Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 04 March 2022

Fish diversity patterns along coastal habitats of the southeastern Galapagos archipelago and their relationship with environmental variables
- Marjorie Riofrío-Lazo 1 ,
- Manuel J. Zetina-Rejón 2 ,
- Leandro Vaca-Pita 3 ,
- Juan Carlos Murillo-Posada 4 &
- Diego Páez-Rosas 1 , 5
Scientific Reports volume 12 , Article number: 3604 ( 2022 ) Cite this article
4238 Accesses
8 Citations
11 Altmetric
Metrics details
- Biodiversity
- Community ecology
- Ichthyology
Coastal habitats are essential for ecological processes and provide important ecosystem services. The Galapagos archipelago has a wide diversity of ichthyofauna which preservation guarantees the functioning of the marine ecosystem. In this study, we used ecological and taxonomic indices as well as multivariate analysis to identify spatiotemporal changes in fish community structure in coastal habitats of San Cristóbal Island in the southeastern Galapagos archipelago. We analyzed how the patterns of variability were related to the abiotic conditions (substrate, sea temperature and depth) of each habitat. Nine sites affected by anthropogenic influence (fishing and tourism) representing different habitats/substrates were sampled. Underwater surveys were conducted during the warm and cold seasons in 2010 and 2011 at transects that varied in depth according to site. Artificial habitat, followed by coral and rocky habitats, had the highest diversity, evenness, and taxonomic distinctness, while mangrove habitats had the lowest values. This was related to the habitat complexity and possible anthropogenic influences. While the diversity patterns were more strongly related to the type of substrate, followed by the combination of substrate and depth, and the sea temperature had less influence. These findings were related to the ecological traits of the fish communities and their mobility between habitats. Temporal changes in fish community diversity and composition were not detected at all sites, suggesting that these species have high fidelity to their habitats and a high environmental tolerance that allows them to persist in their habitats despite strong changes in sea temperature on the Galapagos archipelago.
Similar content being viewed by others
All shallow coastal habitats matter as nurseries for Mediterranean juvenile fish
Potential impacts of marine urbanization on benthic macrofaunal diversity
High taxonomic resolution surveys and trait-based analyses reveal multiple benthic regimes in North Sulawesi (Indonesia)
Introduction.
The Galapagos archipelago has an extensive marine zone that represents a unique hotspot of marine species diversity; these species have colonized this region because of the presence of characteristic marine currents 1 , 2 , 3 . This situation has resulted in diverse marine fish fauna 4 , 5 . There are 128 families of fishes that have been reported in the Galapagos, of which approximately 75 species are endemic 6 , 7 . Approximately 444 fish species have been described 8 , and their distributions are associated with multiple factors, such as resource availability, substrate topography, marine currents, and species behavior 9 , 10 , 11 .
The Equatorial Undercurrent and the South Equatorial current are important in the Galapagos as they supply a macronutrients influx and transport the larvae of different species 6 , 12 . These currents influence the levels of marine productivity, creating a series of regions within the archipelago 13 , 14 , which are distinguished by a mix of Panamanian, Peruvian, Indo-Pacific, and endemic fish species 2 , 11 . The waters of Equatorial Undercurrent are the most nutrient-rich and generate continuous upwellings, mainly in the western region, which contribute to phytoplankton blooms, leading to an increased abundance and diversity of species 13 , 15 .
Productive habitats are also present near the coasts of southeastern islands (Floreana, San Cristóbal, Española), with varying persistence over time 15 , 16 . The zones surrounding the coastlines of islands, inlets, and rocks are relevant habitats in the archipelago where important ecological processes take place 17 , 18 . The coastal habitats, such as rocky reefs, mangroves, coral zones, and sand beaches, on the archipelago are productive areas that supply important ecosystem functions, such as feeding, protection and reproduction areas. A variety of species inhabit these areas through some or all phases of their life span 2 , 12 , 19 , 20 , 21 . Therefore, these habitats are essential as feeding areas, nurseries, and spawning areas and for fish migration with commercial and ecological relevance 22 , 23 , 24 , 25 . Fish frequently depend on distinct habitats with different structural complexities and substrate composition through their life span and visit them seasonally 22 . Habitats are thus linked through species migration 26 .
The preservation of marine biodiversity guarantees the functioning of ecosystems 27 ; therefore, the diminished biodiversity is particularly worrying because it is a difficult process to manage. Some ecological indices have been developed to evaluate the biodiversity of ecosystems, being the indices of diversity, evenness and richness those that are commonly used to compare fish community structures 25 , 28 , 29 . Multivariate analysis is an alternative tool for understanding spatiotemporal changes in biodiversity in different communities 30 . Since the number of species recorded in a site is highly related to sampling effort, these approaches evaluate variations in the taxonomic relatedness between species that could be linked to functional diversity 31 , 32 . Hence, they are used to compare spatiotemporal distributions of species and possible degraded areas 33 , 34 , 35 .
Despite the high ecological importance of the coastal habitats in the Galapagos Islands 17 , 18 , there is a gap regarding the taxonomic diversity evaluation of the fish communities and variability patterns among habitats, which is relevant from the ecosystem management perspective. The zoning of the Galapagos Marine Reserve is focused on protecting marine ecosystems and their biodiversity as well as regulating human activities, such as tourism and artisanal fishing within the archipelago 36 , 37 . However, the zoning system, established in 2000, is still considered preliminary as it does not adequately represent the conservation needs around the marine reserve. Therefore, more data on species diversity and their distribution in the marine ecosystem are required for reconfiguring these zones 17 , 18 .
The fish communities’ composition of the Galapagos is related to its geographical location and the influence of currents and water temperature that generate a remarkable environmental diversity 38 . Factors influencing patterns of variation in fish diversity among different coastal habitat types in the same region of the archipelago have not been thoroughly studied. However, it has been reported that the depth and habitat complexity, described by roughness and number of cavities, determine the structure of fish populations on rocky bottoms in the Galapagos Islands 39 . Furthermore, temperature gradients and the concentration of nutrients in the water (related to anthropogenic influence) likely influence the structure of fish assemblages in rocky habitats 40 . While, in mangrove habitats, the islands' isolation and their location in a convergence zone could influence the composition of fish communities between regions of the Galapagos 25 .
In this study, we compare the fish communities in coastal habitats (coral, rocky, mangrove, oceanic, and artificial) on San Cristóbal Island in the southeastern region of the Galapagos archipelago to determine possible differences in taxonomic diversity and composition among habitats by using ecological and taxonomic indices and multivariate analysis. We assessed which habitats show the highest taxonomic diversity and analyzed how the patterns of diversity variability relate to the abiotic conditions (substrate, depth and temperature) of each habitat. We hypothesized that in a same region of the archipelago, habitat complexity and depth are the variables more influencing fish communities' structure and diversity. While temporally, the seasonal sea temperature could determine the fish diversity patterns in coastal habitats.
The Galapagos Islands are situated approximately 1000 km from mainland Ecuador (Fig. 1 ). This archipelago has 15 major islands and is located within an upwelling system because of the convergence of several oceanic currents 13 . These currents show variations in their strengths throughout the year which result in two different seasons: a warm season (January to May) and a cold season (June to December) with temperatures higher than 25 °C, and between 18 and 24 °C, respectively 41 . This seasonality affects the sea surface temperature around the islands, ranging up to 8 °C between seasons 41 .
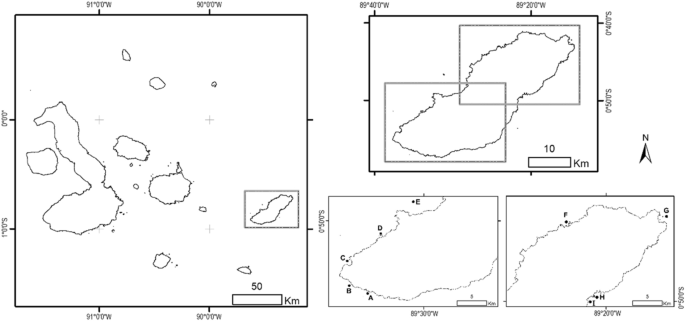
Study area showing the sampling sites (black dots) on the shelf of San Cristóbal Island in the southeastern Galapagos archipelago. (A) Las Negritas (0° 56′ 29.874″ S, 89° 35′ 07.84″ W), (B) La Lobería (0° 55′ 47.25″ S, 89° 36′ 45.65″ W), (C) Karahua (0° 53′ 43.34″ S, 89° 37′ 23.38″ W), (D) Isla Lobos (0° 51′ 23.01″ S, 89° 33′ 55.49″ W), (E) León Dormido (0° 46′ 42.51″ S, 89° 31′ 13.12″ W), (F) La Tortuga (0° 43′ 8.28″ S, 89° 23′ 29.99″ W), (G) Punta Pitt (0° 41′ 58.99″ S, 89° 14′ 42.24″ W), (H) Rosa Blanca-Coral (0° 49′ 43.60″ S, 89° 21′ 14.25″ W) and (I) Rosa Blanca-Mangrove (0° 49′ 51.25″ S, 89° 21′ 41.79″ W). The map was created using ArcGIS 10.5.1 (ESRI, https://www.esri.com ).
We carried out this research in coastal habitats of San Cristóbal Island, which is at the eastern end of the Galapagos archipelago (Fig. 1 ). This island has a shoreline perimeter of approximately 159 km and the subtidal zone can extend to 3 km from the coast, reaching depths of ~ 50 m 42 . More than 90% of the wide shelf is covered by a rocky reef habitat, which is complemented by few patches of mangrove areas and coral reefs 2 , 43 . These characteristics make this site a primary habitat for many fish species during their ontogenetic development, depending on their feeding, growth, reproduction and protection needs 8 . Nine sampling sites distributed throughout the study area were selected according to the type of habitat (Fig. 1 ). We refer to the habitat as the most dominant feature accountable for the environment structural complexity 44 , which can originate both from geological structures (e.g., rocky bottoms) or vegetation (e.g., mangroves).
Rocky habitat is characterized by an irregular sea bottom formed by lava rocks with pronounced rocky elevations producing a variety of caves and fissures. The Isla Lobos islet, Las Negritas and La Lobería represent rocky habitat. Isla Lobos islet is located northwest of the island 0.30 km from the coast; the outer part has an extensive rocky sea bottom with sand patches. Las Negritas, to the southwest of the island, has a very irregular sea bottom, mainly rocky with sand patches, small vegetated areas, and the presence of a few coral colonies from the Pavona genera. Finally, La Lobería, in the southwest of the island, has a wide coral beach and a sea bottom of rocky reefs.
The coral habitat presented an extensive submerged wall with numerous coral colonies of different species, including those of the Pavona and Pocillopora genera. Punta Pitt and Rosa Blanca represent coral habitat. Punta Pitt is a bay located north of the island; the outer part has a sea bottom with a wide coral and algae covering and some rocky formations. To the island's east, Rosa Blanca is a semi-closed coastal area whose outer part of greater depth (about 11.5 m) presents a sea bottom with numerous coral communities accompanied by rocks and sand patches.
The mangrove habitat was in shallow waters (up to 3 m of depth) distinguished by the presence of red mangroves ( Rhizophora mangle L.) and a soft sea bottom mainly consisting of sand. The inshore zone in Rosa Blanca and La Tortuga represent mangrove habitat. At Rosa Blanca, the sea bottom is primarily sandy with few rocky areas. About 95% of the bay is covered by red mangrove trees and 5% by black mangrove ( Avicennia germinans L.). La Tortuga, located to the island's northwest, is a coastal area surrounded by red mangroves and presents a sandy-rocky sea bottom.
The oceanic habitat corresponded to open waters of less than 30 m in depth that were not directly beside the coast but that had an extensive submerged wall composed of compacted volcanic ash. León Dormido (kicker rock) represents oceanic habitat; it is formed by two eroded volcanic tuff rocks located northwest of the island 5 km from the coast. Finally, the artificial habitat was represented by manmade structures constructed of hard substrates. Karahua (sunken ship) represents artificial habitat. This shipwreck locates to the east of the island 0.66 km from the coast, a large reef has formed around the ship's hull, and the sea bottom around it is of a sandy-rocky type.
Data collection
Data were collected during months representative of the warm and cold seasons in 2010 and 2011 by underwater visual surveys using SCUBA diving and snorkeling. All sites were not sampled simultaneously but the number of transects sampled per season was similar among sites (Table 1 ). Censuses were carried out in a total of 180 transects (20 transects at each site) that varied in depth according to site. Dives were usually made at depths of 5 to 12 m in Isla Lobos, Las Negritas, La Lobería, Punta Pitt and Rosa Blanca-coral. The deepest dives (12 to 16 m) were made in León Dormido and Karahua, while shallow dives (1 to 3 m) were made in La Tortuga and the Rosa Blanca-mangrove. The censuses were conducted by a team of three divers early in the morning (08:00 to 10:00) when the exterior lighting favors the identification of species. The transects were linear (2 m wide by 50 m long) and were located at two meters in height, measured from the sea bottom towards the diver. At the León Dormido site, the transects were located at the submerged wall. At mangrove sites, transects were located next to the mangrove fringe to record fish up to 1 m inside the mangrove roots. Two divers moved forward, identifying and counting the species that were within two meters of their perspective (on both left and right sides of the transect). The third diver took pictures of all identified fishes and recorded sea bottom temperature and depth data with a dive computer. This methodology is commonly applied for ichthyofaunal ecological monitoring, as it is a noninvasive and nondestructive technique for gathering data on fish assemblages for further estimates of the density of underwater species 2 , 38 , 45 , 46 . Specialized identification keys 6 were used to identify fish at species level and later corroborated by photo identification based on 47 criteria. Since not all sites were sampled during the same year and the same months, we grouped the data into warm and cold seasons, and analysis was performed as follows.
Data analysis
The numeric abundance was standardized to density by dividing the number of individuals by the number of transects surveyed in each site. We compared the fish community by site and substrate (i.e., rocky, coral, mangrove, oceanic and artificial) during the warm and cold seasons. For comparison purposes with other studies, we assessed the fish community with the most common related diversity indices in fish ecology, Shannon’s diversity index (H′), Pielou’s evenness (J′), the average taxonomic distinctness (Δ+), and the variation in taxonomic distinctness (Λ+), which estimates the taxonomic tree asymmetry 32 . For this, we used the following equations:
where pi is the relative abundance of each species, corresponding to the proportion of individuals of a species concerning the total individuals in the community; H max represents the highest Shannon–Wiener diversity possible value, reached when the species have equal abundances; S is the number of species observed in the sample, and w ij is the weight given to the branch length between species pair i and j in the hierarchical classification. For the taxonomic classification, we used the data retrieved from FishBase ( https://www.fishbase.de/ ) and considered six hierarchies (class, order, family, genus and species) for each taxon. The average taxonomic distinctness (∆+) estimation was based on presence/absence data at each site. In addition, we estimated ∆+ and Λ+ from 1000 simulated subsamples with different numbers of species. We used those estimations to generate 95% probability funnels that were then plotted versus the observed values. In this way, we compared the observed values of ∆+ and Λ+ against the expected values based on random samples. Because the sampling effort does not influence the values of ∆+ and Λ+ , both indicators can be used for comparison with future research 30 , 48 .
In order to identify patterns in the fish community at the sampling sites, a non-metric multidimensional scaling (MDS) analysis was used. MDS is a useful technique to visualize the similarities of data of the same type (e.g., abundance of species). The MDS was carried out by a similarity matrix of Bray–Curtis estimated from a square-root-transformed fish abundance matrix. In the MDS, sampling sites are represented in two-dimensional space, thus the relative distances among sites are in the same rank order. Later, we used an analysis of similarity (ANOSIM) to assess if the community associations do not differ spatially 49 . Additionally, we used a BIOENV test employing Spearman’s rank correlation coefficients to identify which abiotic variables are associated to the fish community structure 30 , 50 . For each sampling site, biotic data consisted of the species abundance and abiotic data were based on a categorical variable for substrate and two continuous variables, depth, and temperature. We used the same biotic matrix employed in the MDS and ANOSIM. We used the similarity percentages (SIMPER) algorithm to determine the species responsible for the differences among groups detected by the MDS 49 . This approach is based on decomposing dissimilarities for measuring the individual species contribution to the overall dissimilarity. Species that accounted for at least 70% of the dissimilarities were identified as responsible for the differences between groups.
To assess statistical differences in the temperature, species richness, diversity of the fish community, evenness, and average taxonomic distinctness values among sites, we performed a Kruskal–Wallis test. We test differences between seasons by a Wilcoxon paired test. The statistical significance was based on the “P-value” at the 0.05 level.
We identified an overall of 43,318 individuals from 75 fish species (Supplementary Table S1 ); 67 of these species were Actinopterygii, and eight were Elasmobranchii. Species were clumped into 61 genera, 36 families, and eight orders, of which Perciformes included 98.54% of the species. The most represented families were Pomacentridae, with six species, and Haemulidae, Scaridae, and Serranidae, with five species each. The species Prionurus laticlavius (Valenciennes, 1846) (17.9%), Thalassoma lucasanum (Gill, 1862) (13.1%) and Halichoeres dispilus (Günther, 1864) (11.1%) constituted the highest share of the whole counted fishes.
The sea temperatures recorded at the sampling sites are shown in Table 1 . The temperature patterns were similar among sites and were significantly higher during the warm season (Wilcoxon paired test, P > 0.05 in all comparisons; Supplementary Fig. S1 and Supplementary Table S2 ). The average (minimum, maximum) temperature during the warm season was 26.8 °C (20–30.5 °C) and that during the cold season was 20.5 °C (16.6–25 °C).
The species richness mean values (± standard deviation) were higher in the warm season (25.6 ± 9) than in the cold season (22.3 ± 7.67), although they did not differ statistically (Kruskal–Wallis test, \(\chi\) 2 = 0.259, df = 1, P = 0.610); Supplementary Fig. S2 and Supplementary Table S2 ). However, we found differences among sites (Kruskal–Wallis test, \(\chi\) 2 = 29.74, df = 8, P = 0.0001; Fig. 2 a). Punta Pitt and Rosa Blanca, which had the presence of coral substrate, had the highest numbers of species during both seasons, while La Lobería, which had rocky substrate, had the lowest species richness in both seasons. Other sites with rocky substrates, such as Negritas and Isla Lobos, had intermediate levels of species richness in both seasons, which were slightly higher than those in La Tortuga and Rosa Blanca, which had mangrove substrates. León Dormido, the oceanic habitat, had lower species richness than La Tortuga and Rosa Blanca (mangrove). Karahua, which had artificial substrate, had a low number of species during the warm season, but the number of species was high during the cold season. The most abundant species at each site are shown in Table 2 . In general, Stegastes beebei (Nichols, 1924), H. dispilus and T. lucasanum were among the most abundant fish species in rocky habitats; Paranthias colonus (Valenciennes, 1846), in oceanic habitat; Stegastes arcifrons (Heller & Snodgrass, 1903), in mangrove habitat; and P. laticlavius and T. lucasanum , in coral and artificial habitats.
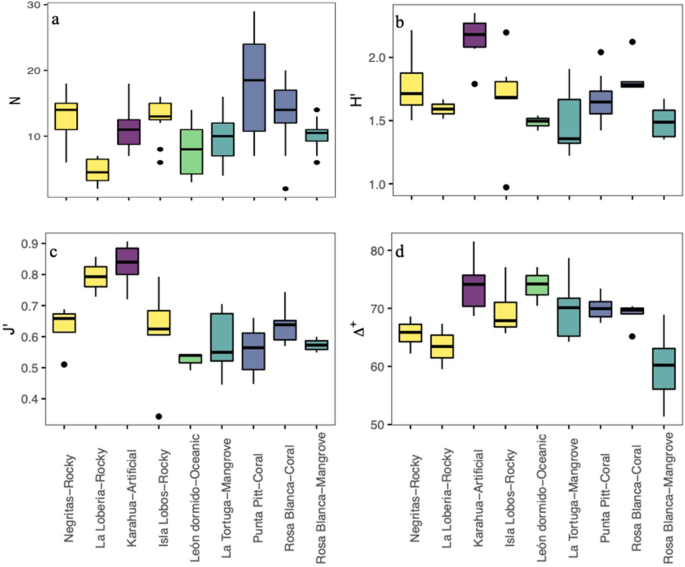
Fish community indices in the different coastal habitat types (sites/substrates) in the southeastern Galapagos Islands: ( a ) species richness (N), ( b ) Shannon index (H′), ( c ) Pielou’s evenness (J′), and ( d ) average taxonomic distinctness (Δ+).
The ecological diversity of the fish community did not show significant differences between seasons by site (Wilcoxon paired test, P > 0.05 in all comparisons; Supplementary Fig. S3 and Supplementary Table S2 ). In most cases, the variation during each season was low, except for the cold season in Isla Lobos and La Tortuga. The mean diversity values per site ranged from 1.3 to 2 decits/ind in the warm season and 1.4 to 2.3 decits/ind in the cold season. However, differences were found among the sites/substrates analyzed (Kruskal–Wallis test, \(\chi\) 2 = 23.51, df = 8, P = 0.0028; Fig. 2 b). In general, the highest diversity values were in the artificial substrate (Karahua), while the lowest values were in the mangrove and oceanic substrates in both seasons (La Tortuga, Rosa Blanca (mangrove) and León Dormido). The evenness also did not show significant differences among seasons (Wilcoxon paired test, P > 0.05 in all comparisons; Supplementary Fig. S4 and Supplementary Table S2 ). The mean evenness values per site ranged from 0.5 to 0.8 in the warm season and 0.4 to 0.9 in the cold season. However, significant differences were found among substrates (Kruskal–Wallis test, \(\chi\) 2 = 25.51, df = 8, P = 0.0013), with the highest evenness value being the artificial substrate and the lowest being the oceanic and mangrove substrates (León Dormido and Rosa Blanca (mangrove); Fig. 2 c).
In relation to the average taxonomic distinctness, we also found no significant differences among seasons per site (Wilcoxon paired test, P > 0.05 in all comparisons; Supplementary Fig. S5 and Supplementary Table S2 ). The mean values per site ranged from 59 to 80 in the warm season and from 57 to 73 in the cold season. However, when comparing the values of average taxonomic distinctness among sites/substrates using a Kruskal–Wallis test, we found statistical differences ( \(\chi\) 2 = 22.4, df = 8, P = 0.0042; Fig. 2 d). The greatest values were in the artificial substrate (Karahua), and the lowest were in the mangrove substrate [Rosa Blanca (mangrove)]. The comparison of average taxonomic distinctness observed and expected values is displayed in Fig. 3 . We found that for all sites, the values were within the 95% probability limits of the simulation, meaning that the observed values were not significantly different from the expected value, except for one mangrove sampling point at the Rosa Blanca site. Additionally, the variation in the average taxonomic distinctness observed values were not different from the expected values, with higher variation and a lower number of species. The values and the variation in the taxonomic distinctness seemed to be very heterogeneous among sites, indicating similarities in the taxonomic composition of the fish community among sites.
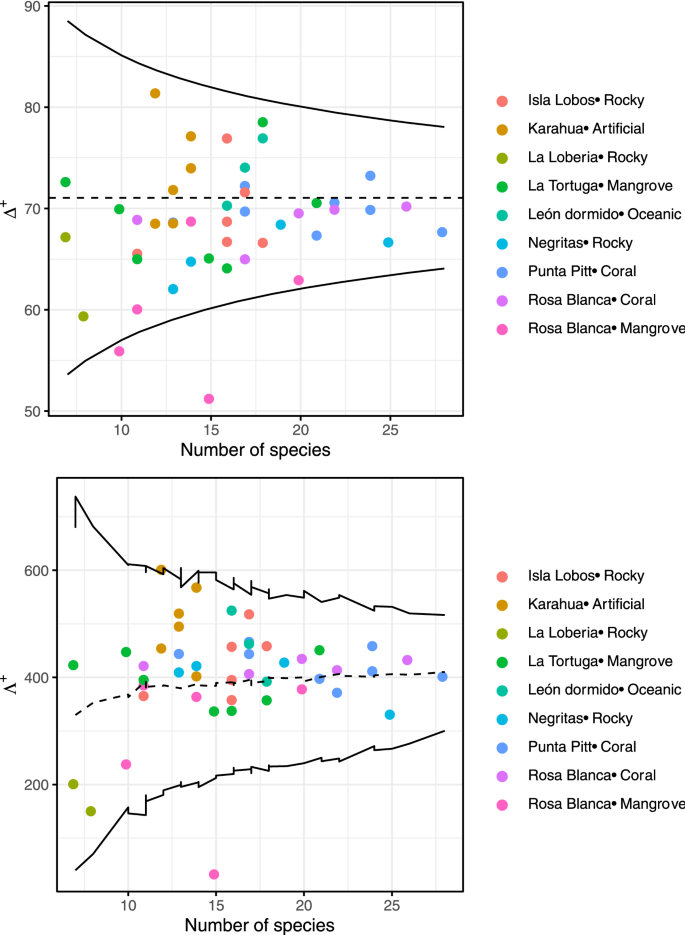
Funnel plot of the simulations of expected and observed average taxonomic distinctness (Δ+) and variation in taxonomic distinctness (Λ ) per site of the fish community in the southeastern Galapagos Islands. Each dot indicates each sample, and its color indicates site. The limits within thin lines indicate 95% of the simulated Δ+ and Λ+ values.
The ecological and taxonomic diversity results contrasted with those obtained by the non-metric multidimensional scaling analysis based on the species composition similarity matrix among sites, which showed an acceptable ordering of spatial variation for several sites with a stress value of 0.2 (Fig. 4 ). Although stress values lower than 0.1 are considered to yield good ordinations results, according to 49 , a stress level of 0.2 could still lead to a usable interpretation in ecological data. The analysis showed that the different groups were mainly related to substrate type. We identified three groups: one included the sampling sites from oceanic and artificial substrates, the second integrated the mangrove areas, and the third included sites from the rocky and coral substrates. The ANOSIM of the species composition between the areas resulted in significant differences (R = 0.714, P = 0.001). The BIOENV test results demonstrated that the substrate had the strongest correlation with the fish community structure (r s = 0.42), followed by the combination of substrate and depth (r s = 0.40), and the combination of substrate, depth and temperature was the least correlated (r s = 0.36). In Fig. 4 , we show how the oceanic and artificial sampling sites were strongly associated with depth and negatively associated with temperature. Conversely, the coral, rocky and mangrove sites were more associated with temperature. From the MDS test results, we identified the fish species contributing more to the dissimilarity between the oceanic-artificial, coral-rocky, and mangrove groups of sampling sites. The average between-group dissimilarities and the species contributing more to these differences are presented in Table 3 . In general, all comparisons showed high values of dissimilarity, at approximately 80%, indicating that the fish community abundance was different between groups. However, species such as P. laticlavius, T. lucasanum, and Apogon atradorsatus were more influential in all comparisons, indicating that the specific abundance of those species was very different among sites.
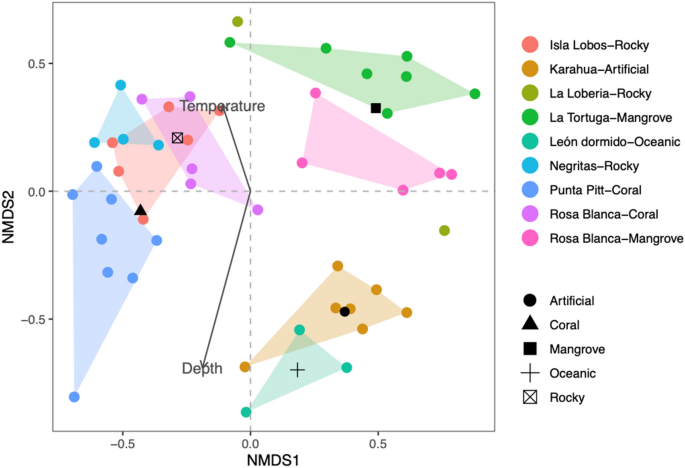
Non-metric multidimensional ordination based on the abundance per sample (dots) of the fish community in the southeastern Galapagos Islands. Convex hulls encloses all samples per site. Additionally, the vectors and centroids of the environmental variables correlate with the fish community structure.
Coastal habitats include productive zones that provide important ecosystem services 26 and essential fish habitats 50 . They are relevant grounds for feeding, nursery and spawning of several fish species with commercial and ecological value in the Galapagos archipelago 2 , 17 , 23 , 24 , 25 . The structural heterogeneity of the coastal habitat as well as the dynamics and exposure to ocean currents are important environmental factors that influence the diversity and abundance of fish species 2 , 10 , 51 , 52 , 53 , 54 . Fish move seasonally through different coastal habitats during their life cycle 22 , 26 , according to their environmental tolerance.
The distribution area of each species is influenced by the different evolutionary processes that have shaped organisms and, consequently, have determined their presence at certain sites 55 . The fish communities in the study area are composed of benthonic and demersal species, which are common in shallow waters with affinities to rocky, coral and sandy bottoms and have distinct zoogeographic affinities. Several of the most abundant species at each sampling site have an affinity for more than one substrate and are present in different types of habitats (i.e., coral, rocky, mangrove, oceanic and artificial), differing only in their percentage of abundance at each type. Some of these species (e.g., Stegastes beebei, S. arcifrons, Thalassoma lucasanum and Prionurus laticlavius) are of Indo-Pacific origin, and some are of wide distribution as Halichoeres dispilus and Paranthias colonus , which is characteristic in the southeastern of the archipelago 2 , 11 .
Fish communities in coral habitats are the most former, complex and diverse in the world, and their abundance and distribution are linked to the environmental traits and the feeding requirements of each species 56 . Our results indicate that the coral habitats have higher species richness and fish abundance, followed by the rocky and mangrove habitats. Both the coral and rocky habitats offer a variety of areas for refuge, feeding, nurturing and reproduction for many fish species 8 , and rocky habitats are particularly extensive on the Galapagos shelf 2 , 18 , 43 . In the same way, mangroves present highly variable physical conditions and provide better essential fish habitat, especially for juvenile blacktip sharks 25 ; however, the species richness is lower than in other coastal habitats and comparable to that reported in mangrove bays on Santa Cruz Island in the center of the archipelago 24 .
Coastal habitats, especially reefs, are greatly diverse, and dominant species are influenced by the environmental variability, affecting the spatial and temporal heterogeneity of the communities 26 . The habitat seasonality, composition and richness, and responses of organisms may be affected by hydrological processes 57 , 58 . We found similar diversity of fish species throughout the year in coastal habitats on San Cristóbal Island, and the diversity seems not to be influenced by seasonality. Both the ecological and taxonomic diversity indices applied here are consistent in these results, suggesting seasonal environmental variability might not significantly influence the temporal and spatial heterogeneity of fish communities. The isolation of the Galapagos archipelago and its location in a convergence zone provides it unique oceanographic conditions 1 , 2 , 3 that determine the specific species composition and biodiversity between regions 2 , 39 , 59 . Coastal habitats fish assemblages are influenced by specific thermal characteristics of each region within an archipelago 59 , as reported, in mangrove and rocky reef habitats in the Galapagos and the Solitary Islands, Australia 2 , 25 , 39 , 60 . Thus, species living around the islands in the same bioregion are adapted to the climatic heterogeneity that might influence a low species turnover between seasons.
Habitat characteristics as depth, heterogeneity (number of cavities), or structural complexity (diversity of substrate types or communities) determine the specific richness between localities on the same island 59 , as reported in fish communities in the Mediterranean or the Galapagos 39 , 61 . The proximity of sampling sites might contribute to the lack of significant differences in fish assemblages among coastal habitats type. The distance between nearby sampling sites in this study ranged from 1 to 21 km (10 km apart on average), with the closest locations being Rosa Blanca-coral and Rosa Blanca-mangrove. Strong connectivity in mangrove and reef habitats has been reported in the Galapagos, the Caribbean, and Indo-Pacific regions 25 , 62 , 63 . However, some authors 64 , 65 have indicated that coral reef fish assemblages are usually not much influenced by nearby assemblages such as soft-bottom or mangrove-fish communities, and rather, seasonal variations are attributed to fish recruitment 66 .
The artificial habitat followed by coral habitats have the highest diversity values, and the diversity tends to be slightly higher in the cold season, although this is not similar at all sites. For example, in Las Negritas (rocky), Punta Pitt (coral), Rosa Blanca (coral) and Rosa Blanca (mangrove), diversity is slightly higher in the warm season, suggesting that new species visit these sites for feeding or reproduction. This is the case for snappers, Lutjanus argentiventris (Peters, 1869) and L. viridis (Valenciennes, 1846), which are only sighted during the warm season. This period coincides with their breeding season from April to May, as reported by 67 on the Pacific coast of Mexico. Another example is P. laticlavius which is twice as abundant during warmer temperatures that is related to higher reproductive activity 68 .
Both environmental and anthropogenic factors influence the fish distribution, abundance, and diversity 33 , 40 . All sampling sites are affected by anthropogenic influences due to fishing and tourist grounds on this region. Although Galapagos artisanal fisheries are regulated by specific strategies to ensure the sustainability of the target species and reduce negative effects on the abundance of other species, it cannot be ruled out that this activity may affect the species diversity in each habitat 69 , 70 . In relation to tourism activities, all are regulated in the visit sites according to the Galapagos Management Plan and include, among others, a maximum number of visitors and certain allowed activities per site. Karahua is the only nonfishing site in this study and has the highest diversity and evenness, suggesting that it may be less impacted than other coastal habitats. However, this site corresponds to an artificial habitat for diving tourism and has different topographic characteristics from the other sites, which could influence the diversity of species and therefore was not comparable. Since fishing sites were not sampled for each coastal habitat in this study, it was not possible to accurately determine fishing effects on the richness and diversity of fish communities.
Higher structural complexity habitats are connected with higher diversity and fish abundances in marine waters 26 , 39 , 71 , 72 . For instance, on the western coast of Sweden and in the Mediterranean Sea, mussel beds and vegetated areas have high fish diversity 73 , 74 , 75 , 76 . On rocky reefs in the Mexican Pacific, rock and coral cover are highly related to dominant fish species and great diversity, while sandy bottoms have less influence on fish diversity 77 . On rocky habitats in the Galapagos Islands, no geographic patterns have been observed to explain the variability of fish abundance, richness, or diversity concerning the structural complexity of the habitat 39 . However, localities with higher roughness and number of cavities linked to a major structural complexity tend to present higher species richness 39 . We find slightly lower diversity values in mangrove habitats than in the other coastal habitats, suggesting geological structures are more important than vegetation in creating the structural complexity in the environments of the Galapagos Islands.
The poor water clarity (higher turbidity) in mangrove habitats may explain the low diversity, since the higher the turbidity, the lower the richness and diversity of fish species 78 . Turbidity modifies penetration and scattering of light 79 , influencing the foraging ability of visual hunting predators 80 . Although turbidity was not measured in this study, and we do not provide any data about it, it is realized that high turbidity may give predation shelter for benthic organisms and small juvenile fish 23 , 78 . Moreover, low visibility may influence the researchers’ ability to detect fish during the samplings, especially when using stereo-Baited Remote Underwater Video stations 25 . Although we had no difficulties in fish observation during the underwater visual surveys, we did not rule out that turbidity could affect the accuracy of the data obtained. Thus, further studies about these effects on mangrove fish communities are recommended.
It is important to consider some aspects that difficult the evaluation of fish communities and their relationship to particular habitats 26 . Fish frequently move between various environments 22 . The number and composition of fish species range with the hour of the day, being half to two-thirds of the species in most fish assemblages of diurnal habits 80 , 81 . Our visual censuses were developed during the same period of the day recording diurnal species. However, to reduce bias in the evaluation of diversity derived from sampling effort, we used a taxonomic diversity index, which is more susceptible to natural environmental variability and less sensitive to sample size variations 30 .
Lower values of taxonomic distinctness (Δ+) indicate more greatly impacted zones 82 , 83 . In this study, the Δ+ values were significantly different among sites, and Rosa Blanca (mangrove) presented the lowest values, indicating that the gatherings of closely related species made up the fish community. This may reduce the ichthyofauna responsiveness to stress factors in the ecosystem 57 , and therefore this area could be more impacted in relation to the other sites. Despite this result and the low diversity and evenness in Rosa Blanca (mangrove), this is one of the sites with higher fish abundance, mainly omnivorous (e.g., S. arcifrons ) and herbivorous (e.g., Scarus ghoban ) fishes which reflects a healthy habitat. This high abundance may be associated with fish mobility via superficial open ocean areas 22 and the connectivity between adjacent habitats as coral reefs that may affect fish assemblages 84 .
Changes in community structure may be little perceptible for detecting in demersal communities 85 . Temporal or spatial community changes are susceptible to the sampling effort, thus multivariate techniques are recommended for evaluating the consistency of this group 34 . According to the MDS and ANOSIM tests, the fish community abundance may be distinct per group of habitats. These groups (oceanic and artificial; mangrove; coral and rocky) are identified by the type of substrate, while the composition of species in each group shows a distinct association strength with environmental variables, like substrate type, depth, and sea temperature.
The species composition in the oceanic and artificial habitats has a positive association with the depth and negative with the sea temperature. In contrast, the species composition in the coral, rocky and mangrove sites are more associated with sea temperature. This trend is related to the ecological traits of fish species but also to the availability resources, since certain fish species with certain set of traits are more associated with some habitats than others expressing their habitat preferences 34 , 72 . For example, the Pacific creole-fish ( Paranthias colonus ), which is the most abundant species in the oceanic habitat, has planktivorous habits that are usually found in deeper waters (from 10 to 70 m) than the other most abundant species in the rest of the sites. The deepest dives were made in oceanic and artificial habitats in 2011, which was a cooler year than 2010. These factors may have favored the abundance of food in these habitats and therefore in a greater abundance of species. On the other hand, species that inhabit shallow, warm waters and graze on algae and invertebrates (e.g., S. beebei, S. arcifrons, T. lucasanum , P. laticlavius, H. dispilus, Xenocys jessiae and Apogon atradorsatus ) are more abundant in the inshore habitats, such as coral, mangrove and rocky sites, which have high structural complexity.
The diversity patterns observed in this study seem to be most strongly related to the type of substrate. The BIOENV test also indicates that the combination of the substrate and depth influences the fish community structure, and the sea temperature has less influence. The habitat complexity and the diversity of sea bottom types are important factors influencing the abundance patterns of many marine species 39 , 71 , 72 , 86 . Depth has also been considered a significant variable influencing fish community structure 34 , 39 , 72 . Habitat structural complexity complements depth, mainly for those species less influenced by depth or where depth and habitat complexity interact to influence fish abundance 72 . Thus, according to the similarity analysis, certain spatial differences in community structure are mainly influenced by three species (i.e., P. laticlavius, T. lucasanum and A. atradorsatus ) with very different abundances between the groups of habitats.
Despite the strong seasonality observed during the sampling years, with the average sea temperatures reaching differences of 6 °C between the warm and cold seasons, no temporal variation was detected in the diversity patterns and community structure of fish at any site. Several studies have demonstrated the influence of sea temperature on diversity patterns in fish communities 25 , 33 , 34 , 40 , 87 . However, our results show that fish communities in the coastal habitats within a region of the Galapagos Islands have a high environmental tolerance, which allows them to persist in their habitat despite drastic seasonal changes in sea temperature. But extreme thermal impacts (i.e., elevated temperatures prevailing for over 12 months) during strong El Niño events could affect fish communities due to the loss of coral and macroalgal beds 88 , 89 . The environmental tolerance, a product of evolutionary processes 55 , allows species to adapt to environmental variability and unpredictability in the productivity of the Galapagos archipelago 11 .
We determined that substrate and depth influence the fish communities’ structure and diversity patterns in the coastal habitats in the southeastern Galapagos archipelago. The community structure differs spatially, and the mangrove habitats have lower diversity values. The diversity patterns are more associated with the substrate type and the habitat's depth and less influenced by the seasonal sea temperature, and fish communities show high environmental tolerance. Although this study did not determine the artisanal fishing influence on the structure of the fish community, fishing might regulate the abundance of species and affect diversity. Therefore, further studies are required to determine these effects in the coastal habitats of the Galapagos Islands.
Witman, J. D. & Smit, F. Rapid community change at a tropical upwelling site in the Galapagos Marine Reserve. Biodivers. Conserv. 12 , 25–45 (2003).
Google Scholar
Edgar, G. J., Banks, S., Fariña, J. M., Calvopiña, M. & Martínez, C. Regional biogeography of shallow reef fish and macro-invertebrate communities in the Galapagos archipelago. J. Biogeogr. 31 , 1107–1124 (2004).
Okey, T. A. et al. A trophic model of a Galápagos subtidal rocky reef for evaluating fisheries and conservation strategies. Ecol. Model. 172 , 383–401 (2004).
Briggs, J. C. & Bowen, B. W. A realignment of marine biogeographic provinces with particular reference to fish distributions. J. Biogeogr. 39 , 12–30 (2012).
Salinas de León, P. et al. Largest global shark biomass found in the northern Galápagos Islands of Darwin and Wolf. PeerJ 4 , e1911. https://doi.org/10.7717/peerj.1911 (2016).
Article CAS PubMed PubMed Central Google Scholar
Humann, P. & DeLoach, N. Reef Fish Identification: Galápagos (ed. Humann, P.) (New World Publications, Inc., 2003).
McCosker, J. E. & Rosenblatt, R. H. The fishes of the Galápagos archipelago: An update. Proc. Calif. Acad. Sci. 61 , 167–195 (2010).
Grove, J. S. & Lavenberg, R. J. The Fishes of the Galapagos Islands (Stanford University Press, 1997).
Allen, G. & Ross-Robertson, D. Fishes of Tropical Eastern Pacific (University of Hawaii Press, 1994).
Ruttenberg, B. I., Haupt, A. J., Chiriboga, A. I. & Warner, R. R. Patterns, causes and consequences of regional variation in the ecology and life history of a reef fish. Oecologia 145 , 394–403 (2005).
ADS PubMed Google Scholar
Bernardi, G. et al. Darwin’s fishes: Phylogeography of Galápagos Islands reef fishes. Bull. Mar. Sci. 90 , 533–549 (2014).
Banks, S., Vera, M. & Chiriboga, A. Establishing reference points to assess long-term change in zooxanthellate coral communities of the northern Galápagos coral reefs. Galapagos Res. 66 , 43–64 (2009).
Palacios, D., Bograd, S., Foley, D. & Schwing, F. Oceanographic characteristics of biological hot spots in the North Pacific: A remote sensing perspective. Deep Sea Res Part II Top. Stud. Oceanogr. 53 , 250–269 (2006).
ADS Google Scholar
Sweet, W. V. et al. Water mass seasonal variability in the Galapagos Archipelago. Deep Sea Res. Part I Oceanogr. Res. Pap. 54 , 2023–2035 (2007).
Schaeffer, B. et al. Phytoplankton biomass distribution and identification of productive habitats within the Galapagos Marine Reserve by MODIS, a surface acquisition system, and in-situ measurements. Remote Sens. Environ. 112 , 3044–3054 (2008).
Witman, J. D., Brandt, M. & Smith, F. Coupling between subtidal prey and consumers along a mesoscale upwelling gradient in the Galapagos Islands. Ecol. Monogr. 80 , 153–177 (2010).
Moity, N. Evaluation of no-take zones in the Galápagos marine reserve, zoning plan 2000. Frontiers. 5 , 244. https://doi.org/10.3389/fmars.2018.00244 (2018).
Article Google Scholar
Lamb, R. W., Smith, F. & Witman, J. D. Consumer mobility predicts impacts of herbivory across an environmental stress gradient. Ecology 101 , e02910. https://doi.org/10.1002/ecy.2910 (2020).
Article PubMed Google Scholar
Edgar, G. J. et al. Conservation of threatened species in the Galapagos Marine Reserve through identification and protection of marine key biodiversity areas. Aquat. Conserv. 18 , 955–968 (2008).
Carrión-Cortez, J. A., Zárate, P. & Seminoff, J. A. Feeding ecology of the green sea turtle ( Chelonia mydas ) in the Galapagos Islands. J. Mar. Biol. Assoc. U. K. 90 , 1005–1013 (2010).
Moity, N., Delgado, B. & Salinas-de-León, P. Correction: Mangroves in the Galapagos islands: Distribution and dynamics. PLoS One 14 , e0212440. https://doi.org/10.1371/journal.pone.0212440 (2019).
Article PubMed PubMed Central Google Scholar
Seitz, R. D., Wennhage, H., Bergström, U., Lipcius, R. N. & Ysebaert, T. Ecological value of coastal habitats for commercially and ecologically important species. ICES J. Mar. Sci. 71 , 648–665 (2014).
Aguaiza, C. The role of mangrove as nursery habitats for coral reef fish species in the Galapagos Islands. MSc Thesis (University of Queensland, 2016).
Llerena-Martillo, Y., Peñaherrera-Palma, C. & Espinoza, E. Fish assemblages in three fringed mangrove bays of Santa Cruz Island, Galapagos Marine Reserve. Rev. Biol. Trop. 66 , 674–687 (2018).
Fierro-Arcos, D. et al. Mangrove fish assemblages reflect the environmental diversity of the Galapagos Islands. Mar. Ecol. Prog. Ser. 664 , 183–205 (2021).
Henseler, C. et al. Coastal habitats and their importance for the diversity of benthic communities: A species-and trait-based approach. Estuar. Coast. Shelf Sci. 226 , 106272. https://doi.org/10.1016/j.ecss.2019.106272 (2019).
Loreau, M. et al. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 294 , 804–808 (2001).
ADS CAS Google Scholar
Menezes, R. F. et al. Variation in fish community structure, richness, and diversity in 56 Danish lakes with contrasting depth, size, and trophic state: Does the method matter?. Hydrobiologia 710 , 47–59 (2013).
Hu, M., Wang, C., Liu, Y., Zhang, X. & Jian, S. Fish species composition, distribution and community structure in the lower reaches of Ganjiang River, Jiangxi, China. Sci. Rep. 9 , 10100. https://doi.org/10.1038/s41598-019-46600-2 (2019).
Article ADS CAS PubMed Google Scholar
Clarke, K. R. & Warwick, R. M. Changes in Marine Communities: An Approach to Statistical Analysis and Interpretation , 2nd ed. (PRIMER-E Ltd, Plymouth Marine Laboratory, 2001).
Warwick, R. M. & Clarke, K. R. New biodiversity measures reveal a decrease in taxonomic distinctness with increasing stress. Mar. Ecol. Prog. Ser. 129 , 301–305 (1995).
Clarke, K. R. & Warwick, R. M. The taxonomic distinctness measure of biodiversity: Weighting of step lengths between hierarchical levels. Mar. Ecol. Prog. Ser. 184 , 21–29 (1999).
Nieto-Navarro, J. T., Zetina-Rejón, M. A., Arreguín-Sánchez, F., Palacios-Salgado, D. & Jordán, F. Changes in fish bycatch during the shrimp fishing season along the eastern coast of the mouth of the Gulf of California. J. Appl. Ichthyol. 29 , 610–616 (2013).
Escobar-Toledo, F., Zetina-Rejón, M. J. & Duarte, L. O. Measuring the spatial and seasonal variability of community structure and diversity of fish by-catch from tropical shrimp trawling in the Colombian Caribbean Sea. Mar. Biol. Res. 11 , 528–539 (2015).
Herrera-Valdivia, E., López-Martínez, J., Castillo Vargasmachuca, S. & García-Juárez, A. R. Diversidad taxonómica y funcional en la comunidad de peces de la pesca de arrastre de camarón en el norte del Golfo de California, México. Rev. Biol. Trop. 64 , 587–602 (2016).
PubMed Google Scholar
Heylings, P., Bensted-Smith, R. & Altamirano, M. Zonificación e historia de la Reserva Marina de Galápagos. In Reserva Marina de Galápagos. Línea Base de la Biodiversidad (eds. Danulat, E. & Edgar, G. J.) 10–21 (Fundación Charles Darwin y Servicio Parque Nacional de Galápagos, 2002).
Edgar, G. J. et al. Bias in evaluating the effects of marine protected areas: The importance of baseline data for the Galapagos Marine Reserve. Environ. Conserv. 3 , 212–218. https://doi.org/10.1017/S0376892904001584 (2004).
Jennings, S., Brierley, A. S. & Walker, J. W. The inshore fish assemblages of the Galápagos archipelago. Biol. Conserv. 70 , 49–57 (1994).
Brito, A., Pérez-Ruzafaga, A. & Bacallado, J. J. Ictiofauna costera de las islas Galápagos: composición y estructura del poblamiento de los fondos rocosos. Res. Cient. Proy. Galápagos TFCM 5 , 61 (1997).
Bruneel, S. et al. Assessing the drivers behind the structure and diversity of fish assemblages associated with rocky shores in the Galapagos Archipelago. J. Mar. Sci. Eng. 9 , 375. https://doi.org/10.3390/jmse9040375 (2021).
Wellington, G. M., Strong, A. E. & Merlen, G. Sea surface temperature variation in the Galápagos Archipelago: A comparison between AVHRR nighttime satellite data and in-situ instrumentation (1982–1998). Bull. Mar. Res. 69 , 27–42 (2001).
Snell, H., Stone, P. & Snell, H. L. A summary of geographical characteristics of the Galapagos Islands. J. Biogeogr. 23 , 619–624 (1996).
Bustamante, R. H., et al . Outstanding marine features of Galápagos. In A Biodiversity Vision for the Galapagos Islands: An Exercise for Ecoregional Planning (eds. Bensted-Smith, R. & Dinnerstein, E.) 60–71 (WWF, 2002).
Airoldi, L. & Beck, M. W. Loss, status and trends for coastal marine habitats of Europe. In Oceanography and Marine Biology: An Annual Review (eds. Gibson, R. N., Atkinson, R. J. A. & Gordon, J. D. M.) vol. 45, 345–405 (Taylor & Francis, 2007).
Carr, M. H., Malone, D. P., Hixon, M. A., Holbrook, S. J. & Schmitt, R. J. How Scuba changed our understanding of nature: underwater breakthrough in reef fish ecology. In Research and Discoveries: The Revolution of Science Through Scuba vol. 39, 157–167 (Smithsonian Contributions to the Marine Sciences, 2013).
Durkacz, S. Assessing the Oceanographic Conditions and Distribution of Reef Fish Assemblages Throughout the Galápagos Islands Using Underwater Visual Survey Methods. MSc Thesis (Texas A & M University, 2014).
Fischer, W. et al . Guía FAO para la identificación de especies para los fines de pesca. Pacífico Centro-Oriental vol. II–III, 648–1652 (FAO, 1995).
Clarke, K. R. & Warwick, R. M. A taxonomic distinctness index and its statistical properties. J. Appl. Ecol. 35 , 523–531 (1998).
Clarke, K. R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 18 , 117–143 (1993).
Rosenberg, A., Binford, T. E., Leathery, S., Hill, R. L. & Bickers, K. Ecosystem approaches to fishery management through essential fish habitat. Bull. Mar. Sci. 66 , 535–542 (2000).
Aburto-Oropeza, O. & Balart, E. F. Community structure of reef fish in several habitats of a rocky reef in the Gulf of California. Mar. Ecol. 22 , 283–305 (2001).
Fulton, C. J., Bellwood, D. R. & Wainwright, P. C. Wave energy and swimming performance shape coral reef fish assemblages. Proc. R. Soc. B 272 , 827–832 (2005).
CAS PubMed Google Scholar
Dominici-Arosemena, A. & Wolff, M. Reef fish community structure in the Tropical Eastern Pacific (Panamá): Living on a relatively stable rocky reef environment. Helgol. Mar. Res. 60 , 287–305 (2006).
Villegas-Sánchez, C. A., Abitia-Cárdenas, L. A., Gutiérrez-Sánchez, F. J. & Galván-Magaña, F. Rocky-reef fish assemblages at San José Island, Mexico. Rev. Mex. Biodivers. 80 , 169–179 (2009).
Wiens, J. J. & Graham, C. H. Niche conservatism: Integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst. 36 , 519–539 (2005).
Glynn, P. Some physical and biological determinants of coral community structure in the eastern Pacific. Ecol. Monogr. 46 , 431–456 (1976).
Ramos-Miranda, J. et al. Changes in four complementary facets of fish diversity in a tropical coastal lagoon after 18 years: A functional interpretation. Mar. Ecol. Prog. Ser. 304 , 1–13 (2005).
Gristina, M., Bahri, T., Fiorentino, F. & Garofalo, G. Comparison of demersal fish assemblages in three areas of the Strait of Sicily under different trawling pressure. Fish. Res. 81 , 60–71 (2006).
Pérez-Ruzafa, A. P., Marcos, C. & Bacallado, J. J. Biodiversidad marina en archipiélagos e islas: patrones de riqueza específica y afinidades faunísticas. Vieraea Folia Scientarum Biologicarum Canariensium. 33 , 455–476 (2005).
Malcolm, H. A., Jordan, A. & Smith, S. D. Biogeographical and cross-shelf patterns of reef fish assemblages in a transition zone. Mar. Biodivers. 40 (3), 181–193 (2010).
García-Charton, J. A. & Pérez-Ruzafa, A. P. Correlation between habitat structure and a rocky reef fish assemblage in the Southwest Mediterranean. Mar. Ecol. 19 (2), 111–128 (1998).
Mumby, P. J. et al. Mangroves enhance the biomass of coral reef fish communities in the Caribbean. Nature 427 , 533–536 (2004).
ADS CAS PubMed Google Scholar
Unsworth, R. K. F. et al. High connectivity of Indo-Pacific seagrass fish assemblages with mangrove and coral reef habitats. Mar. Ecol. Prog. Ser. 353 , 213–224 (2008).
Birkeland, C. & Amesbury, S. S. Fish-transect surveys to determine the influence of neighboring habitats on fish community structure in the tropical Pacific. Co-operation for environmental protection in the Pacific. UNEP Reg. Seas Rep. Stud. 97 , 195–202 (1988).
Thollot, P., Kulbicki, M., & Wantiez, L. Temporal patterns of species composition in three habitats of the St Vincent Bay area (New Caledonia): Coral reefs, soft bottoms and mangroves. In Proceedings International Soc. Reef Studies. 127–137 (1991).
Kulbicki, M. Present knowledge of the structure of coral reef fish assemblages in the Pacific. UNEP Reg. Seas Rep. Stud. 147 , 31–53 (1992).
Cruz-Romero, M., Chávez, E.A., Espino, E. & García, A. Assessment of a snapper complex (Lutjanus spp.) of the eastern tropical Pacific. In Biology, Fisheries and Culture of Tropical Groupers and Snappers (eds. Arreguín-Sánchez, F., Munro, J. L., Balgos, M. C. & Pauly, D.) 324–330 (ICLARM Conf. Proc. 48, 1996).
Aguilar-Santana, F. Biología reproductiva de Prionurus laticlavius (Valenciennes, 1846) (Teleostei: Acanthuridae) en la Costa Sudoccidental del Golfo de California, México. PhD Thesis (Instituto Politécnico Nacional, 2020).
Hall, S. The Effects of Fishing on Marine Ecosystems and Communities (Blackwell Science Ltd., 1999).
Mangi, S. C. & Roberts, C. M. Quantifying the environmental impacts of artisanal fishing gear on Kenya’s coral reef ecosystems. Mar. Pollut. Bull. 52 , 1646–1660 (2006).
Rees, M. J., Jordan, A., Price, O. F., Coleman, M. A. & Davis, A. R. Abiotic surrogates for temperate rocky reef biodiversity: Implications for marine protected areas. Divers. Distrib. 20 (3), 284–296 (2014).
Ferrari, R. et al. Habitat structural complexity metrics improve predictions of fish abundance and distribution. Ecography 41 (7), 1077–1091 (2018).
Pihl, L. & Wennhage, H. Structure and diversity of fish assemblages on rocky and soft bottom shores on the Swedish west coast. J. Fish Biol. 61 , 148–166 (2002).
La Mesa, G., Molinari, A., Gambaccini, S. & Tunesi, L. Spatial pattern of coastal fish assemblages in different habitats in North-western Mediterranean. Mar. Ecol. 32 , 104–114 (2011).
Kristensen, L. D. et al. Establishment of blue mussel beds to enhance fish habitats. Appl. Ecol. Environ. Res. 13 , 783–798 (2015).
Bergström, L., Karlsson, M., Bergström, U., Pihl, L. & Kraufvelin, P. Distribution of mesopredatory fish determined by habitat variables in a predator-depleted coastal system. Mar. Biol. 163 , 201. https://doi.org/10.1007/s00227-016-2977-9 (2016).
Galván-Villa, C. M., Arreola-Robles, J. L., Ríos-Jara, E. & Rodríguez-Zaragoza, F. A. Ensamblajes de peces arrecifales y su relación con el hábitat bentónico de la Isla Isabel, Nayarit, México. Rev. Biol. Mar. Oceanogr. 45 , 311–324 (2010).
Lunt, J. & Smee, D. L. Turbidity alters estuarine biodiversity and species composition. ICES J. Mar. Sci. 77 , 379–387 (2019).
Anthony, K. R., Ridd, P. V., Orpin, A. R., Larcombe, P. & Lough, J. Temporal variation of light availability in coastal benthic habitats: Effects of clouds, turbidity, and tides. Limnol. Oceanogr. 49 , 2201–2211 (2004).
Helfman, G. S. Patterns of community structure in fishes: Summary and overview’. Environ. Biol. Fishes 3 , 129–148 (1978).
Helfman, G. S. Fish behaviour by day, night and twilight. In The Behaviour of Teleost Fishes (ed. Pitcher T.J.) (Springer, 1986).
Warwick, R. M. & Clarke, K. R. Taxonomic distinctness and environmental assessment. J. Appl. Ecol. 35 , 532–543 (1998).
Rogers, S. I., Clarke, K. R. & Reynolds, J. D. The taxonomic distinctness of coastal bottom-dwelling fish communities of the North-east Atlantic. J. Anim. Ecol. 68 , 769–782 (1999).
Robertson, A. I., & Blaber, S. J. M. Plankton, epibenthos and fish communities. In Tropical Mangrove Ecosystems (eds. Robertson, A. I. & Alongi, D. M.) Coastal and Estuarine Studies No. 41, 173–224 (American Geophysical Union, 1992).
Koranteng, K. A. Diversity and stability of demersal species assemblages in the Gulf of Guinea. West Afr. J. Appl. Ecol. 2 , 49–63 (2001).
McCormick, M. I. Comparison of field methods for measuring surface topography and their associations with a tropical reef fish assemblage. Mar. Ecol. Prog. Ser. 112 , 87–96 (1994).
Moraes, L. E., Paes, E., Garcia, A., Möller, O. Jr. & Vieira, J. Delayed response of fish abundance to environmental changes: A novel multivariate time-lag approach. Mar. Ecol. Prog. Ser. 456 , 159–168 (2012).
Edgar, G. J. et al. El Niño, grazers and fisheries interact to greatly elevate extinction risk for Galapagos marine species. Glob. Change. Biol. 16 , 2876–2890 (2010).
Glynn, P. W., Enochs, I. C., Afflerbach, J. A., Brandtneris, V. W. & Serafy, J. E. Eastern Pacific reef fish responses to coral recovery following El Niño disturbances. Mar. Ecol. Prog. Ser. 495 , 233–247 (2014).
Download references
Acknowledgements
We are grateful to the Dirección Parque Nacional Galápagos (DPNG) for the institutional support. We thank the Universidad San Francisco de Quito (USFQ) and Centro Interdisciplinario de Ciencias Marinas from the Instituto Politécnico Nacional (CICIMAR-IPN) for financial and logistical support during the preparation of this manuscript. We also thank the DPNG rangers and volunteers who contributed to the monitoring work and Dr. Leonardo Zurita for help in the preparation of Figure 1 . MJZR thanks the Instituto Politécnico Nacional for the financial support from Project SIP-IPN 20221721 and the fellowships granted through COFAA and EDI. Finally, all authors thank the Galapagos Science Center (GSC) for providing the facilities for information processing.
Author information
Authors and affiliations.
Universidad San Francisco de Quito and Galapagos Science Center, Isla San Cristóbal, Galápagos, Ecuador
Marjorie Riofrío-Lazo & Diego Páez-Rosas
Instituto Politécnico Nacional, Centro Interdisciplinario de Ciencias Marinas (CICIMAR-IPN), La Paz, Baja California Sur, México
Manuel J. Zetina-Rejón
Guía Naturalista en Patrimonio Turístico del Parque Nacional Galápagos, Islas Galápagos, San Cristóbal, Ecuador
Leandro Vaca-Pita
Pontificia Universidad Católica del Ecuador-Sede Manabí, Facultad de Biología, Bahía de Caráquez, Ecuador
Juan Carlos Murillo-Posada
Dirección del Parque Nacional Galápagos, Unidad Técnica Operativa San Cristóbal, Isla San Cristóbal, Galápagos, Ecuador
Diego Páez-Rosas
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: M.R.-L., M.J.Z.-R. and D.P.-R. Methodological design: M.R.-L., M.J.Z.-R. and D.P.-R. Data collection: L.V.-P. and J.C.M.-P. Funding acquisition: J.C.M.-P. and L.V.-P. Formal analysis: M.J.Z.-R. and M.R.-L. Writing—original draft: M.R.-L. Writing—review and editing: M.R.-L., M.J.Z.-R., L.V.-P., J.C.M.-P. and D.P.-R. All authors gave final approval for publication.
Corresponding author
Correspondence to Marjorie Riofrío-Lazo .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Riofrío-Lazo, M., Zetina-Rejón, M.J., Vaca-Pita, L. et al. Fish diversity patterns along coastal habitats of the southeastern Galapagos archipelago and their relationship with environmental variables. Sci Rep 12 , 3604 (2022). https://doi.org/10.1038/s41598-022-07601-w
Download citation
Received : 09 August 2021
Accepted : 16 February 2022
Published : 04 March 2022
DOI : https://doi.org/10.1038/s41598-022-07601-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Global patterns of herbivorous reef fish productivity: the role of prionurus laticlavius in the galápagos.
- Sterling B. Tebbett
- Helen F. Yan
- David R. Bellwood
Coral Reefs (2024)
Patterns of fish occupancy of artificial habitats in the eastern Mediterranean shallow littoral
- Maria-Myrto Ntouni
- Alexis Lazaris
- Evangelos Tzanatos
Marine Biology (2023)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Anthropocene newsletter — what matters in anthropocene research, free to your inbox weekly.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Effects of human disturbance on habitat and fish diversity in Neotropical streams
Contributed equally to this work with: Crislei Larentis, Bruna Caroline Kotz Kliemann, Mayara Pereira Neves, Rosilene Luciana Delariva
Roles Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft
Affiliation Programa de Pós-Graduação em Biologia Comparada, Universidade Estadual de Maringá, Maringá, Paraná, Brazil
Affiliation Programa de Pós-graduação em Ciências Biológicas/Zoologia, Instituto de Biociências, Universidade Estadual Paulista (UNESP), Botucatu, São Paulo, Brazil
Roles Conceptualization, Formal analysis, Methodology, Writing – original draft
Affiliation Programa de Pós-graduação em Biologia Animal, Universidade Federal do Rio Grande do Sul, Porto Alegre, Rio Grande do Sul, Brazil
Roles Conceptualization, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Laboratório de Ictiologia, Ecologia e Biomonitoramentos (LIEB), Universidade Estadual do Oeste do Paraná – UNIOESTE, Cascavel, Paraná, Brazil
- Crislei Larentis,
- Bruna Caroline Kotz Kliemann,
- Mayara Pereira Neves,
- Rosilene Luciana Delariva

- Published: September 9, 2022
- https://doi.org/10.1371/journal.pone.0274191
- Reader Comments
Human pressures have been intensely modifying freshwater ecosystems worldwide. We assessed the effects of human pressure on habitat diversity and primary productivity to understand the consequences on fish fauna in 25 tropical and subtropical streams of two globally important ecoregions: Iguassu and Upper Paraná. We hypothesized that the increased human pressure (urbanization and agriculture) on stream environments, both at the local and catchment scales, directly decreases habitat diversity. We also hypothesized that increased human pressure triggers changes in primary productivity and fish fauna composition and structure. We evaluated the human pressure intensity using the Integrated Disturbance Index and the Rapid Habitat Diversity Assessment protocol, which combines information about land use, land cover and environmental characteristics of the stream catchment and sampling sites. Streams with increased human disturbance had lower habitat diversity, higher primary productivity, and high non-native species abundance. Fish compositional turnover was associated with increased human disturbance. Native and degradation-sensitive fish species, especially endemic ones, were associated with streams with higher habitat diversity and forested cover. Degradation-resistant fishes, mostly non-native species, were associated with streams with higher human disturbance and urban land use. Although human pressure did not affect species richness, Shannon diversity, and Simpson dominance, there were significant effects on numerical abundance and fish species equitability. In this study, human pressure directly affected habitat structure, with indirect consequences for fish fauna, increasing the potential for local extirpation of rare species.
Citation: Larentis C, Kotz Kliemann BC, Neves MP, Delariva RL (2022) Effects of human disturbance on habitat and fish diversity in Neotropical streams. PLoS ONE 17(9): e0274191. https://doi.org/10.1371/journal.pone.0274191
Editor: Frank O. Masese, University of Eldoret, KENYA
Received: September 17, 2021; Accepted: August 23, 2022; Published: September 9, 2022
Copyright: © 2022 Larentis et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript and its Supporting information files.
Funding: The author(s) received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The increase in the human population and the demand for products and services have caused numerous environmental disturbances that strongly affect freshwater ecosystems [ 1 – 3 ]. In rivers and streams, changes in land cover, boosted by agricultural development and urban expansion, are main drivers of environmental degradation [ 4 , 5 ]. Habitat diversity, hydrology, water quality, productivity, and freshwater biodiversity are all threatened [ 6 , 7 ]. Furthermore, human activities are responsible for the introduction of non-native fish species into diverse freshwater environments. This introduction can promote changes in the population dynamics of native species due to competition for food and habitat besides the proliferation of diseases [ 8 , 9 ].
Changes in land cover in stream catchments cause alterations in both the riparian zone and instream habitats, which can lead to habitat homogenization [ 10 ], severely affecting the aquatic biota [ 11 ]. Erosion and sedimentation [ 12 ], soil compaction affecting water infiltration [ 13 , 14 ], and streambed channeling [ 15 ] have been widely observed in stream ecosystems. These physical alterations lead to habitat homogenization, low diversity of food resources, and changes in the structure of the fish fauna [ 16 – 18 ]. Environmental heterogeneity and microhabitat diversity are fundamental to the availability of shelter and food resources for fish species [ 19 ]. These conditions facilitate the existence of diverse species in these streams through utilization of resources in different microhabitats [ 20 , 21 ]. The increased input of nutrients in the water column resulting from urban and agricultural land use causes changes not only in water physic-chemical conditions but also in terms of primary productivity and aquatic biota [ 22 – 24 ]. Nutrient enrichment owing to effluent discharge can intensify biological activity and drastically alter the composition and structure of aquatic food webs. One of the main changes is increased chlorophyll- α (Chl- α ) biomass [ 25 ], which is widely used to measure eutrophication [ 26 ].
Effluent discharge or leaching is much more intense in urban streams, where eutrophication is common [ 23 ], and can be a consequence of the precariousness of sewage disposal, as documented in Brazil [ 27 , 28 ]. Illegal discharge of industrial and domestic sewage in watercourses [ 29 ], and rainwater runoff also contribute to this process [ 30 ]. Eutrophication not only affects freshwater biodiversity but also human health and ecosystem services [ 31 ].
Another worrying factor is the introduction of non-native fish species. This is also considered an important stressor for native assemblages in freshwater environments worldwide [ 32 – 34 ]. In disturbed water courses, non-native species introductions are mainly a result of activities related to aquaculture and aquarism [ 9 , 35 ]. The establishment of non-native fish species can lead to changes in species composition [ 34 ]. These changes are related to an increase in the dominance of more degradation-resistant species, and a decrease and/or loss of species diversity [ 36 ]. Over time, these processes can induce fish fauna homogenization, with a global trend toward biotic homogenization [ 34 ].
Neotropical streams shelter the world’s highest richness and endemism of fishes [ 37 ] and these characteristics are especially relevant in two ecoregions in southern Brazil—Iguassu and Upper Paraná. Such conditions are a result of rapids and waterfalls that occur within these basins, which limit fish distribution upstream, contributing to the high level of endemism in these ecoregions [ 38 ]. Thus, evaluating fish species composition and structure of these ecoregions is important in understanding biogeographic aspects and factors that can affect species distribution. Despite their exceptional diversity and endemism, the streams and tributaries of the Iguassu and Upper Paraná ecoregions have undergone intense anthropogenic transformations. Thus, there is an urgent need to obtain information on fish fauna in headwater streams in the Iguassu and Upper Paraná ecoregions.
In this study, we aimed to assess the effects of human pressure on habitat diversity, primary productivity, and fish fauna composition and structure in 25 Neotropical streams in southern Brazil. We hypothesized that increased human pressure on stream environments, both locally and at catchment scales, decreases habitat diversity and triggers changes in primary productivity, fish species composition, and assemblage structure. We tested the following predictions: i) there is an inverse relationship between habitat diversity and human pressure according to the integrated disturbance index (IDI); ii) streams with low habitat diversity and intense disturbance have higher primary productivity; iii) degradation-resistant species, including non-native ones, are indicators of disturbed streams, and degradation-sensitive and endemic species are indicators of less disturbed streams; iv) species restrictedness highlights endemic and rare species occurring in streams closer to natural conditions; and v) numeric abundance, species richness, and dominance increase with disturbance intensification, and species diversity and equitability decrease in response to this intensification. Considering the regional pool of species, we expect native and endemic species to display specific requirements regarding food, habitat, and ecological conditions. Understanding how human pressure affects stream environments provides useful information for conservation efforts, particularly for endemic species.
Material and methods
Ethics statement.
This study was carried out in strict accordance with protocols in their ethical and methodological aspects for the use of fish. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Universidade Federal do Rio Grande do Sul (Protocol Number CEUA– 32,734). The fish sampling was conducted under license from the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) (Number processes: 25039; 27252). Regarding access to sampling sites, permission was only requested from Instituto Chico Mendes de Conservação da Biodiversidade of the Paraná State for sampling in the Rebio das Perobas; for all the other sites, permission was granted by the private owners.
The study area comprised the Iguassu and Upper Paraná ecoregions, which are globally important because of their species richness and endemism [ 38 ]. The Iguassu ecoregion includes the Iguaçu River Basin and all its tributaries in Brazil above the Iguaçu Falls [ 38 ]. The Upper Paraná ecoregion includes the drainage basin of the Upper Paraná River (comprise Piquiri and Ivaí Basins) and its tributaries above the former Guaíra Falls (Salto de Sete Quedas) [ 37 ].
The Iguaçu (54.820 km 2 ), Piquiri (24.171,70 km 2 ), and Ivaí (36.540 km²) river basins [ 39 ] ( Fig 1 ) are in a region of a humid, subtropical climate (Cfa), as defined by the Köppen climate classification [ 40 ], with hot and humid summers and cold winters. The average annual precipitation varies between 1100 and 2000 mm and the average annual temperatures vary between 11.5 and 25°C [ 38 ]. The Iguaçu River originates in the Serra do Mar and travels across the Paraná Plateau before dropping off at Iguaçu Falls near its confluence with the Paraná River. The altitude varies between 908 m (origin) and 78 m (outfall in the Paraná River) above sea level, with numerous rapids and falls present along its course [ 41 ]. The Piquiri River originates in the Serra do São João at 1237 m altitude, on the third plateau in the south-central region of the state and runs 485 km before reaching the Paraná River [ 42 ]. The Ivaí River is a left-bank tributary of the Paraná River in Paraná State [ 42 ]. This river is formed in the municipality of Prudentópolis by the confluence of the Patos and São João rivers, both in the State Park of Serra da Esperança, on the border between the second and third plateaus of Paraná State [ 42 ]. In these three basins, the predominant land use is livestock pasture and agriculture, with the cultivation of cereals (soybean, corn, wheat) and sugarcane in the sandy soils. The industrial activities are also directly related to agriculture in the interior of Paraná State [ 39 ].
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
The classification of streams as high, medium and low disturbance was according to the Integrated disturbance index values: < 0.09 values—low disturbance (sites with conditions closer to the natural), 0.10 to 0.19—medium disturbance (altered sites), > 0.2—high disturbance (extremely impacted sites). “Raster data obtained from EMBRAPA (Intellectual Property Rights—US Geological Survey), accessed on March 20, 2022. https://www.cnpm.embrapa.br/projetos/relevobr/download/pr/pr.htm ”.
https://doi.org/10.1371/journal.pone.0274191.g001
A total of 25 streams were sampled ( Fig 1 ; S1 Table ) in the Iguaçu (nine streams), Piquiri (ten streams), and Ivaí (six streams) river basins. Sampled streams ranged in size from 1st to 3 rd order [ 43 ] and in land cover gradient from 3 to 80% of reduction of native forest cover.
Land use and land cover characterization
We calculated the different land use and land cover by demarcating the catchment above the sampling point for each stream. The geographical coordinates of the sampling sites were input into Quantum Geographic Information System (QGIS) software (QGIS version 2.18.10). A digital elevation model (DEM) was downloaded from the EMBRAPA Monitoramento por Satélite ( https://www.cnpm.embrapa.br/projetos/relevobr/download/pr/pr.htm ) [ 44 ]. Using the GRASS plugin in QGIS, the DEM raster was opened, and the catchment area for each sampling site was delimited with the ‘r.watershed’ and ‘r.water.outlet’ tools. The land use and land cover data (from 2017) were downloaded from the MapBiomas website ( https://mapbiomas.org/colecoes-mapbiomas-1?cama_set_language=pt-BR ). This raster was used as a base to calculate the different land use and land cover within the polygon of each catchment delimited for the sampling sites. The area (km²) of the following land uses was calculated: urbanized area—paved area, residential and industrial area; agricultural area which included pastures, plantations of annual and perennial crops and silviculture. In relation to land cover, the forested area included areas of riparian forest and remnants of native forest.
Habitat diversity
We applied the rapid habitat diversity assessment (RHDA) protocol, adapted by Callisto et al . [ 45 ], to characterize the habitat diversity of the streams. For this, the Rapid Assessment Protocol (RAP’s) was used, which is a cost-effective bioassessment method because it allows integrated analysis of stream ecosystems through visual inspection of the area. This RAP captures the characteristics of the habitat for rating the degree of impact measured in set scores, determining environmental quality, and indicating the cumulative impacts of multiple stressors [ 45 ]. The RHDA protocol consists of 22 parameters (detailed in the S1 File ). The first 10 were adapted from the Ohio Environmental Protection Agency—USA [ 46 ] and analyze the signals of human pressures in the reach characteristics. The other parameters were adapted from the protocol presented by Hannaford et al . [ 47 ] and assess the environmental characteristics of the sampled site. We used the total score obtained in the RHDA protocol to represent habitat diversity at the sampled sites.
Integrated Disturbance Index
The CDI values range from 0 (no land use in the catchment) to 400 (entire catchment occupied by urban and/or agricultural areas).
This index ranges from 0 to 1, and values close to 1 indicate major disturbances inside the stream channel, in the riparian zone, and/or in the catchment of the sampled sites. Like RHDA, in terms of the IDI values, the stream disturbances were classified into three IDI levels: < 0.09, low disturbance (sites with conditions closer to natural); 0.10–0.19, medium disturbance (altered sites); and > 0.2, high disturbance (extremely impacted sites). We considered high disturbance at IDI > 0.2 because this value included streams with urban land use greater than 20% in their catchment and human interference on the banks and stream channel.
Primary productivity
We used the Chl-α biomass to evaluate primary productivity. Chl-α concentration is an accepted indicator of eutrophication that can be examined to assess if the input of anthropogenic nutrients is affecting an ecosystem [ 50 ]. Chlorophyll-α is a primary indicator and can respond to increasing inputs of nutrients before more serious and irreparable damage occurs, such as loss of submerged aquatic vegetation [ 51 ]. Herein, the Chl-α biomass (μg/L -1 ) was determined from water samples collected at each sampling site. After sampling, 1 L of water from each sample was filtered by a vacuum pump using a fiberglass filter (Merck ® , GF-47 mm). The filters with the retained particles were analyzed in the laboratory using the parameters described for limnological analysis [ 52 ].
Fish assemblage sampling
To verify the composition and diversity of the fish fauna, we sampled three occasions (March—April 2017; July—August 2017, and December 2017—January 2018). In each stream, we conducted fish sampling 50 m reaches using three-pass electrofishing with 40 minutes of effort for each pass. To prevent fish escape, we delimited the reach using blocking nets. After capture, the fish were anesthetized and fixed in 10% formaldehyde. In the laboratory, individuals were identified according to specific identification keys [ 41 , 53 , 54 ]. This study was carried out in strict accordance with protocols in their ethical and methodological aspects for the use of fish. We deposited specimens of all the sampled species in the Coleção Ictiológica do Nupélia (Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura, Universidade Estadual de Maringá—UEM, Paraná State), and in the Coleção Ictiológica of the Universidade Federal do Rio Grande do Sul (UFRGS, Rio Grande do Sul State). The species list with respective vouchers is available only in the online version ( S2 Table ). We also classified the species as native and non-native in each sampling basin (Iguaçu, Piquiri, and Ivaí) according to Baumgartner et al . [ 41 ], Graça, Pavanelli [ 53 ], and Ota et al . [ 54 ] ( S2 Table ).
Statistical analysis
First, we assessed the effects of human pressure (agriculture and urbanization), represented here by the disturbance indices, on environmental conditions of the streams, as portrayed by the physical characteristics of the sampling sites. To investigate the correlation among habitat diversity, IDI, land use, land cover and their possible effects on local primary productivity, we applied Spearman’s correlation analysis using corrplot [ 55 ] and Hmisc [ 56 ] packages. Considering the different scales of variables, the variables were log-transformed with the ‘log’ function. These preliminary analyses are fundamental to understanding how environmental variables interact and avoiding collinearity in the subsequent analysis. Then, how changes in the environmental characteristics of streams affected the fish faunal composition and species distribution and what species would be good indicators of the different stream groups were determined.
To test the influence of environmental variables (explanatory variables) on the spatial distribution of species (response variables), we used distance-based redundancy analysis (dbRDA) [ 57 ], based on the Bray-Curtis distance. This analysis is a form of multivariate multiple regression used to assess the relative importance of each explanatory variable in explaining the differences between the response variables. For this purpose, a square root transformation on the species abundance data was used, thus reducing the weight of the most abundant species in the analysis. The environmental variables were log-transformed at the different scales. To ensure the effectiveness of the variables in the analysis, environmental variables were selected using two criteria. First, all highly correlated variables (Spearman’s r ≥ 0.7, p < 0.05) [ 58 ] were excluded. Second, the variance inflation factor (VIF) was applied to the variables selected by Spearman’s correlation and those with VIF > 10 were excluded [ 59 ]. For VIF > 10, there was severe multicollinearity requiring correction. Nitrate, phosphate, and total nitrogen were excluded due to this process. After the selection of environmental variables, we performed dbRDA with the ‘capscale’ function in the vegan package [ 60 ]. The statistical significance of dbRDA was assessed using a permutation test for dbRDA, using the ‘anova.cca’ function, with 999 permutations, of the vegan package [ 60 ].
To determine whether there were fish species that could be an indicator for each site category according to the IDI levels (> 0.09—low disturbance, 0.10 to 0.19 –medium disturbance, and < 0.2—high disturbance), indicator value analysis was applied (IndVal) [ 61 ]. Indicator values reflect specificity, i.e., the probability of a taxon occurring in a group, and fidelity, i.e., the relative abundance of the taxon in that group. The method of Dufrêne, Legendre [ 61 ] calculates the IndVal index between the species and each site group and then looks for the group corresponding to the highest association value. Finally, the statistical significance of this relationship is tested using a permutation test. IndVal is the default index used to measure the association between a species and a group of sites in ‘multipatt’. However, by default ‘multipatt’ uses an extension of the original Indicator Value method, because the function looks for indicator species of both individual site groups and combinations of site groups, as explained in De Cáceres et al . [ 62 ]. IndVal produces an indicator species value (ISV) that ranges from 0 (absent) to 1 (present in all samples of a particular group). Species that are considered the “best” indicators of a group are those with scores closest to 1, indicating that they are found within their group only and do not occur anywhere else. IndVal is based on the numerical abundance of fish species and was calculated using the ‘multipatt’ function, with 999 permutations, in the indicspecies package version 1.7.8 [ 62 ].
Restrictedness was also calculated using the ‘restrictedness’ function in the funrar package [ 63 ]. This taxonomic metric indicates the presence of rare species at the regional level. The calculation produces a single index per species and is based on a complete dataset containing the presence-absence or relative abundance of species at each site [ 63 ]. Here, we calculated the restrictedness metric using the relative abundance of the species. We measured the numerical abundance (number of individuals by species) and species richness (species number by stream) to calculate the taxonomic diversity indices (Simpson dominance, Joule equitability, and Shannon diversity). We calculated the taxonomic diversity indices usingthe BiodiversityR and vegan packages [ 60 , 64 ]. These indices are based on numeric abundance and facilitate the detection of changes in fish assemblages related to alterations in species abundance and are a useful tool to investigate the effects of human pressure on fish assemblage structure [ 65 ]. The next step was to perform Generalized Linear Mixed Models (GLMMs) with Simpson dominance, Joule equitability, Shannon diversity, the numerical abundance of species, and species richness as response variables, with IDI and the proportion of the numeric abundance of the non-native per native fish species (NNAbu, non-native species abundance/native species abundance) as fixed factors, and basin as a random factor. The NNAbu was included because the presence of non-native species is one of the consequences of human pressure on freshwater environments and has caused numerous alterations in native assemblages [ 9 ]. According to previous correlation analysis results, habitat diversity and IDI are significantly correlated, indicating that only one of these variables should be used in the models. The IDI was used because this index represents the human pressure in the local scale (riparian area) and regional scale (catchment) of the streams. The explanatory variables were log-transformed to standardize the scales. We checked the proper family distribution for each response variable using the function ‘fitdist’ from fitdistrplus package [ 66 ]. Subsequently, GLMMs with beta family distributions were run for Simpson’s dominance and Joule equitability (values bounded between 0 and 1) using the ‘glmmTMB’ function from glmmTMB package [ 67 ]. For Shannon diversity, species richness, and numerical abundance, GLMMs with Gaussian family distribution were run using the ‘lmer’ function from the lme4 package [ 68 ]. Models with an interaction between the effect factors (IDI and NNAbu) and models without interaction were compared using ANOVA to determine if there were differences between the tested models. Additionally, the Akaike information criterion (AIC) [ 69 ] was used to select the best model among the tested models for each response variable [ 70 ]. The residual plots were visually inspected to check the model assumptions and the plots of the models were built using the ggplot2 package [ 71 ].
All analyzes were performed in R programming environment (ver. 3.2.3, R Foundation for Statistical Computing, Vienna, Austria). The level of statistical significance for all analyses was p< 0.05.
Effects of the human pressure on environmental characteristics of streams
Streams with high disturbance were negatively correlated with habitat diversity and forested cover and were positively correlated with urban land use and Chl-α ( Table 1 ; Fig 2 ). Habitat diversity was positively correlated with forested cover and negatively correlated with urban land use and Chl-α ( Fig 2 ). Agricultural land use had no significant relationship with any of the evaluated variables.
The values in the squares represent the correlations, asterisks indicate significant correlations, blank squares indicate no significant correlations.
https://doi.org/10.1371/journal.pone.0274191.g002
https://doi.org/10.1371/journal.pone.0274191.t001
Effects of the human pressure on species composition
A total of 13,615 individuals belonging to 63 species, 12 families, and six orders were sampled. Siluriformes were highlighted with greater species richness (29), followed by Characiformes (23 species). Characidae and Loricariidae were the families with the highest species richness (15 and 10, respectively). Eleven species were classified as non-native ( S2 Table ).
The first two axes of the dbRDA explained 29.36% of the variation, but significant differences were observed only for the dbRDA axis 1 (16,36%) (Axis 1 –F = 4.57; p = 0.048; Axis 2 –F = 3.28; p = 0.338). Temperature, conductivity, and RHDA explained the variability in the composition of fish fauna ( Table 2 ). The first axis was positively correlated with temperature and conductivity, and negatively correlated with RHDA. Among the species that showed a positive correlation with the first axis of the dbRDA, 10 species are native for all sampling basins ( Ancistrus mullerae , Astyanax lacustris , Cambeva davisi , Cambeva aff davisi , Cambeva stawiarski , Corydoras aeneus , Geophagus brasiliensis , Hypostomus derbyi , Ancistrus sp., Hisonotus pachysarkos ), and two species are non-native to the Iguaçu River basin ( Hypostomus ancistroides , Gymnotus sylvius ) ( Fig 3 ). Negative correlation was observed for six native species for all sampling basin ( Neoplecostomus sp. 1, Psalidodon aff. fasciatus , Psalidodon aff. paranae , Psalidodon bockmani , Psalidodon bifasciatus , Phalloceros harpago ) and one non-native species for all sampling basin ( Poecilia reticula ) ( Fig 3 ).
The colors demonstrate the degree of urbanization (red), that is, when the point is red, it presents a high degree of urbanization. The arrows indicate how the variables are related to the dbRDA axes and the underlined variables were statistically significant. IDI- Integrated Disturbance Index, RHDA- Rapid Habitat Diversity Assessment, TDS- Total dissolved solids, TP- Total phosphorus, NH4- Ammonia, TPT- Temperature, CON- Conductivity, DO- Dissolved oxygen, TUR- turbidity. Am = Ancistrus mullerae ; An = Ancistrus sp.; Al = Astyanax lacustris , Cd = Cambeva davisi ; Cf = Cambeva aff. davisi ; Ct = Cambeva stawiarski ; Ca = Corydoras aeneus ; Geophagus brasiliensis ; Gy = Gymnotus sylvius Hp = Hisonotus pachysarkos ; Ha = Hypostomus ancistroides ; Hd = Hypostomus derbyi ; N1 = Neoplecostomus sp. 1; Pb = Psalidodon bifasciatus ; Po = Psalidodon bockmanni ; Pf = Psalidodon aff. fasciatus ; Pp = Psalidodon aff. paranae ; Ph = Phalloceros harpagos ; Pr = Poecilia reticulata . See S2 Table for the code for the other species.
https://doi.org/10.1371/journal.pone.0274191.g003
https://doi.org/10.1371/journal.pone.0274191.t002
Indicator species analysis showed that, among the 63 species considered, only a few species were significantly related with disturbance levels. Four species were indicator species of streams with lower disturbance ( P . bifasciatus , A . minor , C . stawiarski and A . mullerae ), one of the streams with medium disturbance ( R . quelen ), and five were indicator species of streams with high disturbance, non-native, or resilient species ( P . reticulata , H . ancistroides , S . marmoratus , G . brasiliensis and H . derbyi ; Table 3 ). Considering the regional species pool, some species were emphasized as taxonomically rare by the restrictedness metric: Apareiodon vladii , Psalidodon aff. gymnodontus , Bryconamericus ikaa , Callichthys callichthys , Cambeva mboycy , and Hoplias mbigua (all of them with restrictedness = 0.96). Except for Cambeva cf. mboycy , these species occurred in streams with no urban influence.
https://doi.org/10.1371/journal.pone.0274191.t003
Previous influences of human pressure on fish species distribution are also observed in the fish fauna structure ( Fig 4 ; Table 4 ). The numerical abundance of fish species was positively influenced by IDI (t = 2.662; p = 0.014) and NNabu (t = 2.396; p = 0.025) ( Fig 4A and 4B ). In general, streams with higher numerical abundance exhibited great disturbances and non-native fish abundance. Equitability was negatively affected by IDI (z = -1.933; p = 0.053) ( Fig 4C ; Table 4 ). However, species richness, species diversity, and Simpson’s dominance were not significantly affected by IDI and NNAbu.
The blue line represents the best fit of the Generalized Linear Mixed Models (GLMMs) to the data, and the gray shading area indicates the 95% confidence interval. A. Relations among numeric abundance (Abund) and Integrated Disturbance Index (IDI); B. Relations among numeric abundance (Abund) and non-native fish species abundance (NNAbu); C. Relations among equitability (Joule index) and IDI.
https://doi.org/10.1371/journal.pone.0274191.g004
https://doi.org/10.1371/journal.pone.0274191.t004
The results of this study demonstrated an inverse relationship between habitat diversity and disturbance. The intensification of human pressure on stream environments decreased habitat diversity and triggers changes in primary productivity, fish species composition, and assemblage structure. Streams with intense urban land use tended to present high productivity and low habitat diversity, which can culminate in habitat homogenization and eutrophication, drastically reducing the environmental quality in these environments. In disturbed streams, we observed the prevalence of the fish species previously reported in the literature as resistant to degradation; in contrast to the registered in the less disturbed streams, where the more sensitive, rare and endemic species prevailed. This relationship between resistant species and highly disturbed streams has been observed in other studies that evaluated human pressure on stream environments [ 72 , 73 ]. In agreement with the initial hypothesis, the structure of the fish assemblage was affected by human pressure. Specifically, we verified that disturbance intensification tended to increase the numerical abundance and decreased the equitability in these fish assemblages. All these results corroborate that dominance represents a strong predictor of changes in communities induced by the main anthropogenic stressors, as highlighted by Hillebrand et al . [ 74 ].
Effects of land use on stream habitats
Land use for human activities is intrinsically linked to ecological conditions in stream environments. Forest cover removal (on a catchment scale) and riparian forest (on a local scale) increases the input of sediments and nutrients in the stream channel, and intensifies erosion processes, mainly at the stream margins [ 12 ]. Such disturbances caused by human pressure were measured in the current study, presented via the IDI. The negative relationship among disturbances caused by land use (both on catchment and local scale) and habitat diversity in stream environments was clearly shown in the results. Therefore, regardless of the analyzed basins, reduction in habitat diversity can be used as a proxy for anthropogenic effects on stream ecosystems. The findings corroborate other studies showing the consequent homogenization of habitats induced by changes in land use from human activities [ 10 , 16 ].
Primary productivity in streams is directly related to the environmental characteristics (physico-chemical conditions) of these ecosystems [ 75 ]. In our study, the correlations showed that in catchments with high urban land use, forested cover decreased, reducing habitat diversity and increasing primary productivity. In contrast, greater forested cover in streams catchment was positively related to greater habitat diversity. Thus, changes mediated by human pressure (high urban land use and low forested cover) in streams are evidenced by high disturbance (high IDI) and greater primary productivity. Variations in abiotic conditions, such as water temperature, pH, and nutrient load, significantly affect biological productivity in freshwater ecosystems [ 76 ]. In addition, substrate conditions can also influence chemical characteristics, especially in streams [ 76 ]. Primary productivity was generally low in all the sampled streams, but the few high values of productivity that were observed were only recorded in streams with high urban land use in their catchments. The decrease in shading due to the absence or reduction of riparian forest increase the exposure of the water surface to sunlight, and the water temperature increases [ 77 ]. High water temperature together with the nutrient’s enrichment provide conditions for an increase in primary productivity [ 75 , 78 ], which can indicate a highly productive eutrophic state. The trophic state is fundamental to the ecosystem structure and is directly linked to the water quality and biotic integrity of streams [ 75 ]. The observed relationships among RHDA, temperature, conductivity, and dissimilarities in species composition corroborate the relationship between water quality and biotic integrity. Therefore, changes in primary productivity are predicted to mediate the food webs, also driving the composition and structure of the fish fauna.
Individual species responses and identification of indicator species
Sets of distinct fauna that were strongly related to habitat diversity and disturbance gradients were verified in the analyzed streams. Psalidodon bifasciatus and C . stawiarski were mainly related to habitat diversity, corroborating their preference for resources that are more abundant in preserved streams. Psalidodon species feed mainly on plants, algae, and insects, and Cambeva species prefer reaches with riffles, consuming autochthonous food items, mainly insects [ 20 , 79 ]. In contrast, degradation-resistant species, some of them non-native species, such as G . sylvius and H . ancistroides in the Iguaçu Basin [ 41 ], and P . reticulata [ 39 , 53 , 54 ], and even native species, such as P . harpagos , C . aeneus , and A . lacustris , were related to low habitat diversity. Such species can survive in environments with low oxygen levels and are trophic opportunistic, which provides resistance in altered environments [ 80 – 82 ].
Different species were highlighted as indicators in the sampled streams, considering the disturbance level. Astyanax minor and A . mullerae , endemic to the Iguaçu Basin, were indicative of forested stream with low disturbance. These species have requirements for habitat use and food [ 41 ] and have been reported to be sensitive to environmental degradation [ 9 , 83 ]. In addition, P . bifasciatus and C . stawiarski were associated with low-disturbance streams. Rhamdia quelen was indicative of streams with medium disturbance; it is described as a species that lives in pools with sand and mud bottoms and is resistant to environmental variations such as pH and water temperature [ 84 ]. Agricultural land use in catchments generally modifies the substrate composition, reducing the presence of pebbles and gravel and increasing sedimentation. Such changes in the substrate lead to the predominance of soft bottoms in streams [ 85 ], which also alters species composition. The findings reported here corroborate those of other studies [ 86 , 87 ], and reinforce the importance of assessing local and catchment conditions simultaneously with the historical conditions. Furthermore, it is noteworthy that these findings were independent of the analyzed basin and soil types, which indicates the great threat of the loss of ecosystem function to which these environments are exposed.
Degradation-resistant species were highlighted as species indicative of the highly disturbed streams group. Poecilia reticulata has been commonly associated with environments impacted by human activities, as registered in Brazilian streams altered by urbanization [ 3 , 22 , 88 , 89 ]. Hypostomus derbyi , H . ancistroides , and Synbranchus marmoratus are resistant to low oxygen levels because they are considered stomach air-breathing [ 90 , 91 ]. Geophagus brasiliensis is a generalist species that is tolerant to variations in temperature, pH, and low oxygen levels [ 92 ]. These biological traits allow these species to survive in disturbed environments, as indicated by the IndVal results that highlighted them as indicative of streams with high disturbance.
Native and endemic species were highlighted as rare species, by the taxon restrictedness metric, in the basins sampled. For example, Apareiodon vladii is an endemic species of the Piquiri and Ivaí river basins [ 54 ] and was sampled only in a forested stream during this study. Apareiodon species are trophic specialists that mainly feed on vegetal resources, with benthopelagic habit [ 9 ], and prefer habitats with high flow, well-oxygenated waters, and rocky substrates [ 93 ]. Registered only in an agricultural stream of the Ivaí Basin, C . callichthys is widespread in South American rivers [ 54 ], can breathe air to survive in hypoxic and shallow waters [ 94 ], and uses the aquatic vegetation accumulated on the riverbanks and swamps to lay its eggs [ 95 ], characteristics observed at the sampling site of their capture. Hoplias mbigua , P . aff. gymnodontus , B . ikaa , and C . mboycy were sampled from forested streams (except C . mboycy , urban stream) of the Iguaçu Basin and stand out in the regional species pool. Excluding H . mbigua , which is widely registered in freshwater environments in the Paraná–Paraguay system [ 54 ], the other species are considered endemic to the Iguaçu Basin [ 39 ]. Notably, C . mboycy inhabits the reaches with riffles and consume autochthonous food items, mainly insects, which are abundant resources in more preserved streams. In addition, this species was captured in low abundance in a stream that showed urbanization in the basin. This result reinforces the role of the riparian forest, which is fundamental to the physical structure, energy flow, and species diversity of this environment. Additionally, C . mboycy was categorized as endangered [ 96 ], indicating the fragility of this native species and the importance of preserving these streams. It is fundamental to expand the management and conservation efforts in this basin, mainly for the maintenance of riparian forests and habitat diversity, which is essential for preserving this fish fauna.
Assemblage-level responses
High disturbance caused by human pressure in stream environments, such as land use intensification and non-native fish species introduction, positively affected the abundance of fish species. A high abundance of fish species has previously been related to impacted sites [ 9 , 97 ], corroborating our results. Non-native fish species can drive species dominance and cause changes in the original composition of the fish species [ 98 ]. Such alterations could have occurred in the sampled urban streams, where there was a high abundance of P . reticulata . In the case of urbanization, the intensification of disturbances facilitates an increase in degradation-resistant species and a reduction in degradation-sensitive species, leading to the dominance of a few species [ 89 , 99 ]. The existence of different stressors in the same stream or catchment drastically changes the composition and structure of the fish assemblage fish [ 79 ].
Equitability tended to decrease with disturbance intensification probably due the higher abundance of non-native fish species in the streams. Although the evenness index does not reflect whether the dominant species differs in important traits compared to the rare species [ 74 ], here we highlight the occurrence of non-native species resistant to degradation. These non-native species are generalist functional groups [ 72 ] that are degradation-resistant and proliferate rapidly dominating the fish assemblage. Thus, changes resulting from environmental conditions such as eutrophication favor resistant species with consequent changes in interactions and coexistence between species (competition for resources), resulting in reduced equitability. In streams under human pressure, the equitability responds rapidly to the occurred changes, which shows the importance to measure it when evaluating the effects of land use on fish assemblages [ 74 ]. It is worth mentioning that high disturbance was directly related to low habitat diversity and eutrophication (nutrient load + Chl-α), which are considered important factors in determining the structure of fish assemblages in freshwater ecosystems [ 100 ]. Greater disturbance reduces habitat availability for prey and food resources [ 101 ], which can cause an imbalance in species abundance, affecting the equitability and therefore, disruption of food webs in these assemblages, and even ecosystem processes [ 74 ]. Here, we emphasize that the synchronism between habitat degradation caused by land use and the introduction of non-native species enhances the deleterious effects on sensitive species, with consequent homogenization of the biota. In this respect, although the causes of species introduction are sometimes different from those that occur in large systems [ 9 , 36 ], the effects on stream fish structure appear to be convergent.
Herein, this study stands out for comprising representative areas of two ecoregions. Our results suggest some perspectives underlying the current scenario of human impacts and the loss of Neotropical freshwater fish diversity. Considering that small-bodied fish, most of which are exclusive to streams, are considered the largest and most threatened portion of the megadiverse fauna of Neotropical freshwater fish [ 102 ], and that the disturbances reported here are predicted to increase (e.g., with the increase in urban areas), irreversible losses are inevitable. We can infer that regardless of the fish species richness of the basins (the Iguaçu River is comparatively poor in species richness), urbanization is a strong driver of productivity, species composition and structure, which can lead to fauna homogenization. Thus, land use and management decisions, as well as the culture of society, will be decisive in the conservation of stream biodiversity.
In summary, the current study reinforces the important role of forest cover and habitat diversity in maintaining native, endemic, taxonomically rare, and degradation-sensitive fish assemblages in streams. Disturbance intensification drives the increase in primary productivity, as well as alterations in the composition and structure of fish fauna, leading to higher abundance and lower equitability, with the predominance of degradation-resistant species in the disturbed streams. The increase in non-native species abundance in the disturbed streams is also a driver of the higher abundance in the streams that were sampled. Headwater streams shelter a great number of endemic species, registered even in urban streams, a fact that runs contrary to conservation in these water bodies, where intensive disturbance can render endemic species extinct and make way for non-native species. Considering that the evaluated disturbances can lead to extirpation of sensitive species and that these species, mainly in the Iguaçu River Basin, are endemic and taxonomically restricted to this basin, such exclusion can mean their global extinction. Thus, it is necessary to enhance conservation efforts directed toward stream ecosystems to maintain or recover their biodiversity and ecosystem services.
Supporting information
S1 table. characteristics of the sampling areas (streams, surrounding of sampled streams and river basins)..
https://doi.org/10.1371/journal.pone.0274191.s001
S2 Table. Fish species recorded in the streams from Iguaçu, Ivaí and Piquiri river basins, Brazil.
https://doi.org/10.1371/journal.pone.0274191.s002
S1 File. Protocol for Rapid Habitat Diversity Assessment (RHDA, applied in stretches of sampling sites during this study).
https://doi.org/10.1371/journal.pone.0274191.s003
Acknowledgments
We thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship, the UNIOESTE campus Cascavel for the logistical support for the field collection, to the researchers of the Coleção Ictiológica do Nupélia (Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura) and Universidade Federal do Rio Grande do Sul (UFRGS) for confirming the identification of the fish species. Additionally, we thank to Débora Reis de Carvalho and Aymar Orlandi Neto for the statistical support with disturbance indexes and statistical analysis.
- View Article
- Google Scholar
- PubMed/NCBI
- 24. Hauer C, Leitner P, Unfer G, Pulg U, Habersack H, Graf W. The role of sediment and sediment dynamics in the aquatic environment. In: Riverine Ecosystem Management. Cham, Switzerland: Springer Open; 2018. p.151–169.
- 27. Sistema Nacional de Informações sobre Saneamento (SNIS). Painel de Informações Sobre Saneamento [Internet]. Brasil; 2018. http://www.snis.gov.br/ .
- 38. Hales J, Petry P. Freshwater ecoregions of the world (FEOW) [Internet]. 2019. http://feow.org/ecoregions/details/ .
- 39. Pereira MCB, Scroccaro JL, organizers. Série Histórica: Bacias Hidrográficas do Paraná [Internet]. Curitiba: SEMA; 2013. http://pdslitoral.com/wpcontent/uploads/2018/01/Revista_Bacias_Hidrograficas_do_Parana.pdf .
- 41. Baumgartner G, Pavanelli CS, Baumgartner D, Bifi AG, Debona T, Frana VA. Peixes do baixo rio Iguaçu. EDUEM; 2012.
- 42. Maack R. Geografia Física do Estado do Paraná. EDITORA DA UNIVERSIDADE ESTADUAL DE PONTA GROSSA; 2012.
- 44. Miranda EE. Brasil em Relevo. Campinas: Embrapa Monitoramento por Satélite. [Internet]. 2005. http://www.relevobr.cnpm.embrapa.br .
- 46. Environmental Protection Agency (EPA). Biological criteria for the protection of aquatic life. Ohio: Division of Water Quality Monitoring Assessment. Columbus; 1987.
- 48. Kaufmann PR, Levine P, Robison EG, Seeliger C, Peck DV. Quantifying Physical Habitat in Wadeable Streams. EPA/620/R-99/003. U.S. Washington, DC: ENVIRONMENTAL PROTECTION AGENCY; 1999.
- 52. Golterman, H., R. Clymo, M. Ohndtad. Methods for the physical and chemical examination of freshwaters. Blackwell Scientific; 1978.
- 53. Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto Rio Paraná e áreas adjacentes. EDUEM; 2007. https://doi.org/10.1017/CBO9781107415324.004 .
- 55. Wei T, Simko V. R package "corrplot" : Visualization of a Correlation Matrix . (Version 0.88). [Internet]. 2021. https://github.com/taiyun/corrplot .
- 56. Harrell Jr, FE. Hmisc: Harrell Miscellaneous. R package version 4.5–0. [Internet]. 2021. https://CRAN.R-project.org/package=Hmisc .
- 59. Oksanen J, Blanchet FG, Kindt R, Legendre P, O’Hara RB, Simpson GL, et al. Vegan: Community Ecology Package. R package version 1.17–9. [Internet]. 2011. http://CRAN.Rproject.org/package=vegan .
- 60. Oksanen JF, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. Vegan: Community Ecology Package. R package version 2.5–7. [Internet] 2020. https://CRAN.R-project.org/package=vegan .
- 64. Kindt R, Coe R. Tree diversity analysis. A manual and software for common statistical methods for ecological and biodiversity studies. World Agroforestry Centre (ICRAF), Nairobi; 2005.
- 69. Akaike H. Information theory as an extension of the maximum likelihood principle. In: Second International Symposium on Information Theory. Akademiai Kiado; 1973. p. 267–281.
- 71. Wickham H. ggplot2: Elegant Graphics for Data Analysis. SPRINGER-VERLAG; 2016.
- 76. Allan JD, Castillo MM. Stream ecology: structure and function of running waters. SPRINGER; 2007.
- 94. Welcomme RL. Pesca fluvial. Documento Técnico de Pesca N◦ 262. FAO; 1992.
- 96. Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio). Livro Vermelho da Fauna Brasileira Ameaçada de Extinção: Volume I. Brasília, DF: ICMBio/MMA; 2018. http://www.icmbio.gov.br/portal/component/content/article/10187 .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.8(11); 2022 Nov

A review of fish diversity, decline drivers, and management of the Tanguar Haor ecosystem: A globally recognized Ramsar site in Bangladesh
Mst. armina sultana.
a Department of Aquatic Resource Management, Sylhet Agricultural University, Sylhet, Bangladesh
Debasish Pandit
b Department of Oceanography, Khulna Agricultural University, Khulna, Bangladesh
Sanzib Kumar Barman
c Department of Fishery Resources Conservation and Management, Khulna Agricultural University, Khulna, Bangladesh
Kishor Kumar Tikadar
Nishat tasnim, iftekhar ahmed fagun, md. ashraf hussain.
d Department of Fisheries Technology and Quality Control, Sylhet Agricultural University, Sylhet, Bangladesh
Mrityunjoy Kunda
Associated data.
Data will be made available on request.
Tanguar Haor (TH), an ecologically critical area (ECA) and a Ramsar site of worldwide significance, is an essential wetland ecosystem for the Bangladesh’s economic, ecological, social, and cultural aspects. Fish, aquatic plants, amphibians, reptiles, birds, and mammals are notable among the floral and faunal compositions found in this haor . Unfortunately, unsustainable exploitation of its natural resources poses a serious threat to the TH ecosystem. Therefore, the broad objective of this study was to review the status of fish biodiversity along with the driving factors of biodiversity loss and the management issues of the TH ecosystem. A total of 143 species of fishes (137 indigenous and 6 exotic) under 35 families, and 12 orders were documented during the last two decades. Species diversity of the haor has been changed over time due to the effects of climatic, anthropogenic, socioeconomic, and policy related drivers. Furthermore, high dependency on fisheries resources, poverty, and the lack of empowerment to manage the TH fishery were responsible for fish diversity decline. Therefore, ecosystem based co-management through active participation of local community, establishment of balanced fishing tactics, and strengthening alternative livelihoods for highly depended poor harvesters are strongly recommended for the proper management of this valued wetland ecosystem. Furthermore, this review proposes immediate and useful conservation initiatives for the studied wetlands, including comprehensive stock assessment, establishment of gene banks and fish sanctuaries, a combination of input and output control, and regulation with the ECA and RAMSAR guidelines.
- • Tanguar Haor (TH), a Ramsar site of economic and ecological importance, is home of 143 different fish species.
- • The decline in fish diversity of TH is caused by a combination of climatic, anthropogenic, socioeconomic, and policy factors.
- • Almost half of the people living in TH area were extremely poor and dependent on the haor for their livelihoods.
- • Ecosystem-based co-management with active participation from the local communities is imperative for the conservation of TH.
Fish diversity; Livelihood; Management; Ramsar site; Tanguar Haor ; Ecosystems.
1. Introduction
Bangladesh, a floodplain delta of the Brahmaputra, Ganges, and Meghna rivers, features one of the world’s wealthiest and most varied inland aquatic ecosystems, with a diverse fish fauna and vast water resources ( Islam et al., 2015 ; IUCN Bangladesh, 2015a ; Islam and Sultana, 2016 ; Mustafi et al., 2022 ). The nation is one of the world’s top fish producing countries, and during 2019–20 the total fish production was 4.5 million metric ton (MT) ( DoF, 2020 ). Bangladesh has a total inland water area of 6.7 million hectares, with open water capture fisheries accounting for 94% and closed water aquaculture accounting for 6%, respectively ( Hossain, 2014 ). According to the Food and Agriculture Organization (FAO) report, the nation was ranked third position in inland open water capture fisheries production ( FAO, 2020 ). Inland fisheries, encompassing rivers and estuaries, forest water resources in the Sundarbans, beels , Kaptai Lake, floodplains, and haors , are mainly responsible for promoting the fish production of Bangladesh, with approximately 260 freshwater native fish species ( DoF, 2020 ; Mustafi et al., 2022 ; Saha et al., 2021) ).
Typically, haor refers to a saucer-shaped marshy wetland habitats which are flooded for around 7–8 months, resembling a large inland sea during the rainy season ( Pandit et al., 2022 ). The haor basin is a critical ecosystem for fish production both commercially and ecologically ( Salauddin and Islam, 2011 ). It also serves as breeding, nursing, feeding, and overwintering habitats for residents and migratory fish species. For instance, about 154 native fish species covering 35 families and 12 orders inhabit in the haor wetlands ( Pandit et al., 2022 ). In 2018–19, the total fish production of the haor region was 108,880 MT with a productivity of 433 kg/ha ( DoF, 2019 ). Additionally, the growth rate of the fish production from the haor region was 12.11% between FY 2017–18 and FY 2018–19 ( DoF, 2019 ). However, due to overexploitation, habitat fragmentation, siltation of freshwater bodies, and environmental pollution from industries and agricultural chemicals, the percentage of inland capture fisheries to overall fish production had gradually decreased from 62.59% in 1983–84 to 28% in 2013–14 ( DoF, 2019 ).
Bangladesh is an agriculture oriented nation with massive water resources ( Sultana et al., 2016 ) in which Tanguar Haor (TH) is widely renowned freshwater wetland in the country ( Figure 1 ). This haor extends across two upazilas (sub-districts) namely, Tahirpur and Dharmapasha, in Sunamganj District, spanning more than 10,000 ha and serving at least 60,000 people ( IUCN Bangladesh, 2016 ). The floral and faunal compositions in this wetland are renowned for their presence of various species of fishes, aquatic plants, amphibians, reptiles, birds, and mammals. TH is the home to approximately 141 freshwater fish species, and the richness of these fisheries is one of its unique features, paying it the title of ‘mother fishery’. TH’s fish stock was estimated to be 6701 tons ( Ahmed, 2015 ) and this huge fisheries asset’s economic value estimated BDT 151,128,000 (USD 1 = 81 BDT) each year ( Solayman et al., 2018 ). Moreover, this haor is crucial grazing, spawning, breeding, and nursing zone for different freshwater fishes and prawns. It also promptly delivers significant value to the country’s economy by supplying 14% of the annual catch to open-water fisheries in the Sunamganj district and nationally 0.67%, respectively ( Alam et al., 2015 ).

Location of Tanguar Haor in Sunamganj district of northeastern Bangladesh.
Tanguar Haor is one of the six ecologically critical areas (ECAs) of Bangladesh and is a wetland with a global reputation as the Ramsar site ( Tima et al., 2021 ). TH was designated an ECA by the government in 1999 owing to its severe condition as a consequence of detrimental activities of its assets ( GoB, 2004 ). As a result of its global significance, it was chosen as the second Ramsar site of Bangladesh on July 10, 2000 ( IUCN Bangladesh, 2015b ). Beginning in the mid-1990s, the National Conservation Strategy Implementation Project was the government’s first conservation endeavor in TH. However, no notable conservation initiative is currently underway to protect the TH region’s immense fish biodiversity with the participation of fishers and community members ( The Daily Star, 2020 ). Moreover, due to increased fishing pressure and a variety of human activities that promote silting, river pollution, and the loss of natural reproduction and native fish populations in this haor have declined dramatically over the years ( Ahmed, 2008 ). Natural disasters like flash floods and storm surges also threatened Tanguar Haor’s habitat, communities, and livelihood ( Munasinghe, 2000 ). Through several projects, fisheries management practices are currently established in the TH region, but need more intensive care through active community participations as well.
The long-term viability of TH’s fisheries management practices is threatened by anthropogenic, environmental, and climate change/variability, as well as trans-boundary consequences. Biodiversity loss, particularly decline of species diversity, is a significant concern in the nation, and the number of endemic fish species is diminishing year after year in various inland waterbodies owing to a variety of man-made and natural causes ( Pandit et al., 2015a , 2015b , 2021 ; Sultana et al., 2019 ; Akter et al., 2020 ; Barman et al., 2021 ; Saha et al., 2021 ; Das et al., 2022 ; Kamal et al., 2022 ). Except for Alam et al. (2015) there is currently no comprehensive overview study or analysis on the fish assemblages of the TH, causes of fish population decline, or fisheries management techniques. However, Alam et al. (2015) limited their investigation to the fish fauna, risks to TH, geographical and climatic conditions, and the significance of conservation. This treatise therefore aims to review and portray the fish biodiversity, causes of fish diversity decline, conservation approaches, and the barriers of the current fisheries management practices of TH along with some recommendation measures. When it comes to the sustainable management of wetlands and inland waterbodies around the world, fisheries co-management has proven to be a successful tool. Over a decade ago, the co-management approach was alluded to in TH. This review also considers why the diversity of fish in TH declined. Furthermore, the review paper is a first attempt to find out the research gaps from previous works, recent biodiversity condition, and associated threats. The topics like current status, biodiversity value, and related threats have also been compiled that may be helpful for law and policy making organizations in determining future course actions for wise use and sustainable management of TH ecosystem of Bangladesh.
2. Methodology
Following the guidelines of Haddaway et al. (2015) , we conducted a systematic review of the literature. We concentrated on peer-reviewed studies, PhD and Master’s theses, and scientific reports regarding TH that were written in English and published online ( Figure 2 ). The data search was conducted in three comprehensive databases of scholarly publications- Web of Science, Google Scholar and Scopus between January and December 2021. We conducted a preliminary assessment of a subset of articles prior to the main search to determine the search-string combination to utilize. All results were evaluated for relevancy and to avoid papers that were not related to our focus for each search-string. Each string was tested individually. Examples of the search-strings include: water quality parameters, fish diversity, decline factors, management, conservation, livelihood profile and Tanguar Haor . Then, we found 87, 18, 16 peer reviewed papers from google scholar, web of science and scopus, respectively. Finally, we have reviewed 22 papers.

Systematic flow diagram of the articles searched and the process of exclusion.
Relevant studies were identified by a step-by-step approach as shown in Figure 2 using EndNote X9. The step-by-step eligibility criteria were: All studies must include information on the author(s), year of publication and titles, duplicates were removed; article titles and abstracts were screened to include only studies conducted in TH; and the criteria for subsequent inclusion were that the articles, when fully read, considered water quality parameters, fish diversity, driving forces of biodiversity decline, socio-economic profile of engaged fishers, conservation and management strategies of the TH.
3. Results and discussion
3.1. water quality parameters.
Water is the most important component in shaping the land and regulating the climate, as well as provide life support system for all aquatic living organisms ( Tasnim et al., 2022 ). Aquatic life is also impacted by the decline in water quality ( Bhuyain et al., 2022 ; Tasnim et al., 2022 ). Water quality is affected by a wide range of physico-chemical and biological variables, which may have an immediate or passive impact on its quality ( Moses, 1983 ). Within the standard limits, a slight decrease or rise in the concentration of dissolved or suspended materials has a negative impact on the body functions of aquatic organisms. During the monsoon the water quality in the TH area is regular, clean, and transparent. TH has a mesotrophic water quality ( Bhuiyan et al., 2020 ). A few studies have been carried out to determine the different parameters (temperature, pH variation, total dissolved solid, dissolved oxygen, electrical conductivity, nutrient contents, and phytoplanktonic biomass) of the water of TH ( Table 1 ). Based on analyses of different physicochemical and biological parameters, the TH is still devoid of high organic contamination, the water quality is relatively good for fish production, and decline of fish has no direct association with water quality ( Mamun et al., 2013 ; Bhuiyan et al., 2019 ).
Table 1
Water quality parameters of the Tanguar Haor .
3.1.1. Temperature
The water temperature of the TH varied from 22.4 °C to 31.0 °C with an annual mean around 26.0 °C ( Bhuiyan et al., 2019 , 2020 ). Solayman et al. (2018) determined the temperature ranged from 27.2 °C to 29.4 °C with a mean value of 28.28 °C from Rupaboi beel , Muinsakhali beel , Chatrar beel , Boddof beel , and Kolma beel of the haor . Similarly, Mamun et al. (2013) found 27.98 °C as the mean value of water temperature and recorded the minimum value 27.8 °C and the maximum value 28.1 °C.
3.1.2. pH variation
The water pH value of the haor normally fluctuates between 8.1 and 9.7 from December to March and 7.5–7.7 from April to September ( Bhuiyan et al., 2020 ). However, the study of Bhuiyan et al. (2019) recorded the pH values range between 7.2 and 9.7 in the Rauar area of TH throughout the year. Solayman et al. (2018) found the average pH value ranges from 7.3 to 8.7, while Mamun et al. (2013) reported that the average value spans from 6.9 to 7.6. However, the average water pH value in TH is suitable for survival of fish as the pH value 6.5 to 8.5 is considered normal for fisheries ( ECR, 1997 ).
3.1.3. Dissolved oxygen (DO)
Solayman et al. (2018) recorded the average DO content ranged from 4.27 mg/l to 8.56 mg/l. Mamun et al. (2013) discovered 5.5 mg/l as the peak DO value, 4.5 mg/l as the lowest DO value, and 5.02 mg/l as the mean DO value. Surface water with a DO value of 5 mg/l or higher is favorable for fisheries ( ECR, 1997 ) which supports the diversified fisheries of TH.
3.1.4. Total dissolved solid (TDS)
Compared to other waterbodies in Bangladesh, TH has a relatively low TDS (mean = 63.5 mg/l). The TDS value ranges from 51 to 85 mg/l in Rauar area and from 58 to 77 mg/l in watch tower area of TH throughout the year ( Bhuiyan et al., 2019 , 2020 ). However, Mamun et al. (2013) and Solayman et al. (2018) found the ranges from 731 to 1020 mg/l and 670–1036 mg/l, respectively. The authors of both pieces of research opined that the higher TDS value of water may be ascribed to the high dissolved ion content of upstream water and domestic contaminants ( Mamun et al., 2013 ; Solayman et al., 2018 ).
3.1.5. Electrical conductivity
Bhuiyan et al. (2020) measured and recorded the mean electrical conductivity of TH water at 80.75 μS/cm. However, the conductivity value fluctuates from 60 to 110 μS/cm throughout the year ( Bhuiyan et al., 2019 ). On the other hand, the study from Mamun et al. (2013) and Solayman et al. (2018) have found that electrical conductivity ranges from 1044 to 1658 μS/cm with the average value of 1294.4 μS/cm and 1120 to 1460 μS/cm with the average value of 1231 μS/cm, respectively. According to Bhuiyan et al. (2019) , the transparency value found in the TH (Secchi depth 2.48 m) is very compatible with the TDS, conductivity, DO, SRP, and chl-a values measured. Bhuiyan et al. (2019) also compared the TDS and conductivity values with the values measured in other waterbodies of Bangladesh (Khilkhet Beel , Joysagar, and Hakaluki Haor ) and found that the values were consistent with each other. The consistency of the recorded TDS and conductivity values of TH by Mamun et al. (2013) and Solayman et al. (2018) with the values found in other waterbodies.
3.1.6. Water transparency
The Secchi disc depth is a limnological measure that has been incorporated in European legislation as one of the main criteria in assessing water quality ( Domínguez Gómez et al., 2009 ). With a Secchi disk depth of 2.48 m on average, the water transparency of TH remained reasonably steady (2.08–3.0 m) throughout the year ( Bhuiyan et al., 2019 , 2020 ). The fairly low Secchi depth findings depicted that the haor is devoid of biotic and abiotic dissolved materials.
3.1.7. Nutrient content
The nutrient profile analysis is an important part to think about the productivity of any waterbody, although the TH was found to be deficient in nutrients. Different studies have found that the soluble reactive phosphorus (SRP) levels, soluble reactive silicate (SRS) levels, NO 3 –N levels and NH 4 + levels were ranges from 9.76 to 30.05 μg/l, 4.45–16.14 mg/l, 0.25–0.48 mg/l and 270–1380 μg/l, respectively, at the watch tower area of the haor ( Bhuiyan et al., 2020 ). However, the highest concentrations of SRP (30.05 μg/l) SRS (16.14 mg/l), and NH4+ (1380 μg/l) were observed in September, March, and April, respectively ( Bhuiyan et al., 2020 ). In the Rauar area of TH, SRP, SRS, NO 3 –N, and NH 4 + concentrations ranged from 5.43 to 36.43 μg/l, 4–14.58 mg/l, 0.06–0.31 mg/l, and 238–1230 μg/l, respectively ( Bhuiyan et al., 2019 ). Highest amounts of NH 4 + (905 and 1230 μg/l) was found during winter season. According to Bhuiyan et al. (2019) , the highest concentration of NH 4 + in winter season could be due to a presence of higher population of migratory birds during that time. The Watch Tower area has a higher concentration of vital nutrients, such as SRP, NO 3 –N, SRS, and NH 4 + , than the Rauar area ( Bhuiyan et al., 2020 ).

3.1.8. Phytoplanktonic biomass
Phytoplanktonic biomass (Chl-a) concentrations ranged from 1.35 to 8.45 μg/l, while its degradation product pheophytin concentrations ranged from 0.08 to 3.5 μg/l in TH was recorded by Bhuiyan et al. (2019) . A low chl-a value indicates a low phytoplankton standing crop as because of the low concentrations of SRP, SRS, and TDS, occurs ( Bhuiyan et al., 2019 ). Lower phytoplankton biomass in TH might have led in low DO and conductivity.
3.2. Fish biodiversity
In the TH, 143 species of fish (137 indigenous and 6 exotic) belonging 35 families, and 12 orders were documented by several studies ( Hossain et al., 2012 ; Alam et al., 2015 ; Sunny et al., 2020 ; Hussain, 2021 ). Status of indigenous species is presented in Table 2 . Highest numbers (%) of species came from Cypriniformes order, followed by Siluriformes, Anabantiformes, and Perciformes ( Figure 3 ). Mohan et al. (2020) found 39 species belonging to 9 orders and 19 families from the East Kolkata Wetlands the only Ramsar site in the State of West Bengal, India. Cutler et al. (2019) sampled 31 sites within and around the Rapids of Mboungou Badouma and Doume Ramsar site and collected 97 species in 18 families and 9 orders. Karadeniz (2000) surveyed Sultan Sazligi Ramsar site in Turkey and recorded 25 species of mammals, 25 species of mollusks, 5 species of fish, 301 species of birds, and 125 species of algae. Lakshmi et al. (2015) found 78 fish species belong to 14 orders, 37 families and 57 genera from Kolluru Lake of Andhra Pradesh, India. Fahmi-Ahmad et al. (2015) recorded a total of 52 species belonging to 20 families of freshwater fishes from Tasek Bera Ramsar Site, Peninsular Malaysia. Comparing with the above mentioned Ramsar sites around the world, the TH wetland supports higher number of fish species.
Table 2
Status of available indigenous fish species in the Tanguar Haor ( Hossain et al., 2012 ; Alam et al., 2015 ; Sunny et al., 2020 ; Hussain, 2021 ).
Status Code: CR- Critically Endangered, EN- Endangered, VU- Vulnerable, NT- Near Threatened, LC- Least Concern, DD- Data Deficient, NE- Not Evaluated.

Percentage of fish species based on order.
From the last two decades, fish species recorded in different years from TH showed decline in species diversity although these decline in fish diversity are likely due to differences in survey season, method of sampling, number of sites and effort, etc. As, Hossain et al. (2012) recorded 120 indigenous fish species; Alam et al. (2015) found 128 indigenous fish species; Sunny et al. (2020) recorded 75 indigenous fish species and Hussain (2021) found only 56 indigenous species of fish ( Figure 4 ). Sun et al. (2017) studied both Poyang Lake wetland in China and Tanguar Haor wetland in Bangladesh and found that both wetlands have shown a decline in the fish population, with the Tanguar Haor wetland experiencing a bigger decline.

Fish species recorded from Tanguar Haor in different years.
3.3. Drivers of fish diversity decline
TH supports a remarkable array of diversified fish fauna, but these are now facing severe threats because of a combination of different climatic, anthropogenic, socioeconomic, and policy related drivers.
3.3.1. Climatic drivers
3.3.1.1. erratic rainfall.
Erratic rainfall was discovered to be a primary climatic component that was linked to a reduction in fish species diversity in the TH ( Rahaman et al., 2016 ; Sunny et al., 2020 ). After winter, depending on the rainfall the brood fish migration from deeper water to the breeding ground typically occurs between February and March. Between May and October, TH receives more than 80% of its annual total rainfall ( Rahaman et al., 2016 ). Due to unpredictable rainfall from the end of February to the first week of March, there is insufficient water supply in the rivers and beels of the haor ; yet, this immediately encourages fish breeding, especially small indigenous fish species. When there isn’t enough rain, certain fish took part breeding, but their larvae don’t grow because of the scarcity of water ( Aziz et al., 2021 ). Furthermore, the brood fish cannot reach the breeding grounds of the haor in time due to rainfall fluctuation (shortage of rainfall/late rain fall etc.) and shortage of water in the associated rivers and canals ( Akintola, 1995 ). As a result, climate vulnerability due to unpredictable rainfall has impacted on fish breeding activity and diversity compared to a few decades earlier ( Chowdhury et al., 2010 ).
3.3.1.2. Temperature fluctuation
Temperature fluctuations are another important element that influences physiological and ecological processes, which can lead to a decrease in the richness and distribution of fish species in a body of water ( Islam et al., 2016 ; Bhuyain et al., 2022 ). Temperature has a profound influence on all physiological processes, especially reproductive activities including gamete formation, ovulation and spermiation, spawning, embryogenesis, and hatching, as well as larval and juvenile development and survival, which are all linked to fish species diversity. Temperature changes, a fundamental climatic phenomenon, change the physico-chemical characteristics of aquatic ecosystems and plankton productivity, resulting disturbance in fish migration and distribution, which has an indirect but detrimental impact on fish abundance ( Chowdhury et al., 2010 ; Islam et al., 2015 ). Evaporation from open water and evapotranspiration from vegetated surfaces are both affected ( Rahaman et al., 2016 ).
3.3.1.3. Siltation
Siltation in the beels had a great impact on haor fisheries reduction. It results in the degradation of the fish’s natural habitat. Land-use changes in the upper riparian zone may enhance haor sedimentation and disrupt the Jadukata-Patlai River ecosystem ( Alam et al., 2015 ). Flashflood from the upper hilly region of India causes severe siltation in the haor wetland. Due to siltation fish species diversity usually harmed by collapsing the fish migration pathways and the disappearance of small beels that was served as feeding grounds ( Islam et al., 2018 ; Khan, 2011 ). Furthermore, fish larvae’s feeding grounds and migratory routes are restricted due to siltation at haors -river junction places. The water regimes of various aquatic environments, such as beels , rivers, and canals, vary seasonally. Sediment intrusion from upper hill deforestation and its deposition in beels has been observed to diminish light permeability and dissolved oxygen in the water, resulting in lower egg, embryo, and post-larvae survival ( Chapman, 1988 ). Increased turbidity in waterbodies reduces light penetration, which can affect primary productivity, as well as the diversity of fish feeding organisms (secondary production), and eventually, fish species diversity ( Islam et al., 2015 ; Barman et al., 2021 ).
3.3.2. Anthropogenic drivers
3.3.2.1. use of harmful fishing gears.
The most major anthropogenic factor contributing to the decrease of fish species diversity in the TH is the direct use of harmful and illegal fishing gears. Use of synthetic monofilament gill nets ( current jal ), fine-meshed seine nets ( ber jal ), and other illegal fishing devices has become a major worry in the haor in recent years ( Alam et al., 2015 ). Tima et al. (2021) found destructive and prohibited fishing gears such as current jal and fine meshed ber jal used for catching undersized fishes. Anglers can easily catch fish eggs and fry with these fishing gadgets. During FGDs fishermen reported that they putting traps for small fish along their migration route which allowing the fishermen to readily capture fish fry and eggs. Similarly, the broodfish swim close to the haor’s bank at night and are captured by anglers using harpoons (dragging gear). Fish and other aquatic species are exposed to exploitation when those damaging fishing gears and other methods (for example, fishing by de-watering, using chemicals) are used, and the regulatory authority (DoF) has declared these as illegal fishing operations ( Rahaman et al., 2016 ). However, in the absence of adequate governmental oversight, fishermen in haor regions continue to employ dangerous fishing gear and practices as a result, the diversity of fish species has decreased ( Sultana et al., 2016 ). The Department of Fisheries has also set limitations on catching brood fish and juveniles from natural sources in order to protect fish species and their habitats. However, due to a lack of regulatory capability, no noticeable impacts on fish conservation were recorded ( Khan, 2011 ).
3.3.2.2. Development of infrastructures
Establishment of crop dams around this haor is disrupting the water flow and fish movement that may affect natural recruitment and dispersal pattern of this mother fishery ( Alam et al., 2015 ). Construction of road, bridge, culvert, etc. is also disrupting the migratory routes and affected natural recruitment ( Barman et al., 2021 ).
3.3.2.3. Over and indiscriminate fishing
Another anthropogenic activity that has diminished fish species diversity in the haor is overfishing. Overfishing destroys thousands of species at risk which is ultimately responsible for the food and nutrition security of hundreds of millions of people around the world ( Golden et al., 2016 ; Koning et al., 2020 ). Capture fishing and agriculture are the two main sources of income for the residents in the haor . Because of the reduction of wetlands, more than 40% of freshwater fish species are now classified as threatened with extinction at the national level ( IUCN, 2003 ). TH is in danger of losing practically all fish species due to overfishing, which is a severe threat to fish stocks in the haor ( Rahaman et al., 2016 ). Indiscriminate fishing during the breeding season, as well as a reluctance to follow the sustainable fish harvesting method created under the CBSMTH project, were noticed as serious risks to the conservation of fish species in TH ( Alam et al., 2015 ). Overfishing, fishing during ban period, catching undersized fish, fishing at the restricted areas (sanctuary area), and fishing during spawning seasons were recorded by Tima et al. (2021) .
3.3.2.4. Water pollution
Water contamination, which is occasionally caused by coal storage and transportation at Tekerghat point in the TH, is another threat to floral and faunal species. Thousands of boats continuously pollute the water through oil spill and destructive fishing gears which is ultimately affecting the fish population ( Alam et al., 2015 ). Due to aquatic pollution, certain fish species that were available in the TH have now very rare or extinct ( Tima et al., 2021 ).
3.3.2.5. Degradation of swamp forest
The amount of fish produced by TH is clearly diminishing day by day due to the destruction of swamp forest, and the number of other fish species has also decreased significantly. Swamp forests that were once abundant in TH have become extremely rare as a result of removal, cutting, and other anthropogenic pressures, however remnants still exist. The natural regeneration of this forest type is barely noticeable in the marsh, except along the bank of the Foillar beel . The reed beds have also been badly depleted as a result of ongoing over-harvesting for fuel and land conversion to agricultural fields. As a result, certain aquatic species that were once prevalent in the area have become extinct and the integrity of the haor ecosystem was jeopardized due to this process ( GoB, 2004 ). Because swamp forest provides food and shelter for fish, there has been a decline in fish production, species variety, and waterfowl populations during the last few decades ( Alam et al., 2015 ; Islam et al., 2016 ).
3.3.3. Policy related drivers
Fish biodiversity status may be disrupted after the termination of the existing management system because community motivation and system involvement are absent, and insufficient policy frameworks and legislative provisions for biodiversity conservation and protected wetland management ( Alam et al., 2015 ). Tima et al. (2021) found several causes of degradation of fisheries resources of TH as weak enforcement with inadequate surveillance and poor implementation of the legal framework, non-compliance with fishing laws, rules, and policies.
Similar types of drivers were found in other freshwater wetlands in Bangladesh and around the world ( Sun et al., 2017 ; Husen et al., 2019 ; Aziz et al., 2021 ; Amoutchi et al., 2021 ). Erratic rainfall, temperature and siltation have become a significant climatic component that positively correlates with a decrease in the variety of fish species in the Hakaluki Haor , having a considerable impact on fish harvest ( Aziz et al., 2021 ). Amoutchi et al. (2021) observed Ivorian freshwater fish abundance reduction as a consequence of climate change especially change in temperature and rainfall and several anthropogenic activities including gold mining, water withdrawal for human needs, use of small-mesh fishing nets, overfishing, industrial waste discharge, pesticides use for agricultural purposes, obnoxious fishing practices and increase in human population. According to Cooke et al. (2012) , riparian and floodplain habitat degradation, altered hydrology, migration barriers, fisheries exploitation, environmental (climate) change, and introduction of invasive species have made riverine fishes some of the most threatened taxa on the planet. Hydrological alterations were found as the largest threat to fish biodiversity in the Yangtze River basin of China by Fu et al. (2003) . Moyle and Leidy (1992) identified proximate causes of fish species' decline of aquatic ecosystems and divided them into five categories as competition for water, habitat alteration, pollution, introduction of exotic species, and commercial exploitation. Sinha and Khan (2001) recorded major causes of fish decline in the Ganges River and found discharge of wastes generated due to developmental activities including irrigation projects, river course modifications and demographic explosion in the basin, which destroyed floodplains, sloughs, inundation zones, and oxbow lakes. Besides, the hydraulic structures have destroyed the anadromous fisheries ( Tenualosa ilisha , Pangasius pangasius ) of the riverine stretch of the Ganga. Rahaman et al. (2019) recorded climate change as the main driving factor of fish diversity reduction in Meghna River. The overfishing, urea application, annual beel drying, and usage of damaging fishing gears created a clear picture of the anthropogenic factors involved for the decline in fish species diversity in Hakaluki Haor ( Aziz et al., 2021 ). However, overfishing, use of illegal fishing gears (current jal), katha fishing, fishing by dewatering, low water depth in winter, abstraction of water for irrigation, siltation, catching of fry and brood fishes were found as major fish diversity decline factors in different wetlands of Bangladesh ( Sultana et al., 2019 ; Barman et al., 2021 ; Das et al., 2022 ; Kamal et al., 2022 ). The lakes in Pokhara Valley in Nepal were mostly affected by illegal fishing practices, siltation and sedimentation, water pollution, fish habitat loss, increased eutrophication, biological invasion, intensification of agriculture, and development projects ( Husen et al., 2019 ). These drivers have changed the lake’s size, water quality, depth, and availability of natural food, which has had an impact on aquaculture and fisheries in the lake ( Husen et al., 2019 ).
According to Cutler et al. (2019) , freshwater ecosystems face considerable threats that fall into four major categories such as overexploitation, introduced species, habitat destruction, and pollution. The authors added most visible threat to biodiversity of Mboungou Badouma and Doume Ramsar sites of this region is habitat destruction and pollution associated with mining, dams, and timber extraction ( Cutler et al., 2019 ). Kumar et al. (2018) found anthropogenic pressure arising out of alterations of wetland habitats to agricultural lands, habitat destruction, over exploitation, wanton destruction, aquatic pollution, disease, exotic species introduction and overall lack of awareness of biodiversity importance, and absence of proper policy are contributing much to such alarming vulnerability of the rich fish diversity of the East Kolkata Wetlands. Siwakoti and Karki (2009) described various kinds of threats from different Ramsar sites of Nepal and grouped as 1) Destruction and degradation of wetland habitats which includes high rate of drainage and reclamation of wetlands for housing, urban and industrial uses, inappropriate wetland management due to high water pumped for dry season crop, fish harvesting, modification of land use for agriculture; and fragmentation due to encroachment, 2) Loss of wetland ecosystem integrity includes construction of dams, barrage, etc. for hydropower, irrigation and flood management, over-extraction of ground water for domestic and other water requirements, increasing pollution by the use of high doses of pesticides/herbicides and fertilizers in the surrounding agricultural land, industrial waste and domestic sewage; and sedimentation and 3) Depletion of species abundance and diversity includes over harvesting of bio-resources (fishing, grazing, poaching, etc.), destructive harvesting practices (fish bombing, electro-fishing, poisoning, draining, use of small mesh nets, etc.); introduction of invasive alien species of plants which supports the present threats found as responsible for biodiversity depletion of TH.
3.4. Socio-economic profile of the haor inhabitants
3.4.1. livelihood profile.
TH provides versatile opportunities of livelihood for a number of needy people, many of them living under the poverty level, in the form of fishers, farmers, fish traders, transporters, intermediaries, day laborers, etc. More than 70 thousand people in 46 villages around the TH rely on this wetland for their livelihoods, either directly or indirectly ( Sun et al., 2017 ). According to Islam et al. (2014) , nearly half of the inhabitants in the TH region are extremely poor, while the IUCN et al. (2008) survey found that 95 percent of the people in the region struggle for living and 81% rely on the TH for their income.
Fishing practice mainly increases during the monsoon, but most of them engage with agricultural practices in the dry season. Primarily, fishing (30.1%) is the main occupation in TH followed by agricultural farming (12.9%), rearing livestock (8.6%) and businessman (8.6%) were found as the professions which is varied with landmass distribution and season ( Table 3 ). The percentages of fishing profession decrease with the increasing distance from TH ( Islam et al., 2014 ).
Table 3
Leading occupations of the people in the Tanguar Haor region.
3.4.2. Age distribution and farm size
In TH, people of almost all ages are engaged in fishing or other earning activities for supporting their livelihoods. Different researchers have classified them on the basis of age in different ways. However, the majority of middle-aged people are usually observed here in fishing activities. Mamun et al. (2013) found that 45.5% fishers are 31–42 years, 5.5% are under 30 years age and 24.5% fishers are more than 54 years old but Uddin et al. (2015) reported that age of respondents ranged from 19 to 75 years (average 47.6 years). Most of them (42%) were belongs to the old aged group ( Table 4 ). As we found, most of the people in the TH are very needy and living below the poverty level. Most of them are landless or having few land area with an average of 0.4 ha farm size ( Table 4 ) and 50% of them are small farmer while only 4% are large farmer ( Uddin et al., 2015 ).
Table 4
Classification of the respondents based on their farm size and age ( Uddin et al., 2015 ).
3.4.3. Education rate and family size
The distant and time-consuming transportation system, insufficient educational institution, adverse weather condition during dry and monsoon period and poor income of household head has made the low educational rate in this area. About 29%–42% of the population had only an elementary education, while 36%–39.8% were illiterate ( Table 5 ). The literacy rate was determined to be lower than the national average of 74.70% ( BBS, 2020 ). A big family has more people to extract natural resources from TH than a small family. That’s why there is a trend in rural community people of TH area having more children. So that after few years of growing the children can contributes to the family income through extraction of natural resources. More than half of the respondents (54%) had a medium-sized family, compared to 22% who had a small family and 24% who had a large family.
Table 5
Education rate and family size of local people in Tanguar Haor ( Islam et al., 2014 ; Uddin et al., 2015 ).
The average household size in Bangladesh is 4.7 ( BBS, 2020 ), but it is approximately 6 in TH, which is higher than the national average and this is happened as the people are conservative by nature and yet have a primitive view on family planning ( IUCN et al., 2008 ; Islam et al., 2014 ; Uddin et al., 2015 ).
3.4.4. Income and expenditure
Most of the people in TH are hardcore poor and directly or indirectly depend on the TH which is significantly influenced by the season, climatic condition, natural disaster and price fluctuation of their products such as fish, cattle, and agricultural field crops ( Uddin et al., 2015 ). In 2008, the mean annual income of a household in TH was 22,642.44 ± 594.52 BDT. 38,059 BDT. In 2012, the majority of respondents were impoverished (40%) while only 12% were prosperous and 10% were extremely poor ( Table 6 ). However, until 2012, the majority of haor residents were surviving on less than 5000 taka per month. More crucially, about 30% of the households earned between Tk. 1500 and Tk. 3000 per month, while 39% earned between Tk. 3000 and Tk. 5000 per month. Only 2.57% had a monthly income of more than Tk.10000, and nearly 6% had a monthly income of less than 1500 taka. When comparing 2012 to 10–12 years earlier, medium and wealthy people improved by 46.2 % and 50.0 %, respectively, whereas low and extreme poor people fell by 16.7 % and 44.4 % ( Uddin et al., 2015 ; IUCN et al., 2008 ).
Table 6
Changing pattern of monthly income of the respondents in Tanguar Haor ( Uddin et al., 2015 ).
As far as their consumption on food and different things, Table 7 reveals that the average annual expenditure on education is roughly 15% of their wage, followed by 15% for health, 13% for lighting, 6% for cooking fuel, 13% for transportation, and so on. Overall, the survey showed that about 20% of the earnings is spent on food ( IUCN et al., 2008 ; Table 7 ).
Table 7
Classification of household expenditure ( IUCN et al., 2008 ).
3.4.5. Households area and house materials
In rural Bangladesh, almost 28.6% of the families exist underneath the lower poverty line and almost 43.8% of the families live beneath the upper poverty line ( BBS, 2020 ). In Tanguar villages, 2.8% families have no matured male individuals for earning, 13.89% families have no income creating resources, 31% of the families have a land area lower than 10 decimals and almost 49% families have less than 50 decimals of land area ( IUCN et al., 2008 ; Table 8 ).
Table 8
Type of house materials used in Tanguar Haor area ( IUCN et al., 2008 ).
Living in TH area is very difficult because it is one of the remotest areas of Bangladesh. Because of its poor communication infrastructure and vulnerable economic condition of the people limited their access to the major cities. That’s why most of the materials required for building houses in Tanguar area are usually come from the TH itself. Swamp forest supplies structural materials and reeds used for the roofing materials. However, these sources became vulnerable due to over exploitation and extraction over time. In this area only few households had brick walls. Around 21.4% of the people in TH area live in a single room house. The majority of the houses now tin roofs instead of reeds. Bamboo and tin made wall is the most common feature of the households in villages surrounding the TH ( IUCN et al., 2008 ).
3.4.6. Sanitation status, source of drinking water and fuel
Sanitation is a main problem in Tanguar villages. There are mainly three types of latrine. These are sanitary brick built latrines, latrine with only ring slab and open latrines are made on a shaft and hanging is made by 4–8 bamboos, fenced by plastic sheet which is hanged on the haor , river or nearby canal ( Islam et al., 2014 ). Only 7.5–11.6% of the population has access to toilets. Another 12.8%–21.5% use ring-slab or semi-building latrines, while the remaining 71.6–77% use bamboo-made, semi-open, or open latrines to defecate straight into local rivers, canals, and creeks ( Islam et al., 2014 ). Like as the other wetland areas, safe drinking water facility is a more common problem in the haor area as like the other large wetland areas of Bangladesh ( IUCN et al., 2008 ). Though TH is a major waterbody and a Ramsar site, but residents in the area have lack access to safe drinking water. Only 74.2–88.3% people have access to shallow tube-well in TH whereas the national average is 97% ( Islam et al., 2014 ). There is only 1–2 shallow tube well in a village for approximately 600–800 people and it is very hard to collect drinking water in different times like in rainy season or at night. On the other hand, few people like financially well-established set up deep tube-well only for their own family. For the difficulty to access in shallow tube-well around 17.2% people used to drink river or haor water. In the past people of different regions of Bangladesh, using water from ponds, canals, and rivers for household chores was common practices, but in TH, over 77.6% of people still do! ( IUCN et al., 2008 ; Islam et al., 2014 ). As there is no alternative way for cooking food except fuel wood about 50% people of TH used to collect grasses, and 30.1% collect leafs, branches of trees from the swamp forest. Rest of the people use dried cow dung (19.4%) as their daily fuel wood. Use of cow dung as a fuel has a considerable negative impact on the environment ( IUCN et al., 2008 ; Islam et al., 2014 ).
3.4.7. Income determinants of the fishermen in haor region
The income of the fishermen can be affected by several factors, such as the fish catch, age of fishermen, and training program provided in the area ( Ahmed et al., 2021 ). To analyze these factors, Tikadar et al. (2022) carried out a multiple linear regression model in haor area and a summary of that finding is presented in Table 9 . The results show that age of the fishermen, education rate, training program and NGO membership of the fishermen influence their income positively and significantly. Older fishermen tend to have greater experience than the younger. They catch more fish because they are well-versed in the ecology of the floodplains. Additionally, they have better market knowledge, which helps them negotiate a higher price for the fish.
Table 9
Factors influencing the income of the fishermen ( Tikadar et al., 2022 ).
Note: ∗∗∗, ∗∗, ∗ indicate the significance level at 1%, 5% and 10%, respectively.
3.5 . Current management status of Tanguar Haor
In Bangladesh, the Protection and Conservation of Fish Act, 1950, is regarded as the founding document for fisheries management. The purpose of this act was to control the use of current net, fixed engines, explosives, and other dangerous fishing methods by all species in all natural or manmade, open or closed, flowing or stagnant, bodies of water. The Protection and Conservation of Fish Act, 1950 underwent a number of amendments ( Tima et al., 2021 ). Given the economic and ecological significance of the TH natural resources, it demands for key attention of policy makers for the sustainable resource management. Therefore, two major conservation initiatives, namely, the ‘National Conservation Strategy (NCS)’ and the ‘National Environmental Management Action Plan (NEMAP)’ were accomplished by the government of Bangladesh to formulate different management plans for the biologically rich ecosystems in 1990 ( Bevanger et al., 2001 ). In addition, the ‘National Conservation Strategy Implementation Project-1 (NCSIP-1)’ has been started under the NCS, in which the TH wetland ecosystem was one of the most important parts of this project. Then a ‘Tanguar Haor Management Plan (THMP)’ was developed in February 2000, which was also signed by the Ministry of Finance of the Royal Norway for financing this project in 2001 ( Bevanger et al., 2001 ). The purpose of this management plan was to ensure the long-term conservation measures by encouraging the community based sustainable resource management, poverty alleviation through alternative income generations activity, protection of the habitats important for biodiversity maintenance etc. ( Bevanger et al., 2001 ). Moreover, a vital element of the TH management plan was to build a new leasing system to ensure a proper distribution of water resources, as the managing capacity of the fisheries resources in haor is very poor. In this context, a strong community based management plan was developed considering its strong role for fish biodiversity conservation ( Bevanger et al., 2001 ; Islam et al., 2015 ). However, these kinds of new leasing management strategies didn’t see much hope due to unable to maintain a suitable condition for fish and habitat, instead it has increased the conflict among the stakeholders ( Bevanger et al., 2001 ; Islam et al., 2015 ; Newaz and Rahman, 2019 ).
About two decades later of NCS, one more intensive field-based survey was conducted in the TH during 2012–2014. It was done by another project named the ‘Community Based Sustainable Management of TH (CBSMTH)’, especially to make out the fish resources status of the TH and its adjacent beels ( Alam et al., 2015 ). Since the fish sanctuaries serves as an integral part of the conservation process ( Kunda et al., 2022 ), five distinct fish sanctuaries were constructed in this haor under the CBSMTH project in 2011 ( Ahmed, 2013 ; Alam et al., 2015 ). Among them, four sanctuaries were established in four distinct beels , and one more fish sanctuary was recognized in the Patlai River’s Alamer Duar. However, Table 10 illustrates the influence on the TH’s overall fish resources, though an establishment of one more advanced fish sanctuaries was proposed at that time ( Ahmed, 2013 ). Moreover, a significant number of fish protection katha were also established in different beels of the TH wetland, in which the estimated area was about 0.50 acre ( Ahmed, 2011 ). So, we can recognize it as fish sanctuary.
Table 10
Status of fish sanctuaries established in Tanguar Haor under the CBSMTH Project in 2011 ( Ahmed, 2011 ).
Therefore, establishment of the fish sanctuary exhibited a positive impact in terms of overall fish production of this haor system. However, the positive impact of these management systems was limited to a few local communities, wherein the people living a little far from the TH are still remaining away from its core benefits ( Alam et al., 2015 ). Unfortunately, this is because of the poor wetland resource governance systems, which also indicates a very critical issue now-a-days ( Newaz and Rahman, 2019 ). In this situation, the community based co-management system was considered as the sustainable natural resource management through advancing all the local stakeholders ( Islam et al., 2015 ; Newaz and Rahman, 2019 ). However, several studies ( Ahmed, 2011 ; Alam et al., 2015 ) have discussed about the TH considering the biodiversity status, socio-economic evaluation etc., therefore, a very few studies have discoursed the management system of the TH. Hence, Newaz and Rahman (2019) conducted a unique study on the TH in light of the three-phase cycle (2006–2016) of the CBSMTH project, in which the findings demonstrated that the initiative of developing community-based resource management remains a critical task in the TH. Besides, the ineffectiveness of these community systems largely due to the high dependency on resources, lack of guidance at the rural level etc., which is also responsible for poor management strategies ( Newaz and Rahman, 2019 ). Therefore, it would be more clear to discuss the assessment with other Ramsar sites outside Bangladesh by Baker et al. (2021) in Canada. Canada ratified the Ramsar Convention in 1981, and although it went into active in 1975. About 37 Ramsar sites have been identified by Canada since that time ( Baker et al., 2021 ). Therefore, the effectiveness of co-management structures, current management plans, and monitoring agendas were among the sustainability indicators used to evaluate each site. These indicators were created based on the Ramsar Convention Strategic Plan 2016–2024 and the 14 main areas of focus ( Baker et al., 2021 ). Regarding management plans, governance frameworks, and reporting practices at several sites, the assessment results were highly variable. Thus, they had explained that the inadequate monitoring and reporting procedures and outdated management plans were the causes of variation ( Baker et al., 2021 ).
Furthermore, it is certainly unfortunate that the effective sustainable co-management remains a difficult task in the TH, due to the high dependency of resource harvesters on the wetland ecosystems ( Newaz and Rahman, 2019 ). Additionally, any co-management structure must go through a common process of learning by doing, experiencing success and failure along the way, and then undergoing continuous evaluation and development. However, given the previous experiences of both success and failure, government interventions and a coordinated effort by the local community should be sustained for the consolidation of the current governance framework in TH Addressing the needs of the growing human population will be another critical issue for TH fisheries management in the future. Hence, locally appropriate adaptive measures are required to address the social, environmental, and climatic challenges. Robust synchronization among government relevant line agencies, development actors, and stakeholders will be a future direction for addressing these challenges, sustaining the ecosystem, and conserving this rich fish biodiversity ( Alam et al., 2015 ).
3.6. Research gap
The fundamental constraint of this study was that we had to overlook features of communities and their surroundings because our analyses were based on the broad conclusions of published studies. Several generalizations were made. First, because the studies employed various methods/years to assess the status of fish biodiversity, it was unable to make a direct comparison of fish diversity. Second, even if multiple topics were mentioned in the same article, each article was assigned to only one principal theme. For example, we regarded TH to be the key area of interest in most studies conducted in TH. We drew the same conclusions about TH’s various study areas. Finally, there was no effective framework in place to protect TH’s fish biodiversity and natural resources. As a result, the fishing community’s financial situation remained unchanged. Finally, while we are confident that the 22 peer-reviewed publications we covered are a fair and representative sample, due to accessibility and time limits, there were obviously omissions.
3.7. Recommendations for future management
The TH’s natural resources, particularly its fishery resources, are very much important to the local community, particularly the fisherman ( Alam et al., 2015 ). In this context, the community-based fisheries management can benefit to preserve and intensification of this natural resources. However, an effective co-management system mostly takes time to unite the community governments through creating awareness and trusts, building local capacity, preserving informal entree of the poor to resources by creating an effective leasing systems and so on ( IUCN Bangladesh, 2015b ; Newaz and Rahman, 2019 ). In this situation, the management and maintenance of this wetland environment necessitates immediate attention from the appropriate authorities.
However, some specific points are strongly recommended here for the proper management of TH-
- ✓ Increase active participation of the local community to manage Haor fisheries through community motivation and awareness building;
- ✓ Establish a balanced approach tactic of fishing by fishing restriction in different fishing grounds and time;
- ✓ Encourage the use of science-based co-management by making sure that traditional and technological knowledge is incorporated into national policies and wetland management plans to ensure benefits of all resource harvesters;
- ✓ To strengthening the alternative livelihoods-advancement of effective eco-friendly tourism, formation of various income generation activities accompanying with the new market creations, extension of the Vulnerable Group Feeding (VGF) program for the fishers etc. can be considered as an effective management options as well.
General recommendations-
- ✓ Establishment of more effective fish sanctuaries along with the fish protection katha in several parts of the TH system;
- ✓ Develop an early warning system for flash floods in the Haor Basin to decrease damage caused by sudden flood;
- ✓ Hijal, koroch, and other varieties of trees needed to restore for the biological balance of the Haor Basin and protect homesteads areas;
- ✓ More study should be done on the Haor economy and ecology, with an emphasis on identifying problems in various dimensions and exploring prospects in the relevant domains for pragmatic and urgent policy consequences;
- ✓ Government policy and inter-departmental linkage should be well updated and representative to reduce the detrimental effects of ecotourism considering the sustainable haor management, as it is barely accepted from the unwanted influence;
- ✓ Lastly, prepare a master plan for the overall development of Haors , incorporating all areas such as water resources, fisheries, forestry, wetland management, and khas land allocation.
4. Conclusion
The largest wetland area in Bangladesh, TH is home to a wide variety of fish, reeds, algae, and aquatic plants and vegetation. The most clear conclusion regarding correlating trends with fish diversity declines is that it is not possible to identify which parameters exert which influences as multiple drivers are likely in play. As a result, fish diversity is under severe threat of gradual depletion. Several haor management policies and programs have been established by the government and non-governmental organizations, however these are not long-term solutions for haor management due to lack of proper empowering system and awareness of local fishermen. Thus, social recognition, government policy and strategy, nongovernmental activities, and community-based action are all crucial for the conservation of haor biodiversity. This review outlined the research gaps in TH that need to be taken into account in future studies for the sustainable management of TH’s fishery resources. We hoped that the findings of this study would be beneficial to a variety of stakeholders, including the fishing community, local managers, legislators, and TH management authorities. Furthermore, we wish to encourage the national and international communities to organize thorough surveys to evaluate the significance of TH before it undergoes irreparable damage.
Declarations
Author contribution statement.
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Declaration of interest’s statement.
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
- Ahmed M. IUCN, SDC (Swiss Agency for Development and Cooperation); 2008. Ujja Fishing. Protection and Management Report. Fish Migration and Management in Tanguar Haor . TARA (Technical Assistance for Rural Advancement) pp. 1–30. [ Google Scholar ]
- Ahmed M. 2011. Community Led Framework for Estimation of Sustainable Exploitation Level of Fish and Reeds in Tanguar Haor . TARA. Report submitted to IUCN Bangladesh under CBSMTH Project; p. 121. [ Google Scholar ]
- Ahmed M. 2013. Review Status of Existing Sanctuaries and Design and Demonstration of a Site of an Advanced Fish Sanctuary in Tanguar Haor . TARA. Report submitted to IUCN Bangladesh under CBSMTH Project; p. 19. [ Google Scholar ]
- Ahmed M. 2015. Estimation of Sustainable Yield Level of Fisheries Resources in Tanguar Haor . TARA. Report Submitted to IUCN Bangladesh under CBSMTH Project; p. 63. [ Google Scholar ]
- Ahmed M., Saha S.M., Hossain M.E., Khan M.A., Prodhan M.M.H. Assessment of livelihood and food poverty status of the floating fishermen in riverine system of Bangladesh. Soc. Sci. Humanit. Open. 2021; 4 (1) [ Google Scholar ]
- Akintola F.O. In: Cocoa Revolut. Niger., Proceedings of a National Seminar on Revolutionizing Nigeria's Cocoa Industry. Adegeye A.J., Ajayi W.O., editors. University of Ibadan; Ibadan, Nigeria: 1995. Rainfall characteristics and cocoa production in Nigeria. [ Google Scholar ]
- Akter N., Kunda M., Harun-Al-Rashid A., Mazumder S.K., Sultana M.A., Pandit D. Fish biodiversity in the Khiru river of Bangladesh: present status and threats. Int. J. Nat. Soc. Sci. 2020; 7 (4):30–39. [ Google Scholar ]
- Alam A.B.M.S., Badhon M.K., Sarker M.W. IUCN , International Union for Conservation of Nature, Bangladesh Country Office; Dhaka, Bangladesh: 2015. Biodiversity of Tanguar Haor : a Ramsar Site of Bangladesh Volume III: Fish. (pp xii+ 216) [ Google Scholar ]
- Amoutchi A.I., Mehner T., Ugbor O.N., Paul K.E. Fishermen’s perceptions and experiences toward the impact of climate change and anthropogenic activities on freshwater fish biodiversity in Côte d’Ivoire. Discover Sustain. 2021; 2 (1):1–18. [ Google Scholar ]
- Aziz M.S.B., Hasan N.A., Mondol M.M.R., Alam M.M., Haque M.M. Decline in fish species diversity due to climatic and anthropogenic factors in Hakaluki Haor, an ecologically critical wetland in northeast Bangladesh. Heliyon. 2021; 7 (1) [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Baker J., Dupont D., Vasseur L. Exploring Canadian Ramsar sites ecosystem governance and sustainability. Wetlands. 2021; 41 (1):1–11. [ Google Scholar ]
- IUCN Bangladesh . IUCN, International Union for Conservation of Nature, Bangladesh Country Office; Dhaka, Bangladesh: 2015. Red List of Bangladesh Volume 5: Freshwater Fishes. pp xvi+360. [ Google Scholar ]
- IUCN Bangladesh . IUCN, International Union for Conservation of Nature, Bangladesh Country Office; Dhaka, Bangladesh: 2015. Tanguar Haor Management Plan Framework and Guidelines. pp xiv+202. [ Google Scholar ]
- IUCN Bangladesh . IUCN, International Union for Conservation of Nature, Bangladesh Country Office; Dhaka, Bangladesh: 2016. Tanguar: A Decade-Long Conservation Journey. pp viii+62. [ Google Scholar ]
- Barman S.K., Kunda M., Mazumder S.K., Nahiduzzaman M., Barman P.P., Das S.K. Fish-diversity in the Kura river of Bangladesh: patterns and threats. Malays. Appl. Biol. 2021; 50 (3):1–14. [ Google Scholar ]
- BBS . Ministry of Planning, Government of the People’s Republic of Bangladesh; Dhaka: 2020. Statistical Yearbook of Bangladesh Bureau of Statistics; p. 660. [ Google Scholar ]
- Bevanger K., Datta A.K., Eid A.T., Shirin M. 2001. Tanguar haor wetland biodiversity conservation project- an appraisal; p. 37. (NINA Project Report, 16). [ Google Scholar ]
- Bhuiyan M.A.H., Islam S.S., Kowser A., Islam M.R., Kakoly S.A., Asaduzzaman K., Khondker M. Phytoplankton in relation to water quality of Tanguar Haor ecosystem, Bangladesh: I. Rauar station. Dhaka Univ. J. Biol. Sci. 2019; 28 (2):131–138. [ Google Scholar ]
- Bhuiyan M.A.H., Kowser A., Islam S.S., Islam M.R., Mohid M., Kakoly S.A., et al. Phytoplankton in relation to water quality of Tanguar haor ecosystem, Bangladesh: 2. Watch Tower Station. Dhaka Univ. J. Biol. Sci. 2020; 29 (1):9–18. [ Google Scholar ]
- Bhuyain A.S.M.S.R., Barman S.K., Hossain M.M., Khan M.M.H., Mim K.K., Mazumder S.K. Seasonal dynamics of heavy metal concentrations in water and fish from Hakaluki Haor of Bangladesh. Conservation. 2022; 2 (3):473–484. [ Google Scholar ]
- Chapman D.W. Critical review of variables used to define effects of fines in redds of large salmonids. Trans. Am. Fish. Soc. 1988; 117 (1):1–21. [ Google Scholar ]
- Chowdhury M.T.H., Sukhan Z.P., Hannan M.A. Proc. Int. Conf. Environ. Asp. Bangladesh. University of Kitakyushu; Kitakyushu, Japan: 2010. Climate change and its impact on fisheries in Bangladesh; pp. 95–98. [ Google Scholar ]
- Cooke S.J., Paukert C., Hogan Z. Endangered river fish: factors hindering conservation and restoration. Endanger. Species Res. 2012; 17 (2):179–191. [ Google Scholar ]
- Cutler J.S., Mve-Beh J.H., Sullivan J.P., Fermon Y., Sidlauskas B.L. Fish fauna in and around the Rapids of Mboungou Badouma and Doume Ramsar site, Gabon. Check List. 2019; 15 (6):997–1029. [ Google Scholar ]
- Das S.R., Pandit D., Harun-Al-Rashid A., Tasnim N., Kunda M. Impacts of brush pile fishing on fish biodiversity: a case study of the Shari-Goyain River in Bangladesh. Heliyon. 2022; 8 (7) [ PMC free article ] [ PubMed ] [ Google Scholar ]
- DoF . Ministry of Fisheries; Bangladesh: 2019. Yearbook of fisheries Statistics of Bangladesh, 2018-19. Fisheries Resources Survey System (FRSS), Department of Fisheries. [ Google Scholar ]
- DoF . Ministry of Fisheries and Livestock; Bangladesh: 2020. Yearbook of fisheries Statistics of Bangladesh, 2018-19. Fisheries Resources Survey System (FRSS), Department of Fisheries. [ Google Scholar ]
- Domínguez Gómez J.A., Chuvieco Salinero E., Sastre Merlín A. Monitoring transparency in inland water bodies using multispectral images. Int. J. Remote Sens. 2009; 30 (6):1567–1586. [ Google Scholar ]
- ECR . Government of the People’s Republic of Bangladesh, Ministry of Environment and Forest; 1997. The Environment Conservation Rules; pp. 205–207. and Livestock. 36:135. [ Google Scholar ]
- Fahmi-Ahmad M., Rizal S.A., Amirrudin B.A. Ichthyofaunal diversity of Tasek Bera Ramsar site, Pahang, Peninsular Malaysia. J. Wildl. Parks. 2015; 30 :27–43. [ Google Scholar ]
- FAO . Food and Agriculture Organization; Rome: 2020. The state of world Fisheries and Aquaculture 2020. Sustainability in Action. [ Google Scholar ]
- Fu C., Wu J., Chen J., Wu Q., Lei G. Freshwater fish biodiversity in the Yangtze River basin of China: patterns, threats and conservation. Biodivers. Conserv. 2003; 12 (8):1649–1685. [ Google Scholar ]
- GoB, Government of Bangladesh . Ministry of Environment and Forests. Government of the People’s Republic of Bangladesh; 2004. Tanguar Haor Wetland, Biodiversity Conservation Project. [ Google Scholar ]
- Golden C.D., Allison E.H., Cheung W.W., Dey M.M., Halpern B.S., McCauley D.J., et al. Nutrition: fall in fish catch threatens human health. Nature. 2016; 534 (7607):317–320. [ PubMed ] [ Google Scholar ]
- Haddaway N.R., Woodcock P., Macura B., Collins A. Making literature reviews more reliable through application of lessons from systematic reviews. Conserv. Biol. 2015; 29 (6):1596–1605. [ PubMed ] [ Google Scholar ]
- Hossain M.A.R. In: Advances in Fisheries Research in Bangladesh: I. Proc. of 5th Fisheries Conference & Research Fair 2012. 18-19 January 2012, Bangladesh Agricultural Research Council, Dhaka. Wahab M.A., Shah M.S., Hossain M.A.R., Barman B.K., Hoq M.E., editors. Bangladesh Fisheries Research Forum; Dhaka, Bangladesh: 2014. Habitat and fish diversity: Bangladesh perspective, pp 1-26; p. 246. [ Google Scholar ]
- Hossain M.S., Islam M.S., Mondal P., Hoq M.E. Assessment of aquatic natural resources in the Tanguar haor at Sunamgonj, Bangladesh. Bangladesh J. Fish. Res. 2012; 15 (16):81–92. [ Google Scholar ]
- Husen M.A., Gurung T.B., Nepal A.P. Drivers of fisheries and their management in the lakes of Pokhara Valley, Nepal. J. Fish. 2019; 7 (2):706–713. [ Google Scholar ]
- Hussain M.G. Biological diversity status of fish genetic resources at Tanguar Haor wetland in Bangladesh. Bangladesh Mari. J. 2021; 5 (1):193–206. [ Google Scholar ]
- Islam M.J., Sultana M.A. Fishing gears used by the fishermen in wetlands of Chhatak, Sunamganj and sustainable utilization of fishery resources. Int. J. Nat. Sci. 2016; 6 (2):96–103. [ Google Scholar ]
- Islam M.S., Hossain M.S., Hoque M.E., Tusher T.R., Kabir M.H. Study on natural resource management in relation with socio-economic status at Tanguar haor in Sunamgonj district of Bangladesh. Bangladesh J. Environ. Sci. 2014; 26 :59–66. [ Google Scholar ]
- Islam M.A., Islam M.J., Barman S.K., Morshed F., Marine S.S. Study on present status of fish biodiversity in wetlands of Sylhet District, Bangladesh. Agric. For. Fish. 2015; 4 (6):296–299. [ Google Scholar ]
- Islam M.A., Islam M.J., Arefin S., Rashid A., Barman S.K. Factors affecting the fisheries biodiversity of Ratargul swamp forest of Sylhet district, Bangladesh. IOSR J. Environ. Sci. Toxicol. Food Technol. 2016; 10 (1):60–65. [ Google Scholar ]
- Islam M., Rakib M.R., Sufian M., Raihan Sharif A.H.M. Bangladesh I: Climate Change Impacts, Mitigation and Adaptation in Developing Countries. Springer; Cham: 2018. Detection of climate change impacts on the Hakaluki haor wetland in Bangladesh by use of remote sensing and GIS; pp. 195–214. [ Google Scholar ]
- IUCN . International Union for Conservation of Nature; Dhaka, Bangladesh: 2003. Bangladesher Bipanno Bonno Prani. [ Google Scholar ]
- IUCN (International Union for the Conservation of Nature) In: Rich Resources, Poor People- the Paradox of Living in Tanguar Haor , Dhaka, Bangladesh. Haque A.K.E., Kazal M.H., editors. 2008. A survey on resource systems, current use and community profile of Tanguar Haor area; pp. 1–30. [ Google Scholar ]
- Kamal M.A.H.M., Kawsar M.A., Pandit D., Kunda M., Tabassum K., Alam M.T. Fish biodiversity at Kawadighi Haor of northeastern Bangladesh: addressing fish diversity, production and conservation status. Aquat. Sci. Eng. 2022; 37 (3):151–160. [ Google Scholar ]
- Karadeniz N. Sultan Sazligi, Ramsar site in Turkey. Humedales Mediterráneos. 2000; 1 :107–114. [ Google Scholar ]
- Khan S.M.M.H. University of Manitoba; 2011. Participatory Wetland Resource Governance in Bangladesh: an Analysis of Community-Based Experiment in Hakaluki Haor . [ Google Scholar ]
- Koning A.A., Perales K.M., Fluet-Chouinard E., McIntyre P.B. A network of grassroots reserves protects tropical river fish diversity. Nature. 2020; 588 (7839):631–635. [ PubMed ] [ Google Scholar ]
- Kumar B., Kumar S., Biswal A., Dey A., Thakuria J., Hussan A., et al. Present status, abundance and threats of fish diversity on Ramsar site (East Kolkata Wetlands) of West Bengal, India. Int. J. Curr. Microbiol. Appl. Sci. 2018; 7 (7):4000–4007. [ Google Scholar ]
- Kunda M., Ray D., Pandit D., Harun-Al-Rashid A. Establishment of a fish sanctuary for conserving indigenous fishes in the largest freshwater swamp forest of Bangladesh: a community-based management approach. Heliyon. 2022; 8 (5) [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lakshmi B.B., Naidu Y.P., Rao K.R. Ichthyo faunal diversity and conservation of kolleru lake-a Ramsar site in Andhra Pradesh. J. Pharm. Biol. Sci. 2015; 10 (2):13–21. [ Google Scholar ]
- Mamun S.A., Roy S., Rahaman M.S., Jahan M., Islam M.S. Status of fisheries resources and water quality of Tanguar haor. J. Environ. Sci. Nat. Resour. 2013; 6 (1):103–106. [ Google Scholar ]
- Mohan R.R., Nagesh T.S., Das A., Chandrakar S., Saha S., Reddy D.R.K., Anupama R.R. Piscine diversity and its status in East Kolkata wetlands, a Ramsar site of West Bengal, India. J. Entomol. Zool. Stud. 2020; 8 (3):1393–1399. [ Google Scholar ]
- Moses B.S. Ibadan University Press, UNESCO/ICSU; 1983. Introduction to Tropical Fisheries; pp. 102–105. Part. [ Google Scholar ]
- Moyle P.B., Leidy R.A. Conservation Biology. Springer; Boston, MA: 1992. Loss of biodiversity in aquatic ecosystems: evidence from fish faunas; pp. 127–169. [ Google Scholar ]
- Munasinghe M. 2000. Development, Equity and Sustainability (DES) in the Context of Climate Change. (IPCC Expert Meeting in Colombo, 1999). [ Google Scholar ]
- Mustafi S.K., Kunda M., Khan A.K.M.F., Mazumder S.K., Pandit D. Conserving nutrient rich small indigenous species of fish in the wetlands of north-eastern Bangladesh. AACL Bioflux. 2022; 15 (4):2238–2252. [ Google Scholar ]
- Newaz M.W., Rahman S. Wetland resource governance in Bangladesh: an analysis of community-based co-management approach. Environ. Dev. 2019; 32 [ Google Scholar ]
- Pandit D., Kunda M., Harun-Al-Rashid A., Sufian M.A., Mazumder S.K. Present status of fish biodiversity in Dekhar Haor , Bangladesh: a case study. World J. Fish Mar. Sci. 2015; 7 (4):278–287. [ Google Scholar ]
- Pandit D., Kunda M., Islam M.J., Islam M.A., Barman P.P. Assessment of present status of fish diversity in soma Nadi Jalmohal of Sunamganj in Bangladesh. J. Sylhet Agric. Univ. 2015; 2 (1):127–135. [ Google Scholar ]
- Pandit D., Saha S., Kunda M., Harun-Al-Rashid A. Indigenous freshwater ichthyofauna in the Dhanu River and surrounding wetlands of Bangladesh: species diversity, availability, and conservation perspectives. Conservation. 2021; 1 (3):241–257. [ Google Scholar ]
- Pandit D., Shefat S.H.T., Kunda M. In: Small in Scale, Big in Contributions: Advancing Knowledge of Small-Scale Fisheries in Bangladesh. Islam M.M., editor. TBTI Global Publication Series; St. John’s, NL, Canada: 2022. Fish diversity decline threatens small-scale fisheries in the haor basin of Bangladesh. [ Google Scholar ]
- Rahaman M.M., Sajib K.I., Alam I. Impacts of climate change on the livelihoods of the people in Tanguar Haor , Bangladesh. J. Water Resour. Eng. Manag. 2016; 3 (1):1–9. [ Google Scholar ]
- Rahaman M., Ema N.S., Hossain M., Rahman M.M., Hossain Z. Effects of climate change on fisheries biodiversity of the Meghna, Laukhati and Galachipa river in Bangladesh. EurAsia J. BioSci. 2019; 13 (2):1705–1717. [ Google Scholar ]
- Saha S., Nasren S., Pandit D., Mian S. An overview of the biological features, distribution, and conservation of a critically endangered riverine catfish, Bagarius bagarius (Hamilton, 1822), in the natural waters of Bangladesh. Conservation. 2021; 1 (4):350–367. [ Google Scholar ]
- Salauddin M., Islam A.K.M.S. 2011. Identification of Land Cover Changes of the Haor Area of Bangladesh Using Modis Images; pp. 1–7. (3rd International Conference on Water & Flood Management (ICWFM-2011)). [ Google Scholar ]
- Sinha M., Khan M.A. Impact of environmental aberrations on fisheries of the Ganga (Ganges) river. Aquat. Ecosys. Health Manag. 2001; 4 (4):493–504. [ Google Scholar ]
- Siwakoti M., Karki J.B. Conservation status of Ramsar sites of Nepal Tarai: an overview. Bot. Orient. J. Plant Sci. 2009; 6 :76–84. [ Google Scholar ]
- Solayman H.M., Baten M.A., Khan M.B. Status and economic valuation of ecosystem services of Tanguar haor: a wetland of Bangladesh. J. Bangladesh Agric. Univ. 2018; 16 (2):237–243. [ Google Scholar ]
- Sultana M.A., Mazumder S.K., Kunda M. Fishing gears and crafts used in Payra River, Bangladesh. Eur. J. Appl. Sci. 2016; 8 (6):337–346. [ Google Scholar ]
- Sultana M.A., Kunda M., Mazumder S.K. Status and decline causes of fish diversity of Bhawal beel, Bangladesh. Malays. J. Med. Biol. Res. 2019; 6 (2):93–100. [ Google Scholar ]
- Sun C., Zhen L., Miah M.G. Comparison of the ecosystem services provided by China’s Poyang Lake wetland and Bangladesh’s Tanguar Haor wetland. Ecosyst. Serv. 2017; 26 :411–421. [ Google Scholar ]
- Sunny A.R., Reza M., Chowdhury M.A., Hassan M., Baten M., Hasan M., et al. Biodiversity assemblages and conservation necessities of ecologically sensitive natural wetlands of north-eastern Bangladesh. Indian J. Geo. Mar. Sci. 2020; 49 (1):135–148. [ Google Scholar ]
- Tasnim N., Sultana M.A., Tabassum K., Islam M.J., Kunda M. A review of the water quality indices of riverine ecosystem, Bangladesh. Arch. Agric. Environ. Sci. 2022; 7 (1):104–113. [ Google Scholar ]
- The Daily Star Providing permanent support to the people of Tanguar. Haor. 2020 https://www.thedailystar.net/opinion/environment/news/providing-permanent-support-the-people-tanguar-haor-1945997 URL: [ Google Scholar ]
- Tikadar K.K., Islam M.J., Saha S.M., Alam M.M., Barman S.K., Rahman M.A. Livelihood status of small-scale fishermen and determinants of their income: insights from north-eastern floodplains of Bangladesh. Geogr. Sustain. 2022; 3 (3):204–213. [ Google Scholar ]
- Tima T.A., Schneider P., Chanda S.K., Mozumder M.M.H., Hossain M.M., Begum A., Shamsuzzaman M.M. Analyses implementation realities of legal frameworks for sustainable management of Tanguar haor fisheries resources in Bangladesh. Sustainability. 2021; 13 (16):8784. [ Google Scholar ]
- Uddin M.R., Miah M.G.U., Afrad M.S.I., Mehraj H., Mandal M.S.H. Land use change and its impact on ecosystem services, livelihood in Tanguar haor wetland of Bangladesh. Sci. Agric. 2015; 12 :78–88. [ Google Scholar ]
Advertisement
Fish diversity, habitat ecology and their conservation and management issues of a tropical River in Ganga basin, India
- Published: 01 August 2010
- Volume 30 , pages 306–319, ( 2010 )
Cite this article

- Wazir Singh Lakra 1 ,
- Uttam Kumar Sarkar 1 ,
- Rupali Sani Kumar 1 ,
- Ajay Pandey 1 ,
- Vineet Kumar Dubey 1 &
- Om Prakash Gusain 2
1698 Accesses
43 Citations
3 Altmetric
Explore all metrics
In the present communication habitat ecology, species diversity; distribution and different indices of fish biodiversity management were studied in a Central India river (River Betwa, a tributary of River Ganga basin approved under India’s first river linking plan). Correlation between fish species richness with the hydrological attributes showed good relationship and water depth, dissolved oxygen and pH were found the most important variables in shaping fish assemblage. Altogether, sixty-three fish species belonging to 20 families and 45 genera were collected from five sampling stations spread along the upstream, mid stream and lower streams. Cyprinids were the most dominated group represented by 26 species belonging to 15 genera, followed by Bagridae (6 species from 3 genera), and Schilbeidae (4 species from 4 genera). The distribution of fish showed interesting pattern and about 10% species were common to all the sites showing long migration range. Shannon-Weiner diversity index showed considerable variation and ranged from 1.89 to 3.51. Out of 63 species status of 10 species were not known due to data deficit, 29 categorized as lower risk, 14 as vulnerable, 8 as endangered, while the remaining two species were introduced. Our study shows that the River supports considerable diversity of the fishes and is important for conservation and about 34% fish fauna is threatened being either vulnerable or endangered. We assessed that the river supports considerable percentage of food fish (89.47), ornamental fish (49.12%) and sport fish (5.26%). Among the eight major types of fish habitats identified along the entire stretch of river, open river, shallow water and deep pools were habitats contributing maximum diversity. Fish species richness (FSR) were significantly different ( P < 0.05) in all the habitats except channel confluence and scour pool. Trophic niche model may be useful for assessing altered as well as less altered fish habitat of the tropical rivers. Since this river will be interlinked in near future, this study would be useful for conservation planning and management and also for future assessment after interlinking. Issues related to various threats to aquatic environment and conservation management strategies have been discussed.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Fish diversity and limnological parameters influencing fish assemblage pattern in chambal river basin of madhya pradesh, india.
Reconnoitre on ichthyofauna of Mahanadi River of India: shifting diversity down the river continuum and linking ecological traits with patterns in biodiversity
Habitat based fish assemblage and distribution pattern in a large reservoir of peninsular India
Aadland LP (1993) Stream habitat types: their fish assemblages and relationship to flow. North Am J Fish Manag 13:790–806
Article Google Scholar
Adholia UN (1977) Fish fauna of the River Betwa. Geobios 4(6):272–273
Google Scholar
Armantrout NB (1999) Glossary of aquatic habitat inventory terminology. American Fisheries Society, Bethesda
Arunachalam M (1999) Methods for fish habitat inventory in streams/Rivers. In: Proceedings of workshop germplasm inventory and gene banking of freshwater fishes. National Bureau of Fish Genetic Resources, Lucknow, India
Arunachalam M (2000) Assemblage structure of stream fishes in the Western Ghats (India). Hydrobiologia 430:1–31
Bain MB, Stevenson NJ (1999) Aquatic habitat assessment. Asian Fisheries Society, Bethesda
Bayley P, Li H (1994) Riverine fisheries. In: Calow P, Petts GE (eds) The river handbook: hydrological and ecological principles. Blackwell, Boston, pp 251–281
Biswas SP (1993) Manual of methods in fish biology. South Asian Publishers, New Delhi
Boruah S, Biswas SP (2002) Ecohydrology and fisheries of the upper Brahmputra basin. Environmentalist 22:119–131
Bunn SE, Arthington AH (2002) Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environ Manag 30:492–507
Chaube UC (1988) Model study of water use and water balance in Betwa Basin. J Inst Eng Indian Civil Eng Div 69:169–173
Chovance A, Hoffer R, Schiemer F (2003) Fish as bioindicators. In: Market BA, Breure AM, Zechmeiser HG (eds) Bioindicatos and biomonitors, pp 639–675
Colwell (1996) User’s guide to estimates—statistical estimation of species richness and shared species from samples. Version 8. User’s guide and application published at http://viceroy.eeb.uconn.edu/estimates
Copp GH, Bianci Bogutskaya NG, Eros T, Falka I, Ferreira MT, Fox MG, Freyhof J, Gozlan RE, Grabowska J, Kovac V, Moreno-Amich R, Naseka AM, Wiesner C (2005) To be, or not to be, a non-native freshwater fish? Appl Ichthyol 21:242–262
Corbacho C, Sanchez MJ (2001) Patterns of species richness and introduced species in native freshwater fish faunas of a Mediterranean-type basin: the Guadiana River (southwest Iberian Peninsula). Regul Rivers Res Manag 17(6):699–707
Darwall WRT, Vie JC (2005) Identifying important sites for conservation of freshwater biodiversity: extending the species-based approach. Fish Manag Ecol 12:287–293
Das MK (2007) Environment and fish health: a holistic assessment of inland fisheries in India. In: Goswami UC (ed) Natural and anthropogenic hazards on fish and fisheries. Narendra Publishing House, Delhi, pp 137–151
Das SK, Chakrabarty D (2007) The use of fish community structure as a measure of ecological degradation: a case study in two rivers of India. Bio Syst 90:188–196
CAS Google Scholar
Dawson TP, Berry PM, Kampa E (2003) Climate change impacts on freshwater wetland habitat. J Nat Conserv 11:25–30
De Silva SS, Abery NW, Nguyen TTT (2007) Endemic freshwater finfish of Asia: distribution and conservation status. Divers Distrib 13:172–174
Dudgeon D, Arthington AH, Gessner MO, Kawabata ZI, Knowler DJ, Leveque C, Naiman RJ, Prieur-Richard AH, Soto D, Stiassny MLJ, Sullivan CA (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81:163–182
Flores S, Araya PR, Hirt LM (2009) Fish diversity and community structure in a tributary stream of the Parana River. Acta Limnol Bras 21(1):57–66
Froese R, Pauly D (Eds) (2010) FishBase. World Wide Web electronic publication. http://www.Wshbase.org , Cited February 2010
Fu C, Wu J, Chen J, Wu Q, Lei G (2003) Fresh water fish biodiversity in the Yangtze River basin of China: patterns, threats and conservation. Biodivers Conserv 12:1649–1685
Fukushima M, Kameyama S, Kaneko M, Nakao K, Steel EA (2007) Modelling the effects of dams on fresh water fish distribution in Hokkaido, Japan. Freshw Biol 52:1511–1524
Gibbs JP (2000) Wetland loss and biodiversity conservation. Conserv Biol 14(1):314–317
Granado C (2000) Ecologa de communidades el paradigma de los pecces de agua dulce. Universidad de Sevilla Secretariado de Publicaciones, Sevilla
Growns I, Gehrke PC, Astles KL, Pollard DA (2003) A comparison of fish assemblage associated with different riparian vegetation types in the Hawksbury-epan River system. Fish Manag Ecol 10:209–220
Habit E, Belk MC, Tuckfield RC, Parra O (2006) Response of the fish community to human-induced changes in the Biobio River in Chile. Freshw Biol 51:1–11
Jayaram KC (1981) Fresh water fishes of India—hand book. Zoological Survey of India, Calcutta
Jayaram KC (1999) The freshwater fishes of the Indian Region. Narendra Publishing House, Delhi, p 551
Johal MS, Tandon KK, Tyor AK, Rawal YK (2002) Fish diversity in different habitats in the streams of lower Middle Western Himalayas. Polish J Ecol 50:45–56
Kang B, He D, Perrett L, Wang H, Hu W, Deng W, Wu Y (2009) Fish and fisheries in the Upper Mekong: current assessment of the fish community, threats and conservation. Rev Fish Biol Fish 19:465–480
Karr JR, Fausch KD, Angermeier PL, Yant PR, Schlosser IJ (1986) Assessing biological integrity in running waters. A method and its rationale. Illinois Natural History Survey Campaigne. Special Publications, Illinois, 28 pp
Laffaille P, Acou A, Guillouet J, Legult A (2005) Temporal change in European eel, Anguilla anguilla , stock in a small catchment after installation of fish passes. Fish Manag Ecol 12:123–129
Lakra WS, Sarkar UK (2007) Freshwater fish diversity of central India. Edited and published by National Bureau of Fish Genetic Resources, Lucknow, pp 1–183
Leveque C, Balian EV, Martens K (2005) An assessment of animal species diversity in continental waters. Hydrobiologia 542:32–67
Lima-Junior SE, Cardone IB, Goitein R (2006) Fish assemblage structure and aquatic pollution in a Brazilian stream: some limitations of diversity indices and models for environmental impact studies. Ecol Freshw Fish 15(3):284–290
Lobb MD, Orth DJ (1991) Habitat use by an assemblage of fish in a large warm water stream. Trans Am Fish Soc 120:65–78
Manojkumar TG, Kurup BM (2002) Fish habitat diversity and species assemblage structure with reference to five major systems of Kerala In: Boopendranath MR, Meenakumari B, Joseph J, Sankar TV, Pravin P, Edwin L (eds) Proceedings of riverine and reservoir fisheries of India. Society of Fisheries Technologists (India), Cochin, pp 141–150
Mas-Marti E, Garcia-Berthou E, Sabater S, Tomanova S, Monoz I (2010). Comparing fish assemblages and trophic ecology of permanent and intermittent researches in a Mediterranean stream. Hydrobiologia. doi: 10.1007/s10750-010-0292-x
Mishra DN, Moza U (1997) Changing senario of fish and fisheries of River Yamuna—part II. In: Vass KK, Sinha M (eds) Changing perspectives of inland fisheries. Proceedings of the national seminar, march 16–17, 1997, Inland Fisheries Society of India, Barrackpore, pp 57–62
Morita K, Morita SH, Yamamoto S (2009) Effects of habitat fragmentation by damming on salmonid fish: lessons from white-spotted Charr in Japan. Ecol Res 24(4):711–722
Motita K, Yokota A (2002) Population viability of stream-resident salmoids after habitat fragmentation: a case study with white-spotted Charr (Salvelinus leucomaens) . Ecol Modell 155:85–94
NWDA (National water development agency)/Tech.III/122/17/2004. V . http://www.nwda.gov.in
Pandey RP, Mishra SK, Singh R, Ramasastri KS (2008) Streamflow drought severity analysis of Betwa River system (India). Water Res Manag 22:1127–1141
Payne AI, Sinhua R, Singh HR, Huq S (2004) A review of the Ganges basin; its fish and fisheries. In: Welcome RL, Petr R (eds) Proceedings of the second international symposium on the management of large rivers for fisheries, vol 1. Food and Agriculture Organization of the United Nations, Regional Office for Asia and the Pacific; Mekong River Commission (MRC), Fisheries Programme (FP), pp 229–251
Peres-Neto PR (2004) Patterns in the co-occurrence of stream fish metacommunties: the role of site suitability, morphology and phylogeny versus species interactions. Oecologia 140:352–360
Raghavan R, Prasad G, Anvar Ali PH, Pereira B (2008a) Fish fauna of Chalakudy River, part of Western Ghats biodiversity hotspot, Kerala, India: patterns of distribution, threats and conservation needs. Biodivers Conserv 17:3119–3131
Raghavan R, Prasad G, Anvar Ali PH, Pereira B (2008b) Exotic fish species in a global biodiversity hotspot: observations from River Chalakudy, part of Western Ghats, Kerala, India. Biol Invasions 10:37–40
Rahel FJ, Bierwagen B, Taniguchi Y (2008) Managing aquatic species of conservation concern in the face of climate change and invasive species. Conserv Biol 22(3):551–561
Ricciardi A, Rasmussen JB (1999) Extinction rates of North American freshwater fauna. Conserv Biol 13:1220–1222
Sarkar UK, Bain MB (2007) Priority habitats for the conservation of large River fishes in the Ganges River basin. Aquat Conserv Mar Freshw 17:349–359
Sarkar UK, Pathak AK, Lakra WS (2008) Conservation of freshwater fish resources of India: new approaches, assessment and challenges. Biodivers Conserv 17:2495–2511
Sarkar UK, Gupta BK, Lakra WS (2010) Biodiversity, ecohydrology, threat status and conservation priority of freshwater fishes of River Gomti, a tributary of River Ganga (India). Environmentalist 30:3–17
Scrimgeour G, Chambers P (2000) Cumulative effects of pulp mill and municipal effluents on the epilithic biomass and nutrient limitation in a large northern river ecosystem. Can J Fish Aquat Sci 57:1342–1354
Shaffer AJ, Beirne M, Ritchie T, Paradis R, Barry D, Crain P (2009) Fish habitat use response to anthropogenic induced changes of physical processes in the Elwha estuary, Washington, USA. Hydrobiologia 636:179–190
Shahnawaz A, Venkateshwarlu M, Somashekar DS, Santosh K (2010) Fish diversity with relation to water quality of Bhadra River of Western Ghats (India). Environ Monit Assess 161:83–91
Article CAS Google Scholar
Shannon CE, Wiener W (1963) The mathematical theory of communication. University Illinois Press, Urbana pp36.
Specht WL, Paller MH (2004) Macroinvertebrate assessments of 22 locations in SRS streams, in support of the Integrator Operable Unit Program, July–August 2003. WSRC-TR-2004-00482
Sreekantha KV, Ramachandra TV (2005) Fish diversity in Linganamakki Reservoir, Sharavathi River. Ecol Environ Conserv 11:337–348
Srivastava GJ (1988) Fishes of U.P. and Bihar, 3rd edn. Viswa Vidyalaya Prakashan, Varanasi
Statsoft Inc (1999) Electronic statistics textbook. TulsaStatsof, OK. Web: http://www.statsoft.com/textbook/stathome.html
Szollosi-Nagy (2004) In: Proceedings of the United Nations seminar, 25–26 November 2004, Delft, Netherlands
Talwar PK, Jhingran A (1991) Inland fishes of India and adjacent countries, 2 volumes. Oxford and IBH Publishing Co. Pvt. Ltd., New Delhi, xix + 1158 pp
Welcomme RL (1985) River fisheries. FAO Fish Tech Pap 262:1–318
Wichert GA, Rapport DJ (1998) Fish community structure as a measure of degradation and rehabilitation of riperine systems in an agricultural drainage basin. Environ Manag 22(3):425–443
Wolter C, Minow J, Vilcinskas A, Grosch U (2000) Long-term effects of human influence on fish community structure and fisheries in Berlin water: an urban water system. Fish Manag Ecol 7:97–104
Yamamoto S, Morita K, Koizumi I, Maekawa K (2004) Genetic differentiation of white spotted Charr (Salvelinus leucomaens) population after habitat fragmentation: spatial-temporal changes in gene frequencies. Conserv Genet 5:529–538
Download references
Acknowledgments
We are grateful to the Department of Biotechnology (DBT) Govt. of India, New Delhi for financial support to carry out the present investigation.
Author information
Authors and affiliations.
National Bureau of Fish Genetic Resources, Canal Ring Road, Dilkusha, Lucknow, 226002, India
Wazir Singh Lakra, Uttam Kumar Sarkar, Rupali Sani Kumar, Ajay Pandey & Vineet Kumar Dubey
Department of Zoology, HNB Garhwal University, Srinagar, Garhwal, 246174, India
Om Prakash Gusain
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Uttam Kumar Sarkar .
See Table 4 .
Rights and permissions
Reprints and permissions
About this article
Lakra, W.S., Sarkar, U.K., Kumar, R.S. et al. Fish diversity, habitat ecology and their conservation and management issues of a tropical River in Ganga basin, India. Environmentalist 30 , 306–319 (2010). https://doi.org/10.1007/s10669-010-9277-6
Download citation
Published : 01 August 2010
Issue Date : December 2010
DOI : https://doi.org/10.1007/s10669-010-9277-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- River Betwa
- Habitat ecology
- Distribution
- Fish diversity
- Trophic niche
- Conservation
- Find a journal
- Publish with us
- Track your research
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

A Study of Fish Diversity in Bhandara District (MS) India, With Special Emphasis on Pollution and Human Interference in Aquatic Habitats

2013, OIIRJ. Vol. 3 (Sp. Issue), pp. 147-154.
Present study deals with the inventorization of fish species in District Bhandara of Maharashtra India. The study area is well known as a district of lakes. About 30 lakes, ponds, reservoir and some river habitats at rural and forests areas, are explored to collect the fish species and studied their habitat conditions. In previous literature about 38 species are reported in the study area of which all are collected and reported in terms of low, moderate and optimum distribution according to their availability in the nearby markets. During study period 23 more species are collected from the rural water bodies. Identification of species is done by the literature of Day and Jairam. Mostly human interference in the lakes and rivers are mainly responsible for the less distribution of fishes, pollution load and intense hot climatic conditions affects the growth and distribution fishes. Pollution load during the months of summer turns the fish species to develop certain adaptations. The species having more adaptive capabilities showed more in quantities; however some fish fauna is going on the way of scrub down from the study area. Careless management of some lakes and river and agricultural practices in lakes and river basins pollutes the water which creates hazards for eggs and fries to grow up in the adult fishes. Use of certain manures and insecticides in the lake water harms the fish fauna. KEYWORDS: Freshwater fishes, Water bodies, Status, Diversity and Inventorization.
Related Papers
Atul Bobdey
s During the study period 63 species are collected of 8 orders and 17 families, from the water bodies located in Bhandara district. Harvested data indicates the dominance of species family cyprinidae > Ophiocephalidae > Bagaridae > Siluridae>Notopteridae = Ambassidae = Claridae>Nandidae = anabantidae = Osphronemidae = Gobidae = Cichlidae = Anguillidae = Saccobranchidae = Pangasiidae = Sisoridae = Belonidae Mostly human interference in the lakes and rivers are mainly responsible for the less distribution of fishes, pollution load and intense hot climatic conditions affects the growth and distribution fishes. Pollution load during the months of summer turns the fish species to develop certain adaptations. The species having more adaptive capabilities showed more in quantities; however some fish fauna is going on the way of scrub down from the study area. Careless management of some lakes and river and agricultural practices in lakes and river basins pollutes the water which creates hazards for eggs and fries to grow up in the adult fishes. Use of certain manures and insecticides in the lake water harms the fish fauna.
International Journal of Fisheries and Aquatic Studies
Suthakar Isaac
Biodiversity specifies the potential of any aquatic system and also represents its trophic status. It is very much necessary to have a sufficient knowledge of the constituent biota especially for the purpose of conservation and management of the inland water resources such as rivers, reservoirs and ponds. The present study deals with the diversity of fish fauna found in Indrapuri Dam of district Rohtas. The diversity of fish fauna has never been studied in the Indrapuri dam of district Rohtas. In this study, the diversity of freshwater fishes of Indrapuri Dam was studied and assessed from August, 2015 to January, 2016.The aim of the paper was to assess the variety and abundance of the important fish fauna inhabiting in this region. We documented and described 25 freshwater fish species of Indrapuri dam that were belonging to the 5 orders and 12 families and 21 genera. Among them, three species were belonging to the family Bagridae, five species were belonging to Schilbeidae, two spe...
World Journal of Pharmacy and Pharmaceutical Sciences
Abhishek Giri
Shahpura Lake is situated in one of the posh localities of Bhopal, the capital city of the state of Madhya Pradesh. This is also one of the water bodies present in Bhopal and this is a manmade perennial lake and construction during the period of 1974-1975. The latitude of the lake is 23˚12" N and longitude of the lake is 77˚25" E. The lake receives untreated domestic sewage water from measure sewage inlets near Mata Mandir slums and Chunabhatti area. In the present study, physico-chemical parameters viz. water temperature, pH, Dissolved Oxygen, Free CO 2 , Total Alkalinity of water of Shahpura Lake (Bhopal) were analyzed. On the basis of different physicochemical parameters, the status of reservoir is polluted in nature and during the period under study 6 fish species have been recorded.
Devi Prasad Uniyal
Indian Journal of Fisheries
Ajey Pathak
The study presents the status of fish diversity, abundance and habitat structure of Surha Lake, which is a perennial and natural lake fed by the river Ganga. The study was conducted between 2011 to 2013 covering pre and post-monsoon seasons. In total, 4,852 individual fish specimens were collected representing 66 fish species belonging to 23 families. The species diversity comprised 65 species in pre-monsoon and 60 species in post-monsoon season (p<0.05). The highest species diversity was recorded for the family Cyprinidae (22), followed by Bagridae (7). An assessment of conservation status of 66 fish species as per IUCN Red List 2019 criteria listed 6 species under near threatened (NT), 54 under least concern (LC) and two species under vulnerable (VU) category. The study reports several commercially important species under near threatened (Chitala chitala, Labeo pangusia, Ompok bimaculatus, Ompok pabda, , Ailia coila and Bagarius bagarius), which makes Surha Taal an important na...
Journal of Ecological Society, 13/14 …
Rupesh Raut
Devi Prasad
The paper highlights the fish diversity in major wetlands of Mysore district, Karnataka, India and its conservation status. Forty-five species of fishes belonging to 15 families, 31 genera have been identified. Fish species belonging to genus Puntius were more common in many of these lakes. However it was observed that the fish diversity was decreasing since last two years unprecedently mainly due to manifold human activities. Fish diversity in the lakes is becoming rare and about seven species were identified as endangered. Out of the 45 identified fishes, six fish species were identified as threatened species. Tork hudree, also known as Deccan mahseer and seven other species were identified as vulnerable fish species. Though there were 40 species of fish endemic to this region, their number decreased with introduction of more exotic species. Conservation of endemic fishes, propagation of endangered and threatened fishes should be therefore, undertaken to preserve and protect fish ...
Lina Sarkar
Biological Forum – An International Journal
E.D.Israel Oliver king
A study on freshwater fish fauna of Kendrapara district of Odisha, India was provided. A total of 63 species of fishes under 44 genera, 25 families and 8 orders has been recorded. Highest species diversity was observed in the Cyprinidae (33.3%) followed by Bagridae (7.9%). The fish fauna includes 49 least concern (LC), 5 near threatened (NT), 2 data deficient (DD) and 7 not assessed (NA) as per IUCN. The study shows that water bodies of kendrapara district including numerous economical importance food fishes as well as ornamental fishes belongs to freshwater , marine and brackish water habitat. In the study 44 species coming under capture fishery, 45 species has ornamental value, 21 species for culture and 10 species under sports fishery. Water quality of different water bodies are not contaminated as the study shows both the pH & DO are within the tolerance limit of class 'D'. So the water quality of the water bodies under Kendrapara district will be recommended for aquaculture and wild life propagation.
Fishes are the most diversified group among vertebrates, with ca. 33,600 species [3] characterized by their great diversity in morphology, physiology, ecology, life history and behavior. Almost 25% of the global vertebrate diversity accounted by fish is concentrated in the 0.01% of the earth’s water. This tiny fraction of earth’s water supports at least 100000 species out of approximately 1.8 million and almost 6% of all are described species [4]. It is often claimed that freshwater ecosystems arethe most endangered ecosystems in the world [5]. This particular vulnerability of freshwater to fish at global scale reflects the fact that both fishand freshwater are the need of humans and consequently they have been heavily impacted by theiruse and regulation. Asia supports over half of the global human population, with enormous consequent pressures on inland waters and freshwater fish biodiversity [4]. The freshwaters in India are one of the most exploited resources since past many year...
RELATED PAPERS
Lucero Salgado
MUKADIMAH: Jurnal Pendidikan, Sejarah, dan Ilmu-ilmu Sosial
Mustika Permatasari
Revista De Filologia Romanica
Clarinda A Maia
Angewandte Chemie International Edition
Dewi Regita Cahyanti
Folia Geobotanica
Maria Teresa Piedade
Cuadernos de Geografía: Revista Colombiana de Geografía
Risk Management and Healthcare Policy
YAZED ALRUTHIA
GATR GLOBAL JOURNAL OF BUSINESS AND SOCIAL SCIENCE REVIEW
Jerry Lintong
Contrastive Pragmatics
N. Baumgarten
Fiep Bulletin on Line
Marcos Felipe Da Silva Santos
Tommi Auvinen
Marta Torrens
Proceedings of the 12th International Congress on the Archaeology of the Ancient Near East
Doğa Karakaya
The American Journal of Medicine
Dale Bratzler
Journal of the American Chemical Society
Luca Valgimigli
arXiv (Cornell University)
Shen-Fu Tsai
Berichte zur Wissenschaftsgeschichte
Andrea Pető
International Journal of Gynecology & Obstetrics
Wendy Wolfman
Call Girls Mukherjee Nagar, in Delhi
delhi munirka
O DESENVOLVIMENTO DAS FUNÇÕES PSICOLÓGICAS SUPERIORES NA PSICOLOGIA HISTÓRICO-CULTURAL: CONTRIBUIÇÕES À PSICOLOGIA E À EDUCAÇÃO
SONIA BARROCO
Peter Taylor
Critical Care Clinics
Satish Bhagwanjee
Advances in Animal and Veterinary Sciences
Chattida Panprom
Innovaciencia
Nina Kozbagarova
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
- My Shodhganga
- Receive email updates
- Edit Profile
Shodhganga : a reservoir of Indian theses @ INFLIBNET
- Shodhganga@INFLIBNET
- Devi Ahilya Vishwavidyalaya
- Mata Jijabai Government P G Girls College
Items in Shodhganga are licensed under Creative Commons Licence Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0).


COMMENTS
Abstract and Figures. This chapter provides a brief overview of freshwater fish diversity globally, the factors underlying diversity patterns among and within river basins and phylogenetic groups ...
In marine ecosystems, fish species diversity is concentrated in coastal waters (depths of <200 m) that represent <1% of the world's sea surface 36. Marine and freshwater fish diversity also ...
The decline in fish diversity of TH is caused by a combination of climatic, anthropogenic, socioeconomic, and policy factors. ... the review paper is a first attempt to find out the research gaps from previous works, recent biodiversity condition, and associated threats. The topics like current status, biodiversity value, and related threats ...
Abstract. Freshwater fish represent one-fourth of the world's vertebrates and provide irreplaceable goods and services but are increasingly affected by human activities. A new index, Cumulative Change in Biodiversity Facets, revealed marked changes in biodiversity in >50% of the world's rivers covering >40% of the world's continental ...
To identify patterns and drivers of genetic diversity in fishes, we test each of these predictions by determining the relationship between genetic diversity and (i) habitat, (ii) conservation status, and (iii) life‐history characteristics by performing a quantitative review of 463 globally distributed fish species. 2.
We carried out this research in coastal habitats of San Cristóbal Island, which is at the eastern end of the Galapagos archipelago (Fig. 1).This island has a shoreline perimeter of approximately ...
Human pressures have been intensely modifying freshwater ecosystems worldwide. We assessed the effects of human pressure on habitat diversity and primary productivity to understand the consequences on fish fauna in 25 tropical and subtropical streams of two globally important ecoregions: Iguassu and Upper Paraná. We hypothesized that the increased human pressure (urbanization and agriculture ...
RESEARCH PAPER. Marine fish diversity in Tropical America associated with both past and present environmental conditions. Andrea Polanco F., Corresponding Author. ... Snow and Landscape Research WSL, Birmensdorf, Switzerland. Search for more papers by this author. Camille Albouy,
The river Ganges is the largest river in India and the fifth longest in the world. Although, many studies on fish ecology and systematic have been conducted largely to improve fisheries but fish diversity and their distribution pattern from conservation point of view have never been adequately addressed in the Ganges. In this connection, current distribution and abundance of freshwater fishes ...
The fish diversity in the Mahanadi River is in no way exceptional from the current impact of global warming and climate change. ... Review Paper. Review of the Research on the Fish Diversity in ...
For shallow lakes, the most dramatic ecosystem shift is that from a clear-water, macrophyte-dominated regime to a turbid, phytoplankton-dominated regime. Whereas many studies have focussed on the factors that trigger such shifts, it is still unclear how these changes shape fish diversity. In the present study, we characterised the fish communities taxonomically and functionally in Lake Taihu ...
A review of fish diversity, decline drivers, ... Furthermore, the review paper is a first attempt to find out the research gaps from previous works, recent biodiversity condition, and associated threats. The topics like current status, biodiversity value, and related threats have also been compiled that may be helpful for law and policy making ...
Of the total fish diversity known from India, the marine fishes constitute 75.6 percent, comprising of 2443 species belonging to 927 genera, under 230 families of 40 orders. Among the fish diversity-rich areas in the marine waters of India, the Andaman and Nicobar archipelago shows the highest number of species, 1431, followed by the east coast ...
In the present communication habitat ecology, species diversity; distribution and different indices of fish biodiversity management were studied in a Central India river (River Betwa, a tributary of River Ganga basin approved under India's first river linking plan). Correlation between fish species richness with the hydrological attributes showed good relationship and water depth, dissolved ...
The present study is the first of its kind for the river Tons on fish diversity and conservation priority. The study depicted presence of 19 species contributing about 55.88% of total fish diversity published from western Doon valley (Mehta and Gupta, 2007) and about 40.4% of total fish species from entire doon valley (Singh, 1964).
fish diversity was recorded with the help of local fisher man from the study area; the data was collected in all seas ons. Both, direct and indirect methods were applied to find out fish diversity of the area. The diversity indices were analyzed through statistical software PAST version 2.17 C.
The paper highlights the fish diversity in major wetlands of Mysore district, Karnataka, India and its conservation status. Forty-five species of fishes belonging to 15 families, 31 genera have been identified. ... Online International Interdisciplinary Research Journal, {Bi-Monthly}, ISSN2249-9598, Vol-III, Nov 2013 Special Issue A Study of ...
The Shodhganga@INFLIBNET Centre provides a platform for research students to deposit their Ph.D. theses and make it available to the entire scholarly community in open access. ... Studies on the Fish Diversity and Relationships with Zooplanktons in Dhamoi and Gulabpura water Bodies of Jhabua District: Researcher: Ganava, Reena: Guide(s): Khare ...
Their study reveals Wetland as a rich source of fish diversity as it represents fish fauna of Beas and Sutlej river. Kar et al. (2006) focus on the diversity of the fish population in Sone Lake ...
India is one of the megabiodiversity countries in the world and occupies the ninth position. in terms of freshwater megabiodiversity (Mittermeier an d Mittermeier, 1997 ). In India, there are ...