71 Malaria Essay Topic Ideas & Examples
🏆 best malaria topic ideas & essay examples, 📌 simple & easy malaria essay titles, 👍 good essay topics on malaria.
- Descriptive Epidemiology of Malaria These variables allow epidemiologists to understand and describe the health status of a population, identify populations at increased risk of disease, characterize which months and areas have the most and least cases of the disease, […]
- The Global Health Problem of Malaria: A Case Study As both a leading cause of ill health and a barrier to receiving necessary medical care in an emergency, poverty is a significant factor in the availability of healthcare across the world.
- Global Health Issue of Malaria It can be explained due to the higher density of the population in those areas and the low socioeconomic status of most people.
- Malaria: Tropical Medicine and Hygiene Most importantly, it is necessary to note the substantial progress in the global malaria control and elimination effort. However, significant responsibility also lies on the endemic countries that must make internal investments in malaria control […]
- Malaria: Diagnosis and Treatment Mosquitoes, too, have developed resistance to insecticides, which causes the incidence of the disease, eventually contributing to more spread of malaria. Prevention of the causes of malaria is the fundamental responsibility that stops the spread […]
- Malaria: Causes and Treatment The sporozoites that these oocysts release oocysts find a way into the salivary glands of a mosquito. The sporozoite inoculation into a human preserves the life cycle of malaria.
- Malaria Disease Control and Prevention Plasmodium falciparum is the deadliest of the four malaria parasites and causes deaths within a short while if appropriate medication is not sought. Anyone can conduct malaria, especially after exposure to malaria-infested zones like the […]
- Malaria: The Epidemiological Triad The epidemiological triad of the disease presupposes that there is a host, or a human being, an agent, plasmodium falciparum, and the environment, or the areas characterized by the presence of mosquitoes. It becomes a […]
- Malaria and Poor Quality Drugs in Africa The most successful were control and prevention interventions on the island territories; in the meantime, the current state of malaria in large African territories remains unknown.
- Impact of Global Climate Change on Malaria There will be a comparison of the intensity of the changes to the magnitude of the impacts on malaria endemicity proposed within the future scenarios of the climate.
- Malaria and Dichloro-Diphenyl-Trichloroethane: Health, Morality and Economics While every single negative effect that DDT has on the people in the vicinity is to be taken into account and considered a separate legitimate statement against the use of DDT, the fact that the […]
- Malaria: Review and Analysis Malaria is one of the life-threatening infectious diseases whose impacts are experienced in the U.S.healthcare system. Currently, the burden of malaria on the U.S.healthcare systems is relatively high owing to the 2011 disease outcomes.
- Human Diseases: Exploring Malaria The aim of this essay is to explore the concept of malaria as it applies to the category of human diseases Many people in the world are aware of many human diseases.
- Measures of Occurrence and Data Sources in the Incidence of Malaria The review on the clinical findings provides the nature of malaria in relation to its symptoms. Change in the occurrence of malaria can also be detected through the definition of a baseline distribution and climatic […]
- Prevalence of Tuberculosis and Malaria in Africa and Middle East Globally the epidemiological distribution of Malaria and Tuberculosis disease worldwide is greatly skewed with majority of the cases occurring in Africa; 90% of all malaria related deaths for instance take place in Africa which is […]
- The Causes and Management Issues of Malaria The use of a conceptual model to show physiological, social and environmental factors related to the disease provides a clear understanding of the disease.
- The Global Impact of Tuberculosis and Malaria Again the whole of Africa shows the maximum incidence when compared to the rest of the world. The HAART therapy in HIV infections allows the treatment period to be free of TB infection.
- Sylvain Fleury: Global Warming Heats up Need for Malaria Vaccine The central thesis of the author, Sylvain Fleury, is that global warming is one of the major, if not the major, causes of this high spread rage of infectious diseases.
- The Epidemiology of Human Malaria in Africa According to the Global Health Network, the Global Health problem refers to the problems and issues of concern that cut across national health interests and issues, and relates to specific existing experiences and conditions in […]
- Culture & Disease: Malaria in Sub-Saharan Africa Thirdly, a relapse can occur due to the re-emergence of the blood-stage parasites from the parasites in the liver. The female Anopheles mosquito is an important organism in the distribution of the plasmodium, a parasite […]
- Malaria, Leishmaniasis, Dengue Fever and Plague Nowadays, malaria is spread in the territories which are good for malaria mosquitoes’ life, where it is warm and wet; thus, malaria is mostly dislocated in African countries.
- New Malaria Cure: Ethical Issues By investing less expensively in the research and development of the new drug, the company will also be able to develop effective and less expensive medication for many malaria patients worldwide Drug research involves the […]
- Malaria Disease and Drugs in Developing Economies Besides, in some poor regions, the spread of malaria is attributed to factors such as population movement, climatic changes, and resistance to anti-malarial drugs.
- Fighting Against Malaria: Integrated Vector Control The virus of malaria is one of the most common fatal health issues present in the poorest regions of Africa. The implementation of this strategy will let people know what places have to be avoided […]
- Malaria Symptoms and Nursing Preliminary Diagnosis However, the evidence presented in the case study should be enough to analyze and present a preliminary diagnosis of the patient’s condition. This is why the first reaction of the general practitioner was to test […]
- Malaria in Women and Children in Sub-Saharan Africa It is important to note here that, although the whole of Africa has felt the impact of the pandemic, sub-Saharan Africa is the most affected; something that results either due to ignorance or due to […]
- Malaria’s and Agriculture Relationship in Kenya This case study analyses the relationship between malaria and agriculture and some of the measures which have been put in place to lower the occurrence of the disease.
- An Analysis of the Effects and Research for Treating Malaria in Virology
- Developing a Knowledge-Based System for Diagnosis and Treatment of Malaria
- The Discovery Of A New Treatment Against Malaria
- Gender, Race, and Heterogeneous Effects of Epidemic Malaria on Human Capital and Income
- Understanding the Link between Two Illnesses: Malaria and Sickle Cell
- The Issue Of Infectious Disease And How They Are Spread With Cholera And Malaria
- An Analysis of the Most Prevalent and Dangerous Disease Malaria
- Can Benefits from Malaria Eradication Be Increased? Evidence from Costa Rica
- The Effect of Malaria on Settlement and Land Use: Evidence from the Brazilian Amazon
- Co-Designing a Citizen Science Program for Malaria Control in Rwanda
- The History of Malaria and Smallpox and How They Migrated from Western Europe to the United States
- The Role of the Medical Anthropologist in Controlling Malaria in Namibia
- The Importance and Effects of Malaria on People
- Analysis of the Cost of Malaria in Children and Use of Insecticide-treated Bednets in Africa
- The Connection between Malaria and the Sickle Cell Disease
- Agricultural Policy, Migration, and Malaria in the 1930s United States
- An Analysis of the Connection between the Sickle Cell Gene and the Spread of Malaria
- The Treatment of Malaria Using Unconventional Medicine
- The Fight Against Geography: Malaria and Economic Development in Italian Regions
- Epidemiological Trend Of Malaria In Odisha
- The Impact of Deforestation on Malaria Infections in the Brazilian Amazon
- The Ongoing Battle with Making the World Malaria
- The Impact of Malaria Control on Infant Mortality in Kenya
- Immune Response and Imperfect Vaccine in Malaria Dynamics
- The Mosquito Microbiome and Its Impact of Malaria Transmission
- The Effects Of Malaria On African Continent
- Erratum to: Malaria and Economic Evaluation Methods: Challenges and Opportunities
- Biodiversity Conservation and Child Malaria: Microeconomic Evidence from Flores, Indonesia
- The Causes and Management of the Malaria Disease
- The Causes of Malaria and Treatment Options
- An Analysis of the Number of People Affected by Malaria
- The Epidemiological, Socio Economic, and Ecological Implications of Deforestation on Malaria in South West Nigeria
- The Prevalence Of Manifestations Of Falciparum Malaria
- A History of Malaria and Other Diseases Caused by the Mosquito
- The Species of Anopheles Gambia and Their Spreading of Malaria
- Health Implications Of The Western Malaria Mosquito Breeds
- Impact of Malaria Control on Late and Early Infant Mortality in Senegal
- The Clinical Description of Malaria; Causes, Symptoms and Treatment Options
- Advanced Purchase Commitments for a Malaria Vaccine: Estimating Costs and Effectiveness
- The Global Technical Strategy For Malaria 2016-2030
- AgDscam is a Receptor found in Vectors Correlates to Malaria
- The Signs, Symptoms and Prevention Care for Malaria
- The Long-Term Economic Impact of In Utero and Postnatal Exposure to Malaria
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2024, February 28). 71 Malaria Essay Topic Ideas & Examples. https://ivypanda.com/essays/topic/malaria-essay-topics/
"71 Malaria Essay Topic Ideas & Examples." IvyPanda , 28 Feb. 2024, ivypanda.com/essays/topic/malaria-essay-topics/.
IvyPanda . (2024) '71 Malaria Essay Topic Ideas & Examples'. 28 February.
IvyPanda . 2024. "71 Malaria Essay Topic Ideas & Examples." February 28, 2024. https://ivypanda.com/essays/topic/malaria-essay-topics/.
1. IvyPanda . "71 Malaria Essay Topic Ideas & Examples." February 28, 2024. https://ivypanda.com/essays/topic/malaria-essay-topics/.
Bibliography
IvyPanda . "71 Malaria Essay Topic Ideas & Examples." February 28, 2024. https://ivypanda.com/essays/topic/malaria-essay-topics/.
- Health Promotion Research Topics
- Evidence-Based Practice Titles
- Hygiene Essay Topics
- Immunization Paper Topics
- Influenza Topics
- Swine Flu Questions
- Antibiotic Ideas
- Pandemic Ideas


Choose Your Test
Sat / act prep online guides and tips, 3 strong argumentative essay examples, analyzed.
General Education

Need to defend your opinion on an issue? Argumentative essays are one of the most popular types of essays you’ll write in school. They combine persuasive arguments with fact-based research, and, when done well, can be powerful tools for making someone agree with your point of view. If you’re struggling to write an argumentative essay or just want to learn more about them, seeing examples can be a big help.
After giving an overview of this type of essay, we provide three argumentative essay examples. After each essay, we explain in-depth how the essay was structured, what worked, and where the essay could be improved. We end with tips for making your own argumentative essay as strong as possible.
What Is an Argumentative Essay?
An argumentative essay is an essay that uses evidence and facts to support the claim it’s making. Its purpose is to persuade the reader to agree with the argument being made.
A good argumentative essay will use facts and evidence to support the argument, rather than just the author’s thoughts and opinions. For example, say you wanted to write an argumentative essay stating that Charleston, SC is a great destination for families. You couldn’t just say that it’s a great place because you took your family there and enjoyed it. For it to be an argumentative essay, you need to have facts and data to support your argument, such as the number of child-friendly attractions in Charleston, special deals you can get with kids, and surveys of people who visited Charleston as a family and enjoyed it. The first argument is based entirely on feelings, whereas the second is based on evidence that can be proven.
The standard five paragraph format is common, but not required, for argumentative essays. These essays typically follow one of two formats: the Toulmin model or the Rogerian model.
- The Toulmin model is the most common. It begins with an introduction, follows with a thesis/claim, and gives data and evidence to support that claim. This style of essay also includes rebuttals of counterarguments.
- The Rogerian model analyzes two sides of an argument and reaches a conclusion after weighing the strengths and weaknesses of each.
3 Good Argumentative Essay Examples + Analysis
Below are three examples of argumentative essays, written by yours truly in my school days, as well as analysis of what each did well and where it could be improved.
Argumentative Essay Example 1
Proponents of this idea state that it will save local cities and towns money because libraries are expensive to maintain. They also believe it will encourage more people to read because they won’t have to travel to a library to get a book; they can simply click on what they want to read and read it from wherever they are. They could also access more materials because libraries won’t have to buy physical copies of books; they can simply rent out as many digital copies as they need.
However, it would be a serious mistake to replace libraries with tablets. First, digital books and resources are associated with less learning and more problems than print resources. A study done on tablet vs book reading found that people read 20-30% slower on tablets, retain 20% less information, and understand 10% less of what they read compared to people who read the same information in print. Additionally, staring too long at a screen has been shown to cause numerous health problems, including blurred vision, dizziness, dry eyes, headaches, and eye strain, at much higher instances than reading print does. People who use tablets and mobile devices excessively also have a higher incidence of more serious health issues such as fibromyalgia, shoulder and back pain, carpal tunnel syndrome, and muscle strain. I know that whenever I read from my e-reader for too long, my eyes begin to feel tired and my neck hurts. We should not add to these problems by giving people, especially young people, more reasons to look at screens.
Second, it is incredibly narrow-minded to assume that the only service libraries offer is book lending. Libraries have a multitude of benefits, and many are only available if the library has a physical location. Some of these benefits include acting as a quiet study space, giving people a way to converse with their neighbors, holding classes on a variety of topics, providing jobs, answering patron questions, and keeping the community connected. One neighborhood found that, after a local library instituted community events such as play times for toddlers and parents, job fairs for teenagers, and meeting spaces for senior citizens, over a third of residents reported feeling more connected to their community. Similarly, a Pew survey conducted in 2015 found that nearly two-thirds of American adults feel that closing their local library would have a major impact on their community. People see libraries as a way to connect with others and get their questions answered, benefits tablets can’t offer nearly as well or as easily.
While replacing libraries with tablets may seem like a simple solution, it would encourage people to spend even more time looking at digital screens, despite the myriad issues surrounding them. It would also end access to many of the benefits of libraries that people have come to rely on. In many areas, libraries are such an important part of the community network that they could never be replaced by a simple object.
The author begins by giving an overview of the counter-argument, then the thesis appears as the first sentence in the third paragraph. The essay then spends the rest of the paper dismantling the counter argument and showing why readers should believe the other side.
What this essay does well:
- Although it’s a bit unusual to have the thesis appear fairly far into the essay, it works because, once the thesis is stated, the rest of the essay focuses on supporting it since the counter-argument has already been discussed earlier in the paper.
- This essay includes numerous facts and cites studies to support its case. By having specific data to rely on, the author’s argument is stronger and readers will be more inclined to agree with it.
- For every argument the other side makes, the author makes sure to refute it and follow up with why her opinion is the stronger one. In order to make a strong argument, it’s important to dismantle the other side, which this essay does this by making the author's view appear stronger.
- This is a shorter paper, and if it needed to be expanded to meet length requirements, it could include more examples and go more into depth with them, such as by explaining specific cases where people benefited from local libraries.
- Additionally, while the paper uses lots of data, the author also mentions their own experience with using tablets. This should be removed since argumentative essays focus on facts and data to support an argument, not the author’s own opinion or experiences. Replacing that with more data on health issues associated with screen time would strengthen the essay.
- Some of the points made aren't completely accurate , particularly the one about digital books being cheaper. It actually often costs a library more money to rent out numerous digital copies of a book compared to buying a single physical copy. Make sure in your own essay you thoroughly research each of the points and rebuttals you make, otherwise you'll look like you don't know the issue that well.

Argumentative Essay Example 2
There are multiple drugs available to treat malaria, and many of them work well and save lives, but malaria eradication programs that focus too much on them and not enough on prevention haven’t seen long-term success in Sub-Saharan Africa. A major program to combat malaria was WHO’s Global Malaria Eradication Programme. Started in 1955, it had a goal of eliminating malaria in Africa within the next ten years. Based upon previously successful programs in Brazil and the United States, the program focused mainly on vector control. This included widely distributing chloroquine and spraying large amounts of DDT. More than one billion dollars was spent trying to abolish malaria. However, the program suffered from many problems and in 1969, WHO was forced to admit that the program had not succeeded in eradicating malaria. The number of people in Sub-Saharan Africa who contracted malaria as well as the number of malaria deaths had actually increased over 10% during the time the program was active.
One of the major reasons for the failure of the project was that it set uniform strategies and policies. By failing to consider variations between governments, geography, and infrastructure, the program was not nearly as successful as it could have been. Sub-Saharan Africa has neither the money nor the infrastructure to support such an elaborate program, and it couldn’t be run the way it was meant to. Most African countries don't have the resources to send all their people to doctors and get shots, nor can they afford to clear wetlands or other malaria prone areas. The continent’s spending per person for eradicating malaria was just a quarter of what Brazil spent. Sub-Saharan Africa simply can’t rely on a plan that requires more money, infrastructure, and expertise than they have to spare.
Additionally, the widespread use of chloroquine has created drug resistant parasites which are now plaguing Sub-Saharan Africa. Because chloroquine was used widely but inconsistently, mosquitoes developed resistance, and chloroquine is now nearly completely ineffective in Sub-Saharan Africa, with over 95% of mosquitoes resistant to it. As a result, newer, more expensive drugs need to be used to prevent and treat malaria, which further drives up the cost of malaria treatment for a region that can ill afford it.
Instead of developing plans to treat malaria after the infection has incurred, programs should focus on preventing infection from occurring in the first place. Not only is this plan cheaper and more effective, reducing the number of people who contract malaria also reduces loss of work/school days which can further bring down the productivity of the region.
One of the cheapest and most effective ways of preventing malaria is to implement insecticide-treated bed nets (ITNs). These nets provide a protective barrier around the person or people using them. While untreated bed nets are still helpful, those treated with insecticides are much more useful because they stop mosquitoes from biting people through the nets, and they help reduce mosquito populations in a community, thus helping people who don’t even own bed nets. Bed nets are also very effective because most mosquito bites occur while the person is sleeping, so bed nets would be able to drastically reduce the number of transmissions during the night. In fact, transmission of malaria can be reduced by as much as 90% in areas where the use of ITNs is widespread. Because money is so scarce in Sub-Saharan Africa, the low cost is a great benefit and a major reason why the program is so successful. Bed nets cost roughly 2 USD to make, last several years, and can protect two adults. Studies have shown that, for every 100-1000 more nets are being used, one less child dies of malaria. With an estimated 300 million people in Africa not being protected by mosquito nets, there’s the potential to save three million lives by spending just a few dollars per person.
Reducing the number of people who contract malaria would also reduce poverty levels in Africa significantly, thus improving other aspects of society like education levels and the economy. Vector control is more effective than treatment strategies because it means fewer people are getting sick. When fewer people get sick, the working population is stronger as a whole because people are not put out of work from malaria, nor are they caring for sick relatives. Malaria-afflicted families can typically only harvest 40% of the crops that healthy families can harvest. Additionally, a family with members who have malaria spends roughly a quarter of its income treatment, not including the loss of work they also must deal with due to the illness. It’s estimated that malaria costs Africa 12 billion USD in lost income every year. A strong working population creates a stronger economy, which Sub-Saharan Africa is in desperate need of.
This essay begins with an introduction, which ends with the thesis (that malaria eradication plans in Sub-Saharan Africa should focus on prevention rather than treatment). The first part of the essay lays out why the counter argument (treatment rather than prevention) is not as effective, and the second part of the essay focuses on why prevention of malaria is the better path to take.
- The thesis appears early, is stated clearly, and is supported throughout the rest of the essay. This makes the argument clear for readers to understand and follow throughout the essay.
- There’s lots of solid research in this essay, including specific programs that were conducted and how successful they were, as well as specific data mentioned throughout. This evidence helps strengthen the author’s argument.
- The author makes a case for using expanding bed net use over waiting until malaria occurs and beginning treatment, but not much of a plan is given for how the bed nets would be distributed or how to ensure they’re being used properly. By going more into detail of what she believes should be done, the author would be making a stronger argument.
- The introduction of the essay does a good job of laying out the seriousness of the problem, but the conclusion is short and abrupt. Expanding it into its own paragraph would give the author a final way to convince readers of her side of the argument.

Argumentative Essay Example 3
There are many ways payments could work. They could be in the form of a free-market approach, where athletes are able to earn whatever the market is willing to pay them, it could be a set amount of money per athlete, or student athletes could earn income from endorsements, autographs, and control of their likeness, similar to the way top Olympians earn money.
Proponents of the idea believe that, because college athletes are the ones who are training, participating in games, and bringing in audiences, they should receive some sort of compensation for their work. If there were no college athletes, the NCAA wouldn’t exist, college coaches wouldn’t receive there (sometimes very high) salaries, and brands like Nike couldn’t profit from college sports. In fact, the NCAA brings in roughly $1 billion in revenue a year, but college athletes don’t receive any of that money in the form of a paycheck. Additionally, people who believe college athletes should be paid state that paying college athletes will actually encourage them to remain in college longer and not turn pro as quickly, either by giving them a way to begin earning money in college or requiring them to sign a contract stating they’ll stay at the university for a certain number of years while making an agreed-upon salary.
Supporters of this idea point to Zion Williamson, the Duke basketball superstar, who, during his freshman year, sustained a serious knee injury. Many argued that, even if he enjoyed playing for Duke, it wasn’t worth risking another injury and ending his professional career before it even began for a program that wasn’t paying him. Williamson seems to have agreed with them and declared his eligibility for the NCAA draft later that year. If he was being paid, he may have stayed at Duke longer. In fact, roughly a third of student athletes surveyed stated that receiving a salary while in college would make them “strongly consider” remaining collegiate athletes longer before turning pro.
Paying athletes could also stop the recruitment scandals that have plagued the NCAA. In 2018, the NCAA stripped the University of Louisville's men's basketball team of its 2013 national championship title because it was discovered coaches were using sex workers to entice recruits to join the team. There have been dozens of other recruitment scandals where college athletes and recruits have been bribed with anything from having their grades changed, to getting free cars, to being straight out bribed. By paying college athletes and putting their salaries out in the open, the NCAA could end the illegal and underhanded ways some schools and coaches try to entice athletes to join.
People who argue against the idea of paying college athletes believe the practice could be disastrous for college sports. By paying athletes, they argue, they’d turn college sports into a bidding war, where only the richest schools could afford top athletes, and the majority of schools would be shut out from developing a talented team (though some argue this already happens because the best players often go to the most established college sports programs, who typically pay their coaches millions of dollars per year). It could also ruin the tight camaraderie of many college teams if players become jealous that certain teammates are making more money than they are.
They also argue that paying college athletes actually means only a small fraction would make significant money. Out of the 350 Division I athletic departments, fewer than a dozen earn any money. Nearly all the money the NCAA makes comes from men’s football and basketball, so paying college athletes would make a small group of men--who likely will be signed to pro teams and begin making millions immediately out of college--rich at the expense of other players.
Those against paying college athletes also believe that the athletes are receiving enough benefits already. The top athletes already receive scholarships that are worth tens of thousands per year, they receive free food/housing/textbooks, have access to top medical care if they are injured, receive top coaching, get travel perks and free gear, and can use their time in college as a way to capture the attention of professional recruiters. No other college students receive anywhere near as much from their schools.
People on this side also point out that, while the NCAA brings in a massive amount of money each year, it is still a non-profit organization. How? Because over 95% of those profits are redistributed to its members’ institutions in the form of scholarships, grants, conferences, support for Division II and Division III teams, and educational programs. Taking away a significant part of that revenue would hurt smaller programs that rely on that money to keep running.
While both sides have good points, it’s clear that the negatives of paying college athletes far outweigh the positives. College athletes spend a significant amount of time and energy playing for their school, but they are compensated for it by the scholarships and perks they receive. Adding a salary to that would result in a college athletic system where only a small handful of athletes (those likely to become millionaires in the professional leagues) are paid by a handful of schools who enter bidding wars to recruit them, while the majority of student athletics and college athletic programs suffer or even shut down for lack of money. Continuing to offer the current level of benefits to student athletes makes it possible for as many people to benefit from and enjoy college sports as possible.
This argumentative essay follows the Rogerian model. It discusses each side, first laying out multiple reasons people believe student athletes should be paid, then discussing reasons why the athletes shouldn’t be paid. It ends by stating that college athletes shouldn’t be paid by arguing that paying them would destroy college athletics programs and cause them to have many of the issues professional sports leagues have.
- Both sides of the argument are well developed, with multiple reasons why people agree with each side. It allows readers to get a full view of the argument and its nuances.
- Certain statements on both sides are directly rebuffed in order to show where the strengths and weaknesses of each side lie and give a more complete and sophisticated look at the argument.
- Using the Rogerian model can be tricky because oftentimes you don’t explicitly state your argument until the end of the paper. Here, the thesis doesn’t appear until the first sentence of the final paragraph. That doesn’t give readers a lot of time to be convinced that your argument is the right one, compared to a paper where the thesis is stated in the beginning and then supported throughout the paper. This paper could be strengthened if the final paragraph was expanded to more fully explain why the author supports the view, or if the paper had made it clearer that paying athletes was the weaker argument throughout.

3 Tips for Writing a Good Argumentative Essay
Now that you’ve seen examples of what good argumentative essay samples look like, follow these three tips when crafting your own essay.
#1: Make Your Thesis Crystal Clear
The thesis is the key to your argumentative essay; if it isn’t clear or readers can’t find it easily, your entire essay will be weak as a result. Always make sure that your thesis statement is easy to find. The typical spot for it is the final sentence of the introduction paragraph, but if it doesn’t fit in that spot for your essay, try to at least put it as the first or last sentence of a different paragraph so it stands out more.
Also make sure that your thesis makes clear what side of the argument you’re on. After you’ve written it, it’s a great idea to show your thesis to a couple different people--classmates are great for this. Just by reading your thesis they should be able to understand what point you’ll be trying to make with the rest of your essay.
#2: Show Why the Other Side Is Weak
When writing your essay, you may be tempted to ignore the other side of the argument and just focus on your side, but don’t do this. The best argumentative essays really tear apart the other side to show why readers shouldn’t believe it. Before you begin writing your essay, research what the other side believes, and what their strongest points are. Then, in your essay, be sure to mention each of these and use evidence to explain why they’re incorrect/weak arguments. That’ll make your essay much more effective than if you only focused on your side of the argument.
#3: Use Evidence to Support Your Side
Remember, an essay can’t be an argumentative essay if it doesn’t support its argument with evidence. For every point you make, make sure you have facts to back it up. Some examples are previous studies done on the topic, surveys of large groups of people, data points, etc. There should be lots of numbers in your argumentative essay that support your side of the argument. This will make your essay much stronger compared to only relying on your own opinions to support your argument.
Summary: Argumentative Essay Sample
Argumentative essays are persuasive essays that use facts and evidence to support their side of the argument. Most argumentative essays follow either the Toulmin model or the Rogerian model. By reading good argumentative essay examples, you can learn how to develop your essay and provide enough support to make readers agree with your opinion. When writing your essay, remember to always make your thesis clear, show where the other side is weak, and back up your opinion with data and evidence.
What's Next?
Do you need to write an argumentative essay as well? Check out our guide on the best argumentative essay topics for ideas!
You'll probably also need to write research papers for school. We've got you covered with 113 potential topics for research papers.
Your college admissions essay may end up being one of the most important essays you write. Follow our step-by-step guide on writing a personal statement to have an essay that'll impress colleges.

Christine graduated from Michigan State University with degrees in Environmental Biology and Geography and received her Master's from Duke University. In high school she scored in the 99th percentile on the SAT and was named a National Merit Finalist. She has taught English and biology in several countries.
Ask a Question Below
Have any questions about this article or other topics? Ask below and we'll reply!
Improve With Our Famous Guides
- For All Students
The 5 Strategies You Must Be Using to Improve 160+ SAT Points
How to Get a Perfect 1600, by a Perfect Scorer
Series: How to Get 800 on Each SAT Section:
Score 800 on SAT Math
Score 800 on SAT Reading
Score 800 on SAT Writing
Series: How to Get to 600 on Each SAT Section:
Score 600 on SAT Math
Score 600 on SAT Reading
Score 600 on SAT Writing
Free Complete Official SAT Practice Tests
What SAT Target Score Should You Be Aiming For?
15 Strategies to Improve Your SAT Essay
The 5 Strategies You Must Be Using to Improve 4+ ACT Points
How to Get a Perfect 36 ACT, by a Perfect Scorer
Series: How to Get 36 on Each ACT Section:
36 on ACT English
36 on ACT Math
36 on ACT Reading
36 on ACT Science
Series: How to Get to 24 on Each ACT Section:
24 on ACT English
24 on ACT Math
24 on ACT Reading
24 on ACT Science
What ACT target score should you be aiming for?
ACT Vocabulary You Must Know
ACT Writing: 15 Tips to Raise Your Essay Score
How to Get Into Harvard and the Ivy League
How to Get a Perfect 4.0 GPA
How to Write an Amazing College Essay
What Exactly Are Colleges Looking For?
Is the ACT easier than the SAT? A Comprehensive Guide
Should you retake your SAT or ACT?
When should you take the SAT or ACT?
Stay Informed
Get the latest articles and test prep tips!
Looking for Graduate School Test Prep?
Check out our top-rated graduate blogs here:
GRE Online Prep Blog
GMAT Online Prep Blog
TOEFL Online Prep Blog
Holly R. "I am absolutely overjoyed and cannot thank you enough for helping me!”
Home — Essay Samples — Nursing & Health — Public Health Issues — Malaria
Essays About Malaria
This page is designed to help you explore a variety of essay types and topics related to Malaria, encouraging you to tap into your creativity and personal interests to craft a compelling essay.
Essay Types and Topics
Argumentative essay.
- The impact of climate change on the spread of Malaria
- The role of government policies in combating Malaria
Example paragraph: The increasing global temperatures have been linked to the proliferation of Malaria in regions previously unaffected. This essay will explore the connection between climate change and the spread of Malaria, highlighting the urgency for environmental action to address this public health issue.
Example paragraph: It is evident that climate change has significant implications for the spread of Malaria. As such, it is imperative for governments and communities to prioritize environmental policies and initiatives to mitigate the impact of climate change on public health.
Compare and Contrast Essay
- The differences in Malaria prevalence between developed and developing countries
- Comparing the effectiveness of different treatment methods for Malaria
Descriptive Essay
- Painting a vivid picture of the impact of Malaria on a community
- Describing the life cycle of the Malaria parasite
Persuasive Essay
- Persuading readers to support increased funding for Malaria research and prevention
- Advocating for the use of specific strategies to combat Malaria
Narrative Essay
- Sharing a personal experience with Malaria and its impact
- Creating a fictional story that illustrates the challenges of Malaria in a community
Engagement and Creativity
As you explore these essay topics, remember that your personal interests and creativity can greatly enhance the quality of your writing. Engage with the subject matter and allow your unique perspective to shine through in your essays. Your passion for the topic can make a significant difference in capturing the reader's attention.
Educational Value
Each essay type offers unique opportunities for you to develop and showcase different skills. Argumentative essays can sharpen your analytical thinking, while persuasive essays can enhance your ability to craft compelling arguments. Compare and contrast essays provide a platform for honing your critical analysis skills, and narrative essays offer a space for creative storytelling. Descriptive essays, on the other hand, allow you to refine your descriptive abilities, painting vivid pictures with words.
Overview of Protozoan Parasite – a Cause of Malaria
Prevention strategies of malaria controlling, made-to-order essay as fast as you need it.
Each essay is customized to cater to your unique preferences
+ experts online
Malaria: Signs, Symptoms, and Methods of Treatment
Prevention and treatment of malaria, malaria facts, malaria free campaign (mfc) in uganda, let us write you an essay from scratch.
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Aproaches of Controlling and Preventing Malaria Transmission
Debunking malaria: facts and fiction, rapid diagnosis of malaria: problems with rdts, relevant topics.
- Eating Disorders
- Drug Addiction
- Childhood Obesity
- Vaccination
- Birth Control
- Teenage Pregnancy
- Dare Program
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
- Meeting report
- Open access
- Published: 27 May 2022
Reflections on the 2021 World Malaria Report and the future of malaria control
- April Monroe 1 , 7 ,
- Nana Aba Williams 2 , 3 ,
- Sheila Ogoma 4 ,
- Corine Karema 5 , 6 &
- Fredros Okumu 7
Malaria Journal volume 21 , Article number: 154 ( 2022 ) Cite this article
12k Accesses
46 Citations
68 Altmetric
Metrics details
The World Malaria Report, released in December 2021, reflects the unique challenges currently facing the global malaria community. The report showed the devastating toll of malaria, with an estimated 627,000 people losing their lives to the disease in 2020. The improved methodological approach used for calculating cause of death for young children revealed a systematic underestimation of disease burden over the past two decades; and that Africa has an even greater malaria crisis than previously known. While countries were able to prevent the worst-case scenarios, the disruptions due to the COVID-19 pandemic revealed how weak health systems and inadequate financing can limit the capacity of the continent to address the malaria challenge. African countries also face a convergence of biological threats that could redefine malaria control, notably widespread pyrethroid resistance and emerging resistance to artemisinin. Despite these challenges, there is cause for optimism in lessons learned from the COVID-19 pandemic, recent acceleration of cutting edge research and development, and new partnerships that encourage leadership from and ownership by affected countries. This article presents key insights from the 2021 World Malaria Report and reflections on the future trajectories: it was informed by an in-depth discussion with leading malaria experts from the World Health Organization (WHO), the Bill & Melinda Gates Foundation, and the U.S. President’s Malaria Initiative (PMI). The discussion took place during the 34th edition of the Ifakara Master Classes, held virtually on December 15th, 2021.
On December 15th, 2021, the 34th edition of the Ifakara Master Classes featured an in-depth discussion on the 2021 World Malaria Report (WMR), released a week earlier [ 1 ]. The discussion unpacked WMR findings and their implications for the future of malaria control. Guest experts included Dr. Pedro Alonso, Director of the Global Malaria Programme (GMP) at the World Health Organization (WHO), Dr. Abdisalan Noor, WHO Head of Strategic Information for Response Unit, Dr. Jennifer Gardy, Deputy Director, Surveillance, Data, and Epidemiology at the Bill & Melinda Gates Foundation, and Dr. Richard Steketee, Deputy Global Malaria Coordinator for the U.S. President’s Malaria Initiative (PMI).
The discussion, which lasted 2 h and 45 min in total, was organized and facilitated by MasterClass hosts Drs. Fredros Okumu (Director of Science, Ifakara Health Institute, Tanzania) and Sheila Ogoma (Technical Director, Clinton Health Access Initiative), and guest hosts, Drs. Corine Karema (Private Consultant and former Director of National Malaria Control Programme, Rwanda) and Nana Aba Williams (Coordinator, MESA Alliance, ISGlobal, Spain). The session began with a brief overview of the 2021 WMR by Dr. Noor, followed by a series of open-ended technical questions posed by the facilitators to the panel of experts about specific aspects of the WMR. The discussion was hosted on Zoom with 320 live participants from the global malaria community, and was live-streamed on YouTube.
A consolidated account of insights and lessons learned from the discussion is presented here. Findings are organized around topics identified a priori by the Master Class facilitators and key themes that emerged through the discussion.
The importance of numbers
The World Malaria Report, released December 2021, reflects the unique challenges facing the global malaria community. The report lays bare the devastating toll of malaria, with an estimated 627,000 people losing their lives to the disease in 2020. The numbers in the report tell two different stories for countries nearing elimination and countries experiencing high burden.
“A growing number of countries with low burden are moving steadily toward elimination, while countries with the highest burden are struggling.” –Dr. Noor
Eleven countries now experience 70% of the world’s malaria burden while 47 now report fewer than 10,000 cases per year. Even before the COVID-19 pandemic, gains against malaria were leveling off, leading to the role out of the High burden, High impact response in 2018 [ 2 ].
Methodological changes
A new statistical method is being used by the WHO, which provides more precise cause-of-death estimates for young children for all diseases, including malaria. In the revised approach, the proportion of childhood deaths attributable to malaria was 7.8%, up from previous estimates of 4.8% [ 3 , 4 ]. The revised approach revealed that there had been a higher number of estimated deaths between 2000 and 2020 than previously recognized and a systematic underestimation across the time series. The revisions also suggest that a higher number of malaria cases (totaling 1.7 billion) and deaths (10.6 million) had been averted in the same period.
The WMR has gotten clearer, and the quality improved consistently since it was first released. However, for most countries, the WHO still relies on modelled estimates derived from verbal autopsies to calculate all-cause mortality and the cause of death fraction for children under-5 to quantify malaria deaths in this age-group before applying a second adjustment to quantify deaths in older children and adults. There is a strong case for improving surveillance as an intervention and investing more heavily in information systems as recommended in the WHO Global Technical Strategy (GTS) 2016–2030 [ 5 ]. These malaria metrics, whether estimates or not, can be powerful advocacy tools and are, therefore, integral for creating compelling narratives of changes over time.
Impact of COVID-19
In addition to increases due to the methodological changes, the COVID-19 pandemic posed significant challenges, and was associated with ~ 47,000 of the ~ 69,000 extra deaths reported in 2020 relative to 2019, [ 1 ]. This includes increases in cases due to disruptions associated with delays in ITN distribution and disruptions in both diagnosis and treatment. Malaria deaths increased by 12% to an estimated 627,000 in 2020, compared to 2019 figures, with more than two-thirds of the additional 69,000 deaths attributable to COVID19-related service disruptions. While the figures are worrying, countries and partners have done well to prevent the worst-case scenarios earlier projected by the WHO and partners [ 6 , 7 , 8 ]; these models had predicted increases in malaria cases and deaths in Africa of as much as two orders of magnitude.
Threats to malaria control in Africa
A range of challenges from biological threats, to preventing severe disease and death in the most remote areas, to fragile and insufficient malaria funding must be addressed to sustain progress.
“ The situation remains precarious, particularly in sub-Saharan Africa where burden remains unacceptably high and a convergence of threats pose added challenges to disease control efforts…Without immediate accelerated action, key 2030 targets of the WHO Global Technical Strategy [ 5 ] for malaria will be missed, and additional ground may be lost.” –Dr. Noor
- Biological threats
While the epidemiology of malaria in Africa is already more challenging and precarious than elsewhere, the situation is compounded by multiple biological and civil threats. Over 122 million people in 21 malaria-endemic countries needed assistance due to health and humanitarian emergencies in 2020–2021 including Ebola outbreaks, armed conflicts, and flooding. Key biological threats in sub-Saharan Africa include anti-malarial drug resistance in the eastern Africa region [ 9 , 10 , 11 ], threats to diagnostics posed by parasite pfhrp2/3 gene deletions (which can cause false negative diagnostic test results) [ 12 , 13 ], resistance of malaria vector mosquitoes to public health insecticides [ 14 , 15 ], and the invasive vector species, Anopheles stephensi in the Horn of Africa [ 16 , 17 , 18 ]. All these factors threaten to undermine malaria control efforts in ways that are not sufficiently understood.
The WHO is tracking biological threats using the WHO threats map [ 19 ]. For pfhrp2/3 gene deletions, there are already new tests, albeit more expensive, which are prequalified by the WHO that can detect these parasites [ 20 ]. Increased investments to improve surveillance of gene deletions is needed and investments in new diagnostics is essential and a cause for optimism. Insecticide resistance remains a significant challenge to be addressed decisively—PBO nets are now recommended, and other new generation nets are being evaluated [ 21 ]. The WHO recognizes A. stephensi as an efficient malaria vector in urban settings [ 22 ], and affected countries and their neighbours should urgently enhance surveillance and deploy novel tools. Given these threats, malaria stakeholders should be open to examining other potentially-transformative approaches such as genetically modified mosquitoes currently in early-stage development [ 23 , 24 ].
Of particular concern is emerging signs of resistance to artemisinin, which is the backbone of current malaria treatment efforts in Africa [ 9 , 10 , 11 ]. Now confirmed in Uganda [ 9 ] and Rwanda [ 10 , 11 ], artemisinin resistance, more accurately described as delayed parasite clearance, is emerging de novo in Africa and does not appear to be linked to the resistance in malaria parasites in south-east Asia, where this problem was first described [ 25 ]. Setting up effective surveillance systems is, therefore, critical to closely track this threat in the region.
Severe malaria and the last mile
Combatting severe malaria is paramount for averting malaria deaths and depends on systems that support prompt treatment, referral for severe disease, and a full course of treatment to clear infection. However, the most severe malaria cases and deaths are often concentrated in areas where health systems are weakest, where prevention practices are most inadequate, and care workers least trained. Effective community-based approaches, particularly training and appropriately compensating community health workers will be key to reaching the unreached and preventing severe disease.
“…This is a Catch 22… if we try to build our health systems to reach the people furthest out, and at the greatest risk, using our least trained, least supplied workers, the system is then going to have to deal with severe malaria because we weren’t able to prevent it in the first place...the question is, how do we take the community outreach, and community health workers on the periphery, and make sure they’re sufficient in scale, have the right skills, and that they are adequately supervised and supplied?” –Dr. Steketee
Funding gap
A consistent feature of global malaria programmes is that less than half of the necessary annual budget is actually available. A total of $3.3 billion was invested in 2020, compared to target of $6.8 billion. Moreover, to reach global targets, investments will need to increase by more than three times by 2030 to 10.3 billion per year. The current system relies on just a small number of major funders and budget needs are unlikely to be met even if these few sources increase their contributions. Further, the relative investment of countries has not increased despite economic growth.
“When you think about what’s stalled, population growth has not stalled, and that will continue, what’s stalled is the money. We’ve been working on efficiencies but there are limits to what we can achieve with efficiency alone.” –Dr. Steketee
The future of malaria control
The malaria situation cannot be effectively tackled using current practices, highlighting the need for a more transformational approach, tailored to different epidemiological contexts. A drastic change in mindset is needed around the disease and its complexities.
“It has not sunk in that we need to do something drastically different. It is a mindset problem, we need to show greater flexibility, and understand we are facing a very complex problem…malaria is a problem to be solved, not simply a task to be performed.” –Dr. Alonso
Lessons learned from the COVID-19 pandemic
There are important opportunities to learn from the COVID-19 pandemic. The pandemic brought the global malaria community together in a way not previously seen, to ensure a buffer against service delivery disruptions.
“It was really heartening to see that when there’s an emergency, we can work effectively across stakeholders to mount an effective response. COVID19 responses have also demonstrated to Ministries of Health that data matters – high-quality real-time data matters.” –Dr. Gardy
The pandemic has also shown that molecular data can provide important information on current and evolving trends over time, and that mathematical models can be valuable for exploring different intervention scenarios, an approach that is now also being utilized in the WHO-backed High burden, High impact response [ 2 ]. Perhaps most promising has been lessons learned from the development of the COVID-19 vaccine.
“...We’ve seen that things like a massive investment in de-risking multiple aspects of the vaccine production pipeline meant that you could very quickly get new products authorized, under Emergency Use Authorizations, and then eventually under full approval for use. We also saw the culmination of decades of work on mRNA vaccines…it’s working better than what we could have imagined. To hear that there’s now an mRNA pipeline for malaria vaccines is very exciting.” –Dr. Gardy
Innovative financing mechanisms will be needed moving forward to ensure sufficient and sustained funding. Resource mobilization seen during the COVID-19 pandemic shows when can be done when a disease is viewed as a global threat.
“COVID-19 may provide an opportunity – when countries in the global north have felt threatened there’s no limit to the money they spend – building on this momentum is a great opportunity to put the health agenda up front. Strengthening health systems is a key issue in the fight against malaria, it may not be considered malaria money, but is key to getting the commodities out.” –Dr. Alonso
RTS,S malaria vaccine
In 2021, the RTS,S malaria vaccine became the first to be approved for widespread use; and the only vaccine currently available for any human malaria parasites. The vaccine is now recommended for children living in areas with moderate to high Plasmodium falciparum transmission. In addition to the modest efficacy demonstrated in earlier clinical trials [ 26 , 27 ] and results of a consensus modelling programme [ 28 ], data from a WHO-backed pilot study in three countries, Kenya, Ghana and Malawi, suggest that the vaccine is feasible to deliver, safe and has a significant public health impact [ 29 ]. When provided in the context of both the expanded programme of childhood immunizations and other malaria control efforts, the vaccine increases access to prevention for vulnerable children—for instance reaching two thirds of children not protected by insecticide-treated nets (ITNs)—and is cost effective in areas with moderate to high transmission. The vaccine programme has already reached more than 900,000 children in three countries and generated among the most robust evidence for a malaria control tool ever.
It will be critical to think comprehensively about malaria control, including the vaccine, to ensure context-appropriate packages of interventions.
“…Putting one tool against another is really unhelpful, it’s bad public health…We have an armamentarium, we have a set of tools, and we need to look at what’s best in a particular circumstance….” –Dr. Alonso
During the evaluation of RTS,S there was a strong partnership between African scientists, the WHO, and several other players working jointly. For example, the Phase III trials were done in 11 different sites across nine African countries [ 26 , 27 ], and the mathematical modelling done to support final decision-making had been conducted jointly by four different research groups [ 28 ]. There is an important opportunity to leverage benefits of such united approaches to improve outcomes for other technologies and malaria control programmes.
“RTS,S forces the malaria community to work with other departments of the ministry of health that are the custodians of the delivery platforms, such as EPI. Therefore, an added benefit of RTS,S is that it will force the malaria community to come out from a siloed space.” –Dr. Alonso
Working across disease portfolios can also maximize efficiencies in health systems.
“The more we can figure out how to work together on delivery platforms, the more we can see benefits across the board and use the limited (funding) envelope more effectively.” –Dr. Gardy
Toward a unified vision and country-led decision-making
Finally, the future of malaria control will require moving toward country-led, unified visions and funding strategies. This includes ensuring evidence-based decisions and centering affected countries in those decisions.
“If a country has the data to show an area would benefit from a fifth round of seasonal malaria chemoprevention, who is anyone on this planet to tell them no? We need to break those attitudes, the lack of empowerment to countries, that lack of evidence-based decision making – only then will we be able to make progress.” –Dr. Alonso
There must also be a more coordinated response from different partners working within countries and a united strategy. This includes movement toward a single national strategic plan, that is costed properly and against which the investments from inside and outside of the country are aligned to achieve the agreed programme goals.
“Let’s get everyone at the table with one single plan, costed, that we all help develop and that we can all invest in. This is truly a partnership.” –Dr. Steketee
The global malaria community is at an inflection point; progress has levelled off and multiple threats confront countries already hardest hit by the disease. A shift in mindset is urgently needed with truly innovative and collaborative approaches to malaria control. Reflecting on the 2021 WMR and its implications for the future, there is a critical opportunity to take-up lessons learned from the COVID-19 pandemic, including what is possible when the world comes together towards a common goal. Cutting edge research and development, as was seen in recent vaccine development, and improved surveillance, can pave the way to more transformational approaches. Finally, and most importantly, the future of malaria control must be led by affected countries, with unified and coordinated support from donors and partners.
Data availability
Not applicable.
WHO. World malaria report. Geneva: World Health Organization; 2021.
Google Scholar
WHO. High burden to high impact: a targeted malaria response. Geneva: World Health Organization; 2018.
Perin J, Mulick A, Yeung D, Villavicencio F, Lopez G, Strong K, et al. Global, regional, and national causes of under-5 mortality in 2000–19: an updated systematic analysis with implications for the sustainable development goals. Lancet Child Adolesc Health. 2021;6:106–15.
Article Google Scholar
WHO. Meeting report of the WHO evidence review group on malaria burden estimation methods. Geneva: World Health Organization; 2018.
WHO. Global technical strategy for malaria 2016–2030. Geneva: World Health Organization; 2015.
WHO. The potential impact of health service disruptions on the burden of malaria: a modelling analysis for countries in sub-Saharan Africa. Geneva: World Health Organization; 2020.
Sherrard-Smith E, Hogan AB, Hamlet A, Watson OJ, Whittaker C, Winskill P, et al. The potential public health consequences of COVID-19 on malaria in Africa. Nat Med. 2020;26:1411–6.
Article CAS Google Scholar
Weiss DJ, Bertozzi-Villa A, Rumisha SF, Amratia P, Arambepola R, Battle KE, et al. Indirect effects of the COVID-19 pandemic on malaria intervention coverage, morbidity, and mortality in Africa: a geospatial modelling analysis. Lancet Infect Dis. 2021;21:59–69.
Balikagala B, Fukuda N, Ikeda M, Katuro OT, Tachibana S, Yamauchi M, et al. Evidence of artemisinin-resistant malaria in Africa. N Engl J Med. 2021;385:1163–71.
Uwimana A, Legrand E, Stokes BH, Ndikumana JM, Warsame M, Umulisa N, et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat Med. 2020;26:1602–8.
Uwimana A, Umulisa N, Venkatesan M, Svigel SS, Zhou Z, Munyaneza T, et al. Association of Plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: an open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infect Dis. 2021;21:1120–8.
Feleke SM, Reichert EN, Mohammed H, Brhane BG, Mekete K, Mamo H, et al. Plasmodium falciparum is evolving to escape malaria rapid diagnostic tests in Ethiopia. Nat Microbiol. 2021;6:1289–99.
Alemayehu GS, Blackburn K, Lopez K, Dieng CC, Lo E, Janies D, et al. Detection of high prevalence of Plasmodium falciparum histidine-rich protein 2/3 gene deletions in Assosa zone, Ethiopia: implication for malaria diagnosis. Malar J. 2021;20:109.
Hancock PA, Hendriks CJM, Tangena J, Gibson H, Hemingway J, Coleman M, et al. Mapping trends in insecticide resistance phenotypes in African malaria vectors. PLoS Biol. 2020;18: e3000633.
Hemingway J. Resistance: a problem without an easy solution. Pestic Biochem Physiol. 2018;151:73–5.
Ahmed A, Khogali R, Elnour MB, Nakao R, Salim B. Emergence of the invasive malaria vector Anopheles stephensi in Khartoum state central Sudan. Parasit Vectors. 2021;14:511.
Sinka M, Pironon S, Massey NC, Longbottom J, Hemingway J, Moyes CL, et al. A new malaria vector in Africa: predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc Natl Acad Sci USA. 2020;117:24900–8.
Takken W, Lindsay S. Increased threat of urban malaria from Anopheles stephensi mosquitoes Africa. Emerg Infect Dis. 2019;25:1431–3.
WHO. Malaria threats map: Tracking biological challenges to malaria control and elimination. Geneva, World Health Organization, 2021 [cited 2022 January 2022]; Available from: https://apps.who.int/malaria/maps/threats/ ?
WHO. Prequalified In Vitro diagnostics. Geneva, World Health Organization, 2022 [cited 2022 January]; Available from: https://extranet.who.int/pqweb/vitro-diagnostics/vitro-diagnostics-lists .
WHO. Prequalification vector control: Prequalified lists of vector control products. Geneva, World Health Organization, 2021 [cited 2021 March]; Available from: https://extranet.who.int/pqweb/vector-control-products .
WHO. Vector alert: Anopheles stephensi invasion and spread: Horn of Africa, the Republic of the Sudan and surrounding geographical areas, and Sri Lanka: information note. Geneva: World Health Organization; 2019.
Gantz VM, Jasinskiene N, Tatarenkova O, Fazekas A, Macias VM, Bier E, et al. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi . Proc Natl Acad Sci USA. 2015;112:E6736–43.
Nolan T. Control of malaria-transmitting mosquitoes using gene drives. Philos Trans R Soc B. 2021;376:20190803.
Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–67.
RTSS Clinical Trials Partnership. First results of phase 3 trial of RTS, S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365:1863–75.
RTSS Clinical Trials Partnership. Efficacy and safety of the RTS, S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. 2014;11: e1001685.
Penny MA, Verity R, Bever CA, Sauboin C, Galactionova K, Flasche S, et al. Public health impact and cost-effectiveness of the RTS, S/AS01 malaria vaccine: a systematic comparison of predictions from four mathematical models. Lancet Med. 2016;386:367–75.
WHO. Malaria policy advisory group (MPAG) meeting report, October 2021. Geneva: World Health Organization; 2021.
Download references
Acknowledgements
We acknowledge all participants for their engagement and for the additional questions raised during the masterclass. We also acknowledge the participants for reviewing the final manuscript and approving it for publication.
There was no funding for this work.
Author information
Authors and affiliations.
Johns Hopkins Center for Communication Programs, Baltimore, USA
April Monroe
MESA Alliance, Barcelona Institute for Global Health (ISGlobal), Barcelona, Spain
Nana Aba Williams
Barcelona Institute for Global Health (ISGlobal), Hospital Clínic-Universitat de Barcelona, Barcelona, Spain
Clinton Health Access Initiative, Boston, USA
Sheila Ogoma
Quality and Equity Healthcare, Kigali, Rwanda
Corine Karema
Swiss Tropical & Public Health Institute, Basel, Switzerland
Ifakara Health Institute, Ifakara, Tanzania
April Monroe & Fredros Okumu
You can also search for this author in PubMed Google Scholar
Contributions
AM and FO drafted the manuscript. NAW, SO and CK reviewed and contributed to the draft. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Fredros Okumu .
Ethics declarations
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Monroe, A., Williams, N.A., Ogoma, S. et al. Reflections on the 2021 World Malaria Report and the future of malaria control. Malar J 21 , 154 (2022). https://doi.org/10.1186/s12936-022-04178-7
Download citation
Published : 27 May 2022
DOI : https://doi.org/10.1186/s12936-022-04178-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- World Malaria Report
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 03 August 2017
- Margaret A. Phillips 1 ,
- Jeremy N. Burrows 2 ,
- Christine Manyando 3 ,
- Rob Hooft van Huijsduijnen 2 ,
- Wesley C. Van Voorhis 4 &
- Timothy N. C. Wells 2
Nature Reviews Disease Primers volume 3 , Article number: 17050 ( 2017 ) Cite this article
78k Accesses
392 Citations
203 Altmetric
Metrics details
- Antimicrobial resistance
- Public health
Malaria is caused in humans by five species of single-celled eukaryotic Plasmodium parasites (mainly Plasmodium falciparum and Plasmodium vivax ) that are transmitted by the bite of Anopheles spp. mosquitoes. Malaria remains one of the most serious infectious diseases; it threatens nearly half of the world's population and led to hundreds of thousands of deaths in 2015, predominantly among children in Africa. Malaria is managed through a combination of vector control approaches (such as insecticide spraying and the use of insecticide-treated bed nets) and drugs for both treatment and prevention. The widespread use of artemisinin-based combination therapies has contributed to substantial declines in the number of malaria-related deaths; however, the emergence of drug resistance threatens to reverse this progress. Advances in our understanding of the underlying molecular basis of pathogenesis have fuelled the development of new diagnostics, drugs and insecticides. Several new combination therapies are in clinical development that have efficacy against drug-resistant parasites and the potential to be used in single-dose regimens to improve compliance. This ambitious programme to eliminate malaria also includes new approaches that could yield malaria vaccines or novel vector control strategies. However, despite these achievements, a well-coordinated global effort on multiple fronts is needed if malaria elimination is to be achieved.
Similar content being viewed by others
A meta-analysis on global change drivers and the risk of infectious disease

A guide to vaccinology: from basic principles to new developments
Bat species assemblage predicts coronavirus prevalence
Introduction.
Malaria has had a profound effect on human lives for thousands of years and remains one of the most serious, life-threatening infectious diseases 1 – 3 . The disease is caused by protozoan pathogens of the Plasmodium spp.; Plasmodium falciparum and Plasmodium vivax , for which humans are the exclusive mammalian hosts, are the most common species and are responsible for the largest public health burden. Malaria is transmitted by the bite of Plasmodium spp.-infected female mosquitoes of the Anopheles genus 1 – 3 . During a blood meal, infected mosquitoes inject — along with their anticoagulating saliva — sporozoites, which are the infective, motile stage of Plasmodium spp. Sporozoites journey through the skin to the lymphatics and into hepatocytes in the liver ( Fig. 1 ). Inside the hepatocyte, a single sporozoite can generate tens of thousands of merozoites (the stage that results from multiple asexual fissions (schizogony) of a sporozoite within the body of the host), which are released into the bloodstream where they enter red blood cells to replicate (erythrocytic schizogony). A fraction of merozoites (those that are sexually committed) also differentiate and mature into male and female gametocytes, which is the stage that infects the mosquito host when it takes a blood meal 4 , 5 . The onset of clinical symptoms generally occurs 7–10 days after the initial mosquito bite. P. vivax and Plasmodium ovale also have dormant forms, called hypnozoites, which can emerge from the liver years after the initial infection 6 , leading to relapse if not treated properly.
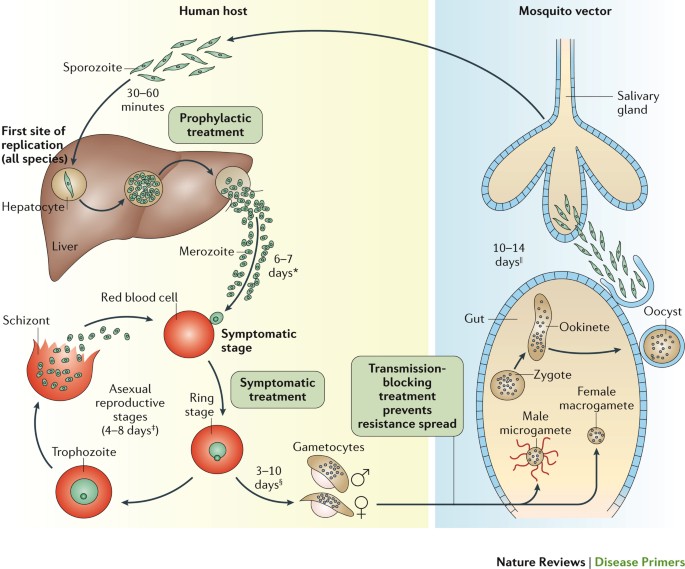
The mosquito vector transmits the Plasmodium spp. parasite in the sporozoite stage to the host during a blood meal. Within 30–60 minutes, sporozoites invade liver cells, where they replicate and divide as merozoites. The infected liver cell ruptures, releasing the merozoites into the bloodstream, where they invade red blood cells and begin the asexual reproductive stage, which is the symptomatic stage of the disease. Symptoms develop 4–8 days after the initial red blood cell invasion. The replication cycle of the merozoites within the red blood cells lasts 36–72 hours (from red blood cell invasion to haemolysis). Thus, in synchronous infections (infections that originate from a single infectious bite), fever occurs every 36–72 hours, when the infected red blood cells lyse and release endotoxins en masse 70 – 72 . Plasmodium vivax and Plasmodium ovale can also enter a dormant state in the liver, the hypnozoite. Merozoites released from red blood cells can invade other red blood cells and continue to replicate, or in some cases, they differentiate into male or female gametocytes 4 , 5 . The transcription factor AP2-G (not shown) has been shown to regulate the commitment to gametocytogenesis. Gametocytes concentrate in skin capillaries and are then taken up by the mosquito vector in another blood meal. In the gut of the mosquito, each male gametocyte produces eight microgametes after three rounds of mitosis; the female gametocyte matures into a macrogamete. Male microgametes are motile forms with flagellae and seek the female macrogamete. The male and female gametocytes fuse, forming a diploid zygote, which elongates into an ookinete; this motile form exits from the lumen of the gut across the epithelium 254 as an oocyst. Oocysts undergo cycles of replication and form sporozoites, which move from the abdomen of the mosquito to the salivary glands. Thus, 7–10 days after the mosquito feeds on blood containing gametocytes, it may be ‘armed’ and able to infect another human with Plasmodium spp. with her bite. Drugs that prevent Plasmodium spp. invasion or proliferation in the liver have prophylactic activity, drugs that block the red blood cell stage are required for the treatment of the symptomatic phase of the disease, and compounds that inhibit the formation of gametocytes or their development in the mosquito (including drugs that kill mosquitoes feeding on blood) are transmission-blocking agents. *Merozoite invasion of red blood cells can be delayed by months or years in case of hypnozoites. ‡ The number of days until symptoms are evident. § The duration of gametogenesis differs by species. || The maturation of sporozoites in the gut of the mosquito is highly temperature-dependent. Adapted with permission from Ref. 255 , Macmillan Publishers Ltd.
PowerPoint slide
The consequences of Plasmodium spp. infection vary in severity depending on the species and on host factors, including the level of host immunity, which is linked to the past extent of parasite exposure 7 , 8 . Malaria is usually classified as asymptomatic, uncomplicated or severe (complicated) 9 ( Box 1 ). Typical initial symptoms are low-grade fever, shaking chills, muscle aches and, in children, digestive symptoms. These symptoms can present suddenly (paroxysms), and then progress to drenching sweats, high fever and exhaustion. Malaria paroxysmal symptoms manifest after the haemolysis of Plasmodium spp.-invaded red blood cells. Severe malaria is often fatal, and presents with severe anaemia and various manifestations of multi-organ damage, which can include cerebral malaria 8 ( Box 1 ). Severe malaria complications are due to microvascular obstruction caused by the presence of red blood cell-stage parasites in capillaries 8 , 10 , 11 . This Primer focuses on our understanding of malaria pathology in the context of parasite and vector biology, progress in diagnostics and new treatments (drugs and vaccines), chemoprotection and chemoprevention.
Box 1: Malaria key terms
Asymptomatic malaria: can be caused by all Plasmodium spp.; the patient has circulating parasites but no symptoms.
Uncomplicated malaria: can be caused by all Plasmodium spp. Symptoms are nonspecific and can include fever, moderate-to-severe shaking chills, profuse sweating, headache, nausea, vomiting, diarrhoea and anaemia, with no clinical or laboratory findings of severe organ dysfunction.
Severe (complicated) malaria: usually caused by infection with Plasmodium falciparum , although less frequently it can also be caused by Plasmodium vivax or Plasmodium knowlesi . Complications include severe anaemia and end-organ damage, including coma (cerebral malaria), pulmonary complications (for example, oedema and hyperpnoeic syndrome 228 ), and hypoglycaemia or acute kidney injury. Severe malaria is often associated with hyperparasitaemia and is associated with increased mortality.
Placental malaria: parasites are present in the placenta, leading to poor outcomes for the fetus and possibly for the mother.
Epidemiology
Human malaria parasites are transmitted exclusively by ∼ 40 species of the mosquito genus Anopheles 12 . During Anopheles spp. mating, males transfer high levels of the steroid hormone 20-hydroxyecdysone to the females, and the presence of this hormone has been associated with favourable conditions for Plasmodium spp. development 13 . Malaria-competent Anopheles spp. are abundant and distributed all over the globe, including the Arctic. However, the efficacy of malaria transmission depends on the vector species and, therefore, varies considerably worldwide; for example, in tropical Africa, Anopheles gambiae is a major and highly efficient vector 14 . The first WHO Global Malaria Eradication Programme (1955–1972) involved, in addition to chloroquine-based treatments, large-scale insecticide campaigns using dichlorodiphenyltrichloroethane (DDT) 15 . This strategy was quite effective against P. falciparum ; although the mosquitoes gradually repopulated DDT-treated areas (because they developed resistance to the insecticide, and the use of DDT itself waned owing to its costs and increasing environmental concerns), these areas have often remained malaria-free and in some cases still are. More-selective vector control approaches, such as the use of insecticide-treated bed nets and indoor residual spraying, have eliminated malaria from several areas (see Diagnosis, screening and prevention, below). However, mosquito resistance to insecticides is a growing concern. Of the 78 countries that monitor mosquito resistance to insecticides, 60 have reported resistance to one or more insecticides since 2010 (Ref. 16 ).
The parasite
Plasmodium spp. are single-celled eukaryotic organisms 17 – 19 that belong to the phylum Apicomplexa, which is named for the apical complex that is involved in host cell invasion. A discussion of the parasite genome and the genetic approaches used to study parasite biology is provided in Box 2 . Of the five human-infective Plasmodium spp., P. falciparum causes the bulk of malaria-associated morbidity and mortality in sub-Saharan Africa, with mortality peaking in the late 1990s at over 1 million deaths annually in the continent 20 ( Fig. 2 ). P. falciparum is associated with severe malaria and complications in pregnancy ( Box 3 ); most malaria-related deaths are associated with this species, which kills ∼ 1,200 African children <5 years of age each day 21 . However, P. falciparum is also found in malarious tropical areas around the world. P. vivax is found in malarious tropical and temperate areas, primarily Southeast Asia, Ethopia and South America, and generally accounts for the majority of malaria cases in Central and South America and in temperate climates. This distribution can be explained by the fact that P. vivax can survive in climatically unfavourable regions and can stay dormant in a hypnozoite form in its human host's liver for many years. Furthermore, many Africans are negative for the Duffy antigen (also known as atypical chemokine receptor 1) on the surface of red blood cells, and this genotype provides protection from P. vivax malaria, as it makes it more difficult for P. vivax to bind to and penetrate red blood cells 22 . However, some cases of P. vivax transmission to Duffy antigen-negative individuals have been reported, which suggests that alternative mechanisms of invasion might be present in some strains, and this might portend the escalation of P. vivax malaria to Africa 23 , 24 . P. ovale is also found in Africa and Asia, but is especially prevalent in West Africa. Two sympatric species exist: P.o. curtisi and P.o. wallikeri 25 . Plasmodium malariae — which can be found worldwide but is especially prevalent in West Africa — causes the mildest infections, although it has been associated with splenomegaly or renal damage upon chronic infection. Plasmodium knowlesi — which was initially considered as a parasite of non-human primates — can not only cause malaria in humans but can also lead to severe and even fatal malaria complications 26 , 27 . The reasons for the emergence of P. knowlesi in humans are not yet fully understood but are possibly linked to land-use changes that have brought humans into close contact with P. knowlesi -infected mosquitoes 28 . Regardless, the possible recent emergence of a form of malaria as a zoonosis poses obvious complications for elimination. In addition, co-infections between P. falciparum and P. vivax have been well-documented and have been reported to occur in up to 10–30% of patients living in areas where both parasites are prevalent 29 , 30 . Mixed infections can also include other species such as P. ovale and P. malariae , and newer diagnostic methods are being developed that will enable better assessment of the frequency and distribution of these types of co-infection (for example, Ref. 31 ).
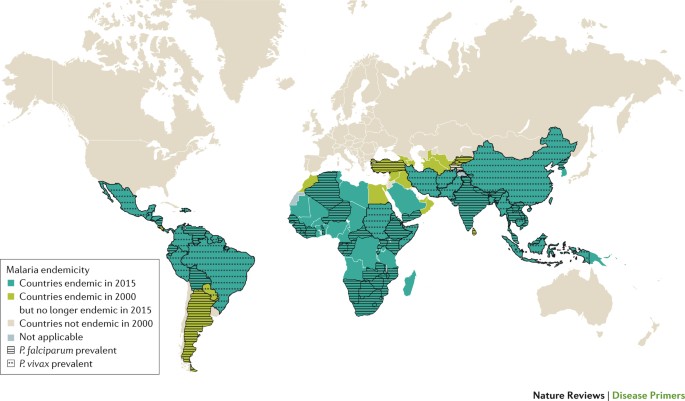
The most-deadly malaria parasite, Plasmodium falciparum , is only found in tropical areas because its gametocytes require 10–18 days at a temperature of >21°C to mate and mature into infectious sporozoites inside the vector 256 . This development timeline is only possible in hot, tropical conditions; where the ambient temperature is lower, mosquitoes can still propagate, but sporozoite maturation is slowed down and, therefore, incomplete, and parasites perish without progeny when the mosquitoes die. Thus, P. falciparum is quite temperature-sensitive; a global temperature rise of 2–3 °C might result in an additional 5% of the world population (that is, several hundred million people) being exposed to malaria 257 . Of note, Plasmodium vivax and Plasmodium ovale can develop in mosquitoes at ambient temperatures as low as 16 °C. The abilities of these parasites to propagate at subtropical temperatures and to remain in the hypnozoite state in the liver are likely to explain their ability to survive dry or cold seasons, and the broader global distribution of these parasites 258 . Countries coded ‘not applicable’ in the Figure were not separately surveyed. Figure based on data from Ref. 16 , WHO.
Box 2: The Plasmodium spp. genome and genomic tools for understanding gene function
Characteristics of the Plasmodium spp. genome
Each haploid genome comprises 23 Mb, which encode the programme for the complex life cycle of the parasite within ∼ 5,500 genes 17 – 19 .
Many genes encode proteins that have similarities to host proteins, many are novel, and many (approximately half) remain annotated as genes with hypothetical or of unknown function.
The Plasmodium spp. genome includes an essential plastid, the apicoplast, which is derived from two sequential endosymbiotic events, and encodes genes from both plant (red algal) and bacterial (cyanobacterium) origin 229 . The bacterial origin of some enzymes encoded by the plastid makes Plasmodium spp. sensitive to some antibacterial agents, whereas the plant-like pathways can be targeted by some herbicides. This plastid is one source of genes that differ from the host and that have been considered as potential drug targets.
Gene transcription across the Plasmodium spp. intra-red blood cell life cycle follows a preprogrammed cyclic cascade during which most genes are expressed at peak levels only once per life cycle 230 – 232 . Genes that encode cell surface proteins involved in host–parasite interactions are the exception.
Gene expression patterns have been reported to lack responses to perturbations. Minimal changes were observed after treatment with antifolates and chloroquine; however, larger changes have been observed for other drug classes 233 , 234 . Species-specific differences in transcription have been observed that seem to be linked to the mammalian host 235 .
Ribosome profiling has demonstrated that transcription and translation are tightly coupled for 90% of genes 236 . Exceptions of translationally upregulated genes are typically found for proteins involved in merozoite egress and invasion.
Epigenetic mechanisms to control gene expression include post-translational histone modifications (methylation and acetylation of the amino terminus are the best-characterized). Many of these modifications have been linked to parasite development 63 , 237 .
Genomic tools
Gene knockouts are possible, but RNA interference-mediated knockdown mechanisms do not function in Plasmodium spp. 238 , 239 .
Regulated RNA aptamer-based approaches have led to methods that enable gene knockouts to be functionally rescued; these methods are key for studying essential genes 238 , 239 .
CRISPR–Cas9-directed genome editing has greatly facilitated the genetic manipulation of Plasmodium falciparum 238 , 239 .
Barcoded mutant Plasmodium berghei libraries have been developed to screen for competitive fitness across tens of mutants in a single mouse 240 .
The in vitro selection of drug-resistant mutant parasites followed by whole-genome sequencing has also become a well-established method for revealing candidate drug targets 241 .
Metabolomics approaches facilitate the understanding of Plasmodium spp. biology, and have been used to profile several antimalarial compounds that have both known and unknown mechanisms of action 242 .

Box 3: Malaria and pregnancy
Pregnant women are more susceptible to Plasmodium spp. infection, particularly in their first pregnancy, as the mother-to-be has not yet acquired immunity to parasites that express the protein variant surface antigen 2-CSA (VAR2CSA) 35 . VAR2CSA on the surface of infected red blood cells facilitates adhesion to chondroitin sulfate A (which is part of placental proteoglycans), leading to red blood cell sequestration in the placenta 7 , 64 . The risk of placental malaria is reduced in multigravid women from endemic areas, who generally have antibodies against VAR2CSA 65 – 67 .
Malaria during pregnancy leads to increased risks to the mother and fetus 36 , 243 . Most studies have focused on sub-Saharan Africa; however, pregnancy-related risks are a problem throughout the world, including in Latin America, where Plasmodium vivax is the dominant causative agent 244 .
Placental malaria might be asymptomatic or clinically mild, but it also leads to an increased risk of death for both the fetus and the mother. It predisposes to miscarriage, stillbirth, preterm delivery and babies with low birth weight whose quality of life will probably be poor because of cognitive, mobility, self-care and sensation limitations; such babies also have a high mortality rate 36 , 243 .
Intermittent preventive treatment with sulfadoxine–pyrimethamine in endemic regions is recommended and is generally administered at each antenatal visit following quickening 108 , although the emergence of resistance is threatening its efficacy 245 .
Treatments for pregnant women must take into account the availability of safety data for the fetus. As a consequence, newer treatments require time to obtain sufficient confirmation of their tolerability in the different trimesters. The WHO recommends quinine sulfate and clindamycin in the first trimester. One study has shown that artemisinin derivatives provide comparable safety to quinine 246 , but, at the time of publication, the results of this study have not yet been incorporated into the WHO guidelines. In the second or third trimester, the WHO recommends artemisinin-based combination therapies 108 .
The treatment of pregnant women with P. vivax , Plasmodium ovale or Plasmodium malariae infection can also include chloroquine, unless resistance is suspected 108 . Women who are at a high risk of relapse can be given weekly chloroquine chemoprophylaxis until after delivery. Follow-up therapy with primaquine against P. vivax and P. ovale hypnozoites is not thought to be safe during pregnancy.
The disease
Malaria remains a major burden to people residing in resource-limited areas in Africa, Asia and Central and South America ( Fig. 2 ). An estimated 214 million cases of malaria occurred in 2015 (Ref. 16 ). Africa bears the brunt of the burden, with 88% of the cases, followed by Southeast Asia (10%), the eastern Mediterranean region (2%) and Central and South America (<1%). Malaria continues to kill over three-times as many people as all armed conflicts; in 2015, there were an estimated 438,000 (Ref. 16 ) — 631,000 (Ref. 20 ) deaths resulting from malaria, compared with an estimated 167,000 deaths due to armed conflicts 32 , 33 . In areas of continuous transmission of malaria, children <5 years of age and the fetuses of infected pregnant women experience the most morbidity and mortality from the disease. Children >6 months of age are particularly susceptible because they have lost their maternal antibodies but have not yet developed protective immunity. In fact, adults and children >5 years of age who live in regions of year-round P. falciparum transmission develop a partial protective immunity owing to repeated exposure to the parasite. There is evidence that immunity against P. vivax is acquired more quickly 34 . Individuals with low protective immunity against P. falciparum are particularly vulnerable to severe malaria. Severe malaria occurs in only 1% of infections in African children and is more common in patients who lack strong immune protection (for example, individuals who live in low-transmission settings, children <5 years of age and naive hosts). Severe malaria is deadly in 10% of children and 20% of adults 7 . Pregnant women are more susceptible to Plasmodium spp. infection because the placenta itself selects for the emergence of parasites that express receptors that recognize the placental vasculature; these receptors are antigens to which pregnant women have not yet become partially immune 7 ( Box 3 ). This vulnerability increases the risk of miscarriage; parasitaemia in the placenta can have adverse effects on the fetus 35 – 37 ( Box 3 ).
Co-infection of Plasmodium spp. with other pathogens — including HIV, Mycobacterium tuberculosis and helminths — is common. HIV-infected adults are at an increased risk of severe malaria and death 38 . The overall prevalence of helminth infection is very high (>50% of the population) in malaria-endemic regions and is associated with increased malaria parasitaemia 39 . Surprisingly, naturally occurring iron deficiency and anaemia protect against severe malaria, which was an unexpected finding 40 , as numerous clinical studies have aimed to fortify children and prevent anaemia by distributing iron supplements 41 .
From 2000 to 2015, the incidence of malaria fell by 37% and the malaria mortality rate fell by 60% globally 16 . The WHO attributes much of this reduction of malaria-associated morbidity and mortality to the scale-up of three interventions: insecticide-treated bed nets (69% of the reduction), artemisinin-based combination therapies (ACTs; 21%) and indoor residual insecticide spraying (10%) 16 (see Diagnosis, screening and prevention, below). Until ACT was introduced, progress in malaria control in most malarious countries was threatened or reversed by the nearly worldwide emergence of chloroquine-resistant and sulfadoxine–pyrimethamine-resistant P. falciparum strains and, more recently, of other resistant Plasmodium spp. ACT has become the antimalarial medicine of choice in most malarious areas, and demonstrates rapid parasite clearance, superior efficacy (compared with other clinically approved drugs) and >98% cure rates (typically defined as the percentage of patients who remain malaria-free for 28 days; re-infection events do not count as a recurrence). ACTs achieve these results even in strains that are resistant to older antimalarials, effectively turning the tide against antimalarial drug resistance. However, the emergence of artemisinin-resistant strains in Southeast Asia threatens the usefulness of ACTs 42 – 45 (see Drug resistance, below).
Mechanisms/pathophysiology
The red blood cell stage.
As previously mentioned, the red blood cell stage of Plasmodium spp. infection is the cause of symptomatic malaria, as red blood cells are the site of abundant parasite replication.
Invasion . Plasmodium spp. parasites gain entry into the red blood cell through specific ligand–receptor interactions mediated by proteins on the surface of the parasite that interact with receptors on the host erythrocyte (mature red blood cell) or reticulocyte (immature red blood cell) 46 ( Fig. 3 ). Whereas P. falciparum can invade and replicate in erythrocytes and reticulocytes, P. vivax and other species predominantly invade reticulocytes, which are less abundant than erythrocytes 47 . Most of the parasite erythrocyte-binding proteins or reticulocyte-binding proteins that have been associated with invasion are redundant or are expressed as a family of variant forms; however, for P. falciparum , two essential red blood cell receptors (basigin and complement decay-accelerating factor (also known as CD55)) have been identified ( Fig. 3 ).
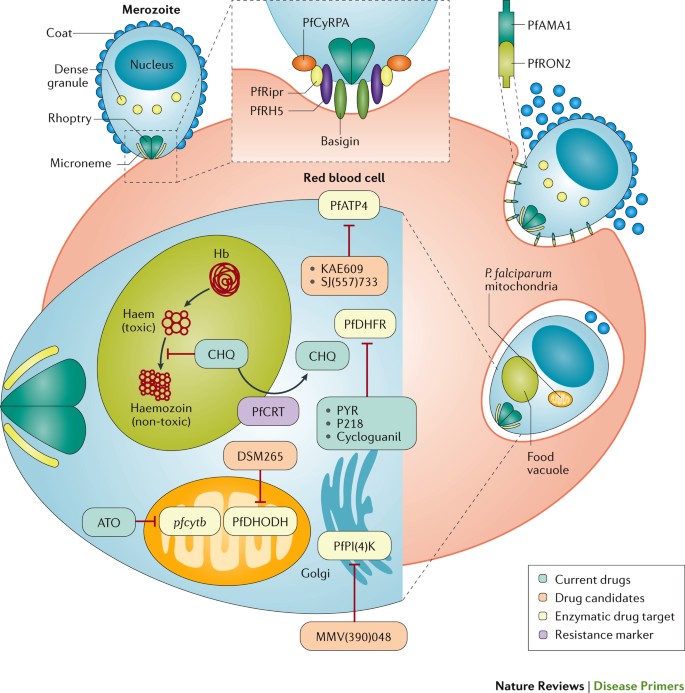
Invasion occurs through a multistep process 259 . During pre-invasion, low-affinity contacts are formed with the red blood cell membrane. Reorientation of the merozoite is necessary to enable close contact between parasite ligands and host cell receptors, and this is then followed by tight junction formation. In Plasmodium falciparum , a forward genetic screen has shown that complement decay-accelerating factor (not shown) on the host red blood cell is essential for the invasion of all P. falciparum strains 260 . The interaction of a complex of P. falciparum proteins (reticulocyte-binding protein homologue 5 (PfRH5), PfRH5-interacting protein (PfRipr) and cysteine-rich protective antigen (PfCyRPA)) with basigin on the red blood cell surface is also essential for the invasion in all strains 261 , 262 . PfRH5 has been studied as a potential vaccine candidate 46 , and antibodies against basigin have been considered as a potential therapeutic strategy 263 . During the PfRH5–PfRipr–PfCyRPA–basigin binding step, an opening forms between the parasite and the red blood cell, and this triggers Ca 2+ release and enables parasite-released proteins to be inserted into the red blood cell membrane. These proteins are secreted from the micronemes (the small secretory organelles that cluster at the apical end of the merozoite) and from the neck of the rhoptries, and include rhoptry neck protein 2 (PfRON2). Binding between PfRON2 and apical membrane antigen 1 (PfAMA1) on the merozoite surface is required to mediate tight junction formation before the internalization process 264 , and PfAMA1 is also being evaluated as a vaccine candidate 265 . Parasite replication within the red blood cell requires the synthesis of DNA, which can be blocked by several antimalarials: pyrimethamine (PYR), P218 and cycloguanil target P. falciparum dihydrofolate reductase (PfDHFR) 266 , and atovaquone (ATO) blocks pyrimidine biosynthesis by inhibiting the expression of the mitochondrial gene pfcytb (which encodes P. falciparum cytochrome b ) and by preventing the formation of oxidized coenzyme Q, which is needed to enable the pyrimidine biosynthetic enzyme dihydroorotate dehydrogenase (PfDHODH) to perform its reaction within the mitochondria 50 . The phase II clinical candidate DSM265 also blocks pyrimidine biosynthesis by directly inhibiting PfDHODH 186 . In addition to DNA synthesis, other processes can be targeted by antimalarial drugs. Chloroquine (CHQ) inhibits haem polymerization in the food vacuole 52 but can be expelled from this compartment by the P. falciparum chloroquine-resistance transporter (PfCRT) 267 . The phase II clinical candidate KAE609 and the preclinical candidate SJ(557)733 both inhibit P. falciparum p-type ATPase 4 (PfATP4), which is required for Na + homeostasis during nutrient acquisition 57 , 183 , 184 . The phase I clinical candidate MMV(390)048 (Ref. 191 ) inhibits P. falciparum phosphatidylinositol 4-kinase (PfPI(4)K), which is required for the generation of transport vesicles that are needed to promote membrane alterations during ingression 58 . Hb, haemoglobin.
Replication . Once Plasmodium spp. gain entry into the red blood cell, they export hundreds of proteins into the host cell cytoplasm and cell surface that modulate the acquisition of nutrients, cell adhesion and sequestration in tissues, and pathogenesis 3 , 48 , 49 . Molecular and cell biology approaches are expanding our understanding of the molecular machinery that is required for the export, as well as the identification and function of the exported proteins.
In the red blood cell, Plasmodium spp. replicate rapidly, and during symptomatic disease the parasites may replicate exponentially to >10 12 parasites per patient. This rapid growth requires sustained pools of nucleotides for the synthesis of DNA and RNA, and as a consequence, many antimalarials target pyrimidine biosynthesis 50 ( Fig. 3 ). Plasmodium spp. are auxotrophic for all of the amino acids they need (that is, they must acquire all of these from food because they cannot synthesize them from precursors). Haemoglobin digestion (in a specialized food vacuole) supplies all amino acids except isoleucine, which must be obtained from other host cell components 51 . Haemoglobin digestion also releases haem, which is toxic to the parasite and, therefore, is polymerized into haemozoin (often called malaria pigment, which is visible as a blue pigment under light microscopy), which is an insoluble crystal that sequesters the toxic metabolite 52 . How haem polymerization is facilitated by the parasite remains unclear. A complex of several proteases and haem detoxification protein (HDP) have been identified in the food vacuole; follow-up in vitro studies have shown that components of this complex (for example, falcipain 2, HDP and lipids) were able to catalyse the conversion of haem into haemozoin 53 . The importance of understanding this mechanism is highlighted by the finding that chloroquine and other antimalarials act by inhibiting haem polymerization 54 ( Fig. 3 ). There is also evidence that the iron (haem-bound or free) liberated in the food vacuole during haemoglobin digestion plays a part in activating the toxicity to the parasite of artemisinin derivatives 42 .
Nutrient uptake by the parasite is coupled to the detrimental accumulation of Na + ; however, the parasite expresses an essential plasma membrane Na + export pump (the cation ATPase P. falciparum p-type ATPase 4 (PfATP4)) that can maintain Na + homeostasis 55 – 57 ( Fig. 3 ). The remodelling of the plasma membrane (membrane ingression) to generate daughter merozoites in the late schizont stage requires P. falciparum phosphatidylinositol 4-kinase (PfPI(4)K) 58 . Both PfPI(4)K and PfATP4 are targets of new drugs that are under development ( Fig. 3 ).
Immune evasion and host immunity
Malaria parasites first encounter the host immune system when sporozoites are injected in the skin (measured to be ∼ 15 per mosquito bite in one study 59 ), where they may be phagocytosed by dendritic cells for antigen presentation in the lymph node draining the skin inoculation site 60 . The chances of transmission are increased when the host is bitten by mosquitoes that carry a larger number of sporozoites, despite the fact that the number of sporozoites that can simultaneously pass through the mosquito's proximal duct is limited by the duct diameter 61 . Sporozoites encounter several other effectors of the immune system, and how a minority of them can reach the liver and infect the hepatocytes is not well understood. Immune evasion in the liver could be in part explained by the ability of sporozoites to suppress the function of Kupffer cells (also known as stellate macrophages, which are the resident macrophages of the liver) and repress the expression of genes that encode MHC class I molecules 62 . Our understanding of the host immunity associated with the red blood cell stage is more complete. Virulence genes in Plasmodium spp. are part of large expanded multigene families that are found in specialized (for example, subtelomeric) regions of the chromosomes 7 , 63 , 64 . These gene families (for example, var genes in P. falciparum ) encode variants of cell surface proteins that function in immune evasion through antigenic variation and also are involved in mediating cytoadherence of infected red blood cells to endothelial cells, which leads to red blood cell sequestration in tissues.
Malaria disease severity — in terms of both parasite burden and the risk of complicated malaria — is dependent on the levels of protective immunity acquired by the human host 65 – 67 , which can help to decrease the severity of symptoms and reduce the risk of severe malaria. Immunity is thought to result from circulating IgG antibodies against surface proteins on sporozoites (thereby blocking hepatocyte invasion) and merozoites (thereby blocking red blood cell invasion). In high-transmission areas where malaria is prevalent year-round, adults develop partially protective immunity. Young infants (<6 months of age) are also afforded some protection, probably from the antibodies acquired from their mother, whereas children from 6 months to 5 years of age have the lowest levels of protective immunity and are the most susceptible to developing high parasitaemia with risks for complications and death (for example, see the study conducted in Kilifi, Kenya 68 ). In low-transmission areas or areas that have seasonal malaria, individuals develop lower levels of protective immunity and typically have worse symptomatic malaria upon infection. This correlation between protective immunity and malaria severity poses a challenge for successful malaria treatment programmes; as the number of infections and the transmission rates decrease, increasing numbers of patients will lose protective immunity and become susceptible to severe disease. The re-introduction of malaria in areas that had been malaria-free for many years could be devastating in the short term and, therefore, well-organized surveillance is required.
Pathogenesis
The predominant pathogenic mechanism is the haemolysis of Plasmodium spp.-infected red blood cells, which release parasites and malaria endotoxin — understood to be a complex of haemozoin and parasite DNA, which trigger Toll-like receptor 9 (TLR9), a nucleotide-sensing receptor involved in the host immune response against pathogens 69 — that leads to high levels of tumour necrosis factor (TNF) production and to clinical symptoms such as fever 70 – 72 . In addition, the membrane of infected red blood cells stiffens, and this loss of deformability contributes to the obstruction of capillaries, which has life-threatening consequences in severe malaria when vital organs are affected 73 .
Parasite factors that influence disease severity . Disease severity and pathogenesis are linked to surface proteins that are expressed by the parasite. In P. falciparum , a major surface antigen is encoded by the var gene family, which contains ∼ 60 members 7 , 11 , 63 , 64 . The majority of the var genes are classified into three subfamilies — A, B and C — on the basis of their genomic location and sequence: the B and C groups mediate binding to host cells via CD36 (also known as platelet glycoprotein 4), whereas the A group genes mediate non-CD36 binding interactions that have been linked to severe malaria, including cerebral malaria 7 , 64 . The var genes encode P. falciparum erythrocyte membrane protein 1 (PfEMP1), with the B and C groups accounting for >80% of PfEMP1 variants. PfEMP1 is the major protein involved in cytoadherence and mediates the binding of infected erythrocytes to the endothelial vasculature. In cerebral malaria, A group PfEMP1 variants mediate the binding of infected erythrocytes to endothelial protein C receptor (EPCR) and intercellular adhesion molecule 1 (ICAM1) in the brain, causing pathology 8 , 11 , 74 , 75 . However, our knowledge of the host cell receptors that are involved in interactions with the infected erythrocytes is probably incomplete. For example, thrombin — which regulates blood coagulation via vitamin K-dependent protein C — can cleave PfEMP1, thereby reversing and preventing the endothelial binding of infected erythrocytes 74 . In pregnancy, the expression of a specific PfEMP1 variant, variant surface antigen 2-CSA (VAR2CSA) — which is not encoded by one of the three main subfamilies — leads to an increased risk of placental malaria 7 , 64 ( Box 3 ).
High parasitaemia levels also seem to correlate with poor outcomes 7 , 75 , and the circulating levels of P. falciparum histidine-rich protein 2 (which is encoded by pfhrp2 ) have been used as a biomarker of parasitaemia that predicts the risks for microvascular obstruction and severe disease 76 . The brain pathology in children with severe malaria has recently been described in detail 77 .
Additionally, P. vivax does not express the same family of var genes that have been found to be strongly associated with endothelium binding and tissue sequestration, which drives severe disease in P. falciparum , and the ability of P. vivax to only invade reticulocytes leads to lower parasite levels 7 .
Host traits that influence disease severity . Malaria has exerted a strong selection pressure on the evolution of the human genome 78 , 79 . Some haemoglobin-encoding alleles that in homozygous genotypes cause severe blood disorders (such as thalassaemia, the earliest described example, and sickle cell disease) have been positively selected in populations living in malaria-endemic areas because heterozygous genotypes protect against malaria 80 . Other inherited haemoglobin abnormalities (for example, mutations affecting haemoglobin C and haemoglobin E) can also provide protection against malaria 81 .
In addition, genetic polymorphisms that affect proteins expressed by red blood cells or that lead to enzyme deficiencies can also be protective against severe disease. The red blood cell Duffy antigen is a key receptor that mediates the invasion of P. vivax through interaction with the Duffy antigen-binding protein on the parasite surface 46 . The genetic inheritance of mutations in ACKR1 (which encodes the Duffy antigen) in Africa is credited with reducing the spread of P. vivax in this continent, although the finding of Duffy antigen-negative individuals who can be infected with P. vivax suggests that we still have an incomplete understanding of the factors involved in P. vivax invasion 82 , 83 . Glucose-6-phosphate dehydrogenase (G6PD) deficiency 78 , 79 provides protection against severe malaria through an unknown mechanism, at least in hemizygous males 84 , but unfortunately also leads to haemolytic anaemia in patients treated with primaquine, which is an 8-aminoquinoline antimalarial and the only agent currently approved for the treatment of latent (liver-stage) P. vivax malaria. The mode of action of primaquine, which is a prodrug, remains unknown.
The mechanisms of malaria protection in these varied genetic disorders have been widely studied 81 . Common findings include increased phagocytosis and elimination by the spleen of infected mutant erythrocytes, which reduces parasitaemia; reduced parasite invasion of mutant red blood cells; reduced intracellular growth rates; and reduced cytoadherence of infected mutant red blood cells. All of these effects increase protection against severe malaria, which is the main driver of human evolution in this case. Some point mutations in the gene that encodes haemoglobin alter the display of PfEMP1 on the surface of infected red blood cells, thereby diminishing cytoadherence to endothelial cells 85 , 86 . This finding highlights the crucial role of cytoadherence in promoting severe disease.
Finally, variability in the response to TNF, which is secreted from almost all tissues in response to malaria endotoxins, has also been proposed as a factor that mediates differential host responses and contributes to severe malaria when levels are high 7 .
Diagnosis, screening and prevention
The WHO criteria for the diagnosis of malaria consider two key aspects of the disease pathology: fever and the presence of parasites 87 . Parasites can be detected upon light microscopic examination of a blood smear ( Fig. 4 ) or by a rapid diagnostic test (RDT) 87 . The patient's risk of exposure (for example, whether the patient lives in an endemic region or their travel history) can assist in making the diagnosis. Furthermore, the clinical expression of Plasmodium spp. infection correlates with the species’ level of transmission in the area. The symptoms of uncomplicated malaria include sustained episodes of high fever ( Box 1 ); when high levels of parasitaemia are reached, several life-threatening complications might occur (severe malaria) ( Box 1 ).
Thin blood films showing Plasmodium falciparum (upper panel) and Plasmodium vivax (lower panel) at different stages of blood-stage development. The images are from methanol-fixed thin films that were stained for 30 minutes in 5% Giemsa. The samples were taken from Thai and Korean patients with malaria: Ethical Review Committee for Research in Human Subjects, Ministry of Public Health, Thailand (reference no. 4/2549, 6 February 2006). The sex symbols represent microgametes (male symbol) and macrogametes (female symbol). ER, early ring stage; ES, early schizont stage; ET, early trophozoite stage; FM, free merozoites; LR, late ring stage; LS, late schizont stage; LT, late trophozoite stage; U, uninfected red blood cell. The slides used were from a previously published study 268 but the images shown have not been previously published. Images courtesy of A.-R. Eruera and B. Russell, University of Otago, New Zealand.
The complications of severe malaria mostly relate to the blocking of blood vessels by infected red blood cells, with the severity and symptoms depending on what organ is affected ( Box 1 ) and to what extent, and differ by age; lung and kidney disease are unusual in children in Africa but are common in non-immune adults.
Parasitaemia . Patients with uncomplicated malaria typically have parasitaemia in the range of 1,000–50,000 parasites per microlitre of blood (however, non-immune travellers and young children who have parasite numbers <1,000 can also present with symptoms). The higher numbers tend to be associated with severe malaria, but the correlation is imprecise and there is no cut-off density. In a pooled analysis of patient data from 61 studies that were designed to measure the efficacy of ACTs (throughout 1998–2012), parasitaemia averaged ∼ 4,000 parasites per microlitre in South America, ∼ 10,000 parasites per microlitre in Asia and ∼ 20,000 parasites per microlitre in Africa 88 . The limit of detection by thick-smear microscopy is ∼ 50 parasites per microlitre 89 . WHO-validated RDTs can detect 50–1,000 parasites per microlitre with high specificity, but many lack sensitivity, especially when compared with PCR-based methods 90 . The ability to detect low levels of parasitaemia is important for predicting clinical relapses, as parasitaemia can increase 20-fold over a 48-hour cycle period. These data are based on measurements in healthy volunteers (controlled human infection models) who were infected at a defined time point with a known number or parasites, and in whom the asymptomatic parasite reproduction was monitored by quantitative PCR up to the point at which the individual received rescue treatment 91 .
In hyperendemic areas (with year-round disease transmission), often many children and adults are asymptomatic carriers of the parasite. In these individuals, the immune system maintains parasites at equilibrium levels in a ‘tug-of-war’. However, parasitaemia in asymptomatic carriers can be extremely high, with reports of levels as high as 50,000 parasites per microlitre in a study of asymptomatic pregnant women (range: 80–55,400 parasites per microlitre) 92 . In addition to the obvious risks for such people, they represent a reservoir for infecting mosquitoes, leading to continued transmission. In clinical studies, the parasitaemia of asymptomatic carriers can be monitored using PCR-based methods, which can detect as few as 22 parasites per millilitre 93 . However, the detection of low-level parasitaemia in low-resource settings requires advanced technology. Loop-mediated isothermal amplification (LAMP) 94 is one promising approach. This type of PCR is fast (10 9 -fold amplification in 1 hour) and does not require thermal cycling, which reduces the requirement for expensive hardware. Versions of this method that do not require electricity are being developed 95 . Nucleic acid-based techniques such as LAMP and PCR-based methods also have the advantage that they can be used to detect multiple pathogens simultaneously and, in theory, identify drug-resistant strains 96 . This approach enables the accurate diagnosis of which Plasmodium spp. is involved, and in the future could lead to the development of multiplexed diagnostics that enable differential diagnosis of the causative pathogens (including bacteria and viruses) in patients who present with fever 97 .
RDTs . RDTs are based on the immunological detection of parasite antigens (such as lactate dehydrogenase (LDH) or histidine-rich protein 2) in the blood, have sensitivities comparable to that of light microscopy examination and have the advantage that they do not require extensive training of the user. These tests provide rapid diagnosis at a point-of-care level in resource-limited settings and can, therefore, substantially improve malaria control. However, occasionally, false-positive results from RDTs can be problematic because they could lead to the wrong perception that antimalarial medicines are ineffective. False-negative test results have been reportedly caused by pfhrp2 gene deletions in P. falciparum strains in South America 98 – 103 . Current data indicate that LDH-targeting RDTs are less sensitive for P. vivax than for P. falciparum 104 , and limited information on the sensitivity of these tests for the rarer species, such as P. ovale or P. malariae , is available. RDTs also offer a great opportunity to track malaria epidemiology; photos taken with mobile phones of the results of the tests can be uploaded to databases (even using cloud-based data architecture 105 ) and provide an automated collection of surveillance data 106 .
Prevention in vulnerable populations
The prevention of Plasmodium spp. infection can be accomplished by different means: vector control, chemoprevention and vaccines. Mosquito (vector) control methods include the following (from the broadest to the most targeted): the widespread use of insecticides, such as DDT campaigns; the use of larvicides; the destruction of breeding grounds (that is, draining marshes and other breeding reservoirs); indoor residual spraying with insecticides (that is, the application of residual insecticide inside dwellings, on walls, curtains or other surfaces); and the use of insecticide-treated bed nets. The use of endectocides has also been proposed; these drugs, such as ivermectin, kill or reduce the lifespan of mosquitoes that feed on individuals who have taken them 107 . However, this approach is still experimental; individuals would be taking drugs that have no direct benefit to themselves (as they do not directly prevent human illness), and so the level of safety data required for the registration of endectocides for this purpose will need to be substantial. Vector control approaches differ in terms of their efficacy, costs and the extent of their effect on the environment. Targeted approaches such as insecticide-treated bed nets have had a strong effect. Chemoprevention is an effective strategy that has been used to reduce malaria incidence in campaigns of seasonal malaria chemoprevention, in intermittent preventive treatment for children and pregnant women, and for mass drug administration 108 . Such antimalarials need to have an excellent safety profile as they are given to large numbers of healthy people. Vaccines excel in eradicating disease, but effective malaria vaccines are challenging because — unlike viruses and bacteria, against which effective vaccines have been developed — protist pathogens (such as Plasmodium spp.) are large-genome microorganisms that have evolved highly effective immune evasion strategies (such as encoding dozens or hundreds of cell surface protein variants). Nevertheless, the improved biotechnological arsenal to generate antigens and improved adjuvants could help to overcome these issues.
Vector control measures . The eradication of mosquitoes is no longer considered an option to eliminate malaria; however, changing the capacity of the vector reservoir has substantial effects on malaria incidence. Long-lasting insecticide-treated bed nets and indoor residual spraying have been calculated to be responsible for two-thirds of the malaria cases averted in Africa between 2000 and 2015 (Ref. 12 ). Today's favoured and more-focused vector control approach involves the use of fine-mazed, sturdy, long-lasting and wash-proof insecticide-treated bed nets 109 . The fabric of these nets is impregnated with an insecticide that maintains its efficacy after ≥20 standardized laboratory washes, and these nets have a 3-year recommended use. Insects are attracted by the person below the net but are killed as they touch the net. However, the efficacy of bed nets is threatened by several factors, including their inappropriate use (for example, for fishing purposes) and behavioural changes in the mosquitoes, which have also begun to bite during the day 110 . The main problem, however, is the increasing emergence of vector resistance to insecticides, especially pyrethroids 110 and, therefore, new insecticides with different modes of action are urgently needed. New insecticides have been identified by screening millions of compounds from the libraries of agrochemical companies, but even those at the most advanced stages of development are still 5–7 years from deployment (see the International Vector Control Consortium website ( http://www.ivcc.com ) and Ref. 111 ) ( Fig. 5 ). Few of these new insecticides are suitable for application in bed nets (because of high costs or unfavourable chemical properties), but some can be used for indoor residual spraying. New ways of deploying these molecules are also being developed, such as improved spraying technologies 112 , timed release to coincide with seasonal transmission and slow-release polymer-based wall linings 113 , 114 .
The categories of compounds that are currently under study are defined in the first column on the left; compounds belonging to these categories have advanced to phase I trials or later stages. New screening hits (developed by Syngenta, Bayer, Sumitomo and the Innovative Vector Control Consortium (IVCC)) are at early research stages and are not expected to be deployed until 2020–2022. Similarly, species-specific approaches to the biological control of mosquitoes are not expected to move forward before 2025. The main data source for this Figure was the IVCC; for the latest updates visit the IVCC website ( www.ivcc.com ). Note that not all compounds listed on the IVCC website are shown in this Figure. The dates reflect the expected deployment dates. AI, active ingredient; CS, capsule suspension; IRS, indoor residual spray; LLIN, long-lasting insecticidal mosquito net; LLIRS, long-lasting indoor residual spray; LSHTM, London School of Hygiene and Tropical Medicine (UK); PAMVERC, Pan-African Malaria Vector Research Consortium. *Clothianidin and chlorfenapyr.
Genetic approaches, fuelled by advances in the CRISPR–Cas9 gene editing technology, represent an exciting area of development for novel insect control strategies. There are currently two main approaches: population suppression, whereby mosquitoes are modified so that any progeny are sterile; and population alteration, whereby mosquitoes are modified so that the progeny are refractory to Plasmodium spp. infection 115 , 116 . Initial approaches to population suppression involved releasing sterile male insects 117 . These strategies have now been developed further, with the release of male insects carrying a dominant lethal gene that kills their progeny 118 , 119 . Gene drive systems can be used for both population suppression and population alteration. These systems use homing endonucleases, which are microbial enzymes that induce the lateral transfer of an intervening DNA sequence and can, therefore, convert a heterozygote individual into a homozygote. Homing endonucleases have been re-engineered to recognize mosquito genes 120 and can rapidly increase the frequency of desirable traits in a mosquito population 121 . Gene drive systems have now been used in feasibility studies to reduce the size of mosquito populations 122 or to make mosquitoes less able to transmit malaria-causing parasites 123 . Another approach is inspired by the finding that Aedes aegypti mosquitoes (the vector for Dengue, yellow fever and Zika viruses) infected with bacteria of the Wolbachia spp. (a parasite that naturally colonizes numerous species of insects) cannot transmit the Dengue virus to human hosts 124 . Symbiont Wolbachia spp. can be modified to make them deleterious to other parasites in the same host, and progress has been made in finding symbionts that can colonize Anopheles spp. mosquitoes 125 , 126 . Although all of the above approaches are very promising, they are still at a very early stage, and the environmental uncertainties associated with the widespread distribution of such technologies, as well as the complex regulatory requirements, provide additional hurdles that will need to be overcome.
Chemoprotection and chemoprevention . Chemoprotection describes the use of medicines (given at prophylactic doses) to temporarily protect subjects entering an area of high endemicity — historically, tourists and military personnel — and populations at risk from emergent epidemics, but is also being increasingly considered for individuals visiting areas that have recently become malaria-free. Chemoprevention, which is often used in the context of seasonal malaria, describes the use of medicines with demonstrated efficacy that are given regularly to large populations at full treatment doses (as some of the individuals treated will be asymptomatic carriers).
Currently, there are three ‘gold-standard’ alternatives for chemoprotection: daily atovaquone–proguanil, daily doxycycline and weekly mefloquine. Mefloquine is the current mainstay drug used to prevent the spread of multidrug-resistant Plasmodium spp. in the Greater Mekong subregion of Southeast Asia, despite having a ‘black box warning’ for psychiatric adverse events; however, an analysis of pooled data from 20,000 well-studied patients found that this risk was small (<12 cases per 10,000 treatments) 127 . An active search to find new medicines that could be useful in chemoprotection, in particular medicines that can be given weekly or even less frequently, is underway. One interesting possibility is the use of long-acting injectable intramuscular combination chemoprotectants, which, if effective, could easily compete with vaccination, if they provided protection with 3–4 injections per year. Such an approach (called pre-exposure prophylaxis) is being studied for HIV infection (which also poses major challenges to the development of an effective vaccine) 128 and may lead to the development of long-acting injectable drug formulations 129 produced as crystalline nanoparticles (to enhance water solubility) using the milling technique.
Chemoprevention generally refers to seasonal malaria chemoprevention campaigns, which target children <5 years of age 130 . In the Sahel region (the area just south of the Sahara Desert, where there are seasonal rains and a recurrent threat of malaria), seasonal malaria chemoprevention with a combination of sulfadoxine–pyrimethamine plus amodiaquine had a strong effect 131 – 135 , with a >80% reduction in the number of malaria cases among children and a >50% reduction in mortality 136 . Although these campaigns are operationally complex — as the treatment has to be given monthly — >20 million children have been protected between 2015 and 2016, at a cost of ∼ US$1 per treatment. A concern about seasonal malaria chemoprevention is the potential for a rebound effect of the disease. Rebound could occur if children lose immunity to malaria while receiving treatment that is later stopped because they reached the age limit, if campaigns are interrupted because of economic difficulties or social unrest (war), or if drug resistance develops. Owing to the presence of resistant strains, a different approach is needed in African areas south of the Equator 137 , and this led to trials of monthly 3-day courses of ACTs in seasonal chemoprevention 135 ; there is an increasing amount of literature on the impressive efficacy of dihydroartemisinin (DHA)–piperaquine to prevent malaria in high-risk groups 138 . To reduce the potential for the emergence of drug resistance, the WHO good practice standards state that, when possible, drugs used for chemoprevention should differ from the front-line treatment that is used in the same country or region 108 , which emphasizes the need for the development of multiple, new and diverse treatments to provide a wider range of options.
Finally, intermittent preventive treatment is also recommended to protect pregnant women in all malaria-endemic areas 108 ( Box 3 ).
Vaccines . Malaria, along with tuberculosis and HIV infection, is a disease in which all components of the immune response (both cellular, in particular, during the liver stage, and humoral, during the blood stage) are involved yet provide only partial protection, which means that developing an effective vaccine will be a challenge. The fact that adults living in high-transmission malarious areas acquire partial protective immunity indicates that vaccination is a possibility. As a consequence, parasite proteins targeted by natural immunity, such as the circumsporozoite protein (the most prominent surface antigen expressed by sporozoites), proteins expressed by merozoites and parasite antigens exposed on the surface of infected red blood cells 139 have been studied for their potential to be used in vaccine programmes 140 . However, experimental malaria vaccines tend to target specific parasite species and surface proteins, an approach that both restricts their use and provides scope for the emergence of resistance. Sustained exposure to malaria is needed to maintain natural protective immunity, which is otherwise lost within 3–5 years 141 , perhaps as a result of the clearance of circulating antibodies and the failure of memory B cells to develop into long-lived plasma B cells. Controlled human infection models 142 – 144 have started to provide a more precise understanding of the early cytokine and T cell responses in naive subjects, emphasizing the role of regulatory T cells in dampening the response against the parasite, which results in the exhaustion of T cells 145 . Vaccine development is currently focusing on using multiple antigens from different stages of the parasite life cycle. Future work will also need to focus on the nature of the immune response in humans and specifically the factors that lead to diminished T cell responses. New generations of adjuvants are needed, possibly compounds that produce the desired specific response rather than inducing general immune stimulation. This is a challenging area of research, as adjuvants often have a completely different efficacy in humans compared with in preclinical animal models.
Currently, there is no vaccine deployed against malaria. The ideal vaccine should protect against both P. falciparum and P. vivax , with a protective, lasting efficacy of at least 75%. The most advanced candidate is RTS,S (trade name: Mosquirix; developed by GlaxoSmithKline and the Program for Appropriate Technology in Health Malaria Vaccine Initiative), which contains a recombinant protein with parts of the P. falciparum circumsporozoite protein combined with the hepatitis B virus surface antigen and a proprietary adjuvant. RTS,S reduced the number of malaria cases by half in 4,358 children 5–17 months of age during the first year following vaccination 146 , preventing 1,774 cases for every 1,000 children also owing to herd immunity, and had an efficacy of 40% over the entire 48 months of follow-up in children who received four vaccine doses over a 4-year period 147 . The efficacy of RTS,S during the entire follow-up period dropped to 26% when children only received three vaccine doses. The efficacy during the first year in 6–12-week-old children was limited to 33%. Thus, the RTS,S vaccine failed to provide long-term protection. Further studies, as requested by the WHO, will be done in pilot implementations of 720,000 children in Ghana, Kenya and Malawi (240,000 in each country, half of whom will receive the vaccine) before a final policy recommendation is made. However, a vaccine with only partial and short-term efficacy could still be used in the fight against malaria. RTS,S could be combined with chemoprevention to interrupt malaria transmission in low-endemic areas 148 . Thus, vaccines that are unable to prevent Plasmodium spp. infection could be used to prevent transmission (for example, by targeting gametocytes) or used as an additional protective measure in pregnant women.
A large pipeline of vaccine candidates is under evaluation ( Fig. 6 ). These include irradiated sporozoites — an approach that maximizes the variety of antigens exposed 149 — and subunit vaccines, which could be developed into multicomponent, multistage and multi-antigen formulations 150 . Although vaccines are typically designed for children, as the malaria map shrinks, both paediatric and adult populations living in newly malaria-free zones will need protection because they would probably lose any naturally acquired immunity and would, therefore, be more susceptible. Indeed, in recent years, there has been a focus on developing transmission-blocking vaccines to drive malaria elimination. This approach has been labelled altruistic, as vaccination would have no direct benefit for the person receiving it, but it would benefit the community; a regulatory pathway for such a novel approach has been proposed 151 , 152 . The most clinically advanced vaccine candidate that is based on this approach is a conjugate vaccine that targets the female gametocyte marker Pfs25 (Ref. 153 ), and other antigens are being tested preclinically. Monoclonal antibodies are another potential tool to provide protection. Improvements in manufacturing and high-expressing cell lines are helping to overcome the major barrier to the use of monoclonal antibodies (high costs) 154 , and improvements in potency and pharmacokinetics are reducing the volume and frequency of administration 155 . Monoclonal antibodies could be particularly useful to safely provide the relatively short-term protection needed in pregnancy. The molecular basis of the interaction between parasites and the placenta is quite well understood; two phase I trials of vaccines that are based on the VAR2CSA antigen are under way 156 , 157 .
The main data source for this Figure was Ref. 269 . Not all vaccines under development are shown in the Figure. AIMV VLP, Alfalfa mosaic virus virus-like particle; AMA1, apical membrane antigen 1; AMANET, African Malaria Network Trust; ASH, Albert Schweitzer Hospital (Gabon); ChAd63, chimpanzee adenovirus 63; CHUV, Centre Hospitalier Universitaire Vaudois (Switzerland); CNRFP, Centre National de Recherche et de Formation sur le Paludisme (Burkina Faso); CS, circumsporozoite protein; CSP, circumsporozoite protein; EBA, erythrocyte-binding antigen; ee, elimination eradication; EP, electroporation; EPA, Pseudomonas aeruginosa exoprotein A; EVI, European Vaccine Initiative; CVac, chemoprophylaxis vaccine; FhCMB, Fraunhofer Center for Molecular Biotechnology (USA); GSK, GlaxoSmithKline; IP, Institut Pasteur (France); INSERM, Institut National de la Santé et de la Recherche Médicale (France); JHU, Johns Hopkins University (USA); KCMC, Kilimanjaro Christian Medical College (Tanzania); KMRI, Kenyan Medical Research Institute; LSHTM, London School of Hygiene and Tropical Medicine (UK); M3V.Ad.PfCA, multi-antigen, multistage, adenovirus-vectored vaccine expressing Plasmodium falciparum CSP and AMA1 antigens; mAb, monoclonal antibody; ME-TRAP multiple epitope thrombospondin-related adhesion protein; MRCG, Medical Research Council (The Gambia); MSP, merozoite surface protein; MVA, modified vaccinia virus Ankara; MUK, Makerere University Kampala (Uganda); NHRC, Navrongo Health Research Centre (Ghana); NIAID, National Institute of Allergy and Infectious Diseases (USA); NIMR, National Institute for Medical Research (UK); NMRC, Naval Medical Research Center (USA); PAMCPH, pregnancy-associated malaria Copenhagen; PATH, Program for Appropriate Technology in Health; PfAMA1-DiCo, diversity-covering Plasmodium falciparum AMA1; PfCelTOS, Plasmodium falciparum cell-traversal protein for ookinetes and sporozoites; PfPEBS, Plasmodium falciparum pre-erythrocytic and blood stage; PfSPZ, Plasmodium falciparum sporozoite; PfSPZ-GA1, genetically attenuated PfSPZ; pp, paediatric prevention; PRIMALVAC, PRIMVAC project (INSERM); PRIMVAC, recombinant var2CSA protein as vaccine candidate for placental malaria; Pfs25, Plasmodium falciparum 25 kDa ookinete surface antigen; PvCSP, Plasmodium vivax circumsporozoite protein; PvDBP, Plasmodium vivax Duffy-binding protein; Rh or RH, reticulocyte-binding protein homologue; SAPN, self-assembling protein nanoparticle; SSI, Statens Serum Institut (Denmark); U., University; UCAP, Université Cheikh Anta Diop (Senegal); UKT, Institute of Tropical Medicine, University of Tübingen (Germany); USAMMRC, US Army Medical Research and Materiel Command; WEHI, Walter and Eliza Hall Institute of Medical Research (Australia); WRAIR, Walter Reed Army Institute of Research (USA). *Sponsors of late-stage clinical trials. ‡ Pending review or approval by WHO prequalification, or by regulatory bodies who are members or observers of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH).
No single drug is ideal against all Plasmodium spp. or all of the manifestations of the disease that occur in different patient populations. Thus, treatment must be tailored to each situation appropriately 108 , 158 . First, the treatment of uncomplicated malaria and that of severe malaria are distinct. In uncomplicated malaria, the treatment of choice is an oral medicine with high efficacy and a low adverse-effect profile. However, the preferred initial therapy in severe malaria requires rapid onset and includes the parenteral administration of an artemisinin derivative, which can rapidly clear the parasites from the blood, and it is also suitable for those patients who have changes in mental status (such as coma) that make swallowing oral medications impossible. For the treatment of malaria during pregnancy, the options are limited to the drugs that are known to be safe for both the expectant mother and the fetus, and different regimens are needed ( Box 2 ). Different drugs are used for different Plasmodium spp., and the choice is usually driven more by drug resistance frequencies (which are lower in P. vivax , P. ovale , P. malariae and P. knowlesi than in P. falciparum ) than by species differences as such. Thus, chloroquine, with its low cost and excellent safety, is used in most cases of non- P. falciparum malaria, where it remains effective, whereas P. falciparum malaria requires newer medicines that overcome resistance issues. The persistence of P. vivax and P. ovale hypnozoites, even after clearance of the stages that cause symptoms, necessitates additional treatments. Only primaquine targets hypnozoites.
P. falciparum malaria
The mainstay treatments for uncomplicated P. falciparum malaria are ACTs: fixed-dose combinations of two drugs, an artemisinin derivative and a quinine derivative 108 ( Box 4 ; Table 1 ).
Owing to its high lipophilicity, artemisinin itself is not the molecule of choice in any stringent regulatory authority-approved combination. Instead, semisynthetic derivatives are used: namely, DHA (the reduced hemiacetal of the major active metabolite of many artemisinin derivatives), artesunate (a succinate prodrug of DHA that is highly water-soluble) or artemether (a methylether prodrug of DHA).
Quinine has been used in medicine for centuries 159 , but it was only in the mid-20th century that a synthetic form was made and the emerging pharmaceutical and government research sectors delivered the next-generation medicines that built on it. The combination partners of choice are 4-aminoquinolines (for example, amodiaquine, piperaquine and pyronaridine) and amino-alcohols (such as mefloquine or lumefantrine); these molecules are believed to interfere with haemozoin formation. There are now five ACTs that have been approved or are close to approval by the FDA, the European Medicines Agency (EMA) or WHO prequalification ( Figs 7 , 8 ; Table 1 ). In pivotal clinical studies, these combinations have proven extremely effective (achieving an adequate clinical and parasitological response (that is, the absence of parasitaemia at day 28 in >94% of patients; for example, see Ref. 160 ), are well-tolerated (as they have been given to >300 million paediatric patients), are affordable (typically under US$1 per dose) and, thanks to ingenious formulations and packaging, are stable in tropical climate conditions.
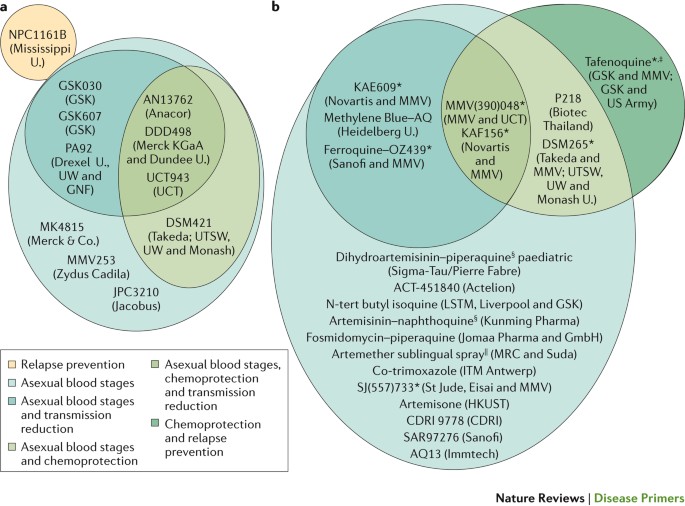
a | Preclinical candidates. b | Compounds or compound combinations that are in clinical development. The multitude of molecules that target only the asexual blood stages reflects the fact that many of these compounds are at an early stage of development, and further assessment of their Target Candidate Profile is still ongoing. KAF156 and KAE609 were discovered in a multiparty collaboration between the Novartis Institute for Tropical Disease (Singapore), the Genomics Institute of the Novartis Research Foundation (GNF; USA), the Swiss Tropical and Public Health Institute, the Biomedical Primate Research Centre (The Netherlands), the Wellcome Trust (UK) and Medicines for Malaria Venture (MMV). DSM265 was discovered through a collaboration involving the University of Texas Southwestern (UTSW; USA), the University of Washington (UW; USA), Monash University (Australia), GlaxoSmithKline (GSK) and MMV. MMV(390)048 was discovered through a collaboration involving the University of Cape Town (UCT; South Africa), the Swiss Tropical and Public Health Institute, Monash University, Syngene and MMV. SJ(557)733 was discovered in a collaboration involving St Jude Children's Research Hospital (USA), Rutgers University (USA), Monash University and MMV. Note that not all compounds are shown in this Figure, and updates can be found on the MMV website ( www.mmv.org) . CDRI, Central Drug Research Institute (India); ITM, Institute of Tropical Medicine; MRC, Medical Research Council; HKUST, Hong Kong University of Science and Technology; U., University. *Part of a combination that aims to be a new single-exposure radical cure (Target Product Profile 1). ‡ Product that targets the prevention of relapse in Plasmodium vivax malaria. § 3-day cure, artemisinin-based combination therapy. || Severe malaria and pre-referral treatment.
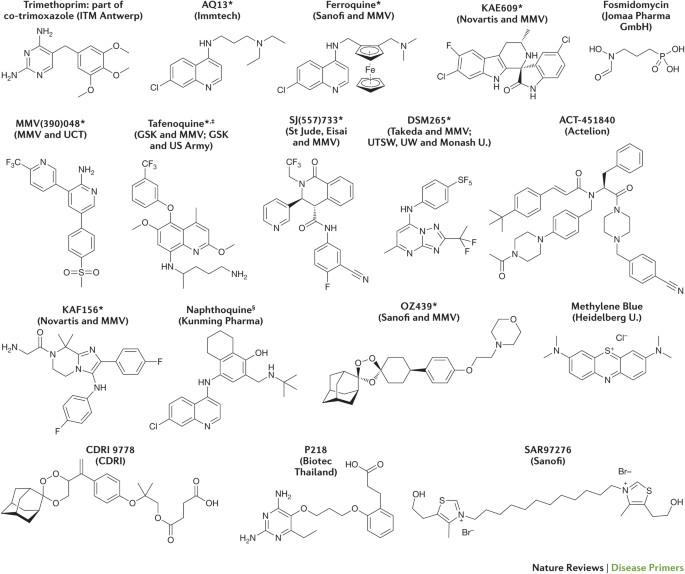
See the Medicines for Malaria Venture (MMV) website ( www.mmv.org ) for updates. CDRI, Central Drug Research Institute (India); GSK, GlaxoSmithKline; ITM, Institute of Tropical Medicine; U., University; UCT, University of Cape Town (South Africa); UTSW, University of Texas Southwestern (USA); UW, University of Washington (USA). *Part of a combination that aims to be a new single-exposure radical cure (Target Product Profile 1). ‡ Product that targets the prevention of relapse in Plasmodium vivax malaria. § 3-day cure, artemisinin-based combination therapy.
Following the results of comprehensive studies in Africa and Asia, the injectable treatment of choice for severe P. falciparum malaria is artesunate 161 – 163 . In the United States, artesunate for intravenous use is available as an Investigational New Drug (IND) through the Centers for Disease Control and Prevention (CDC) malaria hotline and shows efficacies of >90% even in patients who are already unconscious 161 . Sometimes, however, in low-income countries, it is necessary to administer intravenous quinine or quinine while awaiting an artesunate supply. Suppositories of artesunate are in late-stage product development 164 and are already available in Africa as a pre-referral treatment to keep patients alive while they reach a health clinic.
Box 4: Artemisinin
Artemisinin (also known as qinghaosu in China; see the structure) is extracted from the leaves of the Artemisia annua plant.
Youyou Tu was recognized by the 2015 Nobel Prize committee for her contribution to medicine for the discovery of artemisinin, which she achieved by retrieving and following instructions from ancient Chinese texts 247 . Owing to the ability of artemisinin to rapidly reduce parasitaemia and fever, the effect that artemisinin and its derivatives has had on the management of malaria cannot be overstated; since their introduction in the 1970s and their subsequent wider implementation — which was possible particularly owing to the work of Nicholas White and colleagues 248 – 251 — millions of lives have been saved. These drugs seem to be activated by haem-derived iron, and their toxicity is probably mediated through the formation of reactive oxidative radicals 42 . Data indicate that they interfere with phosphatidylinositol-3-phosphate metabolism (which is thought to be involved in the trafficking of haemoglobin to the digestive vacuole 252 ) and provide possible mechanistic insights into the nature of clinically observed artemisinin resistance 253 .
P. vivax malaria
Chloroquine or ACTs are WHO-recommended for uncomplicated P. vivax malaria 108 (although chloroquine is no longer used in several countries, such as Indonesia). As chloroquine-resistant P. vivax is becoming increasingly widespread, particularly in Asia, the use of ACTs is increasing; although only artesunate–pyronaridine is approved for the treatment of blood-stage P. vivax malaria, the other ACTs are also effective and are used off-label. Relapses of P. vivax malaria present a problem in malaria control. Relapse frequencies differ among P. vivax strains; they are high (typically within 3 weeks) in all-year transmission areas, such as Papua New Guinea, but relapse occurs on average after 7 months in areas with a dry or winter season. Some P. vivax strains, such as the Moscow and North Korea strains, are not, in most cases, symptomatic at the time of first infection but become symptomatic only following reactivation of the hypnozoites 165 . Primaquine needs to be administered in addition to the primary treatment to prevent relapse and transmission, which can occur even years after the primary infection. Primaquine treatment, however, requires 14 days of treatment, has gastrointestinal adverse effects in some patients, and is contraindicated in pregnant women and in patients who are deficient in or express low levels of G6PD (as it can cause haemolysis). Tafenoquine 166 , a next-generation 8-aminoquinoline, is currently completing phase III clinical studies. As with patients receiving primaquine, patients receiving tafenoquine will still require an assessment of their G6PD enzyme activity to ensure safe use of the drug and to determine the optimal dose. In phase II studies, tafenoquine was shown to have an efficacy similar to that of primaquine but with a single dose only compared with the 7–14-day treatment with primaquine; higher patient compliance is expected to be a major benefit of a single-dose regimen. The ultimate elimination of P. vivax malaria will be dependent on the availability of safe and effective anti-relapse agents, and is, therefore, a major focus of the drug discovery community.
Drug resistance
The two drugs in ACTs have very different pharmacokinetic profiles in patients. The artemisinin components have a plasma half-life of only a few hours yet can reduce parasitaemia by three-to-four orders of magnitude. By contrast, the 4-aminoquinolines and amino-alcohols have long terminal half-lives (>4 days), providing cure (defined as an adequate clinical and parasitological response) and varying levels of post-treatment prophylaxis. The prolonged half-life of the non-artemisinin component of ACTs has raised concerns in the research community owing to the risk of drug resistance development. However, the effectiveness of the ACTs in rapidly reducing parasitaemia suggests that any emerging resistance has arisen largely as a result of poor clinical practice, including the use of artemisinin derivatives as monotherapy, a lack of patient compliance and substandard medicine quality (including counterfeits); these are all situations in which large numbers of parasites are exposed to a single active molecule 167 . However, resistance to piperaquine 168 and partial resistance to artemisinin 169 (which manifests as a reduced rate of parasite clearance rate rather than a shift in the half-maximal inhibitory concentration (IC 50 )) has been confirmed in the Greater Mekong subregion, as well as resistance to mefloquine and amodiaquine in various parts of the world 170 . Africa has so far been spared, but reports of treatment failure for either artemisinin 171 or ACT 172 in African isolates of P. falciparum have raised concerns. Thus, artemisinin-resistant Plasmodium spp. and insecticide-resistant mosquitoes are major threats to the progress that has been made in reducing the number of malaria-related deaths through current control programmes. It is important to emphasize that progress against malaria has historically been volatile; in many areas, the disease has re-emerged as the efficacy of old drugs has been lost in strains that developed resistance.
Many advances have been made in identifying genetic markers in Plasmodium spp. that correlate with resistance to clinically used drugs ( Table 2 ). These markers enable the research and medical communities to proactively survey parasite populations to make informed treatment choices. Cross-resistance profiles reveal reciprocity between 4-aminoquinolines and amino-alcohols (that is, parasites resistant to one class are also less sensitive to the other). In addition, a drug can exert two opposite selective pressures: one towards the selection of resistant mutants and the other towards the selection of strains that have increased sensitivity to a different drug, a phenomenon known as ‘inverse selective pressure’ (Refs 173 , 174 ). These findings support the introduction of treatment rotation or triple combination therapies as potential future options. Finally, the drug discovery and development pipeline is delivering not only new compounds that have novel modes of action and overcome known resistant strains but also chemicals that have the potential to be effective in a single dose, which could overcome compliance issues. Nevertheless, policymakers need to be on high alert to prevent or rapidly eliminate outbreaks of resistant strains, and to prioritize the development of new treatments.
The drug discovery and development pipeline
The most comprehensive antimalarial discovery portfolio has been developed by the not-for-profit product development partnership Medicines for Malaria Venture (MMV) in collaboration with its partners in both academia and the pharmaceutical industry, with support from donors (mainly government agencies and philanthropic foundations) ( Fig. 7 ). Promising compound series have been identified from three approaches: hypothesis-driven design to develop alternatives to marketed compounds (for example, synthetic peroxides such as ozonides); target-based screening and rational design (for example, screening of inhibitors of P. falciparum dihydroorotate dehydrogenase (PfDHODH)); and phenotypic screening 175 . Phenotypic screening has been the most successful approach to date, in terms of delivering preclinical candidates and identifying — through the sequencing of resistant mutants — novel molecular targets. However, with advances in the understanding of parasite biology and in molecular biology technology, target-based approaches will probably have a substantial role in coming years.
Two combinations — OZ439 (also known as artefenomel) with ferroquine (Sanofi and MMV) and KAF156 with lumefantrine (Novartis and MMV) — are about to begin phase IIb development to test the efficacy of single-dose cure and, in the case of KAF156–lumefantrine, also 2-day or 3-day cures. OZ439 is a fully synthetic peroxide for which sustained plasma exposure is achieved by a single oral dose in humans 176 , 177 ; the hope is that it could replace the three independent doses required for artemisinin derivatives. Ferroquine is a next-generation 4-aminoquinoline without cross-resistance to chloroquine, amodiaquine or piperaquine 178 , 179 . KAF156 is a novel imidazolopiperazine that has an unknown mechanism of action 180 – 182 , but its resistance marker — P. falciparum cyclic amine resistance locus ( pfcarl ) — seems to encode a transporter on the endoplasmic reticulum membrane of the parasite. Interestingly, whereas OZ439 and ferroquine principally affect the asexual blood stages, KAF156 also targets both the asexual liver stage and the sexual gametocyte stage and, therefore, could have an effect on transmission.
Two other compounds, KAE609 (also known as cipargamin 183 , 184 ) and DSM265 (Refs 185 – 188 ), are poised to begin phase IIb and are awaiting decisions on combination partners. KAE609 is a highly potent spiroindolone that provides parasite clearance in patients even more rapidly than peroxides; its assumed mode of action is the inhibition of PfATP4 ( Fig. 3 ), which is encoded by its resistance marker and is a transporter on the parasite plasma membrane that regulates Na + and H + homeostasis. Inhibition of this channel, which was identified through the sequencing of resistant mutants, increases Na + concentrations and pH, resulting in parasite swelling, rigidity and fragility, thereby contributing to host parasite clearance in the spleen in addition to intrinsic parasite killing. In addition, effects on cholesterol levels in the parasite plasma membrane have been noted that are also likely to contribute to parasite killing by leading to an increased rigidity that results in more rapid clearance in vivo 189 . DSM265 is a novel triazolopyrimidine that has both blood-stage and liver-stage activity, and that selectively inhibits PfDHODH ( Fig. 3 ). It was optimized for drug-like qualities from a compound that was identified from a high-throughput screen of a small-molecule library 186 , 190 . DSM265 maintains a serum concentration that is above its minimum parasiticidal concentration in humans for 8 days, and has shown efficacy in both treatment and chemoprotection models in human volunteers in phase Ib trials 185 , 188 .
Within phase I, new compounds are first assessed for safety and pharmacokinetics, and then for efficacy against the asexual blood or liver stages of Plasmodium spp. using a controlled human malaria infection model in healthy volunteers 144 . This model provides a rapid and cost-effective early proof of principle and, by modelling the concentration–response correlation, increases the accuracy of dose predictions for further clinical studies. The 2-aminopyridine MMV(390)048 (also known as MMV048 (Refs 191 , 192 )), SJ(557)733 (also known as (+)-SJ733 (Refs 57 , 193 )) and P218 (Ref. 194 ) are currently progressing through phase I. MMV(390)048 inhibits PfPI(4)K ( Fig. 3 ), and this inhibition affects the asexual liver and blood stages, as well as the sexual gametocyte stage. MMV(390)048 has good exposure in animal models 192 , suggesting that it could potentially be used in a single dose in combination with another drug. SJ(557)733, which is a dihydroisoquinoline, inhibits PfATP4 and is an alternative partner that has a completely different structure from that of KAE609, and it has excellent preclinical safety and development potential. P218 is currently being evaluated for testing in controlled human malaria infection cohorts.
A further eight compounds are undergoing active preclinical development 195 . Of these compounds, four are alternatives to the leading compounds that target established mechanisms: the aminopyrazole PA92 (also known as PA-21A092 (Ref. 196 )) and the thiotriazole GSK030 (also known as GSK3212030A) both target PfATP4; DSM421 (Ref. 197 ) is a triazolopyrimidine alternative to DSM265; and UCT943 (also known as MMV642943) 198 is an alternative to MMV(390)048. Three compounds show novel mechanisms of action or resistance markers: M5717 (also known as DDD498 or DDD107498 (Ref. 199 )) inhibits P. falciparum elongation factor 2 (and, therefore, protein synthesis) and has outstanding efficacy against all parasite life-cycle stages; MMV253 (also known as AZ13721412) 200 is a fast-acting triaminopyrimidine with a V-type ATPase as resistance marker; and AN13762 (also known as AN762) is a novel oxaborole 201 with a novel resistance marker. All of these compounds have been developed through collaborations with MMV.
The eighth compound in active preclinical development, led by Jacobus Pharmaceuticals, is JPC3210 (Ref. 202 ), which is a novel aminocresol that improves upon the historical candidate (WR194965) that was developed by the Walter Reed Army Institute of Research and tested in patients at the time of the development of mefloquine in the 1970s. JPC3210 has an unknown mechanism of action and has potent, long-lasting efficacy in preclinical models, suggesting its potential to be used in a single dose for both treatment and prophylaxis 202 .
Quality of life
Malaria is one among the diseases of poverty. The WHO website states the following: “There is general agreement that poverty not only increases the risk of ill health and vulnerability of people, it also has serious implications for the delivery of effective health-care such as reduced demand for services, lack of continuity or compliance in medical treatment, and increased transmission of infectious diseases” (Ref. 203 ). The socioeconomic burden of malaria is enormous, and although the disease predominantly affects children, it is a serious obstacle to a country's development and economy 204 . Malaria is responsible for annual expenses of billions of euros in some African countries 205 . In many endemic areas, each individual suffers multiple episodes of malaria per year, with each episode causing a loss of school time for children and work time for their parents and guardians. Despite the declining trends in malaria morbidity and mortality, the figures are still disconcertingly high for a disease that is entirely preventable and treatable 16 .
Malaria also has long-term detrimental effects on the non-health-related quality of life of the affected population; it intensifies poverty by limiting education opportunities, as it leads to absenteeism in schools and reduced productivity at work 16 . The effects of acute illness normally drive families to seek urgent attention, which may consist of self-medication, if the disease is familiar to the household. Yet, even an episode of uncomplicated malaria can be potentially fatal, owing to a delay in promptly accessing efficacious antimalarial drugs. As malaria is so familiar to many households, patients — especially children — may be presented late for early diagnosis and treatment in health facilities. Late presentation prolongs morbidity, increases the risk of severe malaria, and deprives the families of income through direct expenses and reduced productivity. Frequent disease episodes experienced in the endemic areas as well as their possible complications can negatively affect child growth and nutrition, shortening the lives of children and family members. The neurological consequences can affect a child's ability to learn and become a self-reliant adult 206 – 208 , as they often occur during an important brain growth phase, when brain areas involved in higher learning (such as planning, decision-making, self-awareness and social sensitivity) mature. Cognitive deficits occurring during the early education years affect the entire family, as they impair the ability of the child to contribute to the well-being of the family as they grow and put additional strain on the parents, who may sometimes have to care for a substantially disabled child and, later, a disabled adult 209 .
The agenda set by the WHO aims for malaria incidence and mortality to decrease by 90% over the next 15 years, with increasing numbers of countries that eliminate the disease 210 . Even if we achieve the ambitious goals set by the WHO, there will still be a child dying of malaria every 10 minutes in 2030. The ACTs are extraordinarily effective, and much of the disease burden could be reduced by the complete deployment and availability of these medicines. There are now two approved ACTs that are specifically designed (taste-masked and sweetened) for paediatric use.
However, the emergence of drug-resistant Plasmodium spp. and insecticide-resistant mosquitoes is a major concern. The first clinical reports of artemisinin resistance came from the Thai–Cambodian border region in the mid-2000s 211 . So far, resistant strains have not spread to Africa, and the severity of the malaria caused by artemisinin-resistant parasites is not different from that of disease caused by wild-type strains. However, if artemisinin derivatives became ineffective, no alternative first-line treatments would be available, as new therapies are still only in phase II clinical trials, and their safety and efficacy will need to be effectively assessed in the field before they can be deployed for widespread clinical use.
Diagnostics
Future diagnostics should address two main issues. First, new diagnostic tests would ideally be non-invasive and not require a blood sample. Many approaches have been piloted, including parasite antigen detection in saliva 212 or urine 213 , the detection of specific volatile chemicals in breath 214 , and direct non-invasive measurements of iron-rich haemozoin in skin blood vessels 215 . Second, diagnostic tests should be able to detect drug-resistant strains directly in the point-of-care setting, rather than in sentinel sites, to provide better treatment and generate more-detailed epidemiological maps 216 . A next-generation amplicon-sequencing method suitable for use in endemic countries would enable the high-throughput detection of genetic mutations in six P. falciparum genes that are associated with resistance to antimalarial drugs, including ACTs, chloroquine and sulfadoxine–pyrimethamine 217 .
Malaria challenges
In addition to the length of the process of discovering and developing new drugs, insecticides and vaccines, in malaria there is the hurdle of the delivery of these new compounds, which first need to obtain approval from all local regulatory authorities. There is a trend for harmonization of the approval requirements among different authorities, with an initiative involving several regional African organizations, for example, to review data on behalf of many countries, similarly to the EMA reviewing files on behalf of all of the European Union countries. These events are paving the way to shorten the time from the end of clinical studies to the day of large-scale deployment, when affected populations will start to reap the benefits.
The move towards elimination and eradication
High-content cellular assays have become available to test inhibitors of transmission and compounds that target hypnozoites 218 , 219 . Discovery efforts for treatment and chemoprotection combinations conform to the malaria Target Product Profiles — a planning tool for therapeutic candidates that is based on FDA guidelines — to ensure that what is delivered has clinical relevance. The MMV has defined 220 and updated 221 Target Candidate Profiles (TCPs), which define the attributes that are required for the ideal medicines and have proven invaluable in guiding single-molecule optimization and decision-making.
The current focus is moving beyond TCP1 (which includes molecules that clear asexual blood-stage parasitaemia); the goal is to deliver compounds that do not simply treat patients and control symptoms but that also have biological activity that disrupts the life cycle of the parasite and hence break the transmission cycle, a step that is necessary in the move towards elimination. Particular areas of interest are anti-relapse agents for P. vivax malaria (TCP3; compounds that target hypnozoites), compounds that kill hepatic schizonts (TCP4) and protect against the onset of symptoms, and gametocytocidal compounds to block transmission (TCP5). Future projects include work on long-lasting endectocides (TCP6), such as ivermectin 107 . The MMV Discovery Portfolio also includes alternative compounds to the clinical frontrunners, molecules with new mechanisms of action (which target, for example, N -myristoyltransferase 222 , coenzyme A biosynthesis 223 , phenylalanyl tRNA synthetase 224 , prolyl 225 tRNA synthetase, plasmepsin V 226 and the Q i site of cytochrome bc 1 (Ref. 227 )) and compounds that seem to be resistance-proof (at least in vitro ).
In conclusion, while much progress has been made towards reducing the burden of malaria, much work remains to be done if these gains are to bring lasting relief to those living under the threat of infection. Without a continued focus on developing new antimalarials and new approaches for diagnosis and vector control, malaria will continue to exert an unacceptable toll on people living in disease endemic areas.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
How to cite this article
Phillips, M. A. et al . Malaria. Nat. Rev. Dis. Primers 3 , 17050 (2017).
Miller, L. H., Ackerman, H. C., Su, X. Z. & Wellems, T. E. Malaria biology and disease pathogenesis: insights for new treatments. Nat. Med. 19 , 156–167 (2013).
CAS PubMed PubMed Central Google Scholar
White, N. J. et al . Malaria. Lancet 383 , 723–735 (2014).
PubMed Google Scholar
Cowman, A. F., Healer, J., Marapana, D. & Marsh, K. Malaria: biology and disease. Cell 167 , 610–624 (2016). References 1–3 comprehensively review malaria biology and the disease.
CAS PubMed Google Scholar
Baker, D. A. Malaria gametocytogenesis. Mol. Biochem. Parasitol. 172 , 57–65 (2010).
Waters, A. P. Epigenetic roulette in blood stream Plasmodium : gambling on sex. PLoS Pathog. 12 , e1005353 (2016).
PubMed PubMed Central Google Scholar
White, N. J. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar. J. 10 , 297 (2011).
Wassmer, S. C. et al . Investigating the pathogenesis of severe malaria: a multidisciplinary and cross-geographical approach. Am. J. Trop. Med. Hyg. 93 , 42–56 (2015). Comprehensively reviews the causes of severe malaria and ongoing research efforts to understand malaria pathophysiology.
Wassmer, S. C. & Grau, G. E. Severe malaria: what's new on the pathogenesis front? Int. J. Parasitol. 47 , 145–152 (2017).
World Health Organization. Severe malaria. Trop. Med. Int. Health 19 (Suppl. 1), 7–131 (2014).
Google Scholar
Dondorp, A. M. & Day, N. P. The treatment of severe malaria. Trans. R. Soc. Trop. Med. Hyg. 101 , 633–634 (2007).
Bernabeu, M. & Smith, J. D. EPCR and malaria severity: the center of a perfect storm. Trends Parasitol. 33 , 295–308 (2017). Reviews the molecular basis of parasite sequestration in the tissues, which leads to the obstruction of the microvasculature and severe disease, and discusses the key role of EPCR in these processes.
Bhatt, S. et al . The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526 , 207–211 (2015). Attempts to identify the relative contribution of different antimalaria measures in reducing the number of malaria cases over the 2000–2015 period.
Mitchell, S. N. et al . Mosquito biology. Evolution of sexual traits influencing vectorial capacity in anopheline mosquitoes. Science 347 , 985–988 (2015).
Sinka, M. E. et al . The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic precis. Parasit. Vectors 3 , 117 (2010).
World Health Organization. Eliminating malaria: learning from the past, looking ahead. WHO http://www.path.org/publications/files/MCP_rbm_pi_rpt_8.pdf (2011).
World Health Organization. World malaria report 2015. WHO http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/ (2015).
Florens, L. et al . A proteomic view of the Plasmodium falciparum life cycle. Nature 419 , 520–526 (2002).
Gardner, M. J. et al . Genome sequence of the human malaria parasite Plasmodium falciparum . Nature 419 , 498–511 (2002). Reports for the first time the P. falciparum genome, which has formed the basis for research into the molecular basis of pathogenesis and parasite biology; a modern-day understanding of the disease would not be possible without this groundbreaking work.
Winzeler, E. A. Advances in parasite genomics: from sequences to regulatory networks. PLoS Pathog. 5 , e1000649 (2009).
Gething, P. W. et al . Mapping Plasmodium falciparum mortality in Africa between 1990 and 2015. N. Engl. J. Med. 375 , 2435–2445 (2016).
Maitland, K. Severe malaria in African children — the need for continuing investment. N. Engl. J. Med. 375 , 2416–2417 (2016).
Miller, L. H., Mason, S. J., Clyde, D. F. & McGinniss, M. H. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy . N. Engl. J. Med. 295 , 302–304 (1976). Describes the discovery that led to a molecular understanding of why most Africans are resistant to infection by P. vivax , thereby explaining the limited penetration of P. vivax in Africa.
Mercereau-Puijalon, O. & Menard, D. Plasmodium vivax and the Duffy antigen: a paradigm revisited. Transfus. Clin. Biol. 17 , 176–183 (2010).
Howes, R. E. et al . Plasmodium vivax transmission in Africa. PLoS Negl. Trop. Dis. 9 , e0004222 (2015).
Sutherland, C. J. et al . Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J. Infect. Dis. 201 , 1544–1550 (2010).
Cox-Singh, J. et al . Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin. Infect. Dis. 46 , 165–171 (2008).
Singh, B. et al . A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 363 , 1017–1024 (2004). Describes the discovery that P. knowlesi , which was previously thought to primarily infect macaques, accounted for over half of the cases of malaria in their study in the Kapit district (Malaysia). Demonstrates for the first time that P. knowlesi should be considered as an emerging infectious disease in humans. Whether transmission via mosquitoes was occurring from monkey to man or from man to man remained an open question.
Brock, P. M. et al . Plasmodium knowlesi transmission: integrating quantitative approaches from epidemiology and ecology to understand malaria as a zoonosis. Parasitology 143 , 389–400 (2016).
Imwong, M., Nakeesathit, S., Day, N. P. & White, N. J. A review of mixed malaria species infections in anopheline mosquitoes. Malar. J. 10 , 253 (2011).
Ginouves, M. et al . Frequency and distribution of mixed Plasmodium falciparum – vivax infections in French Guiana between 2000 and 2008. Malar. J. 14 , 446 (2015).
Srisutham, S. et al . Four human Plasmodium species quantification using droplet digital PCR. PLoS ONE 12 , e0175771 (2017).
Armed Forces Health Surveillance Branch. Update: malaria, U. S. Armed Forces, 2016. MSMR 24 , 2–7 (2017).
IISS: International Institute for Strategic Studies. Armed Conflict Survey 2016 (IISS, 2016).
Mueller, I. et al . Natural acquisition of immunity to Plasmodium vivax: epidemiological observations and potential targets. Adv. Parasitol. 81 , 77–131 (2013).
Ataide, R., Mayor, A. & Rogerson, S. J. Malaria, primigravidae, and antibodies: knowledge gained and future perspectives. Trends Parasitol. 30 , 85–94 (2014).
Desai, M. et al . Epidemiology and burden of malaria in pregnancy. Lancet Infect. Dis. 7 , 93–104 (2007).
McGready, R. et al . Adverse effects of falciparum and vivax malaria and the safety of antimalarial treatment in early pregnancy: a population-based study. Lancet Infect. Dis. 12 , 388–396 (2012).
Cohen, C. et al . Increased prevalence of severe malaria in HIV-infected adults in South Africa. Clin. Infect. Dis. 41 , 1631–1637 (2005).
Mulu, A. et al . Epidemiological and clinical correlates of malaria–helminth co-infections in southern Ethiopia. Malar. J. 12 , 227 (2013).
Gwamaka, M. et al . Iron deficiency protects against severe Plasmodium falciparum malaria and death in young children. Clin. Infect. Dis. 54 , 1137–1144 (2012).
Neuberger, A., Okebe, J., Yahav, D. & Paul, M. Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst. Rev. 2 , CD006589 (2016).
Tilley, L., Straimer, J., Gnadig, N. F., Ralph, S. A. & Fidock, D. A. Artemisinin action and resistance in Plasmodium falciparum . Trends Parasitol. 32 , 682–696 (2016). Comprehensively reviews the emerging threat of artemisinin resistance, covering what is known about the mechanism of action of the drug and the molecular basis of artemisinin resistance.
Woodrow, C. J. & White, N. J. The clinical impact of artemisinin resistance in Southeast Asia and the potential for future spread. FEMS Microbiol. Rev. 41 , 34–48 (2017).
Menard, D. et al . A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N. Engl. J. Med. 374 , 2453–2464 (2016).
Imwong, M. et al . The spread of artemisinin-resistant Plasmodium falciparum in the greater Mekong subregion: a molecular epidemiology observational study. Lancet Infect. Dis. 17 , 491–497 (2017).
Paul, A. S., Egan, E. S. & Duraisingh, M. T. Host–parasite interactions that guide red blood cell invasion by malaria parasites. Curr. Opin. Hematol. 22 , 220–226 (2015). Reviews the molecular basis of parasite invasion.
Lim, C. et al . Reticulocyte preference and stage development of Plasmodium vivax isolates. J. Infect. Dis. 214 , 1081–1084 (2016).
Boddey, J. A. & Cowman, A. F. Plasmodium nesting: remaking the erythrocyte from the inside out. Annu. Rev. Microbiol. 67 , 243–269 (2013).
Spillman, N. J., Beck, J. R. & Goldberg, D. E. Protein export into malaria parasite-infected erythrocytes: mechanisms and functional consequences. Annu. Rev. Biochem. 84 , 813–841 (2015). Reviews the biology associated with red blood cell remodelling upon parasite invasion.
Phillips, M. A. in Neglected Diseases and Drug Discovery (eds Palmer, M. & Wells, T. N. C. ) 65–87 (RCS Publishing, 2011).
Istvan, E. S. et al . Validation of isoleucine utilization targets in Plasmodium falciparum . Proc. Natl Acad. Sci. USA 108 , 1627–1632 (2011).
Wunderlich, J., Rohrbach, P. & Dalton, J. P. The malaria digestive vacuole. Front. Biosci. (Schol. Ed.) 4 , 1424–1448 (2012).
Chugh, M. et al . Protein complex directs hemoglobin-to-hemozoin formation in Plasmodium falciparum . Proc. Natl Acad. Sci. USA 110 , 5392–5397 (2013).
Sigala, P. A. & Goldberg, D. E. The peculiarities and paradoxes of Plasmodium heme metabolism. Annu. Rev. Microbiol. 68 , 259–278 (2014).
Spillman, N. J. et al . Na + regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials. Cell Host Microbe 13 , 227–237 (2013). Identifies the protein target of one of the key new antimalarials that is in clinical development.
Spillman, N. J. & Kirk, K. The malaria parasite cation ATPase PfATP4 and its role in the mechanism of action of a new arsenal of antimalarial drugs. Int. J. Parasitol. Drugs Drug Resist. 5 , 149–162 (2015).
Jimenez-Diaz, M. B. et al . (+)-SJ733, a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance of Plasmodium . Proc. Natl Acad. Sci. USA 111 , E5455–E5462 (2014).
McNamara, C. W. et al . Targeting Plasmodium PI(4)K to eliminate malaria. Nature 504 , 248–253 (2013). Identifies an important new target for drug discovery.
Rosenberg, R., Wirtz, R. A., Schneider, I. & Burge, R. An estimation of the number of malaria sporozoites ejected by a feeding mosquito. Trans. R. Soc. Trop. Med. Hyg. 84 , 209–212 (1990).
Sinnis, P. & Zavala, F. The skin: where malaria infection and the host immune response begin. Semin. Immunopathol. 34 , 787–792 (2012).
Frischknecht, F. et al . Imaging movement of malaria parasites during transmission by Anopheles mosquitoes. Cell. Microbiol. 6 , 687–694 (2004).
Zheng, H., Tan, Z. & Xu, W. Immune evasion strategies of pre-erythrocytic malaria parasites. Mediators Inflamm. 2014 , 362605 (2014).
Duraisingh, M. T. & Horn, D. Epigenetic regulation of virulence gene expression in parasitic protozoa. Cell Host Microbe 19 , 629–640 (2016).
Smith, J. D. The role of PfEMP1 adhesion domain classification in Plasmodium falciparum pathogenesis research. Mol. Biochem. Parasitol. 195 , 82–87 (2014).
Dobbs, K. R. & Dent, A. E. Plasmodium malaria and antimalarial antibodies in the first year of life. Parasitology 143 , 129–138 (2016).
Fowkes, F. J., Boeuf, P. & Beeson, J. G. Immunity to malaria in an era of declining malaria transmission. Parasitology 143 , 139–153 (2016).
Teo, A., Feng, G., Brown, G. V., Beeson, J. G. & Rogerson, S. J. Functional antibodies and protection against blood-stage malaria. Trends Parasitol. 32 , 887–898 (2016).
Marsh, K. & Kinyanjui, S. Immune effector mechanisms in malaria. Parasite Immunol. 28 , 51–60 (2006).
Parroche, P. et al . Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc. Natl Acad. Sci. USA 104 , 1919–1924 (2007).
Karunaweera, N. D., Grau, G. E., Gamage, P., Carter, R. & Mendis, K. N. Dynamics of fever and serum levels of tumor necrosis factor are closely associated during clinical paroxysms in Plasmodium vivax malaria. Proc. Natl Acad. Sci. USA 89 , 3200–3203 (1992).
Vijaykumar, M., Naik, R. S. & Gowda, D. C. Plasmodium falciparum glycosylphosphatidylinositol-induced TNF-α secretion by macrophages is mediated without membrane insertion or endocytosis. J. Biol. Chem. 276 , 6909–6912 (2001).
Wijesekera, S. K., Carter, R., Rathnayaka, L. & Mendis, K. N. A malaria parasite toxin associated with Plasmodium vivax paroxysms. Clin. Exp. Immunol. 104 , 221–227 (1996).
Hosseini, S. M. & Feng, J. J. How malaria parasites reduce the deformability of infected red blood cells. Biophys. J. 103 , 1–10 (2012).
Gillrie M. R. et al . Thrombin cleavage of Plasmodium falciparum erythrocyte membrane protein 1 inhibits cytoadherence. mBio 7 e01120-16 (2016).
Bernabeu, M. et al . Severe adult malaria is associated with specific PfEMP1 adhesion types and high parasite biomass. Proc. Natl Acad. Sci. USA 113 , E3270–E3279 (2016).
Fox, L. L. et al . Histidine-rich protein 2 plasma levels predict progression to cerebral malaria in Malawian children with Plasmodium falciparum infection. J. Infect. Dis. 208 , 500–503 (2013).
Seydel, K. B. et al . Brain swelling and death in children with cerebral malaria. N. Engl. J. Med. 372 , 1126–1137 (2015).
Lopez, C., Saravia, C., Gomez, A., Hoebeke, J. & Patarroyo, M. A. Mechanisms of genetically-based resistance to malaria. Gene 467 , 1–12 (2010).
Piel, F. B. The present and future global burden of the inherited disorders of hemoglobin. Hematol. Oncol. Clin. North Am. 30 , 327–341 (2016).
Elguero, E. et al . Malaria continues to select for sickle cell trait in Central Africa. Proc. Natl Acad. Sci. USA 112 , 7051–7054 (2015).
Kwiatkowski, D. P. How malaria has affected the human genome and what human genetics can teach us about malaria. Am. J. Hum. Genet. 77 , 171–192 (2005).
Cheng, Y. et al . Plasmodium vivax GPI-anchored micronemal antigen (PvGAMA) binds human erythrocytes independent of Duffy antigen status. Sci. Rep. 6 , 35581 (2016).
Gunalan, K. et al . Role of Plasmodium vivax Duffy-binding protein 1 in invasion of Duffy-null Africans. Proc. Natl Acad. Sci. USA 113 , 6271–6276 (2016).
Guindo, A., Fairhurst, R. M., Doumbo, O. K., Wellems, T. E. & Diallo, D. A. X-Linked G6PD deficiency protects hemizygous males but not heterozygous females against severe malaria. PLoS Med. 4 , e66 (2007).
Cholera, R. et al . Impaired cytoadherence of Plasmodium falciparum -infected erythrocytes containing sickle hemoglobin. Proc. Natl Acad. Sci. USA 105 , 991–996 (2008).
Fairhurst, R. M. et al . Abnormal display of PfEMP-1 on erythrocytes carrying haemoglobin C may protect against malaria. Nature 435 , 1117–1121 (2005). References 84 and 86 identify key genetic traits that are associated with protection against P. falciparum malaria.
World Health Organization. Malaria: diagnostic testing. WHO http://www.who.int/malaria/areas/diagnosis/en/ (2016).
Worldwide Antimalarial Resistance Network (WWARN) AL Dose Impact Study Group. The effect of dose on the antimalarial efficacy of artemether–lumefantrine: a systematic review and pooled analysis of individual patient data. Lancet Infect. Dis. 15 , 692–702 (2015). Describes data from the Worldwide Antimalarial Resistance Network (WWARN), which has set up computational tools that measure parasite reduction from human data; the WWARN also collects information on emerging resistance.
Joanny, F., Lohr, S. J., Engleitner, T., Lell, B. & Mordmuller, B. Limit of blank and limit of detection of Plasmodium falciparum thick blood smear microscopy in a routine setting in Central Africa. Malar. J. 13 , 234 (2014).
Azikiwe, C. C. et al . A comparative laboratory diagnosis of malaria: microscopy versus rapid diagnostic test kits. Asian Pac. J. Trop. Biomed. 2 , 307–310 (2012).
McCarthy, J. S. et al . A pilot randomised trial of induced blood-stage Plasmodium falciparum infections in healthy volunteers for testing efficacy of new antimalarial drugs. PLoS ONE 6 , e21914 (2011). Describes the use of the human blood-stage challenge model for drug testing. This model has gone on to be an important tool for the early evaluation of the clinical efficacy of new antimalarial compounds.
Phiri, K. et al . Parasitological clearance rates and drug concentrations of a fixed dose combination of azithromycin–chloroquine in asymptomatic pregnant women with Plasmodium Falciparum parasitemia: an open-label, non-comparative study in sub-Saharan Africa. PLoS ONE 11 , e0165692 (2016).
Imwong, M. et al . Numerical distributions of parasite densities during asymptomatic malaria. J. Infect. Dis. 213 , 1322–1329 (2016).
Notomi, T. et al . Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28 , E63 (2000).
Sema, M. et al . Evaluation of non-instrumented nucleic acid amplification by loop-mediated isothermal amplification (NINA-LAMP) for the diagnosis of malaria in northwest Ethiopia. Malar. J. 14 , 44 (2015).
Pholwat, S. et al . The malaria TaqMan array card includes 87 assays for Plasmodium falciparum drug resistance, identification of species, and genotyping in a single reaction. Antimicrob. Agents Chemother. 61 e00110-17 (2017).
Foundation for Innovative New Diagnostics. Acute febrile syndrome strategy. FiND http://r4d.dfid.gov.uk/PDF/Outputs/FIND/0031-FIND-NMFI-document-print-inhouse.pdf (2012).
Gamboa, D. et al . A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3 : implications for malaria rapid diagnostic tests. PLoS ONE 5 , e8091 (2010).
Akinyi, S. et al . Multiple genetic origins of histidine-rich protein 2 gene deletion in Plasmodium falciparum parasites from Peru. Sci. Rep. 3 , 2797 (2013).
Cheng, Q. et al . Plasmodium falciparum parasites lacking histidine-rich protein 2 and 3: a review and recommendations for accurate reporting. Malar. J. 13 , 283 (2014).
Bharti, P. K. et al . Prevalence of pfhrp2 and/or pfhrp3 gene deletion in Plasmodium falciparum population in eight highly endemic states in India. PLoS ONE 11 , e0157949 (2016).
Deme, A. B. et al . Analysis of pfhrp2 genetic diversity in Senegal and implications for use of rapid diagnostic tests. Malar. J. 13 , 34 (2014).
Parr, J. B. et al . Estimation of Plasmodium falciparum transmission intensity in Lilongwe, Malawi, by microscopy, rapid diagnostic testing, and nucleic acid detection. Am. J. Trop. Med. Hyg. 95 , 373–377 (2016).
Mouatcho, J. C. & Goldring, J. P. Malaria rapid diagnostic tests: challenges and prospects. J. Med. Microbiol. 62 , 1491–1505 (2013).
Soti, D. O. et al . Feasibility of an innovative electronic mobile system to assist health workers to collect accurate, complete and timely data in a malaria control programme in a remote setting in Kenya. Malar. J. 14 , 430 (2015).
Scherr, T. F., Gupta, S., Wright, D. W. & Haselton, F. R. Mobile phone imaging and cloud-based analysis for standardized malaria detection and reporting. Sci. Rep. 6 , 28645 (2016).
Ouédraogo, A. L. et al . Efficacy and safety of the mosquitocidal drug ivermectin to prevent malaria transmission after treatment: a double-blind, randomized, clinical trial. Clin. Infect. Dis. 60 , 357–365 (2015).
World Health Organization. Guidelines for the treatment of malaria, 3rd edn. WHO http://apps.who.int/iris/bitstream/10665/162441/1/9789241549127_eng.pdf (2015).
Chanda, E., Remijo, C. D., Pasquale, H., Baba, S. P. & Lako, R. L. Scale-up of a programme for malaria vector control using long-lasting insecticide-treated nets: lessons from South Sudan. Bull. World Health Organ. 92 , 290–296 (2014).
Ojuka, P. et al . Early biting and insecticide resistance in the malaria vector Anopheles might compromise the effectiveness of vector control intervention in southwestern Uganda. Malar. J. 14 , 148 (2015).
Hemingway, J. et al . Averting a malaria disaster: will insecticide resistance derail malaria control? Lancet 387 , 1785–1788 (2016). Discusses key issues relating to the continued control of the mosquito that transmits malaria.
Knapp, J., Macdonald, M., Malone, D., Hamon, N. & Richardson, J. H. Disruptive technology for vector control: the Innovative Vector Control Consortium and the US Military join forces to explore transformative insecticide application technology for mosquito control programmes. Malar. J. 14 , 371 (2015).
Sibanda, M. & Focke, W. Development of an insecticide impregnated polymer wall lining for malaria vector control. Malar. J. 13 (Suppl. 1), 80 (2014).
Kruger, T., Sibanda, M. M., Focke, W. W., Bornman, M. S. & de Jager, C. Acceptability and effectiveness of a monofilament, polyethylene insecticide-treated wall lining for malaria control after six months in dwellings in Vhembe District, Limpopo Province, South Africa. Malar. J. 14 , 485 (2015).
Burt, A. Heritable strategies for controlling insect vectors of disease. Phil. Trans. R. Soc. B 369 , 20130432 (2014).
Committee on Gene Drive Research in Non-Human Organisms. Recommendations for Responsible Conduct. Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty and Aligning Research with Public Values (National Academies Press, 2016).
Oliva, C. F. et al . Current status and future challenges for controlling malaria with the sterile insect technique: technical and social perspectives. Acta Trop. 132 , S130–S139 (2014).
Bourtzis, K., Lees, R. S., Hendrichs, J. & Vreysen, M. J. More than one rabbit out of the hat: radiation, transgenic and symbiont-based approaches for sustainable management of mosquito and tsetse fly populations. Acta Trop. 157 , 115–130 (2016).
Black, W. C. 4th, Alphey, L. & James, A. A. Why RIDL is not SIT. Trends Parasitol. 27 , 362–370 (2011).
Windbichler, N. et al . A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature 473 , 212–215 (2011).
Esvelt, K. M., Smidler, A. L., Catteruccia, F. & Church, G. M. Concerning RNA-guided gene drives for the alteration of wild populations. eLife 3 , e03401 (2014).
Hammond, A. et al . A CRISPR–Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae . Nat. Biotechnol. 34 , 78–83 (2016).
Gantz, V. M. et al . Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi . Proc. Natl Acad. Sci. USA 112 , E6736–E6743 (2015).
Bull, J. J. & Turelli, M. Wolbachia versus dengue: evolutionary forecasts. Evol. Med. Public Health 2013 , 197–207 (2013).
Wilke, A. B. & Marrelli, M. T. Paratransgenesis: a promising new strategy for mosquito vector control. Parasit. Vectors 8 , 342 (2015).
Wang, S. et al . Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proc. Natl Acad. Sci. USA 109 , 12734–12739 (2012).
Lee, S. J., Ter Kuile, F. O., Price, R. N., Luxemburger, C. & Nosten, F. Adverse effects of mefloquine for the treatment of uncomplicated malaria in Thailand: a pooled analysis of 19, 850 individual patients. PLoS ONE 12 , e0168780 (2017).
Spinner, C. D. et al . HIV pre-exposure prophylaxis (PrEP): a review of current knowledge of oral systemic HIV PrEP in humans. Infection 44 , 151–158 (2016).
Margolis, D. A. & Boffito, M. Long-acting antiviral agents for HIV treatment. Curr. Opin. HIV AIDS 10 , 246–252 (2015).
World Health Organization. WHO policy recommendation: seasonal malaria chemoprevention (SMC) for Plasmodium falciparum malaria control in highly seasonal transmission areas of the Sahel sub-region in Africa. WHO http://www.who.int/malaria/publications/atoz/who_smc_policy_recommendation/en/ (2012).
Cairns, M. et al . Estimating the potential public health impact of seasonal malaria chemoprevention in African children. Nat. Commun. 3 , 881 (2012).
Noor, A. M. et al . Sub-national targeting of seasonal malaria chemoprevention in the Sahelian countries of the Nouakchott initiative. PLoS ONE 10 , e0136919 (2015).
Tagbor, H. et al . Seasonal malaria chemoprevention in an area of extended seasonal transmission in Ashanti, Ghana: an individually randomised clinical trial. Trop. Med. Int. Health 21 , 224–235 (2016).
Tine, R. C. et al . Feasibility, safety and effectiveness of combining home based malaria management and seasonal malaria chemoprevention in children less than 10 years in Senegal: a cluster-randomised trial. Trans. R. Soc. Trop. Med. Hyg. 108 , 13–21 (2014).
Zongo, I. et al . Randomized noninferiority trial of dihydroartemisinin–piperaquine compared with sulfadoxine–pyrimethamine plus amodiaquine for seasonal malaria chemoprevention in Burkina Faso. Antimicrob. Agents Chemother. 59 , 4387–4396 (2015).
Wilson, A. L. & IPTc Taskforce. A systematic review and meta-analysis of the efficacy and safety of intermittent preventive treatment of malaria in children (IPTc). PLoS ONE 6 , e16976 (2011).
Matondo, S. I. et al . High levels of sulphadoxine–pyrimethamine resistance Pfdhfr – Pfdhps quintuple mutations: a cross sectional survey of six regions in Tanzania. Malar. J. 13 , 152 (2014).
Gutman, J., Kovacs, S., Dorsey, G., Stergachis, A. & Ter Kuile, F. O. Safety, tolerability, and efficacy of repeated doses of dihydroartemisinin–piperaquine for prevention and treatment of malaria: a systematic review and meta-analysis. Lancet Infect. Dis. 17 , 184–193 (2017).
Doolan, D. L., Dobano, C. & Baird, J. K. Acquired immunity to malaria. Clin. Microbiol. Rev. 22 , 13–36 (2009).
Hoffman, S. L., Vekemans, J., Richie, T. L. & Duffy, P. E. The march toward malaria vaccines. Am. J. Prev. Med. 49 , S319–S333 (2015).
Keegan, L. T. & Dushoff, J. Population-level effects of clinical immunity to malaria. BMC Infect. Dis. 13 , 428 (2013).
McCarthy, J. S. et al . Experimentally induced blood-stage Plasmodium vivax infection in healthy volunteers. J. Infect. Dis. 208 , 1688–1694 (2013).
Roestenberg, M. et al . Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet 377 , 1770–1776 (2011).
Engwerda, C. R., Minigo, G., Amante, F. H. & McCarthy, J. S. Experimentally induced blood stage malaria infection as a tool for clinical research. Trends Parasitol. 28 , 515–521 (2012).
Wykes, M. N., Horne-Debets, J. M., Leow, C. Y. & Karunarathne, D. S. Malaria drives T cells to exhaustion. Front. Microbiol. 5 , 249 (2014).
RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 386 , 31–45 (2015). Describes a key clinical study that evaluates the efficacy of the only malaria vaccine that is currently at an advanced stage of clinical development.
Penny, M. A. et al . Public health impact and cost-effectiveness of the RTS,S/AS01 malaria vaccine: a systematic comparison of predictions from four mathematical models. Lancet 387 , 367–375 (2015).
Gosling, R. & von Seidlein, L. The future of the RTS, S/AS01 malaria vaccine: an alternative development plan. PLoS Med. 13 , e1001994 (2016). Describes strategies for effectively integrating the RTS,S/AS01 vaccine into malaria control strategies.
Hoffman, S. L. et al . Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J. Infect. Dis. 185 , 1155–1164 (2002).
Draper, S. J. et al . Recent advances in recombinant protein-based malaria vaccines. Vaccine 33 , 7433–7443 (2015).
World Health Organization. WHO Product Development for Vaccines Advisory Committee (PD-VAC) meeting — 2015. WHO http://www.who.int/immunization/research/meetings_workshops/pdvac/en/ (2015).
Nunes, J. K. et al . Development of a transmission-blocking malaria vaccine: progress, challenges, and the path forward. Vaccine 32 , 5531–5539 (2014).
Shimp, R. L. Jr et al . Development of a Pfs25–EPA malaria transmission blocking vaccine as a chemically conjugated nanoparticle. Vaccine 31 , 2954–2962 (2013).
Kelley, B. Industrialization of mAb production technology: the bioprocessing industry at a crossroads. mAbs 1 , 443–452 (2009).
Robbie, G. J. et al . A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob. Agents Chemother. 57 , 6147–6153 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01939899 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02647489 (2016).
Daily, J. P. Malaria 2017: update on the clinical literature and management. Curr. Infect. Dis. Rep. http://dx.doi.org/ 10.1007/s11908-017-0583-8 (2017).
Harrison, N. In celebration of the Jesuit's powder: a history of malaria treatment. Lancet Infect. Dis. 15 , 1143 (2015).
Kinfu, G., Gebre-Selassie, S. & Fikrie, N. Therapeutic efficacy of artemether–lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in northern Ethiopia. Malar. Res. Treat. 2012 , 548710 (2012).
Sinclair, D., Donegan, S., Isba, R. & Lalloo, D. G. Artesunate versus quinine for treating severe malaria. Cochrane Database Syst. Rev. 6 , CD005967 (2012).
Dondorp, A. et al . Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 366 , 717–725 (2005). Describes a key study that demonstrates the efficacy of artesunate for the treatment of malaria and, therefore, supports the clinical use of artesunate.
Dondorp, A. M. et al . Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet 376 , 1647–1657 (2010).
Okebe, J. & Eisenhut, M. Pre-referral rectal artesunate for severe malaria. Cochrane Database Syst. Rev. 5 , CD009964 (2014).
Verhave, J. P. Experimental, therapeutic and natural transmission of Plasmodium vivax tertian malaria: scientific and anecdotal data on the history of Dutch malaria studies. Parasit. Vectors 6 , 19 (2013).
Llanos-Cuentas, A. et al . Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet 383 , 1049–1058 (2014). Describes a key clinical study that demonstrates the efficacy of the only new compound that can prevent P. vivax relapse; tafenoquine and primaquine are the only compounds that have such activity.
White, N. J. Does antimalarial mass drug administration increase or decrease the risk of resistance? Lancet Infect. Dis. 17 , e15–e20 (2017).
Amato, R. et al . Genetic markers associated with dihydroartemisinin–piperaquine failure in Plasmodium falciparum malaria in Cambodia: a genotype–phenotype association study. Lancet Infect. Dis. 17 , 164–173 (2017).
Ariey, F. et al . A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505 , 50–55 (2014).
World Health Organization. World malaria report 2016. WHO http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/ (2016).
Lu, F. et al . Emergence of indigenous artemisinin-resistant Plasmodium falciparum in Africa. N. Engl. J. Med. 376 , 991–993 (2017).
Sutherland, C. J. et al . Pfk13 -independent treatment failure in four imported cases of Plasmodium falciparum malaria treated with artemether–lumefantrine in the United Kingdom. Antimicrob. Agents Chemother. 61 e02382-16 (2017).
Lukens, A. K. et al . Harnessing evolutionary fitness in Plasmodium falciparum for drug discovery and suppressing resistance. Proc. Natl Acad. Sci. USA 111 , 799–804 (2014).
Taylor, A. R. et al . Artemether–lumefantrine and dihydroartemisinin–piperaquine exert inverse selective pressure on Plasmodium falciparum drug sensitivity-associated haplotypes in Uganda. Open Forum Infect. Dis. 4 , ofw229 (2017).
Gamo, F. J. et al . Thousands of chemical starting points for antimalarial lead identification. Nature 465 , 305–310 (2010).
Möhrle, J. J. et al . First-in-man safety and pharmacokinetics of synthetic ozonide OZ439 demonstrates an improved exposure profile relative to other peroxide antimalarials. Br. J. Clin. Pharmacol. 75 , 524–537 (2013).
Phyo, A. P. et al . Antimalarial activity of artefenomel (OZ439), a novel synthetic antimalarial endoperoxide, in patients with Plasmodium falciparum and Plasmodium vivax malaria: an open-label phase 2 trial. Lancet Infect. Dis. 16 , 61–69 (2016). Describes the key phase II clinical study of one of only two new clinical candidates that have reached phase IIb clinical development.
McCarthy, J. S. et al . A phase II pilot trial to evaluate safety and efficacy of ferroquine against early Plasmodium falciparum in an induced blood-stage malaria infection study. Malar. J. 15 469 (2016).
Held, J. et al . Ferroquine and artesunate in African adults and children with Plasmodium falciparum malaria: a phase 2, multicentre, randomised, double-blind, dose-ranging, non-inferiority study. Lancet Infect. Dis. 15 , 1409–1419 (2015).
Leong, F. J. et al . A first-in-human randomized, double-blind, placebo-controlled, single- and multiple-ascending oral dose study of novel imidazolopiperazine KAF156 to assess its safety, tolerability, and pharmacokinetics in healthy adult volunteers. Antimicrob. Agents Chemother. 58 , 6437–6443 (2014).
White, N. J. et al . Antimalarial activity of KAF156 in falciparum and vivax malaria. N. Engl. J. Med. 375 , 1152–1160 (2016).
Kuhen, K. L. et al . KAF156 is an antimalarial clinical candidate with potential for use in prophylaxis, treatment and prevention of disease transmission. Antimicrob. Agents Chemother. 58 , 5060–5067 (2014).
Huskey, S. E. et al . KAE609 (Cipargamin), a new spiroindolone agent for the treatment of malaria: evaluation of the absorption, distribution, metabolism, and excretion of a single oral 300- mg dose of [ 14 C]KAE609 in healthy male subjects. Drug Metab. Dispos. 44 , 672–682 (2016).
White, N. J. et al . Spiroindolone KAE609 for falciparum and vivax malaria. N. Engl. J. Med. 371 , 403–410 (2014).
McCarthy, J. S. et al . Safety, tolerability, pharmacokinetics, and activity of the novel long-acting antimalarial DSM265: a two-part first-in-human phase 1a/1b randomised study. Lancet Infect. Dis. 17 , 626–635 (2017). References 184 and 185 describe key clinical studies that support the efficacy of new antimalarials in the clinical development portfolio.
Phillips, M. A. et al . A long-duration dihydroorotate dehydrogenase inhibitor (DSM265) for prevention and treatment of malaria. Sci. Transl Med . 7 , 296ra111 (2015). Describes the biological activity and product profile of the first inhibitor of PfDHODH to reach clinical development.
Rüeckle, T. et al . A phase IIa proof-of-concept study to assess the efficacy, safety, tolerability and pharmacokinetics of single doses of DSM265 in adult patients with acute, uncomplicated Plasmodium falciparum or vivax malaria mono-infection over a 28-day-extended observation period in Iquitos, Peru. ASTMH http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=2e5199c4-aceb-4d61-8769-342586917c5a&cKey=d7fda50e-36c6-4a28-a76b-96bad2ec308b&mKey=%7bAB652FDF-0111-45C7-A5E5-0BA9D4AF5E12%7d (2015).
Sulyok, M. et al . DSM265 for Plasmodium falciparum chemoprophylaxis: a randomised, double blinded, phase 1 trial with controlled human malaria infection. Lancet Infect. Dis. 17 , 636–644 (2017). Describes the first use of a sporozoite human challenge study to demonstrate the chemopreventive activity of a compound that is under clinical development.
Das, S. et al . Na + influx induced by new antimalarials causes rapid alterations in the cholesterol content and morphology of Plasmodium falciparum . PLoS Pathog. 12 , e1005647 (2016).
Coteron, J. M. et al . Structure-guided lead optimization of triazolopyrimidine-ring substituents identifies potent Plasmodium falciparum dihydroorotate dehydrogenase inhibitors with clinical candidate potential. J. Med. Chem. 54 , 5540–5561 (2011).
Ghidelli-Disse, S. et al . Identification of Plasmodium PI4 kinase as target of MMV390048 by chemoproteomics. Malar. J. 13 , S21 (2014).
Paquet, T. et al . Antimalarial efficacy of MMV390048, an inhibitor of Plasmodium phosphatidylinositol 4-kinase. Sci. Transl Med. 9 eaad9735 (2017).
Ge, J. F. et al . Discovery of novel benzo[ a ]phenoxazine SSJ-183 as a drug candidate for malaria. ACS Med. Chem. Lett. 1 , 360–364 (2010).
Abbat, S., Jain, V. & Bharatam, P. V. Origins of the specificity of inhibitor P218 toward wild-type and mutant PfDHFR: a molecular dynamics analysis. J. Biomol. Struct. Dyn. 33 , 1913–1928 (2015).
Wells, T. N. C., Hooft van Huijsduijnen, R. & Van Voorhis, W. C. Malaria medicines: a glass half full? Nat. Rev. Drug Discov. 14 , 424–442 (2015). Comprehensively reviews the malaria drug discovery pipeline.
Das, S. et al . Rapid reorganization of the parasite plasma membrane in response to a new class of antimalarial drugs. ISMMID http://immid-is.drexelmed.edu/2014_ISMMID_Abstract.pdf (2014).
Phillips, M. A. et al . A triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor with improved drug-like properties for treatment and prevention of malaria. ACS Infect. Dis. 2 , 945–957 (2016).
Le Manach, C. et al . Identification of a potential antimalarial drug candidate from a series of 2-aminopyrazines by optimization of aqueous solubility and potency across the parasite life cycle. J. Med. Chem. 59 , 9890–9905 (2016).
Baragaña, B. et al . A novel multiple-stage antimalarial agent that inhibits protein synthesis. Nature 522 , 315–320 (2015).
Hameed, P. S. et al . Triaminopyrimidine is a fast-killing and long-acting antimalarial clinical candidate. Nat. Commun. 6 , 6715 (2015).
Zhang, Y. K. et al . Synthesis and structure–activity relationships of novel benzoxaboroles as a new class of antimalarial agents. Bioorg. Med. Chem. Lett. 21 , 644–651 (2011).
Chavchich, M. et al . Lead selection of the new aminomethylphenol, JPC-3210 for malaria treatment and prevention. Antimicrob. Agents Chemother. 60 , 3115–3118 (2016).
Basch, P. Textbook of International Health 2nd edn (Oxford Univ. Press, 1999).
Ouattara, A. F. et al . Malaria knowledge and long-lasting insecticidal net use in rural communities of central Cote d’Ivoire. Malar. J. 10 , 288 (2011).
Adetokunbo, O. & Gilles, H. Short Textbook of Public Health, Medicine for the Tropics (BookPower, 2006).
Carter, J. A. et al . Persistent neurocognitive impairments associated with severe falciparum malaria in Kenyan children. J. Neurol. Neurosurg. Psychiatry 76 , 476–481 (2005).
Fernando, S. D., Rodrigo, C. & Rajapakse, S. The ‘hidden’ burden of malaria: cognitive impairment following infection. Malar. J. 9 , 366 (2010).
Kihara, M., Carter, J. A. & Newton, C. R. The effect of Plasmodium falciparum on cognition: a systematic review. Trop. Med. Int. Health 11 , 386–397 (2006).
Holding, P. A. & Snow, R. W. Impact of Plasmodium falciparum malaria on performance and learning: review of the evidence. Am. J. Trop. Med. Hyg. 64 , 68–75 (2001).
World Health Organization. Global technical strategy for Malaria 2016–2030. WHO http://who.int/malaria/areas/global_technical_strategy/en/ (2016).
Noedl, H. et al . Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 359 , 2619–2620 (2008).
Singh, R. et al . Comparison of three PCR-based assays for the non-invasive diagnosis of malaria: detection of Plasmodium parasites in blood and saliva. Eur. J. Clin. Microbiol. Infect. Dis. 33 , 1631–1639 (2014).
Oguonu, T. et al . The performance evaluation of a urine malaria test (UMT) kit for the diagnosis of malaria in individuals with fever in South-east Nigeria: cross-sectional analytical study. Malar. J. 13 , 403 (2014).
Berna, A. Z. et al . Analysis of breath specimens for biomarkers of Plasmodium falciparum infection. J. Infect. Dis. 212 , 1120–1128 (2015).
Lukianova-Hleb, E. et al . Transdermal diagnosis of malaria using vapor nanobubbles. Emerg. Infect. Dis. 21 , 1122–1127 (2015).
World Health Organization. Emergency response to artemisinin resistance in the greater Mekong subregion. Regional framework for action 2013–2015. WHO http://www.who.int/malaria/publications/atoz/9789241505321/en/ (2013).
Rao, P. N. et al . A method for amplicon deep sequencing of drug resistance genes in Plasmodium falciparum clinical isolates from India. J. Clin. Microbiol. 54 , 1500–1511 (2016).
Wells, T. N., Burrows, J. N. & Baird, J. K. Targeting the hypnozoite reservoir of Plasmodium vivax : the hidden obstacle to malaria elimination. Trends Parasitol. 26 , 145–151 (2010). Discusses issues related to targeting the latent stages of P. vivax to address the lack of drug options that are safe in all patients to treat these stages of the parasite.
Chattopadhyay, R. et al . Establishment of an in vitro assay for assessing the effects of drugs on the liver stages of Plasmodium vivax malaria. PLoS ONE 5 , e14275 (2010).
Burrows, J. N., Hooft van Huijsduijnen, R., Möhrle, J. J., Oeuvray, C. & Wells, T. N. C. Designing the next generation of medicines for malaria control and eradication. Malar. J. 12 , 187 (2013). Defines TCPs and Target Product Profiles for malaria.
Burrows, J. N. et al . New developments in anti-malarial target candidate and product profiles. Malar. J. 16 , 26 (2017). Comprehensively analyses the compound properties that are required for the development of effective antimalarials that will cover all species and stages of the disease.
Tate, E. W., Bell, A. S., Rackham, M. D. & Wright, M. H. N -Myristoyltransferase as a potential drug target in malaria and leishmaniasis. Parasitology 141 , 37–49 (2014).
Pett, H. E. et al . Novel pantothenate derivatives for anti-malarial chemotherapy. Malar. J. 14 , 169 (2015).
Kato, N. et al . Diversity-oriented synthesis yields novel multistage antimalarial inhibitors. Nature 538 , 344–349 (2016).
Herman, J. D. et al . The cytoplasmic prolyl-tRNA synthetase of the malaria parasite is a dual-stage target of febrifugine and its analogs. Sci. Transl Med. 7 , 288ra277 (2015).
Russo, I. et al . Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte. Nature 463 , 632–636 (2010).
Nilsen, A. et al . Quinolone-3-diarylethers: a new class of antimalarial drug. Sci. Transl Med. 5 , 177ra137 (2013).
Newton, C. R., Taylor, T. E. & Whitten, R. O. Pathophysiology of fatal falciparum malaria in African children. Am. J. Trop. Med. Hyg. 58 , 673–683 (1998).
Kalanon, M. & McFadden, G. I. Malaria. Plasmodium falciparum and its apicoplast. Biochem. Soc. Trans. 38 , 775–782 (2010). Describes the importance of the apicoplast genome to the malaria parasite and opportunities to target this unique organelle for drug discovery.
Bozdech, Z. et al . The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum . PLoS Biol. 1 , E5 (2003).
Hughes, K. R., Philip, N., Starnes, G. L., Taylor, S. & Waters, A. P. From cradle to grave: RNA biology in malaria parasites. Wiley Interdiscip. Rev. RNA 1 , 287–303 (2010).
Llinas, M., Bozdech, Z., Wong, E. D., Adai, A. T. & DeRisi, J. L. Comparative whole genome transcriptome analysis of three Plasmodium falciparum strains. Nucleic Acids Res. 34 , 1166–1173 (2006).
Ganesan, K. et al . A genetically hard-wired metabolic transcriptome in Plasmodium falciparum fails to mount protective responses to lethal antifolates. PLoS Pathog. 4 , e1000214 (2008).
Hu, G. et al . Transcriptional profiling of growth perturbations of the human malaria parasite Plasmodium falciparum . Nat. Biotechnol. 28 , 91–98 (2010).
Hoo, R. et al . Integrated analysis of the Plasmodium species transcriptome. EBioMedicine 7 , 255–266 (2016).
Caro, F., Ahyong, V., Betegon, M. & DeRisi, J. L. Genome-wide regulatory dynamics of translation in the Plasmodium falciparum asexual blood stages. eLife 3 , e04106 (2014). Describes a comprehensive analysis of the P. falciparum transcriptome over the red blood cell cycle.
PubMed Central Google Scholar
Kirchner, S., Power, B. J. & Waters, A. P. Recent advances in malaria genomics and epigenomics. Genome Med. 8 , 92 (2016). Comprehensively reviews of the role of epigenomics in gene expression in Plasmodium spp.
Lee, M. C. & Fidock, D. A. CRISPR-mediated genome editing of Plasmodium falciparum malaria parasites. Genome Med. 6 , 63 (2014). Reviews genetic approaches to manipulating the P. falciparum genome.
de Koning-Ward, T. F., Gilson, P. R. & Crabb, B. S. Advances in molecular genetic systems in malaria. Nat. Rev. Microbiol. 13 , 373–387 (2015). Reviews genetic approaches to manipulating the Plasmodium spp. genome.
Gomes, A. R. et al . A genome-scale vector resource enables high-throughput reverse genetic screening in a malaria parasite. Cell Host Microbe 17 , 404–413 (2015).
Corey, V. C. et al . A broad analysis of resistance development in the malaria parasite. Nat. Commun. 7 , 11901 (2016).
Allman, E. L., Painter, H. J., Samra, J., Carrasquilla, M. & Llinas, M. Metabolomic profiling of the malaria box reveals antimalarial target pathways. Antimicrob. Agents Chemother. 60 , 6635–6649 (2016).
Manyando, C. et al . Safety of artemether–lumefantrine in pregnant women with malaria: results of a prospective cohort study in Zambia. Malar. J. 9 , 249 (2010).
Yanow, S. K., Gavina, K., Gnidehou, S. & Maestre, A. Impact of malaria in pregnancy as Latin America approaches elimination. Trends Parasitol. 32 , 416–427 (2016).
Harrington, W. E., Morrison, R., Fried, M. & Duffy, P. E. Intermittent preventive treatment in pregnant women is associated with increased risk of severe malaria in their offspring. PLoS ONE 8 , e56183 (2013).
Dellicour, S. et al . First-trimester artemisinin derivatives and quinine treatments and the risk of adverse pregnancy outcomes in Africa and Asia: a meta-analysis of observational studies. PLoS Med. 14 , e1002290 (2017).
Van Voorhis, W. C., Hooft van Huijsduijnen, R. & Wells, T. N. C. Profile of William C. Campbell, Satoshi O®mura, and Youyou Tu, 2015 Nobel Laureates in Physiology or Medicine. Proc. Natl Acad. Sci. USA 112 , 15773–15776 (2015).
White, N. J., Hien, T. T. & Nosten, F. H. A. Brief history of qinghaosu. Trends Parasitol. 31 , 607–610 (2015). Reviews the history of the discovery of artemisinin derivatives.
White, N. J. et al . Averting a malaria disaster. Lancet 353 , 1965–1967 (1999).
White, N. J. & Olliaro, P. L. Strategies for the prevention of antimalarial drug resistance: rationale for combination chemotherapy for malaria. Parasitol. Today 12 , 399–401 (1996).
Hien, T. T. & White, N. J. Qinghaosu. Lancet 341 , 603–608 (1993).
Vaid, A., Ranjan, R., Smythe, W. A., Hoppe, H. C. & Sharma, P. PfPI3K, a phosphatidylinositol-3 kinase from Plasmodium falciparum , is exported to the host erythrocyte and is involved in hemoglobin trafficking. Blood 115 , 2500–2507 (2010).
Mbengue, A. et al . A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature 520 , 683–687 (2015).
Annan, Z. et al . Population genetic structure of Plasmodium falciparum in the two main African vectors. Anopheles gambiae and Anopheles funestus . Proc. Natl Acad. Sci. USA 104 , 7987–7992 (2007).
Josling, G. A. & Llinas, M. Sexual development in Plasmodium parasites: knowing when it's time to commit. Nat. Rev. Microbiol. 13 , 573–587 (2015).
Aly, A. S., Vaughan, A. M. & Kappe, S. H. Malaria parasite development in the mosquito and infection of the mammalian host. Annu. Rev. Microbiol. 63 , 195–221 (2009).
World Health Organization. Climate change and human health. WHO http://www.who.int/globalchange/summary/en/index5.html (2017).
Gething, P. W. et al . Modelling the global constraints of temperature on transmission of Plasmodium falciparum and P. vivax . Parasit. Vectors 4 , 92 (2011).
Weiss, G. E. et al . Revealing the sequence and resulting cellular morphology of receptor–ligand interactions during Plasmodium falciparum invasion of erythrocytes. PLoS Pathog. 11 , e1004670 (2015).
Egan, E. S. et al . Malaria. A forward genetic screen identifies erythrocyte CD55 as essential for Plasmodium falciparum invasion. Science 348 , 711–714 (2015).
Crosnier, C. et al . Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum . Nature 480 , 534–537 (2011). References 260 and 261 identify key receptors involved in P. falciparum invasion.
Volz, J. C. et al . Essential role of the PfRh5/PfRipr/CyRPA complex during Plasmodium falciparum invasion of erythrocytes. Cell Host Microbe 20 , 60–71 (2016). Provides a comprehensive molecular understanding of the role of the receptor basigin in P. falciparum invasion and provides a comprehensive molecular model for the invasion process that highlights the three key steps.
Zenonos, Z. A. et al . Basigin is a druggable target for host-oriented antimalarial interventions. J. Exp. Med. 212 , 1145–1151 (2015).
Srinivasan, P. et al . Binding of Plasmodium merozoite proteins RON2 and AMA1 triggers commitment to invasion. Proc. Natl Acad. Sci. USA 108 , 13275–13280 (2011).
Remarque, E. J., Faber, B. W., Kocken, C. H. & Thomas, A. W. Apical membrane antigen 1: a malaria vaccine candidate in review. Trends Parasitol. 24 , 74–84 (2008).
Yuthavong, Y. et al . Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc. Natl Acad. Sci. USA 109 , 16823–16828 (2012).
Sidhu, A. B., Verdier-Pinard, D. & Fidock, D. A. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science 298 , 210–213 (2002). Describes the identification of the molecular basis for chloroquine resistance.
Lek-Uthai, U. et al . Stronger activity of human immunodeficiency virus type 1 protease inhibitors against clinical isolates of Plasmodium vivax than against those of P. falciparum . Antimicrob. Agents Chemother. 52 , 2435–2441 (2008).
World Health Organization. Rainbow tables. WHO http://www.who.int/immunization/research/development/Rainbow_tables/en/ (2017).
Mishra, N. et al . Emerging polymorphisms in falciparum Kelch 13 gene in northeastern region of India. Malar. J. 15 , 583 (2016).
Spring, M. D. et al . Dihydroartemisinin–piperaquine failure associated with a triple mutant including kelch13 C580Y in Cambodia: an observational cohort study. Lancet Infect. Dis. 15 , 683–691 (2015).
Thuy-Nhien, N. et al . K13-propeller mutations in Plasmodium falciparum populations in malaria endemic regions of Vietnam from 2009 to 2016. Antimicrob. Agents Chemother. 61 , e01578-16 (2017).
Thanh, N. V. et al . Rapid decline in the susceptibility of Plasmodium falciparum to dihydroartemisinin–piperaquine in the south of Vietnam. Malar. J. 16 , 27 (2017).
Sanchez, C. P. et al . Evidence for a pfcrt -associated chloroquine efflux system in the human malarial parasite Plasmodium falciparum . Biochemistry 44 , 9862–9870 (2005).
Valderramos, S. G. & Fidock, D. A. Transporters involved in resistance to antimalarial drugs. Trends Pharmacol. Sci. 27 , 594–601 (2006). Comprehensively reviews transporter mutants that are involved in resistance to the aminoquinoline series of antimalarial drugs (for example, chloroquine).
Borges, S. et al . Genomewide scan reveals amplification of mdr1 as a common denominator of resistance to mefloquine, lumefantrine, and artemisinin in Plasmodium chabaudi malaria parasites. Antimicrob. Agents Chemother. 55 , 4858–4865 (2011).
Humphreys, G. S. et al . Amodiaquine and artemether–lumefantrine select distinct alleles of the Plasmodium falciparum mdr1 gene in Tanzanian children treated for uncomplicated malaria. Antimicrob. Agents Chemother. 51 , 991–997 (2007).
Baliraine, F. N. & Rosenthal, P. J. Prolonged selection of pfmdr1 polymorphisms after treatment of falciparum malaria with artemether–lumefantrine in Uganda. J. Infect. Dis. 204 , 1120–1124 (2011).
Martin, R. E. & Kirk, K. The malaria parasite's chloroquine resistance transporter is a member of the drug/metabolite transporter superfamily. Mol. Biol. Evol. 21 , 1938–1949 (2004).
Ehrhardt, S. et al . Large-scale surveillance of Plasmodium falciparum crt(K76T) in northern Ghana. Antimicrob. Agents Chemother. 51 , 3407–3409 (2007).
Figueiredo, P. et al . Prevalence of pfmdr1 , pfcrt , pfdhfr and pfdhps mutations associated with drug resistance, in Luanda, Angola. Malar. J. 7 , 236 (2008).
Mens, P. F. Ambiguous role of pfcrt K76 in Plasmodium falciparum : a marker of resistance or increased susceptibility. Expert Rev. Anti Infect. Ther. 7 , 409–412 (2009).
Restrepo-Pineda, E., Arango, E., Maestre, A., Rosário, V. E. D. & Cravo, P. Studies on antimalarial drug susceptibility in Colombia, in relation to Pfmdr1 and Pfcrt . Parasitology 135 , 547–553 (2008).
Drew, M. E. et al . Plasmodium food vacuole plasmepsins are activated by falcipains. J. Biol. Chem. 283 , 12870–12876 (2008).
Witkowski, B. et al . A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: a phenotype–genotype association study. Lancet Infect. Dis. 17 , 174–183 (2017).
Download references
Acknowledgements
The authors thank R. Bryant, A. Hill, S. Rees and S. L. Hoffman for their help with the content of Figure 4 and Figure 6 , and S. Duparc for critical reading of the clinical sections of the manuscript.
Author information
Authors and affiliations.
Department of Biochemistry, University of Texas Southwestern Medical Center at Dallas, 5323 Harry Hines Boulevard, Dallas, 75390–9038, Texas, USA
Margaret A. Phillips
Medicines for Malaria Venture, Geneva, Switzerland
Jeremy N. Burrows, Rob Hooft van Huijsduijnen & Timothy N. C. Wells
Tropical Diseases Research Centre, Ndola, Zambia
Christine Manyando
Department of Medicine, Division of Allergy and Infectious Diseases, University of Washington, Center for Emerging and Re-emerging Infectious Diseases, Seattle, Washington, USA
Wesley C. Van Voorhis
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (M.A.P., J.N.B. and W.C.V.V.); Epidemiology (M.A.P. and W.C.V.V.); Mechanisms/pathophysiology (M.A.P.); Diagnosis, screening and prevention (M.A.P., J.N.B., R.H.v.H. and T.N.C.W.); Management (J.N.B., R.H.v.H. and T.N.C.W.); Quality of life (C.M.); Outlook (R.H.v.H. and T.N.C.W.); Overview of Primer (M.A.P.).
Corresponding author
Correspondence to Margaret A. Phillips .
Ethics declarations
Competing interests.
T.N.C.W. is a non-executive director of Kymab in the United Kingdom. Kymab has programmes in malaria that are funded by the Bill & Melinda Gates Foundation. All other authors declare no competing interests.
PowerPoint slides
Powerpoint slide for fig. 1, powerpoint slide for fig. 2, powerpoint slide for fig. 3, powerpoint slide for fig. 4, powerpoint slide for fig. 5, powerpoint slide for fig. 6, powerpoint slide for fig. 7, powerpoint slide for fig. 8, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Phillips, M., Burrows, J., Manyando, C. et al. Malaria. Nat Rev Dis Primers 3 , 17050 (2017). https://doi.org/10.1038/nrdp.2017.50
Download citation
Published : 03 August 2017
DOI : https://doi.org/10.1038/nrdp.2017.50
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Lethal and sublethal impacts of membrane-fed ivermectin are concentration dependent in anopheles coluzzii.
- Monique A. M. Shepherd-Gorringe
- Marie W. Pettit
- Frances M. Hawkes
Parasites & Vectors (2024)
Gene expression analyses reveal differences in children’s response to malaria according to their age
- Kieran Tebben
- Salif Yirampo
- David Serre
Nature Communications (2024)
Histone modification analysis reveals common regulators of gene expression in liver and blood stage merozoites of Plasmodium parasites
- Ashley B. Reers
- Rodriel Bautista
- Evelien M. Bunnik
Epigenetics & Chromatin (2023)
Impact of insecticide resistance on malaria vector competence: a literature review
- Pierre Fongho Suh
- Emmanuel Elanga-Ndille
- Cyrille Ndo
Malaria Journal (2023)
High-throughput analysis of the transcriptional patterns of sexual genes in malaria
- Abel Cruz Camacho
- Neta Regev-Rudzki
Parasites & Vectors (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Home / Essay Samples / Health / Illness / Malaria
Malaria Essay Examples
The impact of malaria disease on africa.
The “Villain in Africa”, otherwise known as Plasmodium or Malaria, is a parasite that affects nearly every continent. This horrific disease kills around one million to two million people each year. The majority of these victims are children in the Saharan parts of Africa. Mosquitos...
The Study Ofthe End of the Malaria-carrying Mosquitoes
Extinction of a species is often thought to be something negative and should be prevented at all costs, but what if we’re talking about the extinction of a “bad” species such as the malaria-carrying mosquitoes, Anopheles gambiae?Malaria is a disease caused by a parasite which...
Insecticides Resistance and Underlying Biological Mechanisms Which Aggravate the Burden of Malaria in Developing Countries
Despite considerable success of malaria control programme in the past, malaria still continues as a major public health problem. The malaria control relies mainly on indoor residual spraying of insecticides, which has become an inspiring fear task due to widespread resistance in malaria vectors, however,...
Crispr Technology as a Solution to Malaria Prevention
Malaria, a disease that is a transmitted person to person through an infected mosquito carrying the gene that allows for the parasite carrying the virus to exist. From a bar graph by Our World In Data, since 2015, about 395, 000 people have died in...
Youyou Tu – a Woman Who Made a Breakthrough in Malaria Treatment
The first women ever to win a Nobel prize in any discipline for China, a brilliant mind, and a crucial discovery in which would ultimately lead to lives saved and a brighter future where we can stand up to Malaria and fight it off. Youyou...
The Correlation Between Food Insecurity and Malaria in Haiti
Over half of Haiti’s population lives in extreme poverty, making it one of the most food insecure countries in the world. Vector-borne diseases that are being spread and transmitted through mosquitos are becoming a public health crisis across the globe. This is especially true in...
Mosquito Borne-diseases and Their Management
Mosquitoes are known as one of the fatal living organisms in the world acting as vectors (living organisms that can transmit infectious diseases between humans or from animals to humans) for different diseases. Their ability to transmit and disseminate disease to humans causes millions of...
Trying to find an excellent essay sample but no results?
Don’t waste your time and get a professional writer to help!
You may also like
- Alcohol Abuse
- Universal Health Care
- Gambling Addiction
- Public Health
- Substance Abuse
- Weight Loss
- Drug Testing
- Childhood Obesity Essays
- Obesity Essays
- Diabetes Essays
- Diabetes Mellitus Essays
- Lung Cancer Essays
- Breast Cancer Essays
- Cancer Essays
- Ebola Virus Essays
- Children With Disabilities Essays
samplius.com uses cookies to offer you the best service possible.By continuing we’ll assume you board with our cookie policy .--> -->