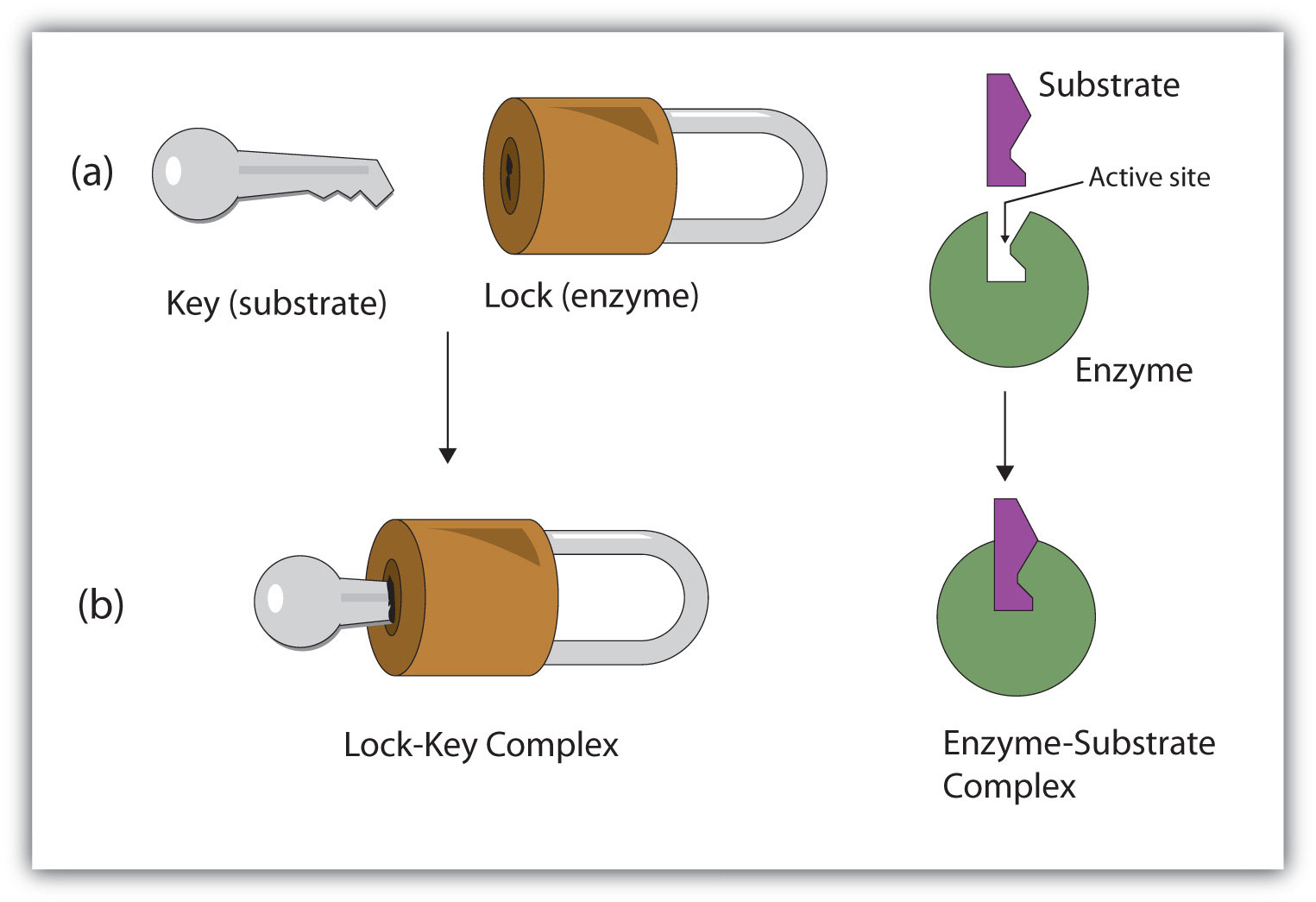
Lock-and-key model
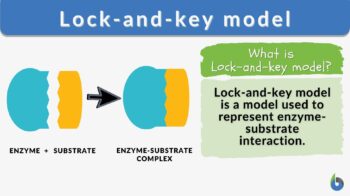
strong>Lock-and-key model n., [lɑk ænd ki ˈmɑdl̩] Definition: a model for enzyme-substrate interaction
Table of Contents

Lock-and-key model Definition
Lock-and-key model is a model for enzyme-substrate interaction suggesting that the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another. In this model, enzymes are depicted as highly specific. They must bind to specific substrates before they catalyze chemical reactions . The term is a pivotal concept in enzymology to elucidate the intricate interaction between enzymes and substrates at the molecular level. In the lock-and-key model, the enzyme-substrate interaction suggests that the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another. Like a key into a lock , only the correct size and shape of the substrate ( the key ) would fit into the active site ( the keyhole ) of the enzyme ( the lock ).
Compare: Induced fit model See also: enzyme , active site , substrate
Lock-and-key vs. Induced Fit Model
At present, two models attempt to explain enzyme-substrate specificity; one of which is the lock-and-key model , and the other is the Induced fit model . The lock and key model theory was first postulated by Emil Fischer in 1894. The lock-and-key enzyme action proposes the high specificity of enzymes. However, it does not explain the stabilization of the transition state that the enzymes achieve. The induced fit model (proposed by Daniel Koshland in 1958) suggests that the active site continues to change until the substrate is completely bound to the active site of the enzyme, at which point the final shape and charge are determined. Unlike the lock-and-key model, the induced fit model shows that enzymes are rather flexible structures. Nevertheless, Fischer’s Lock and Key theory laid an important foundation for subsequent research, such as during the refinement of the enzyme-substrate complex mechanism, as ascribed in the induced fit model. The lock-and-key hypothesis has opened ideas where enzyme action is not merely catalytic but incorporates a rather complex process in how they interact with the correct substrates with precision.
Key Components
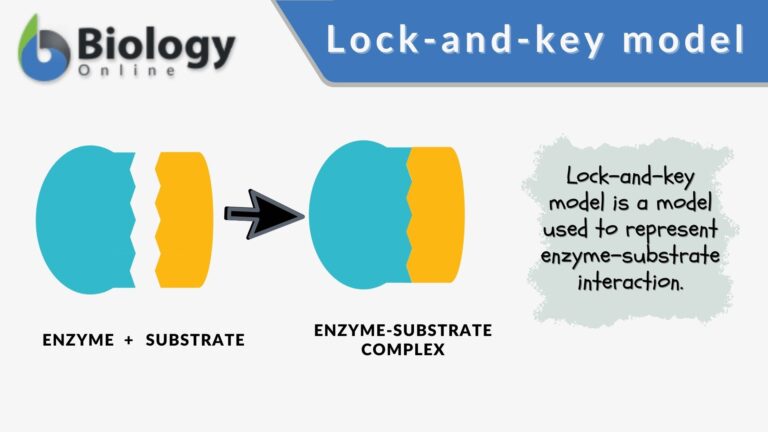
Components of the lock and key model:
- Enzyme : the enzyme structure is a three-dimensional protein configuration, with an active site from where the substrate binds.
- Substrate : often an organic molecule, a substrate possesses a structural feature that complements the geometry of the enzyme’s active site.
In the lock and key model, both the enzymes and the substrates facilitate the formation of a complex that lowers the activation energy needed for a chemical transformation to occur. Such reduction in the activation energy allows the chemical reaction to proceed at a relatively faster rate, making enzymes crucial in various biological and molecular processes.
Lock-and-key Model Examples
Some of the common examples that are often discussed in the context of the Lock and Key Model are as follows:
- Enzyme lactate dehydrogenase with a specific active site for its substrates, pyruvate and lactate. The complex facilitates the interconversion of pyruvate and lactate during anaerobic respiration
- Enzyme carbonic anhydrase with a specific active site for the substrates carbon dioxide and water. The complex facilitates the hydration of carbon dioxide, forming bicarbonate
- Enzyme lysozyme binding with a bacterial cell wall peptidoglycan, which is a vital immune function
Choose the best answer.
Send Your Results (Optional)

- Aryal, S. and Karki, P. (2023). “Lock and Key Model- Mode of Action of Enzymes”. Microbenotes.com. https://microbenotes.com/lock-and-key-model-mode-of-action-of-enzymes/
- Farhana, A., & Lappin, S. L. (2023, May). Biochemistry, Lactate Dehydrogenase . Nih.gov; StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK557536/
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.
Last updated on January 11th, 2024
You will also like...
Ecological Research: Measuring & Analysis
This lesson is about the methods used for ecological research, such as quadrat and transect sampling, canopy fogging, an..
Chromosomes X and Y and Sex Determination
This tutorial looks at sex determination via the sex chromosomes, X and Y. Read it to get more info on X and Y chromosom..

Origins of Life on Earth
Earth was created around 4.5 billion years ago and life began not long after. Primitive life likely possessed the elemen..

Biosecurity and Biocontrol
This lesson explores the impact of biosecurity threats, and why they need to be identified and managed. Examples to incl..
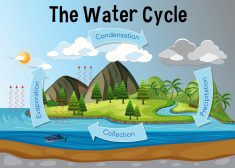
The Water Cycle
The water cycle (also referred to as the hydrological cycle) is a system of continuous transfer of water from the air, s..
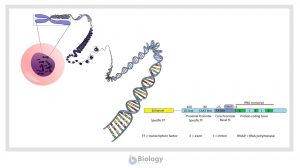
Gene Regulation in Eukaryotes
Learn about the general structure of a eukaryotic gene, the transcription factors, and post-transcriptional regulation....

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

4.7: Enzyme Action
- Last updated
- Save as PDF
- Page ID 178782
Learning Objectives
- To describe the interaction between an enzyme and its substrate.
Enzyme-catalyzed reactions occur in at least two steps. In the first step, an enzyme molecule (E) and the substrate molecule or molecules (S) collide and react to form an intermediate compound called the enzyme-substrate (E–S) complex . (This step is reversible because the complex can break apart into the original substrate or substrates and the free enzyme.) Once the E–S complex forms, the enzyme is able to catalyze the formation of product (P), which is then released from the enzyme surface:
\[S + E \rightarrow E–S \tag{\(\PageIndex{1}\)}\]
\[E–S \rightarrow P + E \tag{\(\PageIndex{2}\)}\]
Hydrogen bonding and other electrostatic interactions hold the enzyme and substrate together in the complex. The structural features or functional groups on the enzyme that participate in these interactions are located in a cleft or pocket on the enzyme surface. This pocket, where the enzyme combines with the substrate and transforms the substrate to product is called the active site of the enzyme (Figure \(\PageIndex{1}\)).
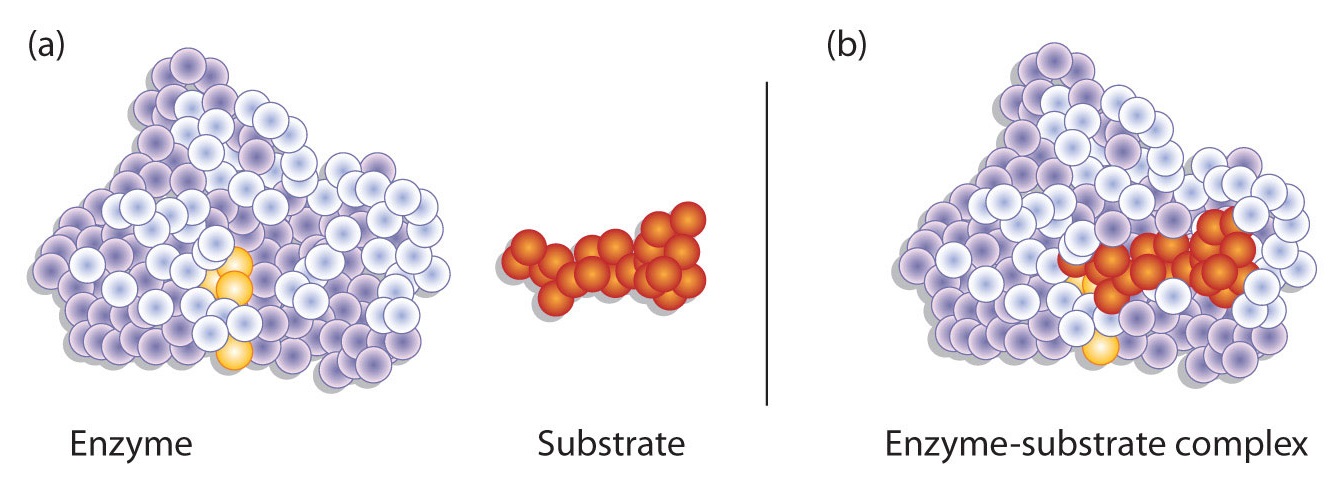
The active site of an enzyme possesses a unique conformation (including correctly positioned bonding groups) that is complementary to the structure of the substrate, so that the enzyme and substrate molecules fit together in much the same manner as a key fits into a tumbler lock. In fact, an early model describing the formation of the enzyme-substrate complex was called the lock-and-key model (Figure \(\PageIndex{2}\)). This model portrayed the enzyme as conformationally rigid and able to bond only to substrates that exactly fit the active site.
Working out the precise three-dimensional structures of numerous enzymes has enabled chemists to refine the original lock-and-key model of enzyme actions. They discovered that the binding of a substrate often leads to a large conformational change in the enzyme, as well as to changes in the structure of the substrate or substrates. The current theory, known as the induced-fit model , says that enzymes can undergo a change in conformation when they bind substrate molecules, and the active site has a shape complementary to that of the substrate only after the substrate is bound, as shown for hexokinase in Figure \(\PageIndex{3}\). After catalysis, the enzyme resumes its original structure.
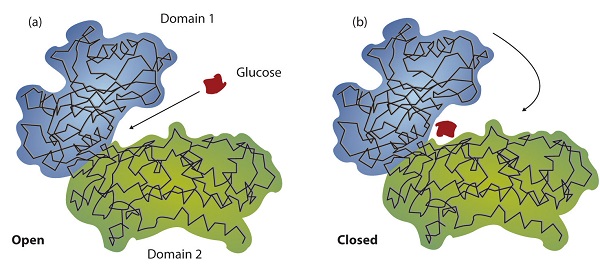
The structural changes that occur when an enzyme and a substrate join together bring specific parts of a substrate into alignment with specific parts of the enzyme’s active site. Amino acid side chains in or near the binding site can then act as acid or base catalysts, provide binding sites for the transfer of functional groups from one substrate to another or aid in the rearrangement of a substrate. The participating amino acids, which are usually widely separated in the primary sequence of the protein, are brought close together in the active site as a result of the folding and bending of the polypeptide chain or chains when the protein acquires its tertiary and quaternary structure. Binding to enzymes brings reactants close to each other and aligns them properly, which has the same effect as increasing the concentration of the reacting compounds.
Example \(\PageIndex{1}\)
- What type of interaction would occur between an OH group present on a substrate molecule and a functional group in the active site of an enzyme?
- Suggest an amino acid whose side chain might be in the active site of an enzyme and form the type of interaction you just identified.
- An OH group would most likely engage in hydrogen bonding with an appropriate functional group present in the active site of an enzyme.
- Several amino acid side chains would be able to engage in hydrogen bonding with an OH group. One example would be asparagine, which has an amide functional group.
Exercise \(\PageIndex{1}\)
- What type of interaction would occur between an COO − group present on a substrate molecule and a functional group in the active site of an enzyme?
One characteristic that distinguishes an enzyme from all other types of catalysts is its substrate specificity . An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality. In contrast, enzymes are much more specific. Some enzymes act on a single substrate, while other enzymes act on any of a group of related molecules containing a similar functional group or chemical bond. Some enzymes even distinguish between D- and L-stereoisomers, binding one stereoisomer but not the other. Urease, for example, is an enzyme that catalyzes the hydrolysis of a single substrate—urea—but not the closely related compounds methyl urea, thiourea, or biuret. The enzyme carboxypeptidase, on the other hand, is far less specific. It catalyzes the removal of nearly any amino acid from the carboxyl end of any peptide or protein.
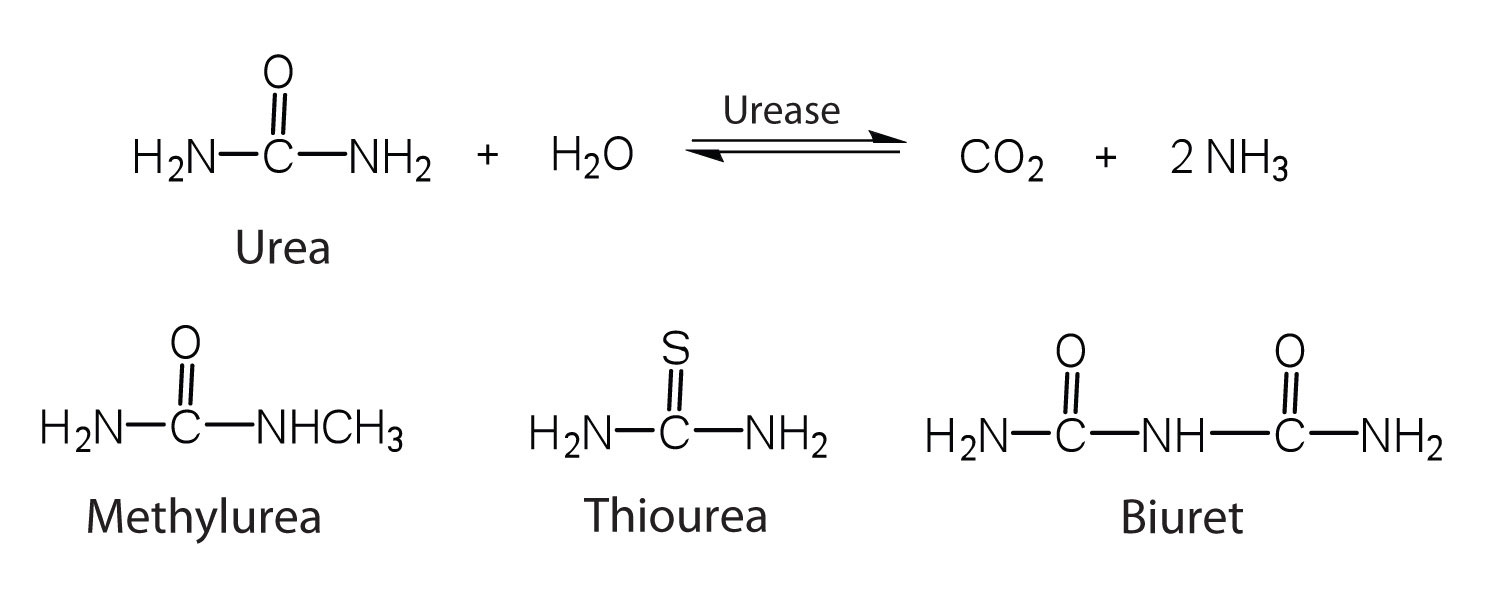
Enzyme specificity results from the uniqueness of the active site in each different enzyme because of the identity, charge, and spatial orientation of the functional groups located there. It regulates cell chemistry so that the proper reactions occur in the proper place at the proper time. Clearly, it is crucial to the proper functioning of the living cell.
A substrate binds to a specific region on an enzyme known as the active site, where the substrate can be converted to product. The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions. The induced-fit model says that an enzyme can undergo a conformational change when binding a substrate. Enzymes exhibit varying degrees of substrate specificity.
Concept Review Exercises
- Distinguish between the lock-and-key model and induced-fit model of enzyme action.
- Which enzyme has greater specificity—urease or carboxypeptidase? Explain.
- The lock-and-key model portrays an enzyme as conformationally rigid and able to bond only to substrates that exactly fit the active site. The induced fit model portrays the enzyme structure as more flexible and is complementary to the substrate only after the substrate is bound.
- Urease has the greater specificity because it can bind only to a single substrate. Carboxypeptidase, on the other hand, can catalyze the removal of nearly any amino acid from the carboxyl end of a peptide or protein.
What type of interaction would occur between each group present on a substrate molecule and a functional group of the active site in an enzyme?
- CH(CH 3 ) 2
- COO −
For each functional group in Exercise 1, suggest an amino acid whose side chain might be in the active site of an enzyme and form the type of interaction you identified.
For each functional group in Exercise 2, suggest an amino acid whose side chain might be in the active site of an enzyme and form the type of interaction you identified.
- hydrogen bonding
- ionic bonding
- dispersion forces
- The amino acid has a polar side chain capable of engaging in hydrogen bonding; serine (answers will vary).
- The amino acid has a negatively charged side chain; aspartic acid (answers will vary).
- The amino acid has a polar side chain capable of engaging in hydrogen bonding; asparagine (answers will vary).
- The amino acid has a nonpolar side chain; isoleucine (answers will vary).
Structural Biochemistry/Protein function/Lock and Key
In the Lock and Key Model, first presented by Emil Fisher, the lock represents an enzyme and the key represents a substrate. It is assumed that both the enzyme and substrate have fixed conformations that lead to an easy fit. Because the enzyme and the substrate are at a close distance with weak attraction, the substrate must need a matching shape and fit to join together. At the active sites, the enzyme has a specific geometric shape and orientation that a complementary substrate fits into perfectly. The theory behind the Lock and Key model involves the complementarity between the shapes of the enzyme and the substrate. Their complementary shapes make them fit perfectly into each other like a lock and a key. According to this theory, the enzyme and substrate shape do not influence each other because they are already in a predetermined perfectly complementary shape. As a result, the substrate will be stabilized. This theory was replaced by the induced fit model which takes into account the flexibility of enzymes and the influence the substrate has on the shape of the enzyme in order to form a good fit.
The active site is the binding site for catalytic and inhibition reaction of the enzyme and the substrate; structure of active site and its chemical characteristic are of specificity for binding of substrate and enzyme. Three models of enzyme-substrate binding are the lock-and-key model, the induced fit model, and the transition-state model. The lock-and-key model assumes that active site of enzyme is good fit for substrate that does not require change of structure of enzyme after enzyme binds substrate.
- Book:Structural Biochemistry
Navigation menu

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

2.7.2: Enzyme Active Site and Substrate Specificity
- Last updated
- Save as PDF
- Page ID 8811

Enzymes catalyze chemical reactions by lowering activation energy barriers and converting substrate molecules to products.
Learning Objectives
- Describe models of substrate binding to an enzyme’s active site.
- The enzyme ‘s active site binds to the substrate.
- Increasing the temperature generally increases the rate of a reaction, but dramatic changes in temperature and pH can denature an enzyme, thereby abolishing its action as a catalyst.
- The induced fit model states an substrate binds to an active site and both change shape slightly, creating an ideal fit for catalysis.
- When an enzyme binds its substrate it forms an enzyme-substrate complex.
- Enzymes promote chemical reactions by bringing substrates together in an optimal orientation, thus creating an ideal chemical environment for the reaction to occur.
- The enzyme will always return to its original state at the completion of the reaction.
- substrate : A reactant in a chemical reaction is called a substrate when acted upon by an enzyme.
- induced fit : Proposes that the initial interaction between enzyme and substrate is relatively weak, but that these weak interactions rapidly induce conformational changes in the enzyme that strengthen binding.
- active site : The active site is the part of an enzyme to which substrates bind and where a reaction is catalyzed.
Enzyme Active Site and Substrate Specificity
Enzymes bind with chemical reactants called substrates. There may be one or more substrates for each type of enzyme, depending on the particular chemical reaction. In some reactions, a single-reactant substrate is broken down into multiple products. In others, two substrates may come together to create one larger molecule. Two reactants might also enter a reaction, both become modified, and leave the reaction as two products.
The enzyme’s active site binds to the substrate. Since enzymes are proteins, this site is composed of a unique combination of amino acid residues (side chains or R groups). Each amino acid residue can be large or small; weakly acidic or basic; hydrophilic or hydrophobic; and positively-charged, negatively-charged, or neutral. The positions, sequences, structures, and properties of these residues create a very specific chemical environment within the active site. A specific chemical substrate matches this site like a jigsaw puzzle piece and makes the enzyme specific to its substrate.
Active Sites and Environmental Conditions
Environmental conditions can affect an enzyme’s active site and, therefore, the rate at which a chemical reaction can proceed. Increasing the environmental temperature generally increases reaction rates because the molecules are moving more quickly and are more likely to come into contact with each other.
However, increasing or decreasing the temperature outside of an optimal range can affect chemical bonds within the enzyme and change its shape. If the enzyme changes shape, the active site may no longer bind to the appropriate substrate and the rate of reaction will decrease. Dramatic changes to the temperature and pH will eventually cause enzymes to denature.
Induced Fit and Enzyme Function
For many years, scientists thought that enzyme-substrate binding took place in a simple “lock-and-key” fashion. This model asserted that the enzyme and substrate fit together perfectly in one instantaneous step. However, current research supports a more refined view called induced fit. As the enzyme and substrate come together, their interaction causes a mild shift in the enzyme’s structure that confirms an ideal binding arrangement between the enzyme and the substrate. This dynamic binding maximizes the enzyme’s ability to catalyze its reaction.

Enzyme-Substrate Complex
When an enzyme binds its substrate, it forms an enzyme-substrate complex. This complex lowers the activation energy of the reaction and promotes its rapid progression by providing certain ions or chemical groups that actually form covalent bonds with molecules as a necessary step of the reaction process. Enzymes also promote chemical reactions by bringing substrates together in an optimal orientation, lining up the atoms and bonds of one molecule with the atoms and bonds of the other molecule. This can contort the substrate molecules and facilitate bond-breaking. The active site of an enzyme also creates an ideal environment, such as a slightly acidic or non-polar environment, for the reaction to occur. The enzyme will always return to its original state at the completion of the reaction. One of the important properties of enzymes is that they remain ultimately unchanged by the reactions they catalyze. After an enzyme is done catalyzing a reaction, it releases its products (substrates).
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- OpenStax College, Biology. October 16, 2013. Provided by : OpenStax CNX. Located at : http://cnx.org/content/m44429/latest...ol11448/latest . License : CC BY: Attribution
- Boundless. Provided by : Boundless Learning. Located at : www.boundless.com//biology/de...llosteric-site. License : CC BY-SA: Attribution-ShareAlike
- cofactor. Provided by : Wiktionary. Located at : en.wiktionary.org/wiki/cofactor. License : CC BY-SA: Attribution-ShareAlike
- coenzyme. Provided by : Wiktionary. Located at : en.wiktionary.org/wiki/coenzyme. License : CC BY-SA: Attribution-ShareAlike
- OpenStax College, Enzymes. October 16, 2013. Provided by : OpenStax CNX. Located at : http://cnx.org/content/m44429/latest...e_06_05_04.jpg . License : CC BY: Attribution
- OpenStax College, Enzymes. October 16, 2013. Provided by : OpenStax CNX. Located at : http://cnx.org/content/m44429/latest...e_06_05_06.jpg . License : CC BY: Attribution
- OpenStax College, Enzymes. October 16, 2013. Provided by : OpenStax CNX. Located at : http://cnx.org/content/m44429/latest...e_06_05_05.jpg . License : CC BY: Attribution
- OpenStax College, Enzymes. October 16, 2013. Provided by : OpenStax CNX. Located at : http://cnx.org/content/m44429/latest...e_06_05_07.jpg . License : CC BY: Attribution
- active site. Provided by : Wikipedia. Located at : en.Wikipedia.org/wiki/active%20site. License : CC BY-SA: Attribution-ShareAlike
- substrate. Provided by : Wiktionary. Located at : en.wiktionary.org/wiki/substrate. License : CC BY-SA: Attribution-ShareAlike
- induced fit. Provided by : Wikipedia. Located at : en.Wikipedia.org/wiki/induced%20fit. License : CC BY-SA: Attribution-ShareAlike
- OpenStax College, Enzymes. October 16, 2013. Provided by : OpenStax CNX. Located at : http://cnx.org/content/m44429/latest...e_06_05_03.jpg . License : CC BY: Attribution
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Mol Sci

Limitations and Extensions of the Lock-and-Key Principle: Differences between Gas State, Solution and Solid State Structures
The lock-and-key concept is discussed with respect to necessary extensions. Formation of supramolecular complexes depends not only, and often not even primarily on an optimal geometric fit between host and guest. Induced fit and allosteric interactions have long been known as important modifications. Different binding mechanisms, the medium used and pH effects can exert a major influence on the affinity. Stereoelectronic effects due to lone pair orientation can lead to variation of binding constants by orders of magnitude. Hydrophobic interactions due to high-energy water inside cavities modify the mechanical lock-and-key picture. That optimal affinities are observed if the cavity is only partially filled by the ligand can be in conflict with the lock-and-key principle. In crystals other forces than those between host and guest often dominate, leading to differences between solid state and solution structures. This is exemplified in particular with calixarene complexes, which by X-ray analysis more often than other hosts show guest molecules outside their cavity. In view of this the particular problems with the identification of weak interactions in crystals is discussed.
1. Introduction
After Emil Fischer coined the lock-and-key picture for the reaction between enzymes and substrates [ 1 ], it became a leading concept for the understanding of intermolecular interactions with proteins, and later for the rational design of drugs. With the advent of supramolecular chemistry the idea gained an enormous momentum, as chemists began to synthetize a large variety of host compounds for practically all possible target guest molecules occurring in nature or in the environment. Although few concepts have played a comparatively important role in chemistry, the lock-and-key principle has limitations and extensions, which often are overlooked.
2. Dependence on the Binding Mechanism/Medium, pH and Stereoelectronic Effects
First of all, there are fundamental differences in the function of the lock-and-key principle in the gas state and in solution; the situation in crystals is again quite different and will be discussed in Section 6 and Section 7 . In solution the presence of a geometrically well-fitting cavity in a receptor is not enough for the binding of a substrate: the price for desolvation of the host and guest prior to complex formation must be paid by compensating non-covalent forces between host and guest, although complete desolvation might not be necessary, and desolvation alone can also contribute to a gain in free energy (see Section 5 on hydrophobic effects). Only in fairly rigid molecular containers [ 2 ], the inside binding of substrates may be controlled solely by the size of the portals. Obviously, the penalty for desolvation can be so large that one must change the reaction medium in order to achieve efficient complexation; a well-known example is the design of receptors for recognition of carbohydrates in water [ 3 , 4 ]. Furthermore, the geometric requirements for an optimal binding between host and guest differ enormously with the different non-covalent interactions [ 5 ]. Coulombic forces, with an r −1 dependence of the binding enthalpy on the distance r between interaction atoms or groups, tolerate much more deviation from a perfect geometric fit than for example dispersive interactions, which fall off with r −6 , and hydrogen bond strength depends significantly on orientation of donor and acceptor.
Solvent effects can be more decisive for complexation strength than size matching. Complexation with crown ethers 18C6 and 18C5 shows that not only the absolute binding energies depend on the medium, essentially as linear function of the cation desolvation free energies of the guest metal ions as shown with a variety of solvents [ 6 ]. Also, the differences between 18C6 and 18C5, which binds weaker due to one hydrogen atom protruding into the cavity, are much smaller in water than in other solvents ( Figure 1 and Table 1 ) [ 7 ].

Complexation of potassium ions with crown ethers 18C6 and 18C5; superimposed structures of the K + -complexes (the K + -ion in the 18C5 complex in red); with binding free energies ΔG in kJ/mol, and differences ΔΔG between them [ 7 ]. Adapted with permission from Raevsky, O.A.; Solovev, V.P.; Solotnov, A.F.; Schneider, H.-J.; Rüdiger, V. Conformation of 18-crown-5 and its influence on complexation with alkali and ammonium cations: Why 18-crown-5 binds more than 1000 times weaker than 18C6. J. Org. Chem. 1996 , 61 , 8113–8116. Copyright 1996 American Chemical Society.
Complexation free energies (in kJ/mol) of crown ethers in different solvents, with differences between 18C6 and 18C5.
Stereoelectronics can play a dominating role in complexation strength. A 1.10-diaza-crown ether ( Figure 2 ) binds metal ions much weaker than expected, due to the unfavourable diaxial orientation of the lone pairs (lp) at nitrogen [ 8 ]. Introduction of a methyl groups at the nitrogen atoms enforces a diequatorial lp orientation, and the binding energy increases to ΔG values expected for such ionophores [ 9 ]. The consequences of a different binding mechanism are illustrated in Figure 3 . Here a change in pH alters the inclusion mode of a ligand in the calix[4]arene host, due to a alternatively dominating ion pair or cation-π interaction [ 10 ].

Stereoelectronics: the 1.10-diaza-crown with R = H (diaxial lone pair (lp) orientation, ( A ) binds K + ions with only ΔG = 10 kJ/mol, with R = Me (diequatorial lp orientation; ( B ) ΔG increases to 26 kJ/mol (in methanol) [ 8 ].

Change of inclusion mode with a calix[4]arene host ( n = 4) as function of the pH [ 10 ].
Electron densities can play a larger role than geometric fitting. Molecular clips and tweezers bear a highly negative surface potential inside; the binding of the preferred guest molecules such as, e.g., NAD + is therefore dictated more by Coulombic forces than by exact fitting [ 11 ]. Ancillary ligands such as tetraaza-cyclododecanes can increase the positive charge at bound highly polarizable lanthanide ions, thereby leading to enhanced sensing affinities towards anions [ 12 ]. Cavitands as those shown in Figure 4 exhibit switching between close “vase” and open “kite” conformations as a function of pH, temperature, and of solvent, with the kite preferred in nonpolar solvents [ 13 ].

Cavitands which switch between close “vase” and open “kite” conformations [ 13 ]. Reprinted from [ 13 ] with permission from VCH/Wiley.
3. Induced Fit
An important extension of the lock-and-key principle was introduced early by Koshland, who proposed that conformational changes in an enzyme, induced by the substrate, can strengthen the binding [ 14 ]. With synthetic hosts binding is often only possible by severe conformational distortions of the host, as demonstrated e.g., with metalloporphyrin cages [ 15 ]. In artificial receptors such an induced fit is particularly obvious if the host is flexible and/or too wide for tight fitting. The resorcarene macrocycle in Figure 5 can bind acetylcholine only in a closed conformation; simultaneously two protons are liberated, thus enabling hydrogen bonds between three phenolic units [ 16 ].

Binding of cholinacetate (Me 3 + N(CH 2 ) 2 OAc) in a resorcarene macrocycle by induced fit (Me groups at + N omitted).
With a calix[6]arene derivative, encapsulation of different charged or neutral species in the hydrophobic cavity is also accompanied by conversion from the 1,3-alternate to the 1,3,5-alternate conformation [ 17 ]. Calix[6]arenes possess a particularly high flexibility; their cavity can by induced fit expand for large ligands or shrink for smaller guest molecules [ 18 ]. Other examples are calix[4]pyrroles which in solution occur in several conformations, but in presence of anions only in the cone conformation ( Figure 6 ); remarkably one finds in crystals mostly the 1,3-alternate form [ 19 , 20 ].

Calix[4]pyrrole in the 1,3-alternate conformation (left side) converts to the cone form by anion binding.
Sometimes a host cavity is only formed by inducing with an added guest the self-assembly of predesigned host parts, leading to so-called capsules [ 21 , 22 , 23 ]. Thus, an assembly of three palladium atoms and two tris -pyridyl ligands is induced by adamantanecarboxylic acid ( Figure 7 a) [ 24 ]; a capsule stabilized by ion pairing forms in presence of e.g., N -methylquinuclidinium cation as guest [ 22 ] ( Figure 7 b); or a steroid as guest induces a host assembly by hydrophobic interactions [ 25 ] ( Figure 7 c).

Self-assembly of predesigned host parts to form capsules, ( a ) with adamantanecarboxylic acid as guest [ 24 ]; ( b ) by ion pairing, with e.g., N -methylquinuclidinium cation as guest [ 22 ]; ( c ) a lipophilic host which self-assembles in presence of a long steroid by hydrophobic interactions [ 25 ].
4. Allosteric Effects
An important extension of the simple lock and key concept is due to allosteric interaction of a second guest component which is not directly acting at the first binding site. A large number of synthetic host guest complexes have been designed which show the typical binding modulation by the presence of a second effector [ 26 , 27 , 28 , 29 ]. This occurs most often, but not necessarily by conformational changes. Figure 8 and Table 2 illustrates the strong influence of an anion as second effector on the binding strength of tetramethylammonium salts in selected calixarenes. NMR analyses verified that the ammonium group is filling the cavity, so that the anion, which forms a strong ion pair with the cation in the apolar solvent chloroform used here, can only bind outside the calix, particularly efficiently with the urea group in the then heterotopic receptor 2 [ 30 ].

Association constants K as (M −1 ) of 1:1 complexes of tetramethylammonium salts Me 4 N + ·X − with hosts 1 and 2 in CDCl 3 , in presence of tosylate, chloride, acetate or trifluoroacetate anions [ 30 ].
Association constants K as (M −1 ) of 1:1 complexes of tetramethylammonium salts Me 4 N + ·X − with hosts 1 and 2 ( Figure 8 ) in CDCl 3 , in presence of tosylate, chloride, acetate or trifluoroacetate anions.
Artificial host compounds can show much stronger allosteric effects than proteins, in which conformational coupling between interacting binding sites is usually much weaker. The example in Figure 9 shows a particularly large ratio K M /K 0 of binding constants with and without second effector; only in the presence of metal ions such as Zn 2+ , a cavity is formed by contraction which binds lipophilic substrates such as dansylamide [ 31 , 32 ].

An example of an allosteric system (L = p -phenyl, M = Zn 2+ , G = dansylamide) in which introduction of metal ions lead to a ratio of binding constants of K M /K 0 >> 100; fluorescence emission occurs only in presence of metal ion [ 31 , 32 ].
5. Hydrophobic Interactions beyond the Lock-and-Key Picture
At first sight it seems that hydrophobic forces, which were traditionally ascribed to an entropy advantage gained by association between lipophilic molecules and subsequent liberation of water molecules, should not lead to particular deviations from the lock-and-key principle: the larger and closer the contact between a host cavity and a guest, the larger will be the number of liberated water molecules. In line with this idea hydrophobic contributions are traditionally evaluated by determination of solvent excluded surfaces. However, there is increasing and recently quantified evidence, that in host guest complexes significant contributions stem from the liberation of high energy water molecules [ 33 , 34 , 35 , 36 ] which in cavities can materialize less than the four hydrogen bonds which exist in bulk water [ 37 ]. Without complexation in a cavity there is only a very small hydrophobic effect, even for saturated compounds [ 38 ]. It has been shown that for essentially closed cavities such as in cucurbiturils the binding free enthalpies with some guest compounds can be completely explained by this non-classical high-energy water effect [ 33 ]. This is particularly so if the host interior offers few non-covalent interactions, as is the case for cucurbiturils, but also for some molecular clips ( Figure 10 ). The higher the number of high-energy water molecules is in a cavity, and the smaller the number of hydrogen bonds of each of these water molecules is, the larger is the energy gain; in accordance to the lock-and-key principle this would be achieved if the fit between host and guest is so perfect that all water molecules are displaced by the guest. However, if the host is large enough to accommodate more water molecules which can develop a satisfactory number of hydrogen bonds the hydrophobic driving force will play a minor role even if there is a perfect fit with a large enough guest which displaces all water molecules. Large hosts such as some cucurbiturils can accommodate a guest molecule and water, which again can exert more or less hydrogen bonds, or even two guest molecules. These possibilities are illustrated in Figure 10 ; complexes with cucurbiturils but also with cyclodextrins or molecular clips exhibit sizeable high-energy water effects [ 33 ]. It has been stressed that also the binding affinity in protein pockets is often not dominated by the lock-and-key principle but by the displacement of free-energetically unfavourable water [ 39 , 40 ].

Host compounds for large hydrophobic binding contributions: cucurbiturils and a molecular clip with four water molecules. Cucur[n]biturils with increasing size: ( a ) Crystal structure of inverted-CB6 with three intracavity water molecules; ( b – d ) Snapshot from molecular dynamics (MD) simulations for ( b ) CB6, ( c ) CB8 and ( d ) CB8·viologen complexes with 4, 14 and six cavity water molecules, respectively. Top : Complexes viewed from the side (CB n atoms in the front removed for clarity); Bottom : Complexes viewed from the top. Reprinted from [ 33 ] with permission from VCH/Wiley.
6. Host and Guest Complexes in the Solid State
In crystals the lattice is stabilized by a multitude of interactions in addition to those between host and guest; the uptake of a guest molecule can lead to a significant change of the solid state structure of the host alone. Metastable different crystalline modifications of the same compound, or polymorphs, are possible in particular if energy differences between molecular conformers and crystal lattice energies are of the same magnitude [ 41 , 42 ]; they are also quite frequent in cocrystals [ 43 ]. Occurrence of polymorphs make the assignment of an optimal host-guest geometry more difficult, but can shed light on the different interaction mechanisms. Isomorphic crystals can show a more unified picture of host and guest complexes, if they offer enough room for ligands, particularly if these are relatively small and if the chemical properties as well as binding mechanisms of different ligands are similar. Such conditions are also typical for complexes with large biomolecules such as proteins, in which the receptor conformation is in addition stabilized by a multitude of interactions. Figure 11 presents an example of a crystal which forms isomorphous structures with a series of linear alcohols [ 44 ]. Interestingly, crystals of inclusion compounds with the guest inside the cavity can often be obtained simply by slow diffusion of guests into the solvent-filled voids of the crystalline sponges [ 45 ], or by exchange of one guest with another one with the complex crystals in the vapour phase [ 46 ].

Example of a crystal of a resorcarene cavitand, containing co-crystalizing trifluorethanol, which forms isomorphous structures with a series of linear alcohols; the refined structure with e.g., n- propanol as ligand shows the relevant electron densities. Reprinted from [ 44 ] with permission of the Royal Society of Chemistry.
The abovementioned similarity between crystals of one receptor with small guest molecules is also the basis of an interesting new method to test selectivities from occupancy factors in a crystal with competing guest molecules [ 44 ]. Thus, isomorphous monoclinic crystals with a resorcarene cavitand and six alcohols were X-rayed without the unnecessary structural refinement; the observed occupancy factors were in close agreement with the relative binding constant ratios of the alcohols. The fully refined structure of the crystal with e.g., n- propanol ( Figure 11 ) shows that the small ligand finds its place without significant distortion of the lattice; comparison with the different alcohols shows an affinity decrease with the increase in the host-guest hydrogen bond distance, which is a function of the alcohol chain length.
7. Intra- and Extra Cavity Complexation in Macrocyclic Receptors/Differences between Solid State, Gas State and Solution Structure
The rather shallow cavity of small calixarenes lead particularly often to extra- (or exo-) cavity complexation, although the simple lock-and-key principle would predict an intra- (or endo-) complex. For complexes between argon and calix[4]arene in the gas state, spectroscopic and quantum-chemical calculations show both orientations, as expected with a preference for the endo-complex ( Figure 12 ) [ 47 , 48 ]. Laser spectroscopic molecular beam experiments and computations of calix[4]arene complexes with a variety of small ligands such as NH 3 , N 2 , CH 4 , and C 2 H 2 indicate also preferred endo complexes, for H 2 O and NH 3 as guest mainly by dipole–dipole interactions, for Ar, N 2 , CH 4 and C 2 H 2 mostly by dispersion forces [ 49 ].

Calix[4]arene complexes with argon; optimized structures of endo-complex and exo-complex. Reprinted from ref. [ 47 , 48 ] with permission of the Royal Society of Chemistry.
That interactions in the solid state are effective also in the gas phase complexes has been aptly discussed by Dalcanale et al. with complexes based on calixarenes or resorcarenes with P=O groups as hydrogen bond acceptors [ 50 ]. Electrospray ionization mass spectrometry (ESI-MS) is a suitable technique to elucidate what happens in the gas state. A major difference is that in the gas phase the outward facing P=O groups are not shielded by neighbouring molecules as in the solid layer, and are therefore amenable to H-bonding with the guest. The complex between the resorcarene cavitand and ethanol ( Figure 13 ) is also a nice example of several supramolecular structures within a crystal, exhibiting hydrogen bonds of EtOH with the two distal P=O groups with a statistical 50% probability; one also observes the synergy of P=O···H–O bonding and CH–π interactions in the cavitand ( Figure 13 a). If as in an isomeric structure ( Figure 13 b) a phenyl group fills the cavity, no C–H···π interaction is possible and also no H-bond to the then outward P=O group; then ethanol is found outside in the crystal lattice. For solid receptor layers, used often for gas detection, the distinction between intracavity vs. extracavity complexation is a particular problem. Location of analytes in the receptor layers can be identified by FT-IR spectroscopy if host and guest diagnostic bands do not overlap due to unspecific adsorption. Unspecific adsorption is characterized by linear adsorption isotherms, in contrast to Langmuir-type isotherms, which deviate significantly from linearity, indicating a specific analyte-layer interaction.

( a ) Resorcarene complexes with ethanol exhibiting two different structures within one crystal (hydrogen bonds of EtOH with the two distal P=O groups with a 50% statistical probability); ( b ) isomeric structure with a phenyl group filling the cavity; ethanol can only bind outside the cavity [ 50 ]. Reprinted from ref. [ 50 ] with permission of the Royal Society of Chemistry.
Complexes with smaller calixarenes show relatively often guest binding outside the cavity, as found e.g., in crystals of the calix[4]arene with toluene; here the guest molecule occupies intermolecular cavities of host channels [ 51 ]. In solution amines in the form of ammonium ions bind to calixarenes or resorcarenes usually as intracavity complexes [ 52 , 53 ], essentially due the cation-π interaction. In the solid state, however, amines bind often to the exo side, or to both sides. Thus, p - tert -butylcalix[4]arene forms with 1,4-butanediamine an inclusion compound with amine side both exo and endo of the cavity [ 54 ]. Both orientations were also found for complexes of amines and calix[6]arene [ 55 ]. In a p-tert -butylcalix[7]arene 1:3 pyridine crystal two pyridines have been found outside the cavity in the crystal lattice, forming a complex/clathrate hybrid [ 56 ]. Crystals of p-tert -butylcalix[8]arene with 8 pyridine molecules in the unit cell show the host macrocycle in an open chairlike conformation, so there is no cavity for the guest molecule [ 57 ].
Metal complexes are frequently bound to the outside of cavities, particularly with the electron-rich outside phenolic parts of calixarenes. For example, p-tert -butylcalix[4]arene coordinates rhodium outside, which allows to bind inside small neutral compounds such as diethylether or small anions such as tetrafluoroborate inside ( Figure 14 ) [ 58 ].

( a ) Crystal structure of a dirhodium p - tert -butylcalix[4]arene complex, with diethylether in the cavity; ( b ) Crystal structure of a triiridinum p-tert- butylcalix[5]arene complex with an encapsulated tetrafluoroborate anion inside [ 58 ]. Reprinted with permission from Staffilani, M.; Hancock, K.S.B.; Steed, J.W.; Holman, K.T.; Atwood, J.L.; Juneja, R.K.; Burkhalter, R.S. Anion binding within the cavity of π-metalated calixarenes J. Am. Chem. Soc. 1997 , 119 , 6324–6335. Copyright 1997 American Chemical Society.
Crystal structures of metal complexes with calix[4]arenes often show metal ions both in- and outside the cavity, e.g., with dinuclear Ti-IV and Ti-III complexes [ 59 ]. Calix[4]bisthiacrowns form with silver an endocyclic disilver complex and with copper exocyclic coordination polymers [ 60 ]. Stacking between the π-surfaces at the outside of 1,3- bis -pyridylmethylcalix[4]arene with different aryl compounds such as perfluoroarene or 1,4-dibromotetrafluorobenzene leads to infinite one-dimensional non-covalent ribbons [ 61 ].
Larger cyclophanes of the type shown in Figure 15 are expected to bind phenyl derivatives in the cavity, as inferred early by Stetter et al . from the formation of a 1:1 crystalline complex with benzene, and from fitting to CPK models [ 62 ]. Later, however, X-ray analysis revealed that the Stetter crystal has the benzene located outside [ 63 ]. Many years later NMR-spectra showed that, in water, benzene in fact does bind within the cavity [ 64 ].

A benzidine-derived cyclophane and its complexation with benzene, expected from Corey–Pauling–Koltun (CPK)-model [ 62 ], and as seen in the crystal by X-ray [ 63 ]; in aqueous solution the benzene is inside [ 64 ]. Adapted from ref. [ 5 ] with permission from Wiley/VCH.
With a complex of europium ion and a (222) cryptand, one can observe the slow movement of the guest out of the cavity to the solution ( Figure 16 ). If one dissolves the solid crystals, which from an earlier X-ray analysis is known to form as expected the inner sphere complex [ 65 ], in water (D 2 O) decomposition occurs into the free metal salt and the protonated ligand. Depending on the pH, two forms of metal complexes with different symmetry appear, as evident from the 1 H-NMR spectra [ 66 ].

Complex of europium ion and a (222) cryptand, crystal structure with the metal ion inside [ 65 ] and structures with the metal in different locations, as observed in solution by NMR spectroscopy [ 66 ]. Partially reprinted from ref. [ 65 ] with permission of the Royal Society of Chemistry.
The triply linked bis -cyclopeptide shown in Figure 17 exhibits remarkable differences between solution and solid state. In aqueous medium the host complexes a sulfate anion with lgK = 6, driven entirely by a gain in entropy. NMR data show that the sulfate is inside the cavity, forming hydrogen bonds to the amide NH groups at the inner surface of the host. In the crystal, however, one finds only water in the cavity, even though the crystals were grown in a solution containing sulfate [ 67 ].

A triply linked bis-cyclopeptide complexing in aqueous solution with high efficiency sulfate ions inside the cavity; in the crystal (right side) only water, no sulfate, is found inside [ 67 ]. Adapted from ref. [ 67 ] with permission from Wiley/VCH.
Cyclodextrin complexes are prone to differ in the solid and solution state, since the hydrophobic effect as important driving force is missing in crystals, and the inside of cyclodextrins offers only C–H bonds for non-covalent interaction, in contrast to the outside and rim. Hydrophilic compounds are said to generally bind with cyclodextrins preferentially outside the cavity [ 68 ]; earlier publications suggested similar possibilities [ 69 ]. Open chain analogues of cyclodextrins often show even more efficient chromatographic enantiorecognition of e.g., drugs [ 70 , 71 , 72 ]. However there are until now not enough conclusive spectroscopic studies for related cyclodextrin complexes in the solid and solution state.
8. Cavity Filling Factors—Conflict with the Lock-and-Key Principle?
Cyclophanes, cavitands and capsules have been shown to bind all kind of organic ligands, particularly those of an aromatic nature, in solution inside the cavity as long as the host leaves enough room for the guest molecule [ 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 ]. However, it has been noted early that there are deviations from the simple lock-and-key picture. Collet et al. found that water-soluble derivatives of cryptophanes, such as 2 in Figure 18 , bind ammonium guest molecules in water not as expected as a function of the size match, but preferred a loose association with smaller ligands [ 81 ]. Similarly, fluorophores composed of smaller phenyl-parts and larger naphthyl-parts bind in water to cyclodextrins, not with the better fitting larger naphthyl part but with the seemingly too small phenyl entity [ 82 ].

Calix[4]arene-carceplex 1 , cryptophane 2 ( n = 2), and carceplex 3 , with volume of the internal cavity, in [Å 3 ] [ 85 ].
Collet et al. showed already in 1993 [ 83 , 84 ] for cryptophanes such as 2 in Figure 18 , that e.g., chloroform binds better than methane, although methane fits geometrically as well in the cavity; they calculated for CHCl 3 in 2 an occupancy factor or packing coefficient (PC) of 0.886, corresponding to a very closely packed crystal; they also observed that the measured free enthalpy and entropy of complexation is comparable with the heat and entropy of crystallization of organic compounds. In contrast, for methane, PC amounts to only 0.348, which is consistent with later systematic evaluations by Rebek et al. [ 85 ] Analyses of many supramolecular complexes in solution, comprising in particular container- and capsule-type hosts have led Rebek et al. to the important generalization, that optimal binding occurs if 55% ± 9% of the space available in a cavity is occupied [ 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 ]. This is in the range of the packing density of organic liquids with a packing coefficient (PC) 0.51 to 0.63. Binding in hosts such as those in Figure 18 is indeed only observed if the PC is between 0.43 and 0.63. Larger packing coefficients of up to 0.70 can be reached if the complex is particularly stabilized by non-covalent interactions; in crystals and the interior of globular proteins the reported PC amounts to 0.66 to 0.77 [ 85 ].
That only a part of the available space is used for filling a cavity seems at first sight to be in conflict with the traditional lock-and-key principle. However, thermal motions, and the vibrational and translatory freedom of movement of host and guest require additional space. Moreover, a complete geometric match between host and guest molecules without any empty space between the complementary van der Waals surfaces can barely exist in the interaction between molecules of different shape and nature, characterized by corners and dimples. The exact calculation of the volume enclosed by the van der Waals surface is also therefore difficult, different methods can lead to variations of up to e.g., 15% [ 96 ]. Molecular dynamics (MD) simulation at 300 K predict e.g., that the volume in cavitands such as in Figure 18 vary over a range of 10% [ 85 ].
Polycyclic aromatic hydrocarbons (PAHs) with high binding affinities resulting from stacking and C–H···π interactions show larger deviation from Rebeks 55% filling factor [ 97 ]. Deviation from the optimal occupation rule was also observed e.g., with deep-cavity cavitand complexes in water [ 98 ]. A crystalline cryptophane complex with xenon exhibits an unusually large packing coefficient of 0.82 instead of 0.55 ± 0.09, with very short Xe···C contacts [ 99 ].
Complexes of an octanuclear cubic coordination cage ( Figure 19 ) in water with a series of aliphatic cyclic ketones show a linear relation between the guest’s surface and the binding ΔG as long as a 55% occupancy is reached [ 100 ]. Whether a crystal contains a guest molecule inside a host cavity can also depend on the preparation mode. With the complex shown in Figure 19 growing crystals from solvents containing excess guest afforded only the empty cage, whereas immersing preformed crystals of the cage in the neat guest cycloundecanone yielded the crystal with the entrapped guest [ 100 ].

Host cage [Co 8 L 12 ](BF 4 ) 16 , complex with cycloundecanone, with a 55% occupancy of the cavity space, Co atoms in green [ 100 ]. With permission from Turega, S.; Cullen, W.; Whitehead, M.; Hunter, C.A.; Ward, M.D. Mapping the internal recognition surface of an octanuclear coordination cage using guest libraries J. Am. Chem. Soc. 2014 , 136 , 8475–8483. Copyright 2014 American Chemical Society.
9. Problems with Identification of Weak Interactions in Crystals
Crystallography has been the most important source for metrical details also of intermolecular bonds [ 101 , 102 ]. The availability of nearly half a million crystal structures in the Cambridge Structural Data Base (CSD) allows identification of the most significant non-covalent interactions also in supramolecular complexes with respect to their geometry [ 103 ]. The combination with computational approaches has led to often reliable generalizations also for weak interactions, although it has been stated that “experimentally found crystal structures of a given compound need not be those of minimal free energy” and that “the choice of relevant intermolecular bonds is sometimes arbitrary” [ 104 ]. This is different in solution or in the gas state: as long there is the commonly observed rapid exchange all occurring structures will reflect the dominating free energies.
That purely statistical evaluations with data bases such as the CSD can be misleading is obvious from the recent controversy about hydrogen bonds with organic fluorine as acceptor. Dunitz et al. found in 5947 crystal structures containing organic fluorine only 37%, or 0.6% with short CF···HX (X = O, N) distances, and therefore concluded in 2004 “Organic Fluorine Hardly Ever Makes Hydrogen Bonds” [ 105 ]. Other crystallographers did find evidence for hydrogen bonds with fluorine; e.g., Mehta and Sen [ 106 ] found with fluorinated polycyclitols H···F distances 2.55 Å and C–H···F angles around 150°; Desiraju et al. [ 107 ] found in layers of polyfluoro-substituted benzenes often 2.23–2.35 Å and C–H···F angles 150–175 Å; some researchers consider 2.41–2.78 Å H···F distances as still typical [ 108 ]. For other halogens (Cl, Br, I) crystal structures seemed to be in agreement with their possibility to act as hydrogen bond acceptor.
For solution and the gas state, all available evidence clearly speaks for fluorine as in fact a much better acceptor than other halogen derivatives [ 109 ], which in view of the electronegativity differences is of course expected in the framework of Pauling’s description of hydrogen bonds. In particular, measurements of equilibrium constants between compounds with a large range of donors and halogen acceptors in solvents such as CCl 4 or CHCl 3 furnished interaction free energies [ 109 ], systematically decreasing from e.g., 7.5 kJ/mol for fluoroalkanes RF to 4.7 kJ/mol for iodoalkanes RI (tested with 1-haloadamantanes with 4-fluorophenol in CCl 4 ), with a systematical dependence on the substitution fragment for all halides [ 110 ]. For binding of fluoro derivatives to proteins, which is important in view of the 20% fluorine occurrence of all drugs, there is also clear indication of relatively strong hydrogen bonding with organic fluorine [ 111 ].
Obviously, the chances to find a significant number of hits in crystals of the thousands of fluorine containing compounds which have been prepared for all kind of reasons amounts to a lottery. The search for weak non-covalent forces in crystals is more promising if no other strong interactions are dominating the lattice: this is the case for example in pure hydrocarbons with e.g., one or more fluorine atoms, or if ones compares similar structures with many of the weak interactions one is looking for. Also, the search in protein databases is more promising, as generally protein complexes are more preselected—nobody will go to the expense of a solid state protein X-ray or NMR analysis if there is no prior evidence or at least hope that e.g., a fluorine generates a particular effect.
10. Conclusions
The lock-and-key principle is still a valuable starting point for the understanding and the design of natural and synthetic supramolecular complexes. Recent examples show the importance of the lock-and-key principle and induced fit also for selectivity in enzymatic reactions [ 112 , 113 ]; how it can apply to the stabilization of transition states has been demonstrated with the bowl-to-bowl inversion of the non-planar corannulene by complexation with a tetracationic cyclophane, accompanied by an induced fit [ 114 ]. As illustrated in Figure 20 only the flat transition state structure of the substrate, not its ground state fits into the host cavity, which leads to a calculated rate increase of inversion by a factor of 10.

Corannulene ( a ) fits to a tetracationic cyclophane host ( b ) only in the flat transition state structure of the substrate, not its ground state, leading to faster inversion of the corannulene [ 114 ]. Reprinted from ref. [ 114 ] with permission from Nature Publishing Group.
As demonstrated in this review the lock-and-key principle underlies important modifications. Optimal geometric fit may be a prerequisite, but high binding affinities depend often on a whole range of other factors, as discussed above. The possible self-inclusion of side groups is also a limitation of the simple lock-and-key concept, as are associations between several host molecules, in which one part of the host is inserted in the cavity of another one. Both interferences depend on the surrounding medium, and can in particular differ in the solid state. Typically, complexes in which the ligand occupies not the cavity of a host but are located outside are more often found in crystals than in solution. Statistical evidence from the analysis of not pre-selected crystal structure databases can be misleading with respect to the identification of very weak interactions. Structures of supramolecular complexes in solution can be evaluated by spectroscopic methods, preferably by NMR spectroscopy. The most often used Nuclear Overhauser Effect (NOE) provide intermolecular distances, but may reflect complexes which exhibit very short distances, and yet are less populated. In contrast to NOE data chemical shifts reflect usually the mixture of all conformers present in solution, according to their stability. Although the accuracy of structure elucidation based on chemical shifts cannot compete with crystallography they can be a useful and time-saving approach for the characterization of host–guest complexes. Both semiempirical and quantum-chemical calculations have been developed for this purpose [ 115 , 116 , 117 , 118 ], recently with emphasis on protein structures [ 119 , 120 , 121 ].
Acknowledgments
The author sincerely thanks Professor Stefan Kubik, Kaiserslautern, for valuable suggestions. He also remembers with gratitude the coworkers whose contributions are mentioned in the references.
Conflicts of Interest
The authors declare no conflict of interest.
Accessibility Links
- Skip to content
- Skip to search IOPscience
- Skip to Journals list
- Accessibility help
- Accessibility Help
Click here to close this panel.
Introduction to enzymes and their applications
Saurabh Bhatia Published September 2018 • Copyright © IOP Publishing Ltd 2018 Pages 1-1 to 1-29
You need an eReader or compatible software to experience the benefits of the ePub3 file format .
Download complete PDF book , the ePub book or the Kindle book
Chapter navigation

- Table of contents
- Next chapter
Export citation and abstract
Permissions.
Get permission to re-use this book
Share this chapter
Affiliations.
Amity institute of Pharmacy, Amity university. Gurgaon, Haryana, India
Published September 2018
Chapter DOI
https://doi.org/10.1088/978-0-7503-1302-5ch1
Books links
Book table of contents
About ePub3
About IOP ebooks
Enzyme catalysis is an area of fundamental importance in different areas. This chapter offers a concise overview to the fundamental principles and mechanisms of action, catalysis inhibition and its pharmaceutical applications. Additionally, this section also covers basics information related with enzymes such as its structure, function and different properties.
This article is available under the terms of the IOP-Standard Books License
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publisher, or as expressly permitted by law or under terms agreed with the appropriate rights organization. Multiple copying is permitted in accordance with the terms of licences issued by the Copyright Licensing Agency, the Copyright Clearance Centre and other reproduction rights organisations.
Permission to make use of IOP Publishing content other than as set out above may be sought at [email protected] .
Saurabh Bhatia has asserted his right to be identified as the author of this work in accordance with sections 77 and 78 of the Copyright, Designs and Patents Act 1988.
1.1. Introduction
The cell is the structural and functional unit of life—the basic building block of living systems. Cells have the capability to effectively utilize biocatalysts, known as enzymes, which have outstanding catalytic efficiency and both substrate and reaction specificity. Enzymes have amazing catalytic power and their high level of specificity for their substrate makes them suitable for biological reactions. They are crucial for cellular metabolism. Each and every chemical reaction that takes place in plants, micro-organisms and animals proceeds at a quantifiable rate as a direct result of enzymatic catalysis. Most of the history of biochemistry is directly or indirectly related to the history of enzyme research. Catalysis in biological systems was initially reported in the early 1800s based on research into the digestion of meat. In this report the catalytic activity of secretions from the stomach, the conversion of starch into sugar by saliva, and various plant extracts were reported.
In 1837, Berzelius documented the catalytic nature of fermentation. In the 1850s Louis Pasteur reported that fermentation was a process initiated by living organisms. During this study it was reported that the fermentation of sugar into alcohol by yeast was catalyzed by ferments. He also hypothesized that these ferments are close to the structure of yeast. These ferments were later called enzymes (in yeast). The key breakthrough in the history of enzymes came in 1897 when Edward Buchner isolated, from yeast cells, the soluble active form of the set of enzymes that catalyzes the fermentation of sugar to alcohol. Emul Fischer reported the first systematic studies on enzyme specificity in the early twentieth century [ 1 ]. Later, in 1926, James Sumner extracted urease in pure crystalline form from jack beans [ 2 ]. He also recognized the protein nature of urease . In 1930, John Northrop and his co-workers crystallized pepsin and trypsin and established them as proteins [ 3 ]. In subsequent years enzymology developed rapidly (table 1.1 ). The important developments during this period are: the elucidation of major metabolic pathways, such as the glycolysis and tricarboxylic acid cycle; the detection of numerous biochemical events of digestion, coagulation, muscular contraction and endocrine function, and their roles in the maintenance, control and integration of complex metabolic processes; the kinetic backgrounds to explain the observations of enzyme action and inhibition; and the development of protocols for examining the structures of functionally sensitive proteins. There has been exhaustive research on enzyme-catalyzed reactions and enzymes involved in cell metabolism. At present, 2000 different enzymes have been recognized, each of which catalyzes a different chemical reaction. Currently, more focus is being directed towards the application of enzymes. The high efficiency of enzymes makes them commercially valuable and their specificity of action is offering diverse advantages in clinical medicine.
Table 1.1. Chronology of enzyme studies.
1.2. Properties of enzymes
Enzymes are the complex protein molecules, often called biocatalysts, which are produced by living cells. They are highly specific both in the reactions that they catalyze and in their choice of reactants, which are known as substrates. An enzyme typically catalyzes a single chemical reaction or a set of closely related reactions [ 4 ]. Side reactions resulting in the wasteful formation of by-products are rare in enzyme-catalyzed reactions, in comparison to uncatalyzed ones. Enzymes can also be defined as soluble, colloidal and organic catalysts that are produced by living cells, but are capable of acting independently of the cells [ 4 ]. Enzymes are currently being used in diverse areas in the food, feed, paper, leather, agriculture and textiles industries, resulting in major cost reductions. Simultaneously, rapid scientific progress is now encouraging the chemistry and pharmacological industries to embrace enzyme technology, a trend supported by concerns regarding energy, raw materials, health and the environment. One of the most common advantages of enzymes is their ability to function continuously even after their removal or separation from the cells. This means that even after the separation of cells from in vivo environments, they continue to work efficiently under in vitro conditions; we can conclude that these biocatalysts remain in an active state even after their isolation. Principally, enzymes are non-toxic, biodegradable and can be produced in ample amounts by micro-organisms for industrial applications. In this chapter, the isolation, production, purification, utilization and application of enzymes (in soluble and immobilized or insoluble form) are discussed in detail. Procedures such as recombinant DNA technology and protein engineering are frequently used to produce more efficient and beneficial enzymes. The industrial production and utilization of enzymes is an important part of industry. Interdisciplinary collaboration between areas such as chemistry, process engineering, microbiology and biochemistry is required to develop the best possible enzyme technology, and eventually to achieve increased production and maintain the enzyme's physico-chemical properties under in vitro environments.
For catalytic action, small quantities of an enzyme are sufficient, where this quantity of enzyme is much smaller in comparison to its substrates. The overall concentration of substrate transformed per mass of enzyme is often very large. Without exception, all enzymes are proteinaceous and exhibit all the properties of a protein. The treatment of enzymes by extreme temperature or extreme pH, or by treatment with other denaturing agents, results in the complete loss of catalytic activity. Structural configurations such as the primary, secondary, tertiary and quaternary structures of enzyme proteins are essential for their catalytic activity. The degree of catalytic activity chiefly depends on the integrity of the enzyme's structure as a protein. As per reports, enzymes have molecular weights ranging from about 12 000 to over 1 million Da. A number of enzymes consist only of polypeptides and contain no chemical groups other than amino acid residues, e.g. pancreatic ribonuclease. Numerous enzymes require a specific, heat stable, low molecular weight organic molecule, known as a co-enzyme. Moreover, a number of enzymes require both a co-enzyme and one or more metal ions for activity. A complete biochemically active compound is formed by the combination of a catalytically active enzyme (also called the protein part) with a co-enzyme or a metal ion—this is called a holoenzyme. The protein part of a holoenzyme is called an apoenzyme. In this arrangement a co-enzyme may bind covalently or noncovalently to the apoenzyme. In certain enzymes the co-enzyme or metal ion is only loosely and transiently bound to the protein. However, in others it is tightly and permanently bound, in which case it is known as a prosthetic group. A prosthetic group signifies a covalently bound co-enzyme. According to reports, co-enzymes and metal ions are stable under heating, while the protein part of an enzyme (the apoenzyme), is denatured by heat.
Prosthetic groups may be classified functionally into two major classes: co-enzymes and co-factors. Co-enzymes may be considered to be biosynthetically related to the vitamins, such as the co-enzyme nicotinamide adenine dinucleotide (NAD) which is vital for cellular energy metabolism and integrates the vitamin niacin into its chemical makeup. Moreover, a co-enzyme may be considered as a co-substrate, experiencing a chemical transformation throughout the enzyme reaction (NAD is reduced to NADH), the reversal of which requires a separate enzyme, perhaps from a different cellular site. Co-enzymes might thus travel intra-cellularly between apo-enzymes and, by transferring chemical groupings, integrate several metabolic processes. Table 1.2 shows a list of the more common co-enzymes and their functions. In contrast to co-enzymes, co-factors, such as pyridoxal phosphate or hem groups, remain with one enzyme molecule and in conjunction complete a cycle of a chemical change brought about by one enzyme turnover [ 5 ]. Other enzymes, such as carboxypeptidase, require metal ions as co-factors, the divalent cations Mg 2+ , Zn 2+ and Mn 2+ being the most common; these are often called enzyme activators [ 6 ]. Table 1.3 lists several enzymes and their respective co-factors.
Table 1.2. Several co-enzymes employed in the transfer of specific atoms or functional groups.
Table 1.3. Several enzymes and their co-factors.
1.3. Catalysis
The role of a catalyst is to increase the speed of a chemical reaction. When the rate of a chemical reaction is governed by a soluble catalyst, which may result in a further increase in the rate of chemical reaction, it is called homogeneous catalysis. In this case catalysis occurs in a solution. When the catalyst is in a separate phase from the reactants, or when catalysis occurs on a insoluble surface or an immobilized matrix, it is known as heterogeneous catalysis. Enzymes are also called biological catalysts. These biological catalysts generally have the properties of homogeneous catalysts, however, a number of enzymes present in membranes are insoluble, and thus are called heterogeneous catalysts. Enzyme specificity is the absolute specificity of protein catalysts to identify and bind to only one or a few molecules. In this process the enzyme carries a defined arrangement of atoms in their active site to bind with the substrate. This active site on the enzyme should have a shape that accurately matches the substrates. Thus specificity is achieved when an enzyme with an active site binds with the chemical reactants (the substrates) at their active sites via weak bond interactions. To undergo a chemical reaction, this active site carries certain residues that form a temporary bond with the chemical reactants, termed the binding site, whereas the catalytic site carries the residues that are responsible for catalysis. Specificity is achieved when a substrate binds to an enzyme that has a defined arrangement of atoms in the active site. An enzyme always catalyzes a single type of chemical reaction, which involves the formation and breakdown of covalent bonds. Since they are specific to one particular reaction, this feature of enzymes is called reaction specificity, also known as absolute reaction specificity, i.e. no by-products are formed.
1.4. The structure of enzymes
Enzymes always act as catalysts and small quantities compared to their substrate are required to considerably increase the rate of chemical reactions, wherein the enzymes themselves experience no overall change [ 7 , 8 ]. In contrast to all true catalysts, an enzyme does not alter the ultimate equilibrium position of a reaction, which is thermodynamically determined, thus merely the rate of completion of equilibrium of a feasible reaction is augmented. In addition to catalytic properties, enzymes exhibit the physico-chemical behavior of proteins: their solubility, electrophoretic properties, electrolytic behaviors and chemical reactivity [ 7 , 8 ]. The primary structural configuration and catalytic action of enzymes is determined by the linear chain of amino acid residues linked via peptide bonds, which constitute a protein molecule. Localized folding of the primary structure is called a secondary structure, whereas the complete folding of the molecule is known as a tertiary structure. In contrast to these structural configurations, a quaternary structure is the agglomeration of several folded chains. The structural features of enzymes are shown in figures 1.1 and 1.2 . In contrast to traditional chemical catalysts, e.g. hydrogen ions, heavy metals or metal oxides, which are most effective in organic solvents, at very high temperatures or at extreme pH values, enzymes operate most efficiently under very mild conditions. When using enzymes, there are certain issues that require attention, such as deviation from homogeneous aqueous solutions, physiological pH and temperature, which can rapidly destroy enzyme activity. However, under normal conditions the increase in reaction rate is rarely matched by their non-protein counterparts.
Figure 1.1. Structural features of enzyme.
Download figure:
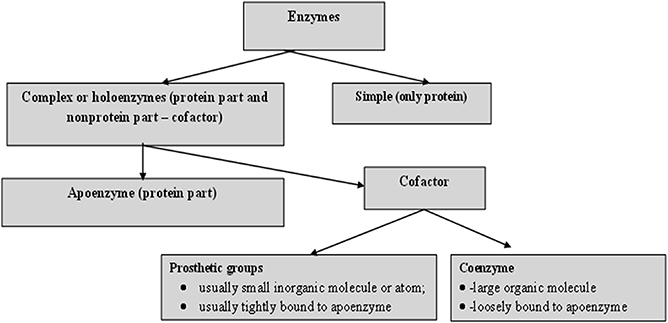
Figure 1.2. Principle components of an enzyme.
1.5. Structural features: primary and secondary structures
Three-dimensional analysis of the amino acid sequence of lysozyme of hen's egg white has demonstrated some features essential for primary structure [ 9 , 10 ]. These are:
- • Molecules derived from a similar source have a similar order of amino acid residues and appear to be random with no obvious predictability.
- • Even though numerous enzymes are intramolecularly crosslinked via disulfide bridges of cysteine, no branching occurs.
Current databases suggest that a small number of amino acids are extra and most are 'functional', i.e. the majority of them co-operatively control the higher orders of structural organization and therefore the catalytic activity. When comparing the primary structures of enzymes performing similar functions, wide structural homologies are detected in their sequence, mainly in the patterns of their nonpolar residues. For example, pancreatic juice contains five inactive precursors (zymogens), namely chymotrypsinogen A, B and C, trypsinogen and proelastase; all of these are activated to the respective proteases by proteolytic cleavage [ 11 ].
1.6. The mechanism of action of enzymes
The mechanism of action is based on a chemical reaction, in which the enzyme binds to the substrate and finally forms an enzyme–substrate complex. This reaction take place in a relatively small area of the enzyme called the active or catalytic site. In other words, the mechanism of enzyme action is based on the nature of the enzyme–substrate interaction, which accounts for the reaction specificity of the biological catalysts. The active or catalytic site of an enzyme is constituted by several amino acids, located at some distance from each other in the peptide chain. These amino acids are brought close together by the folding resulting from the secondary and tertiary structure of the enzymes. Side chains of amino acid residues at the catalytic site provide groups for binding with specific groups of the substrate. Co-factors assist the catalysis. The substrate forms bonds with amino acid residues in the substrate binding domain of the active site. The binding induces a conformational reaction in the active site. During the reaction, the enzyme forms a transition-state complex. As the products of the reaction disassociate, the enzyme returns to the original state. Two different models postulated for the mechanism of enzyme action are given below.
1.6.1. The Fisher template model (lock and key model)
This is a rigid model of the catalytic site, proposed by Emil Fischer in 1894 [ 12 ]. The model explains the interaction between a substrate and an enzyme in terms of a lock and key analogy. In this model, the catalytic site is presumed to be preshaped. The substrate fits as a key fits into a lock. The drawback of this model is the implied rigidity of the catalytic site. The model cannot explain changes in enzyme structure in the presence of allosteric modulators.
1.6.2. Induced fit model
In contrast to the above method, this model suggests a flexible mode for the catalytic site. To overcome the problems of the lock and key model owing to the rigid catalytic site, Koshland [ 13 – 15 ] suggested an induced fit model in 1963. The important feature of this procedure is the flexibility of the active site. In the induced fit model, the substrate induces a conformational change in the active site of the enzyme so that the substrate fits into the active site in the most convenient way so as to promote the chemical reaction. This method suggests competitive inhibition, allosteric modulation and inactivation of enzymes on denaturation.
The Michaelis–Menten theory of enzyme action [ 16 ] offers the basis for most current research on the mechanism of enzyme action. This concept of the enzyme–substrate complex scheme assumes the combination of the enzyme and substrate in phase one (occasionally known as the transition phase) of the enzyme activity and liberation of the enzyme and the products of the catalysis in phase two of the reaction.
1.6.3. Covalent catalysis
Covalent catalysis is evidenced in enzymes capable of forming covalent bonds between the substance and the catalytic group of the active site [ 17 ]. A number of enzymes react with their substrates to form very unstable, covalently joined enzyme–substrate complexes, which undergo further reaction to yield products much more readily than in an uncatalyzed reaction. Several of the enzymes that exhibit covalent catalytic behavior are listed in table 1.4 .
Table 1.4. Various enzymes exhibiting covalent catalytic behavior.
1.7. Catalysis via chymotrypsin
Hummel and Kalnitzky suggested an enzyme mechanism through the depiction of the sequential transition states experienced by the enzyme–substrate complex during catalysis [ 18 ]. Chymotrypsin is a digestive enzyme, responsible for proteolysis (breakdown of proteins and polypeptides) in the duodenum. Chymotrypsin favorably breaks peptide amide bonds (the carboxyl side of the amide bond is a large hydrophobic amino acid). These amino acids contain an aromatic ring in their side chain that fits into a 'hydrophobic pocket' of the enzyme. It is stimulated in the presence of trypsin. Trypsin and chymotrypsin are both serine proteases with high sequence and structural similarities, but with different substrate specificity [ 19 , 20 ].

1.7.1. Intermediary stages of chymotrypsin
As discussed above, chymotrypsin is a protease enzyme that cuts on the C-terminal phenylalanine, tryptophan and tyrosine on peptide chains [ 21 ]. Additionally, it is more specific for aromatic amino acids because of its hydrophobic pocket. Comparable to other serine proteases, chymotrypsin also catalyzes the hydrolysis of certain esters [ 22 ]. The molecular events involved in catalysis are called intermediary enzymology. Chymotrypsin, a protease, favorably accelerates breakdown of peptide bonds in which the aromatic amino acid (Phy, Try, or Trp) or bulky nonpolar R group (Met) contribute a carboxyl group. The synthetic substrate p-nitrophenyl acetate allows colorimetric analysis of chymotrypsin activity, as hydrolysis to p-nitrophenol, which is alkali, changes into the chromophore anionic forms.
1.7.2. Kinetic behavior of α -chymotrypsin
The kinetics of chymotrypsin of p-nitrophenyl acetate can be considered in a 'stop-flow' apparatus. This procedure utilizes substrate quantities of enzymes and measures the events in the first few milliseconds [ 23 ]. The use of p-nitrophenyl acetate as a substrate offers the prospect of investigating solvent effects on both the acylation of the enzyme and the hydrolysis (deacylation) of the acyl enzyme [ 23 ]. The significant features of the slow-flow kinetics of chymotrypsin are:
- - a burst phase featuring rapid liberation of an anion.
- - a subsequent 'steady-state' phase, with slower release of extra anion.
- • A 'charge relay network' acts as a proton shuttle during catalysis by chymotrypsin. The charge relay network of chymotrypsin encompasses three aminoacyl residues that are far apart in a primary structural sense, but close together in a tertiary structural sense. While most of the charged residues of chymotrypsin are present at the surface of the molecule, those of the charge relay network are hidden in the otherwise nonpolar inner side of the protein. These charges transmit residues which activate sequential proton shifts that shuttle protons in the opposite direction. An equivalent series of proton shifts is assumed to accompany the hydrolysis of the physiologic chymotrypsin substrate, e.g. a peptide.
1.7.3. Selective proteolysis in creation of the catalytic sites of enzymes
Various enzymes, hormones and other physiologically active proteins are produced as inactive precursors (zymogens) that are further transformed to the active form by selective enzymatic cleavage (limited proteolysis) of peptide bonds. The final step to activating enzymatic function is limited proteolysis, either in a single activation step or in a consecutive series (cascade). The specificity of each activation reaction is evaluated by the complementarity of the zymogen substrate and the active site of the attacking protease. The arrangement of successive activation reactions is controlled by the specificity of each enzyme, while the extent of amplification of the initial stimulus is evaluated by the effectiveness of each activating step. Zymogen activation produces a prompt and irreversible response to a physiological stimulus, and is capable of initiating new physiological functions. Classical examples are the processes of hormone production, fibrinolysis, complement activation, blood coagulation, supra-molecular assembly, metamorphosis, fertilization and digestion. The zymogens of the pancreatic serine proteases, in particular, have functioned as models for detailed studies of the nature of the molecular changes that are involved in the intense increase in enzymatic activity that results upon incomplete proteolysis of the zymogen.
Specific proteolysis is a common means of activating enzymes and other proteins in biological systems. A number of proteins are manufactured and released in the form of inactive precursor proteins called proproteins. Various enzymes attain full enzymatic activity as they suddenly fold into their characteristic three-dimensional forms. In contrast, other enzymes are produced as inactive precursors that are successively activated by breakdown of one or a few specific peptide bonds. The inactive precursor is known as a zymogen (or a pro-enzyme). In other words, when the proteins are enzymes, the proteins are called pro-ezymes or zymogens (table 1.5 ). An energy source (ATP) is not required for cleavage [ 11 ]. Thus, in comparison to reversible regulation by phosphorylation, even proteins sited outside cells can be triggered by this means. An additional noteworthy difference is that proteolytic activation, in comparison with allosteric control and reversible covalent modification, occurs just once in the life of an enzyme molecule. Transformation of a proprotein to the mature protein includes selective proteolysis. This transforms the proproteins by one or more consecutive proteolytic clips to a arrangement in which the individual activity of the mature protein (its enzymatic activity) is expressed, e.g. the hormone insulin (proinsulin), the digestive enzyme chymotrypsin (chymotrypsinogen), a number of factors for blood clotting and for the blood clot dissolution cascades, and the connective tissue protein collagen (procollagen). Chymotrypsinogen consists of 245 amino acid residues, and is practically devoid of enzymatic activity. As the reaction starts, it is converted into a fully active enzyme. This occurs when the peptide bond joining arginine 15 and isoleucine 16 is cleaved by trypsin. The subsequent active enzyme, known as π-chymotrypsin, then acts on other π -chymotrypsin molecules. Two dipeptides are eliminated to form α -chymotrypsin (the stable form of the enzyme) [ 11 ]. The three subsequent chains in α -chymotrypsin remain interconnected to each another by two interchain disulfide bonds. The outstanding feature of this process is that cleavage of a single specific peptide bond alters the protein from a catalytically inactive form into one that is fully active. The transformation of prochymotrypsin (Pro-CT), a 2,4,5-aminoacyl residue polypeptide, to the active enzyme α -chymotrypsin includes three proteolytic clips and the formation of an active intermediate called π -chymotrypsin ( π -CT) and consequently to the mature catalytically active enzyme α -chymotrypsin ( α -CT). Examples of gastric and pancreatic zymogens are listed in table 1.5 .
Table 1.5. Gastric and pancreatic zymogens.
1.7.4. Kinetic models for enzymes
Generally, enzyme kinetics is defined as the study of the rate of reactions, i.e., how the substrate concentration impacts the velocity of the reaction. Enzyme kinetics involves optimization of bio-catalytic reactions to allow process design and scaling up processes to further increase the production and minimize the overall overhead costs of various procedures. Kinetic investigations in the branch of biochemistry concerned with enzymes can be categorized into three types:
- • Transient-state kinetics : This is the stage of reaction before the steady or rapid-equilibrium state, and involves quick reactions between the enzymes and substrate. These sudden changes in the reaction mixture when the substrate and enzymes are mixed require advance equipment to monitor the reaction before it changes into the steady state. The mechanisms of the reaction are associated with the enzyme structural configuration. Basic steps are involved during an enzyme-catalyzed reaction, which allow the direct study of the intermediates and products formed during a single enzyme cycle, which may further help in direct analysis of individual reaction steps for short times. In this type of reaction a sufficient concentration of enzymes is used to witness the intermediate and product formation.
- • Steady-state kinetics : This is the phase in which the rate of formation of intermediates and the rate of decomposition remain the same, and thus the concentrations of reactive intermediates remain the same. During this reaction substrate concentration is greater than enzyme concentration. The Michaelis–Menten enzyme kinetic (figure 1.3 ) can be considered as the most often studied reaction for several enzymes. For example, chymotrypsin (protease) with a high concentration of substrate achieves maximum velocity of the reaction (called the first order of reaction) but at a certain point the substrate occupies all binding sites of the enzyme, after which further addition of substrate does not increase the rate. This is called the zeroth order of reaction (the steady state). It is the phase in which the enzyme and substrate concentrations cannot be determined using the dissociation constant. Thus steady-state enzyme kinetics is based on the theory that a catalytic reaction remains constant if the reaction is not exposed to continuous changes.
- • Rapid-equilibrium kinetics : This the phase in which both the enzyme and substrate concentrations can be determined using the dissociation constant. During this procedure total enzyme concentration remains constant during the reaction and the concentration is very small compared to the amount of substrate. In this reaction, before the rate-determining reaction, the reactions are in equilibrium with their components, thus this stage is called rapid-equilibrium kinetics.
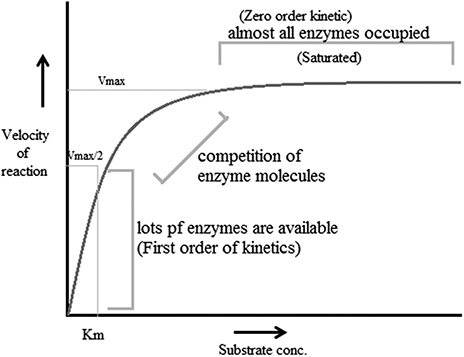
Figure 1.3. The Michaelis–Menten enzyme kinetic.
According to reports, factors that affect enzyme-catalyzed reactions also affect the velocity of a reaction. These factors are called modifiers of enzyme-catalyzed reactions. These modifiers can be divided into two classes: inorganic modifiers (enzyme activators) and organic modifiers (enzyme inhibitors). These factors can have different types of effects on the velocity of the reaction; nevertheless the most vital effect is that they offer many pathways to products, e.g. when one modifier is bound to an enzyme, it alters the rate of reaction and thus forms two rate constants. However, when two modifiers participate, there are five self-regulating equilibria, resulting in three paths for making products.
There are two mechanisms, single-substrate and multiple-substrate, that are helpful in studying the different stages of enzymatic reactions. Understanding these stages helps in understanding the properties of enzymes. Certain enzymes have single substrates (a single substrate binding site), e.g. triosephosphate isomerase, whereas certain enzymes have multiple substrates molecules (multiple binding sites), such as dihydrofolate reductase, and bind with multiple substrates. After the exploration of specific RNA sequences required for RNA replication, new biocatalysts in the form of ribozymes have emerged with the potential to catalyze specific biochemical reactions. There is a misconception about biological catalysts that all biological catalysts are made up of proteins, which is not true; some are RNA-based catalysts (ribozymes and ribosomes). Both are important for many cellular functions. A major difference between enzymes and ribozymes is that RNA-based catalysts are restricted to only a few reactions; however, their reaction mechanisms and kinetics can be studied and classified by similar procedures. Enzyme-based mutation, in particular site-directed mutagenesis, is an important approach to alter genes and investigate the functional and structural features of enzymes, e.g. mutation of the enzyme present in Coprinus cinereus peroxidase offers an understanding of its increased thermostability. Challenges involved in studying cascades of reactions catalyzed by a multi-enzyme, e.g. proteasome involved in the ubiquitin–proteasome pathway, can be overcome by establishing understanding of the complex structure and the respective biochemical reactions. This understanding allows exploration of active sites, intermediate compounds, final products and their interrelation with complex machinery, as well as biochemical reactions. It has been well understood that enzymes that accelerate complex reactions have numerous substrates and involve complex enzyme kinetic mechanisms. As discussed above, most of the biochemical reactions occurring in the body are multi-substrate reactions. In such reactions two substrates are involved and yield two products (figure 1.4 ). These types of reactions involve the transfer of a compound from one compoment to another, e.g. when glucose reacts with ATP in the presence of hexokinase it forms glucose 6-phophaste and ADP. Here, phosphate from ATP is transfered to glucose to form glucose 6 phosphate. The mechanism of catalysis involves two types of reactions: sequential and non-sequential reactions. Sequential reaction results in the formation of a ternary complex. This means that both of the substrates involved in the reaction bind with an enzyme to form the product (figure 1.4 ). Sequential reaction is further divided into two types: the random and compulsory order mechanisms. As the name suggests, in a 'random' mechanism, either substrate can bind first and any product can leave first. In contrast to the random order mechanism, in the compulsory order mechanism the order of binding of the substrate and order of release of the product is specific; this is also called the Theorell–Chance mechanism (figure 1.4 ). In a non-sequential reaction, also called the 'ping-pong' mechanism, formation of ternary complex does not take place. In these types of reactions, when the first substrate binds with enzyme its product is released, and then the second substrate binds and its product is released. Such a reaction is called a double placement reaction. Thus only a single substrate binds at a time; this may be due to the presence of a single binding site on the enzyme. Major differences between the sequential and non-sequential reactions are that the formation of a ternary complex takes place only in the sequential reaction, and that in the sequential reaction both substrates bind to the enzyme and release products, while in the non-sequential mechanism the substrates bind and release their products one after the other (figure 1.4 ).
Figure 1.4. Multi-substrate reactions.
Another type of sequential mechanism is the systematic mechanism, which involves the addition of substrates and formation of products in a specific order.
1.7.5. Enzyme mediated acid–base (general) catalysis
Several protein enzymes use general acid–base catalysis as a way to increase reaction rates [ 26 ]. The amino acid histidine is optimized for this function because it has a pK(a) (where K(a) is the acid dissociation constant) near physiological pH [ 26 ].
When the substrate has been bound at the catalytic site, the charged functional groups of the side chains of neighboring aminoacyl residues may contribute in catalysis by behaving as acidic or basic catalysts. There are two extensive groups of acid–base catalysis by enzymes: general and specific (acid or base) catalysis. Specific acid or specific base catalysis are those reactions in which the reaction rates fluctuate under the influence of changes in H + or H 3 O + concentration, but are independent of the concentrations of the other acids or bases present in the solution. In contrast to specific catalysis, general acid or general base catalysis are the reactions whose rates are very reactive to all acids (proton donors) or bases (proton acceptors) present in the solution. To examine whether a given enzyme-catalyzed reaction is a general or specific acid or base catalysis, the rate of reaction is determined under two sets of circumstances:
- • at different pH values at a constant buffer concentration, and
- • at constant pH values but at different buffer concentrations. Against this background, if the degree of the reaction deviates as a function of pH at a constant buffer concentration, the reaction is specific base/acid catalyzed if the pH is above/below 7.0. If the reaction rate at a constant pH rises as the buffer concentration increases, the reaction is general base/acid catalysis, if the pH is above/below 7.0.
1.7.6. Metallozymes
Almost 25% of all enzymes include tightly bound metal ions or need them for activity. The major role of these metal ions is investigated using techniques such as x-ray crystallography, magnetic resonance imaging (MRI) and electron spin resonance (ESR). A metalloprotein is a protein that contains a metal ion co-factor. Metallozymes contain a certain amount of functional metal ion that is retained during the course of purification [ 27 ]. A metal-activated enzyme binds with metals less firmly, but needs to be activated by addition of metals. Four types of complexes are possible for the tertiary complexes of the catalytic site (Enz), a metal ion (M) and substrate (S) that exhibit 1:1:1 stoichiometry:
All of these complexes are possible for metal-activated enzymes. Metallozymes cannot form the EnzSM complex (substrate–bridge complexes), as the purified enzyme exists as Enz–M. Three generalization can be made:
- • The majority of the kinases (ATP: phosphotransferases) form substrate–bridge complexes of the type enzyme–nucleotide–M.
- • Phosphotransferases (phosphoenolpyruvate or pyruvate used as the substrate), enzymes catalyzing other reactions of phosphoenolpyruvate and carboxylases, form metal bridge complexes (Enz–M–S).
- • A particular enzyme may form one type of bridge complex with one substrate and a different type with another.
The metal ions participate in each of the four mechanisms by which the enzymes are known to accelerate the rates of chemical reaction:
- • Approximation of reactants.
- • Covalent catalysis.
- • General acid–base catalysis.
- • Induction of strain in the enzyme or substrate.
Metal ions are electrophiles (attracted to electrons) and share an electron pair forming a sigma bond. They may also be considered as super acids as they exist in neutral solutions, frequently having a positive charge which is greater than their quantity. Mn 2+ , Ca 2+ and Mg 2+ are the metal ions that are most commonly used in enzymatic catalysis. Two metal ions, iron and manganese are used in the form of haemprotein. Metal ions have the potential to accept electrons via sigma or pi bonds to successively activate electrophiles or nucleophiles. By means of donating electrons, metals can activate nucleophiles or act as nucleophiles themselves. The co-ordination sphere of a metal may bring together the enzyme and substrate or form chelate-producing distortion in either the enzyme or substrate [ 28 ]. A metal ion may also mask a nucleophile and thus avoid an otherwise probable side reaction. Metals can also function as three-dimensional templates for the co-ordination of basic groups on the enzyme or substrate.
1.8. Enzyme inhibition
Enzyme inhibition decreases the activity of an enzyme without significantly disrupting its three-dimensional macromolecular structure. Inhibition is therefore distinct from denaturation and is the result of a specific action by a reagent directed or transmitted to the active site region. When low molecular weight compounds interfere with the activity of enzymes by partially reducing or completely inhibiting the enzyme activity either reversibly or irreversibly, it is known as enzyme inhibition. The compounds responsible for such inhibition are called enzyme inhibitors. To protect the enzyme catalytic site from any change, a ligand binds with a critical side chain in the enzyme. Chemical modification can be performed to test the inhibitor for any drug value. Studies of enzymes can yield much information about the following:
- • A number of drugs useful in medicine, which seem to function because they can inhibit certain enzymes in malfunctioning cells.
- • The convenience of elucidating metabolic pathways in cells.
- • The mechanism of the catalytic activity.
- • The nature of the functional group at the active site.
- • The substrate specificity of the enzyme.
The pharmacological action of drugs is mainly based on enzyme inhibition, e.g. sulfonamides and other antibiotics. In the majority of cases the enzyme inhibited is known. The development of nerve gases, insecticides and herbicides is based on enzyme inhibition studies. There are two major types of enzyme inhibition: reversible and irreversible.
Reversible inhibitors efficiently bind to enzymes by forming weak non-covalent interactions, e.g. ionic bonds, hydrophobic interactions and hydrogen bonds. Reversible inhibitors do not form any strong chemical bonds or reactions with the enzyme, they are formed quickly and can easily be removed, in contrast to irreversible inhibitors. Reversible inhibition includes competitive inhibition, uncompetitive inhibition and noncompetitive inhibition. Irreversible inhibition includes group specific inhibition (reacts only to a certain chemical group), reactive substrate analogs (affinity label) and inhibitors that are structurally similar to the substrate and will bind to the active site, and mechanism-based inhibitors (enzymes transform the inhibitor into a reactive form within the active site).
1.9. Pharmaceutical applications
Currently, enzymes are often utilized for a broad range of applications such as: washing powders (e.g. proteases, lipases, amylases); textile manufacture (amylases and catalase to remove the starch); the leather industry (proteases to hydrolyze proteins); the paper industry; improvement of the environment; food production (enzyme-modified cheese/butter), processing (glucose oxidase for dough strengthening) and preservation; and medical applications. According to current reports, several enzymes are produced industrially and there are significant applications in the food industry (45% of use), detergent industry (35%), textiles industry (10%) and leather industry (3%). Details on the applications of individual enzymes are provided in table 1.6 .
Table 1.6. Industrially produced enzymes from plant sources and their applications.
1.9.1. Diagnostic applications of enzymes
Enzymes have been used widely in diagnostic applications varying from immunoassays to biosensors. Enzyme immunoassay methods hold great promise for application under a wide variety of conditions. Under laboratory conditions they can be as sensitive as radio-immunoassays, but they can also be adapted as simple field screening procedures [ 29 , 30 ]. The examination of enzyme quantity in the extracellular body fluids (blood plasma and serum, urine, digestive juices, amniotic fluid and cerebrospinal fluid) are vital aids to the clinical diagnosis and management of disease. Most enzyme-catalyzed reactions occur within living cells, however, when an energy imbalance occurs in the cells because of exposure to infective agents, bacterial toxins, etc, enzymes 'leak' through the membranes into the circulatory system. This causes their fluid level to be raised above the normal cell level. Estimation of the type, extent and duration of these raised enzyme activities can then furnish information on the identity of the damaged cell and indicate the extent of injury. Enzyme assays can make an important contribution to the diagnosis of diseases, as a minute change in enzyme concentration can easily be measured. Determination of the changes in enzyme level thus offers a greater degree of organ and disease differentiation in comparison to other possible clinico-chemical parameters, e.g. albumin or gamma globulin. Currently, the diagnostic specificity of enzyme tests is such that they are limited primarily to confirming diagnosis, offering data to be weighed alonside other clinical reports, owing to lack of disease specific enzymes. Table 1.7 includes a number of diagnostically important enzymes which are most often examined in clinic laboratories [ 29 , 30 ].
Table 1.7. Diagnostically significant enzymes.
1 B, brain; E, erythrocytes; H, heart muscle; Ht, hepatobiliary tract; I, intestinal mucosa; K, kidney; L, M, skeletal muscle; Pa, pancreas; P1, placenta; Pr, prostate gland; S, saliva.
1.9.1.1. Enzyme examinations in diseases of the liver and biliary
The diseases of the liver and gastrointestinal tract were among the first to which serum enzyme tests were applied. They have proved to be most effective owing to the large size of the organs and the wide range and abundance of enzymes [ 32 – 36 ]. The liver-based enzymes GOT, GPT and AP are examined to evaluate the site and nature of liver disease. LD, GGT, OCT and CHE are also examined. Several enzymes employed in the diagnosis of liver diseases along with their respective levels are listed in table 1.8 .
Table 1.8. Liver diseases and enzymes used in diagnosis [ 32 – 36 ].
1.9.1.2. Enzyme applications in heart disease
According to previous reports, no single enzyme has yet been reported to cure myocardial damage. The discovery of serum glutamine oxalacetic acid transaminase determination (GOT) in 1954 was considered a significant step forward in the diagnosis of acute myocardial infarction. A mixture of results from assays of CPK (creatine phosphokinase), HBD ( α -hydroxybutyrate dehydrogenase) and GOT (glutamine oxalacetic acid transaminase)—each of which has been shown to be elevated in more than 90% of cases—is used for diagnostic purposes [ 37 – 39 ]. The level of CPK starts rising three to four hours after the initial onset of pain, followed in order by GOT and AST (HBD) which appear after approximately eight hours. The maximum levels are reached in the same sequence, CPK after 24 h, LD 1 after 36 h and AST after about two days. The rise in enzyme levels is fairly moderate, AST and CPK increase by four to ten times their respective normal levels and LD 1 is approximately five-fold higher than normal. An enzyme known as hyaluronidase (hyaluronate hydrolysis) has been reported to cure heart attack [ 38 ]. The activity of many enzymes including aldolase, malic dehydrogenase, isomerase and ICD may increase following myocardial infarction [ 38 ].
1.9.1.3. Diagnosis of muscle disease
Skeletal muscle disorders include diseases of the muscle fibers (myopathies) or of the muscle nerves (neurogenic disorders) [ 40 ]. In myopathies CPJ, LD, ALD, GOT and GPT levels are raised. In the case of neurogenic diseases and hereditary diseases, CPK is occasionally raised (2–3 fold) [ 40 ]. Damage to the muscle may be due to extensive muscular exercise, drugs, physical trauma, inflammatory diseases, microbial infection or metabolic dysfunction, or it may be genetically predisposed. In muscular disorders the level of CPK is elevated in serum with the highest frequency and is assayed in the diagnosis of these disorders. An additional useful assayed enzyme is acetyl cholinesterase (AChE), which is significant in regulating certain nerve impulses [ 41 ]. Various pesticides affect this enzyme, so farm labors are frequently tested to be sure that they have not received accidental exposure to significant agricultural toxins. There are number of enzymes that are characteristically used in the clinical laboratory to diagnose diseases. There are highly specific markers for enzymes active in the pancreas, red blood cells, liver, heart, brain, prostate gland and many of the endocrine glands [ 41 ]. From the time when these enzymes became comparatively easy to examine using automated techniques, they have been part of the standard blood tests that veterinarians and medical doctors are likely to need in the diagnosis and treatment/management of diseases.
1.9.2. Enzymes in therapeutics
Enzymes have two significant features that differentiate them from all other types of drugs. First, enzymes frequently bind and act on their targeted sites with high affinity and specificity. Second, enzymes are catalytic and convert numerous target molecules to the desired products. These two important features make enzymes specific and potent drugs that can achieve therapeutic biochemistry in the body that small molecules cannot. These features have resulted in the development of many enzyme-based drugs for a wide range of disorders [ 42 ]. Currently, numerous enzymes are used as therapeutic agents, owing to the following features:
- • High specificity to their substrates.
- • Proficient in producing the desired effect without provoking any side effects.
- • Water soluble.
- • Extremely effective in a biological environment.
Enzymes as therapeutic agents also have some serious disadvantages which restrict their application. Their bulky structure, due to their large molecular weight, excludes them from the intracellular domain. Owing to their high proteinaceous nature they are highly antigenic and are rapidly cleared from blood plasma. Extensive purification from pyrogens and toxins is essential for parenteral enzymes, which increases the cost. Table 1.9 lists some therapeutically important enzymes.
Table 1.9. Therapeutically important enzymes.
1.9.2.1. Enzyme therapy of cancer
In traditional medicine, proteolytic enzymes derived from plant extracts have been used for a long time In addition to proteolytic enzymes from natural resources such as plants, 'modern' enzyme therapy includes pancreatic enzymes. Therapeutically, the use of proteolytic enzymes is partly based on scientific reports and is partly empirical [ 43 ]. Clinical evidence of the use of proteolytic enzymes in cancer studies has typically been obtained with an enzyme preparation comprising a combination of papain, trypsin and chymotrypsin. Earlier reports proved that enzyme therapy can reduce the adverse effects caused by radiotherapy and chemotherapy. There is also a report available that, in some types of tumors, survival may be sustained. The positive effects of systemic enzyme therapy appear to be based on its anti-inflammatory potential. Nevertheless, the exact mechanism of action of systemic enzyme therapy remains unsolved. The proportion of proteinases to antiproteinases, which is regularly used as a prognostic marker in cancer studies, is likely to be influenced by the oral administration of proteolytic enzymes, most likely via induction of the synthesis of antiproteinases. In addition, there are many alterations of cytokine composition during treatment with orally administered enzymes, which might be a sign of the efficacy of enzyme therapy [ 44 ].
Proteases and their inhibitors have long been studied in several tumor systems. However, out of numerous promising serine and metalloproteinase inhibitors, not a single one is included in oncology at present. The present exploration for active antiproteolytic agents is in contrast to the traditional approach, as evidenced by John Beard, who proposed the management of advanced cancer using fresh pancreatic extracts whose antitumor activity was based on their proteolytic potential.
The enzymatic treatment of tumors is based on the idea of denying the abnormal cells their essential metabolic precursors such as amino acids, nucleic acids and folates. A number of enzymes have been examined and evidenced as antitumor agents. l -serine dehydratase, l -arginase, carboxypeptidase G (folate depletion), l -asparaginase, l -methioninase, l -phenylalanine ammonia lyase, l -glutaminase, l -tyrosinase and xanthine oxidase have been studied for their anticancer activity. Enzyme preparations such as asparaginase (amidase), bromelain (protease) and chymotrypsin (protease) have also been studied as cancer treatments (table 1.9 ).
l -asparaginase is the most widely investigated enzyme. It has been reported in treatment against three neoplastic diseases, acute lymphoblastic leukemia, leukemic lymphosarcoma and myeloblastic leukemia. It deprives the cancerous cells of their nutritional asparagine supply. Asparagine is essential for protein synthesis, which takes place inside the cell, and decreased protein synthesis perhaps accounts for the immunosuppression and toxic effects of asparaginase-based treatment.
The prospects of enzyme-based treatment against cancer are very bright, but the difficulties of antigenicity and short circulation time remain to be overcome.
1.9.2.2. Enzymes in thrombolytic treatment
Activation of the blood clotting mechanism during inflammation is part of the body's defense mechanism which requires therapeutic intervention. Under normal physiological conditions there is an equilibrium between blood coagulation (clotting) and fibrinolysis (the process of dissolving the clotted blood) [ 47 ]. Biocatalysts such as enzymes, ribozymes, pro-enzymes, activators and pro-activators are responsible for maintaining equilibrium between clot formation and fibrinolysis. Imbalances in the concentration of these bio-activators may disturb physiology. In the biological process of fibrogenesis, clot formation takes place due to the plasma protein (soluble fibrinogen), which is ultimately converted to insoluble fibrin by the enzyme thrombin. This process is dependent on the conversion of thrombin from prothrombin. This bio-conversion takes place after the cascade of enzymatic reactions which involved certain key biological compounds called clotting factors. A blood clot dissolving enzyme known as plasmin is present in the blood as the pro-enzyme plasminogen. During clot dissolution activators convert the plasminogen to plasmin. This biological process is well regulated by certain process such as vasoconstriction, formation of a fibrin and clot platelet aggregation [ 46 ].
As the body utilizes enzymes in conserving this key balance of homeostasis, in a similar way we can utilize enzymes to repair or restore the homeostatic balance once it is lost. Several reports have shown that one of the best approaches for treating such clinical conditions is the administration of enzymes capable of converting plasminogen to plasmin (the enzyme which dissolves the clot) via intraveneous injection. This type of treatment is called therapeutic thrombolysis or thrombolytic therapy. In this treatment, pharmacological agents are used to medically induce clot breakdown [ 47 ]. Various novel thrombolytic agents have been derived from different sources for therapeutic use, such as from bacteria (streptokinase), the venom of the Malayan pit viper (Arvin), a filamentous fungus Koji mold Aspergillus oryzae (brinase), a South American snake (reptilase) and human urine (urokinase) [ 47 ].
Current advancements in thrombolytic therapy are more focused on the treatment of occlusions (blockages) of blood vessels. These types of therapy can be considered as life-saving and emergency medicine for life-threatening conditions such as myocardial infarction and massive pulmonary embolism, which are the most common reasons for cardiac arrest. This life-saving treatment is more reliable in preventing the blockages of vessels in the lungs and heart. Artery blockage conditions such as pulmonary embolism in the lungs by the formation of a clot creates tension on the right side of the heart, resulting in shortness of breath and chest pain mainly upon breathing in. Enzyme-based thrombolysis for treating massive pulmonary embolism has been considered as an effective approach to dissolving clots in these large vessels. Since surgical removal raises the chances of new blood clot formation that can cause another pulmonary embolism at the same or a different site, it is considered a dangerous practice and thrombolytic therapy is considered the more effective treatment [ 47 ]. Nevertheless, reoccurrence of clot formation or clot re-formation is very common in patients who have undergone enzyme-based thrombolytic treatment. Researchers from various organizations (1971) determined the effectiveness of streptokinase over heparin in reducing the chances of death in acute myocardial infarction patients. Significant results were obtained during this experiment. As discussed above, re-formation of the clot is one of the major concerns in fibrinolytic therapy. Most clinicians start treatment with a high dose of fibrinolytic agents, which is reduced later on. This approach may reduce disease progression for some time, but often increases the chances of clot re-formation. Even after the dissolution of the clot it is very difficult to maintain the same physiologically balanced environment (homeostasis) at the site of damaged tissues and the chance of new clot formation at that particular location is very high. Therefore, fibrinolytic based treatment is always accompanied by anticoagulants, such as heparin [ 46 ].
Major concerns associated with streptokinase therapy are fever, a tendency for bleeding, antigenicity (as with any foreign protein) and the difficulty of determining the proper dose [ 47 ]. Post-enzymatic treatment bleeding is one of the major concerns and it is also a concern when anticoagulants are used alone. According to current research, urokinase (produced in the kidneys and obtained from human urine) is considered safer than streptokinase. For the production of urokinase, 2300 l of urine is required to yield only 29 mg of purified urokinase, thus considering the expense involved in its manufacture, its clinical utilization has been restricted. Other examples are Arvin and reptilase. Utilization of these has been restricted for several reasons, but they are still considered as potential replacements for heparin as anticoagulants. Some researchers have noticed that optimum dose plays an important role and is one of the key factors in determining re-clot formation. Thorough investigation is required to overcome any shortcomings and increase the acceptance of these enzymes in therapeutic use [ 47 ].
1.9.2.3. The role of enzymes in digestive disorders and inflammations
Enzymes play an essential role in the management of various digestive disorders, such as exocrine pancreatic insufficiency [ 48 ]. Supplementation with enzymes may also be advantageous for other conditions associated with poor digestion, such as lactose intolerance. Generally, pancreatic enzymes such as porcine and bovine have been the preferred form of supplementation for exocrine pancreatic insufficiency [ 48 ]. Utilization of microbe-derived lipase has presented promise with reports showing benefits alike to pancreatic enzymes, but with a lower dosage concentration and a broader pH range. The safety and efficacy of enzymes derived from microbial species in the treatment of conditions such as malabsorption and lactose intolerance is promising. Plant-derived enzymes, e.g. bromelain from pineapple, serve as active digestive aids in the breakdown of proteins. Synergistic properties have also been reported using a combination of animal-based enzymes and microbe-derived enzymes or bromelain. Buccal administration of pancreatin (derived from an alcoholic extract of animal pancreas) enhances the enzymatic digestion of starch and proteins in patients with pancreatic cysts and pancreatitis. Pancreatin in combination with lipase is used to treat patients with fatty stools. Hydrolytic enzymes such as papain and fungal extracts ( Aspergillus niger and Aspergillus otyzae ) are used to enhance absorption from the small intestine [ 49 ]. These fungal extracts comprise amylases and proteases along with cellulases, which support the breakdown of the otherwise indigestible fibers of cabbages, etc, and thus reduce dyspepsia and flatulence [ 50 ]. Currently, micro-organisms are used at a large scale for the production of therapeutic enzymes. Among various micro-organisms Saccharomyces cerevisiae, Saccharomyces fragilis, Bacillus subtilis and two Aspergillus species are considered safe by the FDA (USA) for obtaining oral β -galactosidase (from A. oryzae ) which is often used by patients suffering from inherited intestinal disease lactose deficiency [ 51 ]. Children with this genetic disorder children are incapable of digesting milk lactose. Enzymatic preparations such as β -galactosidase catalyze the conversion of lactose to glucose and galactose, which are quickly absorbed by the intestine. Other enzymatic preparations, e.g. penicillinase (from B. subtilis ) are often used to treat hypersensitivity reactions caused by the antibiotic penicillin [ 52 ]. This enzyme catalyzes the conversion of penicillin to penicillanic acid, which is non-immunogenic. In addition, microbial and plant hydrolases are also used to decrease inflammation and edema [ 53 ]. Thrombin, trypsin, chymotrypsin, papain, streptokinase, streptodornase and sempeptidase are under clinical trial investigation. These enzymatic preparations are administered orally and have considerable proteolytic activity in the serum. Streptodornase has also displayed pain-relieving action on systemic injection [ 54 ]. Preparations have also been used to clean dirty wounds and necrotic tissue and to remove debris from second and third degree burns.
1.10. Plants and algae enzyme systems
Plant based foods are usually consumed in their raw form [ 68 ]. This eases the main concern with animal-based enzymes by preserving the integrity of the enzymes themselves. Moreover, plant-based digestive enzymes are effective over a broad scope of pH levels. This range is usually between 3.0 and 9.0, which is highly well-matched with the human gastrointestinal environment [ 55 – 72 ]. Thus plant-based enzymes are compatible for supporting comprehensive digestive health. Protease, amylase, lipase and cellulose are the important enzymes and are present in plants. Protease breaks down protein that can be present in meat, fish, poultry, eggs, cheese and nuts. Amylase assists your body with the breakdown and subsequent absorption of carbohydrates and starches. Lipase aids the digestion of fat. When your diet includes lipase-rich foods, it eases the production burden on the gall bladder, liver and pancreas. Cellulase is present in many fruits and vegetables, and it breaks down food fibers, which increases their nutritional value to our bodies. The presence of cellulase in plant-based sources is important, because it is not naturally present in the human body. Fruits and vegetables are an ideal source for enzymes. They are enzyme-rich and easily consumed without needing to be cooked or processed, ultimately preserving the full functionality of the enzymes. By using plant biotechnology several enzymes can be produced from plants as well algal resources [ 56 – 72 ].
During algal photosynthesis various proteins and enzymes are produced which can be utilized in economic development and environment management, such as in wastewater treatment, production of fine chemicals, and biodiesel production [ 56 – 72 ]. Due to their potential to capture and fix carbon dioxide using solar energy, photosynthetic marine algae are considered as potential models for the production of proteins. It has been recently observed that algal chloroplasts can be transformed for the production recombinant proteins [ 55 ]. Five different classes of recombinant enzymes; xylanase, α-galactosidase, phytase, phosphate anhydrolase, and β-mannanase, D. tertiolecta or C. reinhardtii were in the plastids of D. tertiolecta or C. reinhardtii. Similar strategies should allow for recombinant protein production in many species of marine algae [ 55 ].
- Go to reference in chapter
Export references: BibTeX RIS
B, brain; E, erythrocytes; H, heart muscle; Ht, hepatobiliary tract; I, intestinal mucosa; K, kidney; L, M, skeletal muscle; Pa, pancreas; P1, placenta; Pr, prostate gland; S, saliva.

Fundamentals of Enzyme Engineering pp 111–125 Cite as
Specificity of Enzymes
- Young Je Yoo 5 ,
- Yan Feng 6 ,
- Yong Hwan Kim 7 &
- Camila Flor J. Yagonia 8
- First Online: 13 January 2017
2149 Accesses
The enzymatic reaction starts with the binding of the substrate to the enzyme. When the substrate approaches to the active site of enzyme, the electrostatic microenvironment in the substrate-binding region changes to make the reaction proceeds to form the final products.
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Andrews FH and McLeish MJ. Using site-saturation mutagenesis to explore mechanism and substrate specificity in thiamin diphosphate-dependent enzymes. FEBS J , 2013, 280:6395–6411.
Google Scholar
Antipov E, Cho AE and Klibanov AM. How a single-point mutation in horseradish peroxidase markedly enhances enantioselectivity. J. Am. Chem. Soc. , 2009, 131:11155–11160.
Arnold FH and Volkov AA. Directed evolution of biocatalysts. Curr Opin Chem Biol , 1999, 3:54–59.
Carey FA and Sundberg RJ. Advanced organic chemistry part A: structure and mechanisms. New York, Plenum Press , 1984.
Fitzpatrick P, Ringe D and Klibanov A. Computer assisted modeling of subtilisin enatioselectivity in organic solvent. Biotech.Bioeng , 1992, 40:735–742.
Gao X, Huang F, Feng J, Chen X, Zhang H, Wang Z, Wu Q and Zhu D. Engineering the meso-diaminopimelate dehydrogenase from Symbiobacterium thermophilum by site saturation mutagenesis for d-phenylalanine synthesis. Appl Environ Microbiol , 2013, 79:5078–5081.
Gordon SR, Stanley EJ, Wolf S, Toland A, Wu SJ, Hadidi D, Mills JH, Baker D, Pultz IS and Siegel JB. Computational design of an α-gliadin peptidase. J Am Chem Soc , 2012, 134:20513–20520.
Hilvert D. Design of protein catalysts. Annu. Rev. Biochem., 2013, 82:447–470.
Hopmann KH, Hallberg BM and Himo F. Catalytic mechanism of limonene epoxide hydrolase, a theoretical study. J. Am. Chem. Soc., 2005, 127:14339–14347.
Keith JM, Larrow JF andJacobsen EN. Practical considerations in kinetic resolution reactions. Advanced Synthesis & Catalysis , 2001, 343(1):5–26.
Khare SD, Kipnis Y, Greisen PJ, Takeuchi R, Ashani Y, Goldsmith M, Song Y, Gallaher JL, Silman I, Leader H. Computational redesign of a mononuclear zinc metalloenzyme for organophosphate hydrolysis. Nat Chem Biol , 2012, 8:294–300.
Kiss G, Çelebi-Ölçüm N, Moretti R, Baker D and Houk KN. Computational enzyme design. Angew Chem, Int Ed , 2013, 52:5700–5725.
Manna SK and Mazumdar S. Tuning the substrate specificity by engineering the active site of cytochrome P450cam: A rational approach. Dalton Trans , 2010, 39:3115–3123.
Mouratou B, Kasper P, Gehring H and Christen, P. Conversion of tyrosine phenol-lyase to dicarboxylic amino acid β- lyase, an enzyme not found in nature. J Biol Chem , 1999, 274:1320–1325.
Murphy PM, Bolduc JM, Gallaher JL, Stoddard BL and Baker D. Alteration of enzyme specificity by computational loop remodeling and design. Proc Natl Acad Sci U S A , 2009, 106:9215–9220.
Otten LG, Hollmann F and Arends IW. Enzyme engineering for enantioselectivity: from trial-and-error to rational design? Trends in Biotechnology , 2009, 28(1):46–54.
Rouhi M. Chiral Chemistry: Traditional methods thrive despite numerous hurdles, including tough luck, slow commercialization of catalytic processes. Chemical & Engineering News , 2004, 82:47–62.
Schmid A, Hollmann F, Park JB and Bühler B. The use of enzymes in the chemical industry in Europe. Current Opinion in Biotechnology , 2002, 13:359–366.
Sinclair R, Reid GA and Chapman SK. Re-design of Saccharomyces cerevisiae flavocytochrome b2: Introduction of L-mandelate dehydrogenase activity. Biochem J , 1998, 333:117–120.
Sobolev V, Sorokine A, Prilusky J, Abola EE and Edelman M. Automated analysis of interatomic contacts in proteins. Bioinformatics , 1999, 15:327–332.
Strauss UT, Felfer U and Faber K. Biocatalytic transformation of racemates into chiral building blocks in 100% chemical yield and 100% enantiomeric excess. Tetrahedron-Asymmetry , 1999, 10(1):107–117.
Tyagi S and Pleiss J. Biochemical profiling in silico – Predicting substrate specificities of large enzyme families . J. Biotechnology , 2006, 124:108–116.
Tyka MD, Jung K and Baker D. Efficient sampling of protein conformational space using fast loop building and batch minimization on highly parallel computers. J Comput Chem , 2012, 33:2483–2491.
Watanabe S, Kodaki T and Makino K. Complete reversal of coenzyme specificity of xylitol dehydrogenase and increase of thermostability by the introduction of structural zinc. J. Biol. Chem , 2005, 280:10340–10349.
Wijma HJ and Janssen DB. Computational design gains momentum in enzyme catalysis engineering. FEBS J , 2013, 280:2948–2960.
Wijma HJ, Floor RJ, Bjelic S, Marrink SJ and Baker D. Enantioselective enzymes by computational design and in silico screening. Angewandte Chemie , 2015, 127:3797–3801..
Xie T, Song B, Yue Y, Chao Y and Qian S. Site-saturation mutagenesis of central tyrosine 195 leading to diverse product specificities of an α-cyclodextrin glycosyltransferase from Paenibacillus sp . 602–1. J Biotechnol , 2014, 170:10-16.
Yeon YJ, Park HY and Yoo YJ. Enzymatic reduction of levulinic acid by engineering the substrate specificity of 3-hydroxybutyrate dehydrogenase. Bioresource Technology , 2013, 134:377–380.
Download references
Author information
Authors and affiliations.
School of Chemical and Biological Engineering, Seoul National University, Seoul, Korea
Young Je Yoo
College of Life Science and Biotechnology, Shanghai JiaoTong University, Shanghai, China
School of Energy and Chemical Engineering, Ulsan National Institute of Science and Technology, Ulsan, Korea
Yong Hwan Kim
School of Chemical Engineering, University of San Carlos, Cebu City, Philippines
Camila Flor J. Yagonia
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Young Je Yoo .
Rights and permissions
Reprints and permissions
Copyright information
© 2017 Springer Science+Business Media B.V.
About this chapter
Cite this chapter.
Yoo, Y.J., Feng, Y., Kim, Y.H., Yagonia, C.F.J. (2017). Specificity of Enzymes. In: Fundamentals of Enzyme Engineering. Springer, Dordrecht. https://doi.org/10.1007/978-94-024-1026-6_10
Download citation
DOI : https://doi.org/10.1007/978-94-024-1026-6_10
Published : 13 January 2017
Publisher Name : Springer, Dordrecht
Print ISBN : 978-94-024-1024-2
Online ISBN : 978-94-024-1026-6
eBook Packages : Chemistry and Materials Science Chemistry and Material Science (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Lock and Key Model Enzyme Explained!
by Taha Cheema
Reading about enzymes in IGCSE or O Level Biology syllabus and books might leave you confused about how they work. The “ lock and key model enzyme ” has an elegant and simple explanation. Read on below to find out.
"Just as a key fits a lock, enzymes and substrates are built for each other.”
What is the Lock and Key Model?
In order to explain how enzymes work, we need to be aware of two important terms:
Enzymes: Proteins that help speed up chemical reactions .
Substrates : The chemical compounds that are processed by enzymes (e.g. they can be broken down)
Related: Enzymes
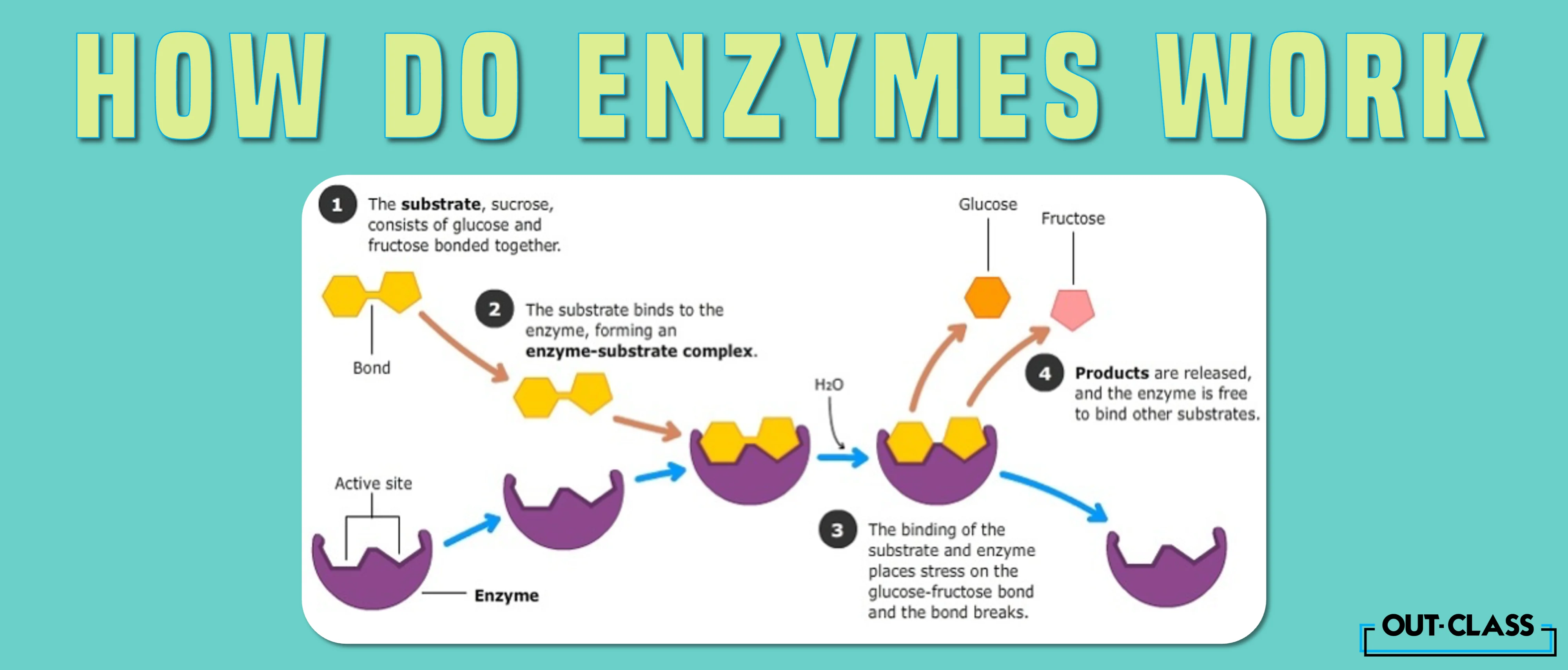
Basics of the Lock and Key Model of Enzyme Action
The basics of the lock and key model enzymes is one of the simplest models of enzyme action to understand. The lock and key model of enzyme action is similar to how we open a lock:
Only one specific key fits and opens a given lock
Similarly, only the correct substrate can fit a given enzyme, allowing it to work
*Note: This unique pairing between enzymes and substrates maintains precision in biological processes . Specific enzymes can focus on their specific reactions and this improves efficiency in maintaining cells !

Using Lock and Key to Describe an Enzyme:
A good way to understand enzyme action is through the lock and key hypothesis:
The substrate can be thought of as a key (substrate = key)
The enzyme can be thought of as the lock (enzyme = lock)
When the substrate enters, it “ activates ” the enzyme, which starts processing the substrate
Other substrates will not be able to “activate” the enzyme, as their shape won’t match
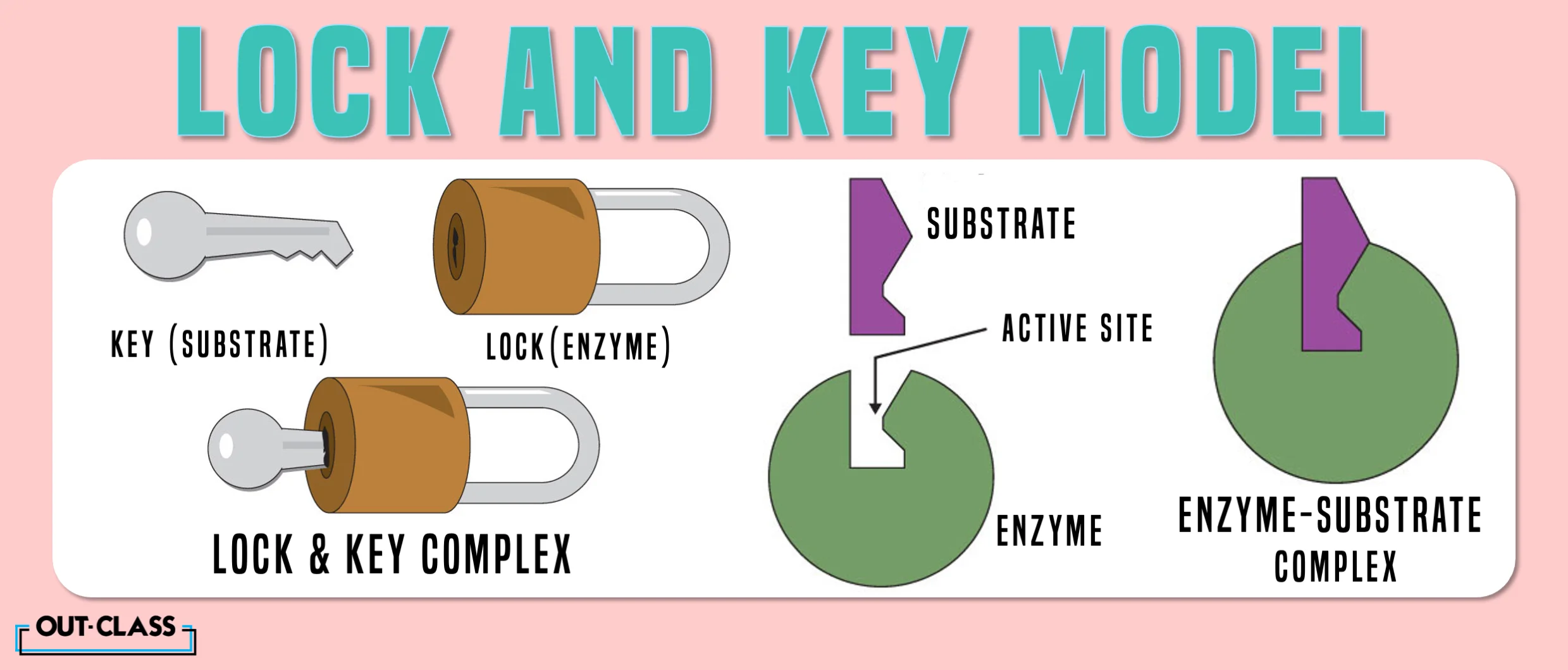
Wrapping-Up
In conclusion, enzymes speed up ( catalyze ) some key chemical reactions in organisms . The theory of lock and key model enzymes says that a given enzyme only interacts with its precise substrates.
We hope that by reading this concept guide, you developed a better understanding of O Level or IGCSE Biology . Another way to clear your concepts is by attempting and practicing with IGCSE or O Level Biology past papers .
Stay tuned to Out-Class for more study guides!
Most Common Repeated Questions:
Unlock the secrets to acing your IGCSE/O Level Biology exams with a sneak peek into the most frequently asked questions that have graced the pages of past papers!
- Explain the ‘lock and key’ hypothesis of enzyme action using a named example (5) [ Oct/Nov 2021]
The change in colour of the apple tissue is due to a series of chemical reactions. An enzyme called PPO acts as a catalyst for one of these reactions. The colour change can be prevented by placing the cut surface of apple tissue in boiling water for a short time immediately after the fruit is cut. Explain this observation using the lock and key hypothesis of enzyme action. (4) [ Oct/Nov 2020]
Q. What are enzymes?
Enzymes are proteins that act as catalysts, speeding up chemical reactions in living organisms. They facilitate these reactions without being consumed or altered themselves.
Related: Enzymes
Q. What is the lock and key model of enzyme action?
The lock and key model is a hypothesis explaining how enzymes interact with substrates. It compares the specificity of enzyme-substrate interactions to a lock that only opens with the correct key. In this model, enzymes (locks) have specific active sites that perfectly fit their substrates (keys).
Q. How does the lock and key model maintain precision in biological processes?
The lock and key model ensures precision by allowing only specific substrates to bind with an enzyme's active site. This specificity prevents enzymes from interacting with inappropriate substrates, maintaining accuracy and efficiency in biological reactions.

If you want to dive right in, let's start with selecting the course you want
What course are you interested in, choose from the list.

18.6 Enzyme Action
Learning objective.
- Describe the interaction between an enzyme and its substrate.
Enzyme-catalyzed reactions occur in at least two steps. In the first step, an enzyme molecule (E) and the substrate molecule or molecules (S) collide and react to form an intermediate compound called the enzyme-substrate (E–S) complex . (This step is reversible because the complex can break apart into the original substrate or substrates and the free enzyme.) Once the E–S complex forms, the enzyme is able to catalyze the formation of product (P), which is then released from the enzyme surface:
Hydrogen bonding and other electrostatic interactions hold the enzyme and substrate together in the complex. The structural features or functional groups on the enzyme that participate in these interactions are located in a cleft or pocket on the enzyme surface. This pocket, where the enzyme combines with the substrate and transforms the substrate to product is called the active site The location on an enzyme where a substrate binds and is transformed to product. of the enzyme ( Figure 18.10 "Substrate Binding to the Active Site of an Enzyme" ). It possesses a unique conformation (including correctly positioned bonding groups) that is complementary to the structure of the substrate, so that the enzyme and substrate molecules fit together in much the same manner as a key fits into a tumbler lock. In fact, an early model describing the formation of the enzyme-substrate complex was called the lock-and-key model A model that portrays an enzyme as conformationally rigid and able to bond only to a substrate or substrates that exactly fit the active site. ( Figure 18.11 "The Lock-and-Key Model of Enzyme Action" ). This model portrayed the enzyme as conformationally rigid and able to bond only to substrates that exactly fit the active site.
Figure 18.10 Substrate Binding to the Active Site of an Enzyme

The enzyme dihydrofolate reductase is shown with one of its substrates: NADP + (a) unbound and (b) bound. The NADP + (shown in red) binds to a pocket that is complementary to it in shape and ionic properties.
Figure 18.11 The Lock-and-Key Model of Enzyme Action

(a) Because the substrate and the active site of the enzyme have complementary structures and bonding groups, they fit together as a key fits a lock. (b) The catalytic reaction occurs while the two are bonded together in the enzyme-substrate complex.
Working out the precise three-dimensional structures of numerous enzymes has enabled chemists to refine the original lock-and-key model of enzyme actions. They discovered that the binding of a substrate often leads to a large conformational change in the enzyme, as well as to changes in the structure of the substrate or substrates. The current theory, known as the induced-fit model A model that says an enzyme can undergo a conformational change when it binds substrate molecules. , says that enzymes can undergo a change in conformation when they bind substrate molecules, and the active site has a shape complementary to that of the substrate only after the substrate is bound, as shown for hexokinase in Figure 18.12 "The Induced-Fit Model of Enzyme Action" . After catalysis, the enzyme resumes its original structure.
Figure 18.12 The Induced-Fit Model of Enzyme Action

(a) The enzyme hexokinase without its substrate (glucose, shown in red) is bound to the active site. (b) The enzyme conformation changes dramatically when the substrate binds to it, resulting in additional interactions between hexokinase and glucose.
The structural changes that occur when an enzyme and a substrate join together bring specific parts of a substrate into alignment with specific parts of the enzyme’s active site. Amino acid side chains in or near the binding site can then act as acid or base catalysts, provide binding sites for the transfer of functional groups from one substrate to another or aid in the rearrangement of a substrate. The participating amino acids, which are usually widely separated in the primary sequence of the protein, are brought close together in the active site as a result of the folding and bending of the polypeptide chain or chains when the protein acquires its tertiary and quaternary structure. Binding to enzymes brings reactants close to each other and aligns them properly, which has the same effect as increasing the concentration of the reacting compounds.
- What type of interaction would occur between an OH group present on a substrate molecule and a functional group in the active site of an enzyme?
Suggest an amino acid whose side chain might be in the active site of an enzyme and form the type of interaction you just identified.
- An OH group would most likely engage in hydrogen bonding with an appropriate functional group present in the active site of an enzyme.
- Several amino acid side chains would be able to engage in hydrogen bonding with an OH group. One example would be asparagine, which has an amide functional group.
Skill-Building Exercise
What type of interaction would occur between an COO − group present on a substrate molecule and a functional group in the active site of an enzyme?
One characteristic that distinguishes an enzyme from all other types of catalysts is its substrate specificity . An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality. In contrast, enzymes are much more specific. Some enzymes act on a single substrate, while other enzymes act on any of a group of related molecules containing a similar functional group or chemical bond. Some enzymes even distinguish between D- and L-stereoisomers, binding one stereoisomer but not the other. Urease, for example, is an enzyme that catalyzes the hydrolysis of a single substrate—urea—but not the closely related compounds methyl urea, thiourea, or biuret. The enzyme carboxypeptidase, on the other hand, is far less specific. It catalyzes the removal of nearly any amino acid from the carboxyl end of any peptide or protein.

Enzyme specificity results from the uniqueness of the active site in each different enzyme because of the identity, charge, and spatial orientation of the functional groups located there. It regulates cell chemistry so that the proper reactions occur in the proper place at the proper time. Clearly, it is crucial to the proper functioning of the living cell.
Concept Review Exercises
Distinguish between the lock-and-key model and induced-fit model of enzyme action.
Which enzyme has greater specificity—urease or carboxypeptidase? Explain.
The lock-and-key model portrays an enzyme as conformationally rigid and able to bond only to substrates that exactly fit the active site. The induced fit model portrays the enzyme structure as more flexible and is complementary to the substrate only after the substrate is bound.
Urease has the greater specificity because it can bind only to a single substrate. Carboxypeptidase, on the other hand, can catalyze the removal of nearly any amino acid from the carboxyl end of a peptide or protein.
Key Takeaways
- A substrate binds to a specific region on an enzyme known as the active site, where the substrate can be converted to product.
- The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions.
- The induced-fit model says that an enzyme can undergo a conformational change when binding a substrate.
- Enzymes exhibit varying degrees of substrate specificity.
What type of interaction would occur between each group present on a substrate molecule and a functional group of the active site in an enzyme?
- CH(CH 3 ) 2
For each functional group in Exercise 1, suggest an amino acid whose side chain might be in the active site of an enzyme and form the type of interaction you identified.
For each functional group in Exercise 2, suggest an amino acid whose side chain might be in the active site of an enzyme and form the type of interaction you identified.
- hydrogen bonding
- ionic bonding
- dispersion forces
- The amino acid has a polar side chain capable of engaging in hydrogen bonding; serine (answers will vary).
- The amino acid has a negatively charged side chain; aspartic acid (answers will vary).
- The amino acid has a polar side chain capable of engaging in hydrogen bonding; asparagine (answers will vary).
- The amino acid has a nonpolar side chain; isoleucine (answers will vary).
Mechanism of Enzyme Action
The mechanism of enzyme action depends upon the two factors, namely enzyme’s specificity and transition state of the reactants or substrates. The enzyme’s specificity is due to its active site, which seems like a small aperture or opening. The enzyme’s active site allows specific binding of an enzyme with the substrate due to residues like –NH 2 , -SH groups etc.
We must have heard that the enzymes are the biocatalysts, but we need to know what biocatalysts do. The participation of enzymes in any biochemical or biological reaction is referred to as “ Catalyzed reaction ”, and they only speed up the reaction upto 10 7 to 10 20 times.
Therefore, enzymes serve as a biocatalyst that only increases the reaction rate or the conversion of reactants into products. It is important to keep in mind that the enzymes are never used up in the reaction, which means they remain free after the release of products.
Enzymes can catalyze the same chemical pathway several times until they get denatured and associate with the inhibitors. In this context, we will study the mechanism of enzyme action through three popular models (lock and key hypothesis, Induced fit model and Michaelis and Menten’s equation.
You will also get to know the difference between the mechanisms of enzyme-catalyzed and uncatalyzed reaction (without enzyme) along with the meaning of some important terms relative to the study of the enzyme’s mechanism.
Content: Mechanism of Enzyme Action
Important terms, lock and key hypothesis, induced fit model, michaelis and menten’s model.
Before proceeding to the mechanism of enzyme action, we must have a brief knowledge of the following terms:
Enzymes : These are the 3-D proteinaceous organic compounds, which function as a “ biocatalyst ” to increase the reaction’s speed. Enzymes are specific due to the presence of a distinct region called an active site of an enzyme .
Enzyme action : It is defined as the enzyme’s activity, which facilitates the catalysis or breakdown of chemical substrates (participating in the reaction) into the desired products. Therefore, the term “Enzyme action” is sometimes interchangeable with the term “ Enzyme catalysis ”.
Enzyme catalysis is necessary for many biological or biochemical pathways to occur or essential for the chemical interconversions that sustain life. Let us look into a few examples of enzyme catalysis:
- Sucrose (disaccharide) converts into two different monosaccharide molecules, i.e. glucose and fructose, via the enzyme action of “ Sucrase ”.
- Glucose (monosaccharide) converts into ethanol (primary alcohol) and atmospheric carbon dioxide via the action of the enzyme “ Zymase ”.
Substrates : In terms of enzymology, the substrates refers to the reactants molecules, which form a temporary association with an enzyme or turn out to form an enzyme-substrate complex ( E-S complex ). Various bonds form between the initial contact of the two, i.e. an enzyme and substrate that releases binding energy to create a perfect fit.
Products : In terms of enzymology, the products refer to the species or molecules form by the conformation changes in the enzyme-substrate complex. The enzymes attain their original state after the release of products, and they are available for the substrate molecules to undergo the same pathway.
The mechanism of enzyme action typically depends upon the activation energy. Enzymes participating in any chemical reaction reduce the activation energy and decreasing the time between the substrate’s interconversion into a product. Therefore, to study the enzyme’s mechanism more in detail, we must know the meaning of the following terms:
Transition state : It refers to the high energy state during which the substrates are in the process of falling into the products. The transition state is the intermediary stage between the substrate and product, which remains unstable , or this stage does not last for long. The substrates require some activation energy to outreach the transition state.
Activation energy : It refers to the minimum energy required for the substrates to get into the transition state and contort the substrate molecules into the desired products. Reactants can form products by utilizing the heat energy from the surroundings. But, the reactants in association with enzymes release products more rapidly.
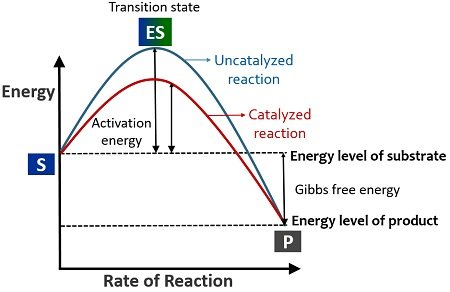
Catalysis : Any chemical reaction, which uses a biocatalyst or heat energy to contort the substrates into products, is the process called catalysis. Substrate transforming into the products solely by the heat energy comes under the category of the uncatalyzed reaction .
Oppositely, substrates contorting into products by the participation of a biocatalyst (enzyme) comes under the category of the catalyzed reaction . Enzymes lower the activation energy or increase the reaction rate (conversion of substrate into a product).
Free energy : In terms of enzymology, free energy or Gibbs free energy is the potential difference between substrates and products’ energy level. It is denoted as ∆G .
It was pioneered by a scientist named Emil Fischer (in 1894), which explains the enzyme’s mechanism. According to this model, an active site is a region of the enzyme, which bears a specific shape or conformation.
Lock and key hypothesis have a simple approach, which says that the particular substrate perfectly fits into the enzyme’s cleft ( active site ) for the reaction to occur. Similarly, the way one specific key fits into the notch of a lock and unlocks it.
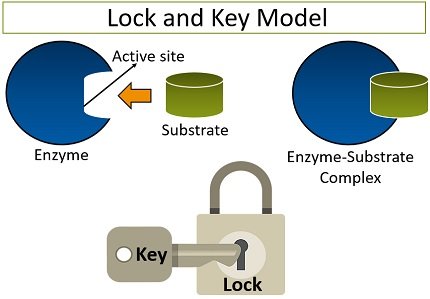
The amino acid residues enable the enzyme’s active site to bind specifically with the substrate. Thus, this model explains an enzyme’s specificity to which only the substrate can bind those with a shape corresponding to an active site’s shape. The lock and key model has many loopholes like:
- This experiment fails to explain the broad specificity of an enzyme.
- It did not explain the binding mechanism of the substrate with an enzyme.
- The lock and key model could not give any information about the mechanism of enzyme catalysis or product formation.
It is the widely accepted model to study the mechanism of enzyme action and pioneered by the scientist Daniel Koshland (in 1959). According to his theory, an active site is a flexible region of the enzyme, which can undergo conformational changes. It is also popular by the name of the hand in glove model .
The induced-fit model explains that the enzyme’s active site possesses two specific locations (buttressing and catalytic site). The substrate initially attaches to the buttressing region , after which the catalytic site brings some conformational changes in the E-S complex. The conformation changes result in the breaking of various bonds between the two and cause the product’s formation.
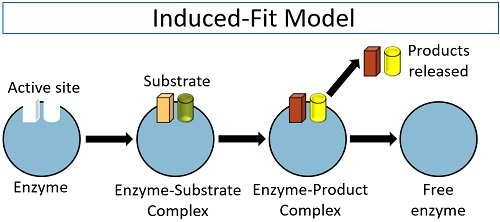
After the catalysis, the enzyme becomes free to carry out the new cycle of converting the substrates into the products. Thus, the induced fit model compensates for the lock and key theory’s loopholes by explaining the broad specificity of an enzyme and the catalysis of the reaction.
Leonor Michaelis and Maud Menten gave an equation in 1913 to explain the mechanism of enzyme action. It depends upon the lowering of activation energy . According to Michaelis Menten’s equation, the enzyme-substrate complex can reversibly dissociate into (enzyme plus substrate) and further proceed to give (enzyme plus product).
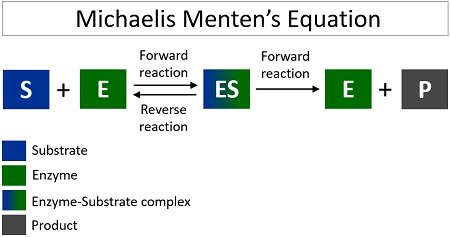
In a catalyzed reaction or an enzyme’s presence, the substrate rapidly reaches the transition state due to decreased activation energy. The enzyme reduces the energy required (activation energy) for the substrate to form products. Conversely, the substrates take more time to reach the transition state and form products without an enzyme catalyst.
The Gibbs free energy will not change even by the participation of an enzyme. Therefore, the Gibbs energy for both catalyzed and uncatalyzed reactions will remain the same . Thus, this model also explains the speed of the reaction.
We can conclude that the mechanism of enzyme action is to lower the activation energy or speed up the substrate’s interconversion into a product. No chemical reaction does not utilize enzymes, unlike substrates, or the enzymes remain free to carry out more chemical interconversions.
Related Topics:
- Difference Between Passive and Active Transport
- Gram Staining
- Spoilage of Fish
- Vitamin B12
- Autoclave Sterilization
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
Start typing and press enter to search
Published by the Moscow Institute of Physics and Technology With the support of Yandex and Microsoft
Time in Lobnya , Moscow Oblast, Russia now
- Tokyo 03:01AM
- Beijing 02:01AM
- Kyiv 09:01PM
- Paris 08:01PM
- London 07:01PM
- New York 02:01PM
- Los Angeles 11:01AM
Time zone info for Lobnya
- The time in Lobnya is 8 hours ahead of the time in New York when New York is on standard time, and 7 hours ahead of the time in New York when New York is on daylight saving time.
- Lobnya does not change between summer time and winter time.
- The IANA time zone identifier for Lobnya is Europe/Moscow.
Time difference from Lobnya
Sunrise, sunset, day length and solar time for lobnya.
- Sunrise: 05:11AM
- Sunset: 07:47PM
- Day length: 14h 36m
- Solar noon: 12:29PM
- The current local time in Lobnya is 29 minutes ahead of apparent solar time.
Lobnya on the map
- Location: Moscow Oblast, Russia
- Latitude: 56.027. Longitude: 37.468
- Population: 62,000
Find best places to eat in Lobnya
- Best pizza restaurants in Lobnya
- Best restaurants with desserts in Lobnya
- Best breakfast restaurants in Lobnya
The 50 largest cities in Russia

IMAGES
VIDEO
COMMENTS
Lock-and-key vs. Induced Fit Model. At present, two models attempt to explain enzyme-substrate specificity; one of which is the lock-and-key model, and the other is the Induced fit model.The lock and key model theory was first postulated by Emil Fischer in 1894.The lock-and-key enzyme action proposes the high specificity of enzymes.
In 1894, Emil Fischer suggested that the specificity of an enzyme towards its substrate is based on the two components exhibiting complementary geometric shapes that fit perfectly like a 'key in a lock'. This simple 'lock and key' analogy succinctly conceptualized the essence of enzyme substrate interaction where the 'lock ...
Lock and Key Model. A German scientist, Emil Fischer postulated the lock and key model in 1894 to explain the enzyme's mode of action. Fischer's theory hypothesized that enzymes exhibit a high degree of specificity towards the substrate. This model assumes that the active site of the enzyme and the substrate fit perfectly into one another ...
The Induced Fit Model Builds upon the Lock-and-Key Hypothesis. This lock-and-key model served the biochemical community well for over 50 years. However, while this model adequately explained how substrates that are too large to fit within the confines of the active site would fail to act as substrates, it did not explain how small substrates, for instance water, often acted as non-substrates ...
The hypothesis that enzyme specificity results from the complementary nature of the substrate and its active site was first proposed by the German chemist Emil Fischer in 1894, and became known as Fischer's 'lock and key hypothesis', whereby only a key of the correct size and shape (the substrate) fits into the keyhole (the active site) of ...
Figure 1 The 'lock and key' model of enzyme action. Fischer's powerful model explained the experimental observations produced by researchers at the time and remained the accepted theory for 60 years. As new experimental techniques allowed researchers to probe enzyme action more closely, a number of experimental observations emerged that ...
similar structure. The specificity of an enzyme with a substrate can be explained by "Lock and key" model. In this model, the lock and key correspond to the enzyme and the substrate, respectively, and only the correctly shaped key can fit into the key hole (active site). This theory is based on the "rigid enzyme" model
Figure \(\PageIndex{2}\): The Lock-and-Key Model of Enzyme Action. (a) Because the substrate and the active site of the enzyme have complementary structures and bonding groups, they fit together as a key fits a lock. (b) The catalytic reaction occurs while the two are bonded together in the enzyme-substrate complex.
In 1894, Emil Fisher discovered that glycolytic enzymes are able to distinguish between sugar stereoisomers. Based upon that discovery, he formulated the lock-and-key hypothesis (Fischer 1894), which proposed that enzymes recognize their substrates just as a lock receives a key.That is, only in the case of exact geometric complementarity between the substrate (key) and enzyme (lock) is the ...
The theory behind the Lock and Key model involves the complementarity between the shapes of the enzyme and the substrate. Their complementary shapes make them fit perfectly into each other like a lock and a key. According to this theory, the enzyme and substrate shape do not influence each other because they are already in a predetermined ...
In this explainer, we will learn how to describe the properties of enzymes and outline the lock-and-key theory of enzyme action. All chemical reactions require an input of energy to get started, called the activation energy. Catalysts speed up the rate of reactions without being used up themselves. Organisms need to expend their energy wisely ...
The enzyme 's active site binds to the substrate. Increasing the temperature generally increases the rate of a reaction, but dramatic changes in temperature and pH can denature an enzyme, thereby abolishing its action as a catalyst. The induced fit model states an substrate binds to an active site and both change shape slightly, creating an ...
1. Introduction. After Emil Fischer coined the lock-and-key picture for the reaction between enzymes and substrates [], it became a leading concept for the understanding of intermolecular interactions with proteins, and later for the rational design of drugs.With the advent of supramolecular chemistry the idea gained an enormous momentum, as chemists began to synthetize a large variety of host ...
'Lock and key' hypothesis of enzyme specificity. Harden and Young: 1901-3: Methods for the derivation of kinetic rate laws; principle of enzyme-substrate complex. ... Enzyme specificity is the absolute specificity of protein catalysts to identify and bind to only one or a few molecules. In this process the enzyme carries a defined ...
In the lock and key hypothesis close lock and key hypothesis Model which compares the specificity of enzymes with a key and its lock., the shape of the active site matches the shape of its ...
The specificity of an enzyme with a substrate can be explained by "Lock and key" model. In this model, the lock and key correspond to the enzyme and the substrate, respectively, and only the correctly shaped key can fit into the key hole (active site). This theory is based on the "rigid enzyme" model focused on the geometric ...
The lock and key model is a hypothesis explaining how enzymes interact with substrates. It compares the specificity of enzyme-substrate interactions to a lock that only opens with the correct key. In this model, enzymes (locks) have specific active sites that perfectly fit their substrates (keys). Q. How does the lock and key model maintain ...
Figure 18.11 The Lock-and-Key Model of Enzyme Action. (a) Because the substrate and the active site of the enzyme have complementary structures and bonding groups, they fit together as a key fits a lock. (b) The catalytic reaction occurs while the two are bonded together in the enzyme-substrate complex. Working out the precise three-dimensional ...
After the catalysis, the enzyme becomes free to carry out the new cycle of converting the substrates into the products. Thus, the induced fit model compensates for the lock and key theory's loopholes by explaining the broad specificity of an enzyme and the catalysis of the reaction. Michaelis and Menten's Model
Andrei Raigorodskii Research areas: Combinatorial geometry, geometric graphs, random graphs, extremal graph and hypergraph theory, probability and linear algebra in combinatorics, Ramsey theory in combinatorics and geometry. E-mail: mraigor [at] yandex [dot] ru Affiliation: 1. Lomonosov Moscow State University, Mechanics and Mathematics Faculty, Department of Math.
Find company research, competitor information, contact details & financial data for ZTI METALLPAK, OOO of Lobnya, Moscow region. Get the latest business insights from Dun & Bradstreet.
The aim of this journal is to publish original, high-quality research articles from a broad range of interests within combinatorics, number theory and allied areas Moscow Journal of Combinatorics and Number Theory
Exact time now, time zone, time difference, sunrise/sunset time and key facts for Lobnya, Moscow Oblast, Russia.