An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMJ Case Rep


Case Report
Atypical presentation of molar pregnancy, ream langhe.
Department of Obstetrics and Gynaecology, Our Lady of Lourdes Hospital, Drogheda, Ireland
Bogdan Alexandru Muresan
Nor azlia abdul wahab.
The classic features of molar pregnancy are irregular vaginal bleeding, hyperemesis, enlarged uterus for gestational age and early failed pregnancy. Less common presentations include hyperthyroidism, early onset pre-eclampsia or abdominal distension due to theca lutein cysts. Here, we present a case of molar pregnancy where a woman presented to the emergency department with symptoms of acute abdomen and was treated as ruptured ectopic pregnancy. The woman underwent laparoscopy and evacuation of retained products of conception. Histological examination of uterine curettage confirmed the diagnosis of a complete hydatidiform mole. The woman was discharged home in good general condition with a plan for serial beta-human chorionic gonadotropin (beta-hCG) follow-up. Complete follow-up includes use of contraception and follow-up after beta-hCG is negative for a year.
Molar pregnancies, a premalignant form of gestational trophoblastic neoplasia, are characterised by an overgrowth of fetal chorionic tissue within the uterus. 1 In the USA, molar pregnancy occurs in 1 in 1000–1200 pregnancies and in 1 in 600 therapeutic abortions. 2 In the UK, the incidence is estimated at 1/714 live births. Currently, there is no database that records gestational events in the Irish population. 3
Molar pregnancies are categorised as partial hydatidiform mole or complete hydatidiform mole based on genetic and histological features. 4 Complete hydatidiform moles usually occur as a result of duplication of a single sperm following fertilisation of an ‘empty’ oocyte (75%–80% of complete hydatidiform moles). They are diploid and entirely of male origin with no evidence of fetal tissues. Less commonly (20%–25%), complete hydatidiform moles can arise following dispermic fertilisation of an empty oocyte. In partial hydatidiform molar pregnancies, the trophoblast cells have two sets of paternal haploid genes and one set of maternal haploid genes (90%). They occur as a result of fertilisation of an oocyte by two sperms at the same time. In partial hydatidiform moles, there is usually evidence of fetal tissues. 4 5
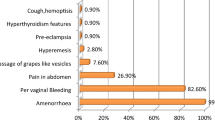
Molar pregnancy is usually presented with painless vaginal bleeding, 6 or sometimes it is associated with abdominal pain and morning sickness, along with enlarged uterus for gestational age. 4 Less commonly, women might present with signs of hyperthyroidism, early onset pre-eclampsia or with acute respiratory failure or neurological symptoms. 4 Women at risk of molar pregnancy, particularly complete hydatidiform mole, are those at the extremes of the reproductive age: girls <15 years and women >45 years. 7 Furthermore, the risk of molar pregnancy is increased in women with a previous history of molar pregnancy. 8
Molar pregnancy can be strongly suggested by ultrasound. 9–11 However, the definitive diagnosis requires histological examination of products of conception. 4 In complete hydatidiform mole, the ultrasound typically shows an absent gestational sac and a complex echogenic intrauterine mass with cystic spaces. The ultrasound diagnosis of a partial hydatidiform mole is difficult as it may resemble a normal conception. 5 12
Molar pregnancy should be treated by suction evacuation for complete hydatidiform mole or medical evacuation if fetal parts are identified. 4 Following evacuation, women should be followed by weekly beta-human chorionic gonadotropin (beta-hCG) estimation until it reaches an undetectable level. 4
Here, we present a case of molar pregnancy, where a woman presented to the emergency department (ED) with symptoms of acute abdomen and was treated as a ruptured ectopic pregnancy. However, histological examination of uterine curettage confirmed the diagnosis of a complete hydatidiform mole.
Case presentation
A 33-year-old woman with a history of sudden onset of right iliac fossa pain and mild vaginal bleeding following 8 weeks amenorrhoea and a positive pregnancy test was brought to the ED by ambulance. The woman was sixth gravid with a history of two uncomplicated vaginal deliveries and three miscarriages, which were treated by evacuation of retained products of conception (ERPC). Eight days prior to the admission, she had vaginal bleeding with passage of clots but she had not presented to the hospital. Her medical history was unremarkable. On examination she was pale, in severe pain, with a temperature of 37°C, pulse rate of 108 beats per minute and blood pressure of 123/73 mm Hg. On abdominal examination, the abdomen was observed to be very distended, tender and rigid. Vaginal examination was not carried out due to the excruciating pain. Abdominal ultrasound examination was performed with difficulty, and showed some free fluid in the abdominal cavity. Two large-bore cannula were inserted and blood was sent urgently for full blood count (FBC), serum electrolytes, renal and liver function tests (LFTs), beta-hCG titre and blood group and cross-matched of 2 units. Her blood results showed iron deficiency anaemia and haemoglobin was 6.1 g/dL.
In light of these symptoms and low haemoglobin level, 1 unit of O-negative red cells was transfused and a decision for emergency laparoscopy was made to rule out ruptured ectopic pregnancy. At the laparoscopy, both fallopian tubes were mildly dilated and blood was dripping from the fimbrial ends of the tubes, which could possibly have been retrograde bleeding from the uterine cavity. There were no tubal masses and the uterus was very bulky: approximately the size of 17 weeks gestation. In view of the bulky size and ‘spongy’ consistency of the uterus, and absence of tubal mass, a transabdominal scan was performed intraoperatively after releasing the pneumoperitoneum. This showed an enlarged uterine cavity with a snowstorm appearance, suggesting a molar pregnancy. Following this, a decision was made for ERPC under ultrasound guidance with suction curette. Endometrial tissues were sent urgently for histological examination.
On day 1 postoperative, the patient developed fever and complained of upper abdominal pain and an orange discolouration of urine. On examination, her abdomen was distended and tender in the right hypochondrial region. A septic infectious screen was performed and intravenous antibiotics were administered; 750 mg cefuroxime, 500 mg metronidazole and 4.5 g piperacillin/tazobactam 8 hourly were commenced as per unit protocol and in collaboration with the microbiologist. A further 2 units of red cells were transfused once fever subsided. The results of the blood culture revealed Gram-negative bacilli growth. Her LFTs showed elevated liver enzyme of alanine transaminase (ALT) (or serum glutamic-pyruvic transaminase (SGPT)) 185 IU/L and bilirubin level of 45 mg/dL. Her liver and biliary tract scan was unremarkable apart from a well-circumscribed hyperechoic lesion of 1 cm with no vascularity (probably a haemangioma).
Forty-eight hours later, her haemoglobin increased to 10 g/dL and repeated LFT showed decreased liver enzyme of ALT (SGPT) 97 IU/L, and bilirubin levels of 11 mg/dL. Her serial beta-hCG dropped from 225 000 IU/L preoperatively to 27 000 IU/L on the fourth postoperative day. Her histological examination result confirmed the diagnosis of complete hydatidiform mole. She showed significant improvement in her condition and LFT results of ALT (SGPT) 52 IU/L, and bilirubin of 6 mg/dL. She was discharged home on the fifth postoperative day with a plan for a weekly beta-hCG and LFT and a follow-up abdominal scan in 3 months’ time. The woman was debriefed about the condition, and information was supplemented with a patient information leaflet. By the third week postoperative, her LFT level return to a normal level and beta-hCG level decreased to 1300 IU/L. She will continue to be monitored weekly until her beta-hCG reaches an undetectable level.
Investigations
FBC, LFT, renal function test, beta-hCG and transabdominal ultrasound.
Differential diagnosis
Ruptured ectopic pregnancy.
Laparoscopy and ERPC.
Outcome and follow-up
The patient was discharged in good condition with a plan for weekly estimation of serum beta-hCG and repeat abdominal ultrasound in 3 months. Complete follow-up includes use of contraception and follow-up after beta-hCG is negative for a year.
The classic features of molar pregnancy are irregular vaginal bleeding, hyperemesis, enlarged uterus for gestational age and early failed pregnancy. Less common presentations include hyperthyroidism, early onset pre-eclampsia or abdominal distension due to theca lutein cysts. Furthermore, women can present with acute respiratory failure or neurological symptoms such as seizures. 4 These symptoms would more commonly be associated with early pre-eclampsia and eclampsia. However, the possibility of metastatic gestational trophoblastic disease (GTD) should also be borne in mind. The particularities of the case are the presenting symptoms.
A review of the literature revealed few cases of molar pregnancies with an atypical clinical picture. A case of complete hydatidiform mole in a 20-year-old II gesta I para woman was reported. 13 The patient underwent excision of a haemorrhagic left ovarian cyst. Histopathological examination revealed a haemorrhagic corpus luteum with a single microscopic focus of detached atypical trophoblast, without chorionic villi. She then had a left salpingo-oophorectomy for persistently elevated hCG, which led to the final diagnosis of complete hydatidiform mole arising in the ovary. Short tandem genetic analysis confirmed the diagnosis of complete hydatidiform mole. 13 Another case of partial molar pregnancy was a 23-year-old primigravida woman who presented with atypical pre-eclampsia (high blood pressure 160/100 mm Hg, proteinuria of 3.4 g in 24 hours, headache, photophobia and anasarca) at 16 weeks gestation. 14 Another primigravida woman presented to ED with abdominal pain and vaginal bleeding and passing of a large, grape-like vesicular mass with multiple negative urine pregnancy tests. These negative tests, which were likely caused by the ‘high-dose hook effect’, delayed the management of the condition until the development of pulmonary choriocarcinoma at the time of diagnosis. 15
Our patient presented with severe lower abdominal pains and pelvic bleeding with a positive pregnancy test, all in the context of an un-booked pregnancy. Severe abdominal pains are not specific to the classic picture of molar pregnancy. This could be caused by the distension of the fallopian tubes and peritoneal irritation. The above symptomatology can mimic a wide range of pathologies like miscarriage, threatened miscarriage, ectopic pregnancy and placental abruption (in second trimester). A good differential diagnosis is important. In this case, the bedside transabdominal ultrasound that was performed in the ED was very difficult due to patient’s acute symptoms and lack of cooperation but was enough to raise a suspicion of haemoperitoneum, once free fluid was see in the abdominal cavity.
Intraoperative findings did not show any large lutein cyst causing the abdominal distension. There were small corpus lutea in otherwise normal ovaries. Large bowels were slightly dilated but not massively to suggest megacolon caused by Clostridium perfringens , which is known to be a gas-producing bacteria. Hence, the rigid distended abdomen at presentation of this woman was most likely from C. perfringens septicaemia, and particularly the intraoperative effect on her body.
High values of LFTs can suggest metastasis in a case of complete molar pregnancy and the patient has to be thoroughly investigated in order to outrule such complications. The patient had a chest X-ray, abdominal ultrasound scan and abdomino-pelvis CT, and metastasis were outruled. In this patient’s case, raised LFTs can be a sign of organ failure in the context of posible septicaemia.
Following the laparoscopy and uterine evacuation procedure, the patient developed fever. Blood cultures results showed Gram-negative bacilli growth, which was C. perfringens . The infection could be due to bacterial proliferation in the necrotic trophoblastic tissue or bacteria that originated from the vaginal flora and ascended into the cervix and the uterus, establishing the infection. However, C. perfringens is not a common flora in the vagina. Infection with C. perfringens is very rare. One of the sources of infection with this organism is from illegal abortion, hence this point should be explored by clinicians dealing with such a case.
There was a case report where a hysterectomy had to be performed after septicaemia with C. perfringens following suction dilatation and curettage for molar pregnancy. 16 This patient was reported to recover well after the hysterectomy. Fortunately, in our case, the patient was treated early with intravenous antibiotics and responded well to the treatment.
Molar pregnancies can present in atypical form. The diagnosis of GTD in the first trimester requires high clinical suspicion. Early diagnosis and management of the condition is associated with decreased risk of morbidity and mortality.
Learning points
- High index of suspicion is the key to diagnosing molar pregnancy, especially if it presents in atypical form.
- Early recognition of the condition saves lives and decreases morbidity.
- Molar pregnancy should be included in the differential diagnosis of vaginal bleeding and abdominal pain in the first trimester.
Acknowledgments
Authors would like to thank the patient for her cooperation.
Contributors: RL: conception and design of study, acquisition of data. RL and AM: analysis and/or interpretation of data, drafting the manuscript. NA and EA: revising the manuscript critically for important intellectual content. All authors approved the final version of the manuscript to be published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Global health
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 2018, Issue
- Atypical presentation of molar pregnancy
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Ream Langhe ,
- Bogdan Alexandru Muresan ,
- Etop Akpan ,
- Nor Azlia Abdul Wahab
- Department of Obstetrics and Gynaecology , Our Lady of Lourdes Hospital , Drogheda , Ireland
- Correspondence to Dr Ream Langhe, reamlanghe{at}yahoo.co.uk
The classic features of molar pregnancy are irregular vaginal bleeding, hyperemesis, enlarged uterus for gestational age and early failed pregnancy. Less common presentations include hyperthyroidism, early onset pre-eclampsia or abdominal distension due to theca lutein cysts. Here, we present a case of molar pregnancy where a woman presented to the emergency department with symptoms of acute abdomen and was treated as ruptured ectopic pregnancy. The woman underwent laparoscopy and evacuation of retained products of conception. Histological examination of uterine curettage confirmed the diagnosis of a complete hydatidiform mole. The woman was discharged home in good general condition with a plan for serial beta-human chorionic gonadotropin (beta-hCG) follow-up. Complete follow-up includes use of contraception and follow-up after beta-hCG is negative for a year.
- obstetrics and gynaecology
- reproductive medicine
https://doi.org/10.1136/bcr-2018-225545
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Background
Molar pregnancies, a premalignant form of gestational trophoblastic neoplasia, are characterised by an overgrowth of fetal chorionic tissue within the uterus. 1 In the USA, molar pregnancy occurs in 1 in 1000–1200 pregnancies and in 1 in 600 therapeutic abortions. 2 In the UK, the incidence is estimated at 1/714 live births. Currently, there is no database that records gestational events in the Irish population. 3
Molar pregnancies are categorised as partial hydatidiform mole or complete hydatidiform mole based on genetic and histological features. 4 Complete hydatidiform moles usually occur as a result of duplication of a single sperm following fertilisation of an ‘empty’ oocyte (75%–80% of complete hydatidiform moles). They are diploid and entirely of male origin with no evidence of fetal tissues. Less commonly (20%–25%), complete hydatidiform moles can arise following dispermic fertilisation of an empty oocyte. In partial hydatidiform molar pregnancies, the trophoblast cells have two sets of paternal haploid genes and one set of maternal haploid genes (90%). They occur as a result of fertilisation of an oocyte by two sperms at the same time. In partial hydatidiform moles, there is usually evidence of fetal tissues. 4 5
Molar pregnancy is usually presented with painless vaginal bleeding, 6 or sometimes it is associated with abdominal pain and morning sickness, along with enlarged uterus for gestational age. 4 Less commonly, women might present with signs of hyperthyroidism, early onset pre-eclampsia or with acute respiratory failure or neurological symptoms. 4 Women at risk of molar pregnancy, particularly complete hydatidiform mole, are those at the extremes of the reproductive age: girls <15 years and women >45 years. 7 Furthermore, the risk of molar pregnancy is increased in women with a previous history of molar pregnancy. 8
Molar pregnancy can be strongly suggested by ultrasound. 9–11 However, the definitive diagnosis requires histological examination of products of conception. 4 In complete hydatidiform mole, the ultrasound typically shows an absent gestational sac and a complex echogenic intrauterine mass with cystic spaces. The ultrasound diagnosis of a partial hydatidiform mole is difficult as it may resemble a normal conception. 5 12
Molar pregnancy should be treated by suction evacuation for complete hydatidiform mole or medical evacuation if fetal parts are identified. 4 Following evacuation, women should be followed by weekly beta-human chorionic gonadotropin (beta-hCG) estimation until it reaches an undetectable level. 4
Here, we present a case of molar pregnancy, where a woman presented to the emergency department (ED) with symptoms of acute abdomen and was treated as a ruptured ectopic pregnancy. However, histological examination of uterine curettage confirmed the diagnosis of a complete hydatidiform mole.
Case presentation
A 33-year-old woman with a history of sudden onset of right iliac fossa pain and mild vaginal bleeding following 8 weeks amenorrhoea and a positive pregnancy test was brought to the ED by ambulance. The woman was sixth gravid with a history of two uncomplicated vaginal deliveries and three miscarriages, which were treated by evacuation of retained products of conception (ERPC). Eight days prior to the admission, she had vaginal bleeding with passage of clots but she had not presented to the hospital. Her medical history was unremarkable. On examination she was pale, in severe pain, with a temperature of 37°C, pulse rate of 108 beats per minute and blood pressure of 123/73 mm Hg. On abdominal examination, the abdomen was observed to be very distended, tender and rigid. Vaginal examination was not carried out due to the excruciating pain. Abdominal ultrasound examination was performed with difficulty, and showed some free fluid in the abdominal cavity. Two large-bore cannula were inserted and blood was sent urgently for full blood count (FBC), serum electrolytes, renal and liver function tests (LFTs), beta-hCG titre and blood group and cross-matched of 2 units. Her blood results showed iron deficiency anaemia and haemoglobin was 6.1 g/dL.
In light of these symptoms and low haemoglobin level, 1 unit of O-negative red cells was transfused and a decision for emergency laparoscopy was made to rule out ruptured ectopic pregnancy. At the laparoscopy, both fallopian tubes were mildly dilated and blood was dripping from the fimbrial ends of the tubes, which could possibly have been retrograde bleeding from the uterine cavity. There were no tubal masses and the uterus was very bulky: approximately the size of 17 weeks gestation. In view of the bulky size and ‘spongy’ consistency of the uterus, and absence of tubal mass, a transabdominal scan was performed intraoperatively after releasing the pneumoperitoneum. This showed an enlarged uterine cavity with a snowstorm appearance, suggesting a molar pregnancy. Following this, a decision was made for ERPC under ultrasound guidance with suction curette. Endometrial tissues were sent urgently for histological examination.
On day 1 postoperative, the patient developed fever and complained of upper abdominal pain and an orange discolouration of urine. On examination, her abdomen was distended and tender in the right hypochondrial region. A septic infectious screen was performed and intravenous antibiotics were administered; 750 mg cefuroxime, 500 mg metronidazole and 4.5 g piperacillin/tazobactam 8 hourly were commenced as per unit protocol and in collaboration with the microbiologist. A further 2 units of red cells were transfused once fever subsided. The results of the blood culture revealed Gram-negative bacilli growth. Her LFTs showed elevated liver enzyme of alanine transaminase (ALT) (or serum glutamic-pyruvic transaminase (SGPT)) 185 IU/L and bilirubin level of 45 mg/dL. Her liver and biliary tract scan was unremarkable apart from a well-circumscribed hyperechoic lesion of 1 cm with no vascularity (probably a haemangioma).
Forty-eight hours later, her haemoglobin increased to 10 g/dL and repeated LFT showed decreased liver enzyme of ALT (SGPT) 97 IU/L, and bilirubin levels of 11 mg/dL. Her serial beta-hCG dropped from 225 000 IU/L preoperatively to 27 000 IU/L on the fourth postoperative day. Her histological examination result confirmed the diagnosis of complete hydatidiform mole. She showed significant improvement in her condition and LFT results of ALT (SGPT) 52 IU/L, and bilirubin of 6 mg/dL. She was discharged home on the fifth postoperative day with a plan for a weekly beta-hCG and LFT and a follow-up abdominal scan in 3 months’ time. The woman was debriefed about the condition, and information was supplemented with a patient information leaflet. By the third week postoperative, her LFT level return to a normal level and beta-hCG level decreased to 1300 IU/L. She will continue to be monitored weekly until her beta-hCG reaches an undetectable level.
Investigations
FBC, LFT, renal function test, beta-hCG and transabdominal ultrasound.
Differential diagnosis
Ruptured ectopic pregnancy.
Laparoscopy and ERPC.
Outcome and follow-up
The patient was discharged in good condition with a plan for weekly estimation of serum beta-hCG and repeat abdominal ultrasound in 3 months. Complete follow-up includes use of contraception and follow-up after beta-hCG is negative for a year.
The classic features of molar pregnancy are irregular vaginal bleeding, hyperemesis, enlarged uterus for gestational age and early failed pregnancy. Less common presentations include hyperthyroidism, early onset pre-eclampsia or abdominal distension due to theca lutein cysts. Furthermore, women can present with acute respiratory failure or neurological symptoms such as seizures. 4 These symptoms would more commonly be associated with early pre-eclampsia and eclampsia. However, the possibility of metastatic gestational trophoblastic disease (GTD) should also be borne in mind. The particularities of the case are the presenting symptoms.
A review of the literature revealed few cases of molar pregnancies with an atypical clinical picture. A case of complete hydatidiform mole in a 20-year-old II gesta I para woman was reported. 13 The patient underwent excision of a haemorrhagic left ovarian cyst. Histopathological examination revealed a haemorrhagic corpus luteum with a single microscopic focus of detached atypical trophoblast, without chorionic villi. She then had a left salpingo-oophorectomy for persistently elevated hCG, which led to the final diagnosis of complete hydatidiform mole arising in the ovary. Short tandem genetic analysis confirmed the diagnosis of complete hydatidiform mole. 13 Another case of partial molar pregnancy was a 23-year-old primigravida woman who presented with atypical pre-eclampsia (high blood pressure 160/100 mm Hg, proteinuria of 3.4 g in 24 hours, headache, photophobia and anasarca) at 16 weeks gestation. 14 Another primigravida woman presented to ED with abdominal pain and vaginal bleeding and passing of a large, grape-like vesicular mass with multiple negative urine pregnancy tests. These negative tests, which were likely caused by the ‘high-dose hook effect’, delayed the management of the condition until the development of pulmonary choriocarcinoma at the time of diagnosis. 15
Our patient presented with severe lower abdominal pains and pelvic bleeding with a positive pregnancy test, all in the context of an un-booked pregnancy. Severe abdominal pains are not specific to the classic picture of molar pregnancy. This could be caused by the distension of the fallopian tubes and peritoneal irritation. The above symptomatology can mimic a wide range of pathologies like miscarriage, threatened miscarriage, ectopic pregnancy and placental abruption (in second trimester). A good differential diagnosis is important. In this case, the bedside transabdominal ultrasound that was performed in the ED was very difficult due to patient’s acute symptoms and lack of cooperation but was enough to raise a suspicion of haemoperitoneum, once free fluid was see in the abdominal cavity.
Intraoperative findings did not show any large lutein cyst causing the abdominal distension. There were small corpus lutea in otherwise normal ovaries. Large bowels were slightly dilated but not massively to suggest megacolon caused by Clostridium perfringens , which is known to be a gas-producing bacteria. Hence, the rigid distended abdomen at presentation of this woman was most likely from C. perfringens septicaemia, and particularly the intraoperative effect on her body.
High values of LFTs can suggest metastasis in a case of complete molar pregnancy and the patient has to be thoroughly investigated in order to outrule such complications. The patient had a chest X-ray, abdominal ultrasound scan and abdomino-pelvis CT, and metastasis were outruled. In this patient’s case, raised LFTs can be a sign of organ failure in the context of posible septicaemia.
Following the laparoscopy and uterine evacuation procedure, the patient developed fever. Blood cultures results showed Gram-negative bacilli growth, which was C. perfringens . The infection could be due to bacterial proliferation in the necrotic trophoblastic tissue or bacteria that originated from the vaginal flora and ascended into the cervix and the uterus, establishing the infection. However, C. perfringens is not a common flora in the vagina. Infection with C. perfringens is very rare. One of the sources of infection with this organism is from illegal abortion, hence this point should be explored by clinicians dealing with such a case.
There was a case report where a hysterectomy had to be performed after septicaemia with C. perfringens following suction dilatation and curettage for molar pregnancy. 16 This patient was reported to recover well after the hysterectomy. Fortunately, in our case, the patient was treated early with intravenous antibiotics and responded well to the treatment.
Molar pregnancies can present in atypical form. The diagnosis of GTD in the first trimester requires high clinical suspicion. Early diagnosis and management of the condition is associated with decreased risk of morbidity and mortality.
Learning points
High index of suspicion is the key to diagnosing molar pregnancy, especially if it presents in atypical form.
Early recognition of the condition saves lives and decreases morbidity.
Molar pregnancy should be included in the differential diagnosis of vaginal bleeding and abdominal pain in the first trimester.
Acknowledgments
Authors would like to thank the patient for her cooperation.
- Hu L , et al
- ↵ Department of Health . Diagnosis, staging and treatment of patients with gestational trophoblastic disease. National Clinical Guideline . 13 , 2015 .
- ↵ Royal College of Obstetricians and Gynaecologists . The management of gestational trophoblastic disease . London : RCOG Guidelines , 2010 .
- Cotran RS ,
- Fausto N , et al
- Sebire NJ ,
- Foskett M ,
- Fisher RA , et al
- Fisher RA ,
- Foskett M , et al
- Fowler DJ ,
- Lindsay I ,
- Seckl MJ , et al
- Paradinas F , et al
- Soto-Wright V ,
- Bernstein M ,
- Goldstein DP , et al
- Berkowitz RS , et al
- Kuroki LM ,
- Hopeman MM , et al
- Barrón Rodríguez JL ,
- Piña Saucedo F ,
- Clorio Carmona J , et al
- Hunter CL ,
- Lekovic JP ,
Contributors RL: conception and design of study, acquisition of data. RL and AM: analysis and/or interpretation of data, drafting the manuscript. NA and EA: revising the manuscript critically for important intellectual content. All authors approved the final version of the manuscript to be published.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
Advertisement
Molar Pregnancy: Epidemiology, Diagnosis, Management, Surveillance
- Family Planning (A Roe and S Sonalkar, Section Editors)
- Published: 19 February 2022
- Volume 11 , pages 133–141, ( 2022 )
Cite this article

- Alice J. Darling ORCID: orcid.org/0000-0002-4708-9247 1 ,
- Benjamin B. Albright 2 ,
- Kyle C. Strickland 3 &
- Brittany A. Davidson 2
495 Accesses
Explore all metrics
Purpose of Review
This review describes recommendations for the diagnosis and management of molar pregnancy, with focus on emerging evidence in recent years, particularly as it pertains to nuances of diagnosis, risk stratification, and surveillance of post-molar malignant trophoblastic disease.
Recent Findings
Topics discussed include advances in histopathologic diagnosis of molar pregnancy to standardize analysis, most recent estimations of post-molar pregnancy malignancy, and updated surveillance guidelines.
Hydatidiform molar pregnancy, resulting from an abnormal fertilization event, is the proliferation of abnormal pregnancy tissue with malignant potential. With increased availability of first trimester ultrasound, early detection of molar pregnancy has increased. While challenging to diagnose radiologically and histologically at early stages, standardization of tissue analysis allows improved detection and increased accuracy of incidence estimate for both complete and partial molar pregnancy. Treatment of molar pregnancy requires evacuation of tissue. Prophylactic chemotherapy or repeat curettage have been explored but not favored. As new molecular markers are sought, our ability to predict malignant transformation following molar pregnancies will allow for more streamlined surveillance. Recent data support a reduction in the length of surveillance following normalization of human chorionic gonadotropin levels after evacuation.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Recurrent Molar Pregnancy
Clinicopathological Study of Gestational Trophoblastic Disease (GTD) in a Tertiary Care Hospital
Evaluation of a routine second curettage for hydatidiform mole: a cohort study
Papers of particular interest, published recently, have been highlighted as:, • of importance.
• Albright BB, Shorter JM, Mastroyannis SA, Ko EM, Schreiber CA, Sonalkar S. Gestational trophoblastic neoplasia after human chorionic gonadotropin normalization following molar pregnancy: a systematic review and meta-analysis. Obstet Gynecol. 2020;135(1):12–23. https://doi.org/10.1097/AOG.0000000000003566 . A systematic review and meta-analysis of post-molar gestational trophoblastic neoplasia incidence. This review found a very low (64/18,357, 0.35%, 95% CI 0.27-0.45%) cumulative incidence of GTN development after hCG normalization following a complete molar pregnancy. This rate was even lower for partial moles (5/14,864, 0.03%, 95% CI 0.01-0.08%) .
Article Google Scholar
Seckl MJ, Sebire NJ, Fisher RA, Golfier F, Massuger L, Sessa C. Gestational trophoblastic disease: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2013;24(6):vi39–50. https://doi.org/10.1093/annonc/mdt345 .
• Ngan HYS, Seckl MJ, Berkowitz RS, Xiang Y, Golfier F, Sekharan PK, et al. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynecol Obstet. 2018;143(S2):79–85. https://doi.org/10.1002/ijgo.12615 . FIGO Cancer Report of 2018 reviewing Gestational Trophoblastic Disease. Includes updated FIGO guidelines-most notably removing elevated hCG at ≥6 months after uterine evacuation from GTN diagnostic criteria and specifying hCG followup intervals including reduced surveillance length after partial molar pregnancy .
Soper JT. Gestational Trophoblastic Disease: Current Evaluation and Management. Obstet Gynecol. 2021;137(2):355–70. https://doi.org/10.1097/aog.0000000000004240 .
Brown J, Naumann RW, Seckl MJ, Schink J. 15 years of progress in gestational trophoblastic disease: scoring, standardization, and salvage. Gynecol Oncol. 2017;144(1):200–7. https://doi.org/10.1016/J.YGYNO.2016.08.330 .
Lurain JR. Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol. 2010;203(6):531–9. https://doi.org/10.1016/J.AJOG.2010.06.073 .
Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. The Lancet. 2010;376(9742):717–29. https://doi.org/10.1016/S0140-6736(10)60280-2 .
Berkowitz RS, Goldstein DP. Current management of gestational trophoblastic diseases. Gynecol Oncol. 2009;112(3):654–62. https://doi.org/10.1016/J.YGYNO.2008.09.005 .
Article CAS Google Scholar
Maisenbacher MK, Merrion K, Kutteh WH. Single-nucleotide polymorphism microarray detects molar pregnancies in 3% of miscarriages. Fertil Steril. 2019;112(4):700–6. https://doi.org/10.1016/j.fertnstert.2019.06.015 .
Cozette C, Scheffler F, Lombart M, Massardier J, Bolze PA, Hajri T, et al. Pregnancy after oocyte donation in a patient with NLRP7 gene mutations and recurrent molar hydatidiform pregnancies. J Assist Reprod Genet. 2020;37(9):2273–7. https://doi.org/10.1007/s10815-020-01861-z .
Eagles N, Sebire NJ, Short D, Savage PM, Seckl MJ, Fisher RA. Risk of recurrent molar pregnancies following complete and partial hydatidiform moles. Hum Reprod. 2015;30(9):2055–63. https://doi.org/10.1093/humrep/dev169 .
Gockley AA, Melamed A, Joseph NT, Clapp M, Sun SY, Goldstein DP, et al. The effect of adolescence and advanced maternal age on the incidence of complete and partial molar pregnancy. Gynecol Oncol. 2016;140(3):470–3. https://doi.org/10.1016/J.YGYNO.2016.01.005 .
Savage PM, Sita-Lumsden A, Dickson S, Iyer R, Everard J, Coleman R, et al. The relationship of maternal age to molar pregnancy incidence, risks for chemotherapy and subsequent pregnancy outcome. J Obstet Gynaecol. 2013;33(4):406–11. https://doi.org/10.3109/01443615.2013.771159 .
Sebire NJ, Foskett M, Fisher RA, Rees H, Seckl M, Newlands E. Risk of partial and complete hydatidiform molar pregnancy in relation to maternal age. BJOG Int J Obstet Gynaecol. 2002;109(1):99–102. https://doi.org/10.1111/j.1471-0528.2002.t01-1-01037.x .
Sato A, Usui H, Shozu M. ABO blood type compatibility is not a risk factor for gestational trophoblastic neoplasia development from androgenetic complete hydatidiform moles. Am J Reprod Immunol. 2020;83(6):e13237. https://doi.org/10.1111/aji.13237 .
Shamshiri Milani H, Abdollahi M, Torbati S, Asbaghi T, Azargashb E. Risk Factors for hydatidiform mole: is husband’s job a major risk factor?. Asian Pac J Cancer Prev. 2017;18(10):2657–62. https://doi.org/10.22034/apjcp.2017.18.10.2657 .
Berkowitz RS, Bernstein MR, Harlow BL, Rice LW, Lage JM, Goldstein DP, et al. Case-control study of risk factors for partial molar pregnancy. Am J Obstet Gynecol. 1995;173(3 Pt 1):788–94. https://doi.org/10.1016/0002-9378(95)90342-9 .
Melamed A, Gockley AA, Joseph NT, Sun SY, Clapp MA, Goldstein DP, et al. Effect of race/ethnicity on risk of complete and partial molar pregnancy after adjustment for age. Gynecol Oncol. 2016;143(1):73–6. https://doi.org/10.1016/j.ygyno.2016.07.117 .
Sundvall L, Lund H, Niemann I, Jensen UB, Bolund L, Sunde L. Tetraploidy in hydatidiform moles. Hum Reprod. 2013;28(7):2010–20. https://doi.org/10.1093/humrep/det132 .
Sebire NJ, Savage PM, Seckl MJ, Fisher RA. Histopathological features of biparental complete hydatidiform moles in women with NLRP7 mutations. Placenta. 2013;34(1):50–6. https://doi.org/10.1016/j.placenta.2012.11.005 .
Fisher RA, Hodges MD, Newlands ES. Familial recurrent hydatidiform mole: a review. J Reprod Med. 2004;49(8):595–601.
Google Scholar
King JR, Wilson ML, Hetey S, Kiraly P, Matsuo K, Castaneda AV et al. Dysregulation of placental functions and immune pathways in complete hydatidiform moles. Int J Mol Sci. 2019;20(20). https://doi.org/10.3390/ijms20204999 .
Fisher RA, Maher GJ. Genetics of gestational trophoblastic disease. Best Pract Res Clin Obstet Gynaecol. 2021;74:29–41. https://doi.org/10.1016/j.bpobgyn.2021.01.004 .
Soellner L, Begemann M, Degenhardt F, Geipel A, Eggermann T, Mangold E. Maternal heterozygous NLRP7 variant results in recurrent reproductive failure and imprinting disturbances in the offspring. Eur J Hum Genet. 2017;25(8):924–9. https://doi.org/10.1038/ejhg.2017.94 .
Sun SY, Melamed A, Joseph NT, Gockley AA, Goldstein DP, Bernstein MR, et al. Clinical presentation of complete hydatidiform mole and partial hydatidiform mole at a regional trophoblastic disease center in the United States over the past 2 decades. Int J Gynecol Cancer. 2016;26(2):367–70. https://doi.org/10.1097/IGC.0000000000000608 .
Sun SY, Melamed A, Goldstein DP, Bernstein MR, Horowitz NS, Moron AF, et al. Changing presentation of complete hydatidiform mole at the New England Trophoblastic Disease Center over the past three decades: does early diagnosis alter risk for gestational trophoblastic neoplasia?. Gynecol Oncol. 2015;138(1):46–9. https://doi.org/10.1016/J.YGYNO.2015.05.002 .
Winder AD, Mora AS, Berry E, Lurain JR. The “hook effect” causing a negative pregnancy test in a patient with an advanced molar pregnancy. Gynecol Oncol Rep. 2017;21:34–6. https://doi.org/10.1016/j.gore.2017.06.008 .
Li P, Koch CD, El-Khoury JM. Perimenopausal woman with elevated serum hCG and abdominal pain. Clin Chim Acta. 2021;522:141–3. https://doi.org/10.1016/j.cca.2021.08.018 .
Ross JA, Unipan A, Clarke J, Magee C, Johns J. Ultrasound diagnosis of molar pregnancy. Ultrasound. 2018;26(3):153–9. https://doi.org/10.1177/1742271x17748514 .
Savage JL, Maturen KE, Mowers EL, Pasque KB, Wasnik AP, Dalton VK, et al. Sonographic diagnosis of partial versus complete molar pregnancy: a reappraisal. J Clin Ultrasound. 2017;45(2):72–8. https://doi.org/10.1002/jcu.22410 .
Ronnett BM. Hydatidiform moles: ancillary techniques to refine diagnosis. Arch Pathol Lab Med. 2018;142(12):1485–502. https://doi.org/10.5858/arpa.2018-0226-RA .
Hui P, Buza N, Murphy KM, Ronnett BM. Hydatidiform moles: genetic basis and precision diagnosis. Annu Rev Pathol. 2017;12:449–85. https://doi.org/10.1146/annurev-pathol-052016-100237 .
Madi JM, Braga A, Paganella MP, Litvin IE, Wendland EM. Accuracy of p57(KIP)(2) compared with genotyping to diagnose complete hydatidiform mole: a systematic review and meta-analysis. BJOG. 2018;125(10):1226–33. https://doi.org/10.1111/1471-0528.15289 .
Zheng XZ, Qin XY, Chen SW, Wang P, Zhan Y, Zhong PP, et al. Heterozygous/dispermic complete mole confers a significantly higher risk for post-molar gestational trophoblastic disease. Mod Pathol. 2020;33(10):1979–88. https://doi.org/10.1038/s41379-020-0566-4 .
Lin LH, Maestá I, St Laurent JD, Hasselblatt KT, Horowitz NS, Goldstein DP, et al. Distinct microRNA profiles for complete hydatidiform moles at risk of malignant progression. Am J Obstet Gynecol. 2021;224(4):372.e1-e30. https://doi.org/10.1016/j.ajog.2020.09.048 .
Braga A, Maestá I, Rocha Soares R, Elias KM, Custódio Domingues MA, Barbisan LF, et al. Apoptotic index for prediction of postmolar gestational trophoblastic neoplasia. Am J Obstet Gynecol. 2016;215(3):336.e1-.e12. https://doi.org/10.1016/j.ajog.2016.04.010 .
Padrón L, Rezende Filho J, Amim Junior J, Sun SY, Charry RC, Maestá I, et al. Manual compared with electric vacuum aspiration for treatment of molar pregnancy. Obstet Gynecol. 2018;1-. https://doi.org/10.1097/AOG.0000000000002522 .
Curry SL, Hammond CB, Tyrey L, Creasman WT, Parker RT. Hydatidiform mole: diagnosis, management, and long-term followup of 347 patients. Obstet Gynecol. 1975;45(1):1–8.
CAS Google Scholar
• Zhao P, Lu Y, Huang W, Tong B, Lu W. Total hysterectomy versus uterine evacuation for preventing post-molar gestational trophoblastic neoplasia in patients who are at least 40 years old: a systematic review and meta-analysis. BMC Cancer. 2019;19(1):13. https://doi.org/10.1186/s12885-018-5168-x . A systematic review and meta-analysis which demonstrated a risk reduction in post-molar GTN of more than 80% in patients ≥40 years old following hysterectomy compared to those receiving uterine evacuations for molar pregnancy treatment .
Giorgione V, Bergamini A, Cioffi R, Pella F, Rabaiotti E, Petrone M, et al. Role of surgery in the management of hydatidiform mole in elderly patients: a single-center clinical experience. Int J Gynecol Cancer. 2017;27(3):550–3. https://doi.org/10.1097/igc.0000000000000903 .
Eysbouts YK, Massuger L, IntHout J, Lok CAR, Sweep F, Ottevanger PB. The added value of hysterectomy in the management of gestational trophoblastic neoplasia. Gynecol Oncol. 2017;145(3):536–42. https://doi.org/10.1016/j.ygyno.2017.03.018 .
Yamamoto E, Nishino K, Niimi K, Watanabe E, Oda Y, Ino K, et al. Evaluation of a routine second curettage for hydatidiform mole: a cohort study. Int J Clin Oncol. 2020;25(6):1178–86. https://doi.org/10.1007/s10147-020-01640-x .
Yamamoto E, Trinh TD, Sekiya Y, Tamakoshi K, Nguyen XP, Nishino K, et al. The management of hydatidiform mole using prophylactic chemotherapy and hysterectomy for high-risk patients decreased the incidence of gestational trophoblastic neoplasia in Vietnam: a retrospective observational study. Nagoya J Med Sci. 2020;82(2):183–91. https://doi.org/10.18999/nagjms.82.2.183 .
Wang Q, Fu J, Hu L, Fang F, Xie L, Chen H, et al. Prophylactic chemotherapy for hydatidiform mole to prevent gestational trophoblastic neoplasia. Cochrane Database of Syst Rev. 2017;9:CD007289-CD. https://doi.org/10.1002/14651858.CD007289.pub3 .
Jiao LZ, Wang YP, Jiang JY, Zhang WQ, Wang XY, Zhu CG, et al. Clinical significance of centralized surveillance of hydatidiform mole. Zhonghua Fu Chan Ke Za Zhi. 2018;53(6):390–5. https://doi.org/10.3760/cma.j.issn.0529-567x.2018.06.006 .
Braga A, Biscaro A, do Amaral Giordani JM, Viggiano M, Elias KM, Berkowitz RS, et al. Does a human chorionic gonadotropin level of over 20,000 IU/L four weeks after uterine evacuation for complete hydatidiform mole constitute an indication for chemotherapy for gestational trophoblastic neoplasia?. Eur J Obstet Gynecol Reprod Biol. 2018;223:50–5. https://doi.org/10.1016/j.ejogrb.2018.02.001 .
Ngu SF, Ngan HYS. Surgery including fertility-sparing treatment of GTD. Best Pract Res Clin Obstet Gynaecol. 2021;74:97–108. https://doi.org/10.1016/j.bpobgyn.2020.10.005 .
Zilberman Sharon N, Maymon R, Melcer Y, Jauniaux E. Obstetric outcomes of twin pregnancies presenting with a complete hydatidiform mole and coexistent normal fetus: a systematic review and meta-analysis. BJOG. 2020;127(12):1450–7. https://doi.org/10.1111/1471-0528.16283 .
Lin LH, Maestá I, Braga A, Sun SY, Fushida K, Francisco RPV, et al. Multiple pregnancies with complete mole and coexisting normal fetus in North and South America: A retrospective multicenter cohort and literature review. Gynecol Oncol. 2017;145(1):88–95. https://doi.org/10.1016/j.ygyno.2017.01.021 .
• Albright BB, Myers ER, Moss HA, Ko EM, Sonalkar S, Havrilesky LJ. Surveillance for gestational trophoblastic neoplasia following molar pregnancy: a cost-effectiveness analysis. Am J Obstet Gynecol. 2021. https://doi.org/10.1016/j.ajog.2021.05.031 . Cost-effectiveness analysis of post-molar GTN surveillance finding reduction or elimination of hCG surveillance would be cost effective and clinically reasonable given the rarity of malignant following hCG normalization. Additionally, found a single hCG test 3 months after uterine evacuation was a cost-effective alternative .
Massad LS, Abu-Rustum NR, Lee SS, Renta V. Poor compliance with postmolar surveillance and treatment protocols by indigent women. Obstet Gynecol. 2000;96(6):940–4. https://doi.org/10.1016/s0029-7844(00)01064-4 .
Blok LJ, Frijstein MM, Eysbouts YK, Custers J, Sweep F, Lok C, et al. The psychological impact of gestational trophoblastic disease: a prospective observational multicentre cohort study. BJOG. 2021. https://doi.org/10.1111/1471-0528.16849 .
Jewell EL, Aghajanian C, Montovano M, Lewin SN, Baser RE, Carter J. Association of ß-hCG surveillance with emotional, reproductive, and sexual health in women treated for gestational trophoblastic neoplasia. J Womens Health (Larchmt). 2018;27(3):387–93. https://doi.org/10.1089/jwh.2016.6208 .
Stafford L, McNally OM, Gibson P, Judd F. Long-term psychological morbidity, sexual functioning, and relationship outcomes in women with gestational trophoblastic disease. Int J Gynecol Cancer. 2011;21(7):1256–63. https://doi.org/10.1097/IGC.0b013e3182259c04 .
Coyle C, Short D, Jackson L, Sebire NJ, Kaur B, Harvey R, et al. What is the optimal duration of human chorionic gonadotrophin surveillance following evacuation of a molar pregnancy? A retrospective analysis on over 20,000 consecutive patients. Gynecol Oncol. 2018;148(2):254–7. https://doi.org/10.1016/j.ygyno.2017.12.008 .
Management of Gestational Trophoblastic Disease: Green-top Guideline No. 38 - June 2020. BJOG. 2021;128(3):e1-e27. https://doi.org/10.1111/1471-0528.16266 .
Horowitz NS, Eskander RN, Adelman MR, Burke W. Epidemiology, diagnosis, and treatment of gestational trophoblastic disease: a Society of Gynecologic Oncology evidenced-based review and recommendation. Gynecol Oncol. 2021;163(3):605–13. https://doi.org/10.1016/j.ygyno.2021.10.003 .
Lybol C, Sweep FC, Ottevanger PB, Massuger LF, Thomas CM. Linear regression of postevacuation serum human chorionic gonadotropin concentrations predicts postmolar gestational trophoblastic neoplasia. Int J Gynecol Cancer. 2013;23(6):1150–6. https://doi.org/10.1097/IGC.0b013e31829703ea .
Hardman S. Use of hormonal contraception after hydatidiform mole. BJOG Int J Obstet Gynaecol. 2016;123(8):1336. https://doi.org/10.1111/1471-0528.13691 .
Gaffield ME, Kapp N, Curtis KM. Combined oral contraceptive and intrauterine device use among women with gestational trophoblastic disease. Contraception. 2009;80(4):363–71. https://doi.org/10.1016/j.contraception.2009.03.022 .
Dantas PRS, Maestá I, Filho JR, Junior JA, Elias KM, Howoritz N, et al. Does hormonal contraception during molar pregnancy follow-up influence the risk and clinical aggressiveness of gestational trophoblastic neoplasia after controlling for risk factors? Gynecol Oncol. 2017;147(2):364–70. https://doi.org/10.1016/j.ygyno.2017.09.007 .
Braga A, Maestá I, Short D, Savage P, Harvey R, Seckl M. Hormonal contraceptive use before hCG remission does not increase the risk of gestational trophoblastic neoplasia following complete hydatidiform mole: a historical database review. BJOG Int J Obstet Gynaecol. 2016;123(8):1330–5. https://doi.org/10.1111/1471-0528.13617 .
Morbidity and Mortality Weekly Report: Classifications for Intrauterine Devices. Centers for Disease Control and Prevention. 2010. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr59e0528a6.htm . Accessed 25 Oct 2021.
Tuncer ZS, Bernstein MR, Goldstein DP, Lu KH, Berkowitz RS. Outcome of pregnancies occurring within 1 year of hydatidiform mole. Obstet Gynecol. 1999;94(4):588–90. https://doi.org/10.1016/S0029-7844(99)00395-6 .
Joneborg U, Coopmans L, van Trommel N, Seckl M, Lok CAR. Fertility and pregnancy outcome in gestational trophoblastic disease. Int J Gynecol Cancer. 2021;31(3):399–411. https://doi.org/10.1136/ijgc-2020-001784 .
Vargas R, Barroilhet LM, Esselen K, Diver E, Bernstein M, Goldstein DP, et al. Subsequent pregnancy outcomes after complete and partial molar pregnancy, recurrent molar pregnancy, and gestational trophoblastic neoplasia: an update from the New England Trophoblastic Disease Center. J Reprod Med. 2014;59(5–6):188–94.
Matsui H, Iitsuka Y, Suzuka K, Seki K, Sekiya S. Subsequent pregnancy outcome in patients with spontaneous resolution of HCG after evacuation of hydatidiform mole: comparison between complete and partial mole. Hum Reprod. 2001;16(6):1274–7. https://doi.org/10.1093/humrep/16.6.1274 .
Abu-Rustum NR, Yashar CM, Bean S, Bradley K, Campos SM, Chon HS, et al. Gestational Trophoblastic Neoplasia, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(11):1374–91. https://doi.org/10.6004/jnccn.2019.0053 .
Download references
Author information
Authors and affiliations.
Department of Obstetrics & Gynecology, Duke University, Durham, NC, USA
Alice J. Darling
Department of Obstetrics & Gynecology, Division of Gynecologic Oncology, Duke University, Durham, NC, USA
Benjamin B. Albright & Brittany A. Davidson
Department of Pathology, Duke University, Durham, NC, USA
Kyle C. Strickland
You can also search for this author in PubMed Google Scholar
Contributions
AD: Project design, literature review, manuscript draft, critical revision, final approval. BA: Project conception and design, literature review, critical revision, final approval. KS: Collecting and preparing specimens for manuscript figure, critical revision, final approval. BD: Project conception and design, literature review, critical revision, final approval.
Ethics declarations
Ethics approval.
Not applicable, this article does not contain any studies with human or animal subjects performed by any of the authors.
Consent to Participate
Not applicable.
Consent for Publication
Conflict of interest.
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Family Planning
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Darling, A.J., Albright, B.B., Strickland, K.C. et al. Molar Pregnancy: Epidemiology, Diagnosis, Management, Surveillance. Curr Obstet Gynecol Rep 11 , 133–141 (2022). https://doi.org/10.1007/s13669-022-00327-6
Download citation
Accepted : 09 February 2022
Published : 19 February 2022
Issue Date : June 2022
DOI : https://doi.org/10.1007/s13669-022-00327-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Molar pregnancy
- Hydatidiform mole
- Complete mole
- Partial mole
- Gestational Trophoblastic Disease
- Surveillance
- Find a journal
- Publish with us
- Track your research
Atypical presentation of molar pregnancy
Affiliation.
- 1 Department of Obstetrics and Gynaecology, Our Lady of Lourdes Hospital, Drogheda, Ireland.
- PMID: 30262528
- PMCID: PMC6169626
- DOI: 10.1136/bcr-2018-225545
The classic features of molar pregnancy are irregular vaginal bleeding, hyperemesis, enlarged uterus for gestational age and early failed pregnancy. Less common presentations include hyperthyroidism, early onset pre-eclampsia or abdominal distension due to theca lutein cysts. Here, we present a case of molar pregnancy where a woman presented to the emergency department with symptoms of acute abdomen and was treated as ruptured ectopic pregnancy. The woman underwent laparoscopy and evacuation of retained products of conception. Histological examination of uterine curettage confirmed the diagnosis of a complete hydatidiform mole. The woman was discharged home in good general condition with a plan for serial beta-human chorionic gonadotropin (beta-hCG) follow-up. Complete follow-up includes use of contraception and follow-up after beta-hCG is negative for a year.
Keywords: obstetrics and gynaecology; pregnancy; reproductive medicine.
© BMJ Publishing Group Limited 2018. No commercial re-use. See rights and permissions. Published by BMJ.
Publication types
- Case Reports
- Abdominal Pain / etiology
- Blood Transfusion
- Cholangiopancreatography, Endoscopic Retrograde
- Chorionic Gonadotropin, beta Subunit, Human / blood
- Hydatidiform Mole / complications
- Hydatidiform Mole / diagnosis*
- Hydatidiform Mole / therapy
- Laparoscopy
- Ultrasonography
- Uterine Hemorrhage / etiology
- Uterine Neoplasms / complications
- Uterine Neoplasms / diagnosis*
- Uterine Neoplasms / therapy
- Chorionic Gonadotropin, beta Subunit, Human
- Open access
- Published: 03 September 2022
Molar pregnancy with a coexisting living fetus: a case series
- Reda Hemida ORCID: orcid.org/0000-0003-0841-0242 1 ,
- Eman Khashaba 2 &
- Khaled Zalata 3
BMC Pregnancy and Childbirth volume 22 , Article number: 681 ( 2022 ) Cite this article
4997 Accesses
5 Citations
1 Altmetric
Metrics details
Coexistence of molar pregnancy with living fetus represents a challenge in diagnosis and treatment. The objective of this study to present the outcome of molar pregnancy with a coexisting living fetus who were managed in our University Hospital in the last 5 years.
We performed a retrospective analysis of patients who presented with molar pregnancy with a coexisting living fetus to our Gestational Trophoblastic Clinic, Mansoura University, Egypt from September, 2015 to August, 2020. Clinical characteristics of the patients, maternal complications as well as fetal outcome were recorded. The patients and their living babies were also followed up at least 6 months after delivery.
Twelve pregnancies were analyzed. The mean maternal age was 26.0 (SD 4.1) years and the median parity was 1.0 (range 0–3). Duration of the pregnancies ranged from 14 to 36 weeks. The median serum hCG was 165,210.0 U/L (range 7662–1,200,000). Three fetuses survived outside the uterus (25%), one of them died after 5 months because of congenital malformations. Histologic diagnosis was available for 10 of 12 cases and revealed complete mole associated with a normal placenta in 6 cases (60%) and partial mole in 4 cases (40%). Maternal complications occurred in 6 cases (50%) with the most common was severe vaginal bleeding in 4 cases (33.3%). There was no significant association between B-hCG levels and maternal complications ( P = 0.3).
Maternal and fetal outcomes of molar pregnancy with a living fetus are poor. Counseling the patients for termination of pregnancy may be required.
Trial registration
The study was approved by Institutional Research Board (IRB), Faculty of Medicine, Mansoura University (number: R.21.10.1492).
Peer Review reports
Introduction
Hydatidiform mole is a rare complication of early pregnancy characterized by disordered proliferation of trophoblastic epithelium and villous edema. It includes complete (CHM) and partial (PHM) hydatidiform moles [ 1 , 2 ]. Partial hydatidiform mole arises as a result of dispermic fertilization of a haploid oocyte, which produces a triploid set of chromosomes and is commonly associated with congenital fetal malformations [ 3 ]. Hydatidiform moles are usually presented with first trimester vaginal bleeding, passage of vesicles, abdominal pain, excessive nausea and vomiting, and rapid abdominal enlargement [ 1 ]. Hyperthyroidism and preeclampsia may be present in some cases of complete hydatidiform moles [ 4 , 5 ]. Human chorionic gonadotropin (hCG) level is elevated but the level in CHM is higher than PHM [ 6 ].
Although complete hydatidiform moles can be easily diagnosed using routine ultrasound assessments early in the first trimester by appearance of snow-storm appearance of the placenta; PHM may mimic missed or incomplete abortion [ 7 ].
During management of molar pregnancy with a coexisting living fetus; the gynecologist should remind that there are three different types. The most common is twin pregnancy with one normal fetus with a normal placenta and a CHM; the second type is twin pregnancy with a normal fetus and placenta and a PHM; and the third, and most uncommon, is a singleton pregnancy consisting of a normal fetus and a placenta with PM changes [ 8 ]. The latter type was reported to occur in 0.005 to 0.01% of all pregnancies [ 9 ]. It is sometimes called “Sad Fetus Syndrome” [ 7 ]. Pregnancy with a PHM and a normal fetus evolves to a viable fetus in less than 25% of cases [ 8 ]. Such pregnancy has little tendency to invade the myometrium and distant metastasis [ 6 ].
Coexistence of molar changes with an apparently healthy fetus is unusual in a case of familial recurrent hydatidiform mole (FRHM). It should be differentiated from mesenchymal dysplasia by morphologic features and immunohistochemistry [ 10 ].
To the best of our knowledge, there are no available international guidelines for management of molar pregnancy with a living fetus. The available publications are mostly case reports, so the authors prepared this manuscript to present the experience of our University GTD referral clinic in the management and outcome of these rare cases.

Patients and methods
In this case series; a retrospective analysis of the patients presented with molar pregnancy with a coexisting living fetus to Gestational Trophoblastic Clinic, Mansoura University, Egypt in 5 years (from September, 2015 to August, 2020). The data of the patients were extracted from the computer and paper files. We included all cases above 18 years with diagnosed molar pregnancy with a living fetus based on clinical, ultrasound, and serum human chorionic gonadotropin (hCG) criteria. The patients who refused to give initial permission to use their data in future research where excluded from the study.
Clinical characteristics including age, parity, obstetric history, gestational age, presenting symptoms, serum hCG on initial diagnosis, and family history, were all recorded. Mode of termination of pregnancy (miscarriage, induction of abortion, hysterotomy, vaginal, or caesarean delivery) was also reported.
Maternal complications during pregnancy, labor, and puerperium were described. Fetal outcomes (miscarriage, congenital fetal malformations, prematurity, or normal) were reported. The patients and living babies were followed up at least for 6 months after delivery .
The study was approved by Institutional Research Board (IRB), Faculty of Medicine, Mansoura University (number: R.21.10.1492). The excel data and figures are anonymous.
Statistical analysis
Data entry and analysis was done using SPSS program, version 23.0 (IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp.) was used to analyze the findings. The qualitative data were described in number and percentage. The quantitative data with normal distribution were described in mean and the standard deviation ( \(\pm\) SD). Discrete variables were summarized in median and range. Contingency coefficient Chi square was used to compare nominal variables. The statistical significance was considered when P value was less than 0.05.
From September 2015 to August, 2020; twelve cases of molar pregnancy with living fetus were managed in our hospital. The mean maternal age was 26.0 ( \(\pm\) SD 4.1) years while median parity was 1.0 (range 0–3). Duration of pregnancy ranged from 14 to 36 weeks. The median serum hCG at time of diagnosis was 165,210.0 U/L (range 7662–1,200,000). Ultrasound reports showed well-defined multicystic snowstorm-like mass connecting with placenta (Fig. 1 ). Amniocentesis was performed in one case and revealed a normal diploid female karyotype. During antenatal follow up, the patients who had no complications and requested to undergo conservative treatment were given two injections of 12 mg of betamethasone 24 h apart from 28 weeks of gestation to prevent respiratory distress syndrome.
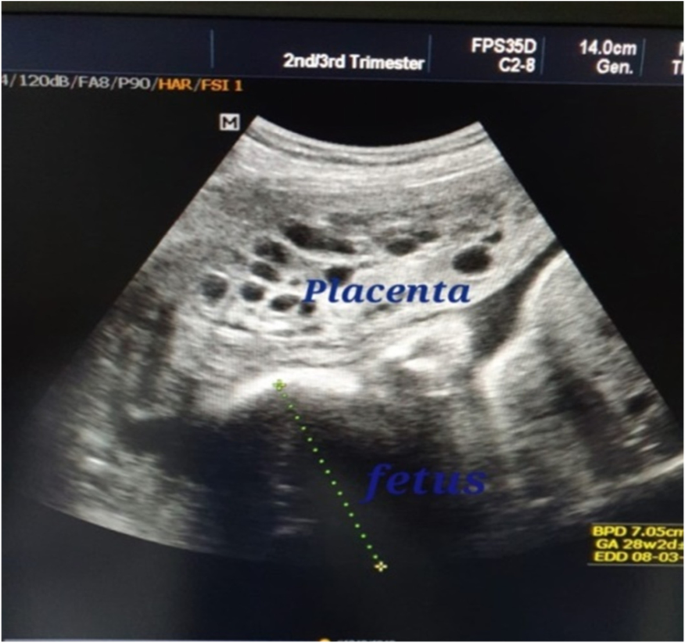
Ultrasound picture of pregnancy of the case (Z) at 28 weeks showing normal fetus with multiple variable-sized vesicles that cannot be separated from another placenta. The fetus is looking morphologically normal
The fetal outcomes are shown in Table 1 ; as can be noticed that fetuses survived outside the uterus in three cases (25%). The first two cases were delivered by caesarean delivery at 33 and 36 weeks of gestation after development of persistent abdominal pain and dyspnea with marked abdominal enlargement. Polyhydramnios was excluded by ultrasound examination. The third case delivered vaginally at 36 weeks of a neonate with multiple congenital anomalies namely hydrocephalus and macroglossia who died after 5 months. Seven cases continued their pregnancy beyond 20 weeks; five of them delivered prematurely (71.4%). only one case of them survived after neonatal care admission.
For the cases who were subjected to cesarean delivery or hysterectomy; the presence of multiple “grape” vesicles on the maternal surface of the placenta was observed.
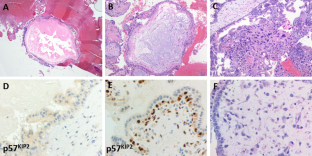
Histologic diagnosis was available for 10 of 12 cases and revealed complete mole associated with a normal placenta in 6 cases (60%) and partial mole in 4 cases (40%)( Figs. 2 and 3 ). Immunohistochemistry for P57 gene was performed on two cases (Figs. 2 and 3 ). The first delivered a phenotypically normal alive female baby and its placenta was misdiagnosed as PHM by morphological evaluation but the cytotrophoblast was negative for p57 immunestaining. The second case was diagnosed as dichorionic twins early in pregnancy that was terminated at 14 weeks of gestation because of severe vaginal bleeding. The fetus was phenotypically normal, placenta was histologically normal, and its cytotrophoblast was positive for p57. In addition, there was large amount of molar tissues that was negative for p57 immunostaining demonstrating a diagnosis of a CHM. These data suggest that this conception consists of a dichorionic twins with a living fetus with normal placenta and a CHM (Figs. 2 and 3 ).
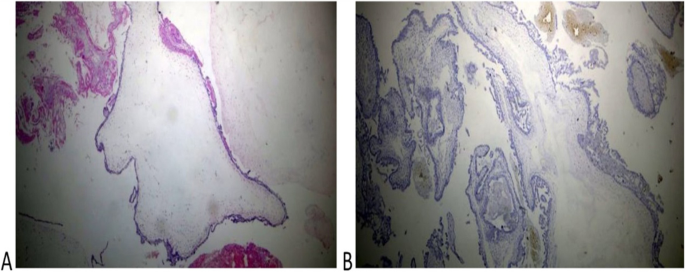
Histopathological examination of the coexistent molar tissues of the case (Z): A Complete hydatidiform mole. The picture shows a dilated trophoblastic villous with cistern formation and trophoblastic epithelium hyperplasia (H&E × 100). B Complete hydatidiform mole. The picture shows a negative reaction to p57 IHC (Peroxidase × 100)
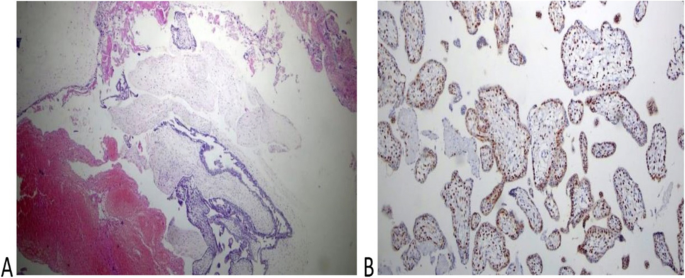
Histopathology of the placenta of the second twin of the case (H): A The picture shows normal trophoblastic villi (H&EX100). B P57 immunestaining of the same case shows a diffuse positive reaction in both trophoblastic and stromal cells (Peroxidase × 100)
Maternal complications occurred in 6 cases (50%) with the most common was severe uterine bleeding that was observed in 4 cases (33.3%). Other maternal complications are listed in Table 1 .
Moreover, three of our patients had familial recurrent hydatidiform mole (FRHM).Two of them are sisters. Genetic study through DNA sequencing confirmed NLRP7 mutations that were previously reported [ 11 ]. One of them experienced molar pregnancy with living fetus 3 times when she was aged 25, 27, and 28 years old.
We did not find a significant association between B-hCG level (when considered less than 500,000 and equal or more than 500,000 Unit/liter) and occurrence of maternal complications ( P = 0.3).
Coexistent molar pregnancy with a living fetus represents a diagnostic and management challenge particularly when the couple is interested to continue pregnancy. In a literature review published by Kawasaki et al. [ 8 ]; eighteen cases of molar pregnancies a coexisting living fetus were reported. The mean gestational age at delivery was 24.5 weeks, and only four fetuses could survive outside the uterus (22.2%) indicating a poor fetal outcome. On karyotyping; placenta was diploid in ten cases, indicating that they may be a CHM in a twin pregnancy or associated placental mesenchymal dysplasia that was also reported by Hojberg et al. [ 12 ].
The patients with molar pregnancy with coexistent living fetus who were managed in our university hospital in the last 5 years were presented in this report. Among the 12 reviewed pregnancies; three fetuses survived outside the uterus (25%). However, one of them died after 5 months because of congenital malformations that were reported by other authors [ 3 ]. The overall fetal survival in our series is less than reported in the literature review [ 8 ]. Moreover, Giorgione et al. [ 13 ] reported that overall neonatal survival in their series was 45% (5 of 11); the difference may be related to different patient criteria and neonatal care facilities in different hospitals. Among seven pregnancies continued beyond 20 weeks; five ended in premature deliveries (71.4%), which is much higher than the reported global incidence of prematurity allover pregnancies of 11% [ 14 ].
Amniocentesis is recommended for cases undergoing conservative treatment to exclude chromosomal abnormalities [ 15 ], however, it was performed only in one case in our series. Nine ladies refused the procedure for fear of complications while early pregnancy termination before time of amniocentesis was performed for two patients. In other series [ 13 ], prenatal invasive procedures were performed in 8 of 13 cases (62%). The acceptability of the pregnant ladies to perform prenatal invasive procedures differs from a community to another.
We reported occurrence of maternal complications in 50% of the studied cases; the commonest was severe vaginal bleeding. Although Sánchez-Ferrer et al. [ 15 ] concluded that termination of pregnancy is not indicated if the fetus is normal and continuation to birth is possible in nearly 60% of cases with no increase in maternal risks when the patient is closely monitored after birth until B-hCG is negative. The difference may be due to different number of cases in each study.
Moreover, two of the managed cases (16.7%) were complicated with early-onset preeclampsia and subsequently the pregnancy was terminated at 22 and 14 weeks of gestation. This finding was also reported by Kawasaki et al. [ 8 ]. In our series, we observed one case of complete mole that progressed to GTN (8.3%) and was successfully treated with single-agent chemotherapy, which is similar to a previous case scenario reported by Peng et al. [ 16 ].
The diagnostic challenge of a case of molar pregnancy with a coexisting living fetus is to differentiate two different conditions; singleton conception with a partial mole and dizygotic twins consisting of normal fetus with a complete mole. If the ultrasound picture of a normal fetus of an appropriate size for its gestational age together with an abnormal cystic placenta, a twin pregnancy consisting of a normal fetus and a CHM should be suspected [ 17 ]. Shaaban et al. [ 18 ] suggested that the peculiar “twin peak” sign in ultrasound, in which chorionic tissues extend into the inter-twin membrane, forming a triangular echogenic structure that intervenes the normal twin sac and the molar pregnancy, confirming the presence of a dichorionic twin gestation.
P57 immunestaining was performed in 2 cases of molar pregnancy with apparently normal fetus (Figs. 2 and 3 ) that confirmed these cases had a dichorionic twin pregnancy consisting of a complete mole with a co-twin of normal fetus and placenta. However, these cases may have been misdiagnosed as partial mole especially when the first ultrasound was done late in pregnancy. Other authors also reported cases of term deliveries of a complete hydatidiform mole with a coexisting living fetus [ 16 , 19 , 20 ].
We did not find a significant association between B-hCG level (when considered less than 500,000 and equal or more than 500,000 Unit/liter) and occurrence of maternal complications ( P = 0.3). This finding was in agree with Chale-Matsau et al. [ 21 ] who concluded that the β-hCG levels do not always correlate with disease severity and prognosis in patients with GTD.
With respect to the reported maternal and fetal complications in our study and other reports, it is necessary to fully inform the pregnant woman of the possible maternal and fetal complications, such as preeclampsia, hyperthyroidism, vaginal bleeding, and theca lutein ovarian cysts. The probability of postpartum development into persistent trophoblastic disease is also high.
The limitations of this study are its retrospective design, limited number of cases, and availability immunohistochemical study of only two cases.
Maternal and fetal outcome of molar pregnancy with a living fetus is poor. The incidence of prematurity is high (71.4%). Counseling of the patients for termination of pregnancy may be need. A global guideline for management is required.
Availability of data and materials
Original data and materials are available on request after contacting the corresponding author.
Abbreviations
Gestational trophoblastic neoplasia
Complete hydatidiform mole
Partial hydatidiform mole
B subunit of human chorionic gonadotropin
Standard deviation
Lurain JR. Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol. 2010;203(6):531–9.
Article Google Scholar
Seckl M, Sebire N, Fisher R, Golfier F, Massuger L, Sessa C, et al. Gestational trophoblastic disease: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl_6):vi39–50.
Stevens FT, Katzorke N, Tempfer C, Kreimer U, Bizjak GI, Fleisch MC, Fehm TN. Gestational Trophoblastic Disorders: An Update in 2015. Geburtshilfe Frauenheilkd. 2015;75(10):1043–50.
Article CAS Google Scholar
Walkington L, Webster J, Hancock BW, Everard J, Coleman RE. Hyperthyroidism and human chorionic gonadotrophin production in gestational trophoblastic disease. Br J Cancer. 2011;104(11):1665–9.
Iriyama T, Wang G, Yoshikawa M, et al. Increased LIGHT leading to sFlt-1 elevation underlies the pathogenic link between hydatidiform mole and preeclampsia. Sci Rep. 2019;9(1):10107.
Soper JT. Gestational Trophoblastic Disease: Current Evaluation and Management. Obstet Gynecol. 2021;137(2):355–70.
Rathod AD, Pajai SP, Gaddikeri A. Partial mole with a coexistent viable fetus—a clinical dilemma: a case report with review of literature. J South Asian Feder Obstet Gynaecol. 2014;6:51–5.
Kawasaki K, Kondoh E, Minamiguchi S, Matsuda F, Higasa K, Fujita K, Mogami H, Chigusa Y, Konishi I. Live-born diploid fetus complicated with partial molar pregnancy presenting with pre-eclampsia, maternal anemia, and seemingly huge placenta: A rare case of confined placental mosaicism and literature review. J Obstet Gynaecol Res. 2016;42(8):911–7.
Smith HO, Kohorn E, Cole LA. Choriocarcinoma and gestational trophoblastic disease. Obstet Gynecol Clin North Am. 2005;32(4):661–84.
Sebire NJ, Fisher RA. Partly molar pregnancies that are not partial moles: additional possibilities and implications. Pediatr Dev Pathol. 2005;8(6):732–3.
Rezaei M, Suresh B, Bereke E, Hadipour Z, Aguinaga M, Qian JH, Bagga R, Fardaei M, Hemida R, Jagadeesh S, Majewski J, Slim R. Novel pathogenic variants in NLRP7, NLRP5 and PADI6 in patients with recurrent hydatidiform moles and reproductive failure. Clin Genet. 2021;99(6):823–8.
Højberg KE, Aagaard J, Henriques U, Sunde L. Placental vascular malformation with mesenchymal hyperplasia and a localized chorioangioma. A rarity simulating partial mole. Pathol Res Pract. 1994;190(8):808–13. https://doi.org/10.1016/S0344-0338(11)80429-2 (discussion 814).
Article PubMed Google Scholar
Giorgione V, Cavoretto P, Cormio G, Valsecchi L, Vimercati A, De Gennaro A, Rabaiotti E, Candiani M, Mangili G. Prenatal Diagnosis of Twin Pregnancies with Complete Hydatidiform Mole and Coexistent Normal Fetus: A Series of 13 Cases. Gynecol Obstet Invest. 2017;82(4):404–9.
Walani SR. Global burden of preterm birth. Int J Gynaecol Obstet. 2020;150(1):31–3.
Sánchez-Ferrer ML, Ferri B, Almansa MT, Carbonel P, López-Expósito I, Minguela A, Abad L, Parrilla JJ. Partial mole with a diploid fetus: case study and literature review. Fetal Diagn Ther. 2009;25(3):354–8.
Peng HH, Huang KG, Chueh HY, Adlan AS, Chang SD, Lee CL. Term delivery of a complete hydatidiform mole with a coexisting living fetus followed by successful treatment of maternal metastatic gestational trophoblastic disease. Taiwan J Obstet Gynecol. 2014;53(3):397–400.
Kutuk MS, Ozgun MT, Dolanbay M, Batukan C, Uludag S, Basbug M. Sonographic findings and perinatal outcome of multiple pregnancies associating a complete hydatiform mole and a live fetus: a case series. J Clin Ultrasound. 2014;42(8):465–71.
Shaaban AM, Rezvani M, Haroun RR, et al. Gestational Trophoblastic Disease: Clinical and Imaging Features. Radiographics. 2017;37(2):681–700.
Sasaki Y, Ogawa K, Takahashi J, Okai T. Complete hydatidiform mole coexisting with a normal fetus delivered at 33 weeks of gestation and involving maternal lung metastasis: a case report. J Reprod Med. 2012;57(7–8):301–4 (PMID: 22838245).
PubMed Google Scholar
Malhotra N, Deka D, Takkar D, Kochar S, Goel S, Sharma MC. Hydatidiform mole with coexisting live foetus in dichorionic twin gestation. Eur J Obstet Gynecol Reprod Biol. 2001;94:301–3.
Chale-Matsau B, Mokoena S, Kemp T, Pillay TS. Hyperthyroidism in molar pregnancy: β-HCG levels do not always reflect severity. Clin Chim Acta. 2020;511:24–7.
Download references
Acknowledgements
Not applicable.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding was received.
Author information
Authors and affiliations.
Gynecologic Oncology Unit, Department of Obstetrics and Gynecology, Mansoura University, 35111 Elgomhuria street, Mansoura, Egypt
Reda Hemida
Department of Community Medicine, Faculty of Medicine, Mansoura University, Mansoura, Egypt
Eman Khashaba
Department of Pathology, Faculty of Medicine, Mansoura University, Mansoura, Egypt
Khaled Zalata
You can also search for this author in PubMed Google Scholar
Contributions
R.H: Conception of Idea, collection of data, and editing manuscript. E.K: Statistical analysis and editing manuscript. K.Z: Pathology revision, preparation of figures, and revision of manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Reda Hemida .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by Institutional Research Board (IRB), Faculty of Medicine, Mansoura University (number: R.21.10.1492). An informed consent for participation in the study was obtained from all participants.
All experiments were performed in accordance with relevant guidelines and regulations.
Consent for publication
An informed consent for publication in an online open-access journal was obtained from the study participants.
Competing interests
No competing interests that are directly or indirectly related to the work submitted for publication.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hemida, R., Khashaba, E. & Zalata, K. Molar pregnancy with a coexisting living fetus: a case series. BMC Pregnancy Childbirth 22 , 681 (2022). https://doi.org/10.1186/s12884-022-05004-3
Download citation
Received : 14 February 2022
Accepted : 26 August 2022
Published : 03 September 2022
DOI : https://doi.org/10.1186/s12884-022-05004-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Molar pregnancy
- Living fetus
BMC Pregnancy and Childbirth
ISSN: 1471-2393
- Submission enquiries: [email protected]
- General enquiries: [email protected]

Complete hydatidiform molar pregnancy
Citation, doi, disclosures and case data.
At the time the case was submitted for publication Dennis Odhiambo Agolah had no recorded disclosures.
Presentation
Primigravida with eight weeks amenorrhea. PDT positive with one week per vaginal bleeding. To rule out H. Mole
Patient Data
A heterogeneous, moderate content that is diffusely interspersed with multifocal anechoic areas (cystic vesicles) within the proximal endometrial cavity resulting in the usual honey-comb appearance is noted.
The endometrial/myometrial interface is relatively obliterated however, the content is hypovascular on color flow Doppler interrogation and no obvious solid fetal parts or bony areas are seen. The cervix and proximal vaginal vault grossly look normal. The left ovary showed two small-sized follicles within its parenchyma which are most likely theca lutein.
3 case questions available
Q: Does a honey-comb, bunch of grapes, or snow-storm appearance a sonographic feature of hydatidiform molar pregnancy? show answer
Q: Does H. Mole show abnormally elevated B-HCG serum titer levels at the laboratory evaluation? show answer
Q: What other possible differential diagnosis is likely in a case as this? show answer
A: Retained non-viable products of conception.
Case Discussion
In the setting of a positive pregnancy test and amenorrhea as such, the sonographic features above strongly hints towards a complete hydatidiform molar pregnancy . Laboratory beta HCG serum levels correlation yielded elevated values at 7873.06 mIU/mL.
- Carol M Rumack, Stephanie R Wilson, MD, Deborah Levine et al. Diagnostic Ultrasound. (2014) ISBN: 9780323374903 - Google Books
- Hai Dettmering. Molar Pregnancy. (2021) ISBN: 9798516666971 - Google Books
8 public playlists include this case
- Usg by Saravana Kumar
- A Obgyn viva Waseem by Waseem Mehmood Nizamani
- OB by Jennifer Powell
- PAH RQ 10 by Rachele Quested
- Gynaecology cases by Aimua Harriet
- Efilm long cases by Aimua Harriet
- IMM TOACS by Aman Raj Kumar
- gyne-new by Jyoti Panwar
Related Radiopaedia articles
- Complete hydatidiform mole
- Molar pregnancy
- Theca lutein cyst
Promoted articles (advertising)
How to use cases.
You can use Radiopaedia cases in a variety of ways to help you learn and teach.
- Add cases to playlists
- Share cases with the diagnosis hidden
- Use images in presentations
- Use them in multiple choice question
Creating your own cases is easy.
- Case creation learning pathway
ADVERTISEMENT: Supporters see fewer/no ads
By Section:
- Artificial Intelligence
- Classifications
- Imaging Technology
- Interventional Radiology
- Radiography
- Central Nervous System
- Gastrointestinal
- Gynaecology
- Haematology
- Head & Neck
- Hepatobiliary
- Interventional
- Musculoskeletal
- Paediatrics
- Not Applicable
Radiopaedia.org
- Feature Sponsor
- Expert advisers

Follow us on :

Take a look at the Recent articles
Molar pregnancy-case report, marina yuabova.
La Guardia Community College, City University of New York, NY, USA
E-mail : aa
DOI: 10.15761/CCRR.1000430
Molar pregnancy is formed as a result of divergent fertilization process that leads to production of atypical tissue within the uterus. It categorized in two groups: partial and complete.
Complete mole involves absence of the embryo, where partial mole demonstrates presence of fetal parts. Molar pregnancy does not result in viable fetus, early detection and treatment is essential for positive outcome.
Patient has presented with typical signs and symptoms for molar pregnancy including vaginal spotting with dark brown grape like substance, law hemoglobin, fundal height greater then expected (as for gestational age), nausea and vomiting.
Writer will present case of molar pregnancy, risk factors, typical presentation and treatment modality.
moles, hydatidiform moles, molar pregnancy
Introduction
Hydatidiform mole is a product of anomalous conceptions, with prevalence about 1 in 500-1000 pregnancies [6]. All cases could be categorized in to two groups, complete or partial hydatidiform mole. In complete molar pregnancy diffuse swelling of chorionic villi and disseminated thromboplastic hyperplasia without embryo or fetal tissues is characteristic. These cases commonly have a diploid karyotype [4]. In opposite, partial molar pregnancy displays central swelling of the chorionic villi and thromboplastic hyperplasia. Some parts of fetal and embryonic tissues are commonly present [7]. All molar pregnancies included defective ovum flawed maternal chromosomal deoxyribonucleic acid, suggesting only of hydatidiform mole has been identified, one of which is advanced maternal age and history of previous molar pregnancies [7]. Despite the fact there are some evidence accessible in regards to definitive risk of consecutive hydatidiform moles after previous partial or complete moles [3,5]. In cases where fetuses with partial moles are pinpointed, they consistently bear association with congenital anomalies, such as cleft lip and syndactyly [5].
Case Report
A 19-year-old female patient reported to OB-GYN clinic for annual examination during the initial interview she complained of very unusual menstrual bleeding, which began 3 days ago, patient has also reported abdominal cramping, nausea, vomiting and lower back pain. Patient admitted to be sexually active but was using calendar method as birth control. Her last menstrual period was exactly 5 weeks ago. Patients past medical and family history are unremarkable. She denied to have any allergies, denied drinking alcoholic beverages but admitted to be an active smoker; she currently smokes 0.5 packs of cigarettes per day. The patient was alert, oriented and in obvious distress. Her temperature was 98.6 F, blood pressure 119/64 mmHg, heart rate 123 bpm, respiratory rate of 16 breaths per min with a pulse oximetry of 98% on room air. On physical exam her skin was cool and clammy and patient's breathing was mildly labored, with thread peripheral pulses. Her fundal height was 2 cm below umbilicus. Abdomen was soft and mildly tender on lower quadrants bilaterally. Her lower extremities were WNL. During Pelvic examination reported loose discharge of blood, clots and a large amount of brown-colored grapelike material. The cervical OS was dilated to approximately 2cm with some cervical motion tenderness. After patient has been transferred to ER blood was collected and sent to lab for analysis, laboratory results as follows: hemoglobin of 8.6 g/dL, hematocrit of 7.5%, white blood count at 16,000 with 78% neutrophils and 5% bands, platelets at 123,000, international normalized ratio of 1.5, and bicarbonate of 14 mmol/L. Bun was elevated at 38 mg/dL and creatinine was 0.7 mg/dL. Beta HCG was 360,514 mIU/mL. IV line was initiated; patient has received 1000 cc bolus of 0.9% of sodium chloride, which followed with infusion rate at 125 cc per hour. Case has been discussed with OB consult and Pelvic Sonogram was performed. Pelvic- sonogram reviled a cloud like image, with absence for heartbeat. Patient was transferred to OR rapidly and two units of crossed matched blood were infused. Dilatation and curettage was performed in OR.
Surgical pathology confirmed a complete hydatidiform mole. Patient’s recovery was unremarkable; patient was discharged home after 48 hours. The patient was instructed on importance of using reliable method of birth control and monitoring of levels of HCG (first 48 hours post evacuation, weekly until hcg <5 miu/ml, then monthly X 6-12 months).
Molar pregnancies are classified as nonviable conceptions and are medically termed hydatidiform moles [3]. They are masses of cysts or benign tumors with a grape-like appearance that grow rapidly in the womb [3]. The abnormality is caused by a problem at conception, manifested by an excessive presence of placenta with little or no fetal development [10].
Hydatidiform moles are the most common form of benign gestational trophoblastic disease [4]. Often fatal in past centuries, significant medical advances in recent years now permit most women with moles to be cured [6].
Depending on the imbalance of genetic material in the pregnancy, the two major types of hydatidiform moles are classified as either complete or partial [1].
Forming when the sperm fertilizes an egg having no chromosomal or genetic material, a complete molar pregnancy is characterized by the presence of the placenta without an embryo [3].
Normally, the fertilized ovum would die and not implant itself in the womb [3]. In rare instances, this egg implants, triggering the growth of the placenta and the production of human chorionic gonadotrophin (HCG), the pregnancy hormone, therefore all symptoms of pregnancy will be present [5].
Partial-molar pregnancies are formed when; a normal ovum is fertilized by two sperms [3-5]. Instead of forming twins, the excessive presence of chromosomal material and trophoblastic tissue prevents normal fetal development [3]. The fetus does not survive more than three months and dies in the uterus [5-7].
As moles are rare, epidemiological studies vary in reporting incidences [6]. Vassilakos [11] states that the frequencies of moles vary by race and occur more among Asian women. Age is a known factor as higher rates of moles occur in women over the age of 40 and under the age of 20 [4,5,8,11], as well as in women under 16 and over 50 [10].
Women with either mole type have symptoms of vaginal bleeding, nausea and vomiting, and can present hyperthyroidism or preeclampsia [1-5].
Routine first trimester ultrasound examination can identify partial or complete molar pregnancy. The most of cases present as missed pregnancy during ultrasonic examination [10]. Histopathological examinations of products of conception are presently gold standard for diagnosis of gestational molar pregnancy. Abnormally high HCG blood levels and overly large uterine size suggestive of molar pregnancy and will warrant further clinical evaluation [2].
Patients who are diagnosed with molar pregnancy must be evaluated for possible complications such as: overactive thyroid, anemia, and toxemia of pregnancy. Patients should have a complete examination and laboratory testing [6].
After any medical complications have been addressed, a decision must e made concerning the best method of evacuation. Suction curettage is the optimal method of evacuation, regardless of uterine size, in patients who wish to retain reproductive function, because it carries a significantly lower risk of excessive bleeding, infection, and retained molar tissue then methods involving induction with oxytocin or prostaglandin. Rh immune globulin should be given to patient with RH conflict [7].
Patients are monitored to prevent the recurrence of benign moles and the development of malignant neoplasia, which can metastasize to the brain, liver or lungs [3]. Chest x-rays and the analysis of HCG levels for six months to one year are necessary [5]. Recurring moles are treated with methotrexate, a low-level chemotherapy [7].
The American College of Obstetricians and Gynecologists has recommended that after evacuation of a mole, serum HCG levels should be monitored every 1-2 weeks in all patients while the levels are elevated and then at monthly intervals for an additional 6 months once the levels become undetectable (5MIU per milliliter) [9].
- Dey M, Dhawan M (2011) Critical care management of molar pregnancy in a peripheral set-up. Med J Armed Forces India 67: 385-387. [Crossref]
- Hydatidiform mole and choriocarcinoma UK information and support service. (2013, July 1). RetrievedAugust9, 2014, from http://www.hmole-chorio.org.uk/faqs.html
- HardingM (2013, October 18)Hydatidiform mole | Health | Patient.co.uk. RetrievedAugust12, 2014, from http://www.patient.co.uk/health/hydatidiform-mole
- Hernandez E (2013, 2021 Copyright OAT. All rights reservneoplasia. Retrieved August 10, 2014, from http://emedicine.medscape.com/article/279116-overview
- Hill A (2011, July 26) Molar pregnancy. Retrieved August 15, 2014, from http://www.obgyn.net/molar-pregnancy
- LurainJR (2010) Gestational trophoblastic disease I: Epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol 203: 531-539. [Crossref]
- Molar pregnancy - NHS Choices (2012, September 18) RetrievedAugust9, 2014, from http://www.nhs.uk/conditions/Molar-pregnancy/Pages/Introduction.aspx
- MooreLE, HernandezE (2012, January 30)Hydatidiform mole. RetrievedAugust9, 2014, from http://emedicine.medscape.com/article/254657-overview
- Ross S, Berkowitz M, Goldstein DP (2009). Molar pregnancy. The new England Journal of Medicine, 360.
- Sebire,N.J., & Seckl,M.J. (2008). Gestational trophoblastic disease: Current management of hydatidiform mole.British Medical Journal,337 (aug15 1), 453-458. doi:10.1136/bmj.a1193
- Vassilakos,P. (2012, August 17).Pathology of molar pregnancy. RetrievedAugust9, 2014, from http://www.gfmer.ch/Books/Reproductive_health/Mole.html
Editorial Information
Editor-in-chief.
Andy Goren University of Rome "G.Marconi"
Article Type
Publication history.
Received date: August 15, 2018 Accepted date: August 23, 2018 Published date: August 29, 2018
©2018 Yuabova M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Yuabova M (2018) Molar Pregnancy-Case report. Clin Case Rep Rev 4: doi: 10.15761/CCRR.1000430.
Corresponding author
Assistant Professor, La Guardia Community College, City University of New York, NY, USA
Journal Home
- Article Info
- Author Info
- Figures & Data
- Privacy Policy
- Terms & conditions
© 2017 Copyright OAT. All rights reserved.
- Reference Manager
- Simple TEXT file
People also looked at
Case report article, case report: a rare case of bilateral molar natal teeth in a term newborn.

- Division of Neonatology and Newborn Medicine, Department of Pediatrics, Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States
Reports of natal and neonatal teeth have been well documented in the literature, though the presence of natal primary molars remains rare, and a clear management strategy does not exist. This is a case study of a female newborn delivered at a gestational age of 41 weeks 1 day to a 31-year-old G1P1001 mother. Apgar scores were 8 and 9 at 1 and 5 min, respectively. The infant was delivered by cesarean section and was admitted to the Mass General Hospital Newborn Nursery, where she received routine care. The patient had two posterior molars, which warranted consultation for oral maxillofacial surgery. Due to the gross mobility of the natal teeth and the risk of aspiration in a small breastfeeding newborn, the decision was made to extract the natal teeth immediately. Understanding the management of natal teeth is important for the pediatrician.
Reports of natal and neonatal teeth have been well documented in the literature in the last 70 years, although the presence of natal primary molars remains rare. Natal teeth are those that are already present at birth and neonatal teeth are those that erupt during the first 30 days of birth and are biologically normal. Natal teeth are reported to be more abundant than neonatal teeth ( 1 , 2 ). Natal teeth, when present, present as mandibular primary incisors 85% of the time, maxillary incisors 11% of the time, and posterior teeth 4% of the time ( 3 ).
The etiology of natal and neonatal teeth continues to remain unknown despite suggestive causative factors, including maternal exposure to environmental toxins, hypovitaminosis, infection, endocrinal disturbances, and hereditary transmission of an autosomal dominant gene ( 4 – 8 ). In some studies, natal or neonatal molars identified may be associated with systemic conditions or syndromes (i.e., histiocytosis X, cleft lip and palate, Pfeiffer syndrome); however, there are no current studies that directly link a causative relationship between natal teeth and syndromes ( 9 – 11 ). Common presentations of natal teeth that warrant further evaluation include tooth mobility, difficulty with breast latch or suckle, and concern for aspiration ( 12 – 14 ). Ulceration of the ventral surface of the tongue, also known as Riga–Fede disease, is a known complication that can occur in the presence of natal or neonatal teeth, and thus it is important that these are evaluated upon initial observation ( 14 – 16 ). There is controversy over the prevalence of occurrence of natal and neonatal teeth in male and female infants. Some systematic reviews have reported no significant difference between male and female infants in tooth morphology ( 1 , 2 , 17 ); however, other authors report higher occurrence of natal teeth in female newborns compared to male newborns ( 2 , 18 ).
We present a noteworthy case of a 2-day-old infant who was born with two maxillary posterior molar natal teeth. According to the American Academy of Pediatric Dentistry, the average age for primary maxillary first molars to erupt is between 11 and 18 months ( 19 , 20 ). The two teeth in this case may represent the premature eruption of maxillary primary molars, which is a rare finding in newborns.
Birth summary
A female newborn was delivered at a gestational age of 41 weeks 1 day to a 31-year-old G1P1001 mother. Apgar scores were 8 and 9 at 1 and 5 min, respectively. The infant was delivered by cesarean section due to concerns of category 2 tracing and fetal intolerance to labor. The physical exam at the time of birth was unremarkable. The newborn patient received vitamin K IM and erythromycin ointment at the time of birth. The patient was admitted to the Mass General Hospital Newborn Nursery, where she received routine care. Routine newborn screenings (metabolic, hearing, bilirubin, cardiac) were performed for 36 h after birth. Both parents consented to Hepatitis B vaccination. The newborn weighed 3,070 g (12.61 percentile) and was 50.8 cm in length (38.87 percentile). Her head circumference was not measured at the time of birth. The only imaging before birth was performed during the first trimester.
Family history
Family history was negative for hyperbilirubinemia in newborns, congenital heart disease, childhood kidney disease, and developmental dysplasia of the hip. The mother has a history of acquired left-sided partial hearing loss. There is no family history of dental or bone abnormalities.
Oral maxillofacial surgery management
Oral and maxillofacial surgery was consulted. A discussion of the risks and benefits of not removing the teeth was had before the parents chose to remove the teeth. Upon extraoral examination, the newborn appeared otherwise healthy and non-syndromic. An intraoral examination revealed the white crowns of two erupted primary maxillary first molars. Natal teeth #B and #I presented with sharp cusp tips, thin fragile enamel, and grade III mobility (see Figure 1 ). Due to the mobility of the teeth, the mother had difficulties with feeding and latching. Ultimately, given the difficulty feeding and future risk of aspiration, the parents opted to have the teeth removed. The teeth were removed using oral glucose and benzocaine topical anesthesia paste with a curved snap. Of note, the crown was removed without evidence of root formation. Hemostasis was easily achieved after extraction and the newborn patient tolerated the procedure well.
Figure 1 . Intraoral examination revealed the white crowns of two erupted primary maxillary first molars. Natal tooth B (Left image) was fully erupted with class III mobility. Natal tooth I (Right image) was partially erupted with class III mobility. Of note, the newborn has Ebstein Pearls, on the midline of the soft palate.
The presence of erupted teeth at birth or the eruption of teeth within 30 days after birth is considered a rare occurrence, with an incidence of 0.1% ( 20 ). In the case of a 2-day-old female infant delivered via cesarean section at 41 weeks of gestation, with no abnormalities detected at routine pregnancy screenings, the presence of two fully formed maxillary molar teeth were noted at birth.
According to the American Academy of Pediatric Dentistry, the average age for primary maxillary first molars to erupt is between 11 and 18 months ( 19 , 20 ). In this study, maxillary molars were present from birth. The case of this 2-day-old infant is noteworthy in that the location of the two natal teeth were maxillary primary molars, as the majority of cases of natal teeth described in the literature are incisors ( 21 ). The management of incisors and molars varies. Incisors are often removed since there is little risk of removing them. The management of molars, however, varies depending on tooth maturity. Immature molars should be removed, as they are less likely to develop normally. However, mature molars should not be removed. Maturity is typically identified via radiographs or histology. However, the mobility of the teeth can also give a good estimation of the level of maturity of the teeth. The teeth in our study are most likely immature, given the high degree of mobility. Mature teeth, on the other hand, do not typically present with mobility. Lastly, this newborn was born with two molars. This is consistent with the other literature, where the average number of natal molars is one or two ( 21 ). One of the highest numbers of natal molars was documented in a study, where the baby was born with eight natal molars, four in the maxillary site and four in the mandibular site ( 22 ). In that study of eight natal molars, the patient required resuscitation, which was not an issue for our patient, likely given the number of teeth.
In some studies, natal or neonatal molars have been suggested to be associated with systemic conditions or syndromes (i.e., histiocytosis X); however, there are no current studies that directly link a causative relationship between natal teeth and syndromes ( 9 ). There are some disorders associated with natal teeth, but given the rarity, there are no conditions that are highly linked with molars specifically. In addition to systemic conditions and developmental disorders, natal teeth have been associated with the congenital defect known as cleft lip and palate, which is inherited in a multifactorial manner ( 23 , 24 ). Currently, there is no known etiology of natal teeth. Some theories suggest that the development of natal teeth is a response to febrile states, endocrine disturbances, nutritional deficiencies, or a response to congenital syphilis ( 23 ). None of the above scenarios were present in this case, given our extent of knowledge of maternal health during gestation.
Finally, it is important to note that the management of natal teeth must be approached on a case-by-case basis. Erupted natal teeth may represent premature primary dentition. For this reason, they should be preserved with close monitoring by a pediatric dentist and pediatrician to ensure that they do not become an aspiration risk or impair feeding ( 20 ). However, in the present case, grade III mobility of the erupted teeth suggested that the natal teeth were immature and that not removing the teeth posed an increased risk for aspiration, especially given the small diameter of the infant airway. Ultimately, there was a decision by the parents of the newborn to remove the teeth. In our case, the teeth were causing issues with latching during breastfeeding, ultimately compromising fetal nutrition. Though this newborn did not present with ulcerations or trauma to the tongue, if the teeth remained in the mouth, there could be trauma to the adjacent soft tissue. Other complications of natal teeth can include pain and lacerations of the mother's breast, and the potential risk for hemorrhage, particularly in the setting of vitamin K deficiency ( 20 ).
Hemorrhage often occurs at the time of extraction and is a rarer occasion nowadays given current practices. Now, before teeth extraction, a consultation with a pediatrician is recommended to make sure that the newborn has received vitamin K prophylaxis after birth ( 20 ). Previously, a physician would wait until the 10th day for tooth extraction to avoid excessive hemorrhage. However, today, newborns are routinely given 1.0 mg of vitamin K after birth, which removes the need to wait until the 10th day ( 21 ). In addition, the removal of a natal tooth may impact the future development of primary and permanent dentition. Monitoring by a pediatric dentist and oral surgeon is important to ensure the normal development of primary and permanent dentition, and to exclude any other dental abnormalities.
Understanding the management of natal teeth is important for the pediatrician. While the cause of natal teeth is not fully understood, the positioning of the teeth can determine the need for removal, especially if the teeth are immature. In a case of a maxillary molar with grade III mobility, known complications such as problems with breastfeeding are common; therefore, the decision was made to remove the teeth.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
BV: Conceptualization, Writing – original draft, Writing – review & editing. LA: Writing – original draft, Writing – review & editing. SV: Writing – original draft, Writing – review & editing.
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank Dr. Romano-Clarke for her mentorship and oversight of this project. We would also like to thank the Oral Maxillofacial Surgery team for their assistance in managing this patient. Finally, we would like to express our gratitude toward the patient and their family, without whom this paper would not be possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wang C-H, Lin Y-T, Lin Y-TJ. A survey of natal and neonatal teeth in newborn infants. J Formos Med Assoc . (2017) 116(3):193–6. doi: 10.1016/j.jfma.2016.03.009
PubMed Abstract | Crossref Full Text | Google Scholar
2. Mhaske S, Yuwanati MB, Mhaske A, Ragavendra R, Kamath K, Saawarn S. Natal and neonatal teeth: an overview of the literature. Int Sch Res Notices . (2013) 2013. doi: 10.1155/2013/956269
Crossref Full Text | Google Scholar
3. Neville BW, Damm DD, Allen CM, Chi AC. Oral & Maxillofacial Pathology . 4th ed. St. Louis, MO: Elsevier (2016).
Google Scholar
4. Bjuggren G. Premature eruption in the primary dentition–a clinical and radiological study. Sven Tandlak Tidskr . (1973) 66(4):343–55.4520015
PubMed Abstract | Google Scholar
5. Hals E. Natal and neonatal teeth: histologic investigations in two brothers. Oral Surg Oral Med Oral Pathol . (1957) 10(5):509–21. doi: 10.1016/0030-4220(57)90011-7
6. Bigeard L, Hemmerle J, Sommermater J. Clinical and ultrastructural study of the natal tooth: enamel and dentin assessments. ASDC J Dent Child . (1996) 63(1):23–31.8655747
7. Anderson R. Natal and neonatal teeth: histologic investigation of two black females. ASDC J Dent Child . (1982) 49:300–3.6956592
8. Alaluusua S, Kiviranta H, Leppäniemi A, Hölttä P, Lukinmaa P-L, Lope L, et al. Natal and neonatal teeth in relation to environmental toxicants. Pediatr Res . (2002) 52(5):652–5. doi: 10.1203/00006450-200211000-00008
9. Galassi MS, Santos-Pinto L, Ramalho L. Natal maxillary primary molars: case report. J Clin Pediatr Dent . (2004) 29(1):41–4. doi: 10.17796/jcpd.29.1.12156uq4m6u1t7r7
10. Kadam M, Kadam D, Bhandary S, Hukkeri RY. Natal and neonatal teeth among cleft lip and palate infants. Natl J Maxillofac Surg . (2013) 4(1):73. doi: 10.4103/0975-5950.117883
11. Alvarez M, Crespi P, Shanske A. Natal molars in Pfeiffer syndrome type 3: a case report. J Clin Pediatr Dent . (1993) 18(1):21–4.8110608
12. Basavanthappa NN, Kagathur U, Basavanthappa RN, Suryaprakash ST. Natal and neonatal teeth: a retrospective study of 15 cases. Eur J Dent . (2011) 5(02):168–72. doi: 10.1055/s-0039-1698875
13. DeSeta M, Holden E, Siddik D, Bhujel N. Natal and neonatal teeth: a review and case series. Br Dent J . (2022) 232(7):449–53. doi: 10.1038/s41415-022-4091-3
14. Goho C. Neonatal sublingual traumatic ulceration (Riga-Fede disease): reports of cases. ASDC J Dent Child . (1996) 63(5):362–4.8958351
15. Hegde R. Sublingual traumatic ulceration due to neonatal teeth (Riga-Fede disease). J Indian Soc Pedod Prev Dent . (2005) 23(1):51–2. doi: 10.4103/0970-4388.16031
16. Padmanabhan MY, Pandey RK, Aparna R, Radhakrishnan V. Neonatal sublingual traumatic ulceration—case report & review of the literature. Dent Traumatol . (2010) 26(6):490–5. doi: 10.1111/j.1600-9657.2010.00926.x
17. Adekoya-Sofowora C. Natal and neonatal teeth: a review. Niger Postgrad Med J . (2008) 15(1):38–41. doi: 10.4103/1117-1936.180932
18. Markou I, Kana A, Arhakis A. Natal and neonatal teeth: a review of the literature. Balkan J Stomatol . (2012) 16(3):132–40.
19. Logan WH, Kronfeld R. Development of the human jaws and surrounding structures from birth to the age of fifteen years. J Am Dent Assoc . (1933) 20(3):379–428. doi: 10.14219/jada.archive.1933.0080
20. American Academy of Pediatric Dentistry. “Management considerations for pediatric oral surgery and oral pathology”. The Reference Manual of Pediatric Dentistry . Chicago, IL: American Academy of Pediatric Dentistry (2022). p. 485–94. Available online at: https://www.aapd.org/globalassets/media/policies_guidelines/bp_oralsurgery.pdf (Accessed January 29, 2024).
21. Brandt SK, Shapiro SD, Kittle PE. Immature primary molar in the newborn. Pediatr Dent . (1983) 5(3):210–3.6579501
22. Kaufman J. An infant born with teeth. Med Rec . (1892) 42:589.
23. Massler M, Savara BS. Natal and neonatal teeth: a review of twenty-four cases reported in the literature. J Pediatr . (1950) 36(3):349–59. doi: 10.1016/S0022-3476(50)80105-1
24. Gorlin RJ. Syndromes of the Head and Neck. Syndromes with Unusual Dental Findings . Oxford: Oxford University Press (1976).
Keywords: decision-making, cranio-maxillofacial surgery, prevention, risk factor(s), tooth development, natal teeth
Citation: Varriano B. M., Ades L. and Vaughan S. R. (2024) Case Report: A rare case of bilateral molar natal teeth in a term newborn. Front. Dent. Med 5:1336865. doi: 10.3389/fdmed.2024.1336865
Received: 11 November 2023; Accepted: 28 March 2024; Published: 25 April 2024.
Reviewed by:
© 2024 Varriano, Ades and Vaughan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: B. M. Varriano [email protected]
This article is part of the Research Topic
Improving Children's Oral Health

IMAGES
VIDEO
COMMENTS
Molar pregnancies, a premalignant form of gestational trophoblastic neoplasia, are characterised by an overgrowth of fetal chorionic tissue within the uterus. 1 In the USA, molar pregnancy occurs in 1 in 1000-1200 pregnancies and in 1 in 600 therapeutic abortions. 2 In the UK, the incidence is estimated at 1/714 live births.
The classic features of molar pregnancy are irregular vaginal bleeding, hyperemesis, enlarged uterus for gestational age and early failed pregnancy. Less common presentations include hyperthyroidism, early onset pre-eclampsia or abdominal distension due to theca lutein cysts. Here, we present a case of molar pregnancy where a woman presented to the emergency department with symptoms of acute ...
Molar pregnancy is a complication in early pregnancies, with a rate of 0.1% in the western countries. The complete hydatidiform mole has a 2-4% risk to convert to choriocarcinoma and 15% risk converting to invasive mole (malignant trophoblastic disease). That is why it is very important to closely follow up these pregnancies for at least 6-12 ...
After a molar pregnancy, patients and their partners commonly express concern about the potential for a molar pregnancy in the future. 52 Case series indicate that most patients with a molar ...
The typical clinical presentation of complete molar pregnancies has changed with the advent of high-resolution ultrasonography. Most moles are now diagnosed in the first trimester before the onset of the classic signs and symptoms. [ 30, 31, 32] Vaginal bleeding. The most common classic symptom of a complete mole is vaginal bleeding.
Hydatidiform molar pregnancy, resulting from an abnormal fertilization event, is the proliferation of abnormal pregnancy tissue with malignant potential. ... Patients with NLRP7 mutation have a 1.8% probability of obtaining a normal pregnancy . Rare cases of families with NLRP7 or KHDC3L ... Clinical presentations of molar pregnancies have ...
The classic features of molar pregnancy are irregular vaginal bleeding, hyperemesis, enlarged uterus for gestational age and early failed pregnancy. Less common presentations include hyperthyroidism, early onset pre-eclampsia or abdominal distension due to theca lutein cysts. Here, we present a case of molar pregnancy where a woman presented to ...
Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am ... Haloub K, Freij M. Recurrent hydatidiform molar pregnancy: a case report of 5 consecutive molar pregnancies complicated by HELLP and DIC, and review of literature. ...
The diagnostic challenge of a case of molar pregnancy with a coexisting living fetus is to differentiate two different conditions; singleton conception with a partial mole and dizygotic twins consisting of normal fetus with a complete mole. ... Lurain JR. Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and ...
Case Discussion. In the setting of a positive pregnancy test and amenorrhea as such, the sonographic features above strongly hints towards a complete hydatidiform molar pregnancy. Laboratory beta HCG serum levels correlation yielded elevated values at 7873.06 mIU/mL.
Case Series. Atypical Presentations of Molar Pregnancy Diagnostic Roles of Imaging, β-Human Chorionic Gonadotropin Measurement, and p57 Immunostaining. ... If the sonographic appearance of the pregnancy is atypical, GTD should be included in the differential diagnosis. Additional nonimaging criteria such as serial quantitative β-human ...
A history of prior molar pregnancy increases the risk of a repeat molar pregnancy to 1%, which is approximately 10 times the risk for the general population. 7,10,11 The risk of a complete mole is the highest at extremes of maternal age (<15 years and >35 years), and data suggest that ova from older women are more susceptible to abnormal ...
Molar pregnancy ( Case Presentation) Feb 1, 2020 • Download as PPTX, PDF •. 4 likes • 1,204 views. Musanna Nabi Chowdhury. Gynaecology Clinical Presentation. Health & Medicine. 1 of 39. Download now. Molar pregnancy ( Case Presentation) - Download as a PDF or view online for free.
Pregnancy of a hydatidiform mole with a coexistent live fetus in most cases is a complete molar pregnancy. Partial molar pregnancy with fetus is rare and almost always ends in miscarriage due to triploid fetus. Case Presentation: In this case study, we present a 19-year-old woman who presented with acute vaginal bleeding and pelvic discomfort ...
Parveen et al.6 reported a case of partial molar pregnancy with normal karyotype of the fetus ending with a live birth on term. Case presentation This is a 22-year-old lady, pregnant at 16 weeks of gestation who is admitted to SBAGAL with evidence of dead fetus in utero and suspected partial molar pregnancy with hCG>10,000IU/ml and vaginal ...
Writer will present case of molar pregnancy, risk factors, typical presentation and treatment modality. Key Words. moles, hydatidiform moles, molar pregnancy. Introduction. Hydatidiform mole is a product of anomalous conceptions, with prevalence about 1 in 500-1000 pregnancies [6].
Differential diagnosis of molar pregnancy Threatend abortion Points in favor: 1. h/o amenorrhoa for 8-12 weeks 2. Aymptoms of early pregnancy 3.Per vaginal bleeding Points against: 1.Expulsion of Grape like vesicles per vagina. 2.Blood is mixed with gelatinous fluid in case of molar pregnancy.
The Supreme Court heard oral arguments in another major abortion access case as it grapples with the aftermath of reversing Roe v. Wade. Follow here for live audio of the arguments, news updates ...
"They have tried to make this case be about the broader debate for access to abortion in cases of unwanted pregnancy, but that's not what this case is about at all," Prelogar said. "Idaho ...
Discussion. The presence of erupted teeth at birth or the eruption of teeth within 30 days after birth is considered a rare occurrence, with an incidence of 0.1% ().In the case of a 2-day-old female infant delivered via cesarean section at 41 weeks of gestation, with no abnormalities detected at routine pregnancy screenings, the presence of two fully formed maxillary molar teeth were noted at ...