An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
InformedHealth.org [Internet]. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006-.


InformedHealth.org [Internet].
In brief: what types of studies are there.
Last Update: September 8, 2016 ; Next update: 2024.
There are various types of scientific studies such as experiments and comparative analyses, observational studies, surveys, or interviews. The choice of study type will mainly depend on the research question being asked.
When making decisions, patients and doctors need reliable answers to a number of questions. Depending on the medical condition and patient's personal situation, the following questions may be asked:
- What is the cause of the condition?
- What is the natural course of the disease if left untreated?
- What will change because of the treatment?
- How many other people have the same condition?
- How do other people cope with it?
Each of these questions can best be answered by a different type of study.
In order to get reliable results, a study has to be carefully planned right from the start. One thing that is especially important to consider is which type of study is best suited to the research question. A study protocol should be written and complete documentation of the study's process should also be done. This is vital in order for other scientists to be able to reproduce and check the results afterwards.
The main types of studies are randomized controlled trials (RCTs), cohort studies, case-control studies and qualitative studies.
- Randomized controlled trials
If you want to know how effective a treatment or diagnostic test is, randomized trials provide the most reliable answers. Because the effect of the treatment is often compared with "no treatment" (or a different treatment), they can also show what happens if you opt to not have the treatment or diagnostic test.
When planning this type of study, a research question is stipulated first. This involves deciding what exactly should be tested and in what group of people. In order to be able to reliably assess how effective the treatment is, the following things also need to be determined before the study is started:
- How long the study should last
- How many participants are needed
- How the effect of the treatment should be measured
For instance, a medication used to treat menopause symptoms needs to be tested on a different group of people than a flu medicine. And a study on treatment for a stuffy nose may be much shorter than a study on a drug taken to prevent strokes .
“Randomized” means divided into groups by chance. In RCTs participants are randomly assigned to one of two or more groups. Then one group receives the new drug A, for example, while the other group receives the conventional drug B or a placebo (dummy drug). Things like the appearance and taste of the drug and the placebo should be as similar as possible. Ideally, the assignment to the various groups is done "double blinded," meaning that neither the participants nor their doctors know who is in which group.
The assignment to groups has to be random in order to make sure that only the effects of the medications are compared, and no other factors influence the results. If doctors decided themselves which patients should receive which treatment, they might – for instance – give the more promising drug to patients who have better chances of recovery. This would distort the results. Random allocation ensures that differences between the results of the two groups at the end of the study are actually due to the treatment and not something else.
Randomized controlled trials provide the best results when trying to find out if there is a cause-and-effect relationship. RCTs can answer questions such as these:
- Is the new drug A better than the standard treatment for medical condition X?
- Does regular physical activity speed up recovery after a slipped disk when compared to passive waiting?
- Cohort studies
A cohort is a group of people who are observed frequently over a period of many years – for instance, to determine how often a certain disease occurs. In a cohort study, two (or more) groups that are exposed to different things are compared with each other: For example, one group might smoke while the other doesn't. Or one group may be exposed to a hazardous substance at work, while the comparison group isn't. The researchers then observe how the health of the people in both groups develops over the course of several years, whether they become ill, and how many of them pass away. Cohort studies often include people who are healthy at the start of the study. Cohort studies can have a prospective (forward-looking) design or a retrospective (backward-looking) design. In a prospective study, the result that the researchers are interested in (such as a specific illness) has not yet occurred by the time the study starts. But the outcomes that they want to measure and other possible influential factors can be precisely defined beforehand. In a retrospective study, the result (the illness) has already occurred before the study starts, and the researchers look at the patient's history to find risk factors.
Cohort studies are especially useful if you want to find out how common a medical condition is and which factors increase the risk of developing it. They can answer questions such as:
- How does high blood pressure affect heart health?
- Does smoking increase your risk of lung cancer?
For example, one famous long-term cohort study observed a group of 40,000 British doctors, many of whom smoked. It tracked how many doctors died over the years, and what they died of. The study showed that smoking caused a lot of deaths, and that people who smoked more were more likely to get ill and die.
- Case-control studies
Case-control studies compare people who have a certain medical condition with people who do not have the medical condition, but who are otherwise as similar as possible, for example in terms of their sex and age. Then the two groups are interviewed, or their medical files are analyzed, to find anything that might be risk factors for the disease. So case-control studies are generally retrospective.
Case-control studies are one way to gain knowledge about rare diseases. They are also not as expensive or time-consuming as RCTs or cohort studies. But it is often difficult to tell which people are the most similar to each other and should therefore be compared with each other. Because the researchers usually ask about past events, they are dependent on the participants’ memories. But the people they interview might no longer remember whether they were, for instance, exposed to certain risk factors in the past.
Still, case-control studies can help to investigate the causes of a specific disease, and answer questions like these:
- Do HPV infections increase the risk of cervical cancer ?
- Is the risk of sudden infant death syndrome (“cot death”) increased by parents smoking at home?
Cohort studies and case-control studies are types of "observational studies."
- Cross-sectional studies
Many people will be familiar with this kind of study. The classic type of cross-sectional study is the survey: A representative group of people – usually a random sample – are interviewed or examined in order to find out their opinions or facts. Because this data is collected only once, cross-sectional studies are relatively quick and inexpensive. They can provide information on things like the prevalence of a particular disease (how common it is). But they can't tell us anything about the cause of a disease or what the best treatment might be.
Cross-sectional studies can answer questions such as these:
- How tall are German men and women at age 20?
- How many people have cancer screening?
- Qualitative studies
This type of study helps us understand, for instance, what it is like for people to live with a certain disease. Unlike other kinds of research, qualitative research does not rely on numbers and data. Instead, it is based on information collected by talking to people who have a particular medical condition and people close to them. Written documents and observations are used too. The information that is obtained is then analyzed and interpreted using a number of methods.
Qualitative studies can answer questions such as these:
- How do women experience a Cesarean section?
- What aspects of treatment are especially important to men who have prostate cancer ?
- How reliable are the different types of studies?
Each type of study has its advantages and disadvantages. It is always important to find out the following: Did the researchers select a study type that will actually allow them to find the answers they are looking for? You can’t use a survey to find out what is causing a particular disease, for instance.
It is really only possible to draw reliable conclusions about cause and effect by using randomized controlled trials. Other types of studies usually only allow us to establish correlations (relationships where it isn’t clear whether one thing is causing the other). For instance, data from a cohort study may show that people who eat more red meat develop bowel cancer more often than people who don't. This might suggest that eating red meat can increase your risk of getting bowel cancer. But people who eat a lot of red meat might also smoke more, drink more alcohol, or tend to be overweight. The influence of these and other possible risk factors can only be determined by comparing two equal-sized groups made up of randomly assigned participants.
That is why randomized controlled trials are usually the only suitable way to find out how effective a treatment is. Systematic reviews, which summarize multiple RCTs , are even better. In order to be good-quality, though, all studies and systematic reviews need to be designed properly and eliminate as many potential sources of error as possible.
- German Network for Evidence-based Medicine. Glossar: Qualitative Forschung. Berlin: DNEbM; 2011.
- Greenhalgh T. Einführung in die Evidence-based Medicine: kritische Beurteilung klinischer Studien als Basis einer rationalen Medizin. Bern: Huber; 2003.
- Institute for Quality and Efficiency in Health Care (IQWiG, Germany). General methods . Version 5.0. Cologne: IQWiG; 2017.
- Klug SJ, Bender R, Blettner M, Lange S. Wichtige epidemiologische Studientypen. Dtsch Med Wochenschr 2007; 132:e45-e47. [ PubMed : 17530597 ]
- Schäfer T. Kritische Bewertung von Studien zur Ätiologie. In: Kunz R, Ollenschläger G, Raspe H, Jonitz G, Donner-Banzhoff N (eds.). Lehrbuch evidenzbasierte Medizin in Klinik und Praxis. Cologne: Deutscher Ärzte-Verlag; 2007.
IQWiG health information is written with the aim of helping people understand the advantages and disadvantages of the main treatment options and health care services.
Because IQWiG is a German institute, some of the information provided here is specific to the German health care system. The suitability of any of the described options in an individual case can be determined by talking to a doctor. informedhealth.org can provide support for talks with doctors and other medical professionals, but cannot replace them. We do not offer individual consultations.
Our information is based on the results of good-quality studies. It is written by a team of health care professionals, scientists and editors, and reviewed by external experts. You can find a detailed description of how our health information is produced and updated in our methods.
- Cite this Page InformedHealth.org [Internet]. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006-. In brief: What types of studies are there? [Updated 2016 Sep 8].
In this Page
Informed health links, related information.
- PubMed Links to PubMed
Recent Activity
- In brief: What types of studies are there? - InformedHealth.org In brief: What types of studies are there? - InformedHealth.org
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

Community Blog
Keep up-to-date on postgraduate related issues with our quick reads written by students, postdocs, professors and industry leaders.
Types of Research – Explained with Examples
- By DiscoverPhDs
- October 2, 2020

Types of Research
Research is about using established methods to investigate a problem or question in detail with the aim of generating new knowledge about it.
It is a vital tool for scientific advancement because it allows researchers to prove or refute hypotheses based on clearly defined parameters, environments and assumptions. Due to this, it enables us to confidently contribute to knowledge as it allows research to be verified and replicated.
Knowing the types of research and what each of them focuses on will allow you to better plan your project, utilises the most appropriate methodologies and techniques and better communicate your findings to other researchers and supervisors.
Classification of Types of Research
There are various types of research that are classified according to their objective, depth of study, analysed data, time required to study the phenomenon and other factors. It’s important to note that a research project will not be limited to one type of research, but will likely use several.
According to its Purpose
Theoretical research.
Theoretical research, also referred to as pure or basic research, focuses on generating knowledge , regardless of its practical application. Here, data collection is used to generate new general concepts for a better understanding of a particular field or to answer a theoretical research question.
Results of this kind are usually oriented towards the formulation of theories and are usually based on documentary analysis, the development of mathematical formulas and the reflection of high-level researchers.
Applied Research
Here, the goal is to find strategies that can be used to address a specific research problem. Applied research draws on theory to generate practical scientific knowledge, and its use is very common in STEM fields such as engineering, computer science and medicine.
This type of research is subdivided into two types:
- Technological applied research : looks towards improving efficiency in a particular productive sector through the improvement of processes or machinery related to said productive processes.
- Scientific applied research : has predictive purposes. Through this type of research design, we can measure certain variables to predict behaviours useful to the goods and services sector, such as consumption patterns and viability of commercial projects.

According to your Depth of Scope
Exploratory research.
Exploratory research is used for the preliminary investigation of a subject that is not yet well understood or sufficiently researched. It serves to establish a frame of reference and a hypothesis from which an in-depth study can be developed that will enable conclusive results to be generated.
Because exploratory research is based on the study of little-studied phenomena, it relies less on theory and more on the collection of data to identify patterns that explain these phenomena.
Descriptive Research
The primary objective of descriptive research is to define the characteristics of a particular phenomenon without necessarily investigating the causes that produce it.
In this type of research, the researcher must take particular care not to intervene in the observed object or phenomenon, as its behaviour may change if an external factor is involved.
Explanatory Research
Explanatory research is the most common type of research method and is responsible for establishing cause-and-effect relationships that allow generalisations to be extended to similar realities. It is closely related to descriptive research, although it provides additional information about the observed object and its interactions with the environment.
Correlational Research
The purpose of this type of scientific research is to identify the relationship between two or more variables. A correlational study aims to determine whether a variable changes, how much the other elements of the observed system change.
According to the Type of Data Used
Qualitative research.
Qualitative methods are often used in the social sciences to collect, compare and interpret information, has a linguistic-semiotic basis and is used in techniques such as discourse analysis, interviews, surveys, records and participant observations.
In order to use statistical methods to validate their results, the observations collected must be evaluated numerically. Qualitative research, however, tends to be subjective, since not all data can be fully controlled. Therefore, this type of research design is better suited to extracting meaning from an event or phenomenon (the ‘why’) than its cause (the ‘how’).
Quantitative Research
Quantitative research study delves into a phenomena through quantitative data collection and using mathematical, statistical and computer-aided tools to measure them . This allows generalised conclusions to be projected over time.

According to the Degree of Manipulation of Variables
Experimental research.
It is about designing or replicating a phenomenon whose variables are manipulated under strictly controlled conditions in order to identify or discover its effect on another independent variable or object. The phenomenon to be studied is measured through study and control groups, and according to the guidelines of the scientific method.
Non-Experimental Research
Also known as an observational study, it focuses on the analysis of a phenomenon in its natural context. As such, the researcher does not intervene directly, but limits their involvement to measuring the variables required for the study. Due to its observational nature, it is often used in descriptive research.
Quasi-Experimental Research
It controls only some variables of the phenomenon under investigation and is therefore not entirely experimental. In this case, the study and the focus group cannot be randomly selected, but are chosen from existing groups or populations . This is to ensure the collected data is relevant and that the knowledge, perspectives and opinions of the population can be incorporated into the study.
According to the Type of Inference
Deductive investigation.
In this type of research, reality is explained by general laws that point to certain conclusions; conclusions are expected to be part of the premise of the research problem and considered correct if the premise is valid and the inductive method is applied correctly.
Inductive Research
In this type of research, knowledge is generated from an observation to achieve a generalisation. It is based on the collection of specific data to develop new theories.
Hypothetical-Deductive Investigation
It is based on observing reality to make a hypothesis, then use deduction to obtain a conclusion and finally verify or reject it through experience.

According to the Time in Which it is Carried Out
Longitudinal study (also referred to as diachronic research).
It is the monitoring of the same event, individual or group over a defined period of time. It aims to track changes in a number of variables and see how they evolve over time. It is often used in medical, psychological and social areas .
Cross-Sectional Study (also referred to as Synchronous Research)
Cross-sectional research design is used to observe phenomena, an individual or a group of research subjects at a given time.
According to The Sources of Information
Primary research.
This fundamental research type is defined by the fact that the data is collected directly from the source, that is, it consists of primary, first-hand information.
Secondary research
Unlike primary research, secondary research is developed with information from secondary sources, which are generally based on scientific literature and other documents compiled by another researcher.

According to How the Data is Obtained
Documentary (cabinet).
Documentary research, or secondary sources, is based on a systematic review of existing sources of information on a particular subject. This type of scientific research is commonly used when undertaking literature reviews or producing a case study.
Field research study involves the direct collection of information at the location where the observed phenomenon occurs.
From Laboratory
Laboratory research is carried out in a controlled environment in order to isolate a dependent variable and establish its relationship with other variables through scientific methods.
Mixed-Method: Documentary, Field and/or Laboratory
Mixed research methodologies combine results from both secondary (documentary) sources and primary sources through field or laboratory research.

An abstract and introduction are the first two sections of your paper or thesis. This guide explains the differences between them and how to write them.

Statistical treatment of data is essential for all researchers, regardless of whether you’re a biologist, computer scientist or psychologist, but what exactly is it?

Scientific misconduct can be described as a deviation from the accepted standards of scientific research, study and publication ethics.
Join thousands of other students and stay up to date with the latest PhD programmes, funding opportunities and advice.

Browse PhDs Now

Stay up to date with current information being provided by the UK Government and Universities about the impact of the global pandemic on PhD research studies.

An academic transcript gives a breakdown of each module you studied for your degree and the mark that you were awarded.

Ellie is a final year PhD student at the University of Hertfordshire, investigating a protein which is implicated in pancreatic cancer; this work can improve the efficacy of cancer drug treatments.

Dr Williams gained her PhD in Chemical Engineering at the Rensselaer Polytechnic Institute in Troy, New York in 2020. She is now a Presidential Postdoctoral Fellow at Cornell University, researching simplifying vaccine manufacturing in low-income countries.
Join Thousands of Students
News alert: UC Berkeley has announced its next university librarian
Secondary menu
- Log in to your Library account
- Hours and Maps
- Connect from Off Campus
- UC Berkeley Home
Search form
Research methods--quantitative, qualitative, and more: overview.
- Quantitative Research
- Qualitative Research
- Data Science Methods (Machine Learning, AI, Big Data)
- Text Mining and Computational Text Analysis
- Evidence Synthesis/Systematic Reviews
- Get Data, Get Help!
About Research Methods
This guide provides an overview of research methods, how to choose and use them, and supports and resources at UC Berkeley.
As Patten and Newhart note in the book Understanding Research Methods , "Research methods are the building blocks of the scientific enterprise. They are the "how" for building systematic knowledge. The accumulation of knowledge through research is by its nature a collective endeavor. Each well-designed study provides evidence that may support, amend, refute, or deepen the understanding of existing knowledge...Decisions are important throughout the practice of research and are designed to help researchers collect evidence that includes the full spectrum of the phenomenon under study, to maintain logical rules, and to mitigate or account for possible sources of bias. In many ways, learning research methods is learning how to see and make these decisions."
The choice of methods varies by discipline, by the kind of phenomenon being studied and the data being used to study it, by the technology available, and more. This guide is an introduction, but if you don't see what you need here, always contact your subject librarian, and/or take a look to see if there's a library research guide that will answer your question.
Suggestions for changes and additions to this guide are welcome!
START HERE: SAGE Research Methods
Without question, the most comprehensive resource available from the library is SAGE Research Methods. HERE IS THE ONLINE GUIDE to this one-stop shopping collection, and some helpful links are below:
- SAGE Research Methods
- Little Green Books (Quantitative Methods)
- Little Blue Books (Qualitative Methods)
- Dictionaries and Encyclopedias
- Case studies of real research projects
- Sample datasets for hands-on practice
- Streaming video--see methods come to life
- Methodspace- -a community for researchers
- SAGE Research Methods Course Mapping
Library Data Services at UC Berkeley
Library Data Services Program and Digital Scholarship Services
The LDSP offers a variety of services and tools ! From this link, check out pages for each of the following topics: discovering data, managing data, collecting data, GIS data, text data mining, publishing data, digital scholarship, open science, and the Research Data Management Program.
Be sure also to check out the visual guide to where to seek assistance on campus with any research question you may have!
Library GIS Services
Other Data Services at Berkeley
D-Lab Supports Berkeley faculty, staff, and graduate students with research in data intensive social science, including a wide range of training and workshop offerings Dryad Dryad is a simple self-service tool for researchers to use in publishing their datasets. It provides tools for the effective publication of and access to research data. Geospatial Innovation Facility (GIF) Provides leadership and training across a broad array of integrated mapping technologies on campu Research Data Management A UC Berkeley guide and consulting service for research data management issues
General Research Methods Resources
Here are some general resources for assistance:
- Assistance from ICPSR (must create an account to access): Getting Help with Data , and Resources for Students
- Wiley Stats Ref for background information on statistics topics
- Survey Documentation and Analysis (SDA) . Program for easy web-based analysis of survey data.
Consultants
- D-Lab/Data Science Discovery Consultants Request help with your research project from peer consultants.
- Research data (RDM) consulting Meet with RDM consultants before designing the data security, storage, and sharing aspects of your qualitative project.
- Statistics Department Consulting Services A service in which advanced graduate students, under faculty supervision, are available to consult during specified hours in the Fall and Spring semesters.
Related Resourcex
- IRB / CPHS Qualitative research projects with human subjects often require that you go through an ethics review.
- OURS (Office of Undergraduate Research and Scholarships) OURS supports undergraduates who want to embark on research projects and assistantships. In particular, check out their "Getting Started in Research" workshops
- Sponsored Projects Sponsored projects works with researchers applying for major external grants.
- Next: Quantitative Research >>
- Last Updated: Apr 25, 2024 11:09 AM
- URL: https://guides.lib.berkeley.edu/researchmethods
- Anxiety Disorder
- Bipolar Disorder
- Schizophrenia
- Adjustment Disorder
- Agoraphobia
- Antisocial Personality Disorder
- Borderline Personality Disorder
- Childhood ADHD
- Dissociative Identity Disorder
- Narcissistic Personality Disorder
- Oppositional Defiant Disorder
- Panic Attack
- Postpartum Depression
- Schizoaffective Disorder
- Seasonal Affective Disorder
- Sex Addiction
- Social Anxiety
- Specific Phobias
- Teenage Depression
- Black Mental Health
- Emotional Health
- Sex & Relationships
- Understanding Therapy
- Workplace Mental Health
- My Life with OCD
- Caregivers Chronicles
- Empathy at Work
- Sex, Love & All of the Above
- Parent Central
- Mindful Moment
- Mental Health News
- Live Town Hall: Mental Health in Focus
- Inside Mental Health
- Inside Schizophrenia
- Inside Bipolar
- ADHD Symptoms Quiz
- Anxiety Symptoms Quiz
- Autism Quiz: Family & Friends
- Autism Symptoms Quiz
- Bipolar Disorder Quiz
- Borderline Personality Test
- Childhood ADHD Quiz
- Depression Symptoms Quiz
- Eating Disorder Quiz
- Narcissim Symptoms Test
- OCD Symptoms Quiz
- Psychopathy Test
- PTSD Symptoms Quiz
- Schizophrenia Quiz
- Attachment Style Quiz
- Career Test
- Do I Need Therapy Quiz?
- Domestic Violence Screening Quiz
- Emotional Type Quiz
- Loneliness Quiz
- Parenting Style Quiz
- Personality Test
- Relationship Quiz
- Stress Test
- What's Your Sleep Like?
- Find Support
- Suicide Prevention
- Drugs & Medications
- Find a Therapist
Research 101: Understanding Research Studies
One of the secrets of science is to understand the language of science, and science’s primary language is the research study . Research studies allow scientists to communicate with one another and share results of their work. There are many different kinds of research and many varying fields of research. And although journals were designed to help professionals communicate such research findings with one another, many times professionals in one field don’t significantly interact with (or are even aware of) researchers in a different field than themselves (e.g., a neuropsychologist may not keep up on the same research findings as a neurologist). This article reviews the major types of research done in the social, behavioral and brain sciences and provides some guideposts to better evaluate the context in which to place new research.
Types of Research
The basis of a scientific research study follows a common pattern:
- Define the question
- Gather information and resources
- Form hypotheses
- Perform an experiment and collect data
- Analyze the data
- Interpret the data and draw conclusions
- Publish results in a peer-reviewed journal
While there are dozens of types of research, most research done falls into one of five categories: clinical case studies; small, non-randomized studies or surveys; large, randomized clinical studies; literature reviews; and meta-analytic studies. Studies can also occur in widely varying fields, from psychology, pharmacology and sociology (what I’ll call “behavioral and treatment studies”), to genetics and brain scans (what I’ll call “organic studies”) to animal studies. Some fields contribute results that are more instantly relevant, while others’ results may help researchers develop new tests or treatments decades from now.
Clinical Case Studies
A clinical case study involves reporting on a single case (or series of cases) that the researcher or clinician has tracked over a period of some significant time (usually months or even years). Many times, such case studies emphasize a narrative or more subjective approach, but may also include objective measures. For instance, a researcher might publish a case study about the positive effects of cognitive-behavioral psychotherapy for a person with depression. The researcher measured the client’s level of depression with an objective measure such as the Beck Depression Inventory, but also describes in detail the client’s progress with specific cognitive-behavioral techniques , such as doing regular “homework” or keeping a journal of one’s thoughts.
The clinical case study is a very good research design for generating and testing hypotheses that may be used in larger studies. It is also a very good manner for disseminating the effectiveness of specific or novel techniques for individuals, or for those that may have a fairly uncommon set of diagnoses. However, generally a clinical case study’s results are not able to be generalized to a broader population. A case study is therefore of limited value to the general population.
Small Studies and Survey Research
There’s no specific criteria that differentiates a “small study” from a “large study,” but I place any non-randomized study in this category, as well as pretty much all survey research. Small studies are generally conducted on student populations (because students are often required to be a research subject for their university psychology classes), involve less than 80 to 100 participants or subjects, and often lack at least one of the core, important research components most often found in larger studies. This component can be the lack of true randomization of subjects, a lack of heterogeneity (e.g., no diversity in the population being studied), or a lack of a control group (or a relevant control group, e.g. a placebo control).
Most survey research also falls into this category, because it also lacks one of these core research components. For instance, a lot of survey research asks participants to identify themselves as having a particular problem, and if they do, then they fill out the survey. While this will almost guarantee the researchers interesting results, it’s also not very generalizable.
The upshot is that while these studies often provide interesting insights and information that can be used for future research, people shouldn’t read too much into these research findings. They are important data points in our overall understanding of the subject. When you take 10 or 20 of these data points and string them together, they should provide a fairly clear and consistent picture about the topic. If the results don’t provide such a clear picture, then there is likely more work to be done in the subject area before meaningful conclusions can be made. Literature reviews and meta-analyses (discussed below) help professionals and individuals better understand such findings over time.
Large, Randomized Studies
Large, randomized studies that draw from diverse populations and include relevant, appropriate control groups are considered the “gold standard” in research. So why aren’t they done more often? Such large studies, often done at multiple geographic locations, are very expensive to run because they include dozens of researchers, research assistants, statisticians, and other professionals as well as hundreds, and sometimes thousands, of subjects or participants. But the findings from such research are robust and can be generalized to others far more easily, so their value to research is important.
Large studies are not immune to problems found in other kinds of research. It’s just that the problems tend to have a much smaller effect, if there are any, since the number of subjects is so large and mixed (heterogeneous). When properly designed and using accepted statistical analyses, large research studies provide both individuals and professionals with solid findings that they can act upon.
Literature Reviews
A literature review is pretty much what it describes. Virtually all peer-reviewed, published research includes what might be called a “mini literature review” in its introduction. In this section of a study, the researchers review previous studies to put the current study into some context. “Research X found 123, Research Y found 456, so we hope to find 789.”
Sometimes, however, the number of studies in a particular area of study is so large and covers so many results that it’s difficult to understand exactly what our understanding is at the moment. To help give researchers a better understanding and context for future research, a literature review may be conducted and published as its own “study.” This will basically be a comprehensive, large-scale review of all studies in a particular area published within the past 10 or 20 years. The review will describe the research efforts, expand on specific findings, and may draw some general conclusions that can be gleaned from such a global review. These reviews are usually fairly subjective and are mainly for other professionals. Their use to the general public is limited and they almost never produce new findings of interest.
Meta-Analytic Studies
A meta-analysis is similar to a literature review in that it seeks to examine all previous research in a very specific topic area. However, unlike a literature review, a meta-analytic study takes the review one important step further – it actually pulls together all of the previous study’s data and analyzes it with additional statistics to draw global conclusions about the data. Why bother? Because so much research is published in many fields that it’s virtually impossible for an individual to draw any solid conclusions from the research without such a global review that pulls together all that data and statistically analyzes it for trends and solid findings.
The key to meta-analytic studies is to understand that researchers can alter the results of such a review by being particular (or not very particular) about the kinds of studies they include in their review. If, for instance, the researchers decide to include non-randomized studies in their review, they will often get different findings than if they hadn’t included them. Sometimes researchers will require certain statistical procedures to have been performed in order for the study to be included, or certain data thresholds to be met (e.g., we’ll only examine studies that had more than 50 subjects). Depending upon what criteria researchers choose to include in their meta-analysis, it will effect the results of the meta-analysis.
Meta-analytic studies, when done properly, are important contributions to our scientific knowledge and understanding. When a meta-analysis is published, it generally acts as a new foundation for other studies to build upon. It also synthesizes a great deal of previous knowledge into a more digestable Chunk of Knowledge for everyone.
Three General Categories of Research
While we’ve discussed the five general types of research in behavioral and mental health, there are also three other categories to consider.
Behavioral & Treatment Studies
Behavioral or treatment studies examine specific behaviors, treatments or therapies and see how they work on people. In psychology and sociology, most research conducted is of this nature. Such research provides direct insights into human behavior or therapeutic techniques that may be of value for treating a specific kind of disorder. This kind of research also helps us better understand a specific health or mental health concern, and how it manifests itself in a certain group of people (e.g., teenagers versus adults). This is the most “actionable” type of research – research that professionals and individuals can take action based upon its findings.
Organic Studies
Research that examines brain structures, neurochemical reactions via PET or other brain imaging techniques, gene research, or research that examines other organic structures in a human body falls under this category. In most cases, such research helps further our understanding of the human body and how it functions, but doesn’t provide immediate insight or help in dealing with a problem today, or suggest new treatments that will be readily available. For instance, researchers often publish findings about how a particular gene may be correlated with a specific disorder. While such findings may eventually lead to some sort of medical test being developed for the disorder, it may be a decade or two before a finding of this nature translates into an actual test or new treatment method.
While such research is vitally important to our eventual better understanding of how our brains and bodies function, research in this category tends not to have much importance today for people dealing with a mental disorder or mental health problem.
Animal Studies
Research is sometimes conducted on an animal to better understand how a specific organ system (such as the brain) reacts to changes, or how an animal’s behavior may be altered by specific social or environmental changes. Animal research, mostly on rats, in the 1950’s and 1960’s focused on studying animal behavior which, in psychology, led to the field of behaviorism and behavior therapy . More recently, the focus of animal studies has been on their biological makeup, to examine certain brain structures and genes related to health or mental health issues.
While certain animals have organ systems that may be very similar to human organ systems, results from animal studies are not automatically generalizable to humans. Animal studies are therefore of limited value to the general population. Research news based upon an animal study generally means any possible significant treatments from such a study are at least a decade or more away from being introduced. In many cases, no specific treatments are developed from animal studies, instead they are used to better understand how a human organ system functions or reacts to a change.
Research in the social sciences and in pharmacology is important because it helps us not only better understand human behavior (both normal and dysfunctional behavior), but also to find more effective and less time-consuming treatments to help with a person is suffering from an emotional or mental health issue.
The best kind of research – large-scale, randomized studies – are also the most rare because of their cost and the amount of resources needed to undertake them. Smaller-scale studies also contribute important data points along the way, inbetween the larger studies, while meta-analyses and literature reviews helps us gain a more global perspective and understanding of our knowledge so far.
While animal research and studies into the brain’s structures and genes are important to contributing to our overall better understanding of how our brains and bodies function, behavioral and treatment research provide concrete data that can generally be used immediately to help people improve their lives.
Last medically reviewed on May 17, 2016
Read this next
JK Rowling broke out medical science to defend her controversial stance on trans women. Responding to twitter
When we're feeling stressed, studies show that one of the best things we can do is to help someone else. Try these simple suggestions for being of…
Even before the nationwide lockdowns, there were far too many people in the U.S. with not enough to eat. The p
I suspect that when most people think about single parents, they think about single mothers. And, yes, single
In the latest example of tone-deaf celeb activism, several celebs including Julianne Moore, Sara Paulson, and
No good comes from constantly refreshing your newsfeed for the latest on COVID-19. Here are 10 things you can do that aren't related to coronavirus.
On Facebook a few days ago, a friend posted that there was no toilet paper anywhere in the town where I live.
If someone lacks food and water, we know the body will suffer. But what about when they lack a sense of belong
Are you an ABA service provider (a BCBA, BCaBA, or other clinician providing ABA services)? Does part of your
To trust someone you love is important. Otherwise, you’ll be forever doubting that person, creating serious
Research Study Types
There are many different types of research studies, and each has distinct strengths and weaknesses. In general, randomized trials and cohort studies provide the best information when looking at the link between a certain factor (like diet) and a health outcome (like heart disease).
Laboratory and Animal Studies
These are studies done in laboratories on cells, tissue, or animals.
- Strengths: Laboratories provide strictly controlled conditions and are often the genesis of scientific ideas that go on to have a broad impact on human health. They can help understand the mechanisms of disease.
- Weaknesses: Laboratory and animal studies are only a starting point. Animals or cells are not a substitute for humans.
Cross-Sectional Surveys
These studies examine the incidence of a certain outcome (disease or other health characteristic) in a specific group of people at one point in time. Surveys are often sent to participants to gather data about the outcome of interest.
- Strengths: Inexpensive and easy to perform.
- Weaknesses: Can only establish an association in that one specific time period.
Case-Control Studies
These studies look at the characteristics of one group of people who already have a certain health outcome (the cases) and compare them with a similar group of people who do not have the outcome (the controls). An example may be looking at a group of people with heart disease and another group without heart disease who are similar in age, sex, and economic status, and comparing their intakes of fruits and vegetables to see if this exposure could be associated with heart disease risk.
- Strengths: Case-control studies can be done quickly and relatively cheaply.
- Weaknesses: Not ideal for studying diet because they gather information from the past, which can be difficult for most people to recall accurately. Furthermore, people with illnesses often recall past behaviors differently from those without illness. This opens such studies to potential inaccuracy and bias in the information they gather.
Cohort Studies
These are observational studies that follow large groups of people over a long period of time, years or even decades, to find associations of an exposure(s) with disease outcomes. Researchers regularly gather information from the people in the study on several variables (like meat intake, physical activity level, and weight). Once a specified amount of time has elapsed, the characteristics of people in the group are compared to test specific hypotheses (such as a link between high versus low intake of carotenoid-rich foods and glaucoma, or high versus low meat intake and prostate cancer).
- Strengths: Participants are not required to change their diets or lifestyle as may be with randomized controlled studies. Study sizes may be larger than other study types. They generally provide more reliable information than case-control studies because they don’t rely on information from the past. Cohort studies gather information from participants at the beginning and throughout the study, long before they may develop the disease being studied. As a group, many of these types of studies have provided valuable information about the link between lifestyle factors and disease.
- Weaknesses: A longer duration of following participants make these studies time-consuming and expensive. Results cannot suggest cause-and-effect, only associations. Evaluation of dietary intake is self-reported.
Two of the largest and longest-running cohort studies of diet are the Harvard-based Nurses’ Health Study and the Health Professionals Follow-up Study.
If you follow nutrition news, chances are you have come across findings from a cohort called the Nurses’ Health Study . The Nurses’ Health Study (NHS) began in 1976, spearheaded by researchers from the Channing Laboratory at the Brigham and Women’s Hospital, Harvard Medical School, and the Harvard T.H. Chan School of Public Health, with funding from the National Institutes of Health. It gathered registered nurses ages 30-55 years from across the U.S. to respond to a series of questionnaires. Nurses were specifically chosen because of their ability to complete the health-related, often very technical, questionnaires thoroughly and accurately. They showed motivation to participate in the long-term study that required ongoing questionnaires every two years. Furthermore, the group provided blood, urine, and other samples over the course of the study.
The NHS is a prospective cohort study, meaning a group of people who are followed forward in time to examine lifestyle habits or other characteristics to see if they develop a disease, death, or some other indicated outcome. In comparison, a retrospective cohort study would specify a disease or outcome and look back in time at the group to see if there were common factors leading to the disease or outcome. A benefit of prospective studies over retrospective studies is greater accuracy in reporting details, such as food intake, that is not distorted by the diagnosis of illness.
To date, there are three NHS cohorts: NHS original cohort, NHS II, and NHS 3. Below are some features unique to each cohort.
NHS – Original Cohort
- Started in 1976 by Frank Speizer, M.D.
- Participants: 121,700 married women, ages 30 to 55 in 1976.
- Outcomes studied: Impact of contraceptive methods and smoking on breast cancer; later this was expanded to observe other lifestyle factors and behaviors in relation to 30 diseases.
- A food frequency questionnaire was added in 1980 to collect information on dietary intake, and continues to be collected every four years.
- Started in 1989 by Walter Willett, M.D., M.P.H., Dr.P.H., and colleagues.
- Participants: 116,430 single and married women, ages 25 to 42 in 1989.
- Outcomes studied: Impact on women’s health of oral contraceptives initiated during adolescence, diet and physical activity in adolescence, and lifestyle risk factors in a younger population than the NHS Original Cohort. The wide range of diseases examined in the original NHS is now also being studied in NHSII.
- The first food frequency questionnaire was collected in 1991, and is collected every four years.
- Started in 2010 by Jorge Chavarro, M.D., Sc.M., Sc.D, Walter Willett, M.D., M.P.H., Dr.P.H., Janet Rich-Edwards, Sc.D., M.P.H, and Stacey Missmer, Sc.D.
- Participants: Expanded to include not just registered nurses but licensed practical nurses (LPN) and licensed vocational nurses (LVN), ages 19 to 46. Enrollment is currently open.
- Inclusion of more diverse population of nurses, including male nurses and nurses from Canada.
- Outcomes studied: Dietary patterns, lifestyle, environment, and nursing occupational exposures that may impact men’s and women’s health; the impact of new hormone preparations and fertility/pregnancy on women’s health; relationship of diet in adolescence on breast cancer risk.
From these three cohorts, extensive research has been published regarding the association of diet, smoking, physical activity levels, overweight and obesity, oral contraceptive use, hormone therapy, endogenous hormones, dietary factors, sleep, genetics, and other behaviors and characteristics with various diseases. In 2016, in celebration of the 40 th Anniversary of NHS, the American Journal of Public Health’s September issue was dedicated to featuring the many contributions of the Nurses’ Health Studies to public health.
Growing Up Today Study (GUTS)
In 1996, recruitment began for a new cross-generational cohort called GUTS (Growing Up Today Study) —children of nurses from the NHS II. GUTS is composed of 27,802 girls and boys who were between the ages of 9 and 17 at the time of enrollment. As the entire cohort has entered adulthood, they complete annual questionnaires including information on dietary intake, weight changes, exercise level, substance and alcohol use, body image, and environmental factors. Researchers are looking at conditions more common in young adults such as asthma, skin cancer, eating disorders, and sports injuries.
Randomized Trials
Like cohort studies, these studies follow a group of people over time. However, with randomized trials, the researchers intervene with a specific behavior change or treatment (such as following a specific diet or taking a supplement) to see how it affects a health outcome. They are called “randomized trials” because people in the study are randomly assigned to either receive or not receive the intervention. This randomization helps researchers determine the true effect the intervention has on the health outcome. Those who do not receive the intervention or labelled the “control group,” which means these participants do not change their behavior, or if the study is examining the effects of a vitamin supplement, the control group participants receive a placebo supplement that contains no active ingredients.
- Strengths: Considered the “gold standard” and best for determining the effectiveness of an intervention (e.g., dietary pattern, supplement) on an endpoint such as cancer or heart disease. Conducted in a highly controlled setting with limited variables that could affect the outcome. They determine cause-and-effect relationships.
- Weaknesses: High cost, potentially low long-term compliance with prescribed diets, and possible ethical issues. Due to expense, the study size may be small.
Meta-Analyses and Systematic Reviews
A meta-analysis collects data from several previous studies on one topic to analyze and combine the results using statistical methods to provide a summary conclusion. Meta-analyses are usually conducted using randomized controlled trials and cohort studies that have higher quality of evidence than other designs. A systematic review also examines past literature related to a specific topic and design, analyzing the quality of studies and results but may not pool the data. Sometimes a systematic review is followed by conducting a meta-analysis if the quality of the studies is good and the data can be combined.
- Strengths: Inexpensive and provides a general comprehensive summary of existing research on a topic. This can create an explanation or assumption to be used for further investigation.
- Weaknesses: Prone to selection bias, as the authors can choose or exclude certain studies, which can change the resulting outcome. Combining data that includes lower-quality studies can also skew the results.
A primer on systematic review and meta-analysis in diabetes research
Terms of use.
The contents of this website are for educational purposes and are not intended to offer personal medical advice. You should seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The Nutrition Source does not recommend or endorse any products.
Our websites may use cookies to personalize and enhance your experience. By continuing without changing your cookie settings, you agree to this collection. For more information, please see our University Websites Privacy Notice .
Neag School of Education
Educational Research Basics by Del Siegle
Types of Research
How do we know something exists? There are a numbers of ways of knowing…
- -Sensory Experience
- -Agreement with others
- -Expert Opinion
- -Scientific Method (we’re using this one)
The Scientific Process (replicable)
- Identify a problem
- Clarify the problem
- Determine what data would help solve the problem
- Organize the data
- Interpret the results
General Types of Educational Research
- Descriptive — survey, historical, content analysis, qualitative (ethnographic, narrative, phenomenological, grounded theory, and case study)
- Associational — correlational, causal-comparative
- Intervention — experimental, quasi-experimental, action research (sort of)
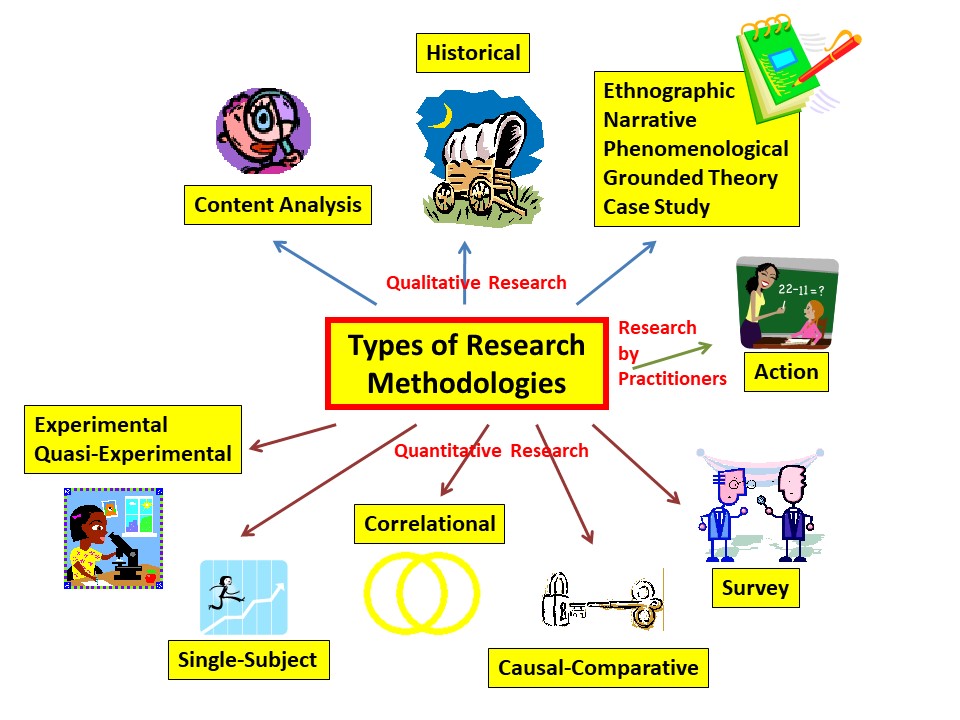
Researchers Sometimes Have a Category Called Group Comparison
- Ex Post Facto (Causal-Comparative): GROUPS ARE ALREADY FORMED
- Experimental: RANDOM ASSIGNMENT OF INDIVIDUALS
- Quasi-Experimental: RANDOM ASSIGNMENT OF GROUPS (oversimplified, but fine for now)
General Format of a Research Publication
- Background of the Problem (ending with a problem statement) — Why is this important to study? What is the problem being investigated?
- Review of Literature — What do we already know about this problem or situation?
- Methodology (participants, instruments, procedures) — How was the study conducted? Who were the participants? What data were collected and how?
- Analysis — What are the results? What did the data indicate?
- Results — What are the implications of these results? How do they agree or disagree with previous research? What do we still need to learn? What are the limitations of this study?
Del Siegle, PhD [email protected]
Last modified 6/18/2019
- Chamberlain University Library
- Chamberlain Library Core
Finding Types of Research
- Evidence-Based Research
On This Guide
About this guide, understand evidence-based practice, identify research study types.
- Quantitative Studies
- Qualitative Studies
- Meta-Analysis
- Systematic Reviews
- Randomized Controlled Trials
- Observational Studies
- Literature Reviews
- Finding Research Tools This link opens in a new window
Throughout your schooling, you may need to find different types of evidence and research to support your course work. This guide provides a high-level overview of evidence-based practice as well as the different types of research and study designs. Each page of this guide offers an overview and search tips for finding articles that fit that study design.
Note! If you need help finding a specific type of study, visit the Get Research Help guide to contact the librarians.
What is Evidence-Based Practice?
One of the requirements for your coursework is to find articles that support evidence-based practice. But what exactly is evidence-based practice? Evidence-based practice is a method that uses relevant and current evidence to plan, implement and evaluate patient care. This definition is included in the video below, which explains all the steps of evidence-based practice in greater detail.
- Video - Evidence-based practice: What it is and what it is not. Medcom (Producer), & Cobb, D. (Director). (2017). Evidence-based practice: What it is and what it is not [Streaming Video]. United States of America: Producer. Retrieved from Alexander Street Press Nursing Education Collection
Quantitative and Qualitative Studies
Research is broken down into two different types: quantitative and qualitative. Quantitative studies are all about measurement. They will report statistics of things that can be physically measured like blood pressure, weight and oxygen saturation. Qualitative studies, on the other hand, are about people's experiences and how they feel about something. This type of information cannot be measured using statistics. Both of these types of studies report original research and are considered single studies. Watch the video below for more information.

Study Designs
Some research study types that you will encounter include:
- Case-Control Studies
- Cohort Studies
- Cross-Sectional Studies
Studies that Synthesize Other Studies
Sometimes, a research study will look at the results of many studies and look for trends and draw conclusions. These types of studies include:
- Meta Analyses
Tip! How do you determine the research article's study type or level of evidence? First, look at the article abstract. Most of the time the abstract will have a methodology section, which should tell you what type of study design the researchers are using. If it is not in the abstract, look for the methodology section of the article. It should tell you all about what type of study the researcher is doing and the steps they used to carry out the study.
Read the book below to learn how to read a clinical paper, including the types of study designs you will encounter.
- Search Website
- Library Tech Support
- Services for Colleagues
Chamberlain College of Nursing is owned and operated by Chamberlain University LLC. In certain states, Chamberlain operates as Chamberlain College of Nursing pending state authorization for Chamberlain University.
- Clinical Trials
About Clinical Studies
Research: it's all about patients.
Mayo's mission is about the patient, the patient comes first. So the mission and research here, is to advance how we can best help the patient, how to make sure the patient comes first in care. So in many ways, it's a cycle. It can start with as simple as an idea, worked on in a laboratory, brought to the patient bedside, and if everything goes right, and let's say it's helpful or beneficial, then brought on as a standard approach. And I think that is one of the unique characteristics of Mayo's approach to research, that patient-centeredness. That really helps to put it in its own spotlight.
At Mayo Clinic, the needs of the patient come first. Part of this commitment involves conducting medical research with the goal of helping patients live longer, healthier lives.
Through clinical studies, which involve people who volunteer to participate in them, researchers can better understand how to diagnose, treat and prevent diseases or conditions.
Types of clinical studies
- Observational study. A type of study in which people are observed or certain outcomes are measured. No attempt is made by the researcher to affect the outcome — for example, no treatment is given by the researcher.
- Clinical trial (interventional study). During clinical trials, researchers learn if a new test or treatment works and is safe. Treatments studied in clinical trials might be new drugs or new combinations of drugs, new surgical procedures or devices, or new ways to use existing treatments. Find out more about the five phases of non-cancer clinical trials on ClinicalTrials.gov or the National Cancer Institute phases of cancer trials .
- Medical records research. Medical records research involves the use of information collected from medical records. By studying the medical records of large groups of people over long periods of time, researchers can see how diseases progress and which treatments and surgeries work best. Find out more about Minnesota research authorization .
Clinical studies may differ from standard medical care
A health care provider diagnoses and treats existing illnesses or conditions based on current clinical practice guidelines and available, approved treatments.
But researchers are constantly looking for new and better ways to prevent and treat disease. In their laboratories, they explore ideas and test hypotheses through discovery science. Some of these ideas move into formal clinical trials.
During clinical studies, researchers formally and scientifically gather new knowledge and possibly translate these findings into improved patient care.
Before clinical trials begin
This video demonstrates how discovery science works, what happens in the research lab before clinical studies begin, and how a discovery is transformed into a potential therapy ready to be tested in trials with human participants:
How clinical trials work
Trace the clinical trial journey from a discovery research idea to a viable translatable treatment for patients:
See a glossary of terms related to clinical studies, clinical trials and medical research on ClinicalTrials.gov.
Watch a video about clinical studies to help you prepare to participate.
Let's Talk About Clinical Research
Narrator: This presentation is a brief introduction to the terms, purposes, benefits and risks of clinical research.
If you have questions about the content of this program, talk with your health care provider.
What is clinical research?
Clinical research is a process to find new and better ways to understand, detect, control and treat health conditions. The scientific method is used to find answers to difficult health-related questions.
Ways to participate
There are many ways to participate in clinical research at Mayo Clinic. Three common ways are by volunteering to be in a study, by giving permission to have your medical record reviewed for research purposes, and by allowing your blood or tissue samples to be studied.
Types of clinical research
There are many types of clinical research:
- Prevention studies look at ways to stop diseases from occurring or from recurring after successful treatment.
- Screening studies compare detection methods for common conditions.
- Diagnostic studies test methods for early identification of disease in those with symptoms.
- Treatment studies test new combinations of drugs and new approaches to surgery, radiation therapy and complementary medicine.
- The role of inheritance or genetic studies may be independent or part of other research.
- Quality of life studies explore ways to manage symptoms of chronic illness or side effects of treatment.
- Medical records studies review information from large groups of people.
Clinical research volunteers
Participants in clinical research volunteer to take part. Participants may be healthy, at high risk for developing a disease, or already diagnosed with a disease or illness. When a study is offered, individuals may choose whether or not to participate. If they choose to participate, they may leave the study at any time.
Research terms
You will hear many terms describing clinical research. These include research study, experiment, medical research and clinical trial.
Clinical trial
A clinical trial is research to answer specific questions about new therapies or new ways of using known treatments. Clinical trials take place in phases. For a treatment to become standard, it usually goes through two or three clinical trial phases. The early phases look at treatment safety. Later phases continue to look at safety and also determine the effectiveness of the treatment.
Phase I clinical trial
A small number of people participate in a phase I clinical trial. The goals are to determine safe dosages and methods of treatment delivery. This may be the first time the drug or intervention is used with people.
Phase II clinical trial
Phase II clinical trials have more participants. The goals are to evaluate the effectiveness of the treatment and to monitor side effects. Side effects are monitored in all the phases, but this is a special focus of phase II.
Phase III clinical trial
Phase III clinical trials have the largest number of participants and may take place in multiple health care centers. The goal of a phase III clinical trial is to compare the new treatment to the standard treatment. Sometimes the standard treatment is no treatment.
Phase IV clinical trial
A phase IV clinical trial may be conducted after U.S. Food and Drug Administration approval. The goal is to further assess the long-term safety and effectiveness of a therapy. Smaller numbers of participants may be enrolled if the disease is rare. Larger numbers will be enrolled for common diseases, such as diabetes or heart disease.
Clinical research sponsors
Mayo Clinic funds clinical research at facilities in Rochester, Minnesota; Jacksonville, Florida; and Arizona, and in the Mayo Clinic Health System. Clinical research is conducted in partnership with other medical centers throughout the world. Other sponsors of research at Mayo Clinic include the National Institutes of Health, device or pharmaceutical companies, foundations and organizations.
Clinical research at Mayo Clinic
Dr. Hugh Smith, former chair of Mayo Clinic Board of Governors, stated, "Our commitment to research is based on our knowledge that medicine must be constantly moving forward, that we need to continue our efforts to better understand disease and bring the latest medical knowledge to our practice and to our patients."
This fits with the term "translational research," meaning what is learned in the laboratory goes quickly to the patient's bedside and what is learned at the bedside is taken back to the laboratory.
Ethics and safety of clinical research
All clinical research conducted at Mayo Clinic is reviewed and approved by Mayo's Institutional Review Board. Multiple specialized committees and colleagues may also provide review of the research. Federal rules help ensure that clinical research is conducted in a safe and ethical manner.
Institutional review board
An institutional review board (IRB) reviews all clinical research proposals. The goal is to protect the welfare and safety of human subjects. The IRB continues its review as research is conducted.
Consent process
Participants sign a consent form to ensure that they understand key facts about a study. Such facts include that participation is voluntary and they may withdraw at any time. The consent form is an informational document, not a contract.
Study activities
Staff from the study team describe the research activities during the consent process. The research may include X-rays, blood tests, counseling or medications.
Study design
During the consent process, you may hear different phrases related to study design. Randomized means you will be assigned to a group by chance, much like a flip of a coin. In a single-blinded study, participants do not know which treatment they are receiving. In a double-blinded study, neither the participant nor the research team knows which treatment is being administered.
Some studies use an inactive substance called a placebo.
Multisite studies allow individuals from many different locations or health care centers to participate.
Remuneration
If the consent form states remuneration is provided, you will be paid for your time and participation in the study.
Some studies may involve additional cost. To address costs in a study, carefully review the consent form and discuss questions with the research team and your insurance company. Medicare may cover routine care costs that are part of clinical trials. Medicaid programs in some states may also provide routine care cost coverage, as well.
When considering participation in a research study, carefully look at the benefits and risks. Benefits may include earlier access to new clinical approaches and regular attention from a research team. Research participation often helps others in the future.
Risks/inconveniences
Risks may include side effects. The research treatment may be no better than the standard treatment. More visits, if required in the study, may be inconvenient.
Weigh your risks and benefits
Consider your situation as you weigh the risks and benefits of participation prior to enrolling and during the study. You may stop participation in the study at any time.
Ask questions
Stay informed while participating in research:
- Write down questions you want answered.
- If you do not understand, say so.
- If you have concerns, speak up.
Website resources are available. The first website lists clinical research at Mayo Clinic. The second website, provided by the National Institutes of Health, lists studies occurring in the United States and throughout the world.
Additional information about clinical research may be found at the Mayo Clinic Barbara Woodward Lips Patient Education Center and the Stephen and Barbara Slaggie Family Cancer Education Center.
Clinical studies questions
- Phone: 800-664-4542 (toll-free)
- Contact form
Cancer-related clinical studies questions
- Phone: 855-776-0015 (toll-free)
International patient clinical studies questions
- Phone: 507-284-8884
- Email: [email protected]
Clinical Studies in Depth
Learning all you can about clinical studies helps you prepare to participate.
- Institutional Review Board
The Institutional Review Board protects the rights, privacy, and welfare of participants in research programs conducted by Mayo Clinic and its associated faculty, professional staff, and students.
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Postdoctoral Fellowships
- Training Grant Programs
- Publications
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

1.3: Types of Research Studies and How To Interpret Them
- Last updated
- Save as PDF
- Page ID 59269

- Alice Callahan, Heather Leonard, & Tamberly Powell
- Lane Community College via OpenOregon
The field of nutrition is dynamic, and our understanding and practices are always evolving. Nutrition scientists are continuously conducting new research and publishing their findings in peer-reviewed journals. This adds to scientific knowledge, but it’s also of great interest to the public, so nutrition research often shows up in the news and other media sources. You might be interested in nutrition research to inform your own eating habits, or if you work in a health profession, so that you can give evidence-based advice to others. Making sense of science requires that you understand the types of research studies used and their limitations.
The Hierarchy of Nutrition Evidence
Researchers use many different types of study designs depending on the question they are trying to answer, as well as factors such as time, funding, and ethical considerations. The study design affects how we interpret the results and the strength of the evidence as it relates to real-life nutrition decisions. It can be helpful to think about the types of studies within a pyramid representing a hierarchy of evidence, where the studies at the bottom of the pyramid usually give us the weakest evidence with the least relevance to real-life nutrition decisions, and the studies at the top offer the strongest evidence, with the most relevance to real-life nutrition decisions .

Figure 2.1. Hierarchy of research design and levels of scientific evidence with the strongest studies at the top and the weakest at the bottom.
The pyramid also represents a few other general ideas. There tend to be more studies published using the methods at the bottom of the pyramid, because they require less time, money, and other resources. When researchers want to test a new hypothesis , they often start with the study designs at the bottom of the pyramid , such as in vitro, animal, or observational studies. Intervention studies are more expensive and resource-intensive, so there are fewer of these types of studies conducted. But they also give us higher quality evidence, so they’re an important next step if observational and non-human studies have shown promising results. Meta-analyses and systematic reviews combine the results of many studies already conducted, so they help researchers summarize scientific knowledge on a topic.
Non-Human Studies: In Vitro & Animal Studies
The simplest form of nutrition research is an in vitro study . In vitro means “within glass,” (although plastic is used more commonly today) and these experiments are conducted within flasks, dishes, plates, and test tubes. One common form of in vitro research is cell culture. This involves growing cells in flasks and dishes. In order for cells to grow, they need a nutrient source. For cell culture, the nutrient source is referred to as media. Media supplies nutrients to the cells in vitro similarly to how blood performs this function within the body. Most cells adhere to the bottom of the flask and are so small that a microscope is needed to see them. The cells are grown inside an incubator, which is a device that provides the optimal temperature, humidity, and carbon dioxide (CO2CO2) concentrations for cells and microorganisms. By imitating the body's temperature and CO2CO2 levels (37 degrees Celsius, 5% CO2CO2), the incubator allows cells to grow even though they are outside the body.
A limitation of in vitro research compared to in vivo research is that it typically does not take digestion or bioavailability into account. This means that the concentration used might not be physiologically possible (it might be much higher) and that digestion and metabolism of what is being provided to cells may not be taken into account. Cell-based in vitro research is not as complex of a biological system as animals or people that have tissues, organs, etc. working together as well.
Since these studies are performed on isolated cells or tissue samples, they are less expensive and time-intensive than animal or human studies. In vitro studies are vital for zooming in on biological mechanisms, to see how things work at the cellular or molecular level. However, these studies shouldn’t be used to draw conclusions about how things work in humans (or even animals), because we can’t assume that the results will apply to a whole, living organism.

Animal studies are one form of in vivo research, which translates to “within the living.” Rats and mice are the most common animals used in nutrition research. Animals are often used in research that would be unethical to conduct in humans. Another advantage of animal dietary studies is that researchers can control exactly what the animals eat. In human studies, researchers can tell subjects what to eat and even provide them with the food, but they may not stick to the planned diet. People are also not very good at estimating, recording, or reporting what they eat and in what quantities. In addition, animal studies typically do not cost as much as human studies.
There are some important limitations of animal research. First, an animal’s metabolism and physiology are different from humans. Plus, animal models of disease (cancer, cardiovascular disease, etc.), although similar, are different from human diseases. Animal research is considered preliminary, and while it can be very important to the process of building scientific understanding and informing the types of studies that should be conducted in humans, animal studies shouldn’t be considered relevant to real-life decisions about how people eat.
Observational Studies
Observational studies in human nutrition collect information on people’s dietary patterns or nutrient intake and look for associations with health outcomes. Observational studies do not give participants a treatment or intervention; instead, they look at what they’re already doing and see how it relates to their health. These types of study designs can only identify correlations (relationships) between nutrition and health; they can’t show that one factor causes another. (For that, we need intervention studies, which we’ll discuss in a moment.) Observational studies that describe factors correlated with human health are also called epidemiological studies . 1
Epidemiology is defined as the study of human populations. These studies often investigate the relationship between dietary consumption and disease development. There are three main types of epidemiological studies: cross-sectional, case-control, and prospective cohort studies.

One example of a nutrition hypothesis that has been investigated using observational studies is that eating a Mediterranean diet reduces the risk of developing cardiovascular disease. (A Mediterranean diet focuses on whole grains, fruits and vegetables, beans and other legumes, nuts, olive oil, herbs, and spices. It includes small amounts of animal protein (mostly fish), dairy, and red wine. 2 ) There are three main types of observational studies, all of which could be used to test hypotheses about the Mediterranean diet:
- Cohort studies follow a group of people (a cohort) over time, measuring factors such as diet and health outcomes. A cohort study of the Mediterranean diet would ask a group of people to describe their diet, and then researchers would track them over time to see if those eating a Mediterranean diet had a lower incidence of cardiovascular disease.
- Case-control studies compare a group of cases and controls, looking for differences between the two groups that might explain their different health outcomes. For example, researchers might compare a group of people with cardiovascular disease with a group of healthy controls to see whether there were more controls or cases that followed a Mediterranean diet.
- Cross-sectional studies collect information about a population of people at one point in time. For example, a cross-sectional study might compare the dietary patterns of people from different countries to see if diet correlates with the prevalence of cardiovascular disease in the different countries.
There are two types of cohort studies: retrospective and prospective. Retrospective studies look at what happened in the past, and they’re considered weaker because they rely on people’s memory of what they ate or how they felt in the past. Prospective cohort studies, which enroll a cohort and follow them into the future, are usually considered the strongest type of observational study design.
Most cohort studies are prospective. Initial information is collected (usually by food frequency questionnaires) on the intake of a cohort of people at baseline, or the beginning. This cohort is then followed over time (normally many years) to quantify health outcomes of the individual within it. Cohort studies are normally considered to be more robust than case-control studies, because these studies do not start with diseased people and normally do not require people to remember their dietary habits in the distant past or before they developed a disease. An example of a prospective cohort study would be if you filled out a questionnaire on your current dietary habits and are then followed into the future to see if you develop osteoporosis. As shown below, instead of separating based on disease versus disease-free, individuals are separated based on exposure. In this example, those who are exposed are more likely to be diseased than those who were not exposed.

Using trans-fat intake again as the exposure and cardiovascular disease as the disease, the figure would be expected to look like this:

There are several well-known examples of prospective cohort studies that have described important correlations between diet and disease:
- Framingham Heart Study : Beginning in 1948, this study has followed the residents of Framingham, Massachusetts to identify risk factors for heart disease.
- Health Professionals Follow-Up Study : This study started in 1986 and enrolled 51,529 male health professionals (dentists, pharmacists, optometrists, osteopathic physicians, podiatrists, and veterinarians), who complete diet questionnaires every 2 years.
- Nurses Health Studies : Beginning in 1976, these studies have enrolled three large cohorts of nurses with a total of 280,000 participants. Participants have completed detailed questionnaires about diet, other lifestyle factors (smoking and exercise, for example), and health outcomes.
Observational studies have the advantage of allowing researchers to study large groups of people in the real world, looking at the frequency and pattern of health outcomes and identifying factors that correlate with them. But even very large observational studies may not apply to the population as a whole. For example, the Health Professionals Follow-Up Study and the Nurses Health Studies include people with above-average knowledge of health. In many ways, this makes them ideal study subjects, because they may be more motivated to be part of the study and to fill out detailed questionnaires for years. However, the findings of these studies may not apply to people with less baseline knowledge of health.
We’ve already mentioned another important limitation of observational studies—that they can only determine correlation, not causation. A prospective cohort study that finds that people eating a Mediterranean diet have a lower incidence of heart disease can only show that the Mediterranean diet is correlated with lowered risk of heart disease. It can’t show that the Mediterranean diet directly prevents heart disease. Why? There are a huge number of factors that determine health outcomes such as heart disease, and other factors might explain a correlation found in an observational study. For example, people who eat a Mediterranean diet might also be the same kind of people who exercise more, sleep more, have a higher income (fish and nuts can be expensive!), or be less stressed. These are called confounding factors ; they’re factors that can affect the outcome in question (i.e., heart disease) and also vary with the factor being studied (i.e., Mediterranean diet).
Intervention Studies
Intervention studies , also sometimes called experimental studies or clinical trials, include some type of treatment or change imposed by the researcher. Examples of interventions in nutrition research include asking participants to change their diet, take a supplement, or change the time of day that they eat. Unlike observational studies, intervention studies can provide evidence of cause and effect , so they are higher in the hierarchy of evidence pyramid.
Randomization: The gold standard for intervention studies is the randomized controlled trial (RCT) . In an RCT, study subjects are recruited to participate in the study. They are then randomly assigned into one of at least two groups, one of which is a control group (this is what makes the study controlled ).
Randomization is the process of randomly assigning subjects to groups to decrease bias. Bias is a systematic error that may influence results. Bias can occur in assigning subjects to groups in a way that will influence the results. An example of bias in a study of an antidepressant drug is shown below. In this nonrandomized antidepressant drug example, researchers (who know what the subjects are receiving) put depressed subjects into the placebo group, while "less depressed" subjects are put into the antidepressant drug group. As a result, even if the drug isn't effective, the group assignment may make the drug appear effective, thus biasing the results as shown below.

This is a bit of an extreme example, but even if the researchers are trying to prevent bias, sometimes bias can still occur. However, if the subjects are randomized, the sick and the healthy people will ideally be equally distributed between the groups. Thus, the trial will be unbiased and a true test of whether or not the drug is effective.

Here is another example. In an RCT to study the effects of the Mediterranean diet on cardiovascular disease development, researchers might ask the control group to follow a low-fat diet (typically recommended for heart disease prevention) and the intervention group to eat a Mediterranean diet. The study would continue for a defined period of time (usually years to study an outcome like heart disease), at which point the researchers would analyze their data to see if more people in the control or Mediterranean diet had heart attacks or strokes. Because the treatment and control groups were randomly assigned, they should be alike in every other way except for diet, so differences in heart disease could be attributed to the diet. This eliminates the problem of confounding factors found in observational research, and it’s why RCTs can provide evidence of causation, not just correlation.
Imagine for a moment what would happen if the two groups weren’t randomly assigned. What if the researchers let study participants choose which diet they’d like to adopt for the study? They might, for whatever reason, end up with more overweight people who smoke and have high blood pressure in the low-fat diet group, and more people who exercised regularly and had already been eating lots of olive oil and nuts for years in the Mediterranean diet group. If they found that the Mediterranean diet group had fewer heart attacks by the end of the study, they would have no way of knowing if this was because of the diet or because of the underlying differences in the groups. In other words, without randomization, their results would be compromised by confounding factors, with many of the same limitations as observational studies.
Placebo: In an RCT of a supplement, the control group would receive a placebo—a “fake” treatment that contains no active ingredients, such as a sugar pill. The use of a placebo is necessary in medical research because of a phenomenon known as the placebo effect. The placebo effect results in a beneficial effect because of a subject’s belief in the treatment, even though there is no treatment actually being administered. An example would be an athlete who consumes a sports drink and runs the 100-meter dash in 11.00 seconds. The athlete then, under the exact same conditions, drinks what he is told is "Super Duper Sports Drink" and runs the 100-meter dash in 10.50 seconds. But what the athlete didn't know was that Super Duper Sports Drink was the Sports Drink + Food Coloring. There was nothing different between the drinks, but the athlete believed that the "Super Duper Sports Drink" was going to help him run faster, so he did. This improvement is due to the placebo effect.

Blinding is a technique to prevent bias in intervention studies. In a study without blinding, the subject and the researchers both know what treatment the subject is receiving. This can lead to bias if the subject or researcher has expectations about the treatment working, so these types of trials are used less frequently. It’s best if a study is double-blind , meaning that neither the researcher nor the subject knows what treatment the subject is receiving. It’s relatively simple to double-blind a study where subjects are receiving a placebo or treatment pill because they could be formulated to look and taste the same. In a single-blind study , either the researcher or the subject knows what treatment they’re receiving, but not both. Studies of diets—such as the Mediterranean diet example—often can’t be double-blinded because the study subjects know whether or not they’re eating a lot of olive oil and nuts. However, the researchers who are checking participants’ blood pressure or evaluating their medical records could be blinded to their treatment group, reducing the chance of bias.
Open-label study:

Single-blinded study:

Double-blinded study:

Like all studies, RCTs and other intervention studies do have some limitations. They can be difficult to carry on for long periods of time and require that participants remain compliant with the intervention. They’re also costly and often have smaller sample sizes. Furthermore, it is unethical to study certain interventions. (An example of an unethical intervention would be to advise one group of pregnant mothers to drink alcohol to determine its effects on pregnancy outcomes because we know that alcohol consumption during pregnancy damages the developing fetus.)
VIDEO: “ Not all scientific studies are created equal ” by David H. Schwartz, YouTube (April 28, 2014), 4:26.
Meta-Analyses and Systematic Reviews
At the top of the hierarchy of evidence pyramid are systematic reviews and meta-analyses . You can think of these as “studies of studies.” They attempt to combine all of the relevant studies that have been conducted on a research question and summarize their overall conclusions. Researchers conducting a systematic review formulate a research question and then systematically and independently identify, select, evaluate, and synthesize all high-quality evidence that relates to the research question. Since systematic reviews combine the results of many studies, they help researchers produce more reliable findings. A meta-analysis is a type of systematic review that goes one step further, combining the data from multiple studies and using statistics to summarize it, as if creating a mega-study from many smaller studies . 4
However, even systematic reviews and meta-analyses aren’t the final word on scientific questions. For one thing, they’re only as good as the studies that they include. The Cochrane Collaboration is an international consortium of researchers who conduct systematic reviews in order to inform evidence-based healthcare, including nutrition, and their reviews are among the most well-regarded and rigorous in science. For the most recent Cochrane review of the Mediterranean diet and cardiovascular disease, two authors independently reviewed studies published on this question. Based on their inclusion criteria, 30 RCTs with a total of 12,461 participants were included in the final analysis. However, after evaluating and combining the data, the authors concluded that “despite the large number of included trials, there is still uncertainty regarding the effects of a Mediterranean‐style diet on cardiovascular disease occurrence and risk factors in people both with and without cardiovascular disease already.” Part of the reason for this uncertainty is that different trials found different results, and the quality of the studies was low to moderate. Some had problems with their randomization procedures, for example, and others were judged to have unreliable data. That doesn’t make them useless, but it adds to the uncertainty about this question, and uncertainty pushes the field forward towards more and better studies. The Cochrane review authors noted that they found seven ongoing trials of the Mediterranean diet, so we can hope that they’ll add more clarity to this question in the future. 5
Science is an ongoing process. It’s often a slow process, and it contains a lot of uncertainty, but it’s our best method of building knowledge of how the world and human life works. Many different types of studies can contribute to scientific knowledge. None are perfect—all have limitations—and a single study is never the final word on a scientific question. Part of what advances science is that researchers are constantly checking each other’s work, asking how it can be improved and what new questions it raises.
Attributions:
- “Chapter 1: The Basics” from Lindshield, B. L. Kansas State University Human Nutrition (FNDH 400) Flexbook. goo.gl/vOAnR , CC BY-NC-SA 4.0
- “The Broad Role of Nutritional Science,” section 1.3 from the book An Introduction to Nutrition (v. 1.0), CC BY-NC-SA 3.0
References:
- 1 Thiese, M. S. (2014). Observational and interventional study design types; an overview. Biochemia Medica , 24 (2), 199–210. https://doi.org/10.11613/BM.2014.022
- 2 Harvard T.H. Chan School of Public Health. (2018, January 16). Diet Review: Mediterranean Diet . The Nutrition Source. https://www.hsph.harvard.edu/nutritionsource/healthy-weight/diet-reviews/mediterranean-diet/
- 3 Ross, R., Gray, C. M., & Gill, J. M. R. (2015). Effects of an Injected Placebo on Endurance Running Performance. Medicine and Science in Sports and Exercise , 47 (8), 1672–1681. https://doi.org/10.1249/MSS.0000000000000584
- 4 Hooper, A. (n.d.). LibGuides: Systematic Review Resources: Systematic Reviews vs Other Types of Reviews . Retrieved February 7, 2020, from //libguides.sph.uth.tmc.edu/c.php?g=543382&p=5370369
- 5 Rees, K., Takeda, A., Martin, N., Ellis, L., Wijesekara, D., Vepa, A., Das, A., Hartley, L., & Stranges, S. (2019). Mediterranean‐style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews , 3 . doi.org/10.1002/14651858.CD009825.pub3
- 6Levin K. (2006) Study design III: Cross-sectional studies. Evidence - Based Dentistry 7(1): 24.
- Figure 2.3. The hierarchy of evidence by Alice Callahan, is licensed under CC BY 4.0
- Research lab photo by National Cancer Institute on Unsplas h ; mouse photo by vaun0815 on Unsplash
- Figure 2.4. “Placebo effect example” by Lindshield, B. L. Kansas State University Human Nutrition (FNDH 400) Flexbook. goo.gl/vOAnR

Types of Research Studies and How To Interpret Them
The field of nutrition is dynamic, and our understanding and practices are always evolving. Nutrition scientists are continuously conducting new research and publishing their findings in peer-reviewed journals. This adds to scientific knowledge, but it’s also of great interest to the public, so nutrition research often shows up in the news and other media sources. You might be interested in nutrition research to inform your own eating habits, or if you work in a health profession, so that you can give evidence-based advice to others. Making sense of science requires that you understand the types of research studies used and their limitations.
The Hierarchy of Nutrition Evidence
Researchers use many different types of study designs depending on the question they are trying to answer, as well as factors such as time, funding, and ethical considerations. The study design affects how we interpret the results and the strength of the evidence as it relates to real-life nutrition decisions. It can be helpful to think about the types of studies within a pyramid representing a hierarchy of evidence, where the studies at the bottom of the pyramid usually give us the weakest evidence with the least relevance to real-life nutrition decisions, and the studies at the top offer the strongest evidence, with the most relevance to real-life nutrition decisions .
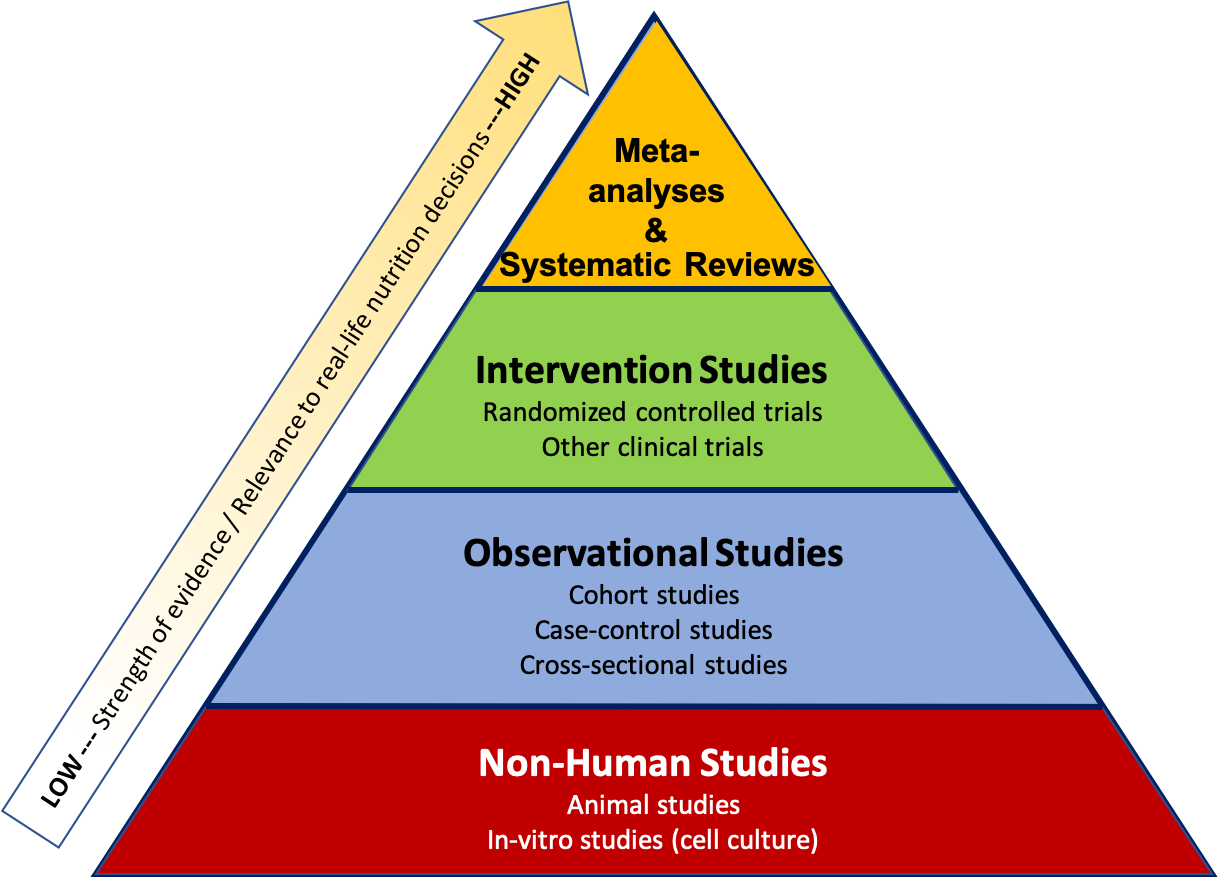
Figure 2.3. The hierarchy of evidence shows types of research studies relative to their strength of evidence and relevance to real-life nutrition decisions, with the strongest studies at the top and the weakest at the bottom.
The pyramid also represents a few other general ideas. There tend to be more studies published using the methods at the bottom of the pyramid, because they require less time, money, and other resources. When researchers want to test a new hypothesis , they often start with the study designs at the bottom of the pyramid , such as in vitro, animal, or observational studies. Intervention studies are more expensive and resource-intensive, so there are fewer of these types of studies conducted. But they also give us higher quality evidence, so they’re an important next step if observational and non-human studies have shown promising results. Meta-analyses and systematic reviews combine the results of many studies already conducted, so they help researchers summarize scientific knowledge on a topic.
Non-Human Studies: In Vitro & Animal Studies
The simplest form of nutrition research is an in vitro study . In vitro means “within glass,” (although plastic is used more commonly today) and these experiments are conducted within flasks, dishes, plates, and test tubes. These studies are performed on isolated cells or tissue samples, so they’re less expensive and time-intensive than animal or human studies. In vitro studies are vital for zooming in on biological mechanisms, to see how things work at the cellular or molecular level. However, these studies shouldn’t be used to draw conclusions about how things work in humans (or even animals), because we can’t assume that the results will apply to a whole, living organism.
Animal studies are one form of in vivo research, which translates to “within the living.” Rats and mice are the most common animals used in nutrition research. Animals are often used in research that would be unethical to conduct in humans. Another advantage of animal dietary studies is that researchers can control exactly what the animals eat. In human studies, researchers can tell subjects what to eat and even provide them with the food, but they may not stick to the planned diet. People are also not very good at estimating, recording, or reporting what they eat and in what quantities. In addition, animal studies typically do not cost as much as human studies.
There are some important limitations of animal research. First, an animal’s metabolism and physiology are different from humans. Plus, animal models of disease (cancer, cardiovascular disease, etc.), although similar, are different from human diseases. Animal research is considered preliminary, and while it can be very important to the process of building scientific understanding and informing the types of studies that should be conducted in humans, animal studies shouldn’t be considered relevant to real-life decisions about how people eat.
Observational Studies
Observational studies in human nutrition collect information on people’s dietary patterns or nutrient intake and look for associations with health outcomes. Observational studies do not give participants a treatment or intervention; instead, they look at what they’re already doing and see how it relates to their health. These types of study designs can only identify correlations (relationships) between nutrition and health; they can’t show that one factor causes another. (For that, we need intervention studies, which we’ll discuss in a moment.) Observational studies that describe factors correlated with human health are also called epidemiological studies . 1
One example of a nutrition hypothesis that has been investigated using observational studies is that eating a Mediterranean diet reduces the risk of developing cardiovascular disease. (A Mediterranean diet focuses on whole grains, fruits and vegetables, beans and other legumes, nuts, olive oil, herbs, and spices. It includes small amounts of animal protein (mostly fish), dairy, and red wine. 2 ) There are three main types of observational studies, all of which could be used to test hypotheses about the Mediterranean diet:
- Cohort studies follow a group of people (a cohort) over time, measuring factors such as diet and health outcomes. A cohort study of the Mediterranean diet would ask a group of people to describe their diet, and then researchers would track them over time to see if those eating a Mediterranean diet had a lower incidence of cardiovascular disease.
- Case-control studies compare a group of cases and controls, looking for differences between the two groups that might explain their different health outcomes. For example, researchers might compare a group of people with cardiovascular disease with a group of healthy controls to see whether there were more controls or cases that followed a Mediterranean diet.
- Cross-sectional studies collect information about a population of people at one point in time. For example, a cross-sectional study might compare the dietary patterns of people from different countries to see if diet correlates with the prevalence of cardiovascular disease in the different countries.
Prospective cohort studies, which enroll a cohort and follow them into the future, are usually considered the strongest type of observational study design. Retrospective studies look at what happened in the past, and they’re considered weaker because they rely on people’s memory of what they ate or how they felt in the past. There are several well-known examples of prospective cohort studies that have described important correlations between diet and disease:
- Framingham Heart Study : Beginning in 1948, this study has followed the residents of Framingham, Massachusetts to identify risk factors for heart disease.
- Health Professionals Follow-Up Study : This study started in 1986 and enrolled 51,529 male health professionals (dentists, pharmacists, optometrists, osteopathic physicians, podiatrists, and veterinarians), who complete diet questionnaires every 2 years.
- Nurses Health Studies : Beginning in 1976, these studies have enrolled three large cohorts of nurses with a total of 280,000 participants. Participants have completed detailed questionnaires about diet, other lifestyle factors (smoking and exercise, for example), and health outcomes.
Observational studies have the advantage of allowing researchers to study large groups of people in the real world, looking at the frequency and pattern of health outcomes and identifying factors that correlate with them. But even very large observational studies may not apply to the population as a whole. For example, the Health Professionals Follow-Up Study and the Nurses Health Studies include people with above-average knowledge of health. In many ways, this makes them ideal study subjects, because they may be more motivated to be part of the study and to fill out detailed questionnaires for years. However, the findings of these studies may not apply to people with less baseline knowledge of health.
We’ve already mentioned another important limitation of observational studies—that they can only determine correlation, not causation. A prospective cohort study that finds that people eating a Mediterranean diet have a lower incidence of heart disease can only show that the Mediterranean diet is correlated with lowered risk of heart disease. It can’t show that the Mediterranean diet directly prevents heart disease. Why? There are a huge number of factors that determine health outcomes such as heart disease, and other factors might explain a correlation found in an observational study. For example, people who eat a Mediterranean diet might also be the same kind of people who exercise more, sleep more, have higher income (fish and nuts can be expensive!), or be less stressed. These are called confounding factors ; they’re factors that can affect the outcome in question (i.e., heart disease) and also vary with the factor being studied (i.e., Mediterranean diet).
Intervention Studies
Intervention studies , also sometimes called experimental studies or clinical trials, include some type of treatment or change imposed by the researcher. Examples of interventions in nutrition research include asking participants to change their diet, take a supplement, or change the time of day that they eat. Unlike observational studies, intervention studies can provide evidence of cause and effect , so they are higher in the hierarchy of evidence pyramid.
The gold standard for intervention studies is the randomized controlled trial (RCT) . In an RCT, study subjects are recruited to participate in the study. They are then randomly assigned into one of at least two groups, one of which is a control group (this is what makes the study controlled ). In an RCT to study the effects of the Mediterranean diet on cardiovascular disease development, researchers might ask the control group to follow a low-fat diet (typically recommended for heart disease prevention) and the intervention group to eat a Mediterrean diet. The study would continue for a defined period of time (usually years to study an outcome like heart disease), at which point the researchers would analyze their data to see if more people in the control or Mediterranean diet had heart attacks or strokes. Because the treatment and control groups were randomly assigned, they should be alike in every other way except for diet, so differences in heart disease could be attributed to the diet. This eliminates the problem of confounding factors found in observational research, and it’s why RCTs can provide evidence of causation, not just correlation.
Imagine for a moment what would happen if the two groups weren’t randomly assigned. What if the researchers let study participants choose which diet they’d like to adopt for the study? They might, for whatever reason, end up with more overweight people who smoke and have high blood pressure in the low-fat diet group, and more people who exercised regularly and had already been eating lots of olive oil and nuts for years in the Mediterranean diet group. If they found that the Mediterranean diet group had fewer heart attacks by the end of the study, they would have no way of knowing if this was because of the diet or because of the underlying differences in the groups. In other words, without randomization, their results would be compromised by confounding factors, with many of the same limitations as observational studies.
In an RCT of a supplement, the control group would receive a placebo —a “fake” treatment that contains no active ingredients, such as a sugar pill. The use of a placebo is necessary in medical research because of a phenomenon known as the placebo effect. The placebo effect results in a beneficial effect because of a subject’s belief in the treatment, even though there is no treatment actually being administered.
For example, imagine an athlete who consumes a sports drink and then runs 100 meters in 11.0 seconds. On a different day, under the exact same conditions, the athlete is given a Super Duper Sports Drink and again runs 100 meters, this time in 10.5 seconds. But what the athlete didn’t know was that the Super Duper Sports Drink was the same as the regular sports drink—it just had a bit of food coloring added. There was nothing different between the drinks, but the athlete believed that the Super Duper Sports Drink was going to help him run faster, so he did. This improvement is due to the placebo effect. Ironically, a study similar to this example was published in 2015, demonstrating the power of the placebo effect on athletic performance. 3
Figure 2.4. An example of the placebo effect
Blinding is a technique to prevent bias in intervention studies. In a study without blinding, the subject and the researchers both know what treatment the subject is receiving. This can lead to bias if the subject or researcher have expectations about the treatment working, so these types of trials are used less frequently. It’s best if a study is double-blind , meaning that neither the researcher nor the subject know what treatment the subject is receiving. It’s relatively simple to double-blind a study where subjects are receiving a placebo or treatment pill, because they could be formulated to look and taste the same. In a single-blind study , either the researcher or the subject knows what treatment they’re receiving, but not both. Studies of diets—such as the Mediterranean diet example—often can’t be double-blinded because the study subjects know whether or not they’re eating a lot of olive oil and nuts. However, the researchers who are checking participants’ blood pressure or evaluating their medical records could be blinded to their treatment group, reducing the chance of bias.
Like all studies, RCTs and other intervention studies do have some limitations. They can be difficult to carry on for long periods of time and require that participants remain compliant with the intervention. They’re also costly and often have smaller sample sizes. Furthermore, it is unethical to study certain interventions. (An example of an unethical intervention would be to advise one group of pregnant mothers to drink alcohol to determine its effects on pregnancy outcomes, because we know that alcohol consumption during pregnancy damages the developing fetus.)
VIDEO: “ Not all scientific studies are created equal ” by David H. Schwartz, YouTube (April 28, 2014), 4:26.
Meta-Analyses and Systematic Reviews
At the top of the hierarchy of evidence pyramid are systematic reviews and meta-analyses . You can think of these as “studies of studies.” They attempt to combine all of the relevant studies that have been conducted on a research question and summarize their overall conclusions. Researchers conducting a systematic review formulate a research question and then systematically and independently identify, select, evaluate, and synthesize all high-quality evidence that relates to the research question. Since systematic reviews combine the results of many studies, they help researchers produce more reliable findings. A meta-analysis is a type of systematic review that goes one step further, combining the data from multiple studies and using statistics to summarize it, as if creating a mega-study from many smaller studies . 4
However, even systematic reviews and meta-analyses aren’t the final word on scientific questions. For one thing, they’re only as good as the studies that they include. The Cochrane Collaboration is an international consortium of researchers who conduct systematic reviews in order to inform evidence-based healthcare, including nutrition, and their reviews are among the most well-regarded and rigorous in science. For the most recent Cochrane review of the Mediterranean diet and cardiovascular disease, two authors independently reviewed studies published on this question. Based on their inclusion criteria, 30 RCTs with a total of 12,461 participants were included in the final analysis. However, after evaluating and combining the data, the authors concluded that “despite the large number of included trials, there is still uncertainty regarding the effects of a Mediterranean‐style diet on cardiovascular disease occurrence and risk factors in people both with and without cardiovascular disease already.” Part of the reason for this uncertainty is that different trials found different results, and the quality of the studies was low to moderate. Some had problems with their randomization procedures, for example, and others were judged to have unreliable data. That doesn’t make them useless, but it adds to the uncertainty about this question, and uncertainty pushes the field forward towards more and better studies. The Cochrane review authors noted that they found seven ongoing trials of the Mediterranean diet, so we can hope that they’ll add more clarity to this question in the future. 5
Science is an ongoing process. It’s often a slow process, and it contains a lot of uncertainty, but it’s our best method of building knowledge of how the world and human life works. Many different types of studies can contribute to scientific knowledge. None are perfect—all have limitations—and a single study is never the final word on a scientific question. Part of what advances science is that researchers are constantly checking each other’s work, asking how it can be improved and what new questions it raises.
Self-Check:
Attributions:
- “Chapter 1: The Basics” from Lindshield, B. L. Kansas State University Human Nutrition (FNDH 400) Flexbook. goo.gl/vOAnR , CC BY-NC-SA 4.0
- “ The Broad Role of Nutritional Science ,” section 1.3 from the book An Introduction to Nutrition (v. 1.0), CC BY-NC-SA 3.0
References:
- 1 Thiese, M. S. (2014). Observational and interventional study design types; an overview. Biochemia Medica , 24 (2), 199–210. https://doi.org/10.11613/BM.2014.022
- 2 Harvard T.H. Chan School of Public Health. (2018, January 16). Diet Review: Mediterranean Diet . The Nutrition Source. https://www.hsph.harvard.edu/nutritionsource/healthy-weight/diet-reviews/mediterranean-diet/
- 3 Ross, R., Gray, C. M., & Gill, J. M. R. (2015). Effects of an Injected Placebo on Endurance Running Performance. Medicine and Science in Sports and Exercise , 47 (8), 1672–1681. https://doi.org/10.1249/MSS.0000000000000584
- 4 Hooper, A. (n.d.). LibGuides: Systematic Review Resources: Systematic Reviews vs Other Types of Reviews . Retrieved February 7, 2020, from //libguides.sph.uth.tmc.edu/c.php?g=543382&p=5370369
- 5 Rees, K., Takeda, A., Martin, N., Ellis, L., Wijesekara, D., Vepa, A., Das, A., Hartley, L., & Stranges, S. (2019). Mediterranean‐style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews , 3 . https://doi.org/10.1002/14651858.CD009825.pub3
- Figure 2.3. The hierarchy of evidence by Alice Callahan, is licensed under CC BY 4.0
- Research lab photo by National Cancer Institute on Unsplas h ; mouse photo by vaun0815 on Unsplash
- Figure 2.4. “Placebo effect example” by Lindshield, B. L. Kansas State University Human Nutrition (FNDH 400) Flexbook. goo.gl/vOAnR
Experiments that are conducted outside of living organisms, within flasks, dishes, plates, or test tubes.
Research that is conducted in living organisms, such as rats and mice.
In nutrition, research that is conducted by collecting information on people’s dietary patterns or nutrient intake to look for associations with health outcomes. Observational studies do not give participants a treatment or intervention; instead, they look at what they’re already doing and see how it relates to their health.
Relationships between two factors (e.g., nutrition and health).
Research that follows a group of people (a cohort) over time, measuring factors such as diet and health outcomes.
Research that compares a group of cases and controls, looking for differences between the two groups that might explain their different health outcomes.
Research that collects information about a population of people at one point in time.
Looking into the future.
Looking at what happened in the past.
Factors that can affect the outcome in question.
Research that includes some type of treatment or change imposed by the researchers; sometimes called experimental studies or clinical trials.
The gold standard for intervention studies, because the research involves a control group and participants are randomized.
A “fake” treatment that contains no active ingredients, such as a sugar pill.
The beneficial effect that results from a subject's belief in a treatment, not from the treatment itself.
technique to prevent bias in intervention studies, where either the research team, the subject, or both don’t know what treatment the subject is receiving.
Neither the research team nor the subject know what treatment the subject is receiving.
Either the research team or the subject know what treatment is being given, but not both.
Researchers formulate a research question and then systematically and independently identify, select, evaluate, and synthesize all high-quality evidence from previous research that relates to the research question.
A type of systematic review that combines data from multiple studies and uses statistical methods to summarize it, as if creating a mega-study from many smaller studies.
Nutrition: Science and Everyday Application, v. 1.0 Copyright © 2020 by Alice Callahan, PhD; Heather Leonard, MEd, RDN; and Tamberly Powell, MS, RDN is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
Share This Book
- Bipolar Disorder
- Therapy Center
- When To See a Therapist
- Types of Therapy
- Best Online Therapy
- Best Couples Therapy
- Best Family Therapy
- Managing Stress
- Sleep and Dreaming
- Understanding Emotions
- Self-Improvement
- Healthy Relationships
- Student Resources
- Personality Types
- Guided Meditations
- Verywell Mind Insights
- 2024 Verywell Mind 25
- Mental Health in the Classroom
- Editorial Process
- Meet Our Review Board
- Crisis Support
Introduction to Research Methods in Psychology
Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
:max_bytes(150000):strip_icc():format(webp)/IMG_9791-89504ab694d54b66bbd72cb84ffb860e.jpg)
Emily is a board-certified science editor who has worked with top digital publishing brands like Voices for Biodiversity, Study.com, GoodTherapy, Vox, and Verywell.
:max_bytes(150000):strip_icc():format(webp)/Emily-Swaim-1000-0f3197de18f74329aeffb690a177160c.jpg)
There are several different research methods in psychology , each of which can help researchers learn more about the way people think, feel, and behave. If you're a psychology student or just want to know the types of research in psychology, here are the main ones as well as how they work.
Three Main Types of Research in Psychology
stevecoleimages/Getty Images
Psychology research can usually be classified as one of three major types.
1. Causal or Experimental Research
When most people think of scientific experimentation, research on cause and effect is most often brought to mind. Experiments on causal relationships investigate the effect of one or more variables on one or more outcome variables. This type of research also determines if one variable causes another variable to occur or change.
An example of this type of research in psychology would be changing the length of a specific mental health treatment and measuring the effect on study participants.
2. Descriptive Research
Descriptive research seeks to depict what already exists in a group or population. Three types of psychology research utilizing this method are:
- Case studies
- Observational studies
An example of this psychology research method would be an opinion poll to determine which presidential candidate people plan to vote for in the next election. Descriptive studies don't try to measure the effect of a variable; they seek only to describe it.
3. Relational or Correlational Research
A study that investigates the connection between two or more variables is considered relational research. The variables compared are generally already present in the group or population.
For example, a study that looks at the proportion of males and females that would purchase either a classical CD or a jazz CD would be studying the relationship between gender and music preference.
Theory vs. Hypothesis in Psychology Research
People often confuse the terms theory and hypothesis or are not quite sure of the distinctions between the two concepts. If you're a psychology student, it's essential to understand what each term means, how they differ, and how they're used in psychology research.
A theory is a well-established principle that has been developed to explain some aspect of the natural world. A theory arises from repeated observation and testing and incorporates facts, laws, predictions, and tested hypotheses that are widely accepted.
A hypothesis is a specific, testable prediction about what you expect to happen in your study. For example, an experiment designed to look at the relationship between study habits and test anxiety might have a hypothesis that states, "We predict that students with better study habits will suffer less test anxiety." Unless your study is exploratory in nature, your hypothesis should always explain what you expect to happen during the course of your experiment or research.
While the terms are sometimes used interchangeably in everyday use, the difference between a theory and a hypothesis is important when studying experimental design.
Some other important distinctions to note include:
- A theory predicts events in general terms, while a hypothesis makes a specific prediction about a specified set of circumstances.
- A theory has been extensively tested and is generally accepted, while a hypothesis is a speculative guess that has yet to be tested.
The Effect of Time on Research Methods in Psychology
There are two types of time dimensions that can be used in designing a research study:
- Cross-sectional research takes place at a single point in time. All tests, measures, or variables are administered to participants on one occasion. This type of research seeks to gather data on present conditions instead of looking at the effects of a variable over a period of time.
- Longitudinal research is a study that takes place over a period of time. Data is first collected at the beginning of the study, and may then be gathered repeatedly throughout the length of the study. Some longitudinal studies may occur over a short period of time, such as a few days, while others may take place over a period of months, years, or even decades.
The effects of aging are often investigated using longitudinal research.
Causal Relationships Between Psychology Research Variables
What do we mean when we talk about a “relationship” between variables? In psychological research, we're referring to a connection between two or more factors that we can measure or systematically vary.
One of the most important distinctions to make when discussing the relationship between variables is the meaning of causation.
A causal relationship is when one variable causes a change in another variable. These types of relationships are investigated by experimental research to determine if changes in one variable actually result in changes in another variable.
Correlational Relationships Between Psychology Research Variables
A correlation is the measurement of the relationship between two variables. These variables already occur in the group or population and are not controlled by the experimenter.
- A positive correlation is a direct relationship where, as the amount of one variable increases, the amount of a second variable also increases.
- In a negative correlation , as the amount of one variable goes up, the levels of another variable go down.
In both types of correlation, there is no evidence or proof that changes in one variable cause changes in the other variable. A correlation simply indicates that there is a relationship between the two variables.
The most important concept is that correlation does not equal causation. Many popular media sources make the mistake of assuming that simply because two variables are related, a causal relationship exists.
Psychologists use descriptive, correlational, and experimental research designs to understand behavior . In: Introduction to Psychology . Minneapolis, MN: University of Minnesota Libraries Publishing; 2010.
Caruana EJ, Roman M, Herandez-Sanchez J, Solli P. Longitudinal studies . Journal of Thoracic Disease. 2015;7(11):E537-E540. doi:10.3978/j.issn.2072-1439.2015.10.63
University of Berkeley. Science at multiple levels . Understanding Science 101 . Published 2012.
By Kendra Cherry, MSEd Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
- Privacy Policy

Home » Research Methodology – Types, Examples and writing Guide
Research Methodology – Types, Examples and writing Guide
Table of Contents

Research Methodology
Definition:
Research Methodology refers to the systematic and scientific approach used to conduct research, investigate problems, and gather data and information for a specific purpose. It involves the techniques and procedures used to identify, collect , analyze , and interpret data to answer research questions or solve research problems . Moreover, They are philosophical and theoretical frameworks that guide the research process.
Structure of Research Methodology
Research methodology formats can vary depending on the specific requirements of the research project, but the following is a basic example of a structure for a research methodology section:
I. Introduction
- Provide an overview of the research problem and the need for a research methodology section
- Outline the main research questions and objectives
II. Research Design
- Explain the research design chosen and why it is appropriate for the research question(s) and objectives
- Discuss any alternative research designs considered and why they were not chosen
- Describe the research setting and participants (if applicable)
III. Data Collection Methods
- Describe the methods used to collect data (e.g., surveys, interviews, observations)
- Explain how the data collection methods were chosen and why they are appropriate for the research question(s) and objectives
- Detail any procedures or instruments used for data collection
IV. Data Analysis Methods
- Describe the methods used to analyze the data (e.g., statistical analysis, content analysis )
- Explain how the data analysis methods were chosen and why they are appropriate for the research question(s) and objectives
- Detail any procedures or software used for data analysis
V. Ethical Considerations
- Discuss any ethical issues that may arise from the research and how they were addressed
- Explain how informed consent was obtained (if applicable)
- Detail any measures taken to ensure confidentiality and anonymity
VI. Limitations
- Identify any potential limitations of the research methodology and how they may impact the results and conclusions
VII. Conclusion
- Summarize the key aspects of the research methodology section
- Explain how the research methodology addresses the research question(s) and objectives
Research Methodology Types
Types of Research Methodology are as follows:
Quantitative Research Methodology
This is a research methodology that involves the collection and analysis of numerical data using statistical methods. This type of research is often used to study cause-and-effect relationships and to make predictions.
Qualitative Research Methodology
This is a research methodology that involves the collection and analysis of non-numerical data such as words, images, and observations. This type of research is often used to explore complex phenomena, to gain an in-depth understanding of a particular topic, and to generate hypotheses.
Mixed-Methods Research Methodology
This is a research methodology that combines elements of both quantitative and qualitative research. This approach can be particularly useful for studies that aim to explore complex phenomena and to provide a more comprehensive understanding of a particular topic.
Case Study Research Methodology
This is a research methodology that involves in-depth examination of a single case or a small number of cases. Case studies are often used in psychology, sociology, and anthropology to gain a detailed understanding of a particular individual or group.
Action Research Methodology
This is a research methodology that involves a collaborative process between researchers and practitioners to identify and solve real-world problems. Action research is often used in education, healthcare, and social work.
Experimental Research Methodology
This is a research methodology that involves the manipulation of one or more independent variables to observe their effects on a dependent variable. Experimental research is often used to study cause-and-effect relationships and to make predictions.
Survey Research Methodology
This is a research methodology that involves the collection of data from a sample of individuals using questionnaires or interviews. Survey research is often used to study attitudes, opinions, and behaviors.
Grounded Theory Research Methodology
This is a research methodology that involves the development of theories based on the data collected during the research process. Grounded theory is often used in sociology and anthropology to generate theories about social phenomena.
Research Methodology Example
An Example of Research Methodology could be the following:
Research Methodology for Investigating the Effectiveness of Cognitive Behavioral Therapy in Reducing Symptoms of Depression in Adults
Introduction:
The aim of this research is to investigate the effectiveness of cognitive-behavioral therapy (CBT) in reducing symptoms of depression in adults. To achieve this objective, a randomized controlled trial (RCT) will be conducted using a mixed-methods approach.
Research Design:
The study will follow a pre-test and post-test design with two groups: an experimental group receiving CBT and a control group receiving no intervention. The study will also include a qualitative component, in which semi-structured interviews will be conducted with a subset of participants to explore their experiences of receiving CBT.
Participants:
Participants will be recruited from community mental health clinics in the local area. The sample will consist of 100 adults aged 18-65 years old who meet the diagnostic criteria for major depressive disorder. Participants will be randomly assigned to either the experimental group or the control group.
Intervention :
The experimental group will receive 12 weekly sessions of CBT, each lasting 60 minutes. The intervention will be delivered by licensed mental health professionals who have been trained in CBT. The control group will receive no intervention during the study period.
Data Collection:
Quantitative data will be collected through the use of standardized measures such as the Beck Depression Inventory-II (BDI-II) and the Generalized Anxiety Disorder-7 (GAD-7). Data will be collected at baseline, immediately after the intervention, and at a 3-month follow-up. Qualitative data will be collected through semi-structured interviews with a subset of participants from the experimental group. The interviews will be conducted at the end of the intervention period, and will explore participants’ experiences of receiving CBT.
Data Analysis:
Quantitative data will be analyzed using descriptive statistics, t-tests, and mixed-model analyses of variance (ANOVA) to assess the effectiveness of the intervention. Qualitative data will be analyzed using thematic analysis to identify common themes and patterns in participants’ experiences of receiving CBT.
Ethical Considerations:
This study will comply with ethical guidelines for research involving human subjects. Participants will provide informed consent before participating in the study, and their privacy and confidentiality will be protected throughout the study. Any adverse events or reactions will be reported and managed appropriately.
Data Management:
All data collected will be kept confidential and stored securely using password-protected databases. Identifying information will be removed from qualitative data transcripts to ensure participants’ anonymity.
Limitations:
One potential limitation of this study is that it only focuses on one type of psychotherapy, CBT, and may not generalize to other types of therapy or interventions. Another limitation is that the study will only include participants from community mental health clinics, which may not be representative of the general population.
Conclusion:
This research aims to investigate the effectiveness of CBT in reducing symptoms of depression in adults. By using a randomized controlled trial and a mixed-methods approach, the study will provide valuable insights into the mechanisms underlying the relationship between CBT and depression. The results of this study will have important implications for the development of effective treatments for depression in clinical settings.
How to Write Research Methodology
Writing a research methodology involves explaining the methods and techniques you used to conduct research, collect data, and analyze results. It’s an essential section of any research paper or thesis, as it helps readers understand the validity and reliability of your findings. Here are the steps to write a research methodology:
- Start by explaining your research question: Begin the methodology section by restating your research question and explaining why it’s important. This helps readers understand the purpose of your research and the rationale behind your methods.
- Describe your research design: Explain the overall approach you used to conduct research. This could be a qualitative or quantitative research design, experimental or non-experimental, case study or survey, etc. Discuss the advantages and limitations of the chosen design.
- Discuss your sample: Describe the participants or subjects you included in your study. Include details such as their demographics, sampling method, sample size, and any exclusion criteria used.
- Describe your data collection methods : Explain how you collected data from your participants. This could include surveys, interviews, observations, questionnaires, or experiments. Include details on how you obtained informed consent, how you administered the tools, and how you minimized the risk of bias.
- Explain your data analysis techniques: Describe the methods you used to analyze the data you collected. This could include statistical analysis, content analysis, thematic analysis, or discourse analysis. Explain how you dealt with missing data, outliers, and any other issues that arose during the analysis.
- Discuss the validity and reliability of your research : Explain how you ensured the validity and reliability of your study. This could include measures such as triangulation, member checking, peer review, or inter-coder reliability.
- Acknowledge any limitations of your research: Discuss any limitations of your study, including any potential threats to validity or generalizability. This helps readers understand the scope of your findings and how they might apply to other contexts.
- Provide a summary: End the methodology section by summarizing the methods and techniques you used to conduct your research. This provides a clear overview of your research methodology and helps readers understand the process you followed to arrive at your findings.
When to Write Research Methodology
Research methodology is typically written after the research proposal has been approved and before the actual research is conducted. It should be written prior to data collection and analysis, as it provides a clear roadmap for the research project.
The research methodology is an important section of any research paper or thesis, as it describes the methods and procedures that will be used to conduct the research. It should include details about the research design, data collection methods, data analysis techniques, and any ethical considerations.
The methodology should be written in a clear and concise manner, and it should be based on established research practices and standards. It is important to provide enough detail so that the reader can understand how the research was conducted and evaluate the validity of the results.
Applications of Research Methodology
Here are some of the applications of research methodology:
- To identify the research problem: Research methodology is used to identify the research problem, which is the first step in conducting any research.
- To design the research: Research methodology helps in designing the research by selecting the appropriate research method, research design, and sampling technique.
- To collect data: Research methodology provides a systematic approach to collect data from primary and secondary sources.
- To analyze data: Research methodology helps in analyzing the collected data using various statistical and non-statistical techniques.
- To test hypotheses: Research methodology provides a framework for testing hypotheses and drawing conclusions based on the analysis of data.
- To generalize findings: Research methodology helps in generalizing the findings of the research to the target population.
- To develop theories : Research methodology is used to develop new theories and modify existing theories based on the findings of the research.
- To evaluate programs and policies : Research methodology is used to evaluate the effectiveness of programs and policies by collecting data and analyzing it.
- To improve decision-making: Research methodology helps in making informed decisions by providing reliable and valid data.
Purpose of Research Methodology
Research methodology serves several important purposes, including:
- To guide the research process: Research methodology provides a systematic framework for conducting research. It helps researchers to plan their research, define their research questions, and select appropriate methods and techniques for collecting and analyzing data.
- To ensure research quality: Research methodology helps researchers to ensure that their research is rigorous, reliable, and valid. It provides guidelines for minimizing bias and error in data collection and analysis, and for ensuring that research findings are accurate and trustworthy.
- To replicate research: Research methodology provides a clear and detailed account of the research process, making it possible for other researchers to replicate the study and verify its findings.
- To advance knowledge: Research methodology enables researchers to generate new knowledge and to contribute to the body of knowledge in their field. It provides a means for testing hypotheses, exploring new ideas, and discovering new insights.
- To inform decision-making: Research methodology provides evidence-based information that can inform policy and decision-making in a variety of fields, including medicine, public health, education, and business.
Advantages of Research Methodology
Research methodology has several advantages that make it a valuable tool for conducting research in various fields. Here are some of the key advantages of research methodology:
- Systematic and structured approach : Research methodology provides a systematic and structured approach to conducting research, which ensures that the research is conducted in a rigorous and comprehensive manner.
- Objectivity : Research methodology aims to ensure objectivity in the research process, which means that the research findings are based on evidence and not influenced by personal bias or subjective opinions.
- Replicability : Research methodology ensures that research can be replicated by other researchers, which is essential for validating research findings and ensuring their accuracy.
- Reliability : Research methodology aims to ensure that the research findings are reliable, which means that they are consistent and can be depended upon.
- Validity : Research methodology ensures that the research findings are valid, which means that they accurately reflect the research question or hypothesis being tested.
- Efficiency : Research methodology provides a structured and efficient way of conducting research, which helps to save time and resources.
- Flexibility : Research methodology allows researchers to choose the most appropriate research methods and techniques based on the research question, data availability, and other relevant factors.
- Scope for innovation: Research methodology provides scope for innovation and creativity in designing research studies and developing new research techniques.
Research Methodology Vs Research Methods
About the author.
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

How to Cite Research Paper – All Formats and...

Data Collection – Methods Types and Examples

Delimitations in Research – Types, Examples and...

Research Paper Format – Types, Examples and...

Research Process – Steps, Examples and Tips

Research Design – Types, Methods and Examples
- 1-888-SNU-GRAD
- Daytime Classes

The Top 3 Types of Dissertation Research Explained

Preparing for your doctoral dissertation takes serious perseverance. You’ve endured years of studies and professional development to get to this point. After sleepless nights and labor-intensive research, you’re ready to present the culmination of all of your hard work. Even with a strong base knowledge, it can be difficult — even daunting — to decide how you will begin writing.
By taking a wide-lens view of the dissertation research process , you can best assess the work you have ahead of you and any gaps in your current research strategy. Subsequently, you’ll begin to develop a timeline so you can work efficiently and cross that finish line with your degree in hand.

What Is a Dissertation?
A dissertation is a published piece of research on a novel topic in your chosen field. Students complete a dissertation as part of a doctoral or PhD program. For most students, a dissertation is the first substantive piece of academic research they will write.
Because a dissertation becomes a published piece of academic literature that other academics may cite, students must defend it in front of a board of experts consisting of peers in their field, including professors, their advisor, and other industry experts.
For many students, a dissertation is the first piece of research in a long career full of research. As such, it’s important to choose a topic that’s interesting and engaging.
Types of Dissertation Research
Dissertations can take on many forms, based on research and methods of presentation in front of a committee board of academics and experts in the field. Here, we’ll focus on the three main types of dissertation research to get you one step closer to earning your doctoral degree.
1. Qualitative
The first type of dissertation is known as a qualitative dissertation . A qualitative dissertation mirrors the qualitative research that a doctoral candidate would conduct throughout their studies. This type of research relies on non-numbers-based data collected through things like interviews, focus groups and participant observation.
The decision to model your dissertation research according to the qualitative method will depend largely on the data itself that you are collecting. For example, dissertation research in the field of education or psychology may lend itself to a qualitative approach, depending on the essence of research. Within a qualitative dissertation research model, a candidate may pursue one or more of the following:
- Case study research
- Autoethnographies
- Narrative research
- Grounded theory
Although individual approaches may vary, qualitative dissertations usually include certain foundational characteristics. For example, the type of research conducted to develop a qualitative dissertation often follows an emergent design, meaning that the content and research strategy changes over time. Candidates also rely on research paradigms to further strategize how best to collect and relay their findings. These include critical theory, constructivism and interpretivism, to name a few.
Because qualitative researchers integrate non-numerical data, their methods of collection often include unstructured interview, focus groups and participant observations. Of course, researchers still need rubrics from which to assess the quality of their findings, even though they won’t be numbers-based. To do so, they subject the data collected to the following criteria: dependability, transferability and validity.
When it comes time to present their findings, doctoral candidates who produce qualitative dissertation research have several options. Some choose to include case studies, personal findings, narratives, observations and abstracts. Their presentation focuses on theoretical insights based on relevant data points.
2. Quantitative
Quantitative dissertation research, on the other hand, focuses on the numbers. Candidates employ quantitative research methods to aggregate data that can be easily categorized and analyzed. In addition to traditional statistical analysis, quantitative research also hones specific research strategy based on the type of research questions. Quantitative candidates may also employ theory-driven research, replication-based studies and data-driven dissertations.
When conducting research, some candidates who rely on quantitative measures focus their work on testing existing theories, while others create an original approach. To refine their approach, quantitative researchers focus on positivist or post-positivist research paradigms. Quantitative research designs focus on descriptive, experimental or relationship-based designs, to name a few.
To collect the data itself, researchers focus on questionnaires and surveys, structured interviews and observations, data sets and laboratory-based methods. Then, once it’s time to assess the quality of the data, quantitative researchers measure their results against a set of criteria, including: reliability, internal/external validity and construct validity. Quantitative researchers have options when presenting their findings. Candidates convey their results using graphs, data, tables and analytical statements.

3. Mixed-Method
Many PhD candidates also use a hybrid model in which they employ both qualitative and quantitative methods of research. Mixed dissertation research models are fairly new and gaining traction. For a variety of reasons, a mixed-method approach offers candidates both versatility and credibility. It’s a more comprehensive strategy that allows for a wider capture of data with a wide range of presentation optimization.
In the most common cases, candidates will first use quantitative methods to collect and categorize their data. Then, they’ll rely on qualitative methods to analyze that data and draw meaningful conclusions to relay to their committee panel.
With a mixed-method approach, although you’re able to collect and analyze a more broad range of data, you run the risk of widening the scope of your dissertation research so much that you’re not able to reach succinct, sustainable conclusions. This is where it becomes critical to outline your research goals and strategy early on in the dissertation process so that the techniques you use to capture data have been thoroughly examined.
How to Choose a Type of Dissertation Research That’s Right for You
After this overview of application and function, you may still be wondering how to go about choosing a dissertation type that’s right for you and your research proposition. In doing so, you’ll have a couple of things to consider:
- What are your personal motivations?
- What are your academic goals?
It’s important to discern exactly what you hope to get out of your doctoral program . Of course, the presentation of your dissertation is, formally speaking, the pinnacle of your research. However, doctoral candidates must also consider:
- Which contributions they will make to the field
- Who they hope to collaborate with throughout their studies
- What they hope to take away from the experience personally, professionally and academically
Personal Considerations
To discern which type of dissertation research to choose, you have to take a closer look at your learning style, work ethic and even your personality.
Quantitative research tends to be sequential and patterned-oriented. Steps move in a logical order, so it becomes clear what the next step should be at all times. For most candidates, this makes it easier to devise a timeline and stay on track. It also keeps you from getting overwhelmed by the magnitude of research involved. You’ll be able to assess your progress and make simple adjustments to stay on target.
On the other hand, maybe you know that your research will involve many interviews and focus groups. You anticipate that you’ll have to coordinate participants’ schedules, and this will require some flexibility. Instead of creating a rigid schedule from the get-go, allowing your research to flow in a non-linear fashion may actually help you accomplish tasks more efficiently, albeit out of order. This also allows you the personal versatility of rerouting research strategy as you collect new data that leads you down other paths.
After examining the research you need to conduct, consider more broadly: What type of student and researcher are you? In other words, What motivates you to do your best work?
You’ll need to make sure that your methodology is conducive to the data you’re collecting, and you also need to make sure that it aligns with your work ethic so you set yourself up for success. If jumping from one task to another will cause you extra stress, but planning ahead puts you at ease, a quantitative research method may be best, assuming the type of research allows for this.
Professional Considerations
The skills you master while working on your dissertation will serve you well beyond the day you earn your degree. Take into account the skills you’d like to develop for your academic and professional future. In addition to the hard skills you will develop in your area of expertise, you’ll also develop soft skills that are transferable to nearly any professional or academic setting. Perhaps you want to hone your ability to strategize a timeline, gather data efficiently or draw clear conclusions about the significance of your data collection.
If you have considerable experience with quantitative analysis, but lack an extensive qualitative research portfolio, now may be your opportunity to explore — as long as you’re willing to put in the legwork to refine your skills or work closely with your mentor to develop a strategy together.
Academic Considerations
For many doctoral candidates who hope to pursue a professional career in the world of academia, writing your dissertation is a practice in developing general research strategies that can be applied to any academic project.
Candidates who are unsure which dissertation type best suits their research should consider whether they will take a philosophical or theoretical approach or come up with a thesis that addresses a specific problem or idea. Narrowing down this approach can sometimes happen even before the research begins. Other times, candidates begin to refine their methods once the data begins to tell a more concrete story.
Next Step: Structuring Your Dissertation Research Schedule
Once you’ve chosen which type of dissertation research you’ll pursue, you’ve already crossed the first hurdle. The next hurdle becomes when and where to fit dedicated research time and visits with your mentor into your schedule. The busyness of day-to-day life shouldn’t prevent you from making your academic dream a reality. In fact, search for programs that assist, not impede, your path to higher levels of academic success.
Find out more about SNU’s online and on-campus education opportunities so that no matter where you are in life, you can choose the path that’s right for you.

Want to learn more about SNU's programs?
Request more information.
Have questions about SNU, our program, or how we can help you succeed. Fill out the form and an enrollment counselor will reach out to you soon!
Subscribe to the SNU blog for inspirational articles and tips to support you on your journey back to school.
Recent blog articles.

Adult Education
A Mother’s Reflections from the Side of the Road: Strategies to Become Unstoppable

Professional and Graduate Studies Graduates Inspire Future Generations

Physician Assistant Program on Track for January 2025 Start Date

Exploring the Path to Teaching: Addressing Common Questions
Have questions about SNU or need help determining which program is the right fit? Fill out the form and an enrollment counselor will follow-up to answer your questions!
Text With an Enrollment Counselor
Have questions, but want a faster response? Fill out the form and one of our enrollment counselors will follow-up via text shortly!
Your Account
Manage your account, subscriptions and profile.
MyKomen Health
ShareForCures
In Your Community
In Your Community
View resources and events in your local community.
Change your location:

Susan G. Komen®
One moment can change everything.
Types of Research Studies
Epidemiology studies.
Epidemiology is the study of the patterns and causes of disease in people.
The goal of epidemiology studies is to give information that helps support or disprove an idea about a possible link between an exposure (such as alcohol use) and an outcome (such as breast cancer) in people.
The 2 main types of epidemiology studies are:
- Observational studies ( prospective cohort or case-control )
Randomized controlled trials
Though they have the same goal, observational studies and randomized controlled trials differ in:
- The way they are conducted
- The strengths of the conclusions they reach
Observational studies
In observational studies, the people in the study live their daily lives as they choose. They exercise when they want, eat what they like and take the medicines their doctors prescribe. They report these activities to researchers.
There are 2 types of observational studies:
Prospective cohort studies
Case-control studies.
A prospective cohort study follows a large group of people forward in time.
Some people will have a certain exposure (such as alcohol use) and others will not.
Researchers compare the different groups (for example, they might compare heavy drinkers, moderate drinkers, light drinkers and non-drinkers) to see which group is more likely to develop an outcome (such as breast cancer).
In a case-control study, researchers identify 2 groups: cases and controls.
- Cases are people who already have an outcome (such as breast cancer).
- Controls are people who do not have the outcome.
The researchers compare the 2 groups to see if any exposure (such as alcohol use) was more common in the history of one group compared to the other.
In randomized controlled trials (randomized clinical trials), researchers divide people into groups to compare different treatments or other interventions.
These studies are called randomized controlled trials because people are randomly assigned (as if by coin toss) to a certain treatment or behavior.
For example, in a randomized trial of a new drug therapy, half the people might be randomly assigned to a new drug and the other half to the standard treatment.
In a randomized controlled trial on exercise and breast cancer risk, half the participants might be randomly assigned to walk 10 minutes a day and the other half to walk 2 hours a day. The researchers would then see which group was more likely to develop breast cancer, those who walked 10 minutes a day or those who walked 2 hours a day.
Many behaviors, such as smoking or heavy alcohol drinking, can’t be tested in this way because it isn’t ethical to assign people to a behavior known to be harmful. In these cases, researchers must use observational studies.
Patient series
A patient series is a doctor’s observations of a group of patients who are given a certain treatment.
There is no comparison group in a patient series. All the patients are given a certain treatment and the outcomes of these patients are studied.
With no comparison group, it’s hard to draw firm conclusions about the effectiveness of a treatment.
For example, if 10 women with breast cancer are given a new treatment, and 2 of them respond, how do we know if the new treatment is better than standard treatment?
If we had a comparison group of 10 women with breast cancer who got standard treatment, we could compare their outcomes to those of the 10 women on the new treatment. If no women in the comparison group responded to standard treatment, then the 2 women who responded to the new treatment would represent a success of the new treatment. If, however, 2 of the 10 women in the standard treatment group also responded, then the new treatment is no better than the standard.
The lack of a comparison group makes it hard to draw conclusions from a patient series. However, data from a patient series can help form hypotheses that can be tested in other types of studies.
Strengths and weaknesses of different types of research studies
When reviewing scientific evidence, it’s helpful to understand the strengths and weaknesses of different types of research studies.
Case-control studies have some strengths:
- They are easy and fairly inexpensive to conduct.
- They are a good way for researchers to study rare diseases. If a disease is rare, you would need to follow a very large group of people forward in time to have many cases of the disease develop.
- They are a good way for researchers to study diseases that take a long time to develop. If a disease takes a long time to develop, you would have to follow a group of people for many years for cases of the disease to develop.
Case-control studies look at past exposures of people who already have a disease. This causes some concerns:
- It can be hard for people to remember details about the past, especially when it comes to things like diet.
- Memories can be biased (or influenced) because the information is gathered after an event, such as the diagnosis of breast cancer.
- When it comes to sensitive topics (such as abortion), the cases (the people with the disease) may be much more likely to give complete information about their history than the controls (the people without the disease). Such differences in reporting bias study results.
For these reasons, the accuracy of the results of case-control studies can be questionable.
Cohort studies
Prospective cohort studies avoid many of the problems of case-control studies because they gather information from people over time and before the events being studied happen.
However, compared to case-control studies, they are expensive to conduct.
Nested case-control studies
A nested case-control study is a case-control study within a prospective cohort study.
Nested case-control studies use the design of a case-control study. However, they use data gathered as part of a cohort study, so they are less prone to bias than standard case-control studies.
All things being equal, the strength of nested case-control data falls somewhere between that of standard case-control studies and cohort studies.
Randomized controlled trials are considered the gold standard for studying certain exposures, such as breast cancer treatment. Similar to cohort studies, they follow people over time and are expensive to do.
Because people in a randomized trial are randomly assigned to an intervention (such as a new chemotherapy drug) or standard treatment, these studies are more likely to show the true link between an intervention and a health outcome (such as survival).
Learn more about randomized clinical trials , including the types of clinical trials, benefits, and possible drawbacks.
Overall study quality
The overall quality of a study is important. For example, the results from a well-designed case-control study can be more reliable than those from a poorly-designed randomized trial.
Finding more information on research study design
If you’re interested in learning more about research study design, a basic epidemiology textbook from your local library may be a good place to start. The National Cancer Institute also has information on epidemiology studies and randomized controlled trials.
Animal studies
Animal studies add to our understanding of how and why some factors cause cancer in people.
However, there are many differences between animals and people, so it makes it hard to translate findings directly from one to the other.
Animal studies are also designed differently. They often look at exposures in larger doses and for shorter periods of time than are suitable for people.
While animal studies can lay the groundwork for research in people, we need human studies to draw conclusions for people.
All data presented within this section of the website come from studies done with people.
Joining a research study
Research is ongoing to improve all areas of breast cancer, from prevention to treatment.
Whether you’re newly diagnosed, finished breast cancer treatment many years ago, or even if you’ve never had breast cancer, there may be breast cancer research studies you can join.
If you have breast cancer, BreastCancerTrials.org in collaboration with Susan G. Komen® offers a custom matching service that can help find a studies that fit your needs. You can also visit the National Institutes of Health’s website to find a breast cancer treatment study.
If you’re interested in being part of other studies, talk with your health care provider. Your provider may know of studies in your area looking for volunteers.
Learn more about joining a research study .
Learn more about clinical trials .
Learn what Komen is doing to help people find and participate in clinical trials .
Updated 12/16/20
TOOLS & RESOURCES
In Your Own Words
How has having breast cancer changed your outlook?
Share Your Story or Read Others
Purdue Online Writing Lab Purdue OWL® College of Liberal Arts
Welcome to the Purdue Online Writing Lab

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
The Online Writing Lab at Purdue University houses writing resources and instructional material, and we provide these as a free service of the Writing Lab at Purdue. Students, members of the community, and users worldwide will find information to assist with many writing projects. Teachers and trainers may use this material for in-class and out-of-class instruction.
The Purdue On-Campus Writing Lab and Purdue Online Writing Lab assist clients in their development as writers—no matter what their skill level—with on-campus consultations, online participation, and community engagement. The Purdue Writing Lab serves the Purdue, West Lafayette, campus and coordinates with local literacy initiatives. The Purdue OWL offers global support through online reference materials and services.
A Message From the Assistant Director of Content Development
The Purdue OWL® is committed to supporting students, instructors, and writers by offering a wide range of resources that are developed and revised with them in mind. To do this, the OWL team is always exploring possibilties for a better design, allowing accessibility and user experience to guide our process. As the OWL undergoes some changes, we welcome your feedback and suggestions by email at any time.
Please don't hesitate to contact us via our contact page if you have any questions or comments.
All the best,
Social Media
Facebook twitter.
Cookie consent
We use our own and third-party cookies to show you more relevant content based on your browsing and navigation history. Please accept or manage your cookie settings below. Here's our cookie policy

- Form Builder Signups and orders
- Survey maker Research and feedback
- Quiz Maker Trivia and product match
- Find Customers Generate more leads
- Get Feedback Discover ways to improve
- Do research Uncover trends and ideas
- Marketers Forms for marketing teams
- Product Forms for product teams
- HR Forms for HR teams
- Customer success Forms for customer success teams
- Business Forms for general business
- Form templates
- Survey templates
- Quiz templates
- Poll templates
- Order forms
- Feedback forms
- Satisfaction surveys
- Application forms
- Feedback surveys
- Evaluation forms
- Request forms
- Signup forms
- Business surveys
- Marketing surveys
- Report forms
- Customer feedback form
- Registration form
- Branding questionnaire
- 360 feedback
- Lead generation
- Contact form
- Signup sheet
- Help center Find quick answers
- Contact us Speak to someone
- Our blog Get inspired
- Our community Share and learn
- Our guides Tips and how-to
- Updates News and announcements
- Brand Our guidelines
- Partners Browse or join
- Careers Join our team
- → The 8 types of market research and ho...
The 8 types of market research and how to use them
There are eight types of marketing research you can try to stay ahead of the competition. Learn more about marketing research methods and how to use them.

Latest posts on Tips
Typeform | 05.2024
Typeform | 04.2024
“If you keep doing what you’ve always done, you’ll keep getting what you’ve always got.”
Doesn’t sound too threatening if you’ve always been successful, right?
Continuing to do what you’ve always done means you’ll fall behind—and probably fade to darkness—to where all the forgotten brands go.
Take Kodak. They were a major player in photography for decades—remember? When digital photography boomed, Kodak kept doing what they always did. Their business floundered and people forgot about them. Well, everyone apart from Pitbull.
Now, look at Fujifilm, one of Kodak’s biggest competitors. They did the opposite and looked for ways to apply their expertise in film to the technology of the new millennium instead. Their company is still going strong.
The same goes for research. If you’re doing the same old types of market research, speaking to the same old people, and doing the same old tired surveys—you’re already behind.
How do you decide what kind of market research you need to do? It all comes down to what you need to know and what your business goals are.
In this article, we’ll explain the various types of market research you can use to solve issues and challenges in your business. We’ll throw you a freebie, too, and provide some market research tips about when to use each strategy.
Let’s get you ahead of the curve.
1. Brand research

Brand research helps with creating and managing a company’s brand, or identity. A company’s brand is the images, narratives, and characteristics people associate with it.
When to use it
Brand research can be used at every stage in a business’s lifecycle, from creation to new product launches and re-branding. There are at least seven types of brand research:
Brand advocacy: How many of your customers are willing to recommend your brand?
Brand awareness : Does your target market know who you are and consider you a serious option?
Brand loyalty: Are you retaining customers?
Brand penetration: What is the proportion of your target market using your brand?
Brand perception : What do people think of as your company’s identity or differentiating qualities?
Brand positioning: What is the best way to differentiate your brand from others in the consumer’s mind and articulate it in a way that resonates?
Brand value: How much are people willing to pay for an experience with your brand over another?
How to do it
A researcher will use several types of market research methods to assess your and your competitors’ strengths and weaknesses. Generally, they will conduct competitor research, both qualitative and quantitative, to get a picture of the overall marketplace. Focus groups and interviews can be used to learn about their emotions and associations with certain brands.
Market research surveys are useful to determine features and benefits that differentiate you from competitors . These are then translated into emotionally compelling consumer language.
2. Campaign effectiveness
This type of market research is designed to evaluate whether your advertising messages are reaching the right people and delivering the desired results. Successful campaign effectiveness research can help you sell more and reduce customer acquisition costs.
It’s estimated people see up to 5,000 advertising messages each day. That means attention is a scarce resource, so campaign effectiveness research should be used when you need to spend your advertising dollars effectively.
Campaign effectiveness research depends on which stage of the campaign you use it in (ideally, it’s all of them!). Quantitative research can be conducted to provide a picture of how your target market views advertising and address weaknesses in the advertising campaign.
3. Competitive analysis

Competitive analysis allows you to assess your competitors’ strengths and weaknesses in the marketplace, providing you with fuel to drive a competitive advantage.
No business exists in a vacuum—competitive analysis is an integral part of any business and market plan. Whether you’re just getting started, moving into a new market, or doing a health check of your business, a competitive analysis will be invaluable.
A researcher will typically choose a few of your main competitors and analyze things like their marketing strategy, customer perceptions, revenue or sales volume, and so on.
Secondary sources such as articles, references, and advertising are excellent sources of competitive information; however, primary research, such as mystery shopping and focus groups, can offer valuable information on customer service and current consumer opinions.
4. Consumer insights
Consumer insights research does more than tell you about who your customers are and what they do. It reveals why customers behave in certain ways and helps you leverage that to meet your business goals.
Knowing your customers deeply is integral to creating a strategic marketing plan. This type of market research can help you anticipate consumer needs, spark innovation, personalize your marketing, solve business challenges, and more.
Consumer insights research should be specific to your business—it’s about getting to know your target audience and customers. Various market research methods can be used, such as interviews, ethnography, survey research, social monitoring, and customer journey research.
Here are some of the characteristics you should understand through consumer insights research:
Purchase habits
Interests, hobbies, passions
Personal and professional information
How they consume media and advertising
5. Customer satisfaction research
Customer satisfaction research is a type of market research that measures customers’ experiences with products or services, specifically looking at how those meet, exceed, or fail to live up to their expectations.
Customer satisfaction is a strong indicator of customer retention and overall business performance. Successful customer satisfaction research should help you understand what your customers like, dislike, and feel needs improvement. You can use this type of market research to look at the quality and design of products, speed and timeliness of delivery, staff and service reliability, knowledge, and friendliness, market price, and value for money.
There are several ways to measure customer satisfaction, most commonly using surveys. An NPS or Voice of the Customer Survey can help you measure customer loyalty. Customer Effort Scoring measures how satisfied people are with customer service or problem resolution. CSAT is any survey that measures customer satisfaction , typically measured using Likert scale surveys . They can be conducted at different points in the customer experience, allowing deeper insight into that moment.
6. Customer segmentation research

Customer segmentation studies aim to divide markets or customers into smaller groups or personas with similar characteristics to enable targeted marketing. By understanding how people in each category behave, you can understand how each influences revenue.
Customer segmentation research is best used if you’re ready to give customers individualized experiences. Not every customer in your target market is the same. The more you understand each specific persona, the easier it is to focus on delivering personalized marketing, build loyal relations, price products effectively, and forecast how new products and services will perform in each segment.
Market researchers use four characteristics to segment customers.
Demographics: demographic information such as age, gender, family status, education, household income, occupation and so on
Geography: where people live, from cities and countries to whether they are city dwellers or suburbanites
Psychographics: socioeconomic status, class, lifestyle, personality traits, generation, interests, hobbies, etc.
Behavior: brand affinity, consumption and shopping habits, spending, etc.
A researcher will identify your current customers and collect data about them through various market research methods, such as surveys, database research, website analytics, interviews, and focus groups. The aim is to gather as much information as possible.
7. Product development
Market research for product development involves using customer knowledge to inform the entire process of creating or improving a product, service, or app and bringing it to market.
Innovation is hard work. A quick Google will tell you that 80–95% of new products fail every year. Conducting market research for product and app development helps minimize the risk of a new product or change going bust as it enters the market. There are three stages where you can use market research:
Conception: The moment you’re thinking about adding something new, market research can find market opportunities and provide insights into customer challenges or their jobs-to-be-done, so you can find a way to fill the gap.
Formation: Once you have an idea, market researchers can help you turn it into a concept that can be tested. You can learn more about strategizing pricing, testing advertising and packaging, value proposition, and so on.
Introduction: Market research can help you gauge attitudes toward the product once it’s in the market and adapt your messaging as it rolls out.
Keep making the product better or find opportunities to introduce it to new markets.
Product development research will utilize different market research methods, depending on the goal of the research. A researcher could present focus groups with product concepts and listen to their opinions, conduct interviews to learn more about their pain points, or perform user testing to see how they interact with an app or website.
8. Usability testing
Usability testing is concerned with understanding how customers use your products in real time. It can involve physical products, like a new blender, or digital products like a website or app.
Usability testing is helpful when you need to detect problems or bugs in early prototypes or beta versions before launching them. It typically costs far less to test a product or service beforehand than to pull a flawed product off the shelves or lose sales because of poor functionality.
There are several types of usability tests, which vary based on whether you’re testing a physical or digital product.
Journey testing involves observing the customer experience on an app or website and monitoring how they perform. This type of study can be done online
Eye tracking studies monitor where people’s eyes are drawn. Generally, they are conducted on websites and apps, but can also be done in stores to analyze where people look while shopping
Learn ability studies quantify the learning curve over time to see which problems people encounter after repeating the same task
Click tracking follows users’ activity on websites to evaluate the linking structure of a website
Checklist testing involves giving users tasks to perform and recording or asking them to review their experience
Combining types of market research with Typeform
When it comes to market research, you need to ask yourself what business challenge or question you’re trying to address. Then, select the appropriate methods and tools, such as market research automation , to simplify your process.From there, the world of useful data and actionable insights will open to you.

About the author
We're Typeform - a team on a mission to transform data collection by bringing you refreshingly different forms.
Liked that? Check these out:

How to generate leads in 2020: from basic to beast mode
Tried all the basic ways to generate leads? Feel like you need some inspiration to fill your email list with hot leads? Well, look no further. Here are our top tips to get more leads (with expert opinions!)
Kirsty Finlayson | 10.2019

The data on data (and how to collect it better)
In 10+ years, our customers have collected over 2.9 billion responses with over 8 million forms. So we have a lot of data... about data collection—and tips for how to do it better. Read the report to learn more.
Typeform | 03.2024

Survey design: Best practices and 15 expert tips
Beautiful surveys perform better. Read our 15 top tips to make more effective surveys and create a bigger impact with your business.
Lydia Kentowski | 01.2024
- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
Scientists welcome new rules on marijuana, but research will still face obstacles
Rhitu Chatterjee

For decades, researchers in the U.S. had to use only marijuana grown at a facility located in Oxford, Mississippi. A few other approved growers have been added in recent years. Brad Horrigan/Hartford Courant/Tribune News Service via Getty Images hide caption
For decades, researchers in the U.S. had to use only marijuana grown at a facility located in Oxford, Mississippi. A few other approved growers have been added in recent years.
As the Biden administration moves to reclassify marijuana as a less dangerous drug, scientists say the change will lift some of the restrictions on studying the drug.
But the change won't lift all restrictions, they say, neither will it decrease potential risks of the drug or help users better understand what those risks are.
Marijuana is currently classified as a Schedule I controlled substance , which is defined as a substance with no accepted medical use and a high potential for abuse. The Biden administration proposed this week to classify cannabis as a Schedule III controlled substance, a category that acknowledges it has some medical benefits.
The current Schedule I status imposes many regulations and restrictions on scientists' ability to study weed, even as state laws have made it increasingly available to the public.
"Cannabis as a Schedule I substance is associated with a number of very, very restrictive regulations," says neuroscientist Staci Gruber at McLean Hospital and Harvard Medical School. "You have very stringent requirements, for example, for storage and security and reporting all of these things."
These requirements are set by the Food and Drug Administration, the Drug Enforcement Administration, the Institutional Review Board and local authorities, she says. Scientists interested in studying the drug also have to register with the DEA and get a state and federal license to conduct research on the drug.
"It's a burdensome process and it is certainly a process that has prevented a number of young and rather invested researchers from pursuing [this kind of work]," says Gruber.
Reclassifying the drug as Schedule III puts it in the same category as ketamine and Tylenol with codeine. Substances in this category have accepted medical use in the United States, have less potential for abuse than in higher categories and abuse could lead to low to moderate levels of dependence on the drug.
This reclassification is "a very, very big paradigm shift," says Gruber. "I think that has a big trickle down effect in terms of the perspectives and the attitudes with regard to the actual sort of differences between studying Schedule III versus Schedule I substances."
Gruber welcomes the change, particularly for what it will mean for younger colleagues. "For researchers who are looking to get into the game, it will be easier. You don't have to have a Schedule I license," she says. "That's a big deal."
The rescheduling of cannabis will also "translate to more research on the benefits and risks of cannabis for the treatment of medical conditions," writes Dr. Andrew Monte in an email. He is associate director of Rocky Mountain Poison and Drug Safety and an emergency physician and toxicologist at the University of Colorado School of Medicine.
"This will also help improve the quality of the research since more researchers will be able to contribute," he adds.

Senate Democrats hold a press conference on Wednesday pitching new, less strict marijuana laws. From left are Senators Cory Booker of N.J., Majority Leader Chuck Schumer of N.Y., and Ron Wyden of Oregon. Tom Williams/CQ-Roll Call, Inc via Getty Imag hide caption
Senate Democrats hold a press conference on Wednesday pitching new, less strict marijuana laws. From left are Senators Cory Booker of N.J., Majority Leader Chuck Schumer of N.Y., and Ron Wyden of Oregon.
But the change in classification won't significantly expand the number of sources for the drug for researchers, says Gruber. For 50 years, researchers were allowed to use cannabis from only one source – a facility at the University of Mississippi. Then, in 2021, the DEA started to add a few more companies to that list of approved sources for medical and scientific research.
While she expects more sources to be added in time, she and many of the researchers she knows have yet to benefit from the recently added sources, as most have limited products available.
"And what we haven't seen is any ability for researchers –cannabis researchers, clinical researchers – to have the ability to study products that our patients and our recreational consumers or adult consumers are actually using," she adds. "That remains impossible."

Shots - Health News
Rare and mysterious vomiting illness linked to heavy marijuana use.
There is very little known information about what is in cannabis products on the market today. Some studies show that the level of THC, the main intoxicant in marijuana, being sold to consumers today is significantly higher than what was available decades ago, and high THC levels are known to pose more health risks.
And Monte cautions that the reclassification itself doesn't mean that cannabis has no health risks. Monte and his colleagues have been documenting some of those risks in Colorado by studying people who show up in the emergency room after consuming cannabis. Intoxication and cyclical vomiting ( cannabinoid hyperemesis syndrome ) and alarming psychiatric symptoms such as psychosis are among the top problems bringing some marijuana users to the hospital.
Research on cannabis has been lacking surveillance of these kinds of impacts for decades, he says. And rescheduling the drug will not fill that "gaping hole in risk surveillance," he writes.
- drug policy
- legal marijuana
- Scientific research
- biden administration
Appointments at Mayo Clinic
Meditation: a simple, fast way to reduce stress.
Meditation can wipe away the day's stress, bringing with it inner peace. See how you can easily learn to practice meditation whenever you need it most.
If stress has you anxious, tense and worried, you might try meditation. Spending even a few minutes in meditation can help restore your calm and inner peace.
Anyone can practice meditation. It's simple and doesn't cost much. And you don't need any special equipment.
You can practice meditation wherever you are. You can meditate when you're out for a walk, riding the bus, waiting at the doctor's office or even in the middle of a business meeting.
Understanding meditation
Meditation has been around for thousands of years. Early meditation was meant to help deepen understanding of the sacred and mystical forces of life. These days, meditation is most often used to relax and lower stress.
Meditation is a type of mind-body complementary medicine. Meditation can help you relax deeply and calm your mind.
During meditation, you focus on one thing. You get rid of the stream of thoughts that may be crowding your mind and causing stress. This process can lead to better physical and emotional well-being.
Benefits of meditation
Meditation can give you a sense of calm, peace and balance that can benefit your emotional well-being and your overall health. You also can use it to relax and cope with stress by focusing on something that calms you. Meditation can help you learn to stay centered and keep inner peace.
These benefits don't end when your meditation session ends. Meditation can help take you more calmly through your day. And meditation may help you manage symptoms of some medical conditions.
Meditation and emotional and physical well-being
When you meditate, you may clear away the information overload that builds up every day and contributes to your stress.
The emotional and physical benefits of meditation can include:
- Giving you a new way to look at things that cause stress.
- Building skills to manage your stress.
- Making you more self-aware.
- Focusing on the present.
- Reducing negative feelings.
- Helping you be more creative.
- Helping you be more patient.
- Lowering resting heart rate.
- Lowering resting blood pressure.
- Helping you sleep better.
Meditation and illness
Meditation also might help if you have a medical condition. This is most often true if you have a condition that stress makes worse.
A lot of research shows that meditation is good for health. But some experts believe there's not enough research to prove that meditation helps.
With that in mind, some research suggests that meditation may help people manage symptoms of conditions such as:
- Chronic pain.
- Depression.
- Heart disease.
- High blood pressure.
- Irritable bowel syndrome.
- Sleep problems.
- Tension headaches.
Be sure to talk to your healthcare professional about the pros and cons of using meditation if you have any of these or other health conditions. Sometimes, meditation might worsen symptoms linked to some mental health conditions.
Meditation doesn't replace medical treatment. But it may help to add it to other treatments.
Types of meditation
Meditation is an umbrella term for the many ways to get to a relaxed state. There are many types of meditation and ways to relax that use parts of meditation. All share the same goal of gaining inner peace.
Ways to meditate can include:
Guided meditation. This is sometimes called guided imagery or visualization. With this method of meditation, you form mental images of places or things that help you relax.
You try to use as many senses as you can. These include things you can smell, see, hear and feel. You may be led through this process by a guide or teacher.
- Mantra meditation. In this type of meditation, you repeat a calming word, thought or phrase to keep out unwanted thoughts.
Mindfulness meditation. This type of meditation is based on being mindful. This means being more aware of the present.
In mindfulness meditation, you focus on one thing, such as the flow of your breath. You can notice your thoughts and feelings. But let them pass without judging them.
- Qigong. This practice most often combines meditation, relaxation, movement and breathing exercises to restore and maintain balance. Qigong (CHEE-gung) is part of Chinese medicine.
- Tai chi. This is a form of gentle Chinese martial arts training. In tai chi (TIE-CHEE), you do a series of postures or movements in a slow, graceful way. And you do deep breathing with the movements.
- Yoga. You do a series of postures with controlled breathing. This helps give you a more flexible body and a calm mind. To do the poses, you need to balance and focus. That helps you to focus less on your busy day and more on the moment.
Parts of meditation
Each type of meditation may include certain features to help you meditate. These may vary depending on whose guidance you follow or who's teaching a class. Some of the most common features in meditation include:
Focused attention. Focusing your attention is one of the most important elements of meditation.
Focusing your attention is what helps free your mind from the many things that cause stress and worry. You can focus your attention on things such as a certain object, an image, a mantra or even your breathing.
- Relaxed breathing. This technique involves deep, even-paced breathing using the muscle between your chest and your belly, called the diaphragm muscle, to expand your lungs. The purpose is to slow your breathing, take in more oxygen, and reduce the use of shoulder, neck and upper chest muscles while breathing so that you breathe better.
A quiet setting. If you're a beginner, meditation may be easier if you're in a quiet spot. Aim to have fewer things that can distract you, including no television, computers or cellphones.
As you get more skilled at meditation, you may be able to do it anywhere. This includes high-stress places, such as a traffic jam, a stressful work meeting or a long line at the grocery store. This is when you can get the most out of meditation.
- A comfortable position. You can practice meditation whether you're sitting, lying down, walking, or in other positions or activities. Just try to be comfortable so that you can get the most out of your meditation. Aim to keep good posture during meditation.
- Open attitude. Let thoughts pass through your mind without judging them.
Everyday ways to practice meditation
Don't let the thought of meditating the "right" way add to your stress. If you choose to, you can attend special meditation centers or group classes led by trained instructors. But you also can practice meditation easily on your own. There are apps to use too.
And you can make meditation as formal or informal as you like. Some people build meditation into their daily routine. For example, they may start and end each day with an hour of meditation. But all you really need is a few minutes a day for meditation.
Here are some ways you can practice meditation on your own, whenever you choose:
Breathe deeply. This is good for beginners because breathing is a natural function.
Focus all your attention on your breathing. Feel your breath and listen to it as you inhale and exhale through your nostrils. Breathe deeply and slowly. When your mind wanders, gently return your focus to your breathing.
Scan your body. When using this technique, focus attention on each part of your body. Become aware of how your body feels. That might be pain, tension, warmth or relaxation.
Mix body scanning with breathing exercises and think about breathing heat or relaxation into and out of the parts of your body.
- Repeat a mantra. You can create your own mantra. It can be religious or not. Examples of religious mantras include the Jesus Prayer in the Christian tradition, the holy name of God in Judaism, or the om mantra of Hinduism, Buddhism and other Eastern religions.
Walk and meditate. Meditating while walking is a good and healthy way to relax. You can use this technique anywhere you're walking, such as in a forest, on a city sidewalk or at the mall.
When you use this method, slow your walking pace so that you can focus on each movement of your legs or feet. Don't focus on where you're going. Focus on your legs and feet. Repeat action words in your mind such as "lifting," "moving" and "placing" as you lift each foot, move your leg forward and place your foot on the ground. Focus on the sights, sounds and smells around you.
Pray. Prayer is the best known and most widely used type of meditation. Spoken and written prayers are found in most faith traditions.
You can pray using your own words or read prayers written by others. Check the self-help section of your local bookstore for examples. Talk with your rabbi, priest, pastor or other spiritual leader about possible resources.
Read and reflect. Many people report that they benefit from reading poems or sacred texts and taking a few moments to think about their meaning.
You also can listen to sacred music, spoken words, or any music that relaxes or inspires you. You may want to write your thoughts in a journal or discuss them with a friend or spiritual leader.
- Focus your love and kindness. In this type of meditation, you think of others with feelings of love, compassion and kindness. This can help increase how connected you feel to others.
Building your meditation skills
Don't judge how you meditate. That can increase your stress. Meditation takes practice.
It's common for your mind to wander during meditation, no matter how long you've been practicing meditation. If you're meditating to calm your mind and your mind wanders, slowly return to what you're focusing on.
Try out ways to meditate to find out what types of meditation work best for you and what you enjoy doing. Adapt meditation to your needs as you go. Remember, there's no right way or wrong way to meditate. What matters is that meditation helps you reduce your stress and feel better overall.
Related information
- Relaxation techniques: Try these steps to lower stress - Related information Relaxation techniques: Try these steps to lower stress
- Stress relievers: Tips to tame stress - Related information Stress relievers: Tips to tame stress
- Video: Need to relax? Take a break for meditation - Related information Video: Need to relax? Take a break for meditation
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Meditation: In depth. National Center for Complementary and Integrative Health. https://nccih.nih.gov/health/meditation/overview.htm. Accessed Dec. 23, 2021.
- Mindfulness meditation: A research-proven way to reduce stress. American Psychological Association. https://www.apa.org/topics/mindfulness/meditation. Accessed Dec. 23, 2021.
- AskMayoExpert. Meditation. Mayo Clinic. 2021.
- Papadakis MA, et al., eds. Meditation. In: Current Medical Diagnosis & Treatment 2022. 61st ed. McGraw Hill; 2022. https://accessmedicine.mhmedical.com. Accessed Dec. 23, 2021.
- Hilton L, et al. Mindfulness meditation for chronic pain: Systematic review and meta-analysis. Annals of Behavioral Medicine. 2017; doi:10.1007/s12160-016-9844-2.
- Seaward BL. Meditation. In: Essentials of Managing Stress. 5th ed. Jones & Bartlett Learning; 2021.
- Seaward BL. Managing Stress: Principles and Strategies for Health and Well-Being. 9th ed. Burlington, Mass.: Jones & Bartlett Learning; 2018.
Products and Services
- A Book: Mayo Clinic Handbook for Happiness
- A very happy brain
- Alternative cancer treatments: 11 options to consider
- Brain tumor
- Brain Tumor
- What is a brain tumor? A Mayo Clinic expert explains
- Brain tumor FAQs
- Living with Brain Tumors
- Long Term Brain Cancer Survivor
- Mayo Clinic Minute: Meditation is good medicine
- Meditation 2.0: A new way to meditate
- Parkinson's disease
- Punk Guitarist Survives Brain Tumor
- Guided meditation video
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Meditation A simple fast way to reduce stress
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
Pan-cancer analysis for the prognostic and immunological role of CD47: interact with TNFRSF9 inducing CD8 + T cell exhaustion
- Open access
- Published: 08 May 2024
- Volume 15 , article number 149 , ( 2024 )
Cite this article
You have full access to this open access article

- Hongxin Liang ORCID: orcid.org/0000-0002-8481-4903 1 ,
- Yong Zheng 2 ,
- Zekai Huang 3 , 4 ,
- Jinchi Dai 4 ,
- Lintong Yao 5 ,
- Daipeng Xie 4 ,
- Duo Chen 6 ,
- Hongrui Qiu 4 ,
- Huili Wang 4 ,
- Jinhang Leng 4 ,
- Ziming Tang 5 ,
- Dongkun Zhang 4 &
- Haiyu Zhou ORCID: orcid.org/0000-0002-3328-6792 1 , 4
The research endeavors to explore the implications of CD47 in cancer immunotherapy effectiveness. Specifically, there is a gap in comprehending the influence of CD47 on the tumor immune microenvironment, particularly in relation to CD8 + T cells. Our study aims to elucidate the prognostic and immunological relevance of CD47 to enhance insights into its prospective utilities in immunotherapeutic interventions.
Differential gene expression analysis, prognosis assessment, immunological infiltration evaluation, pathway enrichment analysis, and correlation investigation were performed utilizing a combination of R packages, computational algorithms, diverse datasets, and patient cohorts. Validation of the concept was achieved through the utilization of single-cell sequencing technology.
CD47 demonstrated ubiquitous expression across various cancer types and was notably associated with unfavorable prognostic outcomes in pan-cancer assessments. Immunological investigations unveiled a robust correlation between CD47 expression and T-cell infiltration rather than T-cell exclusion across multiple cancer types. Specifically, the CD47-high group exhibited a poorer prognosis for the cytotoxic CD8 + T cell Top group compared to the CD47-low group, suggesting a potential impairment of CD8 + T cell functionality by CD47. The exploration of mechanism identified enrichment of CD47-associated differentially expressed genes in the CD8 + T cell exhausted pathway in multiple cancer contexts. Further analyses focusing on the CD8 TCR Downstream Pathway and gene correlation patterns underscored the significant involvement of TNFRSF9 in mediating these effects.
A robust association exists between CD47 and the exhaustion of CD8 + T cells, potentially enabling immune evasion by cancer cells and thereby contributing to adverse prognostic outcomes. Consequently, genes such as CD47 and those linked to T-cell exhaustion, notably TNFRSF9, present as promising dual antigenic targets, providing critical insights into the field of immunotherapy.
Avoid common mistakes on your manuscript.
1 Introduction
The advent of immunotherapies has brought about significant advancements in the survival rates of cancer patients. The upregulation of various immune checkpoints serves as a key mechanism facilitating tumor immune evasion and represents a primary barrier that hampers the effectiveness of immune-based treatments. CD47, a pivotal checkpoint of innate immunity, emerges as a focal point of investigation. Delving into the intricate interplay between CD47 and the tumor immune microenvironment (TIME) to delineate a comprehensive understanding of tumor immune evasion pathways holds promise for the development of tailored therapeutic interventions and the identification of novel immune targets. These endeavors are poised to address challenges associated with immune therapy resistance, ultimately enhancing the survival outcomes of individuals afflicted with malignancies.
CD47 is a transmembrane glycoprotein belonging to the immunoglobulin superfamily, known to interact with signal regulatory protein α(SIRPα). The CD47/SIRPα axis functions to impede myosin accumulation, thereby initiating the "Do not eat me" signal to evade phagocytosis by macrophages [ 1 , 2 ] and suppress innate immunity [ 3 , 4 , 5 ]. Overexpression of CD47 in various tumor cell types has been identified as a mechanism for evading innate immunity[ 4 ], correlating with diminished survival outcomes and reduced responsiveness to conventional therapies[ 6 , 7 , 8 ]. Targeting the CD47/SIRPα axis has been a focal point of investigation, with therapeutic strategies including monoclonal antibodies (McAb), bispecific antibodies, fusion proteins, combination chemotherapies, and immunotherapies, such as CD47 McAb and fusion proteins incorporating SIRPα(e.g., TTI-621/2). These approaches have demonstrated notable clinical efficacy and are currently under evaluation in phase II or III clinical trials[ 9 , 10 , 11 ].
Significantly, the CD47/SIRPα axis exerts influence on adaptive immunity as well. Studies have revealed that inhibition of the CD47/SIRPα axis can directly enhance T-cell responses or act through modulation of myeloid cells [ 12 , 13 ]. Notably, a subset of CD8 + T cells has been identified to express SIRPα[ 14 ], and disrupting the CD47/SIRPα interaction can overcome resistance to CD8-mediated immunotherapy [ 15 , 16 ]. Additionally, CD47 agonists have demonstrated the ability to promote antigen presentation and facilitate cross-priming of T-lymphocytes [ 17 , 18 ]. Preclinical mouse models have underscored the role of CD47 monoclonal antibodies in T-cell cross-priming, with a lack of therapeutic response observed in T-cell-deficient mice but rescued in wild-type counterparts [ 19 ]. Combining radiotherapy with CD47 blockade has been shown to enhance antitumor immunity by directly impacting CD8 + T cells [ 20 ]. The intricate interplay between the tumor microenvironment (TME), tumor immune evasion, cancer prognosis, and therapeutic responses has been elucidated [ 21 , 22 ], highlighting the significance of targeting the CD47/SIRPα axis to impede phagocytosis and counteract innate immunity checkpoints associated with tumor immune evasion. Nonetheless, a comprehensive understanding of the implications of adaptive immunity in regulating the CD47 checkpoint and its impact on antitumor T-cell immunity remains an imperative area for further exploration.
A promising target of interest is the tumor necrosis factor (TNF) receptor superfamily member TNFRSF9 (CD137, TNFRSF9). TNFRSF9 expression is specifically induced through the interaction between the T cell receptor (TCR) and the major histocompatibility complex (MHC) [ 23 ]. Identified as a characteristic marker of tumor-reactive T cell subsets within the tumor microenvironment (TME), TNFRSF9 is notably absent on static T cells present in peripheral blood [ 24 , 25 ]. DSP107, a fusion protein, exhibits dual immune regulatory capabilities by binding to both targets, thereby stimulating innate and adaptive immune responses and showcasing potent antitumor effects. Through the natural trimerization of DSP107 via the 4-1BBL trimerization domain, binding to CD47 on cancer cells disrupts the CD47-SIRPα interaction. This interaction also facilitates the immobilization of DSP107 on the cancer cell surface, enabling the delivery of the 4-1BBL-4-1BB costimulatory signal to T cells localized within the tumor microenvironment. The dual immunomodulatory mechanism of DSP107 is strategically formulated to activate both innate and adaptive immune responses at the tumor site, ultimately enhancing antitumor immunity [ 26 ].
This study conducted a comprehensive analysis of the prognostic and immunological implications of CD47 in pan-cancer, with a specific focus on its association with CD8 + T cell exhaustion. The investigation aimed to elucidate the potential impact of CD47 interaction with TNFRSF9 within the tumor immune microenvironment (TIME) on CD8 + T cell functionality and its consequent effect on the prognosis of patients with malignant tumors. Leveraging a range of bioinformatics tools, the analysis encompassed differential expression assessments, prognosis evaluations, immune cell infiltration patterns, and pathway enrichment analyses across various cancer types. Furthermore, the co-expression relationship between CD47 and the pivotal gene TNFRSF9 was validated utilizing single-cell sequencing data. The research design and technical approach are well-structured and reasonable.
2.1 Analysis tools and data collection
XENA-TCGA GTEx
TCGA ( https://portal.gdc.cancer.gov/ ) and GTEx handling were consolidated by the Toil process in UCSC XENA ( https://xenabrowser.net/datapages/ ). Data (V8.0) conversion: Transcripts per million reads format RNAseq data in TPM format and log2 conversion for analysis and comparison. GTEx, The Genotype-Tissue Expression ( https://www.gtexportal.org/home/ ). After log2 transformation, RNAseq data in TPM (transcripts per million reads) format was examined and contrasted. [ 27 ]
A comprehensive OMICS cancer data analysis web portal is located in Ualcan ( http://ualcan.path.uab.edu/ ). The expression level of CD47 was normalized as transcript per million reads. P < 0.05 was considered statistically significant [ 28 ].
TIMER2.0, Tumor IMmune Estimation Resource ( https://cistrome.shinyapps.io/timer/ ) is a database for comprehensive analysis of tumor-infiltrating immune cells [ 29 ]. The TIMER database consists of 10897 samples from 32 TCGA cancer types to evaluate immune infiltrate abundance.
TIDE [ 30 ] ( http://tide.dfci.harvard.edu/ ) stands for Tumor Immune Dysfunction and Exclusion. It is a computational framework developed to evaluate the potential of tumor immune escape from the gene expression profiles of cancer samples. The TIDE score computed for each tumor sample can be a surrogate biomarker to predict response to immune checkpoint blockade, including anti-PD1 and anti-CTLA4 for melanoma and NSCLC. The highly scored genes in TIDE signatures also present potential regulators of tumor immune escape and resistance to cancer immunotherapies [ 31 ].
STRING database [ 32 ]: (string-db.org) is a protein interaction network database based on public database and literature information. It gathers several public databases, including UniProt, KEGG, NCBI, and Gene Ontology, to integrate these data and generate a comprehensive protein interaction network database.
TISCH collected data from Gene Expression Omnibus (GEO) [ 33 ] and Array Express [ 34 ] to formulate its scRNA-seq atlas [ 35 ], including 79 databases and 2045746 cells from tumor patients and healthy donors. Data sets were processed uniformly to allow for clarification of the TME components at both the single-cell and annotated cluster levels.
HPA[ 36 ] ( https://www.proteinatlas.org ).
The HPA database (Human Protein Atlas) is based on proteomics, transcriptomics, and systems biology data to map tissues, cells, and organs. It includes not only tumor tissue, but also normal tissue protein expression, and can also check the survival curve of tumor patients.
P value less than 0.05 was statistically significant. Significance markers: NS, P ≥ 0.05; *, p < 0.05; * *, p < 0.01; * * *, p < 0.001.
2.2 Analysis of differential CD47 expression in average, tumor stages, and protein levels
Differential CD47 expression levels between tumors and normal tissues adjacent to TCGA cancer types were analyzed using R (version 3.6.3) and R packages (mainly GGGlot2 [version 3.3.3]) from the XENA-TCGA GTEx resource. Furthermore, Protein levels between tumors and adjacent normal tissues were also investigated using the UALCAN interactive web resource. Survival curves were presented using predictive analysis. SurvMiner [version 0.4.9] and Survival package [version 3.2–10] were used (grouped by p-best). The type of prognosis was OS (Overall Survival), DSS (Disease-Specific Survival), and prognostic data were also obtained from a Cell article [ 37 ], and finally verified by immunohistochemistry of HPA database.
2.3 Analysis of tumor immune and immunosuppressive cell infiltration and comparative biomarker analysis
Using the TIMER2 server, we analyzed the correlation between tumor infiltration and CD47 expression, with four immunosuppressive cell types promoting T-cell rejection, MDSCs, CAF, M2-TAM, and Treg across 39 TCGA cancers. The Spearman partial rho value and p < 0.05 were used for correlation analysis. The results of the study used the algorithm with the best positive results. In addition, we used the GSVA R package [version 1.34.0] [ 38 ] to explore correlations between the expression of CD47 and the infiltration of 23 types of immune cells [ 39 ] in TCGA cancers. Then, the overall predictive power of CD47 was compared with standardized biomarkers of tumor immune response in terms of treatment response outcome and OS, and the correlation analysis of CD47 with other immune checkpoints, MHC class molecules and other immune-related molecules was explored.
2.4 Analysis of CD47 on cytotoxic CD8 + T cell infiltration influenced tumor prognosis
We used the GSVA R package [version 1.34.0] [ 38 , 39 ] to explore the difference in Cytotoxic T-cell infiltration in the different CD47 expression situations in TCGA pan-cancer. The median method was used to divide patients into CD47- high group and CD47-low group. We also use the TIDE algorithm to assess the effects of CD47 on Cytotoxic CD8 + T cell [ 31 ].
2.5 Analysis of pathway
We identify DEG between high and low-expression CD47 clusters using the DESeq2 R package [1.26.0 version] [ 40 ]. Division of patients into high and low CD47 groups by median method. These different genes were enriched in the pathway via the R package cluster profile [ 41 , 42 ] [3.14.3 version] (for SEEA analysis) [ 42 ]. [version 3.14.3] (for GSEA analysis) [ 42 ]. Reference gene set: H.all.v7.2.symbols.GMT [Hallmarks]. The species is Homo sapiens. Gene set databases came from MSigDB Collections [ 43 ]. It included the BIOCARTA subset of CP (browse 292 gene sets); KEGG subset of CP (browse 186 gene sets); PID subset of CP (browse 196 gene sets); REACTOME subset of CP (browse 1615 gene sets); WikiPathways subset of CP (browse 664 gene sets). Significance: It is generally considered that the conditions of False Discovery Rate (FDR) < 0.25 and p.adjust < 0.05. Visualization: GGploT2 [version 3.3.3]. Furthermore, CBNplot [ 44 ] was used to investigate molecular regulatory connections. in PID_CD8_TCR_DOWNSTREAM_PATHWAY, exhibiting a Bayesian network inference approach. STRING database was used to detect the PPI (Protein–Protein Interaction Networks), showing the interactions of CD47 protein and core protein of that pathway in biological systems.
2.6 Analysis of co-expression.
We used Software: R (version 3.6.3) to analyze the correlation of CD47 and T-cell exhaustion in TCGA pan-cancer. The data sets were level 3 HTSeq—FPKM RNAseq data format. Visualization of the results is provided by the R package: GGploT2 [version 3.3.3]. Besides, we used TISCH to determine whether CD47 was mainly expressed primarily on CD8 + Tex and whether CD47 has a relationship with the CD8 + Tex gene expressed primarily on CD8 + Tex. The results were presented in the stacked value bar chart (bars of superimposed proportions) to see the expression levels of CD47 and TNFRSF9 in different cohorts through software R (version 3.6.3) and GGPLOT2 [version 3.3.3] (visualization).
3.1 Study flowchart
Figure 1 illustrates the schematic diagram of the present study. The study encompasses clinic prognosis and gene data analyses.
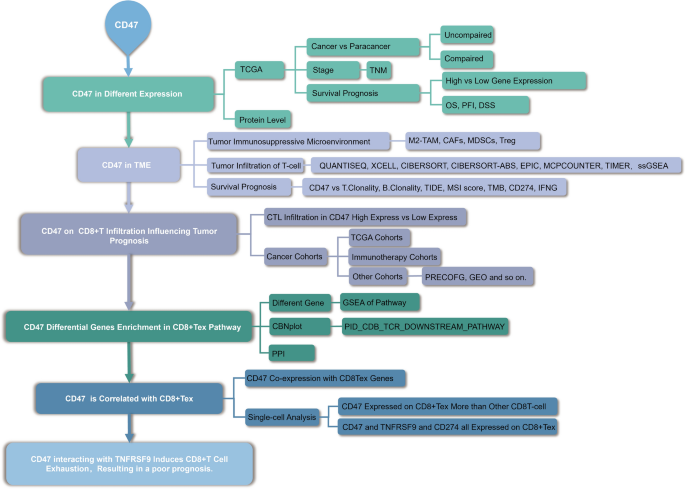
The workflow of the study. TCGA, The Cancer Genome Atlas; TME, Tumor microenvironment; TNM, Tumor Node Metastasis; TAMs, tumor-associated macrophages; CAFs, cancer-associated fibroblasts; aDC (activated DC); B cells; CD8 T cells; CTL(Cytotoxic T cells); DC; Eosinophils; iDC (immature DC); Macrophages; Mast cells; Neutrophils; NK CD56bright cells; NK CD56dim cells; NK cells; pDC (Plasmacytoid DC); T cells; T helper cells; Tcm (T central memory); Tem (T effector memory); Tfh (T follicular helper); Tgd (T gamma delta); Th1 cells; Th17 cells; Th2 cells; Treg, regulatory T cells; CAFs, cancer-associated fibroblasts; MDSCs, myeloid-derived suppressor cells; M2-TAMs; M2 subtype of tumor-associated macrophages. MSI, Microsatellite instability; TMB, Tumor mutational burden; CD274, Cluster of differentiation 274; IFNG, interferon-γ; 4-1BB(TNFRSF9), TNF receptor superfamily member 9; CD8 + Tex, exhausted CD8 T lymphocyte cell
3.2 Abnormal expression of CD47 in pan-cancer patients is associated with tumor stages and poor prognosis
We looked into CD47's carcinogenic potential using the XENA-TCGA GTEx pan-cancer database. When comparing nearly all cancer types to normal tissue, we discovered that CD47 gene expression was higher in the former. (ACC, BRCA, BLCA, CHOL, COAD, DLBC, ESCA, GBM, HNSC, KIRC, KIRP, LAML, LGG, LIHC, LUAD, OV, PAAD, PRAD, READ, SARC, SKCM, STAD, THCA, THYM, UCEC, UCS) (Fig. 2 a). Additionally, we delved deeper into the CD47 expression of paired samples within the XENA-TCGA database, yielding identical outcomes to the XENA-TCGA GTEx pan-cancer database across various cancer types including BRCA, CHOL, COAD, ESCA, HNSC, KIRC, LIHC, PAAD, STAD, THCA, and UCEC. (Supplementary Fig. 1a).
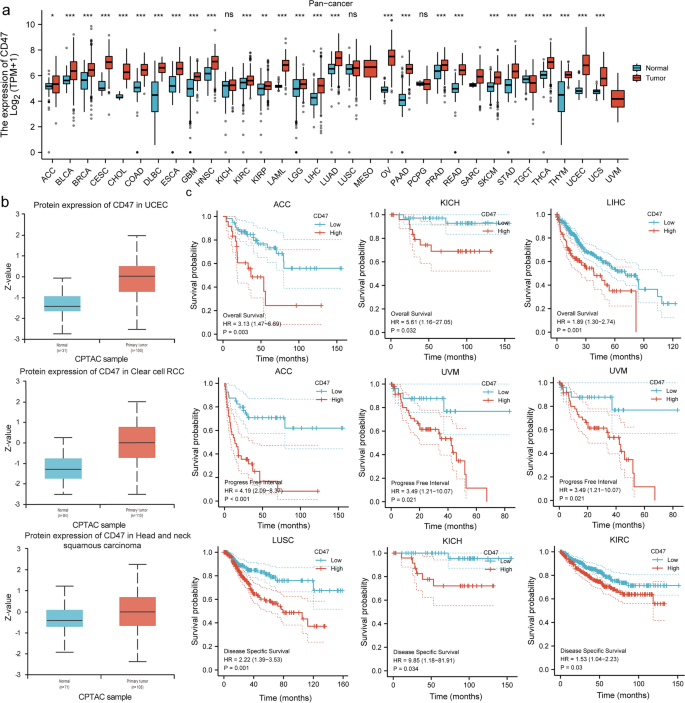
CD47 is aberrantly overexpressed and is associated with poor cancer prognoses. ( A ) Boxplots showing differential CD47 expression levels (log2FPKM + 1)/ (log2TPM + 1) between tumors in the XENA-TCGA_GTEx database. Box plots showing differential CD47 expression levels (log2FPKM + 1)/ (log2TPM + 1) between tumor and adjacent normal tissues (Paired Patient) across the TCGA database. CD47 is expressed differently in multiple cancers. B Boxplots illustrating the varying levels of CD47 expression (protein) among tumors in the CPTAC database. C Kaplan–Meier curves of cumulative survival differences between TCGA cancer cohorts with high and those with low expression levels of CD47. The presentation showcases TCGA cancers that exhibit statistically significant variations among the cohorts. UCSC XENA ( https://xenabrowser.net/datapages/ ) by the Toil process unified TCGA RNAseq TPM format data processing. (GTEx)The Genotype-Tissue Expression; Significance representation: ns, p ≥ 0.05; *, p < 0.05; **, p < 0.01; ***, p < 0.001
Significantly, we noted an increase in CD47 protein expression in HNSC, PAAD, UCEC, RCC, and OV compared to the normal levels, as indicated by the UALCAN database (Fig. S2b, Supplementary Fig. 1b). Moreover, in cancer, the expression of CD47 was elevated in advanced tumor stages. For example, patients with M1 stage lung squamous cell carcinoma malignancy expressed more CD47 than patients with M0 stage. The same trend's outcomes were observed in THCA, UCEC, PRAD, KIRC, KIRP, and LIHC. (Supplementary Fig. 1c). Subsequently, we discovered a correlation between excessive CD47 expression and reduced overall survival in ACC, BRCA, LIHC, and KICH; decreased PFI in ACC, LUSC, UVM, and decreased DSS in ACC, LUSC, LGG, and KICH. All of these findings point to CD47 could be an early biomarker for cancer detection, staging, and monitoring. (Fig. 2 c, Supplementary Fig. 1d). Lastly, the Human Protein Atlas (HPA) database was used to detect the expression of CD47 in human normal tissues. Representative IHC images of CD47 expression in BRCA, HNSC, LUSC, OV, SKCM. (Supplementary Fig. 1e). From the protein expression level, it was again proved that CD47 was highly expressed in tumor tissues, especially on the cell membranes of tumor cells.
3.3 CD47 is related to tumor immune evasion through infiltration by T lymphocyte cells
Due to its association with tumor immunity evasion, we assessed the associations between CD47 expression levels and the infiltration of MDSCs, CAFs, M2-TAMs, and Treg cells through six algorithms (QUANTISEQ, XCELL, CIBERSORT, CIBERSORT-ABS, TIDE, MCPCOUNTER). These types of immune cells could promote T-cell exclusion. Treg and CAF in BRCA-LumA, Treg and CAF in LICH, Treg, MDSC, and CAF in PRAD, and Treg and CAF in THYM were found to positively correlate with CD47 expression. (r > 0.2, p < 0.05, every cell type ≥ 2 algorithms positive, and at least two types of these cells are positive) (Fig. 3 a).

The differential expression of CD47 in tumor microenvironment. A , B The heatmap chart showed correlations of CD47 expression with infiltration by different immune cell types and different immunosuppressive cell types in various TCGA cancer types. C The differential expression of CD47 in Pan-cancer was predominantly associated with CD8 + T cells, CD4 + T cells, DC cells, and macrophages, as demonstrated by the lollipop. Correlation is depicted with a purity-corrected partial ( D ) Bar plot showing the biomarker relevance of CD47 compared to standardized cancer immune evasion biomarkers in immune checkpoint blockade (ICB) sub-cohorts. The AUC was utilized to assess the predictive efficacy of the test biomarkers in determining the ICB response status. Spearman’s rho values and statistical significance were used. (A. B TIMER database) (C. R3.6.3 ssGSEA). Significance representation: ns, p ≥ 0.05; *, p < 0.05; **, p < 0.01; ***, p < 0.001
Subsequently, we employ the identical cognitive approach to identify the association with T cell infiltration and expression of CD47 through seven different algorithms (QUANTISEQ, XCELL, CIBERSORT, CIBERSORT-ABS, EPIC, MCPCOUNTER, TIMER). The results showed that CD47 expression was positively correlated with infiltration of CD8 + T cell in almost all cancer types. (BLCA, BRCA, BRCA-Basal, COAD, DLBC, ESCA, KIRC, KIRP, LIHC, LUSC, PAAD, PRAD, READ, SKCM, SKCM-Metastasis, STAD, TGCT, THCA, UVM) (r > 0.2, p < 0.05, at least two types of these cells are positive, and at least two types of calculation methods). Interestingly, the infiltration of CD8 + T cell effector memory is comparatively lower in the majority of cancer species compared to other types such as CD8 + T cell central memory and CD8 + T cell naive. As for CD4 + T cell, there were still a lot of CD4 + Tcells closely related to CD47, but it depends on the types of CD4 + T cell. The presence of CD47 in the majority of cancer types was observed to have a positive correlation with the infiltration of CD4 + T cell memory resting and CD4 + T cell Th2, in contrast to CD4 + T cell (non-regulatory) and CD4 + T cell Th1(Fig. 3 b).
We further explored which types of T-cell infiltration in tumors were most associated with CD47 using another way. It is worth mentioning that the correlation between CD8 + T cells infiltration and CD47 is the highest in these cancer types (COAD, DLBC, ESCA, HNSC-HPV-, LIHC, LUAD, LUSC, PAAD, STAD) (Fig. 3 b). CD47 expression had strong positive correlations with T cell infiltration in various cancer types including BLCA, CHOL, COADREAD, DLBC, GBM, HNSC, KIRC, KIRP, LAML, LUSC, PAAD, PRAD, SKCM, TGCT, READ, STAD, UCS, and UVM. The T cell subtypes that displayed significant associations included T helper cells, CD8 + T cells, CD4 T cells, Cytotoxic cells, Th1, Th2, Th17, as well as Tgd, Tcm, Tem, and TFH. (Fig. 3 c, Supplementary Fig. 2a). Furthermore, it suggested a strong association between the infiltration of CD8 + T cells and CD47 in numerous cancer types (DLBC, ESCA, LUSC, OV, SKCM, STAD, TGCT, THCA, UCS, UVM). (r > 0.2, p < 0.05).
Then, we assessed CD47 biomarker relevance by comparing CD47 with standardized biomarkers based on its response outcomes to ICB sub-cohorts and OS predictive ability. Interestingly, we found that in 16 of the 25 ICB sub-cohorts, CD47 alone had an area greater than 0.5% under the AUC. CD47 was predicted to be more valuable than TMB, T. Clonality, B. Clonality, and MSI. Seven, nine, seven, and 13 ICB subgroups had more significant AUC values than 0.5. However, CD47 is lower than CD274, TIDE, IFNG, CD8, and Merck18. Based on these results, it is strongly indicated that CD47 plays a pivotal role in the immune microenvironment of tumors and exhibits a strong association with T-cell infiltration(Fig. 3 d). Lastly, We analysed the correlation of CD47 in pan-cancer with other immune checkpoints including immunoinhibitor molecule, MHCmolecule and immunostimula molecule. We found CD47 had strong correlation with CD274, LAG3, IDO1 and TNF receptor superfamily in pan-cancer(r > 0.2, p < 0.05)( Supplementary Fig. 2b).
3.4 CD47 on CD8 + T cells infiltration had an impact on tumor prognosis.
Drawing from our prior findings, it can be inferred that the presence of CD8 + T cells exhibited a strong correlation with CD47. Interestingly, we found that in the CD47 High group, the level of Cytotoxic CD8 + T cell was more frequently observed in BLCA, BRCA, CESC, COAD, COADREAD, ESAD, ESCA, GBM, HNSC, KIRC, LUSC, OV, SKCM, STAD, TGCT, THCA, UCS types compared to the CD47 low group based on TCGA database. (Fig. 4 a, Supplemental Fig. 3a) Then we further identified the same conclusion in Multiple immunotherapy cohorts (Nathanson2017_CTLA4-OS, Gide2019_PD1-OS, Gide2019_PD1 + CTLA4-OS, Miao2018_ICB-OS, Mariathasan2018_PDL1-OS, Riaz2017_PD1-OS, Liu2019_PD1-OS Zhao2019_PD1-OS, VanAllen2015_CTLA4-OS) (Supplemental Fig. 3b).
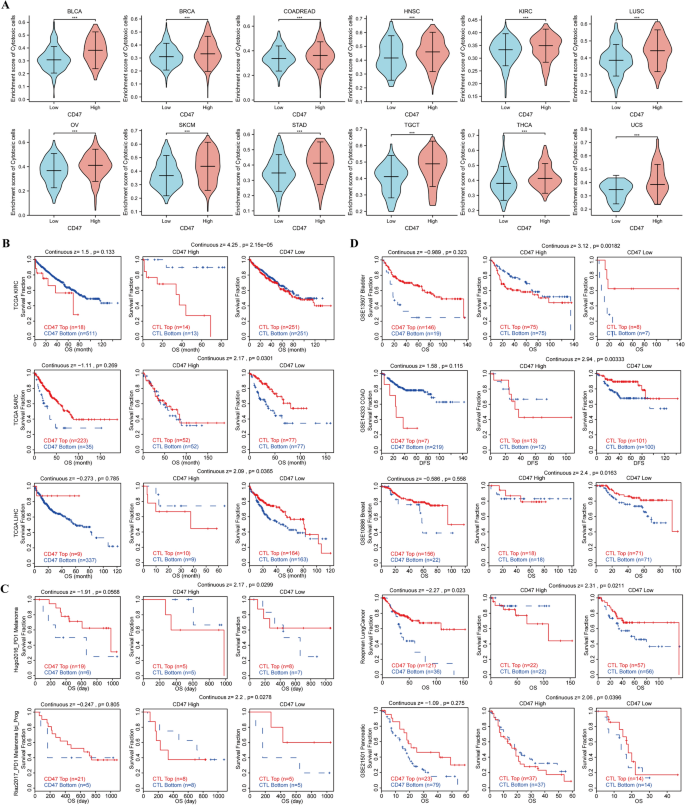
CD47 on CD8 + T cells Infiltration Influenced Tumor Prognosis. A The violin diagram illustrates the disparity in CD47 expression within the infiltration of Cytotoxic CD8 + T-cell. Kaplan–Meier curves (the first picture on the left) of survival ratios as a measure of the B TCGA cohorts C immunotherapeutic response (immune checkpoint blockade) between cancer cohorts D GEO and other cohorts with high and those with low expression levels of CD47. The remainder (the second and third picture on the left) of the graph illustrates the prognosis for the two different sets of CTL expressions with CD47-high group and CD47-low group in above cohorts. Only cancers with statistically significant differences between the cohorts are presented. Significance representation: ns, p ≥ 0.05; *, p < 0.05; **, p < 0.01; ***, p < 0.001
Furthermore, it was observed that the Cytotoxic CD8 + T cell Top group had a poorer prognosis in CD47-high group than the CD47-low group (Fig. 4 b). Especially in these cohorts, high Cytotoxic CD8 + T cell infiltration did not suggest a better prognosis. In the same, this phenomenon was also shown in GSE13507@PRECOG Bladder, GSE10886@PRECOG Breast, Roepman Lung Cancer @PRECOG cohorts, GSE17536 Colorectal OS, E-MTAB-3267-Kidney, GSE31684-Bladder, OV GSE31245@PRECOG, GSE49997 OV, METABRIC BreastLumA, Prostate GSE16560@PRECOG, Gide2019-PD1 + CTLA4 Melanomas (Fig. 4 c, Supplemental Fig. 3c). The same results happened in KIRC, SARC, LIHC in TCGA database (Fig. 4 d). It is well known that T cell dysfunction can negatively impact the prognosis even in the presence of cytotoxic CD8 + T cell, while T cell rejection might negatively impact the prognosis because of the absence of cytotoxic CD8 + T cell infiltration. Consequently, we strongly suggest that CD47 might impair CD8 + T cell function and so negatively impact tumor patients' prognosis.
3.5 CD47 differential genes enrichment in CD8 + Tex pathway.
We categorized the TCGA cohort data into high-expression group and low-expression group, and performed pathway enrichment analysis for both groups of differently expressed genes (p < 0.05). We found that some of the same pathways are present in cancers(Fig. 5 a), which includes PID CD8 TCR Downstream Pathway in BLCA, BRCA, ESAD, ESCA, GBM, KIRP, LIHC, LUSC, OV, PRAD, SKCM, STAD, UCS, UVM; PID CD8 TCR Pathway in BLCA, BRCA, ESCA, GBM, LIHC, LUSC, OV, PRAD, SKCM, STAD, TGCT, UCS, UVM; WP T cell Antigen Receptor TCR Signal Pathway in BLCA, BRCA, ESCA, GBM, KIRP, LIHC, LUSC, OV, PRAD, SKCM, STAD, TGCT, UCS, UVM; BIOCARTA CTLA4 Pathway and WP Cancer Immunotherapy By PD1 Blockade in BLCA, BRCA, ESCA, LIHC, LUSC, OV, PRAD, SKCM, STAD, TGCT, UCS, UVM. Furthermore, CD8 + Tcell exhaustion correlated pathways comprise IL2, IL10, IL12, IL17, INF-gamma, T cell, TCR, and JAK-STAT.
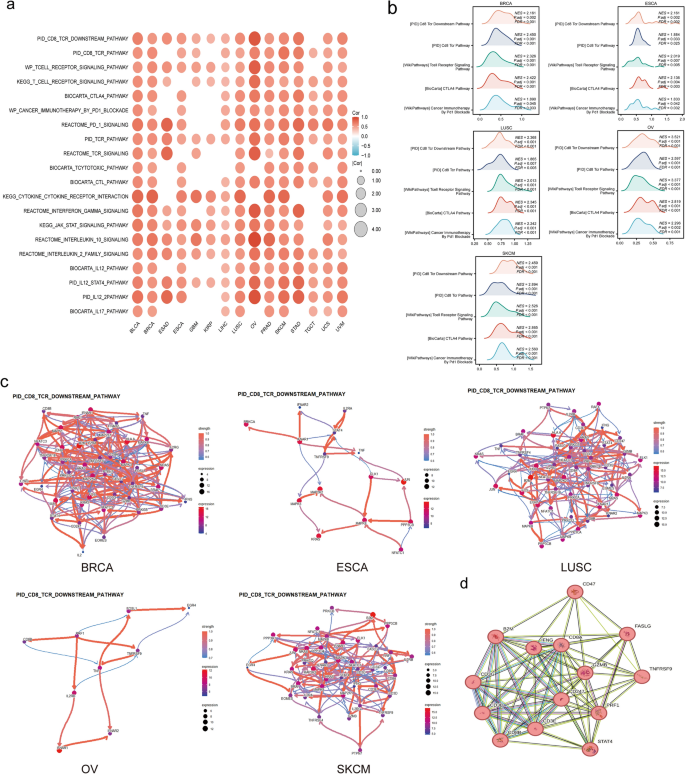
CD47 Differential Genes Enriched in CD8 + Tex Pathway. ( A ) GSEA enrichment analysis results based on CD47 differentially expressed genes in Pan-cancer. ( B ) The mountain map showcased the path enrichment outcomes, encompassing PID CD8 TCR Downstream Pathway, PID CD8 TCR Pathway, WP Tcell Receptor Signal Pathway, BIOCARTA CTLA4 Pathway, and WP Cancer Immunotherapy by PD1 Blockade, along with the gene regulatory network for the PID CD8 TCR Downstream Pathway. ( D ) The PPI network demonstrated the correlation between the CD47 protein and the central protein of PID CD8 TCR Downstream Pathway. The representation of significance is as follows: ns, p ≥ 0.05; *, p < 0.05; **, p < 0.01; ***, p < 0.001
Furthermore, mountain maps presented a visualization of the above first 5 pathways enrichment results (Fig. 5 b), in BRCA, ESCA, LUSC, OV, and SKCM to further demonstrate the distribution of corresponding numbers of differential genes enriched. The figure illustrates that NES (normalized enrichment score) exhibited positivity, with the majority of the differential genes exhibiting enrichment in the high-expression group. We explore further the gene regulatory network of PID CD8 TCR Downstream Pathway (Fig. 5 c, Supplemental Fig. 4a), we discovered that TNFRSF9 and CD8A were expressed at a high level in above all cancers. Taking into account the regulatory networks deduced from the enriched outcomes, we can direct our attention towards a regulatory pathway extending from TNFRSF9 to IL2RA/B/G, via CD8A. Subsequently, we employ PPI(Fig. 5 d) to determine the association between the CD47 protein and the proteins linked to the core-enriched molecules. The findings indicated a direct interaction between CD47 protein and CD8A, TNFRSF9, IFNG, B2M, and GZMB. In light of our observations, we deduced that the activation of the CD47 within the signaling pathway could potentially exert a crucial influence on the regulation of CD8 + T cell functionality—a phenomenon that may potentially collaborate with TNFRSF9.
3.6 CD47 expression is related to the CD8 + Tex in pan-cancer.
We delved deeper into the connection between CD47 and the exhaustion of T-cells. The co-expression heat map showed a relationship between CD47 expression in pan-cancer and T-cell exhausted genes. [ 45 ] CD274, IDO1, CTLA4, ICOS, TIGIT, IL10, TNFRSF9, HAVCR2 exhibited significant co -expression with CD47 in BLCA, BRCA, CESC, COAD, READ, CRC, ESCA, ESCC, GBM, HNSC, LUSC, OSCC, OV, SKCM, STAD, TGCT, THCA, UCS; NFKB1, GRB2, NFATC3, YY1, NFATC2IP, PRDM1, and FOXO1 in other cancers. Additionally, it was demonstrated that TNFRSF9 ranked among the top three genes in the tumors listed below: BRCA, COAD, ESCA, ESAD, ESCC, GBM, LUSC, OV, and DLBC(r > 0.5 but not the top); whereas CD274 held the top three positions in BLCA, BRCA, CESC, COAD, READ, CRC, LAML, LGG, LUAD, OSCC, PCPG, PRAD, READ, SARC, SKCM, STAD, THCA, UCS (Supplemental Table 1). (Fig. 6 a, b, Supplemental 4c).
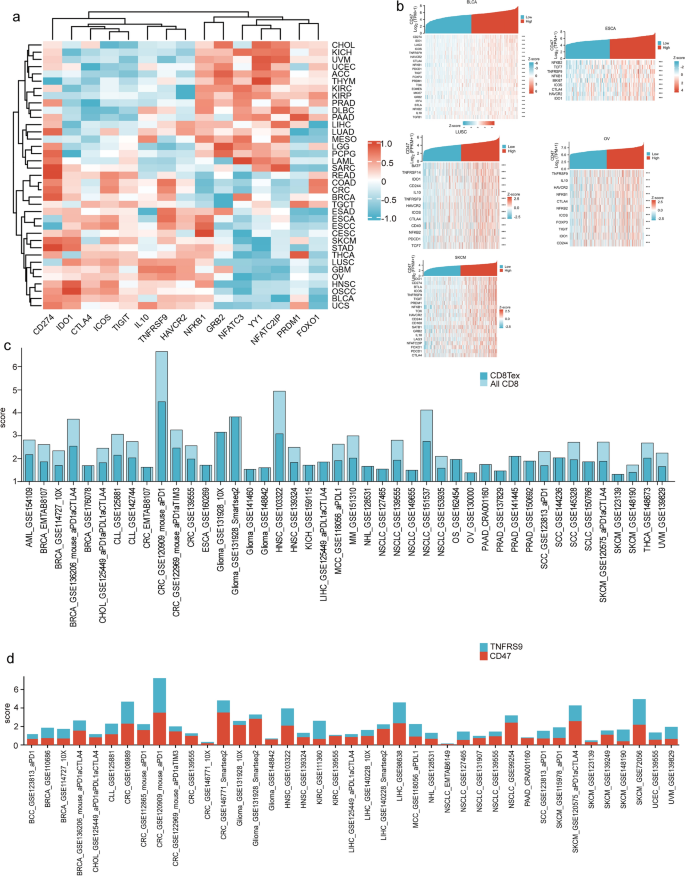
CD47 expression is related to the CD8 + Tex in many cancer types. A , B Co-expression heat map shows the relationship between the expression of CD47 in pan-cancer and the exhausted genes of T cells, especially TNFRSF9, CD274, IDO1, and ICOS. C The bar chart displayed a wide range of CD47 expression in CD8 + Tex and CD8 + T cell from a single-cell database in a pan-cancerous environment. ( D ) The histogram illustrates the manifestation of CD47 and TNFRSF on CD8 + Tex.Significance representation: ns, p ≥ 0.05; *, p < 0.05; **, p < 0.01; ***, p < 0.001
After that, we used the single-cell analysis to evaluate the expression of CD47 in CD8 + Tex and CD8 + T cells from the TISCH database and used the embedded bar chart to show the Data distribution. We found CD47 mainly expressed on CD8 + Tex(> 50%) in AML, BRCA, CHOL, CLL, CRC, ESCA, Glioma, HNSC, KICH, LIHC, MCC, MM, NHL, NSCLC, OS, OV, PRAD, SCC, SCLC, SKCM, THCA, UVM (Fig. 6 c). And we further detect the expression of TNFRSF9 and CD47. It was observed that both of them exhibited expression on CD8 + Tex in BRCA, BCC, CHOL, CLL, CRC, Glioma, KIRC, LIHC, MCC, NHL, NSCLC, PAAD, SKCM, UCEC, and UVM (Fig. 6 d). The findings indicate that the association of CD47 and TNFRSF9 with CD8 + Tex in pan-cancer suggests their involvement in the dysregulation of CD8 + Tex in this type of cancer.
4 Discussion
These findings highlight a strong association between CD47 and the initiation and progression of multiple cancer types. Previous studies have also documented functional associations between CD47 and tumorigenesis, including its role in maintaining immune system homeostasis [ 2 , 46 ]. Furthermore, our investigation focuses on examining the correlation between CD47 expression levels and various parameters such as prognosis [ 48 , 49 ], the tumor microenvironment (TME) [ 50 ], immune escape mechanisms [ 51 ], adaptive immunity [ 47 ], and notably, T-cell exhaustion [ 52 ], within the pan-cancer landscape of TCGA. The substantial heterogeneity and distinctive clinical characteristics observed across different cancer types and subtypes carry significant implications for further research and clinical applications.
In this study, we investigated the oncogenic role of CD47 within TCGA dataset. Our results reveal that CD47 mRNA or protein levels exhibit notable overexpression across a broad spectrum of TCGA cancers, prominently including BRCA, CHOL, COAD, ESCA, HNSC, KIRC, LIHC, PAAD, STAD, THCA, and UCEC. Furthermore, high levels of CD47 were associated with advanced tumor staging or poorer prognosis in ACC, HNSC, KICH, KIRC, LGG, LICH, LUSC, OV, PAAD, RCC, THCA, UCEC, and UVM. These findings corroborate existing clinical and preclinical evidence highlighting elevated CD47 expression in cancer and its correlation with high-risk tumor features [ 45 , 53 , 54 , 55 ]. Our observations suggest CD47 as a potential biomarker for cancer diagnosis, staging, and post-treatment monitoring.
Subsequently, we evaluated the relationship between the expression levels of CD47 and the infiltration of four immunosuppressive cell populations. Specifically, these cell types, including CAFs, Tregs, M2-TAMs, and MDSCs, have been recognized as biomarkers associated with T-cell exclusion within the tumor microenvironment (TME) [ 56 , 57 , 58 , 59 ].The interaction between CAFs and CD47 involves the receptor THBS2/THBS3 on CAFs interacting with CD47 expressed on cancer cells, promoting further cancer progression. Another receptor on the cell membrane of CAFs, MDK, interacts with NCL/SDC2/SDC on cancer cells, potentially serving as therapeutic targets [ 60 ]. Regarding the current understanding of the relationship between regulatory T cells (Tregs) and CD47, research has indicated that the ligand of CD47, SIRPγ, is expressed on T cells and varies with differentiation. Some studies have shown that although SIRPγ is expressed on Tregs, it does not participate in their suppressive function [ 61 ]. Regarding M2-TAMs, reprogramming TAMs into pro-inflammatory M1 macrophages or inhibiting the M2 polarization of macrophages can disrupt the interaction between tumor cells and macrophages, thereby influencing immune function. Furthermore, it has been reported that the CD47/SIRPα axis plays a crucial role in mediating the interaction involving MDSCs in this context [ 62 ]. Inhibition of CXCR2 in G-MDSCs augments the efficacy of CD47 blockade in promoting melanoma tumor cell clearance [ 63 ]. Interestingly, we observed a robust correlation between the expression levels of immunosuppressive cells and CD47 in BRCA-LumA, LICH, LUAD, PRAD, and THYM. (≥ 2 immunosuppressive cell types, every cell type ≥ 2 calculated method positive, r > 0.2 and p < 0.05). Therefore, we hypothesize that one of the primary mechanisms through which CD47 regulates tumor immune evasion, tumor progression, and metastasis is through T-cell rejection.
Consequently, our investigation into the association between CD47 and T-cell infiltration utilized seven distinct algorithms (QUANTISEQ, XCELL, CIBERSORT, CIBERSORT-ABS, EPIC, MCPCOUNTER, TIMER, including ≥ 2 immune types, every cell type ≥ 2 calculated method positive, r > 0.2, p < 0.05). These findings demonstrated significant positive correlations in BLCA, BRCA, BRCA-Basal, COAD, DLBC, ESCA, KIRC, KIRP, LIHC, LUSC, PAAD, PRAD, READ, SKCM, SKCM-Metastasis, STAD, TGCT, THCA, UVM. CD47 blockade has been implemented in various models and clinical trials to enhance phagocytosis, augment T cell infiltration into tumors, and reduce tumor burden both in vitro and in vivo [ 64 , 65 ]. For example, the co-delivery nanocarrier aCD47-DMSN was designed by encapsulating DOX within the mesoporous cavity of MSN and adsorbing aCD47 onto the MSN surface. Following intravenous administration, aCD47-DMSN exhibited robust antitumor efficacy by enhancing the infiltration of CD8 + T cells into the tumor. These results offer another hypothesis that CD47 plays a role in activating dysfunctional T-cell phenotypes to regulate immune escape mechanisms and patient outcomes [ 66 , 67 , 68 ].
While the prognostic implications of CD47-mediated CD8 + T cell activity are yet to be fully elucidated, our focus was on examining the relationship between CD47 expression and levels of infiltrating cytotoxic CD8 + T cells. Analysis of the TCGA database revealed a notable increase in cytotoxic CD8 + T cell infiltration in BLCA, BRCA, CESC, COAD, COADREAD, ESAD, ESCA, GBM, HNSC, KIRC, LUSC, OV, SKCM, STAD, TGCT, THCA, and UCS when comparing the CD47 high and low expression groups. Subsequently, we investigated the potential impact of these findings on treatment response and patient outcomes. Interestingly, no significant differences in prognosis were observed based on CD47 expression status alone. Thus, we further assessed the prognostic implications of cytotoxic CD8 + T cell infiltration in both high and low CD47 expression groups. Notably, in cases where CD47 expression was high, we also explored the prognostic significance of cytotoxic CD8 + T cell infiltration in both the CD47-high and CD47-low subgroups across BLCA, BRCA, COAD, KIRC, LIHC, LUNG CANCER (Adeno, Large, Squamous), OV, PAAD, PRAD, ARC, SKCM, TCGA, PRECOG, and the METABIC cohort. It is widely recognized that the efficacy of immunotherapy is closely associated with the infiltration of cytotoxic CD8 + T cells [ 66 , 67 , 68 ] (Supplemental Fig. 3a), and good Cytotoxic CD8 + T cell infiltration usually represents a better prognosis [ 69 ]. CD47 has been shown to impede the recruitment and activation of effector T cells, leading to intratumoral immunosuppressive effects. By blocking CD47, the suppression of cytotoxic CD8 + T cell function is alleviated, thereby sustaining the anti-tumor response. This blockade operates indirectly by thwarting immunosuppressive signals expressed in antigen-presenting cells or by shielding tumor-infiltrating cytotoxic CD8 + T cells from the local tumor microenvironment's irradiation [ 20 ]. Hence, the expression level of CD47 was associated with dysfunctional T-cells in those cohorts.
Then, utilizing TCGA data, we stratified these tumors into high and low-CD47 expression groups and identified differentially expressed genes between the two cohorts based on median CD47 expression levels. These genes were subjected to pathway enrichment analysis to uncover the correlation between high CD47 expression and immunomodulatory effects. High CD47 expression in BLCA, BRCA, ESAD, ESCA, GBM, KIRP, LIHC, LUSC, OV, PRAD, SKCM, STAD, UCS, and UVM was associated with pathways involving interactions among CD8 + T cells, cytokines, and key immunomodulators such as PD-1, CTLA4, IL-10, INF gamma, and cytotoxic CD8 + T cells. These findings suggest that CD47 may play a crucial role in modulating adaptive immunity, particularly in the context of CD8 + T cell exhaustion. Notably, PD-1, a well-established checkpoint pathway present in both immune and tumor cells, was among the pathways implicated in this immunomodulatory network [ 70 ]. The blockade of PD-1 signaling influences the TME [ 71 ] by initiating an immune response within tumor cells. Pathways induced by PD-1 blockade in cancer immunotherapy have been closely linked to the upregulation of CD47 expression, indicating that elevated CD47 levels post PD-1 inhibitor treatment lead to a reduction in CD8 + T cells, ultimately contributing to drug resistance. Targeting these factors represents a promising adjunctive strategy for patients undergoing anti-programmed death-1 receptor (PD-1)/anti-programmed death-ligand 1 (PD-L1) therapy. Additionally, IL-10 has been demonstrated to contribute to T cell dysfunction. CD8 + T cells play a pivotal role in immune response, metastasis, and tumorigenesis [ 72 ]. A notable association was observed between IL-2 levels and the prognosis of patients diagnosed with pan-cancer [ 73 , 74 ]. However, our analysis did not reveal a significant correlation with T-dysfunction-related pathways in other cancer types. This discrepancy could potentially be attributed to limited data availability or other factors, such as the absence of a key regulatory gene like LAYN that modulates T cell function, or variations in the TME [ 75 ].
To enhance comprehension of the regulatory network, we focused on the enriched pathway-PID CD8 TCR DOWNSTREAM PATHWAY. Utilizing Bayesian network inference through CBNplot, we identified heightened expression of TNFRSF9 and established a regulatory pathway connecting CD8A, IL2RA/B/G to TNFRSF9 in BRCA, ESCA, LUSC, OV, and SKCM. This led to the inference that CD47 might modulate the CD8 TCR DOWNSTREAM pathway, inducing CD8 + T cell exhaustion, potentially in conjunction with TNFRSF9. Subsequently, employing protein–protein interaction (PPI) analysis (Fig. 5 d), we elucidated a direct interaction between the CD47 protein and the proteins associated with the core-enriched molecules. Furthermore, investigation into the CD47 ligand SIRPA and the PPI network of the pathway core genes revealed its association with IL2, IL2RA, CD8A, B2M, IFNG, and GZMB, thereby substantiating our deduction. (Supplemental Fig. 4b) (all combined score > 0.3).
Additionally, we conducted an analysis of the expression profile and co-expression patterns of the T-cell depletion gene set [ 43 ] CD47 across pan-cancer cohorts. Notably, CD274, IDO1, CTLA4, ICOS, TIGIT, IL10, TNFRSF9, and HAVCR2 demonstrated significant co-expression with CD47 in Group 1 (BLCA, BRCA, CESC, COAD, READ, CRC, ESCA, ESCC, GBM, HNSC, LUSC, OSCC, OV, SKCM, STAD, TGCT, THCA, UCS), while NFKB1, GRB2, NFATC3, YY1, NFATC2IP, PRDM1, and FOXO1 exhibited co-expression in other cancer types designated as Group 2. Noteworthy findings included TNFRSF9 ranking among the top three co-expressed genes in BRCA, COAD, ESCA, ESAD, ESCC, GBM, LUSC, OV, and DLBC (with correlation coefficients exceeding 0.5 but not ranking first), and CD274 ranking among the top three in BLCA, BRCA, CESC, COAD, READ, CRC, LAML, LGG, LUAD, OSCC, PCPG, PRAD, READ, SARC, SKCM, STAD, and THCA. These observations suggest potential functional partnerships between these genes and CD47 across various cancer types, a phenomenon also corroborated by previous studies in select cancer types [ 24 , 76 , 77 ].
The TME is widely known for its substantial heterogeneity [ 78 ]. Utilizing the TISCH single-cell database, we explored the impact of CD47 on the TME. Notable variations in immune cell profiles were observed across primary and metastatic tumor sites. Specifically, our analysis revealed that CD47 was prominently expressed on CD8 + exhausted T cells (CD8 + Tex), exhibiting significantly higher expression levels compared to other CD8 + T cell subsets in AML, BRCA, CHOL, CLL, CRC, ESCA, Glioma, HNSC, KICH, LIHC, MCC, MM, NHL, NSCLC, OS, OV, PRAD, SCC, SCLC, SKCM, THCA, and UVM (Fig. 6 c). Furthermore, in BRCA, CHOL, CLL, CRC (COADREAD), GBM, HNSC, KIRC, LIHC, MCC, NHL, NSCLC, PAAD, SCC, SKCM, UCEC, and UVM, both CD47 and TNFRSF9 were co-expressed on CD8 + exhausted T cells. These findings suggest a potential functional partnership between CD47 and TNFRSF9 in regulating CD8 + exhausted T cells, particularly in BRCA, ESCA, LUSC, OV, and SKCM (Supplemental Table 2).
Currently, extensive research efforts are focused on the development of dual-targeting strategies involving CD47, with notable progress in the development of CD47 and TNFRSF9 targeting fusion protein (DSP107) [ 24 , 79 ], PD1 and CD47 bispecific fusion molecules, bispecific antibody CD47xPD-L1, CD47 x HER2, CD47xICAM-1, and other approaches [ 80 , 81 , 82 , 83 , 84 ]. These novel strategies not only aim to mitigate the hematologic toxicity and adverse effects associated with CD47 monoclonal antibodies but also seek to enhance the anti-tumor efficacy of CD47 blockade. By elucidating the cell-intrinsic mechanisms regulated by CD47, we gain insights into its role in promoting tumor biology. Collectively, these advancements position CD47 as a promising therapeutic target in the treatment of both solid and non-solid tumors.
5 Limitation
In our study, we encountered negative and opposite findings in certain types of cancers. The majority of the studies utilized public databases available on the internet, which may have contained slightly outdated data. Future research should focus on utilizing real-world cohorts and conducting multicenter data analysis to enhance the robustness and relevance of the findings. Additionally, the small sample size and dearth of reliable data contributed to limitations in the generalizability of the results. Moreover, methodological deficiencies, such as constraints in the technology and instruments used for data collection, further weakened the study's overall findings. The high expression levels of CD47 and TNFRSF9 in cancer cells and their correlation can be demonstrated through multiple immunofluorescence staining. Additionally, utilizing WB-PCR, it can be shown that CD47 wild-type cancer cells express a higher amount of T cell depletion factors compared to CD47 knockout cancer cells. Through T cell co-culture experiments, it can be established that CD47 knockout cancer cells exhibit reduced T cell depletion compared to wild-type cancer cells. The analysis revealed a poor prognosis in the low CD47 expression group in SKCM. Moreover, the expression of CD47 in adjacent tissues was observed to be higher than in cancer tissues, as evidenced in KICH and LUSC. These findings suggest potential factors contributing to these observations, including intrinsic tumor heterogeneity, the tumor immune microenvironment, specifically telomere Tex cell richness [ 85 , 86 ], or data insufficiency, warranting further experimental validation and investigation.
6 Conclusions
CD47 plays a pivotal role in the TME, prognosis, and immunotherapy not only through its interactions with macrophages but also with tumor-infiltrating T-lymphocyte cells, particularly CD8 + T cells across various cancer types. The interaction between CD47 and TNFRSF9 triggers exhaustion in CD8 + T cells, leading to an unfavorable prognosis. A therapeutic approach targeting both CD47 and TNFRSF9 has the potential to activate both innate and adaptive immune responses, presenting a significant advancement in treatment modalities, especially for patients with BRCA, ESCA, LUSC, OV, and SKCM.
Data availability
The datasets provided in this study can be found in online repositories.
Abbreviations
TNFRSF9 (tumor necrosis factor receptor superfamily member 9), CD137, ILA
Adrenocortical carcinoma
Acute lymphoblastic leukemia
Area under curve
Basal cell carcinoma
A subset of cancer pathway( icluding 292 gene sets) https://maayanlab.cloud/Harmonizome/dataset/Biocarta
Bladder urothelial carcinoma
Breast invasive carcinoma
LumA positive pathological type of breast invasive carcinoma
SRP114962 cohort of non-small cell lung cancer
Cancer associated fibroblasts
Ayesian network plots for enrichment analysis
PD-L1 (Programmed death-ligand 1)
Cervical squamous cell carcinoma and endocervical adenocarcinoma
Cholangiocarcinoma
Chronic lymphocytic leukemia
Colon adenocarcinoma
Cancer pathway
Colerectal cancer
Cytotoxic T lymphocytes
Cytotoxic T-lymphocyte-associated protein 4
Differential Expression Analysis
Lymphoid neoplasm diffuse large B-cell lymphoma
A Novel Bi-Functional Fusion Protein That Combines Inhibition of CD47 with Targeted Activation of 4-1BB
Disease-Specific Survival
Esophageal adenocarcinoma
Esophageal carcinoma
Esophageal cell squamous carcinoma
Fc gamma receptor IIIa
False discovery rate
Fragments Per Kilobase of exon model per Million mapped fragments
Glioblastoma multiforme
Gene Expression Omnibus ( https://www.ncbi.nlm.nih.gov/geo/ )
Growth factor receptor bound protein 2
Genotype-Tissue Expression Project ( https://commonfund.nih.gov/GTEx )
Hepatitis A virus cellular receptor 2
Human epidermal growth factor receptor 2
High-throughput sequence analysis ( https://pypi.org/project/HTSeq/ )
Intercellular Cell Adhesion Molecule-1
Immune checkpoint blockade
CD278 (Inducible Co-Stimulator)
Indoleamine 2,3-dioxygenase 1
Interferon γ
Interleukin
Janus kinase
Kyoto Encyclopedia of Genes and Genomes ( https://www.genome.jp/kegg/ )
Kidney chromophobe
Kidney renal clear cell carcinoma
Kidney renal papillary cell carcinoma
Acute myeloid leukemia
Brain lower grade glioma
Liver hepatocellular carcinoma
Lung adenocarcinoma
Lung squamous cell carcinoma
M2-tumor-associated macrophages
Monoclonal antibody
Merkel cell carcinoma
Myeloid-derived suppressor cells
Mesothelioma
Molecular Taxonomy of Breast Cancer International Consortium ( https://www.mercuriolab.umassmed.edu/metabric )
Main histocompatibility complex
Microsatellite instability
Molecular Signatures Database ( https://www.gsea-msigdb.org/gsea/msigdb/ )
Nuclear factor of activated T cells 2 interacting protein
Nuclear factor of activated T cells 3
Nuclear factor κB
Non-Hodgkin lymphoma
No significance
Non-small cell lung cancer
EMTAB6149 cohort of non-small cell lung cancer
Overall survival
Oral squamous cell carcinoma
Ovarian serous cystadenocarcinoma
Pancreatic adenocarcinoma
Pheochromocytoma and paraganglioma
Programmed cell death protein 1
Progress Free Interval
A subset of cancer pathway( icluding 196 gene sets)
Protein–Protein Interaction Networks
Prostate adenocarcinoma
PR domain zinc finger protein 1
PREsentation and Characterization Of Growth-data ( https://precog.stanford.edu/ )
Phosphatase and tensin homolog
A subset of cancer pathway (browse 1615 gene sets) https://reactome.org/
Pectum adenocarcinoma
Squamous cell carcinoma
System of Environmental Economic Accounting
Signal regulatory protein α
Skin cutaneous melanoma
Stomach adenocarcinoma
Signal transducer and activator of transcription
A database of functional protein association networks
The Cancer Genome Atlas
Central memory T cell
T cell receptor
Effector memory T cell
Exhausted T cell
Follicular helper T cell
Testicular germ cell tumors
Gamma delta T cell
T helper cells
Thyroid carcinoma
Tumor immune dysfunction and exclusion
Translocation induced circling mutation
Tumor immune microenvironment
Tumor immune estimation resource
Tumor Immune Single-cell Hub
Toll-like receptors
Tumor mutational burden
Tumor microenvironment
Tumor necrosis factor
Transcripts per million reads
Regulatory T cell
Uterine corpus endometrial carcinoma
Uterine carcinosarcoma
Uveal melanoma
A subset of cancer pathway (icluding 664 gene sets)
Xena.ucsc.edu
Tsai RK, Discher DE. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol. 2008;180:989–1003.
Article CAS PubMed PubMed Central Google Scholar
Li D, et al. SLAMF3 and SLAMF4 are immune checkpoints that constrain macrophage phagocytosis of hematopoietic tumors. Sci Immunol. 2022;7:eabj5501.
CAS PubMed Google Scholar
Okazawa H, et al. Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J Immunol. 2005;174:2004–11.
Article CAS PubMed Google Scholar
Yang H, et al. Engineering macrophages to phagocytose cancer cells by blocking the CD47/SIRPɑ axis. Cancer Med. 2019;8:4245–53.
Article PubMed PubMed Central Google Scholar
Andrechak JC, Dooling LJ, Discher DE. The macrophage checkpoint CD47: SIRPα for recognition of “self” cells: from clinical trials of blocking antibodies to mechanobiological fundamentals. Philos Trans R Soc Lond B Biol Sci. 2019;374:20180217.
Bouwstra R, et al. CD47 expression defines efficacy of rituximab with CHOP in non-germinal center B-cell (Non-GCB) diffuse large b-cell lymphoma patients (DLBCL), but not in GCB DLBCL. Cancer Immunol Res. 2019;7:1663–71.
Uger R, Johnson L. Blockade of the CD47-SIRPα axis: a promising approach for cancer immunotherapy. Expert Opin Biol Ther. 2020;20:5–8.
Li Y, et al. Overexpression of CD47 predicts poor prognosis and promotes cancer cell invasion in high-grade serous ovarian carcinoma. Am J Transl Res. 2017;9:2901–10.
CAS PubMed PubMed Central Google Scholar
Feng R, Zhao H, Xu J, Shen C. CD47: the next checkpoint target for cancer immunotherapy. Crit Rev Oncol Hematol. 2020;152: 103014.
Article PubMed Google Scholar
Petrova PS, et al. TTI-621 (SIRPα Fc): a CD47-blocking innate immune checkpoint inhibitor with broad antitumor activity and minimal erythrocyte binding. Clin Cancer Res. 2017;23:1068–79.
Puro RJ, et al. Development of AO-176, a next-generation humanized anti-CD47 antibody with novel anticancer properties and negligible red blood cell binding. Mol Cancer Ther. 2020;19:835–46.
van Duijn A, Van der Burg SH, Scheeren FA. CD47/SIRPα axis: bridging innate and adaptive immunity. J Immunother Cancer. 2022;10:e004589.
Cham LB, et al. Immunotherapeutic blockade of CD47 inhibitory signaling enhances innate and adaptive immune responses to viral infection. Cell Rep. 2020;31: 107494.
Strizova Z, et al. Tumoral and peritumoral NK cells and CD8(+) T cells of esophageal carcinoma patients express high levels of CD47. Sci Rep. 2020;10:13936.
Barkal AA, et al. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat Immunol. 2018;19:76–84.
van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer. 2016;16:219–33.
Dheilly E, et al. Tumor-directed blockade of CD47 with bispecific antibodies induces adaptive antitumor immunity. Antibodies. 2018;7:3.
Tseng D, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci USA. 2013;110:11103–8.
Liu X, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21:1209–15.
Soto-Pantoja DR, et al. CD47 in the tumor microenvironment limits cooperation between antitumor T-cell immunity and radiotherapy. Can Res. 2014;74:6771–83.
Article CAS Google Scholar
Hong W, Xue M, Jiang J, Zhang Y, Gao X. Circular RNA circ-CPA4/ let-7 miRNA/PD-L1 axis regulates cell growth, stemness, drug resistance and immune evasion in non-small cell lung cancer (NSCLC). J Exp Clin Cancer Res. 2020;39:149.
Anand P, et al. Single-cell RNA-seq reveals developmental plasticity with coexisting oncogenic states and immune evasion programs in ETP-ALL. Blood. 2021;137:2463–80.
Chester C, Sanmamed MF, Wang J, Melero I. Immunotherapy targeting 4–1BB: mechanistic rationale, clinical results, and future strategies. Blood. 2018;131:49–57.
Bagheri S, Safaie Qamsari E, Yousefi M, Riazi-Rad F, Sharifzadeh Z. Targeting the 4–1BB costimulatory molecule through single chain antibodies promotes the human T-cell response. Cell Mol Biol Lett. 2020;25:28.
Chu DT, et al. An update on anti-CD137 antibodies in immunotherapies for cancer. Int J Mol Sci. 2019;20:1822.
Cendrowicz E, et al. DSP107 combines inhibition of CD47/SIRPα axis with activation of 4–1BB to trigger anti-cancer immunity. J Exp Clin Cancer Res CR. 2022;41:97.
Vivian J, et al. Toil enables reproducible, open source, extensive biomedical data analyses. Nat Biotechnol. 2017;35:314–6.
Chandrashekar DS, et al. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–58.
Li T et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res 48, W509-w514 (2020).
Fu J, et al. Large-scale public data reuse to model immunotherapy response and resistance. Genome Med. 2020;12:21.
Jiang P, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24:1550–8.
Szklarczyk et al. Nucleic acids research 47.D1 (2018): D607-D613.2
Sun D, et al. TISCH: a comprehensive web resource enabling interactive single-cell transcriptome visualization of tumor microenvironment. Nucleic Acids Res. 2021;49:D1420-d1430.
Athar A, et al. ArrayExpress update—from bulk to single-cell expression data. Nucleic Acids Res. 2019;47:D711-d715.
Barrett T, et al. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res. 2013;41:D991-995.
Liu J, et al. An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell. 2018;173:400-416.e411.
Hanzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinf. 2013;14:7.
Article Google Scholar
Bindea G, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–95.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–7.
Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Guo NL, Wan YW. Network-based identification of biomarkers co-expressed with multiple pathways. Cancer Inform. 2014;13:37–47.
PubMed PubMed Central Google Scholar
Sato N, Tamada Y, Yu G, Okuno Y. CBNplot : Bayesian network plots for enrichment analysis. Bioinformatics. 202 https://doi.org/10.1093/bioinformatics/btac175 .
Yu L, et al. Significance of CD47 and its association with tumor immune microenvironment heterogeneity in ovarian cancer. Front Immunol. 2021;12: 768115.
Hayat SMG, et al. CD47: role in the immune system and application to cancer therapy. Cell Oncol. 2020;43:19–30.
Chen SH, et al. Dual checkpoint blockade of CD47 and PD-L1 using an affinity-tuned bispecific antibody maximizes antitumor immunity. J Immunother Cancer. 2021;9:e003464.
Zhang W, et al. Advances in anti-tumor treatments targeting the CD47/SIRPα axis. Front Immunol. 2020;11:18.
Jiang Y, et al. Noninvasive imaging evaluation of tumor immune microenvironment to predict outcomes in gastric cancer. Ann Oncol. 2020;31:760–8.
Pan Y, et al. Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer. J Hematol Oncol. 2019;12:124.
Shimizu A, et al. Exosomal CD47 plays an essential role in immune evasion in ovarian cancer. Mol Cancer Res. 2021;19:1583–95.
Jiang TT, et al. Clinical response to anti-CD47 immunotherapy is associated with rapid reduction of exhausted bystander CD4(+) BTLA (+) T cells in tumor microenvironment of mycosis fungoides. Cancers. 2021;13:5982.
Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15:81–94.
Yang Y, Yang Z, Yang Y. Potential Role of CD47-Directed Bispecific Antibodies in Cancer Immunotherapy. Front Immunol. 2021;12: 686031.
Yu WB, Ye ZH, Chen X, Shi JJ, Lu JJ. The development of small-molecule inhibitors targeting CD47. Drug Discov Today. 2021;26:561–8.
Hanley CJ, Thomas GJ. T-cell tumor exclusion and immunotherapy resistance: a role for CAF targeting. Br J Cancer. 2020;123:1353–5.
Verzella D, et al. GADD45β loss ablates innate immunosuppression in cancer. Can Res. 2018;78:1275–92.
Vonderheide RH, Bear AS. Tumor-derived myeloid cell chemoattractants and T cell exclusion in pancreatic cancer. Front Immunol. 2020;11: 605619.
Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anti-cancer therapy. Nat Rev Clin Oncol. 2019;16:356–71.
Logtenberg MEW, et al. Glutaminyl cyclase is an enzymatic modifier of the CD47- SIRPα axis and a target for cancer immunotherapy. Nat Med. 2019;25:612–9.
Carvalho RF, do Canto LM, Abildgaard C, et al. Single-cell and bulk RNA sequencing reveal ligands and receptors associated with worse overall survival in serous ovarian cancer. Cell Commun Signal. 2022;20(1):176. https://doi.org/10.1186/s12964-022-00991-4 .
Dehmani S, Nerrière-Daguin V, Néel M, et al. SIRPγ-CD47 interaction positively regulates the activation of human T cells in situation of chronic stimulation. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.732530 .
Zhou Y, Qian M, Li J, et al. The role of tumor-associated macrophages in lung cancer: from mechanism to small molecule therapy. Biomed Pharmacother. 2024;170: 116014. https://doi.org/10.1016/j.biopha.2023.116014 .
Banuelos A, Zhang A, Berouti H, et al. CXCR2 inhibition in G-MDSCs enhances CD47 blockade for melanoma tumor cell clearance. Proc Natl Acad Sci USA. 2024;121(5): e2318534121. https://doi.org/10.1073/pnas.2318534121 .
Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-hodgkin lymphoma. Cell. 2010;142:699–713. https://doi.org/10.1016/j.cell.2010.07.044 .
Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21:1209–15. https://doi.org/10.1038/nm.3931 .
Papalampros A, et al. Unique spatial immune profiling in pancreatic ductal adenocarcinoma with enrichment of exhausted and senescent t cells and diffused CD47-SIRPα expression. Cancers. 2020;12:1825.
Borst J, Ahrends T, Bąbała N, Melief CJM, Kastenmüller W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18:635–47.
Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol. 2019;234:8509–21.
Yu Y, et al. Association of long noncoding RNA biomarkers with clinical immune subtype and prediction of immunotherapy response in patients with cancer. JAMA Netw Open. 2020;3: e202149.
Xie F, Xu M, Lu J, Mao L, Wang S. The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol Cancer. 2019;18:146.
Luchini C, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol. 2019;30:1232–43.
Yu AI, et al. Gut microbiota modulate CD8 T Cell responses to influence colitis-associated tumorigenesis. Cell Rep. 2020;31: 107471.
Propper DJ, Balkwill FR. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol. 2022;19:237–53.
Lan T, Chen L, Wei X. Inflammatory cytokines in cancer: comprehensive understanding and clinical progress in gene therapy. Cells. 2021;10:100.
Pan JH, et al. LAYN Is a prognostic biomarker and correlated with immune infiltrates in gastric and colon cancers. Front Immunol. 2019;10:6.
Hsieh RC, et al. ATR-mediated CD47 and PD-L1 up-regulation restricts radiotherapy-induced immune priming and abscopal responses in colorectal cancer. Science Immunol. 2022;7:eabl9330.
Lian S, et al. Simultaneous blocking of CD47 and PD-L1 increases innate and adaptive cancer immune responses and cytokine release. EBio Med. 2019;42:281–95.
Google Scholar
Biffi G, Tuveson DA. Diversity, and biology of cancer-associated fibroblasts. Physiol Rev. 2021;101:147–76.
A. Saeed et al. Phase 1 dose escalation study of DSP107, a first - in - class CD47 and 4–1BB targeting fusion protein, in combination with atezolizumab in patients with advanced solid tumors. The 2023 ASCO (American Society of Clinical Oncology) Annual Meeting, Chicago, American, June 2–6, 2023; 2632.
H.P. Rui et al. D3L-001, a novel bispecific antibody targeting HER2 and CD47, demonstrates potent preclinical efficacy in solid tumors. AACR (American Association for cancer research) Annual Meeting 2023, Orlando, Florida, April 14–19, 2023; 1873.
X. Chauchet et al. NI-2901, an affinity-optimized CD47xPD-L1 bispecific antibody for dual immune checkpoint blockade. AACR (American Association for cancer research) Annual Meeting 2023, Orlando, Florida, April 14–19, 2023; 2951.
S.M. Liu et al. A novel pegylated bispecific antibody-drug conjugate (P-BsADCpbadc) targeting cancers co-expressing PD-L1 and CD47. AACR (American Association for cancer research) Annual Meeting 2023, Orlando, Florida, April 14–19, 2023; 6307.
M. Ma et al. BSI-508, a novel bispecific fusion molecule targeting PD1 and CD47 for cancer immunotherapy. AACR (American Association for cancer research) Annual Meeting 2023, Orlando, Florida, April 14–19, 2023; 2958.
O.K. Wang et al. VBI-002, a CD47xICAM-1 bispecific antibody for the treatment of hepatocellular carcinoma, melanoma and non-small cell lung cancers. AACR (American Association for cancer research) Annual Meeting 2023, Orlando, Florida, April 14–19, 2023; 6334.
Zheng L, et al. Pan-cancer single-cell landscape of tumor-infiltrating T cells. Science. 2021;374:abe6474.
Zhang Z, et al. Pan-cancer landscape of T-cell exhaustion heterogeneity within the tumor microenvironment revealed a progressive roadmap of hierarchical dysfunction associated with prognosis and therapeutic efficacy. EBio Med. 2022;83: 104207.
CAS Google Scholar
Download references
Acknowledgements
We extend our gratitude to the public databases utilized in our investigation, whose platform facilitated and assisted in the uploading of their valuable datasets.
This work was supported by [Science and Technology Program of Guangzhou, China] (Grant numbers [202201011052]).
Author information
Authors and affiliations.
Guangdong Provincial People’s Hospital, Guangdong Cardiovascular Institute, Guangdong Academy of Medical Sciences, Guangzhou, 510100, China
Hongxin Liang & Haiyu Zhou
Department of Anesthesiology, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, 510080, China
The First School of Clinical Medicine, Guangdong Medical University, Zhanjiang, 524023, China
Zekai Huang
Department of Thoracic Surgery, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, 510080, China
Zekai Huang, Jinchi Dai, Daipeng Xie, Hongrui Qiu, Huili Wang, Hao Li, Jinhang Leng, Dongkun Zhang & Haiyu Zhou
Southern Medical University, Guangzhou, 510515, China
Lintong Yao & Ziming Tang
Department of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory Medicine and Beijing Chao-Yang Hospital, Capital Medical University, Beijing, 100020, China
You can also search for this author in PubMed Google Scholar
Contributions
HX Liang, Yong Zheng, and ZK Huang were major contributors who conceived the main idea. JC Dai, LT Yao and DP Xie helped design the study; Duo Chen and Hao Li helped with data collection. HL Wang and HR Qiu helped adjust the image format; JH Leng and ZM Tang helped write the manuscript; All authors approved the submitted version.
Corresponding authors
Correspondence to Dongkun Zhang or Haiyu Zhou .
Ethics declarations
Ethics approval and consent to participate.
Approval was granted by the Ethics Committee at Guangdong Provincial People’s Hospital. (No KY2023-137-01) The research conducted here adhered to the principles outlined in the Declaration of Helsinki.
Competing interests
All of the authors state that there are no conflicts of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1 (zip 95 kb), additional file2 (tif 10061 kb), additional file3 (tif 131045 kb), additional file4 (tif 3808 kb), additional file5 (tif 4244 kb), additional file6 (tif 27286 kb), additional file7 (tif 13340 kb), additional file8 (tif 14377 kb), rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Liang, H., Zheng, Y., Huang, Z. et al. Pan-cancer analysis for the prognostic and immunological role of CD47: interact with TNFRSF9 inducing CD8 + T cell exhaustion. Discov Onc 15 , 149 (2024). https://doi.org/10.1007/s12672-024-00951-z
Download citation
Received : 13 December 2023
Accepted : 27 March 2024
Published : 08 May 2024
DOI : https://doi.org/10.1007/s12672-024-00951-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- CD8 + T cells
- T-cell exhausted
Advertisement
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 07 May 2024
Understanding caregiver burden and quality of life in Kerala’s primary palliative care program: a mixed methods study from caregivers and providers’ perspectives
- Arsha Kochuvilayil 1 &
- Ravi Prasad Varma 1
International Journal for Equity in Health volume 23 , Article number: 92 ( 2024 ) Cite this article
Metrics details
Family caregivers are vital for long-term care for persons with serious health-related suffering in Kerala. Long-term caregiving and ageing may become burdensome and detrimental to patients and caregivers. We compared the caregiver burden and quality-of-life of ageing caregivers with younger caregivers. We also explored the palliative care nurses’ perceptions of the family caregivers’ issues.
We did a mixed method study focusing on two groups: (i) three in-depth interviews and a cross-sectional survey among 221 caregivers of palliative care patients in five randomly selected panchayats (most peripheral tier of three-tier local self-government system in India concerned with governance of a village or small town) of Kollam district, Kerala, as part of development and validation of the Achutha Menon Centre Caregiver Burden Inventory; (ii) five in-depth interviews with purposively selected primary palliative care nurses as part of a study on local governments and palliative care. We used a structured interview schedule to collect cross-sectional data on sociodemographic and caregiving-related characteristics, caregiver burden, and health-related quality of life using the EuroQol EQ5D5L and interview guidelines on caregiver issues tailored based on participant type for qualitative interviews.
Older caregivers comprised 28.1% of the sample and had significantly poorer health and quality-of-life attributes. More senior caregivers experiencing caregiver burden had the lowest mean scores of 0.877 (Standard deviation (SD 0.066, 95% confidence intervals (CI) 0.854–0.899) followed by younger caregivers with high burden (0.926, SD 0.090, 95% CI 0.907–0.945), older caregivers with low burden (0.935, SD 0.058, 95% CI 0.912–0.958) and younger caregivers with low burden (0.980, SD 0.041, 95% CI 0.970–0.990). Caregivers faced physical, psychological, social, and financial issues, leading to a caregiver burden. The relationships between the palliative care nurses and family caregivers were complex, and nurses perceived caregiver burden, but there were no specific interventions to address this.
In our study from Kollam, Kerala, three out of ten caregivers of palliative care patients were 60 years of age or older. They had significantly lower health-related quality of life, particularly if they perceived caregiver burden. Despite being recognized by palliative care nurses, caregiver issues were not systematically addressed. Further research and suitable interventions must be developed to target such problems in the palliative care programme in Kerala.
Norman Daniels “We should not allow misfortune to beget injustice” [ 1 ].
In Low- or Middle-Income Countries (LMIC), when a person becomes bedridden or homebound due to chronic illness or injury, family members are likely to be tasked with caring for a dependent [ 2 ]. State involvement still needs to improve in such situations, but the local government (LG) driven primary palliative care programme in Kerala state, India, has been functioning for nearly 30 years as a well-acknowledged approach for community-based sustainable palliative care [ 3 , 4 , 5 ]. Governance in India comprises powers that are divided between a central government of the country, more regional state governments with separate legislatures, and local governments with locally elected representatives. Local governments oversee governance administration and developmental activities within specific jurisdictions like villages or towns, overseeing local infrastructure and services. Health is considered a subject of interest for the state governments. Kerala initiated decentralization reforms in several sectors including health care, where substantial funds and many functions were transferred to local governments [ 6 ]. The Kerala primary palliative care programme evolved with the support of local governments. Bedridden or homebound patients with serious health-related concerns requiring long-term symptom management are usually registered under this programme [ 5 ]. Pain and symptom management, psychological support for patient and family and provision of assistive aids and medicines are integral parts of the services rendered [ 3 , 4 ]. However, even in this setting palliative care patients are highly dependent on others, primarily family caregivers, for their daily activities [ 4 ]. Family caregivers also help with medical and nursing care requirements [ 7 ]. Consequently, palliative care nurses often train family caregivers on simple and practical strategies of caregiving [ 4 ]. Thus, family caregivers play an integral role in translating programme services into better outcomes for the patient.
At times, for some such caregivers, this caregiving can become a burden, a multidimensional form of distress affecting their physical, psychological, social and financial well-being [ 2 , 8 , 9 ]. Perceived caregiver burden is associated with increased mortality, [ 10 , 11 , 12 ] poor health outcomes, including anxiety and depression [ 13 , 14 ] and reduced quality-of-life among family caregivers [ 15 ]. Several studies have explored caregiver burden and associated factors [ 16 , 17 ], but few studies have looked at these issues from the providers’ perceptive in LMIC [ 18 ]. Palliative care nurses have a limited understanding of caregiver burden and related issues. Patients remain the focus of care, while caregivers and their issues may go largely unnoticed [ 19 ].
Caregivers themselves may be sufferers of chronic diseases. This may be particularly true of Kerala, where the population aged 60 and above comprised 16.5% of the people in 2021 anisre expected to reach 20.9% by 2031 in Kerala [ 20 ]. Ageing caregivers may experience an increased impact of the consequences of caregiving along with physiological ageing, isolation and comorbidities [ 21 ]. With advancing age, multimorbidity is common among the ageing population [ 22 ]. Changing family structures due to migration and the increased number of women entering the workforce lead to many households having only ageing persons. Caring for a bedridden or homebound person by an ageing spouse is likely to be high in the Kerala population. Most such caregivers see ‘caregiving’ as their responsibility and feel obligated to provide care for their dependent. Spouse caregivers frequently report being more stressed and burdened compared to adult-child caregivers [ 9 ]. Ageing spousal carers may be at risk of increased cognitive impairment, loneliness, sadness, and anxiety compared to demographically matched ageing non-caregivers [ 23 ]. Also, our earlier analysis of depression among women caregivers had shown increasing odds of depression for higher age groups. These initial results underscore the significance of considering age as a potential factor that may contribute to varying experiences of burden among caregivers [ 13 ]. Age is usually treated as a confounder in studies on caregiving and adjusted at the time of analysis, and age-specific findings are not often reported [ 24 ]. Recently, however, research attention to the importance of ageing on caregiving outcomes is increasing [ 25 ]. There is a clear need to explore differences in experiences and needs of different age groups within the caregiver population so that targeted interventions and support strategies may be developed.
The World Health Organization in 2002 had recommended that services for chronic care should foster continuity of care and personal connection between the caregiver and the care recipient [ 26 ]. This will require functional relationships between the palliative care nurses and family caregivers, necessitating effective communication and rapport building by the nurse [ 27 ]. How the programme and its frontline representative, the palliative care nurse, perceive family caregivers, the caregiving role and caregiver issues are not adequately explored. A 2019 palliative care policy document from Kerala mentions caregiver support but this is still in a very early stage in the programme [ 28 ]. In this context, we studied the caregiver burden and quality of life of caregivers aged 60 years or above compared to younger caregivers of palliative care patients in Kerala. We also explored the perspectives of palliative care nurses on family caregiver issues in home care settings and whether these perspectives are reflected in the services offered by the nurses and the programme.
The palliative care programme
All panchayats in Kerala have a home care team that is led by a trained palliative care nurse. The nurse conducts periodic home visits along with the field staff of the local primary health centre, elected LG members and community volunteers. Each palliative care nurse schedules the home visits, directs patient health assessment and management and maintains several registers, one of which is the nominal register with patient name, contact information, diagnosis, and remarks on main service provision (e.g., catheter change, wound dressing etc.). We used the patient register of selected panchayats to identify patients and contact their caregivers for enrolment in the study.
The details of the sampling strategy for the cross-sectional survey have been published earlier [ 8 ].. The basis for sample size was adequacy for factor analysis– a sample size of 200 was deemed adequate for factor analysis with 25 items, achieving an item-to-participant ratio of at least 1:8 [ 34 ]. As male caregivers were very few, all male caregivers as reported by palliative care nurses were approached. Women caregivers were selected purposively from the list of patients in each panchayat palliative care registry to represent both cancer and non-cancer conditions.
Regarding sample size for the in-depth interviews, the primary objective of the in-depth interviews with caregivers was scrutiny of the representation of caregiver burden domains identified from the literature, and no new domains emerged after three interviews. For palliative care nurses, perceptions of caregiver burden were first identified and coded from literature and a draft thematic framework was prepared a priori. The first nurse interviewed belonged to the panchayats selected for the quantitative survey. During that interview, the interviewer (AK) felt that the nurse was fully aware of the caregiver issues encountered during the cross-sectional survey by the investigator and was giving responses conforming to the interviewer’s expectations. Therefore, four remaining nurses were purposively selected from panchayats in the same district that were not part of the quantitative study. Interviews were conducted to explore new categories and themes and data collection was stopped when no new categories emerged for two interviews.
Design and data collection techniques
An integrative knowledge synthesis using mixed methods was carried out using analysis of a cross-sectional survey and qualitative exploration using in-depth interviews. This analysis used data from two study components done by the investigators, one on caregivers of palliative care patients and one on palliative care nurses. Table 1 summarizes the participant profile and data collection techniques for each study component.
Data collection from caregivers
The caregiver survey and interviews took place between January and February 2020. The investigators collected data for a study on developing and validating a Caregiver Burden Inventory in early 2020, published earlier [ 8 ]. The portion of that data used here comprised three in-depth interviews (IDI) with caregivers of palliative care patients and cross-sectional survey data of caregiver burden and related issues of 221 caregivers in five randomly selected panchayats in Kollam district, Kerala, India. This analysis focused on a comparison of findings of the cross-sectional survey on the caregivers aged above 60 years with younger or middle-aged caregivers aged between 18 and 59 years. All family caregivers of patients registered under the palliative care programme, aged 18 and above, who identified themselves as the primary caregivers and are providing care for not less than three months were included in the study. Those caregivers having a condition that limits their participation in the study and those caring for a critically ill care recipient during the study period are excluded from the study. An interview schedule was used to collect the sociodemographic information, care recipient and caregiver issues, and caregiver burden based on the Achutha Menon Centre Caregiver Burden Inventory, a nine-item inventory for assessing caregiver burden that had two domains– (i) physical, psychological, and spiritual aspects and (ii) financial aspects. Each item was scored on a 4-point Likert scale from zero to three. A caregiver could potentially score between zero (lowest possible burden level) and 27 (highest possible burden score). Quality of life also was assessed using the Malayalam version of the EuroQol EQ-5D 5-level version (EQ5D5L) [ 29 ]. We used the EQ-5D-5L Indian value set to convert responses to utility values [ 30 ]. The EQ-5D-5L is a widely accepted five-dimension HRQoL measure that covers mobility, self-care, usual activities, pain, anxiety/depression, and overall health state. It is easy to apply in younger and older populations and persons with less education [ 31 ]. It has good psychometric properties and the index values and dimensions have been found to strongly correlate with other measures of global health indicators, physical/functional health, pain, daily activities, and clinical/biological variables [ 32 ].
Data collection from palliative care nurses
The researchers were part of a team working on decentralization and health in Kerala, in which one of the researched themes was the primary palliative care programme [ 33 ]. One of the themes selected for enquiry was caregiver issues. Five primary palliative care nurses (Table 1 ) with at least one year experience were purposively selected and interviewed to get an insightful account of their experiences with caregiver issues. Interviews were conducted telephonically due to COVID-19-related restrictions in 2020 and early 2021.
Data analysis
To assess the validity of the EQ-5D-5L, we performed internal consistency checks and factor analysis of the five items of the EQ-5D-5L for the whole sample and the two age groups of interest separately (up to 59 years and 60 years and above). We extracted one factor from observed item values using principal axis factoring with direct oblimin rotation and correlated it with the utility scores obtained from the Indian value set of the EQ-5D-5L.
For the quantitative data, general characteristics and caregiver issues were summarised as frequencies and proportions or means and standard deviations, along with 95 per cent confidence intervals. Burden scores were converted to a categorical variable using tertiles, and labelled as low, moderate and high burden. Chi-square or Fisher exact tests were done to compare proportions. Analysis of variance (ANOVA) and posthoc Bonferroni tests were done to compare means. IBM SPSS version 25 was used for the quantitative analysis. Qualitative analysis was done manually.
All recordings of the IDIs were translated to English and initially coded by the same researcher (AK) who maintained an audit trail to map the interview transcripts and related codes to categories and themes. The approach to coding and categorising was inductive for the caregiver interviews and deductive for the palliative care nurse interviews. Information extracted from the literature review was used to generate a codebook for qualitative analysis to portray caregiver issues and perspectives of the nurse. The search was limited to articles in English, and title and abstract mention of caregiver issues along with provider perspective. Both investigators reviewed the shortlisted papers, and prepared codes, categories and themes through an iterative process. Existing codes were verified and additional codes, if any, were explored through triangulation with transcripts from caregiver issues mentioned by palliative care nurses in the main decentralization study. (See Additional file 1 ) After describing the findings based on this approach, we referred to Eva Kittay’s critique of Daniels and Nussbaum, based on the burden of caregiving and its effect on the caregiver’s opportunities while interpreting our findings from the study [ 35 ].
Subjectivities of the researchers
AK conducted all interviews and both investigators were involved in the analysis and interpretations. Both investigators hold basic biomedical degrees and subsequently public health qualifications. The research experience of both researchers has been predominantly post-positivist. We believe that our experiences around epidemiological surveys would have shaped the data collection and interpretations in a predominantly biomedical perspective with some consideration of social determinants shaped by our experience level. However, our ongoing engagement with palliative care and caregivers’ issues also brings in some relational approaches and interpretations characteristic of literature on caring.
Ethical aspects
All prospective study participants were assured of their autonomy, benefits and risks, privacy and confidentially and non-effect on care or benefits before obtaining informed consent. Informed consent, written or electronically documented, was obtained from all study participants. The Institutional Ethics Committee of the Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum cleared all tools of the scale development phase. (Letter number SCTIMST/IEC/1444/NOVEMBER-2019 dated 14 November 2019). The proposal and tools of the palliative care nurse interviews, part of the decentralization project, were reviewed and cleared by the institutional ethics committee of Health Action by People Thiruvananthapuram. (IEC EC2/P1/SEP/2020/HAP dated 10 December 2020). While these were originally independent studies, clearance was obtained from the Institutional Ethics Committee of the Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum (Letter number SCTIMST/IEC/2048/MAY-2023 dated 17 June 2023) for a synthesis exercise as part of formative research for a forthcoming study on caregiver burden assessment and intervention.
Validity of the EQ-5D-5L in our study sample
We report the Cronbach’s alpha for internal consistency, the eigen value for the extracted factor, the factor loadings of the extracted factor onto each item of the EQ-5D-5L and Pearson’s correlation coefficient between the extracted factor and utility scores in Table 2 . Internal consistency was moderate to good, eigenvalue was more than one and there was a high correlation between the factor derived from observed values and utility score values taken from the India value set. Factor loadings for pain/ discomfort and anxiety/ depression were relatively higher in the younger age group while for usual activities, factor loadings were higher in the older caregiver group.
Findings from cross-sectional survey among caregivers
Palliative care recipients had various diagnoses ranging from stroke (23.9%), to cancer (12.8%) followed by other conditions. The mean age of the caregivers was 51.2 years (Standard Deviation (SD 12.7). The mean age of the older group was 66.2 years (SD 7.1) and of younger or middle-aged caregivers was 45.3 (SD 9.0). Caregiver ages ranged from 25 to 88 years. Demographic characteristic of the caregivers according to their age category is given the Table 3 . Most caregivers were women, but in the older age group, the proportion of men was significantly higher. Older caregivers were significantly less educated and less likely to be married, but the social class was comparable.
Table 4 depicts the distribution of variables related to caregiving. Nearly all caregivers in both groups were the sole caregiver for their care recipient. A significantly higher proportion of older caregivers were giving care to their spouses. Care requirements were significantly higher for the care recipients of younger caregivers, but most other variables were comparable. A higher proportion of older caregivers reported being satisfied with their caregiving activities.
Older caregivers reported poorer states for all variables related to self-reported morbidity and quality of life attributes measured using the EQ5D5L, except for self-care. (Table 5 ) Nearly three-fourths of older caregivers reported mobility issues; over half had pain or felt anxious or depressed.
The mean EQ-5D-5L utility score for the caregivers was 0.936 (SD 0.078, 95% CI 0.926–0.947). On comparing the caregiver’s age and burden experienced with the utility score, we found that the burden level impacted the perceived quality of life, irrespective of the caregiver’s age. As shown in Fig. 1 , younger caregivers generally had a better quality of life than older caregivers, and those with low caregiver burden had better utility scores than those with moderate to high levels of caregiver burden. Younger caregivers who perceived a high burden level had lower mean utility scores (0.926, SD 0.090, 0.907–0.945) than younger caregivers who perceived a low burden (0.980, SD 0.041, 0.970–0.990). Likewise, older caregivers who perceived a higher burden level had a lower mean utility score (0.877, SD 0.066, 0.854–0.899) than their counterparts with a low burden (0.935, SD 0.058, 0.912–0.958). Except for the difference in means between older caregivers with low burden and younger caregivers with moderate to high burden, all mean differences were statistically significant. ( p < 0.001)
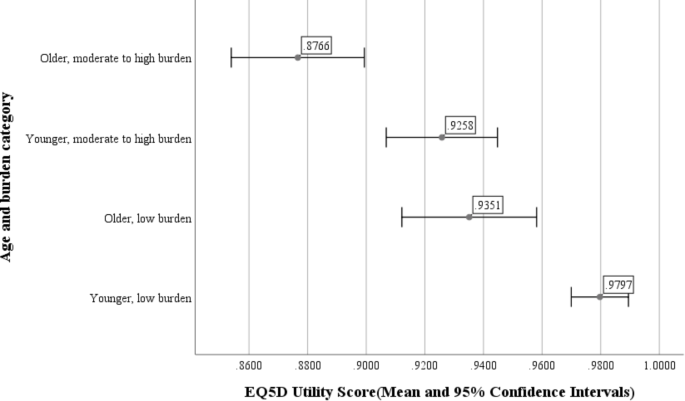
Means and 95% confidence intervals of EQ5D5L utility scores for caregivers grouped based on age category and burden level
Table 6 maps the support these dyads received regarding palliative care nurse visits, assistive devices, food kits or support from non-governmental charitable organisations. The frequency of nurse visits (monthly or above) was determined almost exclusively by patient need and was not associated with caregiver burden level. Among other forms of support, receiving food kits from the LG was found to be significantly higher when high levels of caregiving burden were present.
Themes from in depth interviews with caregivers
“i do everything for her/him”.
All caregivers mentioned doing “everything” for the care recipient, including all activities of daily living, medications, and procedures like skin care.
CG1: “I do everything for her…I bathe her… take her to the toilet…help her to change her dress…. Give her food. Everything…”. CG2: “I’ve cared for my husband for the last 10 years. he is entirely dependent on me… everything…I clean him…bathe him… give him food…everything”.
Caregiving became physically and psychologically demanding
Doing “everything” involved physically demanding activities that reportedly led to chronic body pain for the caregiver.
CG2: “…a constant pain on my legs… I always lift him alone, there will not be anybody home…”.
Other issues mentioned included sleep deprivation, financial and job-related issues, and limitations to social participation due to caregiving. Care recipients could also have a temperament that made caregiving challenging.
CG2: “He is always very angry. He always shouts at me and my son… I’m always worried… I do not know what to do…”.
Care team is only patient-focused, caregiver issues are not addressed
The palliative care team when they visit would do patient-centred procedures, dispense medicines, and provide advice for improving patient care.
CG3: “…people from health (services) come once a month and change the urine tube…. They give medicines also…”.
Some advice provided could not be implemented, often due to affordability issues.
CG2: “…they give instructions about how to do physiotherapy…but it is no use…once in a month we used to call a physiotherapist…but it is expensive…”.
Themes from palliative care nurse interviews
We shortlisted 17 articles for further analysis. Four were from Kerala and the rest were from outside India. Caregiver issues highlighted included burden, burnout, and health and wellbeing-related issues. Four themes on the care provider perspective were initially decided upon, namely: (i) exposition of caregiver burden by providers (ii) nature of family caregiver-health provider relationships (iii) factors that enable or hinder caregiver support from providers (iv) specific interventions that foster caregiver endurance.
Each provider interview took about 40 min, ranging from 35 to 50 min. Open codes from documents were binned into existing categories in the schema or new categories were added, if felt necessary. (See Additional file 1 ) No codes fell into the theme “specific interventions that foster caregiver endurance”. Brief descriptions of the findings were as follows:
Accurate exposition of the caregiver burden by palliative care nurses
All nurses highlighted the “burden” experienced by the family caregivers, mainly expressed as socioeconomic deprivation and challenges.
“Issues like no secure house, no food due to lack of income… patients who cannot buy expensive medication and continue their treatment… bystanders struggling for their children’s education…” (PN2– when reporting quotes abbreviation PN indicates participant attribute - palliative care nurse). “They talk about the difficulties of not being able to go to work leaving their Amma (mother)” (PN1).
Added to this were disruptions and conflicts that the caregivers must handle along with the caregiving role.
Caregivers cannot sleep, they cannot look after their home and other household works, they cannot do their own activities like taking care of children (PN1).
Nurses often found themselves encountering conflicts, either between the caregiver and the patient or among family members taking the main caregiver responsibility. Sometimes patient behaviours were distressing for caregivers.
Sometimes patients will be so “violent” because of their condition; sometimes the patient’s condition is so bad… This also reflects on the caregivers. This affects them and they may also become frustrated. (PN1)
The caregiver role often limited the caregivers to their homes and restricted their social life. Societal perceptions of caring often deepened this social restriction. Nurses clearly described difficulties associated with long-term caregiving including physical pain, psychological distress, individual life disruptions, economic, and social challenges. Some caregivers had become sick from the long haul of physical exhaustion.
I know caregivers like these…so desperate and hopeless… (PN5)
Nurses also felt that caregivers often neglect their well-being and prioritise their patient’s care.
Disparate relationships between caregivers and health providers and the system
Nurse representations of caregiver-provider relationships were complex, ranging from excellent cordiality to open conflicts. Nurses were at times “being like a family member” and at other times involved in verbal altercations and in extreme situations, involvement of law enforcement when neglect of the care recipient was perceived. A consistent part of the relationship, however, was the instrumental contribution expected from the caregiver in caring for the care recipient. Family caregivers were taken for granted as resource persons for caring for the patient and interactions mostly involved general instructions on caregiving or specific training for skin care, wound care, or catheter care. Some task-shifting often happened from the nurses to capable caregivers.
“We made them do these in front of us… The caregiver has taken care of the patient so well.” (PN1, mentioning an example of caregiver education for wound dressing).
Referral for palliative care itself might be perceived by family members as further care was largely up to themselves. It would often take multiple visits to discern all such concerns.
“…they also share their concerns… as palliative (is understood as) end-of-life care…so these makes them worried…” (PN5).
The first time they won’t say everything… after numerous visits, they tell us everything (PN3)
When disagreements were encountered, nurses tried to resolve them by working for a healthy relationship between the caregiver and the care recipient. A somewhat stereotypical portrayal of caregiving emerged in the discourse, where caregiving was a moral imperative of the family, often women. The “best” caregivers were those who fulfilled this expected role well.
“I strongly believe that we should take care of our own parents” (PN2).
“There are no issues or problems for caregivers who are not working” (PN2)
“She is a widow…has two kids…the patient is her late husband’s mother…she (caregiver) is working… she does everything for her patient; only after that she leaves for work… When we visit the patient…it’s so clean and we never feel it’s a room of a bedridden patient…there are caregivers like this” (PN3).
Some caregivers were hesitant to build relationships with palliative care nurses. Nurses too might choose against investing time and visits for getting better acquainted with the caregiver. Caregivers who were demanding and making decisions independent of the nurse were considered problematic.
“They (caregivers) “torture” us by making calls to the panchayat member (the elected LG representatives who helm the programme)…” (PN4).
Families perceived as neglecting the care recipient were labelled as outright problematic. At times, nurses tend to establish an authoritarian role in such instances.
“I say to them if you did not take care of your parents, your seven generations will suffer…” (From additional codes as indicated in the additional file, said by a nurse based on the spiritual belief on results of bad deeds being passed on to future generations) (See Additional file 1 ). “I say, “If you didn’t take care of them, I will inform to (the elected LG representatives) and doctor…If… your mother is lying in (urine and faeces), then you will be taken by police” (From additional codes) (See Additional file 1 ).
But palliative care nurses were often the first in the health system to recognize patient negligence and abuse by the family.
Caregivers who followed their instructions well and include nurses in treatment-related decisions were considered dependable. Yet, once good communication and rapport were established, caregivers often began to consider the nurse “like family” and this was highly valued by nurses, who mentioned several “friendships” that continued long after the death of the patient.
“(When her) daughter (finished school) she (caregiver) asked me which (field of education) is good for her daughter… now, following my advice, the daughter is doing nursing in the district hospital.” (PN3).
Systemic factors often hinder caregiver support
By systemic factors, we mean programmatic focus on the patient, lack of training, lack of time and limited attention to support schemes involving caregiver issues and burden. As such, there were no caregiver-specific initiatives or systematic documentation of caregiver issues. Caregiver support when existed was reactive rather than proactive. Caregivers were mostly given instructional support and/ or instrumental assistance for aiding patient care like medicines, cotton pads, gauze, catheters, Ryle’s tubes, or mobility aids. Communication and consoling were perceived as the main form of intervention by palliative care nurses.
“Their (caregivers) blood pressure will increase because of this lack of sleep. So, during our home visit we will check their BP also…” (PN1).
However, nurses informed eligible caregivers and families about beneficial schemes (‘ Ashwasakiranam ’, a state government-initiated financial assistance scheme for primary caregivers of palliative patients with cancer) or helpful charity organizations, if any.
Lack of time was the main impediment in addressing caregiver issues. Additionally, inadequate training and resources for giving caregiver support were also mentioned. Nurses suggested some systemic failures in recognizing the medical and social issues of caregivers.
“Some of the caregivers, have issues like CKD (chronic kidney disease), cancers or heart problems, but we cannot register them with the palliative care programme.” (PN5).
The main LG support specifically mentioning caregivers was the annual Kudumbasangamom (family gathering) with some recreational programmes, that too in the pre-pandemic days. Some LGs had schemes for self-employment generation for patients or caregivers, to make some products that could be sold for money. LGs support for hosting such schemes was patchy.
“But there was no adequate support from our panchayat for selling their product or purchasing the raw materials…no support for promoting these initiatives.” (PN1).
In this mixed methods study, we attempted to compare caregiver issues between older and younger caregivers in the palliative care program in Kerala. We also tried to document provider-side perspectives on family caregiver issues as articulated by palliative care nurses. The family caregiver issues we identified included physical, psychological, social, and financial issues, much like those reported by Ferrell and Wittenberg in their review of family caregiver trials in cancer patients [ 36 ]. As expected, older caregivers were more susceptible to health-related problems at this age. Irrespective of age, those who experienced a higher burden level had poorer quality of life. When combined, with higher burden experience, older caregivers had the poorest quality of life. This might be brought on by the physical demands of providing care as well as the ageing process’s effects on health.
The absence of any specific service or programme that enables caregiver endurance or any mention of systematic documentation of caregiver issues is a programmatic shortcoming. Nurses gave more attention to patients with skilled care needs and the level of caregiver burden was probably not a factor in determining their visits. Nurses’ tendency for “non-inviting interactions” with family members of patients, by prioritising medical and technical tasks, has been reported earlier from institutional settings [ 37 ]. But nurses recognised most caregiver issues and mentioned insufficient time to address them. Healthcare providers in similar programmes may not even have time for meeting their personal needs due to work demands [ 19 ]. Nurse perceptions about caregiving-related challenges mentioned social determinants of health but also mirrored prevalent socio-cultural and patriarchal norms. Family caregiver-centric studies are rare from LMIC, but available studies reflected socioeconomic deprivation and intense gender-role-driven concentration of caregiving in women [ 38 ]. Nurses however actively tried to improve the family caregivers’ skills in caregiving. This is important to prevent and delay burnout [ 39 ]. Additionally, they provide psychological support, often bonding well with caregivers long after they are bereaved [ 40 ]. Receiving interventions like food kits was significantly higher when the perceived caregiver burden was high. Caregiver burden is multi-dimensional and includes financial difficulties [ 8 ]. LGs generally focus more on the poorest and this finding is expected. The interventions remain basic, but it is promising that LGs can prioritise families with high caregiver burdens for interventions.
Poor households might disproportionately access the LG-run palliative care service, as the services are free of cost. Such households may already have high burden due to pre-existing structural and social disadvantages. Yet, even if caregiving was not causal for the problems expressed, the perceived burden would still be detrimental to quality of life. The directive principles of state policy of the constitution of India clearly list the fundamental rights of citizens and the responsibility of the state to protect citizens unable to access the minimal provisions for social and economic well-being. These principles also mention the autonomy of LGs [ 41 ]. It is thus a moral requirement of the LG-run palliative care programme to focus on the needs of families in addition to the patients.
Our findings draw attention to an important element of long-term care that is somewhat neglected– caregiver impact. Caregiving is a moral responsibility between individuals and at the collective level, as all individuals need care and are dependent at some point in their lives. But caregiving is a mix of reward and burden. Caregivers remain seen as a means to an end when in reality the caregiver is also an end in herself or himself. Allocation of caregiving responsibility is heavily gendered, rendering it as a form of inequity. Potential disadvantages of women may get compounded when she gets restricted to the caregiver role– lesser education, or work opportunities, and often treated as if she is unemployed or not doing economically productive work– leading to depression and a low sense of worth. Another aspect of caregiving that has implications for equity is the way society often works, based on normative or normal people. This may become unfair to suffering people as well as their caregivers, and the burden may be considered inevitable. The family caregiver is not a biological extension of the care recipient’s situation, to be moulded to sustain the biological functions of the care recipient. Neither is caregiving by a family member a law of nature that cannot be changed. This is a situation shaped by relationships between people and societies and the values and practices thereof. Moral requirements of caregiving should also consider what is lost to a caregiver and provide respect for the caregiver. Solutions may be explored by forming partnerships between the caregiver and others and by tapping into existing community resources. This has to happen without diminishing the relationship between the caregiver and the care recipient [ 26 ].
Norman Daniels proposes a lifespan approach of justice that may be useful to consider in this setting [ 42 ]. As individuals get older, their needs changes. When the society itself in an ageing society, that too brings in a new set of needs. In such a situation, reasoning has to be applied on how competing needs are to be met. Competing needs would be between different age groups or between care recipients and those giving care. Some needs would inevitable not be met when social obligations are to be met, but there should be fairness in the terms involved, and adequate social support to prevent issues like burnouts. Identifying beneficial interventions will remain an ethical challenge due to three aspects: (i) the vulnerability of the care recipient should not be exploited (Daniels); (ii) the voice of the caregiver has to be used for meeting the needs of the care recipient, as the capabilities of the latter have diminished (Kittay); (iii) the caregiver too has interests that would often be diminished (Kittay). The caregiver burden is disproportionately a woman’s issue because most of the caregiving work is rendered by women, many of whom are older persons. Discussions of fairness and equity often focus on fair distribution of goods like education and health. As Kittay points out in response to Norman Daniels and Nussbaum, conventional approaches to justice focusing on fair sharing of goods and aiming for equality of opportunity or capability do not talk about fair sharing of burden. In ageing societies, considerations of the distribution of burden may be as important as the distribution of goods.
The CARE framework refers to caregivers as “hidden patients” and recommends a framework comprising Caregiver well-being, Advanced care planning, Respite, and Education for planning to address caregiver issues [ 43 ] The first attribute in addressing family caregiver-related issues is an assessment of need. Symptom severity of care recipients, marginalized families and caregivers with significant psychosocial issues have been suggested as potential indicators of high caregiver issues [ 44 , 45 ]. The deployment of tools like carer support needs assessment tool might help identify support needs and decrease caregiver strain [ 46 ]. Newer modalities like an app-based assessment are being tested in Sweden for family caregivers of patients with dementia [ 47 ]. Examples of successful caregiver interventions from LMIC countries are generally few. The trials covered in the review by Ferrell and Wittenberg were mostly from high-income countries [ 36 ]. In New Zealand three themes of advice for caregivers were considered most useful by providers– caring for oneself physically, emotionally, and spiritually; learning practical skills; and knowing what to expect and plan for as the family member’s health declines [ 48 ]. Researchers from the Netherlands recommended appreciation, information, practical support, and opportunities for time off (like respite care) as useful to lessen caregiver problems [ 49 ]. An intervention based on group sessions for caregivers in South Korea also showed promising physical and psychological outcomes [ 50 ].
Most of these examples are based on individual-level interventions. Krieger et al. reported the need for comprehensive caregiver support at two levels– the individual caregiver level, and the system level [ 51 ]. The United States of America (USA) has had several legislative and programmatic structures aimed at minimizing caregiver distress [ 52 ]. Caregivers of veterans in the USA have specific support like training, financial support, and assistance of a caregiver support coordinator, although Zebrak mentions about the lack of coordination between such policies [ 53 , 54 ]. The National Health Service in the United Kingdom has some specific measures to support caregivers [ 55 ]. The National Institute for Health and Care Excellence, UK has included an assessment of caregivers’ quality-of-life in economic evaluation in its health technology evaluation manual published in January 2022 [ 56 , 57 ].
The primary palliative care programme in Kerala is run by the LGs with support from the health department. Each LG unit sets aside resources from its annual fund allocation to support the wages of the palliative care nurse, travel costs, and costs of equipment, materials, and drugs for home-based care. Additional community-based resources are also mobilised by some LGs. In Kerala, the decentralized health system and the agency available with LGs for extending welfare measures to the needy using locally identified resources offers promise for good interventions [ 6 ]. Caregiver training and certification could be done, and a list of authorised paid caregiver schemes could be piloted, with efforts to include men in the initiative [ 58 ]. Facilities for respite care [ 59 ] may offer some personal space and time for caregivers, or additional appropriate practical help [ 60 ] could be offered. Building the competency of caregivers could extend to self-care in addition to patient care [ 39 ]. The formation of caregiver peer groups could be another intervention that facilitates information sharing, coping and increased social interactions [ 39 , 61 ]. A specialist support service like a caregiver support coordinator or group could be initiated by the district-level health structures of the National Health Mission or the LG or by NGOs. Despite limited evidence of the success of such interventions on a large scale, it is useful to remember the economic value of family caregivers to the health system and community [ 45 ].
Limitations
The quantitative data being cross-sectional, the temporality of the associations we saw cannot be ascertained. Poor health may cause poor quality of life and that may precipitate caregiver burden rather than burden resulting in poor quality of life. However, the implication for the health system remains somewhat the same– poor health, poor quality of life and high caregiver burden need attention whatever the order of their occurrence. Another limitation of the study is the lack of direct interaction with palliative nurses due to COVID-19-related restrictions. The interviews were possibly influenced by the previous experience of the researchers on caregiver issues. Physical visits to the settings and interactions with a wider group of stakeholders from the health department, the LG and other community representatives would have provided richer descriptions of caregiver issues and more quintessential details of caregiver-provider/ system interactions. At the analysis stage, we did not do a multivariable analysis to account for potential confounding or effect modification as data were not primarily collected to explore these aspects. The largely deductive qualitative analysis based on a priori themes is another limitation. As our focus was on validating our literature-generated construct of caregiver burden, we did not explore the experiences of elderly caregivers at that stage of the study and this is a drawback of this synthesis. Yet, we feel that our findings offer some insights that can be used to inform future research in this area.
Caregivers aged 60 years or above made up three out of ten caregivers, with over half caring for their spouse, in this study setting. This is one of the first studies using Indian values of EQ5D5L utility scores for studying the quality of life of caregivers. Older caregivers reported a poor health-related quality-of-life and were experiencing a dual burden of caregiving and poor health, also having chronic health issues needing to take care of others while having to take care of others. The complex dynamics of caregiving by elderly caregivers have not been explored much, suggesting opportunities for future studies to explore these issues and develop targeted interventions for their specific needs. Potential interventions could be Respite care and support services for older caregivers that could offer temporary relief and help caregivers take breaks from caregiving responsibilities. Peer support groups could be another approach that can help caregivers to cope better with the burden. Also, comprehensive geriatric health and wellness programmes encompassing preventive, promotive, curative, rehabilitative and palliative care that jointly cater to patients and caregivers together are needed in settings with high ageing and chronic health conditions.
Data availability
The corresponding author will provide the transcripts, data set and analysis of this current work on reasonable request.
Abbreviations
Analysis of variance
Five level EQ-5D version of EuroQol
In-depth interviews
Low- or Middle-Income Countries
Local Government
Palliative Nurse
Standard Deviation
United Kingdom
United States of America
Daniels N. Just health: Meeting health needs fairly. New York: Cambridge University Press; 2007. p.13.
Ugargol AP, Bailey A. Family caregiving for older adults: gendered roles and caregiver burden in emigrant households of Kerala, India. Asian Popul Stud. 2018;14:194–210.
Article Google Scholar
Abdul Azeez EP, Anbu Selvi G. What determines the sustainability of community-based palliative care operations? Perspectives of the social work professionals. Asian Soc Work Policy Rev. 2019;13:334–42.
Philip RR, Philip S, Tripathy JP, Manima A, Venables E. Twenty years of home-based palliative care in Malappuram, Kerala, India: a descriptive study of patients and their care-givers. BMC Palliat Care. 2018;17:26.
Article PubMed PubMed Central Google Scholar
Sallnow L, Kumar S, Numpeli M. Home-based palliative care in Kerala, India: the neighbourhood network in palliative care. Prog Palliat Care. 2010;18:14–7.
Anju R, Sadanandan R, Vijayakumar K, Raman Kutty V, Soman B, Ravindran RM, et al. Decentralisation, health and sustainable development goal 3. Public Health Action. 2023;13(Suppl 1):51–6.
Article CAS PubMed PubMed Central Google Scholar
Reinhard SC, Given B, Petlick NH, Bemis A. Supporting Family Caregivers in Providing Care. In: Hughes RG, editor. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008. http://www.ncbi.nlm.nih.gov/books/NBK2665/ Accessed 15 Mar 2023.
Kochuvilayil A, Varma RP. Caregiver Burden among Informal caregivers in the Kerala Palliative Care Program: Development and Validation of the Achutha Menon Centre-Caregiver Burden Inventory. J Palliat Med. 2021;24:1197–205.
Article PubMed Google Scholar
Oldenkamp M, Hagedoorn M, Slaets J, Stolk R, Wittek R, Smidt N. Subjective burden among spousal and adult-child informal caregivers of older adults: results from a longitudinal cohort study. BMC Geriatr. 2016;16:208.
Roth DL, Haley WE, Hovater M, Perkins M, Wadley VG, Judd S. Family caregiving and all-cause mortality: findings from a population-based propensity-matched analysis. Am J Epidemiol. 2013;178:1571–8.
Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health effects Study. JAMA. 1999;282:2215–9.
Article CAS PubMed Google Scholar
Perkins M, Howard VJ, Wadley VG, Crowe M, Safford MM, Haley WE, et al. Caregiving strain and all-cause mortality: evidence from the REGARDS study. J Gerontol B Psychol Sci Soc Sci. 2013;68:504–12.
Kochuvilayil A, Varma RP. Factors Associated with Screening positive for Depression among women caregivers of primary Palliative Care patients in Kerala, India. J Palliat Care. 2022;37:510–17.
Govina O, Vlachou E, Kalemikerakis I, Papageorgiou D, Kavga A, Konstantinidis T. Factors Associated with anxiety and depression among family caregivers of patients undergoing Palliative Radiotherapy. Asia Pac J Oncol Nurs. 2019;6:283–91.
Srivastava G, Tripathi RK, Tiwari SC, Singh B, Tripathi SM. Caregiver Burden and Quality of Life of Key Caregivers of patients with dementia. Indian J Psychol Med. 2016;38:133–6.
Devassy SM, Green D, Cheguvera N, Yohannan SV, Grills N, Joubert L. The lived experience of carers who assist people with disability in Ernakulam, Kerala, India. Disabil Rehabil. 2022;44:6660–7.
Usha K. PA26 unmet needs and stress among caregivers of bedridden stroke patients in north Kerala - a community based study. BMJ Support Palliat Care. 2015;5(Suppl 1):A27.
Krutter S, Schaffler-Schaden D, Essl-Maurer R, Wurm L, Seymer A, Kriechmayr C, et al. Comparing perspectives of family caregivers and healthcare professionals regarding caregiver burden in dementia care: results of a mixed methods study in a rural setting. Age Ageing. 2020;49:199–207.
Useros MV, Espín AA, Parra EC, Martínez AB. Family caregivers: nurses’ perception and attitudes. Social Med. 2012;6:151–61.
Google Scholar
Kerala State Planning Board: Economic Review. 2021. 2022. https://spb.kerala.gov.in/sites/default/files/2022-03/ECNO_%20ENG_21_%20Vol_1.pdf . Accessed 15 Mar 2023.
Neri AL, Yassuda MS, Fortes-Burgos AC, Mantovani EP, Arbex FS, de Souza Torres SV, et al. Relationships between gender, age, family conditions, physical and mental health, and social isolation of elderly caregivers. Int Psychogeriatr. 2012;24:472–83.
Pati S, Agrawal S, Swain S, Lee JT, Vellakkal S, Hussain MA, et al. Non communicable disease multimorbidity and associated health care utilization and expenditures in India: cross-sectional study. BMC Health Serv Res. 2014;14:451.
Lavela SL, Ather N. Psychological health in older adult spousal caregivers of older adults. Chronic Illn. 2010;6:67–80.
Tough H, Brinkhof MWG, Siegrist J, Fekete C, SwiSCI Study Group. Social inequalities in the burden of care: a dyadic analysis in the caregiving partners of persons with a physical disability. Int J Equity Health. 2019;19:3.
Melo LA, Jesus ITM, Orlandi FS, et al. Frailty, depression, and quality of life: a study with elderly caregivers. Rev Bras Enferm. 2020;73Suppl(3):e20180947.
World Health Organization: Ethical choices in long-term care: What does justice require? Pp 19. Geneva: World Health Organization. 2002. https://apps.who.int/iris/bitstream/handle/10665/42614/9291562285.pdf;sequence=1 Accessed 15 Mar 2023.
Jika BM, Khan HT, Lawal M. Exploring experiences of family caregivers for older adults with chronic illness: a scoping review. Geriatr Nurs. 2021;42:1525–32.
Government of Kerala: Kerala State Policy on Palliative Care. 2019. https://palliumindia.org/wp-content/uploads/2020/05/Kerala-State-Palliative-Care-Policy-2019.pdf Accessed 15 Mar 2023.
Herdman M, Fox-Rushby J, Rabin R, Badia X, Selai C. Producing other language versions of the EQ-5D. In: Brooks R, Rabin R, de Charro F, editors. The measurement and valuation of health status using EQ-5D: a European perspective. Dordrecht: Kluwer; 2003. pp. 183–90.
Chapter Google Scholar
Jyani G, Sharma A, Prinja S, Kar SS, Trivedi M, Patro BK, et al. Development of an EQ-5D value set for India using an Extended Design (DEVINE) study: the Indian 5-Level version EQ-5D Value Set. Value Health. 2022;25:1218–26.
Marten O, Brand L, Greiner W. Feasibility of the EQ-5D in the elderly population: a systematic review of the literature. Qual Life Res. 2022;31:1621–37.
Feng YS, Kohlmann T, Janssen MF, Buchholz I. Psychometric properties of the EQ-5D-5L: a systematic review of the literature. Qual Life Res. 2021;30:647–73.
Kochuvilayil A, Rajalakshmi S, Krishnan A, Vijayanand SM, Kutty VR, Iype T, et al. Palliative care management committees: a model of collaborative governance for primary health care. Public Health Action. 2023;13:12–8.
Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60:34–42.
Kittay EF. Can contractualism justify state-supported long-term care policies? Or, I’d rather be some mother’s child. A reply to Nussbaum and Daniels. Ethical choices in long-term care. What Does Justice Require. 2002:77–83.
Wittenberg E, Buller H, Ferrell B, Koczywas M, Borneman T. Understanding Family Caregiver Communication to provide family-centered Cancer Care. Semin Oncol Nurs. 2017;33:507–16.
Söderström IM, Benzein E, Saveman BI. Nurses’ experiences of interactions with family members in intensive care units. Scand J Caring Sci. 2003;17:185–92.
Kipp W, Chacko S, Laing L, Kabagambe G. Adolescent reproductive health in Uganda: issues related to access and quality of care. Int J Adolesc Med Health. 2007;19:383–93.
Solli H, Hvalvik S. Nurses striving to provide caregiver with excellent support and care at a distance: a qualitative study. BMC Health Serv Res. 2019;19:893.
Salin S, Kaunonen M, Astedt-Kurki P. Nurses’ perceptions of their relationships with informal carers in institutional respite care for older people. Nurs Res Pract. 2013;2013:967084.
PubMed PubMed Central Google Scholar
Choudhry S, Khosla M, Mehta PB. The Oxford Handbook of the Indian Constitution. 1st ed. Oxford: Oxford University Press; 2016.
Book Google Scholar
Daniels N. Just health: Meeting health needs fairly. New York: Cambridge University Press; 2007. p.162.
Holliday AM, Quinlan CM, Schwartz AW. The hidden patient: the CARE framework to care for caregivers. J Family Med Prim Care. 2022;11:5–9.
Valero-Cantero I, Wärnberg J, Carrión-Velasco Y, Martínez-Valero FJ, Casals C, Vázquez-Sánchez MÁ. Predictors of sleep disturbances in caregivers of patients with advanced cancer receiving home palliative care: a descriptive cross-sectional study. Eur J Oncol Nurs. 2021;51:101907.
Hudson PL, Thomas K, Trauer T, Remedios C, Clarke D. Psychological and social profile of family caregivers on commencement of palliative care. J Pain Symptom Manage. 2011;41:522–34.
Aoun SM, Grande G, Howting D, Deas K, Toye C, Troeung L, et al. The impact of the carer support needs assessment tool (CSNAT) in community palliative care using a stepped wedge cluster trial. PLoS ONE. 2015;10:e0123012.
Kabir ZN, Leung AYM, Grundberg Å, Boström AM, Lämås K, Kallström AP, et al. Care of family caregivers of persons with dementia (CaFCa) through a tailor-made mobile app: study protocol of a complex intervention study. BMC Geriatr. 2020;20:305.
Angelo JK, Egan R, Reid K. Essential knowledge for family caregivers: a qualitative study. Int J Palliat Nurs. 2013;19:383–8.
Bijnsdorp FM, Pasman HRW, Boot CRL, van Hooft SM, van Staa A, Francke AL. Profiles of family caregivers of patients at the end of life at home: a Q-methodological study into family caregiver’ support needs. BMC Palliat Care. 2020;19:51.
Han EJ, Park M, Park S, Giap TT, Han D. Randomized Controlled Trial of the Caregiver Orientation for Mobilizing Personal Assets and Strengths for Self-Care (COMPASS) for Caregiving Journey: A National Family Caregiver Support Program in a Long-Term Care Insurance System. J Am Med Dir Assoc. 2020;21:1906-13.e3.
Krieger T, Feron F, Dorant E. Two-level multi-methodological evaluation of a new complex primary support programme for stroke care-givers in Germany. Ageing Soc. 2022;42:1–31.
Rozario PA, Palley E. When the private sphere goes public: exploring the issues facing family caregiver organizations in the development of long-term care policies. Soc Work Public Health. 2008;23:49–68.
Smith VA, Lindquist J, Miller KEM, Shepherd-Banigan M, Olsen M, Campbell-Kotler M, et al. Comprehensive Family Caregiver Support and Caregiver Well-Being: preliminary evidence from a Pre-post-survey Study with a non-equivalent Control Group. Front Public Health. 2019;7:122.
Zebrak KA, Campione JR. The Effect of National Family Caregiver Support Program Services on Caregiver Burden. J Appl Gerontol. 2021;40:963–71.
National Health Service: Support and benefits for carers. (2018) https://www.nhs.uk/conditions/social-care-and-support-guide/support-and-benefits-for-carers/ Accessed 20 Mar 2023.
Pennington BM. Inclusion of Carer Health-Related Quality of Life in National Institute for Health and Care Excellence appraisals. Value Health. 2020;23:1349–57.
Dawoud D, Lamb A, Moore A, Bregman C, Rupniewska E, Paling T, et al. Capturing what matters: updating NICE methods guidance on measuring and valuing health. Qual Life Res. 2022;31:2167–73.
Cross ER, Emanuel L. Providing inbuilt economic resilience options: an obligation of comprehensive cancer care. Cancer 2008113(12 Suppl):3548–55. https://doi.org/10.1002/cncr.23943 .
Roberts E, Struckmeyer KM. The impact of Respite Programming on Caregiver Resilience in Dementia Care: a qualitative examination of Family Caregiver perspectives. Inquiry. 2018;55:46958017751507.
PubMed Google Scholar
Simon C, Kumar S, Kendrick T. Who cares for the carers? The district nurse perspective. Fam Pract. 2002;19:29–35. https://doi.org/10.1093/fampra/19.1.29 .
Greenwood N, Habibi R, Mackenzie A, Drennan V, Easton N. Peer support for carers: a qualitative investigation of the experiences of carers and peer volunteers. Am J Alzheimers Dis Other Demen. 2013;28:617–26.
Download references
Acknowledgements
The authors thank the Department of Health and Family Welfare, Government of Kerala, the Local Self Government Department of the Government of Kerala and the Panchayats for granting permission to undertake the study.
AK received partial financial support from the project Local Government and Health in Kerala, implemented by Health Action by People (HAP), Thiruvananthapuram, Kerala, for conducting the palliative care nurse interviews. The Local Government and Health project was supported by the Health Systems Transformation Platform, through a financial contribution from the Sir Ratan Tata Trust. The funders had no role in data collection and analysis or preparation of the manuscript.
Author information
Authors and affiliations.
Achutha Menon Centre for Health Science Studies, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, Thiruvananthapuram, Kerala, India
Arsha Kochuvilayil & Ravi Prasad Varma
You can also search for this author in PubMed Google Scholar
Contributions
AK conducted the interviews, undertook the analysis and wrote the first draft of the manuscript. RPV supervised the work and contributed to the conceptualization, design, analysis and revision of the manuscript. Both authors have reviewed and approved the manuscript in its present form including the revisions.
Corresponding author
Correspondence to Ravi Prasad Varma .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethics approval and consent to participate
All study participants provided written or electronic documentation of their informed consent. The study draws on data from two previous studies conducted by the researchers. Both previous studies were cleared by the respective Institutional Ethics Committee (Letter dated November 14, 2019, with number SCTIMST/IEC/1444; and letter dated 10 December 2020, numbered IEC EC2/P1/SEP/2020/HAP). The synthesis is part of the formative work towards the doctoral dissertation of Dr Arsha Kochuvilayil and the protocol and tools were reviewed and cleared by the Institutional Ethics Committee of the Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum. (Letter number SCTIMST/IEC/2048/MAY-2023 dated 17 June 2023).
Consent for publication
Not applicable.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Additional File 1. Coding Schema for Coding Palliative Care Nurse Interviews.
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Kochuvilayil, A., Varma, R.P. Understanding caregiver burden and quality of life in Kerala’s primary palliative care program: a mixed methods study from caregivers and providers’ perspectives. Int J Equity Health 23 , 92 (2024). https://doi.org/10.1186/s12939-024-02155-x
Download citation
Received : 23 March 2023
Accepted : 18 March 2024
Published : 07 May 2024
DOI : https://doi.org/10.1186/s12939-024-02155-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Family caregivers
- Caregiver burden
- Quality of life
- Utility scores
- Palliative nurse
- Palliative care
International Journal for Equity in Health
ISSN: 1475-9276
- General enquiries: [email protected]
This paper is in the following e-collection/theme issue:
Published on 8.5.2024 in Vol 26 (2024)
Application of Patient-Reported Outcome Measurements in Adult Tumor Clinical Trials in China: Cross-Sectional Study
Authors of this article:

Original Paper
- Yan Jia 1, 2 * ;
- Qi Li 1, 2 * ;
- Xiaowen Zhang 1 , MS ;
- Yi Yan 3 ;
- Shiyan Yan 4 , PhD ;
- Shunping Li 5 , PhD ;
- Wei Li 6 , PhD ;
- Xiaowen Wu 7 , PhD ;
- Hongguo Rong 1, 8 * , PhD ;
- Jianping Liu 1, 8 , PhD
1 Center for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
2 Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
3 School of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
4 College of Acupuncture and Massage, Beijing University of Chinese Medicine, Beijing, China
5 Centre for Health Management and Policy Research, Shandong University, Shandong, China
6 International Research Center for Medicinal Administration, Peking University, Beijing, China
7 Peking University Cancer Hospital & Institute, Peking University, Beijng, China
8 Institute for Excellence in Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
*these authors contributed equally
Corresponding Author:
Hongguo Rong, PhD
Center for Evidence-Based Chinese Medicine
Beijing University of Chinese Medicine
No. 11 Beisanhuan East Road, Chaoyang District
Beijing, 100029
Phone: 86 (10)64286757
Email: [email protected]
Background: International health policies and researchers have emphasized the value of evaluating patient-reported outcomes (PROs) in clinical studies. However, the characteristics of PROs in adult tumor clinical trials in China remain insufficiently elucidated.
Objective: This study aims to assess the application and characteristics of PRO instruments as primary or secondary outcomes in adult randomized clinical trials related to tumors in China.
Methods: This cross-sectional study identified tumor-focused randomized clinical trials conducted in China between January 1, 2010, and June 30, 2022. The ClinicalTrials.gov database and the Chinese Clinical Trial Registry were selected as the databases. Trials were classified into four groups based on the use of PRO instruments: (1) trials listing PRO instruments as primary outcomes, (2) trials listing PRO instruments as secondary outcomes, (3) trials listing PRO instruments as coprimary outcomes, and (4) trials without any mention of PRO instruments. Pertinent data, including study phase, settings, geographic regions, centers, participant demographics (age and sex), funding sources, intervention types, target diseases, and the names of PRO instruments, were extracted from these trials. The target diseases involved in the trials were grouped according to the American Joint Committee on Cancer Staging Manual, 8th Edition .
Results: Among the 6445 trials examined, 2390 (37.08%) incorporated PRO instruments as part of their outcomes. Within this subset, 26.82% (641/2390) listed PRO instruments as primary outcomes, 52.72% (1260/2390) as secondary outcomes, and 20.46% (489/2390) as coprimary outcomes. Among the 2,155,306 participants included in these trials, PRO instruments were used to collect data from 613,648 (28.47%) patients as primary or secondary outcomes and from 74,287 (3.45%) patients as coprimary outcomes. The most common conditions explicitly using specified PRO instruments included thorax tumors (217/1280, 16.95%), breast tumors (176/1280, 13.75%), and lower gastrointestinal tract tumors (173/1280, 13.52%). Frequently used PRO instruments included the European Organisation for Research and Treatment of Cancer Quality of Life Core Questionnaire–30, the visual analog scale, the numeric rating scale, the Traditional Chinese Medicine Symptom Scale, and the Pittsburgh Sleep Quality Index.
Conclusions: Over recent years, the incorporation of PROs has demonstrated an upward trajectory in adult randomized clinical trials on tumors in China. Nonetheless, the infrequent measurement of the patient’s voice remains noteworthy. Disease-specific PRO instruments should be more effectively incorporated into various tumor disease categories in clinical trials, and there is room for improvement in the inclusion of PRO instruments as clinical trial end points.
Introduction
Patient-reported outcome (PRO) instruments are defined as any report regarding a patient’s health status obtained directly from the patient, excluding interpretation of the patient’s responses by clinicians or other individuals [ 1 ]. PRO data consist of information obtained directly from patients concerning their health status, symptoms, treatment adherence, physical and social functioning, health-related quality of life, and satisfaction with health care [ 2 - 4 ]. Serving as noninvasive, comprehensive, and patient-centered metrics, PROs play a pivotal role in enhancing patient engagement, facilitating informed clinical decisions, and improving patient-clinician communication [ 5 - 9 ]. High-quality PRO measures examined in rigorous trials can evaluate treatment effectiveness, assess patient adherence to treatment, guide drug research, and inform health care policies [ 2 , 5 ]. In addition, some PRO instruments could supplement safety data and contribute to the assessment of tolerability (eg, Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events [PRO-CTCAE]) [ 2 , 5 ].
In particular, PROs are valuable end points in trials of disabling, chronic, and incurable conditions because they systematically capture the patients’ perspectives in a scientifically rigorous way [ 3 , 10 , 11 ]. Recognizing their importance, clinical trials focused on tumors are increasingly incorporating PRO instruments as primary or secondary outcomes [ 12 - 15 ]. The European Commission has indicated the priority of preventing cancer and ensuring a high quality of life for patients with cancer within the framework of Europe’s Beating Cancer Plan [ 16 ]. The incorporation of PROs in clinical trials offers distinct advantages, including improvements in health-related quality of life, patient-clinician communication, and economic benefits from reduced health care use [ 17 - 20 ]. To uphold best practices in tumor clinical trials that use PROs, several methodological recommendations have emerged in recent years, such as SPIRIT-PRO (Standard Protocol Items: Recommendations for Interventional Trials–Patient-Reported Outcome), CONSORT-PRO (Consolidated Standards of Reporting Trials–Patient-Reported Outcome), SISAQOL (Setting International Standards in Analysing Patient-Reported Outcomes and Quality of Life Endpoints), and other relevant guidelines [ 2 - 4 , 21 ]. However, PRO measures often receive lower priority in the design of oncology-related clinical trials when compared to survival, imaging, and biomarker-related outcomes [ 22 ].
In China, PROs are increasingly being used in clinical trials, but there are challenges as well. A cross-sectional survey of interventional clinical trials conducted in China revealed that only 29.7% of the included trials listed PRO instruments as primary or secondary outcomes [ 23 ]. Moreover, there is a notable absence of comprehensive assessments evaluating the application of PRO instruments in tumor clinical trials in China. Unlike previous cross-sectional studies that encompassed all types of clinical trials, our study primarily examined adult tumor clinical trials in China that have listed PRO instruments as primary or secondary outcomes, referencing the methodologies and reporting patterns of a previous study [ 23 ]. We extracted the registration information of adult randomized clinical trials conducted in China to systematically analyze the application of PRO instruments in tumor clinical trials, aiming to evaluate the application of PRO instruments in adult tumor clinical trials in China and provide potential directions for further investigation.
Study Design
This cross-sectional study was designed to describe the characteristics of adult tumor clinical trials conducted in China between January 1, 2010, and June 30, 2022, that listed PRO instruments as primary or secondary outcomes. All clinical trials should be registered, and data of clinical trials were collected from 2 clinical trial registries, namely ClinicalTrials.gov and the Chinese Clinical Trial Registry, with public disclosure. We conducted data retrieval and export in July 2022. The clinical trials covered 34 provincial-level administrative regions in accordance with the 2019 version of China’s administrative divisions. We further sought to describe the PRO instruments frequently used in trials encompassing diverse target tumor conditions.
Data Collection Strategy
This study focused on interventional randomized clinical trials conducted in China involving participants aged ≥18 years ( Figure 1 ). Duplicate trials with 2 registration identification numbers were treated as a single trial (ClinicalTrials.gov records were retained). The evaluation of tumor clinical trials included three types of information: (1) basic information (registration number, registration date, scientific name, recruiting country, and other information), (2) key information (outcome, target disease, and age and sex of participants), and (3) characteristic information (main sponsor’s location, study settings, number of setting centers, study stage, funding source, and intervention type).

Data Classification
PRO instruments were defined by the US Food and Drug Administration in 2009 [ 1 ] as any report about a patient’s health status obtained directly from the patient, excluding interpretation of the patient’s response by clinicians or other individuals. Trials using PRO instruments as primary or secondary outcomes were considered PRO trials. On the basis of a previous study of PRO labeling of new US Food and Drug Administration–approved drugs (2016-2020) [ 24 ], eligible trials were classified into four groups: (1) trials that listed PRO instruments as primary outcomes, (2) trials that listed PRO instruments as secondary outcomes, (3) trials that listed PRO instruments as coprimary outcomes, and (4) trials without any mention of PRO instruments.
Statistical Analysis
Data related to the characteristics of the included trials (clinical phase, study setting, participant age and sex, region of the primary sponsor, setting center, number of PROs, funding source, and type of intervention) were extracted independently by 2 authors with a predesigned data extraction table. Owing to the varied categories and wide variation of target diseases, we classified similar target diseases based on classifications from the American Joint Committee on Cancer Staging Manual, 8th Edition ( Multimedia Appendix 1 ). On the basis of this categorization of diseases, we consolidated the PRO instruments used in each trial to identify those used most frequently. We conducted quantitative analysis only on items that listed the names of PRO instruments for a more detailed understanding of the commonly used evaluation tools. All data analyses were performed using Stata (version 14.0; StataCorp LLC).
Ethical Considerations
According to the Common Rule (45 CFR part 46) of the US Department of Health and Human Services (Office for Human Research Protections), this study is exempt from institutional review board approval and the requirement for informed patient consent because it did not involve clinical data or human participants. This study followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting guidelines designed for observational studies in epidemiology.
Trial Characteristics
Table 1 presents a comprehensive overview of the included trials. The study included 7251 tumor-focused randomized controlled trials conducted in China between January 1, 2010, and June 30, 2022. Of these 7251 trials, 3276 (45.18%) were sourced from ClinicalTrials.gov, and 3975 (54.82%) were identified from the Chinese Clinical Trial Registry. Of these 7251 trials, after excluding 806 (11.12%) trials (n=5, 0.6% duplicates; n=465, 57.7% non-Chinese trials; n=321, 39.8% trials involving children; and n=15, 1.9% trials with incomplete reports), 6445 (88.88%) eligible trials were identified for analysis.
a The early phase trials included a clinical pretest as well as phase 0 and phase 1 trials.
b Diagnostic new technique clinical study, inspection technology, and trials involving multiple phases.
c Rehabilitation center, nursing home, campus, centers for disease control, home, and research institute.
d The trials were conducted in China, but their sponsor was based overseas.
e N/A: not applicable.
f Combination trials were funded partly by industry and partly by nonindustry institutions, such as universities, hospitals, and so on.
Of the 2,155,306 participants recruited in all included trials, 139,297 (6.46%) were involved in trials with PRO instruments as primary outcomes, 400,064 (18.56%) in trials with PRO instruments as secondary outcomes, and 74,287 (3.45%) in trials with PRO instruments as coprimary outcomes. Among the 6445 trials included, 2390 (37.08%) used PRO instruments as either primary or secondary outcomes, while 4055 (62.92%) did not use any PRO instrument.
The majority of the studies (6098/6445, 94.62%) did not impose any age restrictions on participants (children were excluded). In trials involving PROs, the proportion of older participants (aged >65 y; 42/2390, 1.78%) was slightly higher than in those without PROs (100/6445, 1.55%). Among all trials that incorporated PRO measurements, 17.15% (410/2390) included only female participants, while 4.48% (107/2390) included only male participants. Furthermore, in trials involving only female participants, the vast majority (974/1000, 97.4%) studied breast and female reproductive organ tumors. In trials exclusively involving male participants, more than half (135/267, 50.5%) centered around male genital organ tumors.
Regarding trial phases, of the 6445 clinical trials, early phase trials were the most prevalent (n=1317, 20.43%), followed by phase 3 trials (n=1004, 15.58%), phase 2 trials (n=873, 13.56%), and phase 4 trials (n=779, 12.09%). Of the 2390 PRO-related trials, early phase trials were again the most common (n=575, 24.06%), followed by phase 3 trials (n=284, 11.88%), phase 4 trials (n=269, 11.26%), and phase 2 trials (n=218, 9.12%).
Most of the trials (6034/6445, 93.62%) were conducted in hospitals, with hardly any (3/6445, 0.05%) conducted in community settings. More than half of the primary sponsors were located in eastern China (3745/6445, 58.11%), followed by northern (797/6445, 12.37%) and southern (682/6445, 10.58%) China, while 18.85% (1215/6445) of the primary sponsors were situated in other regions of China, such as the southwestern, central, northwestern, and northeastern regions. Similar patterns were observed for studies involving PROs. The majority of the major sponsors (1916/2390, 80.17%) originated from the eastern, northern, and southern regions of China, while 19.79% (473/2390) hailed from the southwestern, central, northeastern, and northwestern regions. There were differences in the proportions of PRO trials were noted among different provinces; the distribution of PRO instruments across Chinese provinces can be found in Multimedia Appendix 2 .
Moreover, 87.29% (5626/6445) of the trials were single-center trials, and only 11.11% (716/6445) were multicenter trials. Similar phenomena were observed for PRO-related studies, but multicenter trials accounted for a slightly higher percentage (312/2390, 13.05%). Of the 2390 PRO trials, 2144 (89.71%) used 1 to 3 PRO instruments, followed by 4 to 6 (n=218, 9.12%) and 7 to 9 (n=25, 1.05%) PRO instruments. The majority of the trials were nonindustry-funded trials (5443/6445, 84.45%), while 11.67% (752/6445) were industry-funded trials.
Table 2 shows the frequency of intervention types used across different trial classifications. The data indicated that more than a third of the included trials used drugs as the intervention (2496/6445, 38.73%), followed by combination therapies (1350/6445, 20.95%) and surgery (1044/6445, 16.2%). Among clinical trials involving drug interventions, nearly four-tenths (989/2496, 39.62%) used PRO instruments as their outcomes. Trials using drugs as the intervention exhibited a higher incidence of using PRO instruments as their primary or coprimary outcomes (468/989, 47.32%) compared to trials using other intervention types.
a PRO: patient-reported outcome.
b Other interventions included acupuncture, physical exercise, and psychosocial treatment.
Conditions and Participants
The annual counts of tumor clinical trials are listed in Figure 2 . During the study period—from January 1, 2010, to June 30, 2022—the number of tumor clinical trial registrations exhibited a consistent upward trajectory, paralleled by a commensurate increase in the number of clinical trials related to PROs.

Figures 3 and 4 depict the distribution of trial counts and corresponding participant numbers across different tumor types, respectively, wherein PROs served as outcomes. Among the 2390 tumor-related trials that used PRO instruments as primary or secondary outcomes, the top 5 tumors were thorax (448/2390, 18.74%), upper gastrointestinal tract (306/2390, 12.8%), lower gastrointestinal tract (300/2390, 12.55%), breast (289/2390, 12.09%), and head and neck (177/2390, 7.41%) tumors. Trials regarding female reproductive organ (168/2390, 7.03%) and hepatobiliary system (146/2390, 6.11%) tumors were also frequently observed. Male genital organ tumors (56/2390, 2.34%), central nervous system tumors (51/2390, 2.13%), endocrine system tumors (47/2390, 1.97%), and urinary tract tumors (33/2390, 1.38%) all accounted for proportions ranging from 1% to 5%, and hematologic malignant tumors (22/2390, 0.92%), neuroendocrine tumors (14/2390, 0.59%), bone tumors (8/2390, 0.33%), skin tumors (4/2390, 0.17%), ophthalmic tumors (2/2390, 0.08%), and soft tissue sarcoma (1/2390, 0.04%) constituted <1% of the trials.

Among the 613,648 participants enrolled in these PRO trials, 134,940 (22%) were diagnosed with lower gastrointestinal tract tumors, 131,470 (21.42%) with upper gastrointestinal tract tumors, and 79,068 (12.88%) with thorax tumors. Furthermore, there were a number of patients with breast tumors (63,238/613,648, 10.31%), female reproductive organ tumors (440,975/613,648, 6.68%), head and neck tumors (35,642/613,648, 5.81%), or hepatobiliary system tumors (22,044/613,648, 3.59%), each involving >10,000 patients. By contrast, conditions with <10,000 participants encompassed central nervous system tumors (8897/613,648, 1.45%), endocrine system tumors (8472/613,648, 1.38%), male genital organ tumors (8357/613,648, 1.36%), urinary tract tumors (6784/613,648, 1.11%), neuroendocrine tumors (3539/613,648, 0.58%), hematologic malignant tumors (2629/613,648, 0.43%), bone tumors (825/613,648, 0.13%), skin tumors (311/613,648, 0.05%), ophthalmic tumors (274/613,648, 0.04%), and soft tissue sarcoma (266/613,648, 0.04%).
PRO Instruments Used in Clinical Trials
Table 3 presents the number of explicitly specified PROs where trials precisely listed the names of the PRO instruments and the number of implicitly specified PROs where trials referenced patients’ subjective feelings without specifying the instruments used, separately for the 3 trial types. Specifically, the trial that specified the PRO instruments used was classified into “explicitly specified PROs,” and the trial that did not specify the instruments used was classified into “implicitly specified PROs.” It was evident that in primary and coprimary outcome trial sets, a greater number of trials explicitly listed the PRO instruments compared to those that did not specify the instruments used. Among the 3 trial types, the coprimary outcome category exhibited the highest proportion of explicitly specified PROs (339/489, 69.3%).
Tables 4 - 6 display the frequency of use of PRO scales for different diseases under the 3 categories. In trials using PRO instruments as coprimary outcomes, the visual analog scale (VAS) and the numeric rating scale (NRS) were the most commonly used scales for various tumors. For trials using PRO instruments as primary outcomes, the VAS was the most commonly used scale for various diseases. For trials using PRO instruments as secondary outcomes, the most commonly used scale for each disease was the European Organisation for Research and Treatment of Cancer Quality of Life Core Questionnaire-30 (EORTC QLQ-C30).
a VAS: visual analog scale.
b NRS: numeric rating scale.
c EORTC QLQ-LC43: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Lung Cancer 43.
d SF-36: 36-item Short Form Health Survey.
e PSQI: Pittsburgh Sleep Quality Index.
f IPSS: International Prostate Symptom Score.
g LARS: Low Anterior Resection Syndrome.
h EORTC QLQ-C30: European Organisation for Research and Treatment of Cancer Quality of Life Core Questionnaire-30.
i EORTC QLQ-STO22: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Stomach 22.
j UW-QOL: University of Washington Quality of Life Questionnaire.
k QoR-40: Quality of Recovery-40.
l IDS: Involvement-Detachment Scale.
m IIEF-15: International Index of Erectile Function-15.
n QoR-15: Quality of Recovery-15.
o TCMSS: Traditional Chinese Medicine Symptom Scale.
p N/A: not applicable.
a EORTC QLQ-C30: European Organisation for Research and Treatment of Cancer Quality of Life Core Questionnaire-30.
b FACT-L: Functional Assessment of Cancer Therapy–Lung.
c EORTC QLQ-LC13: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Lung Cancer 13.
d FACT-B: Functional Assessment of Cancer Therapy–Breast.
e EORTC QLQ-BR23: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Breast Cancer 23.
f VAS: visual analog scale.
g EORTC QLQ-OES18: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Oesophageal Cancer 18.
h EORTC QLQ-H&N35: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Head and Neck Cancer 35.
i NRS: numeric rating scale.
j EORTC QLQ-CX24: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Cervical Cancer 24.
k EORTC QLQ-HCC18: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Hepatocellular Carcinoma 18.
l FACT-P: Functional Assessment of Cancer Therapy–Prostate.
m BPI-SF: Brief Pain Inventory–Short Form.
n FACT-G: Functional Assessment of Cancer Therapy–General.
o QoR-40: Quality of Recovery-40.
p SF-36: 36-item Short Form Health Survey.
q QoR-15: Quality of Recovery-15.
r WHOQOL-BREF: World Health Organization Quality of Life Brief Version.
s EORTC QLQ-PAN26: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Pancreatic Cancer 26.
t FACIT: Functional Assessment of Chronic Illness Therapy.
u HF-QOL: Hand-Foot Skin Reaction and Quality of Life.
v N/A: not applicable.
w EORTC QLQ-OPT30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Ophthalmic Cancer 30.
c EORTC QLQ-C30: European Organisation for Research and Treatment of Cancer Quality of Life Core Questionnaire-30.
d QoR-15: Quality of Recovery-15.
e TNSS: Total Nasal Symptom Score.
f BCS: Bruggemann Comfort Scale.
g PSQI: Pittsburgh Sleep Quality Index.
h ICIQ-SF: International Consultation on Incontinence Questionnaire–Short Form.
i FACT-P: Functional Assessment of Cancer Therapy–Prostate.
j HADS: Hospital Anxiety and Depression Scale.
k EORTC IADL-BN32: European Organisation for Research and Treatment of Cancer Instrumental Activities of Daily Living in Patients With Brain Tumors-32.
l N/A: not applicable.
m SAS: Self-Rating Anxiety Scale.
n SDS: Self-Rating Depression Scale.
To analyze the overall application of scales in explicitly specified PROs by condition, we examined the specific PRO instruments used in trials that explicitly mentioned the PRO instruments as primary or secondary outcomes ( Table 7 ). Of the 1280 trials, 321 (25.08%) used the EORTC QLQ-C30 ( Multimedia Appendix 3 ), which was the most commonly used PRO scale. Of note, the EORTC QLQ-C30 was the most commonly used scale in trials concerning lower gastrointestinal tract, upper gastrointestinal tract, head and neck, female reproductive organ, hepatobiliary system, bone, neuroendocrine, skin, and ophthalmic tumors as well as hematologic malignancies. In addition, the VAS was used in 24.77% (317/1280) of the trials ( Multimedia Appendix 3 ), predominating in trials involving thorax, breast, male genital organ, endocrine system, central nervous system, and urinary tract tumors. The NRS was also frequently used (169/1280, 13.2%) in cancer trials. More targeted scales have been used for different tumor diseases; for example, the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ)–Head and Neck Cancer 35 (36/101, 35.6%) was more common in head and neck tumor trials, the EORTC QLQ–Oesophageal Cancer 18 (15/140, 10.7%) and the EORTC QLQ–Stomach 22 (14/140, 10%) were frequently observed in upper gastrointestinal cancer trials, the EORTC QLQ–Colorectal Cancer 29 (14/173, 8.1%) scale was prevalent in lower gastrointestinal cancer trials, the EORTC QLQ–Hepatocellular Carcinoma 18 (8/67, 12%) was frequently found in hepatobiliary system tumor trials, the Functional Assessment of Cancer Therapy (FACT)–Lung (21/217, 9.7%) and the EORTC QLQ–Lung Cancer 13 (19/217, 8.8%) commonly featured in thorax tumor trials, the FACT–Breast (29/176, 16.5%) and the EORTC QLQ–Breast Cancer 23 (16/176, 9.1%) were frequently seen in breast cancer trials, the EORTC QLQ–Ovarian Cancer 28 (6/85, 7%) was a typical scale used in female reproductive organ tumor trials, the FACT–Prostate (7/31, 23%) was often used in male genital organ tumor trials, and the FACT–Anemia (1/9, 11%) and the FACT–Lymphoma (1/9, 11%) were common choices in hematologic malignant tumor trials.
b EORTC QLQ-C30: European Organisation for Research and Treatment of Cancer Quality of Life Core Questionnaire-30.
c NRS: numeric rating scale.
d FACT-L: Functional Assessment of Cancer Therapy–Lung.
e EORTC QLQ-LC13: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Lung Cancer 13.
f FACT-B: Functional Assessment of Cancer Therapy–Breast.
g EORTC QLQ-BR23: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Breast Cancer 23.
h EORTC QLQ-CR29: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Colorectal Cancer 29.
i EORTC QLQ-OES18: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Oesophageal Cancer 18.
j EORTC QLQ-STO22: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Stomach 22.
k EORTC QLQ-H&N35: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Head and Neck Cancer 35.
l PG-SGA: Patient-Generated Subjective Global Assessment.
m SDS: Self-Rating Depression Scale.
n EORTC QLQ-OV28: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Ovarian Cancer 28.
o EORTC QLQ-HCC18: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Hepatocellular Carcinoma 18.
p TCMSS: Traditional Chinese Medicine Symptom Scale.
q FACT-P: Functional Assessment of Cancer Therapy–Prostate.
r BPI: Brief Pain Inventory.
s IPSS: International Prostate Symptom Score.
t QoR-15: Quality of Recovery-15.
u QoR-40: Quality of Recovery-40.
v PCSQ: Preparedness for Colorectal Cancer Surgery Questionnaire.
w WHOQOL-BREF: World Health Organization Quality of Life Brief Version.
x FACT-An: Functional Assessment of Cancer Therapy–Anemia.
y FACT-Lym: Functional Assessment of Cancer Therapy–Lymphoma.
z SF-36: 36-item Short Form Health Survey.
aa EORTC QLQ-PAN26: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Pancreatic Cancer 26.
ab N/A: not applicable.
ac HF-QoL: Hand-Foot Skin Reaction and Quality of Life.
ad EORTC QLQ-OPT30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Ophthalmic Cancer 30.
ae PSQI: Pittsburgh Sleep Quality Index.
af BFI: Brief Fatigue Inventory.
Principal Findings
This cross-sectional study depicted the general characteristics of adult tumor clinical trials incorporating PROs in China and analyzed the application of PRO instruments in randomized clinical trials of tumors to provide potential directions for future research and serve as a reference for tumor clinical practice. The findings revealed that a significant proportion, specifically 62.92% (4055/6445) of the included trials, missed the opportunity to capture patients’ subjective evaluations. Of the trials with PRO instruments as end points, 26.82% (641/2390) used PRO instruments as primary outcomes, 52.72% (1260/2390) as secondary outcomes, and 20.46% (489/2390) as coprimary outcomes. The majority of PRO trials (2144/2390, 89.71%) used 1 to 3 PRO instruments. Given that PROs can authentically represent patients’ subjective experiences and evaluations, they should receive heightened emphasis in the context of tumor clinical trials. However, in light of the small proportion of tumor-related randomized clinical trials assessing PROs, policy makers and standard-setting bodies are recommended to further promote the collection of PROs in such trials in China.
This study delved into the yearly distribution of tumor clinical trials, indicating a notable surge in the use of PRO instruments as end points between January 1, 2010, and June 30, 2022. Among the trials incorporating PROs, early phase trials constituted the largest proportion (575/2390, 24.06%), followed by phase 3 (284/2390, 11.88%) and phase 4 (269/2390, 11.26%) trials. A retrospective cross-sectional study suggested a potential correlation between the use of PROs in late-stage trials and improved drug outcomes, such as overall survival [ 25 ]. However, the omission of PROs in late-stage trial results may reduce the value of patient participation in these trials. Previous work has shown that the concern regarding funding for PRO research seems significant, and additional funding was needed—and considered important—to pay for the use of PRO instruments to collect relevant data [ 26 ]. This may also be the reason why, among the included studies, there were few PRO tumor trials funded by industry. Relevant policies could provide more financial support for PRO tumor trials. In addition, our study indicated that the application of PRO instruments was more prevalent in trials involving drug interventions. PRO instruments can serve as valuable tools for assessing patient experiences during treatment, which is an essential aspect of drug discovery [ 27 ], and their absence can result in the exclusion of critical information, such as opportunities for patient-centered support programs and insights into benefit-risk profiles [ 27 ].
In accordance with prior research [ 23 ], our study also identified regional differences in the use of PROs. Tumor trials were more prevalent in the eastern, northern, and southern regions of China—especially in Shanghai, Beijing, Guangdong, and Jiangsu—and the adoption of PRO measurements followed a similar pattern. Conversely, in other regions of China, especially in the northwestern and northeastern regions—such as Qinghai, Tibet and Heilongjiang—both the overall number of tumor clinical trials and those incorporating PRO instruments as end points were conspicuously lower. These results indicated the relationship between the volume of tumor clinical trials and the adoption of PRO tools. In addition, other factors such as economic conditions and medical resources also played an important role in this phenomenon [ 28 ]. Relevant policies can continue to encourage medical resources to be tilted toward rural and less developed areas. Remarkably, the study suggested that in resource-constrained remote regions, simplified applications of PRO instruments may be considered in tumor clinical trials. Moreover, our investigation revealed a lower prevalence of industry-funded trials in tumor clinical trials in China. This discrepancy may be attributed to previous findings that tumor trials were characterized by increased risk and costliness [ 29 ].
This study further found that thorax tumors, breast tumors, and lower gastrointestinal tract tumors were the most common conditions in trials with explicit PRO instruments. This might be related to variances in tumor incidence and different clinical concerns [ 30 ]. In the primary and coprimary outcome trial sets, a higher proportion of trials explicitly listed the PRO instruments as end points compared to those not specifying PROs, underscoring the normative inclination to formalize the acquisition and application of PRO instruments. Adherence to guidelines and standardization of PRO application is essential to maximize the application of PRO trial data, enhance their impact, and minimize research waste [ 31 ]. In particular, studies have shown that the standardized PROs were conducive to making trials or clinical treatments more scientifically rigorous and ethically sound [ 32 - 35 ]. Therefore, the need to standardize the application of PRO instruments remains important, with an increased emphasis on explicitly specifying PRO instruments in clinical trials.
This study analyzed the frequency of the use of PRO instruments in different classifications of trials by medical condition and found that the VAS and the NRS were the most commonly used in trials where PROs were designated as coprimary outcomes. Meanwhile, in all trials that used PRO instruments as outcomes, the VAS and the NRS were consistently prevalent. This prevalence can be attributed to the precision, simplicity, and sensitivity of VAS scores, as well as the ease of use and standardized format of the NRS for assessing subjective indicators [ 36 - 38 ]. In addition, almost 90% of patients with cancer would experience pain during the course of their illness [ 39 ]. The pain is both prevalent and burdensome for patients, but there is a lack of objective evaluation indices available for this purpose [ 40 , 41 ]. Consequently, the VAS emerged as the preferred choice for pain assessment in clinical research. Similarly, the NRS, with its user-friendly nature and standardized format, has been the preferred tool for pain assessment [ 36 - 38 ]. PROs continue to represent the gold standard for evaluating patients’ core pain outcomes [ 42 - 44 ]. In this study, among the trials that used PRO instruments as secondary outcomes, the EORTC QLQ-C30 was the most commonly used (223/606, 36.8%), which might be attributed to the significance of addressing quality-of-life concerns for patients with tumors. This study also scrutinized the prevalent PRO instruments used in various medical conditions and found that the quality-of-life scale was frequently used in clinical trials involving tumors. The high frequency of the EORTC QLQ-C30 and FACT scale groups underscored the widespread application of these instruments in assessing patients’ quality of life in cancer clinical trials in China. Specific modules in the EORTC QLQ scale system, such as the EORTC QLQ–Breast Cancer 23, the EORTC QLQ–Lung Cancer 13, and the EORTC QLQ–Colorectal Cancer 29, have been widely used in various cancer diseases [ 45 , 46 ]. Similarly, specific modules in the FACT scales, such as FACT–Lung (lung cancer), FACT–Breast (breast cancer), and FACT–Prostate (prostate cancer), have exhibited a high rate of use in cancer clinical trials in China. The extensive use of various PRO scales indicates a growing awareness and acceptance of PRO instruments, which, in turn, encourages the development of more effective and reliable PRO instruments. PRO instruments can be divided into universal and disease-specific PRO instruments. Considering the heterogeneity of symptom types in patients with tumors, symptom assessment should be performed for specific diseases [ 47 ]. However, in different tumor trials, the explicitly specified PRO instruments were primarily quality-of-life scales, the VAS, and the NRS, suggesting a need for the application of disease-specific PRO scales for different tumor types in clinical trials. It is suggested that according to the heterogeneity of diseases, experts from different fields should be brought together to develop or improve the disease-specific scale through participatory and consensus approaches under the guidance of relevant guidelines [ 33 , 47 , 48 ]. Acceptance of the scale by a wide range of stakeholders would be beneficial to improve the quality and specificity of the scale [ 48 ]. Training of clinicians and researchers on disease-specific scales is recommended. In addition, regarding the implementation of PRO measurement, it can be attempted as part of routine clinical care delivery for corresponding diseases, as well as continuous quality improvement as a clinical care priority [ 48 ].
This study undertook an in-depth analysis of the fundamental aspects of tumor clinical trials encompassing PROs in China, involving categorizing tumors and assessing the application of specific PRO tools for each tumor type. The findings underscore the critical importance of integrating PRO measures into tumor clinical trials in China and the need to standardize the use of PRO instruments within these trials. In recent years, the Chinese government has attached great importance to the application of PRO instruments in clinical trials. To encourage the patient-centered concept of new drug development and make reasonable use of PRO instruments, the National Medical Products Administration formulated the Guiding Principles for the Application of Patient Reported Outcomes in Drug Clinical Research and Development in 2022. To further promote these guiding principles, the relevant departments can educate researchers about the importance of regulating the application of PRO instruments, provide an interpretation of these principles to researchers, and advise them to follow the guidelines. We encourage researchers to communicate relevant information to regulators in a timely manner to conduct higher-quality clinical trials, such as the background of the study, the type of study, and the scale used. Policy makers should further formulate and implement pertinent policies, and PRO application platforms need to be developed and promoted to accelerate rational use of PROs in tumor clinical trials. It is recommended to define or form an institution or department to coordinate and standardize the use of PROs in clinical trials [ 49 ]. The institution or department can provide researchers with some support, such as methodological guidance for PRO applications, interpretation of relevant guidelines, and guidance on internet technologies. Efforts should also be made to encourage communication and collaboration among policy makers, researchers, and medical institutions to promote the high-quality application of PROs in clinical trials. Furthermore, it is crucial to train clinicians in how to use PRO instruments in clinical practice. Ideally, this training can be part of standard medical education programs in the future. The most successful and effective way of training involved real patient cases and problem-based learning using audio and video clips, which could enable clinicians to know how to use PRO instruments and refer to the PRO data [ 50 ]. Researchers are encouraged to follow relevant guidelines and principles and actively engage in conducting high-quality tumor clinical trials to improve well-established PRO protocols and enrich the array of available PRO instruments, thereby advancing personalized population health. In addition, it is suggested to encourage and provide relevant support to patients who have difficulties in completing the PRO reports [ 51 ].
Limitations
It is important to acknowledge several limitations to this study. First, we excluded trials lacking detailed end point information, which may have introduced bias into the results. Second, the inclusion of trials that have not yet commenced participant recruitment, although necessary for our investigation, may have inflated the reported outcomes. Finally, the exclusion of trials involving children due to their limited expressive ability and the potential influence of parental reporting on outcomes may have introduced bias in the findings.
Conclusions
In China, the incorporation of PROs has demonstrated an upward trajectory in adult randomized clinical trials of tumors in recent years. Nonetheless, the infrequent measurement of the patient’s voice remains noteworthy. This study highlights the need for a more comprehensive evaluation of patients’ experiences in adult tumor clinical trials in China. The incorporation of patients’ subjective feelings in the context of tumor diseases is necessary. Disease-specific PRO instruments should be widely used in different categories of tumor disease. Pertinent policies should be formulated and implemented, and PRO application platforms need to be developed and promoted as well. In addition, researchers should actively engage in conducting high-quality tumor clinical trials. There is room for improvement in the standardization of PROs in China.
Acknowledgments
This work was supported by the high-level traditional Chinese Medicine Key Subjects Construction Project of the National Administration of Traditional Chinese Medicine—Evidence-Based Traditional Chinese Medicine (zyyzdxk-2023249).
Data Availability
The data sets generated and analyzed during this study are available from the corresponding author on reasonable request.
Authors' Contributions
HR and JL conceived of the presented idea. YJ and QL coordinated the data collection and analysis. XW, YY, and YJ performed the data extraction. YJ and QL wrote the first draft of the paper; and SY, SL, WL, and XW provided inputs for subsequent drafts. JL and HR provided comments related to the presentation of the findings and critically reviewed the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
None declared.
Classification of specific diseases.
The number of trials with patient-reported outcomes in each province of China.
Patient-reported outcome tests used most frequently.
- Patrick DL, Burke LB, Powers JH, Scott JA, Rock EP, Dawisha S, et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health. 2007;10 Suppl 2:S125-S137. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Calvert M, Kyte D, Mercieca-Bebber R, Slade A, Chan AW, King MT, the SPIRIT-PRO Group, et al. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO extension. JAMA. Feb 06, 2018;319(5):483-494. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. Feb 27, 2013;309(8):814-822. [ CrossRef ] [ Medline ]
- U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. Oct 11, 2006;4:79. [ FREE Full text ] [ CrossRef ] [ Medline ]
- The Lancet Neurology. Patient-reported outcomes in the spotlight. Lancet Neurol. Nov 2019;18(11):981. [ CrossRef ]
- Marshall S, Haywood K, Fitzpatrick R. Impact of patient-reported outcome measures on routine practice: a structured review. J Eval Clin Pract. Oct 2006;12(5):559-568. [ CrossRef ] [ Medline ]
- Greenhalgh J, Gooding K, Gibbons E, Dalkin S, Wright J, Valderas J, et al. How do patient reported outcome measures (PROMs) support clinician-patient communication and patient care? A realist synthesis. J Patient Rep Outcomes. Dec 2018;2:42. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Chan G, Bezuidenhout L, Walker L, Rowan R. The Impact on Life questionnaire: validation for elective surgery prioritisation in New Zealand prioritisation criteria in orthopaedic surgery. N Z Med J. Apr 01, 2016;129(1432):26-32. [ Medline ]
- Black N. Patient reported outcome measures could help transform healthcare. BMJ. Jan 28, 2013;346:f167. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Tong A, Oberbauer R, Bellini MI, Budde K, Caskey FJ, Dobbels F, et al. Patient-reported outcomes as endpoints in clinical trials of kidney transplantation interventions. Transpl Int. May 20, 2022;35:10134. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Qian Y, Walters SJ, Jacques R, Flight L. Comprehensive review of statistical methods for analysing patient-reported outcomes (PROs) used as primary outcomes in randomised controlled trials (RCTs) published by the UK's Health Technology Assessment (HTA) journal (1997-2020). BMJ Open. Sep 06, 2021;11(9):e051673. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Dai W, Feng W, Zhang Y, Wang XS, Liu Y, Pompili C, et al. Patient-reported outcome-based symptom management versus usual care after lung cancer surgery: a multicenter randomized controlled trial. J Clin Oncol. Mar 20, 2022;40(9):988-996. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kouzy R, Abi Jaoude J, Lin D, Nguyen ND, El Alam MB, Ludmir EB, et al. Patient-reported outcome measures in pancreatic cancer receiving radiotherapy. Cancers (Basel). Sep 02, 2020;12(9):2487. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Singhal U, Skolarus TA, Gore JL, Parry MG, Chen RC, Nossiter J, et al. Implementation of patient-reported outcome measures into health care for men with localized prostate cancer. Nat Rev Urol. May 2022;19(5):263-279. [ CrossRef ] [ Medline ]
- Stover AM, Basch EM. Using patient-reported outcome measures as quality indicators in routine cancer care. Cancer. Feb 01, 2016;122(3):355-357. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Albreht T. Europe's beating cancer plan-a new step towards more comprehensive and equitable cancer control in Europe. Eur J Public Health. Jul 13, 2021;31(3):456-457. [ CrossRef ] [ Medline ]
- Lizán L, Pérez-Carbonell L, Comellas M. Additional value of patient-reported symptom monitoring in cancer care: a systematic review of the literature. Cancers (Basel). Sep 15, 2021;13(18):4615. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Graupner C, Kimman ML, Mul S, Slok AH, Claessens D, Kleijnen J, et al. Patient outcomes, patient experiences and process indicators associated with the routine use of patient-reported outcome measures (PROMs) in cancer care: a systematic review. Support Care Cancer. Feb 2021;29(2):573-593. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Engstrom T, Tanner S, Lee WR, Forbes C, Walker R, Bradford N, et al. Patient reported outcome measure domains and tools used among adolescents and young adults with cancer: a scoping review. Crit Rev Oncol Hematol. Jan 2023;181:103867. [ CrossRef ] [ Medline ]
- Yang LY, Manhas DS, Howard AF, Olson RA. Patient-reported outcome use in oncology: a systematic review of the impact on patient-clinician communication. Support Care Cancer. Jan 2018;26(1):41-60. [ CrossRef ] [ Medline ]
- Coens C, Pe M, Dueck AC, Sloan J, Basch E, Calvert M, et al. International standards for the analysis of quality-of-life and patient-reported outcome endpoints in cancer randomised controlled trials: recommendations of the SISAQOL Consortium. Lancet Oncol. Feb 2020;21(2):e83-e96. [ CrossRef ]
- Basch E, Geoghegan C, Coons SJ, Gnanasakthy A, Slagle AF, Papadopoulos EJ, et al. Patient-reported outcomes in cancer drug development and US regulatory review: perspectives from industry, the food and drug administration, and the patient. JAMA Oncol. Jun 01, 2015;1(3):375-379. [ CrossRef ] [ Medline ]
- Zhou H, Yao M, Gu X, Liu M, Zeng R, Li Q, et al. Application of patient-reported outcome measurements in clinical trials in China. JAMA Netw Open. May 02, 2022;5(5):e2211644. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Gnanasakthy A, Norcross L, DeMuro Romano C, Carson RT. A review of patient-reported outcome labeling of FDA-approved new drugs (2016-2020): counts, categories, and comprehensibility. Value Health. Apr 2022;25(4):647-655. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Haslam A, Herrera-Perez D, Gill J, Prasad V. Patient experience captured by quality-of-life measurement in oncology clinical trials. JAMA Netw Open. Mar 02, 2020;3(3):e200363. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Watkins Bruner D, Bryan CJ, Aaronson N, Blackmore CC, Brundage M, Cella D, et al. Issues and challenges with integrating patient-reported outcomes in clinical trials supported by the National Cancer Institute–sponsored clinical trials networks. J Clin Oncol. Nov 10, 2007;25(32):5051-5057. [ CrossRef ]
- Basch E, Dueck AC. Patient-reported outcome measurement in drug discovery: a tool to improve accuracy and completeness of efficacy and safety data. Expert Opin Drug Discov. Aug 2016;11(8):753-758. [ CrossRef ] [ Medline ]
- Liniker E, Harrison M, Weaver JM, Agrawal N, Chhabra A, Kingshott V, et al. Treatment costs associated with interventional cancer clinical trials conducted at a single UK institution over 2 years (2009-2010). Br J Cancer. Oct 15, 2013;109(8):2051-2057. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. Apr 01, 2019;20(2):273-286. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). Mar 17, 2021;134(7):783-791. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Rivera SC, Kyte DG, Aiyegbusi OL, Slade AL, McMullan C, Calvert MJ. The impact of patient-reported outcome (PRO) data from clinical trials: a systematic review and critical analysis. Health Qual Life Outcomes. Oct 16, 2019;17(1):156. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Di Maio M, Gallo C, Leighl NB, Piccirillo MC, Daniele G, Nuzzo F, et al. Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials. J Clin Oncol. Mar 10, 2015;33(8):910-915. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Howell D, Molloy S, Wilkinson K, Green E, Orchard K, Wang K, et al. Patient-reported outcomes in routine cancer clinical practice: a scoping review of use, impact on health outcomes, and implementation factors. Ann Oncol. Sep 2015;26(9):1846-1858. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Crites JS, Chuang C, Dimmock A, Hwang W, Johannes B, Paranjape A, et al. PROs in the balance: ethical implications of collecting patient reported outcome measures in the electronic health record. Am J Bioeth. Mar 16, 2016;16(4):67-68. [ CrossRef ] [ Medline ]
- Kyte D, Draper H, Calvert M. Patient-reported outcome alerts: ethical and logistical considerations in clinical trials. JAMA. Sep 25, 2013;310(12):1229-1230. [ CrossRef ] [ Medline ]
- Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain. Feb 2003;4(1):2-21. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Caraceni A, Brunelli C, Martini C, Zecca E, De Conno F. Cancer pain assessment in clinical trials. A review of the literature (1999-2002). J Pain Symptom Manage. May 2005;29(5):507-519. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. Jun 2011;41(6):1073-1093. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Hjermstad MJ, Fainsinger R, Kaasa S, European Palliative Care Research Collaborative (EPCRC). Assessment and classification of cancer pain. Curr Opin Support Palliat Care. Mar 2009;3(1):24-30. [ CrossRef ] [ Medline ]
- Santoni A, Santoni M, Arcuri E. Chronic cancer pain: opioids within tumor microenvironment affect neuroinflammation, tumor and pain evolution. Cancers (Basel). Apr 30, 2022;14(9):2253. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Morishita S, Hirabayashi R, Tsubaki A, Aoki O, Fu JB, Onishi H, et al. Relationship between balance function and QOL in cancer survivors and healthy subjects. Medicine (Baltimore). Nov 19, 2021;100(46):e27822. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Comer SD, Zacny JP, Dworkin RH, Turk DC, Bigelow GE, Foltin RW, et al. Core outcome measures for opioid abuse liability laboratory assessment studies in humans: IMMPACT recommendations. Pain. Dec 2012;153(12):2315-2324. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. Dec 2009;146(3):238-244. [ CrossRef ] [ Medline ]
- Younger J, McCue R, Mackey S. Pain outcomes: a brief review of instruments and techniques. Curr Pain Headache Rep. Feb 23, 2009;13(1):39-43. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Wan C, Meng Q, Yang Z, Tu X, Feng C, Tang X, et al. Validation of the simplified Chinese version of EORTC QLQ-C30 from the measurements of five types of inpatients with cancer. Ann Oncol. Dec 2008;19(12):2053-2060. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. Mar 03, 1993;85(5):365-376. [ CrossRef ] [ Medline ]
- Taylor F, Reasner DS, Carson RT, Deal LS, Foley C, Iovin R, et al. Development of a symptom-based patient-reported outcome instrument for functional dyspepsia: a preliminary conceptual model and an evaluation of the adequacy of existing instruments. Patient. Oct 28, 2016;9(5):409-418. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Howell D, Fitch M, Bakker D, Green E, Sussman J, Mayo S, et al. Core domains for a person-focused outcome measurement system in cancer (PROMS-Cancer Core) for routine care: a scoping review and Canadian Delphi Consensus. Value Health. 2013;16(1):76-87. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Eton DT, Beebe TJ, Hagen PT, Halyard MY, Montori VM, Naessens JM, et al. Harmonizing and consolidating the measurement of patient-reported information at health care institutions: a position statement of the Mayo Clinic. Patient Relat Outcome Meas. Feb 10, 2014;5:7-15. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Santana MJ, Haverman L, Absolom K, Takeuchi E, Feeny D, Grootenhuis M, et al. Training clinicians in how to use patient-reported outcome measures in routine clinical practice. Qual Life Res. Jul 2015;24(7):1707-1718. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Riedl D, Lehmann J, Rothmund M, Dejaco D, Grote V, Fischer MJ, et al. Usability of electronic patient-reported outcome measures for older patients with cancer: secondary analysis of data from an observational single center study. J Med Internet Res. Sep 21, 2023;25:e49476. [ FREE Full text ] [ CrossRef ] [ Medline ]
Abbreviations
Edited by A Mavragani; submitted 14.01.23; peer-reviewed by Y Chu, L Guo; comments to author 24.10.23; revised version received 29.10.23; accepted 09.02.24; published 08.05.24.
©Yan Jia, Qi Li, Xiaowen Zhang, Yi Yan, Shiyan Yan, Shunping Li, Wei Li, Xiaowen Wu, Hongguo Rong, Jianping Liu. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 08.05.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete bibliographic information, a link to the original publication on https://www.jmir.org/, as well as this copyright and license information must be included.

IMAGES
VIDEO
COMMENTS
There are various types of scientific studies such as experiments and comparative analyses, observational studies, surveys, or interviews. The choice of study type will mainly depend on the research question being asked. When making decisions, patients and doctors need reliable answers to a number of questions. Depending on the medical condition and patient's personal situation, the following ...
Classification of Types of Research. There are various types of research that are classified according to their objective, depth of study, analysed data, time required to study the phenomenon and other factors. It's important to note that a research project will not be limited to one type of research, but will likely use several.
Here are six common types of research studies, along with examples that help explain the advantages and disadvantages of each: 1. Meta-analysis. A meta-analysis study helps researchers compile the quantitative data available from previous studies. It's an observational study in which the researchers don't manipulate variables.
Types of Research Designs Compared | Guide & Examples. Published on June 20, 2019 by Shona McCombes.Revised on June 22, 2023. When you start planning a research project, developing research questions and creating a research design, you will have to make various decisions about the type of research you want to do.. There are many ways to categorize different types of research.
About Research Methods. This guide provides an overview of research methods, how to choose and use them, and supports and resources at UC Berkeley. As Patten and Newhart note in the book Understanding Research Methods, "Research methods are the building blocks of the scientific enterprise. They are the "how" for building systematic knowledge.
While there are dozens of types of research, most research done falls into one of five categories: clinical case studies; small, non-randomized studies or surveys; large, randomized clinical ...
There are many different types of research studies, and each has distinct strengths and weaknesses. In general, randomized trials and cohort studies provide the best information when looking at the link between a certain factor (like diet) and a health outcome (like heart disease). Laboratory and Animal Studies
Interpret the results. General Types of Educational Research. Descriptive — survey, historical, content analysis, qualitative (ethnographic, narrative, phenomenological, grounded theory, and case study) Associational — correlational, causal-comparative. Intervention — experimental, quasi-experimental, action research (sort of)
This type of information cannot be measured using statistics. Both of these types of studies report original research and are considered single studies. Watch the video below for more information. Study Designs. Some research study types that you will encounter include: Randomized Controlled Trials; Case-Control Studies; Cohort Studies; Cross ...
A meta-analysis is a type of systematic review that goes one step further, combining the data from multiple studies and using statistics to summarize it, as if creating a mega-study from many smaller studies.4. However, even systematic reviews and meta-analyses aren't the final word on scientific questions.
Types of clinical research. There are many types of clinical research: Prevention studies look at ways to stop diseases from occurring or from recurring after successful treatment. Screening studies compare detection methods for common conditions. Diagnostic studies test methods for early identification of disease in those with symptoms.
Research methods are specific procedures for collecting and analyzing data. Developing your research methods is an integral part of your research design. When planning your methods, there are two key decisions you will make. First, decide how you will collect data. Your methods depend on what type of data you need to answer your research question:
Technological: This research looks for ways to improve efficiency in products, processes and production. Scientific: This research measures certain variables to predict behaviors, outcomes and impact. Example: A student working on a doctorate in education studies ways to increase student involvement in the classroom.
Step 2: Choose a type of research design. Within both qualitative and quantitative approaches, there are several types of research design to choose from. Each type provides a framework for the overall shape of your research. ... For practical reasons, many studies use non-probability sampling, but it's important to be aware of the limitations ...
Epidemiology is defined as the study of human populations. These studies often investigate the relationship between dietary consumption and disease development. There are three main types of epidemiological studies: cross-sectional, case-control, and prospective cohort studies. Figure 2.2: Types of epidemiology.
Figure 2.3. The hierarchy of evidence shows types of research studies relative to their strength of evidence and relevance to real-life nutrition decisions, with the strongest studies at the top and the weakest at the bottom. The pyramid also represents a few other general ideas.
An example of this type of research in psychology would be changing the length of a specific mental health treatment and measuring the effect on study participants. 2. Descriptive Research . Descriptive research seeks to depict what already exists in a group or population. Three types of psychology research utilizing this method are: Case studies
This type of research is often used to study cause-and-effect relationships and to make predictions. Qualitative Research Methodology. This is a research methodology that involves the collection and analysis of non-numerical data such as words, images, and observations. This type of research is often used to explore complex phenomena, to gain ...
Here, we'll focus on the three main types of dissertation research to get you one step closer to earning your doctoral degree. 1. Qualitative. The first type of dissertation is known as a qualitative dissertation. A qualitative dissertation mirrors the qualitative research that a doctoral candidate would conduct throughout their studies.
In observational studies, the people in the study live their daily lives as they choose. They exercise when they want, eat what they like and take the medicines their doctors prescribe. They report these activities to researchers. There are 2 types of observational studies: Prospective cohort studies. Case-control studies.
The Online Writing Lab at Purdue University houses writing resources and instructional material, and we provide these as a free service of the Writing Lab at Purdue.
There are eight types of marketing research you can try to stay ahead of the competition. Learn more about marketing research methods and how to use them. ... This type of study can be done online. Eye tracking studies monitor where people's eyes are drawn. Generally, they are conducted on websites and apps, but can also be done in stores to ...
When marijuana becomes a Schedule III instead of a Schedule I substance under federal rules, researchers will face fewer barriers to studying it. But there will still be some roadblocks for science.
A lot of research shows that meditation is good for health. But some experts believe there's not enough research to prove that meditation helps. With that in mind, some research suggests that meditation may help people manage symptoms of conditions such as: Anxiety. Asthma. Cancer. Chronic pain. Depression. Heart disease. High blood pressure.
Purpose The research endeavors to explore the implications of CD47 in cancer immunotherapy effectiveness. Specifically, there is a gap in comprehending the influence of CD47 on the tumor immune microenvironment, particularly in relation to CD8 + T cells. Our study aims to elucidate the prognostic and immunological relevance of CD47 to enhance insights into its prospective utilities in ...
Validity of the EQ-5D-5L in our study sample. We report the Cronbach's alpha for internal consistency, the eigen value for the extracted factor, the factor loadings of the extracted factor onto each item of the EQ-5D-5L and Pearson's correlation coefficient between the extracted factor and utility scores in Table 2.Internal consistency was moderate to good, eigenvalue was more than one and ...
Background: International health policies and researchers have emphasized the value of evaluating patient-reported outcomes (PROs) in clinical studies. However, the characteristics of PROs in adult tumor clinical trials in China remain insufficiently elucidated. Objective: This study aims to assess the application and characteristics of PRO instruments as primary or secondary outcomes in adult ...