
A 55-year-old right-handed woman presented with a 3-year history of cognitive changes. Early symptoms included mild forgetfulness—for example, forgetting where she left her purse or failing to remember to retrieve a take-out order her family placed—and word-finding difficulties. Problems with depth perception affected her ability to back her car out of the driveway. When descending stairs, she had to locate her feet visually in order to place them correctly, such that when she carried her dog and her view was obscured, she had difficulty managing this activity. She struggled to execute relatively simple tasks, such as inserting a plug into an outlet. She lost the ability to type on a keyboard, despite being able to move her fingers quickly. Her symptoms worsened progressively for 3 years, over which time she developed a sad mood and anxiety. She was laid off from work as a nurse administrator. Her family members assumed responsibility for paying her bills, and she ceased driving.
Her past medical history included high blood pressure, Hashimoto’s thyroiditis with thyroid peroxidase antibodies, remote history of migraine, and anxiety. Medications included mirtazapine, levothyroxine, calcium, and vitamin D. She had no history of smoking, drinking alcohol, or recreational drug use. There was no known family history of neurologic diseases.
What Are Diagnostic Considerations Based on the History? How Might a Clinical Examination Help to Narrow the Differential Diagnosis?
Insidious onset and gradual progression of cognitive symptoms over the course of several years raise concern for a neurodegenerative disorder. It is helpful to consider whether or not the presentation fits with a recognized neurodegenerative clinical syndrome, a judgment based principally on familiarity with syndromes and pattern recognition. Onset of symptoms before age 65 should prompt consideration of syndromes in the spectrum of frontotemporal dementia (FTD) and atypical (nonamnesic) presentations of Alzheimer’s disease (AD) ( 1 , 2 ). This patient’s symptoms reflect relatively prominent early dysfunction in visual-spatial processing and body schema, as might be observed in posterior cortical atrophy (PCA), although the history also includes mention of forgetfulness and word-retrieval difficulties. A chief goal of the cognitive examination would be to survey major domains of cognition—attention, executive functioning, memory, language, visual-spatial functioning, and higher somatosensory and motor functioning—to determine whether any domains stand out as more prominently affected. In addition to screening for evidence of focal signs, a neurological examination in this context should assess for evidence of parkinsonism or motor neuron disease, which can coexist with cognitive changes in neurodegenerative presentations.
The patient’s young age and history of Hashimoto’s thyroiditis might also prompt consideration of Hashimoto’s encephalopathy (HE; also known as steroid-responsive encephalopathy), associated with autoimmune thyroiditis. This syndrome is most likely attributable to an autoimmune or inflammatory process affecting the central nervous system. The time course of HE is usually more subacute and rapidly progressive or relapsing-remitting, as opposed to the gradual progression over months to years observed in the present case ( 3 ).
The patient’s mental status examination included the Montreal Cognitive Assessment (MoCA), a brief global screen of cognition ( 4 ), on which she scored 12/30. There was evidence of dysfunction across multiple cognitive domains ( Figure 1 ). She was fully oriented to location, day, month, year, and exact date. When asked to describe a complex scene from a picture in a magazine, she had great difficulty doing so, focusing on different details but having trouble directing her saccades to pertinent visual information. She likewise had problems directing her gaze to specified objects in the room and problems reaching in front of her to touch target objects in either visual field. In terms of other symptoms of higher order motor and somatosensory functioning, she had difficulty demonstrating previously learned actions—for example, positioning her hand correctly to pantomime holding a brush and combing her hair. She was confused about which side of her body was the left and which was the right. She had difficulty with mental calculations, even relatively simple ones such as “18 minus 12.” In addition, she had problems writing a sentence in terms of both grammar and the appropriate spacing of words and letters on the page.
FIGURE 1. Selected elements of a 55-year-old patient’s cognitive examination at presentation a
a BNT-15=Boston Naming Test (15-Item); MoCA=Montreal Cognitive Assessment.
On elementary neurologic examination she had symmetrically brisk reflexes, with spread. She walked steadily with a narrow base, but when asked to pass through a doorway she had difficulty finding her way through it and bumped into the door jamb. Her elemental neurological examination was otherwise normal, including but not limited to brisk, full-amplitude vertical eye movements, normal visual fields, no evidence of peripheral neuropathy, and no parkinsonian signs such as slowness of movement, tremor, or rigidity.
How Does the Examination Contribute to Our Understanding of Diagnostic Considerations? What Additional Tests or Studies Are Indicated?
The most prominent early symptoms and signs localize predominantly to the parietal association cortex: The patient has impairments in visual construction, ability to judge spatial relationships, ability to synthesize component parts of a visual scene into a coherent whole (simultanagnosia or asimultagnosia), impaired visually guided reaching for objects (optic ataxia), and most likely impaired ability to shift her visual attention so as to direct saccades to targets in her field of view (oculomotor apraxia or ocular apraxia). The last three signs constitute Bálint syndrome, which localizes to disruption of dorsal visual networks (i.e., dorsal stream) with key nodes in the posterior parietal and prefrontal cortices bilaterally ( 5 ). She has additional salient symptoms and signs suggesting left inferior parietal dysfunction, including ideomotor limb apraxia and elements of Gerstmann syndrome, which comprises dysgraphia, acalculia, left-right confusion, and finger agnosia ( 6 ). Information was not included about whether she was explicitly examined for finger agnosia, but elements of her presentation suggested a more generalized disruption of body schema (i.e., her representation of the position and configuration of her body in space). Her less prominent impairment in lexical-semantic retrieval evidenced by impaired confrontation naming and category fluency likely localizes to the language network in the left hemisphere. Her impairments in attention and executive functions have less localizing value but would plausibly arise in the context of frontoparietal network dysfunction. At this point, it is unclear whether her impairment in episodic memory mostly reflects encoding and activation versus a rapid rate of forgetting (storage), as occurs in temporolimbic amnesia. Regardless, it does not appear to be the most salient feature of her presentation.
This localization, presenting with insidious onset and gradual progression, is characteristic of a PCA syndrome. If we apply consensus clinical diagnostic criteria proposed by a working group of experts, we find that our patient has many of the representative features of early disturbance of visual functions plus or minus other cognitive functions mediated by the posterior cerebral cortex ( Table 1 ) ( 7 ). Some functions such as limb apraxia also occur in corticobasal syndrome (CBS), a clinical syndrome defined initially in association with corticobasal degeneration (CBD) neuropathology, a 4-repeat tauopathy characterized by achromatic ballooned neurons, neuropil threads, and astrocytic plaques. However, our patient lacks other suggestive features of CBS, including extrapyramidal motor dysfunction (e.g., limb rigidity, bradykinesia, dystonia), myoclonus, and alien limb phenomenon ( Table 1 ) ( 8 ).
a Consensus diagnostic criteria for posterior cortical atrophy per Crutch et al. ( 7 ) require at least three cognitive features and relative sparing of anterograde memory, speech-nonvisual language functions, executive functions, behavior, and personality. Diagnostic criteria for probable corticobasal syndrome per Armstrong et al. ( 8 ) require asymmetric presentation of at least two motor features and at least two higher cortical features. AD=Alzheimer’s disease; CBD=corticobasal degeneration; FDG-PET=[ 18 ]F-fluorodexoxyglucose positron emission tomography; JCD=Jakob-Creutzfeldt disease; LBD=Lewy body disease; PSP=progressive supranuclear palsy; SPECT=single-photon emission computed tomography; TDP=TDP–43 proteinopathy.
TABLE 1. Clinical features and neuropathological associations of posterior cortical atrophy and corticobasal syndrome a
In addition to a standard laboratory work-up for cognitive impairment, it is important to determine whether imaging of the brain provides evidence of neurodegeneration in a topographical distribution consistent with the clinical presentation. A first step in most cases would be to obtain an MRI of the brain that includes a high-resolution T 1 -weighted MRI sequence to assess potential atrophy, a T 2 /fluid-attenuated inversion recovery (FLAIR) sequence to assess the burden of vascular disease and rule out less likely etiological considerations (e.g., infection, autoimmune-inflammatory, neoplasm), a diffusion-weighted sequence to rule out subacute infarcts and prion disease (more pertinent to subacute or rapidly progressive cases), and a T 2 *-gradient echo or susceptibility weighted sequence to examine for microhemorrhages and superficial siderosis.
A lumbar puncture would serve two purposes. First, it would allow for the assessment of inflammation that might occur in HE, as approximately 80% of cases have some abnormality of CSF (i.e., elevated protein, lymphocytic pleiocytosis, or oligoclonal bands) ( 9 ). Second, in selected circumstances—particularly in cases with atypical nonamnesic clinical presentations or early-onset dementia in which AD is in the neuropathological differential diagnosis—we frequently pursue AD biomarkers of molecular neuropathology ( 10 , 11 ). This is most frequently accomplished with CSF analysis of amyloid-β-42, total tau, and phosphorylated tau levels. Amyloid positron emission tomography (PET) imaging, and most recently tau PET imaging, represent additional options that are approved by the U.S. Food and Drug Administration for clinical use. However, insurance often does not cover amyloid PET and currently does not reimburse tau PET imaging. [ 18 ]-F-fluorodeoxyglucose (FDG) PET and perfusion single-photon emission computed tomography imaging may provide indirect evidence for AD neuropathology via a pattern of hypometabolism or hypoperfusion involving the temporoparietal and posterior cingulate regions, though without molecular specificity. Pertinent to this case, a syndromic diagnosis of PCA is most commonly associated with underlying AD neuropathology—that is, plaques containing amyloid-β and neurofibrillary tangles containing tau ( 12 – 15 ).
The patient underwent MRI, demonstrating a minimal burden of T 2 /FLAIR hyperintensities and some degree of bilateral parietal volume loss with a left greater than right predominance ( Figure 2A ). There was relatively minimal medial temporal volume loss. Her basic laboratory work-up, including thyroid function, vitamin B 12 level, and treponemal antibody, was normal. She underwent a lumbar puncture; CSF studies revealed normal cell counts, protein, and glucose levels and low amyloid-β-42 levels at 165.9 pg/ml [>500 pg/ml] and elevated total and phosphorylated tau levels at 1,553 pg/ml [<350 pg/ml] and 200.4 pg/ml [<61 pg/ml], respectively.
FIGURE 2. MRI brain scan of the patient at presentation and 4 years later a
a Arrows denote regions of significant atrophy.
Considering This Additional Data, What Would Be an Appropriate Diagnostic Formulation?
For optimal clarity, we aim to provide a three-tiered approach to diagnosis comprising neurodegenerative clinical syndrome (e.g., primary amnesic, mixed amnesic and dysexecutive, primary progressive aphasia), level of severity (i.e., mild cognitive impairment; mild, moderate or severe dementia), and predicted underlying neuropathology (e.g., AD, Lewy body disease [LBD], frontotemporal lobar degeneration) ( 16 ). This approach avoids problematic conflations that cause confusion, for example when people equate AD with memory loss or dementia, whereas AD most strictly describes the neuropathology of plaques and tangles, regardless of the patient’s clinical symptoms and severity. This framework is important because there is never an exclusive, one-to-one correspondence between syndromic and neuropathological diagnosis. Syndromes arise from neurodegeneration that starts focally and progresses along the anatomical lines of large-scale brain networks that can be defined on the basis of both structural and functional connectivity, a concept detailed in the network degeneration hypothesis ( 17 ). It is important to note that neuropathologies defined on the basis of specific misfolded protein inclusions can target more than one large-scale network, and any given large-scale network can degenerate in association with more than one neuropathology.
The MRI results in this case support a syndromic diagnosis of PCA, with a posteriorly predominant pattern of atrophy. Given the patient’s loss of independent functioning in instrumental activities of daily living (ADLs), including driving and managing her finances, the patient would be characterized as having a dementia (also known as major neurocognitive disorder). The preservation of basic ADLs would suggest that the dementia was of mild severity. The CSF results provide supportive evidence for AD amyloid plaque and tau neurofibrillary tangle (NFT) neuropathology over other pathologies potentially associated with PCA syndrome (i.e., CBD, LBD, TDP-43 proteinopathy, and Jakob-Creutzfeldt disease) ( 13 , 14 ). The patient’s formulation would thus be best summarized as PCA at a level of mild dementia, likely associated with underlying AD neuropathology.
The patient’s symptoms progressed. One year after initial presentation, she had difficulty locating the buttons on her clothing or the food on her plate. Her word-finding difficulties worsened. Others observed stiffness of her right arm, a new symptom that was not present initially. She also had decreased ability using her right hand to hold everyday objects such as a comb, a brush, or a pen. On exam, she was noted to have rigidity of her right arm, impaired dexterity with her right hand for fine motor tasks, and a symmetrical tremor of the arms, apparent when holding objects or reaching. Her right hand would also intermittently assume a flexed, dystonic posture and would sometime move in complex ways without her having a sense of volitional control.
Four to 5 years after initial presentation, her functional status declined to the point where she was unable to feed, bathe, or dress herself. She was unable to follow simple instructions. She developed neuropsychiatric symptoms, including compulsive behaviors, anxiety, and apathy. Her right-sided motor symptoms progressed; she spent much of the time with her right arm flexed in abnormal postures or moving abnormally. She developed myoclonus of both arms. Her speech became slurred and monosyllabic. Her gait became less steady. She underwent a second MRI of the brain, demonstrating progressive bilateral atrophy involving the frontal and occipital lobes in addition to the parietal lobes and with more left > right asymmetry than was previously apparent ( Figure 2B ). Over time, she exhibited increasing weight loss. She was enrolled in hospice and ultimately passed away 8 years from the onset of symptoms.
Does Information About the Longitudinal Course of Her Illness Alter the Formulation About the Most Likely Underlying Neuropathological Process?
This patient developed clinical features characteristic of corticobasal syndrome over the longitudinal course of her disease. With time, it became apparent that she had lost volitional control over her right arm (characteristic of an alien limb phenomenon), and she developed signs more suggestive of basal ganglionic involvement (i.e., limb rigidity and possible dystonia). This presentation highlights the frequent overlap between neurodegenerative clinical syndromes; any given person may have elements of more than one syndrome, especially later in the course of a disease. In many instances, symptomatic features that are less prominent at presentation but evolve and progress can provide clues regarding the underlying neuropathological diagnosis. For example, a patient with primary progressive apraxia of speech or nonfluent-agrammatic primary progressive aphasia could develop the motor features of a progressive supranuclear palsy (PSP) clinical syndrome (e.g., supranuclear gaze impairment, axial rigidity, postural instability), which would suggest underlying PSP neuropathology (4-repeat tauopathy characterized by globose neurofibrillary tangles, tufted astrocytes, and oligodendroglial coiled bodies).
If CSF biomarker data were not suggestive of AD, the secondary elements of CBS would substantially increase the likelihood of underlying CBD neuropathology presenting with a PCA syndrome and evolving to a mixed PCA-CBS. But the CSF amyloid and tau levels are unambiguously suggestive of AD (i.e., very low amyloid-β-42 and very high p-tau levels), the neuropathology of which accounts for not only a vast majority of PCA presentations but also roughly a quarter of cases presenting with CBS ( 18 , 19 ). Thus, underlying AD appears most likely.
NEUROPATHOLOGY
On gross examination, the brain weighed 1,150 g, slightly less than the lower end of normal at 1,200 g. External examination demonstrated mild cortical atrophy with widening of the sulci, relatively symmetrical and uniform throughout the brain ( Figure 3A ). There was no evidence of atrophy of the brainstem or cerebellum. On cut sections, the hippocampus was mildly atrophic. The substantia nigra in the midbrain was intact, showing appropriate dark pigmentation as would be seen in a relatively normal brain. The remainder of the gross examination was unremarkable.
FIGURE 3. Mild cortical atrophy with posterior predominance and neurofibrillary tangles, granulovacuolar degeneration, and a Hirano body a
a Panel A shows the gross view of the brain, demonstrating mild cortical atrophy with posterior predominance (arrow). Panel B shows the hematoxylin and eosin of the hippocampus at high power, demonstrating neurofibrillary tangles, granulovacuolar degeneration, and a Hirano body.
Histological examination confirmed that the neurons in the substantia nigra were appropriately pigmented, with occasional extraneuronal neuromelanin and moderate neuronal loss. In the nucleus basalis of Meynert, NFTs were apparent on hematoxylin and eosin staining as dense fibrillar eosinophilic structures in the neuronal cytoplasm, confirmed by tau immunohistochemistry (IHC; Figure 4 ). Low-power examination of the hippocampus revealed neuronal loss in the subiculum and in Ammon’s horn, most pronounced in the cornu ammonis 1 (CA1) subfield, with a relatively intact neuronal population in the dentate gyrus. Higher power examination with hematoxylin and eosin demonstrated numerous NFTs, neurons exhibiting granulovacuolar degeneration, and Hirano bodies ( Figure 3B ). Tau IHC confirmed numerous NFTs in the CA1 region and the subiculum. Amyloid-β IHC demonstrated occasional amyloid plaques in this region, less abundant than tau pathology. An α-synuclein stain revealed scattered Lewy bodies in the hippocampus and in the amygdala.
FIGURE 4. Tau immunohistochemistry demonstrating neurofibrillary tangles (staining brown) in the nucleus basalis of Meynert, in the hippocampus, and in the cerebral cortex of the frontal, temporal, parietal, and occipital lobes
In the neocortex, tau IHC highlighted the extent of the NFTs, which were very prominent in all of the lobes from which sections were taken: frontal, temporal, parietal and occipital. Numerous plaques on amyloid-β stain were likewise present in all cortical regions examined. The tau pathology was confined to the gray matter, sparing white matter. There were no ballooned neurons and no astrocytic plaques—two findings one would expect to see in CBD ( Table 2 ).
a AD=Alzheimer’s disease; CBD=corticobasal degeneration; CBS=corticobasal syndrome; PCA=posterior cortical atrophy.
TABLE 2. Neuropathological features of this case compared with a case of corticobasal degeneration a
The case was designated by the neuropathology division as Alzheimer’s-type pathology, Braak stage V–VI (of VI), due to the widespread neocortical tau pathology, with LBD primarily in the limbic areas.
Our patient had AD neuropathology presenting atypically with a young age at onset (52 years old) and a predominantly visual-spatial and corticobasal syndrome as opposed to prominent amnesia. Syndromic diversity is a well-recognized phenomenon in AD. Nonamnesic presentations include not only PCA and CBS but also the logopenic variant of primary progressive aphasia and a behavioral-dysexecutive syndrome ( 20 ). Converging lines of evidence link the topographical distribution of NFTs with syndromic presentations and the pattern of hypometabolism and cortical atrophy. Neuropathological case reports and case series suggest that atypical AD syndromes arise in the setting of higher than normal densities of NFTs in networks subserving the functions compromised, including visual association areas in PCA-AD ( 21 ), the language network in PPA-AD ( 22 ), and frontal regions in behavioral-dysexecutive AD ( 23 ). In a large sample of close to 900 cases of pathologically diagnosed AD employing quantitative assessment of NFT density and distribution in selected neocortical and hippocampal regions, 25% of cases did not conform to a typical distribution of NFTs characterized in the Braak staging scheme ( 24 ). A subset of cases classified as hippocampal sparing with higher density of NFTs in the neocortex and lower density of NFTs in the hippocampus had a younger mean age at onset, higher frequency of atypical (nonamnesic) presentations, and more rapid rate of longitudinal decline than subsets defined as typical or limbic-predominant.
Tau PET, which detects the spatial distribution of fibrillary tau present in NFTs, has corroborated postmortem work in demonstrating distinct patterns of tracer uptake in different subtypes of AD defined by clinical symptoms and topographical distributions of atrophy ( 25 – 28 ). Amyloid PET, which detects the spatial distribution of fibrillar amyloid- β found in amyloid plaques, does not distinguish between typical and atypical AD ( 29 , 30 ). In a longitudinal study of 32 patients at early symptomatic stages of AD, the baseline topography of tau PET signal predicted subsequent atrophy on MRI at the single patient level, independent of baseline cortical thickness ( 31 ). This correlation was strongest in early-onset AD patients, who also tended to have higher tau signal and more rapid progression of atrophy than late-onset AD patients.
Differential vulnerability of selected large-scale brain networks in AD and in neurodegenerative disease more broadly remains poorly understood. There is evidence to support multiple mechanisms that are not mutually exclusive, including metabolic stress to key network nodes, trophic failure, transneuronal spread of pathological proteins (i.e., prion-like mechanisms), and shared vulnerability within network regions based on genetic or developmental factors ( 32 ). In the case of AD, cortical hub regions with high intrinsic functional connectivity to other regions across the brain appear to have high metabolic rates across the lifespan and to be foci of convergence of amyloid-β and tau accumulation ( 33 , 34 ). Tau NFT pathology appears to spread temporally along connected networks within the brain ( 35 ). Patients with primary progressive aphasia are more likely to have a personal or family history of developmental language-based learning disability ( 36 ), and patients with PCA are more likely to have a personal history of mathematical or visuospatial learning disability ( 37 ).
This case highlights the symptomatic heterogeneity in AD and the value of a three-tiered approach to diagnostic formulation in neurodegenerative presentations. It is important to remember that not all AD presents with amnesia and that early-onset AD tends to be more atypical and to progress more rapidly than late-onset AD. Multiple lines of evidence support a relationship between the burden and topographical distribution of tau NFT neuropathology and clinical symptomatology in AD, instantiating network-based neurodegeneration via mechanisms under ongoing investigation.
The authors report no financial relationships with commercial interests.
Supported by NIH grants K08 AG065502 (to Dr. Miller) and T32 HL007627 (to Dr. Miller).
The authors have confirmed that details of the case have been disguised to protect patient privacy.
1 Balasa M, Gelpi E, Antonell A, et al. : Clinical features and APOE genotype of pathologically proven early-onset Alzheimer disease . Neurology 2011 ; 76:1720–1725 Crossref , Medline , Google Scholar
2 Mercy L, Hodges JR, Dawson K, et al. : Incidence of early-onset dementias in Cambridgeshire, United Kingdom . Neurology 2008 ; 71:1496–1499 Crossref , Medline , Google Scholar
3 Kothbauer-Margreiter I, Sturzenegger M, Komor J, et al. : Encephalopathy associated with Hashimoto thyroiditis: diagnosis and treatment . J Neurol 1996 ; 243:585–593 Crossref , Medline , Google Scholar
4 Nasreddine ZS, Phillips NA, Bédirian V, et al. : The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment . J Am Geriatr Soc 2005 ; 53:695–699 Crossref , Medline , Google Scholar
5 Rizzo M, Vecera SP : Psychoanatomical substrates of Bálint’s syndrome . J Neurol Neurosurg Psychiatry 2002 ; 72:162–178 Crossref , Medline , Google Scholar
6 Rusconi E : Gerstmann syndrome: historic and current perspectives . Handb Clin Neurol 2018 ; 151:395–411 Crossref , Medline , Google Scholar
7 Crutch SJ, Schott JM, Rabinovici GD, et al. : Consensus classification of posterior cortical atrophy . Alzheimers Dement 2017 ; 13:870–884 Crossref , Medline , Google Scholar
8 Armstrong MJ, Litvan I, Lang AE, et al. : Criteria for the diagnosis of corticobasal degeneration . Neurology 2013 ; 80:496–503 Crossref , Medline , Google Scholar
9 Marshall GA, Doyle JJ : Long-term treatment of Hashimoto’s encephalopathy . J Neuropsychiatry Clin Neurosci 2006 ; 18:14–20 Link , Google Scholar
10 Johnson KA, Minoshima S, Bohnen NI, et al. : Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association . Alzheimers Dement 2013 ; 9:e-1–e-16 Crossref , Medline , Google Scholar
11 Shaw LM, Arias J, Blennow K, et al. : Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer’s disease . Alzheimers Dement 2018 ; 14:1505–1521 Crossref , Medline , Google Scholar
12 Alladi S, Xuereb J, Bak T, et al. : Focal cortical presentations of Alzheimer’s disease . Brain 2007 ; 130:2636–2645 Crossref , Medline , Google Scholar
13 Renner JA, Burns JM, Hou CE, et al. : Progressive posterior cortical dysfunction: a clinicopathologic series . Neurology 2004 ; 63:1175–1180 Crossref , Medline , Google Scholar
14 Tang-Wai DF, Graff-Radford NR, Boeve BF, et al. : Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy . Neurology 2004 ; 63:1168–1174 Crossref , Medline , Google Scholar
15 Victoroff J, Ross GW, Benson DF, et al. : Posterior cortical atrophy: neuropathologic correlations . Arch Neurol 1994 ; 51:269–274 Crossref , Medline , Google Scholar
16 Dickerson BC, McGinnis SM, Xia C, et al. : Approach to atypical Alzheimer’s disease and case studies of the major subtypes . CNS Spectr 2017 ; 22:439–449 Crossref , Medline , Google Scholar
17 Seeley WW, Crawford RK, Zhou J, et al. : Neurodegenerative diseases target large-scale human brain networks . Neuron 2009 ; 62:42–52 Crossref , Medline , Google Scholar
18 Lee SE, Rabinovici GD, Mayo MC, et al. : Clinicopathological correlations in corticobasal degeneration . Ann Neurol 2011 ; 70:327–340 Crossref , Medline , Google Scholar
19 Whitwell JL, Jack CR Jr, Boeve BF, et al. : Imaging correlates of pathology in corticobasal syndrome . Neurology 2010 ; 75:1879–1887 Crossref , Medline , Google Scholar
20 Warren JD, Fletcher PD, Golden HL : The paradox of syndromic diversity in Alzheimer disease . Nat Rev Neurol 2012 ; 8:451–464 Crossref , Medline , Google Scholar
21 Hof PR, Archin N, Osmand AP, et al. : Posterior cortical atrophy in Alzheimer’s disease: analysis of a new case and re-evaluation of a historical report . Acta Neuropathol 1993 ; 86:215–223 Crossref , Medline , Google Scholar
22 Mesulam MM, Weintraub S, Rogalski EJ, et al. : Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia . Brain 2014 ; 137:1176–1192 Crossref , Medline , Google Scholar
23 Blennerhassett R, Lillo P, Halliday GM, et al. : Distribution of pathology in frontal variant Alzheimer’s disease . J Alzheimers Dis 2014 ; 39:63–70 Crossref , Medline , Google Scholar
24 Murray ME, Graff-Radford NR, Ross OA, et al. : Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study . Lancet Neurol 2011 ; 10:785–796 Crossref , Medline , Google Scholar
25 Ossenkoppele R, Lyoo CH, Sudre CH, et al. : Distinct tau PET patterns in atrophy-defined subtypes of Alzheimer’s disease . Alzheimers Dement 2020 ; 16:335–344 Crossref , Medline , Google Scholar
26 Phillips JS, Das SR, McMillan CT, et al. : Tau PET imaging predicts cognition in atypical variants of Alzheimer’s disease . Hum Brain Mapp 2018 ; 39:691–708 Crossref , Medline , Google Scholar
27 Tetzloff KA, Graff-Radford J, Martin PR, et al. : Regional distribution, asymmetry, and clinical correlates of tau uptake on [18F]AV-1451 PET in atypical Alzheimer’s disease . J Alzheimers Dis 2018 ; 62:1713–1724 Crossref , Medline , Google Scholar
28 Xia C, Makaretz SJ, Caso C, et al. : Association of in vivo [18F]AV-1451 tau PET imaging results with cortical atrophy and symptoms in typical and atypical Alzheimer disease . JAMA Neurol 2017 ; 74:427–436 Crossref , Medline , Google Scholar
29 Formaglio M, Costes N, Seguin J, et al. : In vivo demonstration of amyloid burden in posterior cortical atrophy: a case series with PET and CSF findings . J Neurol 2011 ; 258:1841–1851 Crossref , Medline , Google Scholar
30 Lehmann M, Ghosh PM, Madison C, et al. : Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease . Brain 2013 ; 136:844–858 Crossref , Medline , Google Scholar
31 La Joie R, Visani AV, Baker SL, et al. : Prospective longitudinal atrophy in Alzheimer’s disease correlates with the intensity and topography of baseline tau-PET . Sci Transl Med 2020 ; 12:12 Crossref , Google Scholar
32 Zhou J, Gennatas ED, Kramer JH, et al. : Predicting regional neurodegeneration from the healthy brain functional connectome . Neuron 2012 ; 73:1216–1227 Crossref , Medline , Google Scholar
33 Buckner RL, Sepulcre J, Talukdar T, et al. : Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease . J Neurosci 2009 ; 29:1860–1873 Crossref , Medline , Google Scholar
34 Hoenig MC, Bischof GN, Seemiller J, et al. : Networks of tau distribution in Alzheimer’s disease . Brain 2018 ; 141:568–581 Crossref , Medline , Google Scholar
35 Liu L, Drouet V, Wu JW, et al. : Trans-synaptic spread of tau pathology in vivo . PLoS One 2012 ; 7:e31302 Crossref , Medline , Google Scholar
36 Rogalski E, Johnson N, Weintraub S, et al. : Increased frequency of learning disability in patients with primary progressive aphasia and their first-degree relatives . Arch Neurol 2008 ; 65:244–248 Crossref , Medline , Google Scholar
37 Miller ZA, Rosenberg L, Santos-Santos MA, et al. : Prevalence of mathematical and visuospatial learning disabilities in patients with posterior cortical atrophy . JAMA Neurol 2018 ; 75:728–737 Crossref , Medline , Google Scholar
- Jeffrey Maneval , M.D. ,
- Kirk R. Daffner , M.D. ,
- Scott M. McGinnis , M.D.
- Seth A. Gale , M.A., M.D. ,
- C. Alan Anderson , M.D. ,
- David B. Arciniegas , M.D.

- Posterior Cortical Atrophy
- Corticobasal Syndrome
- Atypical Alzheimer Disease
- Network Degeneration
Call our 24 hours, seven days a week helpline at 800.272.3900

- Professionals

- Younger/Early-Onset Alzheimer's
- Is Alzheimer's Genetic?
- Women and Alzheimer's
- Creutzfeldt-Jakob Disease
- Dementia with Lewy Bodies
- Down Syndrome & Alzheimer's
- Frontotemporal Dementia
- Huntington's Disease
- Mixed Dementia
- Normal Pressure Hydrocephalus
- Posterior Cortical Atrophy
- Parkinson's Disease Dementia
- Vascular Dementia
- Korsakoff Syndrome
- Traumatic Brain Injury (TBI)
- Know the 10 Signs
- Difference Between Alzheimer's & Dementia
- 10 Steps to Approach Memory Concerns in Others
- Medical Tests for Diagnosing Alzheimer's
- Why Get Checked?
- Visiting Your Doctor
- Life After Diagnosis
- Stages of Alzheimer's
- Earlier Diagnosis
- Part the Cloud
- Research Momentum
- Our Commitment to Research
- TrialMatch: Find a Clinical Trial
- What Are Clinical Trials?
- How Clinical Trials Work
- When Clinical Trials End
- Why Participate?
- Talk to Your Doctor
- Clinical Trials: Myths vs. Facts
- Can Alzheimer's Disease Be Prevented?
- Brain Donation
- Navigating Treatment Options
- Lecanemab Approved for Treatment of Early Alzheimer's Disease
- Aducanumab Discontinued as Alzheimer's Treatment
- Medicare Treatment Coverage
- Donanemab for Treatment of Early Alzheimer's Disease — News Pending FDA Review
- Questions for Your Doctor
- Medications for Memory, Cognition and Dementia-Related Behaviors
- Treatments for Behavior
- Treatments for Sleep Changes
- Alternative Treatments
- Facts and Figures
- Assessing Symptoms and Seeking Help
- Now is the Best Time to Talk about Alzheimer's Together
- Get Educated
- Just Diagnosed
- Sharing Your Diagnosis
- Changes in Relationships
- If You Live Alone
- Treatments and Research
- Legal Planning
- Financial Planning
- Building a Care Team
- End-of-Life Planning
- Programs and Support
- Overcoming Stigma
- Younger-Onset Alzheimer's
- Taking Care of Yourself
- Reducing Stress
- Tips for Daily Life
- Helping Family and Friends
- Leaving Your Legacy
- Live Well Online Resources
- Make a Difference
- Daily Care Plan
- Communication and Alzheimer's
- Food and Eating
- Art and Music
- Incontinence
- Dressing and Grooming
- Dental Care
- Working With the Doctor
- Medication Safety
- Accepting the Diagnosis
- Early-Stage Caregiving
- Middle-Stage Caregiving
- Late-Stage Caregiving
- Aggression and Anger
- Anxiety and Agitation
- Hallucinations
- Memory Loss and Confusion
- Sleep Issues and Sundowning
- Suspicions and Delusions
- In-Home Care
- Adult Day Centers
- Long-Term Care
- Respite Care
- Hospice Care
- Choosing Care Providers
- Finding a Memory Care-Certified Nursing Home or Assisted Living Community
- Changing Care Providers
- Working with Care Providers
- Creating Your Care Team
- Long-Distance Caregiving
- Community Resource Finder
- Be a Healthy Caregiver
- Caregiver Stress
- Caregiver Stress Check
- Caregiver Depression
- Changes to Your Relationship
- Grief and Loss as Alzheimer's Progresses
- Home Safety
- Dementia and Driving
- Technology 101
- Preparing for Emergencies
- Managing Money Online Program
- Planning for Care Costs
- Paying for Care
- Health Care Appeals for People with Alzheimer's and Other Dementias
- Social Security Disability
- Medicare Part D Benefits
- Tax Deductions and Credits
- Planning Ahead for Legal Matters
- Legal Documents
- ALZ Talks Virtual Events
- ALZNavigator™
- Veterans and Dementia
- The Knight Family Dementia Care Coordination Initiative
- Online Tools
- Asian Americans and Pacific Islanders and Alzheimer's
- Native Americans and Alzheimer's
- Black Americans and Alzheimer's
- Hispanic Americans and Alzheimer's
- LGBTQ+ Community Resources for Dementia
- Educational Programs and Dementia Care Resources
- Brain Facts
- 50 Activities
- For Parents and Teachers
- Resolving Family Conflicts
- Holiday Gift Guide for Caregivers and People Living with Dementia
- Trajectory Report
- Resource Lists
- Search Databases
- Publications
- Favorite Links
- 10 Healthy Habits for Your Brain
- Stay Physically Active
- Adopt a Healthy Diet
- Stay Mentally and Socially Active
- Online Community
- Support Groups
- Find Your Local Chapter
- Any Given Moment
- New IDEAS Study
- RFI Amyloid PET Depletion Following Treatment
- Bruce T. Lamb, Ph.D., Chair
- Christopher van Dyck, M.D.
- Cynthia Lemere, Ph.D.
- David Knopman, M.D.
- Lee A. Jennings, M.D. MSHS
- Karen Bell, M.D.
- Lea Grinberg, M.D., Ph.D.
- Malú Tansey, Ph.D.
- Mary Sano, Ph.D.
- Oscar Lopez, M.D.
- Suzanne Craft, Ph.D.
- About Our Grants
- Andrew Kiselica, Ph.D., ABPP-CN
- Arjun Masurkar, M.D., Ph.D.
- Benjamin Combs, Ph.D.
- Charles DeCarli, M.D.
- Damian Holsinger, Ph.D.
- David Soleimani-Meigooni, Ph.D.
- Donna M. Wilcock, Ph.D.
- Elizabeth Head, M.A, Ph.D.
- Fan Fan, M.D.
- Fayron Epps, Ph.D., R.N.
- Ganesh Babulal, Ph.D., OTD
- Hui Zheng, Ph.D.
- Jason D. Flatt, Ph.D., MPH
- Jennifer Manly, Ph.D.
- Joanna Jankowsky, Ph.D.
- Luis Medina, Ph.D.
- Marcello D’Amelio, Ph.D.
- Marcia N. Gordon, Ph.D.
- Margaret Pericak-Vance, Ph.D.
- María Llorens-Martín, Ph.D.
- Nancy Hodgson, Ph.D.
- Shana D. Stites, Psy.D., M.A., M.S.
- Walter Swardfager, Ph.D.
- ALZ WW-FNFP Grant
- Capacity Building in International Dementia Research Program
- ISTAART IGPCC
- Alzheimer’s Disease Strategic Fund: Endolysosomal Activity in Alzheimer’s (E2A) Grant Program
- Imaging Research in Alzheimer’s and Other Neurodegenerative Diseases
- Zenith Fellow Awards
- National Academy of Neuropsychology & Alzheimer’s Association Funding Opportunity
- Part the Cloud-Gates Partnership Grant Program: Bioenergetics and Inflammation
- Pilot Awards for Global Brain Health Leaders (Invitation Only)
- Robert W. Katzman, M.D., Clinical Research Training Scholarship
- Funded Studies
- How to Apply
- Portfolio Summaries
- Supporting Research in Health Disparities, Policy and Ethics in Alzheimer’s Disease and Dementia Research (HPE-ADRD)
- Diagnostic Criteria & Guidelines
- Annual Conference: AAIC
- Professional Society: ISTAART
- Alzheimer's & Dementia
- Alzheimer's & Dementia: DADM
- Alzheimer's & Dementia: TRCI
- International Network to Study SARS-CoV-2 Impact on Behavior and Cognition
- Alzheimer’s Association Business Consortium (AABC)
- Global Biomarker Standardization Consortium (GBSC)
- Global Alzheimer’s Association Interactive Network
- International Alzheimer's Disease Research Portfolio
- Alzheimer’s Disease Neuroimaging Initiative Private Partner Scientific Board (ADNI-PPSB)
- Research Roundtable
- About WW-ADNI
- North American ADNI
- European ADNI
- Australia ADNI
- Taiwan ADNI
- Argentina ADNI
- WW-ADNI Meetings
- Submit Study
- RFI Inclusive Language Guide
- Scientific Conferences
- AUC for Amyloid and Tau PET Imaging
- Make a Donation
- Walk to End Alzheimer's
- The Longest Day
- RivALZ to End ALZ
- Ride to End ALZ
- Tribute Pages
- Gift Options to Meet Your Goals
- Founders Society
- Fred Bernhardt
- Anjanette Kichline
- Lori A. Jacobson
- Pam and Bill Russell
- Gina Adelman
- Franz and Christa Boetsch
- Adrienne Edelstein
- For Professional Advisors
- Free Planning Guides
- Contact the Planned Giving Staff
- Workplace Giving
- Do Good to End ALZ
- Donate a Vehicle
- Donate Stock
- Donate Cryptocurrency
- Donate Gold & Sterling Silver
- Donor-Advised Funds
- Use of Funds
- Giving Societies
- Why We Advocate
- Ambassador Program
- About the Alzheimer’s Impact Movement
- Research Funding
- Improving Care
- Support for People Living With Dementia
- Public Policy Victories
- Planned Giving
- Community Educator
- Community Representative
- Support Group Facilitator or Mentor
- Faith Outreach Representative
- Early Stage Social Engagement Leaders
- Data Entry Volunteer
- Tech Support Volunteer
- Other Local Opportunities
- Visit the Program Volunteer Community to Learn More
- Become a Corporate Partner
- A Family Affair
- A Message from Elizabeth
- The Belin Family
- The Eliashar Family
- The Fremont Family
- The Freund Family
- Jeff and Randi Gillman
- Harold Matzner
- The Mendelson Family
- Patty and Arthur Newman
- The Ozer Family
- Salon Series
- No Shave November
- Other Philanthropic Activities
- Still Alice
- The Judy Fund E-blast Archive
- The Judy Fund in the News
- The Judy Fund Newsletter Archives
- Sigma Kappa Foundation
- Alpha Delta Kappa
- Parrot Heads in Paradise
- Tau Kappa Epsilon (TKE)
- Sigma Alpha Mu
- Alois Society Member Levels and Benefits
- Alois Society Member Resources
- Zenith Society
- Founder's Society
- Joel Berman
- JR and Emily Paterakis
- Legal Industry Leadership Council
- Accounting Industry Leadership Council
Find Local Resources
Let us connect you to professionals and support options near you. Please select an option below:
Use Current Location Use Map Selector
Search Alzheimer’s Association
- Models of Care Case Studies
The Alzheimer’s Association is committed to connecting clinicians to effective, evidence-based models of care that can be replicated in community settings. Two of these models — the UCLA Alzheimer’s and Dementia Care program and the Age-Friendly Health Systems initiative — are detailed below.
UCLA Alzheimer's and Dementia Care program Age-Friendly Health Systems initiative UCLA Alzheimer’s and Dementia Care program
A dementia-specific model of care that significantly improved the experience for caregivers and people living with the disease.
About the program
The Alzheimer’s Association has partnered with UCLA to replicate the UCLA Alzheimer’s and Dementia Care (ADC) program through a grant from the John A. Hartford Foundation. The program follows a co-management model within the UCLA health system and partners with community-based organizations (CBOs) to provide comprehensive, coordinated, individualized care for people living with Alzheimer’s disease and other dementias.
The goals of the program are to:
- Maximize function, independence and dignity for people living with dementia.
- Minimize caregiver strain and burnout.
- Reduce unnecessary costs through improved care.
To qualify for the program, participants must have a diagnosis of dementia and live outside of a nursing home. The mean age of the first program participants was 82 years old. Almost all of the caregivers were the children (59%) or spouses (41%) of individuals living with Alzheimer’s or other dementias.
Comprehensive care
The ADC program utilizes a co-management model in which a nurse practitioner Dementia Care Specialist (DCS) partners with the participant’s primary care doctor to develop and implement a personalized care plan. The DCS provides support via four key components:
- Conducting in-person needs assessments of individuals living with Alzheimer’s and their caregivers.
- Creating and implementing individualized dementia care plans.
- Monitoring and revising care plans, as needed.
- Providing access 24/7, 365 days a year for assistance and advice to help avoid Emergency Department (ED) visits and hospitalizations.
Community resources
The ADC program also connects caregivers with resources provided by CBOs, including:
- Adult day care.
- Counseling.
- Case management.
- Legal and financial advice.
- Workforce development focused on training families and caregivers.
Program effectiveness
At one year, the quality of care provided by the program as measured by nationally accepted quality measures for dementia was exceedingly high — 92% compared to a benchmark of 38%. As a result, the improvements experienced by both caregivers and patients were significant:
- Ninety-four percent of caregivers felt that their role was supported.
- Ninety-two percent would recommend the program to others.
- Confidence in handling problems and complications of Alzheimer’s and other dementias improved by 79%.
- Caregiver distress related to behavioral symptoms, depression scores and strain improved by 31%, 24% and 15%, respectively.
- Despite disease progression, behavioral symptoms like agitation, irritability, apathy and nighttime behaviors in people living with dementia improved by 22%.
- Depressive symptoms experienced by individuals living with the disease were reduced by 34%.
Cost benefits of the program
An external evaluator compared utilization and cost outcomes and determined that over the course of 3 1/2 years, participants in UCLA’s program had lower total Medicare costs of care ($2,404 per year) relative to those receiving usual care.
In addition to cost savings for individuals and their families, the ADC program reports several financial benefits for health systems, including:
- Hospitalizations: 12% reduction
- ED visits: 20% reduction
- ICU stays: 21% reduction
- Hospital days: 26% reduction
- Hospice in last six months: 60% increase
- Nursing home placement: 40% reduction
UCLA finds that a care program following the ADC model may be able to pay for itself depending on local labor costs, comprehensiveness of billing and local overhead applied to clinical revenue.
To learn more or to contact UCLA about training and replication of the program, visit the UCLA Alzheimer’s and Dementia Care Program website.
Age-Friendly Health Systems initiative
A model of care that incorporates person-centered dementia care into a broader framework for the care of older adults.
About the initiative
Age-Friendly Health Systems is an initiative of The John A. Hartford Foundation and the Institute for Healthcare Improvement (IHI) in partnership with the American Hospital Association (AHA) and the Catholic Health Association of the United States (CHA). Together in 2017, they set a bold vision to build a social movement so all care with older adults is age-friendly care, that:
- Follows an essential set of evidence-based practices.
- Causes no harm.
- Aligns with “What Matters” to the older adult and their family caregivers.
The Age-Friendly Health Systems initiative defines “What Matters” as knowing and aligning care with each older adult’s specific health outcome goals and care preferences including, but not limited to, end-of-life care, and across settings of care.
- Health outcome goals relate to the values and activities that matter most to an individual, help motivate the individual to sustain and improve health, and could be impacted by a decline in health — for example, babysitting a grandchild, walking with friends in the morning, or volunteering in the community. When identified in a specific, actionable, and reliable manner, patients’ health outcome goals can guide decision making.
- Care preferences include the health care activities (e.g., medications, self-management tasks, health care visits, testing, and procedures) that patients are willing and able (or not willing or able) to do or receive.
The 4Ms framework of an Age-Friendly Health System
The 4Ms are not a program, but a framework to guide how care is provided to older adults through every interaction with a health system’s care and services. The 4Ms — What Matters, Medication, Mentation, and Mobility — make the complex care of older adults more manageable because they:
- Identify the core issues that should drive all care and decision making with the care of older adults.
- Organize care and focus on the older adult’s wellness and strengths rather than solely on disease.
- Are relevant regardless of an older adult’s individual disease(s).
- Apply regardless of the number of functional problems an older adult may have, or that person’s cultural, ethnic or religious background.
The 4Ms framework is most effective when all 4Ms are implemented together and are practiced reliably (i.e., for all older adults, in all settings and across settings, in every interaction).
The intention is to incorporate the 4Ms into existing care — rather than layering them on top —to organize the efficient delivery of effective care. This is achieved primarily through redeploying existing health system resources. Many health systems have found they already provide care aligned with one or more of the 4Ms for many of their older adult patients. Much of the effort, then, is to incorporate the other elements and organize care so all 4Ms guide every encounter with an older adult and their family caregivers.
Cost benefits of the initiative
The business case for becoming an Age-Friendly Health System focuses on its financial returns and is stronger when:
- The financial benefits are captured by the health system that is making the investment.
- Utilization and associated expenses of “usual” care are especially burdensome.
- The health system is effective in mitigating those costs.
- The added expense of becoming age-friendly is lower.
See the IHI report, The Business Case for Becoming an Age-Friendly Health System , for guidance on how to make the business case for your health system.
To learn more or to contact IHI about joining the initiative, visit the IHI Age-Friendly Health Systems website.
Health Systems and Medical Professionals Menu
- Alzheimer’s Association Innovation Roundtable (AAIR)
- Prescribing Lecanemab
- Cognitive Screening and Assessment
- Dementia Care Navigation Guiding Principles
- Dementia Care Navigation Roundtable
- Differential Diagnosis of Alzheimer's Disease
- Differential Diagnosis of Creutzfeldt-Jakob Disease
- Differential Diagnosis of Lewy Body Dementia
- Differential Diagnosis of Frontotemporal Dementia
- Differential Diagnosis of Huntington's Disease
- Differential Diagnosis of Korsakoff Syndrome
- Differential Diagnosis of Mixed Dementia
- Differential Diagnosis of Normal Pressure Hydrocephalus
- Differential Diagnosis of Parkinson's Disease Dementia
- Differential Diagnosis of Vascular Dementia
- Disclosure of Diagnosis
- Advanced Imaging and Biomarkers
- Care Planning Visit
- Advanced Care Planning
- Alzheimer's Network (ALZ-NET)
- Guidelines Index
- Clinical Trials Recruiting
- Downloadable Resources for Patients and Caregivers
- I Have Alzheimer's
- Clinical Trials
- Instructional Videos
- Cognitive Assessment Tools
- ISTAART Membership
- Alzheimer's & Dementia Journal
- Continuing Education on Alzheimer's and Dementia
- The Alzheimer’s and Dementia Care ECHO® Program for Health Systems and Medical Professionals
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

When will patients see personalized cancer vaccines?

A molecular ‘warhead’ against disease

Asking the internet about birth control
The brain that defied alzheimer’s.
Genetic mutation found that could shed light on mechanism for disease resistance, lead to new therapies
Neil Osterweil
MGH News and Public Affairs
Illustration by Roy Scott
Aliria Rosa Piedrahita de Villegas should have developed Alzheimer’s disease in her 40s and died from the disease in her 60s because of a rare genetic mutation.
Instead, she lived dementia-free into her 70s, and now her brain is yielding important clues about the pathology of dementia and possible treatments for Alzheimer’s disease.
As researchers at Massachusetts General Hospital and other centers first described in 2019, the woman, from Medellin, Colombia, was a member of an extended family with a mutation in a gene labeled PSEN1. The PSEN1 E280A mutation is autosomal dominant, meaning that only a single copy of the gene is required to cause disease. Carriers of the mutation typically exhibit symptoms of Alzheimer’s in their 40s or 50s, and die from the disease soon after, but this woman did not begin to show signs of Alzheimer’s until her early 70s. She died in 2020 from metastatic melanoma at the age of 77.
The key difference in the Colombian woman’s ability to fend off the disease for three decades appeared to be that in addition to having the PSEN1 E280A mutation, she was also a carrier of both copies of a mutation known as APOE3 Christchurch.
“This exceptional case is an experiment designed by nature that teaches us a way to prevent Alzheimer’s: let’s observe, learn, and imitate nature.” Francisco Lopera, director of the Neuroscience Group of Antioquia in Medellín, Colombia.
The APOE family of genes control production of apolipoproteins, which transport lipids (fats) in blood and other bodily fluids. The APOE2 variant is known to be protective against Alzheimer’s dementia, while the APOE4 variant is linked to an increased risk for the disease.
APOE3, the most common variant, is not typically associated with either reduced or increased risk for Alzheimer’s.
“This is a ground-breaking case for Alzheimer’s disease and has already opened new paths for treatment and prevention, which we’re currently pursuing with some collaborators. This work is now bringing light into some of the mechanisms of resistance to Alzheimer’s disease” says investigator Yakeel T. Quiroz
Quiroz is director of the Multicultural Alzheimer Prevention Program (MAPP ) at Mass General, an associate professor of psychology at Harvard Medical School, and Paul B. and Sandra M. Edgerley MGH Research Scholar 2020-2025 .
As Quiroz and colleagues now report in the neuropathology journal Acta Neuropathologica , the woman did, in fact, have pathologic features of Alzheimer’s disease in her brain, but not in regions of the brain where the hallmarks of Alzheimer’s are typically found.
“This patient gave us a window into many competing forces — abnormal protein accumulation, inflammation, lipid metabolism, homeostatic mechanisms — that either promote or protect against disease progression, and begin to explain why some brain regions were spared while others were not,” says Justin Sanchez, co-first author, and an investigator at MGH Neurology.
Researchers identified in Aliria’s brain a distinct pattern of abnormal aggregation or “clumping” of tau, a protein known to be altered in Alzheimer’s disease and other neurologic disorders.
In this case, the tau pathology largely spared the frontal cortex, which is important for judgment and other “executive” functions, and the hippocampus, which is important for memory and learning. Instead, the tau pathology involved the occipital cortex, the area of the brain at the back of the head that controls visual perception.
The occipital cortex was the only major brain region to exhibit typical Alzheimer’s features, such as chronic inflammation of protective brain cells called microglia, and reduced levels of APOE expression.
“Thus, the Christchurch variant may impact the distribution of tau pathology, modulates age at onset, severity, progression, and clinical presentation of [autosomal dominant Alzheimer’s disease], suggesting possible therapeutic strategies,” the researchers write.
“It is seldom that we have nice surprises while studying familial Alzheimer’s disease brains. This case showed an amazingly clear protected phenotype. I am sure our molecular and pathologic findings will at least suggest some avenues of research and elicit hope for a successful treatment against this disorder.” says co-first author Diego Sepulveda-Falla, research lead at University Medical Center Hamburg-Eppendorf in Hamburg, Germany.
“This exceptional case is an experiment designed by nature that teaches us a way to prevent Alzheimer’s: let’s observe, learn, and imitate nature,” concludes Francisco Lopera, director of the Neuroscience Group of Antioquia in Medellín, Colombia. Lopera is a co-senior author and the neurologist who discovered this family and has been following them for the last 30 years.
Quiroz is a co-senior author of the report, along with Kenneth S. Kosik, University of California, Santa Barbara; Lopera, and Sepulveda-Falla. Sanchez contributed equally to the study.
The study was supported by grants from the National Institutes of Health, MGH Executive Committee on Research (MGH Research Scholar Award), Alzheimer’s Association, the Deutsche Forschungsgemeinschaft, Universidad de Antioquia, the Werner Otto Stiftung, and the Gernam Federal Ministiry of Education and Research.
Share this article
You might like.
Sooner than you may think, says researcher who recently won Sjöberg Prize for pioneering work in field

Approach attacks errant proteins at their roots

Only a fraction of it will come from an expert, researchers say
Forget ‘doomers.’ Warming can be stopped, top climate scientist says
Michael Mann points to prehistoric catastrophes, modern environmental victories
Yes, it’s exciting. Just don’t look at the sun.
Lab, telescope specialist details Harvard eclipse-viewing party, offers safety tips
How dating sites automate racism
Sociologist’s new book finds algorithms that suggest partners often reflect stereotypes, biases
Early onset Alzheimer's disease - a case study
Affiliations.
- 1 Katedra i Zakład Podstawowych Nauk Medycznych, Wydział Nauk o Zdrowiu w Bytomiu, Śląski Uniwersytet Medyczny w Katowicach.
- 2 Katedra i Klinika Neurologii, Wydział Lekarski z Oddziałem Lekarsko-Dentystycznym w Zabrzu, Śląski Uniwersytet Medyczny w Katowicach.
- 3 Górnośląskie Centrum Rehabilitacji Repty, Tarnowskie Góry.
- 4 Faculty of Public Health in Bytom, Medical University of Silesia in Katowice.
- 5 Instytut Psychologii, Wyższa Szkoła Humanitas w Sosnowcu.
- PMID: 34365482
- DOI: 10.12740/PP/OnlineFirst/114122
Dementia syndromes constitute problem not only for the elderly. Early-onset dementia (EOD) starts below the age of 65 years. It accounts for 4-10% of all cases of dementia. EOD has significant psychosocial consequences because it affects people in their most productive years of life, with numerous family, professional and social responsibilities. There are many diseases that have been identified as the cause of the EOD. Among them, the most common are Alzheimer's disease, vascular dementia, fronto-temporal dementia, Lewy body dementia, traumatic brain injury, alcohol related dementia, Huntington's disease, Parkinson's disease, mixed dementia, Creutzfeldt-Jakob disease and Down's syndrome. Most studies have demonstrated Alzheimer's disease as the most common etiology of EOD. The article presents the case of a 33-year-old patient hospitalized in the Department of Neurology in Zabrze, with cognitive dysfunction, speech disordersand featuresof Parkinson's extrapyramidal syndrome that have been progressing for about 15 months. The MR of the head revealed cortical and subcortical atrophy, especially in parietal and temporal lobes. The cerebrospinal fluid examination showed decreased level of β-amyloid and significantly elevated level of H-tau. The patient was diagnosed with early-onset Alzheimer's disease, which was confirmed by genetic testing - the sequence change was identified in the gene for presenilin 1 in a heterozygous system.
Keywords: Alzheimer’s disease; dementia; early-onset dementia.
Publication types
- Case Reports
- Alzheimer Disease*
- Cognitive Dysfunction*
- Lewy Body Disease*
- Parkinson Disease*
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Call for papers
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 34, Issue 1
- Case of early-onset Alzheimer’s disease with atypical manifestation
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0003-1224-4320 Lin Zhu 1 ,
- Limin Sun 2 ,
- Lin Sun 3 , 4 and
- Shifu Xiao 3 , 4
- 1 Department of Rehabilitation Medicine , Shanghai No.3 Rehabilitation Hospital , Shanghai , China
- 2 Department of Rehabilitation Medicine , Huashan Hospital Fudan University , Shanghai , China
- 3 Department of Geriatric Psychiatry , Shanghai Mental Health Center , Shanghai , China
- 4 Alzheimer's Disease and Related Disorders Center, Shanghai Jiao Tong University , Shanghai , China
- Correspondence to Professor Shifu Xiao; xiaoshifu{at}msn.com
Short-term memory decline is the typical clinical manifestation of Alzheimer’s disease (AD). However, early-onset AD usually has atypical symptoms and may get misdiagnosed. In the present case study, we reported a patient who experienced symptoms of memory loss with progressive non-fluent aphasia accompanied by gradual social withdrawal. He did not meet the diagnostic criteria of AD based on the clinical manifestation and brain MRI. However, his cerebrospinal fluid examination showed a decreased level of beta-amyloid 42, and increased total tau and phosphorylated tau. Massive amyloid β-protein deposition by 11C-Pittsburgh positron emission tomography confirmed the diagnosis of frontal variant AD. This case indicated that early-onset AD may have progressive non-fluent aphasia as the core manifestation. The combination of individual and precision diagnosis would be beneficial for similar cases.
- dual (psychiatry)
- cognition disorders
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/gpsych-2020-100283
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Introduction
Clinical report and methods.
Early-onset Alzheimer’s disease (EOAD), which comprises 5% of Alzheimer’s disease (AD), shows a 1.6-year average delay in diagnosis compared with late-onset AD. 1 2 The clinical phenotype of atypical EOAD is heterogeneous, and primary progressive aphasia (PPA) is rarely the initial manifestation of related dementia syndromes. Compared with the progressive non-fluent aphasia (PNFA) related to the language variant phenotype of frontotemporal lobar degeneration (FTLD), molecular imaging studies in patients with primary progressive aphasia suggest the pathological basis of AD. 3 Neurodegeneration uaually starts in a specific neural anatomic networks. The clinical phenotype of PPA can usually infer the type of protein degeneration, which can be used to infer gene mutation. With the development of biomarkers such as genetics, molecular biology, neuroimaging and positron emission tomography (PET), accurate diagnosis can be gradually achieved. In this case study, we describe an AD patient with PNFA as the first symptom.
The patient was a 63-year-old married man, a right-handed businessman, native of Shanghai, with 12 years of school education. He has memory loss and non-fluent speech for 7 years combined with personality changes for 5 years. The patient recovered from hepatitis A 32 years ago and has well-controlled hypertension for 30 years.
The patient’s caregiver described that the patient showed forgetfulness and developed poor pronunciation at the age of 56. His short-term memory has gradually declined as noticed that he repeatedly gave money to customers while selling clothes. He frequently forgot where he parked his bicycle, and it was hard for him to speak a full sentence; his language was vague and short. He was impatient when being asked to repeat a word. Over time, he could only say some single syllables. He evolved into fully aphasia gradually, and his personality also changed gradually. At the age of 59, he could not recognise himself in the mirror and he often hid his shoes because he was worried that they would be stolen. Therefore, his wife had accompanied him to see a neurologist. The physical and neurological examination revealed no remarkable signs. His brain MRI showed mild atrophy in the bilateral frontal lobe ( figure 1A at the age of 59). Fluorodeoxyglucose positron emission tomography (FDG-PET) revealed that glucose metabolism in the bilateral frontal and parietal lobe was declined, and the left side was significant ( figure 1B at the age of 59). The Mini-Mental State Examination (MMSE) score was 18 out of 30 (18/30). At that point, he was diagnosed with cognitive impairment and treated with rivastigmine. After the treatment, his memory improved slightly. In 2017, the neurologist gave him quetiapine and donepezil due to developing visual hallucinations and irritability. The second brain MRI scan revealed increased frontal and temporal atrophy compared with the first one ( figure 1C at the age of 61). The FDG-PET revealed that the cerebral cortical glucose metabolism was further reduced, especially the bilateral frontal and parietal lobes were obvious ( figure 1D at the age of 61).
- Download figure
- Open in new tab
- Download powerpoint
Brain imaging and cognitive score of the patient. (A) The patient’s MRI in May 2015 revealed mild atrophy of the bilateral frontal lobe (at the age of 59). (B) The patient’s FDG-PET in May 2015 revealed that glucose metabolism in the bilateral frontal and parietal lobe was reduced, and the left side was significant (at the age of 59). (C) The patient’s MRI in July 2017 (2 years after the first scan), revealed more atrophy of the bilateral frontal lobe and temporal lobe atrophy occurred (at the age of 61). (D) The patient’s FDG-PET in August 2017 revealed that the cerebral cortical glucose metabolism was reduced more, bilateral frontal and parietal lobes obvious in particular (at the age of 61). (E) The patient’s third MRI in May 2019 (2 years after the second scan) revealed atrophy of the whole cerebral cortex with bilateral frontal lobes, temporal lobe and hippocampus more affected (at the age of 63). (F) The patient’s 11C-PIB PET in May 2019 revealed saliently amyloid deposition in diffuse cortical areas, particularly in the bilateral frontal, parietal, temporal cortices and posterior cingulated gyrus (at the age of 63). (G) Mini-Mental State Examination (MMSE) of the patient. MMSE in May 2015 revealed a total score was 18/30 (at the age of 59). MMSE in May and December 2019 revealed a total score were 3/30 and 2/30; the results showed severe impairments in language and other cognitive areas (at the age of 63). 11C-PIB PET, 11C-Pittsburgh compound B positron emission tomography; FDG-PET, fluorodeoxyglucose positron emission tomography.
In May 2019, the patient’s symptoms aggravated further, which included bad temper, crying often and being more difficult to be looked after. His wife brought him to seek help from a psychiatrist, and he was admitted into the Department of Geriatric Psychiatry of Shanghai Mental Health Center. He underwent routine laboratory tests to exclude non-neurodegenerative and dementia. His neurological examination showed gait abnormality, negative Babinski’s sign, muscular tension hyperactivity, knee jerk reflex hyperactivity and a weak positive right palmar jaw reflex. The MMSE score was 3/30. The patient exhibited severe impairments in orientation (2/10), attention and calculation (1/5), recall (0/6), language (0/8) and visual construction (0/1). The Montreal Cognitive Assessment score was 0 (0/30), which was significantly lower than it was in 2015( figure 1G ). The third brain MRI demonstrated atrophy of the cerebral cortex, especially in the bilateral frontal lobes and hippocampus. The medial temporal lobe atrophy scale was at grade 3 ( figure 1E at the age of 63).
In addition, we tested three pathogenic genes for early-onset AD including amyloid precursor protein, presenilin-1, presenilin-2 genes related to neurocognitive disorders, but no mutation was found. Apolipoprotein E (APOE) genotyping showed APOE ε3/ε3 type. In order to reach a definite diagnosis, the patient underwent 11C-Pittsburgh compound B positron emission tomography (11C-PIB PET) and cerebrospinal fluid (CSF) examination. 11C-PIB PET revealed noticeable amyloid deposition in diffuse cortical areas, particularly in the bilateral frontal, parietal, temporal cortices and posterior cingulated gyrus ( figure 1F at the age of 63). The measured CSF biomarkers showed decreased amyloid β-protein (Aβ) 42 (462 pg/ml; cut-off >562 pg/ml), increased total tau (754 pg/ml; cut-off <370 pg/ml) and increased phosphorylated tau (87.40 pg/ml; cut-off <66.26 pg/ml). Eventually, the diagnosis of frontal variant EOAD was reconfirmed considering the early onset of dementia, the slow progression of symptoms, the absence of focal neurological damage signs and the exclusion of other systemic or brain diseases that could cause dementia. Due to the gastrointestinal adverse reactions of the patient, rivastigmine was suspended. We used memantine 10 mg b.i.d. and donepezil 5 mg q.d. to improve cognition and to control psychobehavioural symptoms and vortioxetine 10 mg q.d. to improve mood. After the treatment and follow-up for 7 months, the patient’s behaviour and mood was improvved significally, and his language expression improved slightly ( figure 1G at the age of 63).
The initial clinical manifestations of the patient included short-term memory decline, poor pronunciation and personality changes at an early stage, followed by behavioural and psychological symptoms of dementia, including hallucinations, delusions of theft, gradual decline in self-care as well as depression. The patient’s brain MRI initially showed mild atrophy of the bilateral frontal lobe. With the progress of the disease, more severe atrophy of the cerebral cortex, temporal lobe and hippocampus appeared besides the further atrophy of the bilateral frontal lobe. The atypical manifestation such as early aphasia, frontal lobe atrophy and personality changes can mislead clinicians in diagnosing frontotemporal lobar degeneration. This is the main reason leading to the misdiagnosis of this patient, which should be taken as a lesson or future reference for clinicians.
According to the current classification schemes, the clinical symptoms were in line with PNFA, which are halting speech by speech sound errors with spared content word comprehension and atrophy of the left frontal lobe. 4 PNFA is one of the primary progressive aphasias. 4 This patient met the diagnostic criteria of frontotemporal dementia, consistent with the early personality changes and cognitive abnormalities. 5 In the past 7 years, the patient’s speech fluency and cognitive function decreased continuously and rapidly. The clinical manifestations could not be explained by typical AD. The CSF phosphorylated tau was slightly higher, and no gene mutations associated with AD were found, which further made it harder to reach the diagnosis. However, the 11C-PIB PET showed heavy and extensive Aβ-amyloid depositions and provided definite pathological evidence of AD. A retrospective study found PNFA with 13%– 31% of cases might have the pathology of AD. 6 The patient met the research diagnostic AT(N) framework of AD, with A: (11C-PIB PET revealed amyloid depositions, CSF Aβ42 decreased), T: (CSF phosphorylated microtubule-associated protein tau increased) and N: (cortical atrophy on MRI, glucose hypometabolism in the bilateral frontal parietal lobe and CSF total microtubule-associated protein tau increased). 7 We use the AD pathological markers as the gold standard to exclude other types of dementia and reach an earlier and more accurate diagnosis. It’s worth pointing out that the patient might have mixed neuropathology. Santos-Santos 6 found that 75% of PNFA or PPA cases may have mixed pathological changes of FTLD and AD. This poses a new challenge for clinicians, suggesting that verified, reliable and accessible biomarkers for diagnosis of FTLD should be developed urgently. Otherwise, the comorbid pathological cases would only be accurately diagnosed after autopsy.
After reaching a clear diagnosis, and according to the China guidelines for the diagnosis and treatment of dementia and cognitive impairment in 2018 and the guidelines for the diagnosis and treatment of AD, 8 the patient was treated with cholinesterase inhibitors and excitatory amino acid receptor antagonists to enhance cognition, and antidepressants were given to relieve his mood. After the treatment, the patient’s symptoms were improved, and his mood was stable. Additionally, the biopsychosocial medical model has become more and more accepted. We should treat the patients with medication and non-drug intervention for patients and their caregivers. Spouses and caregivers of patients with early-onset dementia bear a greater burden and higher depression rates. 9 The speech impairments of this patient appeared early. He was emotionally unstable, grumpy and easy to be tearful, which was alleviated when his wife comforted him. Two weeks later, he was released from the hospital and continued to receive comprehensive rehabilitation treatments. Anyway, providing individualised psychosocial support for patients and their caregivers is very important for improving symptoms and quality of life. 10
Some of PNFAs are due to the underlying pathology of AD, which is more common in EOAD. In the present case, neither clinical examination nor MRI could definitively differentiate FTLD from EOAD. According to AT(N) research framework, we could eventually confirm the neuropathy diagnosis of AD or frontal-variant AD (fvAD), but the previous misdiagnoses were significant. FvAD can lead to social withdrawal and depression. These patients should benefit from accurate diagnosis, medication treatment and individualised psychosocial intervention.
- van Vliet D ,
- de Vugt ME ,
- Bakker C , et al
- Rabinovici GD ,
- Jagust WJ ,
- Furst AJ , et al
- Gorno-Tempini ML ,
- Hillis AE ,
- Weintraub S , et al
- McKhann GM ,
- Albert MS ,
- Grossman M , et al
- Santos-Santos MA ,
- Iaccarino L , et al
- Bennett DA ,
- Blennow K , et al
- Ducharme F ,
- Kergoat M-J ,
- Antoine P , et al
- Ferriero DM ,
- Frisoni GB , et al
Lin Zhu obtained a bachelor’s degree in clinical medicine from Shanghai Jiao Tong University School of Medicine, Shanghai, China in 2006. She is currently working as an attending doctor and psychotherapist at the Neurorehabilitation Department of Shanghai Third Rehabilitation Hospital. After completing clinical training, she started a two-year master program and was certified by the Institute of Psychology of the Chinese Academy of Sciences. In addition, she has also been trained and actively involved in clinical neurological research for half year in the Department of Geriatric Psychiatry of Shanghai Mental Health Center, Shanghai, China. Her main research interest includes the rehabilitation of elderly with psychiatric disorders.
Contributors LZ drafted the case report and manuscript; LMS performed the literature search; LS and SX supervised and revised the manuscript. All authors approved the final manuscript.
Funding This study was supported by a grant of Clinical Research Centre Project of Shanghai Mental Health Centre (CRC2017ZD02) and Scientific Research Program of Shanghai Jing an District Health Committee (2020MS16).
Competing interests None declared.
Patient consent for publication Parental/guardian consent obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Systematic Review
- Open access
- Published: 10 April 2024
Predictors for survival in patients with Alzheimer’s disease: a large comprehensive meta-analysis
- Xiaoting Zheng 1 ,
- Shichan Wang 1 ,
- Jingxuan Huang 1 ,
- Chunyu Li 1 &
- Huifang Shang ORCID: orcid.org/0000-0003-0947-1151 1
Translational Psychiatry volume 14 , Article number: 184 ( 2024 ) Cite this article
Metrics details
- Neuroscience
The prevalence of Alzheimer’s disease (AD) is increasing as the population ages, and patients with AD have a poor prognosis. However, knowledge on factors for predicting the survival of AD remains sparse. Here, we aimed to systematically explore predictors of AD survival. We searched the PubMed, Embase and Cochrane databases for relevant literature from inception to December 2022. Cohort and case-control studies were selected, and multivariable adjusted relative risks (RRs) were pooled by random-effects models. A total of 40,784 reports were identified, among which 64 studies involving 297,279 AD patients were included in the meta-analysis after filtering based on predetermined criteria. Four aspects, including demographic features ( n = 7), clinical features or comorbidities ( n = 13), rating scales ( n = 3) and biomarkers ( n = 3), were explored and 26 probable prognostic factors were finally investigated for AD survival. We observed that AD patients who had hyperlipidaemia (RR: 0.69) were at a lower risk of death. In contrast, male sex (RR: 1.53), movement disorders (including extrapyramidal signs) (RR: 1.60) and cancer (RR: 2.07) were detrimental to AD patient survival. However, our results did not support the involvement of education, hypertension, APOE genotype, Aβ 42 and t-tau in AD survival. Our study comprehensively summarized risk factors affecting survival in patients with AD, provided a better understanding on the role of different factors in the survival of AD from four dimensions, and paved the way for further research.
Introduction
Alzheimer’s disease (AD) is a chronic neurodegenerative disorder with progressive cognitive impairment and is the predominant form of dementia [ 1 , 2 ]. As of 2020, approximately 55 million people worldwide are living with dementia, and that number is predicted to reach 78 million by 2030 [ 3 ]. The mortality of AD increased by 29.28% from 1990 to 2019 with the increase in the aging population [ 4 , 5 ]. Moreover, AD and other dementias were the fourth cause of disability-adjusted life-years (DALYs) for those aged 75 years and older [ 5 ], leading to a tremendous burden on society and caregivers.
In the context of emerging treatments for preclinical AD, despite intensive research and development efforts to identify therapeutic drugs, there is still no effective strategy to stop progression due to insufficient knowledge of the etiology of AD [ 6 ]. Under these circumstances, focusing on influential factors potentiating AD progression since diagnosis is critical for neurologists and patients’ families. Great efforts have been made [ 7 , 8 , 9 , 10 ] to determine predictive factors for survival of AD, and a number of predictors that may worsen the disease prognosis have been identified. Prognostic factors such as age at diagnosis, underweight, extrapyramidal signs (EPS) and psychosis, and history of vascular or heart disease appear to be key players in the progression of AD [ 7 , 8 , 10 , 11 , 12 , 13 , 14 ]. Nutritional status was found to be the exact predictor of an unfavorable course, which was suggested to therefore form part of the clinical evaluation [ 15 ]. One study proposed that combination therapies targeting AD pathophysiology and vascular risk factors might enhance therapeutic effects [ 16 ]. Patients could benefit similarly from remedies that target modifiable factors. However, previous studies which tried to sum up the predictors only kept eyes on a limited dimension of factors, and on account of the research inconsistencies and limited number of studies, the conclusions were inauthentic and the supportive reasons were inadequate [ 7 , 17 , 18 ]. Therefore, an extensive summary is urgently needed, and we performed this meta-analysis to fill this research gap.
With the aim of further understanding the prognostic factors of AD and guiding clinical work from specifically modifiable issues, we designed a systematic meta-analysis to summarize predictive factors for the survival and quality of life of AD patients from various dimensions.
Search strategy
The PubMed, Embase and Cochrane databases were systematically searched from inception to December 2022 by terms “Alzheimer disease OR dementia OR Alzheimer* OR AD OR Dement*” AND “prognosis* OR progress* OR survival OR outcome OR mortality OR death OR hazard” by two independent researchers (XZ and SW). Furthermore, we refined the search scope for case-control or cohort studies by limiting “prospective OR retrospective OR cohort OR case-control OR case control OR consecutive” in the title or abstract. The comprehensive meta-analysis was performed following the Preferred Reporting Item for Systematic Review and Meta-analysis (2020) guidelines [ 19 ] (Supplementary Table S1 ). The titles and abstracts of all retrieved articles were reviewed. We also considered other publications in the full-read reports reference lists as supplementary papers. There were no restrictions applied in the literature search. The protocol for the study was registered with PROSPERO (registration number: CRD42022365357).
Selection criteria
The inclusion criteria were as follows: 1) the diagnostic criteria for AD patients were clearly stated; 2) case-control or cohort studies published in English; 3) the literature reported risk factors for the survival outcome of AD; and 4) the study provided adjusted effect sizes, relative risks (RRs), or hazard ratios (HRs) with 95% confidence intervals (CIs) through multivariate analysis. The exclusion criteria were as follows: 1) duplicate literature without new data; 2) incomplete data or odds ratios (ORs) as effect variables; 3) case reports, conference abstracts, reviews, comments, author replies and editorial materials; 4) studies on animals, cells and genes; and 5) patients diagnosed with any other type of dementia, including but not limited to vascular dementia, frontotemporal dementia, Parkinson’s disease dementia, and dementia with Lewy bodies. Further, considering the search scope of observational studies, we excluded predictors involving only treatment or nursing care to avoid inadequate aggregation.
Data extraction and quality assessment
Data extraction was performed by two independent researchers (XZ and SW). If a study had multiple estimates for the same factor, we only selected the estimates with the most adjusted variables and the longest follow-up time. According to previous survival research, survival was defined as the time when instruments were needed to sustain vital signs or mortality data retrieved from the registration system. For several causes of death, all-cause mortality was chosen to avoid underestimating the real death toll. Only when there were enough studies to conduct meta-analysis (≥3 studies concerning a potential variable) could the adjusted results be extracted. In addition, different studies might use different models or classifications of factors concerning survival and report various estimates in terms of one reference. Given that, we combined the poly-values into an overall value by a random-effects model. For variable inclusion, only categorical data providing the same classification criteria and continuous variables (per year/point increase) were included. The author, publication year, sample size, country, AD diagnostic criteria, included factors, endpoints, mean age, sex ratio, follow-up period, mean disease duration and median survival time were listed.
In addition, we exhibited the Newcastle-Ottawa Scale (NOS) and confounding factors of each eligible study. The endpoints included death, institutionalization, nursing home place (NHP) and cognitive decline (especially rapid decline). For possible factors and endpoints, the combined estimates of items were extracted from four aspects, and the details are described in the Supplementary Materials (Supplementary Table S2 ). All the variables of rating scales, clinical features or comorbidities were considered at diagnosis or at enrollment. Quality assessment was performed by two independent researchers (XZ and SW) using NOS scores. When there was divergence between the other two researchers, a third researcher was consulted to help reach a consensus.
Statistical analysis
To assess the potential impact on survival, the RR with a 95% CI was used as the estimate to be pooled for quantitative synthesis. Due to the adjusted survival time, the HR was considered equal to the RR for analysis, while studies that reported ORs were excluded for their tendency to overestimate the effect size. Four aspects were investigated to probe factors influencing the survival of AD. The primary outcome was the combined adjusted RR and 95% CI for mortality. Additionally, we consolidated the remaining endpoints, including institutionalization, NHP and cognitive decline, into “poor prognosis” as the secondary outcome to represent quality of life.
The multivariable-adjusted estimates and 95% CIs were transformed into log relative risks to calculate combined values using the random-effects model. As a result, those whose effect value was the same as the lower and upper CIs were eliminated to obtain a calculable standard error (SE). For those that provided values with opposite reference objects, we converted them into a unified one to achieve consistency [ 20 ].
Heterogeneity was assessed using the Q test and quantified by the I² metric. I² < 25% indicated no evidence of heterogeneity; 25% < I² < 50% indicated acceptable heterogeneity, and in such cases, the fixed-effects model was adopted for pooled analysis; 50% < I² < 75% indicated possible heterogeneity; and I² > 75% indicated considerable heterogeneity, for which the random-effects model was chosen and further analysis was performed. To explain and reduce heterogeneity, subgroup analysis was applied if necessary. In addition, for heterogeneity that could not be explained, a multivariate sensitivity analysis was performed to examine if the pooled effect size was influenced by sequentially omitting individual studies and to detect the stability of results as well. Meanwhile, a meta-regression ( n ≥ 10) was performed to explore the potential source of heterogeneity using the conservative Hartung–Knapp method [ 21 , 22 ] and to assess the underlying interaction of study characteristics, with the terms age, sex, geographic region, sample size, NOS scores and follow-up period. The Egger test was used to detect potential publication bias, and the trim-and-fill method was constructed for adjustment when significant bias was found.
All of the above statistical analyses were performed using Stata 15.1, with a two-tailed p < 0.05 considered indicative of statistical significance.
Literature retrieval and characteristics
According to the preset retrieval strategy, a total of 40,784 articles were considered from the outset. By excluding 13,876 duplicates and 24,949 records not associated with survival in AD, 1959 potential articles and an additional 12 from reference lists were fully reviewed. A further re-evaluation of each article led to the inclusion of 64 studies [ 11 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 ] concerning 26 probable prognostic factors, which were categorized into four groups, namely, demographic features ( n = 7), clinical features or comorbidities ( n = 13), rating scales ( n = 3) and biomarkers ( n = 3). The detailed search flow diagram is shown in Fig. 1 , and the statistically significant predictors are listed in Table 1 . Moreover, the characteristics of the 64 eligible studies involving 297,279 AD patients are summarized in Supplementary Table S3 . Confounding factors for the included studies are shown in Supplementary Table S4 . The total results are shown in Supplementary Table S5 and the specific forest plots are listed in Supplementary Figs. S1 – 5 .
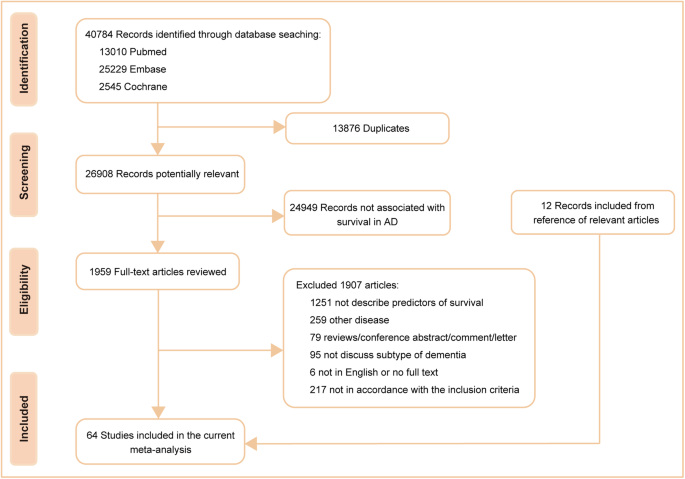
Flowchart of the literature search according to Preferred Reporting Item for Systematic Review and Meta-analysis (PRISMA).
Primary outcomes
Demographic features.
Six factors (age, sex, race, years of education, marital status and smoking) for which there was prior evidence for an association with AD survival were included in the primary analysis (Supplementary Fig. S1 ). We found that there was a poor prognosis for older patients (RR 1.05, 95% CI 1.04–1.07 for baseline age; RR 1.03, 95% CI 1.01–1.05 for age of onset), males (RR 1.58, 95% CI 1.49–1.68) and white patients (RR 1.36, 95% CI 1.21–1.53) (Fig. 2 ). However, years of education (RR 1.00, 95% CI 0.98–1.02), living alone (RR 1.07, 95% CI 0.97–1.19), and smoking (RR 1.00, 95% CI 1.00–1.01) did not show a significant association.
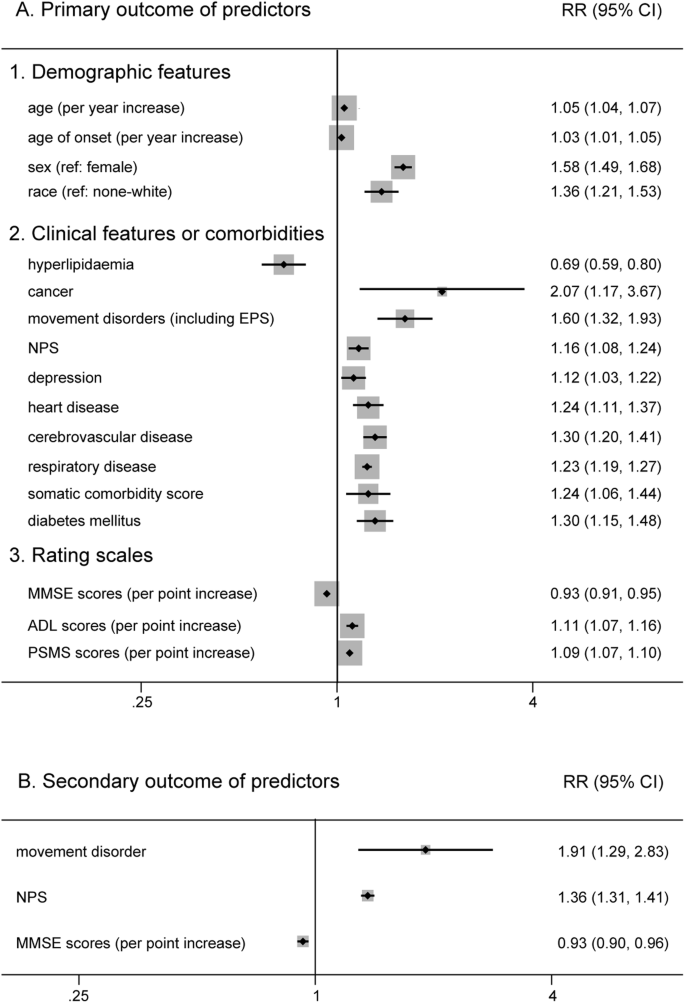
The forest plot displays meta-analysis results of the prognostic factors in AD. AD Alzheimer’s disease, RR relative risk, CI confidence intervals.

Clinical features or comorbidities
Clinical features or comorbidities also play an important role in the prognosis of AD (Supplementary Fig. S2 ). In our analysis, those who had hyperlipidaemia (RR 0.69, 95% CI 0.59–0.80) had a lower risk of death (Fig. 2 ). In contrast, we found that manifestations of movement disorders (including EPS) (RR 1.60, 95% CI 1.32–1.93) and cancer (RR 2.07, 95% CI 1.17–3.67) were more detrimental to AD patient survival. Moreover, other features, such as neuropsychiatric symptoms (NPS) (RR 1.16, 95% CI 1.08–1.24), depression (RR 1.12, 95% CI 1.03–1.22), heart disease (RR 1.24, 95% CI 1.11–1.37), cerebrovascular disease (RR 1.30, 95% CI 1.20–1.41), respiratory disease (RR 1.23, 95% CI 1.19–1.27), diabetes mellitus (RR 1.30, 95% CI 1.15–1.48) and a higher somatic comorbidity score (RR 1.24, 95% CI 1.06–1.44), were associated with poor prognosis (Fig. 2 ). Beyond that, the pooled analysis failed to exhibit a significant outcome in patients with a history of hypertension (RR 1.19, 95% CI 1.00–1.41), wandering or falling (RR 1.39, 95% CI 0.95–2.06) and vascular risk factors (VRF) (RR 1.02, 95% CI 0.93–1.13).
Rating scales
For rating scales (Supplementary Fig. S3 ), patients with higher activity of daily living (ADL) scores (RR 1.11, 95% CI 1.07–1.16) and physical self-maintenance scale (PSMS) scores (RR 1.09, 95% CI 1.07–1.10), which indicated a lack of self-care ability, had an increased risk of death (Fig. 2 ). Similarly, higher Mini-Mental State Examination (MMSE) scores, which indicated relatively good cognitive function, decreased the risk for shorter survival (RR 0.93, 95% CI 0.91–0.95) (Fig. 2 ).
An increasing number of studies have been devoted to elucidating the impact of biomarkers in developing AD rather than survival since diagnosis. However, due to the various cut-off values among different researches, it is not easy to perform quantitative analysis for all biomarkers. Therefore, only three biomarkers were analyzed in the current study (Supplementary Fig. S4 ). We found that neither apolipoprotein E (APOE) ε4 carriers (RR 0.94, 95% CI 0.78–1.14), the level of cerebrospinal fluid (CSF) β-amyloid (Aβ 42 ) (RR 1.09, 95% CI 0.91–1.32) nor total tau protein (t-tau) (RR 1.00, 95% CI 1.00–1.01) had a significant impact on AD patient survival.
Secondary outcome
In the secondary analysis, nine potential factors were calculated (Supplementary Fig. S5 ). We found that movement disorders (including EPS) (RR 1.76, 95% CI 1.11–2.79) and NPS (RR 1.35, 95% CI 1.25–1.46) had a detrimental influence on the prognosis of AD. Same as before, higher MMSE scores (RR 0.93, 95% CI 0.90–0.96) were associated with longer survival (Fig. 2 ). However, age (RR 1.02, 95% CI 0.98–1.06 for baseline age; RR 0.98, 95% CI 0.92–1.05 for age of onset), male sex (RR 0.92, 95% CI 0.81–1.04), living alone (RR 1.67, 95% CI 0.66–4.26), increased ADL scores (RR 1.05, 95% CI 0.96–1.16) and APOE ε4 carrier (RR 0.93, 95% CI 0.72–1.19) had no evident effect on living quality (institutionalization, NHP and cognitive decline) in patients with AD.
Heterogeneity and sensitivity analysis
Heterogeneity exists in some combination of this meta-analysis. In the primary outcome, depression (I 2 = 26.4%), respiratory disease (I 2 = 11.5%), hyperlipidaemia (I 2 < 0.001) and PSMS scores (I 2 = 36.1%) demonstrated unobvious or acceptable heterogeneity. We found that the heterogeneity of movement disorders was reduced by removing the study performed by Stern et al. [ 37 ]. Meanwhile, subgroup analysis was performed to reveal possible heterogeneity among studies, and the heterogeneity for age of onset, sex, cancer, NPS (subdivided into four types: behavioral problems, specific hallucinations or delusions, psychosis, mood disorder, any of the above symptoms), cerebrovascular disease, heart disease (cardiovascular disease), somatic comorbidity score, diabetes mellitus, and ADL scores was reduced to varying degrees (Supplementary Fig. S6 ). Hence, multiple sensitivity analyses for age, race and MMSE scores were performed by removing each study, but there was no change. Furthermore, we carried out meta-regression concerning items of age, sex, geographic region, sample size, NOS scores and follow-up time but failed to explain the source of heterogeneity. For the sensitivity analysis to test the robustness of the overall outcome, there appeared to be no significant difference in the results with any study removed except for depression and cancer (Supplementary Fig. S7 ).
In the secondary outcome, subgroup analysis based on age and NOS scores led to reduced heterogeneity for MMSE scores (Supplementary Fig. S6 ), and the outcome of the combination was stable in the sensitivity analysis (Supplementary Fig. S8 ).
Assessment of publication bias
For the primary analysis, no influences of publication bias on the combined results were identified and the specific items are demonstrated in the Supplementary Materials. Whereas for MMSE scores ( P = 0.040), there exists latent publication bias. Hence, the further trim-and-fill method was used and showed the authenticity and stability of the result (unchanged adjusted RR 0.934, 95% CI 0.917–0.950 for MMSE scores).
For the secondary outcome, the multiple sensitivity analysis exhibited no difference by eliminating any single study (Supplementary Materials). However, for MMSE scores, a bias was observed ( P = 0.001). After the application of the trim-and-fill method, the combined estimate did not change (adjusted RR 0.930, 95% CI 0.915–0.946), which meant that the impact of publication bias was acceptable.
To the best of our knowledge, there has been no meta-analysis summarizing prognostic factors for predicting the survival of AD patients from multiple dimensions. In this study, predictors, including demographic features, clinical features or comorbidities, rating scales and biomarkers, were investigated. In total, 26 probable prognostic factors were finally explored for AD survival, and 17 factors were identified as possibly related to the survival of AD (Fig. 3 ). Among them, hyperlipidaemia and higher MMSE scores were predictors of longer survival. However, males, features of movement disorders (including EPS) and cancer showed a worse prognosis. Moreover, older age, white race, a history of NPS, depression, heart disease, cerebrovascular disease, respiratory disease, higher somatic comorbidity score, diabetes mellitus, higher ADL scores and PSMS scores in patients also impaired AD survival. However, our results did not support the involvement of education, marital status, hypertension, wandering or falling, VRF, APOE genotype, Aβ 42 or t-tau in AD survival. In the secondary analysis, we found that only movement disorders (including EPS), NPS and lower MMSE scores played a meaningful role in the deterioration of progress in AD patients, which was in accordance with our primary analysis. For intervenable symptoms such as NPS and diabetes mellitus, patients may benefit from regular treatments.
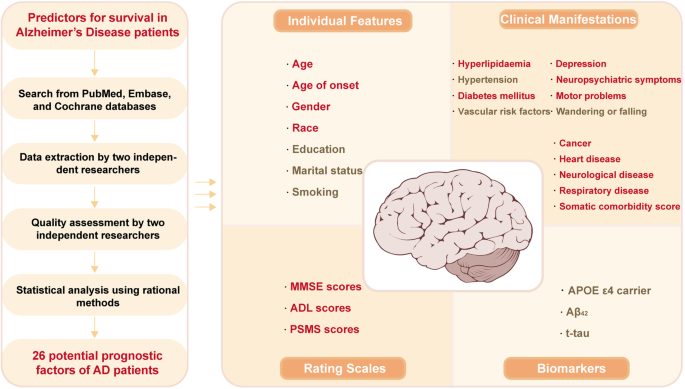
AD Alzheimer’s disease, MMSE The Mini Mental State Examination, ADL Activity of Daily Living, PSMS Physical Self-Maintenance Scale, APOE Apolipoprotein E, CSF cerebrospinal fluid, Aβ β-amyloid, t-tau total tau protein.
Less is known about the clinical value of various factors in AD progression or survival in the past, and many studies have attempted to spell out their associations [ 7 , 8 , 9 , 10 , 11 , 86 , 87 , 88 , 89 , 90 , 91 , 92 ]. Compared with the results of former studies, either accordance or difference was observed in our analysis.
Our findings indicated that hyperlipidaemia was related to longer survival of AD, and yet other VRF, including overall VRF and some separate diseases such as smoking and hypertension, did not show a similar significant association. Earlier research stated that there was no difference in the rate of deterioration between people with and without VRF and assumed that VRF may contribute to the expression of AD initially but was not part of the underlying etiologic process [ 93 , 94 ]. What amazed us was the negative link between hyperlipidaemia and mortality. It should be noted that elevations in blood lipids prolonged the survival of AD patients compared with those without hyperlipidaemia. Hyperlipidaemia has been identified as a risk factor for developing dementia. However, our analysis, which aggregated previous research findings, yielded conflicting results. These findings underscore the complexity of the role of hyperlipidaemia in the occurrence and progression of AD.
Other diseases, such as heart disease and cerebrovascular disease, which have been identified as driving forces in the process of dementia progression, were significantly associated with AD survival. Diabetes mellitus, one of the most prevalent comorbidities, plays an expediting role in disease progression [ 92 , 95 , 96 ], which might originate from an identical source as AD. This is not difficult to accept, as some researchers refer to AD as “diabetes of the brain” or “type-3 diabetes” [ 97 ]. In addition, movement disorders, often accompanied by functional defects, were discovered as a strong predictor of mortality and were associated with adverse outcomes [ 10 , 98 , 99 , 100 ]. In our work, EPS influenced not only the survival of AD patients but also the quality of life since diagnosis, which is not hard to interpret because EPS, such as rigidity, tremor, and postural instability, means a loss of self-care to a certain extent. Similar to most of the following factors, cancer also significantly increased the risk of death in AD. One hypothesis was that the poor prognosis of patients with cancer shortened life expectancy, let alone those before the onset of AD. In terms of other clinical features, behavioral and psychological symptoms are common nonmotor symptoms during the natural history of AD, leading to distress for patients and their caregivers [ 101 ]. NPS and behavioral problems were proven to be detrimental to survival [ 10 , 102 ], and similar results were found for depression, as in our work. In fact, how these manifestations interact remains unknown, and there is still controversy in some studies that disagree with the findings [ 93 ].
For demographic features, some items emerged as significant predictors of mortality. Older age and white race increased the risk of death in our study, which was in accordance with previous studies [ 7 , 103 , 104 ]. Meanwhile, a Framingham study suggested that due to “survival bias”, men appeared to have a lower risk for dementia, in which the included male participants who survived to 65 years old possessed a better physical condition [ 105 ]. The fact remains that once diagnosed with AD or other dementias, having male sex resulted in a worse prognosis compared to having female sex. Similarly, previous studies and our findings showed that a higher education level was not associated with decreased survival in AD [ 87 , 106 ], in contrast to the evidence that a lower level of education was a risk factor for dementia [ 107 ]. The role of marital status should not be ignored, although we did not obtain a meaningful outcome because a former study reported that younger patients living alone exhibited a nearly threefold risk of death than those living with a family [ 108 ]. One explanation was that patients living alone were likely to be diagnosed at a later time than those who lived together with a spouse, which can influence the intervention measures to be taken.
Additionally, we found that cognitive decline and deterioration of personal self-care ability (such as ADL and PSMS scores) were associated with mortality risk in individuals with AD. Previous studies drew the same conclusion as well [ 109 , 110 , 111 , 112 ]. A result from a real-world cohort indicated that poorer baseline cognitive ability and short-term decline in functional ability independently predicted the transition from mild to more severe AD dementia [ 110 ]. Additionally, Aβ42 and tau are generally recognized as diagnostic biomarkers, but few studies have examined whether AD biomarkers are associated with mortality. In our analysis, no difference was observed for APOE ε4 carriers and different levels of CSF biomarkers in disease progression, similar to previous studies [ 113 , 114 , 115 ]. The possible reason was that growing evidence of shared molecular mechanisms between AD and atherosclerosis, showed an association with more cardiovascular mortality [ 34 ]. Notably, it was also reported that AD patients with extreme levels of CSF biomarkers exhibited worse clinical outcomes over time [ 114 ], which might be explained by more advanced disease that contributed to the risk of death. In addition, baseline plasma neurofilament light (NFL) chain was regarded as a predictor of cognitive decline, along with plasma tau in the late mild cognitive impairment (MCI) population [ 116 ].
The primary strength of our meta-analysis lies in its comprehensive and large-scale summary of prognostic factors for predicting survival in patients with AD from four dimensions. Another strength is that more high-quality prospective studies with nearly 300,000 AD patients were included, and stricter inclusion and exclusion criteria were used, which exhibited substantial power in drawing a conclusion. Moreover, we chose the most adjusted variables to decrease the impact of confounding factors that might influence the outcome. Last, a single type of dementia (Alzheimer’s disease) rather than multiple types of dementia was focused on to understand the course of the disease pertinently [ 9 , 92 , 113 , 117 ]. Although some predictors that affected AD survival were identified, these results should be considered with caution due to several limitations. First, we excluded studies that reported different classifications of categorical data or reported ORs as estimate variables to avoid bias. Second, heterogeneity still existed in the analysis of age, race and MMSE scores after the application of multifarious methods, and the generated estimates of clinical type, depression and cancer were not robust in the sensitivity analysis on account of the restricted number of studies. Third, publication bias could not be ignored in that some studies only reported significant results, and the personal characteristics, follow-up time, and sample size varied among studies, although efforts have been made to take that into account. Fourth, the potential relationship between hyperlipidaemia and AD survival could not be interpreted clearly, indicating a need for more research on this topic. Finally, we did not discuss the influence of therapeutic measures on survival in AD patients since observational studies were the major study type within the scope of the search strategy and randomized control studies were incomplete. Similarly, genetic factors were not taken into consideration due to the complicated pathogenesis.
This meta-analysis comprehensively identified intervenable and unmodifiable risk factors for predicting survival in patients with AD from the dimensions of demographic features, clinical features or comorbidities, rating scales and biomarkers.
Data availability
All data analyzed during this study are included in the Supplementary Materials.
Kumar A, Singh A, Ekavali. A review on Alzheimer’s disease pathophysiology and its management: an update. Pharm Rep. 2015;67:195–203.
Article CAS Google Scholar
Du W, Tan J, Xu W, Chen J, Wang L. Association between clusterin gene polymorphism rs11136000 and late-onset Alzheimer’s disease susceptibility: A review and meta-analysis of case-control studies. Exp Ther Med. 2016;12:2915–27.
Article CAS PubMed PubMed Central Google Scholar
Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. Alzheimer’s disease. Lancet. 2021;397:1577–90.
GBD 2019 Ageing Collaborators. Global, regional, and national burden of diseases and injuries for adults 70 years and older: systematic analysis for the Global Burden of Disease 2019 Study. BMJ. 2022;376:e068208.
Google Scholar
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–22.
Article Google Scholar
Cummings J, Lee G, Nahed P, Kambar M, Zhong K, Fonseca J, et al. Alzheimer’s disease drug development pipeline: 2022. Alzheimers Dement. 2022;8:e12295.
Brookmeyer R, Corrada MM, Curriero FC, Kawas C. Survival following a diagnosis of Alzheimer disease. Arch Neurol. 2002;59:1764–7.
Article PubMed Google Scholar
Jagger C, Clarke M, Stone A. Predictors of survival with Alzheimer’s disease: a community-based study. Psychol Med. 1995;25:171–7.
Article CAS PubMed Google Scholar
Brodaty H, Seeher K, Gibson L. Dementia time to death: a systematic literature review on survival time and years of life lost in people with dementia. Int Psychogeriatr. 2012;24:1034–45.
Stern Y, Mayeux R, Sano M, Hauser WA, Bush T. Predictors of disease course in patients with probable Alzheimer’s disease. Neurology. 1987;37:1649–53.
Rountree SD, Chan W, Pavlik VN, Darby EJ, Doody RS. Factors that influence survival in a probable Alzheimer disease cohort. Alzheimers Res Ther. 2012;4:16.
Sousa OV, Amaral TF. Nutritional and functional status in survival of free-living mild Alzheimer’s disease patients. J Cachexia Sarcopenia Muscle. 2017;8:1058–9.
Zhou J, Yu JT, Wang HF, Meng XF, Tan CC, Wang J, et al. Association between stroke and Alzheimer’s disease: systematic review and meta-analysis. J Alzheimers Dis. 2015;43:479–89.
Vázquez Justes D, Dakterzada F, Romero L, Ruiz-Julián M, Paul Arias M, Baraldés Rovira M, et al. Microbleeds and the risk of stroke and mortality in patients with alzheimer disease after 3 years of follow-up. Eur Stroke J. 2021;6:443.
Ousset PJ, Nourhashemi F, Reynish E, Vellas B. Nutritional status is associated with disease progression in very mild Alzheimer disease. Alzheimer Dis Associated Disord. 2008;22:66–71.
Ferrari-Souza JP, Brum WS, Hauschild LA, da Ros LU, Lukasewicz Ferreira PC, Bellaver B, et al. Vascular risk burden is a key player in the early progression of Alzheimer’s disease. Neurobiol Aging. 2024;136:88–98.
Rahmani J, Roudsari AH, Bawadi H, Clark C, Ryan PM, Salehisahlabadi A, et al. Body mass index and risk of Parkinson, Alzheimer, Dementia, and Dementia mortality: a systematic review and dose–response meta-analysis of cohort studies among 5 million participants. Nutritional Neurosci. 2022;25:423–31.
Grønning H, Rahmani A, Gyllenborg J, Dessau R, Høgh P. Does Alzheimer’s disease with early-onset progress faster than with late-onset? A case-control study of clinical progression and cerebrospinal fluid biomarkers. Eur J Neurol. 2012;19:462.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Article PubMed PubMed Central Google Scholar
Orsini N. From floated to conventional confidence intervals for the relative risks based on published dose-response data. Comput Methods Prog Biomed. 2010;98:90–3.
Hartung J, Knapp G. On tests of the overall treatment effect in meta-analysis with normally distributed responses. Stat Med. 2001;20:1771–82.
Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med. 2001;20:3875–89.
Burns A, Lewis G, Jacoby R, Levy R. Factors affecting survival in Alzheimer’s disease. Psychological Med. 1991;21:363–70.
Heyman A, Peterson B, Fillenbaum G, Pieper C. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part XIV: Demographic and clinical predictors of survival in patients with Alzheimer’s disease. Neurology. 1996;46:656–60.
Stern Y, Brandt J, Albert M, Jacobs DM, Liu X, Bell K, et al. The absence of an apolipoprotein epsilon4 allele is associated with a more aggressive form of Alzheimer’s disease. Ann Neurol. 1997;41:615–20.
Claus JJ, van Gool WA, Teunisse S, Walstra GJ, Kwa VI, Hijdra A, et al. Predicting survival in patients with early Alzheimer’s disease. Dement Geriatr Cogn Disord. 1998;9:284–93.
White H, Pieper C, Schmader K. The association of weight change in Alzheimer’s disease with severity of disease and mortality: a longitudinal analysis. J Am Geriatr Soc. 1998;46:1223–7.
McCann JJ, Hebert LE, Li Y, Wolinsky FD, Gilley DW, Aggarwal NT, et al. The effect of adult day care services on time to nursing home placement in older adults with Alzheimer’s disease. Gerontologist. 2005;45:754–63.
Mehta KM, Yaffe K, Pérez-Stable EJ, Stewart A, Barnes D, Kurland BF, et al. Race/ethnic differences in AD survival in US Alzheimer’s Disease Centers. Neurology. 2008;70:1163–70.
Henneman WJ, Sluimer JD, Cordonnier C, Baak MM, Scheltens P, Barkhof F, et al. MRI biomarkers of vascular damage and atrophy predicting mortality in a memory clinic population. Stroke. 2009;40:492–8.
Wattmo C, Wallin AK, Londos E, Minthon L. Risk factors for nursing home placement in Alzheimer’s disease: a longitudinal study of cognition, ADL, service utilization, and cholinesterase inhibitor treatment. Gerontologist. 2011;51:17–27.
Lopez OL, Becker JT, Chang YF, Sweet RA, Aizenstein H, Snitz B, et al. The long-term effects of conventional and atypical antipsychotics in patients with probable Alzheimer’s disease. Am J Psychiatry. 2013;170:1051–8.
Rabins PV, Schwartz S, Black BS, Corcoran C, Fauth E, Mielke M, et al. Predictors of progression to severe Alzheimer’s disease in an incidence sample. Alzheimers Dement. 2013;9:204–7.
Boumenir A, Cognat E, Sabia S, Hourregue C, Lilamand M, Dugravot A, et al. CSF level of β-amyloid peptide predicts mortality in Alzheimer’s disease. Alzheimers Res Ther. 2019;11:29.
Walsh JS, Welch HG, Larson EB. Survival of outpatients with Alzheimer-type dementia. Ann Intern Med. 1990;113:429–34.
Chui HC, Lyness SA, Sobel E, Schneider LS. Extrapyramidal signs and psychiatric symptoms predict faster cognitive decline in Alzheimer’s disease. Arch Neurol. 1994;51:676–81.
Stern Y, Albert M, Brandt J, Jacobs DM, Tang MX, Marder K, et al. Utility of extrapyramidal signs and psychosis as predictors of cognitive and functional decline, nursing home admission, and death in Alzheimer’s disease: Prospective analyses from the predictors study. Neurology. 1994;44:2300–7.
Stern Y, Tang MX, Denaro J, Mayeux R. Increased risk of mortality in Alzheimer’s disease patients with more advanced educational and occupational attainment. Ann Neurol. 1995;37:590–5.
Bowen JD, Malter AD, Sheppard L, Kukull WA, McCormick WC, Teri L, et al. Predictors of mortality in patients diagnosed with probable Alzheimer’s disease. Neurology. 1996;47:433–9.
Geerlings MI, Deeg DJH, Schmand B, Lindeboom J, Jonker C. Increased risk of mortality in Alzheimer’s disease patients with higher education? A replication study. Neurology. 1997;49:798–802.
Stern Y, Tang MX, Albert MS, Brandt J, Jacobs DM, Bell K, et al. Predicting time to nursing home care and death in individuals with Alzheimer disease. J Am Med Assoc. 1997;277:806–12.
Tilvis RS, Strandberg TE, Juva K. Apolipoprotein E phenotypes, dementia and mortality in a prospective population sample. J Am Geriatr Soc. 1998;46:712–5.
Claus JJ, Walstra GJM, Hijdra A, Van Royen EA, Verbeeten B Jr, Van Gool WA. Measurement of temporal regional cerebral perfusion with single-photon emission tomography predicts rate of decline in language function and survival in early Alzheimer’s disease. Eur J Nucl Med. 1999;26:265–71.
Larson EB, Shadlen MF, Wang L, McCormick WC, Bowen JD, Teri L, et al. Survival after Initial Diagnosis of Alzheimer Disease. Ann Intern Med. 2004;140:501–9. +I26
Scarmeas N, Albert M, Brandt J, Blacker D, Hadjigeorgiou G, Papadimitriou A, et al. Motor signs predict poor outcomes in Alzheimer disease. Neurology. 2005;64:1696–703.
Scarmeas N, Brandt J, Albert M, Hadjigeorgiou G, Papadimitriou A, Dubois B, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62:1601–8.
Suh GH, Yeon BK, Shah A, Lee JY. Mortality in Alzheimer’s disease: A comparative prospective Korean study in the community and nursing homes. Int J Geriatr Psychiatry. 2005;20:26–34.
Waring SC, Doody RS, Pavlik VN, Massman PJ, Chan W. Survival among patients with dementia from a large multi-ethnic population. Alzheimer Dis Assoc Disord. 2005;19:178–83.
Carcaillon L, Pérès K, Péré JJ, Helmer C, Orgogozo JM, Dartigues JF. Fast cognitive decline at the time of dementia diagnosis: A major prognostic factor for survival in the community. Dement Geriatr Cogn Disord. 2007;23:439–45.
Scarmeas N, Brandt J, Blacker D, Albert M, Hadjigeorgiou G, Dubois B, et al. Disruptive behavior as a predictor in Alzheimer disease. Arch Neurol. 2007;64:1755–61.
Bruandet A, Richard F, Bombois S, Maurage CA, Masse I, Amouyel P, et al. Cognitive decline and survival in Alzheimer’s disease according to education level. Dement Geriatr Cogn Disord. 2008;25:74–80.
Helzner EP, Scarmeas N, Cosentino S, Tang MX, Schupf N, Stern Y. Survival in Alzheimer disease: a multiethnic, population-based study of incident cases. Neurology. 2008;71:1489–95.
Hatoum HT, Thomas SK, Lin SJ, Lane R, Bullock R. Predicting time to nursing home placement based on activities of daily living scores-a modelling analysis using data on Alzheimer’s disease patients receiving rivastigmine or donepezil. J Med Econ. 2009;12:98–103.
Pavlik VN, Doody RS, Rountree SD, Darby EJ. Vitamin E Use Is Associated with Improved Survival in an Alzheimer’s Disease Cohort. Dement Geriatr Cogn Disord. 2009;28:536–40.
Zhou B, Zhao Q, Teramukai S, Ding D, Guo Q, Fukushima M, et al. Executive function predicts survival in Alzheimer disease: A study in Shanghai. J Alzheimers Dis. 2010;22:673–82.
Musicco M, Palmer K, Russo A, Caltagirone C, Adorni F, Pettenati C, et al. Association between prescription of conventional or atypical antipsychotic drugs and mortality in older persons with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2011;31:218–24.
Go SM, Lee KS, Seo SW, Chin J, Kang SJ, Moon SY, et al. Survival of alzheimer’s disease patients in Korea. Dement Geriatr Cogn Disord. 2013;35:219–28.
Degerman Gunnarsson M, Lannfelt L, Ingelsson M, Basun H, Kilander L. High tau levels in cerebrospinal fluid predict rapid decline and increased dementia mortality in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2014;37:196–206.
Nägga K, Wattmo C, Zhang Y, Wahlund LO, Palmqvist S. Cerebral inflammation is an underlying mechanism of early death in Alzheimer’s disease: A 13-year cause-specific multivariate mortality study. Alzheimers Res Ther. 2014;6:41.
Wattmo C, Londos E, Minthon L. Risk factors that affect life expectancy in Alzheimer’s disease: a 15-year follow-up. Dement Geriatr Cogn Disord. 2014;38:286–99.
Benedictus MR, Prins ND, Goos JDC, Scheltens P, Barkhof F, Van Der Flier WM. Microbleeds, Mortality, and Stroke in Alzheimer Disease The MISTRAL Study. JAMA Neurol. 2015;72:539–45.
Lin FC, Chuang YS, Hsieh HM, Lee TC, Chiu KF, Liu CK, et al. Early statin use and the progression of Alzheimer disease: A total population-based case-control study. Medicine. 2015;94:e2143.
Wattmo C, Londos E, Minthon L. Longitudinal associations between survival in Alzheimer’s disease and cholinesterase inhibitor use, progression, and community-based services. Dement Geriatr Cogn Disord. 2015;40:297–310.
Degerman Gunnarsson M, Ingelsson M, Blennow K, Basun H, Lannfelt L, Kilander L. High tau levels in cerebrospinal fluid predict nursing home placement and rapid progression in Alzheimer’s disease. Alzheimers Res Ther. 2016;8:22.
Nielsen RE, Lolk A, Valentin JB, Andersen K. Cumulative dosages of antipsychotic drugs are associated with increased mortality rate in patients with Alzheimer’s dementia. Acta Psychiatr Scandinavica. 2016;134:314–20.
Mueller C, Huntley J, Stubbs B, Sommerlad A, Carvalho AF, Perera G, et al. Associations of Neuropsychiatric Symptoms and Antidepressant Prescription with Survival in Alzheimer’s Disease. J Am Med Dir Assoc. 2017;18:1076–81.
Black CM, Fillit H, Xie L, Hu X, Kariburyo MF, Ambegaonkar BM, et al. Economic Burden, Mortality, and Institutionalization in Patients Newly Diagnosed with Alzheimer’s Disease. J Alzheimers Dis. 2018;61:185–93.
Chu CS, Li WR, Huang KL, Su PY, Lin CH, Lan TH. The use of antipsychotics is associated with lower mortality in patients with Alzheimer’s disease: A nationwide population-based nested case-control study in Taiwan. J Psychopharmacol. 2018;32:1182–90.
Giil LM, Aarsland D, Hellton K, Lund A, Heidecke H, Schulze-Forster K, et al. Antibodies to multiple receptors are associated with neuropsychiatric symptoms and mortality in Alzheimer’s disease: A longitudinal study. J Alzheimers Dis. 2018;64:761–74.
Ku LJE, Li CY, Sun Y. Can Persistence With Cholinesterase Inhibitor Treatment Lower Mortality and Health-Care Costs Among Patients With Alzheimer’s Disease? A Population-Based Study in Taiwan. Am J Alzheimers Dis other Dement. 2018;33:86–92.
Mueller C, Perera G, Hayes RD, Shetty H, Stewart R. Associations of acetylcholinesterase inhibitor treatment with reduced mortality in Alzheimer’s disease: A retrospective survival analysis. Age Ageing. 2018;47:88–94.
Nielsen RE, Valentin JB, Lolk A, Andersen K. Effects of antipsychotics on secular mortality trends in patients with Alzheimer’s disease. J Clin Psychiatry. 2018;79:17m11595.
Rhodius-Meester HFM, Liedes H, Koene T, Lemstra AW, Teunissen CE, Barkhof F, et al. Disease-related determinants are associated with mortality in dementia due to Alzheimer’s disease. Alzheimers Res Ther. 2018;10:23.
Chen TB, Weng SC, Chou YY, Lee YS, Liang CK, Lin CS, et al. Predictors of Mortality in the Oldest Old Patients with Newly Diagnosed Alzheimer Disease in a Residential Aged Care Facility. Dement Geriatr Cogn Disord. 2019;48:93–104.
Linna M, Vuoti S, Silander K, Hörhammer I, Halminen O, Mikkola T, et al. Impact of Anti-Dementia Medication on the Risk of Death and Causes of Death in Alzheimer’s Disease. J Alzheimers Dis. 2019;71:1297–308.
Chen NC, Liang CK, Yin CH, Lin YT, Lee CC, Chen CL. Effects of Socioeconomic Status on Alzheimer Disease Mortality in Taiwan. Am J Geriatr Psychiatry. 2020;28:205–16.
de Sousa OV, Mendes J, Amaral TF. Nutritional and Functional Indicators and Their Association With Mortality Among Older Adults With Alzheimer’s Disease. Am J Alzheimers Dis other Dement. 2020;35:1533317520907168.
Zhang YQ, Wang CF, Xu G, Zhao QH, Xie XY, Cui HL, et al. Mortality of Alzheimer’s Disease Patients: A 10-Year Follow-up Pilot Study in Shanghai. Can J Neurological Sci. 2020;47:226–30.
Gottesman RT, Kociolek A, Fernandez K, Cosentino S, Devanand DP, Stern Y, et al. Association between Early Psychotic Symptoms and Alzheimer’s Disease Prognosis in a Community-Based Cohort. J Alzheimers Dis. 2021;81:1131–9.
Liew TM. Neuropsychiatric symptoms in early stage of Alzheimer’s and non-Alzheimer’s dementia, and the risk of progression to severe dementia. Age Ageing. 2021;50:1709–18.
Rajamaki B, Hartikainen S, Tolppanen AM. The effect of comorbidities on survival in persons with Alzheimer’s disease: a matched cohort study. BMC Geriatrics. 2021;21:173.
Wattmo C, Blennow K, Hansson O. Cerebrospinal Fluid Biomarker Levels as Markers for Nursing Home Placement and Survival Time in Alzheimer’s Disease. Curr Alzheimer Res. 2021;18:573–84.
Armstrong MJ, Song S, Kurasz AM, Li Z. Predictors of Mortality in Individuals with Dementia in the National Alzheimer’s Coordinating Center. J Alzheimers Dis. 2022;86:1935–46.
Ono R, Uchida K, Nakatsuka K, Megumi M, Fukuda H. Economic Status and Mortality in Patients with Alzheimer’s Disease in Japan: The Longevity Improvement and Fair Evidence Study. J Am Med Dir Assoc. 2022;23:161–4.
van Loenhoud AC, Groot C, Bocancea DI, Barkhof F, Teunissen C, Scheltens P, et al. Association of Education and Intracranial Volume With Cognitive Trajectories and Mortality Rates Across the Alzheimer Disease Continuum. Neurology. 2022;98:e1679–e1691.
PubMed PubMed Central Google Scholar
Felbecker A, Tettenborn B. Influence of Vascular Risk Factors on Cause and Progression of Alzheimer’s Disease. Aktuelle Neurologie. 2016;43:309–17.
Di Santo S, Musicco M. Lifestyle and rate of progression of cognitive decline: Results of the SINDEM cohort study. J Alzheimer’s Dis. 2011;23:S55–S6.
Todd S, Barr S, Roberts M, Passmore AP. Survival in dementia and predictors of mortality: a review. Int J Geriatr Psychiatry. 2013;28:1109–24.
Lee JH. Clinical factors influencing the rate of progression of dementia: A retrospective review from 50 geriatric hospitals in korea. Alzheimers Dement. 2015;11:P524.
Sathe AB, Koh M, Wu HY, Leong I, Ali NB, Chin JJ, et al. Predictors of survival in moderate to advanced dementia: A retrospective review. Ann Acad Med Singap. 2015;44:S411.
Alonso A, Jacobs DR Jr., Menotti A, Nissinen A, Dontas A, Kafatos A, et al. Cardiovascular risk factors and dementia mortality: 40 years of follow-up in the Seven Countries Study. J Neurol Sci. 2009;280:79–83.
van de Vorst IE, Koek HL, de Vries R, Bots ML, Reitsma JB, Vaartjes I. Effect of Vascular Risk Factors and Diseases on Mortality in Individuals with Dementia: A Systematic Review and Meta-Analysis. J Am Geriatr Soc. 2016;64:37–46.
Drachman DA, O’Donnell BF, Lew RA, Swearer JM. The prognosis in Alzheimer’s disease. ‘How far’ rather than ‘how fast’ best predicts the course. Arch Neurol. 1990;47:851–6.
Kalaria RN, Ballard C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord. 1999;13:S115–23.
Wang G. Mortality Of Alzheimer’s Disease Patients In Shanghai: Findings From A Clinic-Based Ten-Year Follow-Up Study. Alzheimers Dement. 2019;15:P827.
Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–94.
Nguyen TT, Ta QTH, Nguyen TKO, Nguyen TTD, Giau VV. Type 3 Diabetes and Its Role Implications in Alzheimer’s Disease. Int J Mol Sci. 2020;21:3165.
Al-Harrasi AM, Iqbal E, Tsamakis K, Lasek J, Gadelrab R, Soysal P, et al. Motor signs in Alzheimer’s disease and vascular dementia: Detection through natural language processing, co-morbid features and relationship to adverse outcomes. Exp Gerontol. 2021;146:111223.
Samson WN, van Duijn CM, Hop WC, Hofman A. Clinical features and mortality in patients with early-onset Alzheimer’s disease. Eur Neurol. 1996;36:103–6.
Lopez OL, Wisnieski SR, Becker JT, Boller F, DeKosky ST. Extrapyramidal signs in patients with probable Alzheimer disease. Arch Neurol. 1997;54:969–75.
Ballard C, Day S, Sharp S, Wing G, Sorensen S. Neuropsychiatric symptoms in dementia: importance and treatment considerations. Int Rev Psychiatry. 2008;20:396–404.
Herrmann N, Harimoto T, Balshaw R, Lanctôt KL, Bacher Y, Bailey P, et al. Risk factors for progression of Alzheimer disease in a Canadian population: The Canadian Outcomes Study in Dementia (COSID). Can J Psychiatry. 2015;60:189–99.
Rountree S, Chan W, Pavlik V, Darby E, Doody R. Factors that influence survival in alzheimer’s patients. Alzheimer’s Dement. 2011;7:S513.
Barr S, Passmore AP, Todd S. Excess mortality associated with Alzheimer’s disease in a community-dwelling population attending a Memory Clinic. Eur Geriatr Med. 2010;1:S58.
Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White R, et al. Lifetime risk of dementia and Alzheimer’s disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49:1498–504.
Paradise M, Cooper C, Livingston G. Systematic review of the effect of education on survival in Alzheimer’s disease. Int Psychogeriatr. 2009;21:25–32.
Yu JT, Xu W, Tan CC, Andrieu S, Suckling J, Evangelou E, et al. Evidence-based prevention of Alzheimer’s disease: systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J Neurol Neurosurg Psychiatry. 2020;91:1201–9.
Wattmo C, Londos E. Predictors Of Mortality In Early- Versus Late-Onset Alzheimer’s Disease: An 18-Year Follow-Up. Alzheimers Dement. 2018;14:P799.
Soto ME, Andrieu S, Cantet C, Reynish E, Ousset PJ, Arbus C, et al. Predictive value of rapid decline in mini mental state examination in clinical practice for prognosis in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;26:109–16.
Reed C, Lebrec J, Andrews JS, Bruno G, Jones RW. Predictors of Disease Progression in Mild Alzheimer’s Disease Dementia Patients – Results From the Geras Real World Cohort and Expedition Trials. Value Health. 2016;19:A425–A6.
Wilson RS, Li Y, Aggarwal NT, McCann JJ, Gilley DW, Bienias JL, et al. Cognitive decline and survival in Alzheimer’s disease. Int J Geriatr Psychiatry. 2006;21:356–62.
Wattmo C, Londos E, Minthon L. Various measures of progression predicting mortality in Alzheimer’s disease. Alzheimers Dement. 2015;11:P293–P4.
Allan CL, Ebmeier KP. The influence of ApoE4 on clinical progression of dementia: A meta-analysis. Int J Geriatr Psychiatry. 2011;26:520–6.
Wallin A, Blennow K, Zetterberg H, Londos E, Minthon L, Hansson O. CSF biomarkers predict a more malignant outcome in Alzheimer’s disease. Eur J Neurol. 2010;17:44.
Liu E, Ashaye A, Travers K, Strand L, Tanna GL, Wyman BT, et al. A prospective, systematic literature review and meta-analyses to evaluate brain amyloid by positron emission tomography(PET) imaging as a biomarker of Alzheimer’s disease (AD) progression. Alzheimers Dement. 2014;10:P353–P4.
Soares H, Feng S, Florian H, Gold M, Lon HK, Mendonca N, et al. Plasma Tau And Neurofilament Light Chain As A Prognostic Biomarker Of Disease Progression In Early Alzheimer’s Disease. Alzheimers Dement. 2018;14:P1038–P9.
Cepoiu-Martin M, Tam-Tham H, Patten S, Maxwell CJ, Hogan DB. Predictors of long-term care placement in persons with dementia: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2016;31:1151–71.
Download references
Acknowledgements
We would like to thank all authors of the original research studies included in the meta-analysis.
This work was supported by the National Key Research and Development Program of China (grant No. 2021YFC2501200) and the Sichuan Science and Technology Program (grant No. 2022ZDZX0023).
Author information
Authors and affiliations.
Department of Neurology, Laboratory of Neurodegenerative Disorders, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu, 610041, China
Xiaoting Zheng, Shichan Wang, Jingxuan Huang, Chunyu Li & Huifang Shang
You can also search for this author in PubMed Google Scholar
Contributions
XZ and CL conceived and designed the study. XZ and SW selected studies and collected data and quality assessment. JH and CL cross-checked the data and quality assessment. XZ, SW and JH contributed to the statistical analysis. XZ wrote the first draft of the manuscript. XZ, SW, JL and HS revised the manuscript.
Corresponding authors
Correspondence to Chunyu Li or Huifang Shang .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary materials, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Zheng, X., Wang, S., Huang, J. et al. Predictors for survival in patients with Alzheimer’s disease: a large comprehensive meta-analysis. Transl Psychiatry 14 , 184 (2024). https://doi.org/10.1038/s41398-024-02897-w
Download citation
Received : 23 January 2023
Revised : 28 March 2024
Accepted : 03 April 2024
Published : 10 April 2024
DOI : https://doi.org/10.1038/s41398-024-02897-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
The Powerful Case for Redefining Alzheimer’s Disease
The signature case of Alzheimer’s disease was a German woman named Auguste whose mental status began to deteriorate to the extent that her husband brought her to a psychiatry clinic in Frankfurt. As is traditional, the scientific literature until very recently referred to Auguste merely by her first name plus the initial of her last name — Auguste D. She was seen at the Frankfurt psychiatric hospital in 1901, and her attending physician at the time was a young 30-something anatomist turned psychiatrist, Alois Alzheimer. Alzheimer came to this case from early interests in the structure of the brain, basically its anatomy and cellular structure. This very structure-based view of brain function was the context he brought to his clinical experiences in psychiatry, a craft he learned under the guidance of Dr. Emil Sioli in Frankfurt-am-Main.

In April 1906, his old mentor Dr. Sioli sent word to Alzheimer that Auguste D. had died. Alzheimer was pleased to learn that Sioli had arranged for an autopsy and had sent tissue from the brain of the deceased woman to Munich for Alzheimer to examine.
Two features of the preparation in particular caught his eye. The first he described in this way: “Throughout the whole cortex … one finds miliar foci, which are caused by deposition of a peculiar substance in the cortex.” The “peculiar substance” we now know was a waxy form of aggregated protein known as amyloid. The “depositions” would come to be known as amyloid plaques. The second feature he described thusly: “very peculiar changes in the neurofibrils … only a tangle of fibrils indicates where a nerve cell had been previously located.” These peculiar neurofibrils are actually aggregates of a different protein known as tau. We now refer to these aggregates as neurofibrillary tangles.
In this way the odd deposits now known as plaques and tangles became tightly linked to a specific form of dementia. Alzheimer made detailed notes on his discovery and took them to his superior, Emil Kraepelin. He was quite certain that the plaques and tangles were the explanation for the highly unusual behavior of Auguste D. Kraepelin apparently liked the idea enough that he suggested that Alzheimer present his findings at a meeting of German psychiatrists in the fall of 1906. Alzheimer agreed and went to Tübingen that fall to announce his discovery.
The original observation was an important case study, but it was elevated to the level of a disease for reasons that were strategic, not scientific.
The story might have ended here as a long-forgotten case study gathering dust in the archives of medicine. Alzheimer’s boss Kraepelin, however, had other ideas. He too was a believer in the idea that psychiatric disease was caused by changes in the physical structure of the brain. The novel plaques and tangles that Alzheimer had found in the brain of Auguste D. neatly fit that philosophy. Kraepelin was very well-known at the time, in part because he was the author of a widely used textbook, “Psychiatrie . ” Kraepelin would periodically update his textbook to include the latest findings (and maybe to sell more books), and, as luck would have it, at the time that Alzheimer published his case, Kraepelin was preparing the eighth edition. To add more support to his own philosophy of the brain, he decided to include the case of Auguste D. in his revision. One might imagine it felt awkward to include a simple case study in a widely used textbook. Kraepelin cleverly solved this problem by elevating the case of Auguste D. to the status of a disease. He called it Alzheimer’s disease, and he included this new condition in the 1910 edition of “Psychiatrie.”
This was a bold and almost reckless move that, in retrospect, had a huge and outsized influence on the field. In most cases, I like it when scientists are bold. Put up a clever argument, and let a smart and informed debate refine it or rebuke it. Either way, science advances. Reckless is not so good. A textbook paragraph is much weightier than the same paragraph in a journal article or a meeting presentation. Textbooks impart a feeling of permanence to an entry. Their contents are imbued with the unspoken assertion that they represent settled art and thus are not easily questioned. Putting Auguste’s condition in a textbook as a new disease comes pretty close to reckless because, on the flimsiest of grounds, Kraepelin was trying to put the “Case Closed” stamp on this telling of what he called Alzheimer’s disease. It was to be the first of three inflations in the definition of Alzheimer’s disease.
Let’s go back and consider Alzheimer’s findings in the brain of Auguste D. Two unusual features occurred together. Unusual deposits, plaques and tangles, were correlated with a highly unusual dementia. One possibility to explain the presence of plaques and tangles in the brain of a person with dementia is the one Alzheimer and Kraepelin favored: The plaques and tangles caused the dementia. That fit well with their philosophies that the function of the brain was governed largely by its structure. It most likely explains why Alzheimer championed this first explanation and why Kraepelin was so eager to promote it. But perhaps Auguste D.’s peculiar dementia caused the brain changes that led to plaques and tangles. The plaques didn’t cause the disease; the disease caused the plaques.

I’ve told the story of Alzheimer and Auguste D. in great detail because it tells us a lot about why the field is stuck and why a successful treatment for Alzheimer’s has been so slow in coming. The original observation was an important case study, but it was elevated to the level of a disease for reasons that were strategic, not scientific. Both Kraepelin and Alzheimer were subscribers to a school of thought that held that the structure of the brain was the key to its function, and that if the structure became littered with abnormal deposits, its function would also become abnormal. Finding plaques and tangles in the brain of a person with dementia fit that idea, and putting it forward as a hypothesis was reasonable. From these origins, however, the two psychiatrists inadvertently biased the thinking of subsequent generations of physicians and scientists. Their assertion that the correlation of plaques and dementia represented a causal relationship — plaques caused the dementia — has proven very hard to shake off, despite the shortage of evidence to support it.
For Alzheimer and Kraepelin, the rare form of early-onset dementia they named Alzheimer’s disease was, they believed, caused by the deposits they had seen in the brain of Auguste D. That first linkage of deposits and dementia was the Trojan horse that released the soldiers of the second inflation. Published in 1976, the article most commonly cited as the manifesto of this effort was written by Robert Katzman and bore the fearsome title “Editorial: The Prevalence and Malignancy of Alzheimer Disease: A Major Killer.” Katzman began his two-page editorial by arguing that there was no really significant difference between the relatively rare condition that was known at the time as Alzheimer’s disease and the far more common condition known as senile dementia. He then went on to argue that dementia was badly underdiagnosed as a cause of death. He estimated that if the cause of death were adjusted to honestly reflect this fact, dementia was arguably “a major killer.” The real purpose of the editorial, however, was to argue for equating senile dementia with Alzheimer’s disease. This was a bit of a stretch.
To bolster his case for their equivalence, Katzman cited earlier clinical speculation that Alzheimer’s disease and senile dementia were similar in their symptoms. He also cited a pair of papers published a few years earlier. Katzman wrote, based on his analysis of these two papers, that when comparing the microscopic appearance of the brain of a person who had died with Alzheimer’s disease with one who had died with the more common senile dementia, “The pathological findings are identical — atrophy of the brain, marked loss of neurons, neurofibrillary tangles, granulovacuolar changes, and neuritic (senile) plaques.”
The problem is that this is not exactly what the two papers had claimed. In fact, five cases with clinical dementia (10 percent of the authors’ subjects) could not be diagnosed with confidence by the microscopic appearance of the brain, and 40 percent had changes that would have led to a non-Alzheimer’s diagnosis. In the end, only “50% were considered to be cases of senile dementia showing the histological features of Alzheimer’s disease.” This is hardly a rock on which to build the claim that senile dementia and Alzheimer’s disease are one and the same.
Katzman’s inflated view of Alzheimer’s disease, however, soon took hold. By 1980, it had earned a place in the third edition of the “Diagnostic and Statistical Manual of Mental Disorders (DSM-III).” Subsequently, attempts were made to precisely define the pathology needed for a diagnosis of Alzheimer’s disease. Eventually, there were so many “authoritative” sources on how to diagnose Alzheimer’s disease that a well-meaning clinician could surely have been forgiven for getting frustrated about what this newly inflated condition really was and whether it applied to the elderly person sitting in his or her office.
To attempt to deal with this, a working group was assembled under the auspices of the National Institute on Aging (NIA) and the Alzheimer’s Association (then known as the Alzheimer’s Disease and Related Disorders Association — ADRDA) to formalize the clinical diagnosis of Alzheimer’s disease. The working group came up with a list of criteria to diagnose what they called “PROBABLE Alzheimer’s Disease [all caps is their emphasis].” But the group went one step further. They established criteria for a diagnosis of “DEFINITE” Alzheimer’s disease. For this score, you first had to have a clinical diagnosis of dementia, using the following five criteria: dementia established by clinical examination; deficits in two or more areas of cognition (problem solving, language, attention, etc.); progressive worsening; no disturbance of consciousness; and onset between ages 40 and 90, most often after age 65. But you also needed “histopathologic [i.e., microscopic] evidence obtained from a biopsy or autopsy.” And what did they consider definitive histopathologic evidence? Remarkably, they didn’t say. Our well-meaning clinician had to wait until the following year when a separate summary of the workshop was published.
The details are important to the aficionado, but it’s the big picture that is important to us. Alzheimer’s disease was to be defined by the presence of plaques. Yet plaques are a feature that is not present in 15 percent of the people with a clinical diagnosis of Alzheimer’s, and a feature that is present in people of all ages including 30 percent of elderly people without any cognitive impairment. If this doesn’t make sense to you, it’s because it doesn’t make sense.
Alzheimer’s disease is defined by the presence of plaques, a feature that is present in people of all ages, including 30 percent of elderly people without any cognitive impairment.
So, who died and made the pathologist king? There was really no good reason for pathology to trump neurology. You can get a definitive diagnosis of any number of complex brain diseases — autism, depression, schizophrenia, epilepsy, and many others — without any pathological study or live imaging of the brain. If a child psychiatrist diagnoses a young boy as having autism, there is no need for a brain scan to test the psychiatrist’s skill. For the purposes of treatment, the child has autism. If a neurologist diagnoses a person as having Parkinson’s, that’s the diagnosis. They don’t wait with bated breath to find out whether there were α-synuclein deposits in the brain. For the purposes of treatment, the person has Parkinson’s disease. Late-life diseases like Parkinson’s and Huntington’s do have characteristic brain abnormalities, but it is the presentation of the clinical symptoms that allows physicians to have confidence in their diagnosis. To be fair, if an autopsy is done and there are no deposits, the diagnosis is questioned, but not rejected . The clinical diagnosis overrules the pathology. As it should.
Then our clinical trials started failing, our antibody trials in particular. In the basic research laboratories of the world, data kept accumulating that violated the expectations of an amyloid-only definition for Alzheimer’s disease biology. In response the NIA began to recognize that there was a “broad consensus … that the criteria should be revised to incorporate state-of-the-art scientific knowledge.” The result was a truly comprehensive review — a compendium of four papers comprising reports of three working groups plus an introductory summary. Coming as it did, a full quarter century after the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) working group met, it would have been an ideal platform to announce the decision to cut the definition of Alzheimer’s disease loose from the presence of plaques. Instead the experts in the field doubled down and bet the store on amyloid. In doing so, they made the entire situation much, much worse.
Writing the first paper was a clear struggle for its authors. The task before the group was to come up with a recommendation for how practicing physicians should decide whether or not a living person, sitting in front of them in their office, had Alzheimer’s disease. But reading their words, it is quite clear they were not about to be drawn into saying in print that amyloid, or any other “biomarker” (like tau), should be used to define Alzheimer’s disease. They made a clean and explicit separation between a clinical diagnosis of Alzheimer’s disease dementia and what they called the “pathophysiological process of Alzheimer’s disease.” What the group essentially said was that in the clinic the presence or absence of amyloid is just a piece of information that can be helpful in reaching a diagnosis, and nothing more.
The second paper in the series was important for the attempt of the working group to define what is known as MCI (mild cognitive impairment). The goal was a useful one: to try to clinically identify Alzheimer’s disease as early as possible so that treatment could begin when the probability for meaningful impact was the greatest. These authors were a separate group of neurologists. They too wrestled with how to incorporate biomarkers into their recommendations. In the end, they conclude, “Considerable work is needed to validate the criteria that use biomarkers and to standardize biomarker analysis for use in community settings.” Like the clinicians in the first paper, they are willing to say that people who have no evidence of amyloid or tau are “unlikely” to have MCI due to Alzheimer’s disease, but they add the caveat that “… such individuals may still have AD, … [but for these patients] … a search for an alternate cause of the MCI syndrome is warranted.” As with the first group, the MCI paper is arguing that while evidence of amyloid and tau may be useful information, it is not definitive.
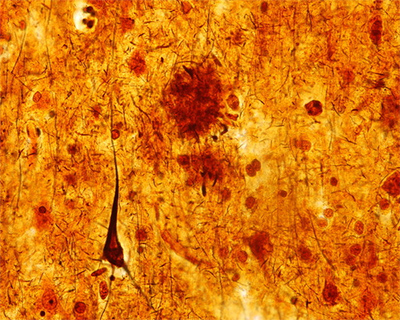
The first two papers had basically said that pathology was only one of several things to consider in reaching a diagnosis. The third paper in the series, however, put the pathophysiology front and center in our definition of Alzheimer’s disease. More than that, however, it exploded our definition of Alzheimer’s disease almost beyond recognition. This is the third inflationary event in the history of Alzheimer’s disease, and unfortunately, compared to the expansions ushered in by Kraepelin and later by Katzman, this third inflation was bigger and more destructive to the field.
It wreaked havoc by “redefining the earliest stages of Alzheimer’s disease.” This redefinition created what was in effect a totally new stage of the disease process: preclinical Alzheimer’s disease. By “preclinical” the authors meant that people with plaques in their brain (or the wrong amount of amyloid in their cerebrospinal fluid) are not healthy people. They already have Alzheimer’s disease. They just haven’t started to show the symptoms yet. In this telling of the story, the 30 percent of elderly people who have plaques but also have normal brain function are not simply healthy people with plaques. They are sick people without symptoms.
This may seem to be just semantics, but it’s actually an incredibly audacious claim. About 1 in every 10 people over the age of 65 have some symptoms of Alzheimer’s disease. That’s 10 percent of the elderly. The other 90 percent have normal, age-appropriate brain function. But we’ve already learned that about a third of the people in this cognitively normal group have significant levels of plaques in their brain. Therefore, according to this new expanded definition, they have preclinical Alzheimer’s disease. The authors are essentially arguing that we should increase our estimates of the total number of cases of Alzheimer’s disease by threefold. Worse still, the authors are making this recommendation despite the fact that there are reasonable doubts as to whether or not amyloid causes Alzheimer’s disease. Ah, you may say, but aren’t those doubts just the rantings of a few crazed misfits at the fringes of the field? Not really. We just read about these same doubts in the first two papers in the series.
The authors of the third paper understood that they were redefining Alzheimer’s disease, and they were clearly conflicted about what they were doing. In the final paragraph they admit, “The definitive studies … are likely to take more than a decade to fully accomplish.” Said in plain language, we have this idea, but we don’t have the data to back it up. Still, we are going to go with our gut, upend both basic and clinical research, and you’re going to have to live with it because most of your grant money comes from the NIA and the Alzheimer’s Association and their names are on this paper.
This is a huge problem because the definition of a disease is one of its most important attributes. Without a precise and accurate definition, there is no way to find a cure for any disease. Sadly, throughout the long history of Alzheimer’s disease research, strategy and politics have overruled science in the push to apply the label of Alzheimer’s disease to an ever-larger fraction of age-related cognitive decline and aging. As a result, we are left with basically no definition — or at least none of any value. Being in this situation, we are effectively blocked from making any real progress toward treatment. For proof of this, one needs to look no further than the unbroken string of expensive clinical trial failures. Our political calculus has overruled our common sense and caused us to stop listening to our own data — a clear example of how not to study a disease.
Karl Herrup is Professor of Neurobiology and an Investigator in the Alzheimer’s Disease Research Center at the University of Pittsburgh School of Medicine. He is the author of “ How Not to Study a Disease: The Story of Alzheimer’s ,” from which this article is adapted.
Case Study Unlocks Clues to Rare Resilience to Alzheimer’s Disease
Posted on May 30th, 2023 by Lawrence Tabak, D.D.S., Ph.D.

Biomedical breakthroughs most often involve slow and steady research in studies involving large numbers of people. But sometimes careful study of even just one truly remarkable person can lead the way to fascinating discoveries with far-reaching implications.
An NIH-funded case study published recently in the journal Nature Medicine falls into this far-reaching category [1]. The report highlights the world’s second person known to have an extreme resilience to a rare genetic form of early onset Alzheimer’s disease. These latest findings in a single man follow a 2019 report of a woman with similar resilience to developing symptoms of Alzheimer’s despite having the same strong genetic predisposition for the disease [2].
The new findings raise important new ideas about the series of steps that may lead to Alzheimer’s and its dementia. They’re also pointing the way to key parts of the brain for cognitive resilience—and potentially new treatment targets—that may one day help to delay or even stop progression of Alzheimer’s.
The man in question is a member of a well-studied extended family from the country of Colombia. This group of related individuals, or kindred, is the largest in the world with a genetic variant called the “Paisa” mutation (or Presenilin-1 E280A ). This Paisa variant follows an autosomal dominant pattern of inheritance, meaning that those with a single altered copy of the rare variant passed down from one parent usually develop mild cognitive impairment around the age of 44. They typically advance to full-blown dementia around the age of 50 and rarely live past the age of 60. This contrasts with the most common form of Alzheimer’s , which usually begins after age 65.
The new findings come from a team led by Yakeel Quiroz , Massachusetts General Hospital, Boston; Joseph Arboleda-Velasquez, Massachusetts Eye and Ear, Boston; Diego Sepulveda-Falla, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; and Francisco Lopera, University of Antioquia, Medellín, Colombia. Lopera first identified this family more than 30 years ago and has been studying them ever since.
In the new case report, the researchers identified a Colombian man who’d been married with two children and retired from his job as a mechanic in his early 60s. Despite carrying the Paisa mutation, his first cognitive assessment at age 67 showed he was cognitively intact, having limited difficulties with verbal learning skills or language. It wasn’t until he turned 70 that he was diagnosed with mild cognitive impairment—more than 20 years later than the expected age for this family—showing some decline in short-term memory and verbal fluency.
At age 73, he enrolled in the Colombia-Boston biomarker research study (COLBOS ). This study is a collaborative project between the University of Antioquia and Massachusetts General Hospital involving approximately 6,000 individuals from the Paisa kindred. About 1,500 of those in the study carry the mutation that sets them up for early Alzheimer’s. As a member of the COLBOS study, the man underwent thorough neuroimaging tests to look for amyloid plaques and tau tangles, both of which are hallmarks of Alzheimer’s.
While this man died at age 74 with Alzheimer’s, the big question is: how did he stave off dementia for so long despite his poor genetic odds? The COLBOS study earlier identified a woman with a similar resilience to Alzheimer’s, which they traced to two copies of a rare, protective genetic variant called Christchurch. This variant affects a gene called apolipoprotein E ( APOE3 ), which is well known for its influence on Alzheimer’s risk. However, the man didn’t carry this same protective variant.
The researchers still thought they’d find an answer in his genome and kept looking. While they found several variants of possible interest, they zeroed in on a single gene variant that they’ve named Reelin-COLBOS . What helped them to narrow it down to this variant is the man also had a sister with the Paisa mutation who only progressed to advanced dementia at age 72. It turned out, in addition to the Paisa variant, the siblings also shared an altered copy of the newly discovered Reelin-COLBOS variant.
This Reelin-COLBOS gene is known to encode a protein that controls signals to chemically modify tau proteins , which form tangles that build up over time in the Alzheimer’s brain and have been linked to memory loss. Reelin is also functionally related to APOE , the gene that was altered in the woman with extreme Alzheimer’s protection. Reelin and APOE both interact with common protein receptors in neurons. Together, the findings add to evidence that signaling pathways influencing tau play an important role in Alzheimer’s pathology and protection.
The neuroimaging exams conducted when the man was age 73 have offered further intriguing clues. They showed that his brain had extensive amyloid plaques. He also had tau tangles in some parts of his brain. But one brain region, called the entorhinal cortex, was notable for having a very minimal amount of those hallmark tau tangles.
The entorhinal cortex is a hub for memory, navigation, and the perception of time. Its degeneration also leads to cognitive impairment and dementia. Studies of the newly identified Reelin-COLBOS variant in Alzheimer’s mouse models also help to confirm that the variant offers its protection by diminishing the pathological modifications of tau.
Overall, the findings in this one individual and his sister highlight the Reelin pathway and brain region as promising targets for future study and development of Alzheimer’s treatments. Quiroz and her colleagues report that they are actively exploring treatment approaches inspired by the Christchurch and Reelin-COLBOS discoveries.
Of course, there’s surely more to discover from continued study of these few individuals and others like them. Other as yet undescribed genetic and environmental factors are likely at play. But the current findings certainly offer some encouraging news for those at risk for Alzheimer’s disease—and a reminder of how much can be learned from careful study of remarkable individuals.
References :
[1] Resilience to autosomal dominant Alzheimer’s disease in a Reelin-COLBOS heterozygous man . Lopera F, Marino C, Chandrahas AS, O’Hare M, Reiman EM, Sepulveda-Falla D, Arboleda-Velasquez JF, Quiroz YT, et al. Nat Med. 2023 May;29(5):1243-1252.
[2] Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: a case report . Arboleda-Velasquez JF, Lopera F, O’Hare M, Delgado-Tirado S, Tariot PN, Johnson KA, Reiman EM, Quiroz YT et al. Nat Med. 2019 Nov;25(11):1680-1683.
Alzheimer’s Disease & Related Dementias (National Institute on Aging/NIH)
“ NIH Support Spurs Alzheimer’s Research in Colombia ,” Global Health Matters, January/February 2014, Fogarty International Center/NIS
“ COLBOS Study Reveals Mysteries of Alzheimer’s Disease ,” NIH Record, August 19, 2022.
Yakeel Quiroz (Massachusetts General Hospital, Harvard Medical School, Boston)
Joseph Arboleda-Velasquez (Massachusetts Eye and Ear, Harvard Medical School, Boston)
Diego Sepulveda-Falla Lab (University Medical Center Hamburg-Eppendorf, Hamburg, Germany)
Francisco Lopera (University of Antioquia, Medellín, Colombia)
NIH Support: National Institute on Aging; National Eye Institute; National Institute of Neurological Disorders and Stroke; Office of the Director
Share this:
- Click to share on Facebook (Opens in new window)
- Click to share on Twitter (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Posted In: News
Tags: Alzheimer’s disease , APOE3 , brain , Christchurch variant , cognitive resilience , Colombia , Colombia-Boston biomarker research study , dementia , genetics , genomics , global health , Paisa mutation , Paisa variant , Presinilin-1 , Reelin-COLBOS gene variant , tau , tau protein
One Comment
The entorhinal cortex is a hub for memory, navigation, and the perception of time. Also pheromone reception. 100mg of healthy adult male facial skin surface lipid pheromone that is normally passed in kissing, allows laughing, singing, and real joy again in an Alzheimer’s patient. The pheromone, the grease on your nose, is always in sight whenever your silly eyes are open. It halts the progression of Alzheimer’s Disease, also FT dementia, Tourette’s, Parry-Romberg, epilepsy, …
Leave a Comment Cancel reply
@nihdirector on twitter, nih on facebook.
Kendall Morgan, Ph.D.
Comments and Questions
If you have comments or questions not related to the current discussions, please direct them to Ask NIH .
You are encouraged to share your thoughts and ideas. Please review the NIH Comments Policy

- Visitor Information
- Privacy Notice
- Accessibility
- No Fear Act
- HHS Vulnerability Disclosure
- U.S. Department of Health and Human Services
- USA.gov – Government Made Easy
Discover more from NIH Director's Blog
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Case Study: A Precision Medicine Approach to Multifactorial Dementia and Alzheimer’s Disease
Mary kay ross.
a Brain Health and Research Institute, Seattle, WA, USA
b Washington University School of Medicine, St. Louis, MO, USA
Kristine L. Lokken
c Department of Psychiatry and Behavioral Neurobiology, University of Alabama-Birmingham, Birmingham, AL, USA
Dale E. Bredesen
d Department of Molecular and Medical Pharmacology, UCLA, Los Angeles, CA, USA
Jared C. Roach
e Institute for Systems Biology, 401 Terry Ave N, Seattle, WA, USA, 98109
Cory C. Funk
Nathan price, noa rappaport, james r. heath.
We report a case of a patient with mixed dementia successfully treated with a personalized multimodal therapy. Monotherapeutics are inadequate for the treatment of Alzheimer’s disease (AD) and mixed dementia; therefore, we approach treatment through an adaptive personalized multimodal program. Many multimodal programs are pre-determined, and thus may not address the underlying contributors to cognitive decline in each particular individual. The combination of a targeted, personalized, precision medicine approach using a multimodal program promises advantages over monotherapies and untargeted multimodal therapies for multifactorial dementia. In this case study, we describe successful treatment for a patient diagnosed with AD, using a multimodal, programmatic, precision medicine intervention encompassing therapies targeting multiple dementia diastheses. We describe specific interventions used in this case that are derived from a comprehensive protocol for AD precision medicine. After treatment, our patient demonstrated improvements in quantitative neuropsychological testing, volumetric neuroimaging, PET scans, and serum chemistries, accompanied by symptomatic improvement over a 3.5-year period. This case outcome supports the need for rigorous trials of comprehensive, targeted combination therapies to stabilize, restore, and prevent cognitive decline in individuals with potentially many underlying causes of such decline and dementia. Our multimodal therapy included personalized treatments to address each potential perturbation to neuroplasticity. In particular, neuroinflammation and metabolic subsystems influence cognitive function and hippocampal volume. In this patient with a primary biliary cholangitis (PBC) multimorbidity component, we introduced a personalized diet that helped reduce liver inflammation. Together, all these components of multimodal therapy showed a sustained functional and cognitive benefit. Multimodal therapies may have systemwide benefits on all dementias, particularly in the context of multimorbidity. Furthermore, these therapies provide generalized health benefits, as many of the factors – such as inflammation – that impact cognitive function also impact other systems.
INTRODUCTION
Recently, there has been increased interest in multimodal interventions for reducing the risk of Alzheimer’s Disease (AD). For example, the World Health Organization [ 1 ] published guidelines to reduce risk for cognitive decline and dementia that emphasize multimodal interventions. Reported lifestyle interventions include increased cognitive, physical, and social activity for reducing risk of cognitive decline [ 2 – 4 ]. Combination therapies and multimodal approaches have produced therapeutic success for chronic illnesses such as cancers, HIV, and cardiovascular disease. These initial successes demonstrate that targeted, multimodal approaches to AD deserve further and more detailed study.
In this paper, we present a single case study with full details of the clinical course and outcome. We describe a successful treatment response for a patient with previously diagnosed AD using a precison medicine, multimodal intervention with specific focus on treating potential contributors such as steroid hormone deficiency, thyroid deficiency, kidney function, liver function, vascular health, tick-borne infections, mercury toxicity, and mycotoxins. Each of these may independently contribute in this individual as an underlying driver of cognitive decline and the AD disease process. In presenting this case, we take a step towards filling a paucity in the literature for methods to evaluate and treat cognitive symptoms secondary to biotoxicity. Treating multimodal disease with potentially synergistic targeted interventions towards each disease modality should be a paradigm for the future of personalized medical treatment of many chronic diseases, particularly those that affect aging individuals.
CASE PRESENTATION
Background information.
A 78-year-old, left-handed retired female physician presented with a one-year history of severe, progressive memory loss, such that her significant other described her memory for recent events as “disastrous.” She described a lifelong mild dyslexia, and amnesia for many events of her early childhood and adolescence. She noted an awareness of mild cognitive problems for approximately 20 years, but these worsened in the year prior to evaluation. She also noted poor recall for movies that she had watched and books that she had read. She noted problems with name recall, and often called her pets by the wrong name. She noticed increased susceptibility to stress and fatigue which seemed to worsen when her son died a tragic death in 2001. At presentation, she reported leading an active life, enjoying golfing, hiking, birding, and gardening. She worked in private practice as a psychiatrist until she retired at the age of 75. She has always been actively involved in many civic associations, and remains active in political circles. She has a family history of dementia and memory issues: her mother developed dementia secondary to hydrocephalus, and her sister developed memory problems but died from an aneurysm at the age of 80. She reported exposure to mold in her partner’s home. She also stated that she has several dogs that she sleeps with in her bed. She lives in New York, does not use tick spray on the dogs, and reports an average of 10 tick bites per year. She had a remote history of Bell’s palsy on the left side of unknown etiology. Her past medical history is also significant for Raynaud’s syndrome, elevated gamma-glutamyl transpeptidase secondary to primary biliary cholangitis (PBC), and elevated mercury. She had elevated inflammatory markers suggesting exposure to biotoxins. She was diagnosed with early AD by her neurologist in 2016. In particular, a fluorodeoxyglucose-positron emission tomography (FDG-PET) scan revealed decreased glucose utilization in the anterior superior precuneus bilaterally and the anterolateral left temporal lobe which is consistent with the earliest manifestations of AD. Her neurologist recommended treatment with donepezil and memantine. She refused both because she had read about their minimal effects on decline and because she had read about anecdotal successes with programmatic treatment.
Mulitmodal Interventions for Alzheimer’s Disease
Recently, there has been increased interest in multimodal interventions for reducing the risk of Alzheimer’s Disease (AD). The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) was the first randomized controlled trial (RCT) demonstrating a beneficial effect of a 2-year multidomain intervention (using nutrition, physical activity, and cognitive training) on cognitive performance in older adults who were at risk for dementia based on vascular parameters [ 2 ]. World Health Organization guidelines include multimodal interventions [ 1 ]. Many others have reported success with lifestyle intervention (increased cognitive, physical, and social activity) in reducing risk of cognitive decline among individuals with mild cognitive impairment (MCI), and with aerobic exercise in improving executive function in those with early stage-cognitive impairment [ 5 , 6 ].
A recent publication by Isaacson et al. [ 7 ] provided further support for the application of multimodal lifestyle interventions to improve cognition and reduce AD and cardiovascular risk scores in patients at risk for AD. The authors used a personalized medicine approach when considering treatment options for patients. They found that multidomain interventions reduced AD and cardiovascular risk scores. In addition, in those seeking prevention, both high and low levels of compliance were associated with improved cognition with individualized multidomain interventions. Patients already exhibiting early cognitive impairment (MCI) showed improved cognition only with high levels of compliance with the individualized multidomain interventions, and cognition declined for those in the low compliance group. Anecdotal sustained improvements have been reported in over 100 patients with AD or MCI using a personalized, precision medicine approach that addresses presumptive contributors to cognitive decline, such as insulin resistance, systemic inflammation, and pathogens [ 8 ], although these are often not reported with detailed descriptions of the interventions and clinical course for each patient. Combination therapies and multimodal approaches have produced therapeutic success for chronic illnesses such as cancer, HIV, and cardiovascular disease [ 3 ]. Because of the complex nature of AD, with many potential and variable contributors, the initial success in prevention trials, and the anecdotal success with cognitive improvement in MCI and AD, a targeted, multimodal approach deserves further detailed study and documentation.
Individuals may vary due to genetics, environment, and lifestyle. Our precision medicine approach considers individual variability by utilizing an extensive evaluation including detailed medical history, lifestyle variables, blood biomarkers, neuroimaging with volumetrics, and quantitative neurocognitive assessments. Practitioners utilizing this approach can further examine whether patients with cognitive issues harbor a significant burden of toxicity contributing to their cognitive decline. Possible multimodal lifestyle interventions for cognitive decline in AD include diet, nutrition, exercise, mindfulness, and stress management. In addition to the effects of lifestyle, epidemiological and pathological studies suggest that there are numerous other underlying drivers such as environmental toxins and chronic infections. Environmental toxins may include mercury from seafood and amalgam fillings or biotoxins such as mycotoxins from mold exposure. Chronic infections could include herpes, gingival disease, COVID-19, or tick born disease such as Lyme disease [ 9 – 11 ]. The recent COVID-19 pandemic has raised the possibility of long-term cognitive effects given the neuroinvasive potential of this novel coronavirus [ 12 ]. Furthermore, pandemic stressors may worsen cognitive issues [ 13 ].
One study showed an 8% increase in hippocampal volume over a 12-week period with a multifaceted lifestyle program [ 14 ]. Additional literature has noted the influence of lifestyle-related factors such as obesity on brain atrophy, and the beneficial effects of physical activity and diet on gray matter volume [ 15 , 16 ]. Physical activity itself has also been linked with reduced burden of amyloid on PET scans [ 17 ]. A systematic review of multiple lifestyle factors including alcohol use and smoking suggested that fMRI also reflects both positive and negative influences of these factors on the physiologic changes reflected by this modality [ 18 ]. A randomized clinical trial of physical activity in 24 elderly women (75–83 years old) assigned either to 3 months of biweekly 90-min sessions focused on aerobic exercise, strength training, and physical therapy versus rest showed that the intervention group had improved glucose metabolism [ 19 ]. This parallels a larger randomized study showing improved hippocampal volumes in a physical activity group versus a passive stretching group on volumetric MR quantification [ 20 ]. A recent Canadian study suggested that a screening imaging program for dementia based in part by modifiable risk factors was financially manageable [ 21 ].
We recommended multimodal interventions for the treatment of this patient. These were personalized, and in addition to diet and exercise interventions, included hormone replacement, DMSA therapy for posisble heavy metal toxicity, Lyme therapy for posisble Borrelia infection, and cognitive training.
DMSA Therapy.
DMSA has a half life of 4 hours and it is important to keep the drug at a steady state so that the bound metals do not become reabsorbed and deposit in other tissues of the body. It is important to note that the order in which treatment progressed was very slow and chelation of heavy metals was actually one of the last therapies. The patient was treated in a progressive order that the chelation of heavy metals was one of the last things she was treated for in an effort to ensure that her gut health was good and that she was stronger and able to handle the chelation of mercury. With a history of PBC there was concern about her ability to sustain good liver detoxification. She was very sensitive to DMSA and noted a decrease in her cognition when she took a dose for provocation. While on chelation she was given herbs that enhance liver detoxification along with a rigorous nutrient regimen. The chelation protocol is: (1) Low Dose DMSA 10 mg/kg is the dose to be taken every 4 hours around the clock for 4 days drinking at least 8 ounces of water with each dose; (2) 10 days off between each 4 day treatment cycle, and (3) during the holiday between oral chelation we recommended patients replenish minerals and nutrients that have been removed with chelation.
Lyme Therapy.
Given dementia symptoms and the patients serologies, medications used for treatment of Lyme are: (1) Cefdinir 300 mg bid; (2) Azithromycin 500 mg qd; and (3) the Byron White herbal regimen.
Cognitive Training.
This patient engages in cognitive training using BrainHQ by Posit Science [ 22 ]. When she first embarked on this program she was unable to do BrainHQ and found it very frusterating. She initially was able to do a brain training program Elevate. She has since graduated to the BrainHQ and is now in the 94 th percentile. She states that she uses her BrainHQ score to help let her know how her cognition is going and it alarms her to any new problems. She is currently using BrainHQ 45 minutes per day.
Baseline Neurological and Cognitive Assessments
The patient underwent memory testing at the age of 65 while attending Canyon Ranch Health Resort. Testing consisted of the Wechsler Memory Scale-III (WMS-III). Results indicated that her Working Memory Index (WMI) was in the average range of ability (WMS-III WMI=108, 70 th percentile); however, her Immediate Memory Index (89, 23 rd percentile), Delayed Memory Index (88, 21 st percentile), and her General Memory Index (88, 21 st percentile) were all in the low average range of ability (see Table 1 ). Interpretation by the health resort clinical psychologist was that her memory was poorer than expected based on her vocation of medical doctor. It was therefore recommended that she undergo more extensive neuropsychological testing.
Hippocampal Volume Measurements. From 2016 to 2019 overall hippocampal volume increased 3%. Timepoints: 1 = 2016; 2 = 2017; 3 = 2019.
However, the patient did not seek further assistance for her memory complaints for several years due to actually forgetting. Her cognitive problems continued to progress insidiously, with an acceleration in cognitive decline starting at approximately age 74. She noted that she was starting to mix up the names of people and pets and that she was starting to have difficulty with navigating spaces, such as having difficulty finding her way back to her table at a restaurant after using the bathroom. An acquaintance encouraged her to look into integrative approaches to brain health, and she began a multimodal program under the supervision of Dr. Ross at what is now the BHRI clinic. She initiated the multimodal program in January 2017 at age 75.
Prior to starting the program, she saw a neurologist in order to obtain confirmatory diagnostic testing. She scored a 28/30 on the MMSE at the initial neurology evaluation in 9/2016. Her neurological exam was normal. She underwent an MRI of the brain in 9/2016 that revealed mild biparietal atrophy with hippocampal cysts on the right side. MRI also revealed a few scattered small foci of hyperintensity in the bilateral hemispheric white matter and paramedian pons, and evidence of a partially empty sella with flattening of the pituitary gland. Volumetric analysis revealed decreased hippocampal volume bilaterally. FDG-PET conducted in 9/2016 indicated mildly decreased FDG activity in the anterior superior precuneus bilaterally as well as in the anterolateral left temporal lobe. The findings were thought to represent early AD pathology and she was referred for neuropsychological testing.
A full neuropsychological evaluation was conducted in 11/2016. She reported to the evaluating neuropsychologist that her overall energy and cognitive problems had improved somewhat over the past few months with the lifestyle changes. Even so, her neuropsychological performances revealed impairments in reaction time, visual organization/constructional skills, and learning/recognition of unstructured information, within the context of estimated high average baseline intellectual capacity. She struggled on the testing with the intial learning of a word list and did not appear to benefit from repetition of the list. In fact, she reported that the words were “dropping out” following the learning trials. Her learning slope was in the borderline-impaired range (7 th percentile). Recognition performance was also notable for a remarkably high number of false positive errors. Learning improved when the verbal material was presented in a contextual format of a story. Reaction time on a computerized measure was slowed, and her approach to drawing a complex geometric figure was disorganized and poorly planned, resulting in inaccuracies in the placement and spacing of the figure details. She performed in the borderline-impaired range on this task (2 nd -5 th percentile). This constellation of findings was felt to be most compatible with early AD.
Clinical Course
A multimodal program was prepared for this patient tailoring the appropriate neutraceuticals and medications to fit this patient’s specific needs. Labs and biomarkers were evaluataed and targeted neutraceuticals were used to optimize levels of hormones, vitamins, glucose, and insulin. Her history of tick bites and Bell’s palsy prompted a workup that revealed a positive Western blot IgM for Lyme disease. She was placed on oral antibiotics and an herbal regimen for 3 months. Her thyroid and sex hormones were suboptimal, so she was placed on bioidentical hormone therapy as well as natural thyroid replacement. She was tested for heavy metals using a pre- and post-provocation test (Doctors Data) that revealed elevated mercury. She initially went to an integrative practice in New York for IV glutathione while she underwent oral chelation [ 23 ]. She was placed on an oral chelation protocol using DMSA. The patient was hospitalized with ehrilichiosis and received IV antibiotics; she states that she noticed an increase in her energy following the antibiotics. She had a home sleep study in 2020 and was found to have moderate sleep apnea. She began CPAP, and now reports even better energy and subjective cognitive function. She also trains her cognitive function with BrainHQ. She also uses a Vielight Gamma, Oura Ring, and MUSE Headband. She meets with a neuro physical therapist via Zoom twice weekly for excise sessions that are coupled with dual tasking. She feels that exercising with dual tasking has contributed greatly to her cognitive rehabilitation. We measured system-wide clinical markers and followed their longitudinal trajectory ( Figure 1 ). The average date of the inflection point towards a positive trend in these markers was in May 2017 five months after start of therapy, suggesting a synergistic and cumulative impact of multimodal interventions within months of commencing therapy.

Trajectories of select clinical chemistries. Hippocampal volume measurements are shown for comparison. Orange line; upper bound of reference. Blue line; lower bound of reference. Multimodal treatment of the entire individual results in subtle changes that impact many physiological subsystems. Although these impacts often change trajectories only within traditional reference ranges, the cumulative effect on well being may be pronounced.
Neuroimaging
The patient’s MRI of the brain on 9/2016 showed a quantified hippocampal volume of 16th percentile on NeuroQuant. Her FDG-PET scan was qualitatively interpreted as having low metabolism in the anterior precuneus and anterolateral temporal lobes. This report also noted a qualitative description of “mild biparietal atrophy.” Neuroquant, the software program that measures these volumes initially has been shown to underestimate hippocampal volume [ 24 ] compared to another FDA-cleared program called Neuroreader, which is also utilized for MRI brain structural quantification [ 25 ]. While each software program compares brain volumes to a normal database, they utilize different databases. It has been published with Neuroreader that its normal database comes from the Alzheimer’s Disease Neuroimaging Initiative Database (ADNI), a well validated and well published research cohort [ 25 ]. It should be noted therefore that while the intial Neuroquant analysis suggested a hippocampal volume at 16 th percentile, the Neuroreader analysis for that same scan showed a more normal value of 54 th percentile. It should also be noted that while the intial FDG PET scan suggested AD based on the parietal hypometabolism, there was no medial temporal lobe hypometabolism reported, although as noted above, there was hypometabolism noted in the precuneus.
The visually interpreted mild biparietal atrophy on the MRI from 2016 was not supported by the quantitative result from Neuroreader, showing a normal parietal lobe volume of 49 th percentile. This discrepancy highlights the need for quantitative evaluations of atrophy, which are usually more accurate than visual evaluations [ 26 ]. Longitudinal evalutions are also key in determining suggestive evidence of AD, since progressive atrophy is noted in conjunction with neurodegenerative disease [ 27 ].
With respect to these longitudinal evaluations, the 2017 brain MRI scan showed a reduction in hippocampal volumes compared to 2016, and this was observed with Neuroquant, as well, although, again, with a lower percentile from that scan at 11 th percentile compared to the still normal hippocampal volume percentile on Neuroreader of 50 th percentile. Additionally, as with the 2016 scan, none of the patient’s brain volumes were found to be abnormally low. It should also be noted that while the Neuroquant percentiles of 11 th or 16 th percentile may be considered abnormally low, the Neuroquant software program uses a 5 th percentile cutoff to determine abnormally low volume,s while Neuroreader uses a 25 th percentile threshold. Overall, the patient’s hippocampal volume from 2016 to 2017 declined on Neuroreader by 3%, which is an abnormal rate of atrophy, as it should normally be declining by only 0.5% per year. Such enhanced rate of decline is a poor prognostic feature in Alzheimer’s patients and in the the risk for conversion from MCI to AD [ 27 ]. However, from 2017 to 2019 the patient’s hippocampal volume to mTIV ratio showed an increase by 8% to the 62nd percentile – up considerably from the 50 th percentile in 2017 ( Table 1 ). Previous publications have shown that the hippocampus is neuroplastic enough to increase in volume when exposed to multimodal personalized treatment programs and lifestyle changes. The volumes for other brain regions, as with 2017 and 2016 studies, were found to be in the normal range. The summary of changes in hippocampal volumes are shown in Figure 2 .

Hippocampal volume changes in the patient from 2016 to 2019. The hippocampal volume decreased from 2016 to 2017 (red line) and then increased between 2017 and 2019 (green line). Volumes were determined with Neuroreader software.
Longitudinal Biomarker Analyses
For each biomarker, we fit a quadratic regression to all longitudinal values. To compute the inflection point (typically the nadir) for each trajectory, we computed the vertex of the parabola resulting from the quadratic regression. In some cases, such as monotonically increasing values (e.g., Vitamin D) we did not compute an inflection point. For the following analytes dates of inflection are: GGT 11/4/16, BUN 7/3/17, alkaline phosphate 12/9/16, homocysteine 9/12/17, ALT 5/15/17, bilirubin 12/21/17. The average inflection date is May 23, 2017. These inflection points group together at approximately the same time (late 2016 through 2017) which is consistent with the timing of full impact of the multimodal intervention.
Improvements in Cognition and Function
As noted above, the patient’s presentation, pattern of cognitive decline, chronicity and progression, neuropsychological testing, MRI volumetrics, and FDG-PET scan results were compatible with a diagnosis of early AD. She has undergone treatment from 2016 to the present. From 2016 until 2020, the patient noted marked subjective improvements in memory. She noted that she was able to remember her golf strokes and those of her friends once again. She no longer failed to feed the parking meters and did not leave her car in the road while still running. Her significant other noted that her memory improved from “disastrous” to “just plain lousy” and ultimately to “normal.”
In the later part of 2019 this patient was able to fly from New York to Seattle unaccompanied and navigated the entire trip on her own with no problem; a patient dignosed with AD in 2016 would most likely not be traveling across the country alone 3 years later. These marked subjective changes were accompanied by objective changes. She consistently used BrainHQ cognitive training; her position in the reference range provided by BrainHQ improved from 9 th to 97 th percentile. Her Montreal Cognitive Assessment (MoCA) was 23 in 10/2017, was stable at 23 in 12/18 and improved to 26 in 11/2019. Her hippocampal volume increased from 50 th to 62 nd percentile, a volume increase of 8%; a positive change this large over this period of time was seen in only 3% of reference AD participants in ADNI [ 28 , 25 ]. Her FDG-PET scan also showed improvement. Her initial FDG-PET revealed mildly decreased FDG activity in the anterior superior precuneus bilaterally as well as in the anterolateral left temporal lobe. The remainder of the brain parenchyma had normal ativity. An interpretation could be that the mild biparietal and hippocampal volume loss with concordant subtle FDG hypometabolism represented the earliest imaging manifestations of underlying AD pathology in her case [ 14 ]. The repeat FDG-PET, which was conducted on the same machine, revealed mild patchy hypometabolism of the superior parietal and anterior temporal lobes along with the superior parietal volume loss which was unchanged from the previous measurement. These findings suggested that age-related changes could account for mild cognitive impairment.
The patient underwent follow-up neuropsychological testing in 11/2019 at the age of 78, after following multimodal treatment recommendations for over three years. Results were compared with her memory testing conducted in 12/2006 at the age of 65 and full neuropsychological testing conducted on 11/2016 at the age of 75 ( Table 2 ).
Neuropsychological Test Performances.
Her estimated intellectual function was stable over time. In 11/2019, she obtained an estimated Full-Scale IQ of 122 which corresponds with the 93 rd percentile and the superior range of functioning. This was considered commensurate with her performance of an estimated Full-Scale IQ of 117 (87 th percentile, high average range) at the 2016 neuropsychological evaluation.
In 11/2019, she scored a 26/30 on the MoCA and a perfect score of 58/58 on the WMS-IV Brief Cognitive Status Evaluation. Her performance on the MoCA showed improvement at this 2019 timepoint in comparison with scores of 23/30 in 2/2017, 23/30 on 10/2017 and 23/30 in 12/2018. Her performance on tests of basic attention, working memory, and processing speed were in the average range (WAIS-IV Digit Span, 75 th percentile; WAIS-IV Coding, 63 rd percentile; Trailmaking Test – Part A, 58 th percentile). These performances reflected slight improvements in comparison to her 2016 evaluation. Performances on measures of both phonemic and semantic verbal fluency were slightly declined in comparison to her 2016 evaluation. She performed at the 32 nd percentile on a test of semantic verbal fluency at the 2019 evaluation, in comparison to the 63 rd percentile although both performances were in the average range of ability. She performed in the low average range on a test of phonemic verbal fluency (FAS, 21 st percentile) at the 2019 evaluation, which was slightly lower than her 2016 performance (39 th percentile).
In terms of visuospatial skills, she had significant difficulty with copying the cube on the MoCA at past evaluations but was able to complete the task with full credit given at the most recent evaluation in 2019. She performed in the average range when copying a complex figure (Rey Complex Figure Test – Copy, 32/36 which is >16 th percentile), reflecting marked improvement in her performance in comparison to the 2016 evaluation (Rey Complex Figure Test – Copy, 24/36 which is 2 nd -5 th percentile) ( Figure 3 ). Her performance on the WAIS-IV Block Design subtest was in the average range (37 th percentile) and consistent with her 2016 performance (50 th percentile).

Organization and detail of Rey figure drawing from 2017 to 2019.
Executive functions were stable over time. She performed in the upper end of the average range on a task of mental flexibility (Trailmaking B Test, 68 th percentile) in 2019 and this was consistent with her 2016 performance (70 th percentile). She easily completed a problem-solving task, achieving all 6 of 6 categories on the Wisconsin Card Sorting Test, with error responses all in the average range. This was also consistent with her 2016 performances. Her ability to learn and immediately recall a complex line drawing was in the high average range for both immediate (86 th percentile) and delayed (92 nd percentile) memory. Although the recall portion of this test was not given at her 2016 evaluation, her performance was in the average range on a similar visual memory task.
Her performance on a verbal list learning task was generally consistent with her performance at the 2016 evaluation. She did improve to learn and recall one additional word, moving her performance to the average range (30 th percentile) at the 2019 evaluation in comparison to her low average (16 th percentile) performance at the 2016 evaluation for short delayed cued recall and long delayed cued recall. Her performance was stable on a story memory task. At the 2019 evaluation, she performed in the average range for learning and memory of auditory information and she was able to retain 83 percent of what she initially learned after a 30-minute delay.
Her most recent cognitive testing indicated generally intact cognitive function across most neurocognitive skills. Her neuropsychological profile indicates general stability over time, with some possible restoration of cognitive function in visuospatial skills, auditory learning and memory, nonverbal memory, and processing speed. Given her high level of intellectual capacity, it is likely that performances in the average range may actually indicate a change in cognition relative to her true abilities. Minor weaknesses continued to be observed in the learning, free recall, and recognition of unstructured information (e.g. word list) and also in phonemic verbal fluency.
Overall, from 2017 to 2019 she manifested measured improvement of cognitive function in visuospatial skills, auditory learning and memory, nonverbal memory, and processing speed, with stability in executive functioning and in other areas of cognition. Stability in cognitive function over a period of 2–3 years for a person diagnosed with early AD is a clinical success. Most patients experience cognitive decline, especially in learning and memory. This patient demonostrated improvements across a variety of cognitive skills.
Given the need for effective AD treatments, it is instructive to identify patients who show improvement or long-term stability, and then evaluate connections between treatments and outcomes. Since AD is a complex multimodal chronic illness, it is essential to evaluate numerous potential contributing factors. Here, we present a case study of multimodal therapy for multimorbid dementia. The patient demonstrated improvements in symptoms, neuropsychological assessments, and brain imaging. An advantage of multimodal intervention is that the health and homeostasis of diverse physiological subsystems can be addressed concurrently. Since many of these subsystems contribute, possibly incrementally, possibly synergistically, or possibly substantially to cognitive function, they all need to be considered. Where appropriate, those that are disease-perturbed should be addressed. In particular, interventions in this individual addressed two key subsystems that influence cognition: neuroinflammation and metabolism. Neuroinflammation appeared to be induced by Borrelia burgdorferi , Ehrlichiosis and heavy metal toxicity – as well as suboptimal nutrient and hormone levels. There is evidence that chronic infection with Borrelia burdorferi such as neuroborreliosis can play a role in the development of AD [ 29 – 31 ]. Our multimodal therapy included personalized treatments to address each of these potentially underlying neuroinflammatory causes. Liver and other metabolic subsystems also influence cognitive function and hippocampal volume [ 32 ]. We introduced a personalized diet that helped reduce liver inflammation due to PBC evidenced by a sustained drop in GGT ( Figure 1 ). Together, all these components of multimodal therapy showed a sustained functional and cognitive benefit. Furthermore, multimodal therpaies may have systemwide benefits as many of the factors – such as inflammation – that impact cognitive function also impact other systems [ 33 ]. Therefore multimodal therapies are particularly appropriate for individuals with multimorbidities.
To some extent, multimodal intervention can be likened to pulling an airplane out of a dive. There may not be an immediate shift from decreasing function to increasing function. Because many factors are changing, one expects a time delay between when one starts to intervene and the lowest point of the curve. Furthermore, one does not necessarily expect all the inflections of all possible assays at exactly the same time, as each physiologic subsystem has distinct dynamics. However, with a comprehensive multimodal program, we do expect them roughly the same time, as we see in these data ( Figure 1 ).
It is essential to take a detailed history and listen to the patient and family members recount the events leading up to the patient’s illness. It is imperative when faced with a patient like this that we consider their entire “exposozome” including their daily living environment. We should consider factors such as mold, in-home toxins, biotoxin exposure, heavy metals, diet, stress management, hormone balance and lifestyle. We believe that an approach of first identifying the many potential contributors to cognitive decline and then applying a personalized, precision medicine approach is essential to designing effective treatments for AD. Such an approach is quite distinct from the typical single drug-centric approach that pre-determines a treatment that is unrelated to the potential etiologic contributors. Chronic illness as it relates to AD is a continuum that starts decades earlier. Cases such as this one suggest that many of these factors can be systematically addressed and reversed. As this is expanded and tested in clinical trials, this represents an exciting approach to 21 st -century medicine and the treatment of chronic disease with a new lens. The COCOA and PREVENTION trials are examples of trials using this approach [ 34 , 35 ].
This case also highlights differences between multiple defintions of “Alzheimer’s disease” in use spanning research and clinical contexts. The Alzheimer’s Association and National Institute of Aging provide a fairly clear framework for the definition of AD to be used in a research context [ 36 ]. This framework requires the documentation of molecular (e.g., amyloid) pathology. We do not know if the patient in this case study has such molecular pathology and cannot definitely classify this patient using the NIA-AA framework. In clinical practice, AD is often diagnosed presumptively, without assays for molecular markers [ 37 ]. The absence of testing for such markers is not always due to diagnostic malpractice; in some cases these tests are not available, are too expensive, are too invasive, would not impace care, or are otherwise contraindicated. Compared to some other diseases, such as infectious diseases, the clinical utility of a confident and precise diagnosis of AD is less. These other diseases have more clearly understood causal pathologies that clearly point to particular monotherapies. One advantage of a personalized multimodal approach for dementia is that it is robust to imprecision in diagnosis and nascent understandings of causalities. Treating multiple possible causes of dementia, personalized based on clinical and molecular evidence, and responding dynamically based on patient response can lead to clinical benefit even in complex cases of mixed dementia.
We have presented a single case example. In our experience, this patient is representative of many whose manifestations and pathophysiology are complex. Each patient has a slightly different presentation and combination of drivers of dementia, but treatments and outcomes via this precison medicine approach share more similarities than differences. The improvement observed in this patient underlines the need for clinical trials to test treatment protocols such as the one described here. The treatment program for this person may provide a guidepost for preventing or reversing the cognitive impairment of others with dementia.
Acknowledgement
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database ( adni.loni.usc.edu ). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at adni.loni.usc.edu .
Funding Statement
Providence St. Joseph Health provided generous funding for the Alzheimer’s Translational Pillar (ATP) at ISB. Analysis was supported by NIH U01AG046139, RF1AG057443, U01AG061359, & R01AG062514.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Personalized Model Unveils Hidden Patterns in Alzheimer’s Progression
A new mathematical model offers hope for better prediction and treatment of Alzheimer’s disease.
Most mathematical models of Alzheimer’s are theoretical, focusing on short term molecular and cellular-level changes that cannot be measured in patients. However, researchers at Duke University School of Medicine and Pennsylvania State University have used real-world data from over 800 people with varying cognitive abilities, to develop the Alzheimer’s Disease Biomarker Cascade (ADBC) model.
This personalized approach, published in the Journal of the Prevention of Alzheimer’s Disease , goes beyond traditional diagnostic methods by incorporating an individual's own biological markers to predict their disease progression.
Subjects were enrolled in the Alzheimer Disease Neuroimaging Initiative (ADNI), a multinational longitudinal study following subjects from normal cognition to mild cognitive impairment, to dementia, with serial cognitive testing, imaging and fluid biomarker data over a period up to two decades.
The ADBC model analyzes participants’ cerebrospinal fluid, brain scans and memory tests to find unique patterns, or clues, about each person’s condition.
The model combines both theory and individual biomarker data to predict how Alzheimer’s might evolve and respond to treatment in individual patients. By analyzing current biological markers, it was able to predict with surprising accuracy how these markers might change in the future for a particular patient.
Researchers say the model opens doors for reclassifying individuals along the Alzheimer’s clinical spectrum and tailoring treatment strategies.

“Alzheimer's disease has long been viewed as a single disorder,” said Jeffrey R. Petrella, MD , a neuroradiologist and director of the Alzheimer’s Imaging Research Laboratory at the Duke University School of Medicine. “This research shows that the disease progresses differently in each person, with unique patterns of biomarker changes.”
Petrella led the team of Duke researchers including Juliet Jiang, Kashyap Sreeram, Sophia Dalziel and Murali Doraiswamy MD, alongside senior study author Wenrui Hao, PhD , professor of mathematics at Penn State, in investigating the feasibility of customizing a causal model of Alzheimer’s disease.
Alzheimer's disease is characterized by changes in the brain including amyloid plaques and neurofibrillary tangles that may harm neurons and affect other types of brain cells. New medications have been successful at reducing amyloid-beta proteins in the brain and slowing memory and thinking decline from Alzheimer’s.
“I would envision using this model in clinical care as part of a precision medicine approach to treatment,” said Petrella. “The model could develop a recommendation of the optimal therapeutic regimen needed to help a patient achieve the best possible result over time while minimizing exposure to side-effects.” Treatment may be one medication or a combination of therapies.
The model identified 14 personalized parameters for each patient. These parameters reflected the growth rates, starting points (latency values), and maximum levels (carrying capacities) of various biomarkers associated with Alzheimer's. Importantly, these parameters differed significantly between individuals categorized by their clinical diagnosis, suggesting they reflect clinically meaningful aspects of the disease process.
When tested against existing data, the ADBC model predicted future biomarker levels with a high degree of accuracy, with an average error rate of just 9% across the entire study group. This accuracy remained strong even when applied to individual patients, with over 80% showing a low error rate in predicting future biomarker points.
The research also revealed a potentially crucial finding. By analyzing the personalized parameters, researchers identified two distinct clusters of patients. These clusters seemed to represent different “endophenotypes,” meaning different underlying biological profiles that influence disease progression.
The researchers emphasize the need for further studies to validate these findings in larger and more diverse community-based patient populations.
The study was supported by the National Science Foundation (DMS-2052676 and DMS-2052685) and the National Institutes of Health (1R35GM146894) with additional funding from the Alzheimer’s Disease Neuroimaging Initiative (U01AG024904) and the Department of Defense.

Newly-Discovered Gene Variant Could Help Prevent or Treat Alzheimer's Disease
Posted: April 10, 2024 | Last updated: April 10, 2024
Newly-Discovered Gene Variant , Could Help Prevent or Treat , Alzheimer's Disease. 'Newsweek' reports that scientists have uncovered a genetic variant that has the potential to reduce the odds of developing Alzheimer's by as much as 70%. The discovery could reportedly lead to new methods to effectively treat or prevent the disease which impacts approximately 5.8 million people in the United States alone. Some genetic variants have been found to have an association with an increased likelihood of developing the disease, while other variants offer protection. . A team from Columbia University found that genes involved with the production of fibronectin play a crucial role in developing Alzheimer's. Healthy individuals usually only have fibronectin present in small amounts along their blood-brain barrier, while those with Alzheimer's have much higher quantities. It's a classic case of too much of a good thing. It made us think that excess fibronectin could be preventing the clearance of [abnormal protein clumps] from the brain, Caghan Kizil, co-leader of the study and professor of neurological sciences at Columbia University's Vagelos College of Physicians and Surgeons, via 'Newsweek'. The team believes that methods aimed at reducing fibronectin could play a crucial role in developing new treatments and preventative measures. . Anything that reduces excess fibronectin should provide some protection, and a drug that does this could be a significant step forward in the fight against this debilitating condition, Caghan Kizil, co-leader of the study and professor of neurological sciences at Columbia University's Vagelos College of Physicians and Surgeons, via 'Newsweek'. Our findings suggest that... we may be able to develop new types of therapies that mimic the gene's protective effect to prevent or treat the disease, Caghan Kizil, co-leader of the study and professor of neurological sciences at Columbia University's Vagelos College of Physicians and Surgeons, via 'Newsweek'. The Columbia University team's findings were published in the journal 'Acta Neuropathologica.'
More for You
Complete vs. Incomplete Proteins: Understanding the Difference
Why Do People Add Plywood Under Their Countertops, And Do You Need To Do It?
Comparison: 2024 Toyota Camry Hybrid Vs 2024 Honda Accord Hybrid
Who is Vladimir Kara-Murza? The new face of Russia’s anti-Putin resistance
14 Black-and-White Dog Breeds That Are Too Cute to Ignore
Tax Day 2024 Freebies and Deals
Emily Mariko Just Shared an Easy 3-Ingredient Cucumber Salad—Here's How to Make It
Naruto Strongest Characters Without Six Paths Powers
Victor Wembanyama put on a show in Memphis
52 Best Online Furniture Stores to Bookmark Now (2024)
New Motorola Edge teased for April 16 announcement — what's a smARTphone?
Fractal pattern identified at molecular scale in nature for first time
If You’re Married, Should You File Taxes Jointly or Separately?
Do Mosquito Lawn Sprays Work?
Three Paramount board members to step down amid merger talks, WSJ reports
“I used to burn those guys up, bro, at 5-9” - Nate Robinson says he dominated Celtics teammates 1-on-1
Fairy Tail: 100 Years Quest Releases First In-Anime Character Artwork
David Hume Kennerly resigns from Gerald Ford foundation board over refusal to honor Liz Cheney with top award
Here's How NOT to Harvest Your Lettuce
The OnePlus Watch 2 just became a lot more adaptable
Skip to main content
- Skip to main menu
- Skip to user menu

Filter News
- All (807,781)
- Topic (765,812)
- Hotbed/Location (736,024)
- Career Advice (3,894)
- Insights (177)
- Webinars (8)
- Podcasts (37)
Vanqua Bio Announces First Patient Dosed in Phase 1 Clinical Trial Evaluating VQ-101, its Small Molecule GCase Activator for GBA-Parkinson’s Disease and Related Disorders
Published: Apr 09, 2024
- VQ-101 is a potent allosteric activator of glucocerebrosidase (GCase) - The Phase 1 program aims to demonstrate VQ-101’s pharmacodynamic effects in healthy volunteers and Parkinson’s patients
CHICAGO, April 09, 2024 (GLOBE NEWSWIRE) -- Vanqua Bio , a clinical-stage biopharmaceutical company dedicated to discovering and developing next-generation medicines for the treatment of neurodegenerative diseases, announced that the first patient has been dosed in a first-in-human Phase 1 clinical study evaluating VQ-101 in healthy individuals and patients with various forms of Parkinson’s disease (PD). VQ-101 is an orally administered brain-penetrant small molecule allosteric activator of the lysosomal enzyme glucocerebrosidase (GCase).
“VQ-101 demonstrated promising efficacy, safety, pharmacokinetics, and target engagement in preclinical studies. The launch of the Phase 1 study exemplifies Vanqua Bio's ability to translate our lysosomal biology and medicinal chemistry expertise into pioneering treatments that are well suited for clinical development,” said Jim Sullivan, PhD, Chief Executive Officer of Vanqua Bio. "This evolution to a clinical-stage company is an important milestone for Vanqua and brings us closer to our goal of addressing unmet patient needs through evidence-based innovation.”
Vanqua is initially developing VQ-101 for the treatment of Parkinson’s disease with mutations in GBA1 (GBA-PD), the gene that encodes GCase. Mutations in GBA1 are the most common genetic risk factor for PD, representing approximately 10% of patients with PD worldwide. Mutations in the GBA1 gene result in a decrease in the activity of GCase. Reductions in the activity of GCase disrupt the function of lysosomes, the recycling centers of the cells, enabling toxic forms of proteins, including alpha synuclein, to accumulate and harm neurons.
In developing VQ-101, Vanqua is adopting a precision-medicine approach that focuses initially on patients with GBA-PD, the largest genetically defined segment of PD. In preclinical studies, Vanqua has demonstrated that VQ-101 activates lysosomal GCase in a live-cell assay in vitro , ex vivo , and in vivo , demonstrates robust pathway engagement, and blocks the accumulation of insoluble alpha synuclein, which is the pathological hallmark of PD.
About the Phase 1 Study The first-in-human study with VQ-101 will be a randomized, double-blind, placebo-controlled single- and multiple-ascending dose study in healthy volunteers and PD patients with or without GBA1 mutations. The study is designed to evaluate VQ-101’s safety and tolerability, pharmacokinetics (PK), and pharmacodynamics (PD).
About Vanqua Bio Founded in 2019 and headquartered in Chicago, Vanqua Bio is a biopharmaceutical company dedicated to discovering and developing next-generation medicines that have the potential to transform the lives of patients with neurodegenerative diseases. Our technology platform utilizes human genetics and patient-derived neuronal cells to identify, validate, and clinically translate novel disease pathways associated with lysosomal dysfunction or aberrant activation of the innate immune system. Initially, we are targeting glucocerebrosidase (GCase) as a potential treatment for Parkinson’s disease (PD). Additional programs address overactivation of the innate immune system in central and peripheral neurodegenerative disorders, including Alzheimer’s disease. For more information, go to www.vanquabio.com .
Media Contact Alyssa Paldo FINN Partners [email protected]
Back to news
Vanqua Bio Announces First Patient Dosed in Phase 1 Clinical Trial Evaluating VQ-101, its Small Molecule GCase Activator for GBA-Parkinson’s Disease and Related Disorders
April 09, 2024 07:30 ET | Source: Vanqua Bio Vanqua Bio
- VQ-101 is a potent allosteric activator of glucocerebrosidase (GCase) - The Phase 1 program aims to demonstrate VQ-101’s pharmacodynamic effects in healthy volunteers and Parkinson’s patients
CHICAGO, April 09, 2024 (GLOBE NEWSWIRE) -- Vanqua Bio , a clinical-stage biopharmaceutical company dedicated to discovering and developing next-generation medicines for the treatment of neurodegenerative diseases, announced that the first patient has been dosed in a first-in-human Phase 1 clinical study evaluating VQ-101 in healthy individuals and patients with various forms of Parkinson’s disease (PD). VQ-101 is an orally administered brain-penetrant small molecule allosteric activator of the lysosomal enzyme glucocerebrosidase (GCase).
“VQ-101 demonstrated promising efficacy, safety, pharmacokinetics, and target engagement in preclinical studies. The launch of the Phase 1 study exemplifies Vanqua Bio's ability to translate our lysosomal biology and medicinal chemistry expertise into pioneering treatments that are well suited for clinical development,” said Jim Sullivan, PhD, Chief Executive Officer of Vanqua Bio. "This evolution to a clinical-stage company is an important milestone for Vanqua and brings us closer to our goal of addressing unmet patient needs through evidence-based innovation.”
Vanqua is initially developing VQ-101 for the treatment of Parkinson’s disease with mutations in GBA1 (GBA-PD), the gene that encodes GCase. Mutations in GBA1 are the most common genetic risk factor for PD, representing approximately 10% of patients with PD worldwide. Mutations in the GBA1 gene result in a decrease in the activity of GCase. Reductions in the activity of GCase disrupt the function of lysosomes, the recycling centers of the cells, enabling toxic forms of proteins, including alpha synuclein, to accumulate and harm neurons.
In developing VQ-101, Vanqua is adopting a precision-medicine approach that focuses initially on patients with GBA-PD, the largest genetically defined segment of PD. In preclinical studies, Vanqua has demonstrated that VQ-101 activates lysosomal GCase in a live-cell assay in vitro , ex vivo , and in vivo , demonstrates robust pathway engagement, and blocks the accumulation of insoluble alpha synuclein, which is the pathological hallmark of PD.
About the Phase 1 Study The first-in-human study with VQ-101 will be a randomized, double-blind, placebo-controlled single- and multiple-ascending dose study in healthy volunteers and PD patients with or without GBA1 mutations. The study is designed to evaluate VQ-101’s safety and tolerability, pharmacokinetics (PK), and pharmacodynamics (PD).
About Vanqua Bio Founded in 2019 and headquartered in Chicago, Vanqua Bio is a biopharmaceutical company dedicated to discovering and developing next-generation medicines that have the potential to transform the lives of patients with neurodegenerative diseases. Our technology platform utilizes human genetics and patient-derived neuronal cells to identify, validate, and clinically translate novel disease pathways associated with lysosomal dysfunction or aberrant activation of the innate immune system. Initially, we are targeting glucocerebrosidase (GCase) as a potential treatment for Parkinson’s disease (PD). Additional programs address overactivation of the innate immune system in central and peripheral neurodegenerative disorders, including Alzheimer’s disease. For more information, go to www.vanquabio.com .
Media Contact Alyssa Paldo FINN Partners [email protected]


IMAGES
COMMENTS
Here, we report a case of a 63-year-old woman (at the time of death) with the clinical history consistent with Alzheimer D, an autopsy with brain histopathology supporting Alzheimer disease (AD), congophylic angiopathy, and Lewy Body pathology, and whose medical genetic testing reveals a novel PSEN2 mutation of adenosine replacing cytosine at ...
Early onset Alzheimer disease (EOAD) is a neurodegenerative dementing disorder that is relatively rare (<1% of all Alzheimer cases). Various genetic mutations of the presenilin 1 (PSEN1) and presenilin 2 (PSEN2) as well as the amyloid precursor protein (APP) gene have been implicated.Mutations of PSEN1 and PSEN2 alter γ-secretase enzyme that cleaves APP resulting in increase in the relative ...
One study adopted 18F-florbetaben PET imaging in Down syndrome patients, suggesting potential role of amyloid imaging in identifying population at risk of dementia. 17 Similar study was conducted on patients with Down syndrome, but with 18F-florbetapir tracer. 18 An attempt to differentiate Down Syndrome pathology from AD has also been made ...
Onset of symptoms before age 65 should prompt consideration of syndromes in the spectrum of frontotemporal dementia (FTD) and atypical (nonamnesic) presentations of Alzheimer's disease (AD) (1, 2). This patient's symptoms reflect relatively prominent early dysfunction in visual-spatial processing and body schema, as might be observed in ...
An NIH-funded case study published recently in the journal Nature Medicine falls into this far-reaching category [1]. The report highlights the world's second person known to have an extreme resilience to a rare genetic form of early onset Alzheimer's disease. These latest findings in a single man follow a 2019 report of a woman with ...
The two most likely diagnoses in this case are the behavioral variant of frontotemporal dementia (bvFTD) 12 and the frontal variant of Alzheimer's disease (fvAD). 13 It can be difficult to ...
For health care professionals, get information about evidence-based case studies for managing Alzheimer's disease and other dementias. Get information and resources for Alzheimer's and other dementias from the Alzheimer's Association. Call our 24 hours, seven days a week helpline at 800.272.3900. menu. About; News;
Case Studies Illustrating Focal Alzheimer's, Fluent Aphasia, Late-Onset Memory Loss and Rapid Dementia. Gamze Balci Camsari, ... Most patients(92%) improved after immunomodulatory therapy. Rapidly Progressive Alzheimer's Disease (rpAD) is another consideration, in a cohort (N=89) of autopsy-confirmed rpAD, ...
Researchers identified in Aliria's brain a distinct pattern of abnormal aggregation or "clumping" of tau, a protein known to be altered in Alzheimer's disease and other neurologic disorders. In this case, the tau pathology largely spared the frontal cortex, which is important for judgment and other "executive" functions, and the ...
Most studies have demonstrated Alzheimer's disease as the most common etiology of EOD. The article presents the case of a 33-year-old patient hospitalized in the Department of Neurology in Zabrze, with cognitive dysfunction, speech disordersand featuresof Parkinson's extrapyramidal syndrome that have been progressing for about 15 months.
Short-term memory decline is the typical clinical manifestation of Alzheimer's disease (AD). However, early-onset AD usually has atypical symptoms and may get misdiagnosed. In the present case study, we reported a patient who experienced symptoms of memory loss with progressive non-fluent aphasia accompanied by gradual social withdrawal. He did not meet the diagnostic criteria of AD based on ...
In this case study, we describe the symptoms, neuropsychological testing, and brain pathology of a man with Alzheimer's disease (AD). AD commonly presents with impairment of memory and language function. In this case, language difficulties were noted more prominently than was memory impairment. Throughout the limbic system and neocortex of ...
The use of antipsychotics is associated with lower mortality in patients with Alzheimer's disease: A nationwide population-based nested case-control study in Taiwan. J Psychopharmacol. 2018;32: ...
By: Karl Herrup. The signature case of Alzheimer's disease was a German woman named Auguste whose mental status began to deteriorate to the extent that her husband brought her to a psychiatry clinic in Frankfurt. As is traditional, the scientific literature until very recently referred to Auguste merely by her first name plus the initial of ...
We conducted a multicenter, nested case-control study of Alzheimer's disease biomarkers in cognitively normal participants who were enrolled in the China Cognition and Aging Study from January ...
Alzheimer's is the most common cause of dementia. It is characterized by a gradual degradation of various physical and cognitive functions[1]. The current fictional case study is about a 42 year old female with early onset Alzheimer's Disease (EOAD) seeking physiotherapy treatment. Evaluation findings include gait abnormalities, decreased balance and decreased cognition.
An NIH-funded case study published recently in the journal Nature Medicine falls into this far-reaching category [1]. The report highlights the world's second person known to have an extreme resilience to a rare genetic form of early onset Alzheimer's disease. These latest findings in a single man follow a 2019 report of a woman with ...
Objective To analyze the clinical evolution of a patient affected by Alzheimer's disease and discuss the repercussions of an early diagnosis. Method Instrumental case study of qualitative and ...
1. Introduction. Alzheimer's disease (AD) (named after the German psychiatric Alois Alzheimer) is the most common type of dementia and can be defined as a slowly progressive neurodegenerative disease characterized by neuritic plaques and neurofibrillary tangles (Figure 1) as a result of amyloid-beta peptide's (Aβ) accumulation in the most affected area of the brain, the medial temporal ...
In this retrospective, multicentre case-control study, we trained, validated, and tested a deep learning algorithm to detect Alzheimer's disease-dementia from retinal photographs using retrospectively collected data from 11 studies that recruited patients with Alzheimer's disease-dementia and people without disease from different countries.
This case also highlights differences between multiple defintions of "Alzheimer's disease" in use spanning research and clinical contexts. The Alzheimer's Association and National Institute of Aging provide a fairly clear framework for the definition of AD to be used in a research context . This framework requires the documentation of ...
Case study. We selected a patient from the database of the geriatric department of the Hospital of Nantes. We were careful to choose a mild amnestic form of AD, diagnosed by an experienced geriatrician based on the criteria of the National Institute on Aging-Alzheimer's Association (McKhann et al., 2011). We simply selected the last patient in ...
A new mathematical model offers hope for better prediction and treatment of Alzheimer's disease. Most mathematical models of Alzheimer's are theoretical, focusing on short term molecular and cellular-level changes that cannot be measured in patients. However, researchers at Duke University School of Medicine and Pennsylvania State University have used real-world data from over 800 people ...
This article describes a qualitative single-case study exploring the marital relationship when one spouse has been diagnosed with Alzheimer's disease (AD). The researchers used a holistic-content narrative analysis to interpret the couple's story, as it was told through three couple interviews spaced two months apart. Guided by the theoretical framework of social constructionism, an in-depth ...
The client is transferred to a long-term care facility. His daughter visits him in the facility until his death, one year later. She has joined a support group to assist other caregivers experiencing the strain and loss of caring for a loved one with Alzheimer's disease. **. Study with Quizlet and memorize flashcards containing terms like Meet ...
b. Alzheimer's disease is a chronic disease that can progress with no set sequence and that has a typical lifespan of 1 to 15 years with the average being 4-8 years. c. Alzheimer's disease is a chronic, progressive disease with a clearly defined course and a typical lifespan of 20 to 30 years. d.
It's a classic case of too much of a good thing. ... protective effect to prevent or treat the disease, Caghan Kizil, co-leader of the study and professor of neurological sciences at Columbia ...
About the Phase 1 Study The first-in-human study with VQ-101 will be a randomized, double-blind, placebo-controlled single- and multiple-ascending dose study in healthy volunteers and PD patients with or without GBA1 mutations. The study is designed to evaluate VQ-101's safety and tolerability, pharmacokinetics (PK), and pharmacodynamics (PD).
About the Phase 1 Study. The first-in-human study with VQ-101 will be a randomized, double-blind, placebo-controlled single- and multiple-ascending dose study in healthy volunteers and PD patients ...